Abstract
Background and aims
Few studies distinguished the independent role of overweight/obesity or their associated-comorbidities in the evolution towards severe forms of COVID-19. Obesity as a unifying risk factor for severe COVID-19 is an emerging hypothesis. The aim of this study was to evaluate whether excessive body weight per se, was a risk factor for developing a severe form of COVID-19.
Patients and methods
We included 131 patients hospitalized for COVID-19 pneumonia in a single center of the internal medicine department in Marseille, France. We recorded anthropometric and metabolic parameters such as fasting glycaemia, insulinemia, HOMA-IR, lipids, and all clinical criteria linked to SARS-CoV-2 infection at the admission. Excess body weight was defined by a BMI ≥ 25 kg/m2. The occurrence of a serious event was defined as a high-debit oxygen requirement over 6 L/min, admission into the intensive care unit, or death.
Results
Among 113 patients, two thirds (n = 76, 67%) had an excess body weight. The number of serious events was significantly higher in excess body weight patients compared to normal weight patients (respectively 25% vs 8%, p = 0.03) although excess body weight patients were younger (respectively 63.6 vs 70.3 years old, p = 0.01). In multivariate analyses, the excess body weight status was the only predictor for developing a serious event linked to SARS-CoV-2 infection, with an odds ratio at 5.6 (95% CI: 1.30–23.96; p = 0.02), independently of previous obesity associated comorbidities. There was a trend towards a positive association between the BMI (normal weight, overweight and obesity) and the risk of serious events linked to COVID-19, with a marked increase from 8.1% to 20% and 30.6% respectively (p = 0.05).
Conclusion
Excess body weight was significantly associated with severe forms of the disease, independently of its classical associated comorbidities. Physicians and specialists in Public Health must be sensitized to better protect people with an excess body weight against SARS-CoV-2 infection.
Keywords: Excess body weight, Overweight, Obesity, COVID-19, SARS-CoV-2, Obesity comorbidities
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and discovered in China in December 2019 has been declared as a pandemic by the World Health Organization (WHO) on March 11th, 2020. At the time of writing, more than 36 million people over the world have been infected with an overall mortality rate of 2.9% [1]. Although the majority of COVID-19 patients presented no or mild symptoms, about 14% of patients developed severe pneumonia with acute respiratory distress syndrome requiring hospitalization, including 5% who need admission into the intensive care unit (ICU) [2].
This difference of COVID-19 clinical presentations led the scientists to identify populations at risk of developing serious forms of the disease. Initial publications showed that some pre-existing conditions could be considered as risk factors for developing severe forms of COVID-19 including age, male gender, diabetes mellitus, cardiovascular disease, arterial hypertension, respiratory disease, renal or hepatic failure [3]. Later, the large New York city study showed that obese people defined by a body mass index (BMI) ≥ 30 kg/m2 accounted for a large proportion of hospitalized patients with SARS-CoV-2 infection [4]. Subsequently, two French studies linked obesity to severe forms of COVID-19 disease. First, a retrospective study in ICU showed that obesity was a risk factor for invasive mechanical ventilation [5]. Then, Caussy et al. showed a significant association between the prevalence of obesity and severe forms of COVID-19 (OR 1.35; 95% CI: 1.08–1.66) and suggested that obesity might be a risk factor of pejorative evolution, increasing the risk of ICU admission [6]. It is well known that obese patients have alterations in respiratory mechanisms [7,8] and an increased risk of comorbidities such as diabetes mellitus, arterial hypertension or cardiovascular diseases. All these conditions predispose obese patients to pneumonia-associated organ failures [9] as previously reported during the 2009 H1N1 pandemic influenza in Mexico, Canada and USA. Sattar et al. recently hypothesized that obesity is a unifying risk factor for severe COVID-19 [10]. Thus, it appears important to decipher the link between excess body weight and its associated comorbidities in the risk to evolve towards severe forms of COVID-19, in order to identify patients at high risk of severe forms of COVID-19 and to better protect them.
Therefore, we studied whether excess body weight per se was an independent risk factor for developing a severe form of COVID-19.
2. Methods
We conducted a retrospective observational monocentric study in La Conception-Hospital in Marseille, France among 131 patients hospitalized for COVID-19. The data included in this study were anonymized, approved and registered at the Health Data Portal of Assistance Publique-Hôpitaux de Marseille under the references RGPD/APHM 2020-65. All raw data are available upon request.
