Abstract
Hepatocellular carcinoma (HCC) is one of the most diagnosed malignancies and a leading cause of cancer-related mortality globally. This is exacerbated by its highly aggressive phenotype, and limitation in early diagnosis and effective therapies. The SUMO-activating enzyme subunit 1 (SAE1) is a component of a heterodimeric small ubiquitin-related modifier that plays a vital role in SUMOylation, a post-translational modification involving in cellular events such as regulation of transcription, cell cycle and apoptosis. Reported overexpression of SAE1 in glioma in a stage-dependent manner suggests it has a probable role in cancer initiation and progression. In this study, hypothesizing that SAE1 is implicated in HCC metastatic phenotype and poor prognosis, we analyzed the expression of SAE1 in several cancer databases and to unravel the underlying molecular mechanism of SAE1-associated hepatocarcinogenesis. Here, we demonstrated that SAE1 is over-expressed in HCC samples compared to normal liver tissue, and this observed SAE1 overexpression is stage and grade-dependent and associated with poor survival. The receiver operating characteristic analysis of SAE1 in TCGA−LIHC patients (n = 421) showed an AUC of 0.925, indicating an excellent diagnostic value of SAE1 in HCC. Our protein-protein interaction analysis for SAE1 showed that SAE1 interacted with and activated oncogenes such as PLK1, CCNB1, CDK4 and CDK1, while simultaneously inhibiting tumor suppressors including PDK4, KLF9, FOXO1 and ALDH2. Immunohistochemical staining and clinicopathological correlate analysis of SAE1 in our TMU-SHH HCC cohort (n = 54) further validated the overexpression of SAE1 in cancerous liver tissues compared with ‘normal’ paracancerous tissue, and high SAE1 expression was strongly correlated with metastasis and disease progression. The oncogenic effect of upregulated SAE1 is associated with dysregulated cancer metabolic signaling. In conclusion, the present study demonstrates that SAE1 is a targetable cancer metabolic biomarker with high potential diagnostic and prognostic implications for patients with HCC.
Keywords: SAE1, hepatocellular carcinoma, SUMOylation, diagnosis, prognosis, metastasis
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most commonly diagnosed cancer and ranks as the third commonest cause of cancer-related mortality, accounting for more than 700,000 fatalities in the world, annually [1]. The major risk factors for HCC include chronic infection of hepatitis B and C viruses (HBV and HCV), cirrhosis, alcohol abuse and non-alcoholic fatty liver disease (NAFLD) [2]. Hepatocarcinogenesis is characterized by dysregulated activation and/or expression of relevant genes in/on the hepatocytes, with resultant oncogene upregulation and tumor suppressor downregulation [3]. The last 5 decades has been characterized by discovery several biomarkers for diagnosis of HCC, including the α-fetoprotein (AFP), AFP-L3 (a heteroplast of AFP), des-γ-carboxyprothrombin (DCP), α-L-fucosidase (AFU), golgi protein 73 (GP73), osteopontin (OPN) and carbohydrate antigen 19-9 (CA19-9), which is globally regarded as diagnostic serological biomarkers for diagnosis of HCC patients. However, due to the clonal evolution, intratumoral and interpatient heterogeneity of HCC [4,5], like AFP, the diagnostic validity and clinical applicability of all these serological biomarkers remain debatable, especially considering their sub-optimal diagnostic specificity and sensitivity for early detection of HCC [6,7].
Similarly, histochemical biomarkers of HCC including glypican-3 (GPC-3), hepatocyte paraffin 1 (Hep Par 1), heat shock protein 70 (HSP70), glutamine synthetase (GS), arginase-1 (Arg-1), cytokeratin 7 and 19 (CK7 and CK19) are also plagued with same weakness in spite of their overall strength [8,9]. Against the background of this diagnostic challenge, the discovery of a biomarker with high and reliable diagnostic and prognostic accuracy and validity remain an unmet need in hepato-oncology clinics. Thus, the exploration for such biomarker in the present study; with the ultimate aim of proffering a therapeutic target, as well as improving the accuracy of diagnosis and efficacy of treatment modality in patients with HCC.
The disease course and progression of HCC is facilitated by altered cellular gene expression with dysregulated metabolism and pathophysiological signaling pathways [3,4,5]. SUMOylation, a post-translational modification that entails addition of small ubiquitin-like modifier (SUMO) groups to target proteins, is involved in numerous cellular events including transcriptional regulation, protein stability, cell cycle and apoptosis [10]. Upregulated expression of SAE1 (SUMO-activating enzyme subunit 1), an essential heterodimeric SUMO-activating effector of SUMOylation, has been implicated in the tumorigenesis and progression of several human malignancies, including in glioma [11], gastric cancer [12] and, more broadly, in Myc-driven carcinomas [13,14]; however, the biological roles of SAE1 in HCC remains underexplored.
In the present study, hypothesizing that SAE1 is implicated in HCC metastatic phenotype and poor prognosis, we investigated the variability of SAE1 expression in several cancer databases and its probable implication in HCC progression. Results presented herein indicate that, compared to normal liver samples, SAE1 is overexpressed in HCC, associated with the enhanced metastatic phenotype, disease progression, and poor prognosis of patients with HCC, thus indicating that SAE1 possesses reliable and clinically-relevant diagnostic value and is a potential novel biomarker of prognosis for HCC.
2. Materials and Methods
2.1. HCC Samples and Cohort Characterization
Clinical samples of patients with HCC were retrieved from the HCC tissue archive of the Taipei Medical University—Shuang Ho Hospital (TMU-SHH), New Taipei, Taiwan. After exclusion of cases with incomplete clinical information and insufficient sample for biomedical assays, only 54 clinical samples were used in the present study. This study was approved by the Institutional Human Research Ethics Review Board (TMU-JIRB No. 201302016) of Taipei Medical University.
2.2. Data Acquisition and Statistical Analysis of HCC
The raw gene expression data of SAE1 and related genes obtained by RNA sequencing (RNA-seq) along with clinical data were downloaded from the freely-accessible Genotype-Tissue Expression (GTEx) (https://gtexportal.org/), The Cancer Genome Atlas (TCGA) (https://xenabrowser.net/) and the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) databases. All data were visualized and analyzed using the GraphPad Prism version 8.0.0 for Windows, (GraphPad Software, San Diego, California USA, www.graphpad.com). Hazard ratios obtained from the analysis of overall and progression-free survival curves in various TCGA databases were visualized using forest plots. STRING version 11.0 (https://string-db.org/) was used for visualization of protein-protein interaction network and functional enrichment analysis.
2.3. Immunohistochemistry
Standard immunohistochemical (IHC) staining and the quantitation of the staining were performed as previously described [15]. Briefly, after de-waxing of the 5μm thick sections using xylene and re-hydration with ethanol, endogenous peroxidase activity was blocked using 3% hydrogen peroxide. This was followed by antigen retrieval, blocking with 10% normal serum, and incubation of the sections with anti-SAE1 (1:500; #ab185552, Abcam, Cambridge, UK), anti-SUMO1 (1:100; #ab32058, Abcam), anti-SUMO2 (1:100; #ab212838, Abcam), and UBC9 (1:100; #ab75854, Abcam) antibodies overnight at 4 °C, followed by goat anti-rabbit IgG (H + L) HRP-conjugated secondary antibody (1:10,000; #65-6120, Thermo Fisher Scientific Inc., Waltham, MA, USA). As chromogenic substrate, Diaminobenzidine (DAB) was used, and the stained sections were counter-stained with Gill’s hematoxylin (Thermo Fisher Scientific, Waltham, MA, USA). The univariate and multivariate analyses were done using the Cox proportional hazards regression model.
