Abstract
Modern subunit vaccines based on homogeneous antigens offer more precise targeting and improved safety compared with traditional whole-pathogen vaccines. However, they are also less immunogenic and require an adjuvant to increase the immunogenicity of the antigen and potentiate the immune response. Unfortunately, few adjuvants have sufficient potency and low enough toxicity for clinical use, highlighting the urgent need for new, potent and safe adjuvants. Notably, a number of natural and synthetic carbohydrate structures have been used as adjuvants in clinical trials, and two have recently been approved in human vaccines. However, naturally derived carbohydrate adjuvants are heterogeneous, difficult to obtain and, in some cases, unstable. In addition, their molecular mechanisms of action are generally not fully understood, partly owing to the lack of tools to elucidate their immune-potentiating effects, thus hampering the rational development of optimized adjuvants. To address these challenges, modification of the natural product structure using synthetic chemistry emerges as an attractive approach to develop well-defined, improved carbohydrate-containing adjuvants and chemical probes for mechanistic investigation. This Review describes selected examples of natural and synthetic carbohydrate-based adjuvants and their application in synthetic self-adjuvanting vaccines, while also discussing current understanding of their molecular mechanisms of action.

Subject terms: Adjuvants, Chemical modification, Carbohydrates, Carbohydrate chemistry, Mechanism of action
Carbohydrate adjuvants potentiate immune responses through diverse mechanisms. This Review highlights carbohydrate-based adjuvants, providing insights into structure–activity relationships and applications in vaccine development, while discussing current knowledge of the mechanisms by which they potentiate and modulate the immune response.
Introduction
Advances in medicine, biotechnology and chemistry and recent insights into immunological mechanisms have enabled current vaccines to be rationally designed and endowed with pure, molecularly defined antigen substructures1. Compared with traditional whole-pathogen vaccines, these subunit vaccines offer advantages in terms of safety and precision, but they are less immunogenic and require the presence of adjuvants for optimal vaccine efficacy. Adjuvants are substances that enhance antigen-specific immune responses by triggering and modulating both the innate and adaptive (acquired) immunity2. They also allow the dose of expensive antigens to be limited, reduce booster immunizations, generate more rapid and durable immune responses, and increase the effectiveness of vaccines in poor responders.
Despite their key role, few sufficiently potent adjuvants with acceptable toxicity for human use are available in licensed vaccines. For more than 70 years, alum (a mixture of diverse aluminium salts) has been the only approved adjuvant in humans and is still one of the most popular in human vaccines. In late 2009, Adjuvant System 04 (AS04), a proprietary combination of alum and the Toll-like receptor 4 (TLR4) ligand monophosphoryl lipid A (MPLA), was approved for the vaccine against human papillomavirus (HPV), Cervarix. However, aluminium adjuvants have relatively low potency and elicit primarily an antibody-mediated T helper 2 (TH2)-type immune response (Box 1), with weak stimulation of cell-mediated immunity. Alum has been shown to act mainly as a delivery system that traps the antigen at the injection site by forming macromolecular aggregates, facilitating its slow release and uptake by antigen-presenting cells3. Nonetheless, the precise mechanism by which aluminium-containing adjuvants enhance the immune response remains poorly understood4. Apart from aluminium salts, the only other licensed adjuvants in human vaccines are oil-in-water emulsions containing squalene (MF59, AS03), in vitro-assembled influenza-virus-like particles (virosomes), and, most recently, the liposome-based Adjuvant System AS01 (ref.5). However, emulsion adjuvants have raised significant concerns owing to their adverse side effects6,7, and their molecular mechanisms of action are not fully defined8. AS01, a liposomal formulation containing MPLA and the saponin natural product QS-21, has only been recently approved for GSK’s malaria (Mosquirix) and shingles (Shingrix) vaccines, benefiting from a synergistic effect of both adjuvants in the early interferon-γ (IFNγ) response that enhanced vaccine immunogenicity9,10.
Therefore, there is still a pressing need for novel, potent and less toxic adjuvants and new formulations for use in subunit vaccines. Classical adjuvant searches focused on improving the strength of the immune response by increasing antibody and/or cytokine production. Current efforts towards enhancing vaccine efficacy centre on the rational development of adjuvants that can elicit optimal, antigen-specific immune responses (responses associated with T helper 1 (TH1) cells or T helper 2 (TH2) cells, see Box 1), including tailored antibody isotype profiles and CD8+ T cell responses11. To identify such adjuvants and select optimal adjuvant–antigen combinations with defined immunological profiles and low toxicity, elucidating their precise pharmacological pathways and molecular mechanisms of action is essential. This mechanistic understanding will, in turn, enable the rational development of future vaccines against various human diseases.
Carbohydrates represent the most widespread class of biomolecules in nature. They play crucial roles in the immune system function and the stimulation of the immune response12 that can be exploited by the chemistry community13. Carbohydrates possess many beneficial properties that make them promising adjuvant candidates, namely, high biocompatibility and tolerability and a strong safety profile14. A variety of natural carbohydrate structures, particularly MPLA and QS-21, have been clinically evaluated as adjuvants and are part of licensed Adjuvant Systems (AS) in human vaccines against HPV (AS04), herpes zoster and malaria (AS01). However, carbohydrate-based immunopotentiators obtained from natural sources are usually difficult to obtain in sufficient quantity, purity and homogeneity. Moreover, although the mechanisms of action of carbohydrate-based adjuvants have been extensively investigated, the molecular bases underlying the adjuvant activity of some of these compounds have not yet been fully elucidated. This is partly owing to the lack of tools to better explore its immune-potentiating effects. This, in turn, has hampered the rational design and development of optimized adjuvants and adjuvant combinations. Furthermore, the complexity and sensitivity of the diverse functionalities of the natural product structure limit the chemical derivatization of the parent compound, leaving limited opportunities for generation of synthetic analogues and structure–activity studies. By contrast, synthetic organic chemistry, including total synthesis and semi-synthetic strategies, offers a more attractive approach to molecularly defined, improved versions of carbohydrate-containing immunostimulants and chemical probes for mechanistic investigation, enabling structural modification of the corresponding natural products with a high level of chemical control.
This Review highlights recent advances in the development of carbohydrate-based adjuvants, including both naturally derived as well as rationally designed, chemically synthesized compounds, and discusses current understanding of the molecular mechanisms of action of the most promising natural and synthetic carbohydrate adjuvants. We focus primarily on adjuvants based on saponin, α-galactosylceramide (α-GalCer), lipopolysaccharide (LPS) and zwitterionic polysaccharide. Polynucleotide (DNA/RNA)-based adjuvants are not discussed here because their immunomodulatory properties rely on the unmethylated cytosine–guanine (CpG) dinucleotides, rather than on the carbohydrate moiety.
Box 1 CD4+ T helper cell subsets.
Naive CD4+ T helper cells interact via their T cell receptors with peptide antigens presented on MHC class II molecules on the antigen-presenting cell surface. Among other factors282,283, activation and differentiation of effector CD4+ T helper cells into distinct subsets depends most predominantly on the nature of the innate immune signals that activated antigen-presenting cells upon antigen encounter, which generates a cytokine milieu that modulates the outcome of the adaptive immune response11,284.
The first subpopulations of effector CD4+ T helper cells discovered were the T helper 1 (TH1) and TH2 subsets285. These are responsible for controlling and eliminating intracellular and extracellular pathogens, respectively285. Adjuvants influence humoral and cell-mediated immune responses by inducing TH1 and TH2 cells to produce TH1-associated or TH2-associated cytokines, thus resulting in TH1 or TH2 immunity. TH1 differentiation is promoted by interferon-γ (IFNγ) and interleukin-12 (IL-12) cytokines, leading to TH1 effector cells, which are best suited for eliminating intracellular pathogens. TH1 cells are characterized by producing IFNγ, a cytokine that activates macrophages, enhancing their microbial killing activity286, and that stimulates production of mouse IgG2a/IgG2b and IgG3 antibody subtypes, which are involved in opsonization and phagocytosis events287. Moreover, IFNγ, together with IL-2, promotes differentiation of CD8+ T lymphocytes into active cytotoxic T cells.
On the other hand, IL-4 induces the differentiation into TH2 effector cells, which are characterized by producing IL-4, IL-5 and IL-13 cytokines288, and are specialized in controlling infections by extracellular parasites. TH2 cytokines stimulate the production of high levels of IgM and non-complement fixing IgG isotypes, such as IgG1 in mice287. Additionally, the TH17 effector cell subset originates from a cytokine milieu containing TGFβ, IL-6 and IL-23 and is characterized by production of IL-17 and IL-22 cytokines. TH17 cells are typically induced in response to extracellular bacteria and fungi and promote production of high-affinity IgG and IgA antibodies, which cooperate with neutrophils and macrophages at barrier tissue sites. Notably, T follicular helper (TFH) lymphocytes are currently considered an activation state of a given effector cell subset, rather than a distinct lineage itself. Thus, TFH cells develop together with TH1, TH2 or TH17 cells to help B cells generate class-switched antibodies289. Finally, regulatory T (Treg) cells promote tolerance towards the recognized antigen, control the immune response and prevent autoimmunity290,291.
Saponin-based adjuvants
Saponins are plant-derived natural products with a range of biological activities that consist of a lipophilic triterpenoid core flanked by one or more oligosaccharide chains (Fig. 1a). While the adjuvant activity of saponins has been widely investigated, including the recently identified Quillaja brasiliensis saponins15, the triterpene glycosides extracted from the bark of the Chilean tree Quillaja saponaria (i.e. QS) have been the primary focus for saponin-based adjuvant research since more than 30 years ago16. Purification by reverse-phase high-performance liquid chromatography (HPLC) of a heterogeneous, adjuvant-active, semi-purified bark extract (i.e. Quil-A) containing more than 20 water-soluble Q. saponaria saponins led to the identification of several QS saponin fractions that elicited humoral and cell-mediated responses, including QS-21, QS-18, QS-17 and QS-7 (ref.17) (Fig. 1a). The main saponin component, QS-18, was found to be highly toxic in mice but saponins QS-7 and QS-21 showed less toxicity. As QS-7 was less abundant, QS-21 was selected and has become the most widely studied saponin adjuvant for the past 25 years18.
Fig. 1. Structures of natural and synthetic QS-based saponin adjuvants and proposed mechanism of action for QS-21-related saponin adjuvants.
a | Structures of saponin natural product adjuvants QS-21, QS-18 and QS-17 derived from the Quillaja saponaria tree17 and summary of structure–adjuvant activity relationships of QS-21 (ref.36). b | Structures of saponin natural product adjuvant QS-7Xyl (ref.17) and summary of QS-7 structure–adjuvant activity relationships29,43. c | Schematic representation of the proposed mechanism of action for QS-21-related saponin adjuvants48. Upon endocytosis, exogenous protein antigens and QS-21 are delivered to dendritic cells (DCs). Following QS-21-mediated disruption of the endosomal membrane, cleaved protein antigens can be further processed into smaller peptide fragments in the cytosol by the proteasome machinery. Degraded peptides are translocated into the endoplasmic reticulum (ER) by transporter molecules, where chaperones facilitate their binding to newly synthesized MHC class I (MHC-I) molecules for vesicular migration through the Golgi to the cell surface. Finally, peptide epitopes exposed on the DC surface in association with MHC-I molecules are presented to naive CD8+ T cells (cross-presentation) through the T cell receptor (TCR). TH, T helper. Part c adapted from ref.47, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), and with permission from ref.53, Elsevier.
QS-21 is not a single compound but a mixture of two isomeric saponins, QS-21-apiose (65% abundance) and QS-21-xylose (35% abundance), that share a glycosylated pseudo-dimeric acyl chain and a branched trisaccharide at the C3 position of the quillaic acid triterpene core, and differ in the terminal sugar of the linear tetrasaccharide that is linked to the C28 carboxyl group of the triterpene19 (Fig. 1a). QS-21 has been the preferred adjuvant in numerous vaccine clinical trials against a variety of cancers18 and infectious diseases20, and vaccine formulations containing QS-21 as an adjuvant have been recently licensed for human use5. QS-21 stimulates both antibody-based and cell-mediated immune responses, eliciting a TH1-biased immune response21 with production of high titres of antibodies (IgG2a and IgG2b, in addition to IgG1), as well as antigen-specific cytotoxic T lymphocytes. However, except its recent approval as part of the AS01 system in GSK’s malaria (Mosquirix)22 and shingles (Shingrix)23 vaccines, the inherent liabilities of QS-21, including scarcity, heterogeneity, hydrolytic instability and dose-limiting toxicity, have limited its clinical advancement as a stand-alone adjuvant.
Structure–activity relationships of QS-21 and synthetic QS variants
To address the inherent issues of QS-21 as an adjuvant and to gain insights into the structural features that are important for activity, a variety of semi-synthetic saponin variants have been developed, yielding important structure–activity relationships (SARs) within the QS saponin family.
One example is the chemical derivatization of the natural product to provide the semi-synthetic saponin adjuvant GPI-0100, which was prepared from QS bark extracts by saponification of the acyl chain, followed by amidation of the branched trisaccharide glucuronic acid with dodecylamine24. Whereas hydrolysis of the acyl chain resulted in deacylated saponins that did not stimulate TH1 immunity or cytotoxic T lymphocyte induction but, rather, TH2 immunity (significant IgG1 antibody levels but poor IgG2a/IgG2b responses)25, replacing the original acyl chain by the dodecylamide at the branched trisaccharide in GPI-0100 restored TH1 immunity and the ability to induce cytotoxic T lymphocyte responses26. Chemical synthesis of the presumably main immunoactive constituents of GPI-0100, based on QS-21 and QS-17/18, confirmed the adjuvant activity of the heterogeneous GPI-0100 adjuvant, albeit at doses five times higher than QS-21 itself27,28. To study further the acylation effect in saponin adjuvant activity, Wang et al. synthesized and immunologically evaluated two C28 pentasaccharide analogues of QS-7 with a single-sugar truncation and showed that acetylation at the 3-O and 4-O positions of the fucosyl unit (Fig. 1b) shifted the immunostimulatory profile from the preferential production of IgG1 antibodies (TH2-biased) to induction of both IgG1 and IgG2a antibody responses (mixed TH1/TH2 immunity)29.
Synthetic chemistry represents a powerful strategy to obtain homogeneous, pure samples of saponin molecules and to develop new saponin analogues with improved properties and therapeutic profiles. Pioneering work by Gin and colleagues led to the first total syntheses of QS-21Api (refs30,31), QS-21Xyl (ref.32) and QS-7Api (ref.33), and to the development of a semi-synthetic sequence34 to a range of chemically stable, amide-containing acyl chain variants of QS-21 (ref.35). These enabled detailed SAR studies of this complex molecule, defining minimal structural requirements for adjuvant activity36 (Fig. 1a).
Strikingly, the terminal sugar within the linear tetrasaccharide37 and the entire branched trisaccharide turned out to be dispensable for adjuvant activity38. Simplification of the acyl chain backbone and modification at the acyl chain terminus did not impair activity35, with the exception of a terminal amino group that abolished adjuvant activity37. Conjugation of the aldehyde-containing immunopotentiator tucaresol at this terminal amine restored activity, although a synergistic enhancement was not observed39. The central glycosidic linkage was quite sensitive to chemical modification, and subtle central linker variations led to QS analogues with drastically different activities that correlated with specific conformational preferences40.
