Abstract
Aim
The coronavirus disease 2019 (COVID-19) pandemic has swept the globe and no specific effective drug has been identified. Drug repurposing is a well-known method to address the crisis in a time-critical fashion. Antipsychotic drugs (APDs) have been reported to inhibit DNA replication of hepatitis B virus, measles virus germination, and HIV infection, along with replication of SARS-CoV and MERS-CoV, both of which interact with host cells as SARS-CoV-2.
Methods
Nineteen APDs were screened using ACE2-HEK293T cell membrane chromatography (ACE2-HEK293T/CMC). Cytotoxicity assay, coronavirus spike pseudotype virus entry assay, surface plasmon resonance, and virtual molecular docking were applied to detect affinity between ACE2 protein and drugs and a potential antiviral property of the screened compounds.
Key findings
After the CMC screening, 8 of the 19 APDs were well-retained on ACE2-HEK293T/CMC column and showed significant antiviral activities in vitro. Three quarters of them belong to phenothiazine and could significantly inhibit the entrance of coronavirus into ACE2-HEK293T cells. Aother two drugs, aripiprazole and tiapride, exhibited weaker inhibition. We selected five of the drugs for subsequent evaluation. All five showed similar affinity to ACE2 and virtual molecular docking demonstrated they bound with different amino acids respectively on ACE2 which SARS-CoV-2 binds to.
Significance
Eight APDs were screened for binding with ACE2, five of which demonstrated potential protective effects against SARS-CoV-2 through acting on ACE2. Although the five drugs have a weak ability to block SARS-CoV-2 with a single binding site, they may provide a synergistic effect in adjuvant therapy of COVID-19 infection.
Keywords: Antipsychotic drugs, Phenothiazines, SARS-CoV-2, ACE2, Drug repurposing
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) has become a global pandemic since the end of 2019 [1]. At present, most countries in the world continue to suffer from the epidemic, and there are yet no effective drugs to combat the novel coronavirus. The coronavirus found in the bronchoalveolar lavage fluid of patients with COVID-19 has been named SARS-CoV-2 [2]. Although it is quite different from the SARS-CoV genetics, it has a similar binding site to SARS-CoV despite amino acid variation in some key residues [3]. Current research suggests that SARS-CoV-2 enters host cells through the ACE2 protein with the S protein on the virus shell. Therefore, it might be possible to prevent and treat COVID-19 pneumonia by blocking or antagonizing ACE2 to prevent the virus from infecting cells [4].
Since the development of new drugs is a long and costly process, the urgency of the pandemic demands that possible therapeutic agents are found among approved drugs; this process is known as drug repurposing or reprofiling. Here we employ this well-known strategy to identify anti-SARS-CoV-2 agents in a time-critical fashion [5].
Antipsychotic drugs (APDs) ameliorate hallucinations and delusions in patients with neuropsychiatric disorders such as schizophrenia and bipolar disorder. Classical phenothiazine APD possesses many different biological effects due to their tricyclic structures. It has been reported that thiazine and phenothiazine scaffolds are often present in compounds with biological properties such as antiproliferative, antibacterial, antipsychotic, anti-inflammatory, antifungal, and antiviral [6,7]. Chlorpromazine is a representative phenothiazine that has frequently been used in vitro to explore its antiviral properties. Studies have suggested that chlorpromazine can inhibit DNA replication of hepatitis B virus (HBV) [8], measles virus germination [9], HIV infection [10], and replication of SARS and human coronavirus 229E in a low micromole range [11]. A study suggested that APDs inhibit SARS-CoV or MERS-CoV, and chlorpromazine and trifluoperazine can inhibit both [12].
It was hypothesized recently that psychiatric patients could be protected from severe forms of COVID-19 by their psychotropic medical treatments. In the GHU PARIS Psychiatrie & Neurosciences college (Sainte-Anne hospital, Paris, France), a lower prevalence of symptomatic and severe forms of COVID-19 infections was seen in psychiatric patients (~4%) compared to health care professionals (~14%). Similar observations have been noted in other psychiatric units in France and other countries [13]. Preclinical and clinical studies reported a high chlorpromazine concentration in the lungs (20–200 times higher than in plasma); this is critical when COVID-19 is considered [14]. We believe that APDs may affect ACE2 potentially to cure this severe viral pneumonia.
