Supplemental Digital Content is available in the text.
Keywords: heart failure; kidney function tests; renal insufficiency, chronic; sodium-glucose transporter 2 inhibitors
Background:
Many patients with heart failure and reduced ejection fraction (HFrEF) have chronic kidney disease that complicates pharmacological management and is associated with worse outcomes. We assessed the safety and efficacy of dapagliflozin in patients with HFrEF, according to baseline kidney function, in the DAPA-HF trial (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure). We also examined the effect of dapagliflozin on kidney function after randomization.
Methods:
Patients who have HFrEF with or without type 2 diabetes and an estimated glomerular filtration rate (eGFR) ≥30 mL·min–1·1.73 m–2 were enrolled in DAPA-HF. We calculated the incidence of the primary outcome (cardiovascular death or worsening heart failure) according to eGFR category at baseline (<60 and ≥60 mL·min–1·1.73 m–2) and used eGFR at baseline as a continuous measure, as well. Secondary cardiovascular outcomes and a prespecified composite renal outcome (≥50% sustained decline eGFR, end-stage renal disease, or renal death) were also examined, along with a decline in eGFR over time.
Results:
Of 4742 patients with a baseline eGFR, 1926 (41%) had eGFR <60 mL·min–1·1.73 m–2. The effect of dapagliflozin on the primary and secondary outcomes did not differ by eGFR category or examining eGFR as a continuous measurement. The hazard ratio (95% CI) for the primary end point in patients with chronic kidney disease was 0.71 (0.59–0.86) versus 0.77 (0.64–0.93) in those with an eGFR ≥60 mL·min–1·1.73 m–2 (interaction P=0.54). The composite renal outcome was not reduced by dapagliflozin (hazard ratio=0.71 [95% CI, 0.44–1.16]; P=0.17) but the rate of decline in eGFR between day 14 and 720 was less with dapagliflozin, –1.09 (–1.40 to –0.77) versus placebo –2.85 (–3.17 to –2.53) mL·min–1·1.73 m–2 per year (P<0.001). This was observed in those with and without type 2 diabetes (P for interaction=0.92).
Conclusions:
Baseline kidney function did not modify the benefits of dapagliflozin on morbidity and mortality in HFrEF, and dapagliflozin slowed the rate of decline in eGFR, including in patients without diabetes.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03036124.
Clinical Perspective.
What Is New?
The sodium-glucose cotransporter 2 inhibitor dapagliflozin slowed the rate of decline in estimated glomerular filtration rate in patients with heart failure with reduced ejection fraction both in patients with and without type 2 diabetes.
There was no difference in the efficacy of dapagliflozin by baseline kidney function in preventing the risk of cardiovascular death or worsening heart failure.
What Are the Clinical Implications?
Patients with heart failure with reduced ejection fraction and impaired kidney function will benefit from the addition of a sodium-glucose cotransporter 2 inhibitor to standard therapies.
Use of sodium-glucose cotransporter 2 inhibitor in this population will slow the progression of kidney dysfunction, but whether this translates into reductions in renal outcomes could not be determined.
Editorial, see p 322
Impaired renal function is common in patients with heart failure and reduced ejection fraction (HFrEF) and up to 50% have chronic kidney disease (CKD) defined as an estimated glomerular filtration rate (eGFR) <60 mL·min–1·1.73 m–2.1 CKD is associated with conditions that lead to the development of heart failure, such as atherosclerosis and hypertension, and common comorbidities in heart failure such as diabetes and anemia, as well. Once heart failure develops, renal function declines and this is associated with a poorer prognosis.2 In part, this may be because the use of many of the therapies known to improve morbidity and mortality in HFrEF is restricted by kidney function and may even be impossible in patients with very low eGFR. Yet, paradoxically, it is patients with CKD who potentially derive the greatest absolute benefit from treatment with pharmacotherapy because of their higher event rates.3
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have recently been shown to improve cardiovascular outcomes in patients with type 2 diabetes.4–7 In addition, they slow the rate of decline in kidney function in these patients and reduce renal morbidity and mortality in patients with type 2 diabetes and kidney dysfunction.8 In the DAPA-HF trial (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure), the SGLT2 inhibitor dapagliflozin reduced the incidence of the primary composite outcome of cardiovascular death or worsening heart failure (HF) in patients who had HFrEF with and without type 2 diabetes.9 In this study, we explored whether the effect of dapagliflozin varied according to baseline renal function. We also examined the effect of dapagliflozin on kidney function and renal outcomes.
Methods
The DAPA-HF trial randomly assigned patients with HFrEF with and without type 2 diabetes in a double-blind, placebo-controlled, event-driven trial.9–11 The SGLT2 inhibitor dapagliflozin at a dose of 10 mg once daily, in addition to standard care, was compared with matching placebo. The design, baseline characteristics, and primary results have been published.9–11 The Ethics Committee of the 410 participating institutions in 20 countries approved the protocol; all patients gave written informed consent. The corresponding author had full access to all the trial data and takes responsibility for its integrity and the data analysis. The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Patients
The trial included patients with HF with a left ventricular ejection fraction ≤40%, ≥18 years of age, New York Heart Association functional class II to IV, and an elevated N-terminal pro-B-type natriuretic peptide level, and who were receiving optimal pharmacological and device therapy. The trial protocol required guideline-recommended medications, including β-blocker, unless contraindicated/not tolerated. The main exclusion criteria included type 1 diabetes, symptomatic hypotension/systolic blood pressure <95 mm Hg, eGFR <30 mL·min–1·1.73 m–2, or “unstable or rapidly progressing renal disease,” in the view of the investigator.
Measurement of Kidney Function and eGFR Subgroup Analysis
Blood samples were taken at randomization, at 14 days, and at 2, 4, 8, and 12 months, and every 4 months thereafter. Creatinine was measured in a central laboratory and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. The prespecified subgroup analysis of the efficacy of dapagliflozin according to baseline eGFR divided patients <60 and ≥60 mL·min–1·1.73 m–2. We also examined the efficacy of dapagliflozin by using eGFR as a continuous measure.
