Abstract
The diverse clinical manifestations of COVID-19 is emerging as a hallmark of the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection. While the initial target of SARS-CoV-2 is the respiratory tract, it is becoming increasingly clear that there is a complex interaction between the virus and the immune system ranging from mild to controlling responses to exuberant and dysfunctional multi-tissue directed autoimmune responses. The immune system plays a dual role in COVID-19, being implicated in both the anti-viral response and in the acute progression of the disease, with a dysregulated response represented by the marked cytokine release syndrome, macrophage activation, and systemic hyperinflammation. It has been speculated that these immunological changes may induce the loss of tolerance and/or trigger chronic inflammation. In particular, molecular mimicry, bystander activation and epitope spreading are well-established proposed mechanisms to explain this correlation with the likely contribution of HLA alleles. We performed a systematic literature review to evaluate the COVID-19-related autoimmune/rheumatic disorders reported between January and September 2020. In particular, we investigated the cases of incident hematological autoimmune manifestations, connective tissue diseases, antiphospholipid syndrome/antibodies, vasculitis, Kawasaki-like syndromes, acute arthritis, autoimmune-like skin lesions, and neurologic autoimmune conditions such as Guillain–Barré syndrome. We screened 6263 articles and report herein the findings of 382 select reports which allow us to conclude that there are 2 faces of the immune response against SARS-CoV-2, that include a benign virus controlling immune response and a many faceted range of dysregulated multi-tissue and organ directed autoimmune responses that provides a major challenge in the management of this viral disease. The number of cases for each disease varied significantly while there were no reported cases of adult onset Still disease, systemic sclerosis, or inflammatory myositis.
Keywords: COVID-19, Autoimmune manifestations, systematic review
1. Introduction
The outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that first emerged in Wuhan (Hubei Province, China) in December 2019 rapidly became a pandemic, with more than 32.7 million cases reported as of September 27, 2020 [1]. The COVID-19 clinical presentation ranges from asymptomatic individuals or mild flu-like symptoms, to a very severe condition with interstitial pneumonia and acute respiratory distress syndrome (ARDS) [2]. In the first phases of the disease, the viral infection triggers a strong immune response which is fundamental for viral clearance, with a cascade of events involving both innate and adaptive immunity that potentially can become harmful when they become dysregulated [3,4]. The immunological alterations associated with different stages of COVID-19 have been described since the earliest reports, and include an elevated number of macrophages, hyperactivation of T cells and the release of increased plasma levels of pro-inflammatory cytokines (e.g. IL-1β, IL-6, TNFα), leading to what is termed as “a cytokine storm” and cytokine release syndrome that appears to be correlated with the severity of disease outcome [5] possibly modifiable with immunomodulating drugs [6]. Several studies enlighten the immunological and clinical similarities between COVID-19 disease and hyperinflammatory diseases, leading to the hypothesis that the SARS-CoV-2 infection might trigger an autoimmune response in genetically predisposed subjects [7,8].
During the past decades, viral infections have been proposed as environmental factors triggering autoimmunity in genetically prone individuals. Respiratory viruses, particularly parainfluenza and coronaviruses, have been associated with the onset of rheumatoid arthritis (RA) [9] while growing evidence describes the occurrence of well-known autoimmune conditions in COVID-19, including autoimmune hemolytic anemia (AIHA), immune thrombocytopenia (ITP), thrombotic events associated with anti-phospholipid antibodies, connective tissue diseases, Kawasaki-like disease, ANCA-associated vasculitis, arthritis, autoimmune-like skin manifestations and neurologic demyelinating syndromes. The enormous number of publications reported in the literature over the past months has made it virtually impossible to follow the literature and derive a meaningful consensus opinion. These thoughts highlight the need for a timely systematic literature review to not only illustrate the data on autoimmune rheumatic conditions described in patients with COVID-19, but also to serve as a summary of what we understand about the potential mechanism(s) involved.
2. Study search strategy and selection
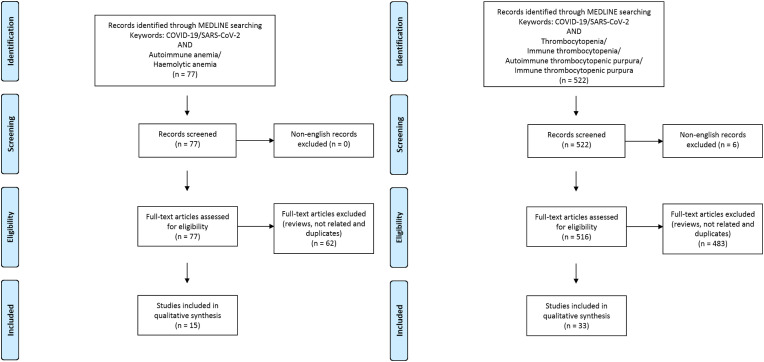
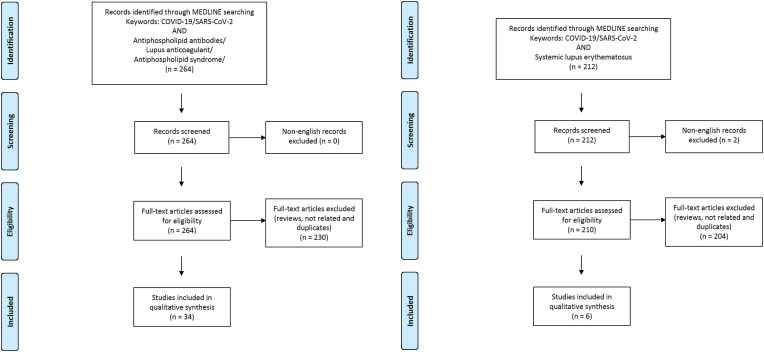
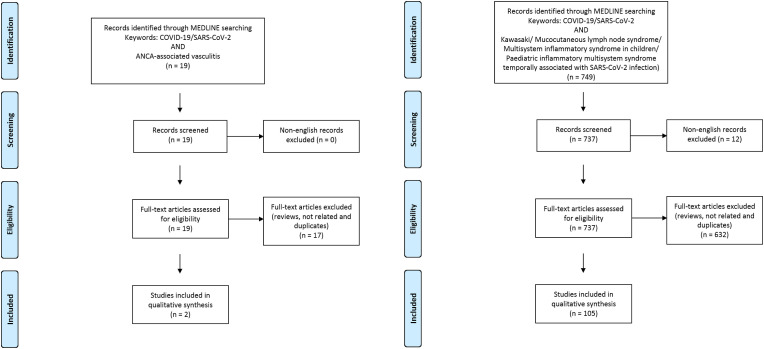
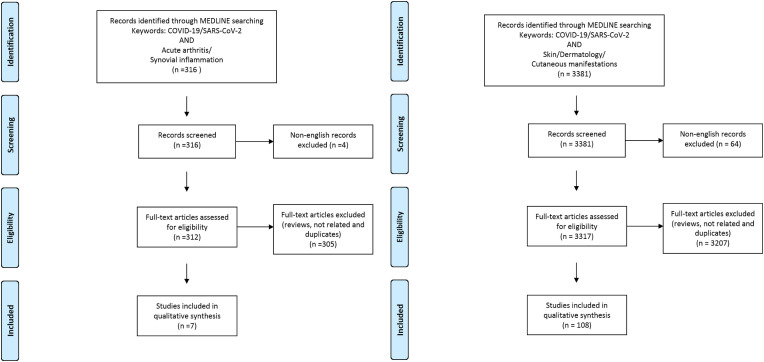
The Medline database was accessed from PubMed and systematically searched for articles published in English between January 1 and September 30, 2020. We followed the search strategy and article selection process illustrated in the flowcharts in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 according to the recommendations of the PRISMA statement [10]. The search strings in title/abstract and the keywords used for each association are detailed in the respective flowchart. Only peer-reviewed articles in English accepted for publication that included case reports and case series were included in this search. Two reviewers (LN and FM) searched all relevant articles independently and summarized them. They discussed any area of uncertainty, screened the full text reports and decided whether these met the inclusion criteria while resolving any disagreement through discussions. Neither of the authors were blind to the journal titles or to the study authors or institutions.
Fig. 1.
Flowcharts show the study selection process according to preferred reporting items for systematic reviews and meta-analyses (PRISMA).
Fig. 2.
Flowcharts show the study selection process according to preferred reporting items for systematic reviews and meta-analyses (PRISMA).
Fig. 3.
Flowcharts show the study selection process according to preferred reporting items for systematic reviews and meta-analyses (PRISMA).
Fig. 4.
Flowcharts show the study selection process according to preferred reporting items for systematic reviews and meta-analyses (PRISMA).
Fig. 5.
Flowcharts show the study selection process according to preferred reporting items for systematic reviews and meta-analyses (PRISMA).
Fig. 6.
Flowcharts show the study selection process according to preferred reporting items for systematic reviews and meta-analyses (PRISMA).
3. Results
3.1. Hematologic manifestations
3.1.1. Autoimmune hemolytic anemia (AIHA)
AIHA is frequently linked to autoimmune diseases, drugs, malignancy and, in rare cases, infections [11]. A few cases of AIHA have been shown to be associated with SARS-CoV-2 infection (Table 1 ) including both warm and cold AIHA. In some cases, patients were affected by pre-existent immune thrombocytopenia, suggesting a susceptible background for hematological dysregulations [[12], [13], [14]]. Molecular mimicry has been proposed to trigger AIHA, with antibodies elicited against viral proteins cross reacting with self-antigens. A putative self-antigen involved could be Ankyrin-1, an erythrocyte membrane protein showing structural similarities with the viral spike protein [15]. In two cases AIHA and idiopathic thrombocytopenic purpura (ITP) presented both during COVID-19 and Evans syndrome were diagnosed [16,17]. In some cases, patients had an indolent B lymphoid malignancy either already known or discovered because of the hemolytic episode. The delay between COVID-19 and hemolytic manifestations ranged from 4 to 13 days [18].
Table 1.
Hemolytic anemia cases associated with SARS-CoV-2 infection.
| Manifestation | Patients number | Sex | Age (years) | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|
| AIHA | 1 warm | F | 46 | IVIg, glucocorticoids and transfusion | Recovered | [12] |
| AIHA | 7 (4 warm, 3 cold) |
4 M 3 F |
median 62 (range 61–89) | Glucocorticoids (5 cases), + rituximab (2 cases), transfusion (2 cases) | Partly recovered | [18] |
| AIHA | 1 cold | F | 46 | None | Death | [13] |
| Evans syndrome | 1 | M | 39 | IVIg | Recovered | [16] |
| AIHA | 1 warm | M | 17 | Glucocorticoids, transfusion | Recovered | [14] |
| AIHA | 1 cold | M | 62 | Transfusion | Recovered | [100] |
| AIHA | 2 cold | F M |
43 63 |
Transfusion None |
Recovered Recovered |
[101] |
| AIHA | 1 warm | M | 56 | IVIg, glucocorticoids and transfusion | Recovered | [102] |
| AIHA | 1 cold | F | 51 | Glucocorticoids | Recovered | [103] |
| Evans syndrome | 1 | F | 23 | Transfusion, glucocorticoids, IVIg, rituximab | Recovered | [17] |
| AIHA | 1 cold | M | 48 | Transfusion | Death | [104] |
| AIHA | 1 warm | F | 13 | Glucocorticoids | Recovered | [105] |
| AIHA | 1 cold | F | 24 | None | Recovered | [106] |
| AIHA | 2 cold | M M |
70 67 |
None None |
Recovered Death |
[107] |
| AIHA | 1 mixed | F | 14 | Glucocorticoids, transfusions, rituximab | Recovered | [25] |
Abbreviations. AIHA: autoimmune hemolytic anemia. F: female. M: male. IVIg: intravenous immunoglobulin.
3.1.2. Immune thrombocytopenic purpura (ITP)
Thrombocytopenia may occur in nearly 30% of cases of coronaviruses infections [19] and a mild reduced platelet count is a common finding in COVID-19. Possible causes include the reduced production due to bone marrow progenitor destruction by direct viral infection or cytokine storm, impaired biogenesis in the lung, platelet consumption due to microthrombi formation or platelet destruction [19]. The latter mechanism could be induced by viral infections through cross-reactivity between viral and platelet proteins, or through platelet coating with antibodies or immune complexes generated during the infection that are recognized by the reticuloendothelial cells and destroyed [20]. ITP has been reported in HKU1 coronavirus (a related but distinct coronavirus) infection [21]. It is therefore not surprising that SARS-CoV-2 also appears to trigger ITP, either during the course of the infection or in some cases weeks after the resolution and appears to be independent of the severity of the disease.
The reported cases of ITP associated with SARS-CoV-2 infection are listed in Table 2 with a limited number of cases occurring at pediatric ages [[22], [23], [24], [25]]. Of note, an association between low platelet counts (for any cause) and mortality has been found in these cases [26,27]. Therefore, when thrombocytopenia is present in the context of SARS-CoV-2 infection, it is important to consider ITP, as the prompt management may significantly improve the prognosis. Flares of previously diagnosed ITP have been described, as in the case of a woman with a previous diagnosis of ITP, receiving immunosuppressive therapy with prednisone (10 mg/daily) and cyclosporine (50 mg/daily) experiencing a severe exacerbation with marked decrease of platelet count during COVID-19 [28]. Another case concerned a 37-year-old woman who was treated with mycophenolate mofetil for ITP secondary to systemic lupus erythematosus and had a flare during mild COVID-19 [29]. ITP exacerbation also occurred in a 34-year-old woman during the second trimester of pregnancy [30] and a de novo disease was diagnosed in another pregnant woman [31]. Among patients affected by ITP, death was determined to be secondary to intracerebral bleeding [32], or respiratory deterioration [33]. One additional case of thrombotic thrombocytopenic purpura (TTP) was diagnosed 9 days after a SARS-CoV-2 infection confirmed by serum tests [34].
Table 2.
Immune thrombocytopenia cases associated with SARS-CoV-2 infection.
| Manifestation | Patients number | Sex | Age (years) | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|
| ITP | 1 | F | 65 | IVIg, platelet transfusion, glucocorticoids, thrombopoietin | Recovered | [108] |
| ITP | 1 | F | 32 | Platelet transfusion, glucocorticoids | Improvement | [109] |
| ITP | 1 | M | 39 | IVIg, glucocorticoids | Recovered | [110] |
| ITP | 3 | 2 M 1 F |
59 66 67 |
Glucocorticoids (2 cases), + IVIg (1 case), platelet transfusion | 2 Improvements 1Death |
[32] |
| ITP | 1 | M | 41 | IVIg | Recovered | [111] |
| ITP | 3 | 1 M 2 F |
50 49 96 |
IVIg | 2 Recovered 1 Death |
[33] |
| ITP | 1 | F | 12 | IVIg, glucocorticoids | Recovered | [22] |
| ITP | 1 | F | 10 | IVIg | Recovered | [23] |
| ITP | 1 | M | 84 | IVIg, glucocorticoids | Recovered | [112] |
| ITP flare | 1 | F | 72 | IVIg, platelet transfusion, glucocorticoids | Recovered | [28] |
| ITP flare | 1 | F | 37 | IVIg, glucocorticoids | Recovered | [29] |
| ITP flare | 1 | F | 34 | IVIg, glucocorticoids | Improvement | [30] |
| ITP | 1 | F | 41 | IVIg, platelet transfusion | Recovered | [31] |
| ITP | 3 | 2 M 1 F |
66 57 79 |
IVIg and thrombopoietin (2 cases). No treatment (1 case) | Recovered | [113] |
| TTP | 1 | F | 57 | IVIg, glucocorticoids, plasma exchange and plasma infusion | Recovered | [34] |
| ITP (1 ITP flare) | 3 | F M M |
57 72 39 |
IVIg IVIg IVIg |
Recovered Recovered Recovered |
[114] |
| ITP | 1 | M | 53 | IVIg, glucocorticoids, platelet transfusion, thrombopoietin | Recovered | [115] |
| ITP | 1 | M | 38 | IVIg, glucocorticoids | Recovered | [116] |
| ITP | 1 | F | 51 | IVIg, glucocorticoids, platelet transfusion, thrombopoietin | Recovered | [117] |
| ITP | 1 | F | 73 | IVIg, glucocorticoids, platelet transfusion | Recovered | [118] |
| ITP | 1 | M | 86 | IVIg, glucocorticoids | Recovered | [119] |
| ITP | 1 | M | 41 | IVIg, glucocorticoids | Recovered | [120] |
| ITP | 14 | 7 M 7 F | Median age 64 | IVIg in 9, glucocorticoids in 7, thrombopoietin in 3 | Recovered, 3 Relapsed | [121] |
| ITP flare | 1 | F | 58 | IVIg, glucocorticoids | Recovered | [122] |
| ITP | 1 | M | 48 | IVIg, glucocorticoids | Recovered | [123] |
| ITP | 1 | F | 29 | Glucocorticoids, platelet transfusion | Recovered | [124] |
| ITP | 3 | F M M |
69 88 31 |
Glucocorticoids Glucocorticoids Glucocorticoids |
Recovered Recovered Recovered |
[125] |
| ITP | 1 | M | 67 | IVIg, glucocorticoids, platelet transfusion, thrombopoietin | Recovered | [126] |
| ITP | 1 | F | 2 | None | Recovered | [127] |
| ITP | 1 | M | 89 | IVIg, glucocorticoids, platelet transfusion | Death | [128] |
| ITP | 1 | M | 22 | IVIg, platelet transfusion | Recovered | [129] |
| ITP | 1 | F | 63 | IVIg | Recovered | [130] |
| ITP | 1 | M | 16 | Glucocorticoids | Improvement | [25] |
Abbreviations. ITP: immune thrombocytopenic purpura. F: female. M: male. IVIg: intravenous immunoglobulins. TTP: thrombotic thrombocytopenic purpura.
In a retrospective single-center study, Chen and Colleagues enrolled 271 patients to determine the association of thrombocytopenia during the delayed-phase of COVID-19, i.e. 14 days after symptoms appeared. Thrombocytopenia occurred in 11.8% of cases, mostly in elderly or in the presence of low lymphocyte count at admission. This was significantly associated with the duration of hospital stay, being mostly transient, lasting less than 7 days. In three patients who developed a dramatic decline in platelet count, without other putative explanations, bone marrow aspiration demonstrated an impaired megakaryocyte maturation, similar to what is observed in ITP. The authors therefore speculated that the delayed-phase platelet decrease might have been immune mediated in these patients [35]. In other cases, the management with immunoglobulins was preferred to glucocorticoids, because of the possible harmful effect of the latter in SARS-CoV-2 infection hypothesized during the first months of the pandemic [36].
3.2. Antiphospholipid antibodies and syndrome
Since the earliest reports, COVID-19 has been associated with coagulation abnormalities and includes a pro-thrombotic state, affecting the prognosis through both arterial and venous thrombotic events [37], observed in up to 31% of patients in intensive care units. Potential underlying mechanisms include immobilization, hypoxia, or disseminated coagulopathy [38]. Serum antiphospholipid antibodies (aPLs), including IgG and IgM anti-cardiolipin (aCL), IgG and IgM anti-beta2-glycoprotein I (β2-GPI) antibodies and lupus anticoagulant (LAC), may be found in up to 12% of young healthy subjects and 18% of elderly people with chronic diseases [39]. Most individuals with aPLs do not experience thrombotic events, for which a “second hit” is probably required to develop the antiphospholipid syndrome (APS), that is based on the confirmation of serum autoantibodies on two or more occasions at least 12 weeks apart. Critical illnesses, infections and aging [40] are known to trigger aPLs, either transiently or chronically with or without the development of thrombosis [41,42]. Table 3 illustrates the cases of aPLs that are either associated with or not associated with thrombosis. These findings seem to suggest an additional role of aPLs or LAC in the pathogenesis of thrombosis (either arterial or venous) in patients with COVID-19.
Table 3.
APL and APS cases associated with SARS-CoV-2 infection.
| Number of patients | Sex | Mean or median age (years) | Number of patients with APL (n° or n°/tot tested) | APL (n°/tot tested) | Manifestations in APL population | Ref. |
|---|---|---|---|---|---|---|
| 3 | 2 M 1 F |
69 65 70 |
3 | aCL IgA 3/3 anti–β2-GPI IgA and IgG 3/3 |
Multiple cerebral infarctions, lower limbs and hand finger ischemia | [131] |
| 2 | 1 M 1 F |
58 29 |
2 | aCL IgG and IgM 2/2 | Splenic infarct, cerebral infarction – peroneal and tibial artery thrombosis | [132] |
| 1 | M | 82 | 1 | aCL IgA, IgM, IgG | Pulmonary embolism | [133] |
| 2 | 2 M | 79 | 1 | aCL IgM | Multiple cerebral infarcts | [134] |
| 1 | F | 49 | 1 | aCL IgG and IgM | Deep vein thrombosis in the four extremities | [135] |
| 1 | M | 72 | 1 | aCL IgM and anti-β2-GPI IgM | ICU patient, no evident thrombosis but signs of endothelial stimulation | [136] |
| 24 | 14 M 10 F | 64.3 | 2 | aCL IgM 2/24 anti-β2-GPI IgM 2/24 |
Venous thromboembolism | [45] |
| 57 | 122 M 28 F | 63 | 50 | LAC 50/57 aCL IgM 1/57 | Thrombotic events | [137] |
| 35 | 24 M 11 F |
56.6 | 31 | LAC 31/34 | Venous thrombosis | [138] |
| 25 | 17 M 8 F | 47.7 | 24 | aCL IgG 13/25 aCL IgM 5/25 aCL IgA 7/25 a-β2-GPI IgG 1/25 a-β2-GPI IgM 0/25 a-β2-GPI IgA 3/25 LAC 23/25 |
Massive pulmonary embolism | [46] |
| 79 | 45 M 34 F | ≈57 | 31 | aCL IgG 4/79 aCL IgM 2/79 aCL IgA 17/79 a-β2-GPI IgG 12/79 a-β2-GPI IgM 1/79 a-β2-GPI IgA 19/79 LAC 2/79 |
Cerebral infarction, myocardial infarction | [44] |
| 35 | 26 M 9 F | 73 | 3 | aCL IgG 1/35 aCL IgM 2/35 aPS/PT IgG 1/35 aPS/PT IgM 2/35 |
Multiple recent microvascular and macrovascular thrombosis at autopsy | [139] |
| 122 | 60 M 62 F | 54.3 | 41 | aCL IgG 15/112 aCL IgM 3/112 aCL IgA 2/121 a-β2-GPI IgG 7/112 a-β2-GPI IgM 8/112 a-β2-GPI IgA 4/121 LAC 16/72 |
10 Thrombosis (venous or arterial) | [140] |
| 31 | 28 M 3 F | 63 | 23 | aCL IgG 6/31 aCL IgM 1/31 aCL IgA 3/31 a-β2-GPI IgG 3/31 a-β2-GPI IgM 1/31 a-β2-GPI IgA 3/31 LAC 21/31 aPS/PT 7/31 |
7 Thrombosis (venous or arterial) | [141] |
| 74 | N.A. | 63.5 | 65 | aCL IgG or aCL IgM or a-β2-GPI IgG or a-β2-GPI IgM or a-β2-GPI IgA 9/74 LAC 63/74 |
5 Thrombosis (venous or arterial) | [142] |
| 86 | 54 M 32 F | 66.6 | 12/31 | aCL IgG or aCL IgM or a-β2-GPI IgG or a-β2-GPI IgM or a-β2-GPI IgA or LAC 12/31 | 5 Acute ischemic strokes | [143] |
| 1 | M | 69 | 1 | aCL IgG and IgM, LAC | Thrombotic microangiopathy | [144] |
| 844 | 405 M 439 F | 59 | 7/9 | aCL IgG or aCL IgM or a-β2-GPI IgG or a-β2-GPI IgM or LAC 7/9 | 7 Acute ischemic strokes | [145] |
| 68 | 34 M 34 F | ≈57 | 30 | aCL IgG 0/62 aCL IgM 1/62 a-β2-GPI IgG 0/62 a-β2-GPI IgM 1/60 LAC 30/68 |
19 Thrombosis (venous or arterial) | [146] |
| 21 | 9 M 12 F | 62 | 12 | aCL IgG 2/21 aCL IgM 3/21 a-β2-GPI IgG 1/21 a-β2-GPI IgM 0/21 LAC 21/31 aPS/PT/annexin IgG or IgM 11/21 |
2 Pulmonary thromboembolisms | [147] |
| 1 | M | 31 | 1 | aCL IgM, LAC | No thrombotic event | [148] |
| 27 | 12 M 15 F | 58 | 7 | aCL IgG or IgM 0/27 a-β2-GPI IgG or IgM a-β2-GPI IgA 1/27 LAC 6/27 |
3 Thrombosis (venous or arterial) | [149] |
| 1 | F | 34 | 1 | LAC | Acute ischemic stroke | [150] |
| 43 | 27 M 16 F | ≈63.2 | 16 | aCL IgG or IgM 0/43 a-β2-GPI IgG or IgM 0/43 LAC 16/43 |
1 Thrombosis | [151] |
| 1 | M | 48 | 1 | aCL IgG and IgM a-β2-GPI IgG and IgM LAC |
APS flare with limb arterial ischemia | [152] |
| 89 | 61 M 7 F | 68 | 64 | aCL IgG or IgM 7/89 a-β2-GPI IgG or IgM 6/89 LAC 59/89 |
6 Deep vein thrombosis, 6 pulmonary embolisms | [153] |
| 33 | 17 M 16 F |
70 | 8 | aCL IgG 5/33 aCL IgM 6/33 a-β2-GPI IgG 2/33 a-β2-GPI IgM 2/33 |
No thrombotic events | [154] |
| 64 | 32 M 32 F | 62 | 64 | aCL IgG or IgM a-β2-GPI IgG or IgM |
N.A. | [155] |
| 19 | 10 M 9 F | 65 | 10 | aCL IgG 2/10 aCL IgM 1/10 aCL IgA 6/10 a-β2-GPI IgG 6/10 a-β2-GPI IgM 0/10 a-β2-GPI IgA 7/10 LAC 1/10 |
4 Acute ischemic strokes | [156] |
| 2369 | N.A. | N.A. | 1 | aPL (not defined) | N.A. | [157] |
| 56 | 33 M 23 F | 66 | 24 | aCL IgG 16/56 aCL IgM 3/56 a-β2-GPI IgG 1/56 a-β2-GPI IgM 4/56 |
N.A. | [158] |
| 56 | N.A. | N.A. | 30 | aCL or a-β2-GPI IgG or IgM 5/56 LAC 25/56 |
N.A. | [43] |
| 1 | M | 31 | 1 | LAC | Acute limb ischemia, myocardial infarction | [159] |
| 1 | F | 30 | 1 | aCL IgG and IgM, a-β2-GPI IgG and IgM, LAC | No thrombotic events, Evans syndrome | [160] |
Abbreviations. aPL: antiphospholipid antibody. APS: antiphospholipid syndrome. M: male. F: female. aCL: anti-cardiolipin antibody. Ig: immunoglobulin. A-β2-GPI: anti-beta2glycoprotein I. N.A.: information not available. ICU: intensive care unit. PTT: partial thromboplastin time. LAC: lupus anticoagulant. aPS/PT: anti phosphatidylserine/prothrombin.
Whether SARS-CoV-2 induces the development of aPLs or acts as a second hit in previously positive patients remains unclear and the clinical significance of aPLs in COVID-19 is still to be elucidated. Some authors have reported a high incidence of LAC in patients with SARS-CoV-2 infection. Thus, for instance, in a cohort of 56 patients, while twenty-five were found to be positive for LAC, five of the 50 patients that were tested had aCL or anti–β2 GPI antibodies, of which three were associated with LAC [43]. APLs may develop in critically ill patients [44], as the reactivity is found in 31 out of 66 patients requiring ICU admission and in none of those in non-critical conditions. The analysis of previous sera revealed that aPLs appear at a median time of 39 days following disease onset, suggesting that critically ill patients with longer disease duration are more likely to develop aPLs.
On the other hand, the association with aPLs is not clear in the analysis of patients with thrombosis. In fact, in a cohort of 785 patients with COVID-19, out of the 24 who had a venous thromboembolism without known risk factors (besides the infection), only two patients were weakly positive for aCL IgM and anti-β2-GPI IgM [45]. On the contrary, in a study of a separate series of patients, a majority of patients with severe thrombotic events had positive aPLs [44,46]. Other authors suggest that these data should be interpreted with caution, as false positive LAC testing might be due to the marked elevation in C-reactive protein (CRP) levels seen in pulmonary or systemic inflammation [47]. Moreover, concomitant therapy with anticoagulants can alter LAC testing [48] and aPL titers are not consistently defined in these studies, making the clinical course difficult to evaluate.
3.3. Systemic lupus erythematosus
A few cases of de novo appearance of systemic lupus erythematosus (SLE) or SLE-like syndrome associated with COVID-19 have been reported. In addition, flares of previously diagnosed SLE have been described. Table 4 illustrates the main clinical manifestations and the outcomes of the reported cases.
Table 4.
Systemic lupus erythematosus cases associated with SARS-CoV-2 infection.
| Patients number | Sex | Age (years) | Manifestations | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|
| 1 | F | 18 | Pericardial tamponade with shock, ventricular dysfunction, pleural serositis, nephritis, ANA, dsDNA, secondary APS, anemia, thrombocytopenia, low complement | Glucocorticoids, plasma exchange, HCQ, anticoagulation | Death | [161] |
| 1 | F | 23 | Nephritis, ANA, dsDNA, low complement, aPL, direct Coombs, varicella-like rash | Glucocorticoids | Death | [162] |
| 1 | F | 85 | Thrombocytopenia, pleural effusion, proteinuria, ANA, low complement, finger vasculitis | Glucocorticoids, HCQ | Improvement | [163] |
| 1 | M | 62 | Nephritis, neuropsychiatric symptoms, lymphopenia, ANA | Glucocorticoids, TCZ | Improvement | [164] |
| 1 (flare) | M | 62 | During flare: low complement, aPL, thrombocytopenia with cerebral hemorrhage, hemolytic anemia | Glucocorticoids, IVIg, rituximab | Death | [165] |
| 1 (flare) | M | 63 | During flare: APS. | Glucocorticoids, HCQ, anticoagulation | Death | [166] |
Abbreviations. F: female. ANA: antinuclear antibodies. dsDNA: anti double strand DNA. APS: antiphospholipid syndrome. HCQ: hydroxychloroquine. M: male. aPL: antiphospholipid antibody. TCZ: tocilizumab. IVIg: intravenous immunoglobulins.
3.4. ANCA-associated vasculitis
A small number of cases of anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis related to COVID-19 have been reported and are listed in Table 5 .
Table 5.
ANCA-associated vasculitis cases related to SARS-CoV-2 infection.
| Patients | Sex | Age (years) | Manifestations | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|
| 2 | M M |
64 46 |
Pauci-immune crescentic glomerulonephritis, MPO-ANCA Focal necrotizing glomerulonephritis, skin vasculitis, c-ANCA |
Glucocorticoids, rituximab Glucocorticoids, rituximab |
Improvement Improvement |
[167] |
| 1 | F | 37 | Pulmonary hemorrhage, PR3-ANCA | Glucocorticoids, plasmapheresis, IVIg | Death | [168] |
Abbreviations. M: male. MPO-ANCA: myeloperoxidase antineutrophil cytoplasmic antibodies. c-ANCA: cytoplasmic antineutrophil cytoplasmic antibodies. PR3-ANCA: proteinase 3 antineutrophil cytoplasmic antibodies. IVIg: intravenous immunoglobulins.
3.5. Kawasaki-like disease
The pediatric population appears to be less affected than adults that develop severe SARS-CoV-2 infection. Thus, the pediatric cases comprise only 1–5% of total COVID-19 cases observed, and those that do become infected generally develop mild disease and low mortality [49]. This has been ascribed to the decreased level of maturity and function (binding affinity) of ACE2, the likely cell receptor for the virus, with reduced virus binding to cells. In addition, differences in the immune response to SARS-CoV-2 was also thought to play a role [50]. Nonetheless, in the early months of 2020, pediatricians began reporting cases of children with fever and signs of systemic inflammation with features in common with Kawasaki disease. Kawasaki disease is an acute and usually self-limiting vasculitis of medium sized vessels, which almost exclusively affects children. In some cases it is complicated by hemodynamic instability, a condition known as Kawasaki disease shock syndrome (KDSS) [51], or by a macrophage activation syndrome (MAS) [52]. In Table 6 Kawasaki disease-like cases are displayed.
Table 6.
Kawasaki-like cases associated with SARS-CoV-2 infection.
| Patients number (confirmed/non confirmed SARS-CoV-2 infection) | Sex | Mean or median age (months/years) | Manifestations | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|
| 1 (confirmed) | F | 6m | Complete KD | ASA, IVIg | Recovered | [169] |
| 1 (confirmed) | M | 5y | Atypical KD | ASA IVIg glucocorticoids |
Recovered | [170] |
| 1 (confirmed) | M | 8y | Complete KD, shock | ASA, IVIg, TCZ | Recovered | [171] |
| 1 (confirmed) | M | 4m | Complete KD | ASA IVIg |
Recovered | [172] |
| 8 (2 confirmed) | 5 M 3F |
8.8y | Atypical KD, shock, toxic shock syndrome symptoms | 8 IVIg, + ASA in 6, glucocorticoids in 5 | 1 Death 7 Recovered 1 Coronary aneurysm |
[65] |
| 4 (all confirmed) | 3 M 1F |
10y | Atypical KD, shock | 1: IVIg, TCZ, anakinra 2: IVIg, TCZ 3: IVIg, TCZ 4: TCZ |
2 Coronary artery abnormalities final outcome unknown | [173] |
| 10 (8 confirmed) | 7 M 3F |
7.5y | 5 complete KD, 5 atypical KD 5 shock |
10 IVIg, + ASA in 2, + glucocorticoids in 8 | Recovered 2 Coronary artery aneurysms |
[59] |
| 21 (19 confirmed) | 9 M 12F |
7.9y | 11 complete KD, 10 atypical KD 12 shock |
21 IVIg, plus ASA in 21, plus glucocorticoids in 10 | Recovered | [60] |
| 16 (11 confirmed) | 8 M 8F |
10y | 10 complete 6 atypical 7 severe (ICU) |
First line: 15 IVIg + ASA 1 HCQ Second line: 4 IVIg again, 1 IVIg + glucocorticoids, 2 glucocorticoids, 1 anakinra, 1 TCZ. |
Improved/Recovered 3 Coronary abnormalities |
[62] |
| 58 (45 confirmed) | 35 M 33F |
9y | 23 fever and inflammatory state. 29 shock 13 complete KD |
IVIg in 41, glucocorticoids in 37, anakinra in 3, infliximab in 8 | 1 Death 8 Coronary artery aneurysms |
[64] |
| 17 (all confirmed) | 8 M 9F |
8y | 8 Complete KD 5 Incomplete KD 13 shock |
4 ASA 14 glucocorticoids 13 IVIg 1 TCZ |
Recovered 1 Coronary artery aneurysm |
[174] |
| 35 (31 confirmed) | 18 M 17F |
10y | 35 Fever and inflammatory state 28 Shock |
35 IVIg, 12 glucocorticoids, 3 anakinra | Recovered | [175] |
| 3 (3 confirmed) | 2 M 1 F |
15.3y | 3 overlapping KD and TSS symptoms | 2 IVIg, 2 ASA, 1 steroid | Recovered 2 Coronary artery dilatations |
[176] |
| 1 confirmed | M | 14y | Atypical KD, shock | Infliximab | Recovered | [177] |
| 44 (confirmed) | 20 M 24 F |
7.3y | 44 Fever 37 gastrointestinal symptoms 22 shock |
42 glucocorticoids, 36 IVIg, 8 anakinra | Recovered 1 Renal replacement therapy |
[63] |
| 33 (confirmed) | 20 M 13 F |
10y | 31 Fever Inflammatory state 21 hypotension |
18 IVIg, 17 glucocorticoids, 12 TCZ | 1 Death 32 Recovered 2 Coronary artery ectasias |
[66] |
| 35 (27 confirmed) | 27 M 8 F |
11y | 33 fever 21 shock |
35 IVIg, glucocorticoids, biologics | 1 Death 34 Recovered 6 Coronary artery aneurysms |
[67] |
| 2 (confirmed) | 2 M | 12y 7y |
Complete KD | 1 steroid, 1 IVIg + steroid | Recovered | [178] |
| 1 (confirmed) | F | 6y | Atypical KD, shock | IVIg, ASA | Recovered | [179] |
| 1 (non-confirmed) | M | 3y | Complete KD | IVIg | N.A. | [180] |
| 1 (non-confirmed) | M | 5y | Atypical KD, shock | IVIg, ASA, glucocorticoids | Recovered | [181] |
| 6 (confirmed) | 1 M 5 F |
8.5y | KD (incomplete), shock | 6 IVIg, 5 glucocorticoids, 1 anakinra | Recovered 1 Coronary artery dilatation |
[182] |
| 20 (19 confirmed) | 10 M 10 F |
10y | Atypical KD, shock | 20 IVIg, + glucocorticoids in 2, + anakinra in 1, + TCZ in 1 | Recovered | [183] |
| 1 (non-confirmed) | F | 3y | Atypical KD | IVIg, ASA | Recovered | [184] |
| 4 (confirmed) | 1 M 3 F |
9.2y | Atypical KD | 4 IVIg, 3 glucocorticoids, 3 ASA |
Recovered | [185] |
| 156 (79 confirmed) | M/F ratio 0.96 | 8y | 66 Atypical KD, 72 ICU | N.A. | N.A. | [186] |
| 15 (at least 12 confirmed) | 11 F 4 F |
8.8y | 13 Atypical KD, 10 shock | 10 IVIg, 5 glucocorticoids, 11 ASA | Recovered 8 Coronary artery abnormalities |
[187] |
| 1 (confirmed) | F | 11y | Atypical KD, shock | IVIg, glucocorticoids, TCZ | Recovered | [188] |
| 1 (confirmed) | M | 16y | Atypical KD, shock | Steroid | Recovered | [189] |
| 1 (confirmed) | M | 10y | Atypical KD, shock | N.A. | Critically ill at last follow up | [190] |
| 33 (confirmed) | 20 M 13 F |
8.6y | 21 Complete KD, 16 shock |
33 IVIg, 29 ASA, 23 steroid, 4 anakinra, 3 TCZ, 1 infliximab | Recovered (16 coronary artery abnormalities) | [191] |
| 1 (non-confirmed) | F | 8y | Atypical KD, shock | IVIg, steroid, ASA | Recovered | [192] |
| 15 (confirmed) | 11 M 4 F |
12y | 15 Atypical KD, shock | 12 IVIg, 2 ASA, 3 steroid, 12 TCZ, 2 anakinra | 1 Death 3 Coronary artery abnormalities |
[68] |
| 99 (95 confirmed) | 53 M |
∼ 33y |
36 KD (complete or atypical) 29 shock |
69 IVIg, 63 steroids | 2 Death 9 Coronary artery aneurysms |
[193] |
| 186 (131 confirmed) | 115 M | 8.3y | 74 KD (complete or atypical), 62 ICU | 144 IVIg, 91 steroid, 14 TCZ/siltuximab, 24 anakinra | 4 Death 15 Coronary artery aneurysms |
[69] |
| 1 (confirmed) | M | 16y | Atypical KD, shock | IVIg, TCZ | Recovered | [194] |
| 1 (confirmed) | M | 9y | MIS-C | Glucocorticoids | Recovered | [195] |
| 1 (confirmed) | F | 35y | Atypical KD | None | Recovered | [196] |
| 1 (confirmed) | F | 36y | KD and shock | Glucocorticoids, IVIg, ASA | Recovered | [197] |
| 6 (3 confirmed) | 3 M 3 F | 8.1y | 4 KD (complete and atypical), 2 myocarditis 3 shock |
None | Recovered | [198] |
| 10 (8 confirmed) | 4 M 6 F | 10.2y | 5 complete KD, 5 atypical KD 4 shock |
9 IVIg, 5 glucocorticoids, 1 TCZ | Recovered 1 Coronary artery aneurysm |
[199] |
| 1 (confirmed) | M | 14y | Atypical KD, shock/MIS-C | IVIg, ASA | Recovered | [200] |
| 1 (confirmed) | M | 6y | Atypical KD | IVIg, ASA | Recovered | [201] |
| 78 | 52 M 26 F | 11y | PIMS-TS 68 shock |
59 IVIg, 57 glucocorticoids, 8 anakinra, 7 infliximab, 3 TCZ, 1 rituximab, 45 ASA | 2 Deaths 18 Coronary artery aneurysms |
[202] |
| 1 (confirmed) | M | 45y | MIS-C | IVIg, TCZ | Recovered | [203] |
| 7 (2 confirmed) | 5 M 2 F | 6.1m | 3 complete KD 4 atypical KD | 7 IVIg, 7 glucocorticoids, 7 ASA, 6 infliximab, 2 anakinra | 1 Death 6 Coronary artery aneurysms |
[204] |
| 1 (confirmed) | F | 19y | Complete KD/MIS-C | IVIg, glucocorticoids, TCZ, colchicine | Recovered | [205] |
| 28 (all confirmed) | 16 M 12 F | 9y | MIS-C | 20 IVIg, 17 glucocorticoids, 5 anakinra, | Recovered 6 Coronary artery abnormalities |
[206] |
| 8 (all confirmed) | N.A. | N.A. | Atypical KD | IVIg, glucocorticoids, ASA | N.A. | [207] |
| 1 (confirmed) | M | 19y | Atypical KD | None | Recovered | [208] |
| 31 (30 confirmed) | 18 M 13 F | 7.6y | MIS-C/KD | 20 IVIg, 21 glucocorticoids, | 1 Death 3 Coronary artery abnormalities |
[209] |
| 1 (confirmed) | M | 16y | PIMS-TS shock | IVIg, glucocorticoids, ASA | Recovered Coronary aneurysm |
[210] |
| 1 (confirmed) | M | 5m | Atypical KD | IVIg, ASA | Recovered Coronary artery abnormalities |
[211] |
| 2 (all confirmed) | N.A. | N.A. | Atypical KD | 2 IVIg | N.A. | [212] |
| 20 (19 confirmed) | 15 M 5 F | 10.6y | PIMS-TS | N.A. | N.A. | [213] |
| 1 (non-confirmed) | F | 7 | PIMS-TS | IVIg, glucocorticoids, ASA | Recovered | [214] |
| 3 (2 confirmed) | 2 M 1 F | 6y | PIMS-TS 2 shock |
IVIg, ASA | Recovered | [215] |
| 570 (565 confirmed) | 316 M 254 F | 8y | MIS-C 202 shock |
424 IVIg, 331 glucocorticoids, 309 ASA | 10 Deaths 95 Coronary artery abnormalities |
[216] |
| 30 (2 confirmed) | 12 M 2 F | 2y | 22 complete KD, 8 atypical KD | 30 IVIg, 14 IVIg + glucocorticoids, | Recovered 2 Coronary artery aneurysms |
[217] |
| 25 (17 confirmed) | 15 M 10 F | 12.5y | MIS-C | 23 IVIg, 20 glucocorticoids, 10 TCZ, 4 infliximab, 1 anakinra | Recovered 7 Coronary artery abnormalities |
[218] |
| 11 (all confirmed) | 9 M 2 F | 59m | PIMS-TS, 5 shock | N.A. | 2 Deaths | [219] |
| 1 (confirmed) | M | 45y | KD | IVIg, TCZ, topical glucocorticoids | Recovered | [220] |
| 1 (confirmed) | F | 13y | MIS-C | IVIg | Recovered | [221] |
| 45 (all confirmed) | 24 M 21 F | 7y | MIS-C | 18 IVIg, 27 glucocorticoids | 5 Deaths | [222] |
| 1 (confirmed) | M | 14y | MIS-C | None | Recovered | [223] |
| 28 (all confirmed) | 14 M 14 F | 11.4y | MIS-C, 23 shock | 20 IVIg, 24 ASA, 27 glucocorticoids | Recovered | [224] |
| 9 (all confirmed) | 4 M 5 F | 12y | MIS-C, 5 shock | 8 IVIg, 6 ASA, 7 TCZ | Recovered | [225] |
| 1 (confirmed) | M | 9y | PIMS-TS | IVIg, glucocorticoids, ASA | Recovered | [226] |
| 6 (all confirmed) | 5 M 1 F | 7.7y | MIS-C, 5 shock | 4 IVIg, 3 ASA, 2 glucocorticoids | 4 Deaths | [227] |
| 3 (all confirmed) | N.A. | N.A. | MIS-C | N.A. | N.A. | [228] |
| 5 (all confirmed) | N.A. | 84.4m | PMIS-TS | None | Recovered | [229] |
| 1 (confirmed) | F | 7m | MIS-C | Glucocorticoids | Death | [230] |
| 2 (1 confirmed) | 2 M | 31m | PIMS-TS | None | 1 Death | [231] |
| 1 (confirmed) | N.A. | N.A. | MIS-C, shock | IVIg, ASA | Recovered | [232] |
| 1 (confirmed) | F | 10y | MIS-C, shock | None | Recovered | [233] |
| 1 (confirmed) | F | 5y | MIS-C, shock | IVIg, ASA | Recovered | [234] |
| 3 (all confirmed) | 1 M 2 F | 8.3y | MIS-C, shock | 2 IVIg, 3 glucocorticoids, 1 TCZ | Recovered | [235] |
| 10 (8 confirmed) | 10 M 6 F | 9.2y | MIS-C, 10 shock | 5 IVIg, 10 glucocorticoids, 2 anakinra | Recovered 4 Coronary artery abnormalities |
[236] |
| 6 (all confirmed) | 2 M 4 F | 6y | MIS-C, 5 shock | 6 IVIg, 6 glucocorticoids | Recovered | [237] |
| 12 (all confirmed) | 9 M 3 F | 8y | MIS-C | N.A. | Recovered 2 Coronary artery abnormalities |
[238] |
| 15 (all confirmed) | 9 M 6 F | 11.5y | MIS-C | 12 IVIg, 3 glucocorticoids, 9 TCZ, 2 anakinra, 2 ASA | 1 Death 1 Coronary artery aneurysm |
[239] |
| 23 (15 confirmed) | 11 M 12 F | 7.2y | MIS-C, 15 shock | 15 IVIg, 22 glucocorticoids | Recovered | [240] |
| 1 (confirmed) | F | 14y | MIS-C, shock | IVIg, glucocorticoids, | Pseudotumor cerebri, improvement | [241] |
| 23 (4 confirmed) | 17 M 6 F | 6.5y | MIS-C, 9 shock | 23 IVIg, 15 glucocorticoids, 2 TCZ | 15 Recovered and no deaths at last follow up | [242] |
| 1 (confirmed) | M | 11y | MIS-C, shock | IVIg, ASA, glucocorticoids, infliximab | Coronary artery aneurysms, temporary pacing for atrioventricular block | [243] |
| 52 (all confirmed) | 31 M 21 F | 10.7y | MIS-C, 25 shock | 28 IVIg, 24 glucocorticoids, 3 anakinra, 1 TCZ, 1 adalimumab, 1 infliximab | 43 Recovered at last follow up | [244] |
| 27 (22 confirmed) | 14 M 13 F | 6y | MIS-C, 12 shock | 19 IVIg, 17 glucocorticoids, 17 ASA | Recovered 3 coronary artery abnormalities |
[245] |
| 3 (all confirmed) | 1 M 2 F | 8.3y | MIS-C, 3 shock | 2 IVIg, 3 glucocorticoids, 3 ASA | Recovered 3 surgery for acute appendicitis 1 coronary artery abnormalities |
[246] |
| 1 (confirmed) | M | 5y | MIS-C, shock | TCZ | Death | [70] |
| 1 (confirmed) | M | 10y | PIMS-TS, shock | IVIg, glucocorticoids, anakinra, ASA | Recovered | [247] |
| 1 (confirmed) | M | 3y | MIS-C | Glucocorticoids | Recovered | [248] |
| 10 (all confirmed) | 3 M 7 F | 9.9y | MIS-C, 1 shock | 7 IVIg, 6 glucocorticoids | Recovered | [249] |
| 33 (18 confirmed) | 19 M 14 F | 2.8y | PIMS-TS, 3 shock | 24 IVIg, 18 glucocorticoids, 3 anakinra | Recovered | [250] |
| 1 (confirmed) | F | 25y | MIS-C, shock | IVIg, ASA | Recovered | [251] |
| 54 (49 confirmed) | 25 M 29 F | 7y | MIS-C | 45 IVIg, 41 glucocorticoids, | Recovered | [252] |
| 1 (confirmed) | F | 15y | MIS-C | IVIg, glucocorticoids, ASA | Recovered Coronary artery aneurysms |
[253] |
| 1 (confirmed) | F | 8y | PIMS-TS | IVIg, glucocorticoids | Recovered | [254] |
| 2 (all confirmed) | 2 F | 11.5y | PIMS-TS, shock | None | Recovered 2 Surgery for acute appendicitis |
[255] |
| 9 (all confirmed) | N.A. | N.A. | MIS-C | N.A. | N.A. | [256] |
| 13 (all confirmed) | 23 M 18 F | ∼8.8y | MIS-C | 8 IVIg, 13 glucocorticoids, 7 anakinra | N.A. | [257] |
| 44 (40 confirmed) | N.A. | N.A. | MIS-C, 19 hepatitis | N.A. | Recovered 6 Coronary artery abnormalities |
[258] |
| 18 (all confirmed) | 14 M 4 F | 7.7y | MIS-C | N.A. | N.A. | [259] |
| 2 (1 confirmed) | 1 M 1 F | 8.5y | PIMS-TS | 1 anakinra | Recovered 1 Coronary artery abnormalities 1 Surgery for acute appendicitis |
[260] |
| 1 (confirmed) | F | 10y | MIS-C, pancreatitis | IVIg, ASA | Recovered | [261] |
| 35 (N.A.) | 22 M 13 F | 8.6y | MIS-C | 19 IVIg, 1 infliximab, glucocorticoids, ASA | Recovered 1 Ileocolic resection with ileostomy |
[262] |
Abbreviations. Confirmed: PCR or serology positive. m: months. F: female. M: male. KD: Kawasaki disease (complete: meeting American Heart Association criteria. Atypical: not fulfilling criteria for complete KD). ASA: acetylsalicylic acid. IVIg: intravenous immunoglobulins. y: years. m: months. LN: lymphadenopathy. TCZ: tocilizumab. KD: Kawasaki disease. KDSS: Kawasaki disease shock syndrome. TSS: toxic shock syndrome. ICU: intensive care unit. MIS-C: multisystem inflammatory syndrome in children. PIMS-TS: pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. N.A.: information not available.
Pediatric COVID-19 cases may develop features similar to Kawasaki disease and this is of particular interest since a role for infectious agents has previously been proposed in the pathogenesis of Kawasaki disease. This is particularly true for respiratory viruses that had previously been reported to be a ‘new’ RNA virus that affects upper respiratory tract detected in the bronchial epithelium [53,54].
The pediatric manifestations described during the pandemic are not entirely representative of Kawasaki disease since the criteria established by the 2017 American Heart Association [55] are infrequently met. As a consequence, the pediatric syndrome associated with SARS-CoV-2 infection has been coined ‘pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection’ (PIMS-TS) in Europe and ‘multisystem inflammatory syndrome in children’ (MIS-C) in the United States [56,57]. Case definitions by the World Health Organization (WHO), Royal College of Pediatrics and Child Health (UK) and Center for Disease Control (CDC, US) are slightly different but all include persistent fever, laboratory evidence of inflammation and single or multi-organ dysfunction following exclusion of other microbial causes. Confirmed SARS-CoV-2 infection is needed for meeting the criteria established by the WHO and CDC [58]. When compared with classical Kawasaki disease, newly diagnosed Kawasaki-like patients were older and had more signs of cardiac involvement, shock and MAS and required adjunctive steroid treatment more frequently [59]. In a different cohort [60], in addition, all patients had gastrointestinal symptoms, which is uncommon in typical Kawasaki disease and 10-fold higher levels of procalcitonin than those reported in classic KDSS [61]. Another study coined the syndrome ‘Kawa-COVID-19’, confirming the fact that it occurs in older age patients who commonly develop gastrointestinal symptoms and hemodynamic failure. These patients also show lower lymphocyte counts, lower platelet count, higher rate of myocarditis and resistance to therapy following a first immunoglobulin course compared to a historical cohort of patients [62]. The high prevalence of gastrointestinal symptoms has also been reported in a series of other cases [63]. Whittaker and colleagues identified three clinical patterns with different manifestations. One with persistent fever and elevated inflammatory markers, but no organ failure or manifestations of Kawasaki disease or toxic shock syndrome. Another with shock, left ventricular dysfunction, elevation of troponin and NT-proBNP. Children with the third pattern fulfilled the American Heart Association diagnostic criteria for Kawasaki disease [64]. Among causes of death, refractory shock and stroke were reported and most patients were on extracorporeal membrane oxygenation (ECMO) support [[65], [66], [67], [68], [69], [70]].
3.6. Arthritis
Only a limited number of cases of acute arthritis have been described in the context of SARS-CoV-2 infection (Table 7 ). In a study by López-González and colleagues, 81 out of 306 patients with proven COVID-19 complained of joint pain at admission, even though none had clear signs of arthritis. Four of them showed crystal-induced acute arthritis during hospitalization, demonstrated by polarized light microscopy analysis of the synovial fluid [71]. Arthritis has been described as an early sign of COVID-19 [72] or occurring after the resolution of the viral infection [73]. In both cases described above, rheumatoid factor and anti-citrullinated peptide antibodies were negative. In one case synovial tissue biopsy was performed, and signs of stromal activation, edema and moderate inflammatory features with perivascular and diffuse infiltrates were observed [72]. In a separate study, three cases of reactive arthritis following SARS-CoV-2 infection were reported; 2 of them completely recovered after NSAIDs therapy [74,75] while one case required intra-articular glucocorticoids injection with moderate improvement [76].
Table 7.
Arthritis cases associated with SARS-CoV-2 infection.
| Manifestation | Patients number | Sex | Mean or median age (years) | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Arthritis | 4 crystal-induced | M | 60.2 | Glucocorticoids (intra-articular or oral), colchicine | Recovered | [71] |
| Arthritis | 1 oligoarthritis | M | 57 | Spontaneous resolution | Recovered | [73] |
| Polyarthritis | 1 | M | 61 | Glucocorticoids, baricitinib | Recovered | [72] |
| Reactive arthritis | 1 | M | 50 | NSAIDs, intra-articular Steroid injection |
Moderate improvement | [76] |
| Crystal-induced arthritis | 4 | N.A. | N.A. | N.A. | N.A. | [71] |
| Reactive arthritis | 1 | F | 37 | Topical NSAIDs | Recovered | [75] |
| Reactive arthritis | 1 | M | 73 | NSAIDs | Recovered | [74] |
Abbreviations: M: male. NSAIDs: non-steroidal anti-inflammatory drugs. N.A.: data not available.
3.7. Skin manifestations
Numerous reports have described several different types of cutaneous manifestations similar to chilblain, urticarial eruptions, diffuse or disseminated erythema among others in COVID-19 patients. Skin lesions reported in COVID-19 patients can be classified into 4 groups: exanthema (varicella-like, papulo-vesicular and morbilliform rash), vascular (chilblain-like, purpuric/petechial and livedoid lesions), urticarial and acro-papular eruption [77]. Skin conditions belonging to the last three mentioned groups, are often described in patients with co-existing autoimmune diseases, especially connective tissue diseases. Criado and colleagues recently discussed the possible mechanisms through which SARS-CoV-2 may exert an action on the skin. Among these are the cytokine release syndrome, the activation of the coagulation and complement systems or the direct entry of the virus following infection of the endothelial cells in dermal blood vessels. Such virus-host interactions may lead to a direct and an indirect damage of the microvasculature of the skin, causing multiple dermatological conditions [78]. According to a recent study, it is possible to establish a temporal relationship between the type of skin involvement and other systemic symptoms and the severity of the disease. Vesicular lesions appear early in the disease course and seem to predate other symptoms, while chilblain-like manifestations are associated with a less severe pulmonary disease and livedo reticularis with the most severe cases of pneumonia [79]. Using the research strategy we employed, we could identify several eligible papers of case reports and case series reporting different kind of autoimmune-like skin lesions in both adults and children with confirmed/suspected COVID-19 disease (Table 8 ).
Table 8.
Autoimmune-like skin lesions in confirmed/suspected COVID-19 patients.
| Skin Lesion | Patients number (sex) | Mean or median age (years) | Treatment for skin lesions | Reference |
|---|---|---|---|---|
| Periorbital erythema | 1 F, 1 M | 46.5 | Aclometasone dipropionate 0.05% ointment; none | [263] |
| Chilblain-like lesions | 3 F, 1 M | 8.25 | None | [264] |
| Purpuric lesions | 1 M, 2 F | 46 | None | [265] |
| Acral/maculopapular and urticarial lesions | 14 M, 12 F | 28 | None | [266] |
| Livedo reticularis | M | 57 | None | [267] |
| Chilblain-like lesions | F | 35 | None | [87] |
| Chilblain-like lesions | 21 F, 19 M | 22 | None | [268] |
| Chilblain-like lesions | 4 M, 3 F | 14 | None | [269] |
| Bullous hemorrhagic vasculitis | M | 79 | Methylprednisolone | [270] |
| Catastrophic acute lower limbs necrosis | M | 83 | N.A. | [271] |
| Papular-purpuric exanthema | F | 39 | Topical glucocorticoids | [272] |
| Purpuric exanthema | M | 59 | N.A. | [273] |
| Purpuric rash | M | 58 | Mometasone furoate cream | [274] |
| Acute maculopapular eruption | M | 34 | Antihistamines | [275] |
| Chilblain lesions/livedo, Maculopapular exanthema, Palpable purpura, Acute urticaria |
5 F, 5 M 6F, 1 M 3 F, 1 M 3 F, 1 M |
39 53 62 54.5 |
N.A. | [276] |
| Urticarial Vasculitis | 1 F, 1 M | 60 | Prednisone, antihistamines | [277] |
| Schamberg's purpura | F | 13 | N.A. | [278] |
| Chilblain-like lesions | 13 F, 17 M | 11 | None | [279] |
| Maculopapular eruptions | 2 M, 5 F | 66.57 | Glucocorticoids (6/7) | [280] |
| Retiform purpura | F | 79 | N.A. | [281] |
| Pernio-like lesions | 155 F, 163 M | 25 | N.A. | [282] |
| Acral necrosis | F | 74 | N.A. | [283] |
| Cutaneous small vessel vasculitis | F | 71 | Betamethasone dipropionate 0.05% cream | [284] |
| Chilblain-like, purpuric, maculopapular lesions | 29 M, 29 F | 14 | N.A. | [285] |
| Cutaneous small vessel vasculitis | F | 83 | Prednisone | [286] |
| Acral purpuric lesions | M | 12 | N.A. | [287] |
| Urticarial lesions | F | 55 | Betamethasone 0.1% ointment, antihistamines | [288] |
| Urticarial rash | 2 F | 35 | Antihistamines | [289] |
| Acral perniosis | 5 M, 1 F | 14 | N.A. | [290] |
| Chilblain lesions | M | 16 | N.A. | [291] |
| Maculopapular rash | 3 F | 73 | None | [292] |
| Chilblain-like lesions | 142 | 27 | N.A. | [293] |
| Acro-ischemia | 3 | N.A. | N.A. | [294] |
| Chilblain-like lesions | M | 23 | N.A. | [295] |
| Vascular acrosyndromes | 2 M, 2 F | 27 | None | [296] |
| Maculopapular rash | F | 37 | None | [297] |
| Petechial skin rash | M | 48 | Betamethasone dipropionate 0.05% cream, loratadine | [298] |
| Urticarial exanthema | M | 61 | Antihistamines | [299] |
| Chilblain-like lesions | 49 M, 46 F | 23 | None | [300] |
| Vascular lesions | 7 | N.A. | None | [301] |
| Chilblain-like lesions | 35 F, 28 M | 14 | N.A. | [302] |
| Digitate Papulosquamous eruption | 1 | N.A. | None | [303] |
| Chilblain-like lesions | 2 F | 31 | N.A. | [304] |
| Maculopapular rash | 2 F, 1 M | 68 | None | [305] |
| Maculopapular/Papulosquamous rash | 6 M, 2 F | 55.6 | N.A. | [306] |
| Maculopapular/Urticarial/Purpuric/Necrotic rash | 33 M, 19 F | 58 | N.A. | [307] |
| Urticarial rash | 2 F | 48 | None | [308] |
| Urticarial rash | M | N.A. | N.A. | [309] |
| Acral vasculitis, Urticarial rash |
6 2 |
N.A. | N.A. | [310] |
| Acro-ischemia | M | 81 | Aspirin | [311] |
| Chilblain-like, Urticarial rash, Maculopapular rash, Livedo/necrosis |
48 F, 23 M 47 F, 26 M 98 F, 78 M 10 F, 11 M |
32.5 48.7 55.3 63.1 |
N.A. | [79] |
| Chilblain-like | 5 F, 14 M | 14 | N.A. | [312] |
| Acral lesions | 42 M, 32 F | 19.66 | N.A. | [313] |
| Urticarial-like | F | 60 | N.A. | [314] |
| Acute urticaria | 1 F, 1 M | 59 | Antihistamines | [315] |
| Oral erosions, petechiae | F | 19 | IVIg, methylprednisolone | [316] |
| Retiform purpura | 1 | N.A. | N.A. | [317] |
| Chilblain-like lesions | 11 M, 6 F | 32 | N.A. | [318] |
| Livedo reticularis | F | 62 | Heparin | [319] |
| Urticarial skin rash, Chilblain-like lesions |
1 F, 1 M F |
59.5 1 |
Glucocorticoids, antihistamines N.A. |
[320] |
| Livedoid retiform purpura | M | 61 | None | [321] |
| Chilblain-like lesions | 13 M, 9 F | 12 | Antihistamines | [322] |
| Chilblain-like lesions | 18 M, 9 F | 14.4 | None | [323] |
| Chilblain-like lesions | 3 F, 3 M | 35 | N.A. | [324] |
| Chilblain-like lesions | 41 | 16 | N.A. | [325] |
| Chilblain-like lesions | F | 48 | None | [326] |
| Acral lesions | 23 M, 13 F | 11.1 | Topical glucocorticoids/antibiotics or none | [327] |
| AGEP-like (exanthematous pustulosis) | M | 33 | N.A. | [328] |
| Urticarial, vesicular, maculopapular, necrotic lesions | 23 | N.A. | N.A. | [329] |
| Urticarial vasculitis | F | 64 | Antihistamines | [330] |
| Grover-like disease | M | 59 | N.A. | [331] |
| Pernio-like eruption | 4 M, 3 F | 33 | Topical/oral glucocorticoids or none | [332] |
| Chilblain-like lesions | M | 10 | Topical glucocorticoids | [333] |
| Acral lesions | 4 F, 1 M | 3 | None | [334] |
| Vascular lesions | 3 F, 7 M | 39.9 | N.A. | [335] |
| Macular eruption with vasculitis | F | 81 | None | [336] |
| Maculopapular rash | 3 F, 1 M | 21.75 | Hydrocortisone, rupatadine and none | [337] |
| Ulcers | 3 M | 65.6 | Antibiotics | [338] |
| Livedo racemosa and retiform purpura | 4 | 55 | Anticoagulation therapy | [339] |
| Enanthem | 6 | 50 | N.A. | [340] |
| Erythema nodosum | M | 42 | Topical glucocorticoids | [341] |
| Anagen effluvium, urticarial lesions and maculopapular rash | F | 35 | Low dose systemic glucocorticoids and antihistamines | [342] |
| Maculopapular, pernio-like, urticarial, vasculitic and petechial skin lesions | 9 M, 1 F | 63 | Glucocorticoids | [343] |
| Pernio-like skin lesions | F | 77 | LMW heparin | [344] |
| Acute urticaria | M | 54 | Topical glucocorticoids, antihistamines | [345] |
| Maculopapular rash | M | 52 | None | [346] |
| Angioedema and urticaria | M | 40 | Antihistamines | [347] |
| Livedo reticularis | F | 34 | None | [348] |
| Erythema nodosum | F | 54 | Naproxen, hydroxyzine | [349] |
| Chilblain-like lesions | 12 M, 12 F | 32 | N.A. | [350] |
| Acro-ischemic lesions | M | 10 | None | [351] |
| Guttate psoriasis | M | 38 | Topical betamethasone 0.025% | [352] |
| Erythematosus rash | M | 69 | Topical glucocorticoids, antihistamines | [353] |
| Auricle perniosis | F | 35 | Methylprednisolone, heparin | [354] |
| Oral vesicles and maculopapular rash | F | 9 | N.A. | [355] |
| Necrotic acral lesions | M | 59 | Tocilizumab | [356] |
| Urticaria and angioedema | F | 46 | Prednisolone, antihistamines | [357] |
| Chilblains and retinal vasculitis | M | 11 | N.A. | [358] |
| Minor aphthae | 1 F, 3 M | 33 | N.A. | [359] |
| Vascular, urticarial and acropapular lesions | 137 | N.A. | N.A. | [360] |
| Petechial and urticarial lesions | F | 33 | Methylprednisolone, antibiotics, anticoagulation therapy | [361] |
| Purpuric rash and maculopapular eruption | F | 42 | N.A. | [362] |
| Maculopapular lesions | F | 57 | None | [363] |
| Chilblain-like lesions | 8 M, 8 F | 10 | N.A. | [364] |
| Chilblain-like, maculopapular exanthema, urticarial, livedo reticularis-like lesions | 13 | N.A. | N.A. | [365] |
| Acrofacial purpura, necrotic ulcerations | 3 F, 18 M | 57 | N.A. | [366] |
| Mucocutaneous manifestations (e.g. aphthous stomatitis, maculopapular acral rash, urticaria) | 304 | N.A. | N.A. | [367] |
| Chilblain-like lesions | 4 M, 5 F | 11 | N.A. | [368] |
Abbreviations: N.A.: information not available. IVIg: immunoglobulins. F: female. M: male.
3.8. Autoimmune-like neurologic disease
Acute inflammatory neuropathies resembling Guillain-Barré syndrome (GBS) have been reported in patients with COVID-19. The inflammatory cascade triggered by SARS-CoV-2 may affect the nervous system and the anosmia (loss of sense of smell) and ageusia (loss of sense of taste) have been reported in up to 60% of the infected patients that corroborates the theory of its neurovirulence [80]. The previously known coronaviruses SARS-CoV and MERS-CoV showed neurotropism, entering the brain via olfactory nerves [81]; the often persistent anosmia and ageusia in COVID-19 patients suggest this new coronavirus is also able to target olfactory neurons. Likewise, these viruses can enter the central nervous system via retrograde axonal transport through other cranial and peripheral nerves [81]. Thus, it is not surprising to note the increasing number of reports on COVID-19 patients with acute immune-mediated like neurologic signs and symptoms, published in the literature since the beginning of the pandemic. A recent systematic review showed that published cases of GBS induced by SARS-CoV-2 reported mostly a sensorimotor, demyelinating GBS with a typical clinical presentation, which is similar to GBS cases due to other etiological factors [82]. Table 9 summarizes the case reports/series identified through our research strategy.
Table 9.
Autoimmune-like neurologic lesions in COVID-19 patients.
| Neurologic condition | Patients number (sex) | Mean or median age (years) | Treatment | Reference |
|---|---|---|---|---|
| Guillain-Barré syndrome | M | 65 | IVIg | [369] |
| Guillain-Barré syndrome | M | 21 | Plasma exchange | [370] |
| Guillain-Barré syndrome | 1 | N.A. | N.A. | [371] |
| Guillain-Barré syndrome | F | 70 | IVIg | [372] |
| Guillain-Barré syndrome | M | 64 | N.A. | [373] |
| Guillain-Barré syndrome | M | 41 | IVIg | [374] |
| Guillain-Barré syndrome | F | 53 | N.A. | [375] |
| Guillain-Barré syndrome | M | 71 | IVIg | [376] |
| Miller-Fisher syndrome | M | 36 | IVIg | [377] |
| Guillain-Barré syndrome | F | 66 | IVIg | [378] |
| Guillain-Barré syndrome | M | 54 | N.A. | [379] |
| Guillain-Barré/Miller-Fisher overlap syndrome AMSAN |
M M |
55 60 |
IVIg IVIg |
[380] |
| Guillain-Barré syndrome | F | 54 | IVIg | [381] |
| Miller-Fisher syndrome | F | 51 | IVIg | [382] |
| Guillain-Barré syndrome with leptomeningeal enhancement |
F | 56 | IVIg | [383] |
| Guillain-Barré syndrome | F | 76 | N.A. | [384] |
| Guillain-Barré syndrome | M | 50 | IVIg | [385] |
| Guillain-Barré syndrome | M | 64 | IVIg | [386] |
| Guillain-Barré syndrome | 1 M, 1 F | 56.5 | IVIg | [387] |
| Guillain-Barré syndrome | M | 72 | IVIg | [388] |
| Guillain-Barré syndrome | 4 M, 1 F | 58.4 | IVIg | [389] |
| Guillain-Barré syndrome | 3 F | 58.6 | IVIg | [390] |
| Guillain-Barrè syndrome | M | 68 | IVIg | [391] |
| Guillain-Barré syndrome | M | ~70 | IVIg | [392] |
| Guillain-Barré syndrome with facial diplegia | M | 58 | IVIg | [393] |
| Guillain-Barré syndrome | M | ~60 | IVIg | [394] |
| Guillain-Barré syndrome | F | 61 | IVIg | [395] |
| Guillain-Barré syndrome with facial diplegia | M | 61 | Prednisone | [396] |
| Guillain-Barré syndrome | M | 54 | IVIg | [397] |
| Guillain-Barré syndrome | M | 57 | IVIg | [398] |
| ADEM | F | 64 | IVIg | [399] |
| ADEM-like condition | M | 71 | N.A. | [400] |
| Guillain-Barré syndrome | F | 66 | IVIg | [401] |
| CIS | F | 42 | N.A. | [402] |
| ADEM | F | 51 | Methylprednisolone I.V., IVIg | [403] |
| ANE | F | 59 | High dose dexamethasone | [404] |
| Acute demyelination | F | 54 | Glucocorticoids | [405] |
| Demyelinating lesions | F | 54 | High dose dexamethasone Antiepileptic therapy |
[406] |
| AMSAN | F | 70 | IVIg | [407] |
| Guillain-Barré syndrome | 7 M | 57 | IVIg | [408] |
| Guillain-Barré syndrome | 1 M, 1 F | 26 | Plasma exchange, I.V. labetalol, IVIg | [409] |
| ANM and AMAN | M | 61 | Methylprednisolone I.V., plasma exchange | [410] |
| Guillain-Barré syndrome | M | 57 | IVIg | [411] |
| AIDP | M | 68 | IVIg | [412] |
| Guillain-Barré syndrome | M | 30 | IVIg, LMW heparin | [413] |
| Guillain-Barré-Strohl syndrome | M | 54 | IVIg | [414] |
| Guillain-Barré syndrome | M | 77 | IVIg | [415] |
| Guillain-Barré syndrome | 4 M, 1 F | 72.6 | IVIg, Methylprednisolone | [416] |
| Guillain-Barré syndrome | F | 67 | Plasma exchange | [417] |
| Guillain-Barré syndrome | M | 49 | IVIg | [418] |
| Miller-Fisher syndrome | M | 63 | None | [419] |
| Guillain-Barré syndrome | M | 49 | IVIg | [420] |
| Guillain-Barré syndrome | M | 11 | IVIg | [421] |
| Guillain-Barré syndrome | M | 15 | IVIg | [422] |
| Miller-Fisher syndrome | M | 31 | IVIg | [423] |
| Guillain-Barré syndrome | M | 75 | I.V. glucocorticoids, IVIg | [424] |
| AMAN | F | 70 | Plasma exchange, IVIg | [425] |
| Miller-Fisher syndrome | M | 61 | Plasma exchange, IVIg | [426] |
| Guillain-Barré syndrome | 11 M, 6 F | 53 | Plasma exchange, IVIg | [427] |
| ATM | M | 24 | I.V. methylprednisolone | [428] |
| Guillain-Barré syndrome | F | 56 | N.A. | [429] |
| Guillain-Barré syndrome | M | 48 | Plasma exchange | [430] |
| Guillain-Barré syndrome | F | 72 | IVIg | [431] |
| Guillain-Barré syndrome | M | 69 | IVIg | [432] |
| Guillain-Barré syndrome | F | 58 | Plasma exchange | [433] |
| Miller-Fisher syndrome, polyneuritis | M M |
50, 39 |
IVIg, acetaminophen | [434] |
| ATM | M | 60 | Methylprednisone | [435] |
| ANM | F | 69 | Methylprednisolone, plasma exchange | [436] |
| ATM | F | 59 | Methylprednisolone | [437] |
| Miller-Fisher syndrome | F | 74 | IVIg | [438] |
| Miller-Fisher syndrome | F | 50 | IVIg | [439] |
| Guillain-Barré syndrome | F | 58 | IVIg | [440] |
Abbreviations: IVIg: intravenous immunoglobulins. N.A.: information not available. M: male. F: female. I.V.: intravenous AMSAN: acute motor sensory axonal neuropathy. ADEM: acute disseminated encephalomyelitis. CIS: clinically isolated syndrome. ANE: acute necrotizing encephalopathy. ATM: acute transverse myelitis. ANM: acute necrotizing myelitis.
4. Discussion
Since February 2020, nearly all the efforts of the international scientific and medical community have been focusing on understanding COVID-19 and our knowledge continues to grow exponentially, but much remains to be elucidated both in terms of pathophysiology and clinical presentation. One of the fundamental issues with regards to COVID-19 is whether the viral infection triggers autoimmunity and is a contributing factor to the risks of complications that develop, or whether patients with autoimmune diseases are at increased risk of infection with SARS-CoV-2 or have a more severe disease outcome. We shall first describe issues related to reports of autoimmune manifestations in COVID-19 patients and subsequently attempt to summarize results of COVID-19 in patients with autoimmune disease. During the pandemic, several autoimmune phenomena have been described to co-occur with or follow COVID-19 and it seems that the inflammatory response is similar in COVID-19 and autoimmunity [7]. In this systematic literature review we analyzed most if not all the reported cases of autoimmune-like manifestations in COVID-19 patients up to September 2020. The results of our research showed a variety of phenomena involving the skin, nervous system, vessels, hematopoietic system and joints. This is relatively not unexpected, considering that viruses are well established triggers of autoimmunity in genetically susceptible individuals. Molecular mimicry, bystander activation and the epitope spreading are well-established proposed mechanisms to explain this link [83]. Moreover, HLA (both class I and class II) and non-HLA polymorphisms are associated with autoimmune diseases and the control of viral infections is largely mediated by the recognition of viral peptides in association with HLA-class I molecules by effector CD8+ T cells [84]. Of note, a recent study identified an association of HLA-DRB1*15:01, HLADQB1*06:02 (MHC-class II) and HLAB*27:07 (MHC-class I) in 99 severe COVID-19 Italian patients [85] and each of these alleles are known to be associated with autoimmunity [86]. Thus, it is possible that COVID-19 patients who express one of these alleles are at increased risk of developing autoimmune-like manifestations.
In light of these considerations, it is likely than the autoimmune manifestations described in COVID-19 represent more the results of the inflammatory cascade and the immune activation triggered by the virus rather than a direct effect of the virus per se. SARS-CoV-2 RNA or proteins have not been detected in the synovia or in the cerebrospinal fluid in COVID-19 patients experiencing arthritis or GBS, however, in chilblain-like lesions immunohistochemical techniques demonstrated the presence of SARS-CoV-2 spike protein in the cytoplasm of cutaneous dermal vessels [87], suggesting a direct role of the virus in conferring damage to the skin and forming skin lesions.
In general, most autoimmune diseases are known to more frequently occur in women compared to men, as estrogens are generally considered as enhancers of immunity while androgens are immunosuppressants, also reflecting the different susceptibility of genders to infections [88]. Our research shows a slightly higher prevalence of hemolytic anemia, immune thrombocytopenia and autoimmune-like skin lesions in women with COVID-19; on the contrary, anti-phospholipid syndrome, Kawasaki-like syndrome and arthritis cases appeared more frequent in men.
Some studies have investigated gender differences in COVID-19. Results of such studies showed that in fact there is no disparity in the prevalence of COVID-19 between men and women, however, male patients have a higher mortality and a more severe disease [89]. The gender-related differences in the spectrum of immune responses might explain both the outcome and the clinical presentation, even in terms of autoimmune-like phenomena.
The different mechanisms by which the virus can induce autoimmunity account for differences in the timing of appearance of clinical manifestation. In fact, some of the clinical manifestations can present at the beginning of the infection [12] and in some cases even in patients with mild COVID-19-related symptoms [33]. Therefore, when evaluating a patient with these manifestations, it is advisable to exclude SARS-CoV-2 positivity. On the other hand, in patients with COVID-19, after weeks from severe infection, seroconversion can induce negative outcomes, as auto-antibodies can be elicited with development of complications, as in some cases of ITP or in the appearance of APLs [35,44].
Another aspect to reflect on is the management of these conditions. Typically, glucocorticoids and immunomodulatory agents represent the gold standard treatment for autoimmune diseases; however, glucocorticoids have been used with caution or completely avoided in COVID-19 patients, as during the first months of the pandemic there were alerts of possible worsening of the viral infection after glucocorticoids, that was the basis for the notes of caution [36]. More recent data, though, support a beneficial role of glucocorticoids, in particular dexamethasone, in the treatment of COVID-19 [90]. We could hypothesize that treating severe cases with glucocorticoids may help reduce the development of autoimmune complications, but a particular attention to the timing of administration has to be considered in order to avoid infection spreading. Infections can trigger autoimmune diseases even after a long latency time, certainly, it would be relevant to investigate whether recovered COVID-19 patients are at greater risk of developing these diseases, indicating that immune dysregulation can be induced even after the infection has resolved.
Patients affected by autoimmune diseases are reasoned to be generally more susceptible to infections, due to the use of the immunosuppressive treatments and particularly when the disease status is not fully under control [91]. In some cases, in fact, these patients can develop disease flares or new autoimmune complications in the context of SARS-CoV-2 infection, as described for patients with pre-existing hemolytic anemia [12], ITP [29,30], and arthritis [71]. It is to be noted that diverse outcomes of SARS-CoV-2 infection in patients with existing autoimmune disease appear in the literature. A possible explanation for these observations relies on the fact that during disease remission these patients have an immune system that is primed to down-regulate the inflammation, while during flares the regulatory mechanisms become dysfunctional enhancing the deleterious effects of the SARS-CoV-2 infection and contributing to the severity of the disease. Since the beginning of the pandemic this subpopulation of patients has been investigated and multiple studies have attempted to assess the risk and outcome of COVID-19 in patients affected by autoimmune diseases [92,93]. On one hand, taking into account the previous considerations, this category of patients should be at higher risk of SARS-CoV-2 infection. On the other hand, they could be protected from a worse disease outcome being under therapy with b-DMARDs or ts-DMARDs, which have been considered useful to treat some features of COVID-19 [94]. According to some data on different cohorts, autoimmune patients do not seem to have an increased risk of SARS-CoV-2 infection compared to the general population and also the disease outcome do not appear to be more severe [95]. In particular, a recently published Italian study investigated the prevalence of SARS-CoV-2 infection in a population affected by inflammatory arthritis and treated with immunosuppressant drugs compared with the general population, without finding a higher prevalence of COVID-19 compared to the general population [96]. Similar results also emerged from a study performed in Spain including a cohort of adult and pediatric patients affected by rheumatic diseases [97]. A preliminary study from the Italian Registry of the Italian Society for Rheumatology showed that immunosuppressive treatments were not significantly associated with an increased risk of intensive care unit admission, or mechanical ventilation, or death [98].
Nonetheless, it is currently impossible to draw any conclusion and it is necessary to be cautious until data on larger cohorts of patients will be available. The efforts of the COVID-19 Global Rheumatology Alliance are focusing in this direction [99].
5. Conclusions
SARS-CoV-2 infection shares features with autoimmune diseases, as it can induce clinical manifestations like Guillain-Barré syndrome, arthritis, antiphospholipid syndrome and chilblain-like lesions. Glucocorticoids, high dose intravenous immunoglobulins, cytokine blockers and immunomodulatory drugs seem to play a relevant role in the management of the disease, as it happens in autoimmune disorders. The number of case reports describing autoimmune-like phenomena in COVID-19 is increasing, and these conditions can involve various organs and systems, thus requiring specific knowledge by specialized physicians and a multidisciplinary approach. It is important to raise awareness about possible long-term complications related to the viral infections and thus the follow-up of recovered COVID-19 patients is encouraged.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interests
None.
Acknowledgements
We would like to thank Mr. Andrea Pederzani for his precious help and contribution to this research.
References
- 1.Gates B. Responding to covid-19 — a once-in-a-century pandemic? N. Engl. J. Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117900. 117900-117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceribelli A., Motta F., De Santis M., Ansari A.A., Ridgway W.M., Gershwin M.E., et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102442. 102442-102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114 doi: 10.1016/j.jaut.2020.102506. 102506-102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R., et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102524. 102524-102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joo Y.B., Lim Y.-H., Kim K.-J., Park K.-S., Park Y.-J. Respiratory viral infections and the risk of rheumatoid arthritis. Arthritis Res. Ther. 2019;21 doi: 10.1186/s13075-019-1977-9. 199-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Liebman H.A., Weitz I.C. Vol. 101. Medical Clinics of North America; 2017. Autoimmune hemolytic anemia; pp. 351–359. [DOI] [PubMed] [Google Scholar]
- 12.Lopez C., Kim J., Pandey A., Huang T., DeLoughery T.G. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br. J. Haematol. 2020;190:31–32. doi: 10.1111/bjh.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zagorski E., Pawar T., Rahimian S., Forman D. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID-19) Br. J. Haematol. 2020 doi: 10.1111/bjh.16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahlster L., Weichert-Leahey N., Trissal M., Grace R.F., Sankaran V.G. COVID-19 presenting with autoimmune hemolytic anemia in the setting of underlying immune dysregulation. Pediatr. Blood Canc. 2020;67 doi: 10.1002/pbc.28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angileri F., Légaré S., Marino Gammazza A., Conway de Macario E., Macario A.J.L., Cappello F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br. J. Haematol. 2020;190:e92–e93. doi: 10.1111/bjh.16883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M., Nguyen C.B., Yeung Z., Sanchez K., Rosen D., Bushan S. Evans syndrome in a patient with COVID-19. Br. J. Haematol. 2020;190:e59–e61. doi: 10.1111/bjh.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadlamudi G., Hong L., Keerthy M. Evans syndrome associated with pregnancy and COVID-19 infection. Case Rep Obstet Gynecol. 2020;2020 doi: 10.1155/2020/8862545. 8862545-8862545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazarian G., Quinquenel A., Bellal M., Siavellis J., Jacquy C., Re D., et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br. J. Haematol. 2020;190:29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou M., Qi J., Li X., Zhang Z., Yao Y., Wu D., et al. The proportion of patients with thrombocytopenia in three human-susceptible coronavirus infections: a systematic review and meta-analysis. Br. J. Haematol. 2020;189:438–441. doi: 10.1111/bjh.16655. [DOI] [PubMed] [Google Scholar]
- 20.Yang M., Ng M.H.L., Li C.K. Thrombocytopenia in patients with severe acute respiratory syndrome (review) Hematology. 2005;10:101–105. doi: 10.1080/10245330400026170. [DOI] [PubMed] [Google Scholar]
- 21.Magdi M., Rahil A. Severe immune thrombocytopenia complicated by intracerebral haemorrhage associated with coronavirus infection: a case report and literature review. Eur J Case Rep Intern Med. 2019;6 doi: 10.12890/2019_001155. 001155-001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel P.A., Chandrakasan S., Mickells G.E., Yildirim I., Kao C.M., Bennett C.M. Severe pediatric COVID-19 presenting with respiratory failure and severe thrombocytopenia. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao H.S., Chason H.M., Fearon D.M. Immune thrombocytopenia (ITP) in a pediatric patient positive for SARS-CoV-2. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1419. [DOI] [PubMed] [Google Scholar]
- 24.Soares A.C.C.V., Loggetto S.R., Manga F.C.M., Faustino L.R., Braga J.A.P. Outcome of SARS-CoV-2 and immune thrombocytopenia in a pediatric patient. Hematol Transfus Cell Ther. 2020 doi: 10.1016/j.htct.2020.1009.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenzweig J.D., McThenia S.S., Kaicker S. SARS-CoV-2 infection in two pediatric patients with immune cytopenias: a single institution experience during the pandemic. Pediatr. Blood Canc. 2020;67 doi: 10.1002/pbc.28503. e28503-e28503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X., Yang Q., Wang Y., Wu Y., Xu J., Yu Y., et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemostasis. 2020;18:1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Z., Chen W., Liang W., Xu C., Sun W., Yi Y. Severe exacerbation of immune thrombocytopenia and COVID-19: the favorable response to corticosteroid-based therapy-a case report. Ann. Hematol. 2020:1–3. doi: 10.1007/s00277-020-04070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merli M., Ageno W., Sessa F., Salvini M., Caramazza D., Mora B., et al. Recurrence of immune thrombocytopenia at the time of SARS-CoV-2 infection. Ann. Hematol. 2020;99:1951–1952. doi: 10.1007/s00277-020-04130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nesr G., Garnett C., Bailey C., Koshy R., Arami S. Immune thrombocytopenia flare with mild COVID-19 infection in pregnancy: a case report. Br. J. Haematol. 2020;190:e146–e148. doi: 10.1111/bjh.16928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang M.W., Nur E., Biemond B.J. Immune thrombocytopenia due to COVID-19 during pregnancy. Am. J. Hematol. 2020;95:E191–E192. doi: 10.1002/ajh.25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bomhof G., Mutsaers P.G.N.J., Leebeek F.W.G., Te Boekhorst P.A.W., Hofland J., Croles F.N., et al. COVID-19-associated immune thrombocytopenia. Br. J. Haematol. 2020;190:e61–e64. doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed M.Z., Khakwani M., Venkatadasari I., Horgan C., Giles H., Jobanputra S., et al. Thrombocytopenia as an initial manifestation of COVID-19; case series and literature review. Br. J. Haematol. 2020;189:1057–1058. doi: 10.1111/bjh.16769. [DOI] [PubMed] [Google Scholar]
- 34.Albiol N., Awol R., Martino R. Autoimmune thrombotic thrombocytopenic purpura (TTP) associated with COVID-19. Ann. Hematol. 2020;99:1673–1674. doi: 10.1007/s00277-020-04097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W., Li Z., Yang B., Wang P., Zhou Q., Zhang Z., et al. Delayed-phase thrombocytopenia in patients with coronavirus disease 2019 (COVID-19) Br. J. Haematol. 2020;190:179–184. doi: 10.1111/bjh.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selmi C., De Santis M., Battezzati P.M., Generali E., Lari S.A., Ceribelli A., et al. Anti-phospholipid antibody prevalence and association with subclinical atherosclerosis and atherothrombosis in the general population. Int. J. Cardiol. 2020;300:209–213. doi: 10.1016/j.ijcard.2019.10.042. [DOI] [PubMed] [Google Scholar]
- 40.Manoussakis M.N., Tzioufas A.G., Silis M.P., Pange P.J., Goudevenos J., Moutsopoulos H.M. High prevalence of anti-cardiolipin and other autoantibodies in a healthy elderly population. Clin. Exp. Immunol. 1987;69:557–565. [PMC free article] [PubMed] [Google Scholar]
- 41.Abdel-Wahab N., Lopez-Olivo M.A., Pinto-Patarroyo G.P., Suarez-Almazor M.E. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus. 2016;25:1520–1531. doi: 10.1177/0961203316640912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdel-Wahab N., Talathi S., Lopez-Olivo M.A., Suarez-Almazor M.E. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2017;27:572–583. doi: 10.1177/0961203317731532. [DOI] [PubMed] [Google Scholar]
- 43.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemostasis. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao M., Zhang Y., Zhang S., Qin X., Xia P., Cao W., et al. Brief report: anti-phospholipid antibodies in critically ill patients with coronavirus disease 2019 (COVID-19) Arthritis Rheum. 2020 doi: 10.1002/art.41425. [DOI] [Google Scholar]
- 45.Galeano-Valle F., Oblitas C.M., Ferreiro-Mazón M.M., Alonso-Muñoz J., del Toro-Cervera J., di Natale M., et al. Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb. Res. 2020;192:113–115. doi: 10.1016/j.thromres.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pineton de Chambrun M., Frere C., Miyara M., Amoura Z., Martin-Toutain I., Mathian A., et al. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: a link with hypercoagulability? J. Intern. Med. 2020 doi: 10.1111/joim.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connell N.T., Battinelli E.M., Connors J.M. Coagulopathy of COVID-19 and antiphospholipid antibodies. J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang N. Response to "Lupus anticoagulant is frequent in patients with Covid-19" (JTH-2020-00483) J. Thromb. Haemostasis. 2020;18:2065–2066. doi: 10.1111/jth.14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 51.Kanegaye J.T., Wilder M.S., Molkara D., Frazer J.R., Pancheri J., Tremoulet A.H., et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W., Gong F., Zhu W., Fu S., Zhang Q. Macrophage activation syndrome in Kawasaki Disease: more common than we thought? Semin. Arthritis Rheum. 2015;44:405–410. doi: 10.1016/j.semarthrit.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Rowley A.H., Baker S.C., Shulman S.T., Garcia F.L., Fox L.M., Kos I.M., et al. RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PloS One. 2008;3 doi: 10.1371/journal.pone.0001582. e1582-e1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowley A.H., Baker S.C., Shulman S.T., Rand K.H., Tretiakova M.S., Perlman E.J., et al. Ultrastructural, immunofluorescence, and RNA evidence support the hypothesis of a "new" virus associated with Kawasaki disease. J. Infect. Dis. 2011;203:1021–1030. doi: 10.1093/infdis/jiq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCrindle Brian W., Rowley Anne H., Newburger Jane W., Burns Jane C., Bolger Anne F., Gewitz M., et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for Health professionals from the American Heart association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 56.https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-risk-assessment-paediatric-inflammatory-multisystem-syndrome-15-May-2020.pdf
- 57.https://emergency.cdc.gov/han/2020/han00432.asp
- 58.Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ (Clinical research ed) 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Zheng Q., Zou L., Wu J., Guo L., Teng L., et al. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFN-γ as biomarkers for early recognition. Pediatr Rheumatol Online J. 2019;17 doi: 10.1186/s12969-018-0303-4. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pouletty M., Borocco C., Ouldali N., Caseris M., Basmaci R., Lachaume N., et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann. Rheum. Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller J., Cantor A., Zachariah P., Ahn D., Martinez M., Margolis K. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020;159:1571–1574. doi: 10.1053/j.gastro.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. J. Am. Med. Assoc. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection: a multi-institutional study from New York city. J. Pediatr. 2020;S0022–3476 doi: 10.1016/j.jpeds.2020.06.045. 0020)30747-30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hameed S., Elbaaly H., Reid C.E.L., Santos R.M.F., Shivamurthy V., Wong J., et al. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. 2020:202543. doi: 10.1148/radiol.2020202543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riollano-Cruz M., Akkoyun E., Briceno-Brito E., Kowalsky S., Posada R., Sordillo E.M., et al. Multisystem inflammatory syndrome in children (mis-C) related to COVID-19: a New York city experience. J. Med. Virol. 2020 doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaushik S., Ahluwalia N., Gangadharan S., Esperenza M., Murthy R., Ofori-Amanfo G., et al. ECMO support in SARS-CoV2 multisystem inflammatory syndrome in children in a child. Perfusion. 2020 doi: 10.1177/0267659120954386. [DOI] [PubMed] [Google Scholar]
- 71.López-González M-d-C, Peral-Garrido M.L., Calabuig I., Tovar-Sugrañes E., Jovani V., Bernabeu P., et al. Case series of acute arthritis during COVID-19 admission. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217914. [DOI] [PubMed] [Google Scholar]
- 72.Alivernini S., Cingolani A., Gessi M., Paglionico A., Pasciuto G., Tolusso B., et al. Comparative analysis of synovial inflammation after SARS-CoV-2 infection. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218315. [DOI] [PubMed] [Google Scholar]
- 73.Yokogawa N., Minematsu N., Katano H., Suzuki T. Case of acute arthritis following SARS-CoV-2 infection. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218281. [DOI] [PubMed] [Google Scholar]
- 74.Saricaoglu E.M., Hasanoglu I., Guner R. The first reactive arthritis case associated with COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Danssaert Z., Raum G., Hemtasilpa S. Reactive arthritis in a 37-year-old female with SARS-CoV2 infection. Cureus. 2020;12 doi: 10.7759/cureus.9698. e9698-e9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ono K., Kishimoto M., Shimasaki T., Uchida H., Kurai D., Deshpande G.A., et al. Reactive arthritis after COVID-19 infection. RMD Open. 2020;6 doi: 10.1136/rmdopen-2020-001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gisondi P., Piaserico S., Bordin C., Alaibac M., Girolomoni G., Naldi L. Cutaneous manifestations of SARS-CoV-2 infection: a clinical update. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Criado P.R., Abdalla B.M.Z., de Assis I.C., van Blarcum de Graaff Mello C., Caputo G.C., Vieira I.C. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? Revision of possible pathophysiologic mechanisms. Inflamm. Res. 2020;69:745–756. doi: 10.1007/s00011-020-01370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galván Casas C., Català A., Carretero Hernández G., Rodríguez-Jiménez P., Fernández-Nieto D., Rodríguez-Villa Lario A., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020:ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Sanctis P., Doneddu P.E., Vigano L., Selmi C., Nobile-Orazio E. Guillain-Barre syndrome associated with SARS-CoV-2 infection. A systematic review. Eur. J. Neurol. 2020;27:2361–2370. doi: 10.1111/ene.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gough S.C.L., Simmonds M.J. The HLA region and autoimmune disease: associations and mechanisms of action. Curr. Genom. 2007;8:453–465. doi: 10.2174/138920207783591690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Novelli A., Andreani M., Biancolella M., Liberatoscioli L., Passarelli C., Colona V.L., et al. HLA; 2020. HLA Allele Frequencies and Susceptibility to COVID-19 in a Group of 99 Italian Patients. (n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cruz-Tapias P.C.J., Anaya J.M. In: Autoimmunity: from Bench to Bedside Bogota (Colombia) Anaya J.M., Shoenfeld Y., Rojas-Villarraga A., et al., editors. El Rosario University Press; 2013. HLA association with autoimmune diseases. [PubMed] [Google Scholar]
- 87.Santonja C., Heras F., Núñez L., Requena L. COVID-19 chilblain-like lesion: immunohistochemical demonstration of SARS-CoV-2 spike protein in blood vessel endothelium and sweat gland epithelium in a polymerase chain reaction-negative patient. Br. J. Dermatol. 2020 doi: 10.1111/bjd.19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Triggianese P., Novelli L., Galdiero M.R., Chimenti M.S., Conigliaro P., Perricone R., et al. Immune checkpoint inhibitors-induced autoimmunity: the impact of gender. Autoimmun. Rev. 2020;19:102590. doi: 10.1016/j.autrev.2020.102590. [DOI] [PubMed] [Google Scholar]
- 89.Jin J.-M., Bai P., He W., Wu F., Liu X.-F., Han D.-M., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Publ. Health. 2020;8 doi: 10.3389/fpubh.2020.00152. 152-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 91.Anaya J.M. The autoimmune tautology. Arthritis Res. Ther. 2010;12:147. doi: 10.1186/ar3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haberman R., Axelrad J., Chen A., Castillo R., Yan D., Izmirly P., et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N. Engl. J. Med. 2020;383:85–88. doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gianfrancesco M.A., Hyrich K.L., Gossec L., Strangfeld A., Carmona L., Mateus E.F., et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. The Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kastritis E., Kitas G.D., Vassilopoulos D., Giannopoulos G., Dimopoulos M.A., Sfikakis P.P. Systemic autoimmune diseases, anti-rheumatic therapies, COVID-19 infection risk and patient outcomes. Rheumatol. Int. 2020;40:1353–1360. doi: 10.1007/s00296-020-04629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Favalli E.G., Agape E., Caporali R. Incidence and clinical course of COVID-19 in patients with connective tissue diseases: a descriptive observational analysis. J. Rheumatol. 2020;47:1296. doi: 10.3899/jrheum.200507. [DOI] [PubMed] [Google Scholar]
- 96.Quartuccio L., Valent F., Pasut E., Tascini C., De Vita S. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population-based study in the first two months of COVID-19 outbreak in Italy. Joint Bone Spine. 2020;87:439–443. doi: 10.1016/j.jbspin.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Michelena X., Borrell H., López-Corbeto M., López-Lasanta M., Moreno E., Pascual-Pastor M., et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin. Arthritis Rheum. 2020;50:564–570. doi: 10.1016/j.semarthrit.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scirè C.A., Carrara G., Zanetti A., Landolfi G., Chighizola C., Alunno A., et al. COVID-19 in rheumatic diseases in Italy: first results from the Italian registry of the Italian Society for Rheumatology (CONTROL-19) Clin. Exp. Rheumatol. 2020;38:748–753. [PubMed] [Google Scholar]
- 99.https://rheum-covid.org/
- 100.Capes A., Bailly S., Hantson P., Gerard L., Laterre P.-F. COVID-19 infection associated with autoimmune hemolytic anemia. Ann. Hematol. 2020;99:1679–1680. doi: 10.1007/s00277-020-04137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huscenot T., Galland J., Ouvrat M., Rossignol M., Mouly S., Sène D., et al. SARS-CoV-2-associated cold agglutinin disease: a report of two cases. Ann. Hematol. 2020;99:1943–1944. doi: 10.1007/s00277-020-04129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hindilerden F., Yonal-Hindilerden I., Akar E., Yesilbag Z., Kart-Yasar K. Severe autoimmune hemolytic anemia in COVID-19 i?nfection, safely treated with steroids. Mediterr J Hematol Infect Dis. 2020;12 doi: 10.4084/MJHID.2020.053. e2020053-e2020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patil N.R., Herc E.S., Girgis M. Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection. Hematol Oncol Stem Cell Ther. 2020;S1658–3876(1620) doi: 10.1016/j.hemonc.2020.06.005. 30116-30113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maslov D.V., Simenson V., Jain S., Badari A. COVID-19 and cold agglutinin hemolytic anemia. TH Open. 2020;4:e175–e177. doi: 10.1055/s-0040-1715791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vega Hernández P., Borges Rivas Y., Ortega Sánchez E., Marqués Cabrero A., Remedios Mateo L., Silvera Roig P., et al. Autoimmune hemolytic anemia in a pediatric patient with severe acute respiratory syndrome coronavirus 2 infection. Pediatr. Infect. Dis. J. 2020;39 doi: 10.1097/INF.0000000000002809. [DOI] [PubMed] [Google Scholar]
- 106.Moonla C., Watanaboonyongcharoen P., Suwanpimolkul G., Paitoonpong L., Jantarabenjakul W., Chanswangphuwana C., et al. Cold agglutinin disease following SARS-CoV-2 and Mycoplasma pneumoniae co-infections. Clin Case Rep. 2020 doi: 10.1002/ccr1003.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jensen C.E., Wilson S., Thombare A., Weiss S., Ma A. Cold agglutinin syndrome as a complication of Covid-19 in two cases. Clin. Infect. Prac. 2020;7–8:100041. doi: 10.1016/j.clinpr.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zulfiqar A.-A., Lorenzo-Villalba N., Hassler P., Andrès E. Immune thrombocytopenic purpura in a patient with covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2010472. e43-e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y., Zhao J., Wu J., Teng Y., Xia X. A rare case of immune thrombocytopenic purpura, secondary to COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen W., Yang B., Li Z., Wang P., Chen Y., Zhou H. Sudden severe thrombocytopenia in a patient in the recovery stage of COVID-19. Lancet Haematol. 2020;7:e624. doi: 10.1016/S2352-3026(20)30175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murt A., Eskazan A.E., Yılmaz U., Ozkan T., Ar M.C. COVID-19 presenting with immune thrombocytopenia: a case report and review of the literature. J. Med. Virol. 2020 doi: 10.1002/jmv.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Humbert S., Razanamahery J., Payet-Revest C., Bouiller K., Chirouze C. COVID-19 as a cause of immune thrombocytopenia. Med. Maladies Infect. 2020;50:459–460. doi: 10.1016/j.medmal.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lorenzo-Villalba N., Zulfiqar A.-A., Auburtin M., Schuhmacher M.H., Meyer A., Maouche Y., et al. Thrombocytopenia in the course of COVID-19 infection. Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001702. 001702-001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Revuz S., Vernier N., Saadi L., Campagne J., Poussing S., Maurier F. Immune thrombocytopenic purpura in patients with COVID-19. Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001751. 001751-001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lévesque V., É Millaire, Corsilli D., Rioux-Massé B., Carrier F.-M. Severe immune thrombocytopenic purpura in critical COVID-19. Int. J. Hematol. 2020;112:746–750. doi: 10.1007/s12185-020-02931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Artru F., Alberio L., Moradpour D., Stalder G. Acute immune thrombocytopaenic purpura in a patient with COVID-19 and decompensated cirrhosis. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayden A., Vyas-Lahar A., Rella V., Rudinskaya A. Severe refractory thrombocytopenia in a woman positive for coronavirus disease 2019 with lupus and antiphospholipid syndrome. Lupus. 2020;29:1472–1474. doi: 10.1177/0961203320940389. [DOI] [PubMed] [Google Scholar]
- 118.Bennett J., Brown C., Rouse M., Hoffmann M., Ye Z. Immune thrombocytopenia purpura secondary to COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.9083. e9083-e9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hindilerden F., Yonal-Hindilerden I., Sevtap S., Kart-Yasar K. Immune thrombocytopenia in a very elderly patient with covid-19. Front. Med. 2020;7 doi: 10.3389/fmed.2020.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deruelle E., Ben Hadj Salem O., Sep Hieng S., Pichereau C., Outin H., Jamme M. Immune thrombocytopenia in a patient with COVID-19. Int. J. Hematol. 2020;112:883–888. doi: 10.1007/s12185-020-02943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mahévas M., Moulis G., Andres E., Riviere E., Garzaro M., Crickx E., et al. Clinical characteristics, management and outcome of COVID-19-associated immune thrombocytopenia: a French multicentre series. Br. J. Haematol. 2020 doi: 10.1111/bjh.17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kondo Y., Kaneko Y., Oshige T., Fukui H., Saito S., Okayama M., et al. Exacerbation of immune thrombocytopaenia triggered by COVID-19 in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218157. [DOI] [PubMed] [Google Scholar]
- 123.Martincic Z., Skopec B., Rener K., Mavric M., Vovko T., Jereb M., et al. Severe immune thrombocytopenia in a critically ill COVID-19 patient. Int. J. Infect. Dis. 2020;99:269–271. doi: 10.1016/j.ijid.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Malik J., Javaid M., Majedi O., Ishaq U., Zahid T. Paying in blood: a case of thrombocytopenia in covid-19. Cureus. 2020;12 doi: 10.7759/cureus.9791. e9791-e9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pascolini S., Granito A., Muratori L., Lenzi M., Muratori P. Coronavirus disease associated immune thrombocytopenia: causation or correlation? J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patel T., Stanton N., Gkikas I., Triantafyllopoulou D.I.D. Severe thrombocytopaenia secondary to COVID-19. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soares A.C.C.V., Loggetto S.R., Manga F.C.M., Faustino L.R., Braga J.A.P. Outcome of SARS-CoV-2 and immune thrombocytopenia in a pediatric patient. Hematol. Trans. Cell Ther. 2020 doi: 10.1016/j.htct.2020.09.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kewan T., Almhana F., Schwartzman L., Daw H., Haddad A. COVID-19 patient with immune thrombocytopenic purpura. Int J Lab Hematol. 2020 doi: 10.1111/ijlh.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pedro Lobos B., Constanza Lobos S., Paola Aravena R. Immune thrombocytopenic purpura associated with coronavirus 19 infection IN an asymptomatic young healthy patient. JAAD Case Rep. 2020;6:1129–1131. doi: 10.1016/j.jdcr.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Levraut M., Ottavi M., Lechtman S., Mondain V., Jeandel P.-Y. Immune thrombocytopenic purpura after COVID-19 infection. Int J Lab Hematol. 2020 doi: 10.1111/ijlh.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2007575. e38-e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hossri S., Shadi M., Hamarsha Z., Schneider R., El-Sayegh D. Clinically significant anticardiolipin antibodies associated with COVID-19. J. Crit. Care. 2020;59:32–34. doi: 10.1016/j.jcrc.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valencia Manrique J., Ghosh K., Boma N. Anticardiolipin antibodies and COVID-19-A case report from America. J. Med. Virol. 2020 doi: 10.1002/jmv.26135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zayet S., Klopfenstein T., Kovẚcs R., Stancescu S., Hagenkötter B. Acute cerebral stroke with multiple infarctions and COVID-19, France, 2020. Emerg. Infect. Dis. J. 2020:26. doi: 10.3201/eid2609.201791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sung J., Anjum S. Coronavirus disease 2019 (COVID-19) infection associated with antiphospholipid antibodies and four-extremity deep vein thrombosis in a previously healthy female. Cureus. 2020;12 doi: 10.7759/cureus.8408. e8408-e8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190 doi: 10.1016/j.thromres.2020.04.014. 62-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bowles L., Platton S., Yartey N., Dave M., Lee K., Hart D.P., et al. Lupus anticoagulant and abnormal coagulation tests in patients with covid-19. N. Engl. J. Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Previtali G., Seghezzi M., Moioli V., Sonzogni A., Cerutti L., Marozzi R., et al. The pathogenesis of thromboembolic disease in covid-19 patients: could be a catastrophic antiphospholipid syndrome? Thromb. Res. 2020;194:192–194. doi: 10.1016/j.thromres.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gatto M., Perricone C., Tonello M., Bistoni O., Cattelan A.M., Bursi R., et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin. Exp. Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- 141.Devreese K.M.J., Linskens E.A., Benoit D., Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Siguret V., Voicu S., Neuwirth M., Delrue M., Gayat E., Stépanian A., et al. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb. Res. 2020;195:74–76. doi: 10.1016/j.thromres.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fan S., Xiao M., Han F., Xia P., Bai X., Chen H., et al. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00806. 806-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shoskes A., Migdady I., Fernandez A., Ruggieri P., Rae-Grant A. Cerebral microhemorrhage and purpuric rash in COVID-19: the case for a secondary microangiopathy. J. Stroke Cerebrovasc. Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105111. 105111-105111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rothstein A., Oldridge O., Schwennesen H., Do D., Cucchiara B.L. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51:e219–e222. doi: 10.1161/STROKEAHA.120.030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reyes Gil M., Barouqa M., Szymanski J., Gonzalez-Lugo J.D., Rahman S., Billett H.H. Assessment of lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19) JAMA network open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17539. e2017539-e2017539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Amezcua-Guerra L.M., Rojas-Velasco G., Brianza-Padilla M., Vázquez-Rangel A., Márquez-Velasco R., Baranda-Tovar F., et al. Presence of antiphospholipid antibodies in COVID-19: case series study. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218100. [DOI] [PubMed] [Google Scholar]
- 148.Yarlagadda K., Mi K., Sendil S., Koons C.L., Komanduri S., Cinicola J.T. A 31-year-old man with COVID-19-associated empyema and lupus anticoagulant. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.926623. e926623-e926623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gutiérrez López de Ocáriz X., Castro Quismondo N., Vera Guerrero E., Rodríguez Rodríguez M., Ayala Díaz R., Martínez López J. Thrombosis and antiphospholipid antibodies in patients with SARS-COV-2 infection (COVID-19) Int J Lab Hematol. 2020 doi: 10.1111/ijlh.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gemcioglu E., Erden A., Davutoglu M., Karabuga B., Kucuksahin O. Acute ischemic stroke in a lupus anticoagulant-positive woman with COVID-19. J. Clin. Rheumatol. 2020;26:236–237. doi: 10.1097/RHU.0000000000001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tvito A., Ben-Chetrit E., Zimmerman F.S., Asher E., Helviz Y. Lupus anticoagulant in patients with COVID-19. Int J Lab Hematol. 2020 doi: 10.1111/ijlh.13334. [DOI] [PubMed] [Google Scholar]
- 152.Maria A.T.J., Diaz-Cau I., Benejean J.-M., Nutz A., Schiffmann A., Biron-Andreani C., et al. Flare of antiphospholipid syndrome in the course of COVID-19. TH Open. 2020;4:e207–e210. doi: 10.1055/s-0040-1716735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ferrari E, Sartre B, Squara F, Contenti J, Occelli C, Lemoel F et al. High prevalence of acquired thrombophilia without prognosis value in Covid‐19 patients. J Am Heart Assoc;0:e017773. [DOI] [PMC free article] [PubMed]
- 154.Pascolini S., Vannini A., Deleonardi G., Ciordinik M., Sensoli A., Carletti I., et al. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin Transl Sci. 2020;29:12908. doi: 10.1111/cts.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hasan Ali O., Bomze D., Risch L., Brugger S.D., Paprotny M., Weber M., et al. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clin. Infect. Dis. 2020:ciaa1496. doi: 10.1093/cid/ciaa1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Y., Cao W., Jiang W., Xiao M., Li Y., Tang N., et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J. Thromb. Thrombolysis. 2020;50:580–586. doi: 10.1007/s11239-020-02182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yasri S., Wiwanitkit V. COVID-19, antiphospholipid syndrome and thrombosis. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620931927. 1076029620931927-1076029620931927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bertin D., Brodovitch A., Beziane A., Hug S., Bouamri A., Mege J.L., et al. Anticardiolipin IgG autoantibody level is an independent risk factor for COVID-19 severity. Arthritis Rheum. 2020 doi: 10.1002/art.41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Renaud-Picard B., Gallais F., Ohana M., Zeyons F., Kretz B., Andre J., et al. Bilateral acute cardioembolic limb ischemia after coronavirus disease 2019 pneumonia in a lung transplant recipient: a case report. Transplant. Proc. 2020;S0041–1345 doi: 10.1016/j.transproceed.2020.06.024. 0020)32588-32584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zarza J., Von Horoch J., Aguayo N., Báez E. Evans syndrome associated with antiphospholipid antibodies in a patient with SARS-COV-2 infection. Hematol Transfus Cell Ther. 2020 doi: 10.1016/j.htct.2020.1008.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mantovani Cardoso E., Hundal J., Feterman D., Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin. Rheumatol. 2020;39:2811–2815. doi: 10.1007/s10067-020-05310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Slimani Y., Abbassi R., El Fatoiki F.-Z., Barrou L., Chiheb S. Systemic lupus erythematosus and varicella-like rash following COVID-19 in a previously healthy patient. J. Med. Virol. 2020 doi: 10.1002/jmv.26513. [DOI] [PubMed] [Google Scholar]
- 163.Bonometti R., Sacchi M.C., Stobbione P., Lauritano E.C., Tamiazzo S., Marchegiani A., et al. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur. Rev. Med. Pharmacol. Sci. 2020;24:9695–9697. doi: 10.26355/eurrev_202009_23060. [DOI] [PubMed] [Google Scholar]
- 164.El Aoud S., Morin C., Lorriaux P., Obert J., Sorial D., Chaabouni T., et al. SARS CoV-2 presenting as lupus erythematosus-like syndrome. Disaster Med. Public Health Prep. 2020:1–9. doi: 10.1017/dmp.2020.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raghavan S., Gonakoti S., Asemota I.R., Mba B. A case of systemic lupus erythematosus flare triggered by severe coronavirus disease 2019. J. Clin. Rheumatol. 2020;26:234–235. doi: 10.1097/RHU.0000000000001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ratner S., Kabani N., Neculiseanu E., Ginzler E. COVID-19 coagulopathy in a patient with systemic lupus erythematosus and antiphospholipid antibodies. J. Clin. Rheumatol. 2020 doi: 10.1097/RHU.0000000000001599. [DOI] [PubMed] [Google Scholar]
- 167.Uppal N.N., Kello N., Shah H.H., Khanin Y., De Oleo I.R., Epstein E., et al. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020 doi: 10.1016/j.ekir.2020.1008.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hussein A., Al Khalil K., Bawazir Y.M. Anti-neutrophilic cytoplasmic antibody (ANCA) vasculitis presented as pulmonary hemorrhage in a positive COVID-19 patient: a case report. Cureus. 2020;12 doi: 10.7759/cureus.9643. e9643-e9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., et al. COVID-19 and kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 170.Rivera-Figueroa E.I., Santos R., Simpson S., Garg P. Incomplete kawasaki disease in a child with covid-19. Indian Pediatr. 2020;57:680–681. doi: 10.1007/s13312-020-1900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Balasubramanian S., Nagendran T.M., Ramachandran B., Ramanan A.V. Hyper-inflammatory syndrome in a child with COVID-19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatr. 2020;57:681–683. doi: 10.1007/s13312-020-1901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Acharyya B.C., Acharyya S., Das D. Indian Pediatr; 2020. Novel Coronavirus Mimicking Kawasaki Disease in an Infant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Waltuch T., Gill P., Zinns L.E., Whitney R., Tokarski J., Tsung J.W., et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am. J. Emerg. Med. 2020;38:2246.e3–2246.e6. doi: 10.1016/j.ajem.2020.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York city. J. Am. Med. Assoc. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Belhadjer Z., Méot M., Bajolle F., Khraiche D., Legendre A., Abakka S., et al. Acute Heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 176.Ng K.F., Kothari T., Bandi S., Bird P.W., Goyal K., Zoha M., et al. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dolinger M.T., Person H., Smith R., Jarchin L., Pittman N., Dubinsky M.C., et al. Pediatric crohn disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J. Pediatr. Gastroenterol. Nutr. 2020;71:153–155. doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Licciardi F., Pruccoli G., Denina M., Parodi E., Taglietto M., Rosati S., et al. SARS-CoV-2–Induced kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 179.Deza Leon M.P., Redzepi A., McGrath E., Abdel-Haq N., Shawaqfeh A., Sethuraman U., et al. COVID-19-Associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. 2020;9:407–408. doi: 10.1093/jpids/piaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Labé P., Ly A., Sin C., Nasser M., Chapelon-Fromont E., Ben Saïd P., et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rauf A., Vijayan A., John S.T., Krishnan R., Latheef A. Multisystem inflammatory syndrome with features of atypical kawasaki disease during COVID-19 pandemic. Indian J. Pediatr. 2020;87:745–747. doi: 10.1007/s12098-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chiotos K., Bassiri H., Behrens E.M., Blatz A.M., Chang J., Diorio C., et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Grimaud M., Starck J., Levy M., Marais C., Chareyre J., Khraiche D., et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann. Intensive Care. 2020;10 doi: 10.1186/s13613-020-00690-8. 69-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yozgat C.Y., Uzuner S., Bursal Duramaz B., Yozgat Y., Erenberk U., Iscan A., et al. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID-19 in a 3-year-old girl. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13770. e13770-e13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Blondiaux E., Parisot P., Redheuil A., Tzaroukian L., Levy Y., Sileo C., et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19. Case Series. Radiol. 2020;297:E283–E288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Belot A., Antona D., Renolleau S., Javouhey E., Hentgen V., Angoulvant F., et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ramcharan T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A.G., et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr. Cardiol. 2020:1–11. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Greene A.G., Saleh M., Roseman E., Sinert R. Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C) Am. J. Emerg. Med. 2020;S0735–6757:30492–30497. doi: 10.1016/j.ajem.2020.05.117. 0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schnapp A., Abulhija H., Maly A., Armoni-Weiss G., Levin Y., Faitatziadou S.M., et al. Introductory histopathological findings may shed light on COVID-19 paediatric hyperinflammatory shock syndrome. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chiu J.S., Lahoud-Rahme M., Schaffer D., Cohen A., Samuels-Kalow M. Kawasaki disease features and myocarditis in a patient with COVID-19. Pediatr. Cardiol. 2020:1–3. doi: 10.1007/s00246-020-02393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Capone C.A., Subramony A., Sweberg T., Schneider J., Shah S., Rubin L., et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J. Pediatr. 2020;S0022–3476 doi: 10.1016/j.jpeds.2020.06.044. 0020)30746-30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dasgupta K., Finch S. A case of pediatric multisystem inflammatory syndrome temporally associated with COVID-19 in south Dakota. South Dakota Med. : J. South Dakota State Med. Assoc. 2020;73:246–251. [PubMed] [Google Scholar]
- 193.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., et al. Multisystem inflammatory syndrome in children in New York state. N. Engl. J. Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Alnashri H., Aljohani N., Tayeb S., Rabie N., AlBenayan E., Alharthi A., et al. A challenging case of multisystem inflammatory syndrome in children related to coronavirus Disease-19 hospitalized under adult medical service. IDCases. 2020;22 doi: 10.1016/j.idcr.2020.e00957. e00957-e00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Giannattasio A., Maglione M., Zenzeri L., Mauro A., Di Mita O., Iodice R.M., et al. A child with a severe multisystem inflammatory syndrome following an asymptomatic COVID-19 infection: a novel management for a new disease? J. Med. Virol. 2020 doi: 10.1002/jmv.26189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chérif M.Y., de Filette J.M.K., André S., Kamgang P., Richert B., Clevenbergh P. Coronavirus disease 2019-related Kawasaki-like disease in an adult: a case report. JAAD Case Rep. 2020;6:780–782. doi: 10.1016/j.jdcr.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am. J. Emerg. Med. 2020;S0735–6757:30542–30548. doi: 10.1016/j.ajem.2020.06.053. 0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ferrero P., Piazza I., Bonino C., Ciuffreda M. Patterns of myocardial involvement in children during COVID-19 pandemic: early experience from northern Italy. Ann. Pediatr. Cardiol. 2020;13:230–233. doi: 10.4103/apc.APC_77_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ouldali N., Pouletty M., Mariani P., Beyler C., Blachier A., Bonacorsi S., et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020;4:662–668. doi: 10.1016/S2352-4642(20)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vari D., Miller J.M., Rellosa N., Srivastava S., Frizzola M., Thacker D. Severe cardiac dysfunction in a patient with multisystem inflammatory syndrome in children associated with COVID-19: retrospective diagnosis of a puzzling presentation. A case report. Prog. Pediatr. Cardiol. 2020;58:101270. doi: 10.1016/j.ppedcard.2020.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cazzaniga M., Baselli L.A., Cimaz R., Guez S.S., Pinzani R., Dellepiane R.M. SARS-COV-2 infection and kawasaki disease: case report of a hitherto unrecognized association. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.00398. 398-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. The Lancet Child & Adolescent Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shaigany S., Gnirke M., Guttmann A., Chong H., Meehan S., Raabe V., et al. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet. 2020;396:e8–e10. doi: 10.1016/S0140-6736(20)31526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vergnano S., Alders N., Armstrong C., Bamber A.R., Bandi S., Evans J.A., et al. Severe refractory Kawasaki disease in seven infants in the COVID-19 era. The Lancet Rheumatol. 2020;2 doi: 10.1016/S2665-9913(20)30231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cogan E., Foulon P., Cappeliez O., Dolle N., Vanfraechem G., De Backer D. Multisystem inflammatory syndrome with complete kawasaki disease features associated with SARS-CoV-2 infection in a young adult. A case report. Front. Med. 2020;7 doi: 10.3389/fmed.2020.00428. 428-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee P.Y., Day-Lewis M., Henderson L.A., Friedman K.G., Lo J., Roberts J.E., et al. Distinct clinical and immunological features of SARS–CoV-2–induced multisystem inflammatory syndrome in children. J. Clin. Inves. 2020:130. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Khan K.S., Ullah I. SARS-CoV-2 causes Kawasaki-like disease in children: cases reported in Pakistan. J. Med. Virol. 2020 doi: 10.1002/jmv.26340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bonnet M., Champagnac A., Lantelme P., Harbaoui B. Endomyocardial biopsy findings in Kawasaki-like disease associated with SARS-CoV-2. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moraleda C., Serna-Pascual M., Soriano-Arandes A., Simó S., Epalza C., Santos M., et al. Multi-inflammatory syndrome in children related to SARS-CoV-2 in Spain. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Regev T., Antebi M., Eytan D., Shachor-Meyouhas Y., Ilivitzki A., Aviel Y.B., et al. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 infection. Pediatr. Infect. Dis. J. 2020;39 doi: 10.1097/INF.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 211.Raut S., Roychowdhoury S., Bhakta S., Sarkar M., Nandi M. Incomplete kawasaki disease as presentation of COVID-19 infection in an infant: a case report. J. Trop. Pediatr. 2020 doi: 10.1093/tropej/fmaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mamishi S., Heydari H., Aziz-Ahari A., Shokrollahi M.R., Pourakbari B., Mahmoudi S., et al. Novel coronavirus disease 2019 (COVID-19) outbreak in children in Iran: atypical CT manifestations and mortality risk of severe COVID-19 infection. J. Microbiol. Immunol. Infect. 2020:30177–30178. doi: 10.1016/j.jmii.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Theocharis P., Wong J., Pushparajah K., Mathur S.K., Simpson J.M., Pascall E., et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2020:jeaa212. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gupta A., Gill A., Sharma M., Garg M. Multi-system inflammatory syndrome in a child mimicking kawasaki disease. J. Trop. Pediatr. 2020 doi: 10.1093/tropej/fmaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Heidemann S.M., Tilford B., Bauerfeld C., Martin A., Garcia R.U., Yagiela L., et al. Three cases of pediatric multisystem inflammatory syndrome associated with COVID-19 due to SARS-CoV-2. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925779. e925779-e925779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Godfred-Cato S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J., et al. COVID-19-Associated multisystem inflammatory syndrome in children - United States, march-July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Iio K., Uda K., Hataya H., Yasui F., Honda T., Sanada T., et al. Kawasaki disease or Kawasaki-like disease: influence of SARS-CoV-2 infections in Japan. Acta Paediatr. 2020 doi: 10.1111/apa.15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Carter M.J., Fish M., Jennings A., Doores K.J., Wellman P., Seow J., et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 219.de Farias ECF, Pedro Piva J, de Mello MLFMF, do Nascimento LMPP, Costa CC, Machado MMM, et al. Multisystem Inflammatory Syndrome Associated With Coronavirus Disease in Children: A Multi-centered Study in Belem, Para, Brazil. Pediatr. Infect. Dis. 2020;39:e374–e376. doi: 10.1097/INF.0000000000002865. [DOI] [PubMed] [Google Scholar]
- 220.Lidder A.K., Pandit S.A., Lazzaro D.R. An adult with COVID-19 kawasaki-like syndrome and ocular manifestations. Am J Ophthalmol Case Rep. 2020;20 doi: 10.1016/j.ajoc.2020.100875. 100875-100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lin J., Lawson E.C., Verma S., Peterson R.B., Sidhu R. Cytotoxic lesion of the corpus callosum in an adolescent with multisystem inflammatory syndrome and SARS-CoV-2 infection. Am. J. Neuroradiol. 2020;41:2017–2019. doi: 10.3174/ajnr.A6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mamishi S., Movahedi Z., Mohammadi M., Ziaee V., Khodabandeh M., Abdolsalehi M.R., et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol. Infect. 2020;148:e196. doi: 10.1017/S095026882000196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Okarska-Napierała M., Zalewska E., Kuchar E. Fever and diarrhea as the only symptoms of multisystem inflammatory syndrome in children (MIS-C) Gastroenterology. 2020;S0016–5085 doi: 10.1053/j.gastro.2020.08.047. 0020)35118-35110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Matsubara D., Kauffman H.L., Wang Y., Calderon-Anyosa R., Nadaraj S., Elias M.D., et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J. Am. Coll. Cardiol. 2020;S0735–1097 doi: 10.1016/j.jacc.2020.08.056. 0720)36488-36483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:928–995.e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Masih M., Moll S., Raza N. Paediatric case of prolonged COVID-19 manifesting as PMIS-TS and atypical Kawasaki. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pereira M.F.B., Litvinov N., Farhat S.C.L., Eisencraft A.P., Gibelli M.A.B.C., Carvalho WBd, et al. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics. 2020;75 doi: 10.6061/clinics/2020/e2209. e2209-e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.DeBiasi R.L., Song X., Delaney M., Bell M., Smith K., Pershad J., et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J. Pediatr. 2020;223:199–203. doi: 10.1016/j.jpeds.2020.05.007. e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wolfler A., Mannarino S., Giacomet V., Camporesi A., Zuccotti G. Acute myocardial injury: a novel clinical pattern in children with COVID-19. Lancet Child Adolesc Health. 2020;4:e26–e27. doi: 10.1016/S2352-4642(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Farias ECFd, Justino M.C.A. Mello MLFMFd. multisystem inflammatory syndrome IN A child associated with coronavirus disease 19 IN the BRAZILIAN amazon: fatal outcome IN an infant. Rev Paul Pediatr. 2020;38 doi: 10.1590/1984-0462/2020/38/2020165. e2020165-e2020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schupper A.J., Yaeger K.A., Morgenstern P.F. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36:1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rojahn A.E., Gammelsrud K.W., Brunvand L.I., Hanche-Olsen T.P., Schistad O., Sæter C.B., et al. Tidsskrift for Den Norske Laegeforening : Tidsskrift for Praktisk Medicin. 2020. Multiorgan inflammatory syndrome associated with SARS-CoV-2 in a child; p. 140. ny raekke. [DOI] [PubMed] [Google Scholar]
- 233.Nguyen D.C., Haydar H., Pace E.R., Zhang X.S., Dobbs K.R. Pediatric case of severe COVID-19 with shock and multisystem inflammation. Cureus. 2020;12 doi: 10.7759/cureus.8915. e8915-e8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bahrami A., Vafapour M., Moazzami B., Rezaei N. Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J. Paediatr. Child Health. 2020 doi: 10.1111/jpc.15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kest H., Kaushik A., DeBruin W., Colletti M., Goldberg D. Multisystem inflammatory syndrome in children (MIS-C) associated with 2019 novel coronavirus (SARS-CoV-2) infection. Case Rep Pediatr. 2020;2020 doi: 10.1155/2020/8875987. 8875987-8875987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Levin T.L., Kurian J., Lee E.Y., Liszewski M.C. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Am. J. Roentgenol. 2020 doi: 10.2214/AJR.20.24032. [DOI] [PubMed] [Google Scholar]
- 237.Diorio C., Henrickson S.E., Vella L.A., McNerney K.O., Chase J., Burudpakdee C., et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS–CoV-2. J. Clin. Inves. 2020:130. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gaitonde M., Ziebell D., Kelleman M.S., Cox D.E., Lipinski J., Border W.L., et al. COVID-19-Related multisystem inflammatory syndrome in children affects left ventricular function and global strain compared with kawasaki disease. J. Am. Soc. Echocardiogr. 2020;33:1285–1287. doi: 10.1016/j.echo.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jhaveri S., Ahluwalia N., Kaushik S., Trachtman R., Kowalsky S., Aydin S., et al. Longitudinal echocardiographic assessment of coronary arteries and left ventricular function following multisystem inflammatory syndrome in children. J. Pediatr. 2020;S0022–3476:30984–30987. doi: 10.1016/j.jpeds.2020.08.002. 0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jain S., Sen S., Lakshmivenkateshiah S., Bobhate P., Venkatesh S., Udani S., et al. Multisystem inflammatory syndrome in children with COVID-19 in Mumbai, India. Indian Pediatr. 2020;57:1015–1019. doi: 10.1007/s13312-020-2026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Verkuil L.D., Liu G.T., Brahma V.L., Avery R.A. Pseudotumor cerebri syndrome associated with MIS-C: a case report. Lancet. 2020;396:532. doi: 10.1016/S0140-6736(20)31725-6. [DOI] [PubMed] [Google Scholar]
- 242.Webb K., Abraham D.R., Faleye A., McCulloch M., Rabie H., Scott C., et al. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health. 2020;4 doi: 10.1016/S2352-4642(20)30272-8. e38-e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Domico M., McCanta A.C., Hunt J.L., Ashouri N., Nugent D., Kelly R.B. High-grade Heart Block requiring transvenous pacing associated with multisystem inflammatory syndrome in children during the COVID-19 pandemic. HeartRhythm Case Rep. 2020 doi: 10.1016/j.hrcr.2020.1008.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Swann O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M., et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Torres J.P., Izquierdo G., Acuña M., Pavez D., Reyes F., Fritis A., et al. Multisystem inflammatory syndrome in children (MIS-C): report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int. J. Infect. Dis. 2020;100:75–81. doi: 10.1016/j.ijid.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lishman J, Kohler C, de Vos C, van der Zalm MM, Itana J, Redfern A, et al. Acute Appendicitis in Multisystem Inflammatory Syndrome in Children With COVID-19. Pediatr. Infect. Dis. J. 2020;39:e472–e473. doi: 10.1097/INF.0000000000002900. [DOI] [PubMed] [Google Scholar]
- 247.Tam H., El Tal T., Go E., Yeung R.S.M. Pediatric inflammatory multisystem syndrome temporally associated with COVID-19: a spectrum of diseases with many names. Can. Med. Assoc. J. 2020;192:E1093–E1096. doi: 10.1503/cmaj.201600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jain M.K., Sahu S.K., Behera J.R., Patnaik S. Multisystem inflammatory syndrome in children associated with COVID 19 treated with oral steroid. Indian J. Pediatr. 2020 doi: 10.1007/s12098-020-03497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Biko D.M., Ramirez-Suarez K.I., Barrera C.A., Banerjee A., Matsubara D., Kaplan S.L., et al. Imaging of children with COVID-19: experience from a tertiary children's hospital in the United States. Pediatr. Radiol. 2020:1–9. doi: 10.1007/s00247-020-04830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Minocha P.K., Phoon C.K.L., Verma S., Singh R.K. Cardiac findings in pediatric patients with multisystem inflammatory syndrome in children associated with COVID-19. Clin. Pediatr. 2020 doi: 10.1177/0009922820961771. [DOI] [PubMed] [Google Scholar]
- 251.Kofman A.D., Sizemore E.K., Detelich J.F., Albrecht B., Piantadosi A.L. A young adult with COVID-19 and multisystem inflammatory syndrome in children (MIS-C)-like illness: a case report. BMC Infect. Dis. 2020;20 doi: 10.1186/s12879-020-05439-z. 716-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jonat B, Gorelik M, Boneparth A, Geneslaw AS, Zachariah P, Shah A, et al. Multisystem Inflammatory Syndrome in Children Associated With Coronavirus Disease 2019 in a Children’s Hospital in New York City: Patient Characteristics and an Institutional Protocol for Evaluation, Management, and Follow-Up. Pediatr. Crit. Care Med. 2020 doi: 10.1097/PCC.0000000000002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nelson C., Ishimine P., Hayden S.R., Correia M., Wardi G. Multisystem inflammatory syndrome in children (mis-C) in an adolescent that developed coronary aneurysms: a case report and review of the literature. J. Emerg. Med. 2020;59:699–704. doi: 10.1016/j.jemermed.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Klocperk A., Parackova Z., Dissou J., Malcova H., Pavlicek P., Vymazal T., et al. Case report: systemic inflammatory response and fast recovery in a pediatric patient with COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01665. 1665-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lang P., Eichholz T., Bakchoul T., Streiter M., Petrasch M., Bösmüller H., et al. Defibrotide for the treatment of PIMS-TS in two pediatric patients. J Pediatric Infect Dis Soc. 2020;9:622–625. doi: 10.1093/jpids/piaa117. piaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kim L., Whitaker M., O'Halloran A., Kambhampati A., Chai S.J., Reingold A., et al. Hospitalization rates and characteristics of children aged <18 Years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 states, march 1-July 25, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;S0092–8674 doi: 10.1016/j.cell.2020.09.016. 0020)31157-31150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cantor A., Miller J., Zachariah P., DaSilva B., Margolis K., Martinez M. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: a single-center report. Hepatology. 2020:n/a. doi: 10.1002/hep.31526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yonker L.M., Neilan A.M., Bartsch Y., Patel A.B., Regan J., Arya P., et al. Pediatric SARS-CoV-2: clinical presentation, infectivity, and immune responses. J. Pediatr. 2020;S0022–3476:31023–31024. doi: 10.1016/j.jpeds.2020.08.037. 0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Harwood R., Partridge R., Minford J., Almond S. Paediatric abdominal pain in the time of COVID-19: a new diagnostic dilemma. J. Surg. Case Rep. 2020;2020:rjaa337. doi: 10.1093/jscr/rjaa337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Stevens J.P., Brownell J.N., Freeman A.J., Bashaw H. COVID-19-Associated multisystem inflammatory syndrome in children presenting as acute pancreatitis. J. Pediatr. Gastroenterol. Nutr. 2020;28 doi: 10.1097/MPG.0000000000002860. [DOI] [PubMed] [Google Scholar]
- 262.Sahn B., Eze O.P., Edelman M.C., Chougar C.E., Thomas R.M., Schleien C.L., et al. Features of intestinal disease associated with COVID-related multisystem inflammatory syndrome in children. J. Pediatr. Gastroenterol. Nutr. 2020 doi: 10.1097/MPG.0000000000002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kalner S., Vergilis I.J. Periorbital erythema as a presenting sign of covid-19. JAAD Case Rep. 2020 doi: 10.1016/j.jdcr.2020.1005.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Colonna C., Monzani N.A., Rocchi A., Gianotti R., Boggio F., Gelmetti C. Chilblain-like lesions in children following suspected COVID-19 infection. Pediatr. Dermatol. 2020;37:437–440. doi: 10.1111/pde.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.García-Legaz Martínez M., Martínez-Doménech Á, Magdaleno-Tapial J., Valenzuela-Oñate C., Partarrieu-Mejías F., Lorca-Spröhnle J., et al. Acute acral cutaneous manifestations during the COVID-19 pandemic: a single-centre experience. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Verheyden M., Grosber M., Gutermuth J., Velkeniers B. Relapsing symmetric livedo reticularis in a patient with COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hubiche T., Le Duff F., Chiaverini C., Giordanengo V., Passeron T. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. Lancet Infect. Dis. 2020;S1473–3099(1420) doi: 10.1016/S1473-3099(20)30518-1. 30518-30511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Colmenero I., Santonja C., Alonso-Riaño M., Noguera-Morel L., Hernández-Martín A., Andina D., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br. J. Dermatol. 2020 doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Negrini S., Guadagno A., Greco M., Parodi A., Burlando M. An unusual case of bullous haemorrhagic vasculitis in a COVID-19 patient. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Del Giudice P., Boudoumi D., Le Guen B., Reverte M., Gutnecht J., Lacour J.P., et al. Catastrophic acute bilateral lower limbs necrosis associated with COVID-19 as a likely consequence of both vasculitis and coagulopathy. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Larrondo J., Cabrera R., Gosch M., Larrondo F., Aylwin M., Castro A. Papular-purpuric exanthem in a COVID-19 patient: clinical and dermoscopic description. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Caputo V., Schroeder J., Rongioletti F. A generalized purpuric eruption with histopathologic features of leucocytoclastic vasculitis in a patient severely ill with COVID-19. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Karaca Z., Yayli S., Çalışkan O. A unilateral purpuric rash in a patient with COVID-19 infection. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13798. e13798-e13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rossi E., Lasagni C., Trakatelli M., Wertzberger Rowan S., Magnoni C. Acute maculopapular eruption in Covid-19 patient: a case report. Dermatol. Ther. 2020;n/a doi: 10.1111/dth.13812. [DOI] [PubMed] [Google Scholar]
- 276.Rubio-Muniz C.A., Puerta-Peña M., Falkenhain-López D., Arroyo-Andrés J., Agud-Dios M., Rodriguez-Peralto J.L., et al. The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.de Perosanz-Lobo D., Fernandez-Nieto D., Burgos-Blasco P., Selda-Enriquez G., Carretero I., Moreno C., et al. Urticarial vasculitis in COVID-19 infection: a vasculopathy-related symptom? J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wollina U. Schamberg-like purpuric eruptions and tonsillitis in mild COVID-19. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13766. e13766-e13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Colonna C., Genovese G., Monzani N.A., Picca M., Boggio F., Gianotti R., et al. Outbreak of chilblain-like acral lesions in children in the metropolitan area of Milan, Italy, during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020;83:965–969. doi: 10.1016/j.jaad.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Reymundo A., Fernáldez-Bernáldez A., Reolid A., Butrón B., Fernández-Rico P., Muñoz-Hernández P., et al. Clinical and histological characterization of late appearance maculopapular eruptions in association with the coronavirus disease 2019. A case series of seven patients. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bosch-Amate X., Giavedoni P., Podlipnik S., Andreu-Febrer C., Sanz-Beltran J., Garcia-Herrera A., et al. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID-19) coagulopathy. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Freeman E.E., McMahon D.E., Lipoff J.B., Rosenbach M., Kovarik C., Takeshita J., et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J. Am. Acad. Dermatol. 2020;83:486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Balestri R., Termine S., Rech G., Girardelli C.R. Late onset of acral necrosis after SARS-CoV-2 infection resolution. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Dominguez-Santas M., Diaz-Guimaraens B., Garcia Abellas P., Moreno-Garcia Del Real C., Burgos-Blasco P., Suarez-Valle A. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Docampo-Simón A., Sánchez-Pujol M.J., Juan-Carpena G., Palazón-Cabanes J.C., Vergara-De Caso E., Berbegal L., et al. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mayor-Ibarguren A., Feito-Rodriguez M., Quintana Castanedo L., Ruiz-Bravo E., Montero Vega D., Herranz-Pinto P. Cutaneous small vessel vasculitis secondary to COVID-19 infection: a case report. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.García-Gil M.F., García García M., Monte Serrano J., Prieto-Torres L., Ara-Martín M. Acral purpuric lesions (erythema multiforme type) associated with thrombotic vasculopathy in a child during the COVID-19 pandemic. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.de Medeiros V.L.S., Silva L.F.T. Follow-up of skin lesions during the evolution of COVID-19: a case report. Arch. Dermatol. Res. 2020:1–4. doi: 10.1007/s00403-020-02091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cepeda-Valdes R., Carrion-Alvarez D., Trejo-Castro A., Hernandez-Torre M., Salas-Alanis J. Cutaneous manifestations in COVID-19: familial cluster of urticarial rash. Clin. Exp. Dermatol. 2020 doi: 10.1111/ced.14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cordoro K.M., Reynolds S.D., Wattier R., McCalmont T.H. Clustered cases of acral perniosis: clinical features, histopathology, and relationship to COVID-19. Pediatr. Dermatol. 2020;37:419–423. doi: 10.1111/pde.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Locatelli A.G., Robustelli Test E., Vezzoli P., Carugno A., Moggio E., Consonni L., et al. Histologic features of long-lasting chilblain-like lesions in a paediatric COVID-19 patient. J. Eur. Acad. Dermatol. Venereol. 2020;34:e365–e368. doi: 10.1111/jdv.16617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sachdeva M., Gianotti R., Shah M., Bradanini L., Tosi D., Veraldi S., et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J. Dermatol. Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.de Masson A., Bouaziz J.-D., Sulimovic L., Cassius C., Jachiet M., Ionescu M.-A., et al. Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J. Am. Acad. Dermatol. 2020;83:667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Suarez-Valle A., Fernandez-Nieto D., Diaz-Guimaraens B., Dominguez-Santas M., Carretero I., Perez-Garcia B. Acro-ischaemia in hospitalized COVID-19 patients. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kolivras A., Dehavay F., Delplace D., Feoli F., Meiers I., Milone L., et al. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tosti G., Barisani A., Queirolo P., Pennacchioli E., Villa L., Lodeserto A.M., et al. Skin signs resembling vascular acrosyndromes during the COVID-19 outbreak in Italy. Clin. Exp. Dermatol. 2020;45:757–758. doi: 10.1111/ced.14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Paolino G., Canti V., Mercuri S.R., Rovere Querini P., Candiani M., Pasi F. Diffuse cutaneous manifestation in a new mother with COVID-19 (SARS-Cov-2) Int. J. Dermatol. 2020;59:874–875. doi: 10.1111/ijd.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Diaz-Guimaraens B., Dominguez-Santas M., Suarez-Valle A., Pindado-Ortega C., Selda-Enriquez G., Bea-Ardebol S., et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820–822. doi: 10.1001/jamadermatol.2020.1741. [DOI] [PubMed] [Google Scholar]
- 299.Quintana-Castanedo L., Feito-Rodríguez M., Valero-López I., Chiloeches-Fernández C., Sendagorta-Cudós E., Herranz-Pinto P. Urticarial exanthem as early diagnostic clue for COVID-19 infection. JAAD Case Rep. 2020;6:498–499. doi: 10.1016/j.jdcr.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Fernandez-Nieto D., Jimenez-Cauhe J., Suarez-Valle A., Moreno-Arrones O.M., Saceda-Corralo D., Arana-Raja A., et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J. Am. Acad. Dermatol. 2020;83:e61–e63. doi: 10.1016/j.jaad.2020.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bouaziz J.D., Duong T., Jachiet M., Velter C., Lestang P., Cassius C., et al. Vascular skin symptoms in COVID-19: a French observational study. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Piccolo V., Neri I., Filippeschi C., Oranges T., Argenziano G., Battarra V.C., et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J. Eur. Acad. Dermatol. Venereol. 2020;34:e291–e293. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sanchez A., Sohier P., Benghanem S., L'Honneur A.-S., Rozenberg F., Dupin N., et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819–820. doi: 10.1001/jamadermatol.2020.1704. [DOI] [PubMed] [Google Scholar]
- 304.Alramthan A., Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin. Exp. Dermatol. 2020;45:746–748. doi: 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gianotti R., Veraldi S., Recalcati S., Cusini M., Ghislanzoni M., Boggio F., et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of milan, Italy. Acta Derm. Venereol. 2020;100 doi: 10.2340/00015555-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Matar S., Oulès B., Sohier P., Chosidow O., Beylot-Barry M., Dupin N., et al. Cutaneous manifestations in SARS-CoV-2 infection (COVID-19): a French experience and a systematic review of the literature. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Askin O., Altunkalem R.N., Altinisik D.D., Uzuncakmak T.K., Tursen U., Kutlubay Z. Cutaneous manifestations in hospitalized patients diagnosed as COVID-19. Dermatol. Ther. 2020 doi: 10.1111/dth.13896. e13896-e13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Annunziata M.C., Patrì A., Ruggiero A., Di Guida A., Menicanti C., Greco V., et al. Cutaneous involvement during COVID-19 pandemic: an emerging sign of infection. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Najafzadeh M., Shahzad F., Ghaderi N., Ansari K., Jacob B., Wright A. Urticaria (angioedema) and COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Gaspari V., Neri I., Misciali C., Patrizi A. COVID-19: how it can look on the skin. Clinical and pathological features in 20 COVID-19 patients observed in Bologna, north-eastern Italy. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Calvão J., Relvas M., Pinho A., Brinca A., Cardoso J.C. Acro-ischaemia and COVID-19 infection: clinical and histopathological features. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.El Hachem M., Diociaiuti A., Concato C., Carsetti R., Carnevale C., Degli Ciofi, Atti M., et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Saenz Aguirre A., De la Torre Gomar F.J., Rosés-Gibert P., Gimeno Castillo J., Martinez de Lagrán Alvarez de Arcaya Z., Gonzalez-Perez R. Novel outbreak of acral lesions in times of COVID-19: a description of 74 cases from a tertiary university hospital in Spain. Clin. Exp. Dermatol. 2020 doi: 10.1111/ced.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rodríguez-Jiménez P., Chicharro P., De Argila D., Muñoz-Hernández P., Llamas-Velasco M. Urticaria-like lesions in COVID-19 patients are not really urticaria - a case with clinicopathological correlation. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.van Damme C., Berlingin E., Saussez S., Accaputo O. Acute urticaria with pyrexia as the first manifestations of a COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020;34:e300–e301. doi: 10.1111/jdv.16523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ciccarese G., Drago F., Boatti M., Porro A., Muzic S.I., Parodi A. Oral erosions and petechiae during SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.26221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Bitar C., Chan M.P., Harms P.W., Fullen D.R., Gudjonsson J.E., Eshaq M., et al. Cutaneous manifestations of hospitalized coronavirus disease 2019 patients: a report of six cases with clinicopathologic features and viral RNA in situ hybridization. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kanitakis J., Lesort C., Danset M., Jullien D. Chilblain-like acral lesions during the COVID-19 pandemic ("COVID toes"): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J. Am. Acad. Dermatol. 2020;83:870–875. doi: 10.1016/j.jaad.2020.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Conforti C., Zalaudek I., Giuffrida R., Zorat F., Grillo A., Colapietro N., et al. “COVID-Mask”: an atypical livedoid manifestation of COVID-19 observed in a Northern Italy hospital. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13701. e13701-e13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Diotallevi F., Campanati A., Bianchelli T., Bobyr I., Luchetti M.M., Marconi B., et al. Skin involvement in SARS-CoV-2 infection: case series. J. Med. Virol. 2020 doi: 10.1002/jmv.26012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Llamas-Velasco M., Muñoz-Hernández P., Lázaro-González J., Reolid-Pérez A., Abad-Santamaría B., Fraga J., et al. Thrombotic occlusive vasculopathy in a skin biopsy from a livedoid lesion of a patient with COVID-19. Br. J. Dermatol. 2020;183:591–593. doi: 10.1111/bjd.19222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Andina D., Noguera-Morel L., Bascuas-Arribas M., Gaitero-Tristán J., Alonso-Cadenas J.A., Escalada-Pellitero S., et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr. Dermatol. 2020;37:406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Garcia-Lara G., Linares-González L., Ródenas-Herranz T., Ruiz-Villaverde R. Chilblain-like lesions in pediatrics dermatological outpatients during the COVID-19 outbreak. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13516. e13516-e13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Landa N., Mendieta-Eckert M., Fonda-Pascual P., Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 Pandemic. Int. J. Dermatol. 2020;59:739–743. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.López-Robles J., de la Hera I., Pardo-Sánchez J., Ruiz-Martínez J., Cutillas-Marco E. Chilblain-like lesions: a case series of 41 patients during the COVID-19 pandemic. Clin. Exp. Dermatol. 2020;45:891–892. doi: 10.1111/ced.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ludzik J., Witkowski A., Hansel D.E., Raess P.W., White K., Leachman S. Case Report: chilblains-like lesions (COVID-19 toes) during the pandemic - is there a diagnostic window? F1000Res. 2020;9:668. doi: 10.12688/f1000research.24766.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Rosés-Gibert P., Gimeno Castillo J., Saenz Aguirre A., De la Torre Gomar F.J., Carnero González L., Martinez de Lagrán Alvarez de Arcaya Z., et al. Acral lesions in a pediatric population during the COVID-19 pandemic: a case series of 36 patients from a single hospital in Spain. World J Pediatr. 2020:1–4. doi: 10.1007/s12519-020-00390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ayatollahi A., Robati R.M., Kamyab K., Firooz A. Late-onset AGEP-like skin pustular eruption following COVID-19: a possible association. Dermatol. Ther. 2020;n/a doi: 10.1111/dth.14275. [DOI] [PubMed] [Google Scholar]
- 329.Rerknimitr P., Theerawattanawit C., Lertpichitkul P., Jantarabenjakul W., Putcharoen O., Puthanakit T., et al. Skin manifestations in COVID-19: the tropics experience. J. Dermatol. 2020;47:e444–e446. doi: 10.1111/1346-8138.15567. [DOI] [PubMed] [Google Scholar]
- 330.Nasiri S., Dadkhahfar S., Abasifar H., Mortazavi N., Gheisari M. Urticarial vasculitis in a COVID-19 recovered patient. Int. J. Dermatol. 2020;59:1285–1286. doi: 10.1111/ijd.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Boix-Vilanova J., Gracia-Darder I., Saus C., Ramos D., Llull A., Santonja C., et al. Grover-like skin eruption: another cutaneous manifestation in a COVID-19 patient. Int. J. Dermatol. 2020;59:1290–1292. doi: 10.1111/ijd.15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Daneshjou R., Rana J., Dickman M., Yost J.M., Chiou A., Ko J. Pernio-like eruption associated with COVID-19 in skin of color. JAAD Case Rep. 2020;6:892–897. doi: 10.1016/j.jdcr.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mohan V., Lind R. Chilblains in COVID-19 infection. Cureus. 2020;12 doi: 10.7759/cureus.9245. e9245-e9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Neri I., Patrizi A., Gabrielli L., Virdi A., Veronesi G., Corsini I., et al. Acral skin eruption observed during SARS-CoV-2 pandemic: possible keratolysis exfoliativa with red palms and soles. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Pangti R., Gupta S., Nischal N., Trikha A. Recognizable vascular skin manifestations of SARS-CoV-2 (COVID-19) infection are uncommon in patients with darker skin phototypes. Clin. Exp. Dermatol. 2020;46:180–182. doi: 10.1111/ced.14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Jamiolkowski D., Mühleisen B., Müller S., Navarini A.A., Tzankov A., Roider E. SARS-CoV-2 PCR testing of skin for COVID-19 diagnostics: a case report. Lancet. 2020;396:598–599. doi: 10.1016/S0140-6736(20)31754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Tatu A.L., Nadasdy T., Bujoreanu F.C. Familial clustering of COVID-19 skin manifestations. Dermatol. Ther. 2020;n/a doi: 10.1111/dth.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Young S., Narang J., Kumar S., Kwizera E., Malik P., Billings S.D., et al. Large sacral/buttocks ulcerations in the setting of coagulopathy: a case series establishing the skin as a target organ of significant damage and potential morbidity in patients with severe COVID-19. Int. Wound J. 2020;17:2033–2037. doi: 10.1111/iwj.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Droesch C., Do M.H., DeSancho M., Lee E.-J., Magro C., Harp J. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol. 2020;156:1–3. doi: 10.1001/jamadermatol.2020.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Jimenez-Cauhe J., Ortega-Quijano D., de Perosanz-Lobo D., Burgos-Blasco P., Vañó-Galván S., Fernandez-Guarino M., et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol. 2020;156:1134–1136. doi: 10.1001/jamadermatol.2020.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Suter P., Mooser B., Pham Huu Thien H.P. Erythema nodosum as a cutaneous manifestation of COVID-19 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Shanshal M. COVID-19 related anagen effluvium. J. Dermatol. Treat. 2020:1–2. doi: 10.1080/09546634.2020.1792400. [DOI] [PubMed] [Google Scholar]
- 343.Potekaev N.N., Zhukova O.V., Protsenko D.N., Demina O.M., Khlystova E.A., Bogin V. Clinical characteristics of dermatologic manifestations of COVID-19 infection: case series of 15 patients, review of literature, and proposed etiological classification. Int. J. Dermatol. 2020;59:1000–1009. doi: 10.1111/ijd.15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Micevic G., Morris J., Lee A.I., King B.A. Pernio-like lesions and coagulopathy in a patient with COVID-19 infection. JAAD Case Rep. 2020 doi: 10.1016/j.jdcr.2020.1008.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Fida M., Mala R., Pupo L., Dibra A., Nasto K. Case report: SARS-CoV-2-induced urticaria or just a concomitance? Dermatol. Ther. 2020 doi: 10.1111/dth.14250. [DOI] [PubMed] [Google Scholar]
- 346.Motohashi I., Takano T., Ie K., Hashimoto Y., Akino S., Okuse C. Development of maculopapular exanthem in a COVID-19 patient. J. Dermatol. 2020;47:e426–e427. doi: 10.1111/1346-8138.15579. [DOI] [PubMed] [Google Scholar]
- 347.Abasaeed Elhag S.A., Ibrahim H., Abdelhadi S. Angioedema and urticaria in a COVID-19 patient: a case report and review of the literature. JAAD Case Rep. 2020;6:1091–1094. doi: 10.1016/j.jdcr.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Khalil S., Hinds B.R., Manalo I.F., Vargas I.M., Mallela S., Jacobs R. Livedo reticularis as a presenting sign of severe acute respiratory syndrome coronavirus 2 infection. JAAD Case Rep. 2020;6:871–874. doi: 10.1016/j.jdcr.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Sipfle Do N., Bridwell Md RE., Roper Do J. Erythema nodosum-like rash in a COVID-19 patient: a case report. Am. J. Emerg. Med. 2020;S0735–6757 doi: 10.1016/j.ajem.2020.07.063. 0720)30658-30656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Stavert R., Meydani-Korb A., de Leon D., Osgood R., Blau J., Luu T. Evaluation of SARS-CoV-2 antibodies in 24 patients presenting with chilblains-like lesions during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020;S0190–9622:32440–32443. doi: 10.1016/j.jaad.2020.08.049. 0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.García-Gil M.F., Monte Serrano J., García García M., Pascual-del-Riquelme J.A., Ara-Martín M. Acro-ischemic lesions associated with extremely elevated D-Dimer in a child during the COVID-19 pandemic. Australas. J. Dermatol. 2020 doi: 10.1111/ajd.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Gananandan K., Sacks B., Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Mizutani Y., Nagai M., Tsuzuku A. Late-onset cutaneous manifestations in a patient with severe COVID-19 infection. J. Dermatol. 2020;47:e347–e348. doi: 10.1111/1346-8138.15520. [DOI] [PubMed] [Google Scholar]
- 354.Proietti I., Tolino E., Bernardini N., Mambrin A., Balduzzi V., Marchesiello A., et al. Auricle perniosis as a manifestation of Covid-19 infection. Dermatol. Ther. 2020;n/a doi: 10.1111/dth.14089. [DOI] [PubMed] [Google Scholar]
- 355.Aghazadeh N., Homayouni M., Sartori-Valinotti J.C. Oral vesicles and acral erythema: report of a cutaneous manifestation of COVID-19. Int. J. Dermatol. 2020;59:1153–1154. doi: 10.1111/ijd.15047. [DOI] [PubMed] [Google Scholar]
- 356.Tammaro A., Chello C., Sernicola A., Magri F., Adebanjo G.A.R., Parisella F.R., et al. Necrotic acral lesions and lung failure in a fatal case of COVID-19. Australas. J. Dermatol. 2020 doi: 10.1111/ajd.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Hassan K. Urticaria and angioedema as a prodromal cutaneous manifestation of SARS-CoV-2 (COVID-19) infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Quintana-Castanedo L., Feito-Rodríguez M., Fernández-Alcalde C., Granados-Fernández M., Montero-Vega D., Mayor-Ibarguren A., et al. Concurrent chilblains and retinal vasculitis in a child with COVID-19. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Dominguez-Santas M., Diaz-Guimaraens B., Fernandez-Nieto D., Jimenez-Cauhe J., Ortega-Quijano D., Suarez-Valle A. Minor aphthae associated with SARS-CoV-2 infection. Int. J. Dermatol. 2020;59:1022–1023. doi: 10.1111/ijd.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Freeman E.E., McMahon D.E., Lipoff J.B., Rosenbach M., Kovarik C., Desai S.R., et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J. Am. Acad. Dermatol. 2020;83:1118–1129. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Farouk S., Sadek A. Cutaneous manifestations of COVID-19: a case report and a new finding from Egypt. Dermatol. Ther. 2020 doi: 10.1111/dth.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Beaupre R., 2nd, Petrie C., Toledo A. Mixed purpuric and maculopapular lesions in a patient with COVID-19: a case report. Clin Pract Cases Emerg Med. 2020;4:349–351. doi: 10.5811/cpcem.2020.6.48617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Ahouach B., Harent S., Ullmer A., Martres P., Bégon E., Blum L., et al. Cutaneous lesions in a patient with COVID-19: are they related? Br. J. Dermatol. 2020;183 doi: 10.1111/bjd.19168. e31-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Rizzoli L., Collini L., Magnano M., Termine S., Barcelli R., Infusino S.D., et al. Chilblain-like lesions during the COVID-19 pandemic: a serological study on a case series. Br. J. Dermatol. 2020;183:782–784. doi: 10.1111/bjd.19348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Méndez Maestro I., Peña Merino L., Udondo González del Tánago B., Aramburu González A., Orbea Sopeña A., Sánchez De Vicente J., et al. Skin manifestations in patients hospitalized with confirmed COVID-19 disease: a cross-sectional study in a tertiary hospital. Int. J. Dermatol. 2020;59:1353–1357. doi: 10.1111/ijd.15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Karagounis T.K., Shaw K.S., Caplan A., Lo Sicco K., Femia A.N. Acrofacial purpura and necrotic ulcerations in COVID-19: a case series from New York City. Int. J. Dermatol. 2020;59:1419–1422. doi: 10.1111/ijd.15181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Nuno-Gonzalez A., Martin-Carrillo P., Magaletsky K., Martin Rios M.D., Herranz Mañas C., Artigas Almazan J., et al. Prevalence of mucocutaneous manifestations, oral and palmoplantar findings in 666 patients with COVID-19 in a field hospital in Spain. Br. J. Dermatol. 2020 doi: 10.1111/bjd.19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Gallizzi R., Sutera D., Spagnolo A., Bagnato A.M., Cannavò S.P., Grasso L., et al. Management of pernio-like cutaneous manifestations in children during the outbreak of COVID-19. Dermatol. Ther. 2020;n/a doi: 10.1111/dth.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J. Clin. Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Hutchins K.L., Jansen J.H., Comer A.D., Scheer R.V., Zahn G.S., Capps A.E., et al. COVID-19–Associated Bifacial weakness with paresthesia subtype of guillain-Barré syndrome. Am. J. Neuroradiol. 2020;41:1707–1711. doi: 10.3174/ajnr.A6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á, Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19. The ALBACOVID Reg. 2020;95 doi: 10.1212/WNL.0000000000009937. e1060-e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Padroni M., Mastrangelo V., Asioli G.M., Pavolucci L., Abu-Rumeileh S., Piscaglia M.G., et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J. Neurol. 2020;267:1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Camdessanche J.P., Morel J., Pozzetto B., Paul S., Tholance Y., Botelho-Nevers E. COVID-19 may induce Guillain-Barré syndrome. Rev. Neurol. (Paris) 2020;176:516–518. doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Farzi M.A., Ayromlou H., Jahanbakhsh N., Bavil P.H., Janzadeh A., Shayan F.K. Guillain-Barré syndrome in a patient infected with SARS-CoV-2, a case report. J. Neuroimmunol. 2020;346 doi: 10.1016/j.jneuroim.2020.577294. 577294-577294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Oguz-Akarsu E., Ozpar R., Mirzayev H., Acet-Ozturk N.A., Hakyemez B., Ediger D., et al. Guillain-barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle Nerve. 2020;62:E54–E57. doi: 10.1002/mus.26992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P., et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e741. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Lantos J.E., Strauss S.B., Lin E. COVID-19–Associated miller Fisher syndrome: MRI findings. Am. J. Neuroradiol. 2020;41:1184–1186. doi: 10.3174/ajnr.A6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ottaviani D., Boso F., Tranquillini E., Gapeni I., Pedrotti G., Cozzio S., et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol. Sci. 2020;41:1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Rana S., Lima A.A., Chandra R., Valeriano J., Desai T., Freiberg W., et al. Novel coronavirus (COVID-19)-Associated guillain-Barré syndrome: case report. J. Clin. Neuromuscul. Dis. 2020;21:240–242. doi: 10.1097/CND.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Assini A., Benedetti L., Di Maio S., Schirinzi E., Del Sette M. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol. Sci. 2020;41:1657–1658. doi: 10.1007/s10072-020-04484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J. Peripher. Nerv. Syst. 2020;25:204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Reyes-Bueno J.A., García-Trujillo L., Urbaneja P., Ciano-Petersen N.L., Postigo-Pozo M.J., Martínez-Tomás C., et al. Miller-Fisher syndrome after SARS-CoV-2 infection. Eur. J. Neurol. 2020 doi: 10.1111/ene.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Sancho-Saldaña A., Lambea-Gil Á, Liesa J.L.C., Caballo M.R.B., Garay M.H., Celada D.R., et al. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin. Med. 2020;20:e93–e94. doi: 10.7861/clinmed.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Marta-Enguita J., Rubio-Baines I., Gastón-Zubimendi I. Fatal Guillain-Barre syndrome after infection with SARS-CoV-2. Neurologia. 2020;35:265–267. doi: 10.1016/j.nrl.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kilinc D., van de Pasch S., Doets A.Y., Jacobs B.C., van Vliet J., Mpj Garssen. Guillain-Barré syndrome after SARS-CoV-2 infection. Eur. J. Neurol. 2020 doi: 10.1111/ene.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Arnaud S., Budowski C., Ng Wing, Tin S., Degos B. Post SARS-CoV-2 guillain-Barré syndrome. Clin. Neurophysiol. 2020;131:1652–1654. doi: 10.1016/j.clinph.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Bigaut K., Mallaret M., Baloglu S., Nemoz B., Morand P., Baicry F., et al. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e785. doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Su X.W., Palka S.V., Rao R.R., Chen F.S., Brackney C.R., Cambi F. SARS-CoV-2-associated Guillain-Barré syndrome with dysautonomia. Muscle Nerve. 2020;62:E48–E49. doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., et al. Guillain-barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Lascano A.M., Epiney J.-B., Coen M., Serratrice J., Bernard-Valnet R., Lalive P.H., et al. SARS-CoV-2 and Guillain-Barré syndrome: AIDP variant with favorable outcome. Eur. J. Neurol. 2020 doi: 10.1111/ene.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Helbok R., Beer R., Löscher W., Boesch S., Reindl M., Hornung R., et al. Guillain-Barré syndrome in a patient with antibodies against SARS-COV-2. Eur. J. Neurol. 2020 doi: 10.1111/ene.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Coen M., Jeanson G., Culebras Almeida L.A., Hübers A., Stierlin F., Najjar I., et al. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav. Immun. 2020;87:111–112. doi: 10.1016/j.bbi.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Chan J.L., Ebadi H., Sarna J.R. Guillain-barré syndrome with facial diplegia related to SARS-CoV-2 infection. Can. J. Neurol. Sci. 2020:1–3. doi: 10.1017/cjn.2020.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Riva N., Russo T., Falzone Y.M., Strollo M., Amadio S., Del Carro U., et al. Post-infectious Guillain-Barré syndrome related to SARS-CoV-2 infection: a case report. J. Neurol. 2020;267:2492–2494. doi: 10.1007/s00415-020-09907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Juliao Caamaño D.S., Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J. Clin. Neurosci. 2020;77:230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Virani A., Rabold E., Hanson T., Haag A., Elrufay R., Cheema T., et al. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00771. e00771-e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Webb S., Wallace V.C., Martin-Lopez D., Yogarajah M. Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Novi G., Rossi T., Pedemonte E., Saitta L., Rolla C., Roccatagliata L., et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e797. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Bracaglia M., Naldi I., Govoni A., Brillanti Ventura D., De Massis P. Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J. Neurol. 2020:1–3. doi: 10.1007/s00415-020-10014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Domingues R.B., Mendes-Correa M.C., de Moura Leite F.B.V., Sabino E.C., Salarini D.Z., Claro I., et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J. Neurol. 2020:1–3. doi: 10.1007/s00415-020-09996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020:1–4. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Dixon L., Varley J., Gontsarova A., Mallon D., Tona F., Muir D., et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7:e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Brun G., Hak J.-F., Coze S., Kaphan E., Carvelli J., Girard N., et al. COVID-19-White matter and globus pallidum lesions: demyelination or small-vessel vasculitis? Neurol Neuroimmunol Neuroinflamm. 2020;7:e777. doi: 10.1212/NXI.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162:1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.El Otmani H., El Moutawakil B., Rafai M.A., El Benna N., El Kettani C., Soussi M., et al. Covid-19 and guillain-Barré syndrome: more than a coincidence! Rev. Neurol. (Paris) 2020;176:518–519. doi: 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Paybast S., Gorji R., Mavandadi S. Guillain-barré syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurol. 2020;25:101–103. doi: 10.1097/NRL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Maideniuc C., Memon A.B. Acute necrotizing myelitis and acute motor axonal neuropathy in a COVID-19 patient. J. Neurol. 2020:1–3. doi: 10.1007/s00415-020-10145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zito A., Alfonsi E., Franciotta D., Todisco M., Gastaldi M., Cotta Ramusino M., et al. COVID-19 and guillain-Barré syndrome: a case report and review of literature. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00909. 909-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Agosti E., Giorgianni A., D'Amore F., Vinacci G., Balbi S., Locatelli D. Is Guillain-Barrè syndrome triggered by SARS-CoV-2? Case report and literature review. Neurol. Sci. 2020:1–6. doi: 10.1007/s10072-020-04553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ameer N., Shekhda K.M., Cheesman A. Guillain-Barré syndrome presenting with COVID-19 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Diez-Porras L., Vergés E., Gil F., Vidal M.J., Massons J., Arboix A. Guillain-Barré-Strohl syndrome and COVID-19: case report and literature review. Neuromuscul. Disord. 2020;30:859–861. doi: 10.1016/j.nmd.2020.08.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.García-Manzanedo S., López de la Oliva Calvo L., Ruiz Álvarez L. Guillain-Barré syndrome after covid-19 infection. Med. Clin. 2020;155 doi: 10.1016/j.medcle.2020.06.019. 366-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Manganotti P., Bellavita G., D’Acunto L., Tommasini V., Fabris M., Sartori A., et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J. Med. Virol. 2020 doi: 10.1002/jmv.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Abrams R.M.C., Kim B.D., Markantone D.M., Reilly K., Paniz-Mondolfi A.E., Gitman M.R., et al. Severe rapidly progressive Guillain-Barré syndrome in the setting of acute COVID-19 disease. J. Neurovirol. 2020;26:797–799. doi: 10.1007/s13365-020-00884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Tiet M.Y., AlShaikh N. Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Ray A. Miller Fisher syndrome and COVID-19: is there a link? BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Liberatore G., De Santis T., Doneddu P.E., Gentile F., Albanese A., Nobile-Orazio E. Clinical Reasoning: a case of COVID-19 associated pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Neurology. 2020 doi: 10.1212/WNL.0000000000010817. [DOI] [PubMed] [Google Scholar]
- 421.Khalifa M., Zakaria F., Ragab Y., Saad A., Bamaga A., Emad Y., et al. Guillain-barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatric Infect Dis Soc. 2020;9:510–513. doi: 10.1093/jpids/piaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Frank C.H.M., Almeida T.V.R., Marques E.A., de Sousa Monteiro Q., Feitoza P.V.S., Borba M.G.S., et al. Guillain-barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J. Trop. Pediatr. 2020 doi: 10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Kopscik M.R., Giourgas B.K., Presley B.C. A case report of acute motor and sensory polyneuropathy as the presenting symptom of SARS-CoV-2. Clin Pract Cases Emerg Med. 2020;4:352–354. doi: 10.5811/cpcem.2020.6.48683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Elkhouly A., Kaplan A.C. Noteworthy neurological manifestations associated with COVID-19 infection. Cureus. 2020;12 doi: 10.7759/cureus.8992. e8992-e8992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 425.Masuccio F.G., Barra M., Claudio G., Claudio S. A rare case of acute motor axonal neuropathy and myelitis related to SARS-CoV-2 infection. J. Neurol. 2020:1–4. doi: 10.1007/s00415-020-10219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Senel M., Abu-Rumeileh S., Michel D., Garibashvili T., Althaus K., Kassubek J., et al. Miller-Fisher syndrome after COVID-19: neurochemical markers as an early sign of nervous system involvement. Eur. J. Neurol. 2020;27:2378–2380. doi: 10.1111/ene.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Foresti C., Servalli M.C., Frigeni B., Rifino N., Storti B., Gritti P., et al. COVID-19 provoking Guillain–Barré syndrome: the Bergamo case series. Eur. J. Neurol. 2020 doi: 10.1111/ene.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Durrani M., Kucharski K., Smith Z., Fien S. Acute transverse myelitis secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a case report. Clin Pract Cases Emerg Med. 2020;4:344–348. doi: 10.5811/cpcem.2020.6.48462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Pelea T., Reuter U., Schmidt C., Laubinger R., Siegmund R., Walther B.W. SARS-CoV-2 associated Guillain-Barré syndrome. J. Neurol. 2020:1–4. doi: 10.1007/s00415-020-10133-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Granger A., Omari M., Jakubowska-Sadowska K., Boffa M., Zakin E. SARS-CoV-2–Associated guillain–Barre syndrome with good response to plasmapheresis. J. Clin. Neuromuscul. Dis. 2020;22 doi: 10.1097/CND.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 431.Civardi C., Collini A., Geda D.J., Geda C. Antiganglioside antibodies in Guillain-Barré syndrome associated with SARS-CoV-2 infection. J. Neurol. Neurosurg. Psychiatr. 2020;91:1361–1362. doi: 10.1136/jnnp-2020-324279. [DOI] [PubMed] [Google Scholar]
- 432.Wada S., Nagasaki Y., Arimizu Y., Shimo M., Matsukuma Y., Okamoto M., et al. Neurological disorders identified during treatment of a SARS-CoV-2 infection. Intern. Med. 2020;59:2187–2189. doi: 10.2169/internalmedicine.5447-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Naddaf E., Laughlin R.S., Klein C.J., Toledano M., Theel E.S., Binnicker M.J., et al. Guillain-barré syndrome in a patient with evidence of recent SARS-CoV-2 infection. Mayo Clin. Proc. 2020;95:1799–1801. doi: 10.1016/j.mayocp.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95 doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 435.Chow C.C.N., Magnussen J., Ip J., Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Sotoca J., Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Chakraborty U., Chandra A., Ray A.K., Biswas P. COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Fernández-Domínguez J., Ameijide-Sanluis E., García-Cabo C., García-Rodríguez R., Mateos V. Miller-Fisher-like syndrome related to SARS-CoV-2 infection (COVID 19) J. Neurol. 2020;267:2495–2496. doi: 10.1007/s00415-020-09912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Manganotti P., Pesavento V., Buoite Stella A., Bonzi L., Campagnolo E., Bellavita G., et al. Miller Fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J. Neurovirol. 2020;26:605–606. doi: 10.1007/s13365-020-00858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Korem S., Gandhi H., Dayag D.B. Guillain-Barré syndrome associated with COVID-19 disease. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237215. [DOI] [PMC free article] [PubMed] [Google Scholar]