Abstract
Background
Chinese herbal medicine (CHM) is thought to be a potential intervention in the treatment of coronavirus disease (COVID-19).
Purpose
This study aimed to investigate the efficacy and safety of CHM or CHM combination therapy for COVID-19.
Study design
Systematic review and meta-analysis
Methods
We searched for relevant studies in the CNKI, CBM, Wanfang Data, PubMed, Cochrane Library, Embase, and other resources from their inception to April 15, 2020. Randomized controlled trials, cohort studies, and case-control studies on CHM or CHM combination therapy for COVID-19 were included. Meta-analysis was performed according to the Cochrane Handbook.
Results
Overall, 19 studies with 1474 patients were included. Meta-analysis showed that the overall clinical effectiveness (OR = 2.67, 95% CI 1.83-3.89, I2 = 0%), improvement in the CT scan (OR = 2.43, 95% CI 1.80-3.29, I2 = 0%), percentage of cases turning to severe/critical (OR = 0.40, 95% CI 0.24-0.67, I2 = 17.1%), reverse transcription-polymerase chain reaction (RT-PCR) negativity rate (OR = 2.55, 95% CI 1.06-6.17, I2 = 56.4%) and disappearance rate of symptoms (fever, cough, and fatigue) were superior by combined CHM treatment of COVID-19. However, there was no statistical difference between the two groups in terms of length of hospital stay (WMD = -0.46, 95% CI -3.87 - 2.95, I2 = 99.5%), and rate of adverse effects (OR = 1.21, 95% CI 0.48-3.07, I2 = 43.5%). The quality of evidence was very low to low.
Conclusion
The combined treatment of COVID-19 with Chinese and Western medicine may be effective in controlling symptoms and reducing the rate of disease progression due to low quality evidence.
Keywords: Chinese herbal medicine, COVID-19, Systematic review, Meta-analysis
Abbreviations: CHM, Chinese herbal medicine; COVID-19, coronavirus disease 2019; RT-PCR, reverse transcription-polymerase chain reaction; WHO, World Health Organization; PHEIC, Public Health Emergency of International Concern; NHC PRC, National Health Commission of the People's Republic of China; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis; AE, adverse effects; CNKI, China National Knowledge Infrastructure; CBM, China Biology Medicine; RCT, randomized controlled trials; CT, computed tomography; RoB, Risk of Bias; NOS, Newcastle-Ottawa Scale; GRADE, Grading of Recommendations Assessment, Development and Evaluation; OR, odds ratios; CI, confidence intervals; WMD, weighted mean differences
Introduction
An unexplained pneumonia has spread rapidly throughout the country since early 2020 (She et al., 2020). The disease is caused by SARS-CoV-2, a novel coronavirus, and presents with manifestations of pneumonia such as cough, fever, fatigue, dyspnea, among others (Fu et al., 2020). On February 11, 2020, the World Health Organization (WHO) named the disease caused by the virus as coronavirus disease (COVID-19) (WHO, 2020a). On January 30, 2020, the WHO declared the outbreak a Public Health Emergency of International Concern (PHEIC) (WHO, 2020b) and as a pandemic on March 11, 2020 (WHO, 2020c). As of April 15, 2020, more than 1,910,000 cases had been diagnosed in 210 countries outside China, with over 123,000 deaths (WHO, 2020d).
Chinese herbal medicine (CHM) was considered important by the health authorities of the country in the fight against COVID-19 (Yang et al., 2020). At the beginning of the COVID-19 outbreak, due to the unknown nature of the disease, there were no effective western medicine treatment protocols and vaccines approved for clinical use. However, in most hospitals in China, traditional Chinese medicine has been used widely and has played an important role in controlling the symptoms of COVID-19, either as monotherapy or in combination with western medicine (Du et al., 2020). Presently, the National Health Commission of the People's Republic of China (NHC PRC) has issued seven editions of the COVID-19 management protocol (NHC, 2020a), with inclusion of CHM in the third edition, which has been continuously optimized in subsequent editions (NHC,2020b). Chinese researches have also developed several clinical practice guidelines for COVID-19 (Ho et al., 2020; Ang et al., 2020). Moreover, a number of systematic reviews on the efficacy of CHM for the treatment of COVID-19 have been conducted (Luo et al., 2020; Wu et al., 2020). However, the majority of these reviews have included indirect evidence or incomplete studies, which provide limited clinical guidance. Therefore, we conducted this systematic review to comprehensively investigate and identify the efficacy and safety of CHM treatment in COVID-19.
The purpose of this systematic review and meta-analysis was to investigate the efficacy and safety of CHM or CHM in combination with other interventions for the treatment of COVID-19, to provide clinicians a basis for the use of CHM, and provide information to other countries regarding the experience of treating COVID-19 with CHM in China.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statements checklist (Moher et al., 2009) and Cochrane handbook for Systematic Reviews (Higgins et al., 2019). Besides, our systematic review protocol was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) on April 27, 2020 and the registration number is INPLASY202040190 (Luo et al., 2020a).
Search strategy
We searched for studies in the China National Knowledge Infrastructure (CNKI), China Biology Medicine (CBM), Wanfang Data, PubMed, Cochrane Library, Embase and Chinese Journal Medical Network (http://medjournals.cn/2019NCP/index.do) databases from their inception to April 15, 2020 (See Supplementary material 1 for detailed search strategy of each database). We also supplemented the search with Google Scholar and the journal preprint services that included ChemRxiv (https://chemrxiv.org/), medRxiv (https://www.medrxiv.org/), BioRxiv (https://www.biorxiv.org/), and SSRN (https://www.ssrn.com/index.cfm/en/). In addition, references in the selected studies were examined to include the literature missed in the main search, and studies included in published systematic reviews were also checked.
Inclusion and exclusion criteria
We included studies if they meet the following criteria: 1) the study population involved COVID-19 patients, and COVID-19 was defined based on the guidelines published by the NHC PRC (NHC, 2020a); 2) the intervention was CHM or CHM in combination with other treatments; 3) the control group was placebo or other therapy; and 4) study types were controlled trials, including randomized controlled trials (RCT), cohort, and case-control studies.
We excluded the literature if: 1) manipulative treatments, such as acupuncture were performed; 2) the language was not English or Chinese; 3) full texts were not available; and 4) duplicated studies were published in English and Chinese.
Study selection
Two researchers (J.L and Y.Z) independently used Endnote X9 for screening of the literature. Articles were first screened based on the title and abstract, and in cases of uncertainty, the full texts were obtained. In case of disagreement between the two researchers, a third researcher (X.N) was consulted. The reasons for exclusion of unqualified studies were recorded. The process of study selection was documented using a PRISMA flow diagram (Moher et al., 2009).
Data collection
Two researchers (J.L and Y.Z) extracted data independently using a predetermined extraction table, and disagreements were resolved by consensus or by consulting a third researcher (X.L). We extracted the following data: 1) basic information; 2) participant's baseline characteristics and inclusion/exclusion criteria; 3) details of intervention and control groups; and 4) outcomes (dichotomous data were number of events and total participants per group; continuous data were presented as mean, standard deviation (SD), and total participants per group).
The primary outcome for this study was the overall clinical effectiveness of therapy [Defined as (number of people cured per group + number of people in remission per group) divided by the total number of people in each group]. Secondary outcomes included 1) improvement in computed tomography (CT) scan findings: number of patients who lost or reduced CT lesions after treatment as a percentage of all patients treated; 2) percentage of cases turning to severe/critical; 3) reverse transcription-polymerase chain reaction (RT-PCR) negativity rate; 4) length of hospital stay; 5) disappearance rate of symptoms (fever, cough, and fatigue): number of patients whose symptoms (fever, cough, and fatigue) disappeared after treatment as a percentage of all patients treated; and 6) rate of adverse effects (AE).
