Abstract
Purpose
The aim of the present article was to briefly summarize current knowledge about the immunomodulatory effects of general anesthetics and the possible clinical effects of this immunomodulation in patients with COVID-19.
Methods
The PubMed, Scopus, and Google Scholar databases were comprehensively searched for relevant studies.
Findings
The novel coronavirus causes a wide spectrum of clinical manifestations, with a large absolute number of patients experiencing severe pneumonia and rapid progression to acute respiratory distress syndrome and multiple organ failure. In these patients, the equilibrium of the inflammatory response is a major determinant of survival. The impact of anesthetics on immune-system modulation may vary and includes both pro-inflammatory and anti-inflammatory effects.
Implications
Inhibition of the development of severe inflammation and/or the enhancement of inflammation resolution by anesthetics may limit organ damage and improve outcomes in patients with COVID-19.
Key words: acute respiratory distress syndrome, anesthetics, COVID-19, inflammation, multiple organ dysfunction syndrome
Graphical abstract
Potent effects of etomidate in patients with COVID-19. ∗Patients intubated without the use of etomidate are anticipated to maintain the pre-induction inflammatory status for the subsequent 24–72 h. ARDS = acute respiratory distress syndrome; MODS = multiple organ dysfunction syndrome; RSI = rapid sequence induction.
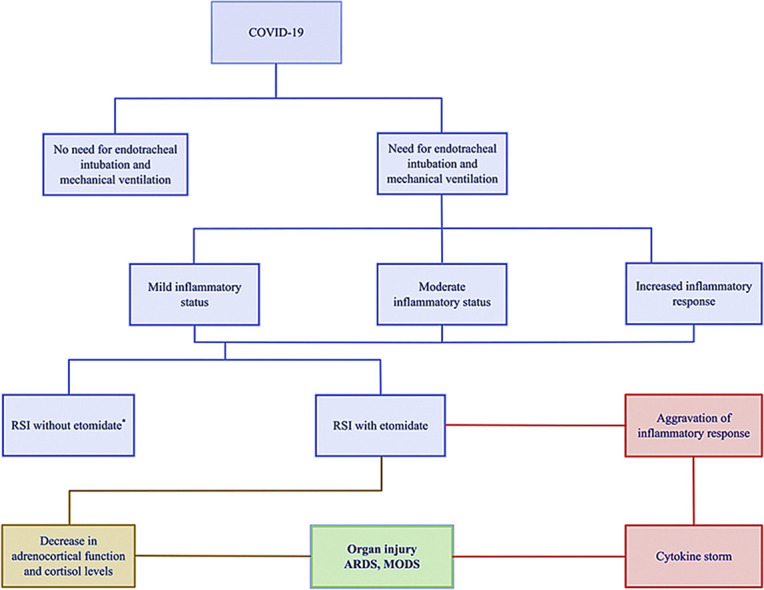
Introduction
Since February 20, 2020, the day of the designation of coronavirus disease by the World Health Organization,1, 2 COVID-19 has spread to include millions worldwide, with confirmed cases increasing again despite austere applied measures. This novel coronavirus causes a wide spectrum of clinical manifestations, with a large absolute number of patients experiencing severe pneumonia and rapid progression to acute respiratory distress syndrome (ARDS) and multiple organ failure.
The virus may spread through the respiratory mucosa and infect other cells, inducing a cytokine storm in the body. Infected patients have high amounts of interleukin-1β, interferon γ, interferon γ–induced protein 10, and monocyte chemotactic protein 1, while a typical patient with pneumonia in whom ARDS develops has a significantly higher neutrophil count, lymphopenia, and more severe cytokine storm than do those without ARDS.1 Moreover, patients requiring admission to the intensive care unit have higher concentrations of inflammatory mediators and cytokines than do those who are not admitted to the intensive care unit, suggesting that the cytokine storm is associated with disease severity.2 In addition, severely ill patients have high levels of pro-inflammatory cytokines, including interleukins 2, 6, 7, and 10; granulocyte colony-stimulating factor; interferon γ–induced protein 10; tumor necrosis factor (TNF)-α; and procalcitonin.2 , 3
Critically ill patients with COVID-19 and acute hypoxemic respiratory insufficiency or failure require early endotracheal intubation and mechanical ventilation. Rapid-sequence induction (RSI), the recommended technique for anesthesia induction, and cautious administration of anesthetic agents may be required. In general, the principles of airway management are like those in more controlled settings, with several drugs being recommended for RSI in patients with COVID-19.4
Although general anesthetics may affect the immune system and may increase morbidity and mortality in patients with COVID-19, surprisingly few attempts to cover this issue have been made. The aim of the present article is to briefly summarize some aspects of current knowledge about the immunomodulatory effects of general anesthetics and the possible clinical effects of this immunomodulation in patients with COVID-19.
Materials and Methods
The PubMed, Scopus, and Google Scholar databases were comprehensively searched for English-language, articles published between 1950 and 2020, using the key terms anesthesia, anesthetics, inflammation, immunomodulation, immune modulation, and proinflammatory. The reference lists of identified articles were searched manually for additional papers eligible for inclusion. Data from articles that were in non-english language were excluded from the review.
Results
A total of 702 articles were identified from the database searches. After the exclusion of 652 articles based on population, outcomes, research method, results of the studies, or duplicates, data from 50 articles (N = 13,786) were included in the present appraisal.
Anesthetic Preferences in Patients with COVID-19
The choices of anesthetics may differ between hospitals, cities, or countries, depending on physicians' preferences and availability. To date, only two published studies have reported on the use of induction agents in COVID-19.5 , 6 Propofol was used in almost all patients, usually combined with other agents, while midazolam and etomidate were used in only a few patients. Neuromuscular blocking agents were used in all patients, with rocuronium being administered in 99% of patients (Table I ). In both studies, the choice of induction agents was usually dictated by hemodynamic considerations and not by the patient's inflammatory status.
Table I.
Demographic characteristics and data on airway management from published studies in patients with COVID-19.