Prespecified Outcomes
The primary outcome of DAPA-HF was the composite of worsening heart failure (HF hospitalization or urgent visit for HF requiring intravenous therapy) or cardiovascular death, whichever occurred first. Prespecified secondary end points included HF hospitalization or cardiovascular death; HF hospitalizations (first and recurrent) and cardiovascular deaths. The prespecified secondary renal outcome was a composite of ≥50% sustained decline eGFR or end-stage renal disease or renal death. Sustained was defined as lasting at least 28 days and end-stage renal disease was defined as a sustained eGFR of <15 mL·min–1·1.73 m–2 or chronic dialysis or renal transplantation. Change from baseline to 8 months in Kansas City Cardiomyopathy Questionnaire-total symptom score12 was examined with the proportion of patients having a ≥5 point increase or decrease in their score at 8 months determined by using logistic regression as previously described.13
One prespecified exploratory end point related to kidney function was the number of cases of doubling of serum creatinine. Doubling of serum creatinine (in comparison with the most recent central laboratory measurement) was adjudicated and defined as a doubling of serum creatinine in comparison with the most recent central laboratory result and could be triggered by a local laboratory result or a central laboratory result. This could represent a chronic or more acute kidney injury.
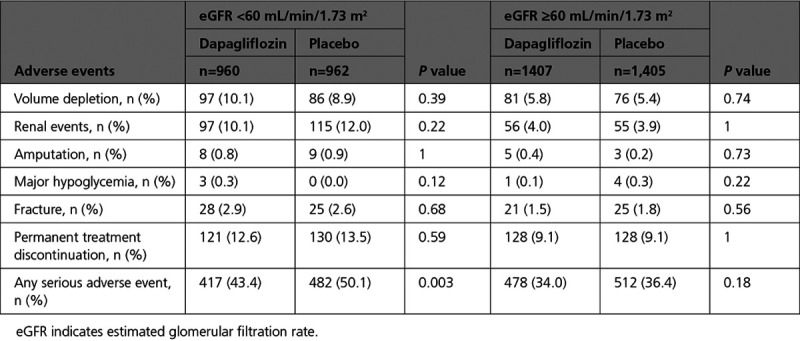
Prespecified safety analyses included any serious adverse event, adverse events related to study drug discontinuation, and adverse events of interest that specifically included renal adverse events.
In addition to these prespecified outcomes, the post hoc outcome of the slope of change from baseline in eGFR over time according to randomized treatment was calculated as described under Statistical Analysis.
Statistical Analysis
Baseline characteristics were summarized as means (SDs), median (interquartile ranges), or percentages. We used the Kaplan-Meier estimate and Cox proportional-hazards models, stratified by diabetes status, and adjusted for history of HF hospitalization (except for all-cause death) and treatment-group assignment to examine the primary and secondary outcomes. The interaction between baseline eGFR and treatment on the primary and secondary outcomes was modeled as a fractional polynomial and graphed.14 The renal composite outcome was evaluated in a Cox model stratified by diabetes status adjusted for baseline eGFR and treatment group. A semiparametric proportional-rates model (described by Lin et al15) was used to analyze total (including recurrent) HF hospitalizations accounting for the risk of cardiovascular death as a terminal event. Repeated-measures mixed-effect models were used to examine the slope of change in eGFR over time according to randomized treatment. These were adjusted for baseline values, visit, randomized treatment, and interaction of treatment and visit with a random intercept and slope per patient with an unstructured covariance structure. There were 2 clear phases to the slope of eGFR, an initial