2.4. SAE1 Knockdown Using CRISPR Interference
Plasmid vectors containing pLV hU6-sgRNA hUbC-dCas9-KRAB-T2a-Puro (Plasmid #71236) was used for SAE1 knockdown in cells by CRISPR interference (CRISPRi). Three SAE1-specific single-guide RNAs (sgRNAs) designed using the online tool CHOPCHOP (http://chopchop.cbu.uib.no/) were synthesized and separately cloned into lenti-dCas9-KRAB. Lentiviruses were packaged and transfected into Huh7 cells. Transfected monoclonal Huh7 cells were selected by 2 μg/mL puromycin. The cell construction with knockdown of SAE1 was verified by genomic sequencing and quantitative real-time PCR. The sgRNA sequences for SAE1 are as follows: sgSAE1#1 (sg#1) 5′-GTGCCACATAAGTG ACCACG-3′, sgSAE1#2 (sg#2) 5′-GGCGACTGCATGTCACGTGA-3′ and sgSAE1#3 (sg#3) 5′-ACGAGGTACT GCGCAGGCGT-3′.
2.5. Real-Time PCR Reaction
Quantitative real-time PCR reaction was performed as previous described in [15] using the following primers: SAE1-FP: 5′-AGGACTGACCATGCTGGATCAC-3′ and SAE1-RP: 5′-CTCAGTGTCC ACCTTCACATCC-3′.
2.6. Western Blot Analysis
Total protein lysate was prepared from cultured HCC cells using ice-cold lysis buffer solution. After boiling at 95 °C for 5 min, immunoblotting was performed. Blots were blocked with 5% non-fat milk in Tris Buffered Saline with Tween 20 (TBST) for 1 h, incubated at 4 °C overnight with specific primary antibodies against SAE1 (1:1000; #13585S, Cell Signaling Technology, Inc., Danvers, MA, USA), CDK4 (1:1000; #2906, Cell Signaling Technology), Cyclin B1 (1:500; Sc-245, Santa Cruz Biotechnology, Dallas, TX, USA), FOXO1 (1:1000; #2880, Cell Signaling Technology), GAPDH (1:500; Sc-47724, Santa Cruz Biotechnology), and KLF9 (1:1000; ab227920, Abcam, Cambridge Inc., Cambridge, UK) in Supplementary Table S1. Thereafter, the polyvinylidene difluoride (PVDF) membranes were washed thrice with TBST, incubated with horseradish peroxidase (HRP)-labeled secondary antibody for 1 h at room temperature and then washed with TBST again before band detection using enhanced chemiluminescence (ECL) Western blotting reagents and imaging with the BioSpectrum Imaging System (UVP, Upland, CA, USA).
2.7. Transwell Matrigel Invasion Assay
After pre-coating the chamber membranes (8 μm, BD Falcon) with Bmatrigel at 4 °C overnight, the wild type (WT) or CRISPRi SAE1-knockdown cells were seeded at a density of 1 × 105 cells per chamber. DMEM with 1% fetal bovine serum (FBS) supplement was added to the upper chamber and DMEM containing 10% FBS added to the lower chamber. Cells were incubated for 48 h. The non-invading cells on the top of membranes was carefully removed using sterile cotton swab, and the invaded cells that penetrate the membrane were fixed in ethanol, followed by crystal violet staining. The number of invaded cells was counted under the microscope in five random fields of vision and representative images photographed.
2.8. Scratch-Wound Migration Assay
Cell migration potential was evaluated using the wound healing assay. Briefly, wild type (WT) or CRISPRi SAE1-knockdown Huh7 cells were seeded onto 6-well plates (1 × 106 cells/well) (Corning Inc., Corning, NY, USA) with complete growth media containing 0.2% FBS, and cultured till 95–100% confluence was attained. That would help cells not going to apoptosis or necrosis, but also no proliferation occurs. The cell monolayers were scratched with sterile yellow pipette tip along the median axes of the culture wells. The cell migration images were captured at the 0 and 16 h time points after denudation, under a microscope with a 10× objective lens, and analyzed with the NIH ImageJ software v1.49 (https://imagej.nih.gov/ij/download.html).
2.9. Statistical Analysis
All assays were performed at least thrice in triplicate. Values are expressed as the mean ± standard deviation (SD). Comparisons between groups were estimated using Student’s t-test for cell line experiments or the Mann–Whitney U-test for clinical data, Spearman’s rank correlation between variables, and the Kruskal–Wallis test for comparison of three or more groups. The Kaplan–Meier method was used for the survival analysis, and the difference between survival curves was tested by a log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 20 (IBM, Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Gene Expression Profile of SAE1 in Pan-Cancer Cohort
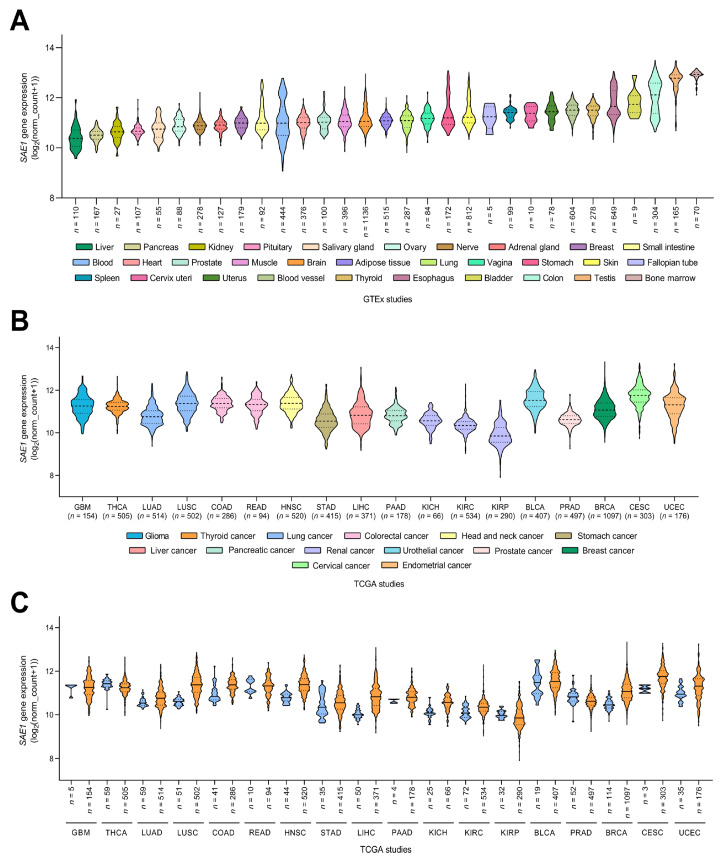
To examine the expression of SAE1 in various tissue types, we analyzed expression data of samples (n = 17,382) derived from non-disease tissues (n = 54) obtained from 948 donors using the Genotype-Tissue Expression (GTEx) project (GTEx Analysis Release V8 (dbGaP Accession phs000424.v8.p2) [16]. The lowest expression of SAE1 was observed in liver (n = 110), pancreas (n = 167), kidney (n = 27) and pituitary (n = 107), in increasing order of magnitude, while testis (n = 165) and bone marrow (n = 70) exhibited the highest SAE1 expression levels (Figure 1A).
