Abstract
In dairy processing environments, many bacterial species adhere and form biofilms on surfaces and equipment, leading to foodborne illness and food spoilage. Among them, Listeria monocytogenes and Pseudomonas spp. could be present in mixed-species biofilms. This study aimed to evaluate the interactions between L. monocytogenes and P. fluorescens in biofilms simulating dairy processing conditions, as well as the capability of P. fluorescens in co-culture to produce the blue pigment in a Ricotta-based model system. The biofilm-forming capability of single- and mixed-cultures was evaluated on polystyrene (PS) and stainless steel (SS) surfaces at 12 °C for 168 h. The biofilm biomass was measured, the planktonic and sessile cells and the carbohydrates in biofilms were quantified. The biofilms were also observed through Confocal Laser Scanning Microscopy analysis. Results showed that only P. fluorescens was able to form biofilms on PS. Moreover, in dual-species biofilms at the end of the incubation time (168 h at 12 °C), a lower biomass compared to P. fluorescens mono-species was observed on PS. On SS, the biofilm cell population of L. monocytogenes was higher in the dual-species than in mono-species, particularly after 48 h. Carbohydrates quantity in the dual-species system was higher than in mono-species and was revealed also at 168 h. The production of blue pigment by P. fluorescens was revealed both in single- and co-culture after 72 h of incubation (12 °C). This work highlights the interactions between the two species, under the experimental conditions studied in the present research, which can influence biofilm formation (biomass and sessile cells) but not the capability of P. fluorescens to produce blue pigment.
Keywords: Listeria monocytogenes, Pseudomonas fluorescens, multi-species, biofilms, blue pigment, dairy product
1. Introduction
Microbial biofilms are three-dimensional structures of various bacteria that adhere to biotic or abiotic surfaces and differentiate into complex communities embedded within extracellular polymeric substances (EPSs) [1]. The relevance of microbial biofilms has been described in different fields including the food industry, where biofilms are responsible for potential food contamination, corrosion, and economic losses [2]. Particularly in the dairy industry, many bacterial species adhere and form biofilms on surfaces and equipment and, among them, Listeria monocytogenes and different species of Pseudomonas [3] are worthy of attention.
Listeria monocytogenes is a ubiquitous pathogen, able to colonize and persist on common surfaces in the food processing environments, thanks to its biofilm formation capability [4]. This psychrotrophic bacterial pathogen can contaminate a wide variety of foods. In particular, several dairy products such as Blue mold cheese, Camembert cheese, and Ricotta have been implicated in listeriosis outbreaks [5]. An increasing trend for human listeriosis cases has been observed from 2009 to 2018 with 2549 cases reported in Europe in 2018 [6]. Moreover, listeriosis has an important socio-economic impact, which can reach approximately €6327 per case [7].
The microbiota of refrigerated foods is dominated by selected microorganisms, such as Pseudomonas spp. In particular, P. fluorescens has been isolated from numerous food products including dairy products, as it can colonize and form biofilms onto surfaces of dairy processing plants [2]. Due to the production of hydrolytic enzymes and pigments such as pyoverdine, pyocianin, and indigoidine [8], P. fluorescens is responsible for food quality decay, food spoilage, reduced shelf-life [9,10], and defects, as in the case of dairy product blue discoloration [11]. In fact, discoloration due to the microbial activity is not an unusual phenomenon and affects several varieties of cheeses and dairy products made from raw or pasteurized milk [12]. Specifically, P. fluorescens produces several pigments, including pyoverdine and indigoidine [11,13]. Pyoverdines are fluorescent siderophores of pseudomonads that play important roles for growth under iron-limiting conditions, permitting their colonization of hosts, from humans to plants [13]. On the other hand, indigoidine is a blue diazadiphenoquinone pigment that can play an important role in tolerance to oxidative stress [14]. Recently, it was found that the pigmentation and the biofilm-forming ability of P. fluorescens are promoted at low incubation temperatures, suggesting their possible involvement in the spread and persistence of these strains in the dairy environment [15,16].
L. monocytogenes and P. fluorescens are able to form biofilms on different surfaces including polystyrene and stainless steel, which are materials commonly used in the food industry [17,18,19]. Dairy plants’ surfaces can be colonized by various microbial species in biofilms, also because of the increase in bacterial tolerance to common sanitizers. Biofilms found in nature are generally formed by two or more microbial species. In fact, multi-species biofilms are commonly encountered in food and food-related environments [20]. Multi-species interactions and the physiological conditions of microorganisms influence the properties of the formed biofilms. Compared to mono-species, mixed-species biofilms are known to provide advantages to microorganisms such as the increase in tolerance against stressful conditions and the capability to degrade organic compounds [21]. However, in multi-species biofilms, microbial cells can interact both positively and negatively [22]. In particular, on the basis of the different bacterial counts and biofilm biomass, the interactions between the two species are evaluated as synergistic (beneficial), neutralistic (no influence), or competitive (deleterious). In fact, when the biomass or the bacterial counts of a dual-species biofilm are greater than the sum of the two single-species biofilms under similar incubation conditions, the interaction is considered synergistic. On the other side, the interaction is evaluated as neutralistic if the biomass or the counts are equivalent, and competitive if they are less than the sum of the two [23].
Although studies on multi-species biofilms are of relevant importance and closer to natural conditions, most of the studies on biofilm formation analyze single-species biofilms [24,25,26]. In fact, only a few studies on bacterial biofilms are based on food model systems that mimic real environments [27,28,29]. Therefore, the objective of this study was to evaluate dual-species biofilms formed by L. monocytogenes and P. fluorescens in a system simulating real conditions encountered in dairy processing by using: (i) surfaces of polystyrene and stainless steel; (ii) L. monocytogenes and blue pigmenting P. fluorescens strains isolated from dairy products; (iii) Ricotta-based dairy model as the growth medium; and (iv) 12 °C as the incubation temperature.
2. Materials and Methods
2.1. Bacterial Strains
Eight strains of Listeria monocytogenes were tested together with one strain of Pseudomonas fluorescens (pf5), isolated from Mozzarella cheese and chosen for its capability to form biofilm and to produce blue pigment on Potato Dextrose Agar, Agar Mascarpone, and Mozzarella cheese [16]. Listeria monocytogenes strains previously isolated from dairy products (LM 1-2-3-4) and dairy plants (LM 5-6-7-8) were characterized and typed in this study. All of the strains were of Italian origin.
The strains were maintained at −80 °C with an anti-freezing agent (glycerol, 20% v/v, Sigma) to preserve the viability of the cells during storage.
2.2. Characterization and Typing of L. monocytogenes Strains
The strains of L. monocytogenes were characterized and typed according to two commonly used techniques: serotyping and Pulsed-Field Gel Electrophoresis (PFGE) [30,31].
Serotyping was carried out according to US Food and Drug Administration (FDA) Bacteriological Analytical Manual [32] using commercial antisera for flagellar and somatic antigens (Denkan Seikem Co. Ltd., Tokyo, Japan), following the manufacturers’ instructions.
PFGE typing was carried out according to the Centers for Disease Control and Prevention PulseNet protocol (CDC, PulseNet Methods PNL04), employing the restriction enzymes ApaI and AscI (New England BioLabs Inc., Ipswich, MA, USA). Salmonella enterica Braenderup H9812 digested with the restriction enzyme XbaI (New England BioLabs) was used as the reference size standard. The separation of restricted DNA fragments was performed for 20–22 h in 1.5% (w/v) Agarose gels in 0.5× Tris-Borate-EDTA buffer (5× TBE diluted in MilliQ water) at 14 °C, 6 V cm−1 with switch times of 4–40 s through CHEF Mapper® XA (Bio-Rad, Hercules, CA, USA). The images were acquired by using ChemiDocTM MP Imaging System (Bio-Rad, CA, USA) and the pulsotypes were analyzed with BioNumerics software version 7.5 (AppliedMaths, Sint-Latem, Belgium).
