Abstract
Although glioblastoma multiforme (GBM) is a widely researched cancer of the central nervous system, we still do not know its full pathophysiological mechanism and we still lack effective treatment methods as the current combination of surgery, radiotherapy, and chemotherapy does not bring about satisfactory results. The median survival time for GBM patients is only about 15 months. In this paper, we present the epidemiology of central nervous system (CNS) tumors and review the epidemiological data on GBM regarding gender, age, weight, height, and tumor location. The data indicate the possible influence of some anthropometric factors on the occurrence of GBM, especially in those who are male, elderly, overweight, and/or are taller. However, this review of single and small-size epidemiological studies should not be treated as definitive due to differences in the survey methods used. Detailed epidemiological registers could help identify the main at-risk groups which could then be used as homogenous study groups in research worldwide. Such research, with less distortion from various factors, could help identify the pathomechanisms that lead to the development of GBM.
Keywords: glioblastoma multiforme, epidemiology, sex, gender, age, overweight, obesity, height
1. Introduction
Glioma is a glial tumor that was first classified soon after its discovery by German pathologist Rudolf Virchow in 1865 [1]. The current World Health Organization (WHO) classification is much more complex and recommends an integrated diagnosis based on a combination of histopathological typing (that distinguishes diffuse astrocytic and oligodendroglial tumors, other astrocytic tumors, ependymal tumors, and other gliomas) and molecular typing which takes into account genetic mutations, e.g., in TERT (telomerase reverse transcriptase), p53 (tumor protein P53), IDH 1 (isocitrate dehydrogenase 1) and IDH 2 (isocitrate dehydrogenases 2), MGMT (O6-alkylguanine DNA alkyltransferase), EGFR (epidermal growth factor receptor), and INI1 (integrase interactor 1) genes, among other genes and molecular markers [2]. The methylation of the MGMT gene promoter and the chromosomal 1p/19q codeletion or mutations in the IDH gene are important clinical information that help not only to determine the type of cancer but also to predict the patient’s condition. A mutation in IDH leads to changes in the reaction catalyzed by the corresponding enzyme, resulting in the production of 2-hydroxyglutarate from α-ketoglutarate. This oncometabolite inhibits α-ketoglutarate-dependent dioxygenases [3,4]. This leads to methylation of histones and DNA 5-methylcytosine hydroxylation. Nevertheless, this effect improves the prognosis of patients with low-grade glioma and glioblastoma multiforme (GBM) [5,6]. MGMT promoter methylation is a prognostic factor for patients with GBM. MGMT is an enzyme that repairs damage caused by TMZ (temozolomide), [7] which means that it is responsible for the drug resistance of GBM cells to commonly used anticancer therapies. MGMT promoter methylation reduces the expression of MGMT and therefore is associated with a better prognosis for GBM patients [8]. The 1p/19q codeletion is typically found in oligodendrogliomas [2], increasing the expression of oncogens and decreasing the expression of anti-oncogens [9].
Despite the already extensive knowledge of the molecular biology of glioma, our knowledge of the pathophysiology of this cancer is still insufficient. Scientists are trying to determine the environmental and individual causes of this type of cancer. Despite an extensive body of work on various potential risk factors (ionizing radiation, alcohol intake, tobacco smoking, the use of cell phones, cytomegalovirus infection, head injuries, and drinking coffee), only ionizing radiation has been fully confirmed to be associated with the formation of glioma [3]. Another very interesting issue is the effect of drugs that nonspecifically inhibit COX (cyclooxygenase), including NSAIDs (nonsteroidal anti-inflammatory drugs) and antihistamines, on the risk of different types of histological gliomas. The researched cases were divided into 3 types: high-grade, anaplastic, and low-grade glioma. The use of NSAIDs was found to be inversely related to the occurrence of all types of glioma. The results with antihistamines were inconclusive. The tumor-promoting effect was present only in the anaplastic subtype and >10 years of drug use in the case of antihistamines. Qiu et al. [10], in their work, recalled that exacerbated COX 2 activity is associated with GBM progression, pointing to its influence on PGE2 (prostaglandin E2) and on the EP receptor (prostaglandin E receptor subtypes). In preclinical studies and in summaries of studies with patients, they confirmed the inhibitory effect of COX 2 inhibitors on tumor progression.
Among the individual factors, particular attention should be paid to the relation between resistance to allergies and immune factors and the likelihood of glioma. Linos et al., in their paper, concluded that there is strong evidence for a curious inverse relation between glioma and atopic disease. The evidence was based on information gained after searching 8 observational studies (with a total of 3450 patients with gliomas). The risk of gliomas was inversely associated with asthma, eczema, and allergy. There was no such coincidence found in the study concerning meningiomas. The authors mentioned the possible influence of the hyperactive immune system accompanying atopy on the inhibition of abnormal glioma cells. The results were unchanged via geographic conditions, study designs, and different atopic conditions. They reflect the protective effect of allergies on glioma growth. The authors pointed out that methodologic limitations are unlikely to explain the observed effect and that there is also considerable need for further prospective studies [11,12]. There are reports on the familial occurrence of glioma, and genetic predisposition to inherited glioma has been observed in 5–10% of cases [13]. Finally, some rare genetic disorders such as types 1 and 2 neurofibromatosis and tuberous sclerosis are also associated with an increased incidence of cancers including glioma [14]. An interesting issue—although rarely mentioned in the literature—is the presence of factors reducing the risk of glioma, such as the occurrence of asthma, genotypes associated with a higher risk of developing asthma, and allergies [11,12].
Equally interesting risk factors in many cancers are also simple anthropometric parameters such as gender [15,16,17], age [18,19,20], body weight [21,22], and height [23,24,25] or, additionally, in the case of central nervous system tumors, anatomical location.
To establish risk factors identifying at-risk groups that should have increased medical supervision, tests based on computed tomography (CT) or magnetic resonance imaging (MRI), which are the basic tools for the detection of gliomas, should be included [26,27,28]. This might help to increase tumor detection rates at an early stage of development, which is particularly important in light of nonspecific symptoms of malignant gliomas, often underestimated or misinterpreted by patients [26,29].
In order to determine at-risk groups and without a full understanding of the pathophysiology of glioma, it seems justified to collect extensive and detailed data on gender, age, height, weight, and histological type in the population of GBM patients. The obtained data could be helpful in creating accurate research assumptions or in faster diagnosis of nonspecific symptoms in specific groups of patients.
This work is a summary of the current data obtained from epidemiological reports available in online databases and aims to highlight the importance of maintaining accurate and reliable epidemiological records regarding gliomas. The PubMed search engine (https://pubmed.ncbi.nlm.nih.gov) was used to find articles for this work, using the following keywords: GBM and epidemiology and glioblastoma or GBM and sex or gender or age or overweight or obesity or height. From the resulting collection of articles, we selected those reviews of epidemiological data that have chapters on gender, age, and tumor location and, at the same time, have been published over the last 5 years (2015–2020). We excluded articles without specific figures as well as those not describing the histological types of tumors. In the case of height and weight, we also included older papers due their high scientific significance.
Importantly, in this paper, we present various statistical data which cannot be compared directly due to differences in definitions, methods of data collection, calculation of indicators, or delays in reporting. Nonetheless, parallel results in research may suggest the existence of certain trends regarding the effect of various GBM risk factors.
2. Characteristics of Glioblastoma Multiforme
Glioblastoma multiforme is a malignant primary tumor of the central nervous system. The incidence of GBM varies depending on the analyzed report, e.g., 3.20 [30], 4.06 [31], 4.17 [32], 4.40 [33], and 4.64 [34] per 100,000 inhabitants. GBM is therefore a rare disease. Alongside this, there is also a very poor prognosis [35]. Despite intensive research in recent years, the survival time has not changed significantly and, on average, does not exceed 15 months [36,37,38,39].
