Supplemental Digital Content is available in the text.
Keywords: diabetes mellitus, empagliflozin, heart failure
Background:
Sodium-glucose cotransporter 2 inhibitors improve outcomes in patients with heart failure with reduced ejection fraction, but additional information is needed about whether glycemic status influences the magnitude of their benefits on heart failure and renal events.
Methods:
Patients with Class II–IV heart failure and a left ventricular ejection fraction ≤40% were randomized to receive empagliflozin (10 mg daily) or placebo in addition to recommended therapy. We prespecified a comparison of the effect of empagliflozin in patients with and without diabetes.
Results:
Of the 3730 patients enrolled, 1856 (50%) had diabetes, 1268 (34%) had prediabetes (hemoglobin A1c [HbA1c] 5.7–6.4%), and 606 (16%) had normoglycemia (HbA1c <5.7%). The risks of the primary outcome (cardiovascular death or hospitalization for heart failure), total hospitalizations for heart failure, and adverse renal outcomes were higher in patients with diabetes, but were similar between patients with prediabetes and normoglycemia. Empagliflozin reduced the risk of the primary outcome in patients with and without diabetes (hazard ratio, 0.72 [95% CI, 0.60–0.87] and 0.78 [95% CI, 0.64–0.97], respectively, P-interaction=0.57). Patients with and without diabetes also did not differ with respect to the effect of empagliflozin on total hospitalizations for heart failure, on the decline in estimated glomerular filtration rate over time, and on the risk of serious adverse renal outcomes. Among these end points, the effects of the drug did not differ in patients with prediabetes or normoglycemia. When analyzed as a continuous variable, baseline HbA1c did not significantly modify the benefits of empagliflozin on the primary outcome (P-interaction=0.40). Empagliflozin did not lower HbA1c in patients with prediabetes or normoglycemia and was not associated with increased risk of hypoglycemia.
Conclusions:
In EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), empagliflozin significantly improved cardiovascular and renal outcomes in patients with heart failure and a reduced ejection fraction, independent of baseline diabetes status and across the continuum of HbA1c.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057977.
Clinical Perspective.
What Is New?
In the placebo-controlled EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), the addition of empagliflozin to recommended heart failure therapy reduced the risk of cardiorenal outcomes in patients with heart failure with reduced ejection fraction with and without diabetes.
The risks of these cardiorenal outcomes were higher in patients with diabetes but were similar between patients with prediabetes and normoglycemia.
These favorable heart failure and renal effects of empagliflozin were consistent in patients with or without diabetes and across the spectrum of A1C.
Empagliflozin did not lower glycohemoglobin in patients without diabetes and was not associated with increased risk of hypoglycemia.
What Are the Clinical Implications?
The heart failure and renal benefits of empagliflozin in patients with heart failure and a reduced ejection fraction are present both in patients with and without diabetes and are not influenced by baseline levels of glycohemoglobin.
Decisions regarding the use of empagliflozin for the treatment of heart failure and a reduced ejection fraction should not be driven by the glycemic status of individual patients.
Editorial, see p 350
Unlike other antihyperglycemic agents, sodium-glucose cotransporter 2 (SGLT2) inhibitors have consistently been shown to reduce the risk of heart failure hospitalizations and serious renal outcomes among patients with diabetes.1–3 These substantial cardio-renal benefits cannot be explained by the antihyperglycemic action of SGLT2 inhibitors. Therefore, it has been suggested that SGLT2 inhibitors exert broad cardioprotective and nephroprotective effects, which would be apparent in patients with or without diabetes.4
In the DAPA-HF trial (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), dapagliflozin was shown to reduce the risk of worsening heart failure events and cardiovascular death independent of diabetes status.5,6 However, the DAPA-HF trial did not comprehensively report the influence of diabetes on the renal effects of dapagliflozin or the influence of prediabetes; furthermore, the trial enrolled primarily patients who had mild to moderate left ventricular systolic dysfunction and increases in natriuretic peptides. EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction) evaluated the effect of empagliflozin in patients with heart failure, including those with more severe left ventricular systolic dysfunction and more severely impaired kidney function, and the trial identified the effect of the drug on renal outcomes as a major outcome variable.7 Empagliflozin reduced the risk of major adverse heart failure and renal outcomes when added to inhibitors of the renin-angiotensin system and β-blockers and regardless of background therapy with mineralocorticoid receptor antagonists and angiotensin receptor/neprilysin inhibitors.8
In this prespecified analysis of EMPEROR-Reduced, we analyzed the efficacy and safety of empagliflozin on heart failure and renal events by baseline diabetes status and across the range of baseline values of glycohemoglobin (HbA1c).
