Abstract
The outbreak of coronaviruses (CoVs) presents an enormous threat to humans. To date, no new therapeutic drugs or vaccines licensed to treat human coronaviruses remain undiscovered. This mini-review briefly reports the number of potential plants widely distributed in Indonesia for further research and development as anti-SARS-CoV-2 agents and the critical targets for SARS-CoV-2 therapy, such as angiotensin-converting enzyme 2 (ACE-2) receptor, spike protein, 3-chymotrypsin-like protease (3CLpro), papain-like protease (PLpro), RNA-dependent RNA polymerase (RdRp), helicase, and serine protease. Indonesia is rich in medicinal plants (herbal); it also has a long history of using plants to treat various hereditary diseases. However, since SARS-CoV-2 is a new disease, it has no history of plant-based treatment anywhere in the world. This mini-review describes natural products from several Indonesian plants that contain compounds that could potentially prevent or reduce SARS-CoV-2 infection, act as potential targeted therapy, and provide new therapeutic strategies to develop SARS-CoV-2 countermeasures.
Keywords: COVID-19, SARS-CoV-2, Therapeutic target, Emodin, Luteolin, Mangroves
COVID-19; SARS-CoV-2; Therapeutic target; Emodin; Luteolin; Mangroves.
1. Introduction
CoVs are a family of positive-stranded RNA viruses that cause life-threatening breathing infections and severe pneumonia in humans (which resulted in a significant epidemic only two decades ago). Presently, three single-stranded RNA (ssRNA) beta-coronaviruses, including SARS (severe acute respiratory syndrome) virus, MERS (Middle East respiratory syndrome) virus, and SARS-CoV-2, have been detected (Masters, 2006; Gorbalenya et al., 2020; Huang et al., 2020).
The World Health Organization (WHO) designated the coronavirus discovered in 2019 as 2019-novel coronavirus or SARS-CoV-2. It has been reported that SARS-CoV-2 is exceptionally homologous to SARS-CoV. Therefore, SARS-CoV-2 is believed to be a close relative of SARS-CoV. Accordingly, the International Virus Classification Commission (ICTV) identified SARS-CoV-2 as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and concurrently, the WHO recognized the disease generated by 2019-nCoV as COVID-19 (Zhu et al., 2020; Chen et al., 2020a, b). At the time this mini-review was written, the number of confirmed cases was 5,488,825, with a mortality rate of 349,095 people in 217 countries or territories (WHO, 2020).
The Department of Clinical Microbiology, Faculty of Medicine, Universitas Indonesia, conducted a preliminary analysis on COVID-19 cases in Indonesia. The Laboratory of Clinical Microbiology (one of the reference laboratories for COVID-19 specimens) received 4167 specimens from March to April 2020, with 582 specimens showing positive results, indicating that the positivity rate was 12.6%. Demographic data revealed that the average age of COVID-19-positive patients was 44.5 years, with an age range of 2–85 years, and 59% were male. Approximately 17% of patients had comorbidities, predominantly cardiovascular disease (58%) and diabetes (37%). The average age of the COVID-19-positive patients reported in that work was similar to that reported in the Singapore case data (42.5 years) but younger than that reported in Wuhan (55.5 years). However, the findings that male sex and cardiovascular disease are COVID-19 comorbidities are concordant with previous studies from other countries (Ibrahim et al., 2020).
Moreover, 8% of the positive patients with COVID-19 were health workers. Another surprising finding was that approximately 22% of COVID-19 patients were asymptomatic, and 34% had mild symptoms, with the remaining 44% exhibiting moderate to serious signs. Although respiratory symptoms are common in patients with COVID-19 (found in 73% of cases), it has been shown that nonspecific symptoms of COVID-19, such as gastrointestinal complaints, lethargy and headaches, cannot be excluded. This fact should definitely be considered due to the transmission risk (Ibrahim et al., 2020).
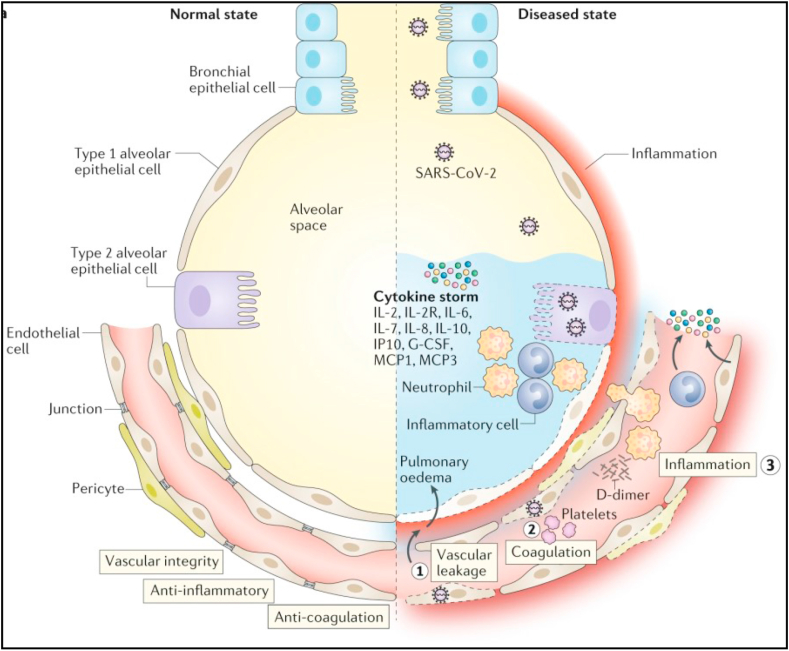
SARS-CoV-2 interacts with cell surface receptors named angiotensin converting enzyme 2 (ACE-2) once it enters the cells of humans (Li et al., 2003; Han et al., 2006; Ge et al., 2013). The penetration of the virus into the human body triggers an immune system reaction with the aim of eliminating the virus. The working immune system consists of various cells, including macrophage cells, neutrophil cells, lymphocytes, dendrite cells, etc., which produce cytokines. These cytokines activate cells and trigger further mechanisms that elicit symptoms of the disease. Example mechanisms in the lungs (Figure 1): S-ACE2 protein binding leads to viral entry – triggers the immune system: (1) Endothelial cell damage occurs, and fluid from the blood vessels enters the alveoli of the lungs, causing symptoms. (2) The damaged endothelium triggers the coagulation system (blood clotting), and a blood clot can form, which is carried through the bloodstream and causes blockage of the blood vessels. (3) An inflammatory reaction (inflammation) occurs, which induces the production of cytokines and leads to a cytokine storm (Teuwen et al., 2020).
Figure 1.
Pathomechanism of the lungs (adapted from Teuwen et al., 2020 with permission).