Highly malignant glioblastoma multiforme is divided into 3 subgroups according to the WHO classification (2016): IDH wild-type GBM (including giant cell GBM, gliosarcoma, and epithelioid GBM), IDH-mutant GBM, and GBM-NOS [2]. The molecular biomarkers of GBM are MGMT promoter methylation, IDH mutation, chromosome 1p/19q deletion, TERT promoter mutation, TP53 mutation, PTEN (Phosphatase and tensin homolog) mutation, CDKN2A (cyclin-dependent kinase inhibitor 2A) deletion, EGFR amplification, PDGFRA (platelet-derived growth factor receptor A) amplification, NF1 (Neurofibromin 1) mutation, MDM2 (Mouse double minute 2 homolog) amplification, and many others [40,41]. Three molecular changes are the most crucial for diagnosing GBM, namely the concurrent gain of whole chromosome 7 and loss of whole chromosome 10 (+7/−10), TERT promoter mutation, and EGFR amplification. These help identify the tumor as GBM even though a histological test may suggest it is a low-grade tumor [42].
Additionally, we distinguish primary and secondary GBM. The former, with a de novo tumor formation, is responsible for 90% of GBM cases and affects mainly elderly patients (above 55). Only 2% of primary GBM cases show mutations in IDH [43]. Secondary cancer develops from astrocytic tumors or oligodendrogliomas and affects younger patients; 80% of secondary GBM cases show mutations in IDH [43] Secondary GBM is also often associated with the mutation of TP53 [44].
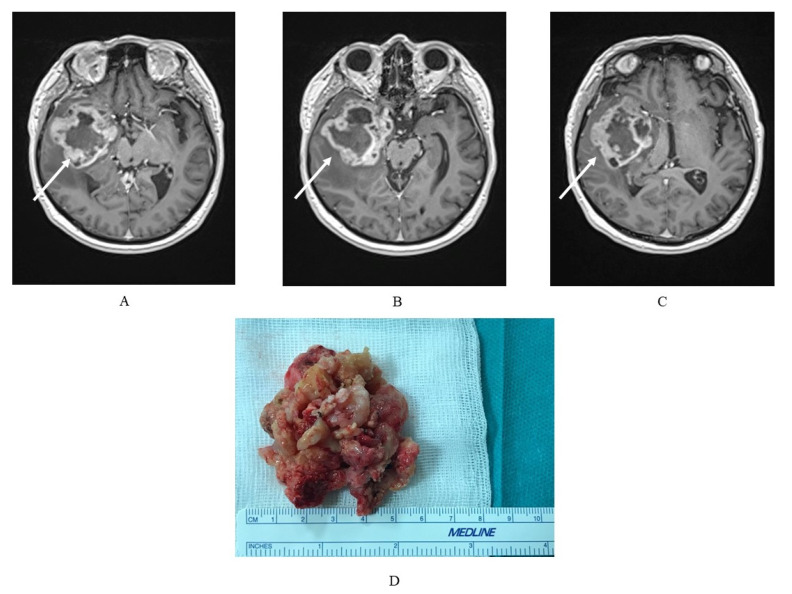
The macroscopic and histological structures of the glioblastoma multiforme are well described by the name of the tumor itself. Macroscopically, the tumor is eminently heterogeneous, with multicolored fields of yellow necrosis, calcifications, and hemorrhagic necrosis [35,45]. Microscopically, it is also heterogeneous, characterized by pleomorphic cells: small, low-diversified, and polynuclear giants with necrosis sites, pseudopalisadium nuclei, and proliferation in the range of micronuclei [45]. In the genetic aspect, the GBM also shows its heterogeneous character (genetic multiform) with various genetic abnormalities [45] (Figure 1).
Figure 1.
(A–C) Magnetic resonance imaging (MRI) exam of a glioblastoma multiforme (GBM) in a 75-year-old patient with a tumor localized in the right temporal lobe with a heterogeneous signal, and, characteristically for GBM, strong, irregular, marginal contrast enhancement and (D) the GBM tumor mass of the same patient, where macroscopically a visible, heterogeneous structure is typical.
Due to the highly aggressive nature of GBM, with a wide range of complications resulting from tumor growth itself and the applied treatment—surgical, chemotherapeutic (marrow suppression, nausea), or radiotherapeutic—improving the quality of life of patients is an essential element. The treatment of GBM should always be accompanied by rehabilitation and psychological support for both the patients themselves and their relatives.
3. The Influence of Gender on the Incidence of Glioblastoma Multiforme
Gender has a significant impact on the functioning of the entire human organism, which results in greater susceptibility or resistance to various diseases. For example, the female gender is a risk factor for osteoporosis or cardiovascular diseases. Sex can also be a factor that determines the prognosis, choice of treatment, or type of screening. Observing gender-specific differences in the prevalence of an individual disease may help design different testing patterns in order to increase the understanding of the causes and development of particular diseases.
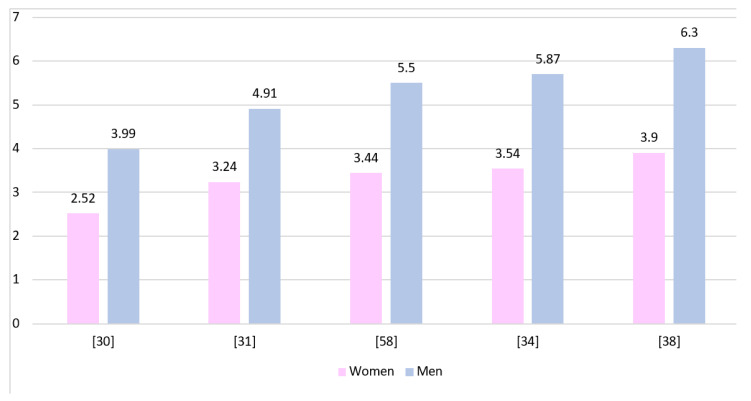
Figure 2 shows the GBM incidence rates in both genders presented by various authors. The rates have been standardized and calculated per 100,000 inhabitants. All presented studies show a higher prevalence of GBM in men, although due to different data collection protocols they cannot be directly compared to each other.
Figure 2.
The incidence rates of GBM presented for both genders and calculated per 100,000 inhabitants of each population.
In order to simplify data analysis, we used the male-to-female sex ratio in the populations presented in the literature. Epidemiological reports describing the occurrence of GBM clearly indicate a higher incidence of this histological type in men (Table 1). The ratio ranged from 1.2 to 2.6 for studies conducted on adult populations, 1.34–2.5 for general populations, and 1–1.5 for children. The increased incidence of GMB in men is associated with differences in the activity of retinoblastoma protein and functioning of cancer stem cells [46].
Table 1.
