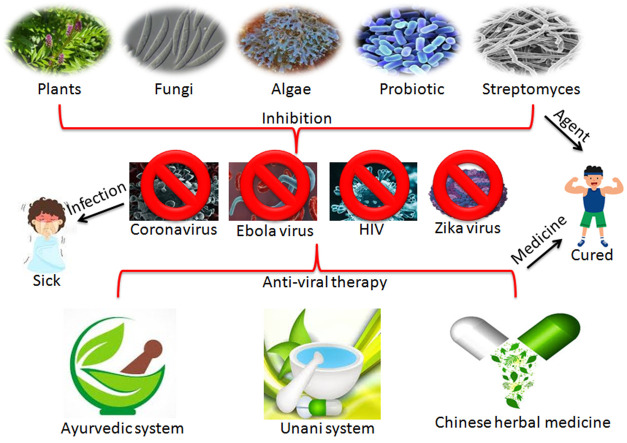
Abstract
In the current scenario, the increasing prevalence of diverse microbial infections as well as emergence and re-emergence of viral epidemics with high morbidity and mortality rates are major public health threat. Despite the persistent production of antiviral drugs and vaccines in the global market, viruses still remain as one of the leading causes of deadly human diseases. Effective control of viral diseases, particularly Zika virus disease, Nipah virus disease, Severe acute respiratory syndrome, Coronavirus disease, Herpes simplex virus infection, Acquired immunodeficiency syndrome, and Ebola virus disease remain promising goal amidst the mutating viral strains. Current trends in the development of antiviral drugs focus solely on testing novel drugs or repurposing drugs against potential targets of the viruses. Compared to synthetic drugs, medicines from natural resources offer less side-effect to humans and are often cost-effective in the productivity approaches. This review intends not only to emphasize on the major viral disease outbreaks in the past few decades and but also explores the potentialities of natural substances as antiviral traits to combat viral pathogens. Here, we spotlighted a comprehensive overview of antiviral components present in varied natural sources, including plants, fungi, and microorganisms in order to identify potent antiviral agents for developing alternative therapy in future.
Abbreviations: AIDS, Acquired immunodeficiency syndrome; CHIKV, Chikungunya virus; CHMs, Chinese herbal medicine; CIN, Cervical intraepithelial neoplasia; COVID-19, Coronavirus disease 2019; DAA, Direct acting antiviral agents; ELISA, Enzyme-linked immunosorbent assay; EPS, Exopolysaccharides; EVD, Ebola virus disease; HA, Hemagglutinin; HAV, Hepatitis A virus; HBV, Hepatitis B virus; HCV, Hepatitis C virus; HIV, Human immunodeficiency virus; HPV, Human papilloma virus; HSV-1, Herpes simplex virus type-1; HSV-2, Herpes simplex virus type-2; MERS-CoV, Middle East Respiratory Syndrome-coronavirus; NA, Neuraminidase; NIV, Nipah virus; ORFs, Open reading frames; PCR, Polymerase chain reaction; RT-PCR, Reverse transcription-polymerase chain reaction; SARS, Severe acute respiratory syndrome; SARS-COV, Severe acute respiratory syndrome coronavirus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; VZV, Varicella zoster virus; ZIKV, Zika virus
Keywords: Antiviral, Alternative therapy, Ethno medicine, Natural sources, Viral diseases
Graphical abstract
1. Introduction
Viral diseases are colossal threat to human and animal population. Emerging viral disease outbreaks have grown rapidly in the recent years and it has created great impact on human life, leading to the sudden increase in mortality rates. Over the past two decades, there have been seven disease epidemics that resulted in huge economic losses in the world, of which Coronavirus disease 2019 (COVID-19), Severe acute respiratory syndrome (SARS), Nipah virus (NIV) disease, West Nile virus disease, Avian Influenza, and Rift Valley fever are caused by viruses. Three modes of viral disease occurrence have been identified such as a) infection to a new host with no transmission, b) spread out to local populations, and c) epidemic or constant host-to-host transmission (Parrish et al., 2008).
Viruses generally consist of DNA or RNA (single/double stranded or positive/negative stranded) as their genetic material which is surrounded by a lipoprotein/glycoprotein covering. Table 1 shows the classification of selected animal viruses with DNA/RNA genomes. Viruses invade host and employ the host metabolic processes as well as generate many copies of viral proteins that produce individual virus. The viral strains eventually get adapted to the host's immune systems. Pre-vaccination was found to be more effective approach. The transmission of virus also depends on the contact of people in a population. Since the viral strains are mutated and are getting adapted, it is difficult to develop the vaccines (Alexander and Kobes, 2011). The antiviral drugs play a very important role in today's life by suppressing the viral transmission and helps in host surviving. Analyzing and understanding the kinetics and dynamics of antiviral drugs aid in controlling the virus during pandemics because the hosts may expose to the infection again. Antivirals are effective in cases where there are no vaccines available for viruses like Influenza virus (Pepin et al., 2013).
Table 1.
Classification of selected animal viruses with DNA/RNA genomes.
| Type of viruses | DNA/RNA material | Family | Virus | Capsid shape | Envelope | Virion size (nm) | Length of genome |
|---|---|---|---|---|---|---|---|
| DNA viruses | dsDNA | Herpesviridae | HSV | Icosahedral | Yes | 200 | 130–230 kbp |
| VZV | Icosahedral | Yes | 150–200 | 125 kb | |||
| Papillomaviridae | HPV | Icosahedral | No | 54–60 | 5–8 kbp | ||
| RT viruses | Reverse transcribing | Retroviridae | HIV | Icosahedral | Yes | 90 | 9 kb |
| Hepadnaviridae | HBV | Icosahedral | Yes | 42 | 3 kbp | ||
| RNA viruses | (+) ssRNA | Coronaviridae | COVID-19 | Spherical | Yes | 120 | 27–32 kb |
| SARS-CoV | Spherical | Yes | 120 | 27–32 kb | |||
| MERS-CoV | Spherical | Yes | 120 | 27–32 kb | |||
| Flaviviridae | Dengue | Icosahedral | Yes | 45 | 11 kb | ||
| ZIKV | Icosahedral | Yes | 50 | 9.7–12 kb | |||
| HCV | Icosahedral | Yes | 50 | 10 kb | |||
| Picornaviridae | HAV | Icosahedral | No | 27 | 7 kb | ||
| Togaviridae | CHIKV | Icosahedral | Yes | 70 | 12 kb | ||
| (−) ssRNA | Filoviridae | Ebola virus | Helical | Yes | 970 | 18–19 kb | |
| Paramyxoviridae | NIV | Helical | Yes | 150 | 18 kb | ||
| Measles | Helical | Yes | 120–150 | 15 kb | |||
| Hantaviridae | Hanta virus | Helical | Yes | 80–120 | 14 kb | ||
| Orthomyxoviridae | Influenza virus | Helical | Yes | 100 | 14 kb |
The degree of virus infection depends on the immunity of human. The immunocompromised hosts are at higher risk of viral infection, thereby creating the situation worse for those people (Ye et al., 2013). The drug usage should be studied properly to analyze the results. Administration of drugs is taken into consideration for predicting the dynamics during epidemic waves. The emergence of pandemic has made every country to contain stockpile of antiviral drugs. These drugs are important because studies showed that these drugs can help in controlling future pandemic. Though it might not cure it, the rate of transmission can be controlled (Becker and Wang, 2011).
Antivirals in combination with other antimicrobials help to combat resistant strains (Villa et al., 2017). Similarly, direct acting antiviral agents (DAA) was very effective in treating hepatitis C virus (HCV) infection. The DAAs constitute a combination of simeprevir, paritaprevir, ritonavir, daclatasvir, ledipasvir, ombitasvir, sofosbuvir, and dasabuvir. The proper intake of food along with the drugs had a great effect (Talavera Pons et al., 2017). Antiviral drugs perform targeted therapy by interacting with viruses' target proteins and the host's immune system (Thomasy and Maggs, 2016). Despite the availability of plethora of antiviral drugs in the market, there is continuous effort by worldwide researchers to identify new therapeutic agents from un/less exploited resources. Those bioactive agents have revealed in vitro and in vivo antiviral potentialities against various groups of viruses. Bioactive agents from natural resources have established a great foundation for designing new therapeutic drugs. It is certainly essential to understand the nature, source of origin, and role of identified active agents as therapeutics. Considering this, the present comprehensive review overviews the effectiveness of antiviral components present in various natural sources (plants, fungi, and prokaryotes) in order to identify potential antiviral agents for developing alternative therapy in future.
2. Major viral diseases outbreaks: an overview
2.1. Zika virus (ZIKV) disease
Zika virus belongs to family Flaviviridae. The virus is transmitted through the bite of infected female mosquitoes, Aedes aegypti and Aedes albopictus. Flaviviruses in human can also lead to many diseases that include West Nile, dengue, yellow fever, tick-borne, and Japanese encephalitis. The route of transmission of ZIKV is through arthropod vectors, central nervous system injury, and hemorrhagic fevers. The infection of ZIKV during pregnancy results in birth defects in new born babies, a condition called microcephaly. In adults, it leads to temporary paralysis. In Flaviviridae family, all members have enveloped virus with single stranded RNA genome and possesses 3 structural proteins envelope, capsid, precursor membrane, and 7 non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). Patients in phase I and II clinical trials are vaccinated with DNA/mRNA vaccine. Symptoms of this infection include skin rashes, headache, joint pain, muscle pain, and fever. The major outbreak took place in Yap Island (2007), South America (2015–2016), and French Polynesia (2013–2014). Guillian-Barre` syndrome and microcephaly are common neurological manifestations of this disease (Lin et al., 2018).
2.2. Nipah virus disease
Nipah virus can be transmitted to humans from animals like bats or pigs. It can also transmit through contaminated food or directly from people to people. It was first recognized in Malaysia (1999), the people who were in contact with sick pigs or contaminations of tissues. Transmission is through unprotective contact or secretions from pigs, and fruits contaminated with secretions of urine by infected fruit bats. Symptoms include fever, headache, myalgia, and acute encephalitis. Incubation period ranges from 4 to 14 days. The diagnosis includes reverse transcription-polymerase chain reaction (RT-PCR) from body fluids and enzyme-linked immunosorbent assay (ELISA). The fruit bats belonging to the family Pteropodidae are the host of NIV. It has also been reported in other animals such as horse, sheep, goats, cats, and dogs. It is a single stranded and non-segmented enveloped RNA virus. The NIV is second member of genus Henipavius belonging to the family Paramyxoviridae. Prevention can be done by reducing overcrowding between animals and avoiding consumption of contaminating foods (Singh et al., 2019).
2.3. SARS-COV
Severe acute respiratory syndrome coronavirus (SARS-COV) belongs to family Coronaviride and order Nidovirales. It causes respiratory or intestinal infections in humans and animals. It is positive sense single stranded RNA virus which has genome size about 30 kb with 14 functional open reading frames (ORFs). Their genome size is larger with respect to all other RNA viruses. Symptoms of this infection include cough, chillness, myalgia, sore throat, rhinorrhea, breathlessness, and diarrhea. Serum test, RT-PCR, and ELISA are the most common tests performed for diagnosing the infected patients. There is no effective antiviral agent identified till date to control SARS-COV (Cheng et al., 2007).
2.4. Herpes genitalis
Herpes genitalis is a sexually transmitted infection caused by herpes simplex virus type-1 (HSV-1) or herpes simplex virus type-2 (HSV-2). They are enveloped DNA virus. The primary mode of transmission is by direct contact. There are some similarities between HSV-1 and HSV-2 based on type of epitopes and antigenic cross reactions. HSV-1 occurs in childhood and HSV-2 occurs during sexual contact. HSV-2 is commonly seen in females. Primary infection results in papular skin, lesion in mucous membrane, swelling in inflammatory regions in vulva, and dysuria. The recurrent infection includes fever, menstruation stress, abortion, and eye lesion. The diagnosis is done by swabbing the infected mucous membrane and then analyzed using polymerase chain reaction (PCR). Another diagnosis includes antibody detection of HSV infection. Acyclovir, valacyclovir, and famciclovir are the first line drugs used for its treatment (Sauerbrei, 2016).
2.5. Measles virus
Measles is caused by Rubella virus. It mainly affects children and pregnant women. The virus belongs to the family Paramyxoviridae and holds single stranded negative sense RNA, encodes 6 structural proteins, and 2 non-structural proteins. Measles occurs only in humans and is transmitted by respiratory droplets, saliva, skin to skin contact, and touching contaminated surface. Incubation period of the virus is 14–18 days. Symptoms include maculopapular rashes, cough, conjunctivitis, fever, and diarrhea. Samples from throat, nasal, and urine are used for analyzing using PCR. Attenuated measles strain is used as a vaccine in the beginning stage of the infection (Kondamudi and Waymack, 2020).
2.6. Human papilloma virus (HPV)
Human papilloma virus disease is a sexually transmitted infection which causes cervical cancer and genital warts. Among various types of HPV, type 16 and 18 are responsible for causing cervical cancer and HPV 6 and 11 cause genital warts. It mostly affects woman and is transmitted through skin to skin contact and infects vagina or anal intercourse. Cervical cancer can be detected by papanicolaou testing; hence changes in squamous epithelium cells should be noted. The changes observed on the abnormal cells are referred as cervical intraepithelial neoplasia (CIN). Depending on the depth of the abnormal cells, it can be classified into 3 types (CIN-1, CIN-2, and CIN-3). CIN-1, CIN-2, and CIN-3 show mild, moderate, and severe dysplasia, respectively. For human papilloma virus, vaccine was developed against the type 6, 11, 16, and 18. It is prophylactic quadrivalent vaccine named gardasil. Another type of vaccine is bivalent vaccine, developed against HPV 16 and 18 (Braaten and Laufer, 2008).
2.7. Acquired immunodeficiency syndrome (AIDS)
AIDS is caused by human immunodeficiency virus (HIV). The virus infects the CD4+ T lymphocytes cells and results in catastrophic effect in the host. When the virus replication is increased it results in cardiovascular disease and infects other organs, resulting in kidney and liver damage. In some cases, tuberculosis plays the major role in activating the disease. Vaccines are developed using X-ray crystallography, cryo electron microscopy, and other technologies including probing the B-cell lineage and genome sequencing (Schwetz and Fauci, 2019).
2.8. Ebola virus disease (EVD)
Ebola virus belongs to family Filoviridae and is transmitted by fruit bats. It is transmitted by infected blood, airborne, and infection through droplet. The EVD can be diagnosed using blood samples, saliva, breast milk, semen, sweat, tears, stool, skin, vaginal, and rectal swabs. The transmission can also be oral such as by consuming uncooked animal food. The production of disease can be through tear, mucous membrane, and skin; which infects immune system and reaches lymph nodes, causing lymphadenopathy and hematogenous spread through liver and spleen resulting in failure of organs. Symptoms can be headache, dysphagia, malaise, dry cough, sore throat, nausea, vomiting, diarrhea, and conjuctival bleeding. Diagnosis is done by RT-PCR and ELISA test by the samples taken from infected persons. Currently, there is no antiviral drug for this virus (Hasan et al., 2019).
2.9. Chicken pox
Chicken pox is caused by varicella zoster virus (VZV) which is also responsible in causing herpes zoster or shingles. It is transmitted by inhaling aerosol droplets from infected patient. Symptoms include small itchy blister that spreads over chest, back, and then spreads through face, resulting in fatigue, fever, headache, and pharyngitis lasy for seven days. It is diagnosed by PCR by the blister fluid samples. Vaccine was introduced in 1995 and it helps in the prevention of the infection (Ayoade and Kumar, 2020).
2.10. Hanta virus disease
Hanta virus causes hemorrhagic fever. It is also called as hanta virus cardio pulmonary syndrome, renal syndrome, and non-pathogenic prospects hill virus. It affects the function of kidney. The virus enters the host by interacting with cell surface integrin receptors and also uses alpha 5 beta 1 receptors to enter into the cell. The infection occurs by direct contact with infected rodents and inhaling virus through lungs. Hanta virus can be differentiated into many types such as Seoul virus from domestic rat, others are black creek canal virus, bayou virus etc. Symptoms include chillness, dizziness, headache, nausea, cough, vomiting, malaise, diarrhea, back pain, abdominal pain, and tachycardia. Diagnosis is based on positive serological test, blood samples detecting viral antigen, viral RNA sequences, serological assays, immunohistochemistry, and PCR. There is no antiviral drug for hanta virus but antipyretics and analgesic are used to control the disease (Mir, 2010).
2.11. COVID-19
Recent emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) belongs to the family Coronaviridae. It has created a great impact throughout the world by its pathogenic nature and named COVID-19 by World Health Organization. The infection was acquired from seafood market in Wuhan, China. The genome of coronavirus consists of positive single stranded RNA of approximately 27–32 kb. The virus has Nsp1–16 (non-structural proteins) genes and others that code for four structural proteins including the envelope protein (E), membrane protein (M), spike protein (S), and the nucleocapsid protein (N) (Schoeman and Fielding, 2019). Symptoms include cough, mild fever, breathlessness, and throat congestion. Detection of the SARS-CoV-2 can be done by RT-PCR. Although few drugs and traditional remedies have been reported to alleviate mild symptoms of COVID-19, there are no medicines or vaccines approved to cure the disease till date. Nevertheless, there are several clinical trials undertaken including antibiotics, vaccines, and natural products proposed for treatment purpose (Bimonte et al., 2020).
2.12. Dengue
Dengue and dengue hemorrhagic fever are caused by the virus that belongs to Flaviviridae family. Flaviviruses infect host by the intermediate vectors like mosquitoes (Aedes aegypti) or ticks. There are four distinct serotypes of dengue viruses (DEN-1, DEN-2, DEN-3, and DEN-4) (Gubler and Clark, 1995). Approximately 2.5 billion people are susceptible at risk for this epidemic disease. Clinically, this disease has an incubation period of 2–7 days and symptoms include rashes, anorexia, cold, flu, nausea, vomiting, and respiratory illness. Laboratory diagnosis includes immunoassay tests and PCR amplification. No vaccines or specific antiviral drugs are available for this disease.
2.13. Chikungunya
Chikungunya, an epidemic threat in the recent years is a mosquito-borne disease in the tropical regions. It is caused by Chikungunya virus (CHIKV), a pathogen of the genus Alphavirus and the family Togaviridae. These are otherwise known as arboviruses as they are arthropod-borne viruses. CHIKV is similar to other alphaviruses including Sindbis viruses and Ross River viruses. Three distinct genotypes including Asian, West African, and East Central South African have been observed so far. CHIKV holds a positive sense single stranded RNA of ~12 kb genome length. The genome analysis revealed that the viral comprise two ORFs. The 5′ORF encodes the nsP1, nsP2, nsP3, and nsP4 non-structural proteins, and the 3′ORF encodes capsid (C), envelope (E1 and E2), and two peptides (E3 and 6K) (Nunes et al., 2015). The acute stage lasts for a week whereas the chronic stage lasts from months to years. The symptoms include fever, arthralgia, rarely causing cardiac, ophthalmic, and neurological disorders. Diagnostic assays include ELISA, IgM antibody levels, and PCR. Treatment includes anti-rheumatic drugs but no vaccines have been discovered yet.
2.14. Influenza
Influenza viruses are significant due to its unavailing presence in the past centuries. The virus belongs to the family Orthomyxoviridae. Three forms namely A, B, and C infect human. Influenza A and B viruses cause relatively high morbidity and mortality compared to the C type. These are enveloped viruses that encompass segmented negative-sense single-stranded RNA. The gene structure contains surface glycoproteins, hemagglutinin (HA), and neuraminidase (NA). Based on the types of HA and NA, a total of 16 HA (H1–16) and 9 NA (N1–9) subtypes are identified in birds. Recent outbreaks in humans contain subtypes H1N1 and H3N2 that are reported to be endemic. The zoonotic spread from birds and swine includes H5N1, H7N9, and H9N2. These have the capabilities to mutate into new forms and produce severe pathological effects (Harris et al., 2017). Symptoms include rapid onset of fever, dry cough, headache, muscle and joint pain, and severe malaise. The diagnostic method comprises influenza-specific RNA by RT-PCR. Treatment includes NA and HA inhibitors with monoclonal antibodies (Nachbagauer and Krammer, 2017) and antiviral drugs.
2.15. Middle East Respiratory Syndrome-coronavirus (MERS-CoV)
MERS-CoV is a zoonotic viral respiratory disease that has infected people with a high mortality rate of nearly 50% in the Middle East (first identified in Saudi Arabia in 2012). The disease is alleged to be contracted from infected camels. Coronaviruses possess enveloped single stranded RNA that is spherical in shape with glycoprotein projections. The genome shows presence of two ORFs namely ORF 1a and 1b coding for non-structural proteins. Structural proteins encode the spike (S), envelope (E), membrane (M), and nucleocapsid (N). Symptoms include mild respiratory disease to severe acute respiratory disease and death. Severe illness can lead to the respiratory failure and may weaken the immune systems, especially with those with renal diseases, cancer, lung diseases, and diabetes. RT-PCR assay has been used as a diagnostic tool to detect the virus. At present, no vaccine or precise treatment is available (Alagaili et al., 2014).
2.16. Hepatitis viral disease
Hepatitis viruses are hepaciviruses that belong to Flaviviridae. These viruses possess a linear and positive sense single stranded RNA genome coding for nearly 10 proteins. There are 7 genotypes encountered till date (genotype 1 to 7). Hepatitis A virus (HAV), a member of hepatovirus is an endemic spread by fecal-oral route. Symptoms include necrosis and inflammation of the liver cells. It includes a positive sense RNA and the genome comprise of about 7500 (nucleotides). The incubation period is approximately 3–5 weeks. Hepatitis B virus (HBV) belongs to Hepadnaviridae family and includes dsDNA virus that replicates via reverse transcription (Stuyver et al., 2000). HCV is transmitted by blood-to-blood contacts and other blood/body fluid contaminants. This is an enveloped single-stranded RNA virus similar to flavivirus. It leads to complications such as liver cirrhosis, liver failure, and liver cancers such as hepatocellular carcinoma. Currently, no treatment is available for HCV infections.
3. Immune mechanisms in viral diseases
Immune system is a complex network of defence mechanism present in living organisms to fight the invading foreign microorganisms and provides protection from diseases. The immune system confers immunity to the organism by eliciting immune responses mediated by specialized immune cells and organs. Once the virus enters the host cells (cytopathic and non-cytopathic), it replicates, kills the infected cells, and invades other cells by releasing cellular contents (Münz et al., 2009).
Innate mechanism in human acts by the interaction of the virus particles with various receptors like endosomal Toll-like receptors, C-type lectin receptors, cytoplasmic retinoic acid-inducible gene I receptors, and Nod-like receptors. Once induced, these receptors produce cytokines and interferons. Following the action encountered by the innate cells like neutrophils and release of pro-inflammatory cytokines, special T cells get induced to respond to the invaders. These cells also persuade B cells to secrete antibodies, which form immune complexes. They further invoke cytotoxic T lymphocytes CD8+ to transfer to the infection site and kill infected cells. Antibody mediated immune responses ie. antigen-antibody complexes induce activation of complement cascade. HIV-1, human cytomegalovirus, and certain other viruses use the host complement control proteins into their virions that create cell lysis (Mengshol et al., 2010).