| Patient Characteristics | No. (%) of Patients (N = 222) |
|---|---|
| Sex5,6 | |
| Male | 147 (66) |
| Female | 75 (34) |
| Medication5,6 | |
| Rocuronium | 220 (99) |
| Propofol | 214 (96) |
| Sufentanil | 99 (45) |
| Fentanyl | 60 (27) |
| Midazolam | 27 (12) |
| Etomidate | 6 (3) |
| Succinylcholine | 2 (1) |
| Oxygen therapy technique5,6 | |
| Noninvasive ventilation | 160 (72) |
| High-flow nasal cannula | 31 (14) |
| Mask with reservoir bag | 21 (9) |
| Regular nasal cannula | 8 (4) |
| Survival | |
| 24 h5,6 | 28 (13) |
| 7 d6 | 10 (5) |
| 30 d6 | 8 (4) |
Immunomodulatory Properties of IV Anesthetic Agents
Innate immunity, the first line of defense, refers to protective mechanisms that are present before infection, and is facilitated by the epithelial membranes, phagocytic cells, dendritic cells (DCs), natural killer cells, and several plasma proteins. The most important cellular reaction of innate immunity is inflammation, which is mediated by DCs and natural killer cells. Adaptive immunity is facilitated by mechanisms that are induced by the recognition of specific pathogen antigens and is mediated primarily by lymphocytes. However, an inflammatory immune response may also be induced by noninfectious stimuli; for example, anesthesia itself may induce an inflammatory response in some patients. Also, endotracheal intubation does not guarantee a complication-free procedure; local inflammatory response secondary to the presence of the endotracheal tube has been described.7
The impact of anesthetics on immune-system modulation may vary and includes both pro- and anti-inflammatory effects.8 Although these effects were first demonstrated >100 years ago, research to date has shown that at concentrations used clinically, different anesthetics affect the functions of the inflammatory response in a diverse manner9 (Table II ). Therefore, the development of therapeutic approaches seems prudent for preventing iatrogenic harm that may lead to dysregulation of this inflammatory process and increased morbidity and mortality.77 The effects of anesthetics on immunomodulation of inflammation are complex, and in patients with COVID-19, the choice and use of these agents must be highly dependent on the immune status, especially in those with obesity, in whom the inflammation-induced synergistic conversion of tissue-resident macrophages to an M1-like phenotype and the expression of cytokines and adipokines by adipocytes will aggravate the inflammatory status.78, 79, 80
Table II.
Immunomodulatory properties of common IV anesthetic agents.
| Agent | Immunomodulation |
|---|---|
| Midazolam | Binds to peripheral receptors on macrophages and modulates their metabolic oxidative responsiveness10 |
| Inhibits human neutrophil function and the activation of mast cells induced by TNF-α11 | |
| Suppresses expression of IL-6 mRNA in human blood mononuclear cells11 | |
| Suppresses monocyte chemotaxis12 | |
| Suppresses the respiratory burst of reactive oxygen species, inhibits NF-κB activation via suppression of IκB-α degradation, and inhibits p38 activation in lipopolysaccharide-stimulated macrophages13 | |
| Suppresses the lipopolysaccharide-stimulated immune responses of human macrophages via translocator protein signaling14 | |
| Reduces extracellular IL-8 accumulation by diminishing its secretion from polymorphonuclear leukocytes, despite constantly high intracellular levels and mRNA expression of IL-815 | |
| Propofol | Suppress oxidative burst formation in TNF-α–primed neutrophils16 |
| Attenuates TNF-α–modulated occludin expression by inhibiting Hif-1α/VEGF/VEGFR-2/ERK signaling pathway in hCMEC/D3 cells17 | |
| Impairs several monocyte and neutrophil functions of the innate immune system, including respiratory burst, chemotaxis, phagocytosis, and polarization18, 19, 20, 21 | |
| Its inhibitory properties on human neutrophils and complement activation may be related to its lipid carrier vehicle18,22 | |
| At least partly inhibits human neutrophil chemotaxis by suppressing the p44/42 mitogen-activated protein kinase pathway18,20 | |
| Has proliferative-suppressing effects in polymorphonuclear leukocytes of critically ill patients who are primarily immunosuppressed23 | |
| Reduces extracellular IL-8 accumulation by diminishing its secretion from polymorphonuclear leukocytes, despite constantly high intracellular levels and mRNA expression of IL-815 | |
| Binds to 5-lipoxygenase and attenuates leukotriene B4 production24 | |
| Produces only cell-mediated immunomodulatory effects on innate immunity that might be generated by its lipid solvent18 | |
| Drug-specifically suppresses neutrophil function and reduces their phagocytic capacity25, 26, 27 | |
| Reduces the phagocytotic capacity of alveolar macrophages and increases their gene expression of pro-inflammatory cytokines (IL-1b, IL-8, IFN-γ, and TNF-α)28,29 | |
| Long-chain triglyceride–diluted propofol inhibits neutrophil superoxide production, reduces the burst activity of neutrophils, and inhibits phagocytosis21,25, 26, 27 | |
| Long-/medium-chain triglyceride–diluted propofol raises the burst activity of neutrophils30 | |
| Reduces the intracellular calcium concentration in neutrophils21 | |
| Chemically resembles the chain-breaking antioxidant a-tocopherol due to its phenolic hydroxyl group, exerting antioxidant properties31 | |
| Ketamine | Inhibition of transcription factor activator protein-1 and NF-κB32 |
| Decreases the production of CRP, TNF-α, and IL-633, 34, 35 | |
| Attenuates lipopolysaccharide-induced liver injury by reducing cyclooxygenase-2, inducible nitric oxide synthase, and NF-κB binding activity33,34,36 | |
| Exerts suppressive effects on the adhesion-molecule expression and oxygen-radical production of human neutrophils37 | |
| Suppresses monocyte chemotaxis12 | |
| Etomidate | The preparation with long-/medium-chain triglycerides increases the respiratory burst activity of polymorphonuclears26,30 |
| Mediates its suppressive effects on polymorphonuclear function by changing cellular amino acid turnover38 | |
| Increases the prevalence of nonresponsiveness to corticotropin in patients with septic shock39 | |
| Suppresses adrenal function, even as a single dose, and increases the possibility of pro-inflammatory cytokine production and secondary infections40, 41, 42, 43, 44, 45, 46, 47 | |
| A single dose of etomidate is associated with increased acute respiratory distress syndrome and multiple organ dysfunction syndrome partly due to an effect of etomidate on the inflammatory response (ie, inhibition of 11β-hydroxylase)48, 49, 50, 51 | |
| Increases the risk for inflammatory organ injury in trauma patients52 | |
| Is associated with increased inflammatory response and worsening of respiratory function53 | |
| Dexmedetomidine | Reduces pro-inflammatory cytokine levels in septic and critically ill patients18,54,55 |
| Significantly decreases leukocyte count, CRP, IL-6, IL-8, and TNF-α levels18,54 | |
| Suppresses the biological behavior of HK-2 cells treated with lipopolysaccharide by down-regulating ALKBH556 | |
| Dexmedetomidine preconditioning protects cardiomyocytes against hypoxia/reoxygenation-induced necroptosis by inhibiting high-mobility group box 1–mediated inflammation57 | |
| Protects against high-mobility group box 1–induced cellular injury by inhibiting proptosis58 | |
| Preemptive administration of dexmedetomidine increases the activity of cervical vagus nerve and has the ability to successfully improve survival in experimental endotoxemia by inhibiting inflammatory cytokine release through α7nAChR-dependent mechanism59 | |
| Enhances resolution of high mobility group box 1 protein-induced inflammation through a vagomimetic action60 | |
| Opioids | Different opioids affect immune function differently, depending on drug factors, host factors, and the duration of exposure61 |
| Morphine, fentanyl, remifentanil, methadone, and codeine present strong immunomodulatory effects62 | |
