Abstract
The clinical spectrum of coronavirus disease 2019 is getting wider with the exponential increase of patients worldwide. Initially described with flu-like symptoms, variable cutaneous manifestations have been reported, with only few histopathological descriptions. Detection of the virus in cutaneous samples has been assessed in very few cases until now, and the causative role of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has not been proven for every type of cutaneous manifestations yet. We aimed to describe histological features of cutaneous eruptions occurring concomitantly to SARS-CoV-2 infection and assess by immunochemistry and in situ hybridization using RNAscope validation techniques the presence of the virus in skin lesions. We retrieved all skin biopsies received in the departments of pathology and dermatopathology, University Hospital of Strasbourg, performed in hospitalized SARS-CoV-2–infected patients presenting concomitant cutaneous manifestations since March 2020. In situ hybridization and immunostaining using a polyclonal SARS nucleocapsid protein antibody were performed on each sample. Skin biopsies from six patients presenting morbilliform eruption concomitant to SARS-CoV-2 infection were available for evaluation. All six samples showed varying degrees of spongiosis, perivascular inflammatory infiltrates of the dermis, and, for some of them, discrete interface dermatitis. In situ hybridization and immunohistochemistry were negative in all cutaneous samples. Morbilliform rash concomitant to SARS-CoV-2 infection is characterized by mild and unspecific histopathological features with no detectable viral RNA and protein and appears then not to be directly caused by the virus. Even if, at least for a few cases, the differential diagnosis with drug hypersensitivity reaction can be difficult, these cutaneous eruptions seem to rather correspond to paraviral rashes.
Keywords: COVID-19, Cutaneous manifestations, Skin, SARS-CoV-2, In situ hybridation
Highlights
-
•
Variable cutaneous manifestations have been reported in patients with COVID-19.
-
•
Morbilliform rash is the most common pattern described.
-
•
Histopathological features of morbilliform rashes are unspecific with no detectable viral RNA and protein of SARS-COV-2.
-
•
The differential diagnosis with drug hypersensitivity reaction can be difficult.
1. Introduction
The coronavirus disease 2019 (COVID-19) has been declared by the World Health Organization as a pandemic in March 2020. Initially described with flu-like symptoms, the clinical spectrum of this infectious disease caused by a new coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is getting wider with the exponential increase of patients worldwide. Cutaneous manifestations in patients with COVID-19 have been first reported by Recalcati [1] in March 2020 as erythematous rash, urticarial, and chickenpox-like vesicles. Since then, many descriptions of skin eruptions occurring concomitantly to SARS-CoV-2 infection have been described, with six main clinical patterns: urticarial rash, confluent maculopapular rash, papulovesicular exanthema, chilblain-like acral pattern, livedo reticularis pattern, and purpuric vasculitis pattern [2]. Maculopapular eruption is the most common pattern described ranging from 23% to 47% of cases [3,4]. Until now, only a few histopathological examinations of skin samples have been reported with usually unspecific pathological lesions such as mild perivascular lymphocytic infiltrate, slight spongiosis, and discreet lichenoid and vacuolar interface dermatitis [5,6]. However, these results remain sparse and require additional database confirmed. Nevertheless, based on these findings, several questions arise regarding the impact and the presence of SARS-CoV-2 in the skin.
We aimed to describe histological features of cutaneous eruption occurring concomitantly to SARS-CoV-2 infection and assess by immunochemistry (IHC) and in situ hybridization (ISH) using RNAscope validation techniques the presence of the virus in skin lesions.
2. Materials and methods
2.1. Clinical data
We retrieved all skin biopsies received in the departments of pathology and dermatopathology, University Hospital of Strasbourg, performed in hospitalized SARS-CoV-2–infected patients presenting concomitant cutaneous manifestations since March 2020. All patients had a nasopharyngeal swab positive for SARS-CoV-2 before the occurrence of rash. Clinical course of the disease and rash, as well as history of recent drug intakes and blood hypereosinophilia, was reported using the patient's medical file. Each skin lesion was clinically examined by dermatologists, directly or by photographs. Each patient has been informed of this study and expressed an oral nonopposition.