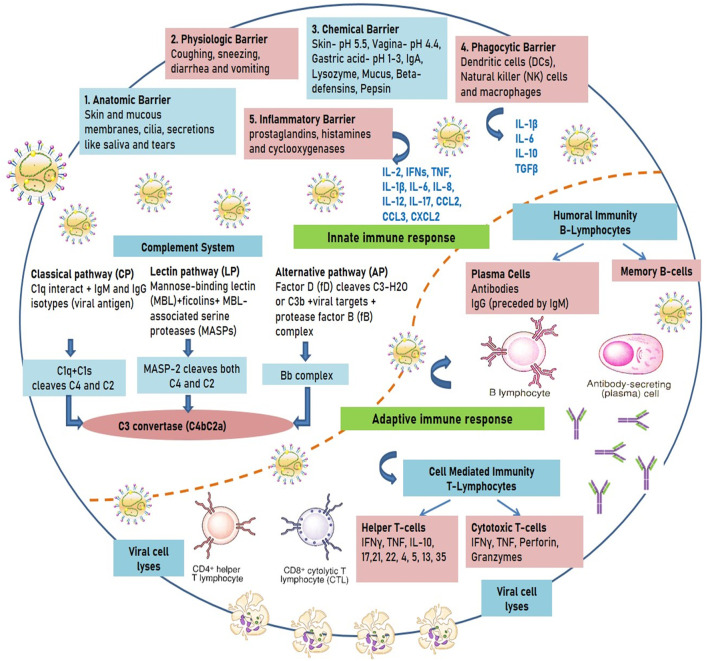
The complement system of the innate immunity includes several factors and cell surface proteins that invoke immune response to the pathogens (Carroll, 2004). Three pathways of complement system are i) classical pathway (viral antigens bound with IgM and IgG interact with C1q and activates 2 serine proteases C1r and C1s, that further cleaves C4 into C4a and C4b to form the C3 convertase-C4bC2a) ii) alternate pathway (triggered by the hydrolysis of C3 that binds to protease factor B. This is cleaved by Factor D to form Bb in order to end the formation of C3 convertase-C4bC2a), and iii) lectin pathway (antigenic substances initiate mannose-binding lectin (MBL) and the ficolins. It forms a complex with MBL-associated serine proteases and cleaves C4 and C2 proteins to form C3 convertase-C4bC2a). These pathways regulate and activate complement factors and unite to form the major C3 component involved in virus pathogenesis (Ricklin et al., 2010). The innate, complement, and the adaptive immune responses are interlinked and are activated by the varying mechanisms, depending on the type of infecting viral particles leading to reduced pathogenesis, regulate inflammatory conditions, and modulating adaptive responses (Fig. 1 ).
Fig. 1.
Immune responses against viral infections.
4. Antivirals from natural sources
Recent researches in etiology have made better understanding of viral diseases. There is a continuous search of natural drugs to target viral proteins. Only limited chemicals are available for treating emerging viral diseases which is a major disadvantage. Therefore, there is an urgent need to unravel the potential antiviral metabolites from varying natural sources.
4.1. Medicinal plants
Medicinal plants produce a variety of bioactive constituents that have the abilities to inhibit the replication cycle of various types of DNA or RNA viruses like HIV, HSV, Influenza virus, Human rhinovirus, Hepatitis B and C virus (HBV and HCV), and Dengue virus. Throughout the globe, medicinal plants act as important components to relieve from various ailments like bacterial, viral, and other infections. To mention a few, bioflavonoids such as Naringin (grape), daidzein (soybean), quercetin (foods and fruits such as green and black tea, apple, onion, citrus, tomato, and some other plants), and hesperetin (citrus) have been reported to fight dengue virus replication (Zandi et al., 2011).
Extracts of plants like Rosa nutkana and Amelanchier alnifolia were found active against enteric coronavirus (Jassim and Naji, 2003). Significant compound glycyrrhizin, found in Glycyrrhiza glabra, has antiviral activity against many viruses such as HBV, HCV, HIV, and HSV infections. Lycorine isolated from Lycoris radiate showed strong anti-SARS-CoV activity. The hot water extracts of Stevia rebaudiana blocked entry of various infectious serotypes of Human Rhinovirus into the permissive cells by an anionic polysaccharide with uronic acid as a major sugar constituent (Mishra et al., 2013).
Essential oils (eucalyptus oil, tea tree oil, and thyme oil) and monoterpenes like isoborneol proved antiviral activities against HSV-1 by inhibiting glycosylation of viral proteins (Astani et al., 2010). Silymarin (from the seeds of Silybum marianum) and catechin (present in green tea extract) inhibited HCV and also displayed anti-inflammatory and immunomodulatory actions (Calland et al., 2012). Table 2 illustrates antiviral properties of various plants associated metabolites against deadly viruses.
Table 2.
Antiviral traits of medicinal plants associated metabolites.
| Name of the compound | Plant | Active against | References |
|---|---|---|---|
| Alkaloids and nitrogenated compounds | |||
| Actinophnine | Actinodaphne hookeri | HSV-1 | Montanha et al. (1995) |
| Atropine | Atropa belladona L. | Enveloped virus | Yamazaki and Tagaya (1980) |
| Biopterin | Crithidia fasciculata | Antiviral activity | Tschesche et al. (1962) |
| Buchapine | Euodia roxburghiana | HIV-1 | Manske and Brossi (1985) |
| Camptothecin | Ophiorrhiza mungos | Herpes virus | Tafur et al. (1976) |
| Canavanin | Carnavalia ensiformis L. | Influenza virus | Pilcher et al. (1955) |
| Caffeine | Theobroma cacao L. and Coffea sp. | Coxsackie-virus, Herpes, Poliovirus, vaccinia, and influenza virus | Yamazaki and Tagaya (1980) |
| Caribine | Hymenocallis arencola | Antiviral activity | Manske and Brossi (1987) |
| Carinatine | Zephyranthes carinata | Antiviral activity | Manske and Brossi (1987) |
| Chelidonine | Chelidonium majus L. | Herpes virus and influenza virus | Manske and Brossi (1987) |
| Cordycepin | Aspergillus nidulans Eidam Wint. Cordyceps militaris | Picornavirus, poliovirus, vaccinia, newcastle disease virus, Herpes simplex, and influenza viruses | Kaij-a-Kamb et al. (1992) |
| Cryptopleurine | Bochneria cylindrica L. Sw. and Cryptocarya pleurosperma | HSV-1 | Cordell (1981); Manske and Brossi (1989) |
| O-demethyl-buchenavianine | Buchenavia capitata | HIV | Vlietinck et al. (1997) |
| Emetine | Cephaelis ipecacuanha A. Rich. | Pseudorabies and Herpes virus | Hanish et al. (1966) |
| Fagaronine | Fagara zanthoxyloides Lam | Retrovirus | Manske and Brossi (1988) |
| Harmaline, Harmine | Peganum harmala | HSV-1 | Rashan (1990) |
| Hypoxanthine | Beta vulgaris | Antiviral activity | Mifflin (1981) |
| Lycorine | Clivia miniata | Antiviral activity | Leven et al. (1983) |
| Michellamines D, Michellamines F | Ancistrocladus korupensis D. Thomas and Gereau | HIV | Hallock et al. (1997) |
| 10-Methoxycamptothecin | Camptotheca acuminata Descene | Adenovirus, Herpes, and vaccinia viruses | Clemens (1977) |
| Odorinol | Aglaia roxburghiana Miq. var. Beddomei | Ranikhet disease virus | Phillipson and Zenk (1980) |
| Oliverine | Polyathia oliveri | HSV-1 | Montanha et al. (1995) |
| Oxostephanine | Stephania japonica | HSV-1 | Montanha et al. (1995) |
| Pachystaudine | Pachypodanthium staudti | HSV-1 | Montanha et al. (1995) |
| Papaverine | Papaver somniferum | CMV, measles, HIV | Manske and Brossi (1990) |
| Psychotrine | Cephaelis acuminata | HIV-1 | Manske and Brossi (1985) |
| Schumannificine | Schumanniophyton magnificum | HIV and HSV | Vlietinck et al. (1997) |
| Taspine | Croton lechleri M. | Avian myeloblastosis virus, Rauscher virus, and Simian sarcoma virus | Manske and Brossi (1990) |
| Homonojirimycin, Deoxymanojirimycin | Omphalea diandra | Homonojirimycin is an inhibitor of several a-glucosidases, Deoxymanojirimycin is an inhibitor of glycoprocessing mannosidase | Kite et al. (1988) |
| Aranotin, Gliotoxin | Arachniotus aureus (Eidam) Schoeter | Coxsackievirus A 21, poliovirus, rhinovirus, influenza virus, and para-influenza virus type 3 | Becker (1980); Miller et al. (1968) |
| Ochropamine and epi-16-Ochropamine | Cabucula erythrocarpa Vatke Mar | Influenza virus | Manske and Brossi (1990) |
| (+)-Glaucine fumarate, (+)-N-Methyllaurotetanine, (+)-Isoboldine, and (−)-Nuciferine HCl | Corydalis cava, Glaucium flavum, Peumus boldo | HSV and picornaviridae | Boustie et al. (1998) |
| Castanospermine, Australine | Castanospermum australe | HIV | Foder and Colasanti (1985) |
| Leurocristina, Periformyline, Perivine, and Vincaleucoblastine | Catharanthus roseus L. G. Don. and C. lanceus Pich | Leurocristina-Mengovirus extracellular virucidal, poliovirus, vaccinia, and influenza viruses Periformyline -poliovirus type 3-Perivine - vaccinia Polio extracellular virucidal activity Vincaleucoblastine - poliovirus vaccinia, and influenza virus | Farnsworth et al. (1968) |
| Columbamine, Berberine, and Palmitine | Annonaceae, Berberis vulgaris, menispermaceae and Papaveraceae | HIV-1 | Manske and Brossi (1990) |
| Narciclasine, Lycoricidine, Pancratistatin, 7-deoxypancratistatin, Acetatos, Isonarciclasine, cis-Dihydronarciclasine, Lycorines, and Pretazettine | Narcissus poeticus L., Lycorine was isolated from Clivia mimiata Regel | Flaviviruses, bunyaviruses, and Poliomyelitis virus | Gabrielsen et al. (1992); Ieven et al. (1982) |
| Buxamine E and Cyclobuxamine H | Buxus sempervirens | HIV-1 reverse transcriptase | Hiller (1987) |
| Triptonines A and Triptonines B | Tripterygium hypoglaucum and Tripterygium wilfordii | HIV | Duan et al. (2000) |
| 5-hydroxynoracronycine and Acrimarine F | Citrus plants | Epstein-Barr virus | Takemura et al. (1995) |
| Fagaronine, Columbamine, and Fulvoplumierin | Plumeria rubra L. | HIV-1 reverse transcriptase | Tan et al. (1991) |
| β-carbolines, furanoquinolines, indolizidines, swainsonine, and castanospermine | Swainsona canescens, Astragalus lentiginosus, Castanospermum australe, Aglaia roxburghiana | DNA viruses | Hudson (1990); Sydiskis et al. (1991); Asano et al. (1996); Erdelmeier et al. (1996); Marchetti et al. (1996) |
| Coumarins | |||
| Calmolide A | Calophyllum lanigerum | HIV | Murray et al. (1982) |
| Coriandrin | Coriandrum sativus | HIV | Towers (1989) |
| Inophyllum B and Inophyllum P | Calophyllum inophyllum Linn. | HIV-1 reverse transcriptase | Patil et al. (1993) |
| Soulatrolide | Calophyllum teysmanii | HIV | Murray et al. (1982) |
| Glycycoumarin and Licopyranocoumarin | Glycyrrhiza glabra | HIV | Vlietinck et al. (1997) |
| Flavonoids | |||
| Acacetin 7-o-(6″-rhamnopyranosyl) β-D-glucopyra-noside) | Chrysanthemum morifolium Ramar (Compositae) | HIV | Qi-Hu et al. (1994) |
| Apigenin | Widely distributed in the plant kingdom | Herpes virus | Béládi et al. (1977) |
| 3,3′ Dimethoxyquercetin | Euphorbia grantii Oliv. and Veronia amygdalina Del. (Compositae) | Picornaviruses and vesicular stomatitis virus | Van Hoof et al. (1989); Rwangabo et al. (1986) |
| Fisetin inactivates | Rhus spp. | Pseudorabies virus | Béládi et al. (1977) |
| O-Glucosyl-7-methyl-5-genistein | Ulex europaeus L. | HSV | Swallow et al. (1975) |
| Glycosil-7-O-luteolin | Matricaria inodora L. (Compositae) | HSV and poliomelytis | Suganda et al. (1983) |
| Hesperetin | Citrus spp. (lemons and sweet oranges) | Vesicular stomatitis | Harborne (1988) |
| Isoquercitrin | Waldsteinia fragarioides Michx. | HSV-1 virus | Karam and Shier (1992) |
| Justicidin B | Phyllanthus acuminatus | Cytomegalovirus and Sindbis virus | Ingham (1983) |
| Kaemferol 3-methyl ether; and Isokaempferide | Solanum sarrachoides | Antiviral activity | Harborne (1988) |
| Luteolin | Widely distributed in the plant kingdom | Pseudorabies virus | Béládi et al. (1977) |
| Luteolin-7-O-glucoside | Matricaria inodora L. (Compositae) | HSV and poliovirus | Béládi et al. (1977) |
| Morin | Chlorophora tinctoria L. Gaud | Pseudorabies virus | Béládi et al. (1977) |
| Naringin | Citrus paradisi Macfad. | Vesicular stomatitis virus | Wacker and Eilmes (1978) |
| Pachypodol (quercetin 3,7,3′-trimethyl ether) | Begonia glabra | Antiviral activity | Cody et al. (1986) |
| Pelargonidin | Pelargonium sp. | Enveloped viruses | Béládi et al. (1977) |
| Quercetin | Chenopodium quinoa | Potato virus X | French and Towers (1992) |
| Quercetin 3-methyl ether | Found as the aglycone in the leaves of Compositae | Antiviral activity | Cody et al. (1986) |
| Quercetin 3-O-(2″-galloyl)-β-D-galactopyranoside | Acer okamotoanum Nakai | HIV-1 integrase | Kim et al. (1998) |
| Quercetagetin | Found in the flowers of many spp. of Compositae | Rauscher murine leukemia and HIV | Cody et al. (1986) |
| Rutin | Fagopyrum esculentum Moench | Pseudorabies and vesicular stomatitis virus | Béládi et al. (1977) |
| Taxifolin | Acacia catechu | Antiviral activity | Harborne (1988) |
| Volkensiflavone | Rhus succedania L. | Influenza B virus | Lin et al. (1997); Lin et al. (1999) |
| Ternatin and Melaternatin | Evodia madagascariensis Baker | HSV-1, HSV-2, adenovirus type 2, poliovirus type 2, and VSV type 2 | Simöes et al. (1990) |
| Afromosin and Formononetin | Wisteria brachybotrys Sieb | Epstein-Barr virus early antigen | Konoshima et al. (1989) |
| Axillarin, Chrysosphenol B, and Chrysosplenol C | Chrysosplenium tosaense | Rhinovirus | Tsuchiya et al. (1985) |
| Lophirone F, Azobechalcone, and Isolophirachalcone | Lophira alata | Epsein-Barr virus early antigen induction test | Murakami et al. (1992) |
| Centaurein and Jacein | Centaurea nigra L. | Herpes virus and poliovirus | Kaij-a-Kamb et al. (1992) |
| 5,7,3,3′,4,5-Hexahydroxyflavone, and 5,7,4′-Trihydroxy-3-glycosylflavone | Befaria cinnamomea | HIV-1 | Mahmood et al. (1993) |
| Agathisflavone, Robustaflavone, Hinokiflavone, Amentoflavone, and Morelloflavone | Rhus succedanea L. and Garcinia multiflora Champ | HIV-1 reverse transcriptase | Lin et al. (1997) |
| 3-O-Methylcalopocarpin, Licoisoflavanone, Glyasperin | Erythrina lysistemon Hutch | HIV | McKee et al. (1997) |
| Macluraxanthone B, Macluraxanthone C, and Dihydrocudraflavone B | Maclura tinctoria | HIV | Groweiss et al. (2000) |
| 7-O-Methyl-glabranine | Tephrosia madrensis | Dengue virus | Sanchez et al. (2000) |
| Wogonin | Scutellaria baicalensis | HBV | Huang et al. (2000) |
| Samarangenin B and Myricetin | Limonium sinense | HSV-1 replication | Lin et al. (2000) |
| Lignans | |||
| Dihydroanhydropodorhizol | Bursera schletchtendalii | HSV-1 | Ayres and Loike (1990) |
| Diphyllin apioside-5-acetate, justicidin A and B, diphyllin, and diphyllin apioside | Justicia procumbens var. leucantha | Vesicular stomatitis virus | Asano et al. (1996) |
| Lignine guaiacyl derivative | Pinus nigra Arnold | HIV | Eberhardt and Young (1996) |
| Deoxypodophyllotoxin, 4′-Dimethylpodophyllotoxin, Podophyllotoxin acetate, Epidophyllotoxin acetate, and β-Peltatin A methyl ether | Juniperus sabina | HSV-1 and vesicular stomatitis virus | Feliciano et al. (1993) |
| Podophyllotoxin, β-Peltatin, Deoxypodophyllotoxin, Picropodophyllotoxin, and α-Peltatin | Podophyllum peltatum | Measles and HSV-1 viruses | McKee et al. (1997); Bedows and Hatfield (1982) |
| Kadsulignan L, Kadsulignan M, and Kadsulignan N | Kadsura coccinea | HIV | Liu and Li (1995) |
| Justicidins A, Justicidins B, Diphyllin, Actigenin, and Trachelogenin | Forsythia intermedia and Ipomoea cairica | HIV-1 | Vlietinck et al. (1998) |
| Schizarin B and taiwanschirin D | Kadsura matsudai | HBV virus | Kuo et al. (2001) |
| Rhinacanthin E and rhinacanthin F | Rhinacanthus nasutus | Influenza virus type A | Kernan et al. (1997) |
| Miscellaneous compounds | |||
| Calcium elenolate | Olea europaea L. | Antiviral activity | Swallow et al. (1975) |
| Castelanone | Castela tweediei | Oncogenic Rous sarcoma virus | Rembold (1989) |
| Chaparrinone | Quassia undulata | Oncogenic Rous sarcoma virus | Rembold (1989) |
| Cochinolide | Homalium cochinchinesis | HSV-1 and -2 | Ishikawa et al. (1998) |
| Curdlan sulphate, Dextran sulphate, and Dextrin sulphate | Dextran sulphate - Viola yedoensis, Dextrin sulphate - Prunella vulgaris and Curdlan sulphate - Alternanthera philoxeroides (Amarantaceae) | HIV | Vlietinck et al. (1998) |
| Glaucarubolone | Quassia simarouba | Oncogenic Rous sarcoma virus | Rembold (1989) |
| D-glucosamine | Dahlis sp., Glycine max (L.) Merr and Phaeseolus aureus Roxb. | Fowl plague, Sindbis and Semliki Forest virus, RNA viruses, HSV, pox virus, NDV-inhibits para influenza 3, and measles | Kaluza et al. (1972) |
| Glucans 1 and Glucans 2 | Nicotania tabacum | Antiviral activity | Rouhier et al. (1995) |
| Pentagalloylglucose | Paeonia albiflora Pallas | HSV | Kaij-a-Kamb et al. (1992) |
| Monoterpenoids, diterpenoids and sesquiterpenoids | |||
| Alloaromadendrol glycosides | Calendula arvensis L. | Vesicular stomatitis virus and rhinovirus (HRV type 1B) | Tommasi et al. (1990) |
| Arbotristosides A,B,C | Nyctanhes arbor-tristis | EMCV and SFV | Rathore et al. (1990) |
| Carnosolic acid and Carnosol | Rosmarinus officinalis L. | HIV protease inhibitors | Pariš et al. (1993) |
| Celafolin A-1, Celaforin B-2, Celaforin B-3, Celaforin C-1, Celaforin D-1, Celaforin D-2, and Celaforin D-3 | Celastrus stephanotiifolius Makino | Epstein-Barr virus | Takaishi et al. (1993) |
| 12-Deoxyphorbol-13(3E,5E-decadienoate) | Excoecaria agallocha | HIV | Erickson et al. (1995) |
| Euglobal T1 | Eucalyptus tereticornis Sm. | Epstein-Barr virus | Kokumai et al. (1991) |
| Euglobal 1, Euglobal 2, and Euglobal 3 | Eucalyptus grandis | Epstein-Barr virus | Takasaki et al. (1990) |
| Halnanolide | Banisteria caapi | Influenza virus A (WS), Newcastle diseases virus, Japanese B encephalitis virus (AZ), and vaccina virus | Cracker and Simon (1986) |
| Liangshanin B and Liangshanin D | Rabdosia liangshanica C.Y. | Hepatitis virus | Fenglei et al. (1989) |
| Nimbinen | Limonoids found in plants of the order Rutales | Antiviral activity | Champagne et al. (1992) |
| Sclerocarpic acid | Glyptopetalum sclerocarpum | HSV 1 and 2 | Sotanaphun et al. (1999) |
| Scoparic acid A, Scoparic acid B, Scoparic acid C, and Scopadulcis acid B | Scoparia dulcis | HSV 1 | Hayashi et al. (1988); Hayashi et al. (1990) |
| Dolabellane | Dolabella californica | Influenza and adenovirus viruses | Piattelli et al. (1995) |
| Safficinolide and Sageone | Salvia officinalis | Vesicular stomatitis virus | Tada et al. (1994) |
| Tripterifordin | Triterygium wilfordii Hook | HIV | Chen et al. (1992) |
| Arennoside, Geniposidic acid, Geniposidic, and Gardenoside | Genipa americana L. | Antiviral activity | Ueda et al. (1991) |
| Xylopinic acid | Xylopia sp. | HIV | Fuller et al. (1996) |
| 12-O-Acetylphorbol-13-Decanoate and 12-O-Decanoylphorbol-13-(2- methyl butyrate) | Croton tiglium | HIV-1 | El-Mekkawy et al. (2000) |
| Phenolic | |||
| 2-O-Caffeoyl-(+)-allohydroxycitric | Spondias mombin | Coxsackie and HSV | Corthout et al. (1992) |
