Abstract
Background
Corticosteroid therapy is used commonly in patients with COVID-19, although its impact on outcomes and which patients could benefit from corticosteroid therapy are uncertain.
Research Question
Are clinical phenotypes of COVID-19 associated with differential response to corticosteroid therapy?
Study Design and Methods
Critically ill patients with COVID-19 from Tongji Hospital treated between January and February 2020 were included, and the main exposure of interest was the administration of IV corticosteroids. The primary outcome was 28-day mortality. Marginal structural modeling was used to account for baseline and time-dependent confounders. An unsupervised machine learning approach was carried out to identify phenotypes of COVID-19.
Results
A total of 428 patients were included; 280 of 428 patients (65.4%) received corticosteroid therapy. The 28-day mortality was significantly higher in patients who received corticosteroid therapy than in those who did not (53.9% vs 19.6%; P < .0001). After marginal structural modeling, corticosteroid therapy was not associated significantly with 28-day mortality (hazard ratio [HR], 0.80; 95% CI, 0.54-1.18; P = .26). Our analysis identified two phenotypes of COVID-19, and compared with the hypoinflammatory phenotype, the hyperinflammatory phenotype was characterized by elevated levels of proinflammatory cytokines, higher Sequential Organ Failure Assessment scores, and higher rates of complications. Corticosteroid therapy was associated with a reduced 28-day mortality (HR, 0.45; 95% CI, 0.25-0.80; P = .0062) in patients with the hyperinflammatory phenotype.
Interpretation
For critically ill patients with COVID-19, corticosteroid therapy was not associated with 28-day mortality, but the use of corticosteroids showed significant survival benefits in patients with the hyperinflammatory phenotype.
Key Words: corticosteroid, COVID-19, phenotype
Abbreviations: HR, hazard ratio; IPTW, inverse probability of treatment weighting; IQR, interquartile range; LCA, latent class analysis; MSCM, marginal structural modeling; NLR, neutrophil to lymphocyte ratio; Pao2, arterial oxygen partial pressure; SARS, severe acute respiratory syndrome; SOFA, Sequential Organ Failure Assessment; SPO2, pulse oxygen saturation; TNF-α, tumor necrosis factor α
FOR EDITORIAL COMMENT, SEE PAGE 1693
In December 2019, COVID-19 was first reported in Wuhan, China, which is caused by severe acute respiratory syndrome coronavirus 2,1 and spread expeditiously to hundreds of countries with massive mortality.2,3 Unfortunately, the specific treatment for COVID-19 to date is limited.
Although the impact of corticosteroid therapy on the clinical outcomes in COVID-19 remained controversial during the viral epidemic in Wuhan, systemic corticosteroid therapy was used widely in clinical practice.4,5 Studies regarding the other severe viral epidemics, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome or influenza, provide conflicting evidence concerning the impact of corticosteroids on clinical outcomes.6,7 Two of the largest studies of influenza A and SARS reported a significant reduction in mortality,8,9 and a recently published commentary supports the use of corticosteroids in COVID-19 because of the similar inflammatory profile of COVID-19.10 Dexamethasone resulted in an absolute lower 28-day mortality among patients with COVID-19 who were receiving either mechanical ventilation or oxygen alone at randomization, but not among those receiving no respiratory support.11
Patient responses to corticosteroids vary; numerous factors impact the effect of corticosteroids therapy, such as disease severity, comorbid illness, and inflammatory conditions.12,13 Therefore, it is difficult to draw decisive conclusions based on a heterogeneous study population. Using an unsupervised machine learning approach,14,15 different combinations of clinical and biological parameters may cluster into novel phenotypes that may respond differently to treatments.16 Whether corticosteroid therapy responds dissimilarly in patients with COVID-19 with different phenotypes has never been explored.
The first objective of our study was to elucidate the association between corticosteroid therapy and 28-day mortality in patients with COVID-19. We also aimed to identify COVID-19 phenotypes with different responses to corticosteroid therapy using an unsupervised machine learning approach.
Methods
Study Design and Participants
We performed a retrospective cohort study in Tongji Hospital, in Wuhan, which was designated to admit only critically ill patients with COVID-19. Patients admitted to eight units between January 2020 and February 2020 who fulfilled the diagnosis of COVID-19 according to World Health Organization interim guidance were included in the present study. All included patients showed a definite outcome (dead or alive) 28 days after hospital admission. We excluded patients who were younger than 18 years or who stayed in the hospital less than 24 hours, as well as patients who had received chronic corticosteroid therapy previously.
The present study was approved by the Research Ethics Commission of Tongji Hospital. Written informed consent was waived by the ethics commission because of the outbreak of COVID-19. Patient-level informed consent was not required.