Figure 1.
Gene expression profile of SAE1 in Pan-Cancer cohort. Violin plots showing the mRNA expression levels of SAE1 in different human tissue types according to GTEx database (A), and in various human cancer types according to TCGA database (B). SAE1 expression levels in adjacent tumor (labeled in blue) and tumor samples (labeled in orange) according to TCGA database (C).
Further exploring the SAE1 mRNA levels in paired tumor-non-tumor samples from patients with one of 18 different cancer types using The Cancer Genome Atlas (TCGA) datasets, we observed that SAE1 was significantly more expressed in liver hepatocellular carcinoma (LIHC, n = 371), compared to their normal tissue counterparts (n = 50) (Figure 1B). The upregulation of SAE1 expression was also found in several other cancer types, including lung squamous cell carcinoma (LUSC, n = 553), colon adenocarcinoma (COAD, n = 327), head and neck squamous cell carcinoma (HNSC, n = 534), kidney chromophobe (KICH, n = 91), breast invasive carcinoma (BRCA, n = 1211), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC, n = 306) and uterine corpus endometrial carcinoma (UCEC, n = 211) (Figure 1C).
3.2. SAE1 Is Overexpressed in HCC and Associated with Disease Progression
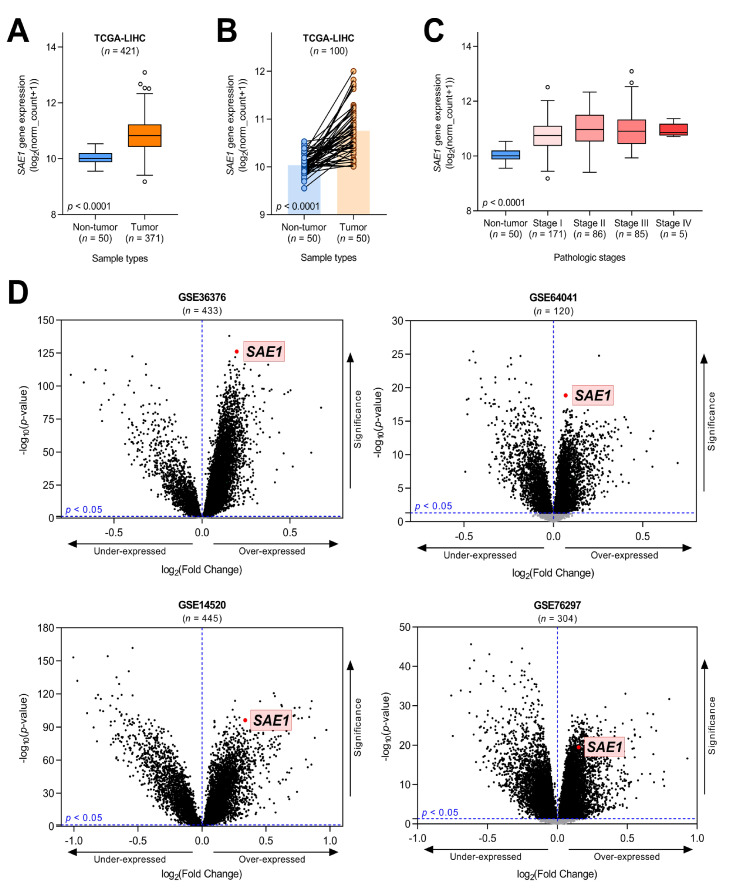
Having demonstrated that SAE1 is significantly more expressed in HCC compared with the non-tumor samples (~1.1-fold, p < 0.0001) (Figure 2A), to minimize probable experimental design-based bias, we excluded unpaired cases (n = 321) and analyzed the expression of SAE1 in only cases with paired tumor–non-tumor samples (n = 100).
Figure 2.
SAE1 is overexpressed in HCC and associated with disease progression. (A) Boxplot showing the mRNA expression levels of SAE1 in HCC and non-HCC samples according to TCGA−LIHC database (circles are outliers), (B) The expression of SAE1 in the same patients, (C) Boxplot showing the SAE1 expression grouped by pathologic stages, (D) Volcano plots indicating SAE1 upregulated according to GEO databases (GSE36376, GSE64041, GSE14520 and GSE76297).
Our results indicate that regardless of excluded cases, the median expression of SAE1 mRNA remained significantly upregulated in the tumor samples (~1.1-fold, p < 0.0001) (Figure 2B). Probing for probable role of SAE1 in disease progression, we demonstrated that SAE1 expression increased with HCC stage, as evidenced by higher expression in advanced stages (stages III/IV) and stage II than in stage I or non-tumor (stages II-IV > stage I >> non-tumor) (p < 0.0001), indicating the increased expression of SAE1 is tumorigenic and disease progression-associated (Figure 2C). Supporting the results above, our analysis of four other HCC cohort datasets downloaded from the Gene Expression Omnibus (GEO): GSE36376 (Park 2012, n = 433), GSE64041 (Makowska 2014, n = 120), GSE14520 (Wang 2009, n = 445), GSE76297 (Wang 2015, n = 304) showed that SAE1 was significantly overexpressed in all these four datasets (Figure 2D), further confirming the overexpression of the gene in TCGA−LIHC. Concordantly, we demonstrated that the expression of SAE1 increased as histologic grade increased (p < 0.0001), was equivocal for gender, and mildly higher in patients aged <60 (Supplementary Figure S1A–C). More so, SAE1 expression in T1 < T2 < T3 < T4 (p = 0.0009), mildly higher in N1 and M1 compared with N0 (p = 0.255) and M0 (p = 0.682), respectively (Supplementary Figure S1D–F). Expectedly, patients with residual tumor (R1 and R2) has higher expression of SAE1 compared with R0 (p = 0.958), and statistically significant upregulation of SAE1 was observed in the deceased compared to those alive (p = 0.025) and equivocal for radiation therapy (Supplementary Figure S1G–I). These results indicate the overexpression of SAE1 in HCC, and its association with disease progression in a stage- and grade-dependent manner.
3.3. The Overexpression of SAE1 Is Associated with Metastasis and Poor Prognosis in Patients with HCC
The eligible subjects in the TMU-SHH HCC cohort were aged from 25 to 85 with median age of 58.24 for patients with high SAE1 (n = 25) and 61.14 for those with low SAE1 (n = 29). 9 (16.67%) were male and 45 (83.33%) were female. Analyzed clinicopathological data, including patients’ demographic (age and gender) and biochemical profile (AFP, lymph node metastasis, tumor stages and survival status) are summarized in Table 1. The cut-off for AFP is based on clinical consensus that a significantly high level of circulating AFP, greater than 400 ng/mL is suggestive of malignancy of the liver. On the other hand, a threshold cut-off of 350 was ascribed for differential expression of SAE1 based on the quick (Q) score derived from the staining intensity and distribution of SAE1 in the clinical samples.