| Tramadol, hydrocodone, oxycodone, and buprenorphine present much weaker or no immunomodulatory capacity62 | |
| Opioids that cross the blood–brain barrier exert more immunomodulatory effects than do opioids that do not cross it63 | |
| Can cause direct sympathetic nervous activation, which may suppress the proliferation and function of some immune cell populations and primary and secondary lymphoid tissues64 | |
| Acute administration of opioids results in either a reduction or no change in adrenocorticotropic hormone or glucocorticoids9,62 | |
| Attenuate the circadian rhythm of adrenocorticotropic hormone and cortisol, leading to consistent increments in circulating levels of these hormones, which might be sufficient to produce immune suppression62 | |
| Impair monocyte and neutrophil function, NK cell–mediated cytotoxicity, lymphocyte and macrophage proliferation, and cytokine release9,62,65,66 | |
| Promote apoptosis by direct activation of the enzymes involved in cell apoptosis (mainly morphine)9,62 | |
| Inhibit leukocyte function by increasing intracellular concentrations of nitric oxide and cyclic adenosine monophosphate, and by inhibiting NF-κB via nitric oxide–dependent mechanisms9,62 | |
| Enhance NK cell cytotoxicity and increase NK and cytotoxic (CD8+) cell counts (mainly fentanyl)18,65 | |
| Produce inhibitory effects on leukocyte migration, NK cell activity, and mitogen-induced lymphocyte proliferation (mainly sufentanil and alfentanil)18,54,67 | |
| Rocuronium | Modulates cytokine production by macrophages/monocytes during the stress response19,59,62 |
| Inhibits apoptosis18,54 | |
| Exerts central sympatholytic effects, including stimulation of cholinergic anti-inflammatory pathways18,54 | |
| Has antinociceptive action involving interactions between pain and immune factors (pro-inflammatory cytokines)18,54 | |
| Inhibits inflammation and pain by suppressing nitric oxide production and enhancing prostaglandin E2 synthesis in endothelial cells68 | |
| Cisatracurium | Decreases the plasma levels of TNF-α and IL-6 by inhibition of nicotinic acetylcholine receptor-α1 in lung injury69 |
| Decreases pulmonary concentrations of IL-1β, IL-6, and IL-8 and serum concentrations of IL-1β and IL-6 in acute respiratory distress syndrome70, 71, 72 | |
| Decreases the expression of high-mobility group box-1 in lung tissues and CD4+ and CD8+ in T-lymphocyte subsets73 | |
| Decreases the expression of TNF-α, IL-6, and high-mobility group box-1 in serum, as well as an alleviation of high-mobility group box-1 protein expression in the diaphragm in early stages of sepsis74 | |
| Decreases total IgE75 | |
| Succinylcholine | Decreases the number of total lymphocytes, total IgE, and CD4/CD8 fractions75 |
| Increases fatty acid–binding protein, insulin, IL-1b, prolactin, S100, calcium-binding protein B, and TNF-α when administered with methohexital76 |
ALKBH = alkylated DNA-repair protein alkB homolog; CRP = C-reactive protein; Hif = hypoxia-inducible factor; HK = hexokinase; IFN = interferon; Ig = immunoglobulin; IL = interleukin; nAChR = nicotinic acetylcholine receptor; NF-κB = nuclear factor κB; NK = natural killer; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor; VEGFR = vascular endothelial growth factor receptor.
Another important contribution of anesthetics is the potent enhancement of inflammation resolution. It is known that endogenous proresolving bioactive mediators can terminate the inflammatory response by promoting the clearance of cellular debris and countering the release of pro-inflammatory cytokines/chemokines.81 A loss of inflammation-resolution mechanisms in infectious diseases sustains pathologic inflammation,79 , 82 and thus, it may also be involved in the preservation of dysregulated inflammation and associated mortality in COVID-19.83, 84, 85 The resolution of inflammation is also stimulated by a pathway involving the arachidonic acid–derived epoxyeicosatrienoic acids (EETs). These mediators modulate ion transport and gene expression, produce vasorelaxation, promote clearance of cellular debris, and activate anti-inflammatory programs to inhibit several key pro-inflammatory cytokines.82 Various anesthetic agents can modify these processes. It is known that leukotrienes are metabolites of arachidonic acid derived from the activity of 5-lipoxygenase.86 Propofol may inhibit 5-lipoxygenase and decrease the production of leukotrienes in DCs and possibly other types of immune and nonimmune cells.24 Also, dexmedetomidine has been shown to up-regulate netrin-1, an orchestrator of inflammation resolution, resulting in the up-regulation of proresolving (lipoxin) and down-regulation of pro-inflammatory (leukotriene B4) humoral mediators.60 The shift of arachidonic acid metabolism to favor inflammation resolution enhances the production of EETs from arachidonic acid by cytochrome P-450 epoxygenases.87 , 88 The enhancement of the production of EETs from arachidonic acid by cytochrome P-450 epoxygenases is very important in patients with low-grade or chronic inflammation, in whom the mitigation of disease severity should be a priority. This mitigation can be further enhanced by the propofol-induced suppression of cyclooxygenase enzyme activity.89 However, ETTs are pulmonary vasoconstrictors and are rapidly metabolized to less-vasoactive dihydroxyeicosatrienoic acids by soluble epoxide hydrolase.82 , 88 , 90, 91, 92 Therefore, the administration of propofol may be more effective in the early stages of inflammation, while in advanced respiratory failure with cytokine storm, propofol may be better administered in combination with a soluble epoxide hydrolase inhibitor (eg, urea, carbamate, or amide derivatives) and a pulmonary vasodilator (eg, IV epoprostenol or inhalational iloprost) to decrease lung inflammation and improve lung function.93 However, these combinations must be further evaluated in well-conducted trials.
Interestingly, EETs appear to affect other organs as well. In the renal cortex, EETs selectively oppose V1 receptor–mediated vasoconstriction, contributing to the relative insensitivity of medullary blood flow to V1-receptor activation. The vasculature of the injured kidney has an impaired vasodilatory response in critically ill patients and loses its autoregulatory behavior.94 Consequently, EETs may have renoprotective effects, which is of great importance considering the increased prevalence of acute kidney injury in patients with COVID-19.95
Several EETs have also been associated with improvement in the recovery of contractile function in isolated perfused mouse hearts96 subjected to global ischemia and reperfusion, and with a reduction in infarct size in intact canine, rat, and mouse hearts.97 Of note, the cardioprotective effects of various EETs are the result of activation of the δ-opioid receptor by the release of the δ-active peptide Met-enkephalin.98 Also, in hypoxic and hemodynamically unstable patients, an increased level of hypoxia-inducible factor 1α may play a role in cardioprotection mediated by EET analogue EET-B in reperfusion.99 , 100 Evidence that the κ-opioid receptor may mediate part of the EET effect,101 while EETs and opioids are acting on similar signaling pathways, comes from the pre- and postconditioning field. All of these findings indicate that the role of opioid receptors in patients with COVID-19 is multifaceted and must be further investigated.98
Inhibition of the development of severe inflammation and the enhancement of inflammation resolution by anesthetics may be a promising alternative approach to limiting severe organ damage and improving outcomes in patients with COVID-19.102 Based on current evidence, midazolam, propofol, ketamine, dexmedetomidine, opioids, rocuronium, cisatracurium, and succinylcholine may have favorable immunomodulatory effects when used in the induction or maintenance of anesthesia. However, etomidate may be harmful, and its use may be questionable.