Risk of Bias
Two researchers (J.L and Y.Z) independently conducted the risk of bias evaluation of the included studies. Disagreements were resolved by consensus or by consulting a third researcher (X.L). For RCTs, we used the Cochrane Risk-of-Bias (RoB) assessment tool (Higgins et al., 2011) consisting of seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. For cohort and case-control studies, we used the Newcastle-Ottawa Scale (NOS) consisting of three domains (selection of exposure, comparability, and assessment of outcome) (Wells et a1., 2003). The maximum score was nine, and studies with scores of seven or more were graded as high quality while those with scores less than seven were considered low quality.
Quality of evidence
We assessed the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt et al., 2008), and classified the studies as “high,” “moderate,” “low,” or “very low” quality. According to the GRADE approach, direct evidence from RCTs begins at high quality, and evidence from observational studies is considered low quality. The quality can be downgraded for five reasons (risk of bias, imprecision, inconsistency, indirectness, and publication bias) and upgraded for three reasons (large magnitude of an effect, dose-response gradient, and effect of plausible residual confounding).
Data synthesis and analyses
We conducted meta-analyses using the STATA version 14.0 software. For dichotomous data, we calculated the odds ratios (OR) with 95% confidence intervals (CI); for continuous data, we calculated weighted mean differences (WMD) with 95% CI. Missing data were dealt with according to the Cochrane Handbook for Systematic Reviews of Interventions. Two-sided P values <0.05 were considered statistically significant. Statistical heterogeneity was assessed with the I² statistic, with values >50% indicating substantial heterogeneity. Subgroup analyses were performed, if heterogeneity was detected, based on the severity of the disease, study type, and complications, and we also considered sensitivity analyses where one study was excluded at a time. Egger test was used to assess publication bias. Qualitative descriptive analysis was used if the heterogeneity of the included studies was large or the data could not be pooled.
Results
Results of study selection
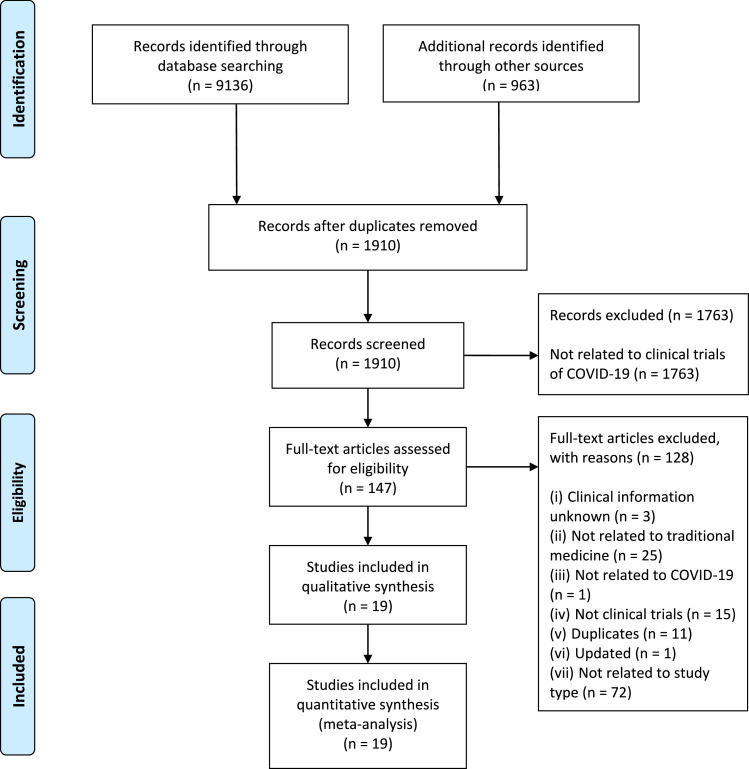
A total of 10,099 records were retrieved and, after elimination of duplicates, the titles and abstracts of 1,910 records were screened. After a final screening, we included 19 studies with 1474 patients (Cheng et al., 2020; Qu et al., 2020; Shi et al., 2020; Xia et al., 2020; Lv et al., 2020; Yao et al., 2020; Xiao et al., 2020; Fu et al., 2020a; Fu et al., 2020b; Yang et al., 2020a; Zhang et al., 2020a; Huang et al., 2020; Duan et al., 2020; Wang et al., 2020; Yang et al., 2020b; Li et al., 2020a; Zhang et al., 2020b; Liu et al., 2020; Ding et al., 2020). The screening process is detailed in Figure 1 .
Figure 1.
Flow chart of study selection.
Characteristics of included studies
All 19 included studies were conducted in China. Six (31.6%) of these were RCTs (Xiao et al., 2020; Fu et al., 2020a; Fu et al., 2020b; Duan et al., 2020; Wang et al., 2020; Ding et al., 2020) and the rest were case-control studies (Cheng et al., 2020; Qu et al., 2020; Shi et al., 2020; Xia et al., 2020; Lv et al., 2020; Yao et al., 2020; Yang et al., 2020a; Zhang et al., 2020a; Huang et al., 2020; Wang et al., 2020; Yang et al., 2020b; Li et al., 2020a; Zhang et al., 2020b; Liu et al., 2020). All research interventions were CHM in combination with other treatments. Basic characteristics are detailed in Table 1 .
Table 1.
Characteristics of included studies.
| Study | Study design | Severity of disease | Original place of patients | Complications | Age (Years) | Numbers of T/C (M/F) | Sample size | Intervention | Course of treatment | |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | |||||||||
| Cheng et al. (2020) | Case-control study | Ordinary | Wuhan | Hypertension, Coronary heart disease, Diabetes, Cerebral infarction | T:55.5 ± 12.3 C:55.8 ± 11.6 |
T:51 (26/25) C:51 (27/24) |
102 | Lianhua Qingwen granules + Routine treatment | Routine treatment | 7d |
| Qu et al. (2020) | Case-control study | NR | Haozhou | NR | T:38.71 ± 0.71 C:38.62 ± 0.52 |
T:40 (25/15) C:30 (16/14) |
70 | Shufeng Jiedu capsule + Arbidol+ Moxifloxacin+Routine treatment | Arbidol + Moxifloxacin+ Routine treatment | 10d |
| Shi et al. (2020) | Case-control study | Mild (2), Ordinary (54), Severe (11) |
Shanghai | Hypertension, Diabetes, Tumor, Fatty liver, Autoimmune liver disease, Complicated with hypertension, diabetes and coronary heart disease, Complicated with hypertension and coronary heart disease | T:47.94 ± 14.46 C:46.72 ± 17.40 |
T:49 (26/23) C:18 (10/8) |
67 | Chinese patent medicine or decoction+ Routine treatment | Routine treatment | NR |
| Xia et al. (2020) | Case-control study | Ordinary (40), Severe (10), Critical (2) |
Hubei | Pulmonary tuberculosis, COPD, Hypertension, Diabetes, Stroke, Chronic nephropathy, Chronic liver disease | T:54.18 ± 13.08 C:53.67 ± 12.70 |
T:34 (17/17) C:18 (6/12) |
52 | Chinese patent medicine or decoction or herb injection+ Arbidol+Moxifloxacin +Routine treatment | Arbidol+Moxifloxacin+Routine treatment | Decoction:5d; Chinese patent medicine:7d; Herb injection:7-10d |
| Lv et al. (2020) | Case-control study | Suspected case | Wuhan | Hypertension, Coronary heart disease, Diabetes, Cerebral infarction | T:59.1 ± 16.56 C:60.2 ± 17.01 |
