Abstract
Background
The net health benefit of using antipsychotics in children and adolescents with ASD is unclear. This review was performed to provide the evidence necessary to inform the Italian national guidelines for the management of ASD.
Methods
We performed a systematic review of randomized controlled trials (RCTs) comparing antipsychotics versus placebo for the treatment of ASD in children and adolescents. For efficacy, acceptability and safety we considered outcomes evaluated by the guideline panel critical and important for decision-making. Continuous outcomes were analyzed by using standardized mean difference (SMD), and dichotomous outcomes by calculating the risk ratio (RR), with their 95% confidence interval (95% CI). Data were analyzed using a random effects model. We used the Cochrane tool to assess risk of bias of included studies. Certainty in the evidence of effects was assessed according to the GRADE approach.
Results
We included 21 RCTs with 1,309 participants, comparing antipsychotics to placebo. Antipsychotics were found effective on “restricted and repetitive interests and behaviors” (SMD − 0.21, 95% CI − 0.35 to − 0.07, moderate certainty), “hyperactivity, inattention, oppositional, disruptive behavior” (SMD − 0.67, 95% CI − 0.92 to − 0.42, moderate certainty), “social communication, social interaction” (SMD − 0.38, 95% CI − 0.59 to − 0.16, moderate certainty), “emotional dysregulation/irritability” (SMD − 0.71, 95% CI − 0.98 to − 0.43, low certainty), “global functioning, global improvement” (SMD − 0.64, 95% CI − 0.96 to − 0.33, low certainty), “obsessions, compulsions” (SMD − 0.30, 95% CI − 0.55 to − 0.06, moderate certainty). Antipsychotics were not effective on “self-harm” (SMD − 0.14, 95% CI − 0.58 to 0.30, very low certainty), “anxiety” (SMD − 0.38, 95% CI − 0.82 to 0.07, very low certainty). Antipsychotics were more acceptable in terms of dropout due to any cause (RR 0.61, 95% CI 0.48 to 0.78, moderate certainty), but were less safe in terms of patients experiencing adverse events (RR 1.19, 95% CI 1.07 to 1.32, moderate certainty), and serious adverse events (RR 1.07, 95% CI 0.48 to 2.43, low certainty).
Conclusions
Our systematic review and meta-analysis found antipsychotics for children and adolescents with ASD more efficacious than placebo in reducing stereotypies, hyperactivity, irritability and obsessions, compulsions, and in increasing social communication and global functioning. Antipsychotics were also found to be more acceptable, but less safe than placebo.
Keywords: Autism spectrum disorder, Antipsychotics, D2 blockers, Systematic review, Meta-analysis, Children, Adolescents, Guidelines
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by persistent impairments in reciprocal social communication and social interactions along with the presence of restricted, repetitive patterns of behaviors, interests, or activities [1]. Two recent studies conducted in Italy reported a prevalence of ASD in children (age range 7–9 years old) of 1.14% and 1.3% [2, 3], consistent with its prevalence in the world, which is reported between 1 and 2% [4]. The male: female ratio is about 4: 1 [5], with 48% of children having intellectual disability [5, 6].
No pharmacological treatment has currently shown to be effective for the treatment of the core symptoms of ASD. In general, pharmacological treatments, combined with psychological interventions, are directed at the treatment of associated symptoms (such as irritability) or coexisting psychiatric conditions (e.g. attention deficit disorder, oppositional disorder, schizophrenia spectrum disorders), which are frequent in patients with ASD [7].
Antipsychotics are used to treat associated comorbidities, such as schizophrenia spectrum disorders (SSD) and behavior disorders [8, 9]. A recent meta-analysis estimated a prevalence rate of SSD symptoms in ASD of 9.6% [10], and vice-versa individuals with SSD have significantly more autistic symptoms than healthy controls [11]. An observational study reported a high incidence of multiple treatment failure when children and adolescents with comorbid ASD and SSD are treated with antipsychotics [12].
A systematic review by Pillay et al. [13] suggested that antipsychotics may be useful in improving core symptoms, particularly stereotyped behaviors and narrow interests. The latest, larger clinical studies have been based on second generation antipsychotics (SGA) such as risperidone, aripiprazole and lurasidone, while older studies studied first-generation antipsychotics (FGA). Few studies compared the effect of two or more antipsychotics [13].
This systematic review was performed within the context of the development of evidence-based guidelines for the diagnosis and management of ASD in children and adolescents for the Italian National Institute of Health (in Italian: Istituto Superiore di Sanità—ISS). This systematic review has been specifically conducted by the Evidence Review Team, based on the manual developed and published by the ISS, to support the ISS autism guidelines panel in formulating recommendations [14, 15]. Following a public application process, the ISS established a multidisciplinary panel, including people with ASD and/or their caregivers, that formulated the following question for its first recommendation:
Should antipsychotics be used for the treatment of children and adolescents with ASD?
Methods
The key elements of the review protocol including participants, intervention, comparator, outcomes, study design (PICOS) were developed by the guideline panel. Panel members and evidence review team members declared conflict of interests and all the process was recorded and it is available for consultation upon request to the study authors.
Population
Children and adolescents aged 0–18 years with a primary diagnosis of ASD. A concurrent secondary diagnosis of another medical condition was not considered as an exclusion criterion.
Intervention
The following list of FGAs and SGAs was selected by panel members to be investigated in the systematic review, with no limitations of dose and administration route: aripiprazole, clozapine, haloperidol, levosulpiride, lurasidone, olanzapine, risperidone, trifluoperazine. We included also studies in which antipsychotics were used as adjunctive treatment (e.g. in addition to behavioral or other pharmacological interventions).
Comparisons
Placebo or no intervention.
Outcomes
To measure the desirable and undesirable effects of the treatment, we assessed the following outcomes:
Restricted and repetitive interests and behavior,
Hyperactivity, inattention, oppositional, disruptive behavior disorders,
Self-harm,
Insomnia,
Social communication, social interaction,
Serious adverse events,
Emotional dysregulation/irritability,
Anxiety,
Adverse events,
Global functioning, global improvement,
Quality of life,
Obsessions, compulsions,
Dropout due to any cause,
Dropout due to adverse events.
These outcomes were deemed to be highly relevant to children and adolescents with ASD by the guideline panel.
Subgroup analyses
We performed subgroup analyses dividing the studies between those that reported Aberrant Behavior Checklist (ABC)—Irritability subscale scores ≥ 18 as the participants’ selection criterion, and those that reported different inclusion criteria or no particular criteria in addition to the diagnosis of ASD. The ABC is a scale empirically developed to assess the effectiveness of psychotropic medications by measuring psychiatric and behavioral disturbances exhibited by individuals with intellectual and developmental disabilities through 5 subscales corresponding to as many domains (irritability [range 0–45], lethargy/social withdrawal [range 0–48], stereotypic behavior [range 0–21], hyperactivity/noncompliance [range 0–48], inappropriate speech [range 0–12], with higher scores indicating worse condition) [13, 16]. We assessed credibility of subgroup effects using the criteria proposed by Sun et al. [17], and we considered it in evaluating the certainty in the evidence of effects [18].
Types of studies included
Randomized controlled trials comparing antipsychotics with placebo or no treatment in the management of ASD were included. Both parallel, crossover and withdrawal design were included. Quasi-randomized trials, such as those allocating by using alternate days of the week, and open label trials were excluded. For trials that had a crossover design only results from the first randomization period were considered, since carry-over effect could not be excluded [19].
Literature search
We performed a comprehensive computer literature search of the CENTRAL, PubMed/Medline, Embase, PsycINFO, Web Of Science databases and of trial registers to find relevant peer reviewed articles on the effect of antipsychotics for children and adolescents with ASD from the date of database inception until January 2019. The full search strategies used are available in Additional file 1 and Additional file 2. No date limit and no language restrictions were used. Finally, we hand-searched references from relevant systematic reviews and included studies to identify any RCT missed by the search strategy.
Study selection and data extraction
Two reviewers (FDC, GD) independently evaluated the retrieved studies for inclusion and assessed the methodological quality of included studies. Information extracted included study characteristics (lead author, publication year, journal), participant characteristics (age range, setting, diagnosis), intervention details (dose ranges, mean doses of study drugs) and outcome measures of interest.
Data analysis
Data were entered and analyzed using STATA 16.1 software. Since different scales were used in the studies, we analyzed data as continuous outcomes using standardized mean difference (SMD) with 95% confidence intervals, utilizing the random effects model, because a certain degree of heterogeneity was expected among trials [20]. In interpreting SMD values, we considered SMD “small” if < 0.40, “moderate” from 0.40 to 0.70, and “large” if > 0.7. We analyzed dichotomous outcomes by calculating the risk ratio (RR) for each trial with the uncertainty in each result being expressed with 95% confidence interval (CI). Heterogeneity between studies has been investigated by the Q-test, by the I-squared statistic (I-squared equal to or more than 50% was considered indicative of heterogeneity), and by visual inspection of the forest plots.
Dealing with missing data
We managed missing data according to Higgins et al. [19]. If dichotomous outcome data were still missing, they were managed according to the intention-to-treat (ITT) principle, and we assumed that patients who dropped out after randomization had a negative outcome. Missing continuous outcome data were either analyzed using the last observation carried forward to the final assessment (LOCF) or on an endpoint basis, including only participants with a final assessment. When p values, t-values, 95% CIs or standard errors were reported in articles, we calculated SDs from their values [20]. If p values, t-values, 95% CIs or standard errors were not reported at the endpoint, SDs were imputed from their baseline values, or, if baseline values were not reported, from the mean value of SDs of individuals randomized to that drug (or to placebo) in the other included studies [21].
Risk of bias and overall certainty of evidence assessment
Two authors independently (GLD, FDC) assessed the risk of bias in the included studies using the Cochrane risk of bias assessment tool [19].
