Abstract
Coronavirus disease 2019 (COVID-19) has rapidly spread around the world causing global public health emergency. In the last twenty years, we have witnessed several viral epidemics such as severe acute respiratory syndrome coronavirus (SARS-CoV), Influenza A virus subtype H1N1 and most recently Middle East respiratory syndrome coronavirus (MERS-CoV). There were tremendous efforts endeavoured globally by scientists to combat these viral diseases and now for SARS-CoV-2. Several drugs such as chloroquine, arbidol, remdesivir, favipiravir and dexamethasone are adopted for use against COVID-19 and currently clinical studies are underway to test their safety and efficacy for treating COVID-19 patients. As per World Health Organization reports, so far more than 16 million people are affected by COVID-19 with a recovery of close to 10 million and deaths at 600,000 globally.
SARS-CoV-2 infection is reported to cause extensive pulmonary damages in affected people. Given the large number of recoveries, it is important to follow-up the recovered patients for apparent lung function abnormalities. In this review, we discuss our understanding about the development of long-term pulmonary abnormalities such as lung fibrosis observed in patients recovered from coronavirus infections (SARS-CoV and MERS-CoV) and probable epigenetic therapeutic strategy to prevent the development of similar pulmonary abnormalities in SARS-CoV-2 recovered patients. In this regard, we address the use of U.S. Food and Drug Administration (FDA) approved histone deacetylase (HDAC) inhibitors therapy to manage pulmonary fibrosis and their underlying molecular mechanisms in managing the pathologic processes in COVID-19 recovered patients.
Keywords: COVID-19, SARS-CoV-2, Pulmonary fibrosis, TGF-β, HDAC inhibitors, Epigenetics
Graphical abstract
1. Background
The latest Coronavirus disease 2019 (COVID-19) situation report from World Health Organization (WHO) indicates that the disease has spread to 213 countries with 16,558,289 confirmed cases and 656,093 deaths as on 29 July 2020. However, it continues to spread rapidly worldwide. COVID-19 is caused by a recently discovered severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This new virus shares its similarity in terms of its genetic makeup and clinical manifestations closely to that of another coronavirus SARS-CoV, which is identified to be responsible for 2013 respiratory illness outbreak [1]. COVID-19 clinical presentations if not managed properly leads to acute respiratory failure and mortality. Further, it has been observed that the older people and people with existing medical concerns such as pneumonia, cardiovascular disease, diabetes and cancer with weakened immune system are at greater risk [2]. In addition to ‘solidarity’ clinical trials for COVID-19 treatments coordinated by the WHO, a large number of country-specific clinical trials are underway to assess the effectiveness of various pre-existing drugs to manage novel coronavirus infection.
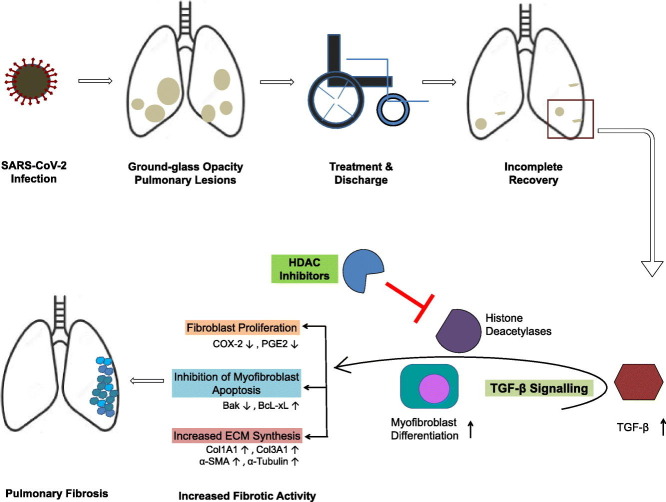
Though currently much of the efforts have been on to help control the coronavirus spread and to identify effective treatment options, data on the post recovery scenario of patients with COVID-19 and apparent development of secondary complications/ailments due to the infection are highly limited and warrant a detailed follow-up study. At present, the worldwide recovery number is at around 10 million, a large number of people who are at risk of development of secondary pathogenesis as a result of damaged lungs and/or incomplete recovery state. In this regard, considerable attempts are being made to understand the pathogenesis of SARS-CoV-2 infection and manifestations caused in various organs. Though the infection affects many vital organs, lung remains the primary and chief organ of target. Some of the characteristic presentations in the lung include bilateral and peripheral ground-glass and consolidative pulmonary opacities [3], unilateral and bilateral pneumonia, moderate to extensive lung lesions [4], thickening of bronchovascular bundles, pulmonary oedema, acute respiratory distress syndrome (ARDS), and pulmonary fibrosis [[5], [6], [7]]. A chest computed tomography (CT) scan has been employed by many studies to describe the characteristic clinical presentations in lung and to assess the severity of lung damage in both adult and aging populations affected due to SARS-CoV-2 infection [[8], [9], [10], [11], [12]]. CT manifestations of COVID-19 include ground glass opacity, consolidation, reticular pattern, crazy paving pattern, gelatinous mucus bronchogram, airway changes such as bronchiectasis and bronchial wall thickening, pleural thickening and effusion, thin curvilinear opacity, occurrence of fibrous stripes, pulmonary vessels dilatation within and around the lesions and solid irregular nodules or nodules with halo sign. Furthermore, manifestations such as lymphadenopathy, pericardial effusion and reversed halo sign are considered to be major risk factors in severe COVID-19 cases [13]. Children are equally susceptible to SARS-CoV-2 infection but Chest CT observations shown to deviate and present atypical characteristics from that of adults [14]. In addition, there is a large population of lung transplant recipients who are at higher risk and the effect of SARS-CoV-2 infection could be detrimental [15]. It is important to note that the clinical manifestations and progression of pulmonary dysfunction in COVID-19 are quite diverse and rapid. Increase in the ground glass density patches and fibrous stripes was observed in a span of 3–14 days on a follow-up Chest CT, implying early CT imaging, including a follow-up imaging could help us track the disease severity with greater clarity [16].