The triterpene C4-aldehyde substituent, hypothesized to play a role in the ability of QS-21 to induce TH1 immunity, was not required for adjuvant activity in truncated synthetic saponins as assessed in terms of antibody response in mice, while the C16-alcohol enhanced activity with higher IgG production38. Subsequently, our multidisciplinary studies involving immunological evaluation and conformational analysis of branched trisaccharide truncated synthetic saponins with targeted modifications at the triterpene have underlined the dispensability of the C4-aldehyde and identified a key role for the C16-hydroxyl group in saponin conformation that correlated with adjuvant activity41, highlighting echinocystic acid as a valuable triterpene source for potent saponin adjuvants further emphasized by its greater affordability and sustainability. Overall, these SAR studies have provided improved, synthetically accessible QS saponins with potent adjuvant activity and non-toxicity in mice, and have enabled the development of saponin probes to gain early insights into their mechanisms of action (see below)42. Most recently, Wang and colleagues have shown that stepwise truncation of the C28 hexasaccharide of QS-7 to a tetrasaccharide retained its ability to induce IgG1 and IgG2a antibodies (TH1 response), albeit at levels lower than the pentasaccharide analogue and QS-21, whereas further sugar truncation led to progressively decreased adjuvant activity, with only the C28 trisaccharide variant inducing significant IgG1 (TH2) antibody response43 (Fig. 1b).
Mechanisms of action
The mechanisms of action of QS-21 are poorly understood, hindering rational development of improved analogues and selection of optimal adjuvant–antigen combinations in future vaccines. A depot effect by which the adjuvant increases the lifetime of the antigen and its presentation to the immune system may not be operative, as prolonged persistence and sustained released of the antigen at the injection site could not be correlated with saponin adjuvant activity21. Binding to TLR2 and TLR4 has also been excluded44. QS-21 has been hypothesized to interact with cell surface lectins through its carbohydrate residues, facilitating antigen uptake into antigen-presenting cells, and to bind amino groups on T cell receptors via imine formation through its triterpene aldehyde, delivering a co-stimulatory signal required for T cell activation and TH1 cellular immunity45.
A number of studies have investigated the mechanisms of action of QS-21 formulated in liposomes and/or in combination with MPLA. In a liposomal formulation injected intramuscularly, QS-21 targeted subcapsular macrophages in the draining lymph nodes, leading to multiple effects: activation of a caspase (i.e. caspase 1), subsequent recruitment and activation of neutrophils and dendritic cells, and, ultimately, induction of antibody and cellular responses, all dependent on myeloid differentiation factor 88 (MyD88), a key signal transduction adapter for interleukin-1 (IL-1)/IL-18 receptors46. A proposed model based on recent data with QS-21 formulated in liposomes involves cholesterol-dependent endocytosis of QS-21 by human monocyte-derived dendritic cells (moDCs), followed by accumulation in and destabilization of the lysosomes. This lysosomal disruption can induce cathepsin B and SYK kinase-mediated activation of moDCs and production of pro-inflammatory cytokines, and could also influence antigen processing and translocation to the cytosol for subsequent cross-presentation47,48 (Fig. 1c). Specifically, saponin-based adjuvants, alone in purified form or formulated with cholesterol and phospholipids in 40-nm cage-like structures (ISCOMATRIX) have been shown to increase antigen cross-presentation in moDCs via endosomal escape and formation of intracellular lipid bodies49. Mechanistically, ISCOMATRIX relies mostly on Quillaja saponins’ adjuvant abilities to (1) induce pro-inflammatory cytokines/chemokines that recruit and activate antigen-presenting cells (dendritic cells), (2) promote antigen trafficking and release into the cytosol and (3) link the innate and adaptive immune responses in vivo in a TLR-independent but MyD88-dependent manner50. In addition to MyD88, NLRP3 inflammasome-related and inflammasome-unrelated IL-18 production/IL-18R signalling modulated innate and adaptive cellular immunity to an ISCOMATRIX vaccine51.
When co-administered with MPLA in mouse antigen-presenting cells, QS-21 was identified as an NLRP3 inflammasome activator, inducing caspase 1-dependent IL-1β/IL-18 production; however, this signalling pathway may inhibit the adjuvant effects of QS-21 in vivo52.
Integrating the previous mechanistic insights, a dual mechanism of action has been recently proposed, whereby QS-21 would act on both T cells and antigen-presenting cells (i.e. dendritic cells) in receptor-mediated and non-receptor-mediated manners, respectively53. According to this model, the triterpene aldehyde group reacts with ε-amino groups, likely from the CD2 receptor on T cells, forming an imine that provides T cells with a co-stimulatory signal required for T cell activation and TH1 immunity45. The proposed mechanism on dendritic cells involves cholesterol-mediated internalization of QS-21 (and exogenous antigens) by interaction with its triterpene and acyl chain, followed by lysosomal destabilization. This leads to dendritic cell activation and pro-inflammatory cytokine production, as well as antigen release to the cytosol for further processing and antigen cross-presentation to CD8 T cells, promoting cytotoxic T lymphocyte responses (Fig. 1c).
Moreover, QS-21-based chemical probes, including radiolabelled and fluorescent synthetic variants, have been utilized in in vivo biodistribution and fluorescence imaging studies to investigate their mechanisms of action. Adjuvant-active saponin probes (co-administered with ovalbumin (OVA) as a prototypic antigen) localized at the injection site and in the draining lymph nodes preferentially over structurally related inactive/attenuated congeners, and the most active fluorescein-labelled saponin was internalized into dendritic cells38. These studies suggest a role for adjuvant-active QS variants in the trafficking of OVA by antigen-presenting cells to the draining lymph nodes, and set the stage for additional investigations with related saponin probes that are ongoing in our group to elucidate the molecular mechanisms of these synthetic saponin adjuvants.
Nonetheless, the variety of structurally distinct saponin adjuvants, some of them devoid of the C4-aldehyde, and the lack of adjuvant activity of other saponin families makes it unlikely that a single, universal mechanism could be operative for QS-21, related synthetic variants and other non-QS saponin adjuvants53. In any case, the identification of the cellular receptor responsible for interaction with aldehyde-containing saponins via imine formation with the C4-aldehyde substituent would provide solid experimental evidence for the suggested role of this group in saponin adjuvant activity, as well as for the proposed mechanism of action of QS-21. The fact that conservative structural modifications and key functionalities such as the aldehyde and hydroxyl groups have such an impact on saponin immunopotentiation hints at mechanisms of action that may only be applicable to closely related saponins with sufficient structural similarity. The established correlation between three-dimensional structure and adjuvant activity implies that saponin conformation is vital for activity and may contribute to proper biodistribution, subcellular localization and/or target binding. This feature, together with the pronounced SAR within this saponin class, suggests a mechanism of action involving interaction with currently unknown (macro)molecular targets.
α-Galactosylceramide-derived adjuvants
α-GalCer (KRN7000) is a marine-sponge-derived synthetic glycolipid with antitumour and immunostimulatory properties. Structurally, it contains a galactose attached through an α-O-glycosidic linkage to a C18 phytosphingosine with an amide-linked, saturated C26 fatty acyl chain54 (Fig. 2a). It binds to the non-polymorphic MHC class I (MHC-I)-like antigen-presenting molecule CD1d on dendritic cells, with its hydrophobic alkyl chains buried inside the CD1d binding groove and its polar portion at the CD1 surface, exposed to the solvent for recognition by the T cell receptor, in an orientation that is determined by conserved polar contacts with defined CD1d residues55. Presentation of α-GalCer bound to CD1d on dendritic cells to the T cell receptor on invariant natural killer T cells (iNKT cells, an unconventional type of T cell lymphocyte expressing a semi-invariant αβ T cell receptor restricted to CD1d and characterized by an invariant α-chain56) leads to iNKT cell activation. While iNKT cells only comprise a small fraction of the overall T cells in the human bloodstream, they represent a special population of lymphocytes owing to their ability to provide rapid response. Thus, these activated iNKT cells immediately produce TH1-type (IFNγ) and TH2-type (IL-4) cytokines upon antigen presentation, stimulating innate and adaptive immune responses57. This unconventional population, characterized by the lack of recognition of classical peptide antigens, limited T cell receptor diversity and scarce abundance, has been shown to induce potent effects and responses through different mechanisms and effector functions. iNKT cells provide new opportunities for T cell immunotherapy, and for the development of defined chemical targets to impact iNKT cell activation and enhance adaptive immune responses in an adjuvant-like fashion58.
Fig. 2. Structures of natural and synthetic α-GalCer-based adjuvants and mechanism of action of α-GalCer.
a | Structure of natural α-galactosylceramide (α-GalCer) and key structural modifications and their impact on activity and cytokine profile production60. b,c | Structures of synthetic α-GalCer variants inducing T helper 2 (TH2)-biased (OCH, panel b)87 and TH1-biased (7DW8-5, panel c)96 responses. d | Schematic representation of invariant natural killer T (iNKT) cell activation and α-GalCer mechanism of action120. α-GalCer presentation on antigen-presenting cell (APC) surface in association with CD1d enables activation of iNKT cells by interaction with their invariant T cell receptor (iTCR). iNKT cells rapidly secrete both pro-inflammatory (TH1) and anti-inflammatory (TH2) cytokines, such as interferon-γ (IFNγ) and interleukin-4 (IL-4), respectively. Depending on several factors, including the nature of the glycolipid antigen, its loading mode into CD1d protein, cytokine milieu, cell types that present the glycolipid antigen, co-stimulatory interactions and frequency of treatment, activated iNKT cells can exhibit a diverse range of responses on other cell types, such as B and T lymphocytes, macrophages, dendritic cells and NK cells. Part d adapted with permission from ref.60, ACS, from ref.63, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), and with permission from ref.120, Future Medicine.
Structure–activity relationships and synthetic α-GalCer variants
Despite the therapeutic potential of α-GalCer, the conflicting activities of the elicited TH1 and TH2 cytokines have limited its clinical efficacy59. This has driven the development of synthetic analogues that can influence NKT cell–dendritic cell interactions and stimulate preferential expression of TH1 or TH2 cytokines by incorporating defined chemical modifications at the galactose and lipid moieties60 (Fig. 2a), guided by the crystal structure of CD1d–α-GalCer61 and the NKT T cell receptor–CD1d–α-GalCer62.
The synthesis and evaluation of these derivatives has enabled accurate SAR studies, identifying key structural features required for TH1-biased and TH2-biased responses (Fig. 2a) and more potent and TH-selective α-GalCer variants (Fig. 2b,c) for use as vaccine adjuvants63.
Early modifications at the sugar moiety revealed that the pyranose form and especially the α-anomeric configuration are essential for adjuvant activity57. Moreover, α-d-fucose (Fuc) and, to a lesser extent, α-glucose, but not α-mannose-linked ceramides, were found to stimulate iNKT cells, with α-GlcCer being less active. These results suggest the importance of the galactose 4′-OH and especially the 2′-OH in immunostimulation, while the 6′-OH is dispensable for activity63. As key positions involved in the interaction with the T cell receptor and in the stability of the CD1d–glycolipid–T cell receptor complex, modifications at the 4′, 3′ and especially 2′ positions (e.g. H (deoxy), F, N3, NH2, NHAc, OMe, O(CH2)2OH = 4′-O-methyl or 4′-O-ethanol-α-GalCer) led to reduced bioactivity64,65 (Fig. 2a). Notable exceptions were the 3-O-sulfo-α-GalCer66,67 and the 4-O-phenylpropyl-α-GalCer analogues68, which induced comparable TH1/TH2 responses, as well as benzyl-modified 4-O variants that promoted TH1-biased immunity69,70. Thus, while deletion or modifications at the 2 position abolished activity, particular variations at the 3-OH (sulfation) and especially the 4-OH (phenyl groups) were less critical for immunostimulation (Fig. 2a).
In contrast to the secondary hydroxyl groups, the galactose 6′-OH is not involved in H-bond formation within the CD1d–α-GalCer–T cell receptor complex. Therefore, modifications of the sugar moiety have mainly focused on this position. In addition to the TH1/TH2-inducing 6′-deoxy analogue (α-FucCer)71, potent TH1-biasing variants of α-GalCer included C6-substituted amides, ureas72 and carbamates, such as NU-α-GalCer and PyrC-α-GalCer, which showed promising antitumour activity in mice73,74.
A variety of other groups have been attached at the C6′ position. These included monosaccharides, for example, the immunologically active α-Gal(1→6)α-GalCer analogue75, amino acids attached through a triazole linker to provide variants (Lys-α-GalCer) that exhibited good selectivity for TH1 responses76, as well as biotin and small fluorophores77,78, yielding immunostimulatory-active analogues for further mechanistic investigation.
Among the diverse galactose-modified analogues, including the more stable and strongly TH1-inducing α-carba-GalCer variant in which the endocyclic oxygen is replaced by a carbon79 (Fig. 2a), only a few elicited modest TH2 profiles, namely, 6-triazole-substituted derivatives80 and 6-O-alkylated analogues bearing long ether-linked aliphatic chains81.
Substitution of the exocyclic glycosidic oxygen by a methylene unit provided the more stable C-glycoside analogue (α-C-GalCer) (Fig. 2a), which exhibited a marked TH1 response (IFNγ and IL-12 cytokines) and superior antimalaria and antimetastatic activity in mice82, but was inactive in human cells83. The thioglycoside analogue α-S-GalCer was also synthesized84, showing no bioactivity in mice, while inducing a TH1 profile in humans85.
A variety of α-GalCer analogues with diverse structural modifications in the lipid moiety have also been synthesized, enabling further SAR studies (Fig. 2a). Selective TH2-inducing analogues were obtained by truncation of the fatty acyl and/or phytosphingosine chains86 and by incorporation of double bonds into the fatty acyl chain, such as the OCH (ref.87) (Fig. 2b) and α-GalCerC20:2 analogues, respectively88. Additional acyl chain modifications that yielded strong TH2-biased cytokine responses included (1) bioisosteric replacement of the acyl chain amide with a triazole, especially in long-chain analogues89, (2) incorporation of an amide in short fatty acid chain derivatives for hydrogen bonding with polar residues in the CD1d pocket (‘anchoring effect’)90,91, (3) incorporation of α-fluorocarbonyl moiety at the acyl chain terminus92 and (4) introduction of a polar chloroacetylamide terminal group in short acyl chain analogues for covalent linkage with CD1d pocket residues93 (Fig. 2a).
Based on activity and structure studies94, introduction of an aromatic group at the fatty acyl chain terminus led to potent TH1 cytokine (IFNγ) secretion95 (Fig. 2a). For example, the fluorophenyl C10 fatty acid derivative 7DW8-5 (ref.96) (Fig. 2c) showed stronger adjuvant activity than α-GalCer in HIV, malaria and influenza vaccines97, while the 4-(4-fluorophenoxy) phenyl undecanoyl analogue (C34) offered superior protective antibacterial and antiviral efficacies98 and more potent adjuvant activity with a Globo-H conjugate cancer vaccine99.
Chemical modifications on the phytosphingosine polar portion (e.g. H (deoxy), F) revealed that both the 4-OH and especially the 3-OH are important for NKT cell activation100–104 (Fig. 2a). A doubly modified α-GalCer variant combining a 4-deoxy-phytosphingosine chain with a hydrocinnamoyl ester on the galactose C6′-OH (AH10-7) induced strong TH1 bias and antitumour iNKT cell responses in mice105, while synthetic diether-containing acyl chain analogues promoted selective TH17 cell responses (IL-17) in vitro106. More recently, a potent photoswitchable analogue incorporating an azobenzene within the acyl chain was developed, which enhanced TH1/TH2 cytokine production upon ultraviolet irradiation107.
Moreover, analogues bearing biotin, NBD fluorophore (NBD-α-GalCer) and benzophenone at the acyl chain terminus were developed to investigate CD1d distribution and glycolipid presentation in dendritic cells, with the latter two showing superior immunostimulatory activity to α-GalCer itself108–110.
Synthetic self-adjuvanting vaccines
Based on the SAR above, α-GalCer variants have been covalently attached to carbohydrate and peptide epitopes to generate synthetic ‘self-adjuvanting’ vaccines incorporating the adjuvant and antigen components in the same molecule.