The main results of the review were presented in’Summary of findings’ (SoF) tables, as recommended by Cochrane [19, 22], using the methodology developed from the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group [23–25]. The confidence in the effect estimates was evaluated in four levels: high, moderate, low, very low. The results were summarized and presented to the panel through the GRADE evidence to decision (EtD) framework [26, 27]. Here we present the results for the following criteria: desirable effects, undesirable effects, and certainty of evidence. The results for the other EtD criteria will be published elsewere [28, 29].
Results
Selected studies
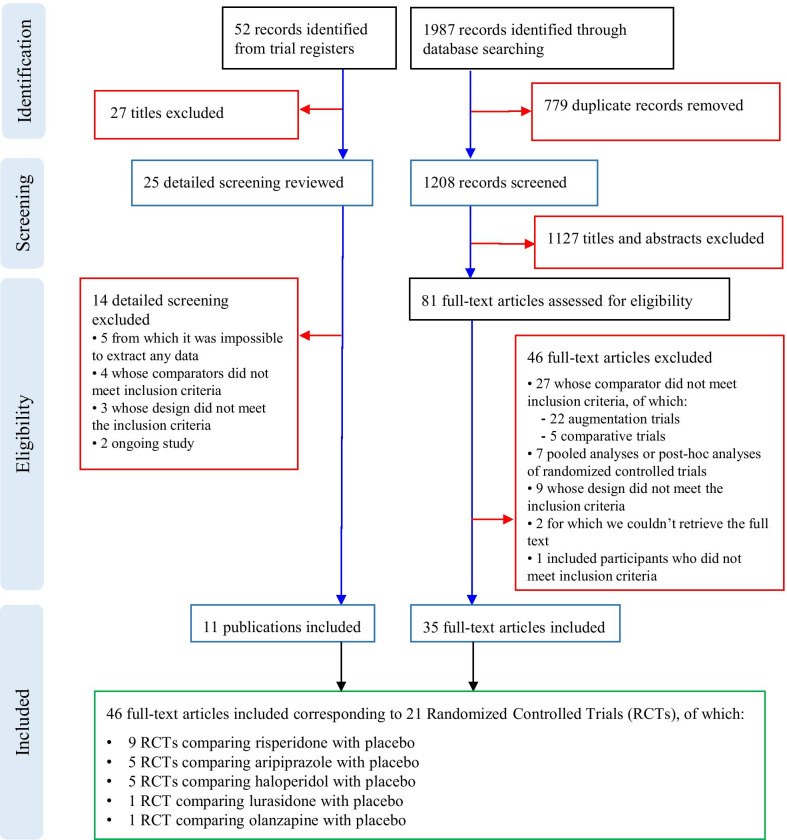
We retrieved from database searching 1987 citations of which 779 were removed, being duplicates. We excluded 1127 records on the basis of titles and abstracts and retrieved 82 documents in full text: 47 studies have been excluded and 35 full-text articles included since satisfied the inclusion criteria. Reasons for the exclusion of the 46 papers: 27 studies whose comparator did not meet inclusion criteria, being augmentation trials without placebo arm [30–51] or trials comparing two pharmacological interventions without placebo arm [52–56]; seven studies were pooled analyses or post-hoc analyses of randomized controlled trials [57–63]; nine studies whose design did not meet the inclusion criteria [64–72]; two studies for which we were not able to retrieve the full-text [73, 74]; one study included only adults [75]. We retrieved 52 records from trial registers, 22 of which evaluated in full text: five trials were excluded since they did not show any result (clinicaltrials.gov identifiers: NCT00147394 [76], NCT00198107 [77], NCT00468130 [78], NCT01171937 [79], NCT00057408 [80]), four trials considered comparators that did not meet inclusion criteria (clinicaltrials.gov identifiers: NCT00080145 [81], NCT00205699 [82], NCT01333072 [83], NCT01844700 [84]), three trial whose design did not meet inclusion criteria (clinicaltrials.gov identifiers: NCT00166595 [85], NCT00691080 [86], NCT00619190 [87]), and two ongoing trials (clinicaltrials.gov identifiers: NCT02574741 [88], NCT03487770 [89]).
Finally, 46 documents, corresponding to 21 RCTs (1309 participants) were included: nine RCTs comparing risperidone with placebo [90–98], five RCTs comparing aripiprazole with placebo [99–103], five RCTs comparing haloperidol with placebo [104–108], one RCT comparing lurasidone with placebo [109], and one RCT comparing olanzapine with placebo [110]. Articles’ selection process is shown in Fig. 1, while full references for included and excluded trials are reported in Additional file 3.
Fig. 1.
PRISMA Flow chart
Included studies characteristics
Six studies (28.6%) included pre-schoolers and school age children, while 15 studies (71.4%) included school-age children and adolescents. The majority were male (83.3%), with a mean age of 8.8 years. The studies included patients diagnosed with autism (71.4%), ASD (23.8%) or PDD-NOS (4.8%), and diagnosis was performed principally through DSM-IV (81.0%) or DSM-III criteria (14.3%). Overall, 318 patients were randomly assigned to aripiprazole, 248 to risperidone, 100 to lurasidone, 67 to haloperidol, 6 to olanzapine, and 545 to placebo. Mean sample size was of 62 (range 11–218). One study recruited patients from Europe, 17 from North America, and three from Asia. Study median duration was eight weeks (Interquartile range: 8–22). Full study characteristics are reported in Table1.
Table 1.
Characteristics of included Randomized Controlled Trials
| Study, Year | Country | Diagnosis | Diagnostic criteria | Severity | Intervention (n) | Control(n) | Study design | Duration of intervention (wks) | Setting | Age mean (SD); range | Female (%) | Mental development | Outcomes | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson, 1984 | USA | Autism | DSM-III | Not reported | Haloperidol 0.5 to 4.0 mg/day (20) | Placebo (20) | Crossover | 14 (2 weeks SB Placebo baseline, 12 weeks DB intervention: placebo/treatment/placebo or treatment/placebo/treatment) | Inpatient | 4.58 (NR); 2.3–6.9 | 27.5 | Not reported | Global changes (CGI-I, CGI-S), Behavior (CPRS), Social Interaction (CPTQ), discrimination learning, adverse events (DOTES), optimal dosages | McNeal Pharmaceuticals; NIMH |
| Anderson, 1989 | USA | Autism | DSM-III | Not reported | Haloperidol 0.25 to 4.0 mg/day (15) | Placebo (30) | Crossover | 14 (2 weeks SB Placebo baseline, 12 weeks DB intervention: treatment/placebo/placebo or placebo/treatment/placebo or placebo/placebo/treatment) | Inpatient | 4.49 (1.16); 2.0–7.6 | 22.2 | Profound to borderline retardation | Global changes (CGI-I, CGI-S), Behavior (CPRS), Social Interaction (CPTQ), discrimination learning | McNeal Pharmaceuticals; NIMH; March of Dimes Birth Defects Foundation grant 12–108 |
| Campbell, 1978 | USA | Autism | Not reported (Kanner and Rutter criteria) | Not reported | Haloperidol 0.5 to 4.0 mg/day, adaptive dose (21) | Placebo (21) | Parallel | 12 (2 weeks SB placebo, 3 weeks DB medication, 5 weeks DB medication plus behavioral therapy, 2 weeks SB placebo plus behavioral therapy) | Inpatient | 4.5 (NR); 2.6–7.2 | 20.0 | Not reported | McNeal Pharmaceutical; Behavior (CPRS, CBI, NGI), adverse events (DOTES), optimal dosages | NIHM |
| Cohen, 1980 | USA | Autism | DSM-III | Not reported | Haloperidol 0.5 to 4.0 mg/day, adaptive dose (Not reported) | Placebo (Not reported) | Crossover | 10 (2 weeks SB placebo, 8 weeks intervention: placebo/Haldoperidol/placebo/Haldoperidol or vice-versa) | Inpatient | 4.7 (NR), 2.1–7.0 | 40.0 | Profound to mild retardation | Behavior, adverse events, optimal dosages | McNeal Pharmaceuticals; NIMH |
| Findling, 2014 | USA | Autism | DSM-IV-TR; ADI-R | CGI-S ≥ 4; ABC-Irritability ≥ 18 | Aripiprazole 2 to 15 mg, adaptive dose (41) | Placebo (44) | Withdrawal | 30 (14 weeks open lable treatment, 16 weeks DB withdrawal) | Specialistic | 10.4 (2.8); 6.0–17.0 | 20.0 | MA ≥ 24 months | ABC, Global changes (CGI-I, CGI-S), quality of life (PedsQL), adverse events (AIMS, BARS, SAS) | Bristol-Myers Squibb; Otsuka Pharmaceuticals Co, Ltd |
| Hellings, 2006 | USA | ASD | DSM-IV | ABC-I > 1SD above given norms for age, gender and setting | Risperidone 1.0 mg/day, fixed dose (NR); Risperidone 1.2 to 2.9 mg/day, adaptive dose (NR) | Placebo (NR) | Crossover | 22 weeks (4 weeks on average SB placebo, 2 weeks DB drug tapering, 4 weeks DB treatment, 2 weeks DB drug tapering, 4 weeks DB treatment, 2 weeks DB drug tapering, 4 weeks on average SB placebo) | Specialistic | 12.0 (2.8); 8.0–15.0 | 33.3 | IQ < 70 | ABC, adverse events (DISCUS) | Janssen Pharmaceutica |
| Hollander, 2006 | USA | ASD | DSM-IV; ADI-R; ADOS | CGI-S ≥ 4 | Olanzapine 2.5 to 20 mg/day, adaptive dose (6) | Placebo (5) | Parallel | 8 | Specialistic | 9.1 (2.5); 6.0–14.8 | 18.1 | Profound retardation to normal | Global changes (CGI-I), compulsion (CY-BOCS), aggression (OASM), adverse events (AIMS, BAS, SAS) | Lilly Research Laboratories |