2.2. Microscopic examination
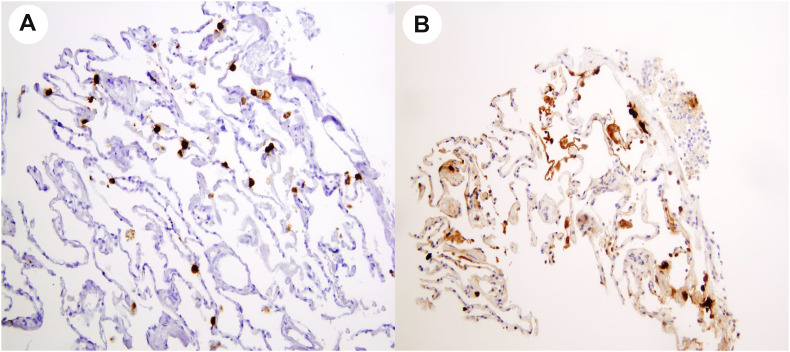
Two experienced pathologists, trained in dermatopathology, blindly analyzed all the cutaneous samples. IHC assays were performed on a Ventana Benchmark ULTRA platform (Ventana Medical Systems, Roche tissue diagnostics, Tucson, AZ, USA) using formalin-fixed paraffin-embedded specimens sectioned at five microns onto positively charged glass slides. IHC antigen retrieval was performed using Cell Conditioning 1 (CC1 – Ventana) for 36 min at 95°C. Specimens were incubated with a polyclonal SARS nucleocapsid protein antibody (200-401-A50; Rockland Immunochemicals, Inc, USA – dilution 1/500) for 32 min at room temperature, followed by revelation with the ultraView Universal DAB detection kit at room temperature. The specimens were then counterstained with hematoxylin [7,8]. For ISH assay, we used RNAscope technology with a SARS-CoV-2 spike probe to COVID-19 coronavirus as previously described [9]. Both assays had been validated in our laboratory on lung samples from SARS-CoV-2–infected patients, where viral RNA and protein were both found in pneumocytes. We used these samples as positive controls (Fig. 1 ).
Fig. 1.
SARS-CoV-2 mRNA detection in pneumocytes by in situ hybridization (A) and viral nucleocapsid protein detection in pneumocytes by immunohistochemistry (B).
3. Results
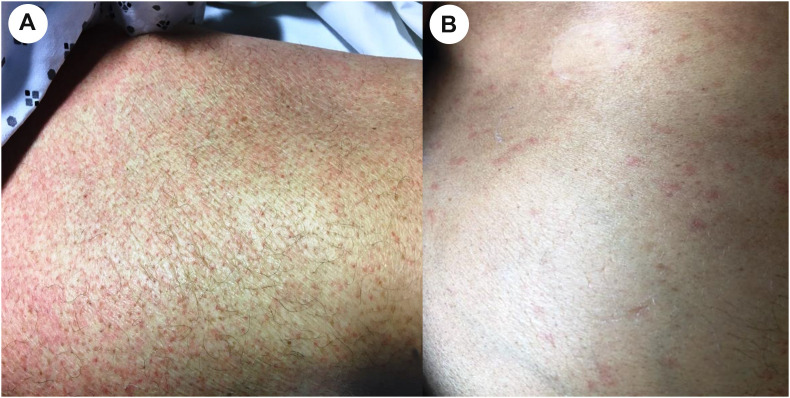
We identified six SARS-CoV-2–infected patients with skin biopsies available for evaluation. Four were performed in intensive care unit–hospitalized patients and two in conventional medicine unit–hospitalized patients (Table 1 ). Clinically, all six patients presented maculopapular rash of the trunk with variable extension to the limbs (Fig. 2 ). The latency between rash and onset of extracutaneous COVID-19 symptoms ranged from 5 to 35 days (mean 23 days). Five patients had been treated with antibiotics during the month preceding the rash. Three of them had blood hypereosinophilia concomitant with their rash. One patient did not use any new medication before the occurrence of the rash.
Table 1.