2.3. Inoculum
The strains were inoculated into Tryptic Soy Broth (TSB, Liofilchem, Roseto, Italy) from fresh microbial cultures, followed by overnight incubation at 37 °C (L. monocytogenes) and 30 °C (P. fluorescens). The bacterial cells were harvested by centrifugation (13,000 rpm for 5 min), washed three times with PBS (Phosphate Buffer Saline) solution, and the Optical Density (OD) of bacterial suspensions was measured by a spectrophotometer Lambda bio 20 (Perkin Elmer, Waltham, MA, USA) to obtain a cell count of about 105 CFU/mL in the growth medium [33]. In order to maintain the same load for the mono- and multi-species inocula, individual bacterial suspensions were diluted in a 1:1 ratio with the medium for the mono-culture, and with the other microbial species for the multi-culture.
2.4. Ricotta-Based Medium Preparation
The Ricotta-based dairy model was prepared following the method described by de Carvalho et al. [34] with some modifications. In detail, 160 g of Ricotta purchased from a local market were diluted in 1 L of distilled water, heated at 42 °C for 50 min in a water bath, and then autoclaved at 121 °C for 15 min. The broth was separated from the solid part by a sterile gauze and stored at 4 °C until use.
2.5. Biofilm Formation on Polystyrene Microplates
To examine the biofilm-forming capability of the strains in mono- and dual-species culture and to select one combination, biofilm formation was assessed on polystyrene surface (PS microtitre plate, Corning incorporated, Kennebunk, ME, USA) for each strain. Then, the eight L. monocytogenes strains were combined with the blue pigmenting P. fluorescens strain. The bacterial suspensions, previously prepared in the Ricotta-based medium, were aliquoted (200 µL) into the wells of PS plates and then incubated at 12 °C for 168 h. Negative control wells contained non-inoculated Ricotta medium. Plates were incubated for 0, 48, 72, 96, and 168 h to allow biofilm formation, and after the incubation period planktonic cells were removed by aspiration and washing the wells with PBS. Thereafter, total biomass (cells plus matrix) was quantified at 590 nm by crystal violet assay, as reported by Rossi et al. [16].
Means and standard deviations of absorbance values derived from five replicates were calculated by subtracting the control value from each mean value.
The blue pigment color appearance during the assay was evaluated visually as presence/absence.
2.6. Biofilm Formation on Stainless Steel and Enumeration of Planktonic and Sessile Cells
To evaluate biofilm formation on stainless steel, AISI 304 coupons (SS coupons, 2 × 2 × 0.1 cm), previously cleaned according to the procedure described by Campana et al. [35] and sterilized at 121 °C for 15 min, were used. L. monocytogenes LM5 strain was chosen as representative of the whole set and combined with P. fluorescens pf5. Each coupon was individually introduced into a sterile glass container, and then 5 mL of Ricotta medium were inoculated with the mono- or dual-species inocula. Also, negative control samples with non-inoculated Ricotta medium were included. The samples were then incubated at 12 °C for 168 h. The assay was performed in triplicate. At each sampling time (0, 48, 72, 96, and 168 h), three sterile glass containers were used. One milliliter of the suspension was taken from each sterile glass container, and serial dilutions were performed to enumerate planktonic cells. The dilutions were distributed on selective media for Pseudomonas spp. (Pseudomonas Agar Base; Oxoid-Thermofisher, Rodano, Italy) and for L. monocytogenes (Agar Listeria according to Ottaviani and Agosti; Biolife, Milano, Italy). The plates were incubated at 30 °C and 37 °C for 48 h, respectively for P. fluorescens and L. monocytogenes, and the number of colonies was counted. The enumeration of cells in biofilms (sessile cells) was performed by rinsing three times the SS coupons with saline solution (0.85% NaCl w/v) in sterile tubes, followed by scraping with two cotton swabs to collect the cells [36]. The swabs were immersed in 10 mL of saline solution, vortexing for 10 s at 230 rpm. Then, tenfold serial dilutions were prepared, and the colonies count was determined on the above mentioned agar media.
The blue pigment color appearance during the assay was evaluated visually as presence/absence.
2.7. EPS Extraction from Biofilms and Total Carbohydrates Quantification
The determination of carbohydrates from biofilms formed on SS coupons was carried out in triplicate for the samples and the incubation times reported in Section 2.6.
The EPSs were extracted as previously reported by Abdallah et al. [37], with some modifications. Briefly, after rinsing, biofilm was scraped from the SS coupon by using cotton swabs that were immersed in 10 mL of saline solution (0.85% NaCl w/v). After that, the solution was sonicated for 5 min at 50 kHz (Starsonic 90 Digit, Liarre, Asti, Italy), the cells were removed by centrifugation (5000× g for 15 min), and the supernatant was collected.
Carbohydrates quantification was carried out following the anthrone method [38] using 1 mL of the EPS extract. Then, 1 mL of the sample was transferred into a Pyrex test tube and 1 mL of cold anthrone reagent (0.1% anthrone solution in 75% sulfuric acid (v/v)) was added. The tubes were placed in a water bath (100 °C, 14 min) and were then cooled at 5 °C for 5 min. Glucose solution (100 mg/L) was used as a standard. The absorbance of the samples at 625 nm was measured, and the results were presented in µg/cm2.
2.8. Confocal Laser Scanning Microscopy (CLSM) Analysis
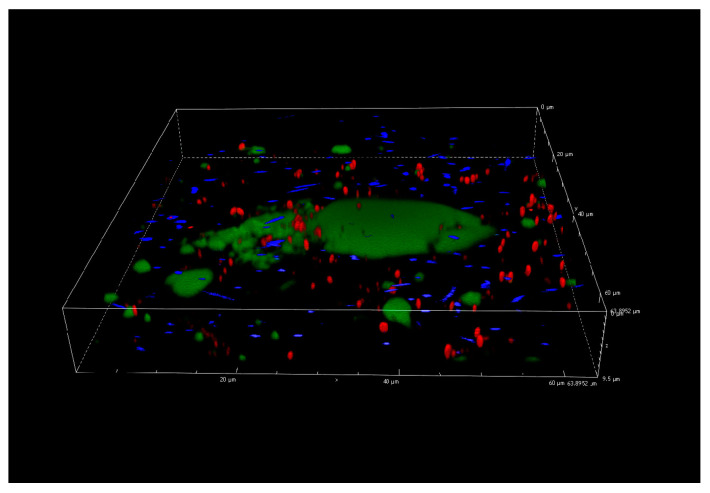
Mono- and dual-species biofilm structures were observed by CLSM according to the method described by Rossi et al. [39]. Briefly, cultures of mono- and dual-species of L. monocytogenes LM5 and P. fluorescens were prepared in Ricotta-based medium as reported in Section 2.3 and were inoculated in eight-well chamber slides (Nunc, Thermo Fisher Scientific, Waltham, MA, USA) at 400 mL per well. A negative control with non-inoculated medium was prepared. The plates were incubated at 12 °C, and the biofilms were allowed to grow for 48, 72, 96, and 168 h without agitation. At each time point, the pre-formed biofilms in each well of the plate were rinsed with sterile water to remove the growth medium and the unattached cells. The biofilms were stained with LIVE/DEAD BacLight Bacterial Viability kit containing the dyes SYTO9 and propidium iodide (PI) according to the manufacturer’s instructions (Molecular Probes, Thermo Fisher Scientific, Eugene, OR, USA). Live bacterial cells appeared fluorescent green, while dead/damaged bacterial cells appeared fluorescent red. The stained biofilms were observed with the Nikon A1R confocal imaging system and controlled by the Nikon NIS Elements software ver. 4.40 (Nikon Corp., Tokyo, Japan), equipped with a Plan Apo 100× oil objective.