| Ichikawa, 2017 | Japan | Autism | DSM-IV-TR; PARS | CGI-S ≥ 4; ABC-I ≥ 18 | Aripiprazole 1 to 15 mg/day, adaptive dose (47) | Placebo (45) | Parallel | 8 | Specialistic | 10.1 (3.2); 6.0–17.0 | 18.5 | IQ 20 to normal | Global changes, (CGI-S, CGAS), ABC, compulsion (CY-BOCS, compulsion scale only), adverse events (C-SSRS, AIMS, DIEPSS, BAS) | Otsuka Pharmaceutical Co., Ltd |
| Kent, 2013 | USA | Autism | DSM-TR; ADI-R | CGI-S ≥ 4; ABC-I ≥ 18 | Risperidone 0.125 to 0.175 mg/day, fixed dose (30); Risperidone 1.25 to 1.75 mg/day, fixed dose (31) | Placebo (35) | Parallel | 32 (6 weeks DB treatment, 26 weeks open lable treatment) | Specialistic | 9.0 (3.1); 5.0–17.0 | 13.0 | MA ≥ 18 months | Global changes (CGI-S, CGI-I), ABC, compulsion (CY-BOCS), adverse events (EPS, SAS, BAS, AIMS) | Johnson & Johnson Pharmaceutical Research & Development, LLC |
| Loebel, 2016 | USA | Autism | DSM-IV-TR; ADI-R | CGI-S ≥ 4; ABC-I ≥ 18 | Lurasidone 20 mg/day, fixed dose (49); Lurasidone 60 mg/day, fixed dose (51) | Placebo (49) | Parallel | 6 | Specialistic | 10.7 (3.0); 6.0–17.0 | 18.2 | Not reported | Global changes (CGI-I, CGI-S), ABC, compulsion (CY-BOCS), caregiver strain (CGSQ), adverse events (AIMS, SAS, BAS) | Sunovion Pharmaceuticals, Inc |
| Luby, 2006 | USA | ASD | DSM-IV | CARS ≥ 30 | Risperidone 0.5 to 1.5 mg/day, adaptive dose (12) | Placebo (12) | Parallel | 26 | Specialistic | 4.0 (1.9); 2.5–6.0 | 26.1 | Not reported | CARS, GARS, adaptive behaviors (CBCL, VABS), communication (PLS-3) | Janssen Pharmaceutica |
| Marcus, 2009 | USA | Autism | DSM-IV-TR; ADI-R | CGI-S ≥ 4; ABC-I ≥ 18 | Aripiprazole 5 mg/day, fixed dose (53); Aripiprazole 10 mg/day, fixed dose (59); Aripiprazole 15 mg/day (54) | Placebo (52) | Parallel | 8 | Specialistic | 9.7 (3.1), 6.0–17.0 | 10.6 | MA ≥ 18 months | CGI-S, ABC, quality of life (PedsQL), compulsion (CY-BOCS), caregiver strain (CGSQ), adverse events (EPS, SAS, BAS, AIMS) | Bristol-Myers Squibb; Otsuka Pharmaceutical Co., Ltd |
| McCraken, 2002 | USA | Autism | DSM-IV; ADI-R | CGI-S ≥ 4; ABC-I ≥ 18 | Risperidone 0.25 to 2.5 (< 45 kg), 0.5 to 3.5 (≥ 45 kg) mg/day, adaptive dose (49) | Placebo (52) | Parallel | 8 | Specialistic | 8.8 (2.7); 5.0–17.0 | 18.8 | MA ≥ 18 months | Global changes (CGI-I), ABC, adverse events (SAS, AIMS) | NIMH; NIH; Korczak Foundation; Janssen Pharmaceutica |
| Nagaraj, 2006 | India | Autism | DSM-IV | Not reported | Risperidone 1.0 mg/day, fixed dose (19) | Placebo (21) | Parallel | 26 | Specialistic | 5.1 (1.7); 2.0–9.0 | 12.8 | IQ ≥ 35 | Global changes (CGAS), CARS, social quotient (VSMS), adverse events (AIMS) | Sun Pharmaceuticals |
| NCT00870727 | USA | PDD-NOS | DSM-IV-TR | CGI-S ≥ 4; ABC-I ≥ 18 | Aripiprazole 2.0 to 20.0 mg/ day, flexible dose (17) | Placebo (16) | Parallel | 8 | Specialistic | 9.6 (2.7); 5.0–17.0 | 21.2 | IQ ≥ 50 | Global changes (CGI-I), ABC, adverse wevents | Indiana University; NIMH; Bristol-Myers Squibb |
| NCT01624675 | Japan | Autism | DSM-IV-TR | CGI-S ≥ 4; ABC-I ≥ 18 | Risperidone 1.0 (< 20 kg weight) or 2.5 (≥ 20 kg weight) mg/day (21) | Placebo (18) | Parallel | 8 | Specialistic | NR (NR); 5.0–17.0 | NR | IQ ≥ 35; MA ≥ 18 months | Global changes (CGI-I, CGI-S, CGAS), ABC, parents satisfaction (PSQ), adverse events | Janssen Pharmaceutical K.K |
| Owen, 2009 | USA | Autism | DSM-IV; ADI-R | CGI-S ≥ 4; ABC-I ≥ 18 | Aripiprazole 2.0 to 15.0 mg/day, adaptive dose (47) | Placebo (51) | Parallel | 8 | Specialistic | 9.3 (3.0); 6.0–17.0 | 12.2 | MA ≥ 18 months | Global changes (CGI-I, CGI-S), ABC, compulsion (CY-BOCS), quality of life (PedsQL), caregiver strain (CGSQ), adverse events (AIMS, BAS, SAS) | Bristol-Myers Squibb; Otsuka Pharmaceutical Co, Ltd; Ogilvy Healthworld Medical Education |
| Remington, 2001 | USA | Autism | DSM-IV | Not reported | Chlomipramine 100 to 150 mg/day (7); Haloperidol 1.0 to 1.5 mg/day (11) | Placebo (7) | Crossover | 22 (1 weeks SB placebo, 21 weeks DB intervention, 3 weeks each one: clomipramine/placebo/haloperidol, placebo/haloperidol/clomipramine, and haloperidol/clomipramine/placebo) | Specialistic | 12.8 (2.4); 10.0–17.0 | 12.0 | Not reported | CARS, adverse events (DOTES, EPS) | Ontario Mental Health Foundation |
| RUPP, 2005 | USA | Autism | DSM-IV; ADI-R | CGI-S ≥ 4; ABC-I ≥ 18 | Risperidone 0.25 to 3.5 (< 45 kg), 0.5 to 4.5 (≥ 45 kg) mg/day, adaptive dose (16) | Placebo (16) | Withdrawal | 8 (3 weeks DB taper, 5 weeks DB placebo) | Specialistic | 9.0 (2.5); 5.0–17.0 | 13.2 | Profound retardation to normal | Relapse (ABC, CGI-I) | NIMH; NIH; Korczak Foundation; Janssen Pharmaceutica |
| Shea, 2004 | Canada | PDD | DSM-IV-TR; CARS | CARS ≥ 30 | Risperidone 0.02 to 0.06 mg/kg/day, adaptive dose (41) | Placebo (39) | Parallel | 8 | Specialistic | 7.5 (2.3); 5.0–12.0 | 22.8 | IQ ≥ 35 | Global change (CGI-C), ABC, VAS, behavior (N-CBRF), adverse events (ESRS) | Janssen-Ortho, Inc., Canada; Johnson & Johnson Pharmaceuticals |
| Troost, 2005 | Netherlands | PDD (Autism, Asperger, PDD-NOS) | DSM-IV-TR; ADI-R | CGI-S ≥ 4; ABC-Irritability ≥ 18 | Risperidone 0.5 to 6.0 mg/day, adaptive dose (12) | Placebo (12) | Withdrawal | 8 (3 weeks DB taper, 5 weeks DB placebo) | Specialistic | 9.1 (2.6); 5.0–17.0 | 8.3 | MA ≥ 18 months | Relapse (ABC, CGI-S) | Not reported |
Legend: ABC: Aberrant Behavior Checklist; ADI-R: Autism Diagnostic Interview—Revised; ADOS: Autism Diagnostic Observation Scale; BASC: Behavioral Assessment System for Children; CARS: Childhood Autism Rating Scale; CDI: Children's Depression Inventory; CGI-I: Clinical Global Impression-Improvement scale; CGI-S: Clinical Global Impression-Severity scale; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; EVT: Expressive Vocabulary Test; NIH: National Institutes, of Health, U.S.; NIMH: National Institute of Mental Health, U.S.; PDDBI: Pervasive Developmental Disorder- Behavioral Inventory; PLS-4: Preschool Language Scale, Fourth Edition; PPVT: Peabody Picture Vocabulary Test; SCQ: Social Communication Questionnaire; SRS: Social Responsiveness Scale; VABS-II: Vineland Adaptive Behavior Scales, Second Edition
To see the results of risk of bias assessment of included studies, see Additional file 4. We assessed publication bias through funnel plots presented in the Additional file 7.
Results and overall certainty of evidence
Forest plots for the main analyses are shown in Additional file 5, while the GRADE evidence profile is shown in Additional file 8.
Antipsychotics probably reduce “hyperactivity, attention deficit, opposition, and disruptive behaviors”, and probably slightly reduce both “restricted and repetitive interests and behaviors” and “obsessions, compulsions”.
Antipsychotics seem to slightly reduce “emotional dysregulation/irritability” and seem to positively influence “global functioning, global improvement”.
There are uncertainties about the effect of antipsychotics on “anxiety” and “self-harm”.
With regard to the safety profile, antipsychotics are likely to increase the risk of “adverse events”, and may induce a slight increase in the incidence of “serious adverse events”.
We found no extractable data regarding “insomnia” and “quality of life” outcomes.
Details about effect estimates and certainty of evidence are reported in Tables 2 and 3: Summary of Findings tables.
Table 2.