2.1. Patients
From March 19th to April 27th 2020, we included patients with SARS-CoV-2 infection confirmed by a positive nasopharyngeal real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) and hospitalized in the COVID-19 units of the internal medicine department in La Conception University Hospital. All the clinical parameters presented in this study were collected at baseline in the emergency department. Patients were secondarily hospitalized according to the severity of the following symptoms: respiratory rate ≥ 25/min; oxygen saturation ≤ 95% measured by fingertip pulse oximeter at rest; blood pressure ≤ 100 mm Hg; impaired consciousness. Inclusion criteria were age greater than 18 years and to be able to get information on weight and height. Weight and height were self-reported. Indeed it was difficult to weigh and measure the height of the patients due to the clinical condition of the patients and to prevent any risk of SARS-CoV-2 transmission. Normal weight was defined as a BMI between 18.5 and 25 kg/m2, excess body weight as a BMI ≥ 25 kg/m2, overweight as a BMI between 25 and 29 kg/m2, and obesity was defined as a BMI ≥ 30 kg/m2. We excluded patients with undernutrition (BMI < 18.5 kg/m2) and hemodialysis patients to avoid confounding factors of serious events. The flow chart of the study is in Fig. 1 . In accordance with the hospital protocol, all patients received the combination of hydroxychloroquine (HCQ) and azithromycin (AZT) except those with a cardiac contraindication. The treatment was started after an electrocardiogram was performed and a cardiologist's opinion was obtained. Thus most patients at the admission (91%) were treated with the combination of HCQ and AZT after a written informed consent, for respectively 10 and 5 days. When it was necessary, a broad spectrum antibiotherapy was administrated to patients.
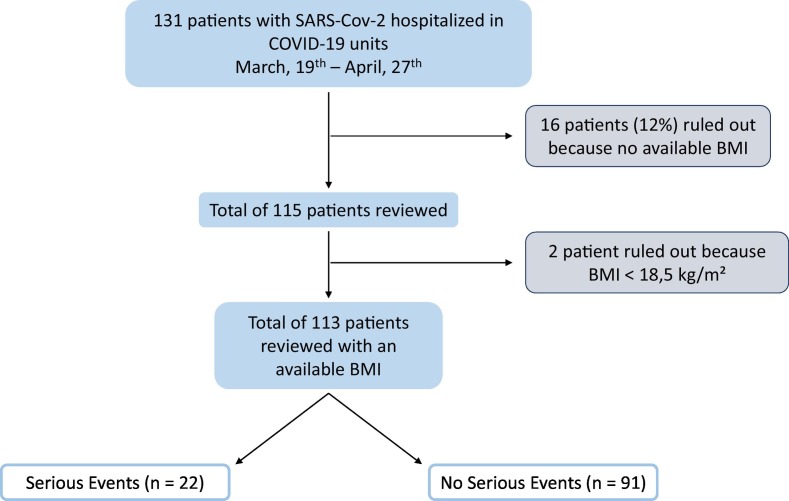
Fig. 1.
Flowchart.
Among the 131 patients, 16 were excluded because missed data on weight or height and 2 more patients were also excluded because they had a body mass index (BMI) below 18.5 kg/m2.
2.2. Data collection
2.2.1. Initial clinical data
The baseline characteristics such as age, gender, tobacco use and BMI were collected in an electronic medical record. Comorbidities and significant treatments such as inhibitors of the renin-angiotensin-aldosterone system (RAAS) (angiotensin-converting enzyme inhibitors and angiotensin receptor blockers), statins and diabetes treatments (oral drugs or insulin) were also recorded: diabetes was defined as a known history of diabetes and hypoglycaemic drugs; arterial hypertension was defined as a known history of hypertension and antihypertensive drugs; chronic respiratory disease was defined as a history of asthma, obstructive sleep apnea or chronic obstructive pulmonary disease and cardiovascular disease was defined as a history of coronary event or peripheral arterial disease. Initial vital constants and initial oxygen requirement on the first day of hospitalization were collected.
2.2.2. Laboratory procedures
2.2.2.1. Confirmation of SARS-CoV-2 infection by RT-PCR methods
Blood samples were collected the first morning of hospitalization in the COVID-19 units after an overnight fast. We measured metabolic parameters (lipids, glycaemia and insulin to calculate the homeostasis model assessment-insulin resistance (HOMA-IR) index and glycated hemoglobin) and inflammatory parameters (C-reactive protein (CRP), ferritin, fibrinogen, procalcitonin and albuminemia).
2.2.3. Pulmonary computed tomography (CT) scans
97 patients out of 113 (26 out of 37 in the normal weight group and 71 out of 76 in the excess body weight group) were evaluated by a CT chest to evaluate signs of SARS-CoV-2 pneumopathy and the severity of lung involvement. The radiologists interpreted the CT chest scans based on the Fleischner Society Nomenclature recommendations on the following criteria: ground glass opacity, consolidation, nodule, reticulation, interlobular septal thickening, crazy-paving pattern, linear opacities, subpleural curvilinear line, bronchial wall thickening, lymph node enlargement, pleural effusion, and pericardial effusion [11].
