Abstract
Severe acute respiratory syndrome coronavirus 2 causes direct damage to the airway epithelium, enabling aspergillus invasion. Reports of COVID-19-associated pulmonary aspergillosis have raised concerns about it worsening the disease course of COVID-19 and increasing mortality. Additionally, the first cases of COVID-19-associated pulmonary aspergillosis caused by azole-resistant aspergillus have been reported. This article constitutes a consensus statement on defining and managing COVID-19-associated pulmonary aspergillosis, prepared by experts and endorsed by medical mycology societies. COVID-19-associated pulmonary aspergillosis is proposed to be defined as possible, probable, or proven on the basis of sample validity and thus diagnostic certainty. Recommended first-line therapy is either voriconazole or isavuconazole. If azole resistance is a concern, then liposomal amphotericin B is the drug of choice. Our aim is to provide definitions for clinical research and up-to-date recommendations for clinical management of the diagnosis and treatment of COVID-19-associated pulmonary aspergillosis.
Introduction
Viral pneumonia increases patients' susceptibility to bacterial and fungal superinfections, including invasive pulmonary aspergillosis (IPA). Influenza-associated pulmonary aspergillosis (IAPA) has complicated the clinical course of many critically ill patients with acute respiratory distress syndrome (ARDS).1, 2 By use of the European Organisation for Research and Treatment of Cancer and the Mycosis Study Group Education and Research Consortium definitions for invasive fungal disease (IFD), it was found that many patients with IAPA could not be classified, leading to missed diagnoses and raising the question of whether current definitions for IFD adequately address all patient populations.3 In December, 2019, COVID-19 emerged from Wuhan, China, and has become pandemic.4 There have been several reports of COVID-19-associated pulmonary aspergillosis (CAPA), raising concerns about this superinfection as an additional contributing factor to mortality.5, 6, 7, 8, 9, 10 Indeed, in a prospective cohort of 108 critically ill patients with ARDS, a higher 30-day mortality was observed in patients with CAPA than in patients without aspergillosis (44% vs 19%), and the association of COVID-19-associated fungal disease with mortality was also supported by another study.11, 12 The population of patients with CAPA harbours many baseline prognostic factors with negative effects on survival,13 which might be further compromised by azole-resistant CAPA, with an increasing number of patients reported in the literature.14, 15, 16
Respiratory viruses cause direct damage to the airway epithelium, enabling aspergillus to invade tissue.17 Furthermore, viral infection hampers ciliary clearance and leads to immune dysfunction or dysregulation, or both, locally or systemically.18 The extent of dysregulation that is associated with ARDS is not yet fully understood, however, some patients develop pronounced immunosuppression, facilitating bacterial and fungal superinfection. Moreover, a distinctive immune-cell event that is observed in patients with COVID-19 is the decrease of T-cell populations, especially in patients with severe disease.19 Decline of lymphocyte counts can be accompanied by defective function. Severe lymphopenia has been established as a factor predicting the risk of invasive mould disease in patients with haematological malignancies.20
Key messages.
-
•
The increasing number of reports on COVID-19-associated invasive pulmonary aspergillosis (CAPA) raise concerns about this superinfection as an additional contributing factor to mortality
-
•
The European Confederation for Medical Mycology and the International Society for Human and Animal Mycology instituted a group of experts to propose consensus criteria for a case definition of CAPA and to provide up-to-date management recommendations for the diagnosis and treatment of patients with CAPA
-
•
Three different grades are proposed (ie, possible, probable, and proven CAPA) to enable researchers to homogeneously classify patients in registries and interventional clinical trials
-
•
Voriconazole or isavuconazole are recommended as first-line treatment for possible, probable, and proven CAPA
-
•
Over time, new insights will be used to further improve the definitions and the management algorithms
Early case series of patients with presumed CAPA indicate that obtaining a diagnosis can be challenging. Although host factors, clinical factors (including radiology), and mycological evidence are often used to diagnose and classify patients with IFD, patients with CAPA might not have host factors and typical radiological features.5, 6, 7, 8, 9, 10, 11 Obtaining mycological evidence of airway-invasive aspergillosis in patients with COVID-19 is complicated by decreased use of diagnostic bronchoscopy, which is necessary to protect health-care workers from aerosol exposure,21, 22 and the low sensitivity of detection of circulating galactomannan in serum.11 Further, detection of aspergillus in specimens of the upper respiratory tract, such as sputum or tracheal aspirate, often does not distinguish between aspergillus colonisation and invasive disease.
Given the challenges that are associated with diagnosis and management of patients with CAPA, there is an urgent need to study the epidemiology and characteristics of this secondary infection. Therefore, the European Confederation for Medical Mycology and the International Society for Human and Animal Mycology instituted a group of experts to propose consensus criteria for a case definition of CAPA and to provide up-to-date management recommendations for the diagnosis and treatment of patients with CAPA.
Methods
Through endorsement of the European Confederation for Medical Mycology, the International Society for Human and Animal Mycology, the INFOCUS Latin American International Society for Human and Animal Mycology associated Working Group, the European Society for Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, and the European Society for Clinical Microbiology and Infectious Diseases Study Group for Infections in Critically-Ill Patients, PK and OAC were assigned to invite experts to participate in this specific set of clinical recommendations in May, 2020. The selection of experts was determined by publication activity associated with CAPA, their personal involvement in patient management, and their affiliation to the participating scientific societies. The group comprised 22 experts from six continents and 14 countries. Small groups were charged with literature review of particular topics and contributing subsections to a draft manuscript. All experts reviewed and commented on the manuscript in several rounds until consensus was achieved, and a final version was circulated for approval.
CAPA characteristics and host factors
IPA is emerging as a serious secondary infection in patients with COVID-19 and ARDS, and two studies have indicated excess mortality rates of 16% and 25% compared with patients without evidence for aspergillosis.11, 12 These excess mortality rates are similar to that found for patients with IAPA, in whom the survival rate in intensive care units (ICUs) was 24% lower than in patients without this secondary infection.1 CAPA diagnosis was associated with ICU mortality in a logistic regression model, even after adjustment for age, need for renal replacement therapy, and sequential organ failure assessment score at ICU admission.11 There are evident similarities between IAPA and CAPA, including high prevalence, absence of classic host factors for invasive fungal infection, similar timing in the disease diagnosis after ICU admission, and the presence of lymphopenia. However, it is unclear whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection itself is the main risk factor for CAPA, or whether additional risk factors, such as corticosteroid therapy, further increase the risk for disease progression. In the study by Bartoletti and colleagues, most patients received anti-interleukin (IL)-6 treatment with tocilizumab, as well as corticosteroids.11 Indeed, chronic corticosteroid treatment was substantially more frequent in patients with CAPA, and corticosteroid use was more frequent in patients who did not survive.11, 12 Furthermore, dexamethasone treatment of patients with severe COVID-19 is likely to increase in the near future, saving many lives, as shown in the RECOVERY trial.23 Dexamethasone treatment and anti-IL-6-directed strategies might, however, also result in an increased susceptibility to superinfections,23, 24 including IPA in patients with severe COVID-19,25 and could lead to increases in CAPA incidence, emphasising the need for guidance.
Imaging
The typical appearance of COVID-19 in patients includes peripheral, bilateral, ground-glass opacities with or without consolidation or visible intralobular lines (ie, so-called crazy paving) in early stages; multifocal ground-glass opacities of rounded morphology with or without consolidation or visible intralobular lines (ie, crazy paving) at peak stage; and reverse halo sign or other findings of organising pneumonia at late stages.26 Additionally, indeterminate and atypical appearance can also occur.
One major difficulty in patients with COVID-19 is the specificity of CT patterns, especially in late stages. As an example, typical widespread ground-glass opacities with non-rounded morphology and non-specific signs could also be associated with an acute lung injury from presumed drug toxicity or even be misinterpreted as pneumocystis pneumonia. Atypical features of COVID-19 can be suggestive of other diseases, particularly other infections, such as lobar or segmental consolidation in the setting of bacterial pneumonia, cavitation from necrotising pneumonia, and tree-in-bud opacities with centrilobular nodules. In this context, many atypical signs of COVID-19 pneumonia can mimic IPA, and vice versa, and radiology alone is not sufficient to define patients with CAPA. There is additional complexity in patients with ARDS, who can present with multiple processes, such as mixed infections or drug toxicities. Indeed, lesions suggestive of IPA can be hidden or mimicked by lung involvement in patients with severe COVID-19. However, use of imaging as a reliable criterion for a case definition of CAPA is debatable, since similar features can be caused by COVID-19 alone (appendix p 3).
Despite all of the limitations previously given, the following statement can be made for critically ill patients with COVID-19: multiple pulmonary nodules or lung cavitation should prompt thorough investigation for IPA, as they are rarely seen with COVID-19 alone and have been described in a small proportion of patients with CAPA to date.12 Frequently observed radiological features of IPA, such as the halo sign, are not sufficient to define CAPA without mycological evidence. This feature is insufficient because the halo sign suggests local infarction, and an intrinsic part of severe COVID-19 is in-situ thrombosis due to endotheliopathy.
