Abstract
The role of skeletal muscle mass in modulating immune response and supporting metabolic stress has been increasingly confirmed. Patients with sarcopenia, characterized by reduced muscle mass and muscle strength, were reported to have poor immune response and metabolic stress when facing acute infection, major surgeries, and other attacks. Based on empirical data, patients with sarcopenia are speculated to have increased infection rates and dismal prognoses amid the current 2019 novel coronavirus disease (COVID-19) epidemic. COVID-19 infection also aggravates sarcopenia because of the increased muscle wasting caused by systematic inflammation and the reduced physical activity and inadequate nutrient intake caused by social isolation. Notably, the interventions targeting skeletal muscle are anticipated to break the vicious circle and benefit the treatment of both conditions. We recommend sarcopenia assessment for populations with advanced age, inactivity, chronic disease, cancers, and nutritional deficiency. Patients with sarcopenia and COVID-19 infection need intensive care and aggressive treatments. The provision of at-home physical activities together with protein supplementation is anticipated to reverse sarcopenia and promote the prevention and treatment of COVID-19. The recommended protocols on nutritional support and physical activities are provided in detail.
Keywords: Novel coronavirus disease, Sarcopenia, SARS-CoV-2
Graphical abstract
The 2019 novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), manifests as systemic disorders, particularly severe pneumonia and acute respiratory distress syndrome [1]. As of December 24 , 2020, a total of 77 530 799 COVID-19 cases have been confirmed worldwide, with 1 724 904 deaths [2]. Although standard diagnostic and prevention methods for COVID-19 have been established, specific or targeted treatments are still lacking. While most researchers have tried to solve the pandemic by studying drugs and developing vaccines, recognition and intervention of adverse physical states, particularly sarcopenia, could be novel but underappreciated methods to promote the treatment of COVID-19.
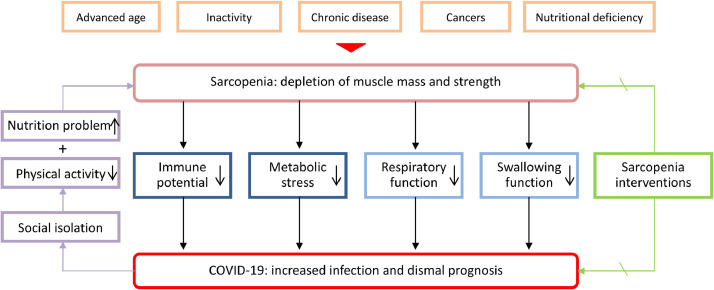
Sarcopenia, defined as the depletion of skeletal muscle mass and muscle strength, is prevalently observed in several physiological and pathologic processes, including aging, inactivity, chronic diseases, cancer progression, and nutritional deficiency (Fig. 1 ) [3], [4], [5]. The prevalence rate of sarcopenia in the older-adult population was approximately 10%, and this rate reached 40% in nursing home residents [6,7]. Notably, the quality and quantity of skeletal muscle mass not only influence motor activity, respiratory function, and swallowing profile, but also affect the immune response and metabolic stress facing acute infection, major surgery, and other attacks [8], [9], [10], [11], [12]. Accordingly, patients with sarcopenia showed compromised function of multiple systems in clinical observations (Fig. 1) [8], [9], [10], [11], [12].
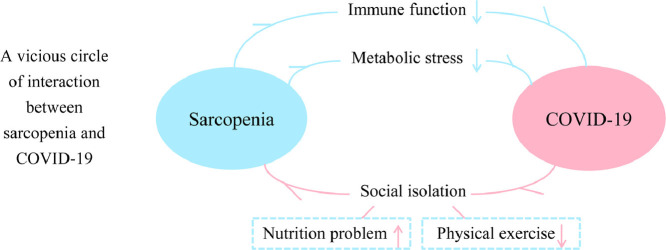
Fig. 1.
A vicious circle of interactions between sarcopenia and COVID-19. Interventions targeting sarcopenia are anticipated to benefit the treatment of both conditions. COVID-19, 2019 novel coronavirus disease.