The excitation/emission for the dyes were 488/525–50 nm and 561.5/595–50 nm for SYTO9 and PI, respectively. The fluorescence of pyoverdine, the siderophore produced by P. fluorescens, was checked with the excitation/emission at 405/460 nm [40].
2.9. Statistical Analysis
Statistical analysis was performed using XLSTAT ver. 2017 (Addinsoft, Paris, France). The data were subjected to analysis of variance (ANOVA), and a Dunnett’s test was employed to compare single- and multi-species results. Statistical significance was achieved at * p < 0.05.
3. Results
3.1. Serotype and Pulsotype of L. monocytogenes Strains
Four serotypes (1/2a, 1/2b, 1/2c, and 4b) were identified among the eight L. monocytogenes strains (Table 1). The most prevalent serotype was 1/2b (for strains isolated from both food and environmental sources), then 1/2a (for food strains) and 1/2c (for environmental strains), followed by 4b (for Mozzarella cheese isolate). A total of eight ApaI and eight AscI PFGE types were distinguished, thus revealing that the strains isolated from food products and environment were genetically different and heterogeneous.
Table 1.
Listeria monocytogenes and Pseudomonas fluorescens strains used in the study.
| Species | Strain Name | Source of Isolation |
Serotype | Pulsotype ApaI | Pulsotype AscI |
|---|---|---|---|---|---|
| L. monocytogenes | LM1 | Gorgonzola cheese | 1/2b | GX6A12.0051 | GX6A16.0071 |
| L. monocytogenes | LM2 | Mozzarella cheese | 4b | GX6A12.0073 | GX6A16.0010 |
| L. monocytogenes | LM3 | Gorgonzola cheese | 1/2a | GX6A12.0032 | GX6A16.0029 |
| L. monocytogenes | LM4 | Caciotta cheese | 1/2a | GX6A12.0390 | GX6A16.0271 |
| L. monocytogenes | LM5 | Environmental | 1/2b | GX6A12.0349 | GX6A16.0255 |
| L. monocytogenes | LM6 | Environmental | 1/2b | GX6A12.0005 | GX6A16.0009 |
| L. monocytogenes | LM7 | Environmental | 1/2c | GX6A12.0373 | GX6A16.0261 |
| L. monocytogenes | LM8 | Environmental | 1/2c | GX6A12.0002 | GX6A16.0007 |
| P. fluorescens | pf5 | Mozzarella cheese |
3.2. Biofilm Formation on Polystyrene Surface
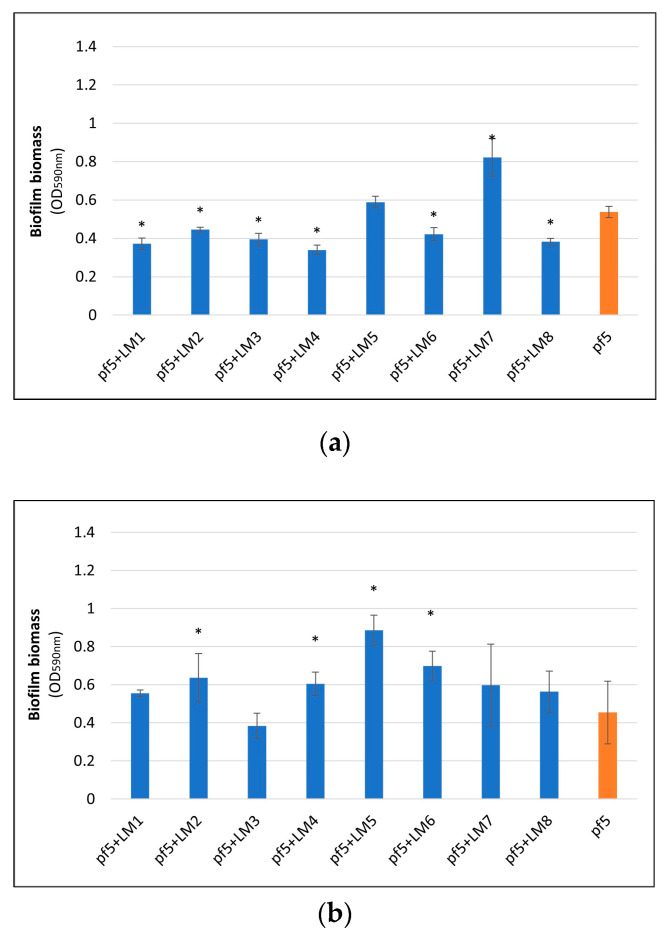
The results of biofilm formation ability of P. fluorescens pf5 in mono- and dual-species with L. monocytogenes strains on PS surface are shown in Figure 1. None of the eight L. monocytogenes strains was able to form biofilms on PS (data not shown). On the other hand, P. fluorescens exhibited good biofilm formation capacity. In fact, the biofilm biomass of P. fluorescens pf5 in single-species increased during incubation time, reaching a maximum value after 168 h of incubation (OD590 nm 1.072 ± 0.167). However, a different behavior was observed for the species in combination, with biofilm biomass variability among the strains. Although at the end of the incubation period biofilms in dual-species systems were significantly lower than the single ones (* p < 0.05), a higher biofilm biomass for some strains was noticed after 72 h, particularly for the combinations P. fluorescens–L. monocytogenes LM5 (Figure 1b). With respect to the blue discoloration, P. fluorescens blue pigment production was monitored during the whole incubation time, and after 72 h the color change of the substrate was observed both in single- and mixed-culture (Supplementary Figure S1).
Figure 1.
Biofilm biomass (OD590 nm) of P. fluorescens pf5 in mono- and dual-species with L. monocytogenes strains on a polystyrene (PS) surface at 12 °C for 168 h. The results are expressed as an average of five replicates and the bars represent the standard deviations. The asterisk (*) indicates statistically significant difference between the mono- and dual-species samples for the same incubation time (* p < 0.05). (a) 48 h, (b) 72 h, (c) 96 h, and (d) 168 h.
Based on the obtained results, the combination L. monocytogenes LM5 and P. fluorescens pf5 was selected for the subsequent analysis.