Mycological evidence
Respiratory samples are the preferred specimens for fungal diagnostics. So far, diagnostic bronchoscopy in patients with COVID-19 has had a small role due to its nature of aerosol generation and high risk of viral transmission.27 However, bronchoscopy allows direct inspection of the trachea and bronchi to identify patients with aspergillus tracheobronchitis.28 Bronchoscopy is primarily indicated in patients with suspected secondary infection, especially if the patient has already tested negative for SARS-CoV-2.
For diagnosis of IPA, bronchoalveolar lavage fluid and lung biopsy samples are the specimens of choice. Tissue culture and tissue microscopy showing invasive growth of septate fungal hyphae of primarily sterile specimens represent the diagnostic gold standard in proving infection. However, biopsies are high-risk procedures in this patient population and, therefore, are avoided by many clinicians (table 1 ).
Table 1.
Pros and cons of diagnostic procedures and their samples in patients with COVID-19
| Pros | Cons | Comments related to CAPA | |
|---|---|---|---|
| Lung biopsy | Provides proof of IPA | Risk of sampling error; scarcely used due to high risk of complications | CT-guided biopsies post mortem have been used as alternative to autopsy29 |
| Bronchoscopy with bronchoalveolar lavage | Allows visualisation of lesions (eg, plaques); bronchoalveolar lavage well validated for the diagnosis of IPA and IAPA; validated specimen for aspergillus antigen test (eg, enzyme immunoassay and lateral flow assay) and PCR; targeted sampling possible | Aerosol generation and contamination of surfaces | In some centres, use is decreased because of risk of nosocomial transmission and SARS-CoV-2 infection of health-care workers;22, 30 SARS-CoV-2 infectiousness correlates with PCR-signal strength, which can be used as guidance on when it's safe to perform bronchoscopy31, 32, 33 |
| Non-bronchoscopic lavage | Obtains material from lower respiratory tract; technique validated for diagnosis of ventilator-associated pneumonia; closed-system sampling | Not fully validated for IPA diagnosis; not fully validated for aspergillus antigen and PCR detection; non-targeted sampling | Suggested as alternative to bronchoalveolar lavage to diagnose CAPA; small number of validation studies12, 34 |
| Tracheal aspirate | Easy to obtain in patients who are intubated | Less representative of lower respiratory tract than is bronchoalveolar lavage; not validated for biomarker detection | Often positive in patients with COVID-19 who are critically ill but can represent upper airway colonisation |
| Sputum | Easy to obtain in most patients | Less representative of lower respiratory tract than is bronchoalveolar lavage; not validated for biomarker detection | Often positive in patients with COVID-19 who are critically ill but can represent upper airway colonisation |
| Serum | Highly indicative for IPA (galactomannan, lateral flow assay, and PCR); validated specimen for galactomannan, lateral flow assay, (1–3)-β-D-glucan, and PCR; easy to obtain | Variable performance in non-neutropenic patients; (1–3)-β-D-glucan not pathogen specific | Commonly negative in CAPA, including proven cases11 |
CAPA=COVID-19-associated invasive pulmonary aspergillosis. IAPA=influenza-associated pulmonary aspergillosis. IPA=invasive pulmonary aspergillosis.
Detection of galactomannan in bronchoalveolar lavage fluid is highly indicative of IPA, as the antigen is released during active fungal growth. To date, galactomannan in bronchoalveolar lavage has been the main diagnostic test to diagnose secondary IPA in patients with severe viral infection, although validation in patients with histologically confirmed COVID-19 is still scarce.35, 36 Detection of galactomannan in bronchoalveolar lavage does not prove tissue invasion, and the likelihood of infection is increased if circulating galactomannan is detected. Unfortunately, the diagnostic yield of serum galactomannan is low in CAPA as, at best, 20% of patients showed positive results, and proven CAPA cases have been reported with negative serum galactomannan.9, 12 This low sensitivity is in line with published performance of serum galactomannan detection in non-neutropenic patients in ICUs but lower than the 65% sensitivity of serum galactomannan in patients with IAPA. Overall, serum galactomannan has decreased value for excluding CAPA.37
Use of not only galactomannan but also another biomarker, namely (1–3)-β-D-glucan, for serum screening might be beneficial. A study unrelated to COVID-19, comparing patients in the ICU with proven or probable IFD with patients with fungal colonisation and without IFD, showed that two consecutive positive test results for serum (1–3)-β-D-glucan generate a specificity of 90%.38 Two consecutive results for serum (1–3)-β-D-glucan might, therefore, increase suspicion of invasive aspergillosis, although (1–3)-β-D-glucan is not specific for aspergillosis and other causes of elevated serum concentration of (1–3)-β-D-glucan need to be excluded.12
The concept of lateral flow assays (LFAs) or lateral flow devices (LFDs) for the diagnosis of IPA was proposed over a decade ago and has been used to successfully test blood and bronchoalveolar lavage.39, 40, 41 The two commercially manufactured LFD (ie, AspLFD, OLM Diagnostics, Newcastle upon Tyne, UK) and LFA (ie, IMMY sona Aspergillus Galactomannan Lateral Flow Assay, IMMY, Norman, OK, USA) tests can be used with a visual reader, which provides a semiquantitative reading and removes subjectivity when interpreting results.41 Performance for the detection of IPA appears to be optimum when testing bronchoalveolar lavage over serum, and the LFA potentially provides superior sensitivity over the LFD.40, 42, 43 Although the lateral flow testing of bronchoalveolar lavage for IPA appears to be reliable, specific data for the diagnosis of CAPA are scarce. When testing non-bronchoscopic lavage or bronchoalveolar lavage from 23 patients with putative CAPA by use of the LFA, agreement with the enzyme immunoassay for galactomannan was excellent (κ=0·702). Although a galactomannan-index threshold of 1·0 provided optimal combined performance (sensitivity=83%; specificity=87%), use of thresholds greater than 1·5 generated specificities greater than 90% and positive likelihood ratios (ie, >10) that were sufficient to confirm CAPA.44 Further multicentre evaluation of lateral flow testing for CAPA is required but data indicate that results are similar to the enzyme immunoassay for galactomannan.12 The lateral flow test is simple and can be done in both containment-level-3 facilities and outside specialist mycology centres, with positivity thresholds equivalent to galactomannan testing of bronchoalveolar lavage and serum.
In 2020, aspergillus PCR was included in consensus guidelines for defining IFD, with the requirement of two positive results providing sufficient specificity to confirm a diagnosis.3 Performance related to CAPA is yet unknown but likely to be similar to other non-haematological populations. Consequently, bronchoalveolar lavage testing is preferable, and although the enhanced sensitivity of PCR means it can detect Aspergillus spp that are colonising or contaminating the airways, PCR testing of bronchoalveolar lavage provides specificity that is at least similar to that of galactomannan testing.45 In the presence of clinical or radiological evidence typical of IPA, a single positive bronchoalveolar lavage result from the infected lobe is likely to be indicative of IPA, and galactomannan testing is usually concordant. As with other biomarkers, detection of aspergillus DNA in the bloodstream of non-haematological populations will likely be low, but PCR positivity is indicative of IPA, although multiple blood positives increase specificity (ie, to >95%).46 Evidence for the testing of non-bronchoscopic lavage (considered to be a blind application of 10–20 mL saline recovered by aspiration via a closed suction system in a patient who is intubated) is scarce in patients with and without CAPA. Efforts have improved methodological standardisation and commercial PCR assays provide quality control and methodological consistency, with the added potential to identify genetic markers that are associated with antifungal resistance.
Current guidelines for invasive aspergillosis and concerns in defining CAPA
Since 2002, consensus definitions have been published by the European Organisation for Research and Treatment of Cancer and the Mycosis Study Group Education and Research Consortium to classify invasive mycoses in patients who are immunosuppressed (table 2 ). This classification relies on host factors, clinical factors (including imaging), and mycological evidence.3 The definitions have been broadly used to classify IPA in patients who are immunosuppressed, especially patients with haematological malignancies, but it became apparent that many critically ill patients could not be classified by use of the criteria, mostly due to an absence of required host factors. In the 2020 update, patients in ICUs were not included as a consensus could not be reached.3 Although the definitions include aspergillus PCR and (1–3)-β-D-glucan as mycological evidence, (1–3)-β-D-glucan is not recommended for use in clinical trials or for defining IPA.3 Furthermore, aspergillus PCR data have been evaluated most extensively in adults with haematological malignancies and stem-cell transplantation, and results might not be transferable to patients in ICUs.3 In 2012, a clinical algorithm was published (AspICU) to help to distinguish aspergillus colonisation from probable or putative IPA in patients in ICUs.47 This clinical algorithm relies on aspergillus culture from lower respiratory tract specimens as an entry criterion and takes host factors and clinical factors into account (table 2). As galactomannan has become an important biomarker for aspergillus diagnosis, it has been recommended to include galactomannan detection as mycological evidence in the AspICU criteria.38, 48 However, with the emergence of IAPA, a substantial proportion of patients with IAPA could not be classified by use of the European Organisation for Research and Treatment of Cancer and the Mycosis Study Group Education and Research Consortium definitions or the AspICU algorithm. Both sets of criteria rely on defined host factors for classification of patients, which might be absent in patients who develop IAPA.1 Furthermore, patients with IAPA can present with atypical pulmonary lesions—for instance, in patients with invasive tracheobronchitis—which preclude classification. Therefore, an expert group proposed definitions for IAPA, distinguishing between invasive aspergillus tracheobronchitis and other pulmonary manifestations of IPA (table 2).49 The case definitions for IAPA rely on an entry criterion of a patient requiring ICU admission for respiratory distress with a temporally related positive influenza test plus mycological evidence, mainly serum and bronchoalveolar lavage galactomannan, to classify patients.