Table 1.
Patient clinicopathological characteristics of TMU-SHH HCC cohort.
| Clinicopathological Variables | Low SAE1 (n = 25) | High SAE1 (n = 29) | p-Value | ||
|---|---|---|---|---|---|
| Gender (n, %) | |||||
| Male | 7 | 28 | 2 | 6.9 | 0.088 |
| Female | 18 | 72 | 27 | 93.1 | |
| Tumor Stage (n, %) | |||||
| I + II | 18 | 72 | 14 | 48.3 | 0.021 * |
| III + IV | 7 | 28 | 15 | 51.7 | |
| Metastasis (n, %) | |||||
| M0 | 18 | 72 | 10 | 48.3 | 0.036 * |
| M1 | 7 | 28 | 19 | 51.7 | |
| Age (n, %) | |||||
| ≤65 | 17 | 68 | 16 | 55.2 | 0.494 |
| >65 | 8 | 32 | 13 | 44.8 | |
| AFP (n, %) | |||||
| <400 ng/mL | 20 | 80 | 10 | 34.5 | 0.379 |
| ≥400 ng/mL | 5 | 20 | 19 | 65.5 | |
| SAE1 (n, %) | |||||
| <350 | 25 | 100 | 0 | 0 | <0.001 * |
| ≥350 | 0 | 0 | 29 | 100 | |
| Survival Status (n, %) | |||||
| Survived | 19 | 76 | 12 | 41.4 | 0.014 * |
| Expired | 2 | 8 | 11 | 37.9 | |
| Lost to follow-up | 4 | 16 | 6 | 20.7 | |
* p-value < 0.05.
Furthermore, employed the Cox proportional hazard model for clinicopathological analysis of SAE1 protein expression, along with disease-specific risk factors, including age, gender, AFP and metastasis in the TMU-SHH HCC cohort (n = 54). Results of both univariate and multivariate analyses revealed that high SAE1 protein expression level is strongly associated with metastasis (Table 2).
Table 2.
Univariate and multivariate analysis of SAE1 expression in TMU-SHH cohort.
| Clinicopathological Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
|
Gender Male vs. Female |
2.050 | 0.262–16.017 | 0.4937 | 0.575 | 0.063–5.278 | 0.6244 |
|
Age, years ≤65 vs. >65 |
1.008 | 0.960–1.057 | 0.7532 | 0.968 | 0.922–1.017 | 0.1944 |
|
AFP, ng/mL <400 vs. ≥400 |
2.375 | 0.725–7.782 | 0.1533 | 1.053 | 0.290–3.821 | 0.9378 |
|
Metastasis M0 vs. M1 |
10.258 | 2.206–47.701 | 0.0030 * | 11.500 | 2.014–65.667 | 0.0060 * |
|
SAE1 Q-Score <350 vs. ≥350 |
1.026 | 1.002–1.051 | 0.0319 * | 1.025 | 1.000–1.049 | 0.0468 * |
* p-value < 0.05.
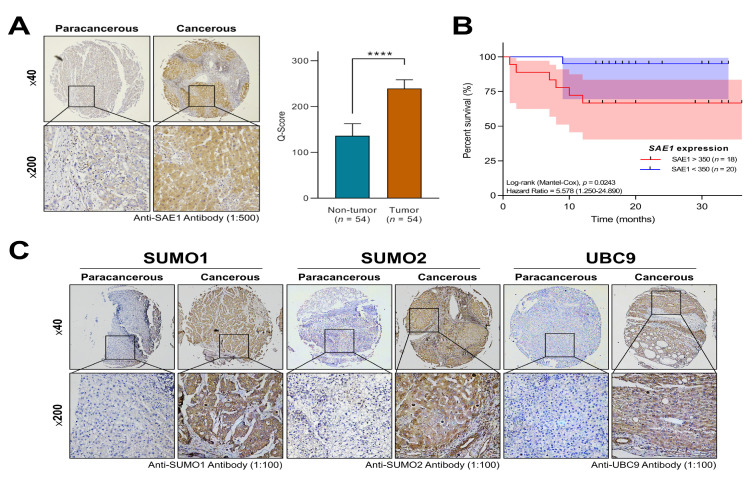
Corroborating the findings from big data analysis, IHC staining of samples from our TMU-SHH HCC cohort (n = 54) showed a 1.85-fold upregulated expression of SAE protein in the cancerous HCC compared to the non-tumor para-cancer liver tissue (p < 0.0001) (Figure 3A).
Figure 3.
The overexpression of SAE1 is associated with metastasis and poor prognosis in patients with HCC. (A) Representative IHC photo-images and graphical representation of SAE1 protein level in paracancerous and cancerous tissues. (**** p < 0.0001) (B) Kaplan-Meier curve of overall survival according to SAE1protein level of TMU-SHH HCC cohort. (C) Representative IHC photo-images of SUMO1, SUMO2, and UBC9 protein levels in paracancerous and cancerous tissues.
Probing for clinical relevance of the observed high expression of SAE1 protein, using the Kaplan-Meier curve for survival analysis, we demonstrated that compared to patients with low SAE1 expression (n = 20), those with high SAE1 expression (n = 18) exhibited worse overall survival ((HR (95%CI): 5.578 (1.250–24.890); p = 0.024)) (Figure 3B). Consistent with the vital role of SUMO proteins and the UBC9 in SUMOylation [17,18,19,20,21], we further demonstrated that similar to SAE1, the expression levels of SUMO1, SUMO2, and UBC9 were upregulated in the HCC tissues compared to their non-tumor paracancerous counterparts (Figure 3C). These results indicate that overexpression of SAE1, a critical component of the SUMOylation complex, is associated with metastasis and poor prognosis in patients with HCC.