3.3. Biofilm Formation on Stainless Steel Surface and Enumeration of Planktonic and Sessile Cells
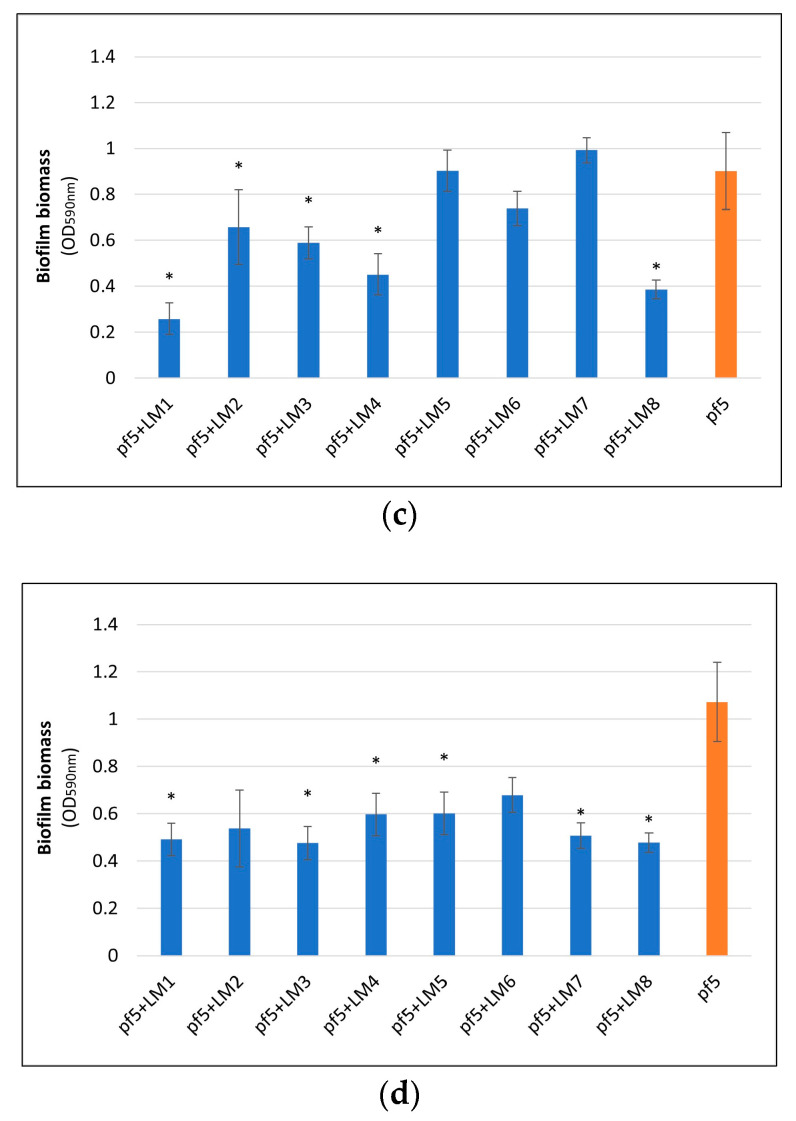
The results of L. monocytogenes LM5 and P. fluorescens pf5 planktonic and sessile cells on SS coupons in both mono-culture and dual-culture conditions, are presented in Figure 2.
Figure 2.
Dynamics of planktonic (a) and sessile (b) cells of L. monocytogenes LM5 and P. fluorescens pf5 in mono- and dual-species conditions on stainless steel (SS) coupons at 12 °C for 168 h. The results represent average values of three replicates and the bars indicates the standard deviations. The asterisk (*) means statistically significant difference between the mono- and dual-species of each strains for the same incubation time (* p < 0.05). L: L. monocytogenes in single-species; L + P: L. monocytogenes in dual-species; P: P. fluorescens in single-species; P + L: P. fluorescens in dual-species.
Regarding the planktonic phenotype (Figure 2a), under mono-species, starting from a load of 5.67 ± 0.08 Log CFU/mL L. monocytogenes reached 8.16 ± 0.06 Log CFU/mL after 48 h, remaining almost stable until the end of the incubation time. However, the presence of P. fluorescens determined a slight but significant (* p < 0.05) decrease of L. monocytogenes counts of about 0.35 and 0.24 Log CFU/mL at 48 and 96 h, respectively. P. fluorescens showed a greater increase in load over time compared to L. monocytogenes: 5.17 ± 0.14 Log CFU/mL at time 0, 7.3 ± 0.06 after 48 h, and 9.15 ± 0.02 Log CFU/mL at 72 h, followed by a load reduction of about 1 Log CFU/mL. P. fluorescens planktonic counts did not differ significantly between mono- and dual-species conditions.
The results regarding the sessile populations (Figure 2b) showed that L. monocytogenes was able to adhere on the SS surface. In fact, L. monocytogenes in mono-species starting from a load of about 1.4 Log CFU/cm2 at time 0 increased up to 3.27 ± 0.07 at 72 h, maintaining these values as almost stable over time. In multi-species conditions, the presence of P. fluorescens statistically (* p < 0.05) increased the pathogen biofilm population after 48 h of incubation, when it reached a sessile load of 3.39 ± 0.36 Log CFU/cm2. However, at the end of the experimental time, L. monocytogenes sessile cells in mixed-culture dropped to 1.4 Log CFU/cm2.
For the sessile population, the culture conditions (mono- or dual-species) did not influence P. fluorescens population level. In fact, it reached a sessile load of 3.58 ± 0.34 Log CFU/cm2 at time 48 h, which decreased during the time reaching 1.4 Log CFU/cm2 after 168 h, with no statistically significant differences among single- and mixed-culture.
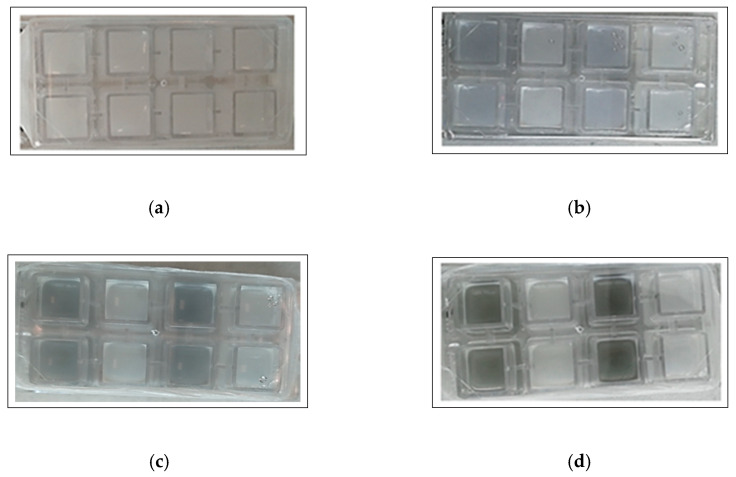
Also, in this experiment, the blue pigment production of P. fluorescens pf5 was observed both in single- and in mixed-culture starting from 72 h (Figure 3), when the highest load of the spoilage bacteria was detected (9.15 ± 0.02 and 8.60 ± 0.21 Log CFU/mL, respectively for pf5 in single- and multi-species conditions).
Figure 3.
Blue pigment color appearance during the assay of biofilm formation on SS coupons using glass container (72 h, 12 °C). From the left: Control, P. fluorescens pf5, L. monocytogenes LM5, and dual-species system.