Table 2.
Comparison of case definitions for patients with possible, probable, putative, or proven IPA by algorithm or group
| Host factors | Clinical factors | Mycological evidence | Comments | |
|---|---|---|---|---|
| EORTC and MSGERC3 | ||||
| Probable invasive aspergillosis | One of the following: recent history of neutropenia (<0·5 × 109 neutrophils per L for >10 days) temporally related to the onset of invasive fungal disease; haematological malignancy; receipt of an allogeneic stem-cell transplant; receipt of a solid organ transplant; prolonged use of corticosteroids (excluding among patients with allergic bronchopulmonary aspergillosis) at a therapeutic dose of ≥0·3 mg/kg corticosteroids for ≥3 weeks in the past 60 days; treatment with other recognised T-cell immunosuppressants, such as calcineurin inhibitors, TNF blockers, lymphocyte-specific monoclonal antibodies, and immunosuppressive nucleoside analogues, during the past 90 days; treatment with recognised inhibitors of B-cell receptor pathway (eg, ibrutinib), possibly BCL2 inhibitors (eg, venetoclax); inherited severe immunodeficiency (eg, chronic granulomatous disease, STAT3 deficiency, or severe combined immunodeficiency); or acute graft-versus-host disease grade III or IV, involving the gut, lungs, or liver, that is refractory to first-line treatment with steroids | Pulmonary aspergillosis (one of the following four patterns on CT: dense, well circumscribed lesions with or without a halo sign, air crescent sign, cavity, or wedge-shaped and segmental or lobar consolidation); or tracheobronchitis (one of the following: tracheobronchial ulceration, nodule, pseudomembrane, plaque, or eschar seen on bronchoscopic analysis) | One of the following: microscopic detection of fungal elements in sputum, bronchoalveolar lavage, bronchial brush, or aspirate indicating a mould; aspergillus recovered by culture of bronchoalveolar lavage or bronchial brush (ie, tracheobronchitis); galactomannan detected in plasma, serum, bronchoalveolar lavage, or cerebrospinal fluid (one of the following: single serum or plasma galactomannan ≥1·0, bronchoalveolar lavage fluid galactomannan ≥1·0, single serum or plasma galactomannan ≥0·7 and bronchoalveolar lavage fluid galactomannan ≥0·8, or cerebrospinal fluid ≥1·0); or aspergillus PCR (one of the following: two or more positive consecutive PCR tests on plasma, serum, or whole blood; or two or more positive PCR tests on bronchoalveolar lavage fluid) | Invasive fungal disease definitions in patients in ICUs were excluded; absence of host factors and radiological features prevent its use for classification of patients with IAPA and CAPA |
| Asp ICU47 | ||||
| Putative IPA | Host risk factors (one of the following: neutropenia [absolute neutrophil count 500/mm3] preceding or at the time of ICU admission, underlying haematological or oncological malignancy treated with cytotoxic agents, glucocorticoid treatment [prednisone equivalent, 20 mg/day], or inborn or acquired immunodeficiency);* or mycological criterion (see mycological evidence) | Compatible signs and symptoms (one of the following: fever refractory to at least 3 days of appropriate antibiotic therapy, recrudescent fever after a period of defervescence of at least 48 h while still on antibiotics and without other apparent cause, pleuritic chest pain, pleuritic rub, dyspnoea, haemoptysis, worsening respiratory insufficiency despite appropriate antibiotic therapy and ventilatory support); and abnormal medical imaging by portable chest x-ray or CT scan of the lungs | Aspergillus-positive culture from lower respiratory tract specimen (entry criterion); and semiquantitative aspergillus-positive culture of bronchoalveolar lavage fluid without bacterial growth, together with a positive cytological smear showing branching hyphae* | For putative IPA classification, one host risk factor, one compatible sign or symptom, abnormal medical imaging, and lower respiratory tract specimen positive for aspergillus are needed (if ≥1 criterion is not met, then the patient is classified as having aspergillus colonisation); modified AspICU criteria include galactomannan above threshold value, even in the absence of host factor (for classifying patients with IAPA) |
| Expert case definitions for IAPA48 | ||||
| Tracheobronchitis (probable) | Influenza-like illness, positive influenza PCR or antigen, and temporal relationship (entry criterion) | Airway plaque, pseudomembrane, or ulcer | At least one of the following: serum galactomannan index >0·5, bronchoalveolar lavage galactomannan index ≥1·0, positive bronchoalveolar lavage culture, positive non-bronchoscopic lavage culture, positive sputum culture, or hyphae in direct microscopy consistent with Aspergillus spp | .. |
| Other pulmonary forms (probable) | Influenza-like illness, positive influenza PCR or antigen, and temporal relationship (entry criterion) | Pulmonary infiltrate (not attributed to another cause) | At least one of the following: serum galactomannan index >0·5, bronchoalveolar lavage galactomannan index ≥1·0, or positive bronchoalveolar lavage culture | .. |
| Other pulmonary forms (probable) | Influenza-like illness, positive influenza PCR or antigen, and temporal relationship (entry criterion) | Cavitating infiltrate (not attributed to another cause) | One of the following: positive sputum culture or positive tracheal aspirate culture | .. |
| Proposed case definition for CAPA (adapted from EORTC and MSGERC3, AspICU47, and expert case definitions of IAPA48) | ||||
| Tracheobronchitis or other pulmonary form (proven) | Patient with COVID-19 needing intensive care and a temporal relationship (entry criterion) | .. | At least one of the following: histopathological or direct microscopic detection of fungal hyphae, showing invasive growth with associated tissue damage; or aspergillus recovered by culture or microscopy or histology or PCR obtained by a sterile aspiration or biopsy from a pulmonary site, showing an infectious disease process | .. |
| Tracheobronchitis (probable) | Patient with COVID-19 needing intensive care and a temporal relationship (entry criterion) | Tracheobronchitis, indicated by tracheobronchial ulceration, nodule, pseudomembrane, plaque, or eschar seen on bronchoscopic analysis | At least one of the following: microscopic detection of fungal elements in bronchoalveolar lavage, indicating a mould; positive bronchoalveolar lavage culture or PCR;† serum galactomannan index >0·5 or serum LFA index >0·5;‡ or bronchoalveolar lavage galactomannan index ≥1·0 or bronchoalveolar lavage LFA index ≥1·0‡ | .. |
| Other pulmonary forms (probable) | Patient with COVID-19 needing intensive care and a temporal relationship (entry criterion) | Pulmonary infiltrate, preferably documented by chest CT, or cavitating infiltrate (not attributed to another cause) | At least one of the following: microscopic detection of fungal elements in bronchoalveolar lavage, indicating a mould; positive bronchoalveolar lavage culture;† serum galactomannan index >0·5 or serum LFA index >0·5;‡ bronchoalveolar lavage galactomannan index ≥1·0 or bronchoalveolar lavage LFA index ≥1·0;‡ two or more positive aspergillus PCR tests in plasma, serum, or whole blood;† a single positive aspergillus PCR in bronchoalveolar lavage fluid (<36 cycles);† or a single positive aspergillus PCR in plasma, serum, or whole blood, and a single positive in bronchoalveolar lavage fluid (any threshold cycle permitted)† | .. |
| Other pulmonary forms (possible)12§ | Patient with COVID-19 needing intensive care and a temporal relationship (entry criterion) | Pulmonary infiltrate, preferably documented by chest CT, or cavitating infiltrate (not attributed to another cause) | At least one of the following: microscopic detection of fungal elements in non-bronchoscopic lavage indicating a mould; positive non-bronchoscopic lavage culture;† single non-bronchoscopic lavage galactomannan index >4·5; non-bronchoscopic lavage galactomannan index >1·2 twice or more; or non-bronchoscopic lavage galactomannan index >1·2 plus another non-bronchoscopic lavage mycology test positive (non-bronchoscopic lavage PCR or LFA) | .. |
CAPA=COVID-19-associated invasive pulmonary aspergillosis. EORTC= European Organisation for Research and Treatment of Cancer. IAPA=influenza-associated pulmonary aspergillosis. ICU=intensive care unit. IPA=invasive pulmonary aspergillosis. LFA=lateral flow assay. MSGERC=Mycoses Study Group Education and Research Consortium.