We screened APDs, tested their affinity for ACE2, and the ability to inhibit the virus surface-anchored spike protein-mediated coronavirus entry, hoping to find effective agents to prevent SARS-CoV-2 infection.
2. Materials and methods
2.1. Drugs and reagents
Dulbecco's modified Eagle's medium (DMEM) high glucose (Cat. No. SH30022.01), and fetal bovine serum (FBS; Cat. No. 16140071) was purchased from HyClone (Logan, UT, USA). Penicillin-streptomycin solution was purchased from Xi'an Hat Biotechnology Co., Ltd. (Xi'an, Shaanxi, China). Cell Counting Kit was purchased from 7Sea Pharmatech Co., Ltd. (Shanghai, China). Chlorpromazine (Chl), perphenazine (Per), fluphenazine (Flu), thioridazine (Thi), promethazine (Pro), aripiprazole (Ari), tiapride (Tia), quetiapine (Que), haloperidol, olanzapine, risperidone, clozapine, succinylcholine chloride, and iohexol were purchased from Meilunbio Co., Ltd. (Dalian, China) and purified to ≥98%. Trifluoperazine (Tri) was obtained from TargetMol Co., Ltd. (Shanghai, China). Diazepam injection was purchased from Jinyao Pharmaceutical Co., Ltd. (Tianjin, China). 2-chlorothiazide was obtained from Xiya Chemical Co., Ltd. (Shandong, China), and magnesium valproate and iopromide were obtained from the National Institute for the Control of Pharmaceutical and Biological Products.
2.2. Cell lines
ACE2 high-expressing HEK293T cells (ACE2hi cells) were cultivated in DMEM high glucose medium containing 10% FBS, 1% penicillin-streptomycin, and 4 μg/mL puromycin, and cultured in an incubator at 37 °C and 5% CO2.
2.3. ACE2-HEK293T/cell membrane chromatography (ACE2-HEK293T/CMC)
The ACE2-HEK293T/CMC column was prepared using ACE2hi-HEK293T cells. Cells were washed three times with saline solution by centrifuging at 800 ×g for 5 min at 4 °C. Tris–HCl (50 mM, pH 7.4) was added before ultrasonication for 30 min and homogenization for 20 min on ice. The resulting homogenate was centrifuged at 2300 ×g for 10 min. The supernatant was centrifuged at 12,000 ×g for 20 min at 4 °C and cell membrane sediment was obtained. The sediment was then mixed with Tris-HCl (50 mM, pH 7.4). The membrane suspension was obtained after the sediment was suspended in saline solution and kept at 4 °C.
The ACE2-HEK293T cell membrane stationary phase (CMSP) was made up of cell membrane and silica. Briefly, 0.05 g of silica was activated at 105 °C for 30 min and was used as a carrier. The suspension was slowly added to activated silica in a vacuum and agitated at 4 °C. The obtained mixture was stirred for 30 min and left overnight at 4 °C to obtain the CMSP. The CMSP was packed into the column by a wet method to yield a CMSP column (10 mm × 3.1 mm, 5 μm).
LC-2010AHT instrument (Shimadzu, Kyoto, Japan) was used under the following conditions:10 mM of Na2HPO4 mobile phase with 0.2 mL/min flow rate, column oven kept at 37 °C, and UV detection at 254 nm.
2.4. Cytotoxicity assay
Cell viability was examined using Cell Counting Kit assays. ACE2hi cells were seeded into 96-well plates at a density of 5 × 103 cells per well and precultured for 24 h. The cells were then treated with different concentrations of each drug (0, 25, 50, and 100 μM) for 24 h. Next, 10 μL of Cell Counting Kit solution was added to each well followed by incubation for 2 h. The relative cell viability was assessed by quantifying light absorbance at 450 nm using a microplate reader. The survival rate of ACE2hi cells was calculated using the following formula:
2.5. The detection of coronavirus spike pseudotype virus entry into ACE2 cells
5 × 104 of ACE2hi cells in 50 μL DMEM per well were seeded into white 96-well plates. The cells were cultured at 37 °C in an incubator containing 5% CO2 for 2 H. medium (25 μL) was removed carefully from 96 wells and 25 μL of medium containing the test drugs was added. Cells were incubated for 2 h, 5 μL of coronavirus spike pseudotype virus was added, cells were incubated at 37 °C in 5% CO2 for 4 h, and 100 μL of complemented DMEM per well was added. After 6–8 h of further incubation, all the culture medium containing the virus was replaced with 200 μL of fresh DMEM and incubated at 37 °C for 48 h. The culture medium was aspirated and 20 μL of cell lysate was added from the Luciferase Assay System to each well. Then, 100 μL of luminescence solution was added to wells before the luciferase chemiluminescence was quantified using a microplate reader at 560 nm. The exposure time was 1 s.