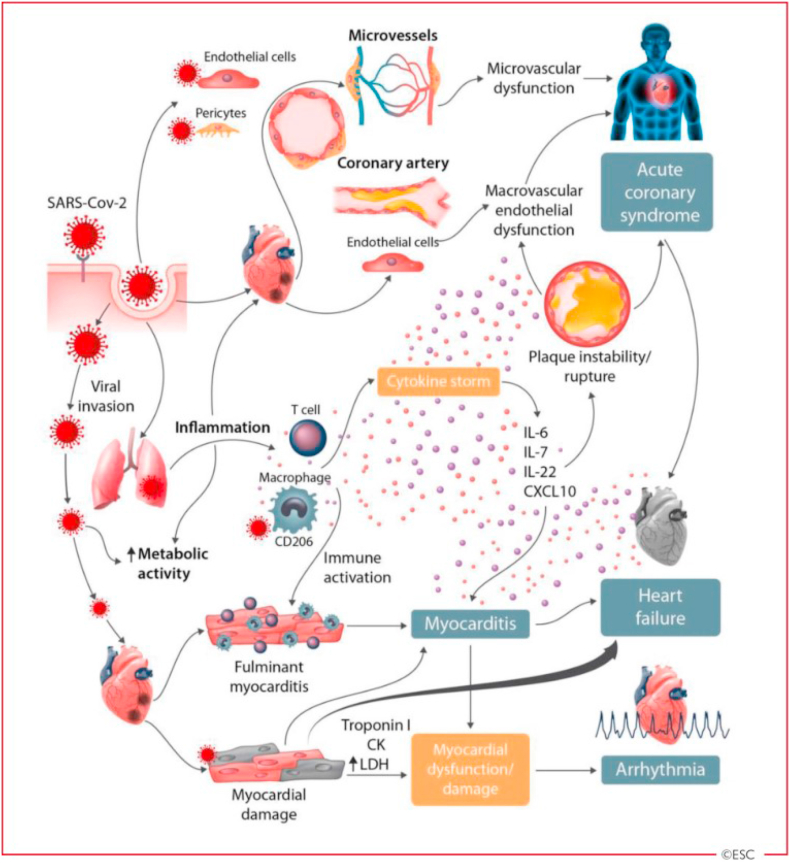
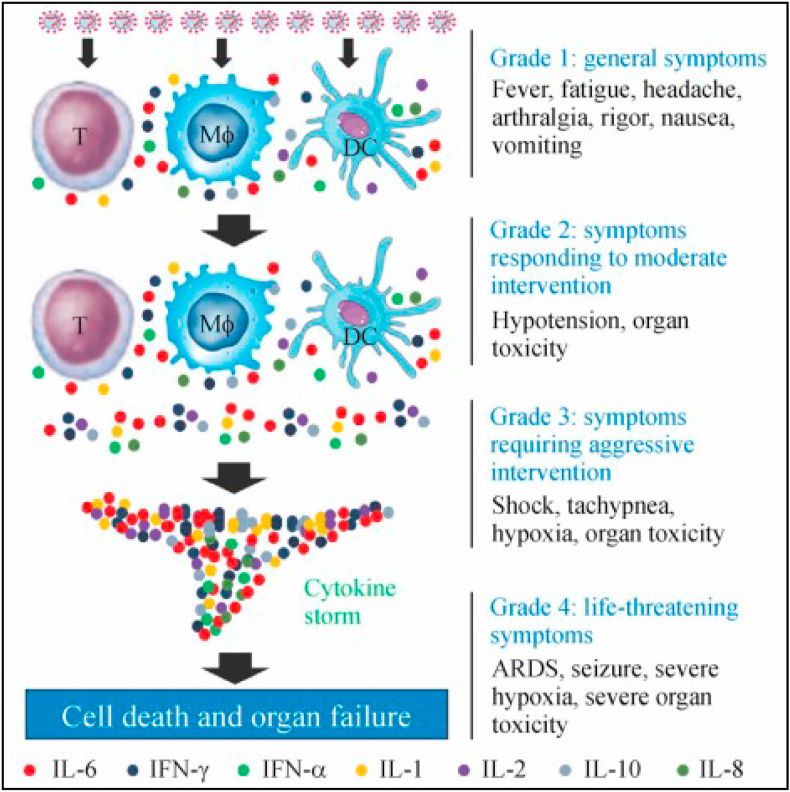
Example mechanisms in the heart (Figure 2): S-ACE2 protein binding leads to viral entry – triggers the immune system: Cytokines are released by macrophages, causing damage to endothelial cells and pericytes, as well as myocyte damage. The resulting damage can lead to myocarditis, acute coronary syndrome, arrhythmia, and heart failure. The symptoms are the same as those of a heart attack (Guzik et al., 2020) and can be mild, moderate, or severe, depending on the amount of cytokines formed. The more cytokines that are formed, the more severe the symptoms will be (illustration in Figure 3) (Zhou et al., 2020a, b).
Figure 2.
Pathomechanism of the heart (adapted Guzik et al., 2020 with permission).
Figure 3.
Cytokine formation related to the severity of COVID-19 symptoms (adapted from Zhou et al., 2020a, b with permission).
There are two therapeutic possibilities for anti-coronavirus therapy (depending on the target): enhancing the human immune system (human cells) and attacking the coronavirus itself. The innate immune response is associated with the human immune system and helps to control the infection by preventing coronavirus replication (Omrani et al., 2014). If the human cell signaling pathways required for virus replication can be blocked, a certain antiviral effect could be achieved.
Coronavirus therapies include preventing viral RNA synthesis through the use of genetic virus material to prevent replication by acting on critical virus enzymes and preventing the binding of virus to human cells (ACE-2 receptor and spike protein) or inhibiting the process of self-assembly of the virus by acting on certain structural proteins, including viral papain-like protease (PLpro), main protease (Mpro/3CLpro, also recognized 3-chymotrypsin-like protease), RNA-dependent RNA polymerase (RdRp), helicase, and serine protease (Yu et al., 2012; Clemente et al., 2019; Wu et al., 2020).
Presently, in silico (computational model) techniques are being used to identify plant compounds that can prevent SARS-CoV-2 infection and replication by predicting their activity against disease targets (Gyebi et al., 2020). For example, methoxyflavonoid compounds such as hesperetin, tangerine, naringenin, and nobiletin have been identified in oranges (Citrus sp.; fruit and skin) (Singh et al., 2020; Utomo et al., 2020), and baikalin, scutellarin, glycyrrhizin, rhoifolin, herbacetin, pectolinarin and galangin compounds have been identified in galangal (Alpinia galanga; rhizome) (Tang et al., 2018; Haslberger et al., 2020; Utomo et al., 2020).
The similarity of the SARS-CoV-2 virus gene sequences with SARS-CoV reaches 79.5%, and the similarity in the manner in which the virus enters humans and passes through the ACE-2 receptor suggests common therapeutic targets (Jia et al., 2005; Zhou et al., 2020a, b). The chemical content (compounds) of some plants has been widely studied, and their activity related to SARS-CoV therapy has been documented, which will speed up evaluations of the activity of these compounds against SARS-CoV-2. Some Indonesian plants contain active compounds that could restrict SARS-CoV-2 infection and replication based on their activity against relevant therapeutic targets. Potentially active compounds have been found in vegetables, crops, and mangroves (Indonesian plants) and show activity (binding) against protein targets such as RBD-S, PD-ACE2, and SARS-CoV-2 protease. These compounds (i.e., hesperidin, flavonoids, curcumin, brazilin, and galangin), show the potential for development as SARS-CoV-2 inhibitors that could be applied in daily life as prophylaxis for COVID-19 (Amin et al., 2019; Azminah et al., 2019; Utomo et al., 2020).
2. Method
Data on COVID-19, herbal medicine and dietary therapy were searched and collected for this miniature review and perspective. We used key search engines, namely, Google Scholar, PubMed, Science Direct and SciFinder. The search keywords used comprised coronavirus; etiology; signs; symptoms; allopathic therapy adjacent to COVID-19; immunomodulatory and anti-influenza herbal activity, SARS-CoV-1, and SARS-CoV-2. The authors appraised, evaluated, and interpreted the selected articles. This perspective reflects the opinion of the authors concerning the use of foods and herbs as preventatives and corresponding therapies against COVID-19.