Only one of these criteria required for diagnosis.
In case of patients with chronic obstructive pulmonary disease or chronic respiratory disease, the PCR or culture results should be confirmed by galactomannan testing to rule out colonisation or chronic aspergillosis. Galactomannan index should be available; galactomannan-index threshold applies to both enzyme immunoassay and LFA.
Visual reader should be used for a primary result and confirmatory galactomannan testing should be sought.
Classification of possible CAPA will most likely be sufficient to initiate antifungal therapy in the clinic but, in line with other consensus statements, it is not recommended for enrolling patients into clinical trials. Possible CAPA could serve as a secondary endpoint in a randomised prophylaxis study. Additional studies are needed to support the specificity of non-bronchoscopic lavage testing. Non-bronchoscopic lavage is considered a blind application of 10–20 mL saline recovered by aspiration via the closed suction system in an intubated patient. Bronchoalveolar lavage and non-bronchoscopic lavage are currently not considered equal for diagnosing CAPA.
At present, there is no generally accepted case definition for patients with CAPA. Case series to date have used modified AspICU criteria, based on clinical, radiological, and mycological criteria (including serum and bronchoalveolar lavage galactomannan), but do not define host factors. This modified set of criteria was previously used to classify patients with IAPA.1 There are similarities between IAPA and CAPA, such as the absence of defined host factors. However, there are also differences between IAPA and CAPA that are possibly related to tropism and pathophysiology of the virus, which could result in varying host susceptibility to IPA and clinical manifestation of disease. Although any indication of aspergillus is highly indicative of IAPA in patients with influenza in ICUs, early case series indicate more heterogeneous manifestations of aspergillus disease in patients with severe COVID-19.
Definition of CAPA for clinical studies
CAPA is defined as IPA in temporal proximity to a preceding SARS-CoV-2 infection. In particular, patients with clinical symptoms that are compatible with COVID-19, confirmed by a positive RT-PCR test, and who develop respiratory insufficiency requiring intensive care, should be considered at high risk for CAPA. We propose the following entry criterion: positive SARS-CoV-2 RT-PCR anytime during 2 weeks between hospital admission and ICU admission or positive RT-PCR within 72–96 h after ICU admission. CAPA might then develop, usually during the following weeks, and the risk for superinfections could be further increased by anti-IL-6-receptor treatment for COVID-19 or corticosteroid treatment for underlying conditions. Until we gain more insight into the pathophysiology of CAPA, we propose three different grades: possible, probable, and proven (table 2; figures 1, 2).
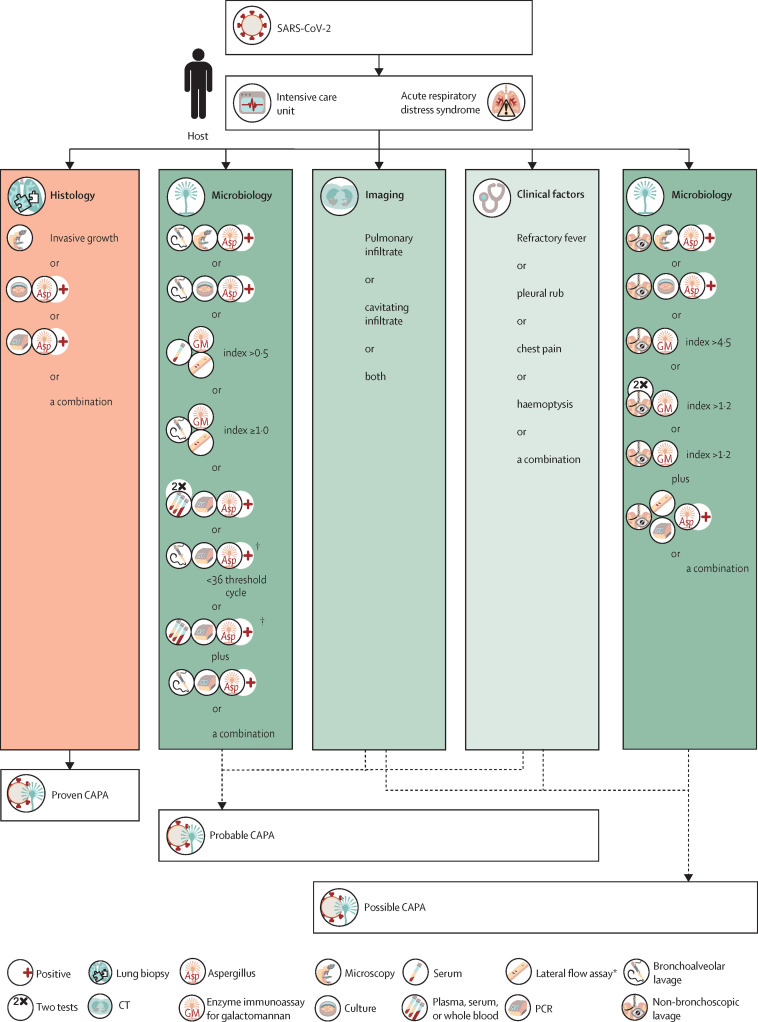
Proven CAPA
Proven CAPA is defined as pulmonary (figure 1 ) or tracheobronchial infection (figure 2 ). It is proven by histopathological or direct microscopic detection, or both, of fungal elements that are morphologically consistent with Aspergillus spp, showing invasive growth into tissues with associated tissue damage, or (with or without) aspergillus recovered by culture or detected by microscopy, in histology studies or by PCR from material that was obtained by a sterile aspiration or biopsy from a pulmonary site, showing an infectious disease (table 2).
Figure 1.
Defining and diagnosing CAPA (pulmonary form)
Classification of possible CAPA will most likely be sufficient to initiate antifungal therapy in the clinic but, in line with other consensus statements, it is not recommended for enrolling patients into clinical trials. Additional studies are needed to confirm the specificity of non-bronchoscopic lavage testing. Bronchoalveolar lavage and non-bronchoscopic lavage are currently not considered equal for diagnosing CAPA. CAPA=COVID-19-associated aspergillosis. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *Visual reader must be used for primary result and confirmatory galactomannan testing should be sought. † In case of patients with chronic obstructive pulmonary disease or chronic respiratory disease, the PCR or culture results should be confirmed by galactomannan testing to rule out colonisation or chronic aspergillosis. Galactomannan index must be available; galactomannan-index threshold applies to both enzyme immunoassay and lateral flow assay.
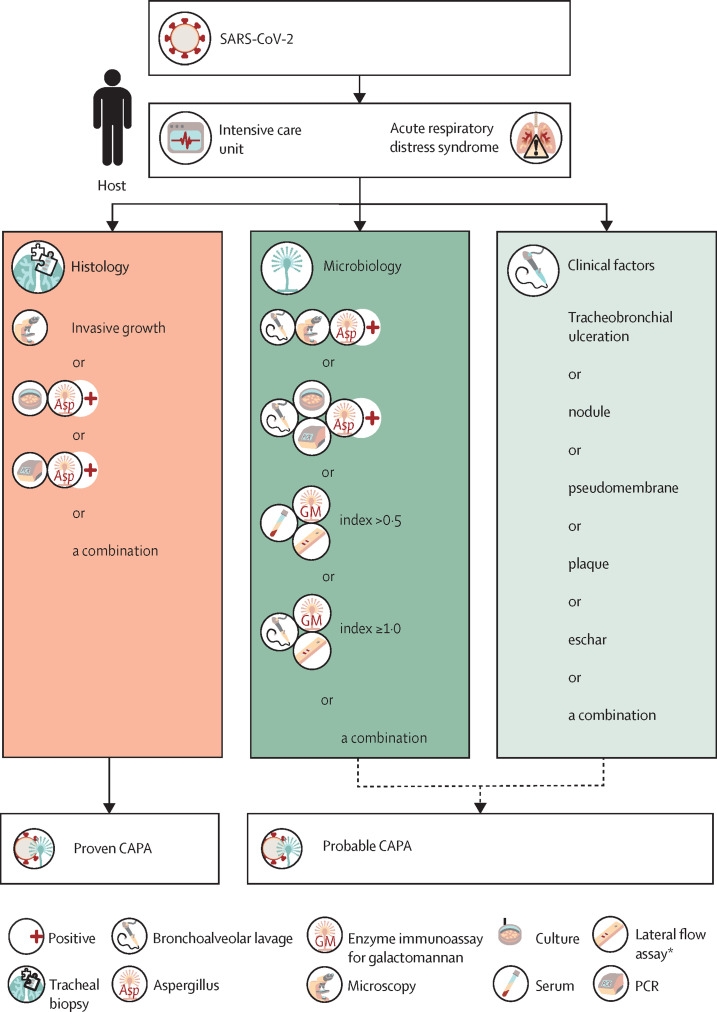
Figure 2.