3.4. SAE1 Is a Reliable Diagnostic and Prognostic Biomarker for HCC
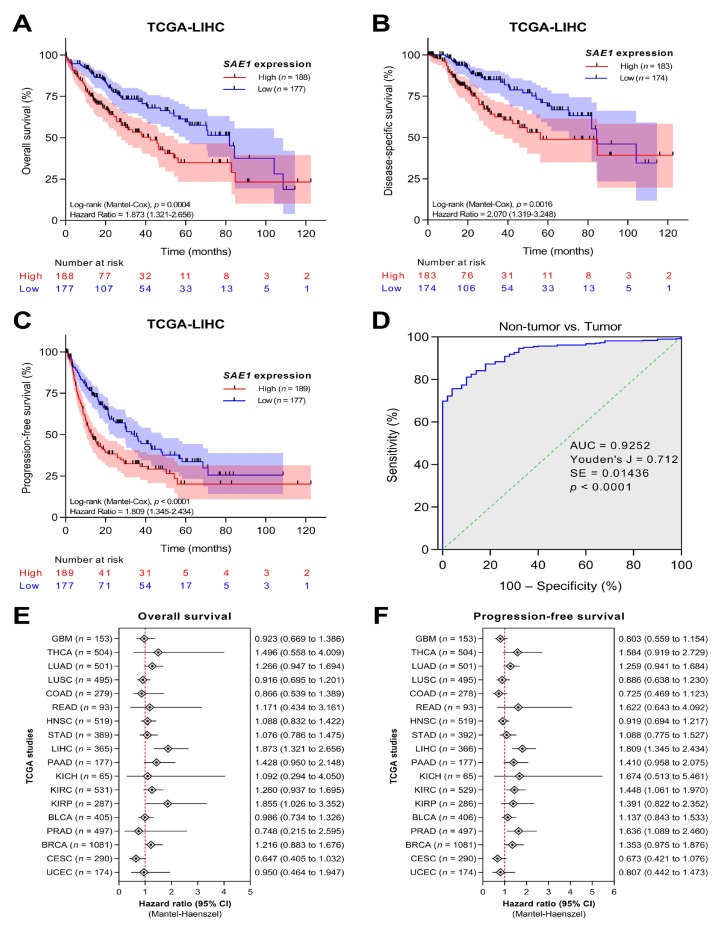
To evaluate the diagnostic and prognostic validity of SAE1 in HCC, we performed a survival analysis of the SAE1 expression-stratified TCGA−LIHC dataset using the Kaplan-Meier plots and receiver operating characteristic (ROC) curves. We demonstrated that patients with high SAE1 expression exhibited worse overall survival (OS) (HR = 1.873, p = 0.0004), disease-survival (DSS) (HR = 2.070, p = 0.0016), and progression-free survival (PFS) (HR = 1.809, p < 0.0001) over a follow-up period of 10 years (Figure 4A–C).
Figure 4.
SAE1 is a reliable diagnostic and prognostic biomarker for HCC. (A–C) Kaplan–Meier curves of overall survival, disease-specific survival and progression-free interval for HCC patients according to TCGA−LIHC database. (D) ROC analysis of SAE1 expression in non-tumor versus tumor. (E,F) Forest plot showing hazard ratio estimates and 95% confidence intervals according to TCGA studies.
In addition, we found that patient with advanced stage HCC exhibited worse OS compared with those in early stage (stage III/IV vs. I/II: p < 0.0001) (Supplementary Figure S2A). Furthermore, from our intergroup analysis of SAE1 expression in HCC vs. normal liver for diagnostic implication, the area under the ROC curve (AUC) was 0.925 (Youden’s J = 0.71, SE = 0.01, p < 0.0001) (Figure 4D), with hazard ratios and 95% confidence intervals of 1.873 (1.321–2.656) and 1.809 (1.345–2.434) for OS and PFS, respectively (Figure 4E,F). More so, compared with SAE1 expression in non-tumor, the AUCs for SAE1 expression in patients with stages I, II, III, and IV were 0.92 (p < 0.0001), 0.93 (p < 0.0001), 0.94 (p < 0.0001), and 1.00 (p = 0.0003), respectively (Supplementary Figure S2B–F).
3.5. SAE1 Upregulates Oncogenic Effectors of Cell Cycle Progression while Downregulating FOXO1-Associated Tumor Suppressing Signaling
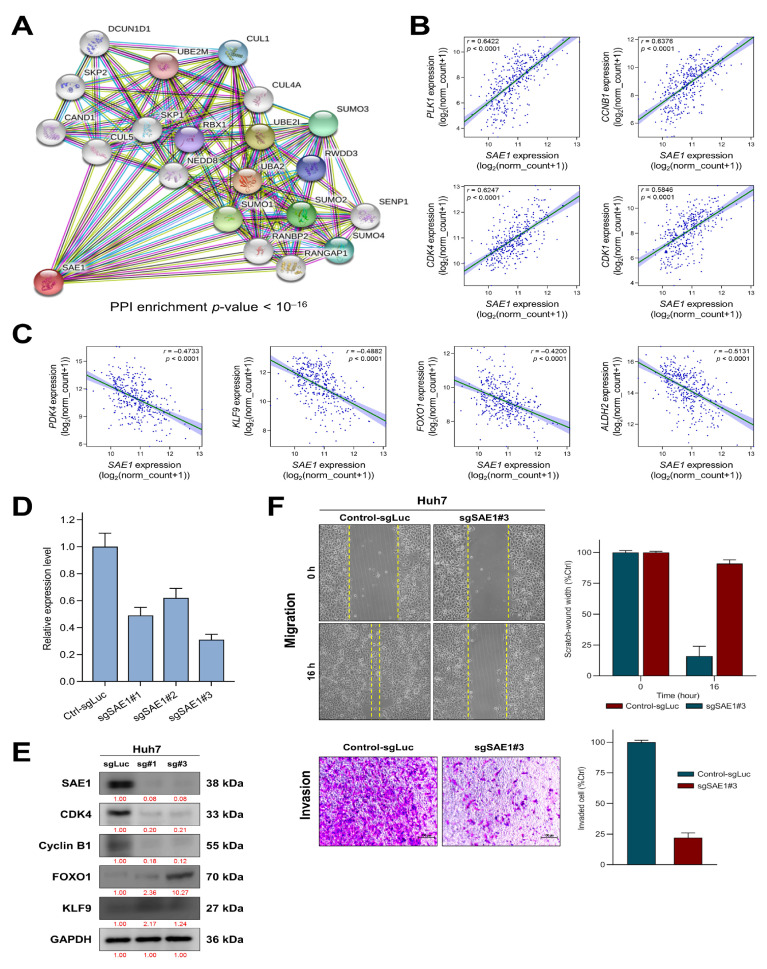
To unravel the underlying molecular mechanism of already documented SAE1-associated hepatocarcinogenesis, we probed for genes concomitantly upregulated or suppressed when SAE1 is upregulated, and SAE1-dependent protein-protein interaction (PPI). Using the STRING database (https://string-db.org) for visualization of probable network of SAE1-associated functional proteins in humans, we found that SAE1 exhibited strong interaction with SUMO proteins such as SAE2 (also called UBA2), SUMO-conjugating enzyme E2I (UBE2I/UBC9) and SUMO specific peptidase 1 (SENP1), neural precursor cell-expressed developmentally down-regulated protein 8 (NEDD8), ubiquitin-conjugating enzyme E2M (UBE2M), RAN GTPase-activating protein 1 (RANGAP1), RAN binding protein 2 (RANBP2), RWD domain containing protein 3 (RWDD3), cullin-4A (CUL4A), cullin-5 (CUL5), cullin-associated NEDD8-dissociated protein 1 (CAND1), RING-box protein 1 (RBX1), S-phase kinase-associated protein 1/2 (SKP1/2), and defective in cullin neddylation 1 domain-containing 1 (DCUN1D1) protein (Figure 5A).
Figure 5.
SAE1 upregulates oncogenic effectors of cell cycle progression while downregulating FOXO1-associated tumor suppressing signaling. (A) The SAE1-involved protein–protein interaction network constructed by STRING database. Dots and line plot showing the expression relationship between SAE1 and (B) oncogenes or (C) tumor suppressor genes. (D) Quantitative real-time PCR analysis validated the CRISPRi knockdown efficacy of sgSAE1s. (E) Western blot data of the effect of sgSAE1#1 (sg#1) and sgSAE1#3 (sg#3) on the expression of SAE1, CDK4, cyclin B1, FOXO1, and KLF9 proteins in Huh7 cells. GAPDH served as loading control. (F) Representative images of the effect of sg#3 on the migration and invasion of Huh7 cells.