T:63 (28/35) C:38 (18/20) | 101 | Lianhua Qingwen granules + Ganciclovir + Moxifloxacin +Routine treatment | Ganciclovir + Moxifloxacin +Routine treatment | 10d |
| Yao et al. (2020) | Case-control study | Ordinary | Wuhan | NR | T:57.1 ± 14.0 C:62.4 ± 12.3 |
T:21 (16/5) C:21 (12/9) |
42 | Lianhua Qingwen granules + Routine treatment | Routine treatment | NR |
| Xiao et al. (2020) | RCT | Mild | Wuhan | NR | T:60.90 ± 8.70 C:62.20 ± 7.50 |
T:100 (64/36) C:100 (66/34) |
200 | Shufeng Jiedu capsule + Arbidol+Nutritional support treatment | Arbidol+ Nutritional support treatment | 14d |
| Fu et al. (2020a) | RCT | Mild, Ordinary |
Guangzhou | Hypertension, Coronary heart disease, Diabetes, Chronic hepatitis B | T:43.26 ± 7.15 C:43.68 ± 6.45 |
T:32 (17/15) C:33 (19/14) |
65 | Toujie Quwen granules* + Moxifloxacin + Ambroxol | Arbidol+Moxifloxacin + Ambroxol | 10d |
| Fu et al. (2020b) | RCT | Ordinary | Guangzhou | Hypertension, Coronary heart disease, Diabetes, Chronic hepatitis | T:45.26 ± 7.25 C:44.68 ± 7.45 |
T:37 (19/18) C:36 (19/17) | 73 | Toujie Quwen granules + Arbidol+ Ambroxol | Arbidol+ Ambroxol | 15d (C: Arbidol 10d,Ambroxol 15 d;T:15d) |
| Yang et al. (2020a) | Case-control study | Ordinary | ShanXi, Hubei | NR | T:50.35 ± 13.37 C:47.17 ± 16.57 |
T:26 (16/10) C:23 (9/14) |
49 | Reyanning Mixture+ Lopinavir+ α-interferon + Arbidol hydrochloride + Ribavirin injection | Lopinavir+ α-interferon + Arbidol hydrochloride + Ribavirin injection | 7d |
| Zhang et al. (2020a) | Case-control study | Ordinary | Wuhan | Hypertension, Coronary heart disease, Diabetes | T:53.7 ± 3.5 C: 55.6 ± 4.2 |
T:22 (9/13) C:23 (10/13) |
45 | Jiaweidayuan decoction + Routine treatment | Routine treatment | 7d |
| Huang et al. (2020) | Case-control study | Ordinary (56), Severe (12) |
Hubei | COPD, Heart disease, Hypertension, Diabetes mellitus | T:60.42 ± 12.84 C: 61.16 ± 13.58 |
T:53 (25/28) C:15 (6/9) |
68 | Pneumonia No.1 Prescription and Pneumonia No.2 Prescription + Routine treatment | Routine treatment | 10d |
| Duan et al. (2020) | RCT | Mild | Hubei | COPD, Diabetes, Hypertension, Pulmonary tuberculosis, Chronic nephropathy, Chronic liver disease, Stroke | T:51.99 ± 13.88 C: 50.29 ± 13.37 |
T:82 (39/43) C:41 (23/18) |
123 | Jinhua Qinggan granules+ Routine treatment | Routine treatment | 5d |
| Wang et al. (2020) | RCT | NR | Hubei | NR | T1(Traditional Chinese medicine and fumigation):39.24 ± 10.01, T2(Traditional Chinese medicine plus fumigation and absorption combined with super dose of vitamin C):54.90 ± 3.61 C: 55.90 ± 3.71 |
T1:10 (5/5), T2:10 (4/6) C:10 (5/5) |
30 | Traditional Chinese medicine and fumigation, Traditional Chinese medicine plus fumigation and absorption combined with super dose of vitamin E |
Ribavirin + vitamin E + folic acid | NR |
| Yang et al. (2020b) | Case-control study | Severe (65), Critical (3) |
Wuhan | Hypertension, Diabetes, COPD, cerebrovascular disease, malignant tumor, Chronic nephropathy, Chronic liver disease | T:61.57 ± 1.84 C:66.46 ± 2.29 |
T:51 (28/23) C:52 (24/28) |
103 | Chinese patent medicine or decoction+ Routine treatment | Routine treatment | NR |
| Li et al. (2020a) | Case-control study | NR | Hubei | NR | T:53.600 ± 0.259 C:50.433 ± 0.338 |
T:30 (15/15) C:30 (13/17) | 60 | Modified Qingfeipaidu decoction + Nutritional support+ Respiratory support+ Arbidol + Lopinavir | Nutritional support+ Respiratory support+ Arbidol + Lopinavir | 3d |
| Zhang et al. (2020b) | Case-control study | Ordinary (44) | Hubei | NR | T:49.05 ± 14.19 C:45.95 ± 14.68 |
T:22 (10/12) C:22 (12/10) | 44 | Xuebijing+ Arbidol + Interferon + support therapy | Arbidol + Interferon + support therapy | 7d |
| Liu et al. (2020) | Case-control study | NR | Beijing | Hypertension, Coronary heart disease, Diabetes, Cirrhosis | T:50.73 (Average) C: 51.75 (Average) |
T:44 (21/23) C:36 (16/20) |
80 | Jinhua Qinggan granules + Oxygen inhalation+ symptomatic and nutritional treatment |
Oxygen inhalation+ symptomatic and nutritional treatment |
7d |
| Ding et al. (2020) | RCT | Mild (21), Ordinary (70), Severe and Critical (9) |
Wuhan | NR | T:54.7 ± 21.3 C:50.8 ± 23.5 |
T:51 (39/12) C:49 (39/10) |
100 | Qingfei Touxie Fuzheng Recipe + Interferon+ Ribavirin+ Carbostyril + Respiratory support | Interferon+ Ribavirin+ Carbostyril Respiratory support | 10d |
Toujie Quwen granules and Pneumonia No.1 Prescription shared the same herbal ingredients; NR: Not Report.
Risk of Bias
Of the six randomized controlled trials included, only two (Fu et al., 2020a; Ding et al., 2020) were considered low risk in the generation of randomized sequences, and both allocation concealment and blinding methods were unclear (Table 2 ).
Table 2.
Risk of bias of included RCTs.
| Study | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|---|
| Xiao et al. (2020) | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
| Fu et al. (2020a) | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
| Fu et al.(2020b) | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
| Duan et al. (2020) | High | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
| Wang et al. (2020) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low |
| Ding et al. (2020) | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
All thirteen case-control studies had a risk of bias score of seven, none of which reported the comparability of cases and controls based on the design or analysis (See Table 3 for details).
Table 3.