Defining and diagnosing CAPA (tracheobronchial form)
CAPA=COVID-19-associated aspergillosis. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *Visual reader must be used for primary result and confirmatory galactomannan testing should be sought. † In case of patients with chronic obstructive pulmonary disease or chronic respiratory disease, the PCR or culture results should be confirmed by galactomannan testing to rule out colonisation or chronic aspergillosis. Galactomannan index must be available; galactomannan-index threshold applies to both enzyme immunoassay and lateral flow assay.
In patients with non-proven CAPA, classification relies on aspergillus culture from the respiratory tract or detection of biomarkers. The challenges in classifying patients with CAPA include distinguishing between airway colonisation and invasive infection, a reluctance to do diagnostic procedures that generate aerosols, restricted validation studies of aspergillus biomarkers in clinical specimens, and few data on aspergillus test performance in patients with COVID-19.
Probable CAPA
Invasive aspergillus tracheobronchitis is classified separately from other pulmonary manifestations as it requires a different diagnostic approach (figure 2). Diagnosis of probable CAPA tracheobronchitis requires observation of tracheobronchial ulceration, nodule, pseudomembrane, plaque, or eschar, alone or in combination, on bronchoscopic analysis and mycological evidence (table 2). Tracheobronchitis can be defined only by visualisation of the tracheal system via bronchoscopy. The diagnosis of probable pulmonary CAPA require a pulmonary infiltrate or nodules, preferably documented by chest CT, or cavitating infiltrate (not attributed to another cause), or both, combined with mycological evidence (figure 1, table 2). For mycological tests and cutoffs, we aimed to comply with other IPA case definitions, if possible.Possible CAPA
Although definitions of proven and probable disease have been shown to be reliable in research, a possible category has been abandoned in most definitions due to the low probability of IPA being present and an absence of consensus.3 However, in the setting of COVID-19 and in view of the challenges that are related to CAPA diagnosis, we propose a possible CAPA category, which enables clinicians to grade patients who have tested positive on samples for which assay validation has not been completed and most likely represents an improvement over empirical antifungal therapy (table 2).50 Possible pulmonary CAPA requires pulmonary infiltrate or nodules, preferably documented by chest CT, or cavitating infiltrate (which is not attributed to another cause) in combination with mycological evidence (eg, microscopy, culture, or galactomannan, alone or in combination) obtained via non-bronchoscopic lavage (figure 1, table 2).12 Detection of galactomannan in non-bronchoscopic lavage is considered to be evidence for CAPA, but proposed cutoff values are based on a single study12 and require further validation. Although classification of possible CAPA will most likely be sufficient to initiate antifungal therapy in the clinical setting, in line with other consensus statements, it is not recommended for enrolling patients into clinical trials. From this category, some diagnostic procedures might be upgraded as further evidence emerges.
Guidance on clinical management of CAPA
Any of the following clinical findings: refractory fever for more than 3 days or a new fever after a period of defervescence of longer than 48 h during appropriate antibiotic therapy, in the absence of any other obvious cause; worsening respiratory status (eg, tachypnoea or increasing oxygen requirements); haemoptysis; and pleural friction rub or chest pain, can trigger diagnostic investigations for CAPA in patients with refractory respiratory failure for more than 5–14 days despite receiving all support recommended for patients with COVID-19 who are critically ill.1 However, the onset of clinical features can be variable and patients can present with CAPA on ICU admission or after. This variation shows the need for regular and persistent testing of patients who meet the inclusion criteria.
The diagnostic investigations can include CT (or possibly repeat CT), which will indicate whether clinical deterioration is due to progression of a pneumonic process that could be compatible with IPA as opposed to other factors, including venous thromboembolic events and cardiac disease. Lung imaging findings should be supplemented with sampling from the lower respiratory tract under appropriate precautions for infection control.51 In patients without clinical response or with progressive nodular infiltrates, CT-guided biopsy or bronchoscopy should be considered if the benefits outweigh the risks for the patient or the risk of transmission. The optimal timepoint for these procedures is unknown, given that there is a substantial risk of complications in patients who are ventilated and that CAPA has been shown to present over a prolonged period (ie, several weeks) during ICU admission.12 In intubated patients, tracheal aspirate and non-bronchoscopic lavage can be regularly obtained, with non-bronchoscopic lavage obtained by use of a closed-suction catheter, reducing the infection risk.34
Respiratory specimens should undergo comprehensive microbiological testing with direct microscopy, high-volume culture,52 PCR that is specific to bacteria and Aspergillus spp, testing for galactomannan in bronchoalveolar lavage or non-bronchoscopic lavage fluid, and, if available, testing with the aspergillus LFA (ie, IMMY sona Aspergillus Galactomannan Lateral Flow Assay) or LFD (ie, AspLFD).40 PCR, galactomannan, and LFA or LFD testing can also be done on serum samples, for which sensitivity is low but specificity is high in non-neutropenic patients, including patients with CAPA, with disease primarily restricted to the airways.
In regions (both within and between countries) with azole resistance or in patients who do not respond to azole therapy, aspergillus isolates should be tested for azole resistance by screening agar (eg, with VIPcheck, Mediaproducts, Groningen, Netherlands), followed up by SensiTitre (Thermo Fisher Scientific, USA), broth microdilution testing, or gradient concentration strips (eg, E-test, bioMérieux, Nürtingen, Germany). Because respiratory cultures can contain both azole-susceptible and azole-resistant isolates during an infection, a minimum of five colonies should be tested.53
In patients who are PCR-positive for SARS-CoV-2, screening with serum galactomannan enzyme immunoassay, or LFA or LFD54 if the enzyme immunoassay for galactomannan is not available, should be considered three times per week, if locally available, until discharge from ICU or defervescence for longer than 7 days with improved lung function. Ideally, this type of screening can be accompanied by regular screening (ie, once per week) of respiratory samples (eg, non-bronchoscopic lavage, tracheal aspirate, or sputum) with culture, PCR, galactomannan (ie, Platelia Aspergillus Ag Kit, Bio-Rad, Hercules, California, USA), or LFA or LFD, with positive tests triggering a CAPA investigation (figures 1, 2). A problem is that biomarkers are usually validated for bronchoalveolar lavage and serum but not for other specimens, including tracheal aspirate, sputum, and non-bronchoscopic lavage (table 1). As these tests have not been validated for these specimens and cutoff values are not established, results should be interpreted with caution and their value in patient classification is uncertain until more scientific evidence becomes available. If patients with COVID-19 also have underlying diseases that put them at high risk of IPA, then we suggest the provided consensus criteria be used as they were specifically developed to challenge the difficulties of non-validated specimens for key tests in CAPA diagnosis.
Antifungal treatment
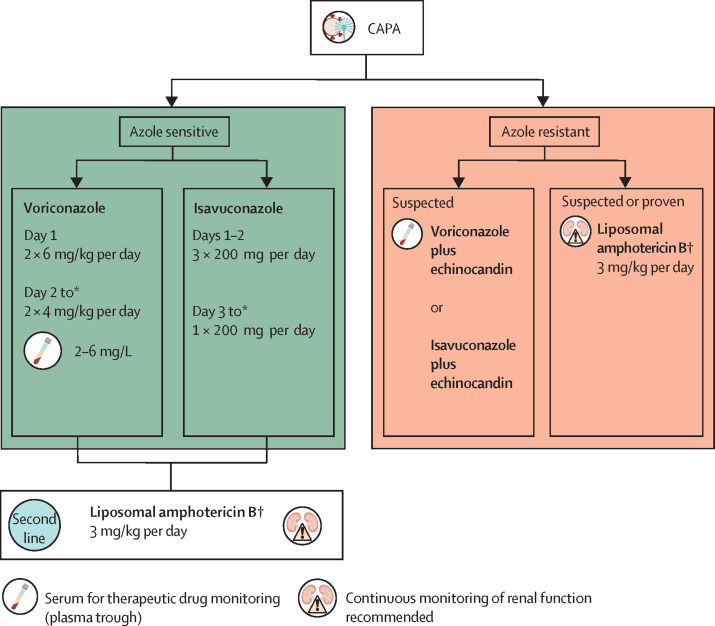
We recommend either voriconazole or isavuconazole as first-line treatment for possible, probable, and proven CAPA.53 The primary focus of IPA treatment during SARS-CoV-2 infection is the management of the lung infection. Since the landmark study in 2002, voriconazole has been the foundational drug for treatment of invasive aspergillosis.55 Although there are still too few cases of documented CAPA to compare the effectiveness of antifungal treatments, there are also no data to suggest that treatment would be different than that for patients without COVID-19. There are, however, multiple specific caveats to these treatment recommendations for CAPA (panel ). Although generally, outside the haematological malignancy setting, voriconazole is the recommended first-line treatment for IPA,53, 67 there are several drawbacks to the use of voriconazole in patients with severe COVID-19, who might warrant alternative first-line treatment options. Besides its narrow therapeutic window,68 drug–drug interactions in particular could reduce the use of voriconazole in the ICU, although all triazoles tend to interact with multiple other drugs.69 Being metabolised via CYP2C19, CYP2C9, and CYP3A4, voriconazole is among the drugs that are most frequently associated with major drug–drug interactions in the ICU setting70 and might show interactions with COVID-19 treatments, such as remdesivir, which is also a substrate for CYP3A4, although its metabolism is primarily mediated by hydrolase activity and the overall effect is not yet fully understood.71 Although few data exist for isavuconazole outside the haematological malignancy setting, isavuconazole—when compared with voriconazole—showed a favourable pharmacokinetic profile and was associated with fewer toxicities.57 Although isavuconazole might be considered an attractive first-line treatment option, it is important to consider that isavuconazole is metabolised via CYP3A4 and could, therefore, be problematic; however, drug–drug interactions are generally less pronounced with isavuconazole than with voriconazole.72, 73 Liposomal amphotericin B is the primary alternative option for treatment of IPA in the ICU;67 however, the drug is nephrotoxic and might result in a further decline of renal function, especially in patients who already have acute kidney injury.74 The concern about renal toxicity is particularly relevant for patients who are infected by SARS-CoV-2, which has shown renal tropism and is a frequent cause of kidney injury.75 Alternative second-line options are posaconazole or echinocandins. Echinocandins should not be used as monotherapy if other options are left, but they can indeed be used for salvage therapy.76 New antifungal classes under development—namely, fosmanogepix, ibrexafungerp, olorofim, and rezafungin—might become future options.77 A proposed treatment path is given in figure 3 . The diagnostic challenges combined with a high prevalence of CAPA cases and the reported excess mortality might justify antifungal prophylaxis trials, similar to those proposed for patients with IAPA;49 other than triazoles, some of the novel antifungals that are under development could become suitable alternatives. However, at present, no antifungal drug with activity against Aspergillus spp has been licensed for prophylactic use in the ICU. For mandatory supportive measures, see the appendix (appendix p 1).