Clinical data of patients with COVID-19 presenting cutaneous manifestations.
| Patients | Hospital unit | Extracutaneous manifestations related to COVID-19 | Cutaneous manifestations occurring concomitantly to COVID-19 | Delay between first COVID-19 extracutaneous symptoms and rash onset (days) | Drugs used before the occurrence of the rash | Delay between initiation of each treatments and the onset of the rash (days) | Hypereosinophilia (>0,5G/L) |
|---|---|---|---|---|---|---|---|
| Patient 1 | ICU | Severe bilateral pneumonia requiring orotracheal intubation | Maculopapular exanthema of trunk and shoulders | 25 | Ceftriaxone Azithromycine Hydroxychloroquine |
25 | Yes |
| Tazocilline Linézolide Valaciclovir |
20 | ||||||
| Patient 2 | ICU | Severe bilateral pneumonia requiring oxygen therapy | Diffuse maculopapular exanthema | 20 | Amoxicilline/clavulanic acid | 20 | No |
| Ceftriaxone Spiramycine Azithromycine Hydroxychloroquine |
3 | ||||||
| Patient 3 | CMU | Flu-like symptoms | Maculopapular exanthema of abdomen and limbs and bilateral livedo of knees Pruritus | 5 | Paracetamol | 0 | No |
| Patient 4 | CMU | Flu-like symptoms | Maculopapular exanthema of trunk and limbs with slight pruritus | 30 | Ceftriaxone Azithromycine |
30 | Yes |
| Patient 5 | ICU | Severe bilateral pneumonia requiring orotracheal intubation | Maculopapular exanthema of trunk and limbs | 35 | Ceftriaxone Azithromycine Linézolide |
35 | No |
| Tazocilline Aciclovir |
25 | ||||||
| Patient 6 | ICU | Severe bilateral pneumonia requiring oxygen therapy | Diffuse maculopapular exanthema Pruritus |
30 | Ceftriaxone Spiramycine Hydroxychloroquine |
20 | Yes |
| Tazocilline Linézolide |
5 |
ICU, intensive care unit; CMU, conventional medicine unit; COVID-19, coronavirus disease 2019.
Fig. 2.
Maculopapular exanthema of the limb (A) and trunk (B).
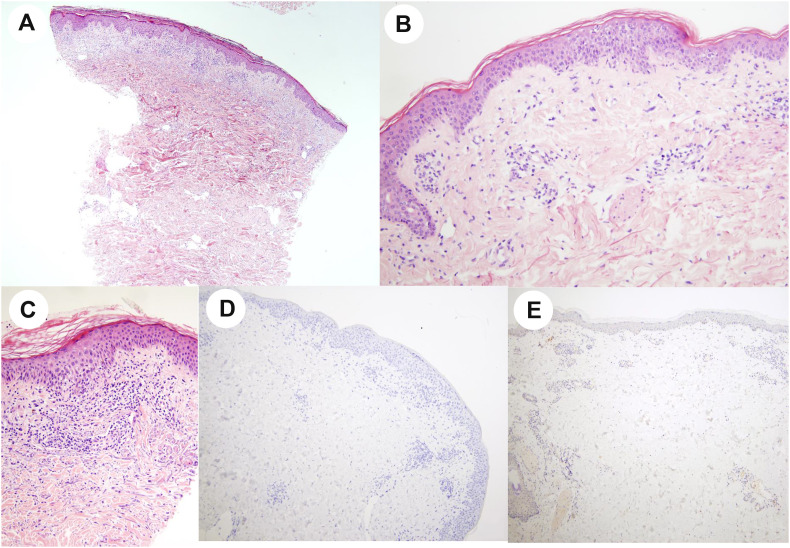
All six samples showed the same histopathological pattern including predominant spongiosis and mild perivascular lymphocytic infiltrate of the dermis associated, for some cases with discrete interface dermatitis with basal cell vacuolization and exceptional apoptotic keratinocytes for one case (Table 2 ). No virally induced cytopathic alterations or intranuclear inclusions were seen (Fig. 3 A–C). ISH and IHC for SARS-CoV-2 were negative in all cutaneous samples (Fig. 3D and E).
Table 2.
Histopathologic clues which could help for the differential diagnosis between viral exanthem and drug-induced reaction.
| Patients | Dermal eosinophilic infiltrate | Vacuolar change of basal keratinocyte | Apoptotic keratinocyte | Lymphocytic exocytosis |
|---|---|---|---|---|
| Patient 1 | Discrete | No | No | No |
| Patient 2 | No | Rare | No | Discrete |
| Patient 3 | No | Rare | No | Discrete |
| Patient 4 | No | Rare | Rare | Discrete |
| Patient 5 | No | Rare | No | Discrete |
| Patient 6 | Discrete | No | No | Discrete |
Fig. 3.
Histopathological features in skin biopsy: Slight spongiosis (A, HE ×4), mild perivascular lymphocytic infiltrate of the dermis (B, HE ×20), and discrete basal cell vacuolization (C, HE ×20). In situ hybridization (ISH) using RNAscope technology (D, ×10) and immunostaining using a polyclonal SARS nucleocapsid protein antibody (E, ×4), both negative.
4. Discussion
Histopathologic evaluation of tissue from SARS-CoV-2–infected patients is critical in advancing our understanding of the pathogenesis of this disease and to assess the distribution of SARS-CoV-2 within different organs and tissue [10]. Until now, the best-described histopathologic features have involved the lungs, which show various degrees of diffuse alveolar damage, severe capillary congestion, and, occasionally, superimposed bronchopneumonia [11]. Postmortem biopsy study reported that various organs such as the liver and heart, even when the presence of SARS-CoV-2 was assessed by real-time reverse transcriptase polymerase chain reaction (real-time RT-PCR) assay, showed only mild, nonspecific histopathologic changes by light microscopic examination [12].
To date, only few studies have been carried out on histopathological features of cutaneous manifestations occurring concomitantly to COVID-19. According to a systematic review published in late June 2020, only 23 cases of skin biopsy were reported to this date [5]. The histopathological features described are usually mild and unspecific and combine various degrees of spongiosis and lymphocytic exocytosis in the epidermis, basal vacuolar changes with interface dermatitis, lymphocytic perivascular and interstitial infiltrate of the dermis associated with dermal congestion, and extravasation of red blood cells [6,[13], [14], [15]]. Viral detection on skin samples using RT-PCR techniques on fresh skin biopsy specimens was performed in two cases. Results were negative [16,17]. A published article provides protocols of immunohistochemical and in situ hybridization assays that can be used for tissue identification of the virus and assessment of its distribution. Authors report that eight autopsies lung samples and one placenta showed cell positivity for SARS-CoV-2. Ten renal biopsies were also evaluated and were all negative [18]. A recent article, published in October 2020, reported histopathological features of seven cases of COVID-19 chilblains demonstrating typical findings of pernio-like lesions (combining variable degrees of lymphocytic vasculitis associated with purpura, superficial and deep perivascular lymphocytic inflammation with perieccrine accentuation, edema, and mild vacuolar interface damage). SARS-CoV-2 immunohistochemistry was positive in endothelial cells, and epithelial cells of eccrine glands and coronavirus particles were found in the cytoplasm of endothelial cells on electron microscopy. These findings could support a causal role of SARS-CoV-2 in chilblain lesions. Given our findings, it could mean that the pathophysiology of COVID-19 cutaneous manifestations is different depending on its type [19].