Risk of bias of included case-control studies.
| Study | Is the case definition adequate | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | Total |
|---|---|---|---|---|---|---|---|---|---|
| Cheng et al. (2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Qu et al. (2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Shi et al. (2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Xia et al. (2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Lv et al. (2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Yao et al. (2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Yang et al. (2020a) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Zhang et al. (2020a) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Huang et al. (2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Yang et al. (2020b) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Li et al. (2020a) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Zhang et al. (2020b) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Liu et al. (2020) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
Primary outcome
Overall clinical effectiveness
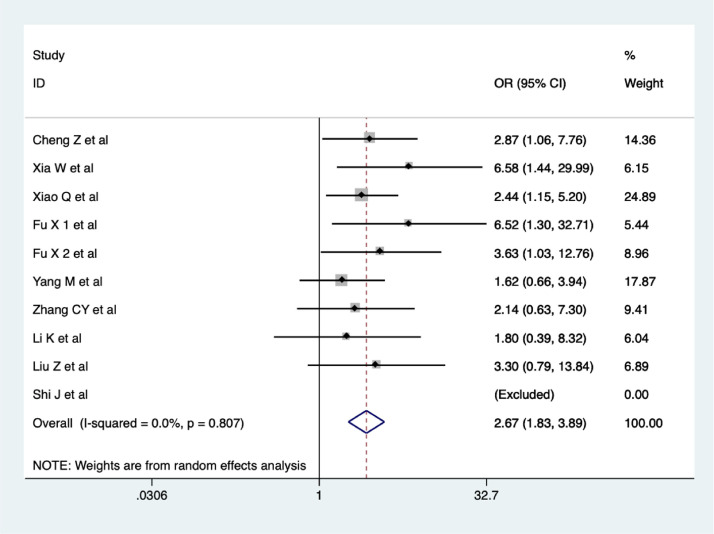
Ten studies (Cheng et al., 2020; Shi et al., 2020; Xia et al., 2020; Xiao et al., 2020; Fu et al., 2020a; Fu et al., 2020b; Yang et al., 2020a; Li et al., 2020a; Zhang et al., 2020b; Liu et al., 2020) reported on the overall clinical effectiveness of CHM treatment. The results of our meta-analysis showed that the overall clinical effectiveness of COVID-19 treatment was better in the CHM combination group than in the control group, and the difference was statistically significant (OR = 2.67, 95% CI 1.83-3.89, I2 = 0%), as shown in Figure 2 . The quality of evidence was low.
Figure 2.
Meta-analysis on the overall clinical effectiveness of COVID-19 treatment in CHM combination group vs. control group.
Secondary outcomes
Improvement in CT scan
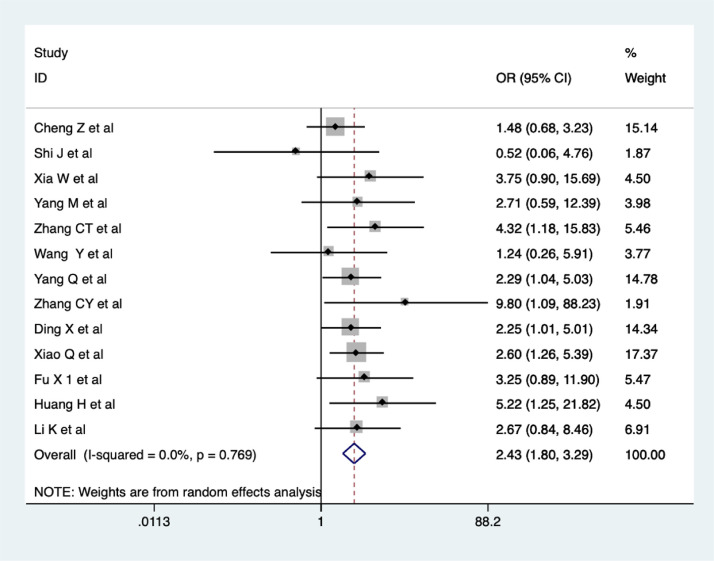
Thirteen trials evaluated the improvements in CT scan findings in the CHM combination and control groups. The meta-analysis revealed a significantly increasing improvement in CT scan findings in the CHM combination group (OR = 2.43, 95% CI 1.80-3.29, I2 = 0%, Figure 3 ). The quality of evidence was low (Cheng et al., 2020; Shi et al., 2020; Xia et al., 2020; Xiao et al., 2020; Fu et al., 2020a; Yang et al., 2020a; Zhang et al., 2020a; Huang et al., 2020; Wang et al., 2020; Yang et al., 2020b; Li et al., 2020a; Zhang et al., 2020b; Ding et al., 2020).
Figure 3.
Meta-analysis on improvement in CT scan in the CHM combination group vs. control group in COVID-19 patients.
Percentage of cases turning to severe/critical
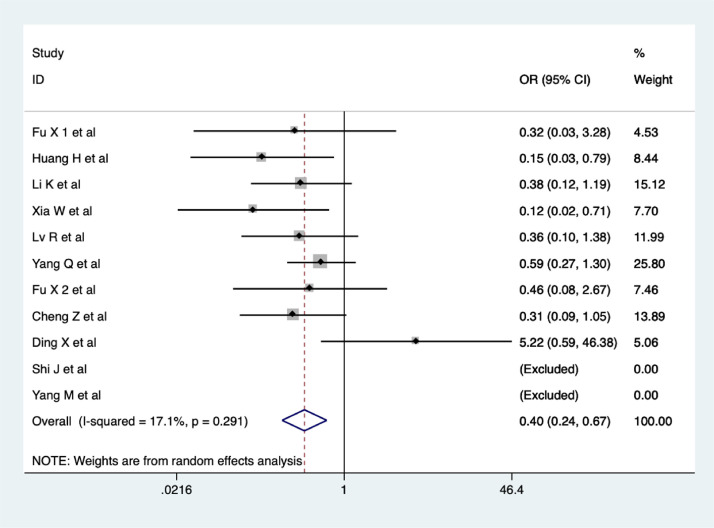
A total of 11 studies reported aggravation in COVID-19 after treatment. Percentage of cases turning to severe/critical was significantly lower in the CHM combination group than in the control group, (OR = 0.40, 95% CI 0.24-0.67, I2 = 17.1%, Figure 4 ). The quality of evidence was low (Fu et al., 2020a; Huang et al., 2020; Li et al., 2020a; Xia et al., 2020; Lv et al., 2020; Yang et al., 2020b; Fu et al., 2020b; Cheng et al., 2020; Ding et al., 2020; Shi et al., 2020; Yang et al., 2020a).
Figure 4.
Meta-analysis of percentage of cases turning to severe/critical in the CHM combination group vs. control group in COVID-19 patients.
RT-PCR negativity rate
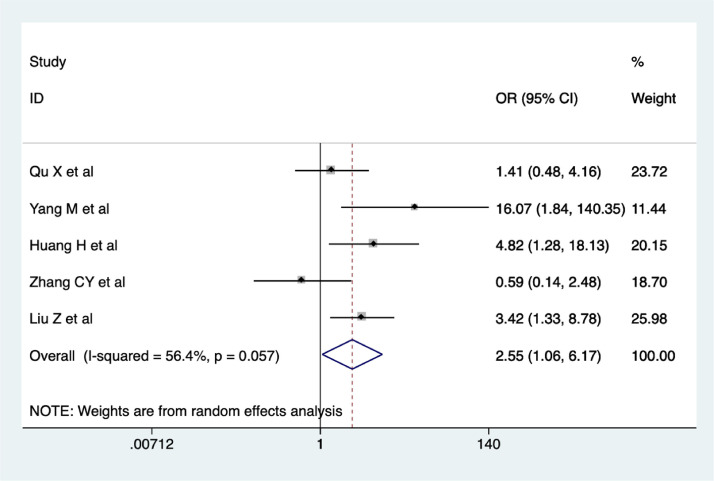
Five studies reported on the RT-PCR negativity rate. The results of our meta-analysis suggested that RT-PCR negativity rate was higher in the CHM combination group than control group (OR = 2.55, 95% CI 1.06-6.17, I2 = 56.4%). As shown in Figure 5 . The quality of evidence was very low (Qu et al., 2020; Yang et al., 2020a; Huang et al., 2020; Zhang et al., 2020b; Liu et al., 2020).
Figure 5.
Meta-analysis of RT-PCR negativity rate in the CHM combination group vs. control group in COVID-19 patients.