Cavallari et al. conjugated the Streptococcus pneumoniae capsule polysaccharide CPS4 to the galactose C6′-OH of α-GalCer, elongated with an amino linker, yielding a carbohydrate-lipid vaccine that induced CPS-specific memory B cells and high-affinity IgG antibodies protective against pneumococcal infection in mice111. Several carbohydrate-based cancer vaccine candidates have been synthesized by attaching tumour-associated threonine-linked Tn (α-GalNAc-O-Thr)112 and sialyl Tn (Neu5Ac(α2→6)α-GalNAc) (STn)113 antigens to the galactose C6 position via amide linkages, providing conjugates that, upon liposomal formulation, elicited strong antigen-specific TH1-type/TH2-type IgG antibodies in mice. Most recently, Li and colleagues prepared a C6′-azido α-GalCer variant to conjugate Tn-MUC1 glycopeptide antigens using click chemistry to give vaccine candidates that induced potent tumour-specific IgG antibody responses114. In another example, an immunostimulatory 6′-deoxy-6′-thio analogue was synthesized and attached to the MHC-I OVA257–264 epitope via disulfide or triazole linkages, yielding vaccine constructs that elicited potent, peptide-specific cytotoxic T lymphocyte responses in vivo, at levels higher than the unconjugated components115.
Notably, Anderson et al. developed a strategy based on an observed reversible rearrangement within the ceramide portion to link, via oxime and/or triazole linkages, peptide antigens to an α-GalCer pro-adjuvant, in which the acyl chain is deliberately rearranged. Upon O→N acyl chain isomerization, the corresponding peptide–glycolipid conjugates enhanced T cell immunity and efficacies in allergy116, cancer117 and influenza118 models.
Mechanisms of action
The adjuvant activity of α-GalCer depends on iNKT cell activation and requires the interaction between the upregulated CD40 co-stimulatory molecule on dendritic cells and CD40L on iNKT cells. Together with the induced increase in pro-inflammatory cytokines, this results in an adjuvant effect that enhances dendritic cell activity and, subsequently, T cell activation (Fig. 2d), including CD8 T cell cross-priming119,120. Moreover, iNKT cell activation enhances B cell activity and antibody production, dependent on the interaction between CD1–α-GalCer complexes on B cells and dendritic cells with T cell receptors on iNKT cells121.
Extensive SAR studies on α-GalCer glycolipids have improved understanding of the molecular mechanisms responsible for controlling iNKT cell activation, enabling rational design of improved synthetic α-GalCer variants for more precise and predictable immune targeting. These mechanisms include the impact of α-GalCer structure on the affinity of the interaction between the T cell receptor and the CD1d–glycolipid complex, and the effects on the kinetics and cellular pathways involved in glycolipid presentation by CD1d. While an early simple model attributed high T cell receptor affinities to TH1 skewing, the affinity of the T cell receptor/CD1d–glycolipid interaction does not fully correlate with the induced cytokine bias. Additionally, other factors, such as the half-life of the ternary complex and the uptake and presentation by antigen-presenting cells, are important for iNKT cell activation and the induced cytokine profile122.
In general, TH1-biased responses seem to be favoured by modifications that stabilize α-GalCer presentation and T cell receptor/CD1d–α-GalCer complexes, namely, O-to-C replacement in the carbasugar and α-C-GalCer variants, and by variations that increase hydrophobicity, such as incorporation of aromatic groups at the 6″ position and at the lipid chains terminus. On the other hand, TH2 immunity is promoted by modifications that decrease the half-life of the ternary complex, slightly disturb ligand presentation or improve glycolipid aqueous solubility, such as invariants with shortened, unsaturated or hydroxylated lipid moieties60. This solubility effect is related to differences in requirements for cellular uptake and intracellular loading of the glycolipids onto CD1d. While TH1-biasing α-GalCer analogues require internalization by antigen-presenting cells and endosomal-dependent presentation by CD1d molecules in plasma membrane lipid rafts, less hydrophobic TH2-skewing α-GalCer analogues can be loaded directly into cell surface CD1d outside of lipid rafts123. Thus, lipid raft localization is likely one major mechanism responsible for cytokine biasing and emerges as a powerful tool in the functional screening of new variants124 for the development of tailored synthetic α-GalCer-based adjuvants.
Lipopolysaccharide-based adjuvants
Lipopolysaccharides, commonly known as endotoxins, represent a wide and highly heterogeneous class of bacterial outer membrane glycolipids from Gram-negative bacteria (e.g. Escherichia coli, Neisseria meningitidis and Salmonella minnesota)125. They are essential for bacterial survival and have endotoxic properties that can be exploited in the context of vaccine adjuvants126. Lipopolysaccharides are sensed by the immune system principally via membrane-bound TLR4, which recognizes lipopolysaccharides extracellularly or within endosomes, inducing a signalling cascade that results in inflammation and the release of pro-inflammatory cytokines. More recently, TLR4-independent lipopolysaccharide recognition systems have also been identified: transient receptor potential channel-dependent sensing of extracellular lipopolysaccharide in neuronal cells and caspase 4/11 as a cytoplasmic sensor of intracellular lipopolysaccharide within the innate immune cells (e.g. macrophages) that leads to secretion of pro-inflammatory cytokines IL-1β and IL-18 and inflammatory cell death127. Thus, lipopolysaccharides trigger innate immunity via caspase-mediated non-canonical inflammasome activation, as well as TLR4 binding128, leading to production of pro-inflammatory cytokines such as tumour necrosis factor (TNF), IL-1 and IL-6.
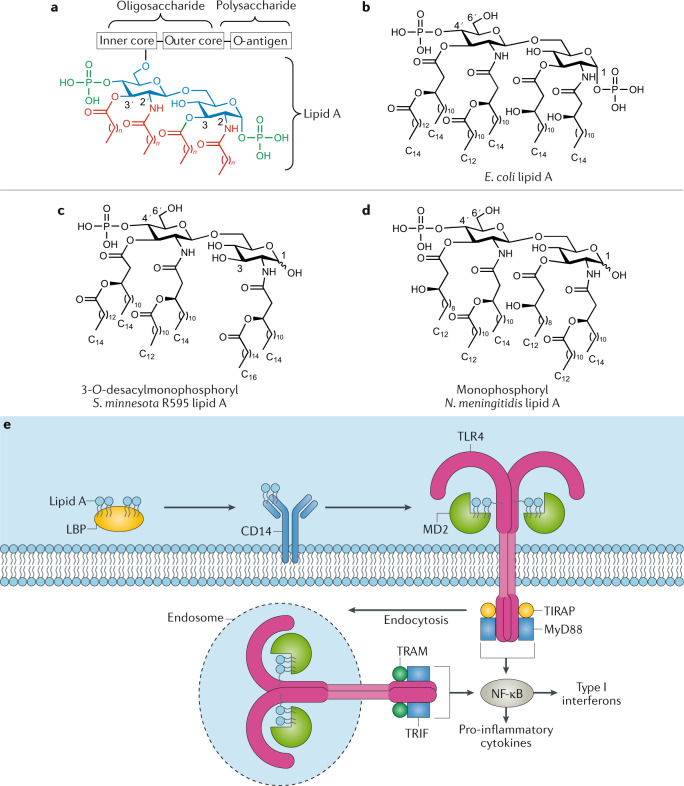
Lipopolysaccharides are composed of an external repeating O-antigen polysaccharide chain, a core oligosaccharide and the highly conserved TLR4-activating lipid A, which comprises a phosphorylated β(1→6)-linked glucosamine disaccharide esterified at C2, C3, C2′ and C3′, with fatty acid chains varying in length and number, depending on the species129 (Fig. 3a,b). Lipid A has potent adjuvant activity, but the massive immune response it induces, characterized by sepsis and septic shock, has limited its clinical use. This has led to the development of potent and less toxic lipid A-based immunomodulators such as the clinically approved MPLA adjuvant130 (Fig. 3c). The bioactivity of lipid A and MPLA is strongly influenced by its heterogeneous chemical composition, comprising a number of carbohydrate functionalities that could influence their properties, as well as TLR4 binding and signalling. To address this limit on their clinical use, synthetic chemistry has emerged as an attractive means to provide practical access to structurally defined derivatives with distinct, potentially improved, clinical and immunological properties.
Fig. 3. Structures of lipid A and monophosphoryl lipid A and its mechanism of action.
a | General structure of lipopolysaccharide (LPS) with its three main domains, lipid A, core oligosaccharide and O-antigen polysaccharide. b | Structure of natural lipid A from Escherichia coli134. c,d | Structures of chemically modified monophosphoryl lipid A from Salmonella minnesota R595 (clinically approved, panel c)147 and from Neisseria meningitidis (panel d)134. e | Schematic representation of LPS/Toll-like receptor 4 (TLR4) signalling pathway and lipid A mechanism of action166. LPS-binding protein (LBP)-associated LPS (or lipid A) is first transferred to CD14 and then delivered to myeloid differentiation factor 2 (MD2), enabling formation of the LPS–MD2–TLR4 ternary complex, which drives dimerization of TLR4 receptors. LPS/TLR4 signalling involves intracellular recruitment of Toll/interlukin-1 (IL-1) receptor domain-containing adapter protein (TIRAP), TRIF-related adapter molecule (TRAM), myeloid differentiation factor 88 (MyD88) and Toll/IL-1 receptor domain-containing adapter inducing interferon-β (TRIF) co-receptors. While the MyD88-dependent pathway promotes pro-inflammatory cytokine expression, the MyD88-independent pathway mediates the induction of type I interferons. Part e adapted with permission from ref.132, Wiley, and with permission from ref.166, Elsevier.
Structure–activity relationship studies and variants of lipid A
Extensive studies using natural and, especially, synthetic lipid A variants with modifications on the sugar, phosphorylation and acyl chain pattern (number and length) have provided key SAR insights that distinguish between agonistic and antagonistic activities, as well as disconnecting lipid A adjuvant activity from its pro-inflammatory toxicity131–134.
Boons and colleagues synthesized several monophosphorylated and diphosphorylated lipid A derivatives by selective acylation of an appropriately functionalized disaccharide intermediate at its C2, C3, C2′ and C3′ positions, revealing important acyl chain features in terms of number (six versus seven), pattern (symmetrical 3+3 over asymmetrical 4+2 arrangement) and length (shorter versus longer) that induce higher TLR4 activation and pro-inflammatory cytokines135. The synthetic monophosphorylated derivatives exhibited less agonistic activity, confirming previous SAR studies136,137, and in agreement with the reduced toxicity shown by MPLA138 (Fig. 3c,d). Moreover, synthetic lipid A analogues containing the core oligosaccharide Kdo (3-deoxy-d-manno-octulosonic acid) units were more active than those lacking this moiety, highlighting its importance for agonistic activity139. Analogues with phosphate-to-carboxylic-acid substitution retained agonistic activity140–142, while monosaccharide deletion decreased lipid A potency but also toxicity, yielding simplified variants with encouraging therapeutic profiles143,144. Collectively, the 3:1 ratio between acyl chains and phosphate groups seems to be important for agonistic activity145. Notably, replacement of the reducing glucosamine unit with an N-acylated aglycon provided aminoalkyl glucosaminide phosphate monosaccharide analogues with adjuvant activity such as RC-529 (Ribi.529)146,147.
Semi-synthetic E. coli-derived MPLA analogues have also been developed by site-selective chemical modification of the lipid pattern (in terms of number, length and position of the acyl chains), the 4′-phosphate and the 6′-hydroxyl group, which was oxidized to the carboxylic acid to then insert an olefin handle for further functionalization. One of such variants bearing a pentenyl moiety at the C6′ position induced a similar cytokine profile to MPLA, highlighting its promising adjuvant activity, and was conjugated to a Tn antigen derivative via thiol–ene coupling to generate a self-adjuvanting vaccine candidate148 (see below). Most recently, rigid α-GlcN(1↔1′)α-Man skewed lipid A mimetics were rationally designed and synthesized, eliciting controllable TLR4 activation and caspase 4/11 oligomerization without inducing caspase 11 protease activity and the associated toxicity149.
Monophosphoryl lipid A
MPLA (3-O-desacyl-4′-monophosphoryl lipid A, MPL from GSK), a detoxified S. minnesota R595 lipid A analogue obtained through hydrolysis of the 1-O-phosphono and (R)-3-hydroxytetradecanoyl groups150 (Fig. 3c), is the most clinically relevant lipid A derivative5. MPLA has been shown to increase antibody and cellular responses without the toxicity associated with lipid A, and has been approved as part of the alum-containing AS04 Adjuvant System in vaccines against HPV151 and hepatitis B152. Successful MPLA combinations with other adjuvants such as QS-21 have also been developed (i.e. AS15, AS02 and, especially, the clinically approved AS01) that induce synergistic immunopotentiating activities and more effective immune responses against a number of infectious diseases and cancers153–155.
Several studies have exploited MPLA as both a carrier and an adjuvant by covalently linking different haptens/antigens to its 6′ position to create a variety of MPLA derivatives. In an early example, a semi-synthetic trinitrophenol–MPLA conjugate, functionalized through an amino chemical handle at the C6′-OH (ref.156), elicited high titres of anti-trinitrophenol IgG antibodies in mice, highlighting MPLA as a T cell-independent carrier for induction of humoral immunity157. An alternative semi-synthetic approach involved derivatization of the C6′-OH of E. coli-derived MPLA to install reactive moieties (azides, alkynes, olefins or thiols) for further conjugation (of the alkene-bearing MPLA derivative) with thiol-containing Tn and TF (Gal(β1→3)α-GalNAc) antigens, providing MPLA–tumour-associated carbohydrate antigen (TACA) conjugates that induced increased production of pro-inflammatory cytokines (IL-6, TNF and IFNγ) in vitro with low toxicity158. Over the past decade, Guo and colleagues have chemically synthesized and evaluated several fully synthetic, self-adjuvanting vaccine candidates134 based on the N. meningitidis159 (Fig. 3d) and E. coli160 MPLA structures conjugated to bacterial antigens161 and TACAs162–164. In mouse immunizations, liposomal formulations of these constructs generally elicited high levels of antigen-specific IgG antibodies that mediated toxicity to the bacterial cell161 and complement-dependent cytotoxicity to the antigen-expressing MCF-7 cancer cell.
Mechanisms of action
The mechanism of action of lipopolysaccharide starts with binding of lipid A to lipopolysaccharide-binding protein, which transfers lipopolysaccharide to the CD14 receptor and then to the co-receptor protein myeloid differentiation factor 2 (MD2), which is associated to TLR4 forming a heterodimer, and directly binds lipid A165,166 (Fig. 3e). The acyl chains of lipid A interact specifically with the MD2 hydrophobic region, while the disaccharide phosphate groups make electrostatic and hydrogen-bond interactions with charged residues in MD2 and TLR4, promoting dimerization of the lipopolysaccharide/MD2/TLR4 complex167,168. This, in turn, results in dimerization of the intracellular Toll/interlukin-1 receptor (TIR) domains of TLR4, initiating the lipopolysaccharide/TLR4 signalling pathway through two distinct intracellular cascades by recruitment of four adapter proteins: MyD88, TIR domain-containing adapter protein (TIRAP; also known as Mal), TRIF-related adapter molecule (TRAM) and Toll/IL-1 receptor domain-containing adapter inducing IFNβ (TRIF)169. The MyD88-dependent pathway involves recruitment of TIRAP and MyD88 to the TIR domain, resulting in early activation of NF-κB, strong production of pro-inflammatory cytokines such as TNF and IL-1β, and TH1 cell responses170. The TRIF/TAM-dependent pathway involves CD14-mediated TLR4/MD2 internalization into endosomes171 and induces late-stage NF-κB activation with lower levels of pro-inflammatory cytokines. It also activates the transcription factor IRF3, which leads to IFNβ and IFN-inducible gene expression with production of type I interferons172,173 (Fig. 3e). More recently, the cysteine protease caspase 4/11 has been discovered as a cytosolic lipopolysaccharide receptor that activates the non-canonical NLRP3 inflammasome pathway via intracellular lipopolysaccharide interaction independent of TLR4 (refs174–176), adding further complexity to the receptor-mediated, lipopolysaccharide-induced cellular activation that triggers innate immunity128. This caspase 11-dependent lipopolysaccharide-sensing pathway and NLRP3 inflammasome activation is associated with adjuvant effects by maximally promoting inflammation via caspase 1-mediated maturation of the pro-inflammatory IL-1 family cytokines (IL-1β and IL-18)128, and by driving pyroptosis, a caspase 1-dependent inflammatory cell death177.