Panel. Specific caveats for CAPA treatment recommendations.
-
•
The route of administration should preferably be intravenous, due to possible malabsorption from gastroparesis in patients in intensive care units.
-
•
Voriconazole treatment (loading dose 6 mg/kg twice a day for two doses, followed by 4 mg/kg twice a day) has a better outcome than does treatment with amphotericin B deoxycholate, especially with its known serious toxicities.55 However, liposomal amphotericin B can be considered for initial therapy if, epidemiologically, drug-resistant patterns support this treatment, before the results of susceptibility testing for voriconazoles are available. The recommended initial dose of liposomal amphotericin B is 3 mg/kg per day.56
-
•
Daily isavuconazole treatment (loading dose 200 mg three times a day for six doses, followed by 200 mg once a day, 12–24 h after the last loading dose) has similar clinical activity to voriconazole but less hepatotoxicity and neurotoxicity and decreased risk of corrected QT-interval prolongation.57, 58
-
•
Echinocandins are not recommended for use as monotherapy in primary invasive aspergillosis;59 but, in combination with an azole, might have some therapeutic advantage in critically ill patients60 and in areas of high prevalence of azole resistance because combination therapy can broaden the coverage until minimal inhibitory concentrations become available.61
-
•
Posaconazole has excellent in-vitro aspergillus activity and has been successfully used as salvage treatment in patients without COVID-19.62
-
•
Itraconazole shows excellent in-vitro aspergillus activity but does not have robust comparative data with established regimens.63
-
•
Polyene treatment for azole-resistant strains is suggested, but new-class agents, such as olorofim, which has US Food and Drug Administration breakthrough therapy designation, should be considered as alternatives, particularly if azole-resistant disease is documented.64 The inositol acylase inhibitor, fosmanogepix, is in clinical trials for invasive aspergillosis,65 and the oral triterpenoid beta-glucan inhibitor, ibrexafungerp,66 is also in clinical trials for invasive aspergillosis and invasive candidiasis. Some of these drugs might be accessible on a named patient basis.
-
•
The optimal duration of therapy is unknown and radiological lung imaging might not be a helpful gauge, but the expert panel suggest 6–12 weeks as a treatment course. However, it seems reasonable to include follow-up lung CT imaging to document the resolution of infiltrates before termination of treatment. In patients who are immunocompromised (eg, with haematological malignancy or receiving immunosuppressive therapy), longer treatment might be necessary than for other patients. Following the galactomannan-index in serum as a measure of therapeutic response might be limited by its poor sensitivity when testing serum in non-neutropenic patients, but attaining follow-up respiratory samples for galactomannan testing could be useful to determine efficacy in patients who are galactomannan positive, which might help to determine treatment duration.
Figure 3.
Recommended treatment for CAPA
CAPA=COVID-19-associated pulmonary aspergillosis. *The optimal duration is unknown, but the expert panel suggests 6–12 weeks as a treatment course. In immunocompromised patients (eg, with haematological malignancy or receiving immunosuppressive therapy), longer treatment might be necessary. † Salvage therapy: caspofungin 70 mg loading dose on the first day followed by 50 mg/day. If body weight is more than 80 kg, then 70 mg loading dose on the first day followed by 70 mg/day.
Therapeutic drug monitoring
Therapeutic drug monitoring is another key component in the treatment of patients with CAPA. Impaired renal or hepatic function, continuous renal replacement therapy, or extracorporeal membrane oxygenation render drug concentrations difficult to predict, and therapeutic drug monitoring is thus recommended for effective and safe drug exposure. Other important aspects affecting drug exposure are protein binding, especially for isavuconazole and posaconazole, and altered drug absorption, distribution, metabolism, and clearance. Furthermore, this population often receives multiple drugs and thus is at increased risk of drug–drug interactions.
We recommend weekly therapeutic drug monitoring in patients with CAPA (ie, twice in the first week) in cases of fully susceptible Aspergillus species, specifically for voriconazole and posaconazole. For voriconazole, a plasma trough concentration of 2–6 mg/L is recommended.53 For posaconazole, the lower threshold is 1 mg/L. An upper limit of 3·75 mg/L for posaconazole is recommended, which appeared safe during phase 3 studies.78, 79 More data are emerging on the use of high-dose posaconazole in the setting of azole-resistant infections.80 By contrast, no isavuconazole target concentration has been defined, but therapeutic drug monitoring might be warranted in patients who are on renal replacement therapy or other extracorporeal treatments and in patients with obesity.81 Therapeutic drug monitoring of liposomal amphotericin B is not warranted.53 In patients with obesity, or during dexamethasone treatment, echinocandins might need therapeutic drug monitoring to ensure doses remain above the minimal effective concentration of the fungus.82, 83
Conclusion
The proposed consensus definitions for CAPA enable researchers to homogeneously classify patients in registries and interventional clinical trials. Moreover, the definitions can be used in daily practice for managing patients with CAPA. The proposed definitions are based on validated tests for IPA to increase the quality of future studies and to distinguish patients with airway colonisation from patients with invasive infection, but they also recognise the need for clinical pragmatism. Our guidance aims to facilitate both clinical trials and clinical management to further improve the understanding of CAPA. Over time, new insights will be used to further improve the definitions and the management algorithms. Future studies on CAPA are needed to elucidate the role of host factors and immunological defence, differentiate pulmonary versus tracheobronchitis phenotypes of aspergillosis, and address an array of diagnosis and management concerns.
Search strategy and selection criteria
Data for this work were identified by searches of MEDLINE, Current Contents, PubMed, Embase, and references from relevant articles using the search string “(Aspergill*)” AND (‘invasive’ OR ‘infection’ OR ‘case’ OR ‘patient’ OR ‘report’) AND (‘COVID*’ OR ‘corona*’), AND (‘SARS CoV 2’) AND (‘Aspergill*’), and “(Aspergill*)” AND (guideline OR treatment OR therapy OR diagnosis OR therapeutic drug monitoring). Only articles published in English between Jan 1, 2000, and Aug 31, 2020, were included.
Acknowledgments
Acknowledgments
We thank Susann Blossfeld for technical support with this manuscript and Ullrich Bethe for critically revising the manuscript. We thank Matteo Oliverio, medical illustrator, for creating the figures.