2.6. Docking studies
Molecular docking studies were carried out using the SYBYL-X 2.0 version to explore the binding mode of the five agents with ACE2. The X-ray crystal structure of ACE2 (PDB code: 6M0J) was downloaded from PDB Bank. Water molecules were removed, and hydrogen was added. Tripos force field and Pullman charge were applied to minimize the molecular energy. Each drug molecule was depicted by the Sybyl/Sketch module (Tripos Inc.), optimized by Powell's method with the Tripos force field with convergence criterion at 0.005 kcal/(Å mol), and assigned using the Gasteiger–Hückel method.
2.7. Surface plasmon resonance assay
For assessment of surface plasmon resonance (SPR), ACE2 protein with a 6-his tag (30 μg/mL) was bonded to a carboxyl sensor chip by capture-coupling. Then, every drug at 5, 10, 20, and 40 μM was injected in a row with PBS running buffer. The interactions between ACE2 and every small molecule were detected using Open SPRTM at 25 °C. The flow rate was 20 μL/min, and the chip was regenerated with hydrochloric acid (pH 2.0). A one-to-one diffusion-corrected model was fitted to the wavelength shifts corresponding to the series of drug concentrations. The data were extracted and analyzed using TraceDrawer software to calculate the K D value.
2.8. Statistical analysis
All the data are presented as mean ± S.E.M. Unpaired t-test was performed using Prism 8. Differences between data sets were considered statistically significant at *P < 0.05, **P < 0.01, and ***P < 0.001.
3. Results
3.1. The screening for antipsychotics using ACE2-HEK293T/cell membrane chromatography
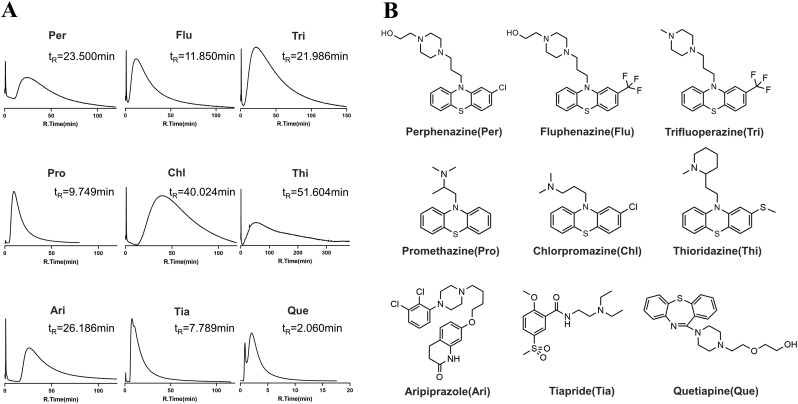
Modern pharmacological studies have shown that combination with receptors or channels on the cell membrane is the first step of drug action. Cell membrane chromatography (CMC) can screen many agents based on this principle [15]. We used ACE2-HEK293T/CMC to select drugs with strong affinity with ACE2. A total of 19 antipsychotics drugs were screened, eight of which showed positive combinations on ACE2-CMC in 10 mM Na2HPO4 ( Fig. 1A ). Agent Que. had little retention in the column and was used as a negative sample to illustrate visually the difference. The graph shows that peaks of the first eight compounds tailed heavily indicating a strong drug interaction with ACE2. Agent Thi had the longest retention time (51.604 min) and Tia showed the weakest combination with ACE2 peaking 7.789 min. Six drugs were phenothiazines, and the other three drugs, Ari, Tia, and Que. belong to phenylpiperazines, benzenesulfonyls, and dibenzothiazepines, respectively ( Fig. 1B). Since the majority of tested compounds showing high affinity are phenothiazines, we expect that the phenothiazine structure might be most suitable for binding with ACE2.