The attenuated pro-inflammatory cytokine production and lower toxicity of MPLA has been associated with the preferential induction of TRIF signalling over the MyD88 pathway178, which is caused by MPLA’s weak ability to form CD14-mediated TLR4/MD2 heterotetramers at the plasma membrane owing to the lack of the 1-O-phosphate group179,180. Additional explanations of MPLA-attenuated toxicity are related to its inability to activate caspase 1, which results in reduced production of pro-inflammatory cytokines such as IL-1 and IL-18 (ref.181), and, alternatively, to its ability to induce high levels of the anti-inflammatory cytokine IL-10 (ref.182). MPLA is able to induce TNF independently of CD14 via MyD88 signalling and to induce TRIF-mediated responses that include upregulation of the co-stimulatory molecule CD86 and IFNβ induction, thus retaining significant lipid A immunostimulatory activity without its associated toxicity. These favourable features have fuelled its successful clinical advancement as an adjuvant for human vaccines153. MPLA is reported to recruit and activate antigen-presenting cells such as dendritic cells to produce cytokines and co-stimulatory molecules that induce IFNγ production by antigen-specific CD4+ T cells, promoting a TH1-skewed or mixed TH1/TH2-type immune response, depending on the co-administered antigen and route of administration183–185. The impaired TLR4 activation by MPLA via TRIF-mediated signalling has been shown to influence CD8+ T cell responses, promoting lower memory CD8+ T cell-mediated protective immunity than lipopolysaccharide in immunized mice186. In non-human primates, MPLA induced systemic expansion of neutrophils and CD14+CD16− monocytes with subsequent migration to the lymph nodes, correlating with local pro-inflammatory gene transcription187.
Zwitterionic polysaccharide adjuvants
In general, carbohydrates have traditionally been considered T cell-independent antigens188, typically triggering the innate immune system and inducing weak antibody responses, without affinity maturation and isotype switching. To achieve T cell-dependent B cell responses, carbohydrate-based epitopes have classically been conjugated to immunocarrier proteins that serve both as a scaffold for multivalent epitope presentation and as a source of CD4+ peptide epitopes for TH cell activation189,190. These activated TH cells provide B lymphocytes with co-stimulatory signals via CD40L/CD40 co-receptor interaction, which, together with the secreted cytokine milieu, ultimately leads to the generation of high-affinity, isotype-switched IgG antibodies and a memory effect.
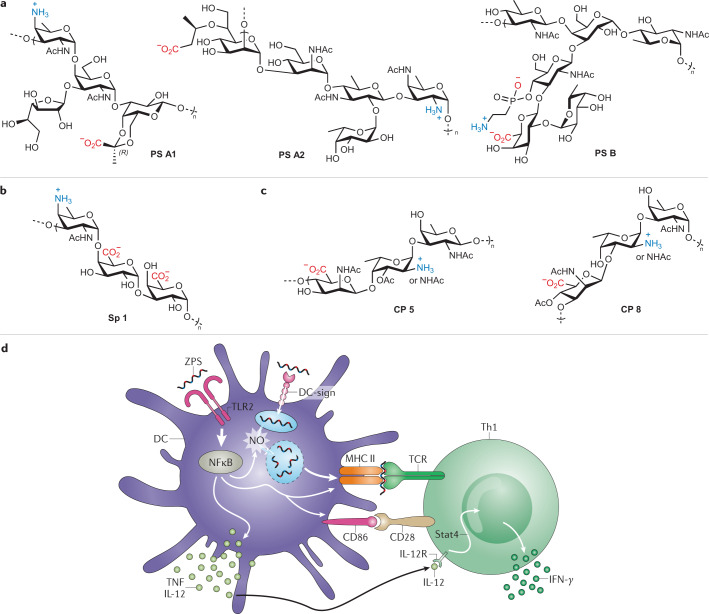
Zwitterionic polysaccharides are immunomodulatory bacterial polysaccharides bearing high density of positively and negatively charged carbohydrate residues191. The most prominent zwitterionic polysaccharides include polysaccharide (PS) A1, PS A2 and PS B, which were isolated from a Bacteroides fragilis strain. These carbohydrate structures have branched repeating units of four, five and six monosaccharides, respectively, with each oligosaccharide unit bearing one amino and carboxylate group, plus an additional phosphonate residue in the case of PS B192,193 (Fig. 4a). The S. pneumoniae type 1 polysaccharide (Sp 1) contains a linear trisaccharide motif comprising a cationic amine-containing monosaccharide, followed by two negatively charged (carboxylate-bearing) sugar residues194 (Fig. 4b). Capsular polysaccharides from Staphylococcus aureus type 5 (CP 5) and type 8 (CP 8) share a similar linear trisaccharide repeating unit with zwitterionic character but differ in acetylation sites and linkages between sugars195 (Fig. 4c).
Fig. 4. Structures of natural zwitterionic polysaccharides and their proposed mechanism of action.
a | Structures of natural zwitterionic polysaccharides (ZPSs) PS A1, PS A2 and PS B from Bacteroides fragilis191. b,c | Structures of natural ZPSs from Streptococcus pneumoniae (type 1, Sp 1, panel b) and Staphylococcus aureus (type 5, CP5, and type 8, CP8, panel c)202. d | Schematic representation of the proposed model for ZPS mechanism of action and crosstalk between innate and adaptive immune compartments215. Within the innate immunity context (left), Toll-like receptor 2 (TLR2)-mediated recognition of zwitterionic polysaccharide induces the activation of the myeloid differentiation factor 88 (MyD88)-dependent pathway in antigen-presenting cells (e.g. dendritic cells), leading to NF-κB-dependent pro-inflammatory cytokine expression (tumour necrosis factor (TNF), interleukin-12 (IL-12)), NO production, and MHC class II (MHC-II) and co-stimulatory molecule expression and upregulation. Adaptive immunity events (right) involving interaction between T cell receptor (TCR) and processed ZPSs presented on MHC-II, along with co-stimulation via CD86/CD28 and IL-12/IL-12R interactions, ultimately trigger ZPS-activated CD4+ T cells to produce the T helper 1 (TH1) cytokine interferon-γ (IFNγ). DC, dendritic cell. Part d adapted with permission from ref.215, Rockefeller University Press.
Unlike other carbohydrate antigens, zwitterionic polysaccharides are considered T cell-dependent antigens, as they can be recognized by and activate TH cells through the following mechanism. Upon processing, presentation of zwitterionic polysaccharide fragments to CD4+ TH cells by antigen-presenting cells occurs on the cell surface in complex with MHC-II molecules. Following interaction through T cell receptors, the resulting activated TH cells produce cytokines and bind to B cells, whose activation leads to IgM-to-IgG class switching and affinity maturation with production of high-affinity IgG antibodies and memory B cells196. However, in addition to their antigen function, zwitterionic polysaccharides have further immunostimulatory properties and their role as adjuvants is well known, especially when co-administered with poorly immunogenic antigens.
The unique structures and activity of these zwitterionic polysaccharides have encouraged researchers to chemically modify natural bacterial PS and to develop synthetic versions and new zwitterionic polysaccharide conjugates that have been investigated in immunological and mechanistic studies.
Structure–activity relationship studies and semi-synthetic variants
Structural studies with naturally derived zwitterionic polysaccharide using NMR and molecular dynamics simulations192,197, as well as circular dichroism198, suggested key features responsible for zwitterionic polysaccharide immunological activity, namely, an α-helical structure and the zwitterionic charge motif presented as alternating positive and negative charges on monosaccharide residues pointing at opposite sides of the helix. These zwitterionic repeating units are required for adjuvant properties, as chemical modifications of the charged amino and carboxylic groups abolished adjuvant activity191,199.
Wack and colleagues introduced, by rational chemical modification, positive charges into naturally anionic group B Streptococcus polysaccharides, generating chemically derived zwitterionic polysaccharide adjuvants that induced activation of antigen-presenting cells via TLR2 and of T cells in vitro200. Notably, co-administration of zwitterionic polysaccharide with tetanus toxoid in mice increased protein-specific IgG antibodies, highlighting its adjuvant activity in vivo. Compared with native polysaccharide conjugates, zwitterionic polysaccharide conjugation to the CRM197 carrier protein elicited higher anti-CRM197 titres, superior TLR2-dependent protective antibody responses against bacterial polysaccharides and enhanced protein-specific but not zwitterionic polysaccharide-specific T cell responses, correlating with its ability to activate TLR2-expressing dendritic cells. However, zwitterionic polysaccharide alone did not induce anti-polysaccharide IgG antibodies, suggesting the need for the protein carrier and T cell help for zwitterionic polysaccharide immunogenicity201.
Zwitterionic polysaccharides have been the subject of several synthetic efforts by the chemistry community, including the chemical synthesis of the zwitterionic oligosaccharide repeating units of Sp1, PS A1, CP5, CP8 (ref.202) and of the Morganella morganii zwitterionic polysaccharide203. The Andreana group synthesized the tetrasaccharide repeating unit of PS A1 using a linear glycosylation strategy204 and has also exploited the immunomodulatory and carrier properties of PS A1 and PS B to construct entirely carbohydrate-based conjugate vaccines incorporating the Tn, TF and STn TACAs205. The synthetic approach towards these conjugates involved site-specific chemical modification of PS A1 by oxidative cleavage of the d-galactofuranose vicinal diol, followed by oxime ligation through the resulting aldehyde with TACAs bearing aminooxy groups at their anomeric position. In their first example, a semi-synthetic Tn–PS A1 construct elicited strong Tn-specific IgG3 antibody responses in mice, highlighting the dual role of PS A1 as both a carrier and an adjuvant206. Subsequently, they prepared an STn–PS A1 conjugate that, upon mouse immunization with an MPLA-derived adjuvant, generated robust cellular immunity with significant IFNγ production and good levels of IgM/IgG antibodies that bound to STn-expressing cancer cells and induced complement-dependent cytotoxicity207. The same synthetic approach was used to access an oxime-linked TF–PS B construct, which generated high titres of TF-specific IgM antibodies and lower levels of IgG1/IgG2b isotypes208, a response differing from the TH1/TH17 immunity induced by the TACA–PS A1 conjugates209.
Recently, Codée and colleagues synthesized Sp1 oligosaccharides encompassing from one to four repeating units (3–12 residues) following an effective stereoselective preglycosylation/postglycosylation-oxidation strategy to assemble the glycan backbone and introduce the carboxylic acid groups. By use of molecular dynamics and NMR, they showed that the observed helical 3D structure, and especially the full helical turn adopted by the nonasaccharides and dodecasaccharides, is required for optimal interaction with anti-Sp1 antibodies, as assessed by binding studies using enzyme-linked immunosorbent assay (ELISA) and saturation transfer difference (STD) NMR210. This study identified the synthetic Sp1 nonamer and dodecamer as structural epitope mimics of the natural polysaccharide, holding promise for vaccine development and mechanistic studies to elucidate the structural basis of the zwitterionic polysaccharide–MHC-II interaction and their molecular mode of action.
Mechanisms of action
Kasper and colleagues first identified and reported a mechanism whereby, upon pinocytosis or receptor-mediated endocytosis in antigen-presenting cells, the zwitterionic polysaccharide PS A is presented by MHC-II molecules analogously to conventional protein-based antigens196,211. Zwitterionic polysaccharides are processed in endosomes, where a nitric-oxide-mediated oxidative burst produces shorter zwitterionic polysaccharide fragments (12–15 kDa, corresponding to ~15 repeating units). These fragments are loaded onto MHC-II molecules, and the zwitterionic polysaccharide–MHC-II complex is presented on the antigen-presenting cell surface for interaction with CD4+ T cell receptors, which results in T cell proliferation and TH1-type cytokine production (Fig. 4d). PS A uptake by dendritic cells has been shown to involve the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) receptor212 and to induce cell maturation with increased expression of co-stimulatory CD80 and CD86 molecules. This, in turn, leads to T cell activation and IFNγ production that requires dendritic cell secretion of IL-12 and activation of the STAT4 transcription factor213.
Interestingly, removal of the zwitterionic charges from PS A has been reported to abolish both in vitro and in vivo MHC-II binding, albeit without impairing antigen-presenting cell-mediated uptake, vesicular trafficking or processing199,214. In addition to DC-SIGN, TLR2 has been identified as a PS A receptor that plays a critical role in initiating the innate immune response and priming the adaptive immune responses to the polysaccharide. Upon TLR2 binding, PS A generates a signalling cascade in antigen-presenting cells that leads to several important events, including activation of NF-κB, production of pro-inflammatory TNF and other molecules that modulate adaptive immunity215.
With these data, an integrated model for PS A-dependent activation of dendritic cells and CD4+ T cells was proposed, linking the innate and adaptive immune system (Fig. 4d). PS A triggers the innate immunity arm in a TLR2-dependent fashion, resulting in NF-κB-dependent production of nitric oxide, which is critical for the processing of DC-SIGN-endocytosed zwitterionic polysaccharide, enabling presentation of short zwitterionic polysaccharide fragments on MHC-II molecules. Interaction between zwitterionic polysaccharide–MHC-II complexes and CD4+ T cell receptors in conjunction with co-stimulation leads to production of IFNγ by CD4+ TH cells, a process enhanced by IL-12 secretion by dendritic cells owing to TLR2 stimulation. Along these lines, TLR2-mediated dendritic cell activation has been proposed to be the mechanism of in vivo adjuvant activity and immunogenicity of natural zwitterionic polysaccharide and zwitterionic-polysaccharide-conjugated vaccines, leading to improved T cell priming and potent immune responses201.
Other carbohydrate-based adjuvants
Mycobacterial carbohydrate adjuvants
Lipoarabinomannans
Lipoarabinomannan is the major mycobacteria cell wall glycolipid component, and comprises an acylated phosphatidyl-myo-inositol anchor glycosylated with a mannan backbone and an arabinan branch that differ between mycobacteria species216. Depending on their lipopolysaccharide structure, lipoarabinomannans have varying immunomodulatory activities217. They can activate MyD88 and complement pathways with production of pro-inflammatory cytokines (TNF, IL-12)218 via interaction with TLR2 and CLRs219 (dectin-2 (ref.220), mannose receptor221, DC-SIGN222) on antigen-presenting cells223, but can also induce anti-inflammatory cytokines (IL-10)224, as reported for mannosylated lipoarabinomannans (ManLAMs)225, which highlights carbohydrate signalling intricacy.
Muramyl dipeptide
Muramyl dipeptide (N-acetylmuramyl-l-alanine-d-isoglutamine) is a glycosylated dipeptide identified from a bacterial cell wall peptidoglycan fraction with adjuvant activity and constitutes the minimal immunoactive component of Freund’s complete adjuvant226. Upon uptake via clathrin-mediated internalization, muramyl dipeptide binds the intracellular receptor NOD2 (ref.227), leading to NF-κB and MAPK activation and induction of pro-inflammatory responses that promote dendritic cell differentiation and enhance CD8+ T cell cross-presentation228. Early in vivo studies with carbohydrate analogues of muramyl dipeptide highlighted the strict structural requirement of the monosaccharide residue for adjuvant activity229. More recently, NOD2 has been shown to recognize naturally occurring muramyl dipeptide variants with modifications in the dipeptide228.