Sarcopenia was reported to be associated with the impaired proliferation of peripheral mononuclear cells, increased ratio of neutrophils to lymphocytes, and damaged homeostasis of natural killer lymphocytes, contributing to immune senescence [12], [13], [14]. Clinical evidence for the impaired immunity in patients with sarcopenia includes the higher incidence of community-acquired and in-hospital pneumonia, increased risk of infectious complications in major surgeries, and dismal survival in various solid cancers [14], [15], [16], [17]. In particular, sarcopenia was recently reported to be associated with a poor response to immune-checkpoint inhibitors in non–small cell lung cancers [17]. A recent prospective study also confirmed the impaired immune response in patients with sarcopenia after esophageal surgery [10]. The main mechanism underlying impaired immunity in patients with sarcopenia refers to the abnormal myokines, such as interleukin (IL)-15, IL-17, and IL-6, which modulate the proliferation and function of both innate and adaptive immune cells [12]. Regarding the metabolic stress during the severe infection, skeletal muscle is catabolized to provide the immune system, liver, and gut with amino acids, especially glutamine [11,18]. Patients with sarcopenia have a decreased availability of such protein mobilization. We speculate that patients with sarcopenia respond poorly to the infection of SARS-CoV-2 because of the impaired immune potential and metabolic stress.
No study has investigated the relationship between sarcopenia and COVID-19. However, some indirect evidence partially supports the adverse impact of sarcopenia on the treatment of COVID-19. First, advanced age, chronic diseases, and cancers, which were important etiologies of sarcopenia, were widely confirmed as risk factors for COVID-19 infection and corresponding mortality [19], [20], [21]. Second, sarcopenia was confirmed to be associated with the incidence of both community-acquired pneumonia and in-hospital pneumonia, which could be analogized to the COVID-19 infection [15,16]. Furthermore, patients with sarcopenia had compromised respiratory muscle strength and respiratory function, which were detrimental in the treatment of severe pneumonia and acute respiratory distress syndrome [9]. Additionally, sarcopenia was demonstrated as a risk factor for aspiration pneumonia in the older-adult population because of the dysfunction of the swallowing muscles, which may exacerbate the condition of bedridden patients with SARS-CoV-2 infection [8]. Considering this evidence, patients with sarcopenia predictably has increased infection rates, greater disease severity, and elevated mortality rates during the COVID-19 pandemic.
Conversely, COVID-19 could be a risk factor for the incidence and progression of sarcopenia because of the reduced physical activity and inadequate protein intake caused by social isolation [22,23]. Both physical exercise and protein-based nutrients have been confirmed as crucial factors in preventing and reversing sarcopenia [3]. The reduced oral protein intake and inactivity predictably aggravate muscle depletion [24]. The inflammatory reaction caused by COVID-19, especially the cytokine storm of interferon-α, interferon-γ, IL-6, IL-12, tumor necrosis factor-α, C-reaction protein, and monocyte chemotactic protein-1 inter observed in severe infection, refers to the elevated metabolic stress and muscle catabolism [25,26]. Theoretically, the interaction between sarcopenia and COVID-19, as shown in Figure 1, could be bidirectional and may form a vicious circle. However, the interventions for sarcopenia are promising in breaking this cycle and benefit the treatment of both conditions.
The diagnosis of sarcopenia could be a vital problem during the pandemic because of the limitation of medical resources and the implementation of social isolation. Patients with advanced age, inactivity, chronic disease, cancers, and nutritional deficiency should be specifically targeted for sarcopenia assessment [5]. The sarcopenia diagnosis consensus established by the European and Asian Working Groups for Sarcopenia (i.e., the combination of decreased muscle strength, muscle mass, and physical status) should be introduced in patients with COVID-19 infection to achieve definite diagnosis and severity classification [3,4]. However, for the community population, the self-measurement of handgrip strength and calf circumference and the use of the SARC-F questionnaire (strength, assistance in walking, rising from a chair, climbing stairs, and falls) could be helpful to detect sarcopenia and permit dynamic surveillance [3,4,27].