Methods
Trial Design
EMPEROR-Reduced (URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057977) was a randomized, double-blind, parallel-group, placebo-controlled, event-driven study. Patients were recruited into EMPEROR-Reduced between April 25, 2017, and November 8, 2019, at 520 centers in 20 countries. The design and conduct of this trial have been published previously.7 The trial was approved by the ethics committee at each study site and all patients provided written informed consent.
Study Patients
Participants included patients ≥18 years or older who had chronic heart failure (New York Heart Association functional Class II, III, or IV) with a left ventricular ejection fraction ≤40%. To enroll patients at increased risk of events, the number of patients with an ejection fraction >30% was limited by requiring that they had been hospitalized for heart failure within 12 months or had exceptionally high levels of NT-proBNP (N-terminal prohormone B-type natriuretic peptide; ie, >1000 pg/mL or >2500 pg/mL in those with an ejection fraction of 31% to 35% or 36% to 40%, respectively); these thresholds were doubled in patients with atrial fibrillation. Patients were receiving all appropriate treatments for heart failure as available and tolerated. Exclusion criteria included symptomatic hypotension, systolic blood pressure of <100 mm Hg or ≥180 mm Hg, or an estimated glomerular filtration rate (eGFR) <20 mL/min/1.73 m2. After a 4- to 28-day screening period, patients who fulfilled eligibility criteria were randomized double-blind (in a 1:1 manner) to receive placebo or empagliflozin 10 mg daily in addition to their usual therapy for heart failure.
For this prespecified subgroup analysis, patients were categorized as having diabetes if they had a history of the diagnosis or if 1 pretreatment HbA1c was at least 6.5% (≥48 mmol/mol). Among those without diabetes, patients were classified as having prediabetes if they had an HbA1c of 5.7% to 6.4%, and they were considered to have normoglycemia if they had all pretreatment HbA1c of <5.7%. Randomization was stratified on the basis of glycemic status at screening (diabetes, prediabetes, or normoglycemia), geographic region (North America, Latin America, Europe, Asia, other), and eGFR (eGFR by the Chronic Kidney Disease Epidemiology Collaboration at screening < or ≥60 mL/min/1.73 m2). After randomization, patients were periodically evaluated for efficacy, vital signs, laboratory tests, and adverse events. These assessments included the Kansas City Cardiomyopathy Questionnaire (KCCQ), systolic blood pressure, hemoglobin, body weight, HbA1c, NT-proBNP, and renal function.
Trial End Points
The primary end point of EMPEROR-Reduced was the time-to-first-event analysis of the combined risk of cardiovascular death or hospitalization for heart failure. This analysis was based on adjudicated events, as assessed by a clinical event committee, which applied prespecified definitions and was blinded to the treatment assignment. The key secondary end points of the study were the total number of adjudicated hospitalizations for heart failure (including first and recurrent events) and the slope of the change in eGFR during double-blind treatment. We prespecified 2 additional assessments of renal function. First, in 966 patients, we analyzed eGFR 23 to 45 days after the withdrawal of the study medication at the end of double-blind treatment. Changes in eGFR from prerandomization to the off-treatment visit allowed for an assessment of the long-term effects of treatment on renal function unconfounded by the presence of an SGLT2 inhibitor. Second, a composite renal end point was defined as the need for chronic dialysis or renal transplant or a ≥40% decrease in eGFR or a sustained eGFR <15 mL/min/1.73 m2 (if the baseline eGFR was ≥30) or <10 mL/min/1.73 m2 (if the baseline eGFR was <30 mL/min/1.73 m2). We evaluated the effects of treatment on the individual components of the primary end point.