Trehalose-6,6′-dimycolate (TDM or cord factor)
TDM comprises an α-d-glucopyranosyl-α-d-glucopyranoside (trehalose) esterified with two α-alkyl, β-hydroxy fatty acid long chains (mycolic acids)230. Its potent adjuvant activity, with induction of pro-inflammatory cytokines and cellular immunity, requires trehalose binding to the Mincle receptor231–233, and is mediated by the macrophage C-type lectin (MCL)234,235. This triggers SYK–CARD9-dependent NF-κB activation, antigen-presenting cell maturation and robust TH1/TH17 responses in vivo236. However, its high reactogenicity makes it unsuitable for human vaccines, leading to the development of simplified, similarly potent and less toxic synthetic Mincle ligands bearing shorter acyl chains. These include trehalose-6,6′-dibehenate237 that is part of the potent T cell priming CAF01 adjuvant formulation238–240, a glucose derivative acylated at 6-OH with 2-tetradecyloctadecanoic acid (GlcC14C18)241 and a synthetic diacyl trehalose variant242. These analogues exemplify how current advances in synthetic chemistry and chemical biology contribute to developing structurally defined, potent adjuvant molecules.
Polysaccharide-based adjuvants
α-Glucans
Dextran (α-1,6-glucan with α-1,3-branches) is an immunostimulatory microbial polysaccharide of glucose that activates NF-κB and induces pro-inflammatory responses14. Acetylated dextran microparticles have been used as antigen–adjuvant carriers, enabling their delivery and acid-mediated release, leading to increased MHC-I/MHC-II antigen presentation243 and enhanced humoral and cellular responses in vivo244. In mouse models, a lymph-node-targeting dextran–CpG adjuvant conjugate enhanced tumour-specific TH1 and cytotoxic T lymphocyte responses to protein vaccines, leading to protective antitumour immunity245,246, highlighting the applicability of dextran as an adjuvant and a carrier for cancer immunotherapy.
β-Glucans
β-Glucans are microorganism-derived linear or branched β-1,3-glucose polysaccharides that bind to C-type lectin dectin-1 and other pattern recognition receptors, such as CR3 on myeloid immune cells, promoting cytokine production and B/T cell activation, leading to enhanced humoral and cellular responses247. Their adjuvant activity stimulates innate and adaptive immunity through different pathways, depending on their source, structure and formulation. Zymosan (a yeast cell wall mixture of β-1,3-glucans) also activates TLR2, inducing NF-κB activation and TNF secretion248, albeit was later reported to induce regulatory antigen-presenting cells and immune tolerance249. Particulate β-glucan activates dendritic cells via dectin-1, inducing cellular and antitumour immune responses, while soluble β-glucan primes neutrophils for complement and CR3-mediated tumoricidal activity250. Notably, β-glucan was shown to reverse immune tolerance and restore cytokine production via dectin-1 (ref.251), highlighting its adjuvant potential for immunotherapy252. Recent studies have exploited β-glucan as a carrier and immune activator to develop synthetic conjugates that increased the immunogenicity of protein-based antigens (CRM197, MUC1), enhancing antigen-specific antibody responses253,254.
Fructans
Inulin is a plant-derived linear β-d-(2→1)polyfructofuranosyl-α-d-glucose polysaccharide that exists as various isoforms, with the insoluble crystalline δ-inulin having been developed as a potent adjuvant (Advax)255,256 in vaccine clinical trials against hepatitis B and influenza257,258. Mechanistically, Advax does not activate NF-κB and/or the inflammasome but the alternative complement pathway, and potentiates the intrinsic activity of the co-administered antigens via TNF signalling, showing low reactogenicity256,259,260. It enhances antigen uptake and presentation by recruiting and priming antigen-presenting cells, leading to a balanced increase of TH1/TH2 antigen-specific cellular and humoral responses259,261.
Mannans
Mannan is a β-1,4-mannose polysaccharide produced by plants and fungi that enhances antigen presentation and TLR4-dependent dendritic cell maturation, potentiating immune responses262. It binds to mannan-binding lectin (MBL) and C-type lectin receptors, activating the complement pathway263. Mannan also activates the inflammasome, leading to caspase 1 and NF-κB activation, with production of IL-1β, IL-6 and TNF264. Mannan structures either in native, oxidized or reduced form have been conjugated to protein-based antigens and carriers as antigen-presenting cell-targeting adjuvant systems, resulting in enhanced antigen uptake and presentation, and increased TH1/TH2 responses265–269. Notably, clinical trials with oxidized mannan–MUC1 vaccine demonstrated its safety and immunogenicity, showing antibody and cellular clinical responses that protected patients with early-stage breast cancer from recurrence270–272.
Chitosan
Chitosan is a partially chemically deacetylated form of chitin (poly β-1,4-N-acetyl-d-glucosamine)273 shown to potentiate humoral and cellular responses to co-administered antigens through a depot effect and by activating macrophages and NK cells via phagocytosis to produce inflammatory cytokines274–276. Chitosan can be internalized via charged-based interactions or receptor-mediated endocytosis, albeit no specific receptors have been identified277. It activates the NLRP3 inflammasome278 and the DNA-sensing cGAS–STING pathway that results in type I interferon-mediated dendritic cell maturation279, with both mechanisms being dependent on phagocytosis and lysosomal destabilization, and necessary for chitosan-promoted TH1 cellular responses277,280. The cGAS–STING and inflammasome pathways are mutually exclusive, depending on the lysosomal disruption induced by distinct chitosan preparations, with a minimal 3,000-Da fully deacetylated chitosan moiety being required for type I interferon responses280. Several chitosan formulations have been studied as carriers and adjuvants in preclinical settings, increasing vaccine delivery and immunogenicity, and potentiating immune responses275,281.
Conclusions
The development of novel, improved adjuvants is a pressing challenge intimately linked to understanding their mechanisms of action. Despite their history and key role, adjuvants have long been used without a real insight into how exactly they potentiate the immune response, and only a few adjuvants have demonstrated sufficient potency and low toxicity to be licensed for human vaccines. This lack of knowledge has hampered the rational design of more potent and safer adjuvants, and demands further mechanistic investigations to fuel vaccine development. Carbohydrates play pivotal roles in nature, being involved in many important events in the context of the immune system. In addition, they are generally biocompatible, safe and well tolerated, which are intrinsic, favourable properties that make carbohydrate structures attractive targets for the development of vaccine adjuvants and immunomodulators. Chemical synthesis is emerging as a powerful approach on this front, providing practical access to homogeneous carbohydrate compounds for adjuvant development, as well as enabling further SAR studies towards improved synthetic analogues. In this Review, we have covered recent advances in natural and synthetic carbohydrate-based adjuvants, including current understanding of their immunopotentiation mechanisms, along with selected applications in vaccines against infectious diseases and cancer. While there are important gaps in our knowledge on the exact mechanisms of action of many adjuvants, particularly saponin-based adjuvants for which no target receptor is currently known, advances in synthetic carbohydrate chemistry and newly developed chemical tools will aid in gaining new insights into the molecular mechanisms underlying saponin immunopotentiation. It is expected that this progress will make it possible for both chemists and immunologists to rationally design and develop novel, carbohydrate-based adjuvants with enhanced efficacy and reduced toxicity for further clinical advancement in human vaccines.
Acknowledgements
Funding from the European Research Council (ERC-2016-STG-716878 ‘ADJUV-ANT VACCINES’ to A.F.-T.) and the Spanish Ministry of Science and Innovation (CTQ2017-87530-R, RYC-2015-17888 to A.F.-T.; Severo Ochoa accreditation SEV-2016-0644 to CIC bioGUNE) is gratefully acknowledged. The authors thank Juan Anguita (CIC bioGUNE) for proofreading the manuscript. A.F.-T. thanks Raquel Fernández for inspiration. C.P. dedicates this work to the memory of Maria Fassari (1959–2020).
Glossary
- Adaptive (acquired) immunity
Evolved arm of the immune system mediated by B and T cells that acts specifically against targeted antigens to eliminate them, developing immunological memory for long-lasting protection.
- Cell-mediated immunity
Involves activation of innate immune cells, T helper and antigen-specific cytotoxic T cells, and release of cytokines in response to antigens to fight against infected or cancer cells.
- Antigen-presenting cells
Group of immune cells including dendritic cells, macrophages and B cells that mediate cellular responses by processing and presenting antigens on their surface for recognition by T cells.
- T helper 1 (TH1) cells
Subpopulation of T helper cells specialized in clearing viruses and bacteria that invade cells. TH1 cells are characterized by producing interferon-γ and are associated with cellular immunity.
- T helper 2 (TH2) cells
Specialized population of T helper cells that regulate antibody-mediated immune responses against extracellular pathogens, such as parasites, by producing mainly interleukin-4 (IL-4), IL-5 and IL-13 cytokines.
- Triterpene glycosides
Amphiphilic natural products found in plants or marine organisms comprising diverse oligosaccharides covalently linked to squalene-derived, polycyclic C30 hydrocarbon cores through O-glycosidic linkages.
- Caspase
Caspases are a family of cysteine protease enzymes that play important roles in various cellular processes, including apoptosis and inflammation. Inflammatory caspases are involved in inflammatory cytokine signalling.
- Lysosomes
Subcellular organelles responsible for degrading proteins and other internalized material. Maturation of endosomes and fusion into lysosomes creates an acidic environment optimal for lysosomal acid hydrolases.
- Pro-inflammatory cytokines
Small proteins secreted by immune cells (e.g. interleukin-1, tumour necrosis factor, interferon-γ) in response to danger signals or non-self stimuli as signalling molecules and immune mediators promoting inflammatory responses to infection.
- Lipid bodies
Functionally active cellular organelles comprising a neutral lipid core surrounded by a phospholipid monolayer. Lipid bodies sequester lipid and proteins, modulating signalling pathways and inflammatory responses.
- Inflammasome
Multiprotein intracellular complex of the innate immune system that activates pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18 in response to microbial infection and cellular damage.
- iNKT cells
Subpopulation of innate-like T cells (natural killer T cells) expressing limited T cell receptor αβ chains that regulate diverse immune responses via rapid, massive inflammatory cytokine release upon α-galactosylceramide/CD1d activation.
- TH17 cell
Specialized population of T helper cells that produce mainly the pro-inflammatory cytokine interleukin-17 and are implicated in immunity against bacterial and fungal infections, and also in certain inflammatory conditions.
- Bacterial outer membrane
Asymmetrical lipid bilayer composed mainly of phospholipids in the inner leaflet and of lipopolysaccharide and proteins in the outer leaflet that is found in Gram-negative bacteria.
- Endosomes
Membrane-delimited intracellular transport vesicles formed by endocytosis that contain material internalized from the plasma membrane. Three main endosome compartments exist: early, late and recycling endosomes.
- Humoral immunity
Arm of the adaptive immunity mediated by antibodies produced by B cells against foreign antigens, triggering specific destructive mechanisms against pathogens and/or cancer cells.
- CD14
Lipopolysaccharide-binding, glycosylphosphatidylinositol-anchored membrane glycoprotein found on myeloid cells that acts as an essential co-receptor (pattern recognition receptor) for lipopolysaccharide detection, leading to monocyte/macrophage activation and innate response initiation.
- MD2
Glycoprotein that binds to the Toll-like receptor 4 (TLR4) extracellular domain, promoting its cell surface translocation and dimerization required for lipopolysaccharide binding by the TLR4/myeloid differentiation factor 2 (MD2) complexes.
- T cell-independent antigens
Antigens represented mainly by polysaccharide antigens that are able to stimulate B cells to produce IgM antibodies without cooperation by T helper cells, and without promoting immunological memory.
- MHC-II molecules
Transmembrane proteins on the surface of professional antigen-presenting cells that present processed antigens to T helper cells, enabling antigen recognition in a ternary complex with the CD4+ T cell receptor.
- CRM197
Genetically modified non-toxic mutant of diphtheria toxin that is widely used as a carrier protein to improve the immunogenicity of carbohydrates and haptens in several clinically approved conjugate vaccines.
- Pattern recognition receptors
Family of cell surface proteins, including Toll-like and C-type lectin receptors, that bind conserved molecular structures in pathogens, playing key roles in innate immune defence.
Author contributions
C.P., R.F. and A.F.-T. researched data for the article and contributed to writing; C.P. and A.F.-T. contributed substantially to discussion of content; C.P. and A.F.-T. contributed to writing and reviewing/editing of the manuscript before submission.
Competing interests
A.F.-T. is co-inventor on patents and patent applications that include synthetic QS-21 variants mentioned in this work. C.P. and R.F. declare no competing interests.
Footnotes
Peer review information
Nature Reviews Chemistry thanks S. van Kasteren, Z. Guo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/26/2021
A Correction to this paper has been published: 10.1038/s41570-021-00273-6
References
- 1.Jones LH. Recent advances in the molecular design of synthetic vaccines. Nat. Chem. 2015;7:952–960. doi: 10.1038/nchem.2396. [DOI] [PubMed] [Google Scholar]
- 2.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 3.Flach TL, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 4.HogenEsch H, O’Hagan DT, Fox CB. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vaccines. 2018;3:51. doi: 10.1038/s41541-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin. Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Fox CB, Haensler J. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev. Vaccines. 2013;12:747–758. doi: 10.1586/14760584.2013.811188. [DOI] [PubMed] [Google Scholar]
- 7.Sobolev O, et al. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat. Immunol. 2016;17:204–213. doi: 10.1038/ni.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Givord C, et al. Activation of the endoplasmic reticulum stress sensor IRE1α by the vaccine adjuvant AS03 contributes to its immunostimulatory properties. NPJ Vaccines. 2018;3:20. doi: 10.1038/s41541-018-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didierlaurent AM, et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines. 2017;16:55–63. doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 10.Coccia M, et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines. 2017;2:25. doi: 10.1038/s41541-017-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann-Leitner E, Leitner W. Adjuvants in the driver’s seat: how magnitude, type, fine specificity and longevity of immune responses are driven by distinct classes of immune potentiators. Vaccines. 2014;2:252–296. doi: 10.3390/vaccines2020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Tejada A, Cañada FJ, Jiménez-Barbero J. Glycans in medicinal chemistry: an underexploited resource. ChemMedChem. 2015;10:1291–1295. doi: 10.1002/cmdc.201500107. [DOI] [PubMed] [Google Scholar]
- 14.Petrovsky N, Cooper PD. Carbohydrate-based immune adjuvants. Expert Rev. Vaccines. 2011;10:523–537. doi: 10.1586/erv.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleck J, et al. Saponins from Quillaja saponaria and Quillaja brasiliensis: particular chemical characteristics and biological activities. Molecules. 2019;24:171. doi: 10.3390/molecules24010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun H-X, Xie Y, Ye Y-P. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 17.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991;146:431–437. doi: 10.4049/jimmunol.146.2.431. [DOI] [PubMed] [Google Scholar]
- 18.Ragupathi G, Gardner JR, Livingston PO, Gin DY. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines. 2011;10:463–470. doi: 10.1586/erv.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soltysik S, Bedore DA, Kensil CR. Adjuvant activity of QS-21 isomers. Ann. N. Y. Acad. Sci. 1993;690:392–395. doi: 10.1111/j.1749-6632.1993.tb44041.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu J, et al. Carbohydrate-based vaccine adjuvants – discovery and development. Expert Opin. Drug Discov. 2015;10:1133–1144. doi: 10.1517/17460441.2015.1067198. [DOI] [PubMed] [Google Scholar]
- 21.Kensil CR. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 22.Laurens MB. RTS,S/AS01 vaccine (Mosquirix™): an overview. Hum. Vaccin. Immunother. 2019;16:480–489. doi: 10.1080/21645515.2019.1669415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharucha T, Ming D, Breuer J. A critical appraisal of ‘Shingrix’, a novel herpes zoster subunit vaccine (HZ/Su or GSK1437173A) for varicella zoster virus. Hum. Vaccin. Immunother. 2017;13:1789–1797. doi: 10.1080/21645515.2017.1317410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marciani DJ, et al. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18:3141–3151. doi: 10.1016/S0264-410X(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 25.Marciani DJ, Pathak AK, Reynolds RC, Seitz L, May RD. Altered immunomodulating and toxicological properties of degraded Quillaja saponaria Molina saponins. Int. Immunopharmacol. 2001;1:813–818. doi: 10.1016/S1567-5769(01)00016-9. [DOI] [PubMed] [Google Scholar]
- 26.Marciani DJ, Reynolds RC, Pathak AK, Finley-Woodman K, May RD. Fractionation, structural studies, and immunological characterization of the semi-synthetic Quillaja saponins derivative GPI-0100. Vaccine. 2003;21:3961–3971. doi: 10.1016/S0264-410X(03)00298-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, Dai Q, Thogaripally P, Zhang P, Michalek SM. Synthesis of QS-21-based immunoadjuvants. J. Org. Chem. 2013;78:11525–11534. doi: 10.1021/jo402118j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P, Škalamera Đ, Sui X, Zhang P, Michalek SM. Synthesis and evaluation of a QS-17/18-based vaccine adjuvant. J. Med. Chem. 2019;62:1669–1676. doi: 10.1021/acs.jmedchem.8b01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Škalamera Đ, Sui X, Zhang P, Michalek SM. Synthesis and evaluation of QS-7-based vaccine adjuvants. ACS Infect. Dis. 2019;5:974–981. doi: 10.1021/acsinfecdis.9b00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, Kim YJ, Navarro-Villalobos M, Rohde BD, Gin DY. Synthesis of the potent immunostimulatory adjuvant QS-21A. J. Am. Chem. Soc. 2005;127:3256–3257. doi: 10.1021/ja0422007. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ, et al. Synthetic studies of complex immunostimulants from Quillaja saponaria: synthesis of the potent clinical immunoadjuvant QS-21Aapi. J. Am. Chem. Soc. 2006;128:11906–11915. doi: 10.1021/ja062364i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng K, et al. Synthesis of QS-21-xylose: establishment of the immunopotentiating activity of synthetic QS-21 adjuvant with a melanoma vaccine. Angew. Chem. Int. Ed. Engl. 2008;47:6395–6398. doi: 10.1002/anie.200801885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng K, Adams MM, Gin DY. Synthesis and structure verification of the vaccine adjuvant QS-7-Api. Synthetic access to homogeneous Quillaja saponaria immunostimulants. J. Am. Chem. Soc. 2008;130:5860–5861. doi: 10.1021/ja801008m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández-Tejada A, Walkowicz WE, Tan DS, Gin DY. Semisynthesis of analogues of the saponin immunoadjuvant QS-21. Methods Mol. Biol. 2017;1494:45–71. doi: 10.1007/978-1-4939-6445-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams MM, et al. Design and synthesis of potent Quillaja saponin vaccine adjuvants. J. Am. Chem. Soc. 2010;132:1939–1945. doi: 10.1021/ja9082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Tejada A, Tan DS, Gin DY. Development of improved vaccine adjuvants based on the saponin natural product QS-21 through chemical synthesis. Acc. Chem. Res. 2016;49:1741–1756. doi: 10.1021/acs.accounts.6b00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chea EK, et al. Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J. Am. Chem. Soc. 2012;134:13448–13457. doi: 10.1021/ja305121q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Tejada A, et al. Development of a minimal saponin vaccine adjuvant based on QS-21. Nat. Chem. 2014;6:635–643. doi: 10.1038/nchem.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Tejada A, et al. Design, synthesis, and immunologic evaluation of vaccine adjuvant conjugates based on QS-21 and tucaresol. Bioorg. Med. Chem. 2014;22:5917–5923. doi: 10.1016/j.bmc.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walkowicz WE, et al. Quillaja saponin variants with central glycosidic linkage modifications exhibit distinct conformations and adjuvant activities. Chem. Sci. 2016;7:2371–2380. doi: 10.1039/C5SC02978C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghirardello M, et al. Exploiting structure–activity relationships of QS-21 in the design and synthesis of streamlined saponin vaccine adjuvants. Chem. Commun. 2020;56:719–722. doi: 10.1039/C9CC07781B. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Tejada A. Design, synthesis and evaluation of optimized saponin variants derived from the vaccine adjuvant QS-21. Pure Appl. Chem. 2017;89:1359–1378. doi: 10.1515/pac-2016-1213. [DOI] [Google Scholar]
- 43.Škalamera Ä, Kim H, Zhang P, Michalek SM, Wang P. Impact of C28 oligosaccharide on adjuvant activity of QS-7 analogues. J. Org. Chem. 2020 doi: 10.1021/acs.joc.0c00359. [DOI] [PubMed] [Google Scholar]
- 44.Pink JR, Kieny MP. 4th Meeting on novel adjuvants currently in/close to human clinical testing: World Health Organization - Organisation Mondiale de la Santé Fondation Mérieux, Annecy, France, 23-25, June 2003. Vaccine. 2004;22:2097–2102. doi: 10.1016/j.vaccine.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today. 2003;8:934–943. doi: 10.1016/S1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- 46.Detienne S, et al. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci. Rep. 2016;6:39475. doi: 10.1038/srep39475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsby I, et al. Lysosome-dependent activation of human dendritic cells by the vaccine adjuvant QS-21. Front. Immunol. 2017;7:663. doi: 10.3389/fimmu.2016.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacaille-Dubois MA. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: a review. Phytomedicine. 2019;60:152905. doi: 10.1016/j.phymed.2019.152905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Den Brok MH, et al. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat. Commun. 2016;7:13324. doi: 10.1038/ncomms13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson NS, et al. ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol. Cell Biol. 2012;90:540–552. doi: 10.1038/icb.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson NS, et al. Inflammasome-dependent and -independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant. J. Immunol. 2014;192:3259–3268. doi: 10.4049/jimmunol.1302011. [DOI] [PubMed] [Google Scholar]
- 52.Marty-Roix R, et al. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem. 2016;291:1123–1136. doi: 10.1074/jbc.M115.683011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marciani DJ. Elucidating the mechanisms of action of saponin-derived adjuvants. Trends Pharmacol. Sci. 2018;39:573–585. doi: 10.1016/j.tips.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Morita M, et al. Structure-activity relationship of α-galactosylceramides against B16-bearing mice. J. Med. Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 55.Girardi E, Zajonc DM. Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunol. Rev. 2012;250:167–179. doi: 10.1111/j.1600-065X.2012.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 57.Kawano T, et al. CD1d-restricted and TCR-mediated activation of V(α)14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 58.Godfrey DI, Uldrich AP, Mccluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat. Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 59.Savage PB, Teyton L, Bendelac A. Glycolipids for natural killer T cells. Chem. Soc. Rev. 2006;35:771–779. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]
- 60.Laurent X, et al. Switching invariant natural killer T (iNKT) cell response from anticancerous to anti-inflammatory effect: molecular bases. J. Med. Chem. 2014;57:5489–5508. doi: 10.1021/jm4010863. [DOI] [PubMed] [Google Scholar]
- 61.Koch M, et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat. Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 62.Borg NA, et al. CD1d–lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 63.Hung J-T, Huang J-R, Yu AL. Tailored design of NKT-stimulatory glycolipids for polarization of immune responses. J. Biomed. Sci. 2017;24:22. doi: 10.1186/s12929-017-0325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wun KS, et al. A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR. J. Exp. Med. 2008;205:939–949. doi: 10.1084/jem.20072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joyce S, et al. Adaptability of the semi-invariant natural killer T-cell receptor towards structurally diverse CD1d-restricted ligands. EMBO J. 2009;28:3579–3590. doi: 10.1038/emboj.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D, et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc. Natl Acad. Sci. USA. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xing GW, et al. Synthesis and human NKT cell stimulating properties of 3-O-sulfo-α/β-galactosylceramides. Bioorg. Med. Chem. 2005;13:2907–2916. doi: 10.1016/j.bmc.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, et al. Introduction of aromatic group on 4′-OH of α-GalCer manipulated NKT cell cytokine production. Bioorg. Med. Chem. 2011;19:2767–2776. doi: 10.1016/j.bmc.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 69.Janssens J, et al. Efficient divergent synthesis of new immunostimulant 4″-modified α-galactosylceramide analogues. ACS Med. Chem. Lett. 2017;8:642–647. doi: 10.1021/acsmedchemlett.7b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janssens J, et al. 4″-O-Alkylated α-galactosylceramide analogues as iNKT-cell antigens: synthetic, biological, and structural studies. ChemMedChem. 2019;14:147–168. doi: 10.1002/cmdc.201800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pauwels N, et al. Divergent synthetic approach to 6′′-modified α-GalCer analogues. Org. Biomol. Chem. 2011;9:8413–8421. doi: 10.1039/c1ob06235b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trappeniers M, et al. 6′-Derivatised α-GalCer analogues capable of inducing strong CD1d-mediated Th1-biased NKT cell responses in mice. J. Am. Chem. Soc. 2008;130:16468–16469. doi: 10.1021/ja8064182. [DOI] [PubMed] [Google Scholar]
- 73.Aspeslagh S, et al. Galactose-modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumour metastasis. EMBO J. 2011;30:2294–2305. doi: 10.1038/emboj.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aspeslagh S, et al. Enhanced TCR footprint by a novel glycolipid increases NKT-dependent tumor protection. J. Immunol. 2013;191:2916–2925. doi: 10.4049/jimmunol.1203134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prigozy TI, et al. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 76.Ma W, et al. Synthesis and biological activities of amino acids functionalized α-GalCer analogues. Bioorg. Med. Chem. 2020;28:115141. doi: 10.1016/j.bmc.2019.115141. [DOI] [PubMed] [Google Scholar]
- 77.Zhou XT, et al. Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6″-amino-6″-deoxy-galactosylceramides. Org. Lett. 2002;4:1267–1270. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]
- 78.Cheng JMH, et al. An improved synthesis of dansylated α-galactosylceramide and its use as a fluorescent probe for the monitoring of glycolipid uptake by cells. Carbohydr. Res. 2011;346:914–926. doi: 10.1016/j.carres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 79.Tashiro T, et al. RCAI-56, a carbocyclic analogue of KRN7000: its synthesis and potent activity for natural killer (NK) T cells to preferentially produce interferon-γ. Tetrahedron Lett. 2007;48:3343–3347. doi: 10.1016/j.tetlet.2007.03.074. [DOI] [Google Scholar]
- 80.Jervis PJ, et al. New CD1d agonists: synthesis and biological activity of 6″-triazole-substituted α-galactosyl ceramides. Bioorg. Med. Chem. Lett. 2012;22:4348–4352. doi: 10.1016/j.bmcl.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung JT, et al. Design and synthesis of galactose-6-OH-modified α-galactosyl ceramide analogues with Th2-biased immune responses. RSC Adv. 2014;4:47341–47356. doi: 10.1039/C4RA08602C. [DOI] [Google Scholar]
- 82.Franck RW, Tsuji M. α-C-galactosylceramides: synthesis and immunology. Acc. Chem. Res. 2006;39:692–701. doi: 10.1021/ar050006z. [DOI] [PubMed] [Google Scholar]
- 83.Li X, Chen G, Garcia-Navarro R, Franck RW, Tsuji M. Identification of C-glycoside analogues that display a potent biological activity against murine and human invariant natural killer T cells. Immunology. 2009;127:216–225. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dere RT, Zhu X. The first synthesis of a thioglycoside analogue of the immunostimulant KRN7000. Org. Lett. 2008;10:4641–4644. doi: 10.1021/ol8019555. [DOI] [PubMed] [Google Scholar]
- 85.Hogan AE, et al. Activation of human invariant natural killer T cells with a thioglycoside analogue of α-galactosylceramide. Clin. Immunol. 2011;140:196–207. doi: 10.1016/j.clim.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Goff RD, et al. Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J. Am. Chem. Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 87.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing Th2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 88.Yu KOA, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc. Natl Acad. Sci. USA. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee T, et al. Synthesis and evaluation of 1,2,3-triazole containing analogues of the immunostimulant α-GalCer. J. Med. Chem. 2007;50:585–589. doi: 10.1021/jm061243q. [DOI] [PubMed] [Google Scholar]
- 90.Inuki S, et al. Isolated polar amino acid residues modulate lipid binding in the large hydrophobic cavity of CD1d. ACS Chem. Biol. 2016;11:3132–3139. doi: 10.1021/acschembio.6b00674. [DOI] [PubMed] [Google Scholar]
- 91.Inuki S, et al. Potent Th2 cytokine bias of natural killer T cell by CD1d glycolipid ligands: anchoring effect of polar groups in the lipid component. Angew. Chem. Int. Ed. Engl. 2018;57:9655–9659. doi: 10.1002/anie.201802983. [DOI] [PubMed] [Google Scholar]
- 92.Kim H, Song H, Park JG, Lee DS, Park SB. Development of α-GalCer analogues with an α-fluorocarbonyl moiety as Th2-selective ligands of CD1d. ACS Med. Chem. Lett. 2019;10:773–779. doi: 10.1021/acsmedchemlett.9b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kishi J, et al. Design and discovery of covalent α-GalCer derivatives as potent CD1d ligands. ACS Chem. Biol. 2020;15:353–359. doi: 10.1021/acschembio.9b00700. [DOI] [PubMed] [Google Scholar]
- 94.Wu D, et al. Design of natural killer T cell activators: structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc. Natl Acad. Sci. USA. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujio M, et al. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J. Am. Chem. Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 96.Li X, et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc. Natl Acad. Sci. USA. 2010;107:13010–13015. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng H, et al. A glycolipid adjuvant, 7DW8-5, enhances the protective immune response to the current split influenza vaccine in mice. Front. Microbiol. 2019;10:2157. doi: 10.3389/fmicb.2019.02157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin KH, et al. In vivo protection provided by a synthetic new alpha-galactosyl ceramide analog against bacterial and viral infections in murine models. Antimicrob. Agents Chemother. 2010;54:4129–4136. doi: 10.1128/AAC.00368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Y-L, et al. Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc. Natl Acad. Sci. USA. 2013;110:2517–2522. doi: 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sidobre S, et al. The T cell antigen receptor expressed by Vα14i NKT cells has a unique mode of glycosphingolipid antigen recognition. Proc. Natl Acad. Sci. USA. 2004;101:12254–12259. doi: 10.1073/pnas.0404632101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leung L, et al. Synthesis and in vivo evaluation of 4-deoxy-4,4-difluoro-KRN7000. Org. Lett. 2008;10:4433–4436. doi: 10.1021/ol801663m. [DOI] [PubMed] [Google Scholar]
- 102.Baek DJ, et al. The 3-deoxy analogue of α-GalCer: disclosing the role of the 4-hydroxyl group for CD1d-mediated NKT cell activation. ACS Med. Chem. Lett. 2011;2:544–548. doi: 10.1021/ml2000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hunault J, et al. 3-Fluoro- and 3,3-difluoro-3,4-dideoxy-KRN7000 analogues as new potent immunostimulator agents: total synthesis and biological evaluation in human invariant natural killer T cells and mice. J. Med. Chem. 2012;55:1227–1241. doi: 10.1021/jm201368m. [DOI] [PubMed] [Google Scholar]
- 104.Golten S, et al. 3,4-Dideoxy-3,3,4,4-tetrafluoro- and 4-OH epimeric 3-deoxy-3,3-difluoro-α-GalCer analogues: synthesis and biological evaluation on human iNKT cells stimulation. Eur. J. Med. Chem. 2019;178:195–213. doi: 10.1016/j.ejmech.2019.05.069. [DOI] [PubMed] [Google Scholar]
- 105.Chennamadhavuni D, et al. Dual modifications of α-galactosylceramide synergize to promote activation of human invariant natural killer T cells and stimulate anti-tumor immunity. Cell Chem. Biol. 2018;25:571–584. doi: 10.1016/j.chembiol.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hwang YS, Yim J, Song H, Park SB. Improved Th17 selectivity of α-galactosylceramide via noncovalent interactions with diether moiety. ACS Med. Chem. Lett. 2019;10:720–725. doi: 10.1021/acsmedchemlett.8b00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trauner D, et al. Optical control of cytokine production using photoswitchable galactosylceramides. Chem. Eur. J. 2019;26:4476–4479. doi: 10.1002/chem.201905279. [DOI] [PubMed] [Google Scholar]
- 108.Sakai T, Naidenko OV, Iijima H, Kronenberg M, Koezuka Y. Syntheses of biotinylated α-galactosylceramides and their effects on the immune system and CD1 molecules. J. Med. Chem. 1999;42:1836–1841. doi: 10.1021/jm990054n. [DOI] [PubMed] [Google Scholar]
- 109.Sakai T, Ehara H, Koezuka Y. Synthesis of NBD-α-galactosylceramide and its immunologic properties. Org. Lett. 1999;1:359–362. doi: 10.1021/ol9900111. [DOI] [PubMed] [Google Scholar]
- 110.Veerapen N, et al. Photoactivable glycolipid antigens generate stable conjugates with CD1d for invariant natural killer T cell activation. Bioconjug. Chem. 2018;29:3161–3173. doi: 10.1021/acs.bioconjchem.8b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cavallari M, et al. A semisynthetic carbohydrate-lipid vaccine that protects against S. pneumoniae in mice. Nat. Chem. Biol. 2014;10:950–956. doi: 10.1038/nchembio.1650. [DOI] [PubMed] [Google Scholar]
- 112.Götze S, et al. Synthesis, liposomal formulation, and immunological evaluation of a minimalistic carbohydrate-α-GalCer vaccine candidate. J. Med. Chem. 2018;61:4918–4927. doi: 10.1021/acs.jmedchem.8b00312. [DOI] [PubMed] [Google Scholar]
- 113.Yin XG, et al. IgG antibody response elicited by a fully synthetic two-component carbohydrate-based cancer vaccine candidate with α-galactosylceramide as built-in adjuvant. Org. Lett. 2017;19:456–459. doi: 10.1021/acs.orglett.6b03591. [DOI] [PubMed] [Google Scholar]
- 114.Chen P-G, et al. Fully synthetic invariant NKT cell-dependent self-adjuvanting antitumor vaccines eliciting potent immune response in mice. Mol. Pharm. 2020;17:417–425. doi: 10.1021/acs.molpharmaceut.9b00720. [DOI] [PubMed] [Google Scholar]
- 115.Compton BJ, et al. Synthesis and activity of 6″-deoxy-6″-thio-α-GalCer and peptide conjugates. Org. Lett. 2015;17:5954–5957. doi: 10.1021/acs.orglett.5b02836. [DOI] [PubMed] [Google Scholar]
- 116.Anderson RJ, et al. A self-adjuvanting vaccine induces cytotoxic T lymphocytes that suppress allergy. Nat. Chem. Biol. 2014;10:943–949. doi: 10.1038/nchembio.1640. [DOI] [PubMed] [Google Scholar]
- 117.Anderson RJ, et al. NKT cell-dependent glycolipid-peptide vaccines with potent anti-tumour activity. Chem. Sci. 2015;6:5120–5127. doi: 10.1039/C4SC03599B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anderson RJ, et al. Augmenting influenza-specific T cell memory generation with a natural killer T cell-dependent glycolipid–peptide vaccine. ACS Chem. Biol. 2017;12:2898–2905. doi: 10.1021/acschembio.7b00845. [DOI] [PubMed] [Google Scholar]
- 119.Fujii SI, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van Kaer L, Parekh VV, Wu L. Invariant NK T cells: potential for immunotherapeutic targeting with glycolipid antigens. Immunotherapy. 2011;3:59–75. doi: 10.2217/imt.10.85. [DOI] [PubMed] [Google Scholar]
- 121.Bai L, et al. Natural killer T (NKT)-B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc. Natl Acad. Sci. USA. 2013;110:16097–16102. doi: 10.1073/pnas.1303218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carreño LJ, Saavedra-Ávila NA, Porcelli SA. Synthetic glycolipid activators of natural killer T cells as immunotherapeutic agents. Clin. Transl. Immunol. 2016;5:e69. doi: 10.1038/cti.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carreño LJ, Kharkwal SS, Porcelli SA. Optimizing NKT cell ligands as vaccine adjuvants. Immunotherapy. 2014;6:309–320. doi: 10.2217/imt.13.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arora P, et al. A rapid fluorescence-based assay for classification of iNKT cell activating glycolipids. J. Am. Chem. Soc. 2011;133:5198–5201. doi: 10.1021/ja200070u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simpson BW, Trent MS. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019;17:403–416. doi: 10.1038/s41579-019-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mazgaeen L, Gurung P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020;21:379. doi: 10.3390/ijms21020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kieser KJ, Kagan JC. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017;17:376–390. doi: 10.1038/nri.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Silipo, A. & Molinaro, A. in Bacterial Lipopolysaccharides (eds Knirel, Y. A. & Valvano, M. A.) 1–20 (Springer, 2011).