3. Targets for blocking the viral entry point
3.1. Angiotensin-converting enzyme 2 (ACE-2)
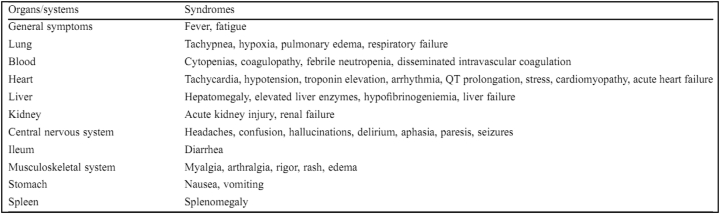
ACE-2 is a receptor that has been confirmed as the entry point for the SARS-CoV-2 virus to infect human cells. Scientists suggest a strong interaction exists between human ACE-2 molecules and SARS-CoV-2 (Xu et al., 2020). ACE-2 receptors are widely present in lung cells (especially lung endothelial cells) and in large numbers in type II alveolar cells in the lungs, epithelial cells in the upper esophagus, enterocytes in the ileum (the last part of the small intestine) and colon (large intestine), epithelial cells in the bile, heart muscle cells, proximal tubules in the kidneys, and urothelial cells in the bladder (Zou et al., 2020), meaning that the SARS CoV-2 virus can attack any organ that has ACE-2 receptors. Symptoms that arise based on the target organ are as follows: in the lungs, it causes shortness–edema–respiratory failure; in the blood, it causes a reduction in the number of blood cells–coagulopathy; in the kidneys, it causes renal failure; and in the heart, it causes tachycardia–arrhythmia and heart failure (Figure 4) (Zhou et al., 2020a, b). ACE-2 is an integral membrane protein type I with an active site domain uncovered outside the cells and has been described in receptor function studies of SARS-CoV-2 (Kuhn et al., 2006). The virus starts the infection by spike protein (S-protein) binding between SARS-CoV-2 and ACE-2 in the host cell (Walls et al., 2020). The interaction of ACE-2 and the viral S-protein enables SARS-CoV-2 to enter the circulation (Belouzard et al., 2009). The SARS-CoV-2 receptor-binding domain (RBD) successfully interacts human ACE2-expressing cells but not with any other receptors, ensuring that in the future, human ACE-2 will be required by the newly evolving SARS-CoV-2 for cell entry (Hoffmann et al., 2020; Letko et al., 2020).
Figure 4.
Signs and symptoms of SARS CoV-2 virus in organs that have ACE-2 receptors (adapted from Zhou et al., 2020a, b with permission).
This infection risk can be prevented or reduced with plant compounds that can interfere with these interactions. Molecular docking studies have shown that baicalin might bind strongly to the ACE-2 enzyme with an expected ΔG (kcal/mol) of –8.46 and potential binding sites at ASN-149, ARG-273, and HIS-505. These binding sites are situated in the hydrophobic area of ACE-2. Based on the potential binding to ACE-2, it can be suggested that baicalin is a promising candidate for 2019-nCoV treatment. Furthermore, molecular docking found that scutellarin has the potential to bind to ACE-2 with a projected ΔG (kcal/mol) of –14.9 and binding sites at GLU-495, UNK-957, and ARG-482 (Chen and Du, 2020). The molecular docking studies of hesperidin with the ACE-2 enzyme demonstrated that hesperidin can bind to ACE-2 with a predicted ΔG (kcal/mol) of –8.3 and binding sites at TYR-613, SER-611, ARG-482, and GLU-479 (Chen and Du, 2020). These results suggest that hesperidin may bind to ACE-2 and thus block 2019-nCoV infection.
A previous study (Takahashi et al., 2015) demonstrated that nicotianamine is a powerful inhibitor of ACE-2, with an IC50 value of 84 nM. The molecular docking studies of nicotinamine to the ACE-2 enzyme showed that nicotinamine has a promising binding affinity to ACE-2, with an approximate ΔG (kcal/mol) of –5.1 and binding sites at ARG-518, GLU-406, SER-409, GLN-522, and GLN-442. These results indicate that nicotinamine might block 2019-nCoV infection by inhibiting ACE-2 (Chen and Du, 2020). The molecular docking results also showed that glycyrrhizin could bind to ACE-2 with an estimated ΔG (kcal/mol) of –9 and binding sites at ARG-559, GLN-388, ARG-393, and ASP-30. Based on the hydrophobic nature of the ACE-2 binding site, the prediction of the binding site of glycyrrhizin is near this site. GLN-388 and ARG-393 are close to the zinc metallopeptidase that might regulate the activity of ACE-2 in cells (Chen and Du, 2020). The docking scores of the binding sites between natural compounds and ACE-2 of SARS-CoV-2 are presented in Table 1.
Table 1.
Docking scores of the natural compounds towards the ACE-2 binding sites of SARS-CoV-2.
| Plant name | Family | Plant part used | Compound name | Binding energy | Binding site | Reference |
|---|---|---|---|---|---|---|
| Scutellaria baicalensis Georgi. | Lamiaceae | – | Baicalin | –8.46 | ASN-149, ARG-273, HIS-505 | Chen and Du (2020) |
| Erigeron breviscapus (Vant.) Hand Mazz. | Asteraceae | – | Scutellarin | –14.9 | GLU-495, UNK-957, ARG-482 | Chen and Du (2020) |
| Citrus aurantium, Citri reticulatae Pericarpium | Rutaceae | – | Hesperidin | –8.3 | TYR-613, SER-611, ARG-482, GLU-479 | Chen and Du (2020) |
| Glycine max | Leguminosae | – | Nicotianamine | –5.1 | ARG-518, GLU-406, SER-409, GLN-522, GLN-442 | Chen and Du (2020) |
| Licorice root (Glycyrrhiza radix) | Fabaceae | Herb | Glycyrrhizin | –9 | ARG-559, GLN-388, ARG-393, ASP-30 | Chen and Du (2020) |
Several edible plants found across Indonesia contain emodin and luteolin compounds, which have the potential to prevent or reduce infection with this virus (Table 2). These compounds are capable of preventing interactions between ACE-2 receptors and the S-protein in SARS-CoV (Miean and Mohamed, 2001; Ho et al., 2007). Edible Indonesian plants, used in folk medicine primarily in tropical regions of the world, are rich sources of emodin and include leaves of Aloe vera (L.) Burm. F. (Xanthorrhoeaceae), roots of Rheum officinale Baill. (Polygonaceae), and seeds of the genus Cassia plants such as Cassia alata L. (Fabaceae) or Senna alata (L.) Roxb. (Fabaceae), Cassia obtusifolia L. (Fabaceae) or roots of Senna obtusifolia (L.) H.S. Irwin & Barneby (Fabaceae), Senna alexandrina Mill. (Fabaceae), and Cassia occidentalis L. (Fabaceae). Plants that contain an abundance of luteolin are the leaves and seeds of celery (Apium graveolens L., Apiaceae), the leaves and flowers of Elephantopus scaber L. (Asteraceae), and the leaves of onions (Allium cepa L., Liliaceae) and broccoli (Brassica oleracea L., Brassicaceae). Other edible plants include the fruit of chili pepper (Capsicum annuum L., Solanaceae), the leaves and fruit of Averrhoa bilimbi L. (Oxalidiaceae), the leaves of kaffir lime (Citrus hystrix D.C., Rutaceae), and the tuber of carrots (Daucuscarota L., Apiaceae). These plants are widely distributed throughout the Indonesian archipelago.
Table 2.