| 2,6-Dihydroxymethoxyisobutylrophenone and 4,6-Dihydroxymethoxyisobutylrophenone | Kunzea ericoides A. Rich. | Antiviral activity | Bloor (1992) |
| Eugenin or Ellagitanin | Syzyium aromatica Merr Paeonia suffruticosa | HSV | Takechi and Tanaka (1982); Takechi and Tanaka (1981) |
| Gentisic acid | Citrus cultivars, Vitus vinifera | Antiviral activity | Van Sumere (1989) |
| Gossypol | Gossypium herbaceum L. | Herpes parainfluenza 3 and influenza viruses | Harborne and Baxter (1993) |
| Guttiferone A,B,C,D, and E | Symphonia globulifera, Garcinia livinstonei, Garcinia ovalifolia and Clusia rosea | HIV | Gustafson et al. (1992) |
| Mallotojaponin and Mallotochromene | Mallotus japonicum | HIV | Van Sumere (1989) |
| Peltalol A | Pothomorpha peltata | HIV-1 | Van Sumere (1989) |
| Pentagalloyl-βD-glucose | Nuphar japonicum | HIV | Porter (1989) |
| Polyphenolic complex | Geranium sanguineum L. | Neuraminidase activity of different influenza virus HINI, H2N2, and H3N2 | Serkedjieva and Manolova (1992) |
| Salicin and Salireposide | Populus trichocarpa | Poliomyelitis and Semliki forest virus | Van Hoof et al. (1989) |
| △-9-Tetrahydrocannabinol | Cannabis sativa L. | HSV-1, HSV-2 | Blevins and Dumic (1980) |
| Woodorien | Woodwardia orientalis | HSV-1 and poliovirus | Xu et al., 2010 |
| Silymarin and Cyanidol | Silybum marianum | Acute viral hepatitis | Swallow et al. (1975) |
| Dibalanocarpol and Balanocarpol | Hopea malibato Foxw | HIV | Hatano et al. (1988) |
| 3,5-di-O-Galloylquinic acid, 3,4,5-tri-O-Caffeoylquinic acid, and 1,3,4-tri-O-Galloylquinic acid | Guiera senegalensis and Securidata longipedunculata | HIV | Van Sumere (1989) |
| (+)-Nortrachelogenin, Genkwanol A, Wilkstrol B, and Daphnodorin B | Wikstroemia indica C. A. Meyer | HIV-1 | Hu et al. (2000) |
| 1,3,4,5-tetra-O-Galloylquinic acid | Lepidobotrys staudtii Engl.) | HIV-1 and HIV-2 | Bokesch et al. (1996) |
| Phenylpropanoids | |||
| Caffeic acid | Coffea arabica | Influenza virus, HSV, vaccinia, and polio viruses | Mølgaard and Ravn (1988) |
| Chlorogenic acid | Coffea arabica | Poliovirus | Mølgaard and Ravn (1988) |
| 3-Methyl-but-2-enyl caffeate | Populus nigra L. | Antiviral activity | Amoros et al. (1994) |
| Usneoidone E, and Usneoidone Z | Brown seaweed Cystoseira usneoides | Antiviral activity | Urones et al. (1992) |
| Verbacoside, Isoverbacoside, Luteoside A, and Luteoside B | Markhamia lutea Seemann ex Baillor | Respiratory syncytial virus | Kernan et al. (1998) |
| Magnolol, Honokiol, and Monoterpenylmagnolol | Magnolia officinalis Rehd. et Wils | Epstein-Barr virus early antigen | Konoshima et al. (1991) |
| Quinones | |||
| Conocurvone | Conospermun incurvum | HIV-1 reverse transcriptase | Decosterd et al. (1993) |
| Juglone | Juglans nigra; Hypericum triquetrifolium | HSV-1 virus and retrovirus | Berg and Labiade (1989) |
| Pseudohypericin | Hypericum triquetrifolium | Retrovirus | Berg and Labiade (1989) |
| Rhinacanthin C and Rhinacanthin D | Rhinacanthus nasutus (L) Kurz | Cytomegalovirus | Sendl et al. (1996) |
| Hypericin and Pseudohypericin | Hypericum perforatum | Retroviruses | Hudson et al. (1993) |
| Tannins | |||
| Agrimoniin | Agrimonia pilosa | Avian myeloblastosis virus | Porter (1989) |
| Coriariin A | Coriaria japonica | HIV | Porter (1989) |
| Procyanidin B2 | Rubus idaeus | HIV | Porter (1989) |
| Camellin B, Gemin D, Chebulagic acid, and Nobotanin B | Chebulagic acid was isolated from Terminalia chebula, gemin D from Geum japonicum, nobotanin B from Tibouchina semicandra | HIV | Vlietinck et al. (1998) |
| Thiophenes and polyacetylenes | |||
| Sidoresmin A | Sirodesmiun diversum | Rhinoviruses | Swallow et al. (1975) |
| Thiarubine-A | Chaenactis douglasii | Cytomegalovirus and Sindbis virus | Hudson et al. (1986a) |
| α-Terthienyl (α-T) ACBP-thiophene | Bidens pilosa, thiophene-A - Chaenactis douglasii, a-Terthienyl and ACBP-thiophene - Tagetes patula | Sindbis virus | Hudson et al. (1986b) |
| Allyl methyl tiosulfinate, Methyl allyl tiosulfinate, Ajoene, and Allicin | Garlic, Allium sativa L. | HSV, parainfluenza virus type 3, vaccinia virus, vesicular stomatitis virus, and human rhinovirus type 2 | Weber et al. (1992) |
| Phenylheptatriyne (PHT), Thiophene-A, Erysolin, and Sulforaphen | Cardaria draba L. Desv. | Mengovirus and newcastle disease virus | Kaij-a-Kamb et al. (1992) |
| Triterpenoids | |||
| β-Aescin | Aesculus hippocastranum L. | Influenza viruses | Hiller (1987) |
| Arjunolic acid | Cochlospermun tinctorium A. Rich. | EBV-EA | Diallo et al. (1989) |
| Chikusetsusaponin | Panax japonicus C.A. Mayer | HIV | Hasegawa et al. (1994) |
| Cucurbitacin F, 23,24-Dihydrocucurbitacin F, 15-oxo-23, 24-Cucurbitacin F, and 15-oxo-Cucurbitacin F | Cowania mexicana | Epstein-Barr virus | Konoshima et al. (1993) |
| Digitoxin | Digitalis purpurea L. | Poliovirus | Koch and Gyorgy (1969) |
| Eichlerianic acid | Cowania Mexicana | Herpes virus type 1 | Hiller (1987) |
| Ganoderiol F and Ganodermanontriol | Ganoderma lucidum | HIV-1 | El-Mekkawy et al. (1998) |
| Gleditsia saponin C | Gleditsia japonica Miquel and Gymnocladus chinensis Baillon | HIV | Konoshima et al. (1995) |
| Gymnocladus saponin G and Glycyrrhizic acid | Glycyrrhiza glabrata L. | HSV 1, vaccinia virus, newcastle disease virus, and vesicular stomatitis virus | Hatano et al. (1988) |
| 3-O-Glucose(1–3) [arabinose 1–4]-glucose-xyloside of 23-hydroxy-protoprimulagenin A 3-O-Glucose(1–3) [arabinose 1–4]-glucose-xyloside of 23-hydroxyproto-primulagenin A | Anagallis arvensis | HSV 1 and poliovirus | Amoros and Girre (1987) |
| Gymnemic acid | Gymnema sylvestre | Anti-influenzal activity | Rao and Cochran (1974) |
| 24-Hydroxydammaran-20,25-dien-3-one | Chisocheton macrophyllus | Epstein-Barr virus | Inada et al. (1993) |
| 1β-Hydroxyaleuritolic acid 3-p-hydroxy-benzoate | Maprounnea Africana | HIV-1 reverse transcriptase | Pengsuparp et al. (1995) |
| (3 β -hydroxyolean-12-en-23,28 dioic acid 23-o-[β-d-glucopyranosyl-28-o-[β-d-glucopyranosyl(1-3)] β-D-gluco-pyranosyl(1-6)] β-D-galactopy-ranoside | Gypsophila capillaris | HSV | Elgamal et al. (1995) |
| Isofouqueierol | Fouquiera splendens Engelm | HSV | Gyorgy and Koch (1969) |
| Lancilactones C | Kadsura lancilimba | HIV | Chen et al. (1999) |
| Lanatoside D | Digitalis lanata Ehrh. | Influenza, Herpes and vaccinia viruses | Koch and Sandor (1969) |
| Methyl ester of wistariasaponin D, Methyl ester of wistariasaponin G, and Methyl ester of dehydrosoyasaponin | Wistaria brachybotrys Sieb | Epstein-Barr | Konoshima et al. (1989) |
| Nigranoic acid | Schisandra sphaerandra Stapf. | HIV | Sun et al. (1996) |
| (22E)-5β-24-Norcholest-22-ene-3 α,4α,11 β,21-tetrol,3,2,1-disulfate | Ophioplocus januarii Luetken | Respiratory syncytial and polio viruses | Roccatagliata et al. (1996) |
| Ouabain | Acokanthera ouabaio Cathel. | Newcastle disease virus | Becher (1976) |
| Saikosaponin-A | Bupleurum falcatum L. | Influenza virus | Hiller (1987) |
| Salaspermic acid | Triterygium wilfordii Hook | HIV | Hiller (1987) |
| Saponin 2 | Anagallis arvensis L. | Herpes virus and poliovirus | Koch and Sandor (1969) |
| Shoeric acid | Strophanthus kombe Oliv | Herpes virus | Kaij-a-Kamb et al. (1992) |
| Strophanthin G | Strophanthus kombe Oliv. | Influenza, Herpes and vaccinia viruses | Kaij-a-Kamb et al. (1992) |
| Suberosol | Polyalthia suberosa Roxburgh Thwaites | HIV | Li et al. (1993) |
| 3-O-trans-Caffeoyltormentic acid | Eriobotrya japonica Lindl.) | Rhinovirus infection | Tommasi et al. (1992) |
| Wistariasaponins A, Wistariasaponins B, and Wistariasaponins C | Wistaria brachybotrys Sieb | Epstein-Barr virus | Konoshima et al. (1989) |
| Zingibroside R1 | Panax zingiberensis | HIV | Hasegawa et al. (1994) |
| 2α-19α-Dihydroxy-3-oxo-12-ursen-28-oic-acid, and Mastinic acid | Geum japonicum | HIV | Hiller (1987) |
| Proscillaridin A and Scillarenin | Urginea scilla Steinh | Influenza, HSV, vaccinia virus, and picornaviruses | Koch and Sandor (1969) |
| Betulinic acid and Platanic acid | Syzigium claviflorum (Roxb.) Wall | HIV | Fujioka et al. (1994) |
| Oleanolic acid and Pomolic acid, Alphitolic acid, Asiantic acid, and Betulinic acid | Oleanolic acid (Prosopis glandulosa, Torr), pomolic acid, alphitolic acid (Rosa woodsii Lindl.), arjunolic acid, asiantic acid, betulinic acid (Syzygium claviflorum Wall) | HIV | Kashiwada et al. (1998) |
| Dammaradienol, Dammaradienol II, Dammarenolic acid, Hydroxydammarenone I, Hydroxyhopanone, Hydroxyoleanolic acid, and Ursonic acid | Balanocarpus heimii King | Herpes virus | Swallow et al. (1975) |
| Epigallocatechin-(4β-8,2β-O-7)-epicatechin, 3-Oxotirucalla-7–24-dien-21oic acid. And Oleanolic acid | Xanthoceras sorbifolia Bunge | HIV-1 | Ma et al. (2000) |
| 1-J3-hydroxyaleuritolic acid-3-p-hydroxybenzoate | Maprounea africana | Reverse transcriptase inhibitors | Cos et al. (2008) |
| Escin | Aesculus chinensis Bge. | HIV | Yang et al. (1999); Xiu-Wuei et al. (1999) |
| Proteins and peptides | |||
| Trichobitacin | Trichosanthes kirilowii | HIV | Mishra et al. (2013) |
| Pokeweed antiviral proteins (PAP) (MRK29, MAP30 and GAP31) | Phytolacca Americana, Momordica charantia, Gelonium multiflorum | HIV-1 | Rajamohan et al. (1999) |
| Panaxagin | Panax ginseng | HIV-1 reverse transcriptase | Ng and Wang (2001) |
| Kalata B1,B2 | Oldenlandia affinis | HIV | Craik et al. (2012) |
| Cyrulin A,B | Chassalia parviflora | HIV | Gustafson et al. (1994) |
| Lunatusin | Phaseolus lunatus | Antiviral activity | Wong and Ng (2005) |
| Vulgarinin | Phaseolus vulgaris | Antiviral activity | Jack and Tzi (2005) |
| Cicerin and Arietin | Cicer arietinum | Antiviral activity | Ye et al. (2002); De Souza et al. (2011) |
| Peptidesa-Mitogenic | Brassica napus | ND-Not determined | Yust (2004) |
| Phaseococcin | Phaseolus coccineus | HIV | Kuczer et al. (2010) |
| Sesquin | Vigna sesquipedalis | HIV | Hultmark et al. (2005) |
4.2. Fungi
Fungi are excellent sources of bioactive metabolites, possessing antiviral properties (Table 3 ). The first antiviral metabolite from fungi Stachybotrys sp. was tested against H1N1 Influenza virus (Moghadamtousi et al., 2015). Compounds isolated from Penicillium sp. were tested for antiviral properties. Trypilepyrazinol acted as an inhibitor against HIV-1 and HCV. (+)-neocitreoviridin showed anti-influenza A virus activity. 3-β-hydroxyergosta-8,14,24(28)-trien-7-one expressed anti-HIV and anti-influenza A activities (Li et al., 2019). Fungi associated compounds such as physcion, neoechinulin D, and dihydroauroglaucin inhibited replication of Influenza A virus (Bovio et al., 2019).
Table 3.
Fungal metabolites against viral pathogens.
| Name of the compound | Organisms | Active against | References |
|---|---|---|---|
| Aphidicolin | Cephalosporium aphidicola | HSV 1 and 2 | Hanson (1972) |
| Hyalodendrin A | Penicillium turbatu | Polio, Coxsackie viruses | Becher (1976) |
| Stachybogrisephenone B, Grisephenone A, and 3,6,8-Trihydroxy-1-methylxanthone | Stachybotrys sp. | Enterovirus-71 | Qin et al. (2014) |
| Halovirs A–E and Simplicilliumtide J | Scytalidium sp. | HSV | Rowley et al. (2003); Youssef et al. (2019) |
| 11a-dehydroxyisoterreulactone A, Arisugacin A, Isobutyrolactone II, and Aspernolide A | Aspergillus terreus SCSGAF0162 | HSV | Nong et al. (2014) |
| Balticolid | Ascomycetous strain 222 | HSV | Shushni et al. (2011) |
| Equisetin | Fusarium heterosporum | HIV | Shushni et al. (2011) |
| Phomasetin | Phoma sp. | HIV | Singh et al. (1999) |
| Integric acid | Xylaria sp. | HIV | Rowley et al. (2004) |
| Stachyflin | Stachybotrys sp. RF-7260 | Influenza virus | Minagawa et al. (2002) |
| Oxoglyantrypine, Norquinadoline A, Deoxynortryptoquivaline, Deoxytryptoquivaline, Tryptoquivaline, and Quinadoline B | Cladosporium sp. | Influenza virus | Peng et al. (2013) |
| Cladosin C | Cladosporium sphaerospermum 2005-01-E3 | Influenza virus | Wu et al. (2014) |
| (Z)-5-(Hydroxymenthyl)-2-(6′)-methylhept-2′-en-2′-yl)-phenol, Diorcinol, and IFV Cordyol C | A. sydowii ZSDS1-F6 | Influenza virus | Wang et al. (2014) |
| Rubrolide S | A. terreus OUCMDZ-1925 | Influenza virus | Zhu et al. (2013) |
| Asperterrestide A | A. terreus SCSGAF0162 | Influenza virus | He et al. (2013) |
| Isoaspulvinone E, Aspulvinone E, and Pulvic acid | A. terreus Gwq-48 | Influenza virus | Gao et al. (2013) |
| Emerimidine A and Emerimidine B | Emericella sp. (HK-ZJ) | Influenza virus | Zhang et al. (2011) |
| Purpurquinone B, Purpurquinone C, Purpuresters A, and TAN-931 | P. purpurogenum JS03-21 | Influenza virus | Wang et al. (2011) |
| Sorbicatechol A and Sorbicatechol B | P. chrysogenum PJX-17 | Influenza virus | Peng et al. (2014) |
| Tetrahydroaltersolanol C and Alterporriol Q | Alternaria sp. ZJ-2008003 | Porcine reproductive and respiratory syndrome | Zheng et al. (2012) |
| Sansalvamide A (43) | Fusarium sp. | Molluscum contagiosum virus | Hwang et al. (1999) |
| 22-O-(N-Me-L-valyl)-21-epiaflaquinolone | Aspergillus sp. XS-20090B15 | Respiratory syncytial virus | Prieto and Castro (2005) |
| B (44) | |||
| Extracts | Agaricus subrufescens | HSV-1 | Bruggemann et al. (2006) |
| GFAHP | Grifola frondosa | HSV | Gu et al. (2007) |
| Beta-glucan-protein | Agaricus subrufescens | HSV | Yamamoto et al. (2013) |
| Aurenitol | Chaetomium coarctatum | Influenza A (H3N2) | Sacramento et al. (2015) |
| Extracts | Lentinula edodes | HPV | Rincão et al. (2012) |
| Polysaccharopeptide | Trametes versicolor | HIV | Collins and Ng (1997) |
| Polysaccharides | Agaricus subrufescens | HPV | Faccin et al. (2007) |
| Extracts | Trametes versicolor | Influenza, HSV | Krupodorova et al. (2014) |
| Adenosine | Cordyceps militaris | HIV protease | Jiang et al. (2011) |
| Velutin | Flammulina velutipes | HIV-reverse transcriptase | Wang and Ng (2001) |
| 4.5 kDa protein | Russula paludosa | HIV protease | Wang et al. (2007) |
| Ganoderic acid | Ganoderma lucidum | HIV protease and HBV | Min et al. (1998) |
| Brefeldin A | Penicillium sp. FKI-7127 | Dengue viruses, ZIKV, and Japanese encephalitis virus | Raekiansyah et al. (2017) |
| Ganodermadiol, applanoxidic acid G triterpenoids, and lucidadiol | Ganoderma pfeifferi Bres. | Influenza virus type A and HSV-1 | Mothana et al. (2003) |
| Cordycepin (also named 3′-deoxyadenosine) | Cordyceps militaris | Influenza viral, HIV-1 RT, Epstein-Barr virus, andRota virus | Yong et al. (2018) |
| Ganodermic acids are A, AM1, B, β, C1, C2,C6, D, Df, DM, E, F, G, H,J,K, Mc, Me, Nf, Mk, N, P, R, S, Sz,T, TR,TQ, X, and Y | Ganoderma lucidum | HIV-1 and HBV | Hsu and Yen (2014) |
| Hispidin and hispolon | Inonotus hispidus (Bull.) P. Karst. | Influenza virus type A and type B | Li and Wang (2006) |
| PSK Krestin and PSP | Trametes versicolor | HIV-1 | Mlinaric et al. (2005) |
| Velutin and Flammulin proteins | Flammulina velutipes | HIV-1 reverse transcriptase | Wang and Ng (2001) |
| Trypilepyrazinol, (+)-neocitreoviridin, and 3β-hydroxyergosta-8,14,24 (28)-trien-7-one | Penicillium sp. | HIV-1, HCV, and Influenza | Li et al. (2019) |
| Physcion, Neoechinulin D, and Dihydroauroglaucin | Eurotium chevalieri | Influenza A virus | Bovio et al. (2019) |
| Isobutyrolactone II | Aspergillus sp. | HSV-1 | Liu et al. (2020) |
A sulphated polysaccharide from Agaricus brasiliensis against HSV-1 and 2, two proteins namely neutral protein bound polysaccharide, acidic protein bound polysaccharide, and triterpenes and laccases of Ganoderma lucidum exhibited anti-HIV-1 protease activity and anti-HIV-1 reverse transcriptase activity (Bishop et al., 2015). GFAHP, a protein from Grifola frondosa inhibited replication of HSV-1(Hassan et al., 2015). Alternaria sp. ZJ-2008003, extracted from Sarcophyton sp. produced tetrahydroaltersolanols C-F and dihydrosolanol A and alterporriols N-R. Tetrahydroaltersolanol C and alterporriol Q showed antiviral activities against the porcine reproductive and respiratory syndrome virus. 11a-Dehydroxyisoterreulactone A from Aspergillus terreus possessed weak antiviral activity against HSV-1 virus. Aspergilli peptides D and E showed inhibitory activities towards HSV-1. Asperterrestide A displayed antiviral activity against H1N1 and H3N2 Influenza virus. Aspergillus sp. derived from Muricellaabnormalis, on fermentation yielded 22-O-(N-methyl-L-valyl)-21-epiaflaquinolone B. It exhibited antiviral activity against human respiratory syncytial virus. Isobutyrolactone II, obtained from another strain of Aspergillus sp. expressed strong antiviral activity towards HSV-1(Liu et al., 2020).
The metabolites halovirs A-E isolated from the marine fungus Scytalidium sp. demonstrated antiviral activity against HSV type-1 and type-2 (Youssef et al., 2019). Equisetin from Fusarium heterosporum, Phomasetin from Phoma sp., Integric acid from Xylaria sp., and Oxoglyantrypine, Norquinadoline A and Tryptoquivaline extracted from Clostridium sp. possessed antiviral activities against HIV.
4.3. Algae
Table 4 shows antiviral attributes of algal metabolites and polysaccharides. Griffithsin and Scytovirin isolated from red and blue-green algae, respectively inhibited HCV (Takebe et al., 2013). The former is also a prominent HIV inhibitor (Besednova et al., 2019). Group I diterpenes like 8α,11-dihydroxy-pachydictyol A, 8β-hydroxy pachydictyol A from Dictyota sp. and diterpenes of Group II including Acetoxypachydiol, 3β-actoxydilophol obtained from Dictyota plectens showed weak antiviral activity. Dolabelladienols A-B extracted from Dictyota pfaffii displayed strong antiviral properties. Bicyclic diterpenes, Crenulidanes from Da-1, and AcDa-1 obtained from D. menstrualis inhibited HIV replication process (Chen et al., 2018).
Table 4.