Potent Effects of Etomidate in Patients with COVID-19
Etomidate is a short-acting IV anesthetic agent used for emergency anesthesia, and for years it has been a drug of choice in RSI and endotracheal intubation.103 Etomidate is a carboxylated imidazole (C14H16N2O2) that binds at a distinct binding site associated with a Cl– ionophore at the γ-aminobutyric acid A receptor. Binding at a distinct site increases the duration for which the Cl– ionophore is open and prolongs the postsynaptic inhibitory effect of γ-aminobutyric acid in the thalamus.
Adrenocortical Inhibition
It is believed that etomidate does not compromise sympathetic tone or myocardial function and produces minimal hemodynamic changes after induction.104 Nonetheless, the advantage of its hemodynamic stability and the disadvantage of its adrenocortical inhibition are not well-proved due to a lack of adequately powered randomized, controlled trials. To date, much of the evidence has been based on systematic reviews and retrospective studies.
In 2008, the results from CORTICUS (Hydrocortisone Therapy for Patients With Septic Shock),105 a multicenter, randomized, double-blind, placebo-controlled trial, suggested that etomidate may prolong the reversal of shock or reduce the likelihood of reversal of shock, raising serious concerns about the use of etomidate in cases of septic shock. Another study reported that etomidate treatment was associated with an increased prevalence of nonresponsiveness to corticotropin in patients with septic shock, with hydrocortisone administration having no effect on outcome in these patients.39 In 2010, Hohl et al40 conducted a systematic review of the effects of a single bolus dose of etomidate on cortisol levels and mortality. The investigators concluded that etomidate suppressed adrenal function transiently. However, whether the supress of the adrenal function had a significant effect on mortality remained unclear. Two years later, Chan et al41 evaluated the effects of a single dose of etomidate on mortality in patients with severe sepsis and septic shock. Despite the existence of possible bias, that meta-analysis reported a statistically significant association between a single dose of etomidate for RSI and mortality, which persisted even after a second sensitivity analysis. Nonetheless, another systematic review and meta-analysis, published in 2015,46 reported no significant correlation between etomidate and mortality in patients with sepsis. The same year, however, a Cochrane database systematic review42 reported that, although there was no conclusive evidence of increases in mortality or health care resource utilization with etomidate use in critically ill patients, the use of etomidate seemed to have increased the risk for adrenal gland dysfunction and multiorgan dysfunction syndrome (MODS). In clinical practice, while etomidate may have fallen from favor over the past 5–10 years because of its effects on adrenal hormone levels affecting adrenoceptor function, it remains an important tool for RSI.
Immunomodulatory Effects
In 2009, Warner et al48 analyzed the data from a clinical trial of prehospital hypertonic saline administration in severely ill trauma patients and reported that a single dose of etomidate for RSI was associated with increased prevalences of ARDS and MODS, partly due to an effect of etomidate on the inflammatory response (ie, inhibition of 11β-hydroxylase). However, apart from the decrease in adrenocortical function and cortisol levels for up to 72 h after a single dose,49 the use of etomidate may be associated with other, more insidious, unintended effects.
The first evidence of the association of etomidate with “late death” was reported in a study in trauma patients conducted in 1983.52 Twenty years later, a retrospective analysis reported that etomidate use was associated with an increased risk for inflammatory organ injury, with patients with an APACHE score of >20 exerting the higher risk for adrenal suppression, ARDS, or MODS.48 This finding was further supported by the results of a substudy of the data from HYPOLYTE (Etomidate Increases Susceptibility to Pneumonia in Trauma Patients),50 a multicenter, randomized, double-blind, placebo-controlled trial of hydrocortisone in trauma patients, which reported that etomidate use was an independent risk factor for hospital-acquired pneumonia on day 28. In a study of the association of etomidate use with mortality in critically ill patients, the investigators reported that etomidate administration was associated with a trend toward a relative increase in mortality.51 However, the small increase in relative risk reported in the literature until 2013 may have been an underestimate, given that some of the previous trials had been underpowered to detect it. In 2014, Hinkewich and Green106 reported an absolute increase in 28-day mortality of 9.2% in trauma patients who received etomidate. Although the difference in mortality was nonsignificant after adjustment for covariates, the investigators stated that the data showed a trend toward increased mortality, advocating that etomidate should be used with caution.
The latest evidence of the immunomodulatory effects of etomidate came out in October 2019, when the results of a planned secondary analysis of PROPPR (Pragmatic Randomized Optimal Platelet and Plasma Ratios),53 a randomized, clinical trial, were reported at the 2019 annual meeting of the American Society of Anesthesiologists. The investigators compared data from patients whose anesthesia was induced with etomidate (n = 87) versus those who received other induction agents (n = 492). Blood samples were taken at admission and at 2, 4, 6, 12, and 24 h, and were screened for 27 cytokines by enzyme-linked immunosorbent assay. Comparison of 30-day clinical outcomes showed an association between etomidate and an increased rate of ARDS (32% vs 16%; P < 0.001) and decreased ventilator-free days (18.5 vs 25 days; P < 0.05). Hospital days, intensive care unit days, and mortality were unaffected (P = 0.33, 0.07, and 0.70, respectively). The researchers concluded that the use of etomidate in the acute setting in trauma patients was associated with increased inflammatory response and worsening of respiratory function.
Should Etomidate Be Used in Patients With COVID-19?
The evidence is alarming and may be extremely important for the management of critically ill patients with COVID-19, in whom the equilibrium of the inflammatory response appears to be a major determinant of survival. Moreover, etomidate has been reported to increase pro-inflammatory cytokine production ex vivo in whole-blood cell cultures challenged with lipopolysaccharide and could therefore prolong the systemic inflammatory response syndrome in patients with COVID-19,43 increasing the possibility of secondary infections and corticosteroid dysfunction.44 , 45 , 107 , 108 On the other hand, the etomidate-mediated decrease in cortisol concentration may decrease monocyte and neutrophil activity and the homing of DCs.109 Furthermore, the decreased cortisol levels increase the risk for inflammation-related complications, such as ARDS and MODS, by impairing the maintenance of vascular tone and endothelial integrity, enhancing the production of phospholipase A2, and impairing leukocyte apoptosis.110 Also, low cortisol increases L-selectin and CD 11b expression on neutrophils and monocytes, which promote chemotaxis, migration of white blood cells, and tissue destruction.48
Another major issue is how etomidate and its possible impact on cytokine/inflammatory proliferation are affected by the concurrent use of corticosteroids. The potent suppression of adrenal function and the increase in pro-inflammatory cytokine production by etomidate typically results in reflex administration of exogenous hydrocortisone, with the goals of improving hemodynamics and suspending the exacerbation of lung injury and MODS (Figure 1). Some evidence suggests that corticosteroids may inhibit the immune response and pathogen clearance. Although septic shock was reported in 7 of 140 patients (5%) with 2019-nCoV included in published reports as of January 29, 2020, evidence then was insufficient for recommending corticosteroid treatment in patients with COVID-19 and ARDS.111 As a result, the World Health Organization in March reported that corticosteroids should not be used in patients with 2019-nCoV–induced lung injury or shock, except in a subset of critically ill patients or in the setting of a clinical trial. However, a study published in May 2020 reported that most (70%) nonsurviving patients with COVID-19 had septic shock, which was significantly higher than the rate of shock in survivors.112 Also, the recommendation in the recent Surviving Sepsis Campaign guideline is to use low-dose corticosteroid therapy (shock reversal), over no corticosteroid therapy, in mechanically ventilated adults with COVID-19 and ARDS (weak recommendation, low-quality evidence).113 Understandably, there is a conflict due to the limited evidence, but the medical community may err toward enthusiasm with corticosteroids, given the profound inflammation. Based on the aforementioned, with etomidate administration, the use of other corticosteroids may be increased compared with the currently recommended dexamethasone, and well-tolerated alternatives, alone or in combination, should be used in RSI in patients with COVID-19.111 , 114 , 115
Conclusions
To date, significant efforts have been made to draw the attention of anesthetists to the importance of appropriate precautions in providing RSI in patients with COVID-19. In this context, the anesthesia provider should keep in mind that a two-phase division is largely suspected in these patients: a first, immune defense–based, protective phase, in which the goal of treatment should be to boost the immune response; and a second, inflammation-driven, damaging phase, during which the immune response should probably be suppressed. Although the evidence regarding this deadly disease remains scarce, the data mentioned herein indicate that further immediate research is warranted for the full clarification of the effects of anesthetics in COVID-19. Considering that systemic inflammation and cytokine storm are associated with adverse outcomes, as well as that reduced inflammation may impair antimicrobial immunity, the authors highly recommend that clinicians monitor patients with COVID-19 receiving anesthetics with anti-inflammatory activity. Although the role of etomidate is questionable, caution in its use is recommended until more evidence is available. It is hoped that this article serves as a template for future explorations and catalyzes new direction toward improved outcomes in patients with COVID-19.