SARS-CoV-2 is a single-stranded RNA virus composed of 16 nonstructural proteins (named as NSP 1–16) whose pathophysiology is not yet fully understood. At the respiratory level, the current hypothesis is that the virus enters the cells via the angiotensin-converting enzyme 2 (ACE2) expressed on type I and II alveolar epithelial cells [20]. Many studies show that ACE2 is expressed in several organs and tissues including the skin, especially in the basal layer of the epidermis, endothelial cells of dermal blood vessels, and eccrine adnexal tissue [21,22]. Moreover, SARS-CoV-2 RNA has been detected in blood samples from a significant number of patients [23]. This brought many authors to assume of a direct viral effect to explain cutaneous manifestations. However, it is now well reported that SARS-CoV-2 infection can cause overactive immune responses that may induce immunopathological conditions, named as cytokine storm [24]. Cytokines could reach not only the lung but all other organs, including the skin. By stimulating inflammatory cells, cytokines could promote eruptions such as erythema, urticarial lesions, vesicles, and others. In this view, rashes may be paraviral due to cytokines [20]. This hypothesis is consistent with the fact that no viral RNA or protein can be detected neither in the lungs after the very early stage of the disease nor in skin lesional samples [16,17]. Histopathological features of cutaneous manifestation reported in our study and in the literature are mild and unspecific. Mild interface dermatitis associated with spongiosis and perivascular inflammatory infiltrate can be observed in various eruptions such as paraviral rash or toxic drug reaction. Indeed, hospitalized patients with SARS-CoV-2 infection are usually treated with several antibiotics (penicillin or azithromycin) and/or antiviral drugs, well known to be responsible of cutaneous toxic reaction.
Drug hypersensitivity reaction and viral infections are the most common cause of morbilliform eruptions, with significant and often problematic clinical overlap. Drug-induced rashes usually develop within three days to three weeks after initiation of a novel drug and may last up to two weeks after cessation of attributable treatment. Blood hypereosinophilia can be a weak argument in favor of drug-induced rash. Histopathological features of these two types of eruptions are also overlapping. Subtle microscopic clues in favor of drug hypersensitivity reaction are eosinophilic infiltrate in the dermis, lymphocytic exocytosis, basal cell damage with apoptotic keratinocytes, and usually more marked lymphocytic dermal infiltrate than in viral exanthema [25]. In our cohort, five patients had a novel drug initiation before the onset of the rash. For three of these patients, the delay between initiation of treatment and rash (comprised between three days and three weeks) could be compatible for drug hypersensitivity cutaneous reaction. Among them, patient 1 and patient 6 had blood hypereosinophilia (Table 1). Under light microscope, patient 1 had slight dermal eosinophilic infiltrate and neither basal cell damage, apoptotic keratinocytes nor lymphocytic exocytosis. Patient 2 had slight lymphocytic exocytosis, rare vacuolar change of basal keratinocyte, and neither dermal eosinophilic infiltrate nor apoptotic keratinocytes. Patient 6 had slight dermal eosinophilic infiltrate and lymphocytic exocytosis with neither basal cell damage nor apoptotic keratinocytes (Table 2). Therefore, for these three patients, there are no clear-cut arguments in favor of drug hypersensitivity reaction rather than viral exanthem. For the three other patients, viral exanthem remains the first hypothesis, with even greater confidence for the patient who did not use any new medication before the occurrence of the rash.
Our study was made on a small cohort of patients and concern only skin biopsy performed on maculopapular rash. To confirm our findings, further investigations on a larger number of patients with various cutaneous manifestations are necessary.
5. Conclusion
Morbilliform rash concomitant to SARS-CoV-2 infection is characterized by mild and unspecific histopathological features with no detectable viral RNA and protein and appears then not to be directly caused by the virus. Even if, at least for a few cases, the differential diagnosis with drug hypersensitivity reaction can be difficult, these cutaneous eruptions seem to rather correspond to paraviral rashes.
Author contributions
All authors whose names appear on the submission contributed substantially to the conception and design of the study, the acquisition of data, or the analysis and interpretation of the data.
Data availability
All available data are published in the current manuscript.
Acknowledgments
The authors thank Nathalie Bervas and Karine Lemarchand (Tetu Bio) for the SARS-CoV antibody. The authors are also grateful to Martine Muckenstrum, Catherine Leberquier, Florence Guenard, and Tuy-Tien Tong for technical assistance.
Footnotes
Funding/Support: None.
Competing interest: The authors have no conflicts of interest to declare.