Algal metabolites and polysaccharides with antiviral activities.
| Antiviral polysaccharide | Organism | Virus | References |
|---|---|---|---|
| Carrageenan | Red alga, Gigartina skottsbergii | Influenza virus, HSV-1, HSV-2, HPV, HRV, and HIV | Vera et al. (2011) |
| Galactan | Red algae, Callophyllis variegate, Agardhiella tenera, Schizymenia binderi, Cryptonemia crenulata | HSV-1, HSV-2, HIV-1, HIV-2, and HAV | Estevez et al. (2001) |
| Alginate | Brown algae, Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, Macrocystis pyrifera | HIV, IAV, and HBV | Jiang et al. (2003) |
| Fucan | Brown algae, Adenocytis utricularis, Undaria pinnatifida, Stoechospermum marginatum, Cystoseira indica, Cladosiphon okamuranus, Fucus vesiculosus | HSV-1, HSV-2, HCMV, VSV, Sindbis virus, and HIV-1 | Patankar et al. (1993) |
| Laminaran | Brown algae, Fucus vesiculosus, Saccharina longicruris, Ascophyllum nodosum | HIV | Rioux et al. (2010) |
| Naviculan | Diatom, Navicula directa | HSV-1 and HSV-2 | Lee et al. (2006) |
| p-KG03 | Microalga, Gyrodinium impudicum | Influenza A virus | Kim et al. (2012) |
| A1 and A2 | Microalga, Cochlodinium polykrikoides | Influenza A and B viruses, RSV-A, RSV-B, and parainfluenza-2 | Hasui et al. (1995) |
| Calcium spirulan | Blue-green alga, Arthrospira platensis | HSV-1, measles, mumps, influenza, polio, Coxsackie, HIV-1 | Hayashi et al. (1996) |
| Nostaflan | Blue-green alga, Nostoc flagelliforme | HSV-1, HSV-2, influenza A virus, and human cytomegalovirus | Kanekiyo et al. (2007) |
| Sea algae extract | Red alga, Schizymenia pacifica | HIV | Nakashima et al. (1987a) |
| Sea weed extract | Acrosiphonia coalita Scagel, Garbary, Golden et Hawkes | HSV-1 and Sindbis virus | Hudson et al. (1999) |
| Sea weed extract | Enteromorpha linza (Linnaeus) J.C. Agardh | HSV-1 and Sindbis virus | Hudson et al. (1999) |
| Sea weed extract | Ulva sp. | HSV-1 and Sindbis virus | Kim et al. (1997) |
| Sea weed extract | Corallina vancouveriensis Yendo | HSV-1 and Sindbis virus | Hudson et al. (1999) |
| Sea weed extract | Analipus japonicus (Harvey) Wynne | HSV-1 | Baba et al. (1988) |
| Sea weed extract | Egregia menziesii | HSV-1 and Sindbis virus | Baba et al. (1988) |
| Sea weed extract | Gracilaria pacifica Abbott | HSV-1 and Sindbis virus | Taylor et al. (1996) |
| Sea weed extract | Nereocystis luetkeana (Mertens) Postels et Ruprecht | HSV-1 | Anani et al. (2000) |
| Sea weeds | Postelsia palmaeformis Ruprecht | HSV-1 | Towers et al. (1997) |
| PLE extracts (hexane, ethanol and water) | Haematococcus pluvialis | HSV-1 | Santoyo et al. (2011) |
| PLE extracts (hexane, ethanol and water) | Dunaliella salina | HSV-2 | Santoyo et al. (2011) |
| Cyanovirin | Nostoc sp. | Influenza A (H1N1) | Smee et al. (2008) |
| β-1,3 glucan | Chlorella vulgaris | Immune stimulator | Spolaore et al. (2006) |
| AcDa-1 | Dictyota menstrualis | HIV | Pereira et al. (2004) |
| SAE (sea algal extract) | Red alga, Schizymenia pacifca | HSV-1 and HSV-2 | Nakashima et al., 1987a, Nakashima et al., 1987b |
| Griffithsin and Scytovirin | Blue-green algae | HCV and HIV inhibitor | Takebe et al. (2013); Besednova et al. (2019) |
| Group I diterpenes like 8α,11-Dihydroxy-pachydictyol A, 8β-Hydroxy pachydictyol A | Dictyota sp. | HIV | Chen et al. (2018) |
| Group II including Acetoxypachydiol, 3β-actoxydilophol | Dictyota plectens | HIV | Chen et al. (2018) |
| Dolabelladienols A-B | Dictyota pfaffii | HIV | Chen et al. (2018) |
| Bicyclic diterpenes, Crenulidanes from Da-1 and AcDa-1 | D. menstrualis | HIV | Chen et al. (2018) |
| Fucoidan | Cladosiphon okamuranus | HIV | Teixeira et al. (2014) |
| Extract | Red alga, Schizymenia pacifica | HIV | Ahmadi et al. (2015) |
| Dieckol | Ecklonia cava | SARS-CoV | Koirala et al. (2017) |
| Ulvan | Ulva armoricana | HIV-reverse transcriptase | Xu et al. (2017); Besednova et al. (2019) |
Fucoidan, a polysaccharide from the marine alga, Cladosiphon okamuranus prevented dengue virus infection (Teixeira et al., 2014). The effect is specific on retroviruses by using heparan sulphate as primary viral receptors (Besednova et al., 2019). Carrageenan, from Gigartina skottsbergii inhibited Influenza virus, HIV, HPV, HSV-1, HSV-2, and dengue virus. Galactan from red algae like Callophyllis variegate and Agardhiella tenera possessed antiviral properties against HIV, HSV-1, -2, Dengue virus, and Hepatitis A virus. Alginate from brown algae inhibited Hepatitis B, Influenza A, and HIV. Fucan from brown algae like Adenocytis utricularis and Undaria pinnatifida expressed antiviral activities against HIV, HSV, Sindbis virus, and Vesicular Stomatitis Indiana virus. The extract of red alga, Schizymenia pacifica exhibited antiviral properties against HIV (Ahmadi et al., 2015).
Calcium spirulan, isolated from Spirulina platensis blocked replication of HSV-1, HIV-1, Influenza A, measles, and mumps virus. Extract of Spirulina maxima reduced HSV-2 infection. Cyanovirin-N, a protein produced by blue-green alga Nostoc ellipsosporum stopped HSV-1 entry into cells by preventing fusion with HSV-1 glycoproteins (Kim et al., 2011). Nostoflan, extracted from Nostoc flagelliforme showed antiviral activities against HSV-1, HSV-2, and Influenza A virus (Thuan et al., 2019). Dieckol isolated from Ecklonia cava prevented cleavage of SARS-CoV 3CL protein and stopped viral replication (Koirala et al., 2017). Ulvan, from Ulva armoricana has been identified to have antiviral properties (Xu et al., 2017). Laminarans or laminarins have been found to play the role of HIV reverse transcriptase and avoid absorption of HIV onto human lymphocytes (Besednova et al., 2019).
4.4. Bacteria
Therapeutic agents from natural resources, particularly bacteria are considered pivotal alternatives of commercially available synthetic drugs. Advancements in genomic technology (identify secondary metabolite gene clusters) and analytical techniques (isolation and purification of compounds) have led the drug discovery approaches to identify novel compounds with antiviral ability. Few noteworthy antiviral drugs isolated so far include surfactins from Bacillus subtilis which display antiviral activities against HSV (Ongena and Jacques, 2008).
Representatives of exopolysaccharides (EPS) producing strains of the genera Streptococcus, Lactococcus, Lactobacillus, Leuconostoc, Pediococcus, and Weissella have been well studied for immunostimulating properties. The EPSs extracted from lactic acid bacteria of the genera Pediococcus, Leuconostoc, and Lactobacillus significantly proved to produce anti-adenovirus effects in cell line studies (Biliavska et al., 2019). Other microbial metabolites like spongouridine, spongothymidine, statins, myriocin, NA255, and cyclosporine were reported to have antiviral activities against HSV1,2, HBV, HIV, influenza virus, HCV, and coronaviruses (Nkongolo et al., 2014). Antiviral attributes of bacteria associated bioactive compounds are summarized in Table 5 .
Table 5.
Antiviral compounds from bacteria.
| Name of the compound | Organisms | Active against | References |
|---|---|---|---|
| Sulfangolid C, soraphen F, epothilon D, and spirangien B, and Kulkenon | Sorangium cellulosum | HIV | Zander et al. (2012) |
| Rhizopodin | Myxococcus stipitatus | HIV | Martinez et al. (2013) |
| Thiangazole, phenalamide A1, and phenoxan | Polyangium species | HIV | Jurkiewicz et al. (1992) |
| Aetheramide A and aetheramide B (10b) | Aetherobacter | HIV | Trowitzsch-Kienast et al. (1992) |
| Ratjadon A (11) and α-pyrone | Sorangium cellulosum | HIV | Gerth et al. (1995) |
| Myxochelins A-F | Angiococcus disciformis | Human cytomegalovirus | Miyanaga et al. (2009) |
| Nannochelin A-C | Nannocystis exedens | Human cytomegalovirus | Kunze et al. (1992) |
| Hyalachelin A-C | Hyalangium minutum | Human cytomegalovirus | Nadmid et al. (2014) |
| Chondramide A-D | genus Chondromyces | EVD | Reichenbach (1988) |
| Noricumazol A-C | Sorangium cellulosum | EVD | Kunze et al. (1991) |
| Labindole A and B, 3-chloro-9H-carbazole, 4-hydroxymethyl-quinoline, and Soraphen A | Labilithrix luteola | HCV | Mulwa et al. (2018) |
| Lanyamycin | Sorangium cellulosum | HCV | Gentzsch et al. (2011) |
| Surfactin | Bacillus amyloliquefaciens | Antiviral activity | Koumoutsi et al. (2004) |
| Bacitracin | Bacillus licheniformis | Antiviral activity | Konz et al. (1997) |
| Lichenysin | Bacillus licheniformis | Antiviral activity | Veith et al. (2004) |
| Locillomycin | Bacillus subtilis1 | Antiviral activity | Luo et al. (2015) |
| Macrolactin A | B. subtilis | HSV | Gustafson et al. (1989) |
| Exopolysaccharides (EPSs) | Pediococcus, Leuconostoc, Lactobacillus | Human adenovirus | Liubov et al. (2019) |
4.5. Actinomycetes
Actinomycetes are present in various environments and are active in the microbial communities. The secondary metabolites of these organisms are potential antiviral agents (Table 6 ). Xiamycin and its methyl ester of Streptomyces sp. GT2002/1503 showed selective anti-HIV-1 activity (Xu et al., 2014). The compound (4S)-4-hydroxy-10-methyl-11-oxo-dodec-2-en-1,4-olide, identified from Streptomyces sp. Smu03 possessed antiviral property over a broad range of Influenza A virus (Li et al., 2018). Antimycin A from Streptomyces kaviengensis inhibited RNA virus families like Togaviridae, Picornaviridae, Bunyaviridae, and western equine encephalitis virus. AhmpatininiBu from Streptomyces sp. CPCC 202950 and 4862F from Streptomyces albosporus I03A-04862 inhibited HIV-1 protease. Narasin from Streptomyces aureofaciens prohibited post-entry stages of viral replication during Dengue virus infection (Teixeira et al., 2014). Other antivirals include daptomycin from Streptomyces roseosporus (Jakubiec-Krzesniak et al., 2018), diffusomycin from Streptomyces sp. KBFP-2025 (Vil et al., 2019), and Sinefungin from Streptomyces griseolus and Streptomyces incarnatus NRRL 8089 (Chen et al., 2017).
Table 6.
Actinobacterial metabolites against viral pathogens.
| Name of the compound | Organism | Active against | References |
|---|---|---|---|
| 9-Methyl strptimidone | Streptomyces sp. S-885 | Poliovirus | Swallow et al. (1975) |
| Rifampin | Streptomyces mediterranei | Vaccinia and pox viruses | De Clercq (1973) |
| Novobiocin | Streptomyces spheroids (Actinomycetales) | Antiviral activity | Murray et al. (1982) |
| Guanine-7-N-oxide | Streptomyces sp. | Rhabdovirus and infectious pancreatic necrovirus | Nakagawa et al. (1985) |
| Antimycin A1a | Streptomyces kaviengensis | Western equine encephalitis virus | Raveh et al. (2013) |
| Xiamycins C-E | Streptomyces sp. #HK18 | Porcine epidemic diarrhea virus, and HIV | Kim et al. 2016; Xu et al. (2014) |
| Pentapeptide 4862F-N,N,N-(trimethylated)-Tyr-L-Leu-L-Val-L-Leu-(dehydrated)-His | Streptomyces albosporus I03A-04862 | HIV-1 | Liu et al. (2012) |
| 4-amino-3-hydroxy-5-(4-methoxyphenyl) pentanoic acid | Streptomyces sp. CPCC 202950 | HIV-1 | Chen et al. (2018) |
| Daptomycin and Nanchangmycin | Streptomyces nanchangensis, Streptomyces roseosporus | ZIKV | Barrows et al. (2016); Pascoalino et al. (2016); Rausch et al. (2017) |
| Chartreusin | Streptomyces chartreusis | Influenza A | Miyakawa et al. (1958) |
| Mannose specific pradimicin-A (PRMA) | Actinomadura hibisca | HIV | Tanabe-Tochikura et al. (1990) |
| Actinohivin | Longispora albida gen. nov, sp. nov | HIV | Chiba et al. (2004); Takahashi et al. (2005) |
| Benzastatin C, a 3-chloro-tetrahydroquinolone alkaloid | Streptomyces nitrosporeus | HSV-1, HSV-2, and vesicular stomatitis virus | Lee et al. (2007) |
| JBIR-68 | Streptomyces sp. RI18 | Influenza virus | Takagi et al. (2010) |
| Methylelaiophylin | Streptomyces melanosporofaciens | Newcastle disease virus | Lee et al. (2011) |
| Furan-2-yl acetate (C6H6O3) | Streptomyces VITSDK1 spp. | Fish nodavirus | Suthindhiran et al. (2011) |
| Di-n-octyl phthalate and bis (2-methylheptyl) phthalate | Streptomyces parvus | HCV | Elnaby et al. (2016) |
| Fattiviracin A1 | Streptomyces microflavus | Antiviral activity | Yokomizo et al. (1998) |
| Musacin C | Streptomyces griseovirdis | Antiviral activity | Schneider et al. (1996) |
| MM461156 | Actinomadura pelletieri | Antiviral activity | Ashton et al. (1990) |
| FK 506 | Streptomyces tsukubaensis | Antiviral activity | Reis et al. (2006) |
| Benzastatin C | Streptomyces nitrosporeus | Antiviral activity | Kuzuyama and Seto (2003); Lee et al. (2007) |
| (4S)-4-hydroxy-10-methyl-11-oxo-dodec-2-en-1,4-olide | Streptomyces sp. Smu03 | Influenza A virus | Li et al. (2018) |
| Ahmpatinini Bu | Streptomyces sp. CPCC 202950 | HIV-1 | Teixeira et al. (2014) |
| 4862F | Streptomyces albosporus I03A-04862 | HIV-1 | Teixeira et al. (2014) |
| Narasin | Streptomyces aureofaciens | Dengue virus | Teixeira et al. (2014) |
4.6. Endophytic bacteria
Endophytes are a group of bacteria and fungi which live inside the host without damaging them. Metabolites obtained from endophytes possess antiviral properties (Table 7 ). Xiamycin A, a distinguished compound extracted from Bruguiera gymnorrhiza mangrove plant, demonstrated selective anti-HIV activity (Christina et al., 2013).
Table 7.
Endophytes derived metabolites with antiviral activities.
| Name of the compound | Organism | Active against | References |
|---|---|---|---|
| Bis (2-methylheptyl) phthalate | Actinomycetes - leaves of Pongamia pinnata | White spot syndrome virus | Rameshthangam and Ramasamy (2007) |
| Xiamycin A | Streptomyces sp. GT 2002/1503 | HIV | Ding et al. (2010) |
| Cytonic acids A and B | Cytonaema sp. | Human cytomegalovirus | Bhardwaj and Agrawal (2014) |
| Valinomycin | Streptomyces tsusimaensis | Coronavirus | Alvin et al. (2014) |
| Altertoxins | Alternaria tenuissima QUE1Se | HIV-1 virus | Bashyal et al. (2014) |
| Aspernidine (A, B), dehydroaustin, emeriphenolicins (A, D), austinol, emerimidine (A, B), austin, and acetoxy dehydroaustin | Emericella sp. (HK-ZJ) | Influenza A virus (H1N1) | Zhang et al. (2009) |
| 2-(Furan-2-yl)-6-(2S,3S,4-trihydroxybutyl) pyrazine | Jishengella endophytica 161,111 | Influenza A virus (H1N1) | Wang et al. (2014) |
4.7. Lichens
Lichens are symbiotic organisms between fungi and algae. Nearly 1100 bioactive metabolites have been isolated from 18,500 lichens, but still numerous organisms are yet to be discovered from different environments. These metabolites generally belong to the classes of polyketides, phenols, terpenoids or quinines. Several research studies indicated the antiviral activities of metabolites (Table 8 ), such as (+)-usnic acid, sekikaic acid, and anthraquinones against arenaviruses, respiratory syncytial virus, and HSV type 1 (Boustie and Grube, 2005; Stocker-Wörgötter, 2008; Zambare and Christopher, 2012; Lai et al., 2013).
Table 8.
Antiviral metabolites from lichens.
| Name of the compound | Organism | Active against | References |
|---|---|---|---|
| Protolichesterinic acid | Cetraria islandica | HIV reverse transcriptase | Van Sumere (1989) |
| Swertifrancheside | Swertia franchetiana | HIV-1 reverse transcriptase | Pengsuparp et al. (1995) |
| Physodalic acid, physodic acid; 3-hydroxy physodic acid, and isophysodic acid | Hypogymnia physodes | Influenza | Pavlovic et al. (2013) |
| Atranorin and fumarprotocetraric acid | Cladonia furcata, Cladonia pyxidata and Cladonia rangiferina | Influenza | Kosanić et al. (2014) |
| Usnic acid and derivatives | Cetraria islandica and Vulpicida canadensis | Influenza A viruses (H1N1 and H3N2) | Sokolov et al. (2014); Shtro et al. (2014); Shtro et al. (2015) |
| α-Methylene-γ-lactone | Lichen Cetraria islandica | HIV-1 reverse transcriptase | Pengsuparp et al. (1995) |
| Depsidone salazinic acid | Parmelia saxatilis (L.) Ach. | Antiviral activity | Omarsdottir et al. (2006) |
| Benzyl depside alectorialic acid | Alectoria nigricans (Ach.) Nyl. | Antiviral activity | Omarsdottir et al. (2006) |
| Anthraquinones, bianthrones, and hypericin derivatives | Parmelia perlata | HSV-1 | Cohen et al. (1996) |
| Sekikaic acid | Ramalina farinacea | Respiratory syncytial virus | Lai et al. (2013) |
5. Complementary and herbal preparations as future therapy
5.1. Indian medicinal plants, Ayurvedic, and Unani systems
Plants are a potential source of antiviral agents. In India, herbal medicines have proved to intensify therapeutic effects against several viral infections like Dengue virus, HBV, HCV, HSV, HIV, and Influenza virus. These natural agents inhibit viral replication and synthesis. These indigenous plants stand alone in Indian tradition and have been recognized worldwide for its beneficial healing effects (Ballabh and Chaurasia, 2007; Pandey et al., 2008).. Some of the common medicinal plants used are shown in Fig. 2 .
Fig. 2.
(a) Indian medicinal plants reported to treat viral diseases such as Measles, Poliomyelitis, Herpes, Influenza, Hepatitis, HIV, Chickenpox, and Yellow fever. (b) Plant extract formulations prepared by Ayurvedic and Unani medicines to combat viral diseases.
An Indian Government initiative, Ayurveda, Yoga and Naturopathy, Unani, Siddha, and Homeopathy (AYUSH) held by the Ministry of Health and Family Welfare, 2014 provides education, awareness, and enhances research to use natural resources that can fight several life threatening diseases. Ayurvedic medicine has been in use since two thousand years. Over 700 herbal drugs were recorded in Ayurveda with reported clinical effects categorised into 50 drug classifications. Also, Unani is recognized as traditional medicine producer, showing therapeutic effects against many infectious diseases. Both the Ayurvedic and Unani systems of medicine have recorded several preparations like decoctions, powders, and liquids of potential plants with immunomodulatory and antiviral properties (Subhose et al., 2005; Patwardhan et al., 2005; Weeks, 2020).
Due to changing lifestyles and requirements for nutrition and immunity to overcome growing infections complementary and herbal medicine can act as best alternatives for chemical drugs. Nutraceutical components and ethnopharmacological preparations play a very important role to fight against viral infections (Kamboj, 2000). India is the largest manufacturer of traditional health products and formulations from medicinal plants. Herbal medicines and other nutrients from food are provided as dietary supplements in the form of pills, capsules, powders, solids or liquid (processed forms). They act as antioxidants, vitamin, and mineral supplements, also alleviate health against respiratory diseases, strengthen the immune system, and protect against the common cold (Mukherjee and Wahile, 2006).
5.2. Chinese herbal medicine (CHMs)
CHMs contain several plant products and preparations which play a tremendous role in treating various ailments (Fig. 3 ). They help to regulate body temperature and detoxify chemical substances in our body. Xiaoqinglong decoction mixture is used in China for respiratory ailments such as asthma, cough, and chronic obstructive pulmonary disease. The mixture consists of wild ginger (Xixin, Asari Radix et Rhizoma), Pinellia ternata (Banxia, Pinelliae Rhizoma), Liquorice root (Gancao, Glycyrrhizae Radix et Rhizoma), Chinese Magnoliavine Fruit (Wuweizi, Schisandrae Chinensis Fructus), dried ginger (Ganjiang, Zingiberis Rhizoma), Cassia Twig (Guizhi, Ramulus Cinnamomi), Chinese Ephedra herb (mahuang, Ephedrae Herba), and white peony root (Baishao, Paeoniae Radix Alba). This herbal extract exhibited antiviral activity against drug-resistant H1N1 virus (Zhen et al., 2018).
Fig. 3.
Chinese herbal medicines used for treating viral infections.
Extracts of Scutellaria baicalensis contain flavonoids such as 5,7,4′-trihydroxy-8-methoxyflavone, baicalein, and 5,7,8,4′-tetrahydroxyflavone. These extracts showed antiviral properties that inhibited the neuraminidase activity of Sendai virus and Infuenza A H5N1 (Hou and Lu, 2009). Houttuynia cordata Thunb is a traditional Chinese medicine used for treating pneumonia and lung-related ailments. It is also found active against SARS-CoV (Lau et al., 2018).
5.3. Other traditional medicines
Maoto is a Japanese herbal medicine used for upper respiratory tract infection. Maoto constitutes extracts obtained from Ephedra herb, Apricot kernel, Cinnamon bark, and Glycyrrhiza root. Maoto expressed antiviral effect against Influenza virus PR8 and H1N1 by inhibiting the V-ATPase present in the endosome and lysosome membranes, thereby preventing the uncoating of the virus and its entry into the cytoplasm (Masui et al., 2017).
Korean Red Ginseng is used as traditional medicine in East Asian countries as it has enhanced pharmacological properties as compared with fresh ginseng (the root of Panax ginseng) because of the steaming process against Respiratory syncytial virus, Rhinovirus, Influenza virus, HIV, Hepatitis virus, Norovirus, Rotavirus, Enterovirus, and Coxsackievirus (Im et al., 2016).
5.4. Enhancing immunity via nutrition
A healthy immune system is the necessity in today's world to combat emerging pathogenic infections. Fig. 4 enlists common nutraceuticals to improve immunity against viral pathogens. Vitamins are the best source of nutrient supplements readily available in plants, fresh fruits, and vegetables. Vitamin C and D hamper speedy recovery of common cold, cough, sore throats, etc., while other vitamins like A, B6, K, and E strengthen the immune system by enhancing inflammatory responses and speed up the biochemical pathways involved in viral destruction. Minerals like zinc, copper, iron, and potassium inhibit pro-inflammatory cytokines and enable the differentiation of T-lymphocytes (Patel et al., 2019). In addition to micronutrients, probiotics not only metabolize food but also wipe out pathogens from the hosts. Herbal home remedies like preparation of decoctions with garlic, ginger, turmeric, pepper, and onions increase flu fighting responses and boost the immune system (Kang et al., 2013; Curtis et al., 2017).
Fig. 4.
Nutraceuticals to improve immunity.
6. Conclusions and future perspectives
Newly emerging viral diseases are serious threat to human health. Recent impact of viral disease outbreaks like COVID-19, SARS, EVD, ZIKV disease, NIV disease, and Influenza viruses have emphasized new drug designing and vaccine development. Though synthetic molecules are available for viral infections, traditional medicines or novel drug formulations from different natural sources benefit better with low complications. Natural resources viz. medicinal plants, bacteria, and fungi have been identified as promising producers of plethora of alkaloids, coumarins, phenolics, flavonoids, lignans, terpenoids, tannins, and peptides which have shown tremendous abilities as antiviral agents and suggested their role in the development of ideal antiviral drugs in future. Indian medicinal plants and Ayurveda have shown beneficial effects against diversified groups of viral diseases. In addition, CHMs and Unani medicines contained several plant products and preparations which played a tremendous role in treating various ailments. These evidences led to investigate further the field of pharmacology in order to strengthen the constant warning of emerging and re-emerging viral infections and develop a state of preparedness in the world. However, plethora of natural resources still requires in depth pharmacological investigations in terms of suggesting their profound roles as therapeutics.