Therapeutic approaches targeting skeletal muscle are anticipated to promote the treatment of COVID-19. Both communities and hospitals should be places for interventions. The role of moderate-intensity exercise in promoting immune function has been widely validated [28,29]. Physical activities, especially aerobic and resistance exercise, also enhance muscle protein synthesis by sensitizing muscle to insulin- or amino acid–mediated anabolic actions [30,31]. Protein support together with physical activity were reported to successfully promote the reservation of skeletal muscle mass [32,33]. The provision of protein intake accompanied by physical exercise is thus promising in promoting immune response and metabolic stress, benefiting the treatment of both sarcopenia and COVID-19 [12,24]. Detailed protocols on nutrition and exercise interventions are discussed below. Notably, patients with sarcopenia and COVID-19 infection should be alert to secondary bacterial infection, pneumonia deterioration, and respiratory failure. Aggressive therapies, including intensive care, antiviral and antibiotic use, and mechanical ventilation, should be planned in advance. Additionally, patients with sarcopenia who are bedridden should be monitored for aspiration during oral or enteral feeding [8]. Agents to promote motility, such as prokinetic medications (metoclopramide or erythromycin) could be administrated appropriately to reduce aspiration [34]. Since patients with sarcopenia were reported to respond effectively to pulmonary rehabilitation, this strategy may be introduced to patients with sarcopenia to promote the prognosis of COVID-19 [35].
Regarding nutritional management during the COVID-19 pandemic, a balanced nutritional formula with high-quality protein (meat, fish, dairy, and eggs, which are rich in leucine) is recommended to promote muscle synthesis [36,37]. The recommended protein intake increases with age, from 0.75 to 0.80 g/kg/d in healthy adults to 1.0 to 1.2 g/kg/d in healthy older adults [37], [38], [39]. For older-adult patients with definite sarcopenia or severe illness, a protein intake of 1.2 to 1.5 g/kg/d should be considered [39]. Increased protein intake (>1.2 g/kg/d) is advised for those people who are exercising and otherwise active [38]. Furthermore, an extra supplementation of protein (10–20 g/d) during the exercise interventions should be considered for reversing sarcopenic status [33,40]. For patients with sarcopenia and COVID-19 infection, nutrition support should fit the increased inflammation reaction and metabolic stress. A calorie support of 25 to 30 cal/kg/d with a protein support of 1.2 to 2.0 g/kg/d should be considered for cases of severe infection [34]. Higher protein support (>2.0 g/kg/d) should be considered for cytokine storm observed in severe COVID-19 infection [25,34]. Notably, the enteral nutrition is superior to the paraenteral nutrition for patients who are critically ill [34]. The supplementation of leucine-enriched whey protein accompanied with vitamin D could be a good formula in promoting muscle wasting in patients with sarcopenia with severe infection [41,42]. All these nutritional supplements could be helpful, although the recommendations cannot be totally achieved.achieved .
Generally, personlized physical activity protocol is recommended during the COVID-19 pandemic. Considering the impaired motor activity in patients with sarcopenia, the physical exercise protocols proposed by Jimenez-Pavon et al. [43] for older adults during the COVID-19 quarantine could be rationally referred. A multicomponent exercise program including aerobic, resistance, balance, coordination, and mobility training exercises should be safe and tolerated. An aerobic exercise of 200 to 400 min/wk distributed among 5 to 7 d, with a minimum of 2 to 3 d/wk of resistance exercise is an appropriate exercise volume. A moderate intensity (40–60% heart rate reserve or 65–75% of maximal heart rate) is anticipated to enhance the protective role of the exercise [43]. Additionally, grouped exercises consisting of 5 min of warm-up, 5 min of strengthening exercises, 5 min of balance exercises, and 5 min of cooldown could be the ideal exercise formula considering its feasibility and effectiveness [33]. Both chair exercises (toe raise, heel raise, knee lift, knee extension, hip flexion, and lateral leg raise) and ankle weight exercises with extra weight are workable without complicated materials [33]. High-intensity exercises that overcome gravity, such as the double-arm pull-downs, sit-to-stands, sit-ups, and push-ups, should be selectively adopted [40]. Resistance band exercises and machine exercises are also valuable if accessible [40]. For patients with severe COVID-19 infection, especially those requiring intensive care, physical exercise should be introduced with subtle and quantified manners. The exercises with assistants or auxiliary appliances should be started with low intensity under surveillance of physicians and generally increased to appropriate levels.