Data Collection
Demographic data, chronic comorbidities, vital signs, and laboratory results obtained within the first 24 h after hospital admission were extracted from electronic medical records. Treatment and outcome data also were recorded. Sequential organ failure assessment (SOFA) scores were calculated to assess the severity of illness by using data from the first 24 h after admission. Because of incomplete measurement and recording of arterial oxygen partial pressure (Pao2), we recorded pulse oxygen saturation (SPO2) instead of Pao2, as well as Fio2 and the mode of ventilation on days 1, 3, 7, 14, and 28 of hospital admission. The main exposure of interest was the administration of IV corticosteroids. We collected data on the date of the initiation of corticosteroid treatments, the type of corticosteroids, the initial and maximum daily dose (expressed in equivalent methylprednisolone dose), and the duration of corticosteroid use. All data were collected by using a case record form modified from the standardized International Severe Acute Respiratory and Emerging Infection Consort.
Outcomes
The primary outcome in the present study was 28-day mortality. Secondary outcomes were the duration of hospital stay and complications during hospitalization, which included ARDS, septic shock, acute kidney injury, acute cardiac injury, and coagulopathy. ARDS was diagnosed based on the Berlin definition; because we used SPO2 instead of Pao2, the oxygenation diagnostic criterion was changed to the ratio of SPO2 to Fio2 < 357 mm Hg.17 Septic shock was defined according to the 2016 Third International Consensus Definition for Sepsis and Septic Shock.18 Acute kidney injury was diagnosed according to the clinical practice guidelines of Kidney Disease: Improving Global Outcomes (KDIGO).19 Acute cardiac injury was diagnosed if serum levels of cardiac biomarkers (hypersensitive troponin I) were more than the 99th percentile upper reference limit. Coagulopathy was defined as a 3-s extension of prothrombin time or a 10-s extension of activated partial thromboplastin time.
Statistical Analysis
Values are presented as the mean ± SD or median (interquartile range [IQR]) for continuous variables as appropriate and as the total number (percentage) for categorical variables. Comparisons between groups were made using the χ 2 test or Fisher exact test for categorical variables and the Student t test or Mann-Whitney U test for continuous variables as appropriate.
We first performed a Cox regression model to characterize the relationship between corticosteroid therapy and 28-day mortality. Based on prior findings,6 baseline variables were selected purposefully into the multivariate Cox proportional hazards regression model as baseline confounders and included age, sex, days from the onset of symptoms to hospital admission, SOFA score on day 1, respiratory rate, hypertension, coronary heart disease, diabetes, COPD, and the method of ventilation on day 1 (0 = no ventilation, 1 = noninvasive ventilation, 2 = invasive ventilation, and 3 = advanced ventilation support [eg, extracorporeal membrane oxygenation, oscillatory, prone]).
Corticosteroid therapy was considered as a time-dependent variable in the present study, except for the above baseline confounders. We reasoned that oxygenation (SPO2 to Fio2) and the model of ventilation during the clinical course of hospital stay may influence the decision regarding the use of corticosteroid therapy and may correlate with outcomes, which were treated as time-dependent confounders in marginal structural modeling (MSCM). By weighting each patient by inverse probability treatment weighting (IPTW), MSCM analysis with IPTW was carried out to correct for baseline and time-dependent confounders. Details of MCSM can be found in e-Appendix 1, e-Table 1, and e-Fig 1. Oxygenation (SPO2 to Fio2) was recorded only on days 1, 3, 7, 14, and 28, and we imputed missing values for the remaining days by the multiple imputation method. We also imputed the method of ventilation between these days by the last observation carried forward method, as described previously.6 Several prespecified subgroup analyses and sensitivity analyses also were performed (e-Appendix 1).
Cluster Analysis
To derive the phenotypes, we selected nine inflammation markers, including WBC count, high-sensitivity C-reactive protein, IL-2R, IL-6, IL-8, IL-10, tumor necrosis factor α (TNF-α), D-dimer, and neutrophil to lymphocyte ratio (NLR), based on previous research and their association with outcome. Those inflammatory biomarkers were measured within the 24 h after hospital admission. The missing data of inflammation markers are summarized in e-Table 2, and the missing values were obtained with the multiple imputation method. Standardized transformation was used for the dataset; additionally, nonnormally distributed variables were log-transformed before standardized transformation. Gap statistics,20 the Calinsky criterion, and the average silhouette method21 were used to determine the optimal number of phenotypes. As soon as the optimal number was determined, we applied consensus k means to identify phenotypically distinct categories in patients with COVID-19, and we developed a biomarker-based parsimonious model to predict the phenotypes. Details can be found in e-Appendix 1. Finally, we conducted MSCM analysis with IPTW in each clinical phenotype. The standardized mean differences and P values were calculated to evaluate the differences between groups or phenotypes, and P < .05 was considered statistically significant. All statistical analyses were performed using RStudio version 1.2.5019 software (RStudio, Inc).