Disappearance rate of symptoms (fever, cough, and fatigue)
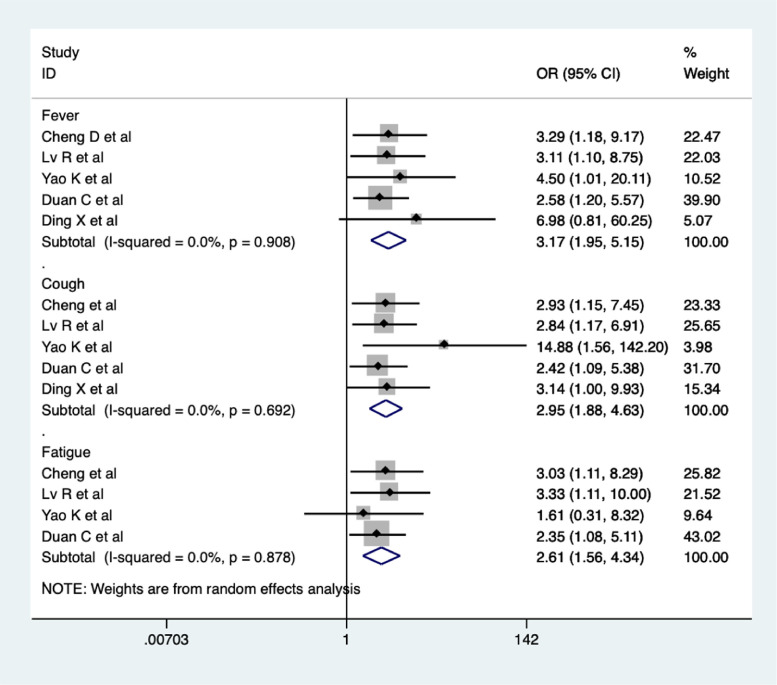
A total of five studies reported the rates of disappearance of fever and cough (Cheng et al., 2020; Lv et al., 2020; Yao et al., 2020; Duan et al., 2020; Ding et al., 2020), and four studies reported the fatigue disappearance rate (Cheng et al., 2020; Lv et al., 2020; Yao et al., 2020; Duan et al., 2020). Meta-analysis suggested that the rates of disappearance of fever (OR = 3.17, 95% CI 1.95-5.15, I2 = 0%), cough (OR = 2.95, 95% CI 1.88-4.63, I2 = 0%), and fatigue (OR = 2.61, 95% CI 1.56-4.34, I2 = 0%) were higher in the CHM combination group compared to the control group (Figure 6 ). The quality of evidence was low.
Figure 6.
Meta-analysis of disappearance rate of symptoms (fever, cough, and fatigue) in the CHM combination group vs. control group in COVID-19 patients.
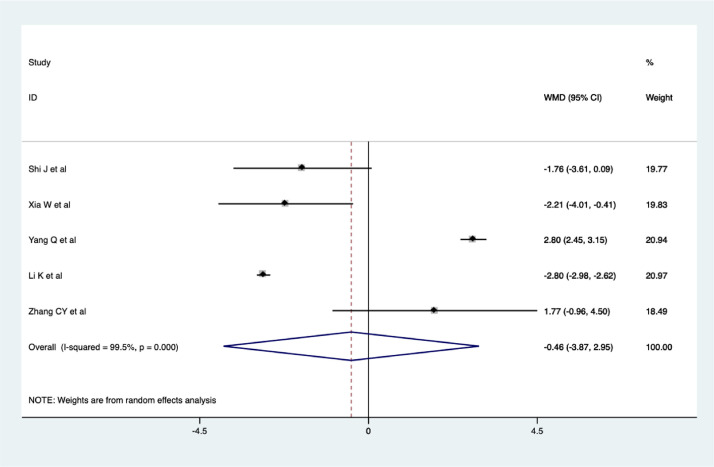
Length of hospital stay
Five studies reviewed the length of hospital stay of patients with COVID-19. The results of the meta-analysis showed that there was no significant difference in the length of hospital stay of COVID-19 patients between the CHM combination and control groups (WMD = -0.46, 95% CI -3.87 - 2.95, I2 = 99.5%), as shown in Figure 7 . The quality of evidence was very low (Shi et al., 2020; Xia et al., 2020; Yang et al., 2020a; Li et al., 2020a; Zhang et al., 2020b).
Figure 7.
Meta-analysis of the length of hospital stay of COVID-19 patients.
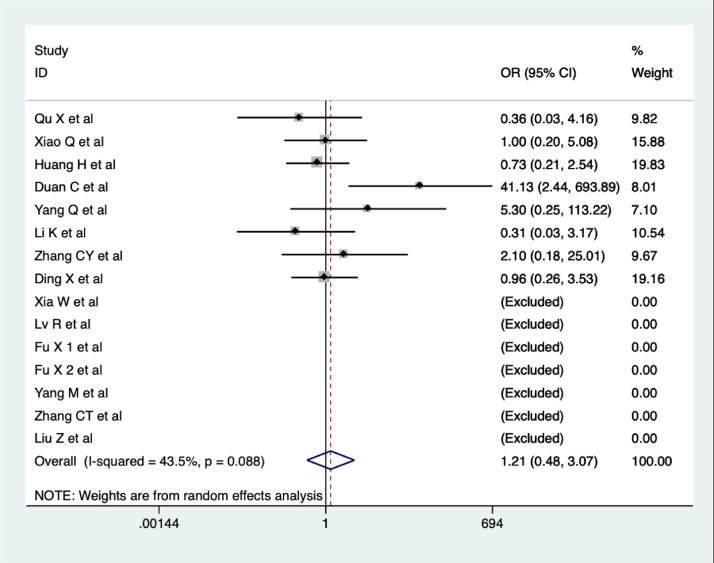
Rate of adverse effects
A total of fifteen studies reported on the rate of adverse effects (AE) (Qu et al., 2020; Xiao et al., 2020; Huang et al., 2020; Duan et al., 2020; Yang et al., 2020b; Li et al., 2020a; Zhang et al., 2020b; Ding et al., 2020; Xia et al., 2020; Lv et al., 2020; Fu et al., 2020a; Fu et al., 2020b; Yang et al., 2020a; Zhang et al., 2020a; Liu et al., 2020). However, eight of these studies reported no adverse events in either group (Qu et al., 2020; Xiao et al., 2020; Huang et al., 2020; Duan et al., 2020; Yang et al., 2020b; Li et al., 2020a; Zhang et al., 2020b; Ding et al., 2020). The main adverse reactions were gastrointestinal reactions such as nausea, abdominal pain, and diarrhea, which could be relieved with rest. Meta-analysis indicated no significant difference in AE between COVID-19 patients in the CHM combination and control groups (OR = 1.21, 95% CI 0.48-3.07, I2 = 43.5%, Figure 8 ). The quality of evidence was low.
Figure 8.
Meta-analysis of AE in COVID-19 patients.
Subgroup and Sensitivity Analyses
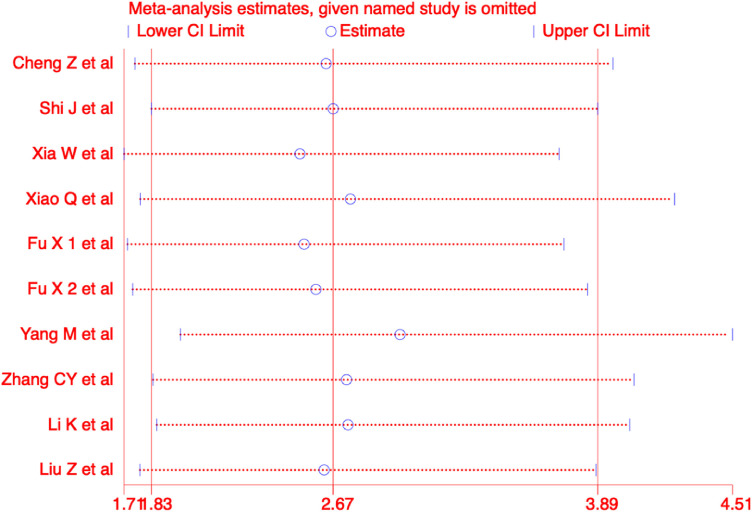
We performed a subgroup analysis of the primary outcome based on study type, disease severity, and presence or absence of comorbidities. The results of the subgroup analyses revealed more effective outcomes in the CHM combination group than control group. Detailed results of the analyses are shown in Table 4 . Furthermore, we performed sensitivity analyses with the use of a metaninf command in the STATA software for overall clinical effectiveness, and the results were not significantly different from those of the primary analysis, as shown in Figure 9 .