decline and then a slower decline. The slope of change in eGFR in each randomization group (expressed as a decrease per mL·min–1·1.73 m–2 were compared between day 0 (randomization) and day 14 and then from day 14 to day 720 of follow-up. In an exploratory analysis, to examine the potential survivor bias introduced into the analyses of eGFR slopes, we also modeled eGFR jointly with all-cause mortality.16 We examined the slope of eGFR in patients with and without diabetes at baseline. Safety analyses were performed in randomly assigned patients who had received at least 1 dose of dapagliflozin or placebo. The interaction between CKD and randomized treatment on the occurrence of the prespecified safety outcomes was tested in a logistic regression model with the baseline CKD group and randomized therapy and their interaction term as the only factors in the model. All analyses were conducted using Stata version 16.1. A P value of <0.05 was considered statistically significant.
Results
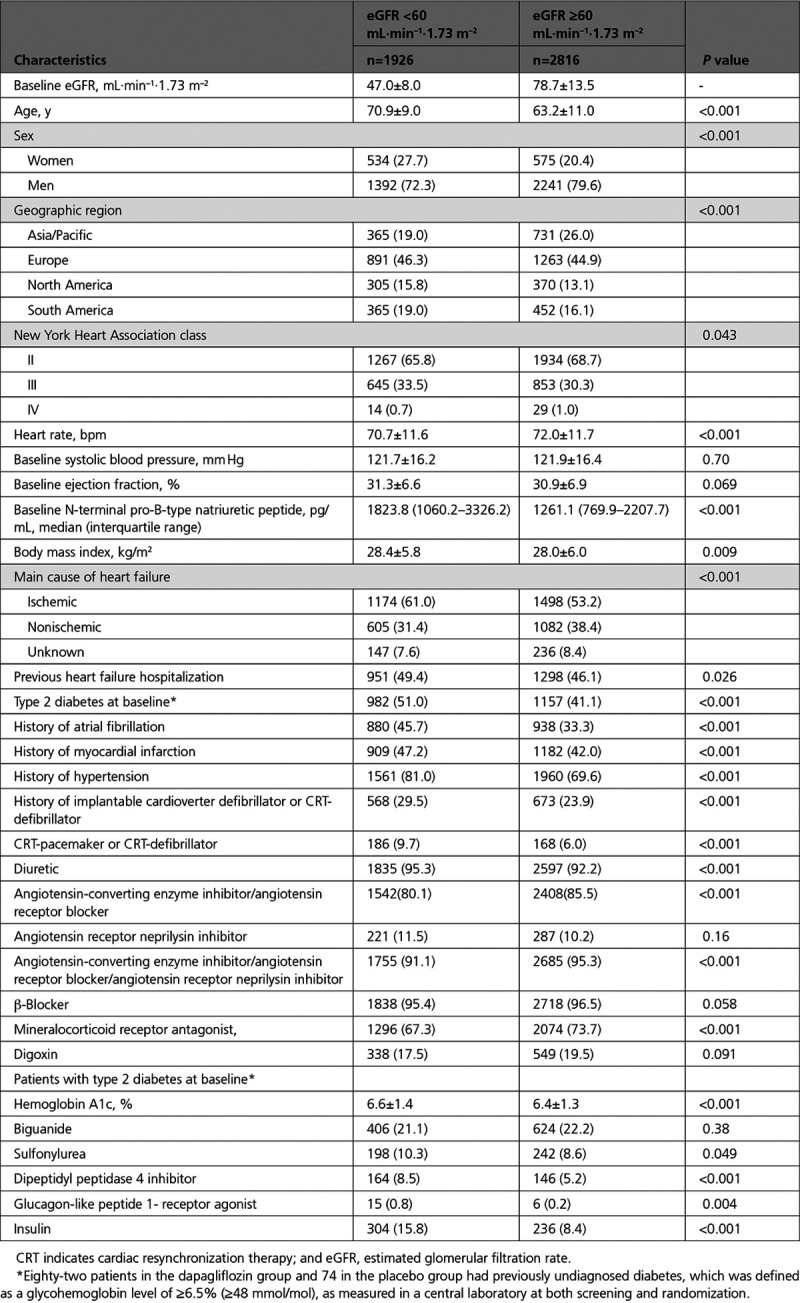
At baseline, an eGFR could be calculated in 4743 patients and 1926 (41%) had a value <60 mL·min–1·1.73 m–2 (Table 1). Participants with lower eGFR were considerably older (71 versus 63 years, respectively), more were women (28% versus 20%), and more had an ischemic cause (61% versus 53%), in comparison with those with an eGFR ≥60 mL·min–1·1.73 m–2. Patients with an eGFR <60 mL·min–1·1.73 m–2 had a higher N-terminal pro-B-type natriuretic peptide, lower heart rate, and more often had a history of atrial fibrillation, myocardial infarction, hypertension, and type 2 diabetes (Table 1). Patients with eGFR <60 mL·min–1·1.73 m–2 were more often treated with a diuretic, but less frequently treated with a renin-angiotensin system blocker or mineralocorticoid receptor antagonist, in comparison with those with an eGFR ≥60 mL·min–1·1.73 m–2. In participants with type 2 diabetes at baseline, patients with a lower eGFR were more likely than individuals with an eGFR ≥60 mL·min–1·1.73 m–2 to be treated with a dipeptidyl peptidase 4 inhibitor and insulin (Table 1).
Table 1.
Baseline Characteristics by Baseline eGFR Groups
Cardiovascular Outcomes According to Baseline eGFR
Primary and Secondary Trial Outcomes
The incidence rates of the primary and secondary outcomes of the trial were higher in those with CKD at baseline (Table 2 and Figure I in the Data Supplement). The efficacy of dapagliflozin in preventing the primary outcome of cardiovascular death or worsening HF did not differ between those with an eGFR of <60 mL·min–1·1.73 m–2 and individuals with an eGFR ≥60 mL·min–1·1.73 m–2 (P for interaction=0.54). The efficacy of dapagliflozin in preventing cardiovascular death, HF hospitalizations, or urgent HF visits, the total HF hospitalizations and all-cause death also did not differ by eGFR group (Table 2). The results were similar when eGFR was treated as a continuous variable, P for interaction=0.77 (Figure 1 and Figure II in the Data Supplement).
Table 2.
Efficacy of Dapagliflozin on the Primary and Secondary Outcomes According to Baseline eGFR
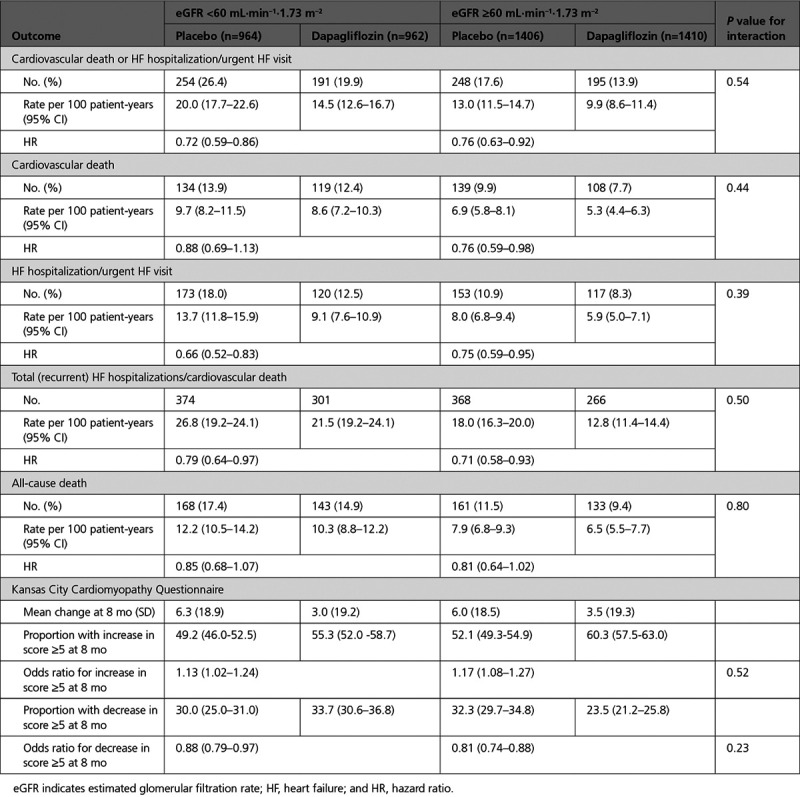
Figure 1.
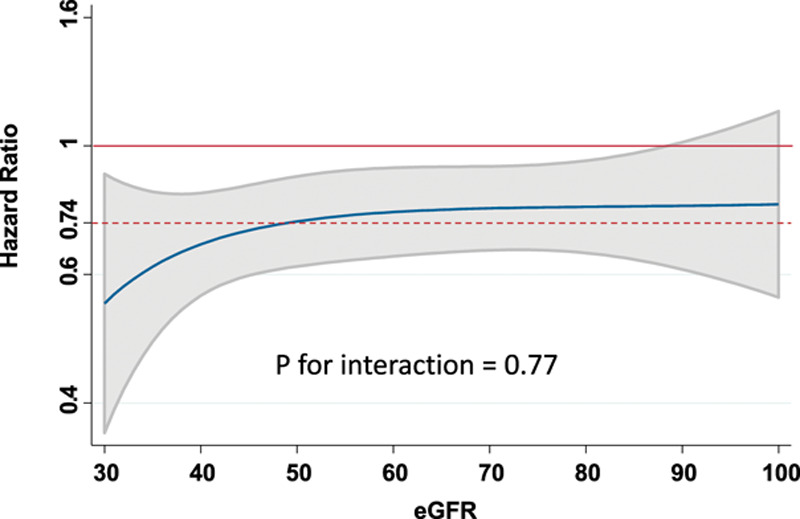
Effect of dapagliflozin on the primary outcome by eGFR at baseline. The blue line represents continuous hazard ratio, and the gray area represents the 95% CI with the overall hazard ratio for the effect of dapagliflozin on the primary outcome given by the dashed red line. eGFR indicates estimated glomerular filtration rate.
Applying the overall relative risk reduction (26%) to the placebo group event rate in those with an eGFR of <60 mL·min–1·1.73 m–2 gave a reduction with dapagliflozin of 52 fewer patients experiencing a primary outcome per 1000 person-years of follow-up. The equivalent absolute risk reduction in patients ≥60 mL·min–1·1.73 m–2 was estimated as 34 fewer patients per 1000 person-years of follow-up. The corresponding reductions in all-cause mortality were 21 and 13, respectively, per 1000 person-years of follow-up.
The proportion of patients with a ≥5 point deterioration in Kansas City Cardiomyopathy Questionnaire score (worsening) was lower in those randomly assigned to dapagliflozin, and the proportion of patients with a ≥5 point improvement in Kansas City Cardiomyopathy Questionnaire score (improvement) was higher in those randomly assigned to dapagliflozin, irrespective of baseline eGFR (Table 2).
Renal Outcomes
Prespecified Composite Renal Outcome
The incidence of the prespecified renal composite outcome was higher in the patients with lower eGFR at baseline than in those with an eGFR ≥60 mL·min–1·1.73 m–2 (Table 3). Although the rate was lower in those randomly assigned to dapagliflozin, the difference was not statistically significant (hazard ratio, 0.71 [95% CI, 0.44–1.16]; P=0.17; Table 3 and Figure 2). The major components of the composite were ≥50% decline in eGFR and the need for sustained dialysis. Although there were fewer patients with a ≥50% decline in eGFR in the dapagliflozin group (n=14) than in the placebo group (n=23), the number of patients started on dialysis was identical in the 2 treatment groups (n=16). No patient received a kidney transplant.
Table 3.
Renal Composite Outcome and Its Components in the DAPA-HF Trial
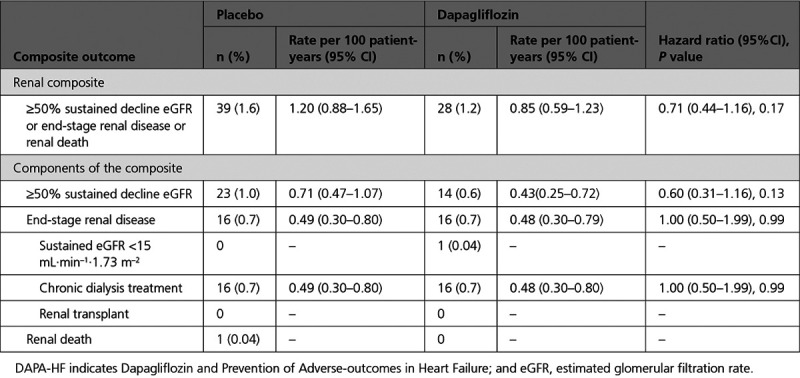
Figure 2.
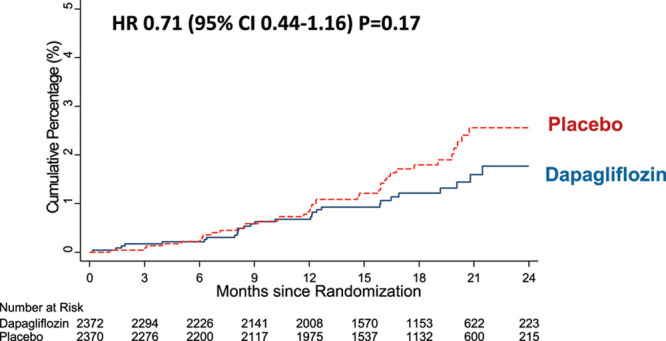
Effect of dapagliflozin on the prespecified renal composite outcome. Renal outcome was a composite of ≥50% sustained decline estimated glomerular filtration rate or end-stage renal disease or renal death in DAPA-HF (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure). HR indicates hazard ratio.
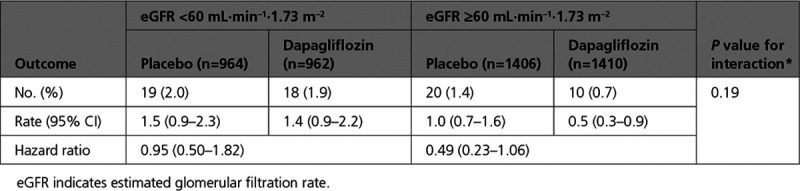
There was no interaction between eGFR group and the effect of dapagliflozin on the renal composite outcome, P for interaction=0.19 (Table 4 and Figure III in the Data Supplement). There were too few events to do a meaningful analysis of the components of the renal composite outcome according to eGFR category.
Table 4.
Renal Composite Outcome by Baseline eGFR
There was also no interaction between baseline diabetes status and the effect of dapagliflozin on the renal composite outcome, P for interaction between baseline diabetes and the effect of randomized treatment=0.87 (Figure IV in the Data Supplement).