Results
Baseline Characteristics
During the study period, a total of 428 patients with COVID-19 were included in the final analysis. Of the study cohort, 280 patients (65.4%) received corticosteroid therapy while hospitalized. The characteristics of the corticosteroid group and the no corticosteroid group are presented in Table 1. In general, patients in the corticosteroid group showed a significantly higher SOFA scores (5.0 [IQR, 2.0-8.0] vs 2.0 [IQR, 1.0-4.0]; P < .0001) with hypercytokinemia characterized by increased levels of IL-2R, IL-6, IL-8, IL-10, and TNF-α. The comorbidities between the two groups were similar. Additional baseline data compared between the groups are presented in e-Table 3.
Table 1.
Demographics and Clinical Characteristics of Patients in the Corticosteroid Group and the No Corticosteroid Group
| Variable | No Corticosteroid | Corticosteroid | P Value | SMD |
|---|---|---|---|---|
| No. | 148 | 280 | . . . | . . . |
| Age, y | 61.5 (49.0-71.0) | 66.0 (55.0-73.0) | .0048 | 0.287 |
| Sex, female | 72 (48.6) | 129 (46.2) | .71 | 0.048 |
| Days from the onset of symptom to hospital admission | 12.0 (8.0-19.3) | 11.0 (8.0-15.3) | .048 | 0.274 |
| Fever (temperature > 37.3°C) | 125 (84.5) | 251 (89.6) | .16 | 0.155 |
| Dyspnea | 89 (60.1) | 179 (63.9) | .51 | 0.078 |
| SOFA score | 2.0 (1.0-4.0) | 5.(2.0-8.0) | < .0001 | 0.620 |
| Comorbidity | . . . | . . . | . . . | . . . |
| Hypertension | 60 (40.5) | 116 (41.6) | .92 | 0.021 |
| Coronary heart disease | 20 (13.6) | 28 (10.0) | .34 | 0.111 |
| Chronic heart failure | 6 (4.1) | 14 (5.0) | .83 | 0.046 |
| Diabetes | 24 (16.2) | 57 (20.5) | .34 | 0.111 |
| COPD | 5 (3.4) | 15 (5.4) | .49 | 0.098 |
| Chronic renal diseases | 2 (1.4) | 2 (0.75) | .91 | 0.063 |
| Liver disease | 1 (0.7) | 9 (3.25) | .19 | 0.185 |
| Malignancy | 5 (3.45) | 8 (2.95) | .77 | 0.029 |
| Immunodeficiency | 2 (1.4) | 4 (1.4) | 1.0 | 0.007 |
| Temperature, °C | 36.6 (36.4-37.2) | 37.0 (36.5-37.9) | < .0001 | 0.116 |
| Heart rate, beats/min | 88.0 (80.0-100.0) | 94.0 (80.0-106.0) | .024 | 0.237 |
| Respiratory rate, breaths/min | 20.0 (20.0-22.0) | 22.0 (20.0-26.5) | < .0001 | 0.314 |
| MAP, mm Hg | 96.7 (89.8-105.7) | 97.3 (89.7-105.0) | .68 | 0.052 |
| SPO2 to Fio2 ratio | 297.0 (237.2-341.4) | 231.7 (100.0-297.0) | < .0001 | 0.591 |
| WBC count, ×109/L | 5.6 (4.2-7.5) | 7.5 (5.2-10.8) | < .0001 | 0.304 |
| Lymphocyte count, ×109/L | 1.1 (0.8-1.5) | 0.7 (0.5-0.9) | < .0001 | 0.741 |
| Platelet count, ×109/L | 211.0 (150.0-288.0) | 176.0 (134.5-237.5) | .0027 | 0.335 |
| Hemoglobin, g/L | 126.0 (111.0-136.0) | 128.0 (115.0-141.0) | .058 | 0.200 |
| Erythrocyte sedimentation rate, mm/h | 28.0 (14.0-55.7) | 38.0 (22.0-60.0) | .033 | 0.157 |
| High-sensitive C-reactive protein, mg/L | 21.3 (4.6-62.8) | 69.5 (33.5-119.9) | < .0001 | 0.158 |
| Procalcitonin, ng/mL | 0.04 (0.03-0.29) | 0.13 (0.06-0.37) | < .0001 | 0.074 |
| IL-1β, pg/mL | 5.0 (5.0-5.0) | 5.0 (5.0-5.0) | .33 | 0.04 |
| IL-2R, U/mL | 579.0 (372.5-873.0) | 948.0 (641.3-1294.8) | < .0001 | 0.444 |
| IL-6, pg/mL | 9.1 (2.0-39.0) | 35.1 (9.8-89.5) | < .0001 | 0.032 |
| IL-8, pg/mL | 10.5 (5.8-20.5) | 20.8 (11.0-43.5) | < .0001 | 0.060 |
| IL-10, pg/mL | 5.0 (5.0-6.2) | 6.7 (5.0-12.2) | < .0001 | 0.201 |
| TNF-α, pg/mL | 7.6 (5.4-10.2) | 9.4 (7.1-12.8) | .0010 | 0.031 |
Data are presented as No. (%) or median (interquartile range) unless otherwise indicated. MAP = mean arterial pressure; SMD = standardized mean difference; SOFA = Sequential Organ Failure Assessment; SPO2 = pulse oxygen saturation; TNF-α = tumor necrosis factor α.