A significant portion of patients recovered from COVID-19 could develop lung fibrosis and therefore a need for rehabilitation and pulmonary rejuvenation. Interestingly, histone deacetylase (HDAC) inhibitors are reported to show promising anti-fibrotic effects mainly through suppressing transforming growth factor beta 1 (TGF-β1) signalling. In this review, we discuss our understanding about the long-term pulmonary abnormalities observed in patients recovered from coronavirus infections (SARS-CoV, MERS-CoV and SARS-CoV-2). Further, we address the U.S. Food and Drug Administration (FDA) approved HDAC inhibitors therapy to manage pulmonary fibrosis and their underlying molecular mechanisms in managing the pathologic processes.
2. Pulmonary abnormalities in patients recovered from SARS-CoV and MERS-CoV infections
As the novel SARS-CoV-2 is genetically similar to previously discovered strains of coronavirus such as SARS-CoV and MERS-CoV, it is important to consider available data on follow-up studies of these previous infections (Table 1 ). At present, for COVID-19 recovered patients, after discharge follow-up data in terms of physiological measurements or imaging to assess the possible secondary damages are unavailable. Considering the fact that the clinical presentations in lungs of SARS-CoV-2 infected people mirror as that of SARS and MERS, it is highly likely that the effect in COVID-19 recovered patients would be on similar lines to that of SARS and MERS.
Table 1.
Overview of long-term pulmonary outcomes of SARS and MERS survivors.
| No. | No. of patients | Age range (years) | Follow-up time period (after first diagnosis) | Computed tomography (CT) findings (% patients) | References |
|---|---|---|---|---|---|
| Severe Acute Respiratory Syndrome (SARS) | |||||
| 1 | 22 | 24–72 | 4 weeks | Irregular linear opacities with or without associated ground-glass opacities (55%) | Gaik C. Ooi 2004 [84] |
| 2 | 24 | 23–70 | 36.5 days | Ground-glass opacity and interstitial thickening (38%), fibrosis (62%) | Gregory E Antonio 2003 [20] |
| 3 | 19 | 22–65 | 25–38 days | Ground-glass opacity (36.8%), fibrosis (63%) | Hsian-He Hsu 2004 [85] |
| 4 | 40 | 42.8 (mean) | 51.8 days ±20.2 | Ground-glass opacity (90%), reticulation (70%), parenchymal band (55%), air trapping (92%), bronchiectasis (18%) | Yeun-Chung Chang 2005 [86] |
| 5 | 100 | – | 2 months | Ground-glass opacity (47%), reticular opacity (29%) | Zheng-yu Jin 2003 [87] |
| 6 | 56 | 21–70 | 3 months | Ground-glass opacity (92.9%), reticulation (87.5%), irregular interlobular septal thickening (89.3%), parenchymal band (50%) | Ka-tak Wong 2004 [21] |
| 7 | 20 | 42.8 (mean) | 140.7 days ±26.7 | Ground-glass opacity (70%), reticulation (50%), parenchymal band (60%), air trapping (92%), bronchiectasis (10%) | Yeun-Chung Chang 2005 [86] |
| 8 | 44 | 21–70 | 6 months | Ground-glass opacity (86.4%), reticulation (92.1%), irregular interlobular septal thickening (92.1%), parenchymal band (43.2%) | Ka-tak Wong 2004 [21] |
| 9 | 57 | 38.1 (mean) | 6 months | Pulmonary abnormalities of varying degree (75.4%) | C K Ng 2004 [88] |
| 10 | 47 | 9.9–16.0 | 6 months | Ground-glass opacity (17%), air trapping (23.4%), fibrosis (6.4%) | A M Li 2004 [89] |
| 11 | 47 | 1.5–17 | 6 months | Ground-glass opacity (14.9%), parenchymal scars (12.8%), air trapping (23.4%) | Winnie C. W. Chu 2005 [24] |
| 12 | 47 | 1.5–17 | 12 months | Ground glass opacity (8.5%), parenchymal scars (17%), air trapping (19%), reticular opacities (6.4%) | Winnie C. W. Chu 2005 [24] |
| 13 | 311 | 38.2 (mean) | 12 months | Lung diffusion abnormalities (27.3%), lung fibrosis (23%) | Lixin Xie 2005 [90] |
| 14 | 8 | 33–73 | Late stage ARDS | Ground-glass opacity (100%), interstitial thickening (100%), fibrosis (37.5%) | Gavin M Joynt 2004 [91] |
| Middle East Respiratory Syndrome (MERS) | |||||
| 1 | 36 | 21–73 | 43 days (median) | Lung fibrosis (33%), ground-glass opacity (5.5%), pleural thickening (5.5%) | Karuna M Das 2017 [25] |
Jiang Gu et al. discussed the possible stage-specific pathogenic changes leading to pulmonary dysfunction upon SARS-CoV infection. It has been stated that pulmonary sequelae of the SARS-CoV infection begins with the onset of edema followed by damage to alveolar epithelia and formation of fibrotic scar. As the disease progress, interstitial and airspace fibrosis and pneumocytic hyperplasia occur. Furthermore, prominent and extensive fibrosis observed in severe SARS [17]. Reports on long-term CT and follow-up studies of SARS recovered patients are quite sparse. In a follow-up study of 71 patients recovered from SARS infection, 4.6% of them showed interstitial abnormality in lungs at around 15 years [18]. As the severity of the infection increased, development of pulmonary fibrosis was observed during SARS infection. Fibrosis progressed rapidly in patients with severe SARS aided by pre-existing medical illness, and required ventilation support along with extended stay in hospital. In this study, patients (mean age-71 years) reported pre-existing cardiovascular disease, chronic obstructive airway disease and chronic liver disease at the time of SARS diagnosis. The duration of the illness was up to 20 days and patients were on ventilation support for an average of 10 days [19]. Most importantly, in another follow-up study, 62% of SARS recovered patients showed development of pulmonary fibrosis. Patients (mean age-39 years) with evidence of fibrosis were hospitalized on an average 22.3 days versus 16.4 days for those with no evidence of fibrosis at follow-up. Other factors such as percent intensive care admission (26.6% vs 11.1%), peak radiographic opacification (13.6% vs 10.8%) and number of abnormal segments on CT scans (10.8 vs 4.7) were higher in fibrosis group when compared with non-fibrosis group [20]. In yet another 15-year follow-up study, which included 78 medical staff infected with SARS, the percentage of pulmonary lesions was noticed to be decreasing but then stabilised and remained for the rest of the period. By the end of the 15-year period, patients who had early diagnosis and treatment showed better recovery from lung damage and superior pulmonary function than patients with late diagnosis, thereby indicating that the improvement in pulmonary function is possible when managed effectively during early phase of infection [18]. Attempts were also made to correlate the chest CT findings with lung function tests and other clinical parameters during the follow-up care (at 3 and 6 months) of patients (mean age-39.4 years) who recovered from severe SARS [21].