Contributors
PK and OAC coordinated the work of the authors and guided the development of the manuscript. All authors contributed to the initial manuscript draft, literature review, compilation of data tables, and interpretation and assessment of recommendations. All authors participated in review and revisions, approved the final manuscript, and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of interests
PK has received non-financial scientific grants from Miltenyi Biotec and the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases; and lecture honoraria from, or is advisor to, Akademie für Infektionsmedizin, Astellas Pharma, European Confederation of Medical Mycology, Gilead Sciences, Gesundheits- und Pflegezentrum Ruesselsheim Academy Ruesselsheim, Merck Sharp and Dohme, Noxxon, and University Hospital of Munich, outside the submitted work. MB has participated in advisory boards or received speaker honoraria from Achaogen, Angelini, Astellas, Bayer, Basilea, BioMeérieux, Cidara, Gilead Sciences, Menarini, Merck Sharp and Dohme, Nabriva, Paratek, Pfizer, Roche, Melinta, Shionogi, Tetraphase, VenatoRx, and Vifor; and has received study grants from Angelini, Basilea, Astellas, Shionogi, Cidara, Melinta, Gilead Sciences, Pfizer, and Merck Sharp and Dohme, outside the submitted work. SCAC has participated in advisory boards or received speaker honoraria from Merck Sharp and Dohme Australia and Gilead Sciences; and has received untied educational grants from Merck Sharp and Dohme Australia and grants from F2G, outside the submitted work. ALC reports grants from Pfizer and Astellas; personal fees from Pfizer, Astellas, and Gilead Sciences; and personal fees and non-financial support from Eurofarma, United Medical-Biotoscana, and Merck Sharp and Dohme, outside the submitted work. MH reports grants from Gilead Sciences and Pfizer, outside the submitted work. NK reports grants from Pfizer and personal fees from Astellas, Gilead Sciences, Merck Sharp and Dohme, and Pfizer, outside the submitted work. CL-F reports grants from Gilead Sciences and Astellas Pharma; personal fees from Gilead Sciences, Merck Sharp and Dohme, Basilea, and Angelini; and other from Gilead Sciences, Astellas Pharma, Merck Sharp and Dohme, and Basilea, outside the submitted work. ROO reports grants from Pfizer and Gilead Sciences, outside the submitted work. DCV is supported by the Fonds de la recherche en sante du Quebec clinician-scientist scholar program (Junior 2); has received research grants from Canadian Institutes of Health Research, CSL Behring Canada, La Fondation du Grand Défi Pierre Lavoie, and US Department of Defense; has received clinical trials funding from Cidara Therapeutics, CSL Behring, and Janssen Pharmaceuticals; and is an advisor to, or has received speaker honoraria from, CSL Behring, Novartis Canada, and UCB Biosciences. L-PZ reports grants from Merck Sharp and Dohme and Pfizer, outside the submitted work. BB reports personal fees from Baxalta, Celgene, Merck Sharp and Dohme, Mundipharma, Johnson and Johnson, Roche, and Takeda; and grants from Celgene, Merck Sharp and Dohme, Roche, Sanofi, Takeda, and AstraZeneca, outside the submitted work. RB has served as a consultant to Astellas Pharma, F2G, Amplyx, Gilead Sciences, Merck Sharp and Dohme, and Pfizer; and has received unrestricted and research grants from Astellas Pharma, Gilead Sciences, Merck Sharp and Dohme, and Pfizer, outside the submitted work, for which contracts were through Radboudumc and all payments were invoiced by Radboudumc. J-PG has participated in advisory boards or received speaker honoraria from Pfizer and Gilead Sciences, outside the submitted work. JRP reports grants from Merck Sharp and Dohme, Astellas, Pfizer, Amplyx, Ampili, and Minnetronix; and other from F2G, Scynexis, and Matinas, outside the submitted work. TFP reports grants to UT Health San Antonio from Cidara and F2G; and consulting fees from Basilea, Mayne, Merck, Pfizer, and Scynexis, outside the submitted work. JFM reports grants from F2G and Pulmocide; consultancy fees from Scynexis; and speaker fees from Gilead Sciences, United Medical, and TEVA, outside the submitted work. LO-Z reports grants from Gilead Sciences, Astellas, Pfizer, Cidara, Scynexis; and personal fees from Gilead Sciences, Astellas, Pfizer, Cidara, F2G, Viracor, and Stendhal, outside the submitted work. PLW reports speaker fees from Gilead Sciences, F2G, Merck Sharp and Dohme, and Pfizer; expert advice fees from Gilead Sciences and F2G; and meeting sponsorship from Bruker, Dynamiker, Launch Diagnostics, and Gilead Sciences. PLW did diagnostic evaluations for Bruker, Dynamiker, and Launch Diagnostics and is a founding member of the European Aspergillus PCR Initiative. PEV reports grants from Mundipharma, F2G, Pfizer, Thermofisher, Cidara, and Gilead Sciences; and non-financial support from IMMY, outside the submitted work. OAC is supported by the German Federal Ministry of Research and Education; is funded by the Deutsche Forschungsgemeinschaft under Germany's Excellence Strategy (CECAD, EXC 2030— 390661388); and has received research grants from, is an advisor to, or received lecture honoraria from Actelion, Allecra Therapeutics, Al-Jazeera Pharmaceuticals, Amplyx, Astellas, Basilea, Biosys, Cidara, Da Volterra, Entasis, F2G, Gilead Sceinces, Grupo Biotoscana, IQVIA, Janssen, Matinas, Medicines Company, MedPace, Melinta Therapeutics, Menarini, Merck Sharp and Dohme, Mylan, Nabriva, Noxxon, Octapharma, Paratek, Pfizer, PSI, Roche Diagnostics, Scynexis, and Shionogi. AC and TP declare no competing interests.
Supplementary Material
References
- 1.Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 2.Vehreschild JJ, Bröckelmann PJ, Bangard C, et al. Pandemic 2009 influenza A(H1N1) virus infection coinciding with invasive pulmonary aspergillosis in neutropenic patients. Epidemiol Infect. 2012;140:18–52. doi: 10.1017/S0950268811002603. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koehler P, Cornely OA, Böttiger BW, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19 associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63:766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi (Basel) 2020;6:91. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangneux JP, Reizine F, Guegan H, et al. Is the COVID-19 pandemic a good time to include aspergillus molecular detection to categorize aspergillosis in ICU patients? A monocentric experience. J Fungi (Basel) 2020;10:105. doi: 10.3390/jof6030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. published online July 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1298. published online Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehler P, Salmanton-García J, Gräfe SK, et al. Baseline predictors influencing the prognosis of invasive aspergillosis in adults. Mycoses. 2019;62:651–658. doi: 10.1111/myc.12926. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed A, Hassan T, Trzos-Grzybowska M, et al. Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: a lethal combination. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.2020.06.005. published online June 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meijer EFJ, Dofferhoff ASM, Hoiting O, Buil JB, Meis JF. Azole-resistant COVID-19-associated pulmonary aspergillosis in an immunocompetent host: a case report. J Fungi (Basel) 2020;6:79. doi: 10.3390/jof6020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghelfenstein-Ferreira T, Saade A, Alanio A, et al. Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU—a case report. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.2020.06.006. published online July 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Short KR, Kasper J, van der Aa S, et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Respir J. 2016;47:954–966. doi: 10.1183/13993003.01282-2015. [DOI] [PubMed] [Google Scholar]
- 18.Herold S, Becker C, Ridge KM, Budinger GR. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur Respir J. 2015;45:1463–1478. doi: 10.1183/09031936.00186214. [DOI] [PubMed] [Google Scholar]
- 19.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanzani M, Vianelli N, Cavo M, Kontoyiannis DP, Lewis RE. Development and internal validation of a model for predicting 60-day risk of invasive mould disease in patients with haematological malignancies. J Infect. 2019;78:484–490. doi: 10.1016/j.jinf.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Wahidi MM, Shojaee S, Lamb CR, et al. The use of bronchoscopy during the coronavirus disease 2019 pandemic: CHEST/AABIP guideline and expert panel report. Chest. 2020;158:1268–1281. doi: 10.1016/j.chest.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houlihan CF, Vora N, Byrne T, et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396:e6–e7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa954. published online July 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis RE, Kontoyiannis DP. Invasive aspergillosis in glucocorticoid-treated patients. Med Mycol. 2009;47(suppl 1):S271–S281. doi: 10.1080/13693780802227159. [DOI] [PubMed] [Google Scholar]