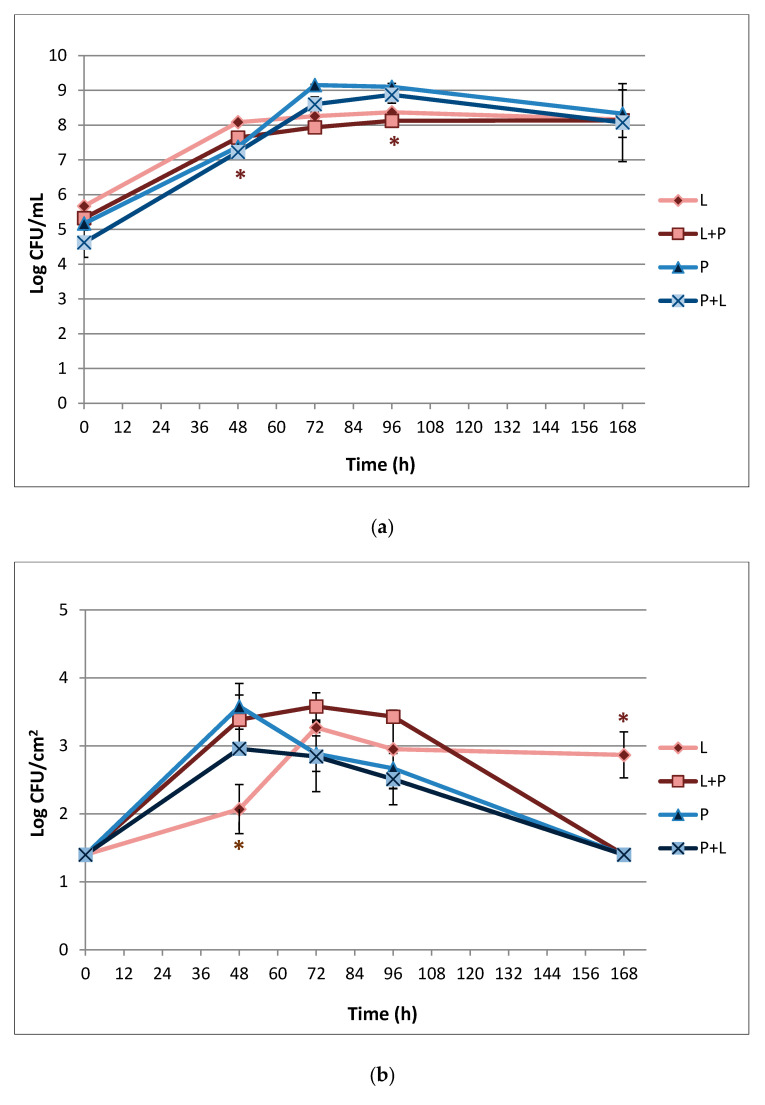
3.4. EPS Analysis by Carbohydrates Quantification
Quantification (µg/cm2) of the carbohydrates extracted from the EPS of P. fluorescens pf5 and L. monocytogenes LM5 biofilms is shown in Figure 4. The total amount of carbohydrates in the biofilms was affected by the time and the species involved in biofilm formation. In single-species biofilms, the biofilm carbohydrates content increased over time with the greatest increase occurring between 48 and 96 h. In fact, at 96 h, values of about 2.49 ± 0.08 and 1.99 ± 0.24 µg/cm2 were observed for P. fluorescens and L. monocytogenes, respectively. Instead, no carbohydrates were revealed at 168 h for both single-species biofilms. The dual-species interactions in biofilms did not have any additive effect on carbohydrate matrix but a statistically significant (* p < 0.05) higher yield than those of the single-species was detected at time 72 h (2.91 ± 0.23 µg/cm2). Remarkably, the carbohydrates of the dual-species biofilms were revealed at high amount (about 2 µg/cm2) also at the end of the experiment (168 h).
Figure 4.
Carbohydrates amount (µg/cm2) from L. monocytogenes LM5 and P. fluorescens pf5 biofilms on SS coupons in mono- and dual-species at 12 °C for 168 h. The results indicate the average of three values and the bars indicate the standard deviation. The asterisk (*) means statistically significant difference between the mono- and dual-species of each strains for the same incubation time (* p < 0.05). P: P. fluorescens in single-species; L: L. monocytogenes in single-species; P + L: L. monocytogenes and P. fluorescens in dual-species.
3.5. Confocal Laser Scanning Microscopy Analysis
Figure 5 describes the dual-species biofilms at the end of the incubation period (168 h, 12 °C), on the basis of CLSM results. No three-dimensional biofilm architecture was revealed but, as for the other incubation times (data not reported), some unexpected evidences made it difficult to analyze the interactions between the two species on biofilm structure. In fact, the figure shows particular green agglomerates with damaged or dead cells (red cells according to PI staining) and detached P. fluorescens cells (blue color of pyoverdine fluorescence). The fact that the agglomerates were not clearly identifiable as cells and that they were present only in the samples with P. fluorescens pf5, suggests that they could depend on blue pigment appearance. In addition, this particular behavior was observed starting from 72 h in correspondence with the blue pigment formation by P. fluorescens (Figure 6).
Figure 5.
Confocal Laser Scanning Microscopy (CLSM) analysis of L. monocytogenes LM5 and P. fluorescens pf5 biofilms in dual-species conditions after 168 h at 12 °C.
Figure 6.
Blue pigment color evolution during the CLSM analysis using the Nunc wells: (a) 48 h, (b) 72 h, (c) 96 h, and (d) 168 h.
Figure 6a–d illustrates the color changes occurred in the CLSM plates during the incubation. Also, in this case, the blue pigment discoloration was revealed starting from 72 h, after which the blue color turned progressively into green/grey, particularly at 168 h.
4. Discussion
The biofilm forming capability of P. fluorescens and L. monocytogenes strains was evaluated in conditions simulating those encountered in a dairy environment. The serotypes of L. monocytogenes strains agree with previous observations, which showed that 1/2a, 1/2b, 1/2c, and 4b are the most common serotypes in foods and environmental sources [30], with 1/2a, 1/2b, and 4b being mainly responsible for listeriosis outbreaks [41].
The results obtained on the PS surface revealed that the L. monocytogenes strains used in this study were not able to form biofilms. Although numerous studies have demonstrated that this pathogen is able to form biofilms on various abiotic and biotic surfaces [42,43,44], also a previous study on mono-species and mixed-species biofilms composed of these two microbial species, reported very low OD values for mono-species L. monocytogenes biofilms, with a maximum value of 0.11 after 96 h of incubation at 20 °C [45]. On the contrary, P. fluorescens pf5 exhibited good biofilm formation capacity, which is an important feature for a spoilage organism. Cells in biofilms are an important risk factor in food environments for the potential contamination of foods and the possibility to become resistant to antimicrobials [20]. Pseudomonas spp., especially P. fluorescens, is a specific spoilage microorganism of dairy products and is frequently isolated from dairy environments [2,46,47].
In general, the population densities of the biofilms on the SS surface observed in our study were lower than those found by other studies [48,49,50], probably due to the conditions adopted to mimic dairy processing environments. In fact, the physical–chemical properties of substrates can affect surface bacterial adhesion and biofilm formation as highlighted by Parkar et al. [51], who observed an attachment decrease of both vegetative cells and spores of thermophilic bacteria on SS surfaces coated with milk proteins.
The fact that L. monocytogenes LM5 was able to adhere to SS and not to PS could be attributable to the affinity established between the charge of the cell surface and the anchoring site. In fact, at low temperatures, L. monocytogenes increases the cell wall hydrophilicity and therefore the affinity to hydrophilic surfaces such as steel or glass [52].
In addition, the results revealed that dual biofilms were colonized to a greater extent by L. monocytogenes LM5 and that dual-species conditions lead to an increase in L. monocytogenes LM5 load compared to mono-species after 2 days of incubation. Stimulation of L. monocytogenes adhesion in mixed-culture biofilms with P. fluorescens was also described by Puga et al. [45], who linked the positive effects on L. monocytogenes to Pseudomonas production of proteinases, able to mobilize essential amino acids. Interestingly, Teh et al. [53] demonstrated that the proteolytic and lipolytic activities of bacterial cells in dairy environments are higher within biofilms than in planktonic cells. Moreover, the exopolymeric substances produced by P. fluorescens may facilitate L. monocytogenes anchorage, attachment, and surface colonization [54,55], providing a thick matrix protection [45]. Our findings are also consistent with the results of Hassan et al. [56], who observed a stronger attachment of L. monocytogenes to surfaces with preexisting Pseudomonas biofilms than to Pseudomonas-free surfaces. In contrast, in another study, the cell density of L. monocytogenes in dual-species biofilms formed at 15 °C for 48 h was lower than that of the single-species [48].
In our study, the interactions between the two microbial species led to a polysaccharides matrix over-production that persisted until the end of the experimental time (168 h). With this respect, Puga et al. [45] also reported that the inclusion of L. monocytogenes in the already established P. fluorescens biofilm increased matrix production. Although L. monocytogenes is considered “a cheater” with a poor matrix production capability, finding shelter inside the matrix of Pseudomonas spp., we observed good amounts of carbohydrates also in single-species L. monocytogenes biofilm.