2.3. Statistical analysis
All results are presented as mean with standard deviation for continuous variables or as frequency with percentage for categorical variables. All the continuous variables were compared between groups with the non-parametric Mann-Whitney U test, or Kruskal-Wallis test depending of the number of BMI groups, and the categorical variables were compared with the Chi-squared or Fisher's exact-test, when appropriate. Several analyses were performed to examine if there was an association between the risk of severe forms of COVID-19 and excess body weight, its comorbidities, pre-admission and admission treatments known to potentially influence the evolution of the disease. We first performed univariate logistic analyses to investigate the association between serious events and the distribution of BMI 1) in 2 groups (BMI between 18.5 and 25 kg/m2 (normal weight group, reference), BMI ≥ 25 kg/m2 (excess body weight group)); and 2) in 3 groups (BMI between 18.5 and 25 kg/m2 (normal weight group, reference), BMI between 25 and 30 kg/m2 (overweight group), BMI ≥ 30 kg/m2 (obese group)). Then, we performed 2 multivariate logistic regression analyses: Model 1 to look for an association between serious events and BMI in 2 groups, age, sex, type 2 diabetes, arterial hypertension, chronic respiratory disease, RAAS inhibitors, statins and hydroxychloroquine and azithromycin combination, and Model 2 with the same parameters but with a distribution of BMI in 3 categories. The variable “cardiovascular disease” was not included in our multivariate analyses because no patient with a history of cardiovascular disease developed a severe form of COVID-19. Results of logistic regression are given as the odds ratio (OR) with the 95% confidence interval (CI). All statistical tests were two-sided and a p-value < 0.05 was considered significant. All statistical analyses were performed using statistical software R version 3.1.0.
2.4. Outcomes
The occurrence of a serious event was defined as a high debit oxygen requirement over 6 L/min, admission into ICU or death. The outcome defined by high debit oxygen requirement was chosen for patients for which there were criteria for ICU admission but for which a panel of physicians, in accordance with the patient's family, decided not to admit them in ICU (n = 3). The characteristics of these 3 patients were: a man of 89 years old, BMI = 25.3 kg/m2 with arterial hypertension and a moderate lung damage on pulmonary CT scan; a woman of 89 years old, BMI = 33.3 kg/m2 with a severe lung damage on pulmonary CT scan; a woman of 73 years old, BMI = 35 kg/m2 with arterial hypertension, dementia and a moderate lung damage on pulmonary CT scan.
3. Results
Among the 113 patients included, one third were normal weight (n = 37, 33%) and two thirds had an excess body weight (n = 76, 67%) and half of whom were obese (n = 36, 47%). General characteristics of patients according to the BMI are presented in Table 1 . Patients with an excess body weight were significantly younger than normal weight patients (63.6 vs 70.3 years old, p = 0.01). Comorbidities (type 2 diabetes, arterial hypertension as well as chronic respiratory diseases) prior to admission were equivalent between the two groups. The prevalence of cardiovascular diseases was higher in the normal weight group (27% vs 12%, p = 0.04). Fasting insulin and glycated hemoglobin levels were significantly higher in the excess body weight group compared to the normal weight group (13.8 vs 10.6 mUI/L, p = 0.04 and 6.5% vs 6%, p = 0.02 respectively). The excess body weight group seems to be more insulinoresistant than the normal weight group with a HOMA-IR index that tends to be higher (respectively 3.84 vs 3.12, p = 0.07). Total cholesterol, LDL-cholesterol (LDL-c) and HDL-cholesterol (HDL-c) levels were decreased in the excess body weight group compared to the normal weight group (3.48 vs 3.89 mmol/L, p = 0.03, 1.95 vs 2.23 mmol/L, p = 0.05 and 0.80 vs 1.06 mmol/L, p = 0.002 respectively). Excess body weight patients were statistically more treated with statins and RAAS inhibitors than normal weight patients (18% vs 0, p = 0.004 and 39% vs 16%, p = 0.01 respectively).
Table 1.
Demographic, clinical and biological characteristics of patients hospitalized with COVID-19 according to BMI.
| All patients |
Normal weight group |
Excess body weight group |
p | |
|---|---|---|---|---|
| n = 113 | n = 37 | n = 76 | ||
| General characteristics | ||||
| Sex ratio, F/M | 55/58 | 17/20 | 38/38 | 0.69 |
| Age, years | 65.4 ± 15.0 | 70.3 ± 16.0 | 63.6 ± 13.9 | 0.01 |
| BMI, kg/m2 | 27.9 ± 6.1 | 22.5 ± 1.8 | 30.9 ± 5.5 | – |
| Current smokers | 10 (9) | 2 (5) | 8 (10) | 0.49 |
| Comorbidities | ||||
| Overweight, 25 ≤ BMI < 30 kg/m2 | 76 (67) | 0 | 76 | – |
| Obesity, BMI ≥ 30 kg/m2 | 36 (32) | 0 | 36 (47) | – |
| Type 2 diabetes | 27 (19) | 8 (21) | 19 (25) | 0.69 |
| Arterial hypertension | 58 (51) | 16 (43) | 42 (55) | 0.23 |
| Cardiovascular diseasea | 19 (17) | 10 (27) | 9 (12) | 0.04 |
| Chronic respiratory diseaseb | 8 (7) | 1 (3) | 7 (9) | 0.26 |
| Metabolic parameters | ||||
| Fasting plasma glucose, mmol/L | 7.03 ± 6.4 | 8.16 ± 10.5 | 6.45 ± 2.0 | 0.21 |
| Fasting insulin, mUI/L | 12.9 ± 11.9 | 10.6 ± 7.5 | 13.8 ± 13.1 | 0.04 |
| HOMA-IR index | 3.63 ± 3.3 | 3.12 ± 2.9 | 3.84 ± 3.4 | 0.07 |
| Glycated hemoglobin, % | 6.36 ± 1.0 | 5.99 ± 0.6 | 6.51 ± 1.1 | 0.02 |
| Total cholesterol, mmol/L | 3.62 ± 0.8 | 3.89 ± 0.7 | 3.48 ± 0.8 | 0.03 |
| Triglycerides, mmol/L | 1.51 ± 0.5 | 1.34 ± 0.5 | 1.60 ± 0.6 | 0.03 |
| LDL-c, mmol/L | 2.04 ± 0.7 | 2.23 ± 0.5 | 1.95 ± 0.7 | 0.05 |
| HDL-c, mmol/L | 0.89 ± 0.4 | 1.06 ± 0.4 | 0.80 ± 0.3 | 0.002 |
| Albumin, g/L | 37.6 ± 5.6 | 36.1 ± 6.6 | 38.4 ± 4.7 | 0.16 |
| Treatments | ||||
| Hypoglycaemic oral drugs | 22 (19) | 6 (16) | 16 (21) | 0.54 |
| Insulin | 12 (10) | 2 (5) | 10 (13) | 0.33 |
| Statins | 14 (13) | 0 | 14 (18) | 0.004 |
| RAAS inhibitors | 36 (32) | 6 (16) | 30 (39) | 0.01 |
Normal weight group defined by an 18.5 ≤ BMI < 25 kg/m2 and excess body weight group defined by a BMI ≥ 25 kg/m2. All data are means ± SD or (%). Mean data were compared between normal weight group and excess body weight group.