Furthermore, we used the cBioPortal for Cancer Genomics (https://www.cbioportal.org/) for the identification of genes with significant positive or negative correlation with SAE1 in the TCGA−LIHC cohort. Our results showed that SAE1 is strongly co-expressed with the cell cycle-related oncogenes PLK1 (r = 0.64, p < 0.0001), CCNB1 (r = 0.64, p < 0.0001), CDK4 (r = 0.58, p < 0.0001) and CDK1 (r = 0.58, p < 0.0001) (Figure 5B), but inversely related to tumor suppressor genes PDK4 (r = −0.47, p < 0.0001), KLF9 (r = −0.47, p < 0.0001), FOXO1 (r = −0.42, p < 0.0001) and ALDH2 (r = −0.5131, p < 0.0001) (Figure 5C). To gain inside into the significance of SAE1 in hepatocarcinogenesis, we knocked down the gene using CRISPRi and validated the knockdown efficacy by real-time PCR analysis. As shown in Figure 5D, sgSAE1#1 and sgSAE1#3 exhibited high knockdown efficacy with roughly 50% and 30%, respectively. Consistent with these data, results of our western blot analysis show that silencing SAE1, elicited upregulated expression of drivers of cancer progression CDK4 and cyclin B1 (a CCNB1 gene product), concomitantly with downregulated tumor suppressors FOXO1 and KLF9 proteins in HCC cell line Huh7 (Figure 5E). Similarly, sgSAE1#3 significantly suppressed the ability of the Huh7 cells to invade (5.2-fold, p < 0.001) or migrate (6.1-fold, p < 0.001) (Figure 5F). These findings suggest that SAE1 upregulates oncogenic effectors of cell cycle progression while downregulating FOXO1-associated tumor suppressing signaling.
3.6. The Oncogenic Effect of Upregulated SAE1 Is Associated with Dysregulated Cancer Metabolic Signalings
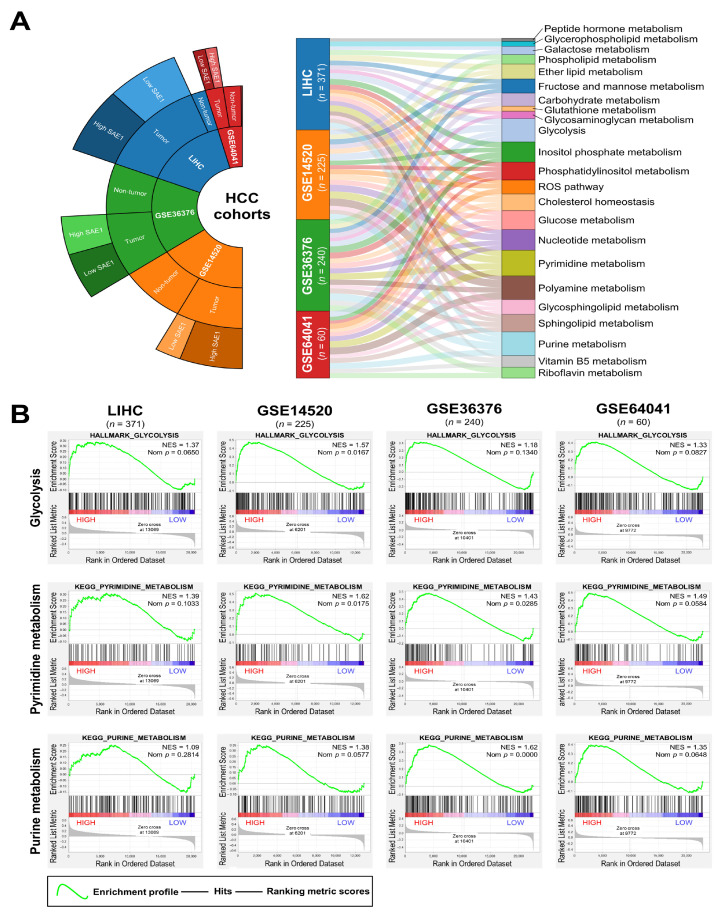
Understanding that several stress signals, including hypoxia, impaired metabolism, nutrient deficiency, DNA damage (genotoxic stress) and dysregulated nucleotide metabolism, facilitate the initiation and development of cancer, and that dysregulated SUMOylation can play a crucial role in the protection of cancer cells from exogenous or endogenous stress signals [21], we investigated likely association of SAE1 expression with hypoxia and impaired metabolism. The results of our gene set enrichment analysis of the LIHC (n = 371), GSE14520 (n = 225), GSE36376 (n = 240), and GSE64041 (n = 60) HCC datasets showed the existence of significant positive correlation between high SAE1 and dysregulated reactive oxygen species (ROS), glycolysis, and cholesterol homeostasis pathways in patients with HCC (Figure 6A). Gene Set Enrichment Analysis (GSEA) plots using HCC datasets implied the upregulated co-expression of SAE1 and biomarkers of glucose metabolism, pyrimidine metabolism, and purine metabolism (Figure 6B). These data do indicate, at least in part, that the oncogenic effect of upregulated SAE1 is associated with dysregulated cancer metabolic signaling.
Figure 6.
The oncogenic effect of SAE1 is associated with dysregulated cancer metabolic signaling. (A) Sankey diagram of the involvement of SAE1 in several metabolic processes in the LIHC, GSE14520, GSE36376, and GSE64041 datasets. (B) Representative GSEA plots showing the association between high SAE1 expression and enriched glycolysis, pyrimidine and purine metabolisms in the LIHC, GSE14520, GSE36376, and GSE64041 datasets.
4. Discussion
Hepatocarcinogenesis entails alteration of cellular gene expression with consequent loss of benignity, acquisition of malignant phenotype and enhancement of the aggressiveness of the resultant cancerous liver cells [3]. Previous studies have shown that post-translational modification such as ubiquitination and SUMOylation, which play very crucial roles in the non-static regulation of protein structure, stability, intracellular localization, activity, function, and interaction with other proteins, are significantly enhanced in HCC [14,17]. Recently, it was reported that the SUMO2-mediated SUMOylation of the supposedly tumor suppressor, liver kinase B1 (LKB1), facilitated hepatocarcinogenesis and disease progression in in vivo mice models and human HCC cohort, especially in hypoxic conditions [17]. Consistent with this report, it has also been shown that hypoxia or exposure to TNF-α upregulated SUMO1 expression and the later enhanced the nuclear translocation and SUMOylation of p65, enhancing HCC cell proliferation, migration, and consequently disease progression [18].