The fast cellular dispersal observed in our study for multi-species biofilms could have been stimulated from the early achievement of high biofilm level (cellular density/matrix threshold) with no extra nutrient supplementation [19,45,50]. As highlighted by the results of L. monocytogenes sessile cells on an SS surface (Figure 2b, Figure 4), the greater detachment rate of the cells in dual-species biofilms compared to the single ones was not associated with a poor EPS production. While Combrouse et al. [57] also did not observe any clear relationship between EPS content and CFU count, in contrast previous works reported a positive correlation between the ability to form biofilms and EPS production [58,59]. EPSs have a key role in maintaining the stability and integrity of biofilm structure via cellular adhesion and cohesion [60] but also other variables related to starvation and nutrient flow cessation [61] influence biofilms detachment.
With respect to the blue pigment production, as previously observed for Mozzarella cheese inoculated with P. fluorescens [16], Ricotta medium blue discoloration was observed at a high load of P. fluorescens pf5 after 72 h of incubation. In agreement with our findings, Andreani et al. [14] observed an evident blue pigment in broth when Pseudomonas counts reached about 7 × 108 CFU/mL, concluding that the blue pigment production took place in the late logarithmic phase. Quintieri et al. [15] reported that in Pseudomonas spp. pigments modulate the transition from planktonic to biofilm state, show antimicrobial effects against other microorganisms, protect cells from oxidative stress, and can act as signaling molecules and virulence factors. Furthermore, Cude et al. [62], in a study conducted on marine Roseobacter Phaeobacter cells, found that indigoidine biosynthesis may provide a significant advantage in the colonization of environmental niches. In addition, the authors suggested that when a surface niche is colonized and quorum is reached, indigoidine biosynthesis is up-regulated to suppress the colonization of competing organisms. However, from our study it is not possible to state that the blue pigment produced by P. fluorescens pf5 possesses antimicrobial activity. In fact, during the blue pigment discoloration in mixed-culture, a strong reduction of L. monocytogenes load was observed only for the sessile cells at 168 h.
The color change from blue to green/grey highlights the possible reduction of indigoidine to leucoindigoidine [16], which is considered a chemical marker of blue discoloration [12]. Although the CLSM analysis did not allow for the detection of the architecture of single- and dual-species biofilms during the incubation time, the agglomerates formation and their interference with samples staining revealed some connection with P. fluorescens indigoidine production. In fact, the indigoidine and leucoindigoidine fluorescence was recorded using a 415 nm excitation and 520 nm emission wavelengths [63], which is near to the range of SYTO 9 used to observe live cells.
5. Conclusions
This study underlines the influence of microbial interactions on biofilms formed under simulated dairy processing conditions. The results showed that L. monocytogenes exhibited the capability to form biofilm only on SS coupons, while P. fluorescens formed biofilm on both PS and SS surfaces. The behavior of planktonic and sessile populations on SS coupons was dependent on the culture conditions. Particularly, the presence of P. fluorescens increased L. monocytogenes sessile population and total EPS carbohydrates amount on SS coupons. Green agglomerates, probably linked to the P. fluorescens blue pigment, were noticed during CLSM analysis and probably interfered with biofilm visualization. Nevertheless, further research is needed to better clarify this point and more studies would be useful to provide more information on the inter-species consortium. In fact, dual-species biofilms are more common than single-species ones in real conditions, show characteristics that could favor L. monocytogenes persistence in food production environments, and for this reason deserve thorough investigation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/10/1/176/s1, Figure S1: Blue pigment production appearance during the assay of biofilm formation of L. monocytogenes strains and P. fluorescens pf5 in mono- and dual-species conditions on PS microtitre plates. (a) 0 h; (b) 24 h; (c) 48 h; (d) 72 h; (e) 96 h; (f) 168 h.
Author Contributions
Conceptualization, A.P.; methodology, C.C.-L. and A.S.; strains provision, F.P.; formal analysis and data curation, F.M.; investigation, F.M., C.R., A.P.C. and L.V.; writing-original draft preparation, F.M. and C.R.; writing—review and editing, A.P. and A.S.; supervision, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kassinger S.J., Van Hoek M.L. Biofilm architecture: An emerging synthetic biology target. Synth. Syst. Biotechnol. 2020;5:1–10. doi: 10.1016/j.synbio.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi C., Chaves-López C., Serio A., Goffredo E., Goga B.T.C., Paparella A. Influence of incubation conditions on biofilm formation by Pseudomonas fluorescens isolated from dairy products and dairy manufacturing plants. Ital. J. Food Saf. 2016;5:5793. doi: 10.4081/ijfs.2016.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira G.S., Lopes D.R.G., Andre C., Silva C.C., Baglinière F., Vanetti M.C.D. Multispecies biofilm formation by the contaminating microbiota in raw milk. Biofouling. 2019;35:819–831. doi: 10.1080/08927014.2019.1666267. [DOI] [PubMed] [Google Scholar]
- 4.Di Ciccio P., Rubiola S., Grassi M.A., Civera T., Abbate F., Chiesa F. Fate of Listeria monocytogenes in the presence of resident cheese microbiota on common packaging materials. Front. Microbiol. 2020;11:1–8. doi: 10.3389/fmicb.2020.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melo J., Andrew P.W., Faleiro M.I. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res. Int. 2015;67:75–90. doi: 10.1016/j.foodres.2014.10.031. [DOI] [Google Scholar]
- 6.European Food Safety Authority and European Centre for Disease Prevention and Control, The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16:5500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrador Z., Gherasim A., López-Vélez R., Benito A. Listeriosis in Spain based on hospitalisation records, 1997 to 2015: Need for greater awareness. Euro Surveill. 2019;24:1800271. doi: 10.2807/1560-7917.ES.2019.24.21.1800271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrascosa C., Millán R., Jaber J.R., Lupiola P., del Rosario-Quintana C., Mauricio C., Sanjuán E. Blue pigment in fresh cheese produced by Pseudomonas fluorescens. Food Control. 2015;54:95–102. doi: 10.1016/j.foodcont.2014.12.039. [DOI] [Google Scholar]
- 9.Martins M., Uelinton M.P., Katharina R., Vanetti M.C.D. Milk-deteriorating exoenzymes from Pseudomonas fluorescens 041 isolated from refrigerated raw milk. Braz. J. Microbiol. 2015;46:207–217. doi: 10.1590/S1517-838246120130859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarei M., Yousefvand A., Maktabi S., Borujeni M.P., Mohammadpour H. Identification, phylogenetic characterisation and proteolytic activity quantification of high biofilm-forming Pseudomonas fluorescens group bacterial strains isolated from cold raw milk. Int. Dairy J. 2020;109:104787. doi: 10.1016/j.idairyj.2020.104787. [DOI] [Google Scholar]