Corticosteroid Therapy
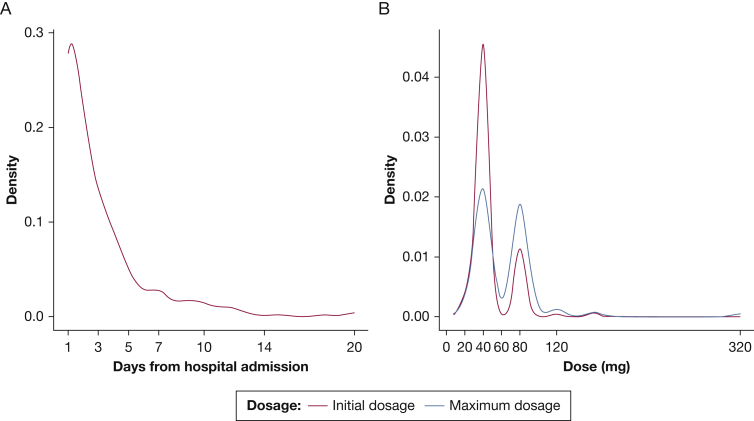
Of the 280 patients administered corticosteroids, 265 patients (94.6%) were administered IV methylprednisolone, whereas IV dexamethasone and hydrocortisone were used in 3.9% and 1.4% of patients, respectively. Corticosteroid therapy was initiated a median of 2.0 days (IQR, 1.0-4.0 days) from hospital admission. The initial and maximum methylprednisolone-equivalent daily doses were 40.0 mg (IQR, 40.0-40.0 mg) and 40.0 mg (IQR, 40.0-80.0 mg), respectively. The median duration for corticosteroid therapy was 6.5 days (IQR, 3.0- 11.0 days) (Table 2). Figure 1 shows the distribution of the time to initiation of corticosteroid therapy from hospital admission (Fig 1A) and the dosage of corticosteroid therapy (Fig 1B).
Table 2.
Corticosteroid Therapy Use Among Patients With COVID-19 (n = 280)
| Variable | Value |
|---|---|
| Drug administered | . . . |
| Methylprednisolone | 265 (94.6) |
| Dexamethasone | 11 (3.9) |
| Hydrocortisone | 4 (1.4) |
| Duration between hospital admission and corticosteroid initiation, d | 2.0 (1.0-4.0) |
| Duration of corticosteroids therapy, d | 6.5 (3.0-11.0) |
| Initial dosage (equivalent methylprednisolone), mg/d | 40.0 (40.0-40.0) |
| Maximum dosage (equivalent methylprednisolone), mg/d | 40.0 (40.0-80.0) |
Data are presented as No. (%) or median (interquartile range).
Figure 1.
A, B, Line graphs showing the distribution of the time to initiation of corticosteroid therapy from hospital admission (A) and dosage of corticosteroid therapy (B).
Clinical Outcomes
Patients who received steroids demonstrated more severe disease and underwent more treatments. Additionally, the corticosteroid group showed significantly higher rates of complications, including ARDS, septic shock, coagulopathy, acute cardiac injury, and acute kidney injury. The 28-day mortality was significantly higher in patients who received corticosteroid therapy than in those who did not (53.9% vs 19.6%; P < .0001). The details are summarized in Table 3.
Table 3.