Doubling of Serum Creatinine
This prespecified exploratory outcome of doubling of serum creatinine relative to the last obtained laboratory value occurred in 43 patients (1.8%) in the dapagliflozin group and 77 patients (3.2%) in the placebo group (hazard ratio, 0.56 [95% CI, 0.39–0.82]; P=0.003; Figure V in the Data Supplement). In those with an eGFR ≥60 mL·min–1·1.73 m–2, 26 patients (1.8%) and 41 (2.9%) patients in the dapagliflozin and placebo groups, respectively, had a doubling of serum creatinine (hazard ratio, 0.62 [95% CI, 0.38–1.01]); in those with an eGFR <60 mL·min–1·1.73 m–2 the numbers were 17 (1.8%) and 36 (3.7%), respectively (hazard ratio, 0.74 [95% CI, 0.26–0.83]; P for interaction=0.74).
Change in eGFR Over Time
Kidney function declined over time in both the placebo group and the dapagliflozin group (Figure 3). There was a small initial decrease in eGFR related to the introduction of dapagliflozin, demonstrated by the change from baseline to day 14. However, after day 14, the rate of decline was steeper in the placebo group than in the dapagliflozin group. Between day 14 and day 720, the change in eGFR in the dapagliflozin group was about one-third of that in the placebo group: change in eGFR mL·min–1·1.73 m–2 per year in the dapagliflozin group –1.09 (95% CI, –1.40 to –0.77) and in the placebo group –2.85 (95% CI, –3.17 to –2.53), P for difference in slopes<0.001. The results from the exploratory joint model where eGFR was modeled jointly with all-cause mortality were not different than the prespecified slope analyses. In patients with and without type 2 diabetes at baseline, we observed similar changes in eGFR over time in the dapagliflozin and placebo groups (P for interaction=0.92; Figure 4).
Figure 3.
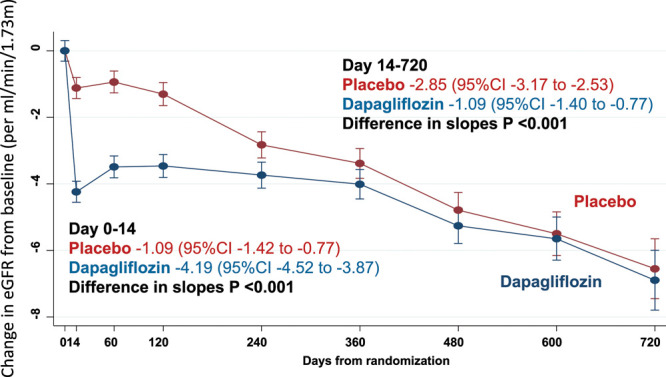
Effect of dapagliflozin on change in eGFR from baseline in DAPA-HF (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure). Slope in eGFR from day 0 to 14 from baseline is the slope per mL·min–1·1.73 m–2 for over 14 days and from 14 to 720 days expressed as a slope per mL·min–1·1.73 m–2 per year. eGFR indicates estimated glomerular filtration rate.
Figure 4.
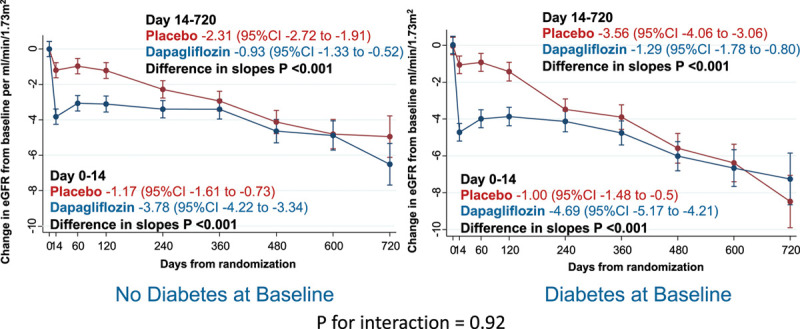
Effect of dapagliflozin, by baseline diabetes status on eGFR. Effect of dapagliflozin by baseline diabetes status (includes 82 patients taking dapagliflozin and 74 patients on placebo with previously undiagnosed diabetes, that is, 2 hemoglobin A1c ≥6.5% [≥48 mmol/mol]) on change in eGFR from baseline in DAPA-HF (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure). Slope in eGFR from day 0 to 14 from baseline is the slope per mL·min–1·1.73 m–2 per over 14 days and from 14 to 720 days expressed as a slope per mL·min–1·1.73 m–2 per year. eGFR indicates estimated glomerular filtration rate.
The same pattern was observed in patients with an eGFR <60 or ≥60 mL·min–1·1.73 m–2 (Figure VI in the Data Supplement).
Safety and Adverse Events
In patients exposed to at least 1 dose of study drug, there were fewer renal adverse events in the group randomly assigned to dapagliflozin: 153 (6.5%) versus 170 (7.2%) in the placebo group (P=0.36). Serious renal adverse events occurred significantly less frequently in those randomly assigned to dapagliflozin: 38 (1.6%) versus 65 (2.7%) in the placebo group (P=0.009). There was no difference in the numbers of individuals stopping study drug because of a renal adverse event (8 in the dapagliflozin group, 9 in the placebo group). Other prespecified safety outcomes are shown in Table 5.
Table 5.
Safety and Tolerability of Dapagliflozin by Baseline eGFR Group
Discussion
In DAPA-HF, the benefits of dapagliflozin on the primary and secondary cardiovascular outcomes were consistent in patients with and without low eGFR, with greater absolute risk reductions in patients with lower eGFR. Although the incidence of the renal composite outcome was numerically lower in patients treated with dapagliflozin, in comparison with placebo, the difference between treatments was not statistically significant. However, dapagliflozin did reduce the risk of doubling serum creatinine relative to the last laboratory measure and of serious renal adverse events, and it attenuated the decrease in eGFR over time in comparison with placebo. This slowing of eGFR decline was observed in patients with and without low eGFR and in those with and without type 2 diabetes.