Changes between the treatment groups in the KCCQ clinical summary score, body weight, blood pressure, hemoglobin and glycohemoglobin, and NT-proBNP were assessed at 52 weeks. Safety analyses included serious adverse events, adverse events leading to discontinuation of study drug, and specified adverse events of interest (hypotension, volume depletion, hypoglycemia, diabetic ketoacidosis, limb amputations, fractures, acute renal failure, and genital and urinary tract infections).
Statistical Analyses
For time to first event analyses, differences between the placebo and empagliflozin groups for the primary end point were assessed for statistical significance using a Cox proportional hazards model, with prespecified covariates of age, sex, geographical region, diabetes status at baseline, left ventricular ejection fraction, and estimated glomerular filtration rate at baseline. These analyses were performed according to the intention-to-treat principle for all randomized patients and included data up to the end of the planned treatment period. For the analysis of total (first and repeated) events, between-group differences were assessed using a joint frailty model, with cardiovascular death as a competing risk. For the analysis of changes in eGFR, KCCQ scores, vital signs, and laboratory measurements, treatment effects were assessed based on changes from baseline using a mixed model for repeated measures. Between-group difference in the slope of change in eGFR were analyzed using a random intercept random slope model. KCCQ as well as eGFR slope and mixed model for repeated measures were analyzed using on-treatment data. The mixed model for repeated measures, the slope model, and the joint frailty model included the same covariates as the Cox model. To assess the consistency of effects across subgroups, subgroup-by-treatment interaction terms were added in the models. Analyses for safety were performed including all the patients who had received at least 1 dose of empagliflozin or placebo.
According to our prespecified statistical plan, analyses of the influence of glycemic status on the effect of empagliflozin on various outcomes and measurements were made primarily by comparing patients with or without diabetes. Analyses were performed to evaluate and compare the effect of empagliflozin in patients with diabetes, prediabetes, and normoglycemia (which were stratification variables in the trial). We also evaluated in exploratory analyses the effect of baseline HbA1c (as a continuous variable) on the effect of empagliflozin on first heart failure hospitalization or cardiovascular death assuming a linear relationship.
All analyses were performed using SAS, version 9.4 (SAS Institute). All P values reported are 2-sided, and P<0.05 was considered as statistically significant in all cases. No adjustments for multiple testing were made.
Data Sharing
Data will be made available on request in adherence with transparency conventions in medical research and through requests to the corresponding author. The executive committee of EMPEROR has developed a comprehensive analysis plan and numerous prespecified analyses, which will be presented in future scientific meetings and publications. At a later time point, the full database will be made available in adherence with the transparency policy of the sponsor (available at https://trials.boehringer-ingelheim.com/transparency_policy.html).
Results
Of the 3730 patients who were randomly assigned to receive either placebo or empagliflozin, 1856 (50%) had diabetes, and of the patients without diabetes, 1268 (34%) had prediabetes and 606 (16%) were normoglycemic. When compared with patients without diabetes, those with diabetes were more likely to have a history of hospitalization for heart failure, New York Heart Association functional Class III symptoms, and a history of hypertension, and a higher proportion had eGFR<60 mL/min/1.73 m2 (Table 1). Patients with prediabetes had baseline characteristics similar to those with normoglycemia (Table I in the Data Supplement). The mean HbA1c was 7.4±1.6% in patients with patients with diabetes, 5.9±0.2% in patients with prediabetes, and 5.3±0.3% in normoglycemic patients. A total of 7.2% of the patients were found to have previously undiagnosed diabetes at baseline. Within each group, the baseline characteristics of the placebo and empagliflozin groups were well-balanced (Table 1 and Table I in the Data Supplement). The median duration of follow-up of 16 months was similar in the 3 groups.