Essentially, SUMOylation constitutes a network of enzymatic activities that elicit formation of isopeptide bond between the glycine at the C-terminal of a SUMO and the lysine residue of a protein substrate through the mediation of heterodimeric SUMO-activating enzyme (SAE1/SAE2 complex), SUMO-conjugating enzyme UBC9, and SUMO E3 ligases [19,20]. Accruing evidence from numerous studies have suggested that dysregulated SAE1 expression and/or activity contributes to uncontrolled cell proliferation, development of cancer, angiogenesis, invasion and metastasis [21]. Against the background of reported implication of SAE1 in SUMOylation and oncogenesis in several malignancies, including glioma and gastric cancer [11,12], and aiming to validate its clinical validity and applicability as a reliable diagnostic and/or prognostic biomarker, the present study explored the expression and of SAE1, an indispensable molecular effector of SUMOylation [22], by probing and analyzing clinicopathological data from our in-house HCC cohort and several HCC databases.
In this study, we demonstrated that SAE1 is differentially expressed in normal and cancerous tissues, including in paired normal liver and HCC samples. More so, we provided evidence that the enhanced expression of SAE1 is both grade- and stage-dependent, indicating a probable role for SAE1 in enhanced onco-aggressiveness and disease progression in patients with HCC. This is consistent with reports demonstrating that SUMOylation-dependent transcriptional sub-programming is required for Myc-driven tumorigenesis, and more so implicating SAE1 in the progression of human glioma and gastric cancer through the activation of SUMOylation-mediated oncogenic signaling pathways [11,12,13].
Additionally, and with clinical relevance, we demonstrated that the overexpression of SAE1 is associated with poor prognosis, as evident in the shorter overall or disease-specific, and relapse-free survival time of patients with high expression of SAE1 in our HCC cohort and freely accessible larger HCC cohorts. These findings are corroborated by recent report that the expression of key components of the SUMO-involved regulatory network including enhanced UBE2I and SAE1 gene expression levels were strongly linked to poor prognosis in HCC [23] and that the SUMOylation pathway is associated with adverse clinical outcome for patients with multiple myeloma [24].
In addition, we demonstrated that underlying the oncogenic and HCC-promoting activity of SAE1 was its ability to upregulate oncogenic effectors of cell cycle progression while downregulating FOXO1-associated tumor suppressing signaling. This is consistent with contemporary knowledge that loss of FOXO1 promotes tumor growth and metastasis [25], and accruing evidence that SUMOs such as SAE1 are essential for the regulation of several cellular processes, including transcriptional regulation, transcript processing, genomic replication and DNA damage repair, where efficiency or inefficiency of the later determines initiation of mitosis or delayed mitotic entry, S-phase arrest, and altered cell cycle progression [26,27,28]; this has significant implication for diseases such as cancer, and suggests that SAE1 is a potential therapeutic target for patients with HCC.
More interestingly, we provided some evidence that SAE1 is a reliable diagnostic biomarker for HCC, with the differential expression of SAE1 in paired normal liver and HCC samples exhibiting an AUC of 0.9252. Similarly, the prognostic relevance of SAE1 expression was shown with stage dependent AUCs ranging from 0.9091 for stage I to 1.00 for stage IV, and Kaplan-Meier plots indicating worse clinical outcome for patients with high SAE1 expression compared to their counterparts with low SAE1 expression. These findings are clinically valid and statistically relevant considering that the ROC curve is a vital tool in disease diagnostics and prognostics, especially where the evaluation of a biomarker’s discriminatory ability is being carried out or for validation of diagnostic and/or prognostic tests. The AUC is the most widely used accuracy index of overall discriminatory power for biomarker identification and validation, such that higher AUC values indicate higher discriminability of a diagnostic or prognostic biomarker or test [29,30].
In conclusion, the present study demonstrates that SAE1 is a targetable SUMO-related molecular biomarker with high potential diagnostic and prognostic implications for patients with HCC.
Acknowledgments
The authors thank all research assistants of the Cancer Translational Research Laboratory and Core Facility Center, Taipei Medical University—Shuang Ho Hospital, for their assistance with the flow cytometry, molecular and cell-based assays. We thank the CRISPR Gene Targeting Core Lab at TMU, TMU Core Facility at TMU in Taiwan for providing technical support.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/10/1/178/s1, Table S1. Specific primary antibodies of western blot in this study. Figure S1. The overexpression of SAE1 is associated with metastasis and poor prognosis in patients with HCC. Boxplots showing the mRNA expression levels of SAE1 according to histologic grade (A), genders (B), age at initial pathologic diagnosis (C), TNM classification (D–F), residual tumor (G), vital status (H) and radiation therapy (I). Figure S2. SAE1 is a reliable diagnostic and prognostic biomarker for HCC. (A) Kaplan-Meier curves of overall survival according to HCC pathologic stages. (B–E) ROC analysis of SAE1 expression in non-tumor versus tumor in stages I, II, III and IV. (F) ROC analysis of SAE1 expression characterized by various clinicopathological factors. Figure S3. Full-size blots of Figure 5E.
Author Contributions
J.R.O.: Study conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing. C.-T.Y., O.A.B., N.V.K., Y.-K.L., W.-H.L.: Data analysis and interpretation. Y.-G.C.: Study conception and design, data analysis and interpretation, final manuscript approval. All authors have read and agreed to the published version of the manuscript.
Funding
This study was also supported by grants from and Taipei Medical University (102TMU-SHH-02) to Wei-Hwa Lee.
Institutional Review Board Statement
This study was conducted in a cohort of patients with HCC cancer at Taipei Medical University Shuang-Ho Hospital, Taipei, Taiwan. The study was reviewed and approved by the institutional review board (TMU-JIRB: 201302016).
Informed Consent Statement
All researchers must ensure that the process of obtaining informed consent from study participants not only conforms to federal, state, and local regulations but also respects each individual’s right to make a voluntary, informed decision.
Data Availability Statement
The datasets used and analyzed in the current study are publicly accessible as indicated in the manuscript.