Summary of Findings (SoF) for the comparison antipsychotics versus placebo – continuous outcomes
| Antipsychotics versus no antipsychotics – continuous outcomes | ||||||
|---|---|---|---|---|---|---|
| Population: children and adolescents with ASD Setting: outpatients and inpatients Intervention: antipsychotics Comparator: no antipsychotics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no Antipsychotics | Risk with Antipsychotics | |||||
| Restricted and repetitive interests and behaviors | – | SMD 0.21 SD lower (0.35 lower to 0.07 lower) | – | 823 (9 RCTs) | ⨁⨁⨁◯ MODERATE a | Antipsychotics probably slightly reduce restricted and repetitive interests and behaviors |
| Hyperactivity, inattention, oppositional, disruptive behavior | – | SMD 0.67 SD lower (0.92 lower to 0.42 lower) | – | 783 (8 RCTs) | ⨁⨁⨁◯ MODERATE a | Antipsychotics probably reduce Hyperactivity, inattention, oppositional, disruptive behavior disorders |
| Self-harm | – | SMD 0.14 SD lower (0.58 lower to 0.30 higher) | – | 77 (1 RCT) | ⨁◯◯◯ VERY LOW b,c | There are some uncertainties about the effect of Antipsychotics on self-harm |
| Social communication, social interaction | – | SMD 0.38 SD lower (0.59 lower to 0.16 lower) | – | 854 (10 RCTs) | ⨁⨁⨁◯ MODERATE a | Antipsychotics probably slightly improve social communication, social interaction |
| Emotional dysregulation/irritability | – | SMD 0.71 SD lower (0.98 lower to 0.43 lower) | – | 879 (9 RCTs) | ⨁⨁◯◯ LOW a,d | Antipsychotics administration may result in a large reduction of emotional dysregulation/irritability |
| Anxiety | – | SMD 0.38 SD lower (0.82 lower to 0.07 higher) | – | 77 (1 RCT) | ⨁◯◯◯ VERY LOW b,c | There are some uncertainties about the effect of Antipsychotics on anxiety |
| Global functioning, global improvement | – | SMD 0.64 SD lower (0.96 lower to 0.33 lower) | – | 839 (10 RCTs) | ⨁⨁◯◯ LOW e,f | Antipsychotics may influence positively Global functioning, global improvement |
| Obsessions, compulsions | – | SMD 0.30 SD lower (0.55 lower to 0.06 lower) | – | 548 (4 RCTs) | ⨁⨁⨁◯ MODERATE g | Antipsychotics probably slightly reduce obsessions, compulsions |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; SMD: Standardised mean difference; RR: Risk ratio
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Explanations
a. Downgraded by one level because most studies showed an unclear risk of bias for selection bias and one study was at high risk for attrition bias
b. Downgraded by one level because the included study was at high risk for selection bias
c. Downgraded by two levels because the sample size is very small (< 400) and the 95%CI for SMD goes from considerable beneficial effects to considerable undesirable effects
d. Downgraded by one level for heterogeneity (I2 = 71.0%), several confidence intervals of the included trial not overlapping and important differences in the point estimates
e. Downgraded by one level because most studies showed an unclear risk for selection bias, two studies were at high risk for reporting bias and one study was at high risk for selection bias
f. Downgraded by one level for heterogeneity (I2 = 75.6%), several confidence intervals of the included trial not overlapping and important differences in the point estimates
g. Downgraded by one level because the 95%CI for SMD goes from considerable beneficial effects to not clinically relevant effects
Table 3.
Summary of Findings (SoF) for the comparison antipsychotics versus placebo –dichotomous outcomes
| Antipsychotics versus no antipsychotics – dichotomous outcomes | ||||||
|---|---|---|---|---|---|---|
| Population: children and adolescents with ASD Setting: outpatients and inpatients Intervention: Antipsychotics Comparator: no Antipsychotics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no Antipsychotics | Risk with Antipsychotics | |||||
| Serious adverse events | 16 per 1.000 | 17 per 1.000 (8 to 39) | RR 1.07 (0.48 a 2.43) | 1057 (13 RCTs) | ⨁⨁◯◯ LOW a,b | Antipsychotics may increase the risk of severe adverse events slightly |
| Adverse events | 657 per 1.000 | 781 per 1.000 (703 to 867) | RR 1.19 (1.07 a 1.32) | 924 (10 RCTs) | ⨁⨁⨁◯ MODERATE c | Antipsychotics probably increase the risk of adverse events |
| Dropout due to any cause | 244 per 1.000 | 149 per 1.000 (117 to 190) | RR 0.61 (0.48 a 0.78) | 1124 (15 RCTs) | ⨁⨁⨁◯ MODERATE d | Antipsychotics probably reduce the risk of dropout due to any cause |
| Drop-out due to adverse events | 39 per 1.000 | 39 per 1.000 (22 to 70) | RR 0.99 (0.55 to 1.79) | 1010 (12 RCTs) | ⨁⨁◯◯ LOW b,e | Antipsychotics may have little or no effect on the risk of dropouts due to adverse events |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; SMD: Standardised mean difference; RR: Risk ratio
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Explanations
a. Downgraded by one level because most studies showed an unclear risk for selection bias, three studies were at high risk for attrition bias, one study was at high risk for selection bias and one study was at high risk for reporting bias
b. Downgraded by one level because the 95%CI for SMD goes from considerable beneficial effects to considerable undesirable effects
c. Downgraded by one level because most studies showed an unclear risk of bias for selection bias and two studies were at high risk for attrition bias
d. Downgraded by one level because most studies showed an unclear risk for selection bias, four studies were at high risk for attrition bias, one study was at high risk for selection bias and one study was at high risk for reporting bias
e. Downgraded by one level because most studies showed an unclear risk for selection bias, four studies were at high risk for attrition bias, one was at high risk for selection bias and one was at high risk for reporting bias
Subgroup effect considerations
Antipsychotics in the subgroup with ABC-Irritability ≥ 18 showed a trend towards a higher efficacy for emotional dysregulation and global functioning, and a trend towards lower efficacy for social communication, social interaction and hyperactivity outcomes. In the same subgroup antipsychotics apparently showed a better safety and tolerability profile (see Additional File 6). However, when considering whether a clear subgroup effect was present or not, we noted that: summary effects for the subgroup versus all were overlapping; nearly all 95% CIs of individual studies were overlapping for all outcomes; there was not a clear suggestion of consistently greater benefits or harms in the subgroup; the p-values for subgroup effects indicated that differences were likely due to chance.
We considered that there were no subgroup effects that are substantially concerning or credible, and certainty of evidence was not downgraded for subgroup effects.
Discussion
We found antipsychotics for children and adolescents with ASD more efficacious, more acceptable, but less safe than placebo.
Antipsychotics are often used as off-label pharmacological treatments for children and adolescents, even though their use in this population is an evidence-based choice for certain conditions [111]. According to a meta-analysis conducted in 2017 by Pillay et al. [13], and similarly to what we found in our review, SGAs were probably effective in reducing irritability, and in producing a slight decrease in social withdrawal, stereotypy and inappropriate speech, as measured by ABC. SGAs also probably increased response rate and decreased global impression of severity. Recent meta-analyses comparing only one drug (i.e. risperidone, aripiprazole, and lurasidone) versus placebo showed all similar results, even if more often did not report significant estimates, probably due to wider confidence intervals, particularly for lurasidone [112–114]. Pillay et al. [13] described narratively head-to-head trials, reporting (1) no differences between aripiprazole and risperidone in main outcomes, (2) mixed results when comparing haloperidol to risperidone or olanzapine. Since the publication of Pillay et al. (2017) systematic review, results from 4 placebo controlled [77, 95, 100, 102] and 2 head-to-head [53, 83] RCTs have been published.
In our study, antipsychotics in short- and medium-term showed good tolerability, as they reduced the risk of dropouts by 39% (moderate certainty), and demonstrated to be relatively safe options, with an increase of 19% in adverse events (moderate certainty) and 9% in serious/severe adverse events (low certainty). Evaluating the long-term safety profile of the antipsychotics was not among the objectives of this systematic review, as we did not perform a systematic review of not-randomized studies. However, it can be stated that, in children and adolescents with ASD: long-term use of risperidone, although generally well-tolerated, has been associated with an increase in plasma glucose, insulin, prolactin and leptin proportional to the dosage; the major problems often resulted from continuous weight gain over time and judged excessive [115–117]; the continued use of aripiprazole has been associated with a decrease in prolactinemia, with a similar risk profile for weight gain [118, 119]. The transition of treatment from risperidone to aripiprazole seems to reduce adverse events such as drowsiness, hyper-prolactinemia and amenorrhoea [120]. The number needed to treat (NNT) evaluated for effectiveness on irritability was lower using risperidone than aripiprazole, but on the other hand the number needed to harm (NNH) for the onset of extrapyramidal symptoms was also lower with risperidone [8]. The use of antipsychotics in general has also been related to hyperuricemia and hyperprolactinemia [121–123].
Study limitations
We have not prospectively registered the protocol for our systematic review; however, the clinical question was formulated by a multidisciplinary panel of experts, and we followed the methodology reported in the manual developed and published by the ISS [14].
After the systematic review was performed and the recommendation formulated by the panel was submitted to public consultation (https://www.osservatorionazionaleautismo.it/attivita-istituzionali/linee-guida/consultazioni-pubbliche), the results of a previously ongoing trial (NCT00198107) [77] were published on clinicaltrial.gov: 40 individuals were randomized to aripiprazole and 41 to placebo for 8 weeks. Data were available for “restricted and repetitive interests and behavior”, “Hyperactivity, inattention, oppositional, disruptive behavior disorders”, “Social communication, social interaction”, “Emotional dysregulation/irritability” (ABC subscales), for “obsessions, compulsions” (CY-BOCS), for “global functioning, global improvement” (ADOS), and for safety and tolerability outcomes (adverse events, dropouts and serious adverse events). Outcome data were consistent with what we found in our meta-analyses; for all these outcomes, except for emotional dysregulation/irritability and global functioning, the certainty of evidence was already rated as moderate. The Evidence Review Team, together with the content expert and the panel chair, considered that the new findings did not change the body of evidence and that there was no need to carry out new statistical analyses nor to reformulate the recommendation for this intervention.