Table 1.
Baseline Characteristics by Diabetes Status
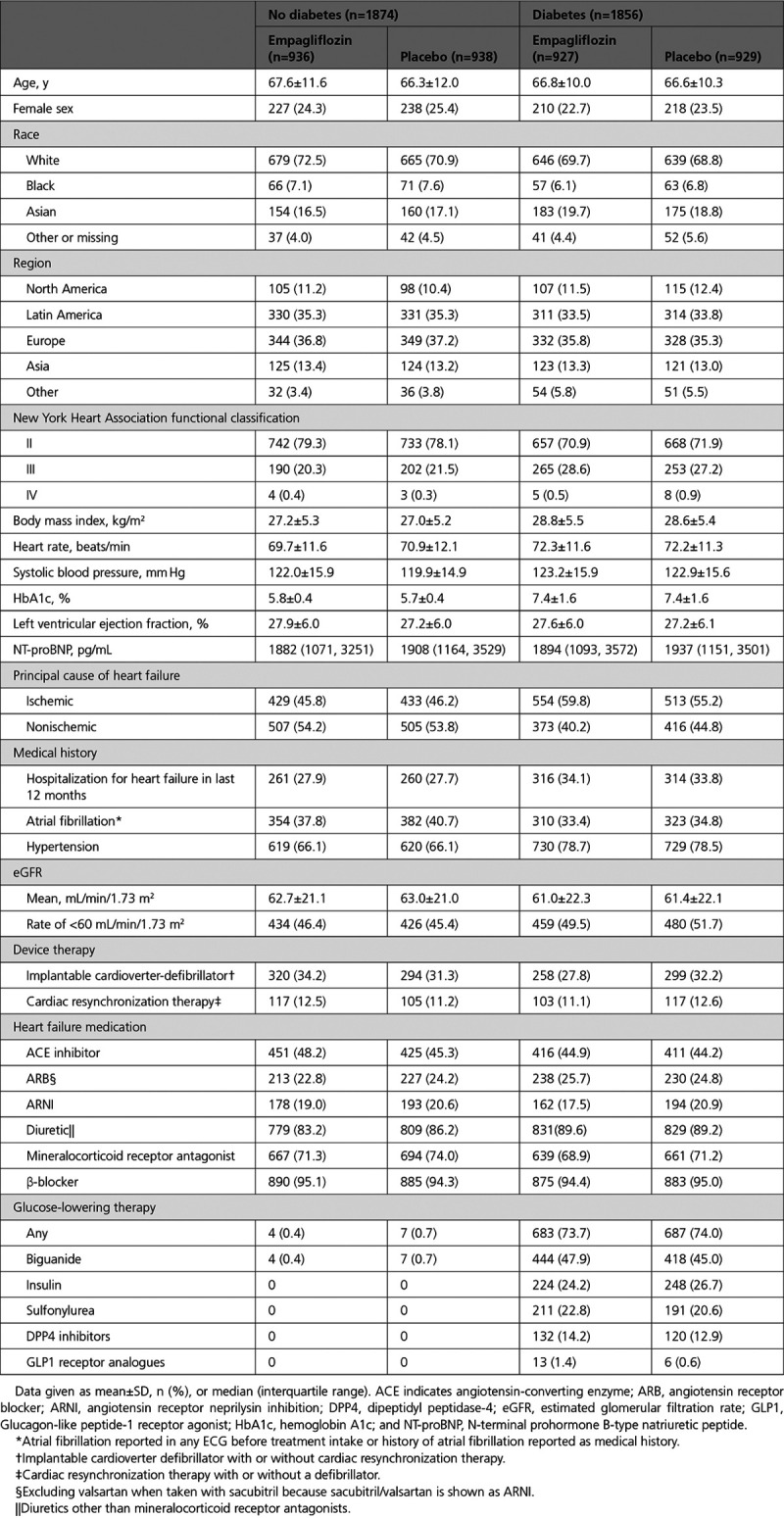
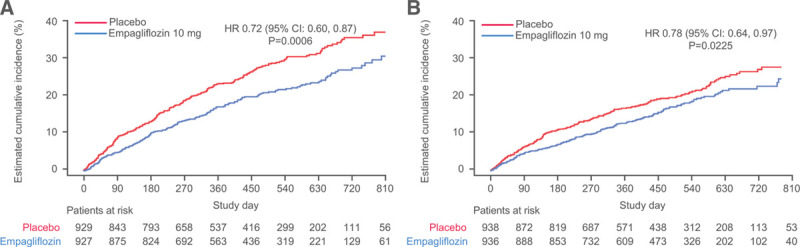
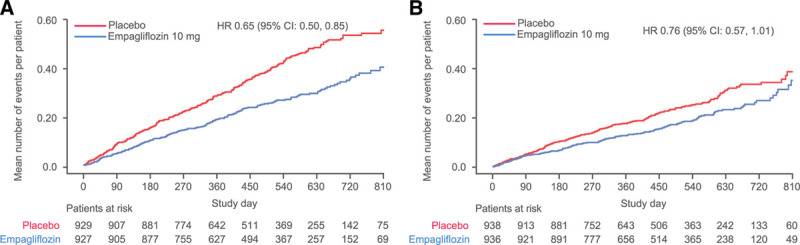
The cumulative incidence curves for the primary outcome and the 2 key secondary outcomes by diabetes status are shown in Figures 1–3. Figure 4 shows the cumulative incidence curves for the renal composite by diabetes status, whereas Figure 5 shows treatment effect of empagliflozin versus placebo on outcomes by glycemic status.
Figure 1.
Effect of empagliflozin on the primary end point of EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction). Time to first event of either cardiovascular death or heart failure hospitalization in (A) patients with diabetes and (B) patients without diabetes. HR indicates hazard ratio.
Figure 3.
Effect of empagliflozin on first and recurrent hospitalizations for heart failure. Effect of empagliflozin on first and recurrent hospitalizations for heart failure in (A) patients with diabetes and (B) patients without diabetes.
Figure 4.
Effect of empagliflozin on renal composite end point. Effect of empagliflozin on renal composite end point in (A) patients with diabetes and (B) patients without diabetes. Composite renal end point is defined as chronic dialysis, renal transplant, sustained reduction of ≥40% eGFR, or sustained eGFR <15 mL/min/1.73 m2 for patients with eGFR ≥30 mL/min/1.73 m2 at baseline (<10 mL/min/1.73 m2 for patients with eGFR <30 mL/min/1.73 m2 at baseline). Dialysis is regarded as chronic if the frequency of dialysis is twice or more per week for at least 90 days. In accordance with usual practice, cumulative incidence plots were truncated when the number of patients being followed in individual subgroups became extremely sparse. HR indicates hazard ratio.
Figure 5.