Fig. 1.
The CMC chromatograms and structures of nine APDs. (A) The CMC chromatograms of nine drugs on the ACE2-CMC column and ACE2-CMC column 10 mm × 2.0 mm; flow rate 0.2 mL/min; column temperature 37 °C; mobile phase 10 mM phosphate-buffer saline; pH 7.4; detection wavelength 254 nm. (B) The structures of the nine compounds.
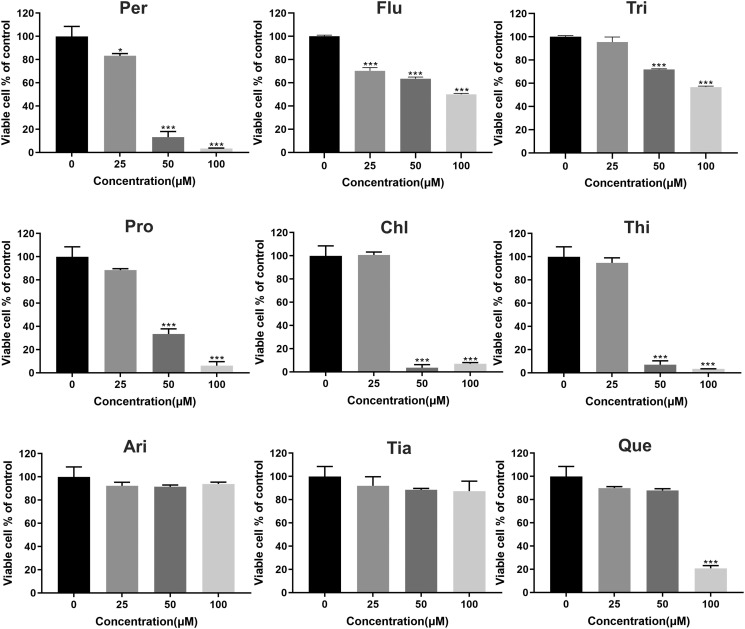
3.2. The effect of the nine antipsychotics on ACE2-293T cell viability
To ensure that the in vitro results were not affected by cytotoxicity, CCK-8 was applied for 24 h to determine the proper concentration. As shown in Fig. 2 , except for Per and Flu, phenothiazines had no significant effect on the activity of ACE2hi cells at 25 μM; however, the cell survival decreased in a dose-dependent manner at concentrations above 25 μM. In contrast, Ari and Tia had no significant effect at concentrations below 100 μM, indicating little cytotoxicity towards ACE2hi cells. The Que. agent showed no significant difference with the control group at concentrations below 50 μM.
Fig. 2.
The cytotoxicity effect of the nine antipsychotic agents. Data are presented as mean ± S.D. ***p < 0.001 compared with control group.
3.3. The antipsychotics suppressed the entrance of coronavirus spike pseudotype virus into ACE2hi cells
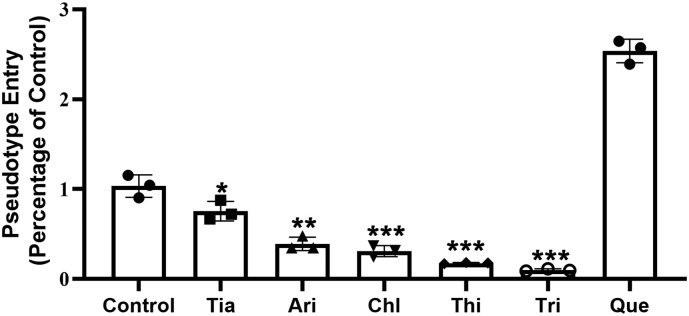
Phenothiazines can be classified into three subclasses according to the structure of the side chain: aliphatic (Chl and Pro), piperidine (Thi), and piperazine (Per, Flu, and Tri) [16]. Considering the structural similarity and cytotoxicity, we selected representative agents from each category to test their inhibition of coronavirus spike pseudotype virus entering ACE2hi cells: Chl, Thi, Tri, Ari, Tia, and Que. These drugs showed no cytotoxicity to cells at 20 μM and this concentration was selected for further testing.