That the benefits of dapagliflozin on the primary and secondary cardiovascular outcomes were consistent in patients with and without low eGFR is important, because these patients are at much higher risk than patients with preserved kidney function (as observed in this study) and often cannot be treated with alternative life-saving therapies.17 Specifically, underutilization of renin-angiotensin system blockers and mineralocorticoid receptor antagonists is well recognized in patients with CKD and, more recently, evidence has been presented that the benefits of β-blockers are attenuated in patients with marked reductions in eGFR.18 Consequently, any treatment that is effective in these high-risk individuals, and well-tolerated, is a potentially important advance in their care. In addition to being effective, dapagliflozin appeared to have an acceptable safety profile in people with CKD. Although patients with CKD experienced more adverse effects of all types than patients without CKD, they did not experience more adverse effects with dapagliflozin in comparison with placebo. Similarly, patients with CKD were more likely to stop study drug than those without CKD, but patients with CKD were no more likely to stop dapagliflozin than placebo, and only ≈13% of patients discontinued dapagliflozin for any reason during follow-up. Our tolerability and safety findings are consistent with those of the CREDENCE trial (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy), the first trial to exclusively enroll patients with type 2 diabetes and CKD8 and the more recent DAPA-CKD trial (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease).19,20
When DAPA-HF was designed initially, the renal benefits of SGLT2 inhibitors had not been established and there was uncertainty about the renal safety of using these agents in HFrEF. We knew that SGLT2 inhibitors had diuretic activity and caused a small decline in eGFR. Therefore, adding a SGLT2 inhibitor on top of conventional diuretics, renin-angiotensin system blockers, and mineralocorticoid receptor antagonists in these patients was a potential concern, especially because many were expected to have CKD at baseline. These concerns were not realized. Although we did not observe a statistically significant reduction in the prespecified renal composite outcome with dapagliflozin, there were a few of these events in DAPA-HF. The effect of dapagliflozin on this outcome was broadly consistent with the effect of other SGLT2 inhibitors on similar composite renal outcomes in trials including patients with type 2 diabetes, taking account of baseline kidney function and duration of follow-up, which are major determinants of the number of renal events observed. Moreover, in the recent EMPEROR-Reduced trial (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction), SGLT2 inhibition did reduce renal events significantly, although that trial used a different renal composite outcome and had more renal events than in DAPA-HF (88 versus 67).21
Although we did not observe a statistically significant reduction in the prespecified renal composite outcome, dapagliflozin did reduce the risk of the doubling of serum creatinine concentration relative to the last visit and serious renal adverse events. The reduced risk of doubling of creatinine concentration is notable, given that previous studies have shown that worsening kidney function identified by even modest increases in creatinine (or equivalent reductions in eGFR) are associated with worse cardiovascular outcomes in patients with HFrEF.3,17,18,22
We also observed a significant reduction in the rate of eGFR decline over time in the dapagliflozin group, an analysis for which we had more statistical power. The rate of decline in eGFR in patients in the placebo group in DAPA-HF was 2.87 (95% CI, 3.19–2.55) mL·min–1·1.73 m–2 per year, which was steeper than in CANVAS (Canagliflozin Cardiovascular Assessment Study; 0.85 mL) and EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; 1.67 mL), but, as expected, less than in CREDENCE (4.7 mL).
More recently, in a large trial using empagliflozin in patients with HFrEF, the rate of decline in eGFR was 2.28 (SD 0.23) mL·min–1·1.73 m–2 per year, which was reduced to 0.55 (0.23) in the SGLT2 inhibitor group.21 Another relevant comparison is the PARADIGM-HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). The annualized rate of decline in eGFR in the enalapril (control) group was 2.04 (2.21–1.88) mL·min–1·1.73 m–2, in comparison with 1.61 (1.77–1.44) in the sacubitril/valsartan group; for comparison, the rate of decline in eGFR in DAPA-HF was reduced to 1.09 (1.41–0.78) mL·min–1·1.73 m–2 per year. Because these 2 treatments are believed to work through distinct and likely complementary mechanisms, it is possible that they might have additive renal benefits in patients with HFrEF.
As expected, our patients with diabetes had a more rapid rate of decline in eGFR than patients without diabetes. We have reported that the benefits of dapagliflozin on cardiovascular outcomes were consistent in patients with and without type 2 diabetes in DAPA-HF.23 The current data extend these findings to the effect of SGLT2 inhibitors on kidney function, providing, we believe, the first evidence that SGLT2 inhibitors may have favorable renal effects in individuals without type 2 diabetes. Other studies of the renal effects of SGLT2 inhibitors in individuals without type 2 diabetes have been small with relatively short follow-up and did not demonstrate a significant effect on albuminuria.24 However, the protective effect of dapagliflozin in patents with CKD, but without diabetes, has been clearly demonstrated by the DAPA-CKD trial (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) and more data on the renal protective effects of SGLT2 inhibitors in individuals without type 2 diabetes will be provided by EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin; URL: https://www.clinicaltrials.gov; Unique identifier: NCT03594110).
The mechanism of the favorable effect of dapagliflozin on eGFR in DAPA-HF is unknown. Although it may be the same as speculated in patients with type 2 diabetes (reduction in intraglomerular pressure attributable to enhanced tubulo-glomerular feedback),24,26–30 it is also possible that prevention of worsening of heart failure may play a role, as appears to be the case with sacubitril/valsartan.31–32 It is likely that there is a detrimental bidirectional interplay between worsening heart failure and worsening renal function in HFrEF, and the preservation of kidney function in these patients is important.
Limitations
The most important limitation of the present analyses is the small number of renal end points that limited our ability to detect a benefit of dapagliflozin on renal outcomes in this population. We are unable to determine the efficacy and safety of dapagliflozin at eGFR levels of <30 mL·min–1·1.73 m–2 because these patients were excluded from the trial. However, our data are consistent with other trials that have enrolled patients with eGFR down to 30 mL·min–1·1.73 m–2. The longer-term trends in eGFR are limited by the relatively short follow-up in trial (median follow-up was 18.2 months). However, our results are consistent with previous trials. Only serious adverse events of interest were collected, and, therefore, we do not have data on more common nonserious adverse events such as mycotic genital infections. Urinary albumin was not collected and therefore we were unable to assess the relationship with other markers of kidney function such as urinary albumin:creatinine ratio. Last, our data do not provide any further information on how the SGLT2 inhibitors preserve kidney function, although they do confirm that the benefits extend to those without type 2 diabetes and those with HFrEF.