An available CT based 84 months data revealed characteristic abnormalities indicating lung fibrosis [22] and these CT presentations observed were very similar to that of CT findings that one would normally see during early days of infection [23]. This could imply that the pulmonary fibrosis could become a persistent challenge in COVID-19 recovered patients and need to be addressed, preferably, at the early stages thereby giving a chance for the lung to heal better and lessen the damage. With reference to the infection in children, the pulmonary abnormalities were persistent in 32% of children even after 12 months since diagnosis [24]. In an another follow-up study of 36 adult patients recovered from MERS, 33% indicated lung fibrosis within a range of 32–320 days post discharge [25].
3. Pathogenesis of lung injury and extracellular matrix development followed by SARS-CoV-2 infection
Though the occurrence of SARS-CoV-2-mediated acute lung injury is becoming increasingly evident, our understanding of the underlying mechanisms of pathogenesis appears to be limited and obscure. The epithelial cells of the nasal cavity represent the primary gateway of SARS-CoV-2 that facilitates the spread of the infection in the respiratory tract. Angiotensin-converting enzyme 2 (ACE2) receptors on the surface of the pulmonary epithelium ensures the entry of SARS-CoV-2 to the upper respiratory region. As the virus migrates to the lower respiratory tract, it infects alveolar type II cells of the lungs [26,27] thereby resulting in the development of edema, degeneration of alveolar epithelial lining, followed by the emergence of hyaline membranes at the damaged alveolar space leading to impaired gas exchange [28]. As the disease progress, a range of host inflammatory responses such as excessive cytokines production and an enhanced influx of inflammatory cells were observed at the site of damage; ultimately resulting in severe lung tissue scarring and fibrosis due to collagen deposition [29,30]. A study by Ling Leng et al. on COVID-19 clinical samples revealed extensive remodelling of the lung extracellular matrix (ECM) in SARS-CoV-2-infected patients. Proteins that have a key role in manifestation of pulmonary fibrosis were identified to be dysregulated. For example, MMP2, MMP8 and cathepsin were found to be upregulated and E-cadherin was downregulated. In addition, the core ECM components of lung basement membrane such as laminins, collagen VI, annexin A2 and fibronectin were highly downregulated [31].
4. Pulmonary fibrosis and COVID-19
The development of scars and its gradual advancement as a result of lung tissue damage leads to fibrotic disease of the lung called pulmonary fibrosis. Further, pulmonary fibrosis is characterized by redox imbalance, alveolar inflammation, inflammatory injury to the alveolar epithelium, dysregulated epithelial-to-mesenchymal transition (EMT), aberrant fibroblast proliferation and differentiation, and excessive accumulation of ECM molecules in the lung parenchyma. All these events lead to poor lung function and eventually respiratory failure [32,33]. Hence, tissue fibrosis is a major contributing cause for morbidity and mortality worldwide.
Fibrotic changes may result from infectious or non-infectious agents. Myofibroblasts are the primary cell type responsible for the fibrotic tissue, which possess enhanced fibrotic, contractile and migratory activities [34]. Following tissue injury, myofibroblasts can be derived from a large variety of cell types including epithelial cells, endothelial cells, and fibroblasts. These cells can promote collagen deposition in the ECM. Unlike in normal wound healing process, the myofibroblast formation coupled with ECM production has been implicated in pulmonary fibrogenesis [35]. Further, these myofibroblasts in turn secrete several mediators, including TGF-β1 to further enhance the fibrotic process. In addition, an imbalance in the expression of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs), which are required for inducing apoptosis in damaged cells and tissue remodelling, is also a major contributing factor for development of pulmonary fibrosis [36].
With the available data so far with regard to COVID-19, it is apparent that pulmonary fibrosis plays an important pathological role in patients recovered from SARS-CoV-2 infection [37]. It has also been discussed that fibrosis being the cause for COVID-19 mortality and suggested for the use of anti-fibrotic agents to treat COVID-19 recovered patients [38]. In one study, about 41% of SARS-CoV-2 infected people developed ARDS and further the risk of progression from ARDS to mortality was increased significantly with patients of advanced age [39]. Given the fact that pulmonary fibrosis is a recognised outcome of ARDS and could cause severe distress long term, it's equally important to explore treatment strategies to address this in COVID-19 recovered patients.
Although it is too early to expect expansive data with regard to follow-up studies of COVID-19 survivors, a latest study from Wuhan has made an attempt to understand pulmonary fibrosis development by following up with CT imaging of COVID-19 recovered patients. The study showed that fibrosis was a likely occurrence, especially with increased severity of the infection. Interestingly, the presence of high inflammatory molecules was observed in these patients [40].
5. Risk factors for pulmonary fibrosis in COVID-19 survivors
The potential risk factors for idiopathic pulmonary fibrosis (IPF) in COVID-19 survivors include advanced age with reduced respiratory capacity, pre-existing comorbidities such as hypertension, diabetes, obesity, cardiovascular disease and ARDS. In addition, COVID-19 severity, prolonged hospitalization and extended ventilation support also increase the risk of development of IPF [37,[41], [42], [43]]. At molecular level, factors that play a role in pulmonary fibrosis development are the extent of reticulation [37] and increased expression of angiotensin converting enzyme 2 (ACE2) in high-risk groups [43]. Notably, patients receiving anti-IL-6 therapy for severe COVID-19 could also be at risk of pulmonary fibrosis, as IL-6 has been shown to be involved in the fibrotic events of the lung [44]. IL-1 is the other regulatory molecule for fibrotic response in pulmonary fibrosis whose increased secretion has been observed in COVID-19 patients [37].
6. TGF-β signalling in pulmonary fibrosis
TGF-β belongs to a family of cytokines and is involved in wide range of cellular functions including, but not limited to, cell growth, proliferation, differentiation and death. TGF-β signalling pathway is known to have central role in pathogenesis of fibrosis by promoting EMT, fibroblast proliferation and differentiation [[45], [46], [47], [48]]. Upon binding of TGF-β1 to its own receptor kinases TGF-β receptor 1 (TGβI) and TGF-β receptor 2 (TGβII), autophosphorylated TGβII phosphorylates TGβI, which subsequently phosphorylates Smad2 and Smad3 and mediates the effect. Of various inflammatory cytokines, interleukin-13 (IL-13) has been shown to have a regulatory role in activation of TGF-β and in turn induction of tissue fibrosis [45]. TGF-β1 has been reported to induce lung fibrosis by activating Smad-dependent and Smad-independent pathways. Through Smad signalling pathways, TGF-β1 has been reported to directly increase the transcription of ECM genes, mainly collagens that favour ECM deposition [[49], [50], [51]]. In addition, MAP Kinases, namely, ERK1/2, JNK and p38 kinase are also involved in TGF-β1-mediated fibrotic response. Interestingly, TGF-β has been shown to dysregulate EGFR signalling by modulating EGFR ligands, which in turn aide in development of fibrosis [52].
Further, evidence of cross talk between Smad and Ras/MEK/ERK cascade influenced by TGF-β provides much needed clarity in understanding pathogenesis of pulmonary fibrosis [53]. Interaction between Smad3 and nucleocapsid protein of SARS-CoV has been shown to induce fibrotic promoter PAI-1 synthesis [54]. Apart from Smad, other downstream proteins that aide and amplify TGF-β response are connective tissue growth factor (CTGF) and fibronectin. Increased expression of CTGF is observed during the pathogenesis of lung fibrosis [55]. Fibronectin expression influences myofibroblast, a highly contractile collagen-producing cell, which plays a major role during fibrotic process [56]. Both CTGF and fibronectin promotes fibroblast proliferation and ECM production. TGF-β directly regulates the expression of alpha smooth muscle actin (α-SMA) in tissue myofibroblasts thereby aiding in tissue granulation [57]. Other matrix proteins regulated by TGF-β during fibrosis are collagen type I and III, and α-tubulin. Therefore, many studies have demonstrated that utilizing proteins [58,59] or small chemicals [60] or microRNAs (miRNAs) [61,62] to disrupt TGF-β production and/or block the associated signal transduction will be beneficial in treating fibrosis.
7. HDACs in pulmonary fibrosis
Although pirfenidone and nintedanib have been approved by the U.S. FDA for treating patients with idiopathic pulmonary fibrosis (IPF), the results are rather disappointing and with no substantial gains in patient recovery. Nintedanib and pirfenidone are shown to reduce the decline of forced vital capacity (FVC) in patients with pulmonary fibrosis [33] and suppress collagen production in activated fibroblasts [63,64]. However, neither of them provides significant relief from the symptoms, nor improves the quality of life. Further, these regimens have adverse effects within the gastrointestinal tract such as diarrhea, nausea and vomiting [64,65]. In addition, treatment with pirfenidone and nintedanib may be associated with photoallergic dermatitis and cardiovascular risk, respectively. Interestingly, pirfenidone, known for its anti-inflammatory property, is being evaluated for use in patients with severe SARS-CoV-2 infection based on the premise that inflammatory cytokine storm and ARDS are the leading cause of mortality in patients with severe COVID-19 [66]. When the treatment strategies fail, the only effective option for these patients is lung transplantation, but comes with its own risks such as organ rejection, secondary complications and being expensive [67]. Due to a possible/likely overflow of patients from pulmonary fibrosis complications as a result of COVID-19 infection is expected, alternative treatment strategies are thereby warranted.
HDACs are known for their role in regulating the transcriptional activity of targeted genes. They act by deacetylation of amino terminal lysine residues of histone and non-histone proteins. HDACs have an all-important role in cellular homeostasis and other fundamental cellular processes such as differentiation, progression and apoptosis. Dysregulation of HDACs activity has been reported in wide range of diseases and therefore an attractive choice for treatment against various diseases including tissue fibrosis and inflammatory diseases [68]. HDACs are reported to play a prime role in regulating intermediates of TGF-β signalling pathway. Increased expression of HDACs has been shown to prompt/trigger fibroblast differentiation to myofibroblasts and in turn increased ECM formation [69,70]. Altered expression of HDACs in the fibroblastic foci of IPF lung has also been observed [71]. Fibroblasts to myofibroblasts differentiation generally mediated by TGF-β1 require HDAC4 [69]. Genes involved in regulation of ECM mediated by TGF-β, particularly through ERK/PI3K pathway have been shown to be controlled by HDACs [72]. HDAC7 found to be involved in repression of key genes required for TGF-β-mediated activation of fibroblasts [73]. Differentiation of myofibroblasts from normal fibroblasts in lung is HDAC4 and Akt-dependent process [70]. Aberrant expression of HDAC6 was observed both in myofibroblasts and type-II alveolar epithelial cells (AECII) in sporadic IPF [71]. Similarly, HDAC8 is also reported to be involved in fibroblast-myofibroblast differentiation during the pathogenesis of IPF [74]. The aforementioned studies clearly indicate that HDACs are capable of epigenetic regulation of TGF-β-mediated gene expression resulting in pulmonary fibrosis. Consequently, exploring the possibility of treating pulmonary fibrosis with HDAC inhibitors is worthwhile.
HDAC inhibitors are quite versatile as they are shown to mitigate myocardial ischemia, atherosclerosis, Alzheimer's disease, including fibrotic diseases (pulmonary fibrosis, renal fibrosis and hepatic cirrhosis) [75]. As outlined in Table 2 , substantial studies are carried out to understand the usefulness of HDAC inhibitors in targeting pulmonary fibrosis particularly modulated by TGF-β-mediated pathways. HDAC inhibitors are shown to suppress TGFβ1-induced profibrotic proteins such as α-SMA, type 1 collagen, fibronectin, CTGF, PAI-1 and CCN1 [74]; likely through epigenetic mechanism/alterations (Table 2). Moreover, effective inhibitions of COX-2 and Fas, and apoptosis resistance in IPF are shown to be mediated by HDACs through epigenetic alterations [76,77]. HDAC inhibitor tubastatin was successful in ameliorating fibrosis in murine bleomycin-induced pulmonary fibrosis model, particularly triggered by TGF-β [78]. In fact, a study comparing the performance of pirfenidone and panobinostat has demonstrated the superior functionality of HDAC inhibitor over pirfenidone in acting against IPF-derived fibroblasts [79].
Table 2.
HDAC inhibitors for pulmonary fibrosis.
| HDAC inhibitors | Target cell type | Regulators/molecules involved | Mechanism of action | Type of fibrosis | References |
|---|---|---|---|---|---|
| Vorinostat and panobinostat | Primary human IPF fibroblasts | COX-2, PGE2, TGF-β1, IL-1 β | Restores COX-2 gene expression repressed by HDAC corepressors. This in turn restores PGE2 expression required for inhibiting fibroblast proliferation | IPF | William R. Coward (2009) [76] |
| Vorinostat | IPF fibroblasts (ILF LL29) | TGF- β 1, α-SMA, MMP1, α-tubulin | Prevents TGF-β-mediated fibroblasts differentiation into myofibroblasts | IPF | Z Wang (2009) [92] |
| Vorinostat | Murine bleomycin-induced pulmonary fibroblasts | Bak, Bcl-xL | Induces apoptosis of myofibroblasts by upregulation of Bak and downregulation of Bcl-xL | MLF | Yan Y Sanders (2014) [93] |
| Vorinostat | Fibroblasts from non-fibrotic lung | TGF- β 1, COX-2, TIA-1 | Upregulates COX-2 expression in TGF-β1-activated fibroblasts and help reduce collagen deposition in fibrosis. COX-2 is an antifibrotic gene | – | Alice Pasini (2018) [94] |
| Vorinostat | Primary human IPF fibroblasts | COL3A1 | Decreases collagen production by repressing type 3 collagen gene expression in fibroblasts | IPF | Xiangyu Zhang (2013) [95] |
| Romidepsin | Primary parenchymal lung fibroblasts & bleomycin-induced murine pulmonary fibroblasts | LOX, CDKN1A, Fn1, COL3A1, COL1A1 | Inhibits fibroblast proliferation and myofibroblast differentiation | IPF | Franco Conforti (2017) [96] |
| Panobinostat | Primary human IPF fibroblasts | pSTAT3, cyclin D1, α-tubulin, Bcl-xL, survivin | Reduces profibrotic phenotypes, induces cell cycle arrest and apoptosis in fibroblasts | IPF | Martina Korfei (2018) [79] |
| Trichostatin A | Human alveolar epithelial cells (A549) (EMT induced) | SFTPC | Attenuates pulmonary fibrosis by restoration of SFTPC gene expression involved in maintaining alveolar integrity and repair | MLF | Chiharu Ota (2015) [97] |
| Trichostatin A | Normal human lung fibroblasts | TGF-β1, α-SMA, collagen I | Blocks Akt-dependent TGF-β1 pathway required for fibroblast-myofibroblast differentiation | – | Weichao Guo (2009) [70] |
| Trichostatin A | Bleomycin-induced rat fibroblasts | HDAC2, p-SMAD2 | Attenuates pulmonary fibrosis | MLF | Qing Ye (2014) [98] |
| Trichostatin A | Primary rat lung fibroblasts | Thy-1, histone acetylation, α-SMA | Restores Thy-1 expression in fibrotic fibroblasts and induces changes in phenotype of the cells | MLF | Yan Y. Sanders (2011) [93] |
| Spiruchostatin A | Primary human IPF fibroblasts | TGF-β1, α-SMA, p21, collagen III | Reduces proliferation of IPF fibroblasts and their biosynthetic activity | IPF | Elizabeth R. Davies (2012) [99] |
| Pracinostat | Primary human IPF fibroblasts | TGF-β1, α-SMA, PGC1α, ACTA2 | Inhibits fibroblasts contractility and ECM deposition | IPF | Dakota L Jones (2019) [73] |
| Panobinostat and valproic acid | Primary human IPF fibroblasts | HDACs, COL1A1,COL3A1, P4HTM | Decreases expression of genes associated with ECM synthesis, proliferation and cell survival | Sporadic IPF | Martina Korfei (2015) [71] |
| Tubastatin | Primary human IPF fibroblasts | TGF-β1/PI3K/Akt, HIF-1α, VEGF, collagen I, HDAC6 | Reduces type-1 collagen gene expression and decreases Akt phosphorylation | IPF | Shigeki Saito (2017) [78] |
IPF, idiopathic pulmonary fibrosis; MLF, murine lung fibrosis; COX-2, cyclooxygenase-2; TGF-β1, transforming growth factor beta 1; IL-1 β, interleukin 1 beta; PGE2, prostaglandin E2; HDAC, histone deacetylase; α-SMA, alpha smooth muscle actin; MMP1, matrix metalloproteinase-1; Bak, Bcl-2 homologous antagonist killer; Bcl-xL, B-cell lymphoma-extra large; TIA-1, T-cell-restricted intracellular antigen 1; COL3A1, collagen type III alpha 1 chain; LOX, lysyl oxidase; CDKN1A, cyclin dependent kinase inhibitor 1A; Fn1, fibronectin-1; COL1A1, collagen type I alpha 1 chain; pSTAT3, phospho-signal transducer and activator of transcription 3; SFTPC, pulmonary surfactant-associated protein C; Akt, protein kinase B; p-SMAD2, phospho- mothers against decapentaplegic homolog 2; Thy-1, cell surface glycoprotein; p21, cyclin-dependent kinase inhibitor; PGC1α, Pparg coactivator 1 alpha; ACTA2, actin alpha 2 smooth muscle; ECM, extracellular matrix; P4HTM, prolyl 4-hydroxylase transmembrane; PI3K, phosphoinositide 3-kinase; HIF-1α, hypoxia-inducible factor 1-alpha; VEGF, vascular endothelial growth factor.
Interestingly, shared similarities have also been discussed in terms of risk factors and epigenetic alterations between IPF and cancer [80]. Furthermore, the use of HDAC inhibitors vorinostat (SAHA), romidepsin, panobinostat and belinostat are approved by U.S. FDA as anti-cancer drugs for select cancer types. And many more are being evaluated at pre-clinical and clinical stages for other malignancies [[81], [82], [83]]. Therefore, the therapeutic potential and clinical application of HDAC inhibitors are well established. As discussed earlier, a number of studies have established a clear link between pulmonary fibrosis and COVID-19 and in some cases attributing COVID-19 mortality to fibrosis. On the other side, research data for efficiently addressing the issue of pulmonary fibrosis in terms of various treatment strategies and the mechanism through which it is affected is available. In terms of understanding the pathogenesis of pulmonary fibrosis, TGF-β seems to assume a central role and shown to modulate range of fibrotic molecules/mediators and thereby influencing the pathogenesis. This could be effectively addressed by targeting TGF-β through epigenetic intervention brought about by HDAC inhibitors. Considering all of the above, HDAC inhibitors treatment could be instrumental in efficiently ameliorating the development of pulmonary fibrosis in COVID-19 recovered patients.
8. Conclusion
The world is under tremendous stress, still struggling to overcome the devastation (suffering and death) caused by SARS-CoV-2 infection. While the dynamics of the disease is being unravelled rapidly and on priority, the data regarding some of the key aspects of COVID-19 recovered patients is limited. Even though number of infection continues to rise around the world, a great number of people are in the recovery phase and are heading home. A large number of COVID-19 affected and recovered patients are of advancing age and are highly unlikely to have mechanisms to recover efficiently. Consequently, effective post recovery management of the illness is critical for full recovery of infected patients with minimum damage and to restore normal functioning. Focus should also be to not let the damage left due to infection further aggravate the problem in COVID-19 survivors owing to inadequate recovery. Among the range of possibilities, recovered patients are more susceptible to development of pulmonary fibrosis. We suggest that COVID-19 recovered patients who show unresolved patchy areas of opacification, interstitial thickening and early signs of fibrosis during the follow-up chest CT after discharge should be considered for HDAC inhibitors treatment to mitigate the effects of a likely development of pulmonary fibrosis. Early intervention with strategic treatments such as HDAC inhibitors addressing the secondary/late consequences of SARS-CoV-2 infection will help reduce complications/mortality and provide better quality of life for COVID-19 recovered patients.
CRediT authorship contribution statement
VR, KS, MK and RS jointly developed the idea for the manuscript; KMP performed the literature search and wrote the manuscript and VR, RS, KS and MK critically revised the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
KMP and VR would like to acknowledge DST-FIST and DST-PURSE Phase 2 (Department of Science & Technology, Government of India) for Science & Technology infrastructure support at the Department of Biochemistry, Bharathidasan University. VR and MK acknowledge RUSA 2.0 Biological Sciences Bharathidasan University. VR would also like to acknowledge a research grant (EEQ/2016/000503) from Science and Engineering Research Board (SERB), Government of India. MK has been supported by the Faculty Recharge Programme, University Grants Commission (UGC-FRP), New Delhi, India. MK would like to acknowledge a research grant (EEQ/2016/000639) and an Early Career Research Award (ECR/2016/000741) from Science and Engineering Research Board (SERB), Government of India. MK acknowledges UGC-SAP and DST-FIST for the infrastructure of the Department of Animal Science, Bharathidasan University.
References
- 1.Wang H., Li X., Li T., et al. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2020 doi: 10.1007/s10096-020-03899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC . Cent. Dis. Control Prev. 2020. Coronavirus disease 2019 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html [Google Scholar]
- 3.Bernheim A., Mei X., Huang M., et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song F., Shi N., Shan F., et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandar V., Mahalaxmi I., Subramaniam M., et al. Follow-up studies in COVID-19 recovered patients - is it mandatory? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang Y., Xu C., Jiang F., et al. Clinical characteristics and changes of chest CT features in 307 patients with common COVID-19 pneumonia infected SARS-CoV-2: a multicenter study in Jiangsu, China. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;96:157–162. doi: 10.1016/j.ijid.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am. J. Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 8.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Cui H., Li K., et al. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr. Radiol. 2020;50:796–799. doi: 10.1007/s00247-020-04656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X., Yu C., Qu J., et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon S.H., Lee K.H., Kim J.Y., et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J. Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding X., Xu J., Zhou J., Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 2020;127 doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Z., Zhang Y., Wang Y., et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan Y.-N., Zhu Y.-Q., Tang L.-L., Qin J. CT features of novel coronavirus pneumonia (COVID-19) in children. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju C.-R., Lian Q.-Y., Zhang J.-H., et al. Recommended prophylactic and management strategies for severe acute respiratory syndrome coronavirus 2 infection in transplant recipients. Chronic Dis Transl Med. 2020 doi: 10.1016/j.cdtm.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Y., Guan H., Zhou S., et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur. Radiol. 2020;30:3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P., Li J., Liu H., et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tse G.M.-K., To K.-F., Chan P.K.-S., et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J. Clin. Pathol. 2004;57:260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonio G.E., Wong K.T., Hui D.S.C., et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228:810–815. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]
- 21.Wong K., Antonio G.E., Hui D.S.C., et al. Severe acute respiratory syndrome: thin-section computed tomography features, temporal changes, and clinicoradiologic correlation during the convalescent period. J. Comput. Assist. Tomogr. 2004;28:790–795. doi: 10.1097/00004728-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Wu X., Dong D., Ma D. Thin-section computed tomography manifestations during convalescence and long-term follow-up of patients with severe acute respiratory syndrome (SARS) Med Sci Monit Int Med J Exp Clin Res. 2016;22:2793–2799. doi: 10.12659/msm.896985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller N.L., Ooi G.C., Khong P.L., et al. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. AJR Am. J. Roentgenol. 2004;182:39–44. doi: 10.2214/ajr.182.1.1820039. [DOI] [PubMed] [Google Scholar]
- 24.Chu W.C.W., Li A.M., Ng A.W.H., et al. Thin-section CT 12 months after the diagnosis of severe acute respiratory syndrome in pediatric patients. AJR Am. J. Roentgenol. 2006;186:1707–1714. doi: 10.2214/AJR.05.0382. [DOI] [PubMed] [Google Scholar]
- 25.Das K.M., Lee E.Y., Singh R., et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27:342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mossel E.C., Wang J., Jeffers S., et al. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372:127–135. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinheimer V.K., Becher A., Tönnies M., et al. Influenza A viruses target type II pneumocytes in the human lung. J. Infect. Dis. 2012;206:1685–1694. doi: 10.1093/infdis/jis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delpino M.V., Quarleri J. SARS-CoV-2 pathogenesis: imbalance in the renin-angiotensin system favors lung fibrosis. Front. Cell. Infect. Microbiol. 2020;10:340. doi: 10.3389/fcimb.2020.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni W., Yang X., Yang D., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care Lond Engl. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leng L., Cao R., Ma J., et al. Pathological features of COVID-19-associated lung injury: a preliminary proteomics report based on clinical samples. Signal Transduct Target Ther. 2020;5:240. doi: 10.1038/s41392-020-00355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer K.C. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expert Rev Respir Med. 2017;11:343–359. doi: 10.1080/17476348.2017.1312346. [DOI] [PubMed] [Google Scholar]
- 33.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet Lond Engl. 2017;389:1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 34.Vallée A., Lecarpentier Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019;9 doi: 10.1186/s13578-019-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardo A., Cabrera S., Maldonado M., Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016;17 doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leask A. COVID-19: is fibrosis the killer? J Cell Commun Signal. 2020;14:255. doi: 10.1007/s12079-020-00569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu M., Liu Y., Xu D., et al. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J. Radiol. 2020;21:746–755. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MGH FLARE June 2 - will COVID-19 cause more IPF? https://us19.campaign-archive.com/?u=ef98149bee3f299584374540a&id=737fad9de0
- 42.Ojo A.S., Balogun S.A., Williams O.T., Ojo O.S. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulm Med. 2020;2020:6175964. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wigén J., Löfdahl A., Bjermer L., et al. Converging pathways in pulmonary fibrosis and Covid-19 - the fibrotic link to disease severity. Respir Med X. 2020;2 doi: 10.1016/j.yrmex.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi T., Tanaka K., Fujita T., et al. Bidirectional role of IL-6 signal in pathogenesis of lung fibrosis. Respir. Res. 2015;16 doi: 10.1186/s12931-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee C.G., Homer R.J., Zhu Z., et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J. Exp. Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minshall E.M., Leung D.Y., Martin R.J., et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am. J. Respir. Cell Mol. Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 47.Khalil N., O’Connor R.N., Flanders K.C., Unruh H. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am. J. Respir. Cell Mol. Biol. 1996;14:131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- 48.Khalil N., Parekh T.V., O’Connor R., et al. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001;56:907–915. doi: 10.1136/thorax.56.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts A.B., Piek E., Böttinger E.P., et al. Is Smad3 a major player in signal transduction pathways leading to fibrogenesis? Chest. 2001;120:43S–47S. doi: 10.1378/chest.120.1_suppl.s43-a. [DOI] [PubMed] [Google Scholar]
- 50.Leask A., Abraham D.J. TGF-beta signaling and the fibrotic response. FASEB J Off Publ Fed Am Soc Exp Biol. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 51.Kandasamy M., Lehner B., Kraus S., et al. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell. Mol. Med. 2014;18:1444–1459. doi: 10.1111/jcmm.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venkataraman T., Frieman M.B. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antivir. Res. 2017;143:142–150. doi: 10.1016/j.antiviral.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe-Takano H., Takano K., Hatano M., et al. DA-Raf-mediated suppression of the Ras—ERK pathway is essential for TGF-β1-induced epithelial-mesenchymal transition in alveolar epithelial type 2 cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao X., Nicholls J.M., Chen Y.-G. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J. Biol. Chem. 2008;283:3272–3280. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen J.T., Knight R.A., Bloor C.A., Spiteri M.A. Enhanced insulin-like growth factor binding protein-related protein 2 (connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am. J. Respir. Cell Mol. Biol. 1999;21:693–700. doi: 10.1165/ajrcmb.21.6.3719. [DOI] [PubMed] [Google Scholar]
- 56.Torr E.E., Ngam C.R., Bernau K., et al. Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype. J. Biol. Chem. 2015;290:6951–6961. doi: 10.1074/jbc.M114.606186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajasekaran S., Vaz M., Reddy S.P. Fra-1/AP-1 transcription factor negatively regulates pulmonary fibrosis in vivo. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi K., Jiang J., Ma T., et al. Pathogenesis pathways of idiopathic pulmonary fibrosis in bleomycin-induced lung injury model in mice. Respir. Physiol. Neurobiol. 2014;190:113–117. doi: 10.1016/j.resp.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Pattarayan D., Rajarajan D., Ayyanar S., et al. C-phycocyanin suppresses transforming growth factor-β1-induced epithelial mesenchymal transition in human epithelial cells. Pharmacol Rep PR. 2017;69:426–431. doi: 10.1016/j.pharep.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 61.Pattarayan D., Thimmulappa R.K., Ravikumar V., Rajasekaran S. Diagnostic potential of extracellular microRNA in respiratory diseases. Clin. Rev. Allergy Immunol. 2018;54:480–492. doi: 10.1007/s12016-016-8589-9. [DOI] [PubMed] [Google Scholar]
- 62.Rajasekaran S., Rajaguru P., Sudhakar Gandhi P.S. MicroRNAs as potential targets for progressive pulmonary fibrosis. Front. Pharmacol. 2015;6:254. doi: 10.3389/fphar.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myllärniemi M., Kaarteenaho R. Pharmacological treatment of idiopathic pulmonary fibrosis - preclinical and clinical studies of pirfenidone, nintedanib, and N-acetylcysteine. Eur Clin Respir J. 2015;2 doi: 10.3402/ecrj.v2.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Margaritopoulos G.A., Vasarmidi E., Antoniou K.M. Pirfenidone in the treatment of idiopathic pulmonary fibrosis: an evidence-based review of its place in therapy. Core Evid. 2016;11:11–22. doi: 10.2147/CE.S76549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kato M., Sasaki S., Nakamura T., et al. Gastrointestinal adverse effects of nintedanib and the associated risk factors in patients with idiopathic pulmonary fibrosis. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-48593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H. A Randomized, Open-label Study to Evaluate the Efficacy and Safety of Pirfenidone in Patients With Severe and Critical Novel Coronavirus Infection. 2020. clinicaltrials.gov
- 67.Pleasants R., Tighe R.M. Management of idiopathic pulmonary fibrosis. Ann. Pharmacother. 2019;53:1238–1248. doi: 10.1177/1060028019862497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang J., Yan H., Zhuang S. Histone deacetylases as targets for treatment of multiple diseases. Clin Sci Lond Engl 1979. 2013;124:651–662. doi: 10.1042/CS20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glenisson W., Castronovo V., Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim. Biophys. Acta. 2007;1773:1572–1582. doi: 10.1016/j.bbamcr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Guo W., Shan B., Klingsberg R.C., et al. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–L870. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korfei M., Skwarna S., Henneke I., et al. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax. 2015;70:1022–1032. doi: 10.1136/thoraxjnl-2014-206411. [DOI] [PubMed] [Google Scholar]
- 72.Barter M.J., Pybus L., Litherland G.J., et al. HDAC-mediated control of ERK- and PI3K-dependent TGF-β-induced extracellular matrix-regulating genes. Matrix Biol J Int Soc Matrix Biol. 2010;29:602–612. doi: 10.1016/j.matbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Jones D.L., Haak A.J., Caporarello N., et al. TGFβ-induced fibroblast activation requires persistent and targeted HDAC-mediated gene repression. J. Cell Sci. 2019;132 doi: 10.1242/jcs.233486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saito S., Zhuang Y., Suzuki T., et al. HDAC8 inhibition ameliorates pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2019;316:L175–L186. doi: 10.1152/ajplung.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoon S., Kang G., Eom G.H. HDAC inhibitors: therapeutic potential in fibrosis-associated human diseases. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20061329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coward W.R., Watts K., Feghali-Bostwick C.A., et al. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol. Cell. Biol. 2009;29:4325–4339. doi: 10.1128/MCB.01776-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang S.K., Scruggs A.M., Donaghy J., et al. Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis. 2013;4:e621. doi: 10.1038/cddis.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saito S., Zhuang Y., Shan B., et al. Tubastatin ameliorates pulmonary fibrosis by targeting the TGFβ-PI3K-Akt pathway. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korfei M., Stelmaszek D., MacKenzie B., et al. Comparison of the antifibrotic effects of the pan-histone deacetylase-inhibitor panobinostat versus the IPF-drug pirfenidone in fibroblasts from patients with idiopathic pulmonary fibrosis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vancheri C., Failla M., Crimi N., Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur. Respir. J. 2010;35:496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 81.Eckschlager T., Plch J., Stiborova M., Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karthik S., Sankar R., Varunkumar K., Ravikumar V. Romidepsin induces cell cycle arrest, apoptosis, histone hyperacetylation and reduces matrix metalloproteinases 2 and 9 expression in bortezomib sensitized non-small cell lung cancer cells. Biomed Pharmacother Biomedecine Pharmacother. 2014;68:327–334. doi: 10.1016/j.biopha.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Karthik S., Sankar R., Varunkumar K., et al. Blocking NF-κB sensitizes non-small cell lung cancer cells to histone deacetylase inhibitor induced extrinsic apoptosis through generation of reactive oxygen species. Biomed Pharmacother Biomedecine Pharmacother. 2015;69:337–344. doi: 10.1016/j.biopha.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 84.Ooi G.C., Khong P.L., Müller N.L., et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230:836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 85.Hsu H.-H., Tzao C., Wu C.-P., et al. Correlation of high-resolution CT, symptoms, and pulmonary function in patients during recovery from severe acute respiratory syndrome. Chest. 2004;126:149–158. doi: 10.1378/chest.126.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang Y.-C., Yu C.-J., Chang S.-C., et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236:1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- 87.Jin Z., You H., Zhang W., et al. Thoracic high resolution CT findings of 100 SARS patients in convalescent period. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:512–515. [PubMed] [Google Scholar]
- 88.Ng C.K., Chan J.W.M., Kwan T.L., et al. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax. 2004;59:889–891. doi: 10.1136/thx.2004.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li A.M., So H.K., Chu W., et al. Radiological and pulmonary function outcomes of children with SARS. Pediatr. Pulmonol. 2004;38:427–433. doi: 10.1002/ppul.20078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie L., Liu Y., Fan B., et al. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir. Res. 2005;6 doi: 10.1186/1465-9921-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joynt G.M., Antonio G.E., Lam P., et al. Late-stage adult respiratory distress syndrome caused by severe acute respiratory syndrome: abnormal findings at thin-section CT. Radiology. 2004;230:339–346. doi: 10.1148/radiol.2303030894. [DOI] [PubMed] [Google Scholar]
- 92.Wang Z., Chen C., Finger S.N., et al. Suberoylanilide hydroxamic acid: a potential epigenetic therapeutic agent for lung fibrosis? Eur. Respir. J. 2009;34:145–155. doi: 10.1183/09031936.00084808. [DOI] [PubMed] [Google Scholar]
- 93.Sanders Y.Y., Tollefsbol T.O., Varisco B.M., Hagood J.S. Epigenetic regulation of thy-1 by histone deacetylase inhibitor in rat lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2011;45:16–23. doi: 10.1165/rcmb.2010-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pasini A., Brand O.J., Jenkins G., et al. Suberanilohydroxamic acid prevents TGF-β1-induced COX-2 repression in human lung fibroblasts post-transcriptionally by TIA-1 downregulation. Biochim Biophys Acta Gene Regul Mech. 2018;1861:463–472. doi: 10.1016/j.bbagrm.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X., Liu H., Hock T., et al. Histone deacetylase inhibition downregulates collagen 3A1 in fibrotic lung fibroblasts. Int. J. Mol. Sci. 2013;14:19605–19617. doi: 10.3390/ijms141019605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conforti F., Davies E.R., Calderwood C.J., et al. The histone deacetylase inhibitor, romidepsin, as a potential treatment for pulmonary fibrosis. Oncotarget. 2017;8:48737–48754. doi: 10.18632/oncotarget.17114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ota C., Yamada M., Fujino N., et al. Histone deacetylase inhibitor restores surfactant protein-C expression in alveolar-epithelial type II cells and attenuates bleomycin-induced pulmonary fibrosis in vivo. Exp. Lung Res. 2015;41:422–434. doi: 10.3109/01902148.2015.1060275. [DOI] [PubMed] [Google Scholar]
- 98.Ye Q., Li Y., Jiang H., et al. Prevention of pulmonary fibrosis via trichostatin A (TSA) in bleomycin induced rats. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2014;31:219–226. [PubMed] [Google Scholar]
- 99.Davies E.R., Haitchi H.M., Thatcher T.H., et al. Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2012;46:687–694. doi: 10.1165/rcmb.2011-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]