The main limitation in the subgroup analysis was that the “all ASDs” studies included a mixed population, while ideally, we would have had groups with and without problem behaviors.
Finally, the use of the EtD framework requires the panel to be familiar with the tool [124]. To overcome this potential limitation, about 2 months before the presentation of the body of evidence on antipsychotics, an EtD framework on a pilot question was presented to the panel [125, 126] for the formulation of a recommendation on the use of polyunsaturated fatty acids. This recommendation will not take part to the Italian guidelines on the diagnosis and treatment of children and adolescents with ASD. In other experiences, panel members have reported that, when familiarity with the EtD framework is achieved, the tool helped them in structuring discussion, saving time, ensuring systematicity in the process of recommendation formulation [127].
Conclusions
We found antipsychotics in children and adolescents with ASD to be significantly more efficacious than placebo in reducing stereotypies, hyperactivity, irritability and obsessions, compulsions, and in increasing social communication and global functioning. Antipsychotics were also found to be significantly more acceptable in terms of dropouts due to any cause, but significantly less safe in terms of patients experiencing adverse events. We found no evidence regarding the impact of antipsychotics on “insomnia” and “quality of life” outcomes in this population. Available evidence on efficacy and safety of antipsychotics in children and adolescents with ASD needs to be evaluated together with evidence on equity, acceptability, feasibility [28], resources required and cost-effectiveness [29] in formulating a recommendation.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist is reported on Additional file 9.
Supplementary information
Additional file 1: Search strategy and results for Systematic Reviews.
Additional file 2: Search strategy and results for Randomized Controlled Trials.
Additional file 3: References for included and excluded trials, with reasons.
Additional file 4: Risk of Bias Summary..
Additional file 5: Forest plots for comparisons between antipsychotics (D2 blockers) and Placebo.
Additional file 6: Forest plots for comparisons between antipsychotics (D2 blockers) and Placebo – subgroup analyses (ABC-Irritability ≥18 vs All ASDs).
Additional file 7: Funnel Plots for outcome with estimates for at least 8 studies.
Additional file 8: GRADE Evidence profile.
Acknowledgements
Members of ISACA guideline working group
Collaborating author names from the ISACA guideline working group: Raffaella Tancredi; Angelo Massagli; Giovanni Valeri; Corrado Cappa; Serafino Buono; Giuseppe Maurizio Arduino; Alessandro Zuddas; Laura Reali; Massimo Molteni; Claudia Felici; Concetta Cordò; Lorella Venturini; Cristina Bellosio; Emanuela Di Tommaso; Sandra Biasci; Clelia M. Duff; Simona Vecchi.
Abbreviations
- ABC
Aberrant Behavior Checklist
- ADHD
attention deficit hyperactivity disorder
- ASD
autism spectrum disorder
- BASC
behavior assessment system for children
- CGI-I
Clinical Global Impression-Improvement scale
- CGI-S
Clinical Global Impression-Severity Scale
- CI
confidence interval
- EtD
evidence to decision
- EVT
expressive vocabulary test
- FGA
first generation antipsychotics
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- ISS
Istituto Superiore di Sanità (Italian National Institute of Health)
- MSEL
Mullen Scales of Early Learning
- PLS
The Preschool Language Scale
- PPVT
Peabody Picture Vocabulary Test
- RCT
randomized controlled trial
- RR
risk ratio
- SGA
Second Generation Antipsychotics
- SMD
Standardized mean difference
- SRS
Social Responsiveness Scale
- VABS
Vineland Adaptive Behavior Scale
Authors’ contributions
Conceptualization: FDC, GLD, GPM, SM, HJS, LA. Data curation: GLD, FDC, FC, ZM, MD, LA. Formal analysis: FDC, GLD, LA. Project administration: MD, FN, HJS, LA, MLS. Supervision: MD, MLS, FN, HJS, LA. Writing – original draft: GLD, FDC, LA. Writing – review & editing: ZM, MD, GPM, FF, MLS, RS, FC, SM, FN, HJS, LA. All authors read and approved final manuscript.
Funding
Italian Ministry of Health Project ‘I disturbi dello spettro autistico: attività previste dal decreto ministeriale del 30.12.2016 – capitolo 4395 (articolo 1, comma 401, legge 28 dicembre 2015, n. 208, recante “Disposizioni per la formazione 661 del bilancio annuale e pluriennale dello Stato (legge di stabilità 2016)”.
Availability of data and materials
All data supporting our findings is contained within the manuscript and the additional files.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Istituto Superiore di Sanità – Autism Spectrum Disorder in Children and Adolescent guideline working group (ISACA guideline working group). Participants to the ISACA guideline working group are listed in the acknowledgments section
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gian Loreto D’Alò and Franco De Crescenzo share co-first authorship
Contributor Information
Gian Loreto D’Alò, Email: gianloretod@gmail.com.
Franco De Crescenzo, Email: f.decrescenzo@deplazio.it.
Laura Amato, Email: l.amato@deplazio.it.
Fabio Cruciani, Email: f.cruciani@deplazio.it.
Marina Davoli, Email: m.davoli@deplazio.it.
Francesca Fulceri, Email: francesca.fulceri@iss.it.
Silvia Minozzi, Email: minozzi.silvia@gmail.com.
Zuzana Mitrova, Email: s.mitrova@deplazio.it.
Gian Paolo Morgano, Email: morganog@mcmaster.ca.
Franco Nardocci, Email: nardoccifranco@gmail.com.
Rosella Saulle, Email: r.saulle@deplazio.it.
Holger Jens Schünemann, Email: holger.schunemann@mcmaster.ca.
Maria Luisa Scattoni, Email: marialuisa.scattoni@iss.it.
On Behalf of the ISACA Guideline Working Group:
Raffaella Tancredi, Angelo Massagli, Giovanni Valeri, Corrado Cappa, Serafino Buono, Giuseppe Maurizio Arduino, Alessandro Zuddas, Laura Reali, Massimo Molteni, Claudia Felici, Concetta Cordò, Lorella Venturini, Cristina Bellosio, Emanuela Di Tommaso, Sandra Biasci, Clelia M. Duff, and Simona Vecchi
Supplementary Information
The online version contains supplementary material available at 10.1186/s12955-021-01669-0.
References
- 1.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013
- 2.Narzisi A, Posada M, Barbieri F, Chericoni N, Ciuffolini D, Pinzino M, et al. Prevalence of Autism Spectrum Disorder in a large Italian catchment area: a school-based population study within the ASDEU project. Epidemiol Psychiatr Sci. 2018;29:e5. doi: 10.1017/S2045796018000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Istituto Superiore di Sanità. Giornata mondiale della Consapevolezza dell’Autismo: in Italia un bimbo ogni 77. 2019. Available at: https://www.iss.it/?p=3421. Accessed 31 Dec 2019.
- 4.Christensen DL, Braun KVN, Baio J, Bilder D, Charles J, Constantino JN, et al. Prevalence and characteristics of Autism Spectrum Disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States. MMWR Surveill Summ. 2012;65(13):1–23. doi: 10.15585/mmwr.ss6513a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postorino V, Fatta LM, Sanges V, Giovagnoli G, De Peppo L, Vicari S, et al. Intellectual disability in Autism Spectrum Disorder: investigation of prevalence in an Italian sample of children and adolescents. Res Dev Disabil. 2016;48:193–201. doi: 10.1016/j.ridd.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 7.Jobski K, Höfer J, Hoffmann F, Bachmann C. Use of psychotropic drugs in patients with autism spectrum disorders: a systematic review. Acta Psychiatr Scand. 2017;135(1):8–28. doi: 10.1111/acps.12644. [DOI] [PubMed] [Google Scholar]
- 8.Fung LK, Mahajan R, Nozzolillo A, Bernal P, Krasner A, Jo B, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(Suppl 2):S124–S135. doi: 10.1542/peds.2015-2851K. [DOI] [PubMed] [Google Scholar]
- 9.Lee ES, Vidal C, Findling RL. A focused review on the treatment of pediatric patients with atypical antipsychotics. J Child Adolesc Psychopharmacol. 2018;28(9):582–605. doi: 10.1089/cap.2018.0037. [DOI] [PubMed] [Google Scholar]
- 10.De Giorgi R, De Crescenzo F, D'Alò GL, Rizzo Pesci N, Di Franco V, Sandini C, et al. Prevalence of non-affective psychoses in individuals with autism spectrum disorders: a systematic review. J Clin Med. 2019 doi: 10.3390/jcm8091304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Crescenzo F, Postorino V, Siracusano M, Riccioni A, Armando M, Curatolo P, et al. Autistic symptoms in schizophrenia spectrum disorders: a systematic review and meta-analysis. Front Psychiatry. 2019;10:78. doi: 10.3389/fpsyt.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downs JM, Lechler S, Dean H, Sears N, Patel R, Shetty H, et al. The association between comorbid autism spectrum disorders and antipsychotic treatment failure in early-onset psychosis: A Historical Cohort Study Using Electronic Health Records. J Clin Psychiatry. 2017;78(9):e1233–e1241. doi: 10.4088/JCP.16m11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillay J, Boylan K, Carrey N, Newton A, Vandermeer B, Nuspl M, et al. First- and Second-Generation Antipsychotics in Children and Young Adults: Systematic Review Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017. Available from http://www.ncbi.nlm.nih.gov/books/NBK442352/PubMed PMID: 28749632. [PubMed]
- 14.Istituto Superiore di Sanità – Centro Nazionale per l’Eccellenza Clinica, la Qualità e la Sicurezza delle Cure. Manuale metodologico per la produzione di linee guida di pratica clinica. 2018. Available at: https://snlg.iss.it/wp-content/uploads/2018/10/MM_v1.2_lug-2018.pdf Accessed 17 Gen 2019.