- 130.Bohannon JK, Hernandez A, Enkhbaatar P, Adams WL. The immunobiology of TLR4 agonists: from endotoxin tolerance to immunoadjuvants. Shock. 2013;40:451–462. doi: 10.1097/SHK.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Needham BD, Trent MS. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat. Rev. Microbiol. 2013;11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Molinaro A, et al. Chemistry of lipid A: at the heart of innate immunity. Chem. Eur. J. 2015;21:500–519. doi: 10.1002/chem.201403923. [DOI] [PubMed] [Google Scholar]
- 133.Cochet F, Peri F. The role of carbohydrates in the lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) signalling. Int. J. Mol. Sci. 2017;18:2318. doi: 10.3390/ijms18112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gao J, Guo Z. Progress in the synthesis and biological evaluation of lipid A and its derivatives. Med. Res. Rev. 2018;38:556–601. doi: 10.1002/med.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Gaekwad J, Wolfert MA, Boons G-J. Modulation of innate immune responses with synthetic lipid A derivatives. J. Am. Chem. Soc. 2007;129:5200–5216. doi: 10.1021/ja068922a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takada H, Kotani S, Lüderitz O. Structural requirements of lipid A for endotoxicity and other biological activities. Crit. Rev. Microbiol. 1989;16:477–523. doi: 10.3109/10408418909104475. [DOI] [PubMed] [Google Scholar]
- 137.Rietschel ET, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 138.Persing DH, et al. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002;10:s32–s37. doi: 10.1016/S0966-842X(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 139.Gaekwad J, et al. Differential induction of innate immune responses by synthetic lipid A derivatives. J. Biol. Chem. 2010;285:29375–29386. doi: 10.1074/jbc.M110.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu W-C, Oikawa M, Fukase K, Suda Y, Kusumoto S. A divergent synthesis of lipid A and its chemically stable unnatural analogues. Bull. Chem. Soc. Jpn. 1999;72:1377–1385. doi: 10.1246/bcsj.72.1377. [DOI] [Google Scholar]
- 141.Mochizuki T, et al. Synthesis and biological activities of lipid A-type pyrancarboxylic acid derivatives. Carbohydr. Res. 2000;324:225–230. doi: 10.1016/S0008-6215(99)00326-2. [DOI] [PubMed] [Google Scholar]
- 142.Akamatsu M, et al. Synthesis of lipid A monosaccharide analogues containing acidic amino acid: exploring the structural basis for the endotoxic and antagonistic activities. Bioorg. Med. Chem. 2006;14:6759–6777. doi: 10.1016/j.bmc.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 143.Matsuura M, Kiso M, Hasegawa A. Activity of monosaccharide lipid A analogues in human monocytic cells as agonists or antagonists of bacterial lipopolysaccharide. Infect. Immun. 1999;67:6286–6292. doi: 10.1128/IAI.67.12.6286-6292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lam C, et al. SDZ MRL 953, a novel immunostimulatory monosaccharidic lipid A analog with an improved therapeutic window in experimental sepsis. Antimicrob. Agents Chemother. 1991;35:500–505. doi: 10.1128/AAC.35.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tamai R, et al. Cell activation by monosaccharide lipid A analogues utilizing Toll-like receptor 4. Immunology. 2003;110:66–72. doi: 10.1046/j.1365-2567.2003.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johnson DA, et al. Synthesis and biological evaluation of a new class of vaccine adjuvants: aminoalkyl glucosaminide 4-phosphates (AGPs) Bioorg. Med. Chem. Lett. 1999;9:2273–2278. doi: 10.1016/S0960-894X(99)00374-1. [DOI] [PubMed] [Google Scholar]
- 147.Johnson DA. Synthetic TLR4-active glycolipids as vaccine adjuvants and stand-alone immunotherapeutics. Curr. Top. Med. Chem. 2008;8:64–79. doi: 10.2174/156802608783378882. [DOI] [PubMed] [Google Scholar]
- 148.D’Alonzo D, et al. A semisynthetic approach to new immunoadjuvant candidates: site-selective chemical manipulation of Escherichia coli monophosphoryl lipid A. Chem. Eur. J. 2016;22:11053–11063. doi: 10.1002/chem.201601284. [DOI] [PubMed] [Google Scholar]
- 149.Adanitsch F, et al. Synthetic glycan-based TLR4 agonists targeting caspase-4/11 for the development of adjuvants and immunotherapeutics. Chem. Sci. 2018;9:3957–3963. doi: 10.1039/C7SC05323A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qureshi N, Mascagni P, Ribi E, Takayama K. Monophosphoryl lipid A obtained from lipopolysaccharides of Salmonella minnesota R595. Purification of the dimethyl derivative by high performance liquid chromatography and complete structural determination. J. Biol. Chem. 1985;260:5271–5278. doi: 10.1016/S0021-9258(18)89017-2. [DOI] [PubMed] [Google Scholar]
- 151.Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev. Vaccines. 2007;6:723–739. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 152.Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev. Vaccines. 2007;6:133–140. doi: 10.1586/14760584.6.2.133. [DOI] [PubMed] [Google Scholar]
- 153.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev. Vaccines. 2011;10:471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 154.Cluff, C. W. in Lipid A in Cancer Therapy. Advances in Experimental Medicine and Biology Vol. 667 (ed. Jeannin, J.-F.) 111–123 (Springer, 2009).
- 155.Fox, C. B., Carter, D., Kramer, R. M., Beckmann, A. M. & Reed, S. G. in Immunopotentiators in Modern Vaccines: Second Edition (eds Schijns, V. E. J. C. & O’Hagan, D. T.) 105–127 (Academic, 2017).
- 156.Myers KR, et al. Preparation and characterization of biologically active 6′-O-(6-aminocaproyl)-4′-O-monophosphoryl lipid A and its conjugated derivative. Bioconjug. Chem. 1992;3:540–548. doi: 10.1021/bc00018a013. [DOI] [PubMed] [Google Scholar]
- 157.Myers KR, et al. Monophosphoryl lipid A behaves as a T-cell-independent type 1 carrier for hapten-specific antibody responses in mice. Infect. Immun. 1995;63:168–174. doi: 10.1128/iai.63.1.168-174.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ziaco M, et al. Development of clickable monophosphoryl lipid A derivatives toward semisynthetic conjugates with tumor-associated carbohydrate antigens. J. Med. Chem. 2017;60:9757–9768. doi: 10.1021/acs.jmedchem.7b01234. [DOI] [PubMed] [Google Scholar]
- 159.Wang Q, Xue J, Guo Z. Synthesis of a monophosphoryl lipid A derivative and its conjugation to a modified form of a tumor-associated carbohydrate antigen GM3. Chem. Commun. 2009 doi: 10.1039/b907351E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tang S, Wang Q, Guo Z. Synthesis of a monophosphoryl derivative of Escherichia coli lipid A and its efficient coupling to a tumor-associated carbohydrate antigen. Chem. Eur. J. 2010;16:1319–1325. doi: 10.1002/chem.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liao G, Zhou Z, Suryawanshi S, Mondal MA, Guo Z. Fully synthetic self-adjuvanting α-2,9-oligosialic acid based conjugate vaccines against group C meningitis. ACS Cent. Sci. 2016;2:210–218. doi: 10.1021/acscentsci.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Q, Zhou Z, Tang S, Guo Z. Carbohydrate-monophosphoryl lipid A conjugates are fully synthetic self-adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem. Biol. 2012;7:235–240. doi: 10.1021/cb200358r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou Z, Liao G, Mandal SS, Suryawanshi S, Guo Z. A fully synthetic self-adjuvanting globo H-based vaccine elicited strong T cell-mediated antitumor immunity. Chem. Sci. 2015;6:7112–7121. doi: 10.1039/C5SC01402F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou Z, Mandal SS, Liao G, Guo J, Guo Z. Synthesis and evaluation of GM2-monophosphoryl lipid A conjugate as a fully synthetic self-adjuvant cancer vaccine. Sci. Rep. 2017;7:11403. doi: 10.1038/s41598-017-11500-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park BS, Lee J-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 167.Park BS, et al. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 168.Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc. Natl Acad. Sci. USA. 2012;109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 170.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 171.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat. Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Karaghiosoff M, et al. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat. Immunol. 2003;4:471–477. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- 173.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 174.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 175.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 176.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gurung P, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mata-Haro V, et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 179.Casella CR, Mitchell TC. Inefficient TLR4/MD-2 heterotetramerization by monophosphoryl lipid A. PLoS ONE. 2013;8:e62622. doi: 10.1371/journal.pone.0062622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miyake K, et al. The attenuated inflammation of MPL is due to the lack of CD14-dependent tight dimerization of the TLR4/MD2 complex at the plasma membrane. Int. Immunol. 2013;26:307–314. doi: 10.1093/intimm/dxt071. [DOI] [PubMed] [Google Scholar]
- 181.Okemoto K, Kawasaki K, Hanada K, Miura M, Nishijima M. A potent adjuvant monophosphoryl lipid A triggers various immune responses, but not secretion of IL-1β or activation of caspase-1. J. Immunol. 2006;176:1203–1208. doi: 10.4049/jimmunol.176.2.1203. [DOI] [PubMed] [Google Scholar]
- 182.Salkowski CA, Detore GR, Vogel SN. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect. Immun. 1997;65:3239–3247. doi: 10.1128/iai.65.8.3239-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.De Becker G, et al. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int. Immunol. 2000;12:807–815. doi: 10.1093/intimm/12.6.807. [DOI] [PubMed] [Google Scholar]
- 184.Ismaili J, et al. Monophosphoryl lipid A activates both human dendritic cells and T cells. J. Immunol. 2002;168:926–932. doi: 10.4049/jimmunol.168.2.926. [DOI] [PubMed] [Google Scholar]
- 185.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cui W, et al. TLR4 ligands lipopolysaccharide and monophosphoryl lipid A differentially regulate effector and memory CD8+ T cell differentiation. J. Immunol. 2014;192:4221–4232. doi: 10.4049/jimmunol.1302569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kwissa M, Nakaya HI, Oluoch H, Pulendran B. Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood. 2012;119:2044–2055. doi: 10.1182/blood-2011-10-388579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Avci FY, Kasper DL. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 2010;28:107–130. doi: 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- 189.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mitchison NA. T-cell–B-cell cooperation. Nat. Rev. Immunol. 2004;4:308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- 191.Tzianabos A, Wang JY, Kasper DL. Biological chemistry of immunomodulation by zwitterionic polysaccharides. Carbohydr. Res. 2003;338:2531–2538. doi: 10.1016/j.carres.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 192.Wang Y, Kalka-Moll WM, Roehrl MH, Kasper DL. Structural basis of the abscess-modulating polysaccharide A2 from Bacteroides fragilis. Proc. Natl Acad. Sci. USA. 2000;97:13478–13483. doi: 10.1073/pnas.97.25.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baumann H, Tzianabos AO, Brisson JR, Kasper DL, Jennings HJ. Structural elucidation of two capsular polysaccharides from one strain of Bacteroides fragilis using high-resolution NMR spectroscopy. Biochemistry. 1992;31:4081–4089. doi: 10.1021/bi00131a026. [DOI] [PubMed] [Google Scholar]
- 194.Lindberg B, Lindqvist B, Lönngren J, Powell DA. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 1. Carbohydr. Res. 1980;78:111–117. doi: 10.1016/S0008-6215(00)83664-2. [DOI] [PubMed] [Google Scholar]
- 195.Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus Types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr. Res. 2005;340:1097–1106. doi: 10.1016/j.carres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 196.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.1016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choi Y-H, Roehrl MH, Kasper DL, Wang JY. A unique structural pattern shared by T-cell-activating and abscess-regulating zwitterionic polysaccharides. Biochemistry. 2002;41:15144–15151. doi: 10.1021/bi020491v. [DOI] [PubMed] [Google Scholar]
- 198.Kreisman LSC, Friedman JH, Neaga A, Cobb BA. Structure and function relations with a T-cell-activating polysaccharide antigen using circular dichroism. Glycobiology. 2007;17:46–55. doi: 10.1093/glycob/cwl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 200.Gallorini S, et al. Introduction of zwitterionic motifs into bacterial polysaccharides generates TLR2 agonists able to activate APCs. J. Immunol. 2007;179:8208–8215. doi: 10.4049/jimmunol.179.12.8208. [DOI] [PubMed] [Google Scholar]
- 201.Gallorini S, et al. Toll-like receptor 2 dependent immunogenicity of glycoconjugate vaccines containing chemically derived zwitterionic polysaccharides. Proc. Natl Acad. Sci. USA. 2009;106:17481–17486. doi: 10.1073/pnas.0903313106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang Q, Overkleeft HS, van der Marel GA, Codée JD. Synthetic zwitterionic polysaccharides. Curr. Opin. Chem. Biol. 2017;40:95–101. doi: 10.1016/j.cbpa.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 203.Keith DJ, Townsend SD. Total synthesis of the congested, bisphosphorylated Morganella morganii zwitterionic trisaccharide repeating unit. J. Am. Chem. Soc. 2019;141:12939–12945. doi: 10.1021/jacs.9b06830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Eradi P, Ghosh S, Andreana PR. Total synthesis of zwitterionic tetrasaccharide repeating unit from Bacteroides fragilis ATCC 25285/NCTC 9343 capsular polysaccharide PS A1 with alternating charges on adjacent monosaccharides. Org. Lett. 2018;20:4526–4530. doi: 10.1021/acs.orglett.8b01829. [DOI] [PubMed] [Google Scholar]
- 205.Nishat S, Andreana P. Entirely carbohydrate-based vaccines: an emerging field for specific and selective immune responses. Vaccines. 2016;4:19. doi: 10.3390/vaccines4020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn–PS A1 conjugates. J. Am. Chem. Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 207.Shi M, Kleski KA, Trabbic KR, Bourgault JP, Andreana PR. Sialyl-Tn polysaccharide A1 as an entirely carbohydrate immunogen: synthesis and immunological evaluation. J. Am. Chem. Soc. 2016;138:14264–14272. doi: 10.1021/jacs.6b05675. [DOI] [PubMed] [Google Scholar]
- 208.Trabbic KR, Bourgault J-P, Shi M, Clark M, Andreana PR. Immunological evaluation of the entirely carbohydrate-based Thomsen-Friedenreich – PS B conjugate. Org. Biomol. Chem. 2016;14:3350–3355. doi: 10.1039/C6OB00176A. [DOI] [PubMed] [Google Scholar]