Abbreviations: BMI = body mass index. F = female. HDL-c = high-density lipoprotein-cholesterol. HOMA-IR = homeostasis model assessment-insulin resistance. LDL-c = low-density lipoprotein-cholesterol. M = male. RAAS = renin-angiotensin-aldosterone system.
Bold value is statistically significant to p-value < 0.05.
Cardiovascular disease: coronary or peripheral arterial disease.
Chronic respiratory disease: asthma, obstructive sleep apnea, chronic obstructive pulmonary disease.
All patients were hospitalized with a positive RT-PCR of SARS-CoV-2 infection and most of them had a chest CT-scan. All the parameters belonging to the COVID-19, at the admission in the COVID-19 units are presented in Table 2 . Excess body weight patients required oxygen therapy more often (82 vs 67%, p = 0.09) and for a longer period (8.2 vs 5.2 days, p = 0.01) than normal weight patients. Excess body weight patients were more often treated by the combination of HCQ and AZT (88% vs 68%, p = 0.01). The CRP levels tended to be higher in the excess body weight group without reaching the significance (89 vs 75 mg/L, p = 0.08). Lung damage on pulmonary CT-scans were more severe in the excess body weight group than in the normal weight group (p = 0.005) with 75% of severe or moderate pneumonia in the excess body weight group compared to 43% in the normal weight group. Finally, the number of serious events was significantly higher in excess body weight patients than in normal weight patients (25% vs 8%, p = 0.03). There was no death in the studied group of 113 patients.
Table 2.
Criteria linked to SARS-CoV-2 infection in patients hospitalized with COVID-19 according to BMI.
| All patients |
Normal weight group |
Excess body weight group |
p | |
|---|---|---|---|---|
| n = 113 | n = 37 | n = 76 | ||
| Baseline clinical parameters | ||||
| Oxygen therapy requirement | 87 (77) | 25 (67) | 62 (82) | 0.09 |
| Heart, rate/min | 87 ± 14 | 86 ± 12 | 88 ± 14 | 0.61 |
| Respiratory, rate/min | 24 ± 7 | 23 ± 5 | 25 ± 7 | 0.16 |
| Hospitalization evolution | ||||
| Hospitalization duration, days | 13.1 ± 6.1 | 13.6 ± 6.9 | 13.0 ± 5.8 | 0.85 |
| Fever duration, days | 3.5 ± 4.5 | 3.7 ± 6.3 | 3.4 ± 3.4 | 0.25 |
| Oxygen therapy requirement, days | 7.2 ± 6.3 | 5.2 ± 6.2 | 8.2 ± 6.1 | 0.01 |
| Broad spectrum antibiotherapy | 86 (76) | 25 (68) | 61 (80) | 0.14 |
| HCQ/AZT treatment combinationa | 92 (81) | 25 (68) | 67 (88) | 0.01 |
| Baseline laboratory results | ||||
| Fibrinogen, g/L | 6.0 ± 1.6 | 5.6 ± 1.8 | 6.2 ± 1.5 | 0.13 |
| Ferritin, μg/L | 850 ± 956 | 872 ± 821 | 836 ± 1045 | 0.78 |
| C-reactive protein, mg/dL | 84 ± 77 | 75 ± 81 | 89 ± 74 | 0.08 |
| Procalcitonin, ng/mL | 0.34 ± 0.7 | 0.46 ± 1.0 | 0.25 ± 0.3 | 0.83 |
| Pulmonary CT-scan | ||||
| Normal | 5 (4) | 2 (5) | 3 (4) | – |
| Mild | 19 (17) | 8 (21) | 11 (14) | – |
| Moderate | 51 (45) | 10 (27) | 41 (54) | – |
| Severe | 22 (19) | 6 (16) | 16 (21) | – |
| Serious events | ||||
| All | 22 (19) | 3 (8) | 19 (25) | 0.03 |
| ICU admission | 19 (17) | 3 (8) | 16 (21) | – |
| High oxygen therapy requirementb | 3 (3) | 0 | 3 (4) | – |
| Death | 0 | 0 | 0 | – |
Normal weight group defined by an 18.5 ≤ BMI < 25 kg/m2 and excess body weight group defined by a BMI ≥ 25 kg/m2. All data are means ± SD or (%). Mean data were compared between normal weight group and excess body weight group.