Conflicts of Interest
The authors declare that they have no potential financial competing interests that may in any way, gain or lose financially from the publication of this manuscript at present or in the future. Additionally, no non-financial competing interests are involved in the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara N., Friedman S.L., Goossens N., Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018;68:526–549. doi: 10.1016/j.jhep.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jee B.A., Choi J.H., Rhee H., Yoon S., Kwon S.M., Nahm J.H., Yoo J.E., Jeon Y., Choi G.H., Woo H.G., et al. Dynamics of Genomic, Epigenomic, and Transcriptomic Aberrations during Stepwise Hepatocarcinogenesis. Cancer Res. 2019;79:5500–5512. doi: 10.1158/0008-5472.CAN-19-0991. [DOI] [PubMed] [Google Scholar]
- 4.Losic B., Craig A.J., Villacorta-Martin C., Martins-Filho S.N., Akers N., Chen X., Ahsen M.E., von Felden J., Labgaa I., D’Avola D., et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat. Commun. 2020;11:291. doi: 10.1038/s41467-019-14050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang A., Zhao X., Yang X.R., Li F.Q., Zhou X.L., Wu K., Zhang X., Sun Q.-M., Cao Y., Zhu H.-M., et al. Circumventing intratumoral heterogeneity to identify potential therapeutic targets in hepatocellular carcinoma. J. Hepatol. 2017;67:293–301. doi: 10.1016/j.jhep.2017.03.005. Erratum in J. Hepatol.2017, 67, 1123. [DOI] [PubMed] [Google Scholar]
- 6.Kotha S., Neong S., Patel K. Serum biomarkers for diagnosis and monitoring viral hepatitis and hepatocellular carcinoma. Expert Rev. Mol. Diagn. 2018;18:713–722. doi: 10.1080/14737159.2018.1496020. [DOI] [PubMed] [Google Scholar]
- 7.Huang T.S., Shyu Y.C., Turner R., Chen H.Y., Chen P.J. Diagnostic performance of alpha-fetoprotein, lens culinaris agglutinin-reactive alpha-fetoprotein, des-gamma carboxyprothrombin, and glypican-3 for the detection of hepatocellular carcinoma: A systematic review and meta-analysis protocol. Syst. Rev. 2013;2:37. doi: 10.1186/2046-4053-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lou J., Zhang L., Lv S., Zhang C., Jiang S. Biomarkers for Hepatocellular Carcinoma. Biomark Cancer. 2017;9:1–9. doi: 10.1177/1179299X16684640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y.X., Yang T.W., Yin J.M., Yang P.X., Kou B.X., Chai M.Y., Liu X.N., Chen D.X. Progress and prospects of biomarkers in primary liver cancer (Review) Int. J. Oncol. 2020;57:54–66. doi: 10.3892/ijo.2020.5035. [DOI] [PubMed] [Google Scholar]
- 10.Geiss-Friedlander R., Melchior F. Concepts in sumoylation: A decade on. Nat. Rev. Mol. Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Liang Z., Xia Z., Wang X., Ma Y., Sheng Z., Gu Q., Shen G., Zhou L., Zhu H., et al. SAE1 promotes human glioma progression through activating AKT SUMOylation-mediated signaling pathways. Cell Commun. Signal. 2019;17:82. doi: 10.1186/s12964-019-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M., Jiang D., Xie X., He Y., Lv M., Jiang X. miR-129-3p inhibits NHEJ pathway by targeting SAE1 and represses gastric cancer progression. Int. J. Clin. Exp. Pathol. 2019;12:1539–1547. [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler J.D., Kahle K.T., Sun T., Meerbrey K.L., Schlabach M.R., Schmitt E.M., Sninnrt S.O., Xu Q., Li M.Z., Hartman Z.C., et al. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science. 2012;335:348–353. doi: 10.1126/science.1212728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J.S., Thorgeirsson S.S. Genome-Scale profiling of gene expression in hepatocellular carcinoma: Classification, survival prediction, and identification of therapeutic targets. Gastroenterology. 2004;127:S51–S55. doi: 10.1053/j.gastro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y.J., Yang C.K., Wei P.L., Huynh T.T., Whang-Peng J., Meng T.C., Hsiao M., Tzeng Y.M., Wu A.T., Yen Y. Ovatodiolide suppresses colon tumorigenesis and prevents polarization of M2 tumor-associated macrophages through YAP oncogenic pathways. J. Hematol. Oncol. 2017;10:60. doi: 10.1186/s13045-017-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consortium G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zubiete-Franco I., García-Rodríguez J.L., Lopitz-Otsoa F., Lopitz-Otsoa F., Serrano-Macia M., Simon J., Fernandez-Tussy F., Barbier-Torres L., Fernandez-Ramos D., Gutierrez-de-Juan V., et al. SUMOylation regulates LKB1 localization and its oncogenic activity in liver cancer. EBioMedicine. 2019;40:406–421. doi: 10.1016/j.ebiom.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Tao X., Zhang J., Wang P., Sha M., Ma Y., Geng X., Feng L., Shen Y., Yu Y., et al. Small ubiquitin-related modifier 1 is involved in hepatocellular carcinoma progression via mediating p65 nuclear translocation. Oncotarget. 2016;7:22206–22218. doi: 10.18632/oncotarget.8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pichler A., Fatouros C., Lee H., Eisenhardt N. SUMO conjugation—A mechanistic view. Biomol. Concepts. 2017;8:13–36. doi: 10.1515/bmc-2016-0030. [DOI] [PubMed] [Google Scholar]
- 20.Tomasi M.L., Tomasi I., Ramani K., Pascale R.M., Xu J., Giordano P., Mato J.M., Lu S.C. S-Adenosyl methionine regulates ubiquitin-conjugating enzyme 9 protein expression and sumoylation in murine liver and human cancers. Hepatology. 2012;56:982–993. doi: 10.1002/hep.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Z.J., Feng Y.H., Gu B.H., Li Y.M., Chen H. The post-translational modification, SUMOylation, and cancer (Review) Int. J. Oncol. 2018;52:1081–1094. doi: 10.3892/ijo.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu R., Fang J., Liu M., Jun A., Liu J., Chen W., Li J., Ma G., Zhang Z., Zhang B., et al. SUMOylation of the transcription factor ZFHX3 at Lys-2806 requires SAE1, UBC9, and PIAS2 and enhances its stability and function in cell proliferation. J. Biol. Chem. 2020;295:6741–6753. doi: 10.1074/jbc.RA119.012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H., Gao S., Chen J., Lou W. UBE2I promotes metastasis and correlates with poor prognosis in hepatocellular carcinoma. Cancer Cell Int. 2020;20:234. doi: 10.1186/s12935-020-01311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driscoll J.J., Pelluru D., Lefkimmiatis K., Fulciniti M., Prabhala R.H., Greipp P.R., Barlogie B., Tai Y.-T., Anderson K.C., Shaughnessy J.D., et al. The sumoylation pathway is dysregulated in multiple myeloma and is associated with adverse patient outcome. Blood. 2010;115:2827–2834. doi: 10.1182/blood-2009-03-211045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko Y.S., Cho S.J., Park J., Kim Y., Choi Y.J., Pyo J.S., Jang B.G., Park J.-W., Kim W.H., Lee B.L. Loss of FOXO1 promotes gastric tumour growth and metastasis through upregulation of human epidermal growth factor receptor 2/neu expression. Br. J. Cancer. 2015;113:1186–1196. doi: 10.1038/bjc.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendriks I.A., Vertegaal A.C. A comprehensive compilation of SUMO proteomics. Nat. Rev. Mol. Cell Biol. 2016;17:581–595. doi: 10.1038/nrm.2016.81. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y., Parvin J.D. Small ubiquitin-like modifier (SUMO) isoforms and conjugation-independent function in DNA double-strand break repair pathways. J. Biol. Chem. 2014;289:21289–21295. doi: 10.1074/jbc.C114.582122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr A.R., Cooper S., Heldt F.S., Butera F., Stoy H., Mansfeld J., Novák B., Bakal C. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat. Commun. 2017;8:14728. doi: 10.1038/ncomms14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan L., Tian L., Liu S. Combining large number of weak biomarkers based on AUC. Stat. Med. 2015;34:3811–3830. doi: 10.1002/sim.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou K.H., Liu A., Bandos A.I., Ohno-Machado L., Rockette H.E. Statistical Evaluation of Diagnostic Performance. Chapman and Hall/CRC; Boca Raton, FL, USA: 2011. p. 33487. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed in the current study are publicly accessible as indicated in the manuscript.