Treatments and Clinical Outcomes Among Patients With COVID-19 in the Corticosteroid and No Corticosteroid Groups
| Variable | No Corticosteroid | Corticosteroid | P Value | SMD |
|---|---|---|---|---|
| Treatments | ||||
| Antiviral treatment | 128 (86.5) | 222 (79.3) | .088 | 0.192 |
| Antibiotics | 88 (59.5) | 261 (93.2) | < .0001 | 0.865 |
| IV immunoglobin | 38 (25.7) | 182 (65.0) | < .0001 | 0.860 |
| Neuromuscular blockade | 6 (4.1) | 42 (15.0) | .0011 | 0.379 |
| High-flow nasal cannula oxygen therapy | 10 (6.8) | 53 (18.9) | .0012 | 0.370 |
| Noninvasive mechanical ventilation | 10 (6.8) | 105 (37.5) | < .0001 | 0.797 |
| Invasive mechanical ventilation | 30 (20.35) | 130 (46.4) | < .0001 | 0.578 |
| ECMO | 6 (4.1) | 16 (5.7) | .61 | 0.077 |
| Renal replacement therapy | 9 (6.1) | 35 (12.5) | .056 | 0.222 |
| Any vasopressor | 26 (17.6) | 129 (46.1) | < .0001 | 0.643 |
| Outcomes | ||||
| ARDS | 37 (25.0) | 177 (63.2) | < .0001 | 0.834 |
| Septic shock | 25 (16.9) | 144 (51.4) | < .0001 | 0.782 |
| Coagulopathy | 11 (7.4) | 85 (30.4) | < .0001 | 0.612 |
| Acute kidney injury | 25 (16.9) | 104 (37.1) | < .0001 | 0.468 |
| Acute cardiac injury | 25 (16.9) | 140 (50.0) | < .0001 | 0.749 |
| 28-day mortality | 29 (19.6) | 151 (53.9) | < .0001 | 0.762 |
| Hospital duration, d | 15.0 (10.0-20.0) | 16.0 (10.0-23.0) | .14 | 0.191 |
Data are presented as No. (%) or median (interquartile range) unless otherwise indicated. ECMO = extracorporeal membrane oxygenation; SMD = standardized mean difference.
Associations Between Corticosteroid Therapy and 28-Day Mortality
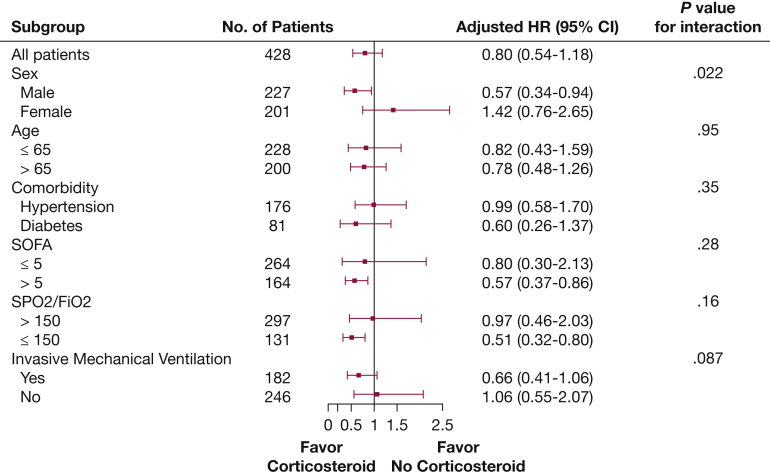
After adjustment for baseline confounders, the Cox proportional hazard model (e-Table 4) showed that corticosteroid therapy was associated with increased 28-day mortality in the overall population (hazard ratio [HR], 1.97; 95% CI, 1.25-3.11; P = .0034) (e-Table 5). However, the association did not remain after using MSCM to account for baseline and time-dependent confounders. Specifically, the MSCM results indicated that no significant association between corticosteroid therapy and 28-day mortality in the COVID-19 population (HR, 0.80; 95% CI, 0.54-1.18; P = .26) (Fig 2). The distribution of IPTW is shown in e-Figure 2, and the SPO2 to Fio2 ratio was the most important predictor of corticosteroid therapy (OR for each 50-point increase, 0.87; 95% CI, 0.84-0.89; P < .0001).
Figure 2.
Forest plot showing subgroup analyses of the association between corticosteroid therapy and 28-day mortality in the original cohort using marginal structural modeling. HR = hazard ratio; SOFA = Sequential Organ Failure Assessment; SPO2 = pulse oxygen saturation.
In the subgroup analyses, the association between corticosteroid therapy and 28-day mortality seemed to be stronger among patients with higher SOFA scores (> 5) and lower SPO2 to Fio2 ratio (< 150 mm Hg), and no interaction was detected. Although the association was stronger in men than in women and the interaction was significant (P = .022), Figure 2 shows the other results of the subgroup analyses. The dose and time of initiation of corticosteroid therapy were not associated with differences in 28-day mortality (e-Table 6). Sensitivity analysis comparing patients who received corticosteroid therapy initiated within the first 7 days with patients in the no corticosteroid therapy group with imputation up to day 7 only did not alter the association (n = 404; HR, 0.76; 95% CI, 0.50-1.14; P = .18).
Derivation and Prediction of Clinical COVID-19 Phenotypes
The consensus κ means clustering models based on the inflammation markers found that a two-class model was the optimal fit with the two distinct phenotypes of COVID-19 (e-Fig 3). Ultimately, 223 patients (52.1%) were classified as having the hypoinflammatory phenotype and 205 patients (47.9%) were classified as having the hyperinflammatory phenotype. The hyperinflammatory phenotype seems to be characterized by elevated levels of proinflammatory cytokines (e-Fig 4), higher SOFA scores, and higher rates of complications than the hypoinflammatory phenotype. Patients in the hyperinflammatory phenotype underwent more treatments, and the 28-day mortality was 15.2% for the hypoinflammatory phenotype and 71.2% for the hyperinflammatory phenotype (P < .0001). The differences between phenotypes are presented in e-Table 7.
Using forward stepwise modeling, the three variables that contributed most to define phenotype were TNF-α, D-dimer, and NLR. Univariate and multivariate analyses showed that the three variables in the model all were predictors of the phenotype (e-Table 5). The area under the receiver operating characteristic curve was 0.96 (95% CI, 0.95-0.98) for the three-variable model (e-Fig 5). When the Youden index was used as the cutoff probability (0.29), the sensitivity of the model was 0.87 and specificity was 0.92.
Corticosteroid Therapy Responds Differently in COVID-19 Phenotypes
The proportion of patients receiving corticosteroid therapy was higher in the hyperinflammatory phenotype than in the hypoinflammatory phenotype (82.4% vs 49.7%; P < .0001). After MSCM, the association between corticosteroid therapy and 28-day mortality was insignificant in patients with the hypoinflammatory phenotype (HR, 1.15; 95% CI, 0.45–2.94; P = .76). Interestingly, corticosteroid therapy was associated with a reduced 28-day mortality in patients with the hyperinflammatory phenotype (HR, 0.51; 95% CI, 0.34–0.78; P = .0018) (e-Table 8), and regardless of the baseline SPO2 to Fio2 ratio and use of invasive mechanical ventilation, the significance of the association persisted (e-Table 9).
Because TNF-α, D-dimer, and NLR contributed most to defining the phenotypes, and the optimal cutoff values for predicting phenotypes were 10.1 pg/mL, 2.0 μg/mL, 6.9, respectively. We grouped patients based on the cutoff value of each variable and found that corticosteroid therapy was associated with a reduced 28-day mortality in patients with D-dimer of > 2.0 μg/mL or NLR of > 6.9 (e-Table 10).
Discussion
The major findings of our study can be summarized as follows: we derived two distinct clinical phenotypes of COVID-19 and concluded that corticosteroid therapy was associated with a reduced 28-day mortality in patients with the hyperinflammatory phenotype and had no impact on the hypoinflammatory phenotype.
Corticosteroid therapy was used commonly for critically ill patients with COVID-19, and the proportion was 74.8% in our study. After using MSCM to account for time-dependent confounders (e-Table 1, e-Fig 1), the significant association between corticosteroid therapy and increased 28-day mortality obtained by Cox proportional hazard model was eliminated. This phenomenon also was observed in two observational studies on influenza A and Middle East respiratory syndrome.6,22 Similar to our findings, prior observational studies identified the lack of harm or improved outcome only after adjustments for multiple confounders. Our results underscore the serious limitations of meta-analyses that incorporate all observational studies without restricting the selection to those that adjust for baseline and time-dependent confounders and provide specifics about corticosteroid treatment. That is, large observational studies with correction for confounders and including analysis of the components of corticosteroid treatment can provide more meaningful data than imprecise meta-analyses.
The insignificant association between corticosteroid therapy and mortality cannot exclude the beneficial effect of corticosteroid therapy among specific patients with COVID-19. In subgroup analyses of the present study, we found that corticosteroid therapy reduced the risk of death in more critically ill patients with COVID-19, such as patients with higher SOFA scores or lower SPO2 to Fio2 ratios, which was similar to previous research of COVID-19 and other severe viral diseases. A single-center retrospective study reported that the methylprednisolone was associated with a reduced risk of death in 84 patients with COVID-19 with ARDS.23 The results of studies on SARS and influenza A both demonstrated that corticosteroids therapy was associated with a lower mortality rate in severe cases with a Pao2 to Fio2 ratio of less than 300 mm Hg.12,24 Regardless of the criteria of severe disease, another large study of SARS drew the same conclusions.9 In addition, although the dose and time of initiation of corticosteroid therapy were not associated with differences in mortality in our study, we thought that, except for disease severity, multiple factors impact response to corticosteroid treatment, such as type of corticosteroid, timing of initiation, dose, method of administration, duration of treatment, and tapering. Analysis of these treatment components provides valuable information to clinicians.
The clinical and biological heterogeneity of critical illness are thought of as dead ends for pharmacotherapy trials, and no single clinical or biological variable was sufficient to distinguish patients from each other. We identified two distinct clinical phenotypes of COVID-19 based on nine selected inflammation markers using consensus κ means clustering models. The association between corticosteroid therapy and 28-day mortality was pronounced in patients with the hyperinflammatory phenotype. From another perspective, according to the level of respiratory support that the patients with COVID-19 were receiving at the time of randomization, a controlled, open-label Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial of dexamethasone divided patients into three groups—patients who were receiving invasive mechanical ventilation, patients who were receiving oxygen alone, and patients who were receiving no respiratory support—and concluded that the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either mechanical ventilation or oxygen alone at randomization.11 Although the inflammatory biomarkers were not measured in the RECOVERY trial of dexamethasone, it is clear that excess inflammation plays a role in the development of pulmonary disease.25 In our study, compared with the hypoinflammatory phenotype, a higher proportion of patients with COVID-19 with the hyperinflammatory phenotype received respiratory support. Both the level of respiratory support and hyperinflammatory status reflect the disease severity. Our study adds hyperinflammatory status as an indication for corticosteroids therapy in COVID-19.
Several potential explanations exist for the beneficial effect of corticosteroids on 28-day mortality in patients with the hyperinflammatory phenotype. Numerous studies have indicated that patients with COVID-19 may have dysregulated inflammation,26, 27, 28 which is similar to that of multifactorial medical ARDS, for which substantial evidence has proven the ability of corticosteroids to downregulate inflammation and improve disease resolution. A review that included eight controlled studies confirmed that glucocorticoid treatment was associated with a significant reduction in markers of systemic inflammation, organ dysfunction scores, and mortality in patients with acute lung injury and ARDS.29 The evidence in support of long corticosteroid treatment in COVID-19 recently was reviewed,8, 9, 10,30 and although the temporal changes of the inflammatory biomarkers during the corticosteroid therapy were lacking in our study, we reasoned that the mechanism of corticosteroid therapy may be similar in the hyperinflammatory phenotype of COVID-19. Besides, a higher proportion of septic shock was observed in patients with the hyperinflammatory phenotype than in those with the hypoinflammatory phenotype (80.0% vs 27.9%; P < .0001), and we cannot ignored the positive effect of corticosteroids on septic shock.31
Because no randomized controlled trails confirmed the beneficial effect of corticosteroid therapy during the outbreak of COVID-19 in Wuhan, no strict indications for corticosteroid therapy in COVID-19 were found in our study. Most physicians evaluate the indications based on previous research regarding other severe viral epidemics. To our knowledge, the reasons for patients not receiving corticosteroids in part were because of the potential risks associated with corticosteroids therapy, such as immunosuppression, secondary infections, long-term complications, and prolonged virus shedding.
Our study is the first to explore the effect of corticosteroid therapy on patients with different inflammatory phenotypes of COVID-19, and we adjusted baseline and time-dependent confounders by MSCM to robust our findings. We also developed a three-variable model to predict phenotype of COVID-19 in our study. Several limitations of the present study should be considered. First, ours was a retrospective observational study; we considered only segmental measured confounders, and the residual measured confounders and unmeasured confounders cannot be included fully. Second, because of incomplete data, we lacked data on other outcomes associated with corticosteroid therapy, such as severe acute respiratory syndrome coronavirus 2 RNA clearance, superinfections, delirium, and GI bleeding, as well as long-term follow-up data for CVOID-19 patients. Third, MSCM requires complete data for time-dependent confounders on each day in the present study. We imputed the missing data using statistical methods, which could mar our findings; however, given that 91.4% of patients initialized corticosteroid therapy within 7 days of hospital admission, we minimized the impact of imputation according to the sensitivity study mentioned above. Fourth, the sample size of our study was relatively small, and our conclusions apply only to critically ill patients with COVID-19. Future studies will require a larger sample size and more heterogeneity patients to explore this issue systematically. Another limitation of the current study is the absence of a uniform regimen of corticosteroid therapy; 45.4% and 40.7% of the patients in our cohort received daily doses of 40 mg and 80 mg methylprednisolone equivalent, respectively. However, in contrast to a previous study that reported the interaction between corticosteroid dose and mortality, such an association was not found in the current study. Finally, the lack of medical resources is obvious, especially in the early stage of the outbreak, and the management protocol of COVID-19 continues to change32; therefore, the effect of corticosteroids on COVID-19 needs to be explored in more randomized studies.