- 11.Reichler S.J., Martin N.H., Evanowski R.L., Kovac J., Wiedmann M., Orsi R.H. A century of gray: A genomic locus found in 2 distinct Pseudomonas spp. is associated with historical and contemporary color defects in dairy products worldwide. Int. J. Dairy Sci. 2019;102:5979–6000. doi: 10.3168/jds.2018-16192. [DOI] [PubMed] [Google Scholar]
- 12.Del Olmo A., Calzada J., Nuñez M. The blue discoloration of fresh cheeses: A worldwide defect associated to specific contamination by Pseudomonas fluorescens. Food Control. 2018;86:359–366. doi: 10.1016/j.foodcont.2017.12.001. [DOI] [Google Scholar]
- 13.Ringel M.T., Brüser T. The biosynthesis of pyoverdines. Microb. Cell. 2018;5:424–437. doi: 10.15698/mic2018.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreani N.A., Carraro L., Martino M.E., Fondi M., Fasolato L., Miotto G., Cardazzo B. A genomic and transcriptomic approach to investigate the blue pigment phenotype in Pseudomonas fluorescens. Int. J. Food Microbiol. 2015;213:88–98. doi: 10.1016/j.ijfoodmicro.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Quintieri L., Fanelli F., Zühlke D., Caputo L., Logrieco A.F., Albrecht D., Riedel K. Biofilm and pathogenesis-related proteins in the foodborne P. fluorescens ITEM 17298 with distinctive phenotypes during cold storage. Front. Microbiol. 2020;11:1–17. doi: 10.3389/fmicb.2020.00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi C., Serio A., Chaves-López C., Anniballi F., Auricchio B., Goffredo E., Cenci-Goga B.T., Lista F., Fillo S., Paparella A. Biofilm formation, pigment production and motility in Pseudomonas spp. isolated from the dairy industry. Food Control. 2018;86:241–248. doi: 10.1016/j.foodcont.2017.11.018. [DOI] [Google Scholar]
- 17.Paz-Méndez A.M., Lamas A., Vázquez B., Miranda J.M., Cepeda A., Franco C.M. Effect of food residues in biofilm formation on stainless steel and polystyrene surfaces by Salmonella enterica strains isolated from poultry houses. Foods. 2017;6:106. doi: 10.3390/foods6120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galié S., García-Gutiérrez C., Miguélez E.M., Villar C.J., Lombó F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018;9:898. doi: 10.3389/fmicb.2018.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ripolles-Avila C., Hascoët A.S., Guerrero-Navarro A.E., Rodríguez-Jerez J.J. Establishment of incubation conditions to optimize the in vitro formation of mature Listeria monocytogenes biofilms on food-contact surfaces. Food Control. 2018;92:240–248. doi: 10.1016/j.foodcont.2018.04.054. [DOI] [Google Scholar]
- 20.Rossi C., Chaves-López C., Serio A., Casaccia M., Maggio F., Paparella A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2020:1–20. doi: 10.1080/10408398.2020.1851169. [DOI] [PubMed] [Google Scholar]
- 21.Røder H.L., Sørensen S.J., Burmølle M. Studying bacterial multispecies biofilms: Where to start? Trends Microbiol. 2016;24:503–513. doi: 10.1016/j.tim.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Lapointe C., Deschênes L., Timothy C.E., Bisaillon Y., Savard T. Interactions between spoilage bacteria in tri-species biofilms developed under simulated meat processing conditions. Food Microbiol. 2019;82:515–522. doi: 10.1016/j.fm.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z., Shan L., Li X., Hu F., Yuan Y., Zhong D., Zhang J. Effects of interspecific interactions on biofilm formation potential and chlorine resistance: Evaluation of dual-species biofilm observed in drinking water distribution systems. J. Water Process. Eng. 2020;38:101564. doi: 10.1016/j.jwpe.2020.101564. [DOI] [Google Scholar]
- 24.Schilcher K., Horswill A.R. Staphylococcal biofilm development: Structure, regulation, and treatment strategies. Microbiol. Mol Biol. Rev. 2020;84:1–36. doi: 10.1128/MMBR.00026-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dygico L.K., Cormac G.M., Grogan G.H., Burgess C.M. The ability of Listeria monocytogenes to form biofilm on surfaces relevant to the mushroom production environment. Int. J. Food Microbiol. 2020;317:108085. doi: 10.1016/j.ijfoodmicro.2019.108385. [DOI] [PubMed] [Google Scholar]
- 26.Handorf O., Pauker V.I., Schnabel U., Weihe T., Freund E., Bekeschus S., Riedel K., Ehlbeck J. Characterization of antimicrobial effects of plasma-treated water (PTW) produced by microwave-induced plasma (MidiPLexc) on Pseudomonas fluorescens biofilms. Appl. Sci. 2020;10:118. doi: 10.3390/app10093118. [DOI] [Google Scholar]
- 27.Haddad S., Elliot M., Savard T., Deschênes L., Smith T., Ells T. Variations in biofilms harbouring Listeria monocytogenes in dual and triplex cultures with Pseudomonas fluorescens and Lactobacillus plantarum produced under a model system of simulated meat processing conditions, and their resistance to benzalkonium chloride. Food Control. 2020:107720. in press. [Google Scholar]
- 28.Wang Y., Hong X., Liu J., Zhu J., Chen J. Interactions between fish isolates Pseudomonas fluorescens and Staphylococcus aureus in dual-species biofilms and sensitivity to carvacrol. Food Microbiol. 2020;91:1–11. doi: 10.1016/j.fm.2020.103506. [DOI] [PubMed] [Google Scholar]
- 29.Ma Z., Stanford K., Xiao M.B., Yan D.N., McAllister T.A. Effects of beef juice on biofilm formation by Shiga toxin–producing Escherichia coli on stainless steel. Foodborne Pathog. Dis. 2020;17:235–242. doi: 10.1089/fpd.2019.2716. [DOI] [PubMed] [Google Scholar]
- 30.Chen J.Q., Regan P., Laksanalamai P., Healey S., Hu Z. Prevalence and methodologies for detection, characterization and subtyping of Listeria monocytogenes and L. ivanovii in foods and environmental sources. Food Sci. Hum. Well. 2017;6:97–120. doi: 10.1016/j.fshw.2017.06.002. [DOI] [Google Scholar]
- 31.Londero A., Costa M., Gallia L., Brusa V., Linaresa L., Prieto M., Leotta G. Characterization and subtyping of Listeria monocytogenes strains from butcher shops. LWT Food Sci. Technol. 2019;113:108363. doi: 10.1016/j.lwt.2019.108363. [DOI] [Google Scholar]
- 32.Donnelly C.W., Diez-Gonzalez F. Guide to foodborne pathogens. In: Labbé R.G., Garcìa S., editors. Listeria monocytogenes. 2nd ed. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2001. pp. 45–74. [Google Scholar]
- 33.Paparella A., Mazzarrino G., Chaves-López C., Rossi C., Sacchetti G., Guerrieri O., Serio A. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016;59:23–31. doi: 10.1016/j.fm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 34.De Carvalho R.J., de Souza G.T., Honório V.G., de Sousa J.P., da Conceição M.L., Maganani M., de Souza E.L. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter co-culture in cheese-mimicking models. Food Microbiol. 2015;52:59–65. doi: 10.1016/j.fm.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Campana R., Casettari L., Fagioli L., Cespi M., Bonacucina G., Baffone W. Activity of essential oil-based microemulsions against Staphylococcus aureus biofilms developed on stainless steel surface in different culture media and growth conditions. Int. J. Food Microbiol. 2017;241:132–140. doi: 10.1016/j.ijfoodmicro.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Dos Santos Rodrigues J.B., de Souza N.T., Scarano J.O.A., de Sousa J.M., Lira M.C., de Figueiredo R.C.B.Q., Magnani M. Efficacy of using oregano essential oil and carvacrol to remove young and mature Staphylococcus aureus biofilms on food-contact surfaces of stainless steel. LWT. 2018;93:293–299. doi: 10.1016/j.lwt.2018.03.052. [DOI] [Google Scholar]