CRediT authorship contribution statement
R. Sagaya Jansi: Investigation, Writing - original draft. Ameer Khusro: Investigation, Writing - original draft. Paul Agastian: Conceptualization, Writing - original draft. Ahmed Alfarhan: Conceptualization, Resources, Supervision. Naif Abdullah Al-Dhabi: Writing - review & editing, Supervision. Mariadhas Valan Arasu: Writing - review & editing, Resources. Rajakrishnan Rajagopal: Writing - review & editing, Resources. Damia Barcelo: Conceptualization, Writing - review & editing, Supervision. Amal Al-Tamimi: Resources, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the support they received from Loyola College and King Saud University for the preparation of this manuscript.
Editor: Lotfi Aleya
References
- Ahmadi A., Zorofchian Moghadamtousi S., Abubakar S., Zandi K. Antiviral potential of algae polysaccharides isolated from marine sources: a review. Biomed. Res. Int. 2015:825203. doi: 10.1155/2015/825203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D., de Wit E., Munster V.J., Hensley L.E., Zalmout I.S., Kapoor A., Epstein J.H., Karesh W.B., Daszak P., Mohammed O.B., Lipkin W.I. Middle East Respiratory Syndrome Coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5(2) doi: 10.1128/mbio.01002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M.E., Kobes R. Effects of vaccination and population structure on influenza epidemic spread in the presence of two circulating strains. BMC Public Health. 2011;11(Suppl. 1):S8. doi: 10.1186/1471-2458-11-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvin A., Miller K.I., Neilan B.A. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol. Res. 2014;169(7–8):483–495. doi: 10.1016/j.micres.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoros M., Girre R.L. Structure of two antiviral triterpene saponins from Anagallis arvensis. Phytochemistry. 1987;26(3):787–791. doi: 10.1016/s0031-9422(00)84787-1. [DOI] [Google Scholar]
- Amoros M., Lurton E., Boustie J., Girre L., Sauvager F., Cormier M. Comparison of the anti-Herpes Simplex Virus activities of propolis and 3-methyl-but-2-enyl caffeate. J. Nat. Prod. 1994;57(5):644–647. doi: 10.1021/np50107a013. [DOI] [PubMed] [Google Scholar]
- Anani K., de Souza C., Akpagana K., Tower G.H.N., Arnason J.T., Gbeassor M., Hudson J.B. Investigation of medicinal plants of Togo for antiviral and antimicrobial activities. Pharm. Biol. 2000;38(1):40–45. doi: 10.1076/1388-0209(200001)38:1;1-b;ft040. [DOI] [PubMed] [Google Scholar]
- Asano J., Chiba K., Tada M., Yoshii T. Antiviral activity of lignans and their glycosides from Justicia procumbens. Phytochemistry. 1996;42(3):713–717. doi: 10.1016/0031-9422(96)00024-6. [DOI] [PubMed] [Google Scholar]
- Ashton R.J., Kenig M.D., Luk K., Planterose D.N., Scott-Wood G. Mm 46115, a new antiviral antibiotic from Actinomadura pelletieri. Characteristics of the producing cultures, fermentation, isolation, physico-chemical and biological properties. J. Antibiot. 1990;43(11):1387–1393. doi: 10.7164/antibiotics.43.1387. [DOI] [PubMed] [Google Scholar]
- Astani A., Reichling J., Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010;24(5):673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoade, F., Kumar, S., 2020. Varicella zoster (chickenpox) [updated 2019 Dec 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-.https://www.ncbi.nlm.nih.gov/books/NBK448191/.
- Ayres D.C., Loike J.D. Cambridge University Press; 1990. Lignans. [DOI] [Google Scholar]
- Baba M., Snoeck R., Pauwels R., de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988;32(11):1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh B., Chaurasia O.P. Traditional medicinal plants of cold desert Ladakh-used in treatment of cold, cough and fever. J. Ethnopharmacol. 2007;112(2):341–345. doi: 10.1016/j.jep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Barrows N.J., Campos R.K., Powell S.T., Prasanth K.R., Schott-Lerner G., Soto-Acosta R., Galarza-Muñoz G., McGrath E.L., Urrabaz-Garza R., Gao J., Wu P., Menon R., Saade G., Fernandez-Salas I., Rossi S.L., Vasilakis N., Routh A., Bradrick S.S., Garcia-Blanco M.A. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host and Microbe. 2016;20(2):259–270. doi: 10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashyal B.P., Wellensiek B.P., Ramakrishnan R., Faeth S.H., Ahmad N., Gunatilaka A.A.L. Altertoxins with potent anti-HIV activity from Alternaria tenuissima QUE1Se, a fungal endophyte of Quercus emoryi. Bioorganic and Medicinal Chemistry. 2014;22(21):6112–6116. doi: 10.1016/j.bmc.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher Y. Springer Verlag, and Wien; New York: 1976. Monographs in Virology: Antiviral Drugs; Mode of Action and Chemotherapy of Viral Infection of Man; pp. 144–148. [PubMed] [Google Scholar]
- Becker Y. Antiviral agents from natural sources. Pharmacol. Ther. 1980;10(1):119–159. doi: 10.1016/0163-7258(80)90011-x. [DOI] [PubMed] [Google Scholar]
- Becker N.G., Wang D. Can antiviral drugs contain pandemic influenza transmission? PLoS One. 2011;6(3):e17764. doi: 10.1371/journal.pone.0017764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedows E., Hatfield G.M. An investigation of the antiviral activity of Podophyllum peltatum. J. Nat. Prod. 1982;45(6):725–729. doi: 10.1021/np50024a015. [DOI] [PubMed] [Google Scholar]
- Béládi I., Pusztai R., Mucsi I., Bakay M., Gábor M. Activity of some flavonoids against viruses. Ann. N. Y. Acad. Sci. 1977;284(1 Third Conference):358–364. doi: 10.1111/j.1749-6632.1977.tb21971.x. [DOI] [PubMed] [Google Scholar]
- Berg A.J.J., Labiade R.P. Methods in plants biochemistry. In: Harborne JB, Plant Phenolic London, Academic Press. Blevins RD, Dumic MP (1980): the effect of D-9-tetrahydrocannabinol on herpes simplex virus replication. J. Gen. Virol. 1989;49:427–431. doi: 10.1099/0022-1317-49-2-427. [DOI] [PubMed] [Google Scholar]
- Besednova N.N., Zvyagintseva T.N., Kuznetsova T.A., Makarenkova I.D., Smolina T.P., Fedyanina L.N., Kryzhanovsky S.P., Zaporozhets T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites. 2019;9(5):87. doi: 10.3390/metabo9050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj A., Agrawal P. A review fungal endophytes: as a store house of bioactive compound. World J. Pharm. Pharm. Sci. 2014;3:228–237. [Google Scholar]
- Biliavska L., Pankivska Y., Povnitsa O., Zagorodnya S. Antiviral activity of exopolysaccharides produced by lactic acid bacteria of the genera Pediococcus, Leuconostoc and Lactobacillus against human adenovirus type 5. Medicina. 2019;55:519. doi: 10.3390/medicina55090519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte S., Crispo A., Amore A., Celentano E., Cuomo A., Cascella M. Potential antiviral drugs for SARS-Cov-2 treatment: preclinical findings and ongoing clinical research. In Vivo. 2020;34:1597–1602. doi: 10.21873/invivo.11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop K.S., Kao C.H.J., Xu Y., Glucina M.P., Paterson R.R.M., Ferguson L.R. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry. 2015;114:56–65. doi: 10.1016/j.phytochem.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Blevins R.D., Dumic M.P. The effect of -9-tetrahydrocannabinol on Herpes Simplex Virus replication. J. Gen. Virol. 1980;49(2):427–431. doi: 10.1099/0022-1317-49-2-427. [DOI] [PubMed] [Google Scholar]
- Bloor S.J. Antiviral phloroglucinols from New Zealand Kunzea species. J. Nat. Prod. 1992;55(1):43–47. doi: 10.1021/np50079a006. [DOI] [PubMed] [Google Scholar]
- Bokesch H.R., McKee T.C., Currens M.J., Gulakowski R.J., McMahon J.B., Cardellina J.H., Boyd M.R. HIV-inhibitory gallotannins from Lepidobotrys staudtii. Nat. Prod. Lett. 1996;8(2):133–136. doi: 10.1080/10575639608043252. [DOI] [Google Scholar]
- Boustie J., Grube M. Lichens-a promising source of bioactive secondary metabolites. Plant Genetic Resources. 2005;3(2):273–287. doi: 10.1079/pgr200572. [DOI] [Google Scholar]
- Boustie J., Stigliani J.L., Montanha J., Amoros M., Payard M., Girre L. Antipoliovirus structure-activity relationships of some aporphine alkaloids. J. Nat. Prod. 1998;61(4):480–484. doi: 10.1021/np970382v. [DOI] [PubMed] [Google Scholar]
- Bovio E., Garzoli L., Poli A., Luganini A., Villa P., Musumeci R., McCormack G.P., Cocuzza C.E., Gribaudo G., Mehiri M., Varese G.C. Marine fungi from the sponge Grantia compressa: biodiversity, chemodiversity, and biotechnological potential. Marine Drugs. 2019;17(4):220. doi: 10.3390/md17040220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten K.P., Laufer M.R. Human Papillomavirus (HPV), HPV-related disease, and the HPV vaccine. Rev. Obstet. Gynecol. 2008;1(1):2–10. [PMC free article] [PubMed] [Google Scholar]
- Bruggemann R., Matsuo Orlandi J., Benati F.J., Faccin L.C., Mantovani M.S., Nozawa C., Linhares R.E.C. Antiviral activity of Agaricus blazei Murrill ss. Heinem extract against human and bovine herpesviruses in cell culture. Braz. J. Microbiol. 2006;37(4):561–565. doi: 10.1590/s1517-83822006000400029. [DOI] [Google Scholar]
- Calland N., Dubuisson J., Rouillé Y., Séron K. Hepatitis C virus and natural compounds: a new antiviral approach? Viruses. 2012;4(10):2197–2217. doi: 10.3390/v4102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004;5(10):981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Champagne D.E., Koul O., Isman M.B., Scudder G.G.E., Neil Towers G.H. Biological activity of limonoids from the rutales. Phytochemistry. 1992;31(2):377–394. doi: 10.1016/0031-9422(92)90003-9. [DOI] [Google Scholar]
- Chen K., Shi Q., Fujioka T., Zhang D.C., Hu C.Q., Jin J.Q., Kilkuskie R.E., Lee K.H. Anti-AIDS agents, 4. Tripterifordin, a novel anti-HIV principle from Tripterygium wilfordii: isolation and structural elucidation. J. Nat. Prod. 1992;55(1):88–92. doi: 10.1021/np50079a013. [DOI] [PubMed] [Google Scholar]
- Chen D.F., Zhang S.-X., Wang H.K., Zhang S.-Y., Sun Q.Z., Cosentino L.M., Lee K.H. Novel anti-HIV Lancilactone C and related triterpenes from Kadsura lancilimba. J. Nat. Prod. 1999;62(1):94–97. doi: 10.1021/np980291d. [DOI] [PubMed] [Google Scholar]
- Chen S., Kinney W.A., Van Lanen S. Nature’s combinatorial biosynthesis and recently engineered production of nucleoside antibiotics in Streptomyces. World J. Microbiol. Biotechnol. 2017;33(4) doi: 10.1007/s11274-017-2233-6. [DOI] [PubMed] [Google Scholar]
- Chen J., Li H., Zhao Z., Xia X., Li B., Zhang J., Yan X. Diterpenes from the marine algae of the genus Dictyota. Marine Drugs. 2018;16(5):159. doi: 10.3390/md16050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H., Inokoshi J., Nakashima H., Ōmura S., Tanaka H. Actinohivin, a novel anti-human immunodeficiency virus protein from an actinomycete, inhibits viral entry to cells by binding high-mannose type sugar chains of gp120. Biochem. Biophys. Res. Commun. 2004;316(1):203–210. doi: 10.1016/j.bbrc.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Christina A., Christapher V., Bhore S.J. Endophytic bacteria as a source of novel antibiotics: an overview. Pharmacogn. Rev. 2013;7(13):11–16. doi: 10.4103/0973-7847.112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M.J. Chemical Rubber Co. Press; Cleveland, Ohio: 1977. Chemoprophylaxis and Virus Infections of Respiratory Tract II; pp. 189–190. [Google Scholar]
- Cody V., Middleton E., Harborne J.B. Alan R Liss; New York: 1986. Plant Flavonoids in Biology and Medicine. [Google Scholar]
- Cohen P.A., Hudson J.B., Towers G.H.N. Antiviral activities of anthraquinones, bianthrones and hypericin derivatives from lichens. Experientia. 1996;52(2):180–183. doi: 10.1007/bf01923366. [DOI] [PubMed] [Google Scholar]
- Collins R.A., Ng T.B. Polysaccharopeptide from Coriolus versicolor has potential for use against human immunodeficiency virus type 1 infection. Life Sci. 1997;60(25) doi: 10.1016/s0024-3205(97)00294-4. PL383–PL387. [DOI] [PubMed] [Google Scholar]
- Cordell G.A. Wiley; New York: 1981. Introduction to Alkaloids: A Biogenetic Approach. [Google Scholar]
- Corthout J., Pieters L., Claeys M., Berghe D.V., Vlietinck A. Antiviral caffeoyl esters from Spondias mombin. Phytochemistry. 1992;31(6):1979–1981. doi: 10.1016/0031-9422(92)80344-e. [DOI] [Google Scholar]
- Cos P., Maes L., Vlietinck A., Pieters L. Plant-derived leading compounds for chemotherapy of human immunodefiency virus (HIV) infection – an update (1998–2007) Planta Med. 2008;74(11):1323–1337. doi: 10.1055/s-2008-1081314. [DOI] [PubMed] [Google Scholar]
- Cracker L.E., Simon J. Harwarth Press, Inc; New York: 1986. Herbs, Spices, and Medicinal Plants2; p. 22. [Google Scholar]
- Craik D.J., Swedberg J.E., Mylne J.S., Cemazar M. Cyclotides as a basis for drug design. Expert Opin. Drug Discovery. 2012;7(3):179–194. doi: 10.1517/17460441.2012.661554. [DOI] [PubMed] [Google Scholar]
- Curtis L.J., Bernier P., Jeejeebhoy K., Allard J., Duerksen D., Gramlich L., Laporte M., Keller H.H. Costs of hospital malnutrition. Clin. Nutr. 2017;36(5):1391–1396. doi: 10.1016/j.clnu.2016.09.009. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Chemical Rubber Co. Press; Cleveland, Ohio: 1973. Selective Inhibitors of Viral Functions; pp. 78–82. [Google Scholar]
- De Souza A.D.R., Medeiros A.C., De Souza M., Wink I.R., Siqueira M.B.C., Ferreira L., Fernandes M.P., Loayza Hidalgo I.L.D.S., Torres Evaluation of the impact of exposure to pesticides on the health of the rural population: vale do Taquari, State of Rio Grande do Sul (Brazil) CiênciaSaúdeColetiva. 2011;16:3519–3528. doi: 10.1590/s1413-81232011000900020. [DOI] [PubMed] [Google Scholar]
- Decosterd L., Parsons I., Gustafson K., Cardellina J., McMahon J., Cragg G., Murata Y., Pannell L., Steiner J., Clardy J., Boyd M. Isolation, structure and synthesis of Conocurvone, a potent, novel HIV-inhibitory naphthoquinone trimer from Conospermum species. Planta Med. 1993;59(S 1):A581. doi: 10.1055/s-2006-959777. [DOI] [Google Scholar]
- Diallo B., Vanhaelen M., Vanhaelen-Fastré R., Konoshima T., Kozuka M., Tokuda H. Studies on inhibitors of skin-tumor promotion. Inhibitory effects of triterpenes from Cochlospermum tinctorium on Epstein-Barr virus activation. J. Nat. Prod. 1989;52(4):879–881. doi: 10.1021/np50064a039. [DOI] [PubMed] [Google Scholar]
- Ding L., Münch J., Goerls H., Maier A., Fiebig H.-H., Lin W.H., Hertweck C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorganic and Medicinal Chemistry Letters. 2010;20(22):6685–6687. doi: 10.1016/j.bmcl.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Duan H., Takaishi Y., Imakura Y., Jia Y., Li D., Cosentino L.M., Lee K.H. Sesquiterpene alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: a new class of potent anti-HIV agents. J. Nat. Prod. 2000;63(3):357–361. doi: 10.1021/np990281s. [DOI] [PubMed] [Google Scholar]
- Eberhardt T., Young R. Assessment of the anti-HIV activity of a pine cone isolate. Planta Med. 1996;62(01):63–65. doi: 10.1055/s-2006-957801. [DOI] [PubMed] [Google Scholar]
- Elgamal M.H.A., Soliman H.S.M., Karawya M.S., Mikhova B., Duddeck H. Isolation of triterpene saponins from Gypsophila capillaris. Phytochemistry. 1995;38(6):1481–1485. doi: 10.1016/0031-9422(94)00900-e. [DOI] [PubMed] [Google Scholar]
- El-Mekkawy S., Meselhy M.R., Nakamurame N., Tezuka Y., Hattori M., Kakiuchi N., Shimotohno K., Kawahata T., Otake T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry. 1998;49(6):1651–1657. doi: 10.1016/s0031-9422(98)00254-4. [DOI] [PubMed] [Google Scholar]
- El-Mekkawy S., Meselhy M.R., Nakamura N., Hattori M., Kawahata T., Otake T. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry. 2000;53(4):457–464. doi: 10.1016/s0031-9422(99)00556-7. [DOI] [PubMed] [Google Scholar]
- Elnaby A.H., Abo-Elala G., Abdel-Raouf U., Abd-elwahab A., Hamed M. Antibacterial and anticancer activity of marine Streptomyces parvus: optimization and application. Biotechnol. Biotechnol. Equip. 2016;30(1):180–191. [Google Scholar]
- Erdelmeier C., Cinatl J., Rabenau H., Doerr H., Biber A., Koch E. Antiviral and antiphlogistic activities of Hamamelis virginiana bark. Planta Med. 1996;62(03):241–245. doi: 10.1055/s-2006-957868. [DOI] [PubMed] [Google Scholar]
- Erickson K.L., Beutler J.A., Cardellina J.H., McMahon J.B., Newman D.J., Boyd M.R. A novel phorbol ester from Excoecaria agallocha. J. Nat. Prod. 1995;58(5):769–772. doi: 10.1021/np50119a020. [DOI] [PubMed] [Google Scholar]
- Estevez J.M., Ciancia M., Cerezo A.S. DL-galactan hybrids and agarans from gametophytes of the red seaweed Gymnogongrus torulosus. Carbohydrate Res. 2001;331:27–41. doi: 10.1016/S0008-6215(01)00015-5. [DOI] [PubMed] [Google Scholar]
- Faccin L.C., Benati F., Rincão V.P., Mantovani M.S., Soares S.A., Gonzaga M.L., Nozawa C., Carvalho Linhares R.E. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Lett. Appl. Microbiol. 2007;45(1):24–28. doi: 10.1111/j.1472-765x.2007.02153.x. [DOI] [PubMed] [Google Scholar]
- Farnsworth N.R., Svoboda G.H., Blomster R.N. Antiviral activity of selected Catharanthus alkaloids. J. Pharm. Sci. 1968;57(12):2174–2175. doi: 10.1002/jps.2600571235. [DOI] [PubMed] [Google Scholar]
- Feliciano A., Gordaliza M., del Corral J., Castro M., García-Grávalos M., Ruiz-Lázaro P. Antineoplastic and antiviral activities of some cyclolignans. Planta Med. 1993;59(03):246–249. doi: 10.1055/s-2006-959660. [DOI] [PubMed] [Google Scholar]
- Fenglei Z., Yunlong X., Handong S. Diterpenoids constituents of Rabdosia liangshanica. Phytochemistry. 1989;28:1671–1674. [Google Scholar]
- Foder G.B., Colasanti B. Vol. 3. Wiley; New York: 1985. Alkaloids, Chemical and Biological Perspectives; pp. 1–90. [Google Scholar]
- French C.J., Towers G.H.N. Inhibition of infectivity of potato virus X by flavonoids. Phytochemistry. 1992;31(9):3017–3020. doi: 10.1016/0031-9422(92)83438-5. [DOI] [Google Scholar]
- Fujioka T., Kashiwada Y., Kilkuskie R.E., Cosentino L.M., Ballas L.M., Jiang J.B., Janzen W.P., Chen I.-S., Lee K.H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994;57(2):243–247. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- Fuller R.W., Cardellina J.H., Boyd M.R. HIV-inhibitory natural products. Diterpene carboxylic acid from fruits of Xylopia sp.1. Nat. Prod. Lett. 1996;8(3):169–172. doi: 10.1080/10575639608044890. [DOI] [Google Scholar]
- Gabrielsen B., Monath T.P., Huggins J.W., Kefauver D.F., Pettit G.R., Groszek G., Hollingshead M., Kirsi J.J., Shannon W.M., Schubert E.M., DaRe J., Ugarkar B., Ussery M.A., Phelan M.J. Antiviral (RNA) activity of selected amaryllidaceae isoquinoline constituents and synthesis of related substances. J. Nat. Prod. 1992;55(11):1569–1581. doi: 10.1021/np50089a003. [DOI] [PubMed] [Google Scholar]
- Gao W., Sun Y., Chen S., Zhang J., Kang J., Wang Y., Wang H., Xia G., Liu Q., Kang Y. Mushroom lectin enhanced immunogenicity of HBV DNA vaccine in C57BL/6 and HBsAg-transgenic mice. Vaccine. 2013;31(18):2273–2280. doi: 10.1016/j.vaccine.2013.02.062. [DOI] [PubMed] [Google Scholar]
- Gentzsch J., Hinkelmann B., Kaderali L., Irschik H., Jansen R., Sasse F., Frank R., Pietschmann T. Hepatitis C virus complete life cycle screen for identification of small molecules with pro- or antiviral activity. Antivir. Res. 2011;89(2):136–148. doi: 10.1016/j.antiviral.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gerth K., Schummer D., Höfle G., Irschik H., Reichenbach H. Ratjadon: a new antifungal compound from Sorangium cellulosum (Myxobacteria) production, physico-chemical and biological properties. J. Antibiot. 1995;48(9):973–976. doi: 10.7164/antibiotics.48.973. [DOI] [PubMed] [Google Scholar]
- Groweiss A., Cardellina J.H., Boyd M.R. HIV-inhibitory prenylated xanthones and flavones from Macluratinctoria. J. Nat. Prod. 2000;63(11):1537–1539. doi: 10.1021/np000175m. [DOI] [PubMed] [Google Scholar]
- Gu C.Q., Li J.W., Chao F., Jin M., Wang X.-W., Shen Z.Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antivir. Res. 2007;75(3):250–257. doi: 10.1016/j.antiviral.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Gubler D.J., Clark G.G. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg. Infect. Dis. 1995;1(2):55–57. doi: 10.3201/eid0102.952004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson K., Roman M., Fenical W. The macrolactins, a novel class of antiviral and cytotoxic macrolides from a deep-sea marine bacterium. J. Am. Chem. Soc. 1989;111(19):7519–7524. doi: 10.1021/ja00201a036. [DOI] [Google Scholar]
- Gustafson K.R., Blunt J.W., Munro M.H.G., Fuller R.W., McKee T.C., Cardellina J.H., McMahon J.B., Cragg G.M., Boyd M.R. The guttiferones, HIV-inhibitory benzophenones from Symphonia globulifera, Garcinia livingstonei, Garcinia ovalifolia and Clusia rosea. Tetrahedron. 1992;48(46):10093–10102. doi: 10.1016/s0040-4020(01)89039-6. [DOI] [Google Scholar]