Interpretation
Corticosteroid therapy is used widely in patients with COVID-19, especially in patients with elevated proinflammatory cytokines. After adjustment for baseline and time-dependent confounders, the use of corticosteroids showed significant survival benefit in critically ill patients with the hyperinflammatory phenotype.
Take-home Points.
Study Question: Are clinical phenotypes of COVID-19 associated with differential response to corticosteroid therapy?
Results: Corticosteroid therapy was not associated significantly with 28-day mortality in the overall population (HR, 0.80; 95% CI, 0.54-1.18; P = .26), but was associated with a reduced 28-day mortality (HR, 0.45; 95% CI, 0.25–0.80; P = .0062) in patients with COVID-19 with the hyperinflammatory phenotype.
Interpretation: Use of corticosteroids showed significant survival benefits in patients with COVID-19 with the hyperinflammatory phenotype.
Acknowledgments
Author contributions: H. Q. is the guarantor of the article and takes responsibility for the integrity of the work, including the data and analysis of article. B. D. and H. Q. conceptualized the research aims and planned the analyses. H. C., J. X., N. S., J. W., S. L., J. Z., J. J., M. M., Y. Y., Y.C., and W. H. collected the clinical data. H. C. and J. X. participated in processing data and performing the statistical analysis. Q. S. wrote part of the manuscript. H. C. wrote the manuscript. All authors contributed to the acquisition, analysis, or interpretation of data. All authors revised the report and approved the final version before submission.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
H. Chen, J. Xie, N. Su, J. Wang, and Q. Sun contributed equally to this manuscript.
FUNDING/SUPPORT: This work was supported by Ministry of Science and Technology of the People’s Republic of China [Grants 2020YFC0843700 and 2020YFC0841300].
Supplementary Data
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabi Y.M., Mandourah Y., Al-Hameed F. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 7.Cao B., Gao H., Zhou B. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med. 2016;44(6):e318–e328. doi: 10.1097/CCM.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Yang S.G., Gu L. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respir Viruses. 2017;11(4):345–354. doi: 10.1111/irv.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Y., Xu Y.X., Wang B. Clinical recommendations from an observational study on MERS: glucocorticoids was benefit in treating SARS patients. Int J Clin Exp Med. 2016;9(5):8865–8873. [Google Scholar]
- 10.Villar J., Confalonieri M., Pastores S.M., Meduri G.U. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor. 2020;2(4) doi: 10.1097/CCE.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horby P., Lim W.S., Emberson J.R. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lansbury L.E., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Shen Lim W. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta-analysis. Crit Care Med. 2020;48(2):e98–e106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 13.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 14.Wilkerson M.D., Hayes D.N. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26(12):1572–1573. doi: 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rindskopf D., Rindskopf W. The value of latent class analysis in medical diagnosis. Stat Med. 1986;5(1):21–27. doi: 10.1002/sim.4780050105. [DOI] [PubMed] [Google Scholar]
- 16.Famous K.R., Delucchi K., Ware L.B. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195(3):331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandharipande P.P., Shintani A.K., Hagerman H.E. Derivation and validation of SpO2/FiO2 ratio to impute for PaO2/FiO2 ratio in the respiratory component of the sequential organ failure assessment score. Crit Care Med. 2009;37(4):1317–1321. doi: 10.1097/CCM.0b013e31819cefa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus Definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 20.Yan M., Ye K. Determining the number of clusters using the weighted gap statistic. Biometrics. 2007;63(4):1031–1037. doi: 10.1111/j.1541-0420.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 21.Lengyel A., Botta-Dukat Z. Silhouette width using generalized mean-A flexible method for assessing clustering efficiency. Ecol Evol. 2019;9(23):13231–13243. doi: 10.1002/ece3.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delaney J.W., Pinto R., Long J. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care. 2016;20:75. doi: 10.1186/s13054-016-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen R.C., Tang X.P., Tan S.Y. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X., Tan Y., Ling Y. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- 26.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azkur A.K., Akdis M., Azkur D. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2020;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meduri G.U., Annane D., Chrousos G.P., Marik P.E., Sinclair S.E. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136(6):1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 30.Yam L.Y., Lau A.C., Lai F.Y., Shung E., Chan J., Wong V. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54(1):28–39. doi: 10.1016/j.jinf.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Annane D., Pastores S.M., Rochwerg B. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017;43(12):1751–1763. doi: 10.1007/s00134-017-4919-5. [DOI] [PubMed] [Google Scholar]
- 32.Xie J., Tong Z., Guan X., Du B., Qiu H., Slutsky A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46(5):837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.