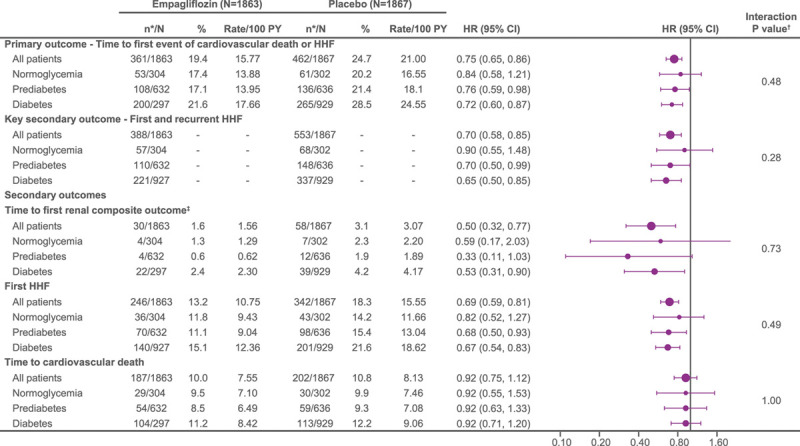
Treatment effect of empagliflozin vs placebo on primary and secondary outcomes in patients with normoglycemia, prediabetes, and diabetes. Recurrent event analyses are based on a joint frailty model accounting for competing risk of cardiovascular death. *n corresponds to number of events in recurrent event analyses and number of patients with event for time-to-first-event analysis. †Interaction P values from trend test assuming ordered categories. The trend test reflects an assumed ordering of the subgroups from normoglycemia to prediabetes to diabetes testing a linear trend across subgroups. ‡Composite renal end point: time to first event of chronic dialysis or renal transplant or sustained reduction of ≥40% estimated glomerular filtration rate (eGFR; Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]cr); or for patients with eGFR (CKD-EPI)cr ≥30 mL/min/1.73 m2 at baseline: sustained eGFR <15 mL/min/1.73 m2; for patients with eGFR (CKD-EPI)cr <30 mL/min/1.73 m2 at baseline: sustained eGFR <10 mL/min/1.73 m2. An eGFR (CDK-EPI)cr reduction is considered sustained if it is determined by 2 or more consecutive postbaseline central laboratory measurements separated by at least 30 days (first to last of the consecutive eGFR values). If there is no additional measurement ≥30 days after the eGFR reduction is observed and the patient dies within 60 days of this measurement, then the eGFR reduction is also considered sustained. HHF indicates hospitalization for heart failure.
Primary Outcome and Total Hospitalizations for Heart Failure
When placebo event rates were considered, the incidence of the primary composite outcome of cardiovascular death or hospitalization for heart failure was ≈40% higher in diabetic patients than in nondiabetic patients (24.6 versus 17.6 per 100 patient-years of follow-up, P<0.001; Figure 1 and Table 2), but there was no difference in risk between patients with prediabetes and those with normoglycemia (18.1 versus 16.6 per 100 patient-years of follow-up, P=0.63; Figure 5).
Table 2.