- Gustafson K.R., Sowder R.C., Henderson L.E., Parsons I.C., Kashman Y., Cardellina J.H., McMahon J.B., Buckheit R.W., Pannell L.K., Boyd M.R. Circulins A and B. Novel human immunodeficiency virus (HIV)-inhibitory macrocyclic peptides from the tropical tree Chassalia parvifolia. J. Am. Chem. Soc. 1994;116(20):9337–9338. doi: 10.1021/ja00099a064. [DOI] [Google Scholar]
- Gyorgy E., Koch A. Heart glycosides in poliovirus host cell interaction. Effect of the time of addition on stimulatory or inhibitory action. Acta Microbiology. 1969;16:197–202. [PubMed] [Google Scholar]
- Hallock Y.F., Manfredi K.P., Dai J.R., Cardellina J.H., Gulakowski R.J., McMahon J.B., Schäffer M., Stahl M., Gulden K.-P., Bringmann G., François G., Boyd M.R. Michellamines D−F, new HIV-inhibitory dimeric naphthylisoquinoline alkaloids, and korupensamine E, a new antimalarial monomer, from Ancistrocladus korupensis. J. Nat. Prod. 1997;60(7):677–683. doi: 10.1021/np9700679. [DOI] [PubMed] [Google Scholar]
- Hanish J., Vajda G., Liona B. The mode of action of emetine. Acta chirurgica Academiae Scientiarum Hungaricae. 1966;7:51–54. [PubMed] [Google Scholar]
- Hanson J.R. Academic Press; New York: 1972. Chemistry of Terpenes and Terpenoids; p. 155. [Google Scholar]
- Harborne J.B. London Chapman and Hall; 1988. The Flavonoids: Advances in Research Since 1980. [Google Scholar]
- Harborne J.B., Baxter H. Burgess Science Press Basingstoke; London: 1993. Phytochemical Dictionary a Handbook of Bioactive Compounds from Plants; pp. 12–567. [Google Scholar]
- Harris K.A., Freidl G.S., Munoz O.S., von Dobschuetz S., De Nardi M., Wieland B., Koopmans M.P.G., Stärk K.D.C., van Reeth K., Dauphin G., Meijer A., de Bruin E., Capua I., Hill A.A., Kosmider R., Banks J., Stevens K., van der Werf S., Enouf V., Breed A.C. Epidemiological risk factors for animal Influenza A viruses overcoming species barriers. Eco Health. 2017;14(2):342–360. doi: 10.1007/s10393-017-1244-y. [DOI] [PubMed] [Google Scholar]
- Hasan S., Ahmad S.A., Masood R., Saeed S. Ebola virus: a global public health menace: a narrative review. Journal of Family Medicine and Primary Care. 2019;8(7):2189–2201. doi: 10.4103/jfmpc.jfmpc_297_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Matsumiya S., Uchiyama M., Kurokawa T., Inouye Y., Kasai R., Ishibashi S., Yamasaki K. Inhibitory effect of some triterpenoid saponins on glucose transport in tumor cells and its application to in vitro cytotoxic and antiviral activities. Planta Med. 1994;60(03):240–243. doi: 10.1055/s-2006-959467. [DOI] [PubMed] [Google Scholar]
- Hassan S.T.S., Masarčíková R., Berchová K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015;67(10):1325–1336. doi: 10.1111/jphp.12436. [DOI] [PubMed] [Google Scholar]
- Hasui M., Matsuda M., Okutani K., Shigeta S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995;17(5):293–297. doi: 10.1016/0141-8130(95)98157-T. [DOI] [PubMed] [Google Scholar]
- Hatano T., Yasuhara T., Miyamoto K., Okuda T. Anti-human immunodeficiency virus phenolics from licorice. Chem. Pharm. Bull. 1988;36(6):2286–2288. doi: 10.1248/cpb.36.2286. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Niwayama S., Hayashi T., Nago R., Ochiai H., Morita N. In vitro and in vivo antiviral activity of scopadulcic acid B from Scoparia dulcis, Scrophulariaceae, against herpes simplex virus type 1. Antivir. Res. 1988;9(6):345–354. doi: 10.1016/0166-3542(88)90036-8. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Hayashi K., Uchida K., Niwayama S., Morita N. Antiviral agents of plant origin. II. Antiviral activity of scopadulcic acid B derivatives. Chem. Pharm. Bull. 1990;38(1):239–242. doi: 10.1248/cpb.38.239. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Hamada J., Hayashi T. A screening strategy for selection of anti-HSV-1 and anti-HIV extracts from algae. Phytother. Res. 1996;10(3):233–237. doi: 10.1002/(sici)1099-1573(199605)10:3<233::aid-ptr824>3.0.co;2-w. [DOI] [Google Scholar]
- He F., Bao J., Zhang X.Y., Tu Z.C., Shi Y.M., Qi S.H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013;76(6):1182–1186. doi: 10.1021/np300897v. [DOI] [PubMed] [Google Scholar]
- Hiller K. In: New results on the structure and biological activity of triterpenoid saponins. Hostettmann K., Lea P.J., editors. Clarendon Press; Oxford: 1987. pp. 167–168. [Google Scholar]
- Hou E.C., Lu Y.X. Primary hepatocarcinoma treated by traditional Chinese medicine combined with transcatheter arterial chemoembolization. Zhongguo Zhongxiyi Jiehe Zazhi. 2009;29:225–227. [PubMed] [Google Scholar]
- Hsu C.L., Yen G.C. The Enzymes. Elsevier; 2014. Ganoderic acid and lucidenic acid (triterpenoid) pp. 33–56. [DOI] [PubMed] [Google Scholar]
- Hu K., Kobayashi H., Dong A., Iwasaki S., Yao X. Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica. Planta Med. 2000;66(6):564–567. doi: 10.1055/s-2000-8601. [DOI] [PubMed] [Google Scholar]
- Huang, R.L., Chen, C.C., Huang, H.L., Chang, C.G., Chen, C.F., Chang, C., Hsieh, M.T., 2000. Anti-Hepatitis B virus effects of wogonin isolated from Scutellaria baicalensis. Planta Medica, 66(8), 694–698. doi:/ 10.1055/s-2000-9775. [DOI] [PubMed]
- Hudson J., Graham E., Fong R., Finlayson A., Towers G. Antiviral properties of thiarubrine-a, a naturally occurring polyine. Planta Med. 1986;52(01):51–54. doi: 10.1055/s-2007-969068. [DOI] [PubMed] [Google Scholar]
- Hudson J., Graham E., Chan G., Finlayson A., Towers G. Comparison of the antiviral effects of naturally occurring thiophenes and polyacetylenes. Planta Med. 1986;52(06):453–457. doi: 10.1055/s-2007-969252. [DOI] [PubMed] [Google Scholar]
- Hudson J.B., Harris L., Towers G.H.N. The importance of light in the anti-HIV effect of hypericin. Antivir. Res. 1993;20(2):173–178. doi: 10.1016/0166-3542(93)90006-5. [DOI] [PubMed] [Google Scholar]
- Hudson J.B., Kim J.H., Lee M.K., Hong Y.K., DeWreede R.E. Multiple antiviral activities in extracts of seaweeds from British Columbia. Pharm. Biol. 1999;37(4):300–306. doi: 10.1076/phbi.37.4.300.5804. [DOI] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 2005;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Hwang Y., Rowley D., Rhodes D., Gertsch J., Fenical W., Bushman F. Mechanism of inhibition of a poxvirus topoisomerase by the marine natural product Sansalvamide A. Mol. Pharmacol. 1999;55(6):1049–1053. doi: 10.1124/mol.55.6.1049. [DOI] [PubMed] [Google Scholar]
- Ieven M., Vlietinick A.J., Berghe D.A. Vanden, Totte J., Dommisse R., Esmans E., Alderweireldt F. Isolation of alkaloids from Clivia miniata regel (Amaryl-lidaceae) J. Nat. Prod. 1982;45(5):564–573. doi: 10.1021/np50023a009. [DOI] [PubMed] [Google Scholar]
- Im K., Kim J., Min H. Ginseng, the natural effectual antiviral: protective effects of Korean Red Ginseng against viral infection. Journal of Ginseng Research. 2016;40(4):309–314. doi: 10.1016/j.jgr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada A., Somekawa M., Murata H., Nakanishi T., Tokuda H., Nishino H., Iwashima A., Darnaedi D., Murata J. Structures and inhibitory effects on Epstein-Barr virus activation of triterpenoids from leaves of Chisocheton macrophyllus King. Chem. Pharm. Bull. 1993;41(3):617–619. doi: 10.1248/cpb.41.617. [DOI] [Google Scholar]
- Ingham J.L. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Springer Vienna; 1983. Naturally occurring isoflavonoids (1855–1981) pp. 1–266. [DOI] [Google Scholar]
- Ishikawa T., Nishigaya K., Uchikoshi H., Chen I.S. Cochinolide, a new γ-alkylidene bicyclic butenolide with antiviral activity, and its β-glucopyranoside from Homaliumcochin chinensis. J. Nat. Prod. 1998;61(4):534–537. doi: 10.1021/np970341z. [DOI] [PubMed] [Google Scholar]
- Jack H.W., Tzi B.N. Vulgarinin, a broad-spectrum antifungal peptide from haricot beans (Phaseolus vulgaris) Int. J. Biochem. Cell Biol. 2005;37:1626–1632. doi: 10.1016/j.biocel.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Jakubiec-Krzesniak K., Rajnisz-Mateusiak A., Guspiel A., Ziemska J., Solecka J. Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Pol. J. Microbiol. 2018;67(3):259–272. doi: 10.21307/pjm-2018-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassim S.A.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95(3):412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Jiang B., Xu X., Li L., Yuan W. Study on ‘911’ anti-HBV effect in HepG2 2115 cells culture. Modern Preventive Medicine. 2003;30:517–518. [Google Scholar]
- Jiang Y., Wong J.H., Fu M., Ng T.B., Liu Z.K., Wang C.R., Li N., Qiao W.T., Wen T.Y., Liu F. Isolation of adenosine, iso-sinensetin and dimethylguanosine with antioxidant and HIV-1 protease inhibiting activities from fruiting bodies of Cordyceps militaris. Phytomedicine. 2011;18(2–3):189–193. doi: 10.1016/j.phymed.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz E., Jansen R., Kunze B., Trowitzsch-Kienast W., Forche E., Reichenbach H., Höfle G., Hunsmann G. Three new potent HIV-1 inhibitors from Myxobacteria. Antivir. Chem. Chemother. 1992;3(4):189–193. doi: 10.1177/095632029200300401. [DOI] [Google Scholar]
- Kaij-a-Kamb M., Amoros M., Girre L. Search for new antiviral agent of plant origin. Pharm. Acta Helv. 1992;67:130–147. [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J. Gen. Virol. 1972;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Kamboj V.P. Herbal medicine. Curr. Sci. 2000;8(1):35–39. [Google Scholar]
- Kanekiyo K., Hayashi K., Takenaka H., Lee J.B., Hayashi T. Anti-Herpes Simplex Virus target of an acidic polysaccharide, Nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007;30(8):1573–1575. doi: 10.1248/bpb.30.1573. [DOI] [PubMed] [Google Scholar]
- Kang E.J., Kim S.Y., Hwang I.-H., Ji Y.J. The effect of probiotics on prevention of common cold: a meta-analysis of randomized controlled trial studies. Korean Journal of Family Medicine. 2013;34(1):2–10. doi: 10.4082/kjfm.2013.34.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam M., Shier T. Isolation and characterization of an antiviral flavonoid from Waldsteinia fragarioides. J. Nat. Prod. 1992;55:1525–1527. doi: 10.1021/np50088a022. [DOI] [PubMed] [Google Scholar]
- Kashiwada Y., Wang H.K., Nagao T., Kitanaka S., Yasuda I., Fujioka T., Yamagishi T., Cosentino L.M., Kozuka M., Okabe H., Ikeshiro Y., Hu C.-Q., Yeh E., Lee K.H. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids1. J. Nat. Prod. 1998;61(9):1090–1095. doi: 10.1021/np9800710. [DOI] [PubMed] [Google Scholar]
- Kernan M.R., Sendl A., Chen J.L., Jolad S.D., Blanc P., Murphy J.T., Stoddart C.A., Nanakorn W., Balick M.J., Rozhon E.J. Two new lignans with activity against influenza virus from the medicinal plant Rhinacanthus nasutus. J. Nat. Prod. 1997;60(6):635–637. doi: 10.1021/np960613i. [DOI] [PubMed] [Google Scholar]
- Kernan M.R., Amarquaye A., Chen J.L., Chan J., Sesin D.F., Parkinson N., Ye Z., Barrett M., Bales C., Stoddart C.A., Sloan B., Blanc P., Limbach C., Mrisho S., Rozhon E.J. Antiviral phenylpropanoid glycosides from the medicinal plant Markhamia lutea. J. Nat. Prod. 1998;61(5):564–570. doi: 10.1021/np9703914. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Huang A.M., Bannister K., Choi T.J., Towers G.H.N., DeWreede R.E., Hudson J.B., Jin H., Hong Y.K. Biological activities of seaweed extracts from British Columbia, Canada, and Korea. Can. J. Bot. 1997;75(10):1656–1660. doi: 10.1139/b97-878. [DOI] [Google Scholar]
- Kim H.J., Woo E.R., Shin C.G., Park H. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. J. Nat. Prod. 1998;61(1):145–148. doi: 10.1021/np970171q. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Vo T.S., Ngo D.H. Marine Medicinal Foods - Implications and Applications, Macro and Microalgae. Elsevier; 2011. Potential application of marine algae as antiviral agents in medicinal foods; pp. 245–254. [DOI] [PubMed] [Google Scholar]
- Kim M., Yim J.H., Kim S.-Y., Kim H.S., Lee W.G., Kim S.J., Kang P.-S., Lee C.-K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012;93(2):253–259. doi: 10.1016/j.antiviral.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Kite G.C., Fellows L.E., Fleet G.W.J., Liu P.S., Scofield A.M., Smith N.G. α-Homonojirimycin [2,6-dideoxy-2,6-imino-d-glycero-l-gulo-heptitol] from Omphalea diandra L.: isolation and glucosidase inhibtion. Tetrahedron Lett. 1988;29(49):6483–6485. doi: 10.1016/s0040-4039(00)82379-5. [DOI] [Google Scholar]
- Koch A., Gyorgy E. Heart glycosides in poliovirus host cell interaction. I. Effect of digitoxin and digitoxin and their aglucons on one step growth curves. Acta. Microbiology. 1969;16:189–196. [PubMed] [Google Scholar]
- Koch A., Sandor G. Heart glycosides in poliovirus host cell interaction III. Chemical Structure and Activity. Acta Microbiology. 1969;16:245–251. [PubMed] [Google Scholar]
- Koirala P., Jung H.A., Choi J.S. Recent advances in pharmacological research on Ecklonia species: a review. Arch. Pharm. Res. 2017;40(9):981–1005. doi: 10.1007/s12272-017-0948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokumai M., Konoshima T., Kozuka M., Haruna M., Ito K. Euglobal T1, a new Euglobal from Eucalyptus tereticornis. J. Nat. Prod. 1991;54(4):1082–1086. doi: 10.1021/np50076a025. [DOI] [Google Scholar]
- Kondamudi, N.P., Waymack, J.R., Measles. (updated 2019 Nov 20). In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2020 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK448068/.
- Konoshima T., Kozuka M., Haruna M., Ito K., Kimura T., Tokuda H. Studies on the constituents of leguminous plants. XII. The structures of new triterpenoid saponins from Wistaria brachybotrys SIEB. et ZUCC. Chem. Pharm. Bull. 1989;37(10):2731–2735. doi: 10.1248/cpb.37.2731. [DOI] [Google Scholar]
- Konoshima T., Kozuka M., Tokuda H., Nishino H., Iwashima A., Haruna M., Ito K., Tanabe M. Studies on inhibitors of skin tumor promotion, IX. Neolignans from Magnolia officinalis. J. Nat. Prod. 1991;54(3):816–822. doi: 10.1021/np50075a010. [DOI] [PubMed] [Google Scholar]
- Konoshima T., Takasaki M., Kozuka M., Haruna M., Ito K., Estes J.R., Lee K.H. Constituents of rosaceous plants. I. Structures of new Triterpenoids from Cowania mexicana. Chem. Pharm. Bull. 1993;41(9):1612–1615. doi: 10.1248/cpb.41.1612. [DOI] [PubMed] [Google Scholar]
- Konoshima T., Yasuda I., Kashiwada Y., Cosentino L.M., Lee K.H. Anti-AIDS agents, 21. Triterpenoid saponins as anti-HIV principles from fruits of Gleditsia japonica and Gymnocladus chinesis, and a structure-activity correlation. J. Nat. Prod. 1995;58(9):1372–1377. doi: 10.1021/np50123a006. [DOI] [PubMed] [Google Scholar]
- Konz D., Klens A., Schörgendorfer K., Marahiel M.A. The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: molecular characterization of three multi-modular peptide synthetases. Chem. Biol. 1997;4(12):927–937. doi: 10.1016/s1074-5521(97)90301-x. [DOI] [PubMed] [Google Scholar]
- Kosanić M., Ranković B., Stanojković T., Rančić A., Manojlović N. Cladonia lichens and their major metabolites as possible natural antioxidant, antimicrobial and anticancer agents. LWT Food Sci. Technol. 2014;59(1):518–525. doi: 10.1016/j.lwt.2014.04.047. [DOI] [Google Scholar]
- Koumoutsi A., Chen X.-H., Henne A., Liesegang H., Hitzeroth G., Franke P., Vater J., Borriss R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 2004;186(4):1084–1096. doi: 10.1128/jb.186.4.1084-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupodorova T., Rybalko S., Barshteyn V. Antiviral activity of Basidiomycete mycelia against influenza type A (serotype H1N1) and herpes simplex virus type 2 in cell culture. Virol. Sin. 2014;29(5):284–290. doi: 10.1007/s12250-014-3486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczer M., Dziubasik K., Midak-Siewirska A., Zahorska R., Łuczak M., Konopińska D. Studies of insect peptides alloferon, any -GS and their analogues. Synthesis and antiherpes activity. J. Pept. Sci. 2010;16(4):186–189. doi: 10.1002/psc.1219. [DOI] [PubMed] [Google Scholar]
- Kunze B., Trowitzsch-Kienast W., Höfle G., Reichenbach H. Nannochelins A, B and C, new iron-chelating compounds from Nannocystis exedens (myxobacteria). Production, isolation, physico-chemical and biological properties. J. Antibiot. 1992;45(2):147–150. doi: 10.7164/antibiotics.45.147. [DOI] [PubMed] [Google Scholar]
- Kuo Y.H., Li S.Y., Huang R.L., Wu M.-D., Huang H.C., Lee K.H. Schizarin B, C, D, and E, four new lignans from Kadsuramatsudai and their antihepatitis activities. J. Nat. Prod. 2001;64(4):487–490. doi: 10.1021/np000261m. [DOI] [PubMed] [Google Scholar]
- Kuzuyama T., Seto H. Diversity of the biosynthesis of the isoprene units. Nat. Prod. Rep. 2003;20(2):171–183. doi: 10.1039/b109860h. [DOI] [PubMed] [Google Scholar]
- Lai D., Odimegwu D., Esimone C., Grunwald T., Proksch P. Phenolic compounds with in vitro activity against respiratory syncytial virus from the Nigerian lichen Ramalina farinacea. Planta Med. 2013;79(15):1440–1446. doi: 10.1055/s-0033-1350711. [DOI] [PubMed] [Google Scholar]
- Lau, S.K.P., Zhang, L., Luk, H.K.H., Xiong, L., Peng, X., Li, K.S.M., He, X., Zhao, P.S.-H., Fan, R.Y.Y., Wong, A.C.P., Ahmed, S.S., Cai, J.P., Chan, J.F.W., Sun, Y., Jin, D., Chen, H., Lau, T.C.K., Kok, R.K.H., Li, W., Woo, P.C.Y., 2018. Receptor usage of a novel bat lineage C betacoronavirus reveals evolution of Middle East Respiratory Syndrome-related coronavirus spike proteins for human dipeptidyl peptidase 4 binding. J. Infect. Dis., 218(2), 197–207. doi:/ 10.1093/infdis/jiy018. [DOI] [PMC free article] [PubMed]
- Lee J.B., Hayashi K., Hirata M., Kuroda E., Suzuki E., Kubo Y., Hayashi T. Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Biol. Pharm. Bull. 2006;29(10):2135–2139. doi: 10.1248/bpb.29.2135. [DOI] [PubMed] [Google Scholar]
- Lee J.G., Yoo I.D., Kim W.G. Differential antiviral activity of Benzastatin C and its dechlorinated derivative from Streptomyces nitrosporeus. Biol. Pharm. Bull. 2007;30(4):795–797. doi: 10.1248/bpb.30.795. [DOI] [PubMed] [Google Scholar]
- Lee S.D., Lee D.W., Ko Y.H. Marmoricola korecus sp. nov. Int. J. Syst. Evol. Microbiol. 2011;61(7):1628–1631. doi: 10.1099/ijs.0.025460-0. [DOI] [PubMed] [Google Scholar]
- Leven M., Van den Berghe D., Vlietinck A.J. Plant antiviral agents. Planta Med. 1983;49:109–114. [PubMed] [Google Scholar]
- Li Y.Q., Wang S.F. Anti-hepatitis B activities of ganoderic acid from Ganoderma lucidum. Biotechnol. Lett. 2006;28(11):837–841. doi: 10.1007/s10529-006-9007-9. [DOI] [PubMed] [Google Scholar]
- Li H.Y., Sun N.-J., Kashiwada Y., Sun L., Snider J.V., Cosentino L.M., Lee K.H. Anti-AIDS agents, 9. Suberosol, a new C31Lanostane-type triterpene and anti-HIV principle from Polyalthia suberosa. J. Nat. Prod. 1993;56(7):1130–1133. doi: 10.1021/np50097a017. [DOI] [PubMed] [Google Scholar]
- Li F., Chen D., Lu S., Yang G., Zhang X., Chen Z., Fan S., Wu S., He J. Anti-influenza A viral butenolide from Streptomyces sp. Smu03 inhabiting the intestine of Elephas maximus. Viruses. 2018;10(7):356. doi: 10.3390/v10070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang Y., Hao X., Li S., Jia J., Guan Y., Peng Z., Bi H., Xiao C., Cen S., Gan M. Broad-spectrum antiviral natural products from the marine-derived Penicillium sp. IMB17-046. Molecules (Basel, Switzerland) 2019;24(15):2821. doi: 10.3390/molecules24152821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.M., Anderson H., Flavin M.T., Pai Y.H.S., Mata-Greenwood E., Pengsuparp T., Pezzuto J.M., Schinazi R.F., Hughes S.H., Chen F.C. In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997;60(9):884–888. doi: 10.1021/np9700275. [DOI] [PubMed] [Google Scholar]
- Lin Y.M., Flavin M.T., Schure R., Chen F.C., Sidwell R., Barnard D.I., Huffmann J.H., Kern E.R. Antiviral activities of biflavonoids. Planta Med. 1999;65(2):120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- Lin L.C., Kuo Y.C., Chou C.J. Anti-Herpes Simplex Virus Type-1 flavonoids and a new flavanone from the root of Limonium sinense. Planta Med. 2000;66(04):333–336. doi: 10.1055/s-2000-8540. [DOI] [PubMed] [Google Scholar]
- Lin H.H., Yip B.S., Huang L.M., Wu S.C. Zika virus structural biology and progress in vaccine development. Biotechnol. Adv. 2018;36(1):47–53. doi: 10.1016/j.biotechadv.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Liu J.S., Li L. Kadsulignans L-N, three dibenzocyclooctadiene lignans from Kadsura coccinea. Phytochemistry. 1995;38(1):241–245. doi: 10.1016/0031-9422(94)00557-a. [DOI] [Google Scholar]