Distribution of edible plants containing emodin or luteolin in Indonesia.
| Species | Tissue | Compound | Reference |
|---|---|---|---|
| Aloe vera | Leaves | Emodin | (Ahlawat and Khatkar, 2011) |
| Rheum officinale | Roots | Emodin | (Hsu and Chung, 2012); (Kuo et al., 2020) |
| Cassia alata or Senna alata | Leaves, stems, fruits | Emodin | (Hennebelle et al., 2009); (Das et al., 2019) |
| Roots | Emodin | (Adedoyin et al., 2015) | |
| Stems | Luteolin | (Hennebelle et al., 2009) | |
| Cassia obtusifolia or Senna obtusifolia | Seeds | Emodin | (Yang et al., 2003) |
| Senna alexandrina | Pods | Emodin | (Elkhidir et al., 2012) |
| Cassia occidentalis | Roots | Emodin | (Chukwujekwu et al., 2006) |
| Apium graveolens | Leaves | Luteolin | (Zhu and Row, 2011); (Shivashri et al., 2013) |
| Elephantopus scaber | The whole plant, aerial part, and roots | Luteolin | (Zuo et al., 2016); (Chang et al., 2011); (Su et al., 2009) |
| Allium cepa | Bulbs | Luteolin | (Singh and Goel, 2015) |
| Brassica oleracea | Leaves | Luteolin | (Miean and Mohamed, 2001); (Schmidt et al., 2010); (Mageney et al., 2017) |
| Capsicum annuum | Fruits | Luteolin | (Miean and Mohamed, 2001); (Materska et al., 2015) |
| Averrhoa bilimbi | Fruits and leaves | Luteolin | (Miean and Mohamed, 2001); (Ramsay and Mueller-Harvey, 2016) |
| Citrus hystrix | Fruits | Luteolin | (Alu'Datt et al., 2017) |
| Daucus carota | Fruits | Luteolin | (Miean and Mohamed, 2001) |
Several studies have reported several mangrove plants that contain emodin or luteolin, as shown in Table 3. Two mangrove species, Kandelia candel and Lumnitzera racemosa, have been reported to contain emodin (Wu et al., 2008; Liu et al., 2010). In Indonesia, K. candel is distributed in northeast Sumatera and west and north Kalimantan, while L. racemosa is widely available throughout Indonesia (Noor et al., 1999). Indonesia is recognized as the major mangrove area in the world and has vast species diversity (Noor et al., 1999). Similarly, luteolin is found in Avicennia marina leaves and aerial roots and in mangroves and is associated with Pongamia pinnata leaves and stems (Sharaf et al., 2000; Momtazi-borojeni et al., 2013). A. marina and P. pinnata are found throughout Indonesia (Noor et al., 1999; Li et al., 2006; Ahmed et al., 2010; Shamsuddin et al., 2013). This potential of these raw materials opens up several non-wood functions of mangrove plants that can help reduce and prevent SARS-CoV-2 infection.
Table 3.
Indonesian mangrove plants containing emodin or luteolin.
| Species | Tissue | Compound | Reference |
|---|---|---|---|
| Kandelia candel | Stem barks | Emodin | (Liu et al., 2010) |
| Lumnitzera racemosa | Stems and leaves | Emodin | (Wu et al., 2008) |
| Avicennia marina | Leaves | Luteolin | (Momtazi-borojeni et al., 2013) |
| Aerial roots | Luteolin | (Sharaf et al., 2000) | |
| Pongamia pinnata | Stem | Luteolin | (Li et al., 2006) |
| Leaves | Luteolin | (Ahmed et al., 2010); (Shamsuddin et al., 2013) |
3.2. Spike protein
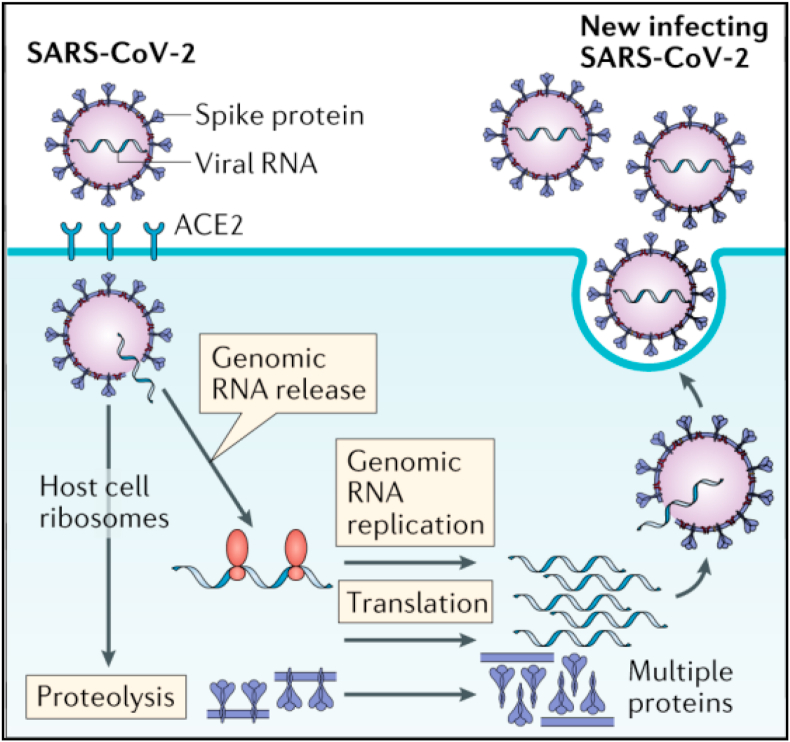
The coronavirus (CoV) family has an abundance of similar spike proteins. This spike protein (S-protein) interacts with host cells, such as pulmonary and parabronchial epithelial cells, and aids in SARS-CoV-2 infection via the epithelial cell membrane (Xia et al., 2020). The surface spike (S) glycoprotein (S-protein) is an essential binding protein for fusion of the virus with the cell membrane of the target cells (host cells) via cellular receptor angiotensin-converting enzyme 2 (ACE-2) (Song et al., 2018). The S-protein has two main functions: the first is binding to the target cell (host cells), which can be imagined as a key in a lock (it must fit or match), and the second is fusion of the viral membrane with the host cell (human), which allows the virus to enter the target cell. Once in the target cell, the RNA from SARS-CoV-2 can be transferred into the host cell to immediately begin virus propagation (Walls et al., 2020), and then the virus will multiply (replicate) and be packaged into new viruses. The new copies of the virus will then leave the target cell and look for other target cells (Figure 5) (Risitano et al., 2020). SARS-CoV-2 is easily transmitted because the S-protein on the virus surface binds quite efficiently to ACE-2 on human cells.
Figure 5.
Mechanism of SARS CoV-2 entry and replication in target cells (adapted from Risitano et al., 2020 with permission).
As shown in a previous study of several potentially active compounds, the docking scores of cannabinoids, rhoifolin, pectolinarin, morin, kaempferol, epigallocatechin gallate, herbacetin, and hesperidin against the S-protein were –10.2, –9.5, –9.8, –8.8, –8.5, –9.8, –8.3, and –10.4, respectively (Tallei et al., 2020). Another investigation showed that hesperidin had a docking score against the S-protein of –9.6 (Utomo et al., 2020). The docking scores of these natural compounds with the binding sites (protein) of the S-protein of SARS-CoV-2 are shown in Table 4.
Table 4.
Docking scores of natural compounds towards the S-protein binding site in SARS-CoV-2.