Disclosures
The authors have indicated that they have no conflicts of interest with regard to the content of this article.
Acknowledgments
EFB declares that she was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI143882 (PI; EFB).
References
- 1.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng L., Qiu H., Wan L., et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan's experience. Anesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao W., Wang T., Jiang B., et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125:28–37. doi: 10.1016/j.bja.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L., Li J., Zhou M., Chen Z. Summary of 20 tracheal intubation by anesthesiologists for patients with severe COVID-19 pneumonia: retrospective case series. J Anesth. 2020;34:599–606. doi: 10.1007/s00540-020-02778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasileiou P.V., Chalkias A., Brozou V., et al. Interleukin-6 as a marker of inflammation secondary to endotracheal intubation in pediatric patients. Inflammation. 2013;36:1533–1538. doi: 10.1007/s10753-013-9696-x. [DOI] [PubMed] [Google Scholar]
- 8.Rossaint J., Zarbock A. Anesthesia-induced immune modulation. Curr Opin Anaesthesiol. 2019;32:799–805. doi: 10.1097/ACO.0000000000000790. [DOI] [PubMed] [Google Scholar]
- 9.Cruz F.F., Rocco P.R., Pelosi P. Anti-inflammatory properties of anesthetic agents. Crit Care. 2017;21:67. doi: 10.1186/s13054-017-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memiş D., Hekimoğlu S., Vatan İ., Yandım T., Yüksel M. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth. 2007;98:550–552. doi: 10.1093/bja/aem017. [DOI] [PubMed] [Google Scholar]
- 11.Nishina K., Akamatsu H., Mikawa K., et al. The inhibitory effects of thiopental, midazolam, and ketamine on human neutrophil functions. Anesth Analg. 1998;86:159–165. doi: 10.1097/00000539-199801000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Krumholz W., Reussner D., Hempelmann G. The influence of several intravenous anaesthetics on the chemotaxis of human monocytes in vitro. Eur J Anaesthesiol. 1999;16:547–549. doi: 10.1046/j.1365-2346.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.N., Son S.C., Lee S.M., et al. Midazolam inhibits proinflammatory mediators in the lipopolysaccharide-activated macrophage. Anesthesiology. 2006;105:105–110. doi: 10.1097/00000542-200607000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Horiguchi Y., Ohta N., Yamamoto S., Koide M., Fujino Y. Midazolam suppresses the lipopolysaccharide-stimulated immune responses of human macrophages via translocator protein signaling. Int Immunopharmacol. 2019;66:373–382. doi: 10.1016/j.intimp.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 15.Galley H.F., Dubbels A.M., Webster N.R. The effect of midazolam and propofol on interleukin-8 from human polymorphonuclear leukocytes. Anesth Analg. 1998;86:1289–1293. doi: 10.1097/00000539-199806000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Weiss M., Buhl R., Medve M., Schneider E.M. Tumor necrosis factor-a modulates the selective interference of hypnotics and sedatives to suppress N-formyl-methionyl-leucyl-phenylalanine-induced oxidative burst formation in neutrophils. Crit Care Med. 1997;25:128–134. doi: 10.1097/00003246-199701000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Ding X., Miao C., Chen J. Propofol attenuated TNF-α-modulated occludin expression by inhibiting Hif-1α/VEGF/VEGFR-2/ERK signaling pathway in hCMEC/D3 cells. BMC Anesthesiol. 2019;19:127. doi: 10.1186/s12871-019-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneemilch C.E., Schilling T., U. Bank Effects of general anaesthesia on inflammation. Best Pract Res Clin Anaesthesiol. 2004;18:493–507. doi: 10.1016/j.bpa.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Fröhlich D., Rothe G., Schwall B., Schmitz G., Hobbhahn J., Taeger K. Thiopentone and propofol, but not methohexitone nor midazolam, inhibit neutrophil oxidative responses to the bacterial peptide FMLP. Eur J Anaesthesiol. 1996;13:582–588. doi: 10.1046/j.1365-2346.1996.d01-405.x. [DOI] [PubMed] [Google Scholar]
- 20.Jensen A.G., Dahlgren C., Eintrei C. Propofol decreases random and chemotactic stimulated locomotion of human neutrophils in vitro. Br J Anaesth. 1993;70:99–100. doi: 10.1093/bja/70.1.99. [DOI] [PubMed] [Google Scholar]
- 21.Mikawa K., Akamatsu H., Nishina K., et al. Propofol inhibits human neutrophil functions. Anesth Analg. 1998;87:695–700. doi: 10.1097/00000539-199809000-00039. [DOI] [PubMed] [Google Scholar]
- 22.Cleary T.G., Pickering L.K. Mechanisms of intralipid effect on polymorphonuclear leukocytes. J Clin Lab Immunol. 1983;11:6. [PubMed] [Google Scholar]
- 23.Pirttinkangas C.O., Perttila J., Salo M. Propofol emulsion reduces proliferative responses of lymphocytes from intensive care patients. Intensive Care Med. 1993;19:299–302. doi: 10.1007/BF01690552. [DOI] [PubMed] [Google Scholar]
- 24.Okuno T., Koutsogiannaki S., Ohba M., et al. Intravenous anesthetic propofol binds to 5-lipoxygenase and attenuates leukotriene B4 production. FASEB J. 2017;31:1584–1594. doi: 10.1096/fj.201601095R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller A., Heller S., Blecken S., Urbaschek R., Koch T. Effects of intravenous anesthetics on bacterial elimination in human blood in vitro. Acta Anaesthesiol Scand. 1998;42:518–526. doi: 10.1111/j.1399-6576.1998.tb05160.x. [DOI] [PubMed] [Google Scholar]
- 26.Weiss M., Birkhan A., Krone M., Schneider E.M. Do etomidate and propofol influence oxygen radical production of neutrophils? Immunopharmacol Immunotoxicol. 1996;18:291–307. doi: 10.3109/08923979609052737. [DOI] [PubMed] [Google Scholar]
- 27.Heine J., Jaeger K., Osthaus A., et al. Anaesthesia with propofol decreases FMLP-induced neutrophil respiratory burst but not phagocytosis compared with isoflurane. Br J Anaesth. 2000;85:424–430. doi: 10.1093/bja/85.3.424. [DOI] [PubMed] [Google Scholar]
- 28.Kotani N., Hashimoto H., Sessler D.I., et al. Intraoperative modulation of alveolar macrophage function during isoflurane and propofol anesthesia. Anesthesiology. 1998;89:1125–1132. doi: 10.1097/00000542-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Kotani N., Hashimoto H., Sessler D.I., et al. Expression of genes for proinflammatory cytokines in alveolar macrophages during propofol and isoflurane anesthesia. Anesth Analg. 1999;89:1250–1256. [PubMed] [Google Scholar]
- 30.Heine J., Jaeger K., Weingaertner N., Scheinichen D., Marx G., Piepenbrock S. Effects of different preparations of propofol, diazepam, and etomidate on human neutrophils in vitro. Acta Anaesthesiol Scand. 2001;45:213–220. doi: 10.1034/j.1399-6576.2001.450213.x. [DOI] [PubMed] [Google Scholar]
- 31.Aarts L., van der Hee R., Dekker I., de Jong J., Langemeijer H., Bast A. The widely used anesthetic agent propofol can replace a-tocopherol as an antioxidant. FEBS Lett. 1995;357:83–85. doi: 10.1016/0014-5793(94)01337-z. [DOI] [PubMed] [Google Scholar]
- 32.Welters I.D., Hafer G., Menzebach A., et al. Ketamine inhibits transcription factors activator protein 1 and nuclear factor-kappab, Interleukin-8 production, as well as CD11b and CD16 expression: studies in human leukocytes and leukocytic cell lines. Anesth Analg. 2010;110:934–941. doi: 10.1213/ANE.0b013e3181c95cfa. [DOI] [PubMed] [Google Scholar]
- 33.Loix S., De Kock M., Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62:47–58. [PubMed] [Google Scholar]
- 34.Hirota K K., Ketamine D. G. Lambert. New uses for an old drug? Br J Anaesth. 2011;107:123–126. doi: 10.1093/bja/aer221. [DOI] [PubMed] [Google Scholar]
- 35.Beilin B., Rusabrov Y., Shapira Y., et al. Low-dose ketamine affects immune responses in humans during the early postoperative period. Br J Anaesth. 2007;99:522–527. doi: 10.1093/bja/aem218. [DOI] [PubMed] [Google Scholar]
- 36.Weiss M., Birkhahn A., Mettler S., Schneider M., Wernet P. Stereoselective suppression of neutrophil function by ketamine? Immunopharm Immunotoxicol. 1995;17:91–107. doi: 10.3109/08923979509052723. [DOI] [PubMed] [Google Scholar]
- 37.Weigand M.A., Schmidt H., Zhao Q., Plaschke K., Martin E., Bardenheuer H.J. Ketamine modulates the stimulated adhesion molecule expression on human neutrophils in vitro. Anesth Analg. 2000;90:206–212. doi: 10.1097/00000539-200001000-00041. [DOI] [PubMed] [Google Scholar]
- 38.Mühling J., Weiss S., Knülle V., Sablotzki A., Dehne M.G., Hempelmann G. Effects of etomidate on free intracellular amino acid concentrations in polymorphonuclear leucocytes in vitro. Acta Anaesthesiol Scand. 2000;44:429–435. doi: 10.1034/j.1399-6576.2000.440412.x. [DOI] [PubMed] [Google Scholar]
- 39.Cuthbertson B.H., Sprung C.L., Annane D., et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009;35:1868–1876. doi: 10.1007/s00134-009-1603-4. [DOI] [PubMed] [Google Scholar]
- 40.Hohl C.M., Kelly-Smith C.H., Yeung T.C., Sweet D.D., Doyle-Waters M.M., Schulzer M. The effect of a bolus dose of etomidate on cortisol levels, mortality, and health services utilization: a systematic review. Ann Emerg Med. 2010;56:105–113. doi: 10.1016/j.annemergmed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Chan C.M., Mitchell A.L., Shorr A.F. Etomidate is associated with mortality and adrenal insufficiency in sepsis: a meta-analysis. Crit Care Med. 2012;40:2945–2953. doi: 10.1097/CCM.0b013e31825fec26. [DOI] [PubMed] [Google Scholar]
- 42.Bruder E.A., Ball I.M., Ridi S., Pickett W., Hohl C. Single induction dose of etomidate versus other induction agents for endotracheal intubation in critically ill patients. Cochrane Database Syst Rev. 2015;1:CD010225. doi: 10.1002/14651858.CD010225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsen B., Hoff G., Wilhelm W., Buchinger H., Wanner G.A., Bauer M. Effect of intravenous anesthetics on spontaneous and endotoxin-stimulated cytokine response in cultured human whole blood. Anesthesiology. 1998;89:1218–1227. doi: 10.1097/00000542-199811000-00023. [DOI] [PubMed] [Google Scholar]
- 44.Bochicchio C.V., Napolitano L.M., Joshi M., et al. Persistent systemic inflammatory response syndrome is predictive of nosocomial infection in trauma. J Trauma. 2002;53:245–251. doi: 10.1097/00005373-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Kwon Y.S., Suh G.Y., Jeon K., et al. Serum cytokines and critical illness-related corticosteroid insufficiency. Intensive Care Med. 2010;36:1845–1851. doi: 10.1007/s00134-010-1971-9. [DOI] [PubMed] [Google Scholar]
- 46.Gu W.J., Wang F., Tang L., Liu J.C. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Chest. 2015;147:335–346. doi: 10.1378/chest.14-1012. [DOI] [PubMed] [Google Scholar]
- 47.Vinclair M., Broux C., Faure P., et al. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34:714–719. doi: 10.1007/s00134-007-0970-y. [DOI] [PubMed] [Google Scholar]
- 48.Warner K.J., Cuschieri J., Jurkovich G.J., Bulger E.M. Single-dose etomidate for rapid sequence intubation may impact outcome after severe injury. J Trauma. 2009;67:45–50. doi: 10.1097/TA.0b013e3181a92a70. [DOI] [PubMed] [Google Scholar]
- 49.Longnecker D.E. Stress free: to be or not to be? Anesthesiology. 1984;61:643–644. [PubMed] [Google Scholar]
- 50.Asehnoune K., Mahe P.J., Seguin P., et al. Etomidate increases susceptibility to pneumonia in trauma patients. Intensive Care Med. 2012;38:1673–1682. doi: 10.1007/s00134-012-2619-8. [DOI] [PubMed] [Google Scholar]
- 51.Sunshine J.E., Deem S., Weiss N.S., et al. Etomidate, adrenal function, and mortality in critically ill patients. Respir Care. 2013;58:639–646. doi: 10.4187/respcare.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watt I., Ledingham I.M. Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anaesthesia. 1984;39:973–981. doi: 10.1111/j.1365-2044.1984.tb08885.x. [DOI] [PubMed] [Google Scholar]
- 53.Lopez E., Bruner E., Kim S., et al. Etomidate in trauma patients is associated with inflammation and pulmonary dysfunction [ASA Abstracts online] http://www.asaabstracts.com/strands/asaabstracts/abstract.htm?year=2019&index=6&absnum=1805 Available at:
- 54.Kurosawa S., Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–277. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 55.Cavalcanti V., Santos C.L., Samary C.S., et al. Effects of short-term propofol and dexmedetomidine on pulmonary morphofunction and biological markers in experimental mild acute lung injury. Respir Physiol Neurobiol. 2014;203:45–50. doi: 10.1016/j.resp.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhu S., Lu Y. Dexmedetomidine suppressed the biological behavior of HK-2 cells treated with LPS by down-regulating ALKBH5. Inflammation. 2020;43:2256–2263. doi: 10.1007/s10753-020-01293-y. [DOI] [PubMed] [Google Scholar]
- 57.Chen J., Jiang Z., Zhou X., et al. Dexmedetomidine preconditioning protects cardiomyocytes against hypoxia/reoxygenation-induced necroptosis by inhibiting HMGB1-mediated inflammation. Cardiovasc Drugs Ther. 2019;33:45–54. doi: 10.1007/s10557-019-06857-1. [DOI] [PubMed] [Google Scholar]
- 58.Ji X., Guo Y., Zhou G., et al. Dexmedetomidine protects against high mobility group box 1-induced cellular injury by inhibiting pyroptosis. Cell Biol Int. 2019;43:651–657. doi: 10.1002/cbin.11140. [DOI] [PubMed] [Google Scholar]
- 59.Xiang H., Hu B., Li Z., Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37:1763–1770. doi: 10.1007/s10753-014-9906-1. [DOI] [PubMed] [Google Scholar]
- 60.Hu J., Vacas S., Feng X., et al. Dexmedetomidine prevents cognitive decline by enhancing resolution of high mobility group box 1 protein-induced inflammation through a vagomimetic action in mice. Anesthesiology. 2018;128:921–931. doi: 10.1097/ALN.0000000000002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20:9–15. [PubMed] [Google Scholar]
- 62.Al-Hashimi M., Scott W.M., Thompson J.P., Lambert D.G. Editor's choice: opioids and immune modulation: more questions than answers. Br J Anaesth. 2013;111:80–88. doi: 10.1093/bja/aet153. [DOI] [PubMed] [Google Scholar]
- 63.Hernandez M., Flores L., Bayer B. Immunosuppression by morphine is mediated by central pathways. J Pharmacol Exp Ther. 1993;267:1336–1341. [PubMed] [Google Scholar]
- 64.Hall D.M., Suo J.L., Weber R.J. Opioid mediated effects on the immune system: sympathetic nervous system involvement. J Neuroimmunol. 1998;83:29–35. doi: 10.1016/s0165-5728(97)00218-x. [DOI] [PubMed] [Google Scholar]
- 65.Jacobs R., Karst M., Scheinichen D., et al. Effects of fentanyl on cellular immune functions in man. Int J Immunopharmacol. 1999;21:445–454. doi: 10.1016/s0192-0561(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 66.Welters I.D., Menzebach A., Goumon Y., et al. Morphine suppresses complement receptor expression, phagocytosis, and respiratory burst in neutrophils by a nitric oxide and mu(3) opiate receptor-dependent mechanism. J Neuroimmunol. 2000;11:139–145. doi: 10.1016/s0165-5728(00)00401-x. [DOI] [PubMed] [Google Scholar]
- 67.Sacerdote P., Gaspani L., Rossoni G., Panerai A.E., Bianchi M. Effect of the opioid remifentanil on cellular immune response in the rat. Int J Immunopharmacol. 2001;1:713–719. doi: 10.1016/s1567-5769(01)00005-4. [DOI] [PubMed] [Google Scholar]
- 68.Baek S.B., Shin M.S., Han J.H., et al. Rocuronium bromide inhibits inflammation and pain by suppressing nitric oxide production and enhancing prostaglandin E2 synthesis in endothelial cells. Int Neurourol J. 2016;20:296–303. doi: 10.5213/inj.1632796.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fanelli V., Morita Y., Cappello P., et al. Neuromuscular blocking agent cisatracurium attenuates lung injury by inhibition of nicotinic acetylcholine receptor-α1. Anesthesiology. 2016;124:132–140. doi: 10.1097/ALN.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 70.Forel J.M., Roch A., Marin V., et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 71.Papazian L., Forel J.M., Gacouin A., et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 72.Alhazzani W., Alshahrani M., Jaeschke R., et al. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17:43. doi: 10.1186/cc12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He T T., Tao J., Wang X., Wang X. Effects of cisatracurium in combination with ventilation on inflammatory factors and immune variations in sepsis rats. Exp Ther Med. 2018;15:4414–4418. doi: 10.3892/etm.2018.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang J., Yang B., Han G., Yang M., Li S. Early administration of cisatracurium attenuates sepsis-induced diaphragm dysfunction in rats. Inflammation. 2015;38:305–311. doi: 10.1007/s10753-014-0034-8. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez Palacios A., Ortiz Ponce M., Rodríguez Pérez A., Schamann Medina F., García Marrero J.A. Modification of mediators of immune reaction after general anaesthesia. Allergol Immunopathol. 2004;32:352–360. doi: 10.1016/s0301-0546(04)79268-x. [DOI] [PubMed] [Google Scholar]
- 76.Stelzhammer V., Rothermundt M., Guest P.C., et al. Proteomic changes induced by anaesthesia and muscle relaxant treatment prior to electroconvulsive therapy. Proteomics Clin Appl. 2011;5:644–649. doi: 10.1002/prca.201100040. [DOI] [PubMed] [Google Scholar]
- 77.Lassen H.C., Henriksen E., Neukirch F., Kristensen H.S. Treatment of tetanus; severe bone-marrow depression after prolonged nitrous-oxide anaesthesia. Lancet. 1956;270:527–530. doi: 10.1016/s0140-6736(56)90593-1. [DOI] [PubMed] [Google Scholar]
- 78.Seidu S., Gillies C., Zaccardi F., et al. The impact of obesity on severe disease and mortality in people with SARS-CoV-2: a systematic review and meta-analysis. Endocrinol Diab, Metab. 2020 doi: 10.1002/edm2.176. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pal A., Gowdy K.M., Oestreich K.J., Beck M., Shaikh S.R. Obesity-driven deficiencies of specialized pro-resolving mediators may drive adverse outcomes during SARS-CoV-2 infection. Front Immunol. 2020;11:1997. doi: 10.3389/fimmu.2020.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chalkias A., Nitsotolis T., Papalexandrou A., Mikros S., Iacovidou N., Xanthos T. Sagittal abdominal diameter may effectively predict future complications and increased mortality in intensive care unit patients with severe sepsis. J Crit Care. 2013;28:964–969. doi: 10.1016/j.jcrc.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 81.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panigrahy D., Gilligan M.M., Huang S., et al. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer. Metastasis Rev. 2020;39:337–340. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chalkias A., Mouzarou A., Samara E., Xanthos T., Ischaki E., Pantazopoulos I. Soluble urokinase plasminogen activator receptor: a biomarker for predicting complications and critical care admission of COVID-19 patients. Mol Diagn Ther. 2020;24:517–521. doi: 10.1007/s40291-020-00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pantazopoulos I., Daniil Z., Moylan M., et al. Nasal high flow use in COPD patients with hypercapnic respiratory failure: treatment algorithm & review of the literature. COPD. 2020;17:101–111. doi: 10.1080/15412555.2020.1715361. [DOI] [PubMed] [Google Scholar]
- 85.Chalkias A., Xanthos T., Papageorgiou E., Anania A., Beloukas A., Pavlopoulos F. Intraoperative initiation of a modified ARDSNet protocol increases survival of septic patients with severe acute respiratory distress syndrome. Heart Lung. 2018;47:616–621. doi: 10.1016/j.hrtlng.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 86.Murphy R.C., Gijón M.A. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 87.Schmelzer K.R., Kubala L., Newman J.W., Kim I.H., Eiserich J.P., Hammock B.D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spector A.A., Fang X., Snyder G.D., Weintraub N.L. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 89.Inada T., Hirota K., Shingu K. Intravenous anesthetic propofol suppresses prostaglandin E2 and cysteinyl leukotriene production and reduces edema formation in arachidonic acid-induced ear inflammation. J Immunotoxicol. 2015;12:261–265. doi: 10.3109/1547691X.2014.938874. [DOI] [PubMed] [Google Scholar]
- 90.Wepler M., Beloiartsev A., Buswell M.D., et al. Soluble epoxide hydrolase deficiency or inhibition enhances murine hypoxic pulmonary vasoconstriction after lipopolysaccharide challenge. Am J Physiol Lung Cell. Mol Physiol. 2016;311:1213–1221. doi: 10.1152/ajplung.00394.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gartung A., Yang J., Sukhatme V.P., et al. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc Natl Acad Sci USA. 2019;116:1698–1703. doi: 10.1073/pnas.1803999116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kandhi S., Zhang B., Froogh G., et al. EETs promote hypoxic pulmonary vasoconstriction via constrictor prostanoids. Am J Physiol Lung Cell. Mol Physiol. 2017;313:350–359. doi: 10.1152/ajplung.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morisseau C., Goodrow M.H., Dowdy D., et al. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA. 1999;96:8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajapakse N.W., Roman R.J., Falck J.R., Oliver J.J., Evans R.G. Modulation of V1-receptor-mediated renal vasoconstriction by epoxyeicosatrienoic acids. Am J Physiol Regul Integr Comp Physiol. 2004;287:181–187. doi: 10.1152/ajpregu.00555.2002. [DOI] [PubMed] [Google Scholar]
- 95.Azam T.U., Shadid H.R., Blakely P., et al. International study of inflammation in COVID-19. Soluble urokinase receptor (SuPAR) in COVID-19-related AKI. J Am Soc Nephrol. 2020;31:2725–2735. doi: 10.1681/ASN.2020060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seubert J.M., Sinal C.J., Graves J., et al. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Motoki A., Merkel M.J., H Packwood W., et al. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol. 2008;295:2128–2134. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gross G.J., Baker J.E., Hsu A., Wu H.E., Falck J.R., Nithipatikom K. Evidence for a role of opioids in epoxyeicosatrienoic acid-induced cardioprotection in rat hearts. Am J Physiol Heart Circ Physiol. 2010;298:2201–2207. doi: 10.1152/ajpheart.00815.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neckář J., Hsu A., Hye Khan M.A., et al. Infarct size-limiting effect of epoxyeicosatrienoic acid analog EET-B is mediated by hypoxia-inducible factor-1α via downregulation of prolyl hydroxylase 3. Am J Physiol Heart Circ Physiol. 2018;315:1148–1158. doi: 10.1152/ajpheart.00726.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Appelberg S., Gupta S., Svensson Akusjärvi S., et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg. Microbes Infect. 2020;9:1748–1760. doi: 10.1080/22221751.2020.1799723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tanaka K., Kersten J.R., Riess M.L. Opioid-induced cardioprotection. Curr Pharm Des. 2014;20:5696–5705. doi: 10.2174/1381612820666140204120311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chalkias A., Koutsovasilis A., Laou E., Papalois A., Xanthos T. Measurement of mean systemic filling pressure after severe hemorrhagic shock in swine anesthetized with propofol-based total intravenous anesthesia: implications for vasopressor-free resuscitation. Acute Crit Care. 2020;35:93–101. doi: 10.4266/acc.2019.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vinson D.R., Bradbury D.R. Etomidate for procedural sedation in emergency medicine. Ann Emerg Med. 2002;39:592–598. doi: 10.1067/mem.2002.123695. [DOI] [PubMed] [Google Scholar]
- 104.Devlin R.J., Kalil D. Etomidate as an induction agent in sepsis. Crit Care Nurs Clin North Am. 2018;30:1–9. doi: 10.1016/j.cnc.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 105.Sprung C.L., Annane D., Keh D., et al. CORTICUS Study Group Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 106.Hinkewich C., Green R. The impact of etomidate on mortality in trauma patients. Can J Anaesth. 2014;61:650–655. doi: 10.1007/s12630-014-0161-6. [DOI] [PubMed] [Google Scholar]
- 107.Spyropoulos V., Chalkias A., Georgiou G., et al. Initial immune response in Escherichia coli, Staphylococcus aureus, and Candida albicans bacteremia. Inflammation. 2020;43:179–190. doi: 10.1007/s10753-019-01108-9. [DOI] [PubMed] [Google Scholar]
- 108.Chalkias A., Spyropoulos V., Koutsovasilis A., Papalois A., Kouskouni E., Xanthos T. Cardiopulmonary arrest and resuscitation in severe sepsis and septic shock: a research model. Shock. 2015;43:285–291. doi: 10.1097/SHK.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 109.Webster J.I., Tonelli L., Sternberg E.M. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 110.Tassopoulos A., Chalkias A., Papalois A., et al. Assessment of post-resuscitation intestinal injury and timing of bacterial translocation in swine anaesthetized with propofol-based total intravenous anaesthesia. Cureus. 2020;12 doi: 10.7759/cureus.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hantoushzadeh S., Norooznezhad A.H. Possible cause of inflammatory storm and septic shock in patients diagnosed with (COVID-19) Arch Med Res. 2020;51:347–348. doi: 10.1016/j.arcmed.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:440–469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Horby P., Lim W.S., Emberson J.R., et al. RECOVERY Collaborative Group, Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sterne J.A.C., Murthy S., Diaz J.V., et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]