In conclusion, during the COVID-19 pandemic, patients with sarcopenia predictably are at higher =than average risk of infection and have poorer prognosis.. This article proposes, to our knowledge for the first time, a vicious circle of interactions between sarcopenia and COVID-19. Interventions targeting sarcopenia are anticipated to benefit the prevention and treatment of COVID-19. Based on the aforementioned discussions, we propose the following recommendations:
-
1)
A balanced nutritional formula including adequate protein intake with regular physical exercise (aerobic and resistance) should be achieved to prevent the development of sarcopenia and promote the community prevention of COVID-19.
-
2)
Older adults, especially those with inactivity, chronic diseases, cancers, and nutritional deficiencies, should be targeted for sarcopenia assessment and classification during the COVID-19 pandemic, and those with definite sarcopenia warrant extra protein support (10–20 g/d).
-
3)
Patients with sarcopenia with SARS-CoV-2 infection could benefit from early introduction of high-quality protein (1.2–2.0 g/kg/d, leucine-enriched) support accompanied with subtle physical exercise, which helps promote immune response and metabolic stress.
-
4)
Patients with sarcopenia with severe COVID-19 infection warrant aggressive treatments, including but not limited to the early administration of intensive care, antiviral and antibiotic use, and mechanical ventilation.
Considering that controlling the pandemic outbreaks could be a long struggle, we anticipate further studies to directly reveal the interaction between sarcopenia and COVID-19 and establish validated protocols to solve these issues.
Acknowledgment
We would like to thank Run Shi (Department of Materials Science and Engineering, Southern University of Science and Technology) Mantang Qiu (Department of Thoracic Surgery, Peking University People's Hospital) for providing help during the research.
Footnotes
This work was supported by Medical and Health Technology Innovation Project of Chinese Academy of Medical Sciences (grant numbers 2018-12M-3-003) (Spatial-Temporal Mapping Analysis on Chinese Cancer Burden). The authors declare no conflict of interest. All research data are shown in the article.
P.W. contributed to the conceptualization, methodology, software, writing (original draft preparation), validation, and supervision of the article. Y.L. contributed to the conceptualization, investigation, and writing (reviewing and editing) of the article. Q.W. contributed to the software, data curation, and visualization of the article.
References
- 1.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO coronavirus disease (COVID-2019) dashboard. Available at: https://covid19.who.int/. Accessed November 29, 2020.
- 3.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21 doi: 10.1016/j.jamda.2019.12.012. 300–7.e2. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Shen Y, Chen J, Chen X, Hou L, Lin X, Yang M. Prevalence and associated factors of sarcopenia in nursing home residents: a systematic review and meta-analysis. J Am Med Dir Assoc. 2019;20:5–13. doi: 10.1016/j.jamda.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48:48–56. doi: 10.1093/ageing/afy106. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki T, Ebihara S, Mori T, Izumi S, Ebihara T. Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int. 2020;20:7–13. doi: 10.1111/ggi.13839. [DOI] [PubMed] [Google Scholar]
- 9.Ohara DG, Pegorari MS, Oliveira Dos Santos NL, de Fátima Ribeiro Silva C, Oliveira MSR, Matos AP. Cross-sectional study on the association between pulmonary function and sarcopenia in Brazilian community-dwelling elderly from the Amazon region. J Nutr Health Aging. 2020;24:181–187. doi: 10.1007/s12603-019-1290-y. [DOI] [PubMed] [Google Scholar]
- 10.Wang PY, Chen XK, Liu Q, Yu YK, Xu L, Liu XB. Highlighting sarcopenia management for promoting surgical outcomes in esophageal cancers: evidence from a prospective cohort study. Int J Surg. 2020;83:206–215. doi: 10.1016/j.ijsu.2020.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr. 1995;62:30–39. doi: 10.1093/ajcn/62.1.30. [DOI] [PubMed] [Google Scholar]
- 12.Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012;4:535–546. doi: 10.18632/aging.100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang PY, Xu LD, Chen XK, Xu L, Yu YK, Zhang RX. Sarcopenia and short-term outcomes after esophagectomy: a meta-analysis. Ann Surg Oncol. 2020;27:3041–3051. doi: 10.1245/s10434-020-08236-9. [DOI] [PubMed] [Google Scholar]