Male-to-female sex ratio in studies on GBM.
| Country | Dominant Sex | Gender Ratio | Years Range | Population Size | Patient Age | Literature |
|---|---|---|---|---|---|---|
| Adults | ||||||
| USA | Men | 1.148 | 1997–2010 | 3759 | >66 | Burton et al., 2015 [47] |
| USA | Men | 1.347 | 1973–2014 | 49,124 | >20 | K. Li et al., 2018 [48] |
| USA | Men | 1.35 | 2004–2013 | 24,262 | All | Xu et al., 2017 [49] |
| USA | Men | 1.364 | 1985–2014 | 25,117 | 18–60+ | Bin Abdulrahman et al., 2019 [50] |
| USA | Men | 1.375 | 2000–2014 | 6,430,706 | >20 | Gittleman et al., 2018 [33] |
| USA | Men | 1.38 | 2004–2015 | 30,767 | 18–70+ | Xie et al., 2018 [51] |
| USA | Men | 1.395 | 2010–2014 | 33,473 | >18 | Bohn et al., 2018 [36] |
| USA | Men | 1.45 | 2001–2011 | 1826 | >19 | Shabihkhani et al., 2017 [52] |
| Denmark | Men | 1.545 | 2009–2014 | 1364 | >18 | Hansen et al., 2018 [38] |
| China Singapore | Men | 1.547 | 2002–2011 | 107 | 13–85 | Cheo et al., 2017 [53] |
| USA | Men | 1.596 | 2000–2008 | 6586 | 18–70 | Tian et al., 2018 [54] |
| Sweden | Men | 1.6 | 2001–2012 | 143 | 18–99 | Bruhn et al., 2018 [37] |
| Germany | Men | 1.629 | 2011–2014 | 2382 | >18 | De Witt Hamer et al., 2019 [55] |
| China Hong Kong | Men | 1.833 | 2003–2005 2010–2012 |
68 | >18 | Chan et al., 2017 [56] |
| India | Men | 2.588 | 2012–2014 | 61 | 15–68 | Ghosh et al., 2017 [57] |
| Adults and children | ||||||
| Spain | Men | 1.338 | 1993–2014 | 463 | 0–74+ | Fuentes-Raspall et al., 2017 [58] |
| USA | Men | 1.359 | 2010–2014 | 56,421 | All | Ostrom et al., 2017 [30] |
| Canada | Men | 1.459 | 2009–2013 | 5830 | All | Walker et al., 2019 [31] |
| France | Men | 1.5 | 2008–2015 | 2053 | All | Fabbro-Peray et al., 2019 [32] |
| England | Men | 1.503 | 2007–2011 | 10,743 | 0–85+ | Brodbelt et al., 2015 [34] |
| Iran | Men | 2.476 | 2011–2016 | 73 | All | Salehpour et al., 2019 [59] |
| Children | ||||||
| USA | Men | 1.102 | 1973–2013 | 154 | 0–18 | Maxwell et al., 2018 [60] |
| USA | Women | 1.049 | 1973–2013 | 252 | 0–5 | Liu et al., 2018 [39] |
| Men | 1.278 | 1973–2013 | 287 | 6–10 | ||
| Men | 1.438 | 1973–2013 | 634 | 11–19 | ||
| USA | Men | 1.455 | 2000–2010 | 302 | <20 | Lam et al., 2018[61] |
Gender ratio—the quantitative ratio of the dominant sex to the other sex in the study group.
Gittleman et al. [33] described a statistically significant increase in the incidence of GBM in men, and according to Walker et al. [31], it is unlikely that the continual predominance of men as patients with malignant histological types of glioma is accidental. Additionally, there are also studies that report the same gender distribution among GBM patients of different geographic origin [36,62]. On this basis, it could be concluded that gender and the resulting biological differences are associated with a higher incidence of GBM among men. This is contrary to the conclusions of Tian et al. [54], who show statistically significant differences in gender distribution between races. There are some studies that also show statistically significant gender-related differences in GBM survival [52,54], although there are others where the differences are not statistically significant [33,34,36]. In the study by Shabihkhani et al. [52], the male gender was found to have a statistically significant risk factor with regard to survival, with a hazard ratio equal to 1.053 (CI (confidence interval) 1.022–1.085), while Tian et al. [54] and Xie et al. [51] indicated a higher survival of female patients with GBM at hazard ratios equal to 0.906 (CI 0.859–0.954) and 0.937 (CI 0.914–0.961), respectively. Gender does not influence the incidence of IDH1 mutation in GBM [63,64]. One study indicates increased TERT promoter mutation in men with GBM [65], although this has not been confirmed in other reports [66,67,68].
4. Effect of Age on the Incidence of Glioblastoma Multiforme
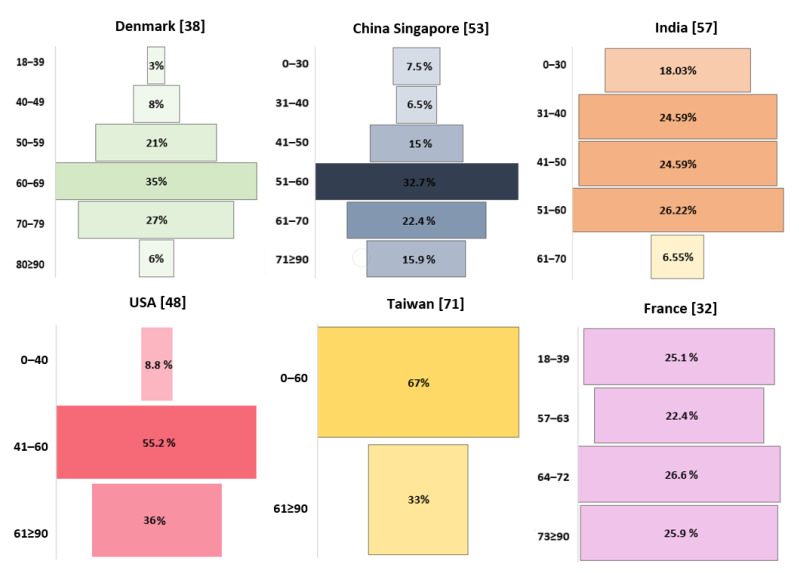
Age, a factor analyzed in research on many diseases, is also a contributing factor of most cancers, with recommendations for specific screening tests for patients of certain ages. Therefore, it seems obvious that age can also significantly influence the incidence of GBM (Figure 3).
Figure 3.
Age-related incidence of GBM.
In the study by Hansen et al., the majority of patients with GBM (83%) were over 50 years old [38], while Cheo et al. and Gosh et al. reported that it was most common in those in their 60s [53,57]. In the study by Li et al., 47.9% of patients were aged 65+ years, with incidences peaking between 75 and 79 years of age, followed by a drop [48].
In children, the frequency of tumors in a central location decreases with age (p < 0.0001) [39]. In addition, an increase in age in the entire population correlates with a decrease in survival rates [38] and is considered a significant risk factor [37,55], with a hazard ratio of 1.54 (CI 1.46–1.62, p < 0.00001) [55]. In a study by Bohn et al. [36], the hazard ratio for 3-year survival in GBM was 2.18 (1.91–2.49) times higher for patients over 50 years of age (p < 0.001).
Age as a significant mortality risk factor was also confirmed in children aged from 6 to 10 years, with a hazard ratio equaling 1.408 (CI: 1.069–1.854, p = 0.01), and in those aged from 11 to 19 years, with a hazard ratio equal to 1.406 (CI: 1.094–1.806, p = 0.0077) [25]. According to Ghosh et al., the median survival rate is highest between the ages of 31 and 40 [57]: 8.8 months for patients under 50 years of age and 4.55 months for those older than 50 (p = 0.016). Other reports confirm that the median survival rate of GBM patients decreases with age [36,57,69], and according to Cheo et al., it is the lowest among those aged 70 and above. Importantly, the patient’s age is associated with IDH mutation. IDH1 mutation is more frequent in younger patients than in older patients [63,64,70], while the opposite is true for TERT promoter mutation [65,66,67,68], PTEN mutation, and MGMT methylation [70]. In young patients with GBM (18–45 years), TP53 mutation is more frequent than in older patients (46 years and above) [70].
5. Glioblastoma Multiforme Incidence Depending on Tumor Location in the Central Nervous System
Most epidemiological reports show GBM tumors in the central nervous system, with the detailed location determined using various methods. Although some works refer to the ICD-O-3 Code (International Statistical Classification of Diseases and Related Health Problems), which facilitates a reliable reading of the results, this practice is not common enough to apply it to every epidemiological report. Some works described the location only in a very general way, e.g., by indicating a hemisphere.