In the control group without any drug, the level for the luciferase luminescence value of spike pseudotype virus-infected ACE2hi cells was defined as 1. Treatment with Chl, Thi, Tri, Ari and Tia reduced the coronavirus spike pseudotype virus entry ratio to 0.76 ± 0.11, 0.39 ± 0.08, 0.31 ± 0.06, 0.18 ± 0.01, 0.10 ± 0.01, respectively ( Fig. 3 ), which indicated that drug treatment had a significant inhibiting effect on the virus entry into ACE2hi cells. Three phenothiazines (Chl, Thi, Tri) had the strongest inhibitory effect; the value for Que. increased to 2.54 ± 0.13 indicating that Que. did not inhibit the virus entry and may promote it. The results correspond to the retention values determined by CMC.
Fig. 3.
The inhibition effect of six representative drugs on the entrance of coronavirus spike pseudotype virus into ACE2 cells. Data are presented as mean ± S.D. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group.
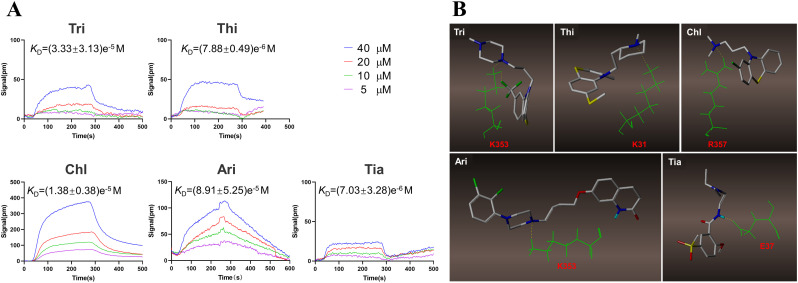
3.4. The binding character of antipsychotics with ACE2
The ability of drugs to inhibit virus entry has been verified. To elucidate binding with the ACE2, an SPR test was applied to determine the K D values. The K D values of the five drugs were (7.03 ± 3.28)e−6 M, (8.91 ± 5.25)e−5 M, (1.38 ± 0.38)e−5 M, (7.88 ± 0.49)e−6 M, and (3.33 ± 3.13)e−5 M, respectively, demonstrating that each drug combined with ACE2 receptor with similarly high affinity ( Fig. 4A ). To identify the critically combined regions, molecular docking was carried out. It was found that every agent binds to one amino acid of ACE2: K353 for Tri, K31 for Thi, R357 for Chl, K353 for Ari, and E37 for Tia ( Fig. 4B). Each of the five drugs occupied one of the binding sites used by SARS-CoV-2; this explains the viral entry inhibition by the five antipsychotics. The involvement of a single binding site also explains the lower affinity observed in the SPR assay.
Fig. 4.
The bind character of five antipsychotic drugs. (A) The SPR analysis of the five antipsychotic drugs. (B) The virtual molecular docking analysis of the five APDs.
4. Discussion
COVID-19 pandemic was declared on 11th March 2020 after the virus appeared in late 2019 [17]. As of the writing of this paper, it has caused more than 770 thousand deaths out of 22 million infected people worldwide. Although many clinical trials with small-molecule drugs and vaccines have been performed or are undergoing, there are still no specific drugs against COVID-19. Therefore, finding the treatment of COVID-19 infection is an urgent matter.
Antipsychotic drugs have been reported to exhibit viral activity through different mechanisms. Epstein–Barr virus (EBV) maintains a lifelong latent presence in human B cells and switching from the latent to the lytic cycle in infected cultured cells allows EBV to replicate and spread. Clozapine was found to inhibit reactivation of the EBV lytic cycle by decreasing the levels of the viral lytic genes BZLF1, BRLF1, and BMLF1 [18]. Thioridazine, another antipsychotic drug, was confirmed to inhibit the cell entry of EBOV by directly interacting with the glycoprotein of EBOV. This suggests that antipsychotic drugs may possess antiviral ability on SARS-CoV-2 [19].