- 26.Lang M, Som A, Mendoza DP, et al. Detection of unsuspected coronavirus disease 2019 cases by computed tomography and retrospective implementation of the Radiological Society of North America/Society of Thoracic Radiology/American College of Radiology consensus guidelines. J Thorac Imaging. 2020;35:346–353. doi: 10.1097/RTI.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 27.Wahidi MM, Lamb C, Murgu S, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. J Bronchology Interv Pulmonol. 2020;27:e52–e54. doi: 10.1097/LBR.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koehler P, Bassetti M, Kochanek M, Shimabukuro-Vornhagen A, Cornely OA. Intensive care management of influenza-associated pulmonary aspergillosis. Clin Microbiol Infect. 2019;25:1501–1509. doi: 10.1016/j.cmi.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Zhou P, Wei Y, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrego A, Pajares V, Fernández-Arias C, Vera P, Mancebo J. Bronchoscopy in patients with COVID-19 with invasive mechanical ventilation: a single-center experience. Am J Respir Crit Care Med. 2020;202:284–287. doi: 10.1164/rccm.202004-0945LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 32.Bullard J, Dust K, Funk D, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa638. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Biesen S, Kwa D, Bosman RJ, Juffermans NP. Detection of invasive pulmonary aspergillosis in COVID-19 with non-directed BAL. Am J Respir Crit Care Med. 2020;202:1171–1173. doi: 10.1164/rccm.202005-2018LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutsaert L, Steinfort N, Van Hunsel T, et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10:71. doi: 10.1186/s13613-020-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antinori S, Rech R, Galimberti L, et al. Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: a diagnostic challenge. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101752. published online May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verweij PE, Gangneux J-P, Bassetti M, et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talento AF, Dunne K, Joyce EA, et al. A prospective study of fungal biomarkers to improve management of invasive fungal diseases in a mixed specialty critical care unit. J Crit Care. 2017;40:119–127. doi: 10.1016/j.jcrc.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 39.Thornton CR. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol. 2008;15:1095–1105. doi: 10.1128/CVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Z, Fu M, Zhang J, Zhou H, Fu Y, Zhou J. Diagnostic accuracy of a novel lateral-flow device in invasive aspergillosis: a meta-analysis. J Med Microbiol. 2015;64:702–707. doi: 10.1099/jmm.0.000092. [DOI] [PubMed] [Google Scholar]
- 41.Jenks JD, Prattes J, Frank J, et al. Performance of the bronchoalveolar lavage fluid aspergillus galactomannan lateral flow assay with cube reader for diagnosis of invasive pulmonary aspergillosis: a multicenter cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1281. published online Aug 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mercier T, Dunbar A, de Kort E, et al. Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: a comparative multicenter study. Med Mycol. 2020;58:444–452. doi: 10.1093/mmy/myz079. [DOI] [PubMed] [Google Scholar]
- 43.Lass-Flörl C, Lo Cascio G, Nucci M, et al. Respiratory specimens and the diagnostic accuracy of aspergillus lateral flow assays (LFA-IMMY): real-life data from a multicentre study. Clin Microbiol Infect. 2019;25:1563.e1–1563.e3. doi: 10.1016/j.cmi.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 44.White PL. ECMM webinar on COVID-19 associated aspergillosis: point of care diagnosis—soon reality? Oct 1, 2020. https://www.youtube.com/watch?v=xY595itDbVo
- 45.White PL, Wingard JR, Bretagne S, et al. Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin Infect Dis. 2015;61:1293–1303. doi: 10.1093/cid/civ507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cruciani M, Mengoli C, Loeffler J, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev. 2019;9 doi: 10.1002/14651858.CD009551.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blot SI, Taccone FS, Van den Abeele AM, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 48.Meersseman W, Lagrou K, Maertens J, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med. 2008;177:27–34. doi: 10.1164/rccm.200704-606OC. [DOI] [PubMed] [Google Scholar]
- 49.Verweij PE, Rijnders BJA, Brüggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heard KL, Hughes S, Mughal N, Moore LSP. COVID-19 and fungal superinfection. Lancet Microbe. 2020;1:e107. doi: 10.1016/S2666-5247(20)30065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koehler P, Cornely OA, Kochanek M. Bronchoscopy safety precautions for diagnosing COVID-19 associated pulmonary aspergillosis—a simulation study. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13183. [DOI] [PubMed] [Google Scholar]
- 52.Marr KA, Datta K, Mehta S, et al. Urine antigen detection as an aid to diagnose invasive aspergillosis. Clin Infect Dis. 2018;67:1705–1711. doi: 10.1093/cid/ciy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(suppl 1):e1–38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Mercier T, Guldentops E, Lagrou K, Maertens J. Prospective evaluation of the turbidimetric β-D-glucan assay and two lateral flow assays on serum in invasive aspergillosis. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa295. published online March 19. [DOI] [PubMed] [Google Scholar]
- 55.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 56.Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial) Clin Infect Dis. 2007;44:1289–1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 57.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 58.Mellinghoff SC, Bassetti M, Dörfel D, et al. Isavuconazole shortens the QTc interval. Mycoses. 2018;61:256–260. doi: 10.1111/myc.12731. [DOI] [PubMed] [Google Scholar]
- 59.Hiemenz JW, Raad II, Maertens JA, et al. Efficacy of caspofungin as salvage therapy for invasive aspergillosis compared to standard therapy in a historical cohort. Eur J Clin Microbiol Infect Dis. 2010;29:1387–1394. doi: 10.1007/s10096-010-1013-0. [DOI] [PubMed] [Google Scholar]
- 60.Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 61.Lestrade PPA, Meis JF, Melchers WJG, Verweij PE. Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clin Microbiol Infect. 2019;25:799–806. doi: 10.1016/j.cmi.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 62.Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 63.Denning DW, Tucker RM, Hanson LH, Stevens DA. Treatment of invasive aspergillosis with itraconazole. Am J Med. 1989;86:791–800. doi: 10.1016/0002-9343(89)90475-0. [DOI] [PubMed] [Google Scholar]
- 64.du Pré S, Beckmann N, Almeida MC, et al. Effect of the novel antifungal drug f901318 (olorofim) on growth and viability of Aspergillus fumigatus. Antimicrob Agents Chemother. 2018;62:e00231–e00238. doi: 10.1128/AAC.00231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao M, Lepak AJ, Marchillo K, et al. APX001 pharmacokinetic/pharmacodynamic target determination against Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2019;63:e02372–e02378. doi: 10.1128/AAC.02372-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis MR, Donnelley MA, Thompson GR., 3rd Ibrexafungerp: a novel oral glucan synthase inhibitor. Med Mycol. 2020;58:579–592. doi: 10.1093/mmy/myz083. [DOI] [PubMed] [Google Scholar]
- 67.Patterson TF, Thompson GR, 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoenigl M, Duettmann W, Raggam RB, et al. Potential factors for inadequate voriconazole plasma concentrations in intensive care unit patients and patients with hematological malignancies. Antimicrob Agents Chemother. 2013;57:3262–3267. doi: 10.1128/AAC.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenks JD, Mehta SR, Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: which drug and when? Med Mycol. 2019;57(suppl 2):S168–S178. doi: 10.1093/mmy/myy052. [DOI] [PubMed] [Google Scholar]
- 70.Baniasadi S, Farzanegan B, Alehashem M. Important drug classes associated with potential drug–drug interactions in critically ill patients: highlights for cardiothoracic intensivists. Ann Intensive Care. 2015;5:44. doi: 10.1186/s13613-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCreary EK, Pogue JM. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infect Dis. 2020;7:a105. doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jenks JD, Salzer HJ, Prattes J, Krause R, Buchheidt D, Hoenigl M. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:1033–1044. doi: 10.2147/DDDT.S145545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoenigl M, Prattes J, Neumeister P, Wölfler A, Krause R. Real-world challenges and unmet needs in the diagnosis and treatment of suspected invasive pulmonary aspergillosis in patients with haematological diseases: an illustrative case study. Mycoses. 2018;61:201–205. doi: 10.1111/myc.12727. [DOI] [PubMed] [Google Scholar]
- 74.Armstrong-James D, Koh M, Ostermann M, Cockwell P. Optimal management of acute kidney injury in critically ill patients with invasive fungal infections being treated with liposomal amphotericin B. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2019-233072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heinz WJ, Buchheidt D, Ullmann AJ. Clinical evidence for caspofungin monotherapy in the first-line and salvage therapy of invasive aspergillus infections. Mycoses. 2016;59:480–493. doi: 10.1111/myc.12477. [DOI] [PubMed] [Google Scholar]
- 77.Kupferschmidt K. New drugs target growing threat of fatal fungi. Science. 2019;366:407. doi: 10.1126/science.366.6464.407. [DOI] [PubMed] [Google Scholar]
- 78.Cornely OA, Duarte RF, Haider S, et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimivrob Chemother. 2016;71:718–726. doi: 10.1093/jac/dkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cornely OA, Robertson MN, Haider S, et al. Pharmacokinetics and safety results from the phase 3 randomized, open-label, study of intravenous posaconazole in patients at risk of invasive fungal disease. J Antimivrob Chemother. 2017;72:3406–3413. doi: 10.1093/jac/dkx263. [DOI] [PubMed] [Google Scholar]
- 80.Schauwvlieghe AFAD, Buil JB, Verweij PE, et al. High-dose posaconazole for azole-resistant aspergillosis and other difficult-to-treat mould infections. Mycoses. 2020;63:122–130. doi: 10.1111/myc.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zurl C, Waller M, Schwameis F, et al. Isavuconazole treatment in a mixed patient cohort with invasive fungal infections: outcome, tolerability and clinical implications of isavuconazole plasma concentrations. J Fungi (Basel) 2020;6:90. doi: 10.3390/jof6020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muilwijk EW, Lempers VJ, Burger DM, et al. Impact of special patient populations on the pharmacokinetics of echinocandins. Expert Rev Anti Infect Ther. 2015;13:799–815. doi: 10.1586/14787210.2015.1028366. [DOI] [PubMed] [Google Scholar]
- 83.Kofla G, Ruhnke M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature. Eur J Med Res. 2011;16:159–166. doi: 10.1186/2047-783X-16-4-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.