According to the study by Fabbro-Peray et al. [32] on 2053 GBM patients in France, 47.1% had a tumor located in the right hemisphere, 45.7% had tumors in the left hemisphere, and 7.2% showed a “central” location. In the study of Ghosh et al., on Indian patients [57], the incidence of a tumor in the right hemisphere was found in 50.81% of cases, in the left hemisphere was found in 39.34%, while in the central location was found in 9.83% of cases. Of course, it should not be concluded that the Indian population is more likely to have GBM in the right hemisphere than the French population, as the latter study was conducted on a considerably smaller group of 61 patients. In addition, a question also arises whether this location description is accurate enough to be used for interstudy comparisons. Another classification adds the category of “other locations”. For example, in the population of 1173 patients in the U.S. aged under 19 years studied by Liu et al. [39], 48.02% of tumors were located in the hemispheres of the brain, 30.16% were in the “brain center”, and 22.83% were in another location. Another study, also in the U.S., showed that 72% of the tumors had a supratentorial location and that only 1.1% were subtentorial, with 29.9% showing a different location [48]. The prevalence of GBM in a supratentorial location (71.6%) was confirmed by a study by Bin Abdulrahman et al. [50]. A study on children by Lam et al. [61] showed that supratentorial tumors accounted for 61.9% of cases and were associated with better survival than subtentorial tumors (p = 0.002).
A common way to describe tumor location is the SEER (Surveillance, Epidemiology, and End Results) system with the following categories: “not a paired site”, when the origin can be attributed to neither side; “right: origin of primary”; “left: origin of primary”; “only one side involved”, when the tumor is unilateral with unknown origin; “bilateral involvement”, used for a bilateral tumor where the side of origin is unknown; and “paired site”, when the tumor has a central site of origin. The SEER system was used in the studies of Xu et al. [49] and Xie et al. [51], with “not a paired site” reported in 16.1% and 16% of cases, “paired site” reported in 1.3% and 0.9%, “one side involvement” reported in 81.3% and 81.6%, and “bilateral involvement” reported in 1.3% and 1.5% of cases, respectively. Both studies were conducted by Chinese teams and covered the same time period, albeit Xie et al.’s [51] analysis covered a period that was two years longer, focusing on the relationship between civil status and survival and including data on the location of the tumor.
The most common way to present location data is to specify the following structures: frontal lobe, temporal lobe, parietal lobe, occipital lobe, cerebellum, brain stem, and other location. Although the percentage distribution of the location in this classification varies between reports (Table 2), one of the recurring trends is the rare occurrence of GBM in the cerebellum, although the work of Shieh et al. [71] contradicts this by indicating that there is a 25% share of this location. Another correlation is the rare occurrence of GBM in the occipital lobe among all brain lobes. A study by Li et al. [72] compared the incidence of GBM in the frontal and temporal lobes in 406 Chinese patients and showed that the tumor location closer to the ventricle is statistically significantly more frequent in the frontal than the temporal lobe. Additionally, the lobes differed in the IDH mutation (p = 0.021) or MGMT promoter methylation status (p = 0.012). Patients with a tumor in the frontal lobe showed significantly shorter survival levels than patients with a tumor in the temporal lobe. However, no difference was observed in the size or sides of the tumor in both lobes. Patients with a tumor crossing the midline and with a central location had worse survival outcomes than others [38].
Table 2.
Summary of percentage distributions of GBM locations in the central nervous.
| Country | Frontal Lobe | Temporal Lobe | Parietal Lobe | Occipital Lobe | Cerebellum | Central | Brainstem | Brain | NOS Chamber | NOS Brain | Overlapping Brain Damage |
Other | Years Range | Population Size | Literature |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA | 38 | 35 | 21 | 6 | - | - | - | - | - | - | - | - | 2010–2014 | 33,473 | Bohn et al., 2018 [36] |
| England | 24.9 | 21.8 | 16.7 | 4.8 | 0.5 | - | 0.4 | - | - | - | - | 30.9 | 2007–2011 | 10,743 | Brodbelt et al., 2015 [34] |
| USA | 25.25 | 23.6 | 16.7 | 4.2 | 0.5 | - | 0.5 | 3.6 | 0.4 | 0.4 | 17.6 | - | 2000–2008 | 6586 | Tian et al., 2018 [54] |
| Denmark | 30 | 32 | 19 | 11 | - | 8 | - | - | - | - | - | - | 2009–2014 | 1364 | Hansen et al., 2018 [38] |
| USA | 22.8 | 22.8 | 54.3 | - | - | - | - | - | - | - | - | - | 2000–2010 | 302 | Lam et al., 2018 [61] |
| India | 42.62 | 9.83 | 9.83 | 4.91 | - | - | - | - | - | - | - | 31.78 | 2012–2014 | 61 | Ghosh et al., 2017 [57] |
| Taiwan | 31 | 13 | 6 | 9 | 25 | - | - | - | - | - | - | 17 | 2005–2016 | 44 | Shieh et al., 2020 [71] |
6. Influence of Overweight and Obesity on the Incidence of Glioblastoma Multiforme
It is estimated that, in 2015, 1.9 billion adults were affected by overweight and another 609 million were obese [73]. In the U.S. alone, 64% of the population is overweight and 28% is obese. The problem of abnormal body weight mainly affects the populations of developed countries, but it is also growing in developing countries [73]. Obesity is associated with many diseases, including cardiovascular diseases, hypertension, and type II diabetes. In recent years, obesity has become also a risk factor for many cancers, including endometrial cancer, kidney cancer, and esophageal adenocarcinoma [74]. Obesity also increases cancer mortality [75]. However, on the other hand, obesity has been shown to reduce the risk of some cancers, including lung cancer [21] and prostate cancer [22].
Studies available on PubMed show that obesity does not affect the risk of morbidity or mortality among GBM patients (Table 3). The first published study in 1989, which considered more than 11,000 patients from Norway, did not show a link between BMI and GBM [76]. This was confirmed by later studies. For example, Jones et al. did not find any correlation between BMI and the overall survival of GBM patients [77]. Wiedmann et al. did not show a correlation between BMI and the risk of GBM and other gliomas [78]. However, it seems that abnormal body weight does affect the processes associated with GBM development. It has been proven that abnormally high BMI at the age of 18 increases the incidence of this type of cancer at a later age [79]. This was confirmed by Little et al. on a group of 1111 patients with glioma, including 694 patients with GBM [80]. Being underweight at 21 years of age was associated with a decrease in the incidence of glioma, and obesity (BMI > 30) at 21 years of age increased the incidence of glioma at a later age—each additional kilogram of weight in the overweight increased the risk of glioma by 4% [80]. At the time of diagnosis, BMI was not associated with the incidence of glioma or GBM. Nevertheless, BMI may serve as a predictive factor. Potharaju et al. showed, in a study on 392 GBM patients that overweight and obesity improved overall survival [81]. On the other hand, a study by Jones et al. on a 3 times larger population of GBM patients showed that BMI does not affect overall survival levels [77].
Table 3.