Abbreviations: AZT = azithromycin. BMI = body mass index. HCQ = hydroxychloroquine. ICU = intensive care unit.
Bold value is statistically significant to p-value < 0.05.
HCQ/AZT: combination of hydroxychloroquine for 10 days and azithromycin for 5 days.
Oxygenotherapy flow rate > 6 L/min.
We then analyzed the association of excess body weight, its comorbidities and pre-admission and admission treatments known to potentially influence the evolution of the disease and the occurrence of a serious event. Univariate and multivariate analyses (Model 1) are shown in Table 3 . The excess body weight appeared the only predictor of occurrence of a serious event linked to SARS-CoV-2 infection, with an OR at 5.58 (95% CI: 1.30–23.96; p = 0.02).
Table 3.
Univariate and multivariate logistic regression analyses for serious events (model 1).a
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Excess body weight, BMI ≥ 25 kg/m2 | 3.78 (1.04–13.72) | 0.04 | 5.58 (1.30–23.96) | 0.02 |
| Age, years | 0.98 (0.95–1.01) | 0.17 | 0.98 (0.94–1.02) | 0.35 |
| Sex | 1.48 (0.57–3.80) | 0.42 | 1.76 (0.61–5.09) | 0.30 |
| Type 2 diabetes | 0.66 (0.20–2.14) | 0.49 | 0.99 (0.21–4.77) | 0.99 |
| Arterial hypertension | 0.75 (0.29–1.90) | 0.54 | 0.93 (0.21–4.10) | 0.92 |
| Chronic respiratory diseaseb | 1.42 (0.27–7.55) | 0.68 | 1.68 (0.23–12.33) | 0.61 |
| RAAS inhibitors | 0.76 (0.27–2.15) | 0.61 | 1.25 (0.24–6.34) | 0.79 |
| Statins | 0.29 (0.04–2.31) | 0.24 | 0.14 (0.01–1.74) | 0.13 |
| HCQ/AZT treatment combinationc | 0.73 (0.23–2.25) | 0.58 | 0.33 (0.08–1.35) | 0.12 |
Abbreviations: AZT = azithromycin. BMI = body mass index. HCQ = hydroxychloroquine. RAAS = renin-angiotensin-aldosterone system.
Bold value is statistically significant to p-value < 0.05.
Univariate and multivariate associations between serious events and BMI between 18.5 and 25 kg/m2 (reference), BMI ≥ 25 kg/m2, age, sex, type 2 diabetes, arterial hypertension, chronic respiratory disease, RAAS inhibitors, statins and HCQ/AZT combination.
Chronic respiratory disease: asthma, obstructive sleep apnea, chronic obstructive pulmonary disease.
HCQ/AZT: combination of hydroxychloroquine for 10 days and azithromycin for 5 days.
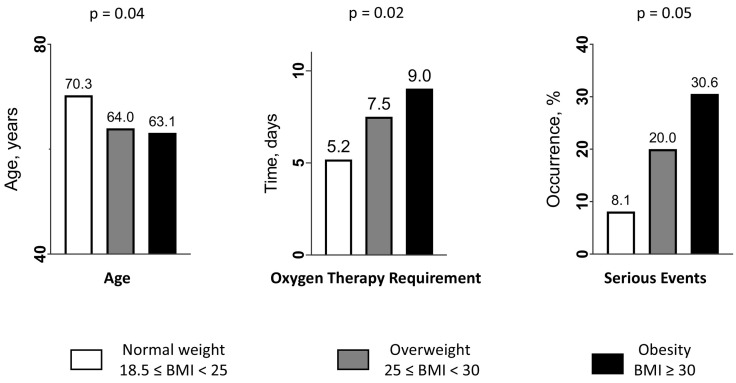
To better understand the relationship between BMI and the risk of serious events, we have split the patients into 3 BMI groups (normal weight, overweight and obese groups) and found a difference (p = 0.05) in the occurrence of a serious event linked to COVID-19 between the 3 groups, with a marked increase between the groups: from 8.1% in the normal weight group to 20% in the overweight group and 30.6% in the obese group (Fig. 2 , right panel). The distribution of patients according to their age, the duration of oxygen therapy requirement and the 3 classes of BMI is illustrated in Fig. 2 (left and middle panels). Moreover, we performed multivariate logistic regression analysis (Model 2) to study separately the association between overweight status (BMI: 25–30 kg/m2) or obesity (BMI ≥ 30 kg/m2), and serious events linked to COVID-19. We found that only obesity remained a statistically independent risk factor for severe forms of COVID-19 with an OR at 9.14 (p = 0.007). There was a trend to borderline significance between overweight status and serious events with an OR at 3.81 (p = 0.09) (Table 4 ).
Fig. 2.