SARS-CoV-2 is believed to enter the host cell by binding to ACE2 on its plasma membrane of the cell and infect it [4,20,21]. Therefore, it might be beneficial to prevent SARS-CoV-2 infection by blocking or antagonizing the ACE2 signaling pathway of susceptible cells [22].
We established an ACE2-HEK293T/CMC model using ACE2hi cells and used it to screen potential anti-SARS-CoV-2 compounds targeting the ACE2 protein. Eight out of nineteen antipsychotic drugs including Thi, Chl, Pro, Ari, Per, Flu, Tri, and Tia were found to be retained on the ACE2-HEK293T/CMC column indicating their potential affinity to ACE2.
To evaluate their antiviral effect on SARS-CoV-2, a cytotoxicity assay was carried out that indicated nearly no cytotoxicity of the above drugs on ACE2hi cells under 25 μM concentration. Based on the structural diversity and cytotoxicity, five antipsychotic drugs were selected for the subsequent antiviral evaluations at 20 μM. It was observed that all five tested drugs inhibited virus entry into ACE2hi cells, indicating their potential antiviral ability and the suitability of the HEK293T/CMC model. And Tri has been identified as the most promising inhibition agent based on the result of the coronavirus spike pseudotype virus assay.
To further confirm the interaction between the antipsychotic drugs and ACE2, SPR and molecular docking was performed. SARS-CoV-2 was reported to bind to ACE2 at R393, R357, K353, Y83, Q42, Y41, D38, E37, E35, K31, D30, and Q24 locations [[23], [24], [25]]. As shown in Fig. 4B, all five drugs bonded to ACE2 at one critical amino acid with two different binding characteristics, demonstrating the possible mechanism for preventing virus entry into the host cells. Among them, Tri and Tia were served as electron acceptor, and Thi, Chl and Ari were served as electron donor during binding with ACE2. The SPR assay also revealed their affinity for ACE2. In general, antipsychotic drugs with phenothiazine scaffolds showed more potential antiviral ability, drugs with cyclic substituents showed greater potential than chain substituents. Since each antipsychotic drug blocked only a small area occupied by SARS-CoV-2, they could not completely prevent cell invasion. While using these drugs individually may not benefit clinically, combined cocktails may be effective due to each drug binding to different amino acids.
Our results suggest that APDs, especially phenothiazines, can significantly block SARS-CoV-2 binding to ACE2. However, we cannot completely exclude the possibility that phenothiazines possess the ability to inhibit the replication of coronaviruses. Krystian Pluta determined a structure-reaction relationship law in which the tightest binders with RNA of HIV were compounds with 3–4 carbons between tricyclic structure and amino group [26]. In our study, Thi and Tri had the strongest inhibitory effect with three carbon atoms between the phenothiazine part and the amino group, and Chl has a weaker effect with two carbon. This result is consistent with the rule mentioned above. Therefore, it is likely that phenothiazines may have dual functions that inhibit binding to ACE2 and virus replication. This double-target involvement might result in a larger effect and deserves research to be demonstrated.
Overall, our study demonstrated that antipsychotic drugs inhibit the entry of SARS-CoV-2 into host cells by targeting ACE2 and offer a possible option for treating SARS-CoV-2 infection.
5. Conclusion
Considering the current coronavirus pandemic, we utilized the drug purposing principle to examine the possible antiviral activity of APDs via binding to ACE2. Five out of nineteen APDs were found to significantly inhibit the coronavirus spike pseudotype virus entry into ACE2hi cells and show parallel affinity to ACE2 protein in vitro. And trifluoperazine was proposed to be most promising drug repurposing in COVID-19. The results indicate that APDs such as thioridazine and trifluoperazine may inhibit SARS-CoV-2 activity by binding to ACE2 when used in drug cocktails or adjuvant therapy.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 81930096, 81903573); the Postdoctoral Research Foundation of China (No. 2018M643682); and the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2020JQ-089).
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciotti M., Angeletti S., Minieri M., Giovannetti M., Benvenuto D., Pascarella S., Sagnelli C., Bianchi M., Bernardini S., Ciccozzi M. COVID-19 Outbreak: An Overview. Chemotherapy. 2019;64:215–223. doi: 10.1159/000507423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Krüger, T. Herrler, S. Erichsen, T.S. Schiergens, G. Herrler, N.H. Wu, A. Nitsche, M.A. Müller, C. Drosten, S. Pöhlmann, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell 181 (2020) 271–280.e278. [DOI] [PMC free article] [PubMed]
- 5.Senanayake S.L. Drug repurposing strategies for COVID-19. Future Drug Discov. 2020;2:FDD40. [Google Scholar]
- 6.Choudhary S., Silakari O., Singh P.K. Key updates on the chemistry and biological roles of thiazine scaffold: a review. Mini-Rev. Med. Chem. 2018;18:1452–1478. doi: 10.2174/1389557518666180416150552. [DOI] [PubMed] [Google Scholar]
- 7.Morak-Młodawska B., Jeleń M. New biological properties of neuroleptic phenothiazines. Pol. Merkur. Lekarski. 2007;23:459–461. [PubMed] [Google Scholar]
- 8.Hirschman S.Z., Garfinkel E. Inhibition of hepatitis B DNA polymerase by intercalating agents. Nature. 1978;271:681–683. doi: 10.1038/271681a0. [DOI] [PubMed] [Google Scholar]
- 9.Bohn W., Rutter G., Hohenberg H., Mannweiler K. Inhibition of measles virus budding by phenothiazines. Virology. 1983;130:44–55. doi: 10.1016/0042-6822(83)90116-2. [DOI] [PubMed] [Google Scholar]
- 10.Hewlett I., Lee S., Molnar J., Foldeak S., Pine P.S., Weaver J.L., Aszalos A. Inhibition of HIV infection of H9 cells by chlorpromazine derivatives. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997;15:16–20. doi: 10.1097/00042560-199705010-00003. [DOI] [PubMed] [Google Scholar]
- 11.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Ch. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jr., Jahrling P.B., Laidlaw M., Johansen L.M., Lear-Rooney C.M., Glass P.J., Hensley L.E., Frieman M.B. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Ch. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plaze M., Attali D., Petit A.C., Blatzer M., Simon-Loriere E., Vinckier F., Cachia A., Chrétien F., Gaillard R. Repurposing of chlorpromazine in COVID-19 treatment: the reCoVery study. Encephale. 2020;46:S35–s39. doi: 10.1016/j.encep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickel M.H., Graber B.E., Moor M. Distribution of chlorpromazine and imipramine in adipose and other tissues of rats. Life Sci. 1983;33:2025–2031. doi: 10.1016/0024-3205(83)90742-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen R., Liu X., Jin S., Lin J., Liu J. Machine learning for drug-target interaction prediction. Molecules. 2018;23 doi: 10.3390/molecules23092208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Y., Che D., Wei D., Wang C., Xie Y., Zhang K., Cao J., Fu J., Zhou N., He H. Phenothiazine antipsychotics exhibit dual properties in pseudo-allergic reactions: activating MRGPRX2 and inhibiting the H(1) receptor. Mol. Immunol. 2019;111:118–127. doi: 10.1016/j.molimm.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson A.G., Gaffy C.B., Weseli J.R., Gorres K.L. Inhibition of Epstein-Barr virus lytic reactivation by the atypical antipsychotic drug clozapine. Viruses. 2019;11:450. doi: 10.3390/v11050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Ren J., Fry E.E., Xiao J., Townsend A.R., Stuart D.I. Structures of Ebola virus glycoprotein complexes with tricyclic antidepressant and antipsychotic drugs. J. Med. Chem. 2018;61:4938–4945. doi: 10.1021/acs.jmedchem.8b00350. [DOI] [PubMed] [Google Scholar]
- 20.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y., Gong Y., Xiao H., Fan Z., Tan S., Wu G., Tan W., Lu X., Fan C., Wang Q., Liu Y., Zhang C., Qi J., Gao G.F., Gao F., Liu L. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368:1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 24.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pluta K., Morak-Młodawska B., Jeleń M. Recent progress in biological activities of synthesized phenothiazines. Eur. J. Med. Chem. 2011;46:3179–3189. doi: 10.1016/j.ejmech.2011.05.013. [DOI] [PubMed] [Google Scholar]