Summary of studies on the association of BMI with the risk of GBM.
| Country | Tested Rate | Observations | Population Size | Literature |
|---|---|---|---|---|
| Norway | Risk factor | No significant interactions | 11,144 | Helseth et al., 1989 [76] |
| Norway | Risk factor | No significant interactions | 3102 | Wiedmann et al., 2017 [78] |
| USA | Risk factor | BMI at 18 correlates with the incidence rate at a later age | 480 | Moore et al., 2009 [79] |
| USA | Risk factor | BMI at 21 correlates with the incidence rate at a later age | 1111 glioma (group with 694 GBM cases) | Little et al., 2013 [80] |
| USA | Overall survival | No significant interactions | 1259 | Jones et al., 2010 [77] |
| India | Prognostic Marker/overall survival | Obesity/overweight improves prognosis | 392 | Potharaju et al., 2018 [81] |
7. Height as a Risk Factor for GBM Incidence
Height is a risk factor for many cancers. Meta-analyses have shown that it can be associated with colorectal cancer [23], kidney cancer [24], pancreatic cancer [25], prostate cancer [82], and thyroid cancer [24]. This is related to the fact that genes and signaling pathways responsible for high growth also participate in cancer processes [83]. Another important link between height and cancer risk is the level of various hormones responsible for being tall. In particular, cancer incidence is promoted by high levels of insulin-like growth factors (IGF) and growth hormones (GH) [84,85,86] that naturally occur in tall people.
Available studies also show that growth is strongly linked to the incidence of GBM (Table 4), although genetic research has not shown that height has any influence on the frequency of IDH1 mutation in glioma [78]. The first published study, from 1989, shows that each additional 15 cm of height in men is associated with a 36% higher incidence rate of GBM [77]. In women, on the other hand, it was only 18% higher and was not statistically significant. The same correlation was observed by Wiedmann et al., who observed that, in a group of 3102 patients with GBM, both taller men and women were at a higher risk of GBM [78], with specific numbers similar to those reported by Helseth et al. [76]. In another study on a group of 321 GBM patients, Cote et al. [87] observed that every additional inch of height increased the risk of GBM by 7%. Similar results were obtained by Kitahara et al., who showed that, in both sexes, height is a significant risk factor for GBM [88], although much smaller than in the three aforementioned studies. In men, each additional 5 cm of height resulted in an 8% higher risk of GBM, while in women, it was only 4%.
Table 4.
Height as a GBM risk factor: review of the literature.
| Country | Tested Rate | OR | Observations | Population Size | Literature |
|---|---|---|---|---|---|
| Norway | Risk factor | 1.36 (+15 cm) | Positive correlation in men | 6391 (men) | Helseth et al., 1989 [76] |
| Norway | Risk factor | 1.18 (+15 cm) | No statistically significant correlation | 4752 (women) | Helseth et al., 1989 [76] |
| USA, Finland, Australia, Sweden | Risk factor | 1.08 (+5 cm) | Positive correlation in men | 405 (men) | Kitahara et al., 2012 [88] |
| USA, Finland, Australia, Sweden | Risk factor | 1.04 (+5 cm) | Positive correlation in women | 308 (women) | Kitahara et al., 2012 [88] |
| Norway | Risk factor | 1.24 (+10 cm) | Positive corelation | 3102 | Wiedmann et al., 2017 [78] |
| USA | Risk factor | 1.07 (+1 inch) | Positive correlation | 321 | Cote et al., 2018 [87] |
8. Conclusions
Differences in the results from different epidemiological reports may be caused by differences in intrapopulation gender ratios; insufficient sample sizes in relation to population size; different definitions of variables, e.g., in terms of including gliosarcoma or large cell glioma as GBM; or differences in the formulated inclusion and exclusion criteria. Due to the above reasons, these reports should not be directly compared with each other. The analysis of the data collected in this article seems to indicate a higher incidence of GBM in men or an increasing risk of its occurrence with age. Finally, our review indicates the need for more accurate and precise reporting of epidemiological data on the location of GBM tumors.
The use of molecular tests on GBM tumors provides a more accurate determination of the prevalence of each GBM subgroup. The differences in how often these subgroups occur between various studied populations must be compared against environmental conditions. Individual GBM subtypes differ in the initial stages of development due to differences in the molecular and immunological characteristics of the patient as well as in other external factors. The survival curves presented in various reports do not indicate any significant trends for each subgroup. Identical aggressive surgical resections followed by radio- and chemotherapy have resulted in either very long or extremely short survival times, suggesting that only the natural characteristics of the tumor, when analyzed along with that of the host’s, can provide a reasonable prognosis. The fact that this type of tumor occurs relatively frequently with significantly different survival rates led us to review the available literature data. This review shows that further comparative research is required in this field to obtain more standardized information that could be used to increase the efficacy of and to modify the process of the treatment.
Author Contributions
Conceptualization, D.S. and I.B.-B.; investigation, D.S., J.K., P.K., K.K., M.F. and I.G.; writing—original draft preparation, D.S., J.K., P.K., M.F. and K.K.; writing—review and editing, I.G., I.B.-B. and A.M.-M.; supervision, I.B.-B.; funding acquisition, D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the statutory budget of the Department of Biochemistry and Medical Chemistry Pomeranian Medical University in Szczecin, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeAngelis L.M., Mellinghoff I.K. Virchow 2011 or How to ID(H) Human Glioblastoma. J. Clin. Oncol. 2011;29:4473–4474. doi: 10.1200/JCO.2011.37.5873. [DOI] [PubMed] [Google Scholar]
- 2.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classi-fication of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Xu W., Yang H., Liu Y., Yang Y., Wang P., Kim S.H., Ito S., Yang C., Wang P., Xiao M.T., et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhury R., Yeoh K.K., Tian Y.M., Hillringhaus L., Bagg E.A., Rose N.R., Leung I.K.H., Li X.S., Woon E.C.Y., Yang M., et al. The Oncometabolite 2-Hydroxyglutarate Inhibits Histone Lysine Demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanson M., Marie Y., Paris S., Idbaih A., Laffaire J., Ducray F., El Hallani S., Boisselier B., Mokhtari K., Hoang-Xuan K., et al. Isocitrate Dehydrogenase 1 Codon 132 Mutation Is an Important Prognostic Biomarker in Gliomas. J. Clin. Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 6.Houillier C., Wang X., Kaloshi G., Mokhtari K., Guillevin R., Laffaire J., Paris S., Boisselier B., Idbaih A., Laigle-Donadey F., et al. IDH1 or IDH2 Mutations Predict Longer Survival and Response to Temozolomide in Low-Grade Gliomas. Neurology. 2010;75:1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 7.Fan C.H., Liu W.L., Cao H., Wen C., Chen L., Jiang G. O6-Methylguanine DNA Methyltransferase as a Promising Target for the Treatment of Temozolomide-Resistant Gliomas. Cell Death Dis. 2013;4:e876. doi: 10.1038/cddis.2013.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binabaj M.M., Bahrami A., ShahidSales S., Joodi M., Joudi Mashhad M., Hassanian S.M., Anvari K., Avan A. The Prognostic Value of MGMT Promoter Methylation in Glioblastoma: A Meta-Analysis of Clinical Trials. J. Cell. Physiol. 2018;233:378–386. doi: 10.1002/jcp.25896. [DOI] [PubMed] [Google Scholar]