References
- 1.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol JEADV. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 2.Marzano A.V., Cassano N., Genovese G., Moltrasio C., Vena G.A. Cutaneous manifestations in patients with COVID-19: a preliminary review of an emerging issue. Br J Dermatol. 2020;183:431–442. doi: 10.1111/bjd.19264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachdeva M., Gianotti R., Shah M., Bradanini L., Tosi D., Veraldi S. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askin O., Altunkalem R.N., Altinisik D.D., Uzuncakmak T.K., Tursen U., Kutlubay Z. Cutaneous manifestations in hospitalized patients diagnosed as COVID-19. Dermatol Ther. 2020 doi: 10.1111/dth.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Q., Fang X., Pang Z., Zhang B., Liu H., Zhang F. COVID-19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol JEADV. 2020 doi: 10.1111/jdv.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianotti R., Veraldi S., Recalcati S., Cusini M., Ghislanzoni M., Boggio F. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of milan, Italy. Acta Derm Venereol. 2020;100 doi: 10.2340/00015555-3490. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thi Nhu Thao T., Labroussaa F., Ebert N., V’kovski P., Stalder H., Portmann J. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform. Nature. 2020;582:561–565. doi: 10.1038/s41586-020-2294-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhao B., Ni C., Gao R., Wang Y., Yang L., Wei J. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F., Flanagan J., Su N., Wang L.-C., Bui S., Nielson A. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn JMD. January 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth R.F., Xu X., Buja L.M. A call to action: the need for autopsies to determine the full extent of organ involvement associated with COVID-19. Chest. 2020;158:43–44. doi: 10.1016/j.chest.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menter T., Haslbauer J.D., Nienhold R., Savic S., Deigendesch H., Frank S. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian S., Xiong Y., Liu H., Niu L., Guo J., Liao M. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol Off J U S Can Acad Pathol Inc. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Nieto D., Ortega-Quijano D., Segurado-Miravalles G., Pindado-Ortega C., Prieto-Barrios M., Jimenez-Cauhe J. Comment on: cutaneous manifestations in COVID-19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol. 2020;34:e252–e254. doi: 10.1111/jdv.16470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimenez-Cauhe J., Ortega-Quijano D., Carretero-Barrio I., Suarez-Valle A., Saceda-Corralo D., del Real C.M.-G. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol. 26 July 2020 doi: 10.1111/ced.14281. http://onlinelibrary.wiley.com/doi/abs/10.1111/ced.14281 Disponible sur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianotti R., Zerbi P., Dodiuk-Gad R.P. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. May 2020;98:141–143. doi: 10.1016/j.jdermsci.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahouach B., Harent S., Ullmer A., Martres P., Bégon E., Blum L. Cutaneous lesions in a patient with COVID-19: are they related? Br J Dermatol. 2020;183 doi: 10.1111/bjd.19168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez A., Sohier P., Benghanem S., L'Honneur A.-S., Rozenberg F., Dupin N. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819–820. doi: 10.1001/jamadermatol.2020.1704. [DOI] [PubMed] [Google Scholar]
- 18.Best Rocha A., Stroberg E., Barton L.M., Duval E.J., Mukhopadhyay S., Yarid N. Detection of SARS-CoV-2 in formalin-fixed paraffin-embedded tissue sections using commercially available reagents. Lab Investig J Tech Methods Pathol. 2020;100:1485–1489. doi: 10.1038/s41374-020-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colmenero I., Santonja C., Alonso-Riaño M., Noguera-Morel L., Hernández-Martín A., Andina D. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criado P.R., Abdalla B.M.Z., de Assis I.C., van Blarcum de Graaff Mello C., Caputo G.C., Vieira I.C. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? Revision of possible pathophysiologic mechanisms. Inflamm Res Off J Eur Histamine Res Soc Al. août 2020;69:745–756. doi: 10.1007/s00011-020-01370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. June 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet Lond Engl. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2020:1–7. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S., Khandpur S., Arava S., Rath R., Ramam M., Singh M. Assessment of histopathological features of maculopapular viral exanthem and drug-induced exanthem. J Cutan Pathol. December 2017;44:1038–1048. doi: 10.1111/cup.13047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data are published in the current manuscript.