- 15.Morgano GP, Fulceri F, Nardocci F, Barbui C, Ostuzzi G, Papola D, et al. Introduction and methods of the evidence-based guidelines for the diagnosis and management of autism Spectrum disorder by the Italian National Institute of health. Health Qual Life Outcomes. 2020;18(1):81. doi: 10.1186/s12955-020-01320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt JD, Huete JM, Fodstad JC, Chin MD, Kurtz PF. An evaluation of the Aberrant Behavior Checklist for children under age 5. Res Dev Disabil. 2013;34(4):1190–1197. doi: 10.1016/j.ridd.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 18.Welch VA, Akl EA, Pottie K, Ansari MT, Briel M, Christensen R, et al. GRADE equity guidelines 3: considering health equity in GRADE guideline development: rating the certainty of synthesized evidence. J Clin Epidemiol. 2017;90:76–83. doi: 10.1016/j.jclinepi.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. 2. Chichester: Wiley; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59(1):7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH, et al. Presenting results and “Summary of findings” tables. In: Higgins JPT, Green S, et al., editors. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley; 2008. pp. 335–338. [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66(2):158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66(2):173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al.; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016.10.1136/bmj.i2016. [DOI] [PubMed]
- 27.Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. [DOI] [PubMed]
- 28.D’Alò GL, De Crescenzo F, Amato L, Cruciani F, Davoli M, Fulceri F, et al. Acceptability, equity, and feasibility of using Antipsychotics in children and adolescents with autism spectrum disorder: a systematic review. BMC Psychiatry. 2020;20(1):561. doi: 10.1186/s12888-020-02956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Alò GL, Basile M, De Crescenzo F, Amato L, Cicchetti A, Cruciani F, et al. Resources required of using antipsychotics in children and adolescents with autism spectrum disorder: a systematic review and cost-analysis study in the Italian scenario. 2020 (unpublished).
- 30.Akhondzadeh S, Asadabadi M. Risperidone plus celecoxib in children with autistic disorder: a double-blind, randomized trial. Br J Clin Pharmacol. 2012;73(6):983–984. doi: 10.1111/j.1365-2125.2012.04253.x. [DOI] [Google Scholar]
- 31.Akhondzadeh S, Fallah J, Mohammadi MR, Imani R, Mohammadi M, Salehi B, et al. Double-blind placebo-controlled trial of pentoxifylline added to risperidone: effects on aberrant behavior in children with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(1):32–36. doi: 10.1016/j.pnpbp.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Akhondzadeh S, Tajdar H, Mohammadi MR, Mohammadi M, Nouroozinejad GH, Shabstari OL, et al. A double-blind placebo controlled trial of piracetam added to risperidone in patients with autistic disorder. Child Psychiatry Hum Dev. 2008;39(3):237–245. doi: 10.1007/s10578-007-0084-3. [DOI] [PubMed] [Google Scholar]
- 33.Asadabadi M, Mohammadi MR, Ghanizadeh A, Modabbernia A, Ashrafi M, Hassanzadeh E, et al. Celecoxib as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Psychopharmacology. 2013;225(1):51–59. doi: 10.1007/s00213-012-2796-8. [DOI] [PubMed] [Google Scholar]
- 34.Ghaleiha A, Alikhani R, Kazemi MR, Mohammadi MR, Mohammadinejad P, Zeinoddini A, et al. Minocycline as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind placebo-controlled trial. J Child Adolesc Psychopharmacol. 2016;26(9):784–791. doi: 10.1089/cap.2015.0175. [DOI] [PubMed] [Google Scholar]
- 35.Ghaleiha A, Asadabadi M, Mohammadi MR, Shahei M, Tabrizi M, Hajiaghaee R, et al. Memantine as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2013;16(4):783–789. doi: 10.1017/S1461145712000880. [DOI] [PubMed] [Google Scholar]
- 36.Ghaleiha A, Ghyasvand M, Mohammadi MR, Farokhnia M, Yadegari N, Tabrizi M, et al. Galantamine efficacy and tolerability as an augmentative therapy in autistic children: a randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2014;28(7):677–685. doi: 10.1177/0269881113508830. [DOI] [PubMed] [Google Scholar]
- 37.Ghaleiha A, Mohammadi E, Mohammadi MR, Farokhnia M, Modabbernia A, Yekehtaz H, et al. Riluzole as an adjunctive therapy to risperidone for the treatment of irritability in children with autistic disorder: a double-blind, placebo-controlled, randomized trial. Paediatr Drugs. 2013;15(6):505–514. doi: 10.1007/s40272-013-0036-2. [DOI] [PubMed] [Google Scholar]
- 38.Ghaleiha A, Rasa SM, Nikoo M, Farokhnia M, Mohammadi MR, Akhondzadeh S. A pilot double-blind placebo-controlled trial of pioglitazone as adjunctive treatment to risperidone: effects on aberrant behavior in children with autism. Psychiatry Res. 2015;229(1–2):181–187. doi: 10.1016/j.psychres.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 39.Ghanizadeh A, Ayoobzadehshirazi A. A randomized double-blind placebo-controlled clinical trial of adjuvant buspirone for irritability in autism. Pediatr Neurol. 2015;52(1):77–81. doi: 10.1016/j.pediatrneurol.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 40.Ghanizadeh A, Moghimi-Sarani E. A randomized double blind placebo controlled clinical trial of N-acetylcysteine added to risperidone for treating autistic disorders. BMC psychiatry. 2013;13:196. doi: 10.1186/1471-244X-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hajizadeh-Zaker R, Ghajar A, Mesgarpour B, Afarideh M, Mohammadi MR, Akhondzadeh S. l-Carnosine as an adjunctive therapy to risperidone in children with autistic disorder: a randomized, double-blind. Placebo-Controlled Trial J Child Adolesc Psychopharmacol. 2018;28(1):74–81. doi: 10.1089/cap.2017.0026. [DOI] [PubMed] [Google Scholar]
- 42.Hasanzadeh E, Mohammadi MR, Ghanizadeh A, Rezazadeh SA, Tabrizi M, Rezaei F, et al. A double-blind placebo controlled trial of Ginkgo biloba added to risperidone in patients with autistic disorders. Child Psychiatry Hum Dev. 2012;43(5):674–682. doi: 10.1007/s10578-012-0292-3. [DOI] [PubMed] [Google Scholar]
- 43.Moazen-Zadeh E, Shirzad F, Karkhaneh-Yousefi MA, Khezri R, Mohammadi MR, Akhondzadeh S. Simvastatin as an adjunctive therapy to risperidone in treatment of autism: a randomized, double-blind, placebo-controlled clinical trial. J Child Adolesc Psychopharmacol. 2018;28(1):82–89. doi: 10.1089/cap.2017.0055. [DOI] [PubMed] [Google Scholar]
- 44.Mohammadi MR, Yadegari N, Hassanzadeh E, Farokhnia M, Yekehtaz H, Mirshafiee O, et al. Double-blind, placebo-controlled trial of risperidone plus amantadine in children with autism: a 10-week randomized study. Clin Neuropharmacol. 2013;36(6):179–184. doi: 10.1097/WNF.0b013e3182a9339d. [DOI] [PubMed] [Google Scholar]
- 45.Moharreri F, Abdollahian E, Hosseini SA, Mirzadeh M. Comparative study on the effect of risperidone and its combination with naltrexone in pediatric patients with autistic spectrum disorders: a clinical trial study. Int J Pediatrics-Mashhad. 2017;5(12):6375–6382. doi: 10.22038/ijp.2017.18557.1516. [DOI] [Google Scholar]
- 46.Nikoo M, Radnia H, Farokhnia M, Mohammadi MR, Akhondzadeh S. N-acetylcysteine as an adjunctive therapy to risperidone for treatment of irritability in autism: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Clin Neuropharmacol. 2015;38(1):11–17. doi: 10.1097/WNF.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 47.Rezaei M, Moradi A, Tehrani-Doost M, Hassanabadi H, Khosroabadi R. Effects of combining medication and pivotal response treatment on aberrant behavior in children with autism spectrum disorder. Children (Basel). 2018 doi: 10.3390/children5020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rezaei V, Mohammadi MR, Ghanizadeh A, Sahraian A, Tabrizi M, Rezazadeh SA, et al. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1269–1272. doi: 10.1016/j.pnpbp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Scahill L, Aman MG, McDougle CJ, Arnold LE, McCracken JT, Handen B, et al. Trial design challenges when combining medication and parent training in children with pervasive developmental disorders. J Autism Dev Disord. 2009;39(5):720–729. doi: 10.1007/s10803-008-0675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scahill L, McDougle CJ, Aman MG, Johnson C, Handen B, Bearss K, et al. Research Units on Pediatric Psychopharmacology Autism. Effects of risperidone and parent training on adaptive functioning in children with pervasive developmental disorders and serious behavioral problems. J Am Acad Child Adolesc Psychiatry. 2012;51(2):136–146. doi: 10.1016/j.jaac.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calarge CA, Ziegler EE, Del Castillo N, Aman M, McDougle CJ, Scahill L, et al. Iron homeostasis during risperidone treatment in children and adolescents. J Clin Psychiatry. 2015;76(11):1500–1505. doi: 10.4088/JCP.14m09258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghanizadeh A, Sahraeizadeh A, Berk M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum Dev. 2014;45(2):185–192. doi: 10.1007/s10578-013-0390-x. [DOI] [PubMed] [Google Scholar]
- 53.Lamberti M, Siracusano R, Italiano D, Alosi N, Cucinotta F, Di Rosa G, et al. Head-to-head comparison of aripiprazole and risperidone in the treatment of ADHD symptoms in children with autistic spectrum disorder and ADHD: a pilot, open-label. Random Control Study Paediatr Drugs. 2016;18(4):319–329. doi: 10.1007/s40272-016-0183-3. [DOI] [PubMed] [Google Scholar]
- 54.Malone RP, Cater J, Sheikh RM, Choudhury MS, Delaney MA. Olanzapine versus haloperidol in children with autistic disorder: an open pilot study. J Am Acad Child Adolesc Psychiatry. 2001;40(8):887–894. doi: 10.1097/00004583-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry. 2008;17(1):1–8. doi: 10.1007/s00787-007-0620-5. [DOI] [PubMed] [Google Scholar]
- 56.Nicol GE, Yingling MD, Flavin KS, Schweiger JA, Patterson BW, Schechtman KB, et al. Metabolic effects of antipsychotics on adiposity and insulin sensitivity in youths: a randomized clinical trial. JAMA Psychiatry. 2018;75(8):788–796. doi: 10.1001/jamapsychiatry.2018.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aman MG, Hollway JA, McDougle CJ, Scahill L, Tierney E, McCracken JT, et al. Cognitive effects of risperidone in children with autism and irritable behavior. J Child Adolesc Psychopharmacol. 2008;18(3):227–236. doi: 10.1089/cap.2007.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aman MG, Kasper W, Manos G, Mathew S, Marcus R, Owen R, et al. Line-item analysis of the Aberrant Behavior Checklist: results from two studies of aripiprazole in the treatment of irritability associated with autistic disorder. J Child Adolesc Psychopharmacol. 2010;20(5):415–422. doi: 10.1089/cap.2009.0120. [DOI] [PubMed] [Google Scholar]
- 59.Anderson GM, Scahill L, McCracken JT, McDougle CJ, Aman MG, Tierney E, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiat. 2007;61(4):545–550. doi: 10.1016/j.biopsych.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 60.McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162(6):1142–1148. doi: 10.1176/appi.ajp.162.6.1142. [DOI] [PubMed] [Google Scholar]
- 61.Vo LC, Snyder C, McCracken C, McDougle CJ, McCracken JT, Aman MG, et al. No apparent cardiac conduction effects of acute treatment with risperidone in children with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2016;26(10):900–908. doi: 10.1089/cap.2016.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez LE, Adams PB, Uysal S, Hallin A, Campbell M, Small AM. A comparison of live and videotape ratings: clomipramine and haloperidol in autism. Psychopharmacol Bull. 1995;31(2):371–378. [PubMed] [Google Scholar]
- 63.Lewis DW, Couch DM, Marcus RN, Manos G, Mankoski R, Carson WH. Efficacy and safety of flexibly-dosed aripiprazole for the treatment of irritability associated with autistic disorder in children and adolescents (6–17 years). Annals of neurology.2009;66(Supp 13 [hardcopy) Suppl 1 [electronic copy]):S110‐111, Abstract no: 143.
- 64.Aman M, Rettiganti M, Nagaraja HN, Hollway JA, McCracken J, McDougle CJ, et al. Tolerability, safety, and benefits of risperidone in children and adolescents with autism: 21-month follow-up after 8-week placebo-controlled trial. J Child Adolesc Psychopharmacol. 2015;25(6):482–493. doi: 10.1089/cap.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnold LE, Vitiello B, McDougle C, Scahill L, Shah B, Gonzalez NM, et al. Parent-defined target symptoms respond to risperidone in RUPP autism study: customer approach to clinical trials. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1443–1450. doi: 10.1097/00004583-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Caicedo C, Williams SH. Risperidone improves behavior in children with autism. J Fam Pract. 2002;51(11):915. [PubMed] [Google Scholar]
- 67.Crosland KA, Zarcone JR, Lindauer SE, Valdovinos MG, Zarcone TJ, Hellings JA, et al. Use of functional analysis methodology in the evaluation of medication effects. J Autism Dev Disord. 2003;33(3):271–279. doi: 10.1023/A:1024402500425. [DOI] [PubMed] [Google Scholar]
- 68.Gencer O, Emiroglu FN, Miral S, Baykara B, Baykara A, Dirik E. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder. An open label maintenance study. Eur Child Adolesc Psychiatry. 2008;17(4):217–225. doi: 10.1007/s00787-007-0656-6. [DOI] [PubMed] [Google Scholar]
- 69.Kent JM, Hough D, Singh J, Karcher K, Pandina G. An open-label extension study of the safety and efficacy of risperidone in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 2013;23(10):676–686. doi: 10.1089/cap.2012.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novaes CM, Pondé MP, Freire AC. Control of psychomotor agitation and aggressive behavior in patients with autistic disorder: a retrospective chart review. Arq Neuropsiquiatr. 2008;66(3B):646–651. doi: 10.1590/S0004-282X2008000500008. [DOI] [PubMed] [Google Scholar]
- 71.Perry R, Campbell M, Adams P, et al. Long-term efficacy of haloperidol in autistic children: continuous versus discontinuous drug administration. J Am Acad Child Adolesc Psychiatry. 1989;28(1):87–92. doi: 10.1097/00004583-198901000-00016. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez LE, Campbell M, Small AM, Cueva JE, Armenteros JL, Adams PB. A pilot study of clomipramine in young autistic children. J Am Acad Child Adolesc Psychiatry. 1996;35(4):537–544. doi: 10.1097/00004583-199604000-00021. [DOI] [PubMed] [Google Scholar]
- 73.Elizur A, Davidson S. The evaluation of the anti-autistic activity of sulpiride. Curr Ther Res Clin Exp. 1975;18(4):578–584. [PubMed] [Google Scholar]
- 74.Miller B, Wallis H. Mode of action of sulpiride in autistic children. A double blind study. MMW Munch Med Wochenschr. 1979;121(19):667–669. [PubMed] [Google Scholar]
- 75.McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633–641. doi: 10.1001/archpsyc.55.7.633. [DOI] [PubMed] [Google Scholar]
- 76.NCT00147394. Risperidone Pharmacokinetics in Children With Pervasive Developmental Disorder (PDD). First Posted: 7th Sep 2005. Accessed 23rd Jan 2019.
- 77.NCT00198107. Evaluating the Effectiveness of Aripiprazole and D-Cycloserine to Treat Symptoms Associated With Autism. Available at: clinicaltrial.gov; Accessed 18th Dec 2019. Results published on March 29, 2019, after the completion of our systematic review for the ASD guidelines development purpose.
- 78.NCT00468130. Efficacy of Aripiprazole Versus Placebo in the Reduction of Aggressive and Aberrant Behavior in Autistic Children (Abilify). Available at: clinicaltrial.gov; Accessed 18th Dec 2019.
- 79.NCT01171937. Risperidone Treatment In Children With Autism Spectrum Disorder And High Levels Of Repetitive Behavior (ProjectV). First Posted: 29th Jul 2010. Accessed 23rd Jan 2019.
- 80.NCT00057408. A Controlled Study of Olanzapine in Children With Autism. Available at: clinicaltrial.gov; Accessed 18th Dec 2019.
- 81.NCT00080145. RUPP PI PDD: Drug and Behavioral Therapy for Children With Pervasive Developmental Disorders. Available at: clinicaltrial.gov; Accessed 9th Jan 2019.
- 82.NCT00205699. Metabolic Effects of Antipsychotics in Children (MEAC). First Posted: 20th Sep 2005. Accessed 23rd Jan 2019.
- 83.NCT01333072. Biomarkers in Autism of Aripiprazole and Risperidone Treatment (BAART) (BAART). Available at: clinicaltrial.gov; Accessed 22nd January 2019.
- 84.NCT01844700. 1/2-MC4R Genotype and Pediatric Antipsychotic Drug- Induced Weight Gain. Available at: clinicaltrial.gov; Accessed 18th Dec 2019.
- 85.NCT00166595. Pharmacogenetics of Risperidone in Children With Pervasive Developmental Disorder (PDD). Available at: clinicaltrial.gov; Accessed 18th Dec 2019.
- 86.NCT00691080. Understanding Sleep Problems in Children With Autism Spectrum Disorder (REST). Available at: clinicaltrial.gov; Accessed 18th Dec 2019.
- 87.NCT00619190. Study of Aripiprazole to Treat Children and Adolescents With Autism (PAIRS). Available at: clinicaltrial.gov; Accessed 18th Dec 2019.
- 88.NCT02574741. Combination Treatment for Augmenting Language in Children With ASD (PIII). Available at: clinicaltrial.gov; Accessed 18th Dec 2019.
- 89.NCT03487770. Aripiprazole Oral Solution in the Treatment of Children and Adolescents With Autistic Disorder. Available at: clinicaltrial.gov; Accessed 18th Dec 2019.
- 90.Hellings JA, Zarcone JR, Reese RM, Valdovinos MG, Marquis JG, Fleming KK, et al. A crossover study of risperidone in children, adolescents and adults with mental retardation. J Autism Dev Disord. 2006;36(3):401–411. doi: 10.1007/s10803-006-0078-1. [DOI] [PubMed] [Google Scholar]
- 91.Kent JM, Kushner S, Ning X, Karcher K, Ness S, Aman M, et al. Risperidone dosing in children and adolescents with autistic disorder: a double-blind, placebo-controlled study. J Autism Dev Disord. 2013;43(8):1773–1783. doi: 10.1007/s10803-012-1723-5. [DOI] [PubMed] [Google Scholar]
- 92.Luby J, Mrakotsky C, Stalets MM, Belden A, Heffelfinger A, Williams M, et al. Risperidone in preschool children with autistic spectrum disorders: an investigation of safety and efficacy. J Child Adoles Psychopharmacol. 2006;16(5):575–587. doi: 10.1089/cap.2006.16.575. [DOI] [PubMed] [Google Scholar]
- 93.McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 94.Nagaraj R, Singhi P, Malhi P. Risperidone in children with autism: randomized, placebo-controlled, double-blind study. J Child Neurol. 2006;21(6):450–455. doi: 10.1177/08830738060210060801. [DOI] [PubMed] [Google Scholar]
- 95.NCT01624675. A Study to Evaluate the Efficacy and Safety of Risperidone (R064766) in Children and Adolescents With Irritability Associated With Autistic Disorder. First posted: 21st Jun 2012. Accessed 18th Feb 2019.
- 96.Research Units on Pediatric Psychopharmacology (RUPP). Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162(7):1361–9. [DOI] [PubMed]
- 97.Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634–e641. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- 98.Troost PW, Lahuis BE, Steenhuis MP, Ketelaars CE, Buitelaar JK, van Engeland H, et al. Long-term effects of risperidone in children with autism spectrum disorders: a placebo discontinuation study. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1137–1144. doi: 10.1097/01.chi.0000177055.11229.76. [DOI] [PubMed] [Google Scholar]
- 99.Findling RL, Mankoski R, Timko K, Lears K, McCartney T, McQuade RD, et al. A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry. 2014;75(1):22–30. doi: 10.4088/JCP.13m08500. [DOI] [PubMed] [Google Scholar]
- 100.Ichikawa H, Mikami K, Okada T, Yamashita Y, Ishizaki Y, Tomoda A, et al. Aripiprazole in the treatment of irritability in children and adolescents with autism spectrum disorder in Japan: a randomized, double-blind. Placebo-controlled Study Child Psychiatry Hum Dev. 2017;48(5):796–806. doi: 10.1007/s10578-016-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marcus RN, Owen R, Kamen L, Manos G, McQuade RD, Carson WH, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1110–1119. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- 102.NCT00870727. Study of Aripiprazole in the Treatment of Pervasive Developmental Disorders. First posted: 27th Mar 2009. Accessed: 18th Feb 2019.
- 103.Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–1540. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- 104.Anderson LT, Campbell M, Grega DM, Perry R, Small AM, Green WH. Haloperidol in the treatment of infantile autism: effects on learning and behavioral symptoms. Am J Psychiatry. 1984;141(10):1195–1202. doi: 10.1176/ajp.141.10.1195. [DOI] [PubMed] [Google Scholar]
- 105.Anderson LT, Campbell M, Adams P, Small AM, Perry R, Shell J. The effects of haloperidol on discrimination learning and behavioral symptoms in autistic children. J Autism Dev Disord. 1989;19(2):227–239. doi: 10.1007/BF02211843. [DOI] [PubMed] [Google Scholar]
- 106.Campbell M, Anderson LT, Meier M, Cohen IL, Small AM, Samit C, et al. A comparison of haloperidol and behavior therapy and their interaction in autistic children. J Am Acad Child Psychiatry. 1978;17(4):640–655. doi: 10.1016/S0002-7138(09)61017-7. [DOI] [PubMed] [Google Scholar]
- 107.Cohen IL, Campbell M, Posner D, Small AM, Triebel D, Anderson LT. Behavioral effects of haloperidol in young autistic children. An objective analysis using a within-subjects reversal design. J Am Acad Child Psychiatry. 1980;19(4):665–677. doi: 10.1016/S0002-7138(09)60969-9. [DOI] [PubMed] [Google Scholar]
- 108.Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: a double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol. 2001;21(4):440–444. doi: 10.1097/00004714-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 109.Loebel A, Brams M, Goldman RS, Silva R, Hernandez D, Deng L, et al. Lurasidone for the treatment of irritability with autistic disorder. J Autism Dev Disord. 2016;46:1153–1163. doi: 10.1007/s10803-015-2628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hollander E, Wasserman S, Swanson EN, Chaplin W, Schapiro ML, Zagursky K, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16(5):541–548. doi: 10.1089/cap.2006.16.541. [DOI] [PubMed] [Google Scholar]
- 111.Putignano D, Clavenna A, Reale L, Bonati M. The evidence-based choice for antipsychotics in children and adolescents should be guaranteed. Eur J Clin Pharmacol. 2019;75(6):769–776. doi: 10.1007/s00228-019-02641-0. [DOI] [PubMed] [Google Scholar]
- 112.Maneeton N, Maneeton B, Putthisri S, Suttajit S, Likhitsathian S, Srisurapanont M. Aripiprazole in acute treatment of children and adolescents with autism spectrum disorder: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018;14:3063–3072. doi: 10.2147/NDT.S174622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maneeton N, Maneeton B, Putthisri S, Woottiluk P, Narkpongphun A, Srisurapanont M. Risperidone for children and adolescents with autism spectrum disorder: a systematic review. Neuropsychiatr Dis Treat. 2018;14:1811–1820. doi: 10.2147/NDT.S151802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Channing J, Mitchell M, Cortese S. Lurasidone in children and adolescents: systematic review and case report. J Child Adolesc Psychopharmacol. 2018;28(7):428–436. doi: 10.1089/cap.2018.0046. [DOI] [PubMed] [Google Scholar]
- 115.Fallah MS, Shaikh MR, Neupane B, Rusiecki D, Bennett TA, Beyene J. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168–180. doi: 10.1089/cap.2018.0115. [DOI] [PubMed] [Google Scholar]
- 116.Hellings JA, Cardona AM, Schroeder SR. Long-term safety and adverse events of risperidone in children, adolescents, and adults with pervasive developmental disorders. J Mental Health Res Intellectual Disabilities. 2010;3(3):132–144. doi: 10.1080/19315864.2010.494763. [DOI] [Google Scholar]
- 117.Srisawasdi P, Vanwong N, Hongkaew Y, Puangpetch A, Vanavanan S, Intachak B, et al. Impact of risperidone on leptin and insulin in children and adolescents with autistic spectrum disorders. Clin Biochem. 2017;50(12):678–685. doi: 10.1016/j.clinbiochem.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 118.Marcus RN, Owen R, Manos G, Mankoski R, Kamen L, McQuade RD, et al. Safety and tolerability of aripiprazole for irritability in pediatric patients with autistic disorder: a 52-week, open-label, multicenter study. J Clin Psychiatry. 2011;72(9):1270–1276. doi: 10.4088/JCP.09m05933. [DOI] [PubMed] [Google Scholar]
- 119.Ichikawa H, Hiratani M, Yasuhara A, Tsujii N, Oshimo T, Ono H, et al. An open-label extension long-term study of the safety and efficacy of aripiprazole for irritability in children and adolescents with autistic disorder in Japan. Psychiatry Clin Neurosci. 2018;72(2):84–94. doi: 10.1111/pcn.12607. [DOI] [PubMed] [Google Scholar]
- 120.Ishitobi M, Hiratani M, Kosaka H, Takahashi T, Mizuno T, Asano M, et al. Switching to aripiprazole in subjects with pervasive developmental disorders showing tolerability issues with risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):128–131. doi: 10.1016/j.pnpbp.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 121.Roke Y, Buitelaar JK, Boot AM, Tenback D, van Harten PN. Risk of hyperprolactinemia and sexual side effects in males 10–20 years old diagnosed with autism spectrum disorders or disruptive behavior disorder and treated with risperidone. J Child Adolesc Psychopharmacol. 2012;22(6):432–439. doi: 10.1089/cap.2011.0109. [DOI] [PubMed] [Google Scholar]
- 122.Roke Y, van Harten PN, Buitelaar JK, Tenback DE, Quekel LG, de Rijke YB, et al. Bone mineral density in male adolescents with autism spectrum disorders and disruptive behavior disorder with or without antipsychotic treatment. Eur J Endocrinol. 2012;167(6):855–863. doi: 10.1530/EJE-12-0521. [DOI] [PubMed] [Google Scholar]
- 123.Vanwong N, Srisawasdi P, Ngamsamut N, Nuntamool N, Puangpetch A, Chamkrachangpada B, et al. Hyperuricemia in children and adolescents with autism spectrum disorder treated with risperidone: the risk factors for metabolic adverse effects. Front Pharmacol. 2017;7:527. doi: 10.3389/fphar.2016.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moberg J, Oxman AD, Rosenbaum S, Schünemann HJ, Guyatt G, Flottorp S, et al.; GRADE Working Group. The GRADE Evidence to Decision (EtD) framework for health system and public health decisions. Health Res Policy Syst. 2018;16(1):45. 10.1186/s12961-018-0320-2. [DOI] [PMC free article] [PubMed]
- 125.D’Alò GL, De Crescenzo F, Minozzi S, Morgano GP, Mitrova Z, Scattoni ML, et al. Equity, acceptability and feasibility of using polyunsaturated fatty acids in children and adolescents with autism spectrum disorder: a rapid systematic review. Health Qual Life Outcomes. 2020;18(1):101. doi: 10.1186/s12955-020-01354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Crescenzo F, D’Alò GL, Morgano GP, Minozzi S, Mitrova Z, Saulle R, et al. Impact of polyunsaturated fatty acids on patient-important outcomes in children and adolescents with autism spectrum disorder: a systematic review. Health Qual Life Outcomes. 2020;18(1):28. doi: 10.1186/s12955-020-01284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenbaum SE, Moberg J, Glenton C, Schünemann HJ, Lewin S, Akl E, et al. Developing evidence to decision frameworks and an interactive evidence to decision tool for making and using decisions and recommendations in health care. Glob Challenges. 2018;2(9):1700081. doi: 10.1002/gch2.201700081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Search strategy and results for Systematic Reviews.
Additional file 2: Search strategy and results for Randomized Controlled Trials.
Additional file 3: References for included and excluded trials, with reasons.
Additional file 4: Risk of Bias Summary..
Additional file 5: Forest plots for comparisons between antipsychotics (D2 blockers) and Placebo.
Additional file 6: Forest plots for comparisons between antipsychotics (D2 blockers) and Placebo – subgroup analyses (ABC-Irritability ≥18 vs All ASDs).
Additional file 7: Funnel Plots for outcome with estimates for at least 8 studies.
Additional file 8: GRADE Evidence profile.
Data Availability Statement
All data supporting our findings is contained within the manuscript and the additional files.