- 9.Gladitz J., Klink B., Seifert M. Network-Based Analysis of Oligodendrogliomas Predicts Novel Cancer Gene Candidates within the Region of the 1p/19q Co-Deletion. Acta Neuropathol. Commun. 2018;6:49. doi: 10.1186/s40478-018-0544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu J., Shi Z., Jiang J. Cyclooxygenase-2 in Glioblastoma Multiforme. Drug Discov. Today. 2017;22:148–156. doi: 10.1016/j.drudis.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartzbaum J.A., Fisher J.L., Aldape K.D., Wrensch M. Epidemiology and Molecular Pathology of Glioma. Nat. Clin. Pract. Neurol. 2006;2:494–503. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- 12.Linos E., Raine T., Alonso A., Michaud D. Atopy and Risk of Brain Tumors: A Meta-Analysis. J. Natl. Cancer Inst. 2007;99:1544–1550. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 13.Fisher J.L., Schwartzbaum J.A., Wrensch M., Wiemels J.L. Epidemiology of Brain Tumors. Neurol. Clin. 2007;25:867–890. doi: 10.1016/j.ncl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Ohgaki H., Kleihues P. Genetic Alterations and Signaling Pathways in the Evolution of Gliomas. Cancer Sci. 2009;100:2235–2241. doi: 10.1111/j.1349-7006.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S.E., Paik H.Y., Yoon H., Lee J.E., Kim N., Sung M.K. Sex- and Gender-Specific Disparities in Colorectal Cancer Risk. World J. Gastroenterol. 2015;21:5167–5175. doi: 10.3748/wjg.v21.i17.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeCosse J.J., Ngoi S.S., Jacobson J.S., Cennerazzo W.J. Gender and Colorectal Cancer. Eur. J. Cancer Prev. 1993;2:105–115. doi: 10.1097/00008469-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lucca I., Klatte T., Fajkovic H., De Martino M., Shariat S.F. Gender Differences in Incidence and Outcomes of Urothelial and Kidney Cancer. Nat. Rev. Urol. 2015;12:585–592. doi: 10.1038/nrurol.2015.232. [DOI] [PubMed] [Google Scholar]
- 18.Fane M., Weeraratna A.T. How the Ageing Microenvironment Influences Tumour Progression. Nat. Rev. Cancer. 2020;20:89–106. doi: 10.1038/s41568-019-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tricoli J.V., Bleyer A. Adolescent and Young Adult Cancer Biology. Cancer J. 2018;24:267–274. doi: 10.1097/PPO.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 20.Sandiford O.A., Moore C.A., Du J., Boulad M., Gergues M., Eltouky H., Rameshwar P. Advances in Experimental Medicine and Biology. Vol. 1056. Springer; Cham, Switzerland: 2018. Human Aging and Cancer: Role of MiRNA in Tumor Microenvironment; pp. 137–152. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y., Dong J., Sun K., Zhao L., Zhao F., Wang L., Jiao Y. Obesity and Incidence of Lung Cancer: A Meta-Analysis. Int. J. Cancer. 2013;132:1162–1169. doi: 10.1002/ijc.27719. [DOI] [PubMed] [Google Scholar]
- 22.Porter M.P., Stanford J.L. Obesity and the Risk of Prostate Cancer. Prostate. 2005;62:316–321. doi: 10.1002/pros.20121. [DOI] [PubMed] [Google Scholar]
- 23.Song X., Gong X., Zhang T., Jiang W. Height and Risk of Colorectal Cancer. Eur. J. Cancer Prev. 2018;27:521–529. doi: 10.1097/CEJ.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 24.Liang S., Lv G., Chen W., Jiang J., Wang J. Height and Kidney Cancer Risk: A Meta-Analysis of Prospective Studies. J. Cancer Res. Clin. Oncol. 2015;141:1799–1807. doi: 10.1007/s00432-014-1870-5. [DOI] [PubMed] [Google Scholar]
- 25.Aune D., Vieira A.R., Chan D.S.M., Rosenblatt D.A.N., Vieira R., Greenwood D.C., Cade J.E., Burley V.J., Norat T. Height and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies. Cancer Causes Control. 2012;23:1213–1222. doi: 10.1007/s10552-012-9983-0. [DOI] [PubMed] [Google Scholar]
- 26.Alexander B.M., Cloughesy T.F. Adult Glioblastoma. J. Clin. Oncol. 2017;35:2402–2409. doi: 10.1200/JCO.2017.73.0119. [DOI] [PubMed] [Google Scholar]
- 27.Lotan E., Jain R., Razavian N., Fatterpekar G.M., Lui Y.W. State of the Art: Machine Learning Applications in Glioma Imaging. Am. J. Roentgenol. 2019;212:26–37. doi: 10.2214/AJR.18.20218. [DOI] [PubMed] [Google Scholar]
- 28.Pope W.B., Brandal G. Conventional and Advanced Magnetic Resonance Imaging in Patients with High-Grade Glioma. Q. J. Nucl. Med. Mol. Imaging. 2018;62:239–253. doi: 10.23736/S1824-4785.18.03086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed R., Oborski M.J., Hwang M., Lieberman F.S., Mountz J.M. Malignant Gliomas: Current Perspectives in Diagnosis, Treatment, and Early Response Assessment Using Advanced Quantitative Imaging Methods. Cancer Manag. Res. 2014;6:149–170. doi: 10.2147/CMAR.S54726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostrom Q.T., Gittleman H., Liao P., Vecchione-Koval T., Wolinsky Y., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(Suppl. 5):v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker E.V., Davis F.G. CBTR founding affiliates. Malignant Primary Brain and Other Central Nervous System Tumors Diagnosed in Canada from 2009 to 2013. Neuro Oncol. 2019;21:360–369. doi: 10.1093/neuonc/noy195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabbro-Peray P., Zouaoui S., Darlix A., Fabbro M., Pallud J., Rigau V., Mathieu-Daude H., Bessaoud F., Bauchet F., Riondel A., et al. Association of Patterns of Care, Prognostic Factors, and Use of Radiotherapy–Temozolomide Therapy with Survival in Patients with Newly Diagnosed Glioblastoma: A French National Population-Based Study. J. Neurooncol. 2019;142:91–101. doi: 10.1007/s11060-018-03065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gittleman H., Boscia A., Ostrom Q.T., Truitt G., Fritz Y., Kruchko C., Barnholtz-Sloan J.S. Survivorship in Adults with Malignant Brain and Other Central Nervous System Tumor from 2000–2014. Neuro Oncol. 2018;20:VII6–VII16. doi: 10.1093/neuonc/noy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodbelt A., Greenberg D., Winters T., Williams M., Vernon S., Collins V.P. Glioblastoma in England: 2007–2011. Eur. J. Cancer. 2015;51:533–542. doi: 10.1016/j.ejca.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Hanif F., Muzaffar K., Perveen K., Malhi S.M., Simjee S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017;18:3–9. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohn A., Braley A., De La Vega P.R., Carlos Zevallos J., Barengo N.C. The Association between Race and Survival in Glioblastoma Patients in the US: A Retrospective Cohort Study. PLoS ONE. 2018;13:e0198581. doi: 10.1371/journal.pone.0198581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruhn H., Strandéus M., Milos P., Hallbeck M., Vrethem M., Lind J. Improved Survival of Swedish Glioblastoma Patients Treated According to Stupp. Acta Neurol. Scand. 2018;138:332–337. doi: 10.1111/ane.12966. [DOI] [PubMed] [Google Scholar]
- 38.Hansen S., Rasmussen B.K., Laursen R.J., Kosteljanetz M., Schultz H., Nørgård B.M., Guldberg R., Gradel K.O. Treatment and Survival of Glioblastoma Patients in Denmark: The Danish Neuro-Oncology Registry 2009–2014. J. Neurooncol. 2018;139:479–489. doi: 10.1007/s11060-018-2892-7. [DOI] [PubMed] [Google Scholar]
- 39.Liu M., Thakkar J.P., Garcia C.R., Dolecek T.A., Wagner L.M., Dressler E.V.M., Villano J.L. National Cancer Database Analysis of Outcomes in Pediatric Glioblastoma. Cancer Med. 2018;7:1151–1159. doi: 10.1002/cam4.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furgason J.M., Koncar R.F., Michelhaugh S.K., Sarkar F.H., Mittal S., Sloan A.E., Barnholtz-Sloan J.S., Bahassi E.M. Whole Genome Sequence Analysis Links Chromothripsis to EGFR, MDM2, MDM4, and CDK4 Amplification in Glioblastoma. Oncoscience. 2015;2:618–628. doi: 10.18632/oncoscience.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen R., Smith-Cohn M., Cohen A.L., Colman H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics. 2017;14:284–297. doi: 10.1007/s13311-017-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulut O., Kilic G., Netea M.G., Netea M.G. The CIMPACT-NOW Updates and Their Significance to Current Neuro-Oncology Practice L. Neuro Oncol. Pract. 2020;30:1–14. doi: 10.1093/nop/npaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellai M., Piazzi A., Caldera V., Monzeglio O., Cassoni P., Valente G., Schiffer D. IDH1 and IDH2 Mutations, Immunohistochemistry and Associations in a Series of Brain Tumors. J. Neurooncol. 2011;105:345–357. doi: 10.1007/s11060-011-0596-3. [DOI] [PubMed] [Google Scholar]
- 44.Tamimi A.F., Juweid M. Glioblastoma. Codon Publications; Singapore: 2017. Epidemiology and Outcome of Glioblastoma; pp. 143–153. [DOI] [PubMed] [Google Scholar]
- 45.Tataranu L.G., Ciubotaru V., Cazac T.L., Alexandru O., Purcaru O.S., Tache D.E., Artene S.A., Dricu A. Brain Tumors—An Update. InTech; Londou, UK: 2018. Current Trends in Glioblastoma Treatment. [DOI] [Google Scholar]
- 46.Sun T., Warrington N.M., Luo J., Brooks M.D., Dahiya S., Snyder S.C., Sengupta R., Rubin J.B. Sexually Dimorphic RB Inactivation Underlies Mesenchymal Glioblastoma Prevalence in Males. J. Clin. Investig. 2014;124:4123–4133. doi: 10.1172/JCI71048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burton E., Ugiliweneza B., Woo S., Skirboll S., Boaky M. A Surveillance, Epidemiology and End Results-Medicare Data Analysis of Elderly Patients with Glioblastoma Multiforme: Treatment Patterns, Outcomes and Cost. Mol. Clin. Oncol. 2015;3:971–978. doi: 10.3892/mco.2015.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li K., Lu D., Guo Y., Wang C., Liu X., Liu Y., Liu D. Trends and Patterns of Incidence of Diffuse Glioma in Adults in the United States, 1973–2014. Cancer Med. 2018;7:5281–5290. doi: 10.1002/cam4.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H., Chen J., Xu H., Qin Z. Geographic Variations in the Incidence of Glioblastoma and Prognostic Factors Predictive of Overall Survival in US Adults from 2004–2013. Front. Aging Neurosci. 2017;9:352. doi: 10.3389/fnagi.2017.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bin Abdulrahman A.K., Bin Abdulrahman K.A., Bukhari Y.R., Faqihi A.M., Ruiz J.G. Association between Giant Cell Glioblastoma and Glioblastoma Multiforme in the United States: A Retrospective Cohort Study. Brain Behav. 2019;9:e01402. doi: 10.1002/brb3.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie J.C., Yang S., Liu X.Y., Zhao Y.X. Effect of Marital Status on Survival in Glioblastoma Multiforme by Demographics, Education, Economic Factors, and Insurance Status. Cancer Med. 2018;7:3722–3742. doi: 10.1002/cam4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shabihkhani M., Telesca D., Movassaghi M., Naeini Y.B., Naeini K.M., Hojat S.A., Gupta D., Lucey G.M., Ontiveros M., Wang M.W., et al. Incidence, Survival, Pathology, and Genetics of Adult Latino Americans with Glioblastoma. J. Neurooncol. 2017;132:351–358. doi: 10.1007/s11060-017-2377-0. [DOI] [PubMed] [Google Scholar]
- 53.Cheo S.T.T., Lim G.H., Lim K.H.C. Glioblastoma Multiforme Outcomes of 107 Patients Treated in Two Singapore Institutions. Singap. Med. J. 2017;58:41–45. doi: 10.11622/smedj.2016044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian M., Ma W., Chen Y., Yu Y., Zhu D., Shi J., Zhang Y. Impact of Gender on the Survival of Patients with Glioblastoma. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Witt Hamer P.C., Ho V.K.Y., Zwinderman A.H., Ackermans L., Ardon H., Boomstra S., Bouwknegt W., van den Brink W.A., Dirven C.M., van der Gaag N.A., et al. Between-Hospital Variation in Mortality and Survival after Glioblastoma Surgery in the Dutch Quality Registry for Neuro Surgery. J. Neurooncol. 2019;144:313–323. doi: 10.1007/s11060-019-03229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan D.T.M., Hsieh S.Y.P., Lau C.K.Y., Kam M.K.M., Loong H.H.F., Tsang W.K., Poon D.M.C., Poon W.S. Ten-Year Review of Survival and Management of Malignant Glioma in Hong Kong. Hong Kong Med. J. 2017;23:134–139. doi: 10.12809/hkmj164879. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh M., Shubham S., Mandal K., Trivedi V., Chauhan R., Naseera S. Survival and Prognostic Factors for Glioblastoma Multiforme: Retrospective Single-Institutional Study. Indian J. Cancer. 2017;54:362–367. doi: 10.4103/ijc.IJC_157_17. [DOI] [PubMed] [Google Scholar]
- 58.Fuentes-Raspall R., Solans M., Roca-Barceló A., Vilardell L., Puigdemont M., del Barco S., Comas R., García-Velasco A., Astudillo A., Carmona-Garcia M.C., et al. Descriptive Epidemiology of Primary Malignant and Non-Malignant Central Nervous Tumors in Spain: Results from the Girona Cancer Registry (1994–2013) Cancer Epidemiol. 2017;50:1–8. doi: 10.1016/j.canep.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Salehpour F., Mirzaei F., Meshkini A., Parsay S., Salehi S., Asl M.M.B. Trends in Primary Brain Tumors: A 5-Year Retrospective Histologically Confirmed Study in Tabriz, Iran, 2011–2016. Asian J. Neurosurg. 2019;14:427–431. doi: 10.4103/ajns.AJNS_212_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maxwell R., Luksik A.S., Garzon-Muvdi T., Yang W., Huang J., Bettegowda C., Jallo G.I., Terezakis S.A., Groves M.L. Population-Based Study Determining Predictors of Cancer-Specific Mortality and Survival in Pediatric High-Grade Brainstem Glioma. World Neurosurg. 2018;119:e1006–e1015. doi: 10.1016/j.wneu.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 61.Lam S., Lin Y., Zinn P., Su J., Pan I.W. Patient and Treatment Factors Associated with Survival among Pediatric Glioblastoma Patients: A Surveillance, Epidemiology, and End Results Study. J. Clin. Neurosci. 2018;47:285–293. doi: 10.1016/j.jocn.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 62.Shanmugavadivel D., Liu J.F., Murphy L., Wilne S., Walker D. Accelerating Diagnosis for Childhood Brain Tumours: An Analysis of the HeadSmart UK Population Data. Arch. Dis. Child. 2019;105:355–362. doi: 10.1136/archdischild-2018-315962. [DOI] [PubMed] [Google Scholar]
- 63.Nobusawa S., Watanabe T., Kleihues P., Ohgaki H. IDH1 Mutations as Molecular Signature and Predictive Factor of Secondary Glioblastomas. Clin. Cancer Res. 2009;15:6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 64.Bleeker F.E., Atai N.A., Lamba S., Jonker A., Rijkeboer D., Bosch K.S., Tigchelaar W., Troost D., Van Dertop W.P., Bardelli A., et al. The Prognostic IDH1R132 Mutation Is Associated with Reduced NADP+-Dependent IDH Activity in Glioblastoma. Acta Neuropathol. 2010;119:487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong D.E., Woo S.R., Nam H., Nam D.H., Lee J.H., Joo K.M. Preclinical and Clinical Implications of TERT Promoter Mutation in Glioblastoma Multiforme. Oncol. Lett. 2017;14:8213–8219. doi: 10.3892/ol.2017.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon M., Hosen I., Gousias K., Rachakonda S., Heidenreich B., Gessi M., Schramm J., Hemminki K., Waha A., Kumar R. TERT Promoter Mutations: A Novel Independent Prognostic Factor in Primary Glioblastomas. Neuro Oncol. 2015;17:45–52. doi: 10.1093/neuonc/nou158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen H.N., Lie A., Li T., Chowdhury R., Liu F., Ozer B., Wei B., Green R.M., Ellingson B.M., Wang H.J., et al. Human TERT Promoter Mutation Enables Survival Advantage from MGMT Promoter Methylation in IDH1 Wild-Type Primary Glioblastoma Treated by Standard Chemoradiotherapy. Neuro Oncol. 2017;19:394–404. doi: 10.1093/neuonc/now189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kikuchi Z., Shibahara I., Yamaki T., Yoshioka E., Shofuda T., Ohe R., Matsuda K., Saito R., Kanamori M., Kanemura Y., et al. TERT Promoter Mutation Associated with Multifocal Phenotype and Poor Prognosis in Patients with IDH Wild-Type Glioblastoma. Neuro Oncol. Adv. 2020;2:1–10. doi: 10.1093/noajnl/vdaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang K.B., Wang T.T., Woon C.T., Cheah E.S., Moore X.L., Zhu C., Wong M.C. Enhancement of Glioblastoma Radioresponse by a Selective COX-2 Inhibitor Celecoxib: Inhibition of Tumor Angiogenesis with Extensive Tumor Necrosis. Int. J. Radiat. Oncol. 2007;67:888–896. doi: 10.1016/j.ijrobp.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 70.Ferguson S.D., Xiu J., Weathers S.P., Zhou S., Kesari S., Weiss S.E., Verhaak R.G., Hohl R.J., Barger G.R., Reddy S.K., et al. GBM-Associated Mutations and Altered Protein Expression Are More Common in Young Patients. Oncotarget. 2016;7:69466–69478. doi: 10.18632/oncotarget.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shieh L.T., Guo H.R., Chang Y.K., Lu N.M., Ho S.Y. Clinical Implications of Multiple Glioblastomas: An Analysis of Prognostic Factors and Survival to Distinguish from Their Single Counterparts. J. Formos. Med. Assoc. 2020;119:728–734. doi: 10.1016/j.jfma.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 72.Li H.Y., Sun C.R., He M., Yin L.C., Du H.G., Zhang J.M. Correlation Between Tumor Location and Clinical Properties of Glioblastomas in Frontal and Temporal Lobes. World Neurosurg. 2018;112:e407–e414. doi: 10.1016/j.wneu.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 73.Chooi Y.C., Ding C., Magkos F. The Epidemiology of Obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Fang X., Wei J., He X., Lian J., Han D., An P., Zhou T., Liu S., Wang F., Min J. Quantitative Association between Body Mass Index and the Risk of Cancer: A Global Meta-Analysis of Prospective Cohort Studies. Int. J. Cancer. 2018;143:1595–1603. doi: 10.1002/ijc.31553. [DOI] [PubMed] [Google Scholar]
- 75.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 76.Helseth A., Tretli S. Pre-Morbid Height and Weight as Risk Factors for Development of Central Nervous System Neoplasms. Neuroepidemiology. 1989;8:277–282. doi: 10.1159/000110195. [DOI] [PubMed] [Google Scholar]
- 77.Jones L.W., Ali-Osman F., Lipp E., Marcello J.E., McCarthy B., McCoy L., Rice T., Wrensch M., Il’Yasova D. Association between Body Mass Index and Mortality in Patients with Glioblastoma Mutliforme. Cancer Causes Control. 2010;21:2195–2201. doi: 10.1007/s10552-010-9639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiedmann M.K.H., Brunborg C., Di Ieva A., Lindemann K., Johannesen T.B., Vatten L., Helseth E., Zwart J.A. The Impact of Body Mass Index and Height on the Risk for Glioblastoma and Other Glioma Subgroups: A Large Prospective Cohort Study. Neuro Oncol. 2017;19:976–985. doi: 10.1093/neuonc/now272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moore S.C., Rajaraman P., Dubrow R., Darefsky A.S., Koebnick C., Hollenbeck A., Schatzkin A., Leitzmann M.F. Height, Body Mass Index, and Physical Activity in Relation to Glioma Risk. Cancer Res. 2009;69:8349–8355. doi: 10.1158/0008-5472.CAN-09-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Little R.B., Madden M.H., Thompson R.C., Olson J.J., LaRocca R.V., Pan E., Browning J.E., Egan K.M., Nabors L.B. Anthropometric Factors in Relation to Risk of Glioma. Cancer Causes Control. 2013;24:1025–1031. doi: 10.1007/s10552-013-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Potharaju M., Mangaleswaran B., Mathavan A., John R., Thamburaj V., Ghosh S., Ganesh S., Kalvakonda C., Loganathan M., Bapu S., et al. Body Mass Index as a Prognostic Marker in Glioblastoma Multiforme: A Clinical Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2018;102:204–209. doi: 10.1016/j.ijrobp.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 82.Sequoia J.S.P., Wright M.E., McCarron P., Pietinen P., Taylor P.R., Virtamo J., Albanes D. A Prospective Investigation of Height and Prostate Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2006;15:2174–2178. doi: 10.1158/1055-9965.EPI-06-0467. [DOI] [PubMed] [Google Scholar]
- 83.Tripaldi R., Stuppia L., Alberti S. Human Height Genes and Cancer. Biochim. Biophys. Acta Rev. Cancer. 2013;1836:27–41. doi: 10.1016/j.bbcan.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Perry J.K., Liu D.X., Wu Z.S., Zhu T., Lobie P.E. Growth Hormone and Cancer: An Update on Progress. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20:307–313. doi: 10.1097/MED.0b013e328363183a. [DOI] [PubMed] [Google Scholar]
- 85.Watts E.L., Perez-Cornago A., Appleby P.N., Albanes D., Ardanaz E., Black A., Bueno-de-Mesquita H.B., Chan J.M., Chen C., Chubb S.A.P., et al. The Associations of Anthropometric, Behavioural and Sociodemographic Factors with Circulating Concentrations of IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3 in a Pooled Analysis of 16,024 Men from 22 Studies. Int. J. Cancer. 2019;145:3244–3256. doi: 10.1002/ijc.32276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boguszewski C.L., Boguszewski M.C. da S. Growth Hormone’s Links to Cancer. Endocr. Rev. 2019;40:558–574. doi: 10.1210/er.2018-00166. [DOI] [PubMed] [Google Scholar]
- 87.Cote D.J., Downer M.K., Smith T.R., Smith-Warner S.A., Egan K.M., Stampfer M.J. Height, Waist Circumference, Body Mass Index, and Body Somatotype across the Life Course and Risk of Glioma. Cancer Causes Control. 2018;29:707–719. doi: 10.1007/s10552-018-1052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kitahara C.M., Wang S.S., Melin B.S., Wang Z., Braganza M., Inskip P.D., Albanes D., Andersson U., Beane Freeman L.E., Buring J.E., et al. Association between Adult Height, Genetic Susceptibility and Risk of Glioma. Int. J. Epidemiol. 2012;41:1075–1085. doi: 10.1093/ije/dys114. [DOI] [PMC free article] [PubMed] [Google Scholar]