Table 4.
Subgroup analyses of the overall clinical effectiveness.
| Subgroup | Studies | Statistical method | Effect estimate | I-squared |
|---|---|---|---|---|
| Study type | ||||
| RCT | 3 (Xiao et al., 2020; Fu et al., 2020a; Fu et al., 2020b) | OR, Random | 3.06, 95%CI [1.68-5.59] | 0% |
| CCS | 7 (Cheng et al., 2020; Shi et al., 2020; Xia et al., 2020; Yang et al., 2020a; Li et al., 2020a; Zhang et al., 2020b; Liu et al., 2020) | OR, Random | 2.44, 95%CI [1.51-3.96] | 0% |
| Disease severity | ||||
| Mild case | 6 (Cheng et al., 2020; Xiao et al., 2020; Fu et al., 2020a; Fu et al., 2020b; Yang et al., 2020a; Zhang et al., 2020b) | OR, Random | 2.52, 95%CI [1.66-3.83] | 0% |
| Mixed case | 4 (Shi et al., 2020; Xia et al., 2020; Li et al., 2020a; Liu et al., 2020) | OR, Random | 3.40, 95%CI [1.44-8.05] | 0% |
| With or without comorbidities | ||||
| With | 6 (Cheng et al., 2020; Shi et al., 2020; Xia et al., 2020; Fu et al., 2020a; Fu et al., 2020b; Liu et al., 2020) | OR, Random | 3.88, 95%CI [2.17-6.95] | 0% |
| Without | 4 (Xiao et al., 2020; Yang et al., 2020a; Li et al., 2020a; Zhang et al., 2020b) | OR, Random | 2.04, 95%CI [1.25-3.34] | 0% |
Figure 9.
Sensitivity analyses of overall clinical effectiveness in COVID-19 patients.
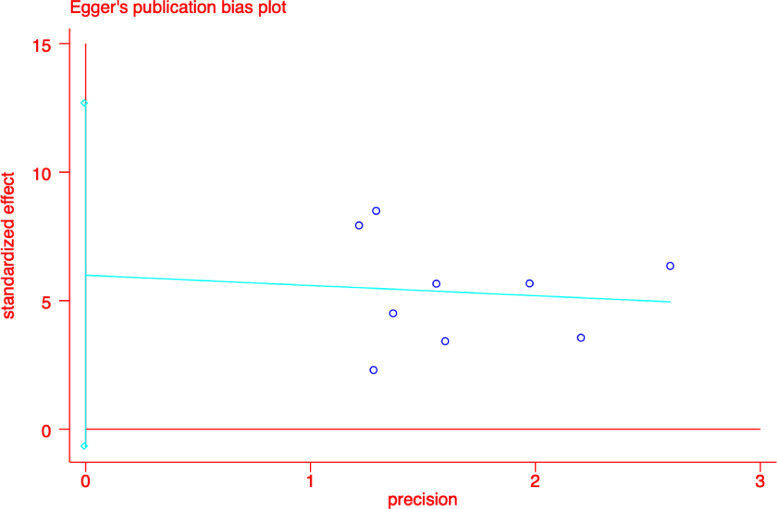
Publication Bias
We used the metabias6 command in the STATA software to test the publication bias. The result did not reveal any publication bias (P >|t| = 0.072) (See Figure 10 for details).
Figure 10.
Publication bias test.
Discussion
Our systematic review suggested that CHM combination therapy was more effective in the treatment of patients with COVID-19, in reducing the rate of aggravation of the condition and control of symptoms. However, there was no difference in parameters of safety and reduction of mortality between the test and control groups in the included studies. The quality of evidence of the included studies was very low to low.
CHM showed good clinical efficacy and immense potential in the treatment of COVID-19 (Zhang et al., 2020c). As a representative dosage form widely used in clinical practice, CHM decoction was found effective in the treatment of COVID-19, probably by alleviating the "cytokine storm". A review (Ren et al., 2020) suggested that its potential mechanism was inhibiting the arachidonic acid metabolism and regulating cytokine levels.
Various decoctions were used in the studies included in this systematic review. Through frequency analysis of all decoctions, we found that EPHEDRAE HERBA (Chinese pinyin: mahuang), ARMENIACAE SEMEN AMARUM (Chinese pinyin: kuxingren) , GLYCYRRHIZAE RADIX ET RHIZOMA (Chinese pinyin: gancao), PINELLIAE RHIZOMA (Chinese pinyin: banxia), and CITRI RETICULATAE PERICARPIUM (Chinese pinyin: chenpin) were the most commonly used Chinese medicines. An in vitro study (Zhong et al., 2018) found that Amygdalin, an active ingredient extracted from ARMENIACAE SEMEN AMARUM could inhibit the expression of interleukin (IL)-17A, IL-23, chemokine (C-C motif) ligand (CCL) 2, and CCL5, and prevent the over activation of NF-κB and p38 MAPK signaling pathways, thereby inhibiting inflammation. An animal study (Lv et al., 2017) suggested that amygdalin inhibited the inflammatory response by reducing the expression of pro-inflammatory cytokines, including IL-1β, IL-6, and tumor necrosis factor (TNF)-α.
In addition to decoction, Chinese patent medicine, the modernized forms of traditional Chinese medicine, have also been widely used in the treatment of COVID-19. This study showed that the most commonly used Chinese patent medicines were Lianhua Qingwen granules and Xuebijing. One study showed that Lianhua Qingwen capsule could inhibit the cytokine storm induced by SARS-COV-2 by inhibiting the expression of TNF- α, IL-6, CCL-2/ monocyte chemoattractant protein (MCP)-1, and C-X-C motif chemokine (CXCL)-10/interferon gamma-induced protein (IP)- 10 (Li et al., 2020b). The capsule was also reported to have antiviral activity in vitro and could inhibit replication of SARS-COV-2 in cells. The results of a RCT showed that routine treatment combined with injection of Xuebijing could effectively improve pneumonia, mortality, and the duration of mechanical ventilation in patients with severe pneumonia (Song et al., 2019). An animal experiment found that Xuebijing could down-regulate the expression of inflammatory cytokines such as IL-6, TNF-α, MCP-1, macrophage chemoattractant protein-2 (MIP-2), and IL-10 (Li et al., 2020c). Xuebijing demonstrated a good down-regulating effect on inflammatory reactions. The above studies showed that the Chinese patent medicines, Lianhua Qingwen and Xuebijing, played an important role in the treatment of COVID-19.
Our study showed that treatment with CHM combination could reduce the symptoms of fever, cough, and fatigue in patients with COVID-19, and did not exclude the placebo effect. However, our study also found that combined CHM treatment could improve CT findings as well as objective outcomes of RT-PCR negativity rate. We are therefore confident of the results of our study. Although our study showed no difference in the length of hospital stay and AE in COVID-19 patients between the CHM combination and control groups, the findings indirectly suggested that the added effect of CHM was the improvement of symptoms of COVID-19.
Subgroup analyses found that both RCTs and case-control studies showed better results in the combined CHM group than the control group. Comorbidities did not influence the effectiveness of combined CHM treatment of patients with COVID-19. At the same time, the combination therapy did not seem to have different effects depending on the severity of COVID-19. However, given the delayed efficacy of CHM, it may not be possible to treat patients with severe disease. This could be considered a limitation of Chinese medicine in the treatment of COVID-19.
The findings of our study are consistent with previously published systematic reviews and meta-analyses. Wu et al. found that integrated treatment with traditional Chinese and western medicines could reduce the conversion rate of severe cases, improve the clinical cure rate, along with certain advantages in relieving cough and fever in patients with COVID-19 (Wu et al., 2020). However, only eight studies were included in the aforementioned review, including one RCT, one before and after trial, two case series studies, and four retrospective studies. Luo et al. suggested that a Chinese herbal formula could be an alternative approach for prevention of COVID-19 in high-risk populations (Luo et al., 2020); however, the conclusions of the study were based on indirect evidence (from SARS and H1N1). Li et al. conducted a systematic review and meta-analysis on CHM for COVID-19 (Li et al., 2020d); however, included only RCTs and quasi-RCTs. Moreover, the primary and secondary outcomes were not similar to our study.
We examined the quality of evidence of the included studies using GRADE approach. Meanwhile, we conducted a systematic and comprehensive literature search and supplemented with the grey literature. However, this study may have the following limitations. First, the low quality of the studies included in this review, especially RCTs, may have affected our results; however, due to the gravity of the COVID-19 outbreak, low-quality clinical trials should be considered. Second, since the authors of the study speak only Chinese and English, literature in other languages were not included, causing a potential language bias. Third, due to the heterogeneity of standard-of-care for COVID-19 in each study was large, potential bias may arise. Lastly, as the COVID-19 epidemic has not yet fully subsided and more large-sample clinical studies are ongoing, evidence from the current analysis is incomplete, and further updates are expected to complement the results of this systematic review after full control of the epidemic (Norris, 2018).
In conclusion, the combined treatment of COVID-19 with Chinese and Western medicines may be effective in controlling symptoms and reducing the rate of disease progression. However, the safety of this combined approach remains unknown and large sample, high quality multicenter randomized controlled trials must be conducted in the future.
Authors’ contributions
L.X.F. conducted data-analysis and drafted the manuscript; N.X.J. designed the study, interpreted the results, and drafted the manuscript; L.J.H. and Z.Y.D. searched the literature, collected the data and assessed the methodological quality of included studies; W.L., H.D.H., L.Y.T., G.J.W. and W.W.X. assisted with the design of PICOs and interpreted the results. C.Y.F. conceived the study and oversought the study implementation; C.Y.L. provided the methodological guidance; L.L. conceived the study, designed the PICOs and interpreted the study results; All authors read and approved the final manuscript.
Funding
Guangdong Provincial Key Laboratory of Research on Emergency in TCM (No. 2017B030314176).
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgement
We thank Mengjuan Ren and Ling Wang, from School of Public Health of Lanzhou University for conducting data extraction. We thank all the authors for their wonderful collaboration.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2020.153282.
Appendix. Supplementary materials
References
- Ang L., Lee H.W., Choi J.Y., Zhang J., Soo L.M. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr Med Res. 2020;9 doi: 10.1016/j.imr.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D.Z., Wang W.J., Li Y., Wu X.D., Zhou B., Song Q.Y. Efficacy analysis on 51 cases of COVID-19 treated with Traditional Chinese Medicine Lianhua Qingwen: a multi-center retrospective study. Tianjin J Tradit Chin Med. 2020:1–6. [Google Scholar]
- Ding X.J., Zhang Y., He D.C., Zhang M.Y., Tan Y.J., Yu A.R., Yu Q., Wu W., Yang W.C., Huang H.S., Liu L. Clinical Effect and Mechanism of Qingfei Touxie Fuzheng Recipe in the Treatment of Novel Coronavirus Pneumonia. Her Med. 2020:1–10. [Google Scholar]
- Du H.Z., Hou X.Y., Miao Y.H., Huang B.S., Liu D.H. Traditional Chinese Medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP) Chin J Nat Med. 2020;18:206–210. doi: 10.1016/S1875-5364(20)30022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C., Xia W.G., Zhen C.J., Sun G.B., Li Z.L., Li Q.L., Li P., Zhang H.L., Yang F.W., Zhang B.L., Liu Q.Q. Clinical observation of Jinhua Qinggan Granules in the Treatment of Novel Coronavirus Pneumonia. J Tradit Chin Med. 2020:1–5. [Google Scholar]
- Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., Li P., Zhou Y., Lin Y.F., Duan Q., Luo G., Fan S., Lu Y., Feng A., Zhan Y., Liang B., Cai W., Zhang L., Du X., Li L., Shu Y., Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X.X., Lin L.P., Tan X.H. Clinical Study on Treatment of Cases of COVID-19 with Toujie Quwen Granules. Chin J Exp Tradit Med Form. 2020:1–6. [Google Scholar]
- Fu X.X., Lin L.P., Tan X.H. Clinical study on 37 case of COVID-19 treated with integrated traditional Chinese and Western Medicine. Tradit Chin Drug Res Clin Pharm. 2020:1–9. [Google Scholar]
- Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H.J., GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., Cochrane Bias Methods Group; Cochrane Statistical Methods Group The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. John Wiley & Sons; ChichesterUK: 2019. www.training.cochrane.org/handbook [Google Scholar]
- Ho L.T.F., Chan K.K.H., Chung V.C.H., Leung T.H. Highlights of traditional Chinese medicine frontline expert advice in the China national guideline for COVID-19. Eur J Integr Med. 2020 doi: 10.1016/j.eujim.2020.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Zhao Y., Zuo X.H., Jin J.S., Guo Y. Treatment of COVID-19 by Pneumonia No.1 Prescription and Pneumonia No.2 Prescription. Acta Chin Med. 2020:1–11. [Google Scholar]
- Li K.Y., An W., Xia F., Chen M., Yang P., Liao Y.L., Xu X., Zhou Q., Fang S.S., Zhang M.W. Observation on clinical effect of modified Qingfeipaidu decoction in treatment of COVID-19. Zhong Cao Yao. 2020;51:2046–2049. [Google Scholar]
- Li R.F., Hou Y.L., Huang J.C., Pan W.Q., Ma Q.H., Shi Y.X., Li C.F., Zhao J., Jia Z.H., Jiang H.M., Zheng K., Huang S.X., Dai J., Li X.B., Hou X.T., Wang L., Zhong N.S., Yang Z.F. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Qian Y., Miao Z., Zheng P., Shi T., Jiang X., Pan L., Qian F., Yang G., An H., Zheng Y. Xuebijing Injection Alleviates Pam3CSK4-Induced Inflammatory Response and Protects Mice From Sepsis Caused by Methicillin-Resistant Staphylococcus aureus. Front. Pharmacol. 2020;11:104. doi: 10.3389/fphar.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu X., Guo L., Li J., Zhong D., Zhang Y., Clarke M., Jin R. Traditional Chinese herbal medicine for treating novel coronavirus (COVID-19) pneumonia: protocol for a systematic review and meta-analysis. Syst Rev. 2020;9:75. doi: 10.1186/s13643-020-01343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.L., Li X.H., Gou C.Y., Li L., Luo X.L., Zhang C., Zhang Y., Zhang J.Y., Jin A.H., Li H.Y., Zeng Y., Li T.Z., Wang X.J. Clinical Observation and evaluation of Jinhua Qinggan Granules in the Treatment of Novel Coronavirus Pneumonia. J Tradit Chin Med. 2020:1–16. doi: 10.19852/j.cnki.jtcm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., Liu J.P. Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Chin J Integr Med. 2020;26:243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, X.F., Ni, X.J., Lin, J.H., Zhang, Y.D.. 2020a. The add-on effect of Chinese herbal medicine on COVID-19: a systematic review and meta-analysis. INPLASY202040190. https://inplasy.com/inplasy-2020-4-0190/. [DOI] [PMC free article] [PubMed]
- Lv J., Xiong W., Lei T., Wang H., Sun M., Hao E., Wang Z., Huang X., Deng S., Deng J., Wang Y. Amygdalin ameliorates the progression of atherosclerosis in LDLreceptor deficientmice. Mol Med Rep. 2017;16:8171–8179. doi: 10.3892/mmr.2017.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv R.B., Wang W.J., Li X. Clinical observation on 63 suspected cases of COVID-19 treated with Traditional Chinese Medicine Lianhua Qingwen. J Tradit Chin Med. 2020:1–5. [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHC . NHC; 2020. Notice on the issuance of the new 7th version of the COVID-19 Diagnosis and Treatment Guideline.http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf [Google Scholar]
- NHC . NHC; 2020. Notice on the issuance of the new 3rd version of the COVID-19 Diagnosis and Treatment Guideline.http://www.nhc.gov.cn/yzygj/s7653p/202001/f492c9153ea9437bb587ce2ffcbee1fa/files/39e7578d85964dbe81117736dd789d8f.pdf [Google Scholar]
- Norris S.L. Meeting public health needs in emergencies – World Health Organization guidelines. J. Evid. Based. Med. 2018;11:133–135. doi: 10.1111/jebm.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X.K., Hao S.L., Ma J.H., Wei G.Y., Song K.Y., Tang C., Gao Y.F., Liang S.Q., Du W.J. Observation on the clinical effect of Shufeng Jiedu Capsule combined with Arbidol Hydrochloride Capsules in the treatment of COVID-19. Zhong Cao Yao. 2020;51:1167–1170. [Google Scholar]
- Ren Y., Yao M.C., Huo X.Q., Gu Y., Zhu W.X., Qiao Y.J., Zhang Y.L. Study on treatment of "cytokine storm" by anti-2019-nCoV prescriptions based on arachidonic acid metabolic pathway. Zhongguo Zhong Yao Za Zhi. 2020;45:1225. doi: 10.19540/j.cnki.cjcmm.20200224.405. 123. [DOI] [PubMed] [Google Scholar]
- She J., Jiang J., Ye L., Hu L., Bai C., Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin Transl Med. 2020;9:19. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Yang Z.G., Ye C., Chen S.S., Lu Y.F., Lv Y., Xu Q.N., Tang B.Z., Yin K.S., Chen X.R. Clinical observation on 49 cases of non-critical coronavirus disease 2019 in Shanghai treated by integrated traditional Chinese and western medicine. Shanghai J Tradit Chin Med. 2020;54:30–35. [Google Scholar]
- Song Y., Yao C., Yao Y., Han H., Zhao X., Yu K., Liu L., Xu Y., Liu Z., Zhou Q., Wang Y., Ma Z., Zheng Y., Wu D., Tang Z., Zhang M., Pan S., Chai Y., Song Y., Zhang J., Pan L., Liu Y., Yu H., Yu X., Zhang H., Wang X., Du Z., Wan X., Tang Y., Tian Y., Zhu Y., Wang H., Yan X., Liu Z., Zhang B., Zhong N., Shang H., Bai C. XueBiJing Injection Versus Placebo for Critically Ill Patients With Severe Community-Acquired Pneumonia: A Randomized Controlled Trial. Crit Care Med. 2019;47:e735–e742. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.L., Yang X.D., Liu Y.P., Zhang J., Feng Y.F., Shang L., Zhang K.W., Cheng Z.J., He X.J. Preliminary clinical effect analysis of the treatment of novel coronavirus pneumonia by internal administration of traditional Chinese medicine plus fumigation and absorption combined with super dose of vitamin C in treating NOVID-19. J Xi'an Jiaotong Univ Med Sci. 2020:1–7. [Google Scholar]
- Wells, G.A., Shea, B., O'Connell, D., Peterson, J., Welch, V., Losos, M., Tugwell, P., 2003. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- WHO . WHO; 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [Google Scholar]
- WHO . WHO; 2020. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)https://www.who.int/news-room/detail/23-01-2020-statement-on-the-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- WHO . WHO; 2020. WHO Director-General's opening remarks at the media briefing on COVID-19.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 [Google Scholar]
- WHO . WHO; 2020. Coronavirus disease (COVID-2019) situation reports-86.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200415-sitrep-86-covid-19.pdf?sfvrsn=c615ea20_6 [Google Scholar]
- Wu Y.Q., Zou L., Yu X., Sun D., Li S.B., Tang L., Yang J.R., Chen X.Y., Wu Y.G., Fang H. Clinical effects of integrated traditional Chinese and western medicine on COVID-19: a systematic review. Shanghai J Tradit Chin Med. 2020:1–8. [Google Scholar]
- Xia W.G., An C.Q., Zhen C.J., Zhang J.X., Huang M., Wang Y., Yang F.W., Duan C., Li Z.L., Liu Q.Q., Zhang B.L. Clinical study on 34 case of COVID-19 treated with integrated traditional Chinese and Western Medicine. J Tradit Chin Med. 2020;61:375–382. [Google Scholar]
- Xiao Q., Jiang Y.J., Wu S.S., Wang Y., An J., Xu W.P., Wu J.J. Value analysis of Shufeng Jiedu Capsule combined with Arbidol Hydrochloride Capsules in the treatment of mild cases of COVID-19. J Emerg Tradit Chin Med. 2020:1–3. [Google Scholar]
- Yang M.B., Dang S.S., Huang S., Li Y.J., Guo Y.L. Multi-center Clinical Observation of Reyanning Mixture in Treatment of Novel Coronavirus Pneumonia. Chin J Exp Tradit Med Form. 2020:1–6. [Google Scholar]
- Yang Q., Sun Q.G., Jiang B., Xu H.J., Luo M., Xie P., Huang W., Cong Z.W. Retrospective clinical study on treatment of COVID-2019 patients with integrated traditional Chinese and Western medicine. Zhong Cao Yao. 2020;51:2050–2054. [Google Scholar]
- Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int J Biol Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K.T., Liu M.Y., Li X., Huang J.H., Cai H.B. Retrospective Clinical Analysis on Treatment of Novel Coronavirus-infected Pneumonia with Traditional Chinese Medicine Lianhua Qingwen. Chin J Exp Tradit Med Form. 2020:1–7. [Google Scholar]
- Zhang C.T., Yang Y., You F.M., Huang Q.S., Gao P.Y., Tang J.Y., Xie C.G., Xiao W., Sun Z.T., Zhang H., Qian B. Clinical Study on COVID-19 from the Perspective of "Yidujiashi" Theory. Pharm Clin Chin Materia Medica. 2020:1–8. [Google Scholar]
- Zhang C.Y., Zhang S., Wang W., Zhang X.Q. Clinical observation of Xuebijing in the treatment of COVID-19. Chin Hosp Pharm J. 2020:1–5. [Google Scholar]
- Zhang Y.S., Cong W.H., Zhang J.J., Guo F.F., Li H.M. Research progress of intervention of Chinese herbal medicine and its active components on human coronavirus. Zhongguo Zhong Yao Za Zhi. 2020;45:1263–1271. doi: 10.19540/j.cnki.cjcmm.20200219.501. [DOI] [PubMed] [Google Scholar]
- Zhong X.Q., Li L., Lu C.J., Lu Y., Wei J.A., Han L. Anti-inflammation Effect of Amygdalin on Macrophage 264.7 Cells Stimulated by Lipopolysaccharide. Tradit Chin Drug Res Clin Pharm. 2018;29:257–263. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.