- 16.Altuna-Venegas S, Aliaga-Vega R, Maguina JL, Parodi JF, Runzer-Colmenares FM. Risk of community-acquired pneumonia in older adults with sarcopenia of a hospital from Callao, Peru 2010–2015. Arch Gerontol Geriatr. 2019;82:100–105. doi: 10.1016/j.archger.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Cao L, Xu S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non–small cell lung cancer patients: a systematic review and meta-analysis. Int Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106907. [DOI] [PubMed] [Google Scholar]
- 18.Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–650. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang W, Guan W, Chen R, Wang W, Li J, Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morley JE, Kalantar-Zadeh K, Anker SD. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11:863–865. doi: 10.1002/jcsm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akseer N, Kandru G, Keats EC, Bhutta ZA. COVID-19 pandemic and mitigation strategies: implications for maternal and child health and nutrition. Am J Clin Nutr. 2020;112:251–256. doi: 10.1093/ajcn/nqaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirwan R, McCullough D, Butler T, Perez de Heredia F, Davies IG, Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. Geroscience. 2020;42:1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meftahi GH, Jangravi Z, Sahraei H, Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging. Inflamm Res. 2020;69:825–839. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell. 2018;17:e12750. doi: 10.1111/acel.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson RJ, Kunz H, Agha N, Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141:568–573. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- 31.Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care. 2009;12:66–71. doi: 10.1097/MCO.0b013e32831cef75. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto Y, Kaji A, Sakai R, Takahashi F, Kawano R, Hamaguchi M. Effect of exercise habit on skeletal muscle mass varies with protein intake in elderly patients with type 2 diabetes: a retrospective cohort study. Nutrients. 2020;12:3220. doi: 10.3390/nu12103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016;103:830–840. doi: 10.3945/ajcn.115.113357. [DOI] [PubMed] [Google Scholar]
- 34.Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Crit Care Med. 2016;44:390–438. doi: 10.1097/CCM.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 35.Jones SE, Maddocks M, Kon SS, Canavan JL, Nolan CM, Clark AL. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70:213–218. doi: 10.1136/thoraxjnl-2014-206440. [DOI] [PubMed] [Google Scholar]
- 36.Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: a randomized, controlled trial. Am J Clin Nutr. 2018;107:217–226. doi: 10.1093/ajcn/nqx028. [DOI] [PubMed] [Google Scholar]
- 37.Traylor DA, Gorissen SHM, Phillips SM. Perspective: protein requirements and optimal intakes in aging: are we ready to recommend more than the recommended daily allowance? Adv Nutr. 2018;9:171–182. doi: 10.1093/advances/nmy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr. 2014;33:929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeuchi I, Yoshimura Y, Shimazu S, Jeong S, Yamaga M, Koga H. Effects of branched-chain amino acids and vitamin D supplementation on physical function, muscle mass and strength, and nutritional status in sarcopenic older adults undergoing hospital-based rehabilitation: a multicenter randomized controlled trial. Geriatr Gerontol Int. 2019;19:12–17. doi: 10.1111/ggi.13547. [DOI] [PubMed] [Google Scholar]
- 41.Lin CC, Shih MH, Chen CD, Yeh SL. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: an open-label, parallel-group study. Clin Nutr. 2020 doi: 10.1016/j.clnu.2020.08.017. S0261–5614:30432–5. [DOI] [PubMed] [Google Scholar]
- 42.Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16:740–747. doi: 10.1016/j.jamda.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez-Pavon D, Carbonell-Baeza A, Lavie CJ. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: special focus in older people. Prog Cardiovasc Dis. 2020;63:386–388. doi: 10.1016/j.pcad.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]