- Liu X., Gan M., Dong B., Zhang T., Li Y., Zhang Y., Fan X., Wu Y., Bai S., Chen M., Yu L., Tao P., Jiang W., Si S. 4862F, a new inhibitor of HIV-1 protease, from the culture of Streptomyces I03A-04862. Molecules (Basel, Switzerland) 2012;18(1):236–243. doi: 10.3390/molecules18010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Frank M., Yu X., Yu H., Tran-Cong N.M., Gao Y., Proksch P. Progress in the Chemistry of Organic Natural Products. vol. 111. Springer International Publishing; 2020. Secondary metabolites from marine-derived fungi from China; pp. 81–153. [DOI] [PubMed] [Google Scholar]
- Luo C., Liu X., Zhou X., Guo J., Truong J., Wang X., Zhou H., Li X., Chen Z. Unusual biosynthesis and structure of Locillomycins from Bacillus subtilis 916. Appl. Environ. Microbiol. 2015;81(19):6601–6609. doi: 10.1128/AEM.01639-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Nakamura N., Hattori M., Kakuda H., Qiao J., Yu H. Inhibitory effects on HIV-1 protease of constituents from the wood of Xanthocerassor bifolia. J. Nat. Prod. 2000;63(2):238–242. doi: 10.1021/np9902441. [DOI] [PubMed] [Google Scholar]
- Mahmood N., Pizza C., Aquino R., De Tommasi N., Piacente S., Colman S., Burke A., Hay A.J. Inhibition of HIV infection by flavanoids. Antivir. Res. 1993;22(2–3):189–199. doi: 10.1016/0166-3542(93)90095-z. [DOI] [PubMed] [Google Scholar]
- Manske R.F., Brossi A. Academic Press; London: 1985. The Alkaloids 24; pp. 153–154. [Google Scholar]
- Manske R.F., Brossi A. Academic Press; London: 1987. The Alkaloids 15; pp. 83–164. [Google Scholar]
- Manske R.F., Brossi A. Academic Press; London: 1988. The Alkaloids 18; pp. 99–216. [Google Scholar]
- Manske R.F., Brossi A. Academic Press; London: 1989. The Alkaloids 34; pp. 211–239. [Google Scholar]
- Manske R.F., Brossi A. Academic Press; London: 1990. The Alkaloids 36; pp. 135–170. [Google Scholar]
- Marchetti M., Pisani S., Pietropaolo V., Seganti L., Nicoletti R., Degener A., Orsi N. Antiviral effect of a polysaccharide from Sclerotium glucanicum towards Herpes Simplex Virus Type 1 infection. Planta Med. 1996;62(04):303–307. doi: 10.1055/s-2006-957889. [DOI] [PubMed] [Google Scholar]
- Martinez J.P., Hinkelmann B., Fleta-Soriano E., Steinmetz H., Jansen R., Diez J., Frank R., Sasse F., Meyerhans A. Identification of myxobacteria-derived HIV inhibitors by a high-throughput two-step infectivity assay. Microb. Cell Factories. 2013;12:85. doi: 10.1186/1475-2859-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S., Nabeshima S., Ajisaka K., Yamauchi K., Itoh R., Ishii K., Soejima T., Hiromatsu K. Maoto, a traditional Japanese herbal medicine, inhibits uncoating of influenza virus. Evidence-Based Complementary and Alternative Medicine: ECAM. 2017 doi: 10.1155/2017/1062065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee T.C., Bokesch H.R., McCormick J.L., Rashid M.A., Spielvogel D., Gustafson K.R., Alavanja M.M., Cardellina J.H., Boyd M.R. Isolation and characterization of new anti-HIV and cytotoxic leads from plants, marine, and microbial organisms. J. Nat. Prod. 1997;60(5):431–438. doi: 10.1021/np970031g. [DOI] [PubMed] [Google Scholar]
- Mengshol J.A., Golden-Mason L., Arikawa T., Smith M., Niki T., McWilliams R., Randall J.A., McMahan R., Zimmerman M.A., Rangachari M., Dobrinskikh E., Busson P., Polyak S.J., Hirashima M., Rosen H.R. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5(3):e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin B.J. Academic Press; New York: 1981. Biochemistry of Plants 5; p. 345. [Google Scholar]
- Miller P.A., Milstrey K.P., Trown P.W. Specific inhibition of viral ribonucleic acid replication by gliotoxin. Science. 1968;159(3813):431–432. doi: 10.1126/science.159.3813.431. [DOI] [PubMed] [Google Scholar]
- Min B.S., Nakamura N., Miyashiro H., Bae K.W., Hattori M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998;46(10):1607–1612. doi: 10.1248/cpb.46.1607. [DOI] [PubMed] [Google Scholar]
- Minagawa K., Kouzuki S., Yoshimoto J.U.N., Kawamura Y., Tani H., Iwata T., Terui Y., Nakai H., Yagi S., Hattori N., Fujiwara T., Kamigauchi T. Stachyflin and Acetylstachyflin, novel anti-influenza A virus substances, produced by Stachybotrys sp. RF-7260. Isolation, structure elucidation and biological activities. J. Antibiot. 2002;55(2):155–164. doi: 10.7164/antibiotics.55.155. [DOI] [PubMed] [Google Scholar]
- Mir M.A. Hantaviruses. Clin. Lab. Med. 2010;30(1):67–91. doi: 10.1016/j.cll.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K.P., Sharma N., Diwaker D., Ganju L., Singh S.B. Plant derived antivirals: a potential source of drug development. Journal of Virology and Antiviral Research. 2013;2:2–9. [Google Scholar]
- Miyakawa T., Anzai O., Shimizu N. Studies on antiviral antibiotics from streptomyces. Japanese Journal of Microbiology. 1958;2(1):53–62. doi: 10.1111/j.1348-0421.1958.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Miyanaga S., Sakurai H., Saiki I., Onaka H., Igarashi Y. Synthesis and evaluation of myxochelin analogues as antimetastatic agents. Bioorganic and Medicinal Chemistry. 2009;17(7):2724–2732. doi: 10.1016/j.bmc.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Mlinaric A., Kac J., Pohleven F. Screening of selected wood-damaging fungi for the HIV-1 reverse transcriptase inhibitors. Acta Pharma. 2005;55:69–79. [PubMed] [Google Scholar]
- Moghadamtousi S.Z., Nikzad S., Kadir H.A., Abubakar S., Zandi K. Potential antiviral agents from marine fungi: an overview. Marine Drugs. 2015;13(7):4520–4538. doi: 10.3390/md13074520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mølgaard P., Ravn H. Evolutionary aspects of caffeoyl ester distribution in dicotyledons. Phytochemistry. 1988;27(8):2411–2421. doi: 10.1016/0031-9422(88)87005-5. [DOI] [Google Scholar]
- Montanha J., Amoros M., Boustie J., Girre L. Anti-herpes virus activity of aporphine alkaloids. Planta Med. 1995;61(05):419–424. doi: 10.1055/s-2006-958128. [DOI] [PubMed] [Google Scholar]
- Mothana R.A.A., Awadh Ali N.A., Jansen R., Wegner U., Mentel R., Lindequist U. Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi. Fitoterapia. 2003;74(1–2):177–180. doi: 10.1016/s0367-326x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- Mukherjee P.K., Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J. Ethnopharmacol. 2006;103(1):25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Mulwa L.S., Jansen R., Praditya D.F., Mohr K.I., Wink J., Steinmann E., Stadler M. Six heterocyclic metabolites from the myxobacterium Labilithrix luteola. Molecules (Basel, Switzerland) 2018;23(3):542. doi: 10.3390/molecules23030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz C., Lünemann J.D., Getts M.T., Miller S.D. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat. Rev. Immunol. 2009;9(4):246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A., Tanaka S., Ohigashi H., Hirota M., Irie R., Takeda N., Tatematsu A., Koshimizu K. Possible anti-tumour promoters: BI- and tetraflavonoids from Lophira alata. Phytochemistry. 1992;31(8):2689–2693. doi: 10.1016/0031-9422(92)83612-3. [DOI] [PubMed] [Google Scholar]
- Murray R.D.H., Mendez J., Brown L. Wiley; Chichester: 1982. The Natural Coumarins. [Google Scholar]
- Nachbagauer R., Krammer F. Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect. 2017;23(4):222–228. doi: 10.1016/j.cmi.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadmid S., Plaza A., Lauro G., Garcia R., Bifulco G., Müller R. Hyalachelins A–C, unusual siderophores isolated from the terrestrial myxobacterium Hyalangium minutum. Org. Lett. 2014;16(16):4130–4133. doi: 10.1021/ol501826a. [DOI] [PubMed] [Google Scholar]
- Nakagawa A., Tomoda H., Hao M., Okano K., Iwai Y., Omura S. Antiviral activities of pentalenolactones. J. Antibiot. 1985;38(8):1114–1115. doi: 10.7164/antibiotics.38.1114. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Kido Y., Kobayashi N., Motoki Y., Neushul M., Yamamoto N. Antiretroviral activity in a marine red alga: reverse transcriptase inhibition by an aqueous extract of Schizymenia pacifica. J. Cancer Res. Clin. Oncol. 1987;113(5):413–416. doi: 10.1007/bf00390034. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Kido Y., Kobayashi N., Motoki Y., Neushul M., Yamamoto N. Purification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob. Agents Chemother. 1987;31(10):1524–1528. doi: 10.1128/aac.31.10.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T.B., Wang H. Panaxagin, a new protein from Chinese ginseng possesses anti-fungal, anti-viral, translation-inhibiting and ribonuclease activities. Life Sci. 2001;68(7):739–749. doi: 10.1016/s0024-3205(00)00970-x. [DOI] [PubMed] [Google Scholar]
- Nkongolo S., Ni Y., Lempp F.A., Kaufman C., Lindner T., Esser-Nobis K., Lohmann V., Mier W., Mehrle S., Urban S. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J. Hepatol. 2014;60(4):723–731. doi: 10.1016/j.jhep.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Nong X.H., Wang Y.F., Zhang X.Y. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus. Mar. Drugs. 2014;12:6113–6124. doi: 10.3390/md12126113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M.R.T., Faria N.R., de Vasconcelos J.M., Golding N., Kraemer M.U.G., de Oliveira L.F., Azevedo R.S.S., da Silva D.E.A., da Silva E.V.P., da Silva S.P., Carvalho V.L., Coelho G.E., Cruz A.C.R., Rodrigues S.G., Vianez J.L.S.G., Jr., Nunes B.T.D., Cardoso J.F., Tesh R.B., Hay S.I., Vasconcelos P.F.C. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med. 2015;13:102. doi: 10.1186/s12916-015-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omarsdottir S., Olafsdottir E.S., Freysdottir J. Immunomodulating effects of lichen-derived polysaccharides on monocyte-derived dendritic cells. Int. Immunopharmacol. 2006;6(11):1642–1650. doi: 10.1016/j.intimp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Ongena M., Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16(3):115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Pandey M.M., Rastogi S., Rawat A.K.S. Indian herbal drug for general healthcare: an overview. The Internet Journal of Alternative Medicine. 2008;6(1):3. [Google Scholar]
- Pariš A., Štrukelj B., Renko M., Turk V., Pukl M., Umek A., Korant B.D. Inhibitory effect of carnosolic acid on HIV-1 protease in cell-free assays. J. Nat. Prod. 1993;56(8):1426–1430. doi: 10.1021/np50098a031. [DOI] [PubMed] [Google Scholar]
- Parrish C.R., Holmes E.C., Morens D.M., Park E.C., Burke D.S., Calisher C.H., Laughlin C.A., Saif L.J., Daszak P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008;72(3):457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoalino B.S., Courtemanche G., Cordeiro M.T., Gil L.H.V.G., Freitas-Junior L. Zika antiviral chemotherapy: identification of drugs and promising starting points for drug discovery from an FDA-approved library. F1000Research. 2016;5:2523. doi: 10.12688/f1000research.9648.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patankar M.S., Oehninger S., Barnett T., Williams R.L., Clark G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993;268(29):21770–21776. [PubMed] [Google Scholar]
- Patel N., Penkert R.R., Jones B.G., Sealy R.E., Surman S.L., Sun Y., Tang L., DeBeauchamp J., Webb A., Richardson J., Heine R., Dallas R.H., Ross A.C., Webby R., Hurwitz J.L. Baseline serum vitamin A and D levels determine benefit of oral vitamin A&D supplements to humoral immune responses following pediatric influenza vaccination. Viruses. 2019;11(10):907. doi: 10.3390/v11100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil A.D., Freyer A.J., Eggleston D.S., Haltiwanger R.C., Bean M.F., Taylor P.B., Caranfa M.J., Breen A.L., Bartus H.R. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993;36(26):4131–4138. doi: 10.1021/jm00078a001. [DOI] [PubMed] [Google Scholar]
- Patwardhan B., Warude D., Pushpangadan P., Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid. Based Complement. Alternat. Med. 2005;2(4):465–473. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic V., Stojanovic I., Jadranin M., Vajs V., Djordjević I., Smelcerovic A., Stojanovic G. Effect of four lichen acids isolated from Hypogymnia physodes on viability of rat thymocytes. Food Chem. Toxicol. 2013;51:160–164. doi: 10.1016/j.fct.2012.04.043. [DOI] [PubMed] [Google Scholar]
- Peng J., Lin T., Wang W., Xin Z., Zhu T., Gu Q., Li D. Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. J. Nat. Prod. 2013;76(6):1133–1140. doi: 10.1021/np400200k. [DOI] [PubMed] [Google Scholar]
- Peng J., Zhang X., Du L., Wang W., Zhu T., Gu Q., Li D. Sorbicatechols A and B, antiviral Sorbicillinoids from the marine-derived fungus Penicillium chrysogenum PJX-17. J. Nat. Prod. 2014;77(2):424–428. doi: 10.1021/np400977e. [DOI] [PubMed] [Google Scholar]
- Pengsuparp T., Cai L., Constant H., Fong H.H.S., Lin L.Z., Kinghorn A.D., Pezzuto J.M., Cordell G.A., Ingolfsdöttir K., Wagner H., Hughes S.H. Mechanistic evaluation of new plant-derived compounds that inhibit HIV-1 reverse transcriptase. J. Nat. Prod. 1995;58(7):1024–1031. doi: 10.1021/np50121a006. [DOI] [PubMed] [Google Scholar]
- Pepin K.M., Riley S., Grenfell B.T. Effects of influenza antivirals on individual and population immunity over many epidemic waves. Epidemiol. Infect. 2013;141(2):366–376. doi: 10.1017/S0950268812000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H., Leaoferreira L., Moussatche N., Teixeira V., Cavalcanti D., Costa L., Diaz R., Frugulhetti I. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1) Antivir. Res. 2004;64(1):69–76. doi: 10.1016/s0166-3542(04)00122-6. [DOI] [PubMed] [Google Scholar]
- Phillipson J.D., Zenk M.H. Academic Press; London: 1980. Indole and Biogenetically Related Alkaloids. [Google Scholar]
- Piattelli M., Tringali C., Neri P., Rocco C. Stereochemistry and conformation of dolabellane diterpenes: an NMR and molecular mechanics study. J. Nat. Prod. 1995;58(5):697–704. doi: 10.1021/np50119a007. [DOI] [Google Scholar]
- Pilcher K.S., Soike K.F., Smith V.H., Trosper F., Folston B. Inhibition of multiplication of Lee influenza virus by canavanine. Exp. Biol. Med. 1955;88(1):79–86. doi: 10.3181/00379727-88-21498. [DOI] [PubMed] [Google Scholar]
- Porter L.J. Methods in Plant Biochemistry. Elsevier; 1989. Tannins; pp. 389–419. [DOI] [Google Scholar]
- Prieto C., Castro J.M. Porcine reproductive and respiratory syndrome virus infection in the boar: a review. Theriogenology. 2005;63(1):1–16. doi: 10.1016/j.theriogenology.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Qi-Hu C., Chen K., Shi Q., Kilkuskie R., Chi-Cheng Y., Lee Y.J. Anti-AIDS agent 10. Acacetin-7-O-β-D-galactopyranoside, an anti-HIV principle from Chrysanthemum morifolium and a structure-activity correlation with some related flavonoids. J. Nat. Prod. 1994;57:42–51. doi: 10.1021/np50103a006. [DOI] [PubMed] [Google Scholar]
- Qin C., Lin X., Lu X., Wan J., Zhou X., Liao S., Tu Z., Xu S., Liu Y. Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2. J. Antibiot. 2014;68(2):121–125. doi: 10.1038/ja.2014.97. [DOI] [PubMed] [Google Scholar]
- Raekiansyah M., Mori M., Nonaka K., Agoh M., Shiomi K., Matsumoto A., Morita K. Identification of novel antiviral of fungus-derived brefeldin A against dengue viruses. Tropical Medicine and Health. 2017;45:32. doi: 10.1186/s41182-017-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamohan F., Venkatachalam T.K., Irvin J.D., Uckun F.M. Pokeweed antiviral protein isoforms PAP-I, PAP-II, and PAP-III depurinate RNA of human immunodeficiency virus (HIV)-1. Biochem. Biophys. Res. Commun. 1999;260(2):453–458. doi: 10.1006/bbrc.1999.0922. [DOI] [PubMed] [Google Scholar]
- Rameshthangam P., Ramasamy P. Antiviral activity of bis(2-methylheptyl)phthalate isolated from Pongamia pinnata leaves against White Spot syndrome virus of Penaeus monodon Fabricius. Virus Res. 2007;126(1–2):38–44. doi: 10.1016/j.virusres.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Rao G.S., Cochran K.W. Antiviral activity of triterpenoid saponins containing acylated b-amyrin aglycones. J. Pharm. Sci. 1974;63:471–473. doi: 10.1002/jps.2600630341. [DOI] [PubMed] [Google Scholar]
- Rashan L.J. In vitro study of the antiviral activity of some b-carboline alkaloids. Fitoterapia LXI. 1990:153–155. [Google Scholar]
- Rathore A., Rivastava V., Srivastava K.C., Tandon J.S. Iridoid glucosides from Nyctanthes arbor-tristis. Phytochemistry. 1990;29(6):1917–1920. doi: 10.1016/0031-9422(90)85040-m. [DOI] [Google Scholar]
- Rausch K., Hackett B.A., Weinbren N.L., Reeder S.M., Sadovsky Y., Hunter C.A., Schultz D.C., Coyne C.B., Cherry S. Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against Zika virus. Cell Rep. 2017;18(3):804–815. doi: 10.1016/j.celrep.2016.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveh A., Delekta P.C., Dobry C.J., Peng W., Schultz P.J., Blakely P.K., Tai A.W., Matainaho T., Irani D.N., Sherman D.H., Miller D.J. Discovery of potent broad spectrum antivirals derived from marine actinobacteria. PLoS One. 2013;8(12):e82318. doi: 10.1371/journal.pone.0082318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach H. Myxobacteria: a source of new antibiotics. Trends Biotechnol. 1988;6(6):115–121. doi: 10.1016/0167-7799(88)90100-x. [DOI] [Google Scholar]
- Reis S.A., Moussatche N., Damaso C.R.A. FK506, a secondary metabolite produced by Streptomyces, presents a novel antiviral activity against Orthopoxvirus infection in cell culture. J. Appl. Microbiol. 2006;100(6):1373–1380. doi: 10.1111/j.1365-2672.2006.02855.x. [DOI] [PubMed] [Google Scholar]
- Rembold H. Economic and Medicinal Plant Research. Elsevier; 1989. The azadirachtins- their potential for insect control; pp. 57–72. [DOI] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincão V.P., Yamamoto K.A., Ricardo N.M.P.S., Soares S.A., Meirelles L.D.P., Nozawa C., Linhares R.E.C. Polysaccharide and extracts from Lentinula edodes: structural features and antiviral activity. Virol. J. 2012;9:37. doi: 10.1186/1743-422X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux L.-E., Turgeon S.L., Beaulieu M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry. 2010;71(13):1586–1595. doi: 10.1016/j.phytochem.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Roccatagliata A.J., Maier M.S., Seldes A.M., Pujol C.A., Damonte E.B. Antiviral sulfated steroids from the ophiuroid Ophioplocus januarii. J. Nat. Prod. 1996;59(9):887–889. doi: 10.1021/np960171a. [DOI] [PubMed] [Google Scholar]
- Rouhier P., Kopp M., Begot V., Bruneteau M., Fritig B. Structural features of fungal β-D-glucans for the efficient inhibition of the initiation of virus infection on Nicotiana tabacum. Phytochemistry. 1995;39(1):57–62. doi: 10.1016/0031-9422(94)00852-k. [DOI] [PubMed] [Google Scholar]
- Rowley D.C., Kelly S., Kauffman C.A., Jensen P.R., Fenical W. Halovirs A-E, new antiviral agents from a marine-derived fungus of the genus Scytalidium. ChemInform. 2003;35(6) doi: 10.1002/chin.200406159. [DOI] [PubMed] [Google Scholar]
- Rowley D.C., Kelly S., Kauffman C.A., Jensen P.R., Fenical W. Synthesis and structure–activity relationships of the halovirs, antiviral natural products from a marine-derived fungus. Bioorganic and Medicinal Chemistry. 2004;12(18):4929–4936. doi: 10.1016/j.bmc.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Rwangabo P., Laekeman G., Claeys M., Totté J., Pieters L., Berghe D., Herman A., Vlietinck A. Phytochemical- and pharmacological investigation of the biologically active fraction from the flowers of Vernonia amygdalina. Planta Med. 1986;52(06):547–548. doi: 10.1055/s-2007-969351. [DOI] [PubMed] [Google Scholar]
- Sacramento C.Q., Marttorelli A., Fintelman-Rodrigues N., de Freitas C.S., de Melo G.R., Rocha M.E.N., Kaiser C.R., Rodrigues K.F., da Costa G.L., Alves C.M., Santos-Filho O., Barbosa J.P., Souza T.M.L. Aureonitol, a fungi-derived tetrahydrofuran, inhibits influenza replication by targeting its surface glycoprotein hemagglutinin. PLoS One. 2015;10(10):e0139236. doi: 10.1371/journal.pone.0139236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez I., Gomez-Garibay F., Taboada J., Ruiz B.H. Antiviral effect of flavonoids on the dengue virus. Phytother. Res. 2000;14(2):89–92. doi: 10.1002/(sici)1099-1573(200003)14:2<89::aid-ptr569>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Santoyo S., Jaime L., Plaza M., Herrero M., Rodriguez-Meizoso I., Ibañez E., Reglero G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2011;24(4):731–741. doi: 10.1007/s10811-011-9692-1. [DOI] [Google Scholar]