| Plant name | Family | Plant part used | Compound name | Binding energy | Binding site (protein) | Reference |
|---|---|---|---|---|---|---|
| Marijuana (Cannabis spp.) | Cannabaceae | – | Cannabinoids | –10.2 | S-protein (6VXX) | Tallei et al. (2020) |
| Rhus plant (Rhus succedanea), Bitter orange (Citrus aurantium), Bergamot (Citrus bergamia), Grapefruit (Citrus paradisi), Lemon (Citrus limon), Lablab beans (Lablab purpureus), Tomato (Lycopersicon esculentum), Artichoke (Cynara scolymus), Bananas (Musa spp.), Grape (Vitis vinifera) | – | – | Rhoifolin | –9.5 | S-protein (6VXX) | Tallei et al. (2020) |
| Plume thistles (Cirsium spp.), Yellow toadflax (Linaria vulgaris) |
– | – | Pectolinarin | –9.8 | S protein (6VXX) | Tallei et al. (2020) |
| Osage orange (Maclura pomifera), Almond (Prunus dulcis), Old fustic (Chlorophora tinctoria), Guava (Psidium guajava) | – | – | Morin | –8.8 | S-protein (6VXX) | Tallei et al. (2020) |
| Kale (Brassica oleracea var. sabellica), Beans (Phaseolus vulgaris), Tea (Camellia sinensis), Spinach (Spinacia oleracea), Broccoli (Brassica oleracea var. Italica) | – | – | Kaempferol | –8.5 | S-protein (6VXX) | Tallei et al. (2020) |
| Tea (Camellia sinensis) (green tea), skin of Apple (Malus domestica), Plum (Prunus domestica), Onion (Allium cepa), Hazelnut (Corylus avellana) | – | – | Epigallocatechin gallate | –9.8 | S-protein (6VXX) | Tallei et al. (2020) |
| Golden root (Rhodiola spp.), Gossypium (Gossypium hirsutum), Common horsetail (Equisetum arvense), Common boneset (Eupatorium perfoliatum) | – | – | Herbacetin | –8.3 | S-protein (6VXX) | Tallei et al. (2020) |
| Citrus fruit (Citrus spp.), Peppermint (Mentha spp.), Yellow Toadflax (Linaria vulgaris) | – | – | Hesperidin | –10.4 | S-protein (6VXX) | Tallei et al. (2020) |
| Citrus sp. | Rutaceae | – | –9.6 | Spike glycoprotein-RBD (6LXT) | Utomo et al. (2020) |
4. Targets for blocking RNA synthesis and viral replication
4.1. 3-chymotrypsin-like cysteine protease (3CLpro)
3CLpro is a crucial enzyme for coronavirus replication (Liu and Wang, 2020). 3CLpro mediates Nsps (nonstructural protein) maturation directly, which is significant in the virus life cycle. Investigations into the structure and catalytic mechanism of 3CLpro have made it an enticing target for anti-coronavirus drug development. Peptide and small molecular inhibitors primarily contain inhibitors of SARS-CoV-2 3CLpro (Pillaiyar et al., 2016).
As presented in a previous study of several potentially active compounds, the best docking scores of cannabinoids, rhoifolin, pectolinarin, morin, kaempferol, epigallocatechin gallate, herbacetin, and hesperidin against 3CLpro were –8, –8.2, –8.2, –7.8, –7.8, –7.8, –7.2, and –8.3, respectively (Tallei et al., 2020).
Hesperidin was found to dose-dependently inhibit the cleavage activity of the 3-C-like protease (3CLpro) of SARS coronavirus in cell-free and cell-based assays, with an IC50 of 8.3 μM (Lin et al., 2005). A previous study also showed that the docked hesperidin compound against 3CLpro was –13.51 (Utomo et al., 2020), and according to a previous investigation, the best hesperidin position against SARS-CoV-2 3CLpro had a score of –10.1 (Chen et al., 2020a, b).
Another investigation reported that the rhoifolin binding score for SARS-CoV 3CLpro was –9.565, and the induced-fit docking result of pectolinarin against SARS-CoV 3CLpro was –8.054. Based on a previous description, the best binding positions against SARS-CoV 3CLpro of morin, kaempferol, and herbacetin had scores of –8.930, –8.526, and –9.263, respectively (Jo et al., 2020). Epigallocatechin gallate has also been reported to prevent the proteolytic property of SARS-CoV 3CLpro (Nguyen et al., 2012). The docking scores of the binding sites of natural compounds towards 3CLpro of SARS-CoV-2 are presented in Table 5.
Table 5.
Docking scores of the natural compounds towards the 3CLpro binding site of SARS-CoV-2.
| Plant name | Family | Plant part used | Compound name | Binding energy | Binding site | Reference |
|---|---|---|---|---|---|---|
| Marijuana (Cannabis spp.) | Cannabaceae | Cannabinoids | –8 | – | Tallei et al. (2020) | |
| Rhus plant (Rhus succedanea), Bitter orange (Citrus aurantium), Bergamot (Citrus bergamia), Grapefruit (Citrus paradisi), Lemon (Citrus limon), Lablab beans (Lablab purpureus), Tomato (Lycopersicon esculentum), Artichoke (Cynara scolymus), Bananas (Musa spp.), Grape (Vitis vinifera) | – | – | Rhoifolin | –8.2 | – | Tallei et al. (2020) |
| – | – | –9.565 | Asp142, Gln166, Gln189, Leu167, Phe140, Thr24, Thr26 | Jo et al. (2020) | ||
| Plume thistles (Cirsium spp.), Yellow toadflax (Linaria vulgaris) |
– | – | Pectolinarin | –8.2 | – | Tallei et al. (2020) |
| – | – | –8.054 | Gln189, Glu166, Gly143, HIE164, His41, His163 | Jo et al. (2020) | ||
| Osage orange (Maclura pomifera), Almond (Prunus dulcis), Old fustic (Chlorophora tinctoria), Guava (Psidium guajava) | – | – | Morin | –7.8 | – | Tallei et al. (2020) |
| – | – | –8.930 | Asp142, Ile188, Glu166 | Jo et al. (2020) | ||
| Kale (Brassica oleracea var. sabellica), Beans (Phaseolus vulgaris), Tea (Camellia sinensis), Spinach (Spinacia oleracea), Broccoli (Brassica oleracea var. Italica) | – | – | Kaempferol | –7.8 | – | Tallei et al. (2020) |
| – | – | –8.526 | Asp142, Ile188, Glu166 | Jo et al. (2020) | ||
| Tea (Camellia sinensis) (green tea), skin of Apple (Malus domestica), Plum (Prunus domestica), Onion (Allium cepa), Hazelnut (Corylus avellana) | – | – | Epigallocatechin gallate | –7.8 | – | Tallei et al. (2020) |
| Golden root (Rhodiola spp.), Gossypium (Gossypium hirsutum), Common horsetail (Equisetum arvense), Common boneset (Eupatorium perfoliatum) | – | – | Herbacetin | –7.2 | – | Tallei et al. (2020) |
| – | – | –9.263 | Asp142, Ile188, Gln189, Glu166, His41 | Jo et al. (2020) | ||
| Citrus fruit (Citrus spp.), Peppermint (Mentha spp.), Yellow Toadflax (Linaria vulgaris) | – | – | Hesperidin | –8.3 | – | Tallei et al. (2020) |
| Citrus sp. | Rutaceae | – | –13.51 | – | Utomo et al. (2020) | |
| –10.1 | – | Chen et al., 2020a, b |
4.2. Papain-like protease (PLpro)
The polyproteins of the coronavirus are expressed after the papain-like protease (PLpro) enzyme plays a critical function in cleaving the polyproteins into fewer products, which are utilized for replicating novel viruses (Wrapp et al., 2020). PLpro controls the cleavage of the N-terminus of the replicase polyprotein to release Nsp1, Nsp2, and Nsp3, which is indispensable for modulating virus replication (Harcourt et al., 2004).
The bioactive compounds from natural sources, including platycodin D, baicalin, sugetriol-3,9-diacetate (from Platycodon grandiflorus, Scutellaria baicalensis, Cyperus rotundus, respectively), phaitanthrin D and 2,2-di (3-indolyl)-3-indolent from Isatis indigotica, catechin compounds ((e)-epigallocatechin gallate and 2 (3,4-dihydroxy phenyl)-2-[[2-(3,4-dihydroxy phenyl)-3,4-dihydro5,7-dihydroxy-2H-1-benzopyran-3-yl]oxy]-3,4-dihydro-2H-1benzopyran-3,4,5,7-tetrol), exhibited strong binding affinity to PLpro protein, illustrating that these compounds could be used for SARS-CoV-2 therapeutics (Wu et al., 2020).
Furthermore, the screening results reported in a previous study revealed that a series of drugs, including antivirals (ribavirin, valganciclovir, and thymidine), antibacterials (chloramphenicol, cefamandole, and tigecycline), muscle relaxants (chlorphenesin carbamate), and antitussives (levodropropizine), might all have a high binding affinity to PLpro. According to the docking model results, ribavirin attached to the active site of the enzyme and was described as a SARS-PLpro inhibitor (PDB code 3e9s). The interaction between ribavirin and the enzyme (hydrophobic interaction and strong hydrogen bonding) signifies that it is a potent PLpro inhibitor (Wu et al., 2020).