- 209.De Silva RA, et al. The entirely carbohydrate immunogen Tn-PS A1 induces a cancer cell selective immune response and cytokine IL-17. Cancer Immunol. Immunother. 2012;61:581–585. doi: 10.1007/s00262-012-1205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang Q, et al. Synthetic, zwitterionic Sp1 oligosaccharides adopt a helical structure crucial for antibody interaction. ACS Cent. Sci. 2019;5:1407–1416. doi: 10.1021/acscentsci.9b00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tzianabos AO, et al. Bacterial pathogens induce abscess formation by CD4+ T-cell activation via the CD28–B7-2 costimulatory pathway. Infect. Immun. 2000;68:6650–6655. doi: 10.1128/IAI.68.12.6650-6655.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bloem K, et al. Interaction of the capsular polysaccharide A from Bacteroides fragilis with DC-SIGN on human dendritic cells is necessary for its processing and presentation to T cells. Front. Immunol. 2013;4:103. doi: 10.3389/fimmu.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 214.Cobb BA, Kasper DL. Characteristics of carbohydrate antigen binding to the presentation protein HLA-DR. Glycobiology. 2008;18:707–718. doi: 10.1093/glycob/cwn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang Q, et al. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J. Exp. Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mishra AK, Driessen NN, Appelmelk BJ, Besra GS. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host–pathogen interaction. FEMS Microbiol. Rev. 2011;35:1126–1157. doi: 10.1111/j.1574-6976.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Doz E, et al. Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J. Biol. Chem. 2007;282:26014–26025. doi: 10.1074/jbc.M702690200. [DOI] [PubMed] [Google Scholar]
- 218.Mazurek J, et al. Divergent effects of mycobacterial cell wall glycolipids on maturation and function of human monocyte-derived dendritic cells. PLoS ONE. 2012;7:e42515. doi: 10.1371/journal.pone.0042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ishikawa E, Mori D, Yamasaki S. Recognition of mycobacterial lipids by immune receptors. Trends Immunol. 2017;38:66–76. doi: 10.1016/j.it.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 220.Yonekawa A, et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity. 2014;41:402–413. doi: 10.1016/j.immuni.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 221.Kang PB, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tallieux L, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Turner J, Torrelles JB. Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018;76:fty026. doi: 10.1093/femspd/fty026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Källenius G, Correia-Neves M, Buteme H, Hamasur B, Svenson SB. Lipoarabinomannan, and its related glycolipids, induce divergent and opposing immune responses to Mycobacterium tuberculosis depending on structural diversity and experimental variations. Tuberculosis. 2016;96:120–130. doi: 10.1016/j.tube.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 225.Yuan C, et al. Mycobacterium tuberculosis mannose-capped lipoarabinomannan induces IL-10-producing B cells and hinders CD4+ Th1 immunity. iScience. 2019;11:13–30. doi: 10.1016/j.isci.2018.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ogawa C, Liu Y-J, Kobayashi KS. Muramyl dipeptide and its derivatives: peptide adjuvant in immunological disorders and cancer therapy. Curr. Bioact. Compd. 2011;7:180–197. doi: 10.2174/157340711796817913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Marina-García N, et al. Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation. J. Immunol. 2009;182:4321–4327. doi: 10.4049/jimmunol.0802197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schenk M, et al. Human NOD2 recognizes structurally unique muramyl dipeptides from Mycobacterium leprae. Infect. Immun. 2016;84:2429–2438. doi: 10.1128/IAI.00334-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Azuma I, et al. Adjuvant activity of carbohydrate analogs of N-acetylmuramyl-L-alanyl-D-isoglutamine on the induction of delayed-type hypersensitivity to azobenzenearsonate-N-acetyl-L-tyrosine in guinea pigs. Infect. Immun. 1981;33:834–839. doi: 10.1128/iai.33.3.834-839.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Marrakchi H, Lanéelle M-A, Daffé M. Mycolic acids: structures, biosynthesis, and beyond. Chem. Biol. 2014;21:67–85. doi: 10.1016/j.chembiol.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 231.Ishikawa E, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin mincle. J. Exp. Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Feinberg H, et al. Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J. Biol. Chem. 2013;288:28457–28465. doi: 10.1074/jbc.M113.497149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Furukawa A, et al. Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc. Natl Acad. Sci. USA. 2013;110:17438–17443. doi: 10.1073/pnas.1312649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Miyake Y, et al. C-type lectin MCL is an FcRγ-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity. 2013;38:1050–1062. doi: 10.1016/j.immuni.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 235.Miyake Y, Oh-hora M, Yamasaki S. C-type lectin receptor MCL facilitates Mincle expression and signaling through complex formation. J. Immunol. 2015;194:5366–5374. doi: 10.4049/jimmunol.1402429. [DOI] [PubMed] [Google Scholar]
- 236.Werninghaus K, et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ–Syk–Card9–dependent innate immune activation. J. Exp. Med. 2009;206:89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schoenen H, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Agger EM, et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS ONE. 2008;3:e3116. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lindenstrøm T, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 2009;182:8047–8055. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 240.Aagaard C, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 2011;17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 241.Decout A, et al. Rational design of adjuvants targeting the C-type lectin Mincle. Proc. Natl Acad. Sci. USA. 2017;114:2675–2680. doi: 10.1073/pnas.1612421114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Holzheimer M, et al. Asymmetric total synthesis of mycobacterial diacyl trehaloses demonstrates a role for lipid structure in immunogenicity. ACS Chem. Biol. 2020;15:1835–1841. doi: 10.1021/acschembio.0c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Frechet JMJ. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc. Natl Acad. Sci. USA. 2009;106:5497–5502. doi: 10.1073/pnas.0901592106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen N, et al. Investigation of tunable acetalated dextran microparticle platform to optimize M2e-based influenza vaccine efficacy. J. Control. Release. 2018;289:114–124. doi: 10.1016/j.jconrel.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang W, An M, Xi J, Liu H. Targeting CpG adjuvant to lymph node via dextran conjugate enhances antitumor immunotherapy. Bioconjug. Chem. 2017;28:1993–2000. doi: 10.1021/acs.bioconjchem.7b00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xu X, et al. Carbohydrate-based adjuvants activate tumor-specific Th1 and CD8+ T-cell responses and reduce the immunosuppressive activity of MDSCs. Cancer Lett. 2019;440–441:94–105. doi: 10.1016/j.canlet.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 247.Brown GD, Gordon S. Fungal β-glucans and mammalian immunity. Immunity. 2003;19:311–315. doi: 10.1016/S1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 248.Sato M, et al. Direct binding of toll-like receptor 2 to zymosan, and zymosan-induced NF-κB activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 2003;171:417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 249.Dillon S, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Qi C, et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood. 2011;117:6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Novakovic B, et al. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell. 2016;167:1354–1368. doi: 10.1016/j.cell.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jin Y, Li P, Wang F. β-glucans as potential immunoadjuvants: a review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine. 2018;36:5235–5244. doi: 10.1016/j.vaccine.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 253.Donadei A, et al. Rational design of adjuvant for skin delivery: conjugation of synthetic β-glucan dectin-1 agonist to protein antigen. Mol. Pharm. 2015;12:1662–1672. doi: 10.1021/acs.molpharmaceut.5b00072. [DOI] [PubMed] [Google Scholar]
- 254.Wang H, et al. β-Glucan as an immune activator and a carrier in the construction of a synthetic MUC1 vaccine. Chem. Commun. 2019;55:253–256. doi: 10.1039/C8CC07691J. [DOI] [PubMed] [Google Scholar]
- 255.Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising β-D-[2 → 1] poly(fructo-furanosyl) α-d-glucose polymers. Glycobiology. 2011;21:595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Petrovsky N, Cooper PD. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015;33:5920–5926. doi: 10.1016/j.vaccine.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gordon D, Kelley P, Heinzel S, Cooper P, Petrovsky N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled phase 1 study. Vaccine. 2014;32:6469–6477. doi: 10.1016/j.vaccine.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gordon DL, et al. Human phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax™ delta inulin adjuvant. Vaccine. 2016;34:3780–3786. doi: 10.1016/j.vaccine.2016.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hayashi M, et al. Advax, a delta inulin microparticle, potentiates in-built adjuvant property of co-administered vaccines. EBioMedicine. 2017;15:127–136. doi: 10.1016/j.ebiom.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Counoupas C, et al. Delta inulin-based adjuvants promote the generation of polyfunctional CD4+ T cell responses and protection against Mycobacterium tuberculosis infection. Sci. Rep. 2017;7:8582. doi: 10.1038/s41598-017-09119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Saade F, Honda-Okubo Y, Trec S, Petrovsky N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sheng KC, et al. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 2006;118:372–383. doi: 10.1111/j.1365-2567.2006.02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gadjeva M, Takahashi K, Thiel S. Mannan-binding lectin — A soluble pattern recognition molecule. Mol. Immunol. 2004;41:113–121. doi: 10.1016/j.molimm.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 264.Lamkanfi M, Malireddi RKS, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J. Biol. Chem. 2009;284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Apostolopoulos V, Pietersz GA, Loveland BE, Sandrin MS, McKenzie IF. Oxidative/reductive conjugation of mannan to antigen selects for T1 or T2 immune responses. Proc. Natl Acad. Sci. USA. 1995;92:10128–10132. doi: 10.1073/pnas.92.22.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ahlén G, et al. Mannosylated mucin-type immunoglobulin fusion proteins enhance antigen-specific antibody and T lymphocyte responses. PLoS ONE. 2012;7:e46959. doi: 10.1371/journal.pone.0046959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Durán-Lobato M, Carrillo-Conde B, Khairandish Y, Peppas NA. Surface-modified P(HEMA-co-MAA) nanogel carriers for oral vaccine delivery: design, characterization, and in vitro targeting evaluation. Biomacromolecules. 2014;15:2725–2734. doi: 10.1021/bm500588x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sirvent S, et al. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce functional regulatory T cells through programmed death ligand 1. J. Allergy Clin. Immunol. 2016;138:558–567.e11. doi: 10.1016/j.jaci.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 269.Wu Y, et al. Reversible mannosylation as a covalent binding adjuvant enhances immune responses for porcine circovirus type 2 vaccine. ACS Omega. 2018;3:17341–17347. doi: 10.1021/acsomega.8b02264. [DOI] [Google Scholar]
- 270.Karanikas V, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J. Clin. Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Apostolopoulos V, et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835] Breast Cancer Res. 2006;8:R27. doi: 10.1186/bcr1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Vassilaros S, et al. Up to 15-year clinical follow-up of a pilot phase III immunotherapy study in stage II breast cancer patients using oxidized mannan–MUC1. Immunotherapy. 2013;5:1177–1182. doi: 10.2217/imt.13.126. [DOI] [PubMed] [Google Scholar]
- 273.Aranaz I, et al. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009;3:203–230. [Google Scholar]
- 274.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25:2085–2094. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Arca HÇ, Günbeyaz M, Şenel S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines. 2009;8:937–953. doi: 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- 276.Li X, et al. Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine. Clin. Dev. Immunol. 2013;2013:387023. doi: 10.1155/2013/387023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Moran HBT, Turley JL, Andersson M, Lavelle EC. Immunomodulatory properties of chitosan polymers. Biomaterials. 2018;184:1–9. doi: 10.1016/j.biomaterials.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 278.Bueter CL, et al. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J. Biol. Chem. 2011;286:35447–35455. doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Carroll EC, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016;44:597–608. doi: 10.1016/j.immuni.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Fong D, et al. Lysosomal rupture induced by structurally distinct chitosans either promotes a type 1 IFN response or activates the inflammasome in macrophages. Biomaterials. 2017;129:127–138. doi: 10.1016/j.biomaterials.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 281.Vasiliev YM. Chitosan-based vaccine adjuvants: incomplete characterization complicates preclinical and clinical evaluation. Expert Rev. Vaccines. 2015;14:37–53. doi: 10.1586/14760584.2015.956729. [DOI] [PubMed] [Google Scholar]
- 282.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Schijns V, et al. Modulation of immune responses using adjuvants to facilitate therapeutic vaccination. Immunol. Rev. 2020;296:169–190. doi: 10.1111/imr.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 286.Su X, et al. Interferon-γ regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat. Immunol. 2015;16:838–849. doi: 10.1038/ni.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 1993;54:229–270. doi: 10.1016/S0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 288.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 289.Ruterbusch M, Pruner KB, Shehata L, Pepper M. In vivo CD4+ T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu. Rev. Immunol. 2020;38:705–725. doi: 10.1146/annurev-immunol-103019-085803. [DOI] [PubMed] [Google Scholar]
- 290.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lucca LE, Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat. Rev. Immunol. 2020;20:680–693. doi: 10.1038/s41577-020-0296-3. [DOI] [PubMed] [Google Scholar]