- Sauerbrei A. Herpes genitalis: diagnosis, treatment and prevention. Geburtshilfe Frauenheilkd. 2016;76(12):1310–1317. doi: 10.1055/s-0042-116494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A., Späth J., Breiding-Mack S., Zeeck A., Grabley S., Thiericke R. New cineromycins and musacins obtained by metabolite pattern analysis of Streptomyces griseoviridis (FH-S 1832). II. Structure elucidation. J. Antibiot. 1996;49(5):438–446. doi: 10.7164/antibiotics.49.438. [DOI] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwetz T.A., Fauci A.S. The extended impact of human immunodeficiency virus/AIDS research. J. Infect. Dis. 2019;219(1):6–9. doi: 10.1093/infdis/jiy441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendl A., Chen J.L., Jolad S.D., Stoddart C., Rozhon E., Kernan M., Nanakorn W., Balick M. Two new naphthoquinones with antiviral activity from Rhinacanthus nasutus. J. Nat. Prod. 1996;59(8):808–811. doi: 10.1021/np9601871. [DOI] [PubMed] [Google Scholar]
- Serkedjieva J., Manolova N. Plant Polyphenols. Springer; US: 1992. Plant polyphenolic complex inhibits the reproduction of influenza and herpes simplex viruses; pp. 705–715. [DOI] [PubMed] [Google Scholar]
- Shtro A.A., Zarubaev V.V., Luzina O.A., Sokolov D.N., Kiselev O.I., Salakhutdinov N.F. Novel derivatives of usnic acid effectively inhibiting reproduction of influenza A virus. Bioorg. Med. Chem. 2014;22(24):6826–6836. doi: 10.1016/j.bmc.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Shtro A.A., Zarubaev V.V., Luzina O.A., Sokolov D.N., Salakhutdinov N.F. Derivatives of usnic acid inhibit broad range of influenza viruses and protect mice from lethal influenza infection. Antiviral Chemistry and Chemotherapy. 2015;24(3–4):92–98. doi: 10.1177/2040206616636992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shushni M.A.M., Singh R., Mentel R., Lindequist U. Balticolid: a new 12-membered macrolide with antiviral activity from an ascomycetous fungus of marine origin. Marine Drugs. 2011;9(5):844–851. doi: 10.3390/md9050844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simöes C.M.O., Amoros M., Girre L., Gleye J., Fauvel M.T. Antiviral activity of ternatin and meliternatin, 3-methoxyflavones from species of Rutaceae. J. Nat. Prod. 1990;53(4):989–992. doi: 10.1021/np50070a036. [DOI] [Google Scholar]
- Singh S.B., Zink D., Polishook J., Valentino D., Shafiee A., Silverman K., Felock P., Teran A., Vilella D., Hazuda D.J., Lingham R.B. Structure and absolute stereochemistry of HIV-1 integrase inhibitor integric acid. A novel eremophilane sesquiterpenoid produced by a Xylaria sp. Tetrahedron Lett. 1999;40(50):8775–8779. doi: 10.1016/s0040-4039(99)01878-x. [DOI] [Google Scholar]
- Singh R.K., Dhama K., Chakraborty S., Tiwari R., Natesan S., Khandia R., Munjal A., Vora K.S., Latheef S.K., Karthik K., Singh Malik Y., Singh R., Chaicumpa W., Mourya D.T. Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies - a comprehensive review. The Veterinary Quarterly. 2019;39(1):26–55. doi: 10.1080/01652176.2019.1580827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D.F., Bailey K.W., Wong M.-H., O’Keefe B.R., Gustafson K.R., Mishin V.P., Gubareva L.V. Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin-N. Antivir. Res. 2008;80(3):266–271. doi: 10.1016/j.antiviral.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov D.N., Kiselev O.I., Salakhutdinov N.F. Bioorganic and medicinal chemistry novel derivatives of usnic acid effectively inhibiting reproduction of influenza A virus. Bioorganic and Medicinal Chemistry. 2014;22(24):6826–6836. doi: 10.1016/j.bmc.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Sotanaphun U., Lipipun V., Suttisri R., Bavovada R. A new antiviral and antimicrobial sesquiterpene from Glyptopetalum sclerocarpum. Planta Med. 1999;65(03):257–258. doi: 10.1055/s-2006-960472. [DOI] [PubMed] [Google Scholar]
- Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101(2):87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- Stocker-Wörgötter E. Metabolic diversity of lichen-forming ascomycetous fungi: culturing, polyketide and shikimate metabolite production, and PKS genes. Nat. Prod. Rep. 2008;25(1):188–200. doi: 10.1039/b606983p. [DOI] [PubMed] [Google Scholar]
- Stuyver L., Rossau R., Zoulim F., Fried M., De Gendt S., Schinazi R.F., Van Geyt C. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J. Gen. Virol. 2000;81(1):67–74. doi: 10.1099/0022-1317-81-1-67. [DOI] [PubMed] [Google Scholar]
- Subhose V., Srinivas P., Narayana A. Basic principles of pharmaceutical science in Ayurveda. Bulletin of the Indian Institute of History of Medicine. 2005;35(2):83–92. [PubMed] [Google Scholar]
- Suganda A.G., Amoros M., Girre L., Fauconnier B. Effets Inhibiteurs de Quelques Extraites Bruts et Semi Purifiés de Plantes Indigènes Françaises sur la Multiplication de l’Herpesvirus Humain 1 et du Poliovirus Humain 2 en Culture Cellulaire. J. Nat. Prod. 1983;46(5):626–632. doi: 10.1021/np50029a006. [DOI] [PubMed] [Google Scholar]
- Sun H., Qiu S., Lin L., Wang Z., Lin Z., Pengsuparp T., Pezzuto J.M., Fong H.H.S., Cordell G.A., Farnsworth N.R. Nigranoic acid, a triterpenoid from Schisandra sphaerandra that inhibits HIV-1 reverse transcriptase. J. Nat. Prod. 1996;59(5):525–527. doi: 10.1021/np960149h. [DOI] [PubMed] [Google Scholar]
- Suthindhiran K., Sarath Babu V., Kannabiran K., Ishaq Ahmed V.P., Sahul Hameed A.S. Anti-fish nodaviral activity of furan-2-yl acetate extracted from marine Streptomyces spp. Nat. Prod. Res. 2011;25(8):834–843. doi: 10.1080/14786419.2010.530599. [DOI] [PubMed] [Google Scholar]
- Swallow D.L., Sc M.A., Fric P. Antiviral agents. Prog. Drug Res. 1975;22:267–270. doi: 10.1007/978-3-0348-7102-0_6. [DOI] [PubMed] [Google Scholar]
- Sydiskis R.J., Owen D.G., Lohr J.L., Rosler K.H., Blomster R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 1991;35(12):2463–2466. doi: 10.1128/aac.35.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Okuno K., Chiba K., Ohnishi E., Yoshii T. Antiviral diterpenes from Salvia officinalis. Phytochemistry. 1994;35(2):539–541. doi: 10.1016/s0031-9422(00)94798-8. [DOI] [Google Scholar]
- Tafur S., Nelson Y., DeLong D., Gordon H. Antiviral components of Ophiorrhiza mungos. Lloydia. 1976;39:261–262. [PubMed] [Google Scholar]
- Takagi M., Motohashi K., Nagai A., Izumikawa M., Tanaka M., Fuse S., Doi T., Iwase K., Kawaguchi A., Nagata K., Takahashi T., Shin-ya K. Anti-influenza virus compound from Streptomyces sp. RI18. Org. Lett. 2010;12(20):4664–4666. doi: 10.1021/ol102007d. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Inokoshi J., Chiba H., Omura S., Tanaka H. Essential regions for antiviral activities of actinohivin, a sugar-binding anti-human immunodeficiency virus protein from an actinomycete. Arch. Biochem. Biophys. 2005;437(2):233–240. doi: 10.1016/j.abb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Takaishi Y., Ohshima S., Nakano K., Tomimatsu T., Tokuda H., Nishino H., Iwashima A. Structures of Sesquiterpene polyol esters from Celastrus stephanotiifolius with potential tumor-promotion inhibitory activity. J. Nat. Prod. 1993;56(6):815–824. doi: 10.1021/np50096a003. [DOI] [PubMed] [Google Scholar]
- Takasaki M., Konoshima T., Shingu T., Tokuda H., Nishino H., Iwashima A., Kozuka M. Structures of euglobal-G1, -G2, and -G3 from Eucalyptus grandis, three new inhibitors of Epstein- Barr virus activation. Chem. Pharm. Bull. 1990;38(5):1444–1446. doi: 10.1248/cpb.38.1444. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Saucedo C.J., Lund G., Uenishi R., Hase S., Tsuchiura T., Kneteman N., Ramessar K., Tyrrell D.L.J., Shirakura M., Wakita T., McMahon J.B., O’Keefe B.R. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS One. 2013;8(5):e64449. doi: 10.1371/journal.pone.0064449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takechi M., Tanaka Y. Purification and characterization of antiviral substance from the bud of Syzygium aromatica. Planta Med. 1981;42(05):69–74. doi: 10.1055/s-2007-971548. [DOI] [PubMed] [Google Scholar]
- Takechi M., Tanaka Y. Antiviral substances from the root of Paeonia species. Planta Med. 1982;45(08):252–253. doi: 10.1055/s-2007-971387. [DOI] [PubMed] [Google Scholar]
- Takemura Y., Ju-ichi M., Ito C., Furukawa H., Tokuda H. Studies on the inhibitory effects of some acridone alkaloids on Epstein-Barr virus activation. Planta Med. 1995;61(04):366–368. doi: 10.1055/s-2006-958104. [DOI] [PubMed] [Google Scholar]
- Talavera Pons S., Boyer A., Lamblin G., Chennell P., Châtenet F.-T., Nicolas C., Sautou V., Abergel A. Managing drug-drug interactions with new direct-acting antiviral agents in chronic hepatitis C. Br. J. Clin. Pharmacol. 2017;83(2):269–293. doi: 10.1111/bcp.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.T., Pezzuto J.M., Kinghorn A.D., Hughes S.H. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J. Nat. Prod. 1991;54(1):143–154. doi: 10.1021/np50073a012. [DOI] [PubMed] [Google Scholar]
- Tanabe-Tochikura A., Tochikura T.S., Yoshida O., Oki T., Yamamoto N. Pradimicin A inhibition of human immunodeficiency virus: attenuation by mannan. Virology. 1990;176(2):467–473. doi: 10.1016/0042-6822(90)90016-k. [DOI] [PubMed] [Google Scholar]
- Taylor R.S.L., Manandhar N.P., Hudson J.B., Towers G.H.N. Antiviral activities of Nepalese medicinal plants. J. Ethnopharmacol. 1996;52(3):157–163. doi: 10.1016/0378-8741(96)01409-2. [DOI] [PubMed] [Google Scholar]
- Teixeira R.R., Pereira W.L., Oliveira A.F.C., da Silva A.M., de Oliveira A.S., da Silva M.L., da Silva C.C., de Paula S.O. Natural products as source of potential dengue antivirals. Molecules (Basel, Switzerland) 2014;19(6):8151–8176. doi: 10.3390/molecules19068151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasy S.M., Maggs D.J. A review of antiviral drugs and other compounds with activity against feline herpesvirus type 1. Vet. Ophthalmol. 2016;19 Suppl 1(Suppl. 1):119–130. doi: 10.1111/vop.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuan N.H., An T.T., Shrestha A., Canh N.X., Sohng J.K., Dhakal D. Recent advances in exploration and biotechnological production of bioactive compounds in three cyanobacterial genera: Nostoc, Lyngbya, and Microcystis. Frontiers in Chemistry. 2019;7:604. doi: 10.3389/fchem.2019.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi N., Pizza C., Conti C., Orsi N., Stein M.L. Structure and in vitro antiviral activity of sesquiterpene glycosides from Calendula arvensis. J. Nat. Prod. 1990;53:830–835. doi: 10.1021/np50070a009. [DOI] [PubMed] [Google Scholar]
- Tommasi N., De Simone F., Pizza C., Mahmood N., Moore P., Conti C., Orsi N., Stein M. Constituents of Eriobotrya japonica. A study of their antiviral propierties. J. Nat. Prod. 1992;55:1067–1073. doi: 10.1021/np50086a006. [DOI] [PubMed] [Google Scholar]
- Towers G.H.N. Photosensitisers from plants and their photodynamic action. Prog. Phytochem. 1989;6:183–189. [Google Scholar]
- Towers G.H.N., Page J., Hudson J.B. New aspects of light-mediated activities of natural products from plants and fungi. Curr. Org. Chem. 1997;1:395–414. [Google Scholar]
- Trowitzsch-Kienast W., Forche E., Wray V., Reichenbach H., Jurkiewicz E., Hunsmann G., Höfle G. Antibiotika aus Gleitenden Bakterien, 45. Phenalamide, neue HIV-1-inhibitor en ausMyxococcus stipitatus Mx s40. Liebigs Annalen Der Chemie. 1992;7:659–664. doi: 10.1002/jlac.1992199201112. [DOI] [Google Scholar]
- Tschesche R., Hess B., Ziegler I., Machleidt H. Über Pteridine, XVII. Trennung von synthetischem Biopterin und Isobiopterin. Justus Liebigs Annalen Der Chemie. 1962;658(1):193–201. doi: 10.1002/jlac.19626580117. [DOI] [Google Scholar]
- Tsuchiya Y., Shimizu M., Hiyama Y., Itoh K., Hashimoto Y., Nakayama M., Horie T., Morita N. Antiviral activity of natural occurring flavonoids in vitro. Chem. Pharm. Bull. 1985;33(9):3881–3886. doi: 10.1248/cpb.33.3881. [DOI] [PubMed] [Google Scholar]
- Ueda S., Iwahashi Y., Tokuda H. Production of anti-tumor-promoting iridoid glucosides in Genipa americana and its cell cultures. J. Nat. Prod. 1991;54(6):1677–1680. doi: 10.1021/np50078a032. [DOI] [PubMed] [Google Scholar]
- Urones J.G., Basabe P., Marcos I.S., Pineda J., Lithgow A.M., Moro R.F., Brito Palma F.M.S., Arau’jo M.E.M., Gravalos M.D.G. Meroterpenes from Cystoseira usneoides. Phytochemistry. 1992;31(1):179–182. doi: 10.1016/0031-9422(91)83031-f. [DOI] [Google Scholar]
- Van Hoof L., Totté J., Corthout J., Pieters L.A., Mertens F., Vanden Berghe D.A., Vlietinck A.J., Dommisse R., Esmans E. Plant antiviral agents, VI. Isolation of antiviral phenolic glucosides from populus cultivar beaupre by droplet counter-current chromatography. J. Nat. Prod. 1989;52(4):875–878. doi: 10.1021/np50064a038. [DOI] [PubMed] [Google Scholar]
- Van Sumere C.F. Methods in Plant Biochemistry. Elsevier; 1989. Phenols and phenolic acids; pp. 29–73. [DOI] [Google Scholar]
- Vera J., Castro J., Gonzalez A., Moenne A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Marine Drugs. 2011;9(12):2514–2525. doi: 10.3390/md9122514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vil V., Terent’ev A.O., Al Quntar A.A.A., Gloriozova T.A., Savidov N., Dembitsky V.M. Oxetane-containing metabolites: origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 2019;103(6):2449–2467. doi: 10.1007/s00253-018-09576-z. [DOI] [PubMed] [Google Scholar]
- Villa T.G., Feijoo-Siota L., Rama J.L.R., Ageitos J.M. Antivirals against animal viruses. Biochem. Pharmacol. 2017;133:97–116. doi: 10.1016/j.bcp.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlietinck A.J., Bruyne D., Berghe V. In: Current Organic Chemistry, Natural Product Chemistry Issue. Atta-ur-Rahman, editor. Benthem Science Publishers; The Netherlands: 1997. Plant substances as antiviral agents; pp. 320–326. [Google Scholar]
- Vlietinck A., De Bruyne T., Apers S., Pieters L. Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med. 1998;64(02):97–109. doi: 10.1055/s-2006-957384. [DOI] [PubMed] [Google Scholar]
- Wacker A., Eilmes H.G. Antivirale wirkung von pffanzeninhaltsstoffen. Arzneimittelforschung. Drug Research. 1978;28:347–349. [PubMed] [Google Scholar]
- Wang H., Ng T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001;68(18):2151–2158. doi: 10.1016/s0024-3205(01)01023-2. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang H.X., Ng T.B. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides. 2007;28(3):560–565. doi: 10.1016/j.peptides.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang Y., Wang W., Fu P., Liu P., Zhu W. Anti-influenza virus polyketides from the acid-tolerant fungus Penicillium purpurogenum JS03-21. J. Nat. Prod. 2011;74(9):2014–2018. doi: 10.1021/np2004769. [DOI] [PubMed] [Google Scholar]
- Wang P., Kong F., Wei J., Wang Y., Wang W., Hong K., Zhu W. Alkaloids from the mangrove-derived actinomycete Jishengella endophytica 161111. Marine Drugs. 2014;12(1):477–490. doi: 10.3390/md12010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber N., Andersen D., North J., Murray B., Lawson L., Hughes B. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58(05):417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- Weeks J. Call to action: announcing the traditional, complementary and integrative health and medicine COVID-19 support registry. J. Altern. Complement. Med. 2020;26(4):256–258. doi: 10.1089/acm.2020.29083.jjw. [DOI] [PubMed] [Google Scholar]
- Wong J.H., Ng T.B. Lunatusin, a trypsin-stable antimicrobial peptide from lima beans (Phaseolus lunatus L.) Peptides. 2005;26(11):2086–2092. doi: 10.1016/j.peptides.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Wu G., Sun X., Yu G., Wang W., Zhu T., Gu Q., Li D. Cladosins A–E, hybrid polyketides from a deep-sea-derived fungus, Cladosporium sphaerospermum. J. Nat. Prod. 2014;77(2):270–275. doi: 10.1021/np400833x. [DOI] [PubMed] [Google Scholar]
- Xiu-Wuei Y., Jing Z., Yu-Xin C., Xue-Hui L., Chao-Mei M., Masao H., Li-He Z. Anti-HIV-1 protease triterpenoid saponins from the seeds of Aesculus chinensis. J. Nat. Prod. 1999;62:1510–1513. doi: 10.1021/np990180u. [DOI] [PubMed] [Google Scholar]
- Xu H.X., Kadota S., Kurokawa M., Shiraki K., Matsumoto T., Namba T. ChemInform abstract: isolation and structure of woodorien, a new glucoside having antiviral activity, from Woodwardia orientalis. ChemInform. 2010;25(19) doi: 10.1002/chin.199419253. [DOI] [PubMed] [Google Scholar]
- Xu D.B., Ye W.W., Han Y., Deng Z.X., Hong K. Natural products from mangrove actinomycetes. Marine Drugs. 2014;12(5):2590–2613. doi: 10.3390/md12052590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.Y., Huang X., Cheong K.L. Recent advances in marine algae polysaccharides: isolation, structure, and activities. Marine Drugs. 2017;15(12):388. doi: 10.3390/md15120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K.A., Galhardi L.C.F., Rincão V.P., Soares S. de A., Vieira Í.G.P., Ricardo N.M.P.S., Nozawa C., Linhares R.E.C. Antiherpetic activity of an Agaricus brasiliensis polysaccharide, its sulfated derivative and fractions. Int. J. Biol. Macromol. 2013;52:9–13. doi: 10.1016/j.ijbiomac.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Yamazaki Z., Tagaya I. Antiviral effects of atropine and caffeine. J. Gen. Virol. 1980;50(2):429–431. doi: 10.1099/0022-1317-50-2-429. [DOI] [PubMed] [Google Scholar]
- Yang X.W., Zhao J., Cui Y.X., Liu X.H., Ma C.M., Hattori M., Zhang L.H. Anti-HIV-1 protease triterpenoid saponins from the seeds of Aesculus chinensis. J. Nat. Prod. 1999;62(11):1510–1513. doi: 10.1021/np990180u. [DOI] [PubMed] [Google Scholar]
- Ye X.E., Ng T.B., Rao P.F. Cicerin and arietin, novel chickpea peptides, with different antifungal potencies. Peptides. 2002;23:817–822. doi: 10.1016/s0196-9781(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Ye M., Beach J., Martin J.W., Senthilselvan A. Occupational pesticide exposures and respiratory health. Int. J. Environ. Res. Public Health. 2013;10(12):6442–6471. doi: 10.3390/ijerph10126442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo K., Miyamoto Y., Nagao K., Kumagae E., Habib E.-S.E., Suzuki K., Harada S., Uyeda M. Fattiviracin A1, a novel antiviral agent produced by Streptomyces microflavus strain no. 2445. II. Biological properties. J. Antibiot. 1998;51(11):1035–1039. doi: 10.7164/antibiotics.51.1035. [DOI] [PubMed] [Google Scholar]
- Yong T., Chen S., Xie Y., Chen D., Su J., Shuai O., Jiao C., Zuo D. Cordycepin, a characteristic bioactive constituent in Cordyceps militaris, Ameliorates hyperuricemia through URAT1 in hyperuricemic mice. Front. Microbiol. 2018;9:58. doi: 10.3389/fmicb.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef F.S., Ashour M.L., Singab A.N.B., Wink M. A comprehensive review of bioactive peptides from marine fungi and their biological significance. Marine Drugs. 2019;17(10):559. doi: 10.3390/md17100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yust M. Rapeseed protein hydrolysates: a source of HIV protease peptide inhibitors. Food Chem. 2004;87(3):387–392. doi: 10.1016/j.foodchem.2003.11.020. [DOI] [Google Scholar]
- Zambare V.P., Christopher L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012;50(6):778–798. doi: 10.3109/13880209.2011.633089. [DOI] [PubMed] [Google Scholar]
- Zander W., Irschik H., Augustiniak H., Herrmann M., Jansen R., Steinmetz H., Gerth K., Kessler W., Kalesse M., Höfle G., Müller R. Sulfangolids, macrolide sulfate esters from Sorangium cellulosum. Chem. Eur. J. 2012;18(20):6264–6271. doi: 10.1002/chem.201100851. [DOI] [PubMed] [Google Scholar]
- Zandi K., Teoh B.T., Sam S.S., Wong P.F., Mustafa M.R., Abubakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011;8:560. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mu J., Feng Y., Kang Y., Zhang J., Gu P.J., Wang Y., Ma L.F., Zhu Y.-H. Broad-spectrum antimicrobial epiphytic and endophytic fungi from marine organisms: isolation, bioassay and taxonomy. Marine Drugs. 2009;7(2):97–112. doi: 10.3390/md7020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Sun S., Zhu T., Lin Z., Gu J., Li D., Gu Q. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry. 2011;72(11−12):1436–1442. doi: 10.1016/j.phytochem.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Zhen G., Jing J., Fengsen L. Traditional Chinese medicine classic herbal formula Xiaoqinglong decoction for acute exacerbation of chronic obstructive pulmonary disease: a systematic review protocol. Medicine. 2018;97(52):e13761. doi: 10.1097/MD.0000000000013761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C.J., Shao C.L., Guo Z.Y., Chen J.F., Deng D.S., Yang K.L., Chen Y.Y., Fu X.M., She Z.G., Lin Y.C., Wang C.Y. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012;75(2):189–197. doi: 10.1021/np200766d. [DOI] [PubMed] [Google Scholar]
- Zhu T., Chen Z., Liu P., Wang Y., Xin Z., Zhu W. New rubrolides from the marine-derived fungus Aspergillus terreus OUCMDZ-1925. J. Antibiot. 2013;67(4):315–318. doi: 10.1038/ja.2013.135. [DOI] [PubMed] [Google Scholar]