Conclusion
In patients with HFrEF, morbidity, mortality, and symptoms were improved by dapagliflozin, in comparison with placebo, regardless of baseline kidney function. The favorable safety and tolerability profile of dapagliflozin in comparison with placebo was not altered by baseline kidney function, and kidney function declined more slowly in patients who received dapagliflozin.
Sources of Funding
The DAPA-HF trial (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure) was funded by AstraZeneca. Dr McMurray is supported by a British Heart Foundation Center of Research Excellence Grant RE/18/6/34217.
Disclosures
Dr Jhund’s employer the University of Glasgow has been remunerated by AstraZeneca for working on the DAPA-HF (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure) and DELIVER trial (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) and speakers fees from AstraZeneca and grants from Boehringer Ingelheim. Dr Solomon reports grants from AstraZeneca, Bellerophon, Celladon, Ionis, Lone Star Heart, Mesoblast, National Institutes of Health/National Heart, Lung, and Blood Institute, Sanofi Pasteur, and Eidos; grants and personal fees from Alnylam, Amgen, AstraZeneca, BMS, Gilead, GSK, MyoKardia, Novartis, Theracos, Bayer, and Cytokinetics; and personal fees from Akros, Corvia, Ironwood, Merck, Roche, Takeda, Quantum Genomics, AoBiome, Janssen, Cardiac Dimensions, Tenaya, and Daichi-Sankyo. Dr Docherty’s employer the University of Glasgow has been remunerated by AstraZeneca for working on the DAPA-HF trial. Dr Anand reported receiving personal fees from AstraZeneca during the conduct of the study and personal fees from Amgen, ARCA, Boston Scientific Corporation, Boehringer Ingelheim, LivaNova, and Zensun outside the submitted work. Dr Heerspink reports consulting for AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundi Pharma, and Retrophin, with a policy that honoraria are paid to his employer. Dr de Boer reported receiving grants from Abbott, Bristol-Myers Squibb, and Novo Nordisk; personal fees from Abbott; and grants and personal fees from Novartis, Roche, and AstraZeneca outside the submitted work. Dr Desai reported receiving personal fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, Merck, Regeneron, and Relypsa and grants and personal fees from AstraZeneca, Alnylam, and Novartis outside the submitted work. Dr Kitakaze reported receiving grants and personal fees from AstraZeneca during the conduct of the study and grants from the Japanese government, the Japan Heart Foundation, and the Japan Agency for Medical Research and Development; grants and personal fees from Asteras, Sanofi, Pfizer, Ono, Novartis, Tanabe-Mitubishi, and Takeda; and personal fees from Daiichi Sankyo, Bayer, Behringer, Kowa, Sawai, Merck Sharp & Dohme, Shionogi, Kureha, Japan Medical Data, Taisho-Toyama, and Toa Eiyo outside the submitted work. Dr Merkely reported receiving personal fees from AstraZeneca and Servier. Dr O’Meara reported consultation and speaker fees being paid to the Montreal Heart Institute Research Center from Amgen, Merck, and Novartis; receiving consultation and speaker fees from AstraZeneca, Bayer, and Boehringer Ingelheim; serving on a steering committee and as a national leader for clinical studies with fees paid to Montreal Heart Institute Research Center from American Regent, AstraZeneca, Cytokinetics, Merck, and Novartis; and clinical trial participation from Amgen, Abbott, American Regent, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eidos, Novartis, Merck, Pfizer, and Sanofi. Dr Schou reported receiving personal fees and nonfinancial support from AstraZeneca and personal fees from Novo Nordisk and Boehringer Ingelheim. Dr Tereshchenko reports personal fees from Servier, AstraZeneca, Pfizer, Novartis, and Boehringer Ingelheim. Dr Verma received financial support from AstraZeneca for the conduct of DAPA-HF at his institute. He has also received grants and personal fees for speaker honoraria and advisory board participation from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, and Merck. He has received grants and personal fees for advisory board participation from Amgen, grants from Bristol-Myers Squibb, personal fees for speaker honoraria and advisory board participation from Eli Lilly, Novo Nordisk, and Sanofi, and personal fees for speaker honoraria from EOCI Pharmacomm Ltd, Novartis, Sun Pharmaceuticals, and Toronto Knowledge Translation Working Group. Dr Inzucchi reports personal fees and nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi/Lexicon, Merck, VTV Therapeutics, and Abbott/Alere, as well as personal fees from AstraZeneca and Zafgen. Dr Køber reports other support from AstraZeneca and personal fees from Novartis and Bristol-Myers Squibb as a speaker. Dr Kosiborod reports personal fees from AstraZeneca; grants, personal fees, and other from AstraZeneca; grants and personal fees from Boehringer Ingelheim; and personal fees from Sanofi, Amgen, NovoNordisk, Merck (Diabetes), Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr Martinez reports personal fees from AstraZeneca. Dr Ponikowski reports personal fees and other from AstraZeneca, Boehringer Ingelheim, Bayer, BMS, Cibiem, Novartis, and RenalGuard; personal fees from Pfizer, Servier, Respicardia, and Berlin-Chemie; other from Amgen; and grants, personal fees, and other from Vifor Pharma. Dr Sabatine reports grants from Bayer, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Pfizer, Poxel, Quark Pharmaceuticals, and Takeda; grants and personal fees from Amgen, AstraZeneca, Intarcia, Janssen Research and Development, The Medicines Company, MedImmune, Merck, and Novartis; and personal fees from Anthos Therapeutics, Bristol-Myers Squibb, CVS Caremark, DalCor, Dynamix, Esperion, IFM Therapeutics, and Ionis. Dr Sabatine is a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women’s Hospital from Abbott, Aralez, Roche, and Zora Biosciences. Drs Langkilde, Bengtsson, and Sjöstrand are full-time employees of AstraZeneca. Dr McMurray s employer the University of Glasgow has been remunerated by AstraZeneca for working on the DAPA-HF and DELIVER trial, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, Abbvie, Novartis, Glaxo Smith Kline, Vifor-Fresenius, Kidney Research UK, and Novartis, and other support from Bayer, DalCor, Pfizer, Merck, Bristol Myers, and Squibb, as well.
Supplemental Materials
Data Supplement Figures I–VI
Supplementary Material
Footnotes
Sources of Funding, see page 307
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.120.050391.
References
- 1.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014; 35:455–469. doi: 10.1093/eurheartj/eht386 [DOI] [PubMed] [Google Scholar]
- 2.Damman K, Masson S, Lucci D, Gorini M, Urso R, Maggioni AP, Tavazzi L, Tarantini L, Tognoni G, Voors A, et al. Progression of renal impairment and chronic kidney disease in chronic heart failure: an analysis from GISSI-HF. J Card Fail. 2017; 23:2–9. doi: 10.1016/j.cardfail.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 3.Beldhuis IE, Streng KW, Ter Maaten JM, Voors AA, Van Der Meer P, Rossignol P, McMurray JJV, Damman K. Renin-angiotensin system inhibition, worsening renal function, and outcome in heart failure patients with reduced and preserved ejection fraction: a meta-analysis of published study data. Circ Hear Fail. 2017; 10:e003588 doi: 10.1161/CIRCHEARTFAILURE.116.003588 [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. doi: 10.1056/NEJMc1600827 [DOI] [PubMed] [Google Scholar]
- 5.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 6.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. ; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019; 380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 7.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39. doi: 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. ; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. ; DAPA-HF Committees and Investigators. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail. 2019; 21:665–675. doi: 10.1002/ejhf.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. ; DAPA-HF Committees and Investigators. The Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail. 2019; 21:1402–1411. doi: 10.1002/ejhf.1548 [DOI] [PubMed] [Google Scholar]
- 12.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000; 35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 13.Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020; 141:90–99. doi: 10.1161/CIRCULATIONAHA.119.044138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royston P, Sauerbrei W. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med. 2004; 23:2509–2525. doi: 10.1002/sim.1815 [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Ser B (Stat Methodol). 2000; 62:711–730. doi: 10.1093/biostatistics/kxv050 [Google Scholar]
- 16.Vonesh E, Tighiouart H, Ying J, Heerspink HL, Lewis J, Staplin N, Inker L, Greene T. Mixed-effects models for slope-based endpoints in clinical trials of chronic kidney disease. Stat Med. 2019; 38:4218–4239. doi: 10.1002/sim.8282 [DOI] [PubMed] [Google Scholar]
- 17.Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, McMurray JJ. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol. 2014; 63:853–871. doi: 10.1016/j.jacc.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 18.Kotecha D, Gill SK, Flather MD, Holmes J, Packer M, Rosano G, Böhm M, McMurray JJV, Wikstrand J, Anker SD, et al. ; Beta-Blockers in Heart Failure Collaborative Group. Impact of renal impairment on beta-blocker efficacy in patients with heart failure. J Am Coll Cardiol. 2019; 74:2893–2904. doi: 10.1016/j.jacc.2019.09.059 [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJV, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa-Rotter R, Chertow GM, Greene T, Held C, Hou FF, et al. ; DAPA-CKD Trial Committees and Investigators. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease [published online November 13, 2020]. Circulation. doi: 10.1161/CIRCULATIONAHA.120.051675 [DOI] [PubMed] [Google Scholar]
- 20.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. ; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 21.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–1424. doi: 10.1056/NEJMoa2022190. https://www.nejm.org/doi/10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 22.Damman K, Solomon SD, Pfeffer MA, Swedberg K, Yusuf S, Young JB, Rouleau JL, Granger CB, McMurray JJ. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM-study programme. Eur J Heart Fail. 2016; 18:1508–1517. doi: 10.1002/ejhf.609 [DOI] [PubMed] [Google Scholar]
- 23.Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020; 323:1353–1368. doi: 10.1001/jama.2020.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, Laverman GD, Lim SK, Di Tanna GL, Reich HN, et al. ; DIAMOND investigators. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020; 8:582–593. doi: 10.1016/S2213-8587(20)30162-5 [DOI] [PubMed] [Google Scholar]
- 25.Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, Lindberg M, McMurray J, Rossing P, Toto R, et al. ; DAPA-CKD Investigators. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant. 2020; 35:274–282. doi: 10.1093/ndt/gfz290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 75:422–434. doi: 10.1016/j.jacc.2019.11.031 [DOI] [PubMed] [Google Scholar]
- 27.Lee MMY, Petrie MC, McMurray JJV, Sattar N. How do SGLT2 (sodium-glucose cotransporter 2) inhibitors and GLP-1 (glucagon-like peptide-1) receptor agonists reduce cardiovascular outcomes?: completed and ongoing mechanistic trials. Arterioscler Thromb Vasc Biol. 2020; 40:506–522. doi: 10.1161/ATVBAHA.119.311904 [DOI] [PubMed] [Google Scholar]
- 28.Sridhar VS, Rahman HU, Cherney DZI. What have we learned about renal protection from the cardiovascular outcome trials and observational analyses with SGLT2 inhibitors? Diabetes Obes Metab. 2020; 22suppl 155–68. doi: 10.1111/dom.13965 [DOI] [PubMed] [Google Scholar]
- 29.Cherney DZI, Heerspink HJL, Frederich R, Maldonado M, Liu J, Pong A, Xu ZJ, Patel S, Hickman A, Mancuso JP, et al. Effects of ertugliflozin on renal function over 104 weeks of treatment: a post hoc analysis of two randomised controlled trials. Diabetologia. 2020; 63:1128–1140. doi: 10.1007/s00125-020-05133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Bommel EJM, Lytvyn Y, Perkins BA, Soleymanlou N, Fagan NM, Koitka-Weber A, Joles JA, Cherney DZI, van Raalte DH. Renal hemodynamic effects of sodium-glucose cotransporter 2 inhibitors in hyperfiltering people with type 1 diabetes and people with type 2 diabetes and normal kidney function. Kidney Int. 2020; 97:631–635. doi: 10.1016/j.kint.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 31.Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018; 138:1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818 [DOI] [PubMed] [Google Scholar]
- 32.Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018; 6:489–498. doi: 10.1016/j.jchf.2018.02.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