Primary and Secondary Outcomes in EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction) by Diabetes Status
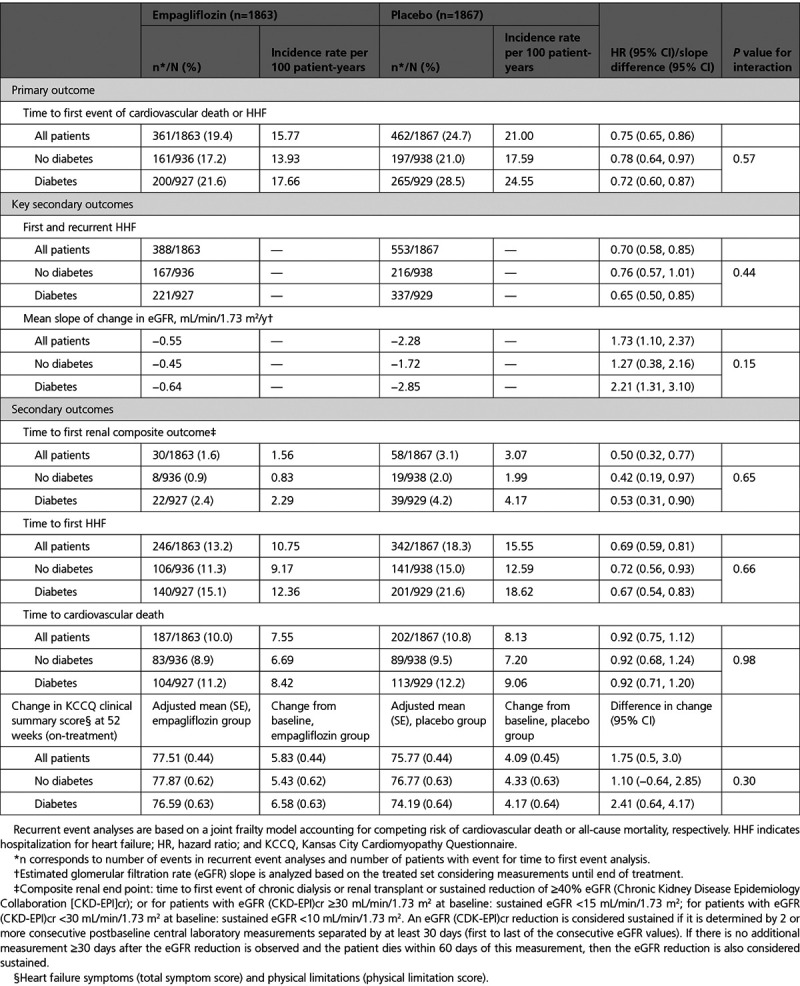
The effect of empagliflozin on the primary outcome variable was not influenced by the presence or absence of diabetes (P-interaction=0.57). In patients with diabetes, the primary outcome occurred in 200 of 927 (21.6%) in the empagliflozin group and 265 of 929 (28.5%) in the placebo group (hazard ratio [HR], 0.72 [95% CI, 0.60–0.87]; Table 2). Among patients without diabetes, the primary outcome occurred in 161 of 936 (17.2%) in the empagliflozin group and 197 of 938 (21%) in the placebo group (HR, 0.78 [95% CI, 0.64–0.97]). Cumulative incidence plots according to treatment are shown for patients with and without diabetes in Figure 1A and 1B. The HRs for the effect of empagliflozin on the risk of cardiovascular death or heart failure hospitalization in patients with prediabetes and in those with normoglycemia were 0.76 (95% CI, 0.59–0.98) and 0.84 (95% CI, 0.58–1.21), respectively (P-trend=0.48); the estimate in patients with normoglycemia was imprecise because it was based on only 114 events (Figure 5). However, when considered as a continuous variable, HbA1c did not influence the effect of empagliflozin on the primary outcome when the relationship was evaluated assuming linearity (P-interaction=0.40; Figure 2).
Figure 2.
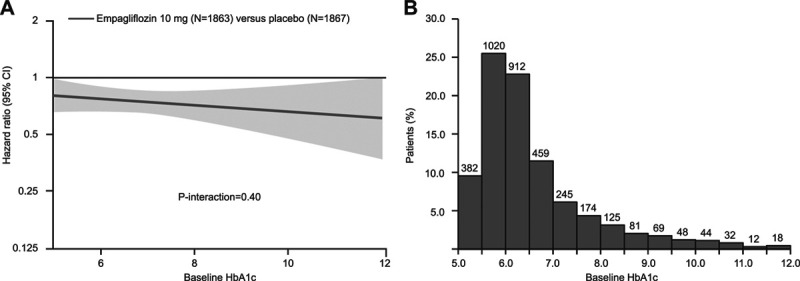
Effect of empagliflozin on the primary outcome of EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction) by baseline glycohemoglobin (HbA1c) as continuous variable and distribution of HbA1c at baseline in the range between 5% and 12%. A, Effect of empagliflozin on the primary outcome of EMPEROR-Reduced by baseline HbA1c as continuous variable. This figure shows the linear association between HbA1c and log hazard ratio for the primary end point. The nonsignificant interaction test (P=0.40) indicates that the slope is not significantly different from zero. However, the display makes assumptions about linearity that are difficult to validