Abstract
Background
Due to variations and the inadequate use of personal protective equipment (PPE), this study aimed to evaluate our enhanced PPE protocols for minimizing doffing contamination.
Methods
Among 3 PPE kits (simple, Level D, and Level C), 30 participants conducted the first simulation in their adapted way and the second following enhanced protocols. After donning, participants performed a 1-minute simulation of direct care on a patient simulator covered with fluorescent powder. For tracking contamination routes between doffing processes, fluorescent powder contamination was examined with ultraviolet lamps in the darkened room.
Results
Participants were mostly registered nurses (N = 27, 90%), female (87%), and on average 31.7 years old with 8.5 years of clinical experience. Among 61 total simulations, 32 had at least 1 contamination (52.5%); “Noticeable” level (40%) at the “hands-fingers” and “shirt” body areas were most frequent. For first and second simulations with identical PPE kits, compared to the first with adapted practice, the second with enhanced protocols showed a significant reduction in doffing contamination rates (72.7% vs 22.7%, P = .0009 for both Level C and D; 77.8% vs 27.8%, P = .0027 for Level D).
Conclusions
Our enhanced protocols could significantly reduce contaminations. More studies are necessary to provide safer PPE protocol options.
Keywords: Health care personnel, Doffing, Safety, Evaluation, Fluorescent powder
Introduction
The correct use of personal protective equipment (PPE) is critical to protect health care personnel (HCP); however, variations and the inadequate use of PPE have been reported.1, 2, 3, 4, 5 Because most authorities’ PPE guidelines are based on limited evidence and mostly on experts’ opinions,6 sometimes conflicts among guidelines can cause confusion for HCP, even for infection preventionists who need to prepare their own PPE protocols customized for their health care facilities. For example, in response to the 2014 Ebola outbreak, the World Health Organization PPE guidelines recommended the avoidance of taping over gloves and gowns/coveralls with concerns that taping might cause the tearing down of PPE while doffing tape,6 while the Centers for Disease Control and Prevention allowed a taping option to prevent wrist skin exposure at that time.7 Similarly, along with the complexity of using several PPE items together, confusion from unstandardized protocol issues, including evolving protocols over time, were reported as practical PPE barriers by infection control leaders from their HCP training experiences for the 2015 Korea Middle East Respiratory Syndrome (MERS) outbreak.8
Moreover, although HCP know that contaminations during PPE removal could transmit infectious pathogens,4 , 9 substantial contaminations during the doffing process have been observed among HCP. Our prior study adopting fluorescent powder and various PPE items reported a 79.2% overall contamination rate during the doffing process among HCP's simulations, as well as an 82% contamination rate in follow-up simulations even after providing individual feedback on previous errors while HCP were trying to avoid their previous mistakes.5 However, scientific evidence to support developing clear PPE protocols and guidelines remain insufficient. Therefore, this study aimed to provide safer PPE protocol options by evaluating our simulation-based enhanced PPE protocols and tracking contamination changes at each doffing step to minimize doffing contamination from emerging infectious pathogens that can cause severe contamination during direct patient contact.
Methods
This experimental simulation study was conducted with the Seoul National University Institutional Review Board's approval (SNU 16-10-012).
Developing the enhanced PPE protocols
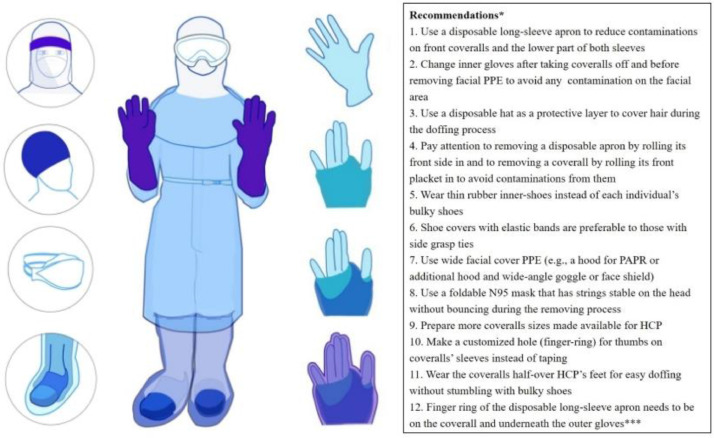
Infection control nurse team leaders from 10 major Korean hospitals who had provided HCP PPE trainings during the 2015 MERS outbreak were invited for an expert panel to identify confusing PPE protocols for each PPE item and possible combinations of several PPE. Following our prior study methods for PPE contamination simulations,5 the confusing PPE protocols identified from the panel were compared in aspects of PPE doffing contamination with 4 standardized HCP (ie, like the term for a standardized patient in simulations, nurses trained to act as assigned nurses at the special isolation unit in order to simulate care for patients with contagious infections) after providing at least 1 minute of direct patient care simulation (eg, physical exam) on a patient simulator, using fluorescent powder and ultraviolet (UV) light. Contamination status was thoroughly examined at every doffing step to track how the contamination level and location changed. Based on these protocol-comparison results, suggestions for enhanced contamination-minimizing protocols were developed (published in Korean, English abstract available; Fig. 1 ).10
Fig 1.
Recommended enhanced protocols for minimizing contamination with full coverage personal protective equipment. Note: Upper left illustration is a face shield alternative to wide goggles; Upper left, second from top is showing a thin inner hat (ideal); Upper left third from top is a foldable N95 respirator underneath wide goggles and a hood; Lower left shows transparent layers with thin-rubber inner shoes, half-over the foot coveralls; Right side images, starting from the top, show inner glove, coveralls’ finger hole over the inner glove, overlapping a disposable long-sleeve apron's finger hole, and a full set of covered outer gloves; powered air purifying respirator, powered air purifying respirator; HCP, healthcare personnel; *Recommendations numbered 1-10 adopted from the previously developed enhanced protocols10; ** & *** Findings from the current study. Illustrated by Ji Hyun Park, PhD.
Simulation evaluating adapted practice and enhanced PPE protocols
Recruitment
Participants were recruited in the order of applications from Seoul National University Hospital by posting recruitment flyers on bulletin boards at units or nearby elevators. Infection control nurses’ participation from the expert panel's hospitals were also welcomed because they were in charge of training HCP on PPE use. Eligible trained HCP were limited to those who were doctors, nurses, radiology technicians, and who had received enough PPE trainings, such as work experience at MERS isolation rooms or PPE training completion(s) for MERS cases. To encourage simulation participation, an incentive (ie, a 50,000 KRW gift card) was provided.
Study procedure
Each participant visited the College of Nursing, Objective Structured Clinical Examination Center for 80-minute simulations during their reserved time slot from February 24 to March 15, 2017. After receiving study information, HCP voluntarily signed consent forms and completed a 20-item prior simulation survey through SurveyMonkey, Inc. (San Mateo, CA) using a tablet computer to collect HCP's demographic information; PPE experience, and beliefs on PPE effectiveness, cumbersomeness, and confidence in use on a 10-level Likert scale (adopted from the prior study5). The survey included 3 open-ended questions about their response-reflections. Participants were then asked to conduct at least 2 instances of PPE simulations among 3 PPE kits: simple kit (disposable long-sleeved apron, surgical mask, and gloves, as commonly used for isolated patients); full-body Level D (coveralls, goggles, N95 respirator, inner gloves, outer gloves, and shoe covers); and full-body Level C (Level D components [with thicker coveralls] plus inner shoe covers, apron, and hood; Powered Air Purifying Respirator was excluded for this simulation because of its complexity for decontamination difficulty between repeated simulations and superiority in minimizing facial contamination). Participants could choose their preferred full-body PPE kit that they were used to for their fully confident PPE performance during the MERS outbreak. Participants, who chose the same full-body kits for 2 times of simulations, conducted the first PPE simulation in their adapted way and the second simulation following enhanced protocols. For the second simulation with enhanced protocols, an additional hood, disposable hat, gloves, thin-rubber inner-shoes, disposable long-sleeved apron, and wider goggles/face shield were prepared as recommended from our prior study.10 After finishing simulations, participants completed a postsimulation survey consisting of 5 open-ended questions to reflect their simulation: confusing parts in donning/doffing; opinions about their contamination reasons; how to prevent their contamination; suggestions for safe PPE use; and better PPE education effectiveness.
Simulation procedure
Adopting the same simulation methods,5 , 10 after donning a PPE kit in a preparation room, HCP entered the adjacent simulation room where they needed to perform a minimum 1-minute simulation of direct patient care on an Emergency Care Simulator (Medical Education Technologies Inc., Sarasota, FL) which was fully covered with sufficient amounts of fluorescent powder (Glo Germ Powder, Glo Germ Company, Moab, UT). To check how much initial contamination occurred on the PPE surfaces right after finishing a simulation, contamination with fluorescent powder was examined with a handheld UV LED lamp in the darkened room. To track contamination during each step of PPE doffing, the UV lamp check was repeated between every doffing process, while participants closed their eyes. All donning and doffing processes were videotaped using two tablet computers by one trained research assistant (RA) and one research aide from opposing angles. All contamination findings were photographed using a high-resolution (ie, digital single-lens reflex) camera. After the first PPE simulation was completed, all contamination was thoroughly removed before the second PPE simulation started.
Simulation environment
Based on the prior studies and the expert panel's experience, the simulation environment was prepared as ideally as possible to support HCP's best performance. Procedure posters for using Level C and D published by the Korean Centers for Disease Control and Prevention were posted on the walls of a preparation room for donning and a simulation room for doffing to aid HCP. If HCP needed a trained observer, the RA played the role. A chair was prepared in case HCP needed one for the stable doffing of the shoe covers. To avoid any cross-contamination from the pump of the hand sanitizer that was observed from the prior study,5 a touch-free, automatic-spray hand sanitizer dispenser was installed. A full-length floor mirror was prepared to help HCP observe their own performance during doffing processes. Because the UV handheld lamp was sometimes not enough for the camera lens to focus on very small fluorescent powder in the darkened room, an additional UV-bulb stand was equipped. To avoid any contamination from discarding PPE in a trash can,5 the largest hazard trash box without a cover was prepared. To minimize flying fluorescent powder in the room's air due to air flows from the central air circulation system, an air purifier with a high efficiency particulate air filter was continuously operated during the simulation. Between simulations, the RA mopped the floor if fluorescent powder was visually seen on surfaces.
Data analysis
Recorded videos and photos were initially coded by the RA, then double-checked and analyzed thoroughly by the principal investigator. Following the prior study's methods,5 fluorescent powder contamination captured by photographs was categorized into 4 contamination levels: “negligible” (very few particles, hard to find), “noticeable” (more contaminated than “negligible,” no problem for finding), “apparent” (easily found with enough amounts of powder, evident contamination), and “severe” (massively contaminated with significant amount). Areas of body contaminations were grouped into 8 categories: scalp-hair-ear, face, neck-collar, shirt, arm-wrist, hands-fingers, pants, and foot-shoes.5 Descriptive statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC). For identical PPE sets, McNemar's test was adopted to compare levels of contaminations between the first simulation of HCP in their adapted way and the second simulation with the enhanced protocols.
Results
Participants were mostly registered nurses (N = 27, 90%; 2 doctors and 1 radiologist), female (87%), with a mean age of 31.7 years (range 25-47), and had 8.5 years of clinical experience on average (standard deviation = 5.7, range 1.5-24). One-third of HCP (N = 10) were infection control nurses and two-thirds were from various units, including intensive care units and an isolation unit. Regarding PPE use experience in the prior-simulation survey, 90% of participants had used a Level D kit and 40% a Level C kit; 47% were unfamiliar with Powered Air Purifying Respirator use, 37% had never used hoods, 23% had never used face shields, and 33% had never used aprons. Participants believed PPE provide protection (means 7.7 ± standard deviation 1.7), were somewhat worried about exposure when using PPE (5.9 ± 2.5), felt cumbersomeness (7.7 ± 1.8), rated their performance moderately (6.7 ± 1.7), and showed better confidence in using general PPE (7.5 ± 1.5) than full-body PPE (5.7 ± 2.2). Through open-ended responses, some participants expressed their previous worries about contracting MERS, poor PPE quality, unsure about their PPE use performance without a buddy system, and a lack of properly sized of PPE with no size options.
A total of 61 simulations were completed by 30 participants (twice per person, except 1 participant with 3 simulations): 18 chose Level D kits for their first and second simulations; 4 chose Level C kits for both simulations; and 8 chose different kits for first and second simulations (1 conducted 3 simulations for all 3 kits). Among all 61 simulations, 32 simulations had at least 1 contamination (52.5%) and “noticeable” level contamination was the most frequent (40%); “hands-fingers” and “shirt” were the most contaminated body areas. From 8 participants’ different kits simulations (simple, n = 7; Level D, n = 5; Level C, n = 5), the simple kit resulted in a 100% contamination rate throughout most body sites (except the pants and feet/shoes) and a more layered kit (ie, Level C) showed less contamination (Table 1 ). From 26 participants who chose the identical kit for first and second simulations, a significant reduction in doffing contaminations was found in the second simulations with enhanced protocols (Fig. 1) compared to the first simulations in their adapted way (McNemar's test, 72.7% vs 22.7%, P = .0009 for both Level C and D; 77.8% vs 27.8%, P = .0027 for Level D). This reduction was possible because enhanced PPE protocols (second simulation) with a long-sleeve apron as the broad outer layer perfectly protected major contaminations on the front coveralls and lower sleeves, compared to adapted practice (first simulation), which usually contaminated those areas severely (Fig. 2 ). Preventing the contamination of the coveralls’ placket made the inner gloves’ surface uncontaminated enough for no consecutive doffing touch contamination.
Table 1.
Summary comparison of contamination results by body sites from different kit simulations
 |
Note: *NC, number of participants who made contamination(s); **NS, number of simulations; *** 1 participant tried Level C accustomed practice, then Level D enhanced protocol (no contamination result); S, severe contamination; A, apparent contamination; N, noticeable contamination; M, minimal contamination (equivalent to the “negligible” level; for the brief abbreviation in this table, the name is modified to “minimal”); numbers in each cell graph are the number of contamination cases; for an insightful understanding of this table, please note that these results were the accumulated contamination results based on the number of contaminated simulations, and each contaminated simulation has variations of contaminated body sites.
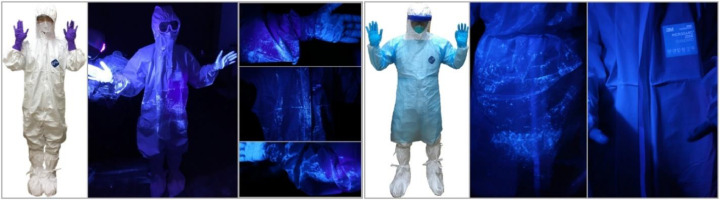
Figure 2.
Consecutive scenes of contamination in the doffing process comparing adapted practice and enhanced protocols. Note: First picture (from left), Level D kit donning completion with accustomed practice; Second picture, checking contamination in the darkened room with UV lights immediately after finishing a minimum 1-minute patient care simulation; Third picture(s), while checking contamination result, severe contamination found on the left glove and the left sleeve of coverall (top), on the front area of coveralls (middle; in particular on the coveralls placket), and on the right glove and the right sleeve of coveralls (bottom); Fourth picture, Level D kit donning completion with enhanced protocols (eg, disposable long-sleeve apron); Fifth picture, severe contamination found on the disposable long-sleeve apron's front surface; Last picture, clean coveralls surface after doffing a disposable apron and outer gloves.
Tracking contamination by UV lamp checking between each doffing step clearly revealed that every touch with outer surfaces brought contamination; for example, from doffing the outer gloves, the inner gloves became more contaminated the more that they touched contaminated front surfaces/plackets of coveralls (Fig. 3 ). Even inner gloves cleaned using disinfectant wipes on contaminated surfaces became re-contaminated with additional touches of contaminated doffing items. Although disinfectant wipes between each doffing step were certainly helpful to diminish contamination amounts from contaminated glove surfaces, fluorescent powder remained between the fingers and fingertips (Fig. 3).
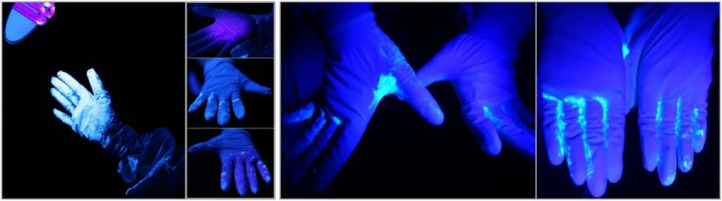
Figure 3.
Consecutive scenes of contamination in doffing gloves with adapted practice. Note: First picture (from left), severely contaminated outer gloves immediately after finishing a 1-minute patient care simulation under a UV-bulb stand; Second picture(s), clean inner glove after doffing outer glove (top), contaminated inner glove (middle: back of gloved hand; bottom: palm) after doffing coverall; Third and fourth pictures, remaining fluorescent powder between fingers and fingertips after cleaning with disinfectant wipes.
Some HCP demonstrated their own effective adapted donning/doffing knowhow; for example, wearing the coveralls half-over their feet made the coveralls come off with less effort. HCP could avoid bending down, which involves the contaminated surface of the coveralls touching the chest to the legs while holding the coveralls with the hands to remove their feet from the coverall; instead, they could take the coveralls off from one foot by stepping on the coveralls on the ground with the other foot (Table 2 ). Thin-rubber, seamless inner shoes recommended by our enhanced protocols (Fig. 1) were helpful to take the coveralls and shoe covers off easily together without stumbling with bulky shoes. Due to the commonly large size of the coveralls, the sagging parts of the coveralls were one of the contamination causes with failures in meticulously rolling down the potentially contaminated surface inside during the coveralls’ doffing, in particular with thick material (Table 2). Among the miscellaneous minor contamination errors which included participants allowing the contaminated surface of the coveralls to flap around, notably, for one female young nurse (who was from a hemodialysis unit, although she met the recruiting criteria of completing the PPE trainings) who wore the N95 respirator loosely (ie, mid-nasal gap) to avoid a latent imprint on her facial surface, fluorescent powder collected enough to be “apparent” level inside the mask around the sides of her nose in both (first and second) simulations (Table 2).
Table 2.
Visual summary of observation results in simulations and suggested recommendations
| Related pictures | Descriptions and comments |
|---|---|
 |
From previous PPE donning/doffing experience, healthcare personnel (HCP) developed their own knowhow to avoid the cumbersome issue of shoes getting stuck inside of the coverall (left picture). HCP wore coveralls half on their shoes (middle picture) and removed them by stepping on the coveralls with the other foot (right picture). This method effectively made HCP take less time and avoid bending down to hold the coveralls to extract their foot in our observation. Worthy of being considered for the recommendations, when the coverall without combined foot-covers is used with additional shoe covers. |
 |
The string type of finger rings which was attached at the end of coverall sleeves were not enough to hold the sleeve covers’ inner gloves; often, the string was too long and wrist skin can be exposed despite the string (left picture). Instead, trained HCP made a thumbhole on a sleeve as a thumb finger ring using scissors; it was more stable and fully covers the gap between inner gloves and sleeves (right picture). |
 |
Most of the inner glove contaminations occurred during the coveralls doffing process by touching contaminated brackets. After opening the front zipper (left picture), then bending down to untie the shoe ties (middle picture) caused the contaminated outer bracket to touch the HCP's inner shirt (right picture). It would be better to untie the shoe cover strings before opening the front zipper of the coveralls. |
 |
The corner of the untucked inner shirt touched the contaminated surface of the coveralls (middle picture) during the doffing process and became contaminated (right picture). The inner shirt needs to be tucked in the inner pant. |
 |
After doffing the hood of the coveralls (left pictures), the sliding down front surface of the hood touched the back hair (middle picture), because it was not folded over easily due to the thick material. It would be better to make sure that the hood part is completely turned down or folded over. In addition, managing hair in a compact way (eg, in a disposable inner hat) would be recommended. |
 |
Leaving the sagging coveralls without rolling them down (left and middle pictures) to put the contaminated surface inside caused contamination on the arm (right picture) while bending down to take the shoes off. It is recommended to make sure the contaminated surface inside by rolling the coveralls down. |
 |
The contaminated stethoscope caused contamination around the ear, hair, mask, and the collar of the shirt. Disinfection of the stethoscope's surface is necessary every time before use. However, it may be better not to use the stethoscope, because it would not be easy to disinfect the full surface of the stethoscope thoroughly. |
 |
While a hood was being removed (left picture), the front end of the hood, which was contaminated by touching the contaminated front surface of the coverall, contaminated the touched hair part (right picture). The end of the hood needs to be carefully folded so as not to touch any part of the body when it is removed. |
 |
Loosely wearing goggles that had a front-head gap (left picture) and loosely wearing an N95 respirator that had a mid-nasal gap (middle picture) caused a collection of fluorescent powder on the curved sides of the participant's nose (right picture). As generally recommended, HCP need to ensure the sealing of an N95 respirator along with their face shape. |
In the postsurvey responses, confusion regarding PPE donning/doffing was mainly from the different styles of PPE items and various combinations of adding or omitting PPE items. Regarding their contamination errors, they reflected unconscious touch with contaminated inner gloves while they were focusing on the other item's doffing process; some suggested changing gloves every step to prevent these contaminations. They also showed concerns about the impermeable, heat-retaining coveralls’ material which causes sweating and the time constraints inside the coverall. Other suggestions to improve the safe use of PPE include a helmet-style fully covered facial mask, spraying disinfectants on the full surface of PPE as the first doffing step, an air-shower after the last doffing step, and the development of universal PPE protocols across health care institutions.
Discussion
This study result presented significantly reduced PPE doffing contaminations with our enhanced protocols, adopting an additional disposable long-sleeved apron which was effective to protect substantial contamination on the front surface/placket of the coveralls and sagged lower sleeves and could be discarded first as the most contaminated layers with outer gloves; by doing so, when the inner gloves touched to open the placket, pull the zipper down, and take the coverall off, they could be less contaminated. If the coveralls’ size properly fits HCP's body size (ie, with different sizes available, the opposite reality of having commonly available one large size of PPE coverall which has caused an ill-fitting issue,8 , 11) with no sagging sleeves, then a common sleeveless disposable apron may be enough to prevent most contaminations from the front coveralls. Despite some potential worries about cumbersomeness or cost, the additional apron would be worthy to reduce contamination during doffing.
As a majority of PPE guidelines for Ebola virus disease recommended,6 , 7 disinfecting the surface of gloves with a disinfectant wipe between consecutive doffing steps was shown to be effective in cleaning most contaminations on the flat surface of the gloves’ palms and backs; however, this study also illustrated the remaining contamination between fingers or fingertips which were not cleaned meticulously. As HCP often missed some surfaces of hands and fingers when they perform hand hygiene quickly,12 HCP were likely to miss some parts of glove surfaces when they clean gloves with a disinfectant wipe during the doffing process. Although there is no evidence for the transmission risk of infectious agents due to uncleaned part of gloves, we may need to address this point for HCP's safer doffing in the PPE use guidelines. Additionally, our tracking of contaminations on gloves in every doffing step showed on-and-off recontaminations and changes in contamination location on gloves as HCP touched different sites of PPE items and cleaned the gloves’ surface with a disinfectant wipe for each doffing process. As contaminated fingers can sequentially transfer virus to several clean surfaces,13 our findings confirmed that each touch of gloved hands can be contaminated again during the doffing after each wipe-disinfecting process. Therefore, as in our enhanced protocols, at least changing the inner gloves before removing facial PPE (ie, mask, goggles) which involves the possibility of facial skin contamination with contaminated inner gloves needs to be recommended, although contamination on the facial skin and its link to infection through the closest mucosal membrane (ie, eyes, nasal cavity, or mouth) was not scientifically examined.
In our experiment, all coveralls in both Level D and C available in Korea did not have attached shoe covers; HCP had to wear coveralls whose legs ended at the ankles, which caused more difficulty for HCP to remove their bulky shoes. This coveralls’ doffing can be different when the coveralls have both shoes covers attached. As the prior simulation study found,5 untied hair can cause minor contaminations; a disposable inner hat in this study with our enhanced protocols was protective to take off the strings of the mask and goggles easily without contamination/entangling with hair. In this study, among disposable inner hats, a form-fitted surgical cap with strings was better than a loose-fitted surgical cap with an elastic band (similar to a shower cap) in terms of not being dragged by mask strings. To protect hair, a thin elastic hair cap (similar to a rubber swimming hat, but thinner) may need to be developed for better safer PPE use.
More protective inner layers of PPE items (ie, thin rubber inner shoes and hat) may be worthy to prevent some snags and contamination during the doffing process. Nonetheless, more layers bring more burdens in time, cost, and effort. HCP may want to proceed doffing quickly after feeling uncomfortable and exhausted within a stifling PPE kit. In fact, according to an international survey on PPE use for coronavirus disease 2019 with 2,711 HCP participating at intensive care units, after longer wearing PPE (median 4 hours), HCP reported heat, thirst, pressure areas, headache, bathroom break difficulty, and severe exhaustion.14 With these PPE use discomforts, HCP are more likely to be eager to doff PPE quickly. However, because hastening of PPE doffing can cause doffing contamination,5 taking each doffing step with meticulous action and vigilance is necessary for ensuring HCP's safety. Further research on balancing doffing safety and lessening burden are necessary by reflecting human physio-psychic aspects and logistics of PPE use environments.
Furthermore, adding or omitting PPE items makes protocols vary depending on the design of each PPE, and changes the points emphasized in protocols. Therefore, developing standardized PPE protocols are all about the details related to available PPE items and styles. Additionally, some HCP may have their own preference for heavier layers of PPE for their safety or lighter layers for their convenience.8 To resolve these detail complexities with different layers and styles, the development of better designed PPE (eg, combining layers into a 1 or 2 pieces) for customizing health care practice with PPE is necessary as participants suggested “need better standardized PPE” in the postsimulation surveys in both the prior study in the United States5 and this study in Korea; then, standardizing PPE protocols would get easier and repeated practicum-based training would make HCP competent in safe PPE use.
Although this study was planned to be conducted with well-trained HCP and best support environment for correct PPE doffing, we found fluorescent powder on both sides of one HCP's nose that was inhaled through the gap between the upper part of N95 respirator and her face, because she did not mold the metal nose clip along the bridge of her nose. It was quite surprising that fluorescent powder particles were clearly found as “apparent” levels on both curved sides of her nose; this visible evidence could be used for emphasizing the importance of mask molding the nose area in HCP trainings. Compared to other participants, she was not used to using full-body PPE (even N95 respirators) daily at her hemodialysis unit after she completed the PPE trainings for back up HCP on MERS preparedness; therefore, we can underline the necessity of regular basis repeated PPE trainings to avoid trained HCP's unguardedness or forgetfulness in PPE use details.
This study has several limitations. First, participants were the small numbers of a convenient sample consisting of trained Korean HCP and infection control nurses who usually train HCP on PPE use, so this result may not be generalizable to untrained HCP, other Korean hospitals, and other countries. In addition, the number of simulations might be insufficient. According to a PPE systematic review which included 9 studies published up to January 2016, the sample size for PPE behavior or trainings should be 60 or more for prospective follow-up HCP cohort studies and randomized controlled studies.15 However, our study which aimed to describe how contamination changes in each doffing step comparing HCP's adapted way of PPE use with our enhanced PPE protocol, could not have over 60 participants, because 30 was the most feasible number for recruiting voluntarily participating, well-trained HCP for MERS. Our study also required more time to conduct simulations due to tracking checks for every doffing step for both participants and research team.
Second, comparison results in our study were not as strictly drawn as in randomized controlled studies. Rather than strict comparisons for before and after or first and second, our study allowed more room for catching meaningful findings to suggest minimizing contamination protocols as an observational, descriptive, and experimental simulation which was a consecutive further study from the prior studies.5 , 10 By allowing more flexibility, we could reflect HCP's knowhow from their countless PPE experience.
Third, HCP might perform better PPE use in the second simulations than the first because the first simulation could be their practice opportunity. However, this practice effect probably did not affect our study outcomes. Because we asked participants to close their eyes while tracking contamination at each doffing step and did not provide any feedback on their contaminations from their first simulation before their second, it is unlikely that participants could improve their PPE donning and doffing even after practicing one time. For example, the HCP who wore N95 respirators loosely had “apparent” level contaminations on both sides of her nose for both simulations.
Last, no scientific evidence exists for simulating pathogens with fluorescent powder. However, fluorescent powder was the best form to test PPE contamination among existing fluorescent products in our preparation test for the prior study10 (unpublished study result). In fact, PPE contamination with covered fluorescent powder during patient care simulations would be more reality-reflective (ie, contamination occurs during patient care) compared to fluorescent lotion/gel or chocolate syrup for HCP PPE training purposes, because intentionally applying these markers on PPE surfaces by HCP4 or trainers usually makes HCP aware of contamination locations upon application. Indeed, because we adopted the fluorescent powder format, we could observe contaminations through the gap of the N95 respirator's upper part, which was probably not demonstrable by lotion or gel formats. Moreover, this study used much fluorescent powder under the worst-case scenarios with maximum contaminations to track doffing contaminations; thus, actual contamination with pathogens in reality would be much less. In addition, our findings are only relevant to full body PPE (Level D and C) for fatal infectious diseases, not useful for standard precautions in routine health care practices.
Conclusions
Using visual tracking for fluorescent powder, this simulation study observed changes in contamination during the doffing process, reported a significant reduction in doffing contamination with our enhanced protocols, and provided several noteworthy points in refining PPE protocols to improve HCP safety. More simulation studies in various HCP populations are necessary for general application as well as to clarify unresolved various combination details (eg, adding or omitting) for different kinds of PPE to support standardizing PPE protocols in disaster preparation for emerging fatal infectious diseases.
Acknowledgment
The authors thank the College of Nursing, Seoul National University for the full support for this study; Min Young Jung (a master's program student) for her diligent RA work; Jayoung L. Park and Seung Hun Ko (undergraduate students) for their assistance as research aides.
Footnotes
Funding: This study was funded for Kang, J. by “the 2016 Seoul National University Invitation Program for Distinguished Scholars.”
Previous presentation: This study was presented at the 22nd Korean Society for Healthcare-associated Infection Control and Prevention Conference in 2017 (May 26, Seoul, Korea) as a poster presentation (# P-10).
Conflicts of interest: All authors have no potential conflicts of interest to disclose.
References
- 1.Zellmer C, Van Hoof S, Safdar N. Variation in health care worker removal of personal protective equipment. Am J Infect Control. 2015;43:750–751. doi: 10.1016/j.ajic.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell R, Roth V, Gravel D, et al. Are health care workers protected? An observational study of selection and removal of personal protective equipment in Canadian acute care hospitals. Am J Infect Control. 2013;41:240–244. doi: 10.1016/j.ajic.2012.04.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John A, Tomas ME, Cadnum JL, et al. Are health care personnel trained in correct use of personal protective equipment. Am J Infect Control. 2016;44:840–842. doi: 10.1016/j.ajic.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Tomas ME, Kundrapu S, Thota P, et al. Contamination of health care personnel during removal of personal protective equipment. JAMA Intern Med. 2015;175:1904–1910. doi: 10.1001/jamainternmed.2015.4535. [DOI] [PubMed] [Google Scholar]
- 5.Kang J, O'Donnell JM, Colaianne B, Bircher N, Ren D, Smith KJ. Use of personal protective equipment among health care personnel: results of clinical observations and simulations. Am J Infect Control. 2017;45:17–23. doi: 10.1016/j.ajic.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Personal protective equipment in the context of filovirus disease outbreak response: rapid advice guideline. 2014. Available at: http://apps.who.int/iris/bitstream/10665/137410/1/WHO_EVD_Guidance_PPE_14.1_eng.pdf?ua=1. Published 2014. Accessed September 26, 2017. [PubMed]
- 7.CDC. Guidance on personal protective equipment to be used by healthcare workers during management of patients with Ebola virus disease in US hospitals, including procedures for putting on (donning) and removing (doffing). CDC. Available at: http://www.cdc.gov/vhf/ebola/hcp/procedures-for-ppe.html?mobile=. 2014. Accessed October 25, 2014.
- 8.Kang J, Kim EJ, Choi JH, et al. Difficulties in using personal protective equipment: training experiences with the 2015 outbreak of Middle East respiratory syndrome in Korea. Am J Infect Control. 2018;46:235–237. doi: 10.1016/j.ajic.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova L, Alfano-Sobsey E, Rutala WA, Weber DJ, Sobsey M. Virus transfer from personal protective equipment to healthcare employees' skin and clothing. Emerg Infect Dis. 2008;14:1291–1293. doi: 10.3201/eid1408.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J. Simulation results for contamination comparisons by various use protocols of personal protective equipment. Korean J Med. 2018;93:41–49. [Google Scholar]
- 11.PROSPECT. Women's personal protective equipment: one size does not fit all. PROSPECT. Available at:https://www.tuc.org.uk/sites/default/files/2016-01299-Leaflet-booklet-Women%27s-PPE–One-Size-Does-Not-Fit-All-Version-26-09-2016%20%282%29.pdf. 2016. Accessed July 30, 2017.
- 12.WHO. WHO guidelines on hand hygiene in health care. WHO. Available at:https://www.who.int/gpsc/5may/tools/9789241597906/en/. 2009. Accessed September 27, 2020.
- 13.Barker J, Vipond IB, Bloomfield SF. Effects of cleaning and disinfection in reducing the spread of norovirus contamination via environmental surfaces. J Hosp Infect. 2004;58:42–49. doi: 10.1016/j.jhin.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Tabah A, Ramanan M, Laupland KB, et al. Personal protective equipment and intensive care unit healthcare worker safety in the COVID-19 era (PPE-SAFE): an international survey. J Crit Care. 2020;59:70–75. doi: 10.1016/j.jcrc.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verbeek JH, Ijaz S, Mischke C, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD011621.pub2. Cd011621. [DOI] [PMC free article] [PubMed] [Google Scholar]