4.3. RNA-dependent RNA polymerase (RdRp)
Coronavirus expresses RNA-dependent RNA polymerase (RdRp) to generate the daughter RNA genome, which is an a significant replication step that catalyzes the synthesis of complementary RNA strands utilizing viral RNA templates (Mullard, 2018). The conserved protein in coronavirus (Nsp12) is an RdRp and the vital enzyme of the coronavirus replication or transcription complex (Subissi et al., 2014). According to a study on SARS-CoV and MERS-CoV inhibitors, Nsp12-RdRp has been shown to be a pivotal drug target. However, the targeted inhibition of Nsp12-RdRp might not induce significant toxicity and adverse impacts on host cells, and no particular inhibitors have been identified so far (Chu et al., 2006).
Some bioactive compounds from natural sources and derivatives with antiviral, anti-inflammatory, and antitumor activities have revealed major binding affinity to RdRp, including betulonal, 2β,30β-dihydroxy-3,4-seco-friedelolactone-27-lactone, 14-deoxy-11,12-didehydroandrographolide, 1,7-dihydroxy-3-methoxyxanthone, and theaflavin 3,3′-di-O-gallate (from Cassine xylocarpa, Andrographis paniculata, Swertia pseudochinensis, and Camellia sinensis, respectively); gnidicin and gniditrin (from Gnidia lamprantha); and andrographolide derivatives (Wu et al., 2020). It has been shown that RNA-dependent RNA polymerase was detected in a mangrove plant, Excoecaria agallocha (Li et al., 2012).
4.4. Helicase
SARS-CoV-2 and other coronaviruses have an RNA helicase enzyme, which is important for viral replication and proliferation. A previous study demonstrated that myricetin and scutellarin compounds are capable of inhibiting helicase enzyme activity (Yu et al., 2012). Several Indonesian plants contain myricetin and scutellarin compounds; myricetin was found in clove plants (Syzygium aromaticum; flowers), duwet plants (Syzygium cumini; leaves), Semarang guava (Syzygium samarangense; leaves), and rosella (Hibiscus sabdariffa; flower petals), while scutellarin was found in Jakatuwa plants (Scoparia dulcis; leaves) and senggugu (Clerodendron serratum; leaves) (Cai and Wu, 1996; Kuo et al., 2004; Ali et al., 2005; Ayyanar and Subash-Babu, 2012; Wu et al., 2012; Wang et al., 2017).
Some bioactive compounds from natural sources have revealed high binding affinity to helicase, including many flavanoids (a-glucosyl hesperidin, hesperidin, rutin, quercetagetin 6-O-bD-glucopyranoside, and homovitexin), xanthones such as 3,5-dimethoxy-1-[(6-O-bDxylopyranosyl-bD-glucopyranosyl)oxy]-9H-xanthen-9-one, kouitchenside H, kouitchenside A, 8,2-dihydroxy-3,4,5-trimethoxy-1 [(6-O-bD-xylopyranosyl-b-D-glucopyranosyl)oxy]-9H-xanthen-9one, kouitchenside D, 1-hydroxy-2,6-dimethoxy-8-[(6-O-bDxylopyranosyl-bD-glucopyranosyl)oxy]-9H-xanthen-9-one and triptexanthoside D from Swertia genus, phyllaemblicin B and phyllaemblinol from Phyllanthus emblica (Wu et al., 2020).
In addition, according to structure-modeling helicase proteins, as described previously, several drugs, including antibacterial (lymecycline, cefsulodine, and rolitetracycline), antifungal (itraconazole), anti-HIV-1 (saquinavir), anticoagulant (dabigatran), and diuretic (canrenoic acid) drugs, have been projected through virtual ligand screening to be helicase inhibitors with high mfScores.
4.5. Serine protease
As previously described, SARS-CoV-2 infection in humans not only requires ACE-2 receptors as an entry point but also involves the binding of the S-protein on the surface of the virus to ACE-2 receptors. At a later stage, the activity of the serine protease enzyme required by TMPRSS2 (a transmembrane glycol) allows for fusion and entry of the target cell to begin infection. The inhibition of serine protease activity is a target in the prevention of viral infections. Recently, it has been shown that in addition to inhibiting interactions with ACE-2 receptors, the inhibition of protease enzymes (especially serine proteases) is also an impending target for controlling SARS-CoV-2 infection. Compounds that inhibit proteases in serine residues (serine protease inhibitors, hereafter referred to as SPIs) are thought to be good candidate drugs to stop the viral life cycle. SARS-CoV-2 employs proteases to facilitate the infection of host cells, making proteases a significant therapeutic target, as they are involved in many vital processes in the propagation of coronaviruses (Clemente et al., 2019; Hoffmann et al., 2020).
Plants are an abundant source of SPI and have been widely studied. SPI compounds derived from plants are generally in the form of proteins or molecules containing proteins (large molecules). Leguminous family plants (Fabaceae, Poaceae, and Solanaceae) are the primary sources of SPI-producing plants. The protein fraction derived from legumes is rich in SPI compounds. Examples of leguminous plants whose seeds contain SPI are peanuts (Arachis hypogaea), soybeans (Glycine max), beans (Phaseolus vulgaris), peas (Pisum sativum), and snore (Crotalaria juncea). In addition to legumes, several Indonesian plants contain SPI compounds, including moringa (Moringa oleifera; leaves and seeds), bitter melon (Momordica charantia; seeds), cucumber (Cucumis sativus; fruit), pumpkin (Cucurbita moschata; fruit), pineapple (Ananas comosus; fruit), sweet potato (Ipomoea batatas; tuber), and potato (Solanum tuberosum; tuber) (Bijina et al., 2011; Srikanth and Chen, 2016). Furthermore, SPI has been reported in the mangrove plant Excoecaria agallocha (Li et al., 2012) and mangrove-associated Derris trifoliata (Bhattacharyya and Babu, 2009).
The Indonesian government encourages the potential use of domestic resources to deal with COVID-19. The efficacy claims of the products available to combat COVID-19 are more focused on the function of maintaining and increasing the human immune system (Hartanti et al., 2020). The future prospects of natural compounds on COVID-19 from Indonesian medicinal plants include, for example, trans-cinnamaldehyde and analogs of Cinnamomum verum as immunomodulators; 6-gingerol, 6-shogaol, and 8-shogaol of Zingiber officinale var. Rubrum as antioxidants (Qadir et al., 2018); curcumin and polysaccharide as immunomodulators; phyllanthin and the related phenolic compounds of Phyllanthus niruri as immunomodulators; and polysaccharides of Oryza sativa as immunomodulators (Yang et al., 2015).
5. Conclusion
In conclusion, all the compounds and plants mentioned in this review have not yet been tested for effectiveness in experimental models relevant to SARS-CoV-2 because this virus was only identified at the beginning of 2020. However, these plants possess the potential to prevent infection or inhibit the replication of SARS-CoV-2 based on the identified therapeutic targets in SARS-CoV-2 and their reaction to SARS-CoV.
The results of the abovementioned computational model (in silico) predictions still need confirmation via laboratory testing and experimentation. Many strategies are being developed by researchers to seek out active antiviral compounds, including those derived from natural plant compounds. The rich biodiversity of Indonesian plants provides a variety of structural compounds from natural materials, providing major capital in the effort to identify suitable drug candidates to combat disease, including SARS-CoV-2. At the same time, this is a call for Indonesian researchers to support drug-independence programs.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the Directorate General of Research and Community Service, the Ministry of Research, and Technology/National Agency for Research and Innovation of the Republic of Indonesia through World-Class Research Program 2020 (No. 214/SP2H/AMD/LT/DRPM/2020).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adedoyin T.A., Joshua I.O., Ofem O.E., Akpan J. Effects of Cassia alata root extract on smooth muscle activity. J. Pharmaceut. Res. Int. 2015:406–418. [Google Scholar]
- Ahlawat K.S., Khatkar B.S. Processing, food applications and safety of Aloe vera products: a review. J. Food Sci. Technol. 2011;48(5):525–533. doi: 10.1007/s13197-011-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F., Sadhu S.K., Ishibashi M. Search for bioactive natural products from medicinal plants of Bangladesh. J. Nat. Med. 2010;64(4):393–401. doi: 10.1007/s11418-010-0424-7. [DOI] [PubMed] [Google Scholar]
- Ali B.H., Al Wabel N., Blunden G. Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: a review. Phytother Res. 2005;19(5):369–375. doi: 10.1002/ptr.1628. [DOI] [PubMed] [Google Scholar]
- Alu'Datt M.H., Rababah T., Alhamad M.N., Al-Mahasneh M.A., Ereifej K., Al-Karaki G. Profiles of free and bound phenolics extracted from Citrus fruits and their roles in biological systems: content, and antioxidant, anti-diabetic and anti-hypertensive properties. Food Funct. 2017;8(9):3187–3197. doi: 10.1039/c7fo00212b. [DOI] [PubMed] [Google Scholar]
- Amin M.S., Saputri F.C., Mun’im A. Inhibition of dipeptidyl peptidase 4 (DPP IV) activity by some Indonesia edible plants. Phcog. J. 2019;11(2):231–236. [Google Scholar]
- Ayyanar M., Subash-Babu P. Syzygiumcumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pacific J. Trop. Biomed. 2012;2(3):240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azminah A., Erlina L., Radji M., Mun’im A., Syahdi R.R., Yanuar A. In silico and in vitro identification of candidate SIRT1 activators from Indonesian medicinal plants compounds database. Comput. Biol. Chem. 2019;83:107096. doi: 10.1016/j.compbiolchem.2019.107096. [DOI] [PubMed] [Google Scholar]
- Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A., Babu C.R. Purification and biochemical characterization of a serine proteinase inhibitor from Derris trifoliata Lour. seeds: insight into structural and antimalarial features. Phytochemistry. 2009;70(6):703–712. doi: 10.1016/j.phytochem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Bijina B., Chellappan S., Krishna J.G., Basheer S.M., Elyas K.K., Bahkali A.H., Chandrasekaran M. Protease inhibitor from Moringa oleifera with potential for use as therapeutic drug and as seafood preservative. Saudi J. Biol. Sci. 2011;18(3):273–281. doi: 10.1016/j.sjbs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Wu C.D. Compounds from Syzygiumaromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996;59(10):987–990. doi: 10.1021/np960451q. [DOI] [PubMed] [Google Scholar]
- Chang C.L., Shen C.C., Ni C.L., Chen C.C. A new sesquiterpene from Elephantopus scaber. Hung Kuang J. 2011;65:49–56. [Google Scholar]
- Chen H., Du Q. 2020. Potential Natural Compounds for Preventing SARS-CoV-2 (2019-nCoV) Infection. [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.W., Yiu C.P.B., Wong K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000 Res. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.K., Gadthula S., Chen X., Choo H., Olgen S., Barnard D.L. Antiviral activity of nucleoside analogues against SARS-coronavirus (SARS-coV) Antivir. Chem. Chemother. 2006;17:285e9. doi: 10.1177/095632020601700506. [DOI] [PubMed] [Google Scholar]
- Chukwujekwu J.C., Coombes P.H., Mulholland D.A., Van Staden J. Emodin, an antibacterial anthraquinone from the roots of Cassia occidentalis. South Afr. J. Bot. 2006;72(2):295–297. [Google Scholar]
- Clemente M., Corigliano M.G., Pariani S.A., Sánchez-López E.F., Sander V.A., Ramos-Duarte V.A. Plant serine protease inhibitors: biotechnology application in agriculture and molecular farming. Int. J. Mol. Sci. 2019;20(6):1345. doi: 10.3390/ijms20061345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K.R., Iwasaki A., Suenaga K., Kato-Noguchi H. Evaluation of phytotoxic potential and identification of phytotoxic substances in Cassia alata Linn. leaves. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019;69(6):479–488. [Google Scholar]
- Elkhidir I.B., Yagi A.I., El Badawi S.M., Yagi S.M. Toxicity of aqueous extract of Senna alexandrina miller pods on Newzealand rabbits. Eur. J. Med. Plants. 2012:252–261. [Google Scholar]
- Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE-2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D'Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Wang D.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyebi G.A., Ogunro O.B., Adegunloye A.P., Ogunyemi O.M., Afolabi S.O. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CLpro): an in silico screening of alkaloids and terpenoids from African medicinal plants. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1764868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D.P., Penn-Nicholson A., Cho M.W. Identification of critical determinants on ACE-2 for SARS-CoV entry and development of a potententry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt B.H., Jukneliene D., Kanjanahaluethai A., Bechill J., Severson K.M., Smith C.M. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J. Virol. 2004;78:13600e12. doi: 10.1128/JVI.78.24.13600-13612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartanti D., Dhiani B.A., Charisma S.L., Wahyuningrumet R. The potential roles of jamu for COVID-19: a learn from the traditional Chinese medicine. Pharm. Sci. Res. 2020;7:12–22. Special Issue on COVID-19. [Google Scholar]
- Haslberger A., Jacob U., Hippe B., Karlic H. Mechanisms of selected functional foods against viral infections with a view on COVID-19: mini review. Funct. Foods Health Dis. 2020;10(5):195–209. [Google Scholar]
- Hennebelle T., Weniger B., Joseph H., Sahpaz S., Bailleul F. Senna alata. Fitoterapia. 2009;80(7):385–393. doi: 10.1016/j.fitote.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S.C., Chung J.G. Anticancer potential of emodin. Biomedicine. 2012;2(3):108–116. doi: 10.1016/j.biomed.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim F., Natasha A., Saharman Y.R., Sudarmono P. Preliminary report of COVID 19 testing: experience on clinical microbiology laboratory Universitas Indonesia in jakarta, Indonesia. New Microb. New Infect. 2020 doi: 10.1016/j.nmni.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J. ACE-2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on dierentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J.H., Radoshitzky S.R., Li W., Wong S.K., Choe H., Farzan M. New Concepts of Antiviral Therapy. Springer; 2006. The SARS Coronavirus Receptor ACE-2 A Potential Target for Antiviral Therapy; pp. 397–418. [Google Scholar]
- Kuo I.P., Lee P.T., Nan F.H. Rheum officinale extract promotes the innate immunity of orange-spotted grouper (Epinephelus coioides) and exerts strong bactericidal activity against six aquatic pathogens. Fish Shellfish Immunol. 2020;102:117–124. doi: 10.1016/j.fsi.2020.04.024. [DOI] [PubMed] [Google Scholar]
- Kuo Y.C., Yang L.M., Lin L.C. Isolation and immunomodulatory effect of avonoids from Syzygium samarangense. Planta Med. 2004;70(12):1237–1239. doi: 10.1055/s-2004-835859. [DOI] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for lineage B β-coronaviruses, including 2019-nCoV. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Li X., Shi C., Deng Z., Fu H., Proksch P. Pongamone A–E, five flavonoids from the stems of a mangrove plant, Pongamia pinnata. Phytochemistry. 2006;67(13):1347–1352. doi: 10.1016/j.phytochem.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Li Y., Yu S., Liu D., Proksch P., Lin W. Inhibitory effects of polyphenols toward HCV from the mangrove plant Excoecaria agallocha L. Bioorg. Med. Chem. Lett. 2012;22(2):1099–1102. doi: 10.1016/j.bmcl.2011.11.109. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005;68(1):36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Cai X.L., Yang H., Xia X.K., Guo Z.Y., Yuan J. The bioactive metabolites of the mangrove endophytic fungus Talaromyces sp. ZH-154 isolated from Kandelia candel (L.) Druce. Planta Med. 2010;76(2):185–189. doi: 10.1055/s-0029-1186047. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang X.J. 2020. Potential Inhibitors for 2019-nCoV Coronavirus M Protease from Clinically Approved Medicines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mageney V., Neugart S., Albach D.C. A guide to the variability of flavonoids in Brassica oleracea. Molecules. 2017;22(2):252. doi: 10.3390/molecules22020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materska M., Konopacka M., Rogoliński J., Ślosarek K. Antioxidant activity and protective effects against oxidative damage of human cells induced by x-radiation of phenolic glycosides isolated from pepper fruits Capsicum annuum L. J. Food Chem. 2015;168(1):546–553. doi: 10.1016/j.foodchem.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49(6):3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- Momtazi-borojeni A.A., Behbahani M., Sadeghi-aliabadi H. Antiproliferative activity and apoptosis induction of crude extract and fractions of Avicennia marina. Iran. J. Basic Med. Sci. 2013;16(11):1203. [PMC free article] [PubMed] [Google Scholar]
- Mullard A. Ebola outbreak prompts experimental drug rollout. Nat. Rev. Drug Discov. 2018;17:460. doi: 10.1038/nrd.2018.114. [DOI] [PubMed] [Google Scholar]
- Nguyen T.T.H., Woo H.J., Kang H.K., Nguyen V.D., Kim Y.M., Kim D.W. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor Y.R., Khazali M., Suryadiputra I.N.N. PKA/WI-IP (Wetlands International-Indonesia Programme); Bogor: 1999. Panduanpengenalan mangrove di Indonesia; p. 220. [Google Scholar]
- Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.H. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J. Med. Chem. 2016;59:6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadir M.M.F., Bhatti A., Ashraf M.U., Sandhu M.A., Anjum S., John P. Immunomodulatory and therapeutic role of Cinnamomum verum extracts in collagen-induced arthritic BALB/c mice. Immunopharmacology. 2018;26(1):157–170. doi: 10.1007/s10787-017-0349-9. [DOI] [PubMed] [Google Scholar]
- Ramsay A., Mueller-Harvey I. Procyanidins from Averrhoa bilimbi fruits and leaves. J. Food Compos. Anal. 2016;47:16–20. [Google Scholar]
- Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Zietz M., Schreiner M., Rohn S., Kroh L.W., Krumbein A. Genotypic and climatic influences on the concentration and composition of flavonoids in kale (Brassica oleracea var. sabellica) Food Chem. 2010;119(4):1293–1299. doi: 10.1021/jf9033909. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A.A., Najiah M., Suvik A., Azariyah M.N., Kamaruzzaman B.Y., Effendy A.W. Antibacterial properties of selected mangrove plants against Vibrio species and its cytotoxicity against Artemia salina. World Appl. Sci. J. 2013;25(2):333–340. [Google Scholar]
- Sharaf M., El-Ansari M.A., Saleh N.A.M. New flavonoids from Avicennia marina. Fitoterapia. 2000;71(3):274–277. doi: 10.1016/s0367-326x(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Shivashri C., Rajarajeshwari T., Rajasekar P. Hepatoprotective action of celery (Apium graveolens) leaves in acetaminophen-fed freshwater fish (Pangasius sutchi) Fish Physiol. Biochem. 2013;39(5):1057–1069. doi: 10.1007/s10695-012-9762-6. [DOI] [PubMed] [Google Scholar]
- Singh B., Singh J.P., Kaur A., Singh N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020:109114. doi: 10.1016/j.foodres.2020.109114. [DOI] [PubMed] [Google Scholar]
- Singh T., Goel R.K. Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. Neurotoxicology. 2015;49:1–7. doi: 10.1016/j.neuro.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Song W., Gui M., Wang X., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14(8) doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S., Chen Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front. Pharmacol. 2016;7:470. doi: 10.3389/fphar.2016.00470. 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Wu X., Chung H.Y., Li Y., Ye W. Antiproliferative activities of five Chinese medicinal herbs and active compounds in Elephantopus scaber. Nat. Prod. Commun. 2009;4(8):1025–1030. [PubMed] [Google Scholar]
- Subissi L., Imbert I., Ferron F., Collet A., Coutard B., Decroly E. SARS-CoV ORF1b-encoded nonstructural proteins 12e16: replicative enzymes as antiviral targets. Antivir. Res. 2014;101 doi: 10.1016/j.antiviral.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Yoshiya T., Yoshizawa-Kumagaye K., Sugiyama T. Nicotianamine is a novel angiotensin-converting enzyme 2 inhibitor in soybean. Biomed. Res. 2015;36(3):219–224. doi: 10.2220/biomedres.36.219. [DOI] [PubMed] [Google Scholar]
- Tallei T.E., Tumilaar S.G., Niode N.J., Fatimawali, Kepel B.J., Idroes R. 2020. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (M) and Spike (S) Glycoprotein Inhibitors: a Molecular Docking Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Xu C., Yagiz Y., Simonne A., Marshall M.R. Phytochemical profiles, and antimicrobial and antioxidant activities of greater galangal [Alpinia galanga (Linn.) Swartz.] flowers. Food Chem. 2018;255:300–308. doi: 10.1016/j.foodchem.2018.02.027. [DOI] [PubMed] [Google Scholar]
- Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo R.Y., Ikawati M., Meiyanto E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprints. Org. 2020:1–8. [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.H., Luan F., He X.D., Wang Y., Li M.X. Traditional uses and pharmacological properties of Clerodendrum phytochemicals. J. Tradit. Complementary Med. 2017;8(1):2438. doi: 10.1016/j.jtcme.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Novel Coronavirus 2019.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Retrieved from. [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Xiao Q., Xu J., Li M.Y., Pan J.Y., Yang M.H. Natural products from true mangrove flora: source, chemistry and bioactivities. Nat. Prod. Rep. 2008;25(5):955–981. doi: 10.1039/b807365a. [DOI] [PubMed] [Google Scholar]
- Wu W.H., Chen T.Y., Lu R.W., Chen S.T., Chang C.C. Benzoxazinoids from Scoparia dulcis (sweet broomweed) with antiproliferative activity against the DU-145 human prostate cancer cell line. Phytochemistry. 2012;83:110–115. doi: 10.1016/j.phytochem.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020:1–3. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.C., Hsieh C.C., Lin W.C. Characterization and immunomodulatory activity of rice hull polysaccharides. Carbohydr. Polym. 2015;124:150–156. doi: 10.1016/j.carbpol.2015.02.025. [DOI] [PubMed] [Google Scholar]
- Yang Y.C., Lim M.Y., Lee H.S. Emodin isolated from Cassia obtusifolia (Leguminosae) seed shows larvicidal activity against three mosquito species. J. Agric. Food Chem. 2003;51(26):7629–7631. doi: 10.1021/jf034727t. [DOI] [PubMed] [Google Scholar]
- Yu M.S., Lee J., Lee J.M., Kim Y., Chin Y.W., Jee J.G., Jeong Y.J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012;22(12):4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Chen S., Chen Z. Advances in COVID-19: the virus, the pathogenesis, and evidence-based control and therapeutic strategies. Front. Med. 2020;14(2):117–125. doi: 10.1007/s11684-020-0773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Row K.H. Box-Behnken design for optimizing extraction of luteolin from celery leaves. J. Liq. Chromatogr. Relat. Technol. 2011;34(12):1036–1049. [Google Scholar]
- Zuo A.X., Wan C.P., Zheng X., Rao G.X. Chemical constituents of Elephantopus scaber. Chem. Nat. Compd. 2016;52(3):484–486. [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.