- 37.Abdallah H.M.I., Asaad G.F.M., Nada S.A., Taha H.S., Seif El-Nasr M.M. Influence of extract derived in-vitro cell suspension cultures of Echinacea purpurea against some immunosuppressive effects. Res. J. Pharm. Biol. Chem. Sci. 2015;6:1136–1143. [Google Scholar]
- 38.Harimawan A., Ting Y.P. Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtilis and their role in bacterial adhesion. Colloids Surf. B. 2016;146:459–467. doi: 10.1016/j.colsurfb.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 39.Rossi C., Chaves-López C., Serio A., Anniballi F., Valbonetti L., Paparella A. Effect of Origanum vulgare essential oil on biofilm formation and motility capacity of Pseudomonas fluorescens strains isolated from discoloured Mozzarella cheese. J. Appl. Microbiol. 2018;124:1220–1231. doi: 10.1111/jam.13707. [DOI] [PubMed] [Google Scholar]
- 40.Martin N.H., Murphy S.C., Ralyea R.D., Wiedmann M., Boor K.J. When cheese gets the blues: Pseudomonas fluorescens as the causative agent of cheese spoilage. Int. J. Dairy Sci. 2011;94:3176–3183. doi: 10.3168/jds.2011-4312. [DOI] [PubMed] [Google Scholar]
- 41.Braga V., Vázquez S., Vico V., Pastorino V., Mota M.I., Legnani M., Schelotto F., Lancibidad G., Varela G. Prevalence and serotype distribution of Listeria monocytogenes isolated from foods in Montevideo-Uruguay. Braz. J. Microbiol. 2017;48:689–694. doi: 10.1016/j.bjm.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendez E., Walker D.K., Vipham J., Trinetta V. The use of a CDC biofilm reactor to grow multi-strain Listeria monocytogenes biofilm. Food Microbiol. 2020;92:103592. doi: 10.1016/j.fm.2020.103592. [DOI] [PubMed] [Google Scholar]
- 43.Skowron K., Brożek K., Łukasik M., Wiktorczyk N., Korkus J., Gospodarek-Komkowska E. Assessment of drug susceptibility and biofilm formation ability by clinical strains of Listeria monocytogenes. Disaster Emerg. Med. J. 2020;5:12–18. doi: 10.5603/DEMJ.a2020.0002. [DOI] [Google Scholar]
- 44.Hossain M.I., Mizana M.F.R., Ashrafudoulla M., Nahara S., Joo H.J., Jahid I.K., Parkc S.H., Kim K.S., Ha S.D. Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBEC™ biofilm device. LWT Food Sci. Technol. 2020;118:108864. doi: 10.1016/j.lwt.2019.108864. [DOI] [Google Scholar]
- 45.Puga C.H., Dahdouh E., SanJose C., Orgaz B. Listeria monocytogenes colonizes Pseudomonas fluorescens biofilms and induces matrix over-production. Front. Microbiol. 2018;9:1706. doi: 10.3389/fmicb.2018.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Circella E., Schiavone A., Barrasso R., Camarda A., Pugliese N., Bozzo G. Pseudomonas azotoformans belonging to Pseudomonas fluorescens Group as causative agent of blue coloration in carcasses of slaughterhouse rabbits. Animals. 2020;10:256. doi: 10.3390/ani10020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caputo L., Quintieri L., Bianchi D.M., Decastelli L., Monaci L., Visconti A., Baruzzi F. Pepsin-digested bovine lactoferrin prevents Mozzarella cheese blue discoloration caused by Pseudomonas fluorescens. Food Microbiol. 2015;46:15–24. doi: 10.1016/j.fm.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Pang X., Yuk H.G. Effects of the colonization sequence of Listeria monocytogenes and Pseudomonas fluorescens on survival of biofilm cells under food-related stresses and transfer to salmon. Food Microbiol. 2019;82:142–150. doi: 10.1016/j.fm.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Pang X., Wong C., Chung H.J., Yuk H.G. Biofilm formation of Listeria monocytogenes and its resistance to quaternary ammonium compounds in a simulated salmon processing environment. Food Control. 2019;98:200–208. doi: 10.1016/j.foodcont.2018.11.029. [DOI] [Google Scholar]
- 50.Papaioannou E., Giaouris E.D., Berillis P., Boziaris I.S. Dynamics of biofilm formation by Listeria monocytogenes on stainless steel under mono-species and mixed-culture simulated fish processing conditions and chemical disinfection challenges. Int. J. Food Microbiol. 2018;267:9–19. doi: 10.1016/j.ijfoodmicro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 51.Parkar S.G., Flint S.H., Palmer J.S., Brooks J.D. Factors influencing attachment of thermophilic bacilli to stainless steel. J. Appl. Microbiol. 2001;90:901–908. doi: 10.1046/j.1365-2672.2001.01323.x. [DOI] [PubMed] [Google Scholar]
- 52.Chavant P., Gaillard-Martinie B., Talon R., Hébraud M., Bernardi T. A new device for rapid evaluation of biofilm formation potential by bacteria. J. Microbiol. Methods. 2007;68:605–612. doi: 10.1016/j.mimet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Teh K.H., Flint S., Palmer J., Andrewes P., Bremer P., Lindsay D. Biofilm− An unrecognised source of spoilage enzymes in dairy products? Int. Dairy J. 2014;34:32–40. doi: 10.1016/j.idairyj.2013.07.002. [DOI] [Google Scholar]
- 54.Aswathanarayan J.B., Vittal R.R. Attachment and biofilm formation of Pseudomonas fluorescens PSD4 isolated from a dairy processing line. Food Sci. Biotechnol. 2014;23:1903–1910. doi: 10.1007/s10068-014-0260-8. [DOI] [Google Scholar]
- 55.Puga C.H., Orgaz B., Muñoz S., SanJose C. Cold stress and presence of Pseudomonas fluorescens affect Listeria monocytogenes biofilm structure and response to chitosan. Mol. Genet. Med. 2015;9:1–6. [Google Scholar]
- 56.Hassan A.N., Birt D.M., Frank J.F. Behavior of Listeria monocytogenes in a Pseudomonas putida biofilm on a condensate-forming surface. J. Food Prot. 2004;67:322–327. doi: 10.4315/0362-028X-67.2.322. [DOI] [PubMed] [Google Scholar]
- 57.Combrouse T., Sadovskaya I., Faille C., Kol O., Guérardel Y., Midelet-Bourdin G. Quantification of the extracellular matrix of the Listeria monocytogenes biofilms of different phylogenic lineages with optimization of culture conditions. J. Appl. Microbiol. 2013;114:1120–1131. doi: 10.1111/jam.12127. [DOI] [PubMed] [Google Scholar]
- 58.Borucki M.K., Peppin J.D., White D., Loge F., Call D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003;69:7336–7342. doi: 10.1128/AEM.69.12.7336-7342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zameer F., Rukmangada M.S., Chauhan J.B., Khanum S.A., Kumar P., Devi T.A., Prasad M.N., Dhananjaya B.L. Evaluation of adhesive and anti-adhesive properties of Pseudomonas aeruginosa biofilms and their inhibition by herbal plants. Iran. J. Microbiol. 2016;8:108–119. [PMC free article] [PubMed] [Google Scholar]
- 60.Simoes M., Pereira M.O., Vieira M.J. Effect of mechanical stress on biofilms challenged by different chemicals. Water Res. 2005;39:5142–5152. doi: 10.1016/j.watres.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 61.Petrova O.E., Sauer K. Escaping the biofilm in more than one way: Desorption, detachment or dispersion. Curr. Opin. Microbiol. 2016;30:67–78. doi: 10.1016/j.mib.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cude W.N., Mooney J., Tavanaei A.A., Hadden M.K., Frank A.M., Christopher A., Gulvik C.A., May A.L., Buchana A. Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine Roseobacter Phaeobacter sp. strain Y4IW. Appl. Environ. Microbiol. 2012;78:4771–4780. doi: 10.1128/AEM.00297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown A.S., Robins K.J., Ackerley D.F. A sensitive single-enzyme assay system using the non-ribosomal peptide synthetase BpsA for measurement of L-glutamine in biological samples. Sci. Rep. 2017;7:41745. doi: 10.1038/srep41745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request.