Distribution of patients according to the age (years), the duration of oxygen therapy requirement (days), the occurrence of a serious event, and per the 3 classes of body mass index (18.5–25 kg/m2 (reference) (in white), 25–30 kg/m2 (in grey), and ≥30 kg/m2 (in black)).
Table 4.
Univariate and multivariate logistic regression analyses for serious events (model 2).a
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Overweight, 25 ≤ BMI < 30 kg/m2 | 2.83 (0.69–11.63) | 0.15 | 3.81 (0.79–18.33) | 0.09 |
| Obesity, BMI ≥ 30 kg/m2 | 4.99 (1.26–19.76) | 0.02 | 9.14 (1.81–46.18) | 0.007 |
| Age, years | 0.98 (0.95–1.01) | 0.17 | 0.99 (0.95–1.03) | 0.48 |
| Sex | 1.48 (0.57–3.80) | 0.41 | 2.25 (0.71–7.08) | 0.17 |
| Type 2 diabetes | 0.66 (0.20–2.14) | 0.49 | 1.04 (0.21–5.15) | 0.96 |
| Arterial hypertension | 0.75 (0.29–1.90) | 0.54 | 0.88 (0.20–3.90) | 0.86 |
| Chronic respiratory diseaseb | 1.42 (0.27–7.55) | 0.68 | 1.44 (0.19–10.80) | 0.72 |
| RAAS inhibitors | 0.76 (0.27–2.15) | 0.61 | 1.13 (0.21–5.91) | 0.89 |
| Statins | 0.29 (0.04–2.31) | 0.24 | 0.12 (0.01–1.57) | 0.11 |
| HCQ/AZT treatment combinationc | 0.73 (0.23–2.25) | 0.59 | 0.40 (0.10–1.70) | 0.22 |
Abbreviations: AZT = azithromycin. BMI = body mass index. HCQ = hydroxychloroquine. RAAS = renin-angiotensin-aldosterone system.
Bold value is statistically significant to p-value < 0.05.
Univariate and multivariate associations between serious events and BMI between 18.5 and 25 kg/m2 (reference), BMI between 25 and 30 kg/m2, BMI ≥ 30 kg/m2, age, sex, type 2 diabetes, arterial hypertension, chronic respiratory disease, RAAS inhibitors, statins and HCQ/AZT combination.
Chronic respiratory disease: asthma, obstructive sleep apnea, chronic obstructive pulmonary disease.
HCQ/AZT: combination of hydroxychloroquine for 10 days and azithromycin for 5 days.
4. Discussion
In the present study, we showed that excess body weight was a strong predictor of severe forms of SARS-CoV-2 infection in patients hospitalized for COVID-19, independently of obesity-related comorbidities such as diabetes, arterial hypertension and chronic respiratory disease. Excess body weight patients were 5.6 times more likely to develop a severe form of COVID-19 than normal weight patients, although the latter were older. Severe forms of COVID-19 were mainly represented by ICU admission since there were no death and few patients requiring high oxygen therapy. Excess body weight patients also had more severe pneumonia on CT-scans, and required a more prolonged oxygen therapy than normal weight patients.
The fact that obesity is a risk factor for severe forms of COVID-19 is a new concept emerging only a few months ago. Data on the distinct role of excess body weight per se or its complications are scarce, probably because of the difficulties to register anthropometric and metabolic data on patients during the acute phase of the pandemic. However, our results on the independent link between excess body weight and severe forms of the COVID-19 are in agreement with those of 2 very recent studies. In the multicentric CORONADO study, conducted in France on 1317 diabetic patients hospitalized for COVID-19, Cariou et al. observed that BMI was the only pre-admission factor that was independently associated with severe forms of the disease (defined by tracheal intubation and/or death within 7 days of admission) [12]. In a study realized in the Italian Veneto region among 92 patients hospitalized for COVID-19, Busetto et al. showed that overweight and obese patients presented more severe forms (defined by assisted ventilation and/or access to ICU or semi-ICU) despite a younger age of about 10 years than normal weight patients, similarly to the present study. More interestingly, when they included two obesity-related comorbidities in their logistic regression analysis (type 2 diabetes and respiratory chronic disease), the authors found that BMI ≥ 25 kg/m2 remained independently linked to the severe forms of the SARS-CoV-2 infection [13]. In the present study, we have studied more parameters in the logistic regression analysis such as arterial hypertension and pre-admission therapies (RAAS inhibitors and statins), that are important factors linked to obesity. The variable “cardiovascular disease” was not included in our multivariate analysis because no patient with a history of cardiovascular disease developed a severe form of COVID-19, although they were older than patients without cardiovascular disease (73 vs 66 years old, p = 0.03).
Our results are in favor of a graded relationship between the BMI and the risk of severe forms of COVID-19. Indeed, when we split the patients into 3 groups according to BMI categories, we showed a trend towards a positive association between the occurrence of a serious event linked to COVID-19 and the 3 groups of BMI with a progressive increase from 8.1% in the normal weight group to 20% in the overweight group and 30.6% in the obese group. This is in accordance with the results of a recent meta-analysis that suggested that there was a linear dose-response association between BMI and COVID-19 severity and mortality [14]. However, when we studied separately the association between overweight or obesity status, and serious events linked to COVID-19, we found a trend to borderline significance (mainly due to a lack of statistical power) between overweight status and serious events with an OR at 3.81 (p = 0.09) and a clear statistically independent association between obesity and severe forms of COVID-19 with an OR at 9.14 (p = 0.007). These latter results are in agreement with those of a very recent work performed by Palaiodimos et al. Indeed, the authors demonstrated that severe obesity (BMI ≥ 35 kg/m2) was independently associated with higher in-hospital mortality, intubation and increasing oxygenation requirement in 200 patients hospitalized for COVID-19 in the Bronx [15]. These results confirm the hypothesis that obesity by itself and not obesity-related comorbidities is the dominant risk factor for severe forms of the COVID-19. Interestingly, the authors also found that patients with a BMI < 25 kg/m2 and patients with a BMI > 35 kg/m2 had a higher in-hospital mortality in comparison to patients with a BMI between 25 and 35 kg/m2, in favor of a J-shape distribution between BMI and mortality. In the present study, we did not find an increased risk of severe forms of the disease in normal weight patients, but there were several differences between the two studies: i) we excluded patients with BMI < 18.5 kg/m2 i.e. with undernutrition, ii) nearly one quarter of the patients of the Bronx study died from COVID-19 but no patient died in the present study reflecting the greater fragility of patients hospitalized for COVID-19 in the Bronx iii) variables included in the multivariable analyses differed between the two studies.
All these results support the hypothesis that excess body weight is the unifying factor for severe forms of the COVID-19 [10]. Metabolic syndrome characterized by abdominal obesity, insulin resistance, atherogenic dyslipidemia and pro-inflammatory and prothrombotic state should predispose to severe forms of COVID-19. However, it seems difficult to explore this potential link in cohorts of patients for whom the metabolic syndrome is not clearly known prior to the disease. Indeed, to define the abdominal obesity, it is almost impossible to measure waist circumference in patients hospitalized in COVID-19 units, because of the critical condition of the majority of the patients with impossibility to stand up and the prohibition to bring a seamstress tape in patients' rooms. Moreover, lipidic (triglycerides and HDL-c) and glycaemic (blood glucose) biological parameters and blood pressure used to define metabolic syndrome are not reliable in the context of acute viral sepsis. For example, the low levels of HDL-c in the whole group of patients (mean 0.89 ± 0.4 mmol/L) were probably linked to the viral SARS-CoV-2 infection as it is well known that viral infections can cause insulin resistance. And even if we found that HDL-c levels were largely decreased in patients who had a serious event compared to those without serious event (0.66 ± 0.2 mmol/L vs 0.94 ± 0.4 mmol/L, p = 0.003, data not shown) as it was previously described [16], the HDL-c levels during the acute phase of the infectious disease do not reflect the pre-admission metabolic syndrome [17]. Similarly, the blood pressure is also uninterpretable in this context of acute sepsis. However, the visceral fat, another key player in metabolic syndrome and insulin resistance, was recently positively associated to serious events in patients hospitalized for COVID-19. Two studies took advantage of the systematic realization of a pulmonary computed tomography to measure visceral fat on the lower slices. Watanabe et al. demonstrated that visceral fat was independently associated with the need of intensive care in 150 patients hospitalized for COVID-19 (OR: 2.474, p = 0.046), but the measure of BMI was lacking in this study [18]. Then, a “proof-of-concept” study, carried out on 30 patients with COVID-19 showed a significant association between the amount of visceral fat and severe forms of COVID-19 (hospitalization in ICU and invasive mechanical ventilation). However, the small number of patients did not allow the inclusion of other risk factors of severe forms of COVID-19 (except age and sex) in multivariate analyses [19].
We did not find any independent association between glucose metabolism-related parameters at admission and severe forms of COVID-19. To our knowledge, no study has measured insulinemia levels and consequently the HOMA-IR index to evaluate the insulin-resistant state in infected patients. We did not find any independent correlation between insulin resistance and an evolution towards a severe form of COVID-19. However, it is not possible to distinguish the preexisting insulin-resistant state seen in dysmetabolic patients, from the insulin resistance linked to inflammation due to COVID-19. The glucose control assessed with the glycated hemoglobin was also not associated with severe forms of COVID-19. These results are in accordance with the CORONADO study [12].
At least two other important mechanisms than excess weight-related comorbidities should be involved to explain the association between excess weight and severe forms of COVID-19: i) an impaired adaptative immune response against SARS-CoV-2 infection, as it has been reported in other viral infections such as influenza virus [20]; and which may be linked to ii) a chronic pro-inflammatory state. Indeed, obesity is characterized by a low-grade inflammation. Although CRP concentration was higher in excess body weight patients, it did not reach statistical significance in our study, but has been shown to be associated with severe forms of COVID-19 and death in several studies [21,22]. Obesity may thus predispose to the harmful inflammatory state involved in severe forms of COVID-19, suggesting that targeting inflammation may possibly constitute a protective strategy in SARS-CoV-2-infected obese patients.
The prevalence of excess body weight in the 113 patients hospitalized for COVID-19 was 67%. This prevalence is higher than those of the general adult population in France which is 49% in the 2 most recent French studies [23,24] and in the world which is 39% [25]. This result is almost identical to those reported by Busetto et al. who found an excess body weight prevalence of 65.2% in the Italian population of patients hospitalized for COVID-19 [13]. For the obesity, defined by an BMI ≥ 30 kg/m2, the prevalence in the present study was 32% similarly to those in the Italian study (31.5%) but clearly higher than the prevalence of 17% in Marseille area [24]. However, this prevalence of obesity in hospitalized European patients for COVID-19 was lower than that of hospitalized patients in the USA which was 41.2% [4].
The present study has several limitations. Firstly, the sample size was relatively small, and did not allow us to perform multiple comparisons tests, thus the results should be viewed with caution. Secondly, the population of hospitalized patients for COVID-19 was not representative of the general infected population, thus results cannot be extrapolated to general population. Thirdly, the data are extracted from a single center, however, it is important to note that during the very acute phase of the SARS-CoV-2 infection, it was difficult to rapidly develop collaborations between different centers in order to record anthropometric and metabolic data. Fourthly, weight and height were collected from self-reported information. Fifthly, the retrospective nature of the study was also a limitation. Larger and prospective studies are needed to better understand the risk factors for a severe form of COVID-19 and to follow the mid- and long-term prognosis of infected patients.
5. Conclusion
Excess bodyweight, which affects a large proportion of the world's population, appears as an independent risk factor for developing severe forms of COVID-19. Physicians and specialists in Public Health must be sensitized to better protect people with an excess body weight against SARS-CoV-2 infection.
Financial support
None.
CRediT authorship contribution statement
SB and GK are the principal investigators of the study. LP, GK, RV and SB contributed to the design and the setting of the study. LP, ML, MK and RC recorded all the patient data. LP, AB, GK, MK, RC, RV and SB wrote the manuscript. RGi made the statistical analyses of the data. RGr, ND and RV made a critical revision of the manuscript. SB is the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data.
Declaration of competing interest
LP, RG, AB, ML, MK, RC, RG, ND, GK, RV and SB have no conflicts of interest linked to this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metabol.2021.154703.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Demographic, clinical characteristics and criteria linked to SARS‐CoV‐2 infection in patients hospitalized with COVID-19 according to BMI (normal weight, overweight and obesity).
References
- 1.World Health Organization, Coronavirus Disease (COVID-19), Situation report - 154 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200622-covid-19-sitrep-154.pdf?sfvrsn=d0249d8d_2 n.d.
- 2.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi 2020;41:145–51. 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caussy C., Pattou F., Wallet F., Simon C., Chalopin S., Telliam C., et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umbrello M., Fumagalli J., Pesenti A., Chiumello D. Pathophysiology and management of acute respiratory distress syndrome in obese patients. Semin Respir Crit Care Med. 2019;40:40–56. doi: 10.1055/s-0039-1685179. [DOI] [PubMed] [Google Scholar]
- 8.Dixon A.E., Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stapleton R.D., Suratt B.T. Obesity and nutrition in acute respiratory distress syndrome. Clin Chest Med. 2014;35:655–671. doi: 10.1016/j.ccm.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattar N., McInnes I.B., McMurray J.J.V. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 11.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 12.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020 doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busetto L, Bettini S, Fabris R, Serra R, Dal Pra' C, Maffei P, et al. Obesity and COVID-19: an Italian snapshot. Obes Silver Spring Md 2020. 10.1002/oby.22918. [DOI] [PMC free article] [PubMed]
- 14.Du Y., Lv Y., Zha W., Zhou N., Hong X. Association of Body mass index (BMI) with critical COVID-19 and in-hospital mortality: a dose-response meta-analysis. Metabolism. 2020;154373 doi: 10.1016/j.metabol.2020.154373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx. New York Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X., Chen D., Wu L., He G., Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin Chim Acta Int J Clin Chem. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirillo A., Catapano A.L., Norata G.D. HDL in infectious diseases and sepsis. Handb Exp Pharmacol. 2015;224:483–508. doi: 10.1007/978-3-319-09665-0_15. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M., et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen A., Bressem K., Albrecht J., Thieß H.-M., Vahldiek J., Hamm B., et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green W.D., Beck M.A. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14:S406–S409. doi: 10.1513/AnnalsATS.201706-447AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdot C, Torres M, Salanave B, Deschamps V. Corpulence des enfants et des adultes en France métropolitaine en 2015. Résultats de l'étude Esteban et évolution depuis 2006. http://invs.santepubliquefrance.fr/beh/2017/13/2017_13_1.html. Bull Epidémiol Hebd 201713234-41.
- 24.Matta J. Prévalence du surpoids, de l'obésité et des facteurs de risqué cardio-métaboliques dans la cohorte Constances. Bull Epidémiol Hebd 201635–36640–6 Hebd. 2016;(35–36):640–6. http://beh.santepubliquefrance.fr/beh/2016/35-36/2016_35.
- 25.World Health Organization Overweight and obesity in 2016. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic, clinical characteristics and criteria linked to SARS‐CoV‐2 infection in patients hospitalized with COVID-19 according to BMI (normal weight, overweight and obesity).