Abstract
Background
To date, 750 000 patients with COVID-19 worldwide have required mechanical ventilation and thus are at high risk of acute brain dysfunction (coma and delirium). We aimed to investigate the prevalence of delirium and coma, and risk factors for delirium in critically ill patients with COVID-19, to aid the development of strategies to mitigate delirium and associated sequelae.
Methods
This multicentre cohort study included 69 adult intensive care units (ICUs), across 14 countries. We included all patients (aged ≥18 years) admitted to participating ICUs with severe acute respiratory syndrome coronavirus 2 infection before April 28, 2020. Patients who were moribund or had life-support measures withdrawn within 24 h of ICU admission, prisoners, patients with pre-existing mental illness, neurodegenerative disorders, congenital or acquired brain damage, hepatic coma, drug overdose, suicide attempt, or those who were blind or deaf were excluded. We collected de-identified data from electronic health records on patient demographics, delirium and coma assessments, and management strategies for a 21-day period. Additional data on ventilator support, ICU length of stay, and vital status was collected for a 28-day period. The primary outcome was to determine the prevalence of delirium and coma and to investigate any associated risk factors associated with development of delirium the next day. We also investigated predictors of number of days alive without delirium or coma. These outcomes were investigated using multivariable regression.
Findings
Between Jan 20 and April 28, 2020, 4530 patients with COVID-19 were admitted to 69 ICUs, of whom 2088 patients were included in the study cohort. The median age of patients was 64 years (IQR 54 to 71) with a median Simplified Acute Physiology Score (SAPS) II of 40·0 (30·0 to 53·0). 1397 (66·9%) of 2088 patients were invasively mechanically ventilated on the day of ICU admission and 1827 (87·5%) were invasively mechanical ventilated at some point during hospitalisation. Infusion with sedatives while on mechanical ventilation was common: 1337 (64·0%) of 2088 patients were given benzodiazepines for a median of 7·0 days (4·0 to 12·0) and 1481 (70·9%) were given propofol for a median of 7·0 days (4·0 to 11·0). Median Richmond Agitation–Sedation Scale score while on invasive mechanical ventilation was –4 (–5 to –3). 1704 (81·6%) of 2088 patients were comatose for a median of 10·0 days (6·0 to 15·0) and 1147 (54·9%) were delirious for a median of 3·0 days (2·0 to 6·0). Mechanical ventilation, use of restraints, and benzodiazepine, opioid, and vasopressor infusions, and antipsychotics were each associated with a higher risk of delirium the next day (all p≤0·04), whereas family visitation (in person or virtual) was associated with a lower risk of delirium (p<0·0001). During the 21-day study period, patients were alive without delirium or coma for a median of 5·0 days (0·0 to 14·0). At baseline, older age, higher SAPS II scores, male sex, smoking or alcohol abuse, use of vasopressors on day 1, and invasive mechanical ventilation on day 1 were independently associated with fewer days alive and free of delirium and coma (all p<0·01). 601 (28·8%) of 2088 patients died within 28 days of admission, with most of those deaths occurring in the ICU.
Interpretation
Acute brain dysfunction was highly prevalent and prolonged in critically ill patients with COVID-19. Benzodiazepine use and lack of family visitation were identified as modifiable risk factors for delirium, and thus these data present an opportunity to reduce acute brain dysfunction in patients with COVID-19.
Funding
None.
Translations
For the French and Spanish translations of the abstract see Supplementary Materials section.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, emerged as a public health threat in December, 2019, in the city of Wuhan, China,1 and a pandemic was subsequently declared by WHO in March, 2020.2 According to the online interactive COVID-19 dashboard, hosted by Johns Hopkins University (Baltimore, MD, USA) by December, 2020, 80 million people had been infected, and 1·7 million people had died as a result of COVID-19 and associated complications. Since the pandemic began, it is estimated that around 1·5 million patients have been admitted to intensive care units (ICUs) worldwide, of whom, 750 000 patients have required mechanical ventilation for viral pneumonia and acute respiratory distress syndrome (ARDS).3, 4
Research in context.
Evidence before this study
We searched MEDLINE and Web of Science for original peer-reviewed cohort studies describing the prevalence of delirium in patients with COVID-19 admitted to intensive care units, published between Jan 1 and Aug 22, 2020, using the search terms “(covid19” AND “ICU” AND “delirium” OR “encephalopathy” AND “prospective“ OR “retrospective” OR “follow-up“ OR “longitudinal)”. Only reports published in English that included at least five participants were considered. Our search yielded one single-centre study that reported delirium or abnormal neurological examination results for 118 (84·3%) of 140 patients with COVID-19 admitted to the intensive care unit (ICU).
Added value of this study
To our knowledge, this cohort study of more than 2000 critically ill patients with COVID-19 from 69 ICUs across 14 countries is the largest study to date to assess the epidemiology of coma and delirium in critically ill patients with COVID-19. The study highlights a high burden of acute brain dysfunction (>80% of patients had coma and >50% developed delirium) that lasted for a median of 2 weeks, which might have implications for survivorship (eg, acquired dementia). The two strongest modifiable predictors of delirium were benzodiazepine infusion (around 60% higher risk of delirium) and family visitation (around 30% lower risk of delirium).
Implications of all the available evidence
Widely adopted guidelines for the management of pain, agitation, delirium, and immobility, and practice frameworks, such as the ABCDEF bundle, have contributed to a substantial reduction in the use of benzodiazepine-based sedation in the past decade (from about 80% to <10%) and in delirium prevalence in mechanically ventilated patients (from approximately 80% to 50%). Delirium has also been shown to be a significant predictor of acquired dementia after critical illness. This study of a large, representative sample of critically ill patients shows that clinicians have reverted to outdated and potentially harmful treatment strategies of deep sedation with widespread use of benzodiazepine infusions, immobilisation, and isolation from families because of the COVID-19 pandemic; these changes in clinical practice are associated with significantly higher prevalence and duration of delirium and coma, portending a major risk for ICU-related dementia and survivorship. Since no pharmaceutical therapies are available for the treatment of COVID-19 at present, this study provides evidence that clinicians should aim to use supportive and proven therapies that avoid deep sedation with benzodiazepine infusions, and facilitate safe in-person or virtual visitation for patients with COVID-19.
COVID-19 has been shown to cause acute organ dysfunction; however, brain dysfunction has not been systematically studied in large representative populations of ICU patients.5, 6, 7 Coma and delirium are serious manifestations of acute brain dysfunction, which often accompany systemic critical illness, and delirium has been associated with poor outcomes in critically ill patients without COVID-19, including mortality, cognitive dysfunction, and subsequent dementia in survivors.8 Previously, delirium has been reported to affect up to 70% of patients who require mechanical ventilation,9 but a 2018 multicentre study that adhered to guideline-recommended practices10 reported delirium prevalence rates of less than 50%.11 Data from smaller cohorts of mechanically ventilated patients with COVID-19 have suggested that these practice guidelines are not being adhered to, and patients are often prescribed higher doses of sedatives and analgesics than patients without COVID-19.12, 13 This approach might be secondary to concerns that the acute respiratory failure due to COVID-19 is markedly different from acute respiratory failure due to bacterial pneumonia or other causes of sepsis,14 reports of propofol shortages, the use of non-ICU trained staff to treat patients during surges in patient numbers, prolonged use of neuromuscular blockade, and concerns regarding propofol infusion syndrome, self-extubation, and proning.15, 16, 17
Although most previous studies of neurological manifestations of COVID-197 did not systematically evaluate patients for delirium despite the availability of validated monitoring tools, one small ICU study reported a delirium prevalence of 84%,12 whereas a delirium prevalence of 11–12% has been reported among the general population of hospitalised patients with COVID-19.18, 19 Although the true prevalence of delirium in critically ill patients with COVID-19 is unknown, patients with COVID-19 are at high risk of delirium due to systemic inflammation and neuroinflammation, other organ system failures, increased risk of thrombosis, and the effects of deep sedative strategies, prolonged mechanical ventilation, and social isolation from families.12, 20, 21
We aimed to elucidate sedation practices, ICU resource constraints, the prevalence of coma, and the prevalence of, and risk factors for delirium in the ongoing pandemic to guide clinicians and mitigate delirium and associated long-term cognitive consequences for patients with COVID-19 in the future.
Methods
Study design and participants
For this multicentre, retrospective cohort study, we invited institutions that were members of the International Research Project for the Humanization of ICU (Proyecto-Humanizando los Cuidados Intensivos [Proyecto HU-CI]) network or Neuro-Intensive Care section of the European Society of Intensive Care Medicine, or had previously collaborated with members of the study steering committee (BTP, RB, GHLC, EWE, and PPP). The steering committee selected 69 sites from 14 countries for inclusion in the study (appendix 3 p 3). Institutions were eligible if patients with COVID-19 were receiving treatment in the ICU, they were routinely assessed for delirium in the ICUs, could secure local regulatory approval, and were willing to commit resources for a 2-week data collection period.
Researchers at Vanderbilt University Medical Center (Nashville, TN, USA), INCLIVA Research Health Institute (Valencia, Spain), and Proyecto HU-CI (Madrid, Spain) coordinated the study.
Between May 12 and 26, 2020, study personnel abstracted data about all patients with COVID-19 who met study eligibility criteria and were admitted to their ICUs before April 28, 2020—a date selected to ensure that at least 28-day outcomes would be available by database closure (May 26, 2020). Beginning with the first eligible patient admitted to their ICUs and continuing with consecutively admitted eligible patients, study personnel at study sites abstracted data on as many eligible patients as possible during the 2-week data collection period. To standardise and synchronise data collection at the sites, we provided training materials (30 min training video, an educational slide set, and a frequently asked questions manual) based on the study protocol.
Patients (aged ≥18 years) were eligible if they were admitted to participating ICUs with SARS-CoV-2 infection. We excluded patients who were moribund (ie, did not survive >24 h) or had life-support measures withdrawn within 24 h of ICU admission; prisoners; patients with pre-existing mental illness (eg, schizophrenia, psychosis, or major depression), neurodegenerative disorders (eg, dementia or Parkinson's disease), congenital or acquired brain damage (eg, stroke in the 2 weeks before ICU admission, subarachnoid haemorrhage, ongoing seizures, anoxic brain injury, or traumatic brain injury); hepatic coma; drug overdose; suicide attempt; or those who were blind or deaf.
The institutional review board of Vanderbilt University Medical Center approved the study. Ethical approval was also obtained at each site from local regulatory boards before data collection. The requirement for written informed consent was waived since data were de-identified and collected retrospectively.
Data collection
De-identified data were extracted from electronic medical records and entered directly into a REDCap database.22 We recorded baseline patient characteristics, including (but not limited to) age on hospital admission, sex, Simplified Acute Physiology Score (SAPS) II within the first 24 h of ICU admission (0–163 points), history of smoking, alcohol abuse, and hearing or vision impairment. A full list of data collected from patients is included in the study protocol.
We also collected hospital stay data and daily ICU data between admission and discharge from the ICU, death, or 21 days after index ICU admission, whichever occurred first. These data included treatment with sedative and analgesic continuous infusions, sedation level (Richmond Agitation-Sedation Scale [RASS],23 Sedation-Agitation Scale,24 or the Glasgow Coma Scale25), delirium (Confusion Assessment method for the ICU [CAM-ICU]26 or the Intensive Care Delirium Screening Checklist [ICDSC], done prospectively by the medical team),27 type of respiratory support (invasive mechanical ventilation, non-invasive mechanical ventilation, high-flow nasal cannula, or low-flow nasal cannula), restraint use, treatment with continuous vasopressors or inotropes, use of antipsychotics, performance of the ABCDEF bundle,10, 28 duration of ICU and hospital stay, and vital status (dead or alive) at 28 days. The ABCDEF bundle is a standard of care bundle of guideline-recommended assessments and practice; it includes six elements: element A (assess, prevent, and manage pain), element B (both spontaneous awakening trials and spontaneous breathing trials), element C (choice of analgesia and sedation), element D (assess, prevent, and manage delirium), element E (early mobility and exercise), and element F (family engagement and empowerment).29 For this study, we only assessed family visitations for the F element of the bundle. We collected data about participating sites, including their relevant practice standards and how they were affected during the COVID-19 pandemic. Mental status was defined as follows: coma was defined as a day when the patients were unresponsive to verbal stimulation (RASS equivalent score –4 or –5 or Glasgow Coma Scale score of <8); patients were considered delirious if they were responsive to verbal stimulation and had a positive delirium assessment scale assessment (CAM-ICU or ICDSC) documented. If a patient was responsive to verbal stimulation but was not delirious, they were considered to be awake without delirium.
Outcomes
The primary outcome was to determine the prevalence of delirium and coma in critically ill patients with COVID-19, and risk factors associated with the development of delirium. We also investigated the predictors of the number of days alive without delirium or coma using a computation of the number of days within the 21-day study period where the patient was alive and free of delirium or coma. This variable was truncated at 21 days because the study design only collected daily assessment data for a maximum of 21 days, ICU discharge or death, whichever occurred first. Days alive without delirium or coma accounts for the contribution of both death and coma, such that patients with more days alive without delirium or coma have a better outcome since they are alive and have more days free of both delirium and coma. The secondary outcome was index ICU length of stay, number of ventilator-free days, and vital status at day 28. Ventilator-free days indicates the number of days the patient was alive and free of the mechanical ventilation (invasive or non-invasive) in a 28-day period. Ventilator-free days account for the contribution of death, such that patients with a higher number of ventilator-free days have a better outcome, since they are alive and breathing without mechanical ventilation.
Statistical analysis
According to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, patient characteristics are expressed as median (IQR) for continuous variables, and n (%) for categorical variables.30
To evaluate risk factors associated with the probability of being delirious the following day, we used a multivariable multinomial regression model, which included baseline risk factors and risk factors on the preceding day. Other states (such as coma, awake without delirium, death, and discharge) were considered as competing risks. Covariates included age, sex, SAPS II score, baseline vision or hearing impairment, smoking status or alcohol abuse, and daily data for respiratory support type, vasopressors, position (prone vs supine), restraint use, deepest sedation level (in RASS equivalents), exposure to sedatives (categorised into three mutually exclusive variables: received benzodiazepine infusions on a given day, received a sedative infusion other than a benzodiazepine on a given day, or received no sedative infusion on a given day), exposure to opioids, antipsychotics, and oral anxiolytics or hypnotics, and performance of the ABCDEF bundle. In contrast to previous publications,29, 31 we used a modification of the ABCDEF bundle performance as a covariate in our model to investigate the contributions of specific elements of the bundle to outcomes in patients with COVID-19 and thus provide areas of focus for clinicians. To study the effect of restricted visitation policies in the ICU secondary to COVID-19, we analysed the performance of the F element of the ABCDEF bundle as a separate covariate. We used the three categories of sedative exposure as a covariate to determine the true impact of the C element (choice of sedative).
To evaluate risk factors for delirium and coma-free days (calculated as the number of days alive without delirium or coma during the 21 days after ICU admission), we used a proportional odds logistic regression model. Since delirium and coma-free days is a continuous, ordinal outcome that is non-normally distributed, a proportional odds logistic regression model for ordinal dependent variables was considered the best choice, as prespecified in the statistical analysis plan. Proportional odds assumptions were evaluated using graphical methods and were met satisfactorily. Covariates for this model were chosen a priori and included baseline variables including age, sex, SAPS II score, baseline vision or hearing impairment, smoking and alcohol abuse, and ICU day 1 data for respiratory support type, vasopressors, position (prone vs supine), restraint use, deepest sedation level (in RASS equivalents), and exposure to sedatives, opioids, antipsychotics, and oral anxiolytics or hypnotics.
We reported our results as adjusted odds ratios (ORs), with 95% CIs.32 For continuous variables, comparisons shown in parentheses in figures correspond with the 75th versus 25th percentile values of that variable. The model for days alive without delirium or coma included all patients for whom delirium or coma assessments were documented on 90% of days spent in the index ICU. The multinomial model further excluded patients who had less than 2 days of index ICU stay because the model used daily covariates from the preceding day. We also did sensitivity analyses that included all patients regardless of the proportion of days with documentation of delirium or coma assessment. Restricted cubic splines for continuous variables were incorporated into the models. To account for correlation between patients at a specific site or repeated measures per patient, we adjusted SEs using Huber-White sandwich estimation.33 Model assumptions were evaluated graphically. Proportional odds assumption was checked using logistic regression with multiple cutoffs.34
Missing data for individual clinical variables were imputed using clinical imputation rules when appropriate, and the remaining missing data (<1%) were imputed using model-based single imputation strategies versus multiple imputation because the amount of missing data was low. In all cases, decisions and processes were documented both in data management and analysis code and in statistical reports.
Before modelling, we did redundancy analyses to assess multicollinearity between independent variables using an adjusted R 2 cutoff of 0·7. When multicollinearity was identified, we eliminated the lowest ranked covariate per a prespecified rank list of covariates listed in the statistical analysis plan.
All analyses were done using R software (version 3.6.2 or above). For the multinomial model the mlogit, mice, sandwich, and aod packages were used. For the proportional odds model the rms, Hmisc, and mice packages were used. All tests were two-sided, and p<0·05 was considered statistically significant.
Role of the funding source
There was no funding source for this study. BTP, RB, and PPP had full access to the data and had final responsibility for the decision to submit for publication.
Results
Between Jan 20 and April 28, 2020, 4530 patients with COVID-19 were admitted to 69 ICUs. Characteristics of the study sites, including changes secondary to the pandemic, are shown in table 1 . The sites were located in 14 countries across Europe, North America, central America, South America, and Africa, with 50% of sites located in Spain (appendix 3 pp 3–7). More than two-thirds of hospitals had more than 500 hospital beds and 94% were teaching hospitals. The majority of sites used the CAM-ICU tool for delirium assessments and the RASS tool for sedation assessments, and had protocols in place for managing delirium. Of the 69 ICUs included, 84% increased their ICU bed capacity with a median increase of 24 beds (IQR 12–39), during the COVID-19 pandemic, and 29 (42%) reported a shortage of adequate resources, especially shortages of ICU providers, personal protective equipment, ventilators, ICU beds, sedatives, and intravenous infusion tubing sets (table 1).
Table 1.
Characteristics of study sites
| Study sites (n=69) | ||
|---|---|---|
| Number of hospital beds | ||
| <500 | 21 (30%) | |
| 500–1000 | 32 (46%) | |
| >1000 | 16 (23%) | |
| Hospital type | ||
| Teaching | 65 (94%) | |
| Non-teaching | 4 (6%) | |
| Location | ||
| Europe | 51 (74%) | |
| North America or central America | 13 (19%) | |
| South America | 3 (4%) | |
| Africa | 2 (3%) | |
| Number of ICU beds before the COVID-19 pandemic | 19 (13–30) | |
| Sites that added additional ICU beds during study period | 58 (84%) | |
| Additional beds added | 24 (12–39) | |
| Protocol in place for identifying delirium | 62 (90%) | |
| Protocol in place for managing delirium | 47 (68%) | |
| Delirium assessment tool | ||
| Confusion Assessment Method for the ICU | 65 (94%) | |
| Intensive Care Delirium Screening Checklist | 4 (6%) | |
| Level of sedation assessment tool | ||
| Richmond Agitation–Sedation Scale | 67 (97%) | |
| Sedation-Agitation Scale | 2 (3%) | |
| Restricted visitation during the study period due to COVID-19 | 68 (99%) | |
| Visitors restricted to certain times of the day | 4/68 (6%) | |
| Visitors restricted to only when a patient is dying | 36/68 (53%) | |
| Visitors completely restricted (no visitors allowed) | 28/68 (41%) | |
| Staff reported facilitated virtual contact* between the patient and family or friends | 66 (96%) | |
| Full duration of patient's stay | 62/66 (94%) | |
| Only in situations when a patient was dying | 4/66 (6%) | |
| Shortage of available resources during the time the study period | 29 (42%) | |
| Shortage of critical care providers | 23/29 (79%) | |
| Shortage of personal protective equipment for providers | 21/29 (72%) | |
| Shortage of ventilators | 16/29 (55%) | |
| Shortage of ICU beds | 15/29 (52%) | |
| Shortage of sedatives | 11/29 (38%) | |
| Shortage of health-care providers | 10/29 (34%) | |
| Shortage of hospital beds | 6/29 (21%) | |
| Shortage of intravenous tubing sets | 5/29 (17%) | |
| Shortage of extracorporeal membrane oxygenation circuit tubing sets | 5/29 (17%) | |
| Shortage of mechanical ventilators | 3/29 (10%) | |
| Shortage of analgesics | 1/29 (3%) | |
| Shortage of vasopressors | 1/29 (3%) | |
Data are n (%), median (IQR), or n/N (%). ICU=intensive care unit.
Via telephone, mobile phone, iPad or tablet, or laptop.
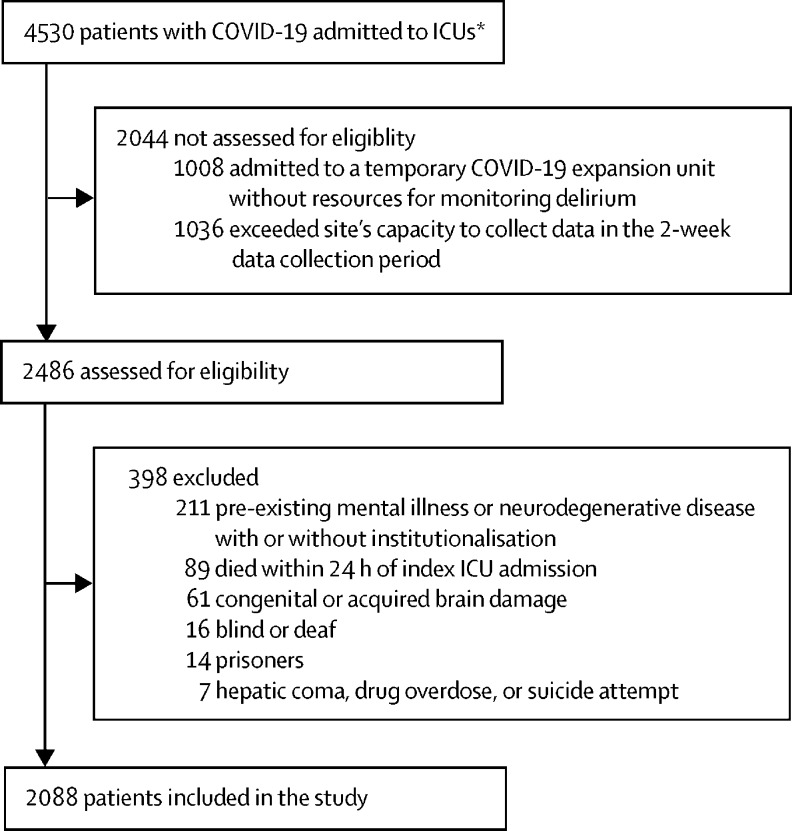
Of the 4530 patients admitted to ICUs, 1008 were not screened because they were admitted to an ICU at a site that did not monitor for delirium and 1036 patients were admitted to sites that did not have capacity to screen all eligible patients and collect data in the 2-week collection period. Thus, sites were able to collect data from 2486 consecutive patients during the 2-week data collection period (appendix 3 p 8). Of the 2486 patients whose medical records were assessed for eligibility, 398 met at least one exclusion criteria, thus 2088 patients were included in the study cohort (figure 1 ). The median age of the cohort was 64·0 years (IQR 54·0 to 71·0), with a median Charlson comorbidity score of 1·0 (0·0 to 2·0), and patients were deemed to be moderately ill on admission (median SAPS II score 40·0 [30·0 to 53·0]; table 2 ). 1497 (71·7%) of 2088 patients were men and 591 (28·3%) were women. 2044 (97·9%) of 2088 patients were admitted with acute respiratory failure, and 1866 (89·4%) required invasive mechanical ventilation, non-invasive mechanical ventilation, or high-flow nasal cannula on the day of ICU admission; 758 (37·1%) had severe ARDS (defined as a partial pressure of arterial oxygen to fraction of inspired oxygen ratio of <100) and 1317 (63·1%) were placed in the prone position for a median of 4·0 days (IQR 2·0 to 6·0). 1827 (87·5%) of 2088 patients required invasive mechanical ventilation at some point during their hospitalisation. Most patients received continuous sedative infusions while on mechanical ventilation: 1337 (64·0%) were given benzodiazepines for a median of 7·0 days (4·0 to 12·0) and 1481 (70·9%) were given propofol for a median of 7·0 days (4·0 to 11·0). Median RASS score while on invasive mechanical ventilation was –4 (IQR –5 to –3) and –5 (–5 to –4) for the first 8 days (appendix 3 p 9).
Figure 1.
Study flow diagram
ICU=intensive care unit. *All patients who were COVID-19 positive and admitted to an ICU from the first reported case in each ICU until April 28th, 2020, were considered for inclusion.
Table 2.
Demographic and clinical characteristics of patients
| Patients (n=2088) | |||
|---|---|---|---|
| Age, years* | 64·0 (54·0–71·0) | ||
| Sex | |||
| Men | 1497 (71·7%) | ||
| Women | 591 (28·3%) | ||
| Race† | |||
| White | 1598 (76·5%) | ||
| Black or African Descent | 145 (6·9%) | ||
| American Indian or Alaska Native | 133 (6·4%) | ||
| Asian | 26 (1·2%) | ||
| Mixed race | 14 (0·7%) | ||
| Other | 172 (8·2%) | ||
| Vision or hearing impairment | 110 (5·3%) | ||
| Current smoker or alcohol abuse | 226 (10·8%) | ||
| Charlson comorbidity score‡ | 1·0 (0·0–2·0) | ||
| Comorbidities on admission | |||
| Congestive heart failure | 139 (6·7%) | ||
| Chronic obstructive pulmonary disease | 241 (11·5%) | ||
| Diabetes | 483 (23·1%) | ||
| Liver disease | 48 (2·3%) | ||
| Renal disease | 134 (6·4%) | ||
| Simplified Acute Physiology Score II§ | 40·0 (30·0–53·0) | ||
| Diagnosis at enrolment | |||
| Acute respiratory distress syndrome | 2044 (97·9%) | ||
| Mild¶ | 236 (11·5%) | ||
| Moderate‖ | 929 (45·5%) | ||
| Severe** | 758 (37·1%) | ||
| Unknown | 121 (5·9%) | ||
| Other†† | 44 (2·1%) | ||
| Respiratory support on intensive care unit admission | |||
| Invasive mechanical ventilation | 1397 (66·9%) | ||
| Non-invasive mechanical ventilation | 173 (8·3%) | ||
| High flow nasal cannula | 296 (14·2%) | ||
| Low flow nasal cannula or no additional oxygen | 222 (10·6%) | ||
| Use of prone positioning | 1317 (63·1%) | ||
| Duration of proning, days | 4·0 (2·0–6·0) | ||
| Use of continuous opioid infusion while on invasive mechanical ventilation‡‡ | |||
| Ever used | 1659 (79·5%) | ||
| Duration of use, days | 11 (7–17) | ||
| Use of continuous sedative infusion while on invasive mechanical ventilation | |||
| Benzodiazepine | |||
| Ever used | 1337 (64·0%) | ||
| Duration of use, days | 7·0 (4·0–12·0) | ||
| Propofol | |||
| Ever used | 1481 (70·9%) | ||
| Duration of use, days | 7·0 (4·0–11·0) | ||
| Dexmedetomidine | |||
| Ever used | 920 (44·1%) | ||
| Duration of use, days | 4·0 (2·0–7·0) | ||
| Clonidine | |||
| Ever used | 191 (9·1%) | ||
| Duration of use, days | 5·0 (2·0–8·0) | ||
| Ketamine | |||
| Ever used | 140 (6·7%) | ||
| Duration of use, days | 4·0 (2·0–6·0) | ||
| Sevoflurane | |||
| Ever used | 47 (2·3%) | ||
| Duration of use, days | 3·0 (1·0–4·0) | ||
Data are median (IQR) or n (%). Some percentages do not sum to 100 because of rounding. Summary statistics were reported for non-missing values. PaO2=partial pressure arterial oxygen. FiO2=fraction of inspired oxygen.
Six patients were aged >90 years and thus their ages were rounded down to 90 years when entered into the database to maintain regulatory rules for de-identified data; in accordance with country regulatory rules, for 44 patients included at two participating sites, age values were rounded to the nearest ten.
Race was recorded as entered into the electronic health record; 56 (2·7%) of 2088 patients had no race reported in the medical record or the participating site could not report their race due to regulatory limitations, thus these patients were included in the other category.
Scores range from 0 to 33, with higher scores indicating a higher burden of coexisting illness.
Scores range from 0 to 163 with higher scores indicating greater severity of illness; a median score of 40 represents a patient population with moderate severity of critical illness.
Defined as a PaO2/FiO2 ratio of 200–300.
Defined as a PaO2/FiO2 ratio of 100–199.
Defined as a PaO2/FiO2 ratio of <100.
Other diagnosis represents patients who were admitted to the hospital and required treatment in the intensive care unit for a reason other than COVID-19, who then tested positive; such diagnoses included diabetic ketoacidosis, acute myocardial infarction, cardiac arrhythmias, post-operative surgery surveillance, acute kidney failure, and acute gastrointestinal bleeding.
Infusions included remifentanil, sufentanil, fentanyl, morphine, and hydromorphone.
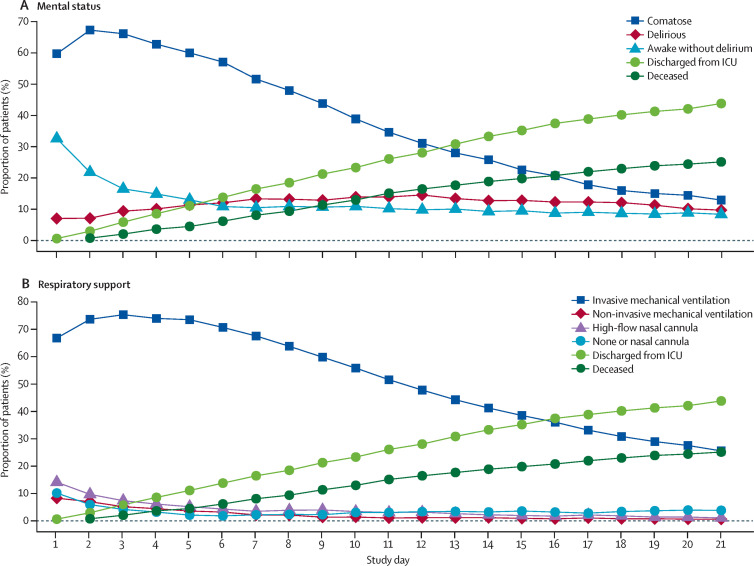
1704 (81·6%) of 2088 patients were comatose for a median of 10·0 days (IQR 6·0–15·0), and 1147 (54·9%) were delirious for a median of 3·0 days (2·0–6·0; table 3 , figure 2 ). Delirium could not be assessed in 313 (15·0%) of 2088 patients due to coma that persisted until death or study day 21. Of 1704 patients with coma, 16 (<1%) were not on concomitant sedation. Acute brain dysfunction (coma or delirium) affected patients for a median of 12·0 days (IQR 7·0 –18·0). During the 21-day study period, patients were alive without delirium or coma for a median of 5·0 days (0·0–14·0). 601 (28·8%) of 2088 patients died within 28 days of admission, with most of those deaths occurring in the ICU.
Table 3.
Patient outcomes
| Patients (n=2088) | ||||
|---|---|---|---|---|
| Coma | ||||
| Prevalence (ever comatose in 21 days) | 1704 (81·6%) | |||
| Coma duration, days* | 10·0 (6·0–15·0) | |||
| Persistently comatose until death or day 21 | 313 (15·0%) | |||
| Delirium† | ||||
| Prevalence (ever delirious in 21 days) | 1147 (54·9%) | |||
| Delirium duration, days* | 3·0 (2·0–6·0) | |||
| Delirium subtypeठ| ||||
| Ever hypoactive | 388/925 (41·9%) | |||
| Hypoactive only delirium duration, days | 2·0 (1·0–4·0) | |||
| Ever hyperactive | 479/925 (51·8%) | |||
| Hyperactive only delirium duration, days | 2·0 (1·0–4·0) | |||
| Acute brain dysfunction (coma or delirium) | ||||
| Coma or delirium duration, days | 12·0 (7·0–18·0) | |||
| Days alive without delirium or coma in 21 days¶ | 5·0 (0·0–14·0) | |||
| Index length of stay in ICU in 28 day period | 14·0 (8·0–25·0) | |||
| Ventilator-free days in 28 day period‖ | 7·0 (0·0–20·0) | |||
| Vital status on day 28 | ||||
| Dead | 601 (28·8%) | |||
| Alive | 1416 (67·8%) | |||
| Unknown | 71 (3·4%) | |||
Data are n (%), median (IQR), or n/N (%). ICU=intensive care unit.
If a patient was both delirious and comatose on the same day, that day was counted for both delirium duration and coma duration outcomes since they were examined as separate outcomes.
Patients were not screened for coma or delirium after discharge from the ICU and thus during the days after index ICU discharge until the day of death or the end of 21 days (whichever occurred first) patients were considered to be awake without delirium.
Ever hypoactive and ever hyperactive delirium categories during the 21-day study period were not mutually exclusive.
Data on delirium subtype not reported for 222 of 1147 patients.
Number of days within the 21-day study period on which patients were alive and free of delirium or coma; this variable was truncated at 21 days because the study design only collected daily assessment data for a maximum of 21 days, ICU discharge or death, whichever happened first.
Number of days patients were alive and did not require mechanical ventilation (invasive or non-invasive) in a 28-day period.
Figure 2.
Mental status and respiratory support status in the 21-day study period (n=2088)
(A) Mental status over time. Coma was defined as a day when the patients were unresponsive to verbal stimulation (Richmond Agitation-Sedation Scale score of –4 or –5 or Glasgow Coma Scale score of <8). Patients were considered delirious if they had a positive delirium assessment scale assessment (Confusion Assessment Method for the Intensive Care Unit or the Intensive Care Delirium Screening Checklist) documented. All other patients were considered awake without delirium. Discharge represents discharge from the intensive care unit. (B) Respiratory status over time. ICU=intensive care unit.
Patients were assessed for pain (ABCDEF bundle element A) at least once on 73% of eligible days, for sedation-agitation level (element C) at least once on 98% of eligible days, and delirium (element D) at least once on 83% of eligible days (table 4 ). Benzodiazepines were avoided (element C) on 52·4% of eligible days a patient was on invasive mechanical ventilation. Less than 25% of eligible patient-days included either of the two components of element B, with spontaneous awakening trials done on 23·8% of eligible days and spontaneous breathing trials done on 22·8% of eligible days. Some type of early mobility (element E), including active range of motion exercises, occurred on 33·9% of eligible days. Only 17% of patient-days included any type of visitation with family or friends (in-person or virtual), and only 8·1% of eligible days involved an in-person visit from family or friends.
Table 4.
ABCDEF bundle performance
| Performance of ABCDEF bundle on days eligible for assessment (n/N [%])* | Performance of ABCDEF bundle on all study days (n=27 022) | ||
|---|---|---|---|
| Element A (assess, prevent, and manage pain) | 19 827/27 022 (73·4%) | 19 827 (73·4%) | |
| Element B | |||
| Spontaneous awakening trial | 5165/21 699 (23·8%) | 5165 (19·1%) | |
| Spontaneous breathing trial | 5174/22 687 (22·8%) | 5174 (19·1%) | |
| Element C | |||
| Assessment of sedation-agitation | 26 501/27 022 (98·1%) | 26 501 (98·1%) | |
| Avoidance of benzodiazepine† | 11 892/22 687 (52·4%) | 11 892 (44·0%) | |
| Element D (assess, prevent, and manage delirium) | 11 044/13 330 (82·9%) | 11 044 (40·9%) | |
| Element E (early mobility and exercise) | 4519/13 330 (33·9%) | 4519 (16·7%) | |
| Element F (family engagement and empowerment) | 4599/27 022 (17·0%) | 4599 (17·0%) | |
| In-person visitation with family or friends | 2192/27 022 (8·1%) | 2192 (8·1%) | |
| Virtual visitation only with family or friends | 2407/27 022 (8·9%) | 2407 (8·9%) | |
n/N (%)=days performed/eligible days. Daily performance of the ABCDEF bundle is shown for all cumulative ICU days for 2075 patients for a total of 27 022 days (13 patients who were only in the ICU for 1 day were excluded). Day of ICU discharge and day of death were not included. ICU=intensive care unit.
Daily performance criteria for each bundle element was defined as follows: element A, at least one pain assessment completed on all available days; element B, a spontaneous awakening trial (ie, daily sedation cessation) was done on days when patients were receiving continuous infusions, and a spontaneous breathing trial was done on days when patients were receiving invasive mechanical ventilation (eligible days included days when patients were on mechanical ventilation and might not have passed safety screen for a spontaneous awakening or breathing trial); element C, at least one agitation-sedation assessment completed on all available days; element D, at least one delirium assessment completed on all days when patients were not in a coma; element E, any exercise or mobility (ie, active range of motion, sit on edge of bed, stand, walk) occurred on all days when patients were not in a coma; element F, in-person visit or virtual connection via an electronic device (eg, cell phone, tablet, or laptop) with family or friends on all available days.
An additional criterion was added for the C element of the bundle for this study: number of days that benzodiazepines were avoided when patients were on invasive mechanical ventilation; clinical practice guidelines recommend avoiding this drug class for routine sedation management, however, this is not typically used to evaluate compliance or performance for this element of the bundle and thus was not included in the criteria used for our modelling.
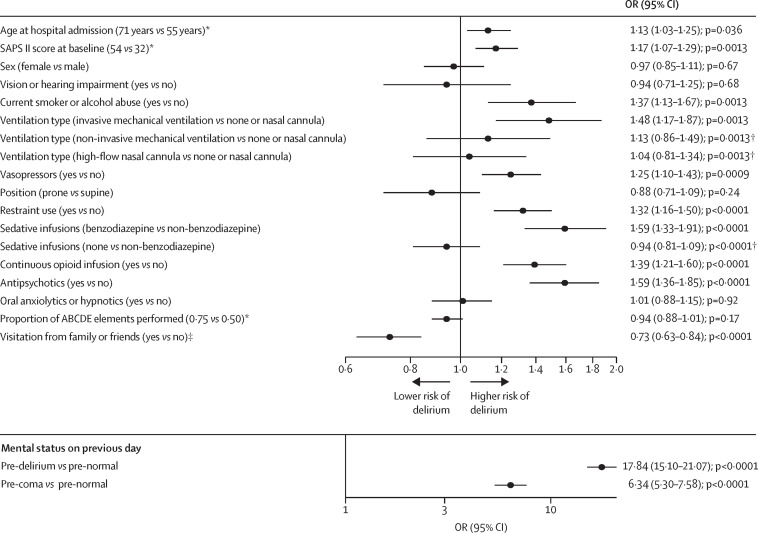
Delirium during the previous day was associated with a 17-times higher risk of being delirious the next day (OR 17·84 [95% CI 15·10–21·07], p<0·0001) and coma during the previous day was associated with a six-times higher risk of coma the next day (6·34 [5·30–7·58], p<0·0001), when compared with being awake without delirium (figure 3 ). Similarly, older age (OR 1·13 [1·03–1·25], p=0·036), higher SAPS II score (1·17 [1·07–1·29], p=0·0013), smoking or alcohol abuse (1·37 [1·13–1·67], p=0·0013), invasive mechanical ventilation (1·48 [1·17–1·87], p=0·0013), vasopressors (1·25 [1·10–1·43], p=0·0009), restraint use (1·32 [1·16–1·50], p<0·0001), antipsychotics (1·59 [1·36–1·85], p<0·0001), and sedative benzodiazepine infusions (1·59 [1·33–1·91], p<0·0001), and continuous opioid infusions (1·39 [1·21–1·60], p<0·0001) were each associated with higher risk of delirium the next day. Family visitation (bundle element F), however, was associated with a 27% lower risk of delirium (OR 0·73 [0·63–0·84], p<0·0001; figure 3).
Figure 3.
Forest plot of daily probability of delirium
All patients who had at least 90% delirium or coma assessments during their index ICU stay and 2 days of ICU stay were included in this analysis (n=2049). Other states, such as comatose, awake without delirium, deceased, and discharged (from index ICU) were considered as competing risks for this multinomial regression analysis. Risk factors with scores greater than 1 (and not crossing 1) were associated with a statistically higher risk of delirium the following day. ICU=intensive care unit. OR=odds ratio. SAPS II=Simplified Acute Physiology Score II. *For all continuous variables (age, SAPS II, proportion of ABCDE elements performed), comparisons shown in parentheses correspond to the 75th vs 25th percentile values for that variable. †p values shown represent the overall p values for the variable and are not associated with the level to level comparisons within these variables, which are represented by the 95% CIs. ‡Bundle element F was assessed separately since COVID-19 presents a unique circumstance in which in-person visitation was restricted at most of the participating sites.
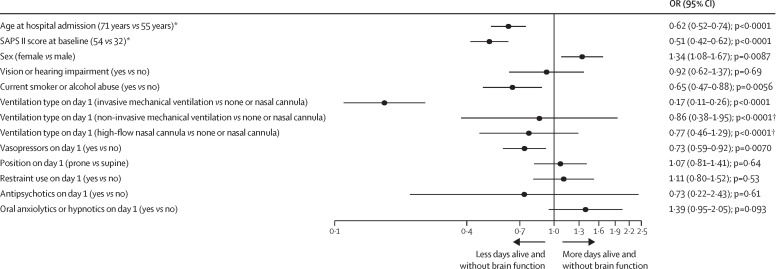
Older age (OR 0·62 [95% CI 0·52–0·74], p<0·0001), higher SAPS II scores (0·51 [0·42–0·62], p<0·0001), smoking or alcohol abuse (0·65 [0·47–0·88], p=0·0056), invasive mechanical ventilation on day 1 (0·17 [0·11–0·26], p<0·0001), and vasopressor use on day 1 (0·73 [0·59–0·92], p=0·0070) were independently associated with fewer delirium and coma-free days, whereas female sex (1·34 [1·08–1·67], p=0·0087) was associated with more delirium-free and coma-free days during the 21-day study period (figure 4 ). Redundancy analysis showed that use of continuous sedation, depth of sedation, and opioid use on day 1 were highly correlated with the use of invasive mechanical ventilation and thus, these variables were not included in the final model.
Figure 4.
Forest plot of days alive and free of coma or delirium
All patients who had at least 90% delirium or coma assessments during their index intensive care unit stay were included in this analysis (n=2062). Risk factors with an OR of less than 1 indicate a negative patient outcome (fewer days alive and free of brain dysfunction coma or delirium). OR=odds ratio. SAPS II=Simplified Acute Physiology Score II. *For all continuous variables (age, SAPS II, proportion of ABCDE elements performed), comparisons shown in parentheses correspond to the 75th vs 25th percentile values for that variable. †p values shown represent the overall p values for the variable and are not associated with the level to level comparisons within these variables, which are represented by the 95% CIs.
Results of sensitivity analyses, which included patients who had more than 10% missing delirium assessments and therefore required imputation, were qualitatively the same as those of the primary regression models (data not shown).
Discussion
In this large, international cohort study of more than 2000 patients with severe COVID-19, acute brain dysfunction (coma or delirium) was more common and more prolonged than observed in other studies of patients with acute respiratory failure without COVID-19.8, 11 Patients with COVID-19 also received treatment with sedatives for a prolonged duration: two-thirds of patients were given benzodiazepines and propofol for a median of 7 days. As a result of the COVID-19 pandemic, many ICUs were operating in resource-constrained environments, and despite demonstrated efficacy in previous studies, evidenced-based strategies, such as light sedation techniques, spontaneous awakening and breathing trials, avoiding benzodiazepines, early mobility, and family visitation, all occurred on fewer than 1 in every 3 days among patients with severe COVID-19.10, 28 We found that risk of delirium among patients with severe COVID-19 was lower when benzodiazepine sedative infusions were avoided and family was present, whereas greater severity of illness and greater respiratory support was associated with a higher risk of delirium.
To our knowledge, this study is the only multisite study to assess critically ill patients with COVID-19 for delirium and coma using validated assessments and is the largest cohort of mechanically ventilated patients with COVID-19 published to date. We found that more than 80% of patients had coma and more than 50% developed delirium. These results build on the initial retrospective reports from Wuhan, China, which reported that 40 (45%) of 88 patients hospitalised with severe COVID-19 had nervous system symptoms, with 13 (15%) having impaired consciousness.7 Similarly, in a small cohort, Helms et al12 reported that 118 (84%) of 140 patients had delirium or abnormal neurological examination that highly correlated with time on ventilation. More recent cohorts of critically ill patients have reported combined durations of coma and delirium of less than a week, however, in this large COVID-19 cohort study, we found that patients had combined acute brain dysfunction for almost 2 weeks. In the MIND-USA study,11 the median duration of coma was was 1 day and that of delirium was 4 days, for a total of 5 days. In contrast, in our cohort, the median duration of coma was 10 days, and 3 days for delirium. We also found that more than 50% of patients had a median of 2 days of hyperactive delirium (IQR 1·0–4·0), which is higher than that reported previously.35 Such prolonged periods of acute brain dysfunction have negative implications for impaired survivorship of patients with COVID-19. Patients with acute brain dysfunction are at high risk of developing ICU-associated dementia and associated post-intensive care syndrome, which affects quality of life8, 36, 37 and should be avoided by using lighter targeted sedation if possible.
Although SARS-CoV-2 infection was initially hypothesised to contribute directly to neurological symptoms, it seems more likely that neurological effects are caused indirectly by factors such as low blood-oxygen levels, coagulopathy, exposure to sedative and analgesic drugs, isolation, and immobility.12, 38, 39 Heavy sedation, especially with benzodiazepines,10, 40 is generally considered to increase risk of delirium and coma during acute illness, and often protocol-driven efforts to minimise sedation when managing acute respiratory failure have led to reductions in acute brain dysfunction.29, 41, 42 Early reports during the pandemic advocated neuromuscular blockade and deep sedation to treat patients with COVID-19 who have ARDS. Our data reflect this management strategy with a high proportion of patients given benzodiazepines (64%) and propofol (71%), both for a median of 7 days. Patient factors, such as increased ventilator-patient dyssynchrony, need for higher positive end expiratory pressures, agitation, and the decision to prone patients, might have also contributed to deeper sedation. The prolonged use of deep sedation could also be secondary to the increased number of ICU patients observed at our sites, the need to utilise non-ICU-trained staff, and inadequate resources with regards to providers, equipment, and sedatives. Our results indicate that regardless of the indication, prolonged sedation is not without consequences, and such findings provide an opportunity to improve care for future patients.
Our results support avoidance of benzodiazepine sedative infusions, which were associated with a 59% higher risk of developing delirium. When possible, health-care providers should adhere to current sedation guidelines for mechanically ventilated patients, even those with COVID-19, which recommend limiting neuromuscular blockade, avoidance of continuous infusions of benzodiazepines, light levels of sedation, frequent awakening and breathing trials, and mobilisation; these practices improve short-term outcomes and might also reduce the risk of post-intensive care syndrome, which affects a high proportion of acute respiratory failure survivors.43, 44, 45 This is in line with earlier recommendations from Fan and colleagues,16 who recommended that clinicians should adhere to evidence-based practices and manage the mechanical ventilation needs and associated needs of patients with COVID-19 in a similar manner to those without the disease. As our ability to care for patients with COVID-19 improve, with better strategies to combat the virus itself and associated sequelae, and health-care systems prepare for subsequent waves of infection, it is crucial for ICU practitioners to move away from deep sedation towards the use of lighter sedation when patient-related factors and logistical issues permit its safe use, for optimum patient recovery.
Critically ill patients with COVID-19 might be uniquely affected by social isolation resulting from restricted visitation in most hospitals during the pandemic. In our cohort, we found family visitation occurred on less than 20% of eligible days, however, when visitation was allowed (virtual or in-person), the risk of delirium the following day significantly decreased (27% lower). Family presence in the ICU has been associated with decreased anxiety, reduced length of stay, and increases in patients' sense of security, satisfaction, and quality of care.28, 46 Patients with severe COVID-19 are at high risk of these sequelae.
Our study had several limitations that warrant consideration. Because all data were collected during a short time period in May, 2020, changes in the routine care of patients with severe COVID-19 might have occurred since study completion. However, since only a few months have passed since the study was completed, the results are likely to remain highly relevant. The retrospective study design meant that clinical assessments, which might be less sensitive than prospective research assessments, were used to detect delirium and coma. Medical teams did these assessments prospectively as part of routine care using validated instruments, and the inclusion of ICUs at 69 sites across 14 countries greatly enhanced the generalisability of study findings. Furthermore, we did not collect any neuroimaging data to further substantiate our findings. We also did not collect data on acute kidney injury, and therefore were unable to evaluate the associations of acute kidney injury and delirium in this population. Additionally, we did not collect data on sedative doses, sedation goals, or the rationale for sedation choices, but can speculate on the basis of reports13 that suggested use of deep sedation and neuromuscular blockade, compounded with resource constraints, led to some of the choices made. The timing of delirium assessments and daily sedation cessation were also not tracked. Although it is possible that we might have overestimated delirium prevalence slightly if done while patients were on sedative medications,47 rapidly resolving sedative-induced delirium is rare (approximately 10%)48 and most cases of delirium persist even with daily awakening trials. Additionally, we did not include patients with known history of brain conditions and doing so might have resulted in an underestimation of delirium prevalence. Patients were not screened for coma or delirium after discharge from the ICU and thus during the days after index ICU discharge until the day of death or the end of 21 days (whichever occurred first) patients were considered to be awake without delirium; as a result, we might have under-reported the duration of delirium. We chose a 2-week period for data collection to allow sites to enrol all patients with COVID-19 who met study eligibility criteria and to ensure that up to 28 days of data would be available by database closure date. Although we enrolled more than 2000 patients, data collection was not possible for 1036 additionally eligible patients. Exclusion of these patients could have led to some bias in our results, although the variability in enrolling patients between study countries did not vary greatly. As in any observational study, we cannot determine causality when examining factors associated with delirium and coma in patients with COVID-19. We did not record whether coma was intentionally drug induced or secondary to a patient's disease process. However, our results are consistent with the findings of numerous previous studies of delirium and coma in acute respiratory patients without COVID-19, and are key for health-care teams to formulate quality improvement decisions for COVID-19 moving forward, using evidence collected before the current pandemic16 and large multinational studies, such as ours, to identify potential modifiable risk factors.
In summary, this large, international study of patients with severe COVID-19 found that delirium and coma are common and often last for twice the duration in this patient population than that in general ICU patients. This prolonged period of acute brain dysfunction is a potential predictor of worse long-term outcomes of these survivors. The overuse of benzodiazepine sedative infusions and lack of family visitation (either in person or virtual) were associated with more delirium and thus, strategies to modify these approaches might mitigate delirium and any associated sequalae.
Data sharing
All de-identified data and the data dictionary will be shared with approval from the COVID-D steering committee and a signed data access agreement. All requests should be sent to brenda.pun@vumc.org. The statistical analysis plan and protocol are publicly available online.
Acknowledgments
Acknowledgments
We used REDCap, a secure online database, supported in part by the National Institutes of Health (TR000445). BTP is supported in part by National Heart Lung and Blood Institute (R01HL14678-01). NEB is supported by the National Institute on Aging of the National Institutes of Health (K76AG054864). TDG received support from the National Institutes of Health (HL135144). EWE is currently receiving grant funding from National Institute on Aging (1R01AG058639-02A1 and 3R01AG058639-02S1) and the Veteran's Administration. PPP is supported by the National Institute of Health (AG061161, AG058639, AG054259 and GM120484). The authors would like to thank all study centres and staff who participated, and also acknowledge the efforts of the nurses, trainees, ancillary staff, and physicians at participating sites who have worked selflessly to care for patients during the COVID-19 pandemic.
Contributors
BTP, RB, GHLC, OMO, WC, RR, B-GKS, SW-L, TDG, and PPP contributed to the literature search, figures, study design, data collection, data analysis, data interpretation, and writing of the manuscript. BTP, RB, GHLC, OMO, WC, RR, B-GKS, SW-L, TDG, and PPP accessed and verified the data. PLS, GL, NEB, EWE, and GL contributed to the study design, data collection, data interpretation, and critical revision of the manuscript. All the authors contributed to data collection and writing of the manuscript.
Declaration of interests
FST reports personal fees from BD, Neuroptics, Zoll, and Nihon Khoden, outside of the submitted work. NEB reports grants from the National Institutes of Health, outside of the submitted work. TDG reports grants from the National Institutes of Health, and personal fees from Haisco Pharmaceutical, outside of the submitted work. EWE reports grants from the National Institutes of Health, the Veteran's Administration, and BioXcel; non-financial support from Eli Lilly; and honoraria from Pfizer and Orion, during the conduct of the study. PPP reports grants from Pfizer, during the conduct of the study. All other authors declare no competing interests.
Contributor Information
COVID-19 Intensive Care International Study Group:
Jacques Creteur, Elisa Govea Bogossian, Lorenzo Peluso, Felipe González-Seguel, Viviane Hidalgo-Calibin, Pablo Carreño-Montenegro, Verónica Rojas, Eduardo Tobar, Antonio Ramírez-Palma, Karen Herrera-Davis, Alexis Ferré, Stéphane Legriel, Thomas Godet, Ugo Fraisse, Bruno Gonçalves, Aurélien Mazeraud, Myrto Tzimou, Frank Rasulo, Silvia Beretta, Mattia Marchesi, Chiara Robba, Denise Battaglini, Paolo Pelosi, Anna Teresa Mazzeo, Alberto Noto, Giuseppe Servillo, Annachiara Marra, Salvatore Lucio Cutuli, Gabriele Pintaudi, Eleonora Stival, Eloisa Sofia Tanzarella, Erik Roman-Pognuz, Chiara Maria Concetta Massaro, Muhammed Elhadi, Lisa Smit, Theresa Olasveengen, Isabel Jesus Pereira, Carla Margarida Teixeira, Alice Santos, Miguel Valente, Cristina Granja, Rita Pereia, João Silva, Blanca Furquet, Mónica García Simón, Daniel A Godoy Torres, Berta Monleón, Esteban Morcillo, Nekane Romero, Ainhoa Serrano, Sara Torrico Sánchez, Francisco Luis Pérez Caballero, Isabel Peña Luna, Ignacio Baeza Gómez, Milagros Calizaya Vargas, Jordi Morillas Pérez, Genís Carrasco Gómez, Ricard Molina Latorre, Sheila Moya Gutiérrez, Irene Patricia Barón Barrera, Cristina Delgado Palacios, Beatriz García Góngora, Laura Labrador Romero, Laura Galarza, Ignacio Catalán-Monzón, Enver Rodriguez-Martínez, Cristina Murcia Gubianas, Ariadna Bellès, María Esther Rodriguez Delgado, Jesús Caballero, Dulce Morales, Andrés Pujol, Jorge Rubio, Eva Álvarez Torres, Estefanía Carvajal Revuelta, Isabel de la Calle Gil, Blanca Fernández Tomás, Berta Gallego Rodríguez, Matilde González Serrano, Paloma LaTorre Andreu, Aris Pérez Lucendo, Elena Abril Palomares, Elena González González, María Cruz Martín Delgado, Carlos Muñoz De Cabo, Pablo T. Aznar, Carlos A. Calvo, Ignacio Garutti, Fernando Higuero, David Martínez-Gascueña, Emilio Maseda, Itziar Insausti, Ana Montero Feijoo, Alejandro Suarez-de-la-Rica, Beatriz Del Moral Barbudo, Yago García Blanco-Traba, Maria Claudia Giménez Santamarina, Alba Gonzalo Millán, Sergio Llorente Damas, David Pestaña Lagunas, Isabel Reyes García, Alejandro Ruiz Perea, Álvaro Ortega Guerrero, María Jesús Mármol Cubillo, David Díaz Muñoz, Silvia García de Castrillón i Ramal, Xavier Andorrà Sunyer, María de las Nieves Noci Moreno, Rosa María Pérez Manrique, Emilio del Campo Molina, María Elena Martínez Quintana, Sol Fernandez-Gonzalo, Gemma Gomà Fernández, Guillem Navarra-Ventura, Anna Baró Serra, Cristina Fuster, Oriol Plans Galván, Diana Gil-Castillejos, Mario Dalorzo González, Francisco Javier Morán Gallego, Irene Paredes Borrachero, Patricia Rodríguez Villamizar, Juan Romeu Prieto, María José Sánchez Carretero, Susana Gallardo Sánchez, Filadelfo Bustos Molina, María Luisa García Pérez, Paula Castello-Mora, Jaume Puig, María Rosa Sanchis-Martin, Carmen Andrea Sanchis-Veryser, María Pilar Vicente-Fernández, Rafael Zaragoza, Laura Lizama, Irene Torres, Cristina Álvarez, Paula Ramírez, Meri Martin Cerezuela, María Jesús Montero, Jose García Cantos, Paola Valls, Nerea Aretxabala Cortajarena, Pablo García Domelo, Laura González Cubillo, Marta Martín Martínez, Inés Pérez Francisco, Yolanda Poveda Hernández, Amaia Quintano Rodero, César Rodriguez Nuñez, Martin Siegemund, Anna Estermann, Núria Zellweger, Imen Ben Saida, Mohamed Boussarsar, Figen Esen, Perihan Ergin Özcan, Christopher Berkey, Christine Harb, Morgan H. Tandy, Ellis Morgan, Karen Shephard, Robert C Hyzy, Michael Kenes, Kristine Nelson, Robert E. Hosse, Katie M. Vance, C. Adrian Austin, Aaron Lerner, Emily Sanders, Robert A Balk, David A Bennett, Andrew R. Vogel, Lucia Chowdhury, Kiran Devulapally, Michelle Woodham, Sarah Cohen, Nihal Patel, Catherine M. Kuza, Mandeep Sing, Spencer Roberson, Kelly Drumright, Sameep Sehgal, Sara C. LaHue, Vanja C. Douglas, and Aarti Sarwal
Supplementary Materials
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus disease (COVID-2019). Situation report–126. May 25, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 3.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 7.Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv. 2020 doi: 10.1101/2020.02.22.20026500. published online Feb 25. (preprint) [DOI] [Google Scholar]
- 8.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 10.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 11.Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506–2516. doi: 10.1056/NEJMoa1808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms J, Kremer S, Merdji H, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapp CM, Zaeh S, Niedermeyer S, Punjabi NM, Siddharthan T, Damarla M. The use of analgesia and sedation in mechanically ventilated patients with COVID-19 ARDS. Anesth Analg. 2020;131:e198–e200. doi: 10.1213/ANE.0000000000005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 15.Hanidziar D, Bittner EA. Sedation of mechanically ventilated COVID-19 patients: challenges and special considerations. Anesth Analg. 2020;131:e40–e41. doi: 10.1213/ANE.0000000000004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcez FB, Aliberti MJR, Poco PCE, et al. Delirium and adverse outcomes in hospitalized patients with COVID-19. J Am Geriatr Soc. 2020;68:2440–2446. doi: 10.1111/jgs.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ticinesi A, Cerundolo N, Parise A, et al. Delirium in COVID-19: epidemiology and clinical correlations in a large group of patients admitted to an academic hospital. Aging Clin Exp Res. 2020;32:2159–2166. doi: 10.1007/s40520-020-01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24:176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 24.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 27.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 28.Davidson JE, Powers K, Hedayat KM, et al. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004–2005. Crit Care Med. 2007;35:605–622. doi: 10.1097/01.CCM.0000254067.14607.EB. [DOI] [PubMed] [Google Scholar]
- 29.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. [Google Scholar]
- 31.Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017;45:171–178. doi: 10.1097/CCM.0000000000002149. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q, Shepherd BE, Li C, Harrell FE., Jr Modeling continuous response variables using ordinal regression. Stat Med. 2017;36:4316–4335. doi: 10.1002/sim.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 34.Vanderbilt Biostatistics Regression modeling strategies. With applications to linear models, survival analysis and logistic regression. http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/RmS
- 35.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 36.Norman BC, Jackson JC, Graves JA, et al. Employment outcomes after critical illness: an analysis of the bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors cohort. Crit Care Med. 2016;44:2003–2009. doi: 10.1097/CCM.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanberg N, Ashton NJ, Andersson LM, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95:e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 40.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Barnes-Daly MA, Pun BT, Harmon LA, et al. Improving health care for critically ill patients using an evidence-based collaborative approach to ABCDEF bundle dissemination and implementation. Worldviews Evid Based Nurs. 2018;15:206–216. doi: 10.1111/wvn.12290. [DOI] [PubMed] [Google Scholar]
- 42.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 43.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 44.Elliott D, Davidson JE, Harvey MA, et al. Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med. 2014;42:2518–2526. doi: 10.1097/CCM.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 45.Marra A, Pandharipande PP, Girard TD, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018;46:1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleinpell R, Heyland DK, Lipman J, et al. Patient and family engagement in the ICU: report from the task force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care. 2018;48:251–256. doi: 10.1016/j.jcrc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Haenggi M, Blum S, Brechbuehl R, Brunello A, Jakob SM, Takala J. Effect of sedation level on the prevalence of delirium when assessed with CAM-ICU and ICDSC. Intensive Care Med. 2013;39:2171–2179. doi: 10.1007/s00134-013-3034-5. [DOI] [PubMed] [Google Scholar]
- 48.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189:658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All de-identified data and the data dictionary will be shared with approval from the COVID-D steering committee and a signed data access agreement. All requests should be sent to brenda.pun@vumc.org. The statistical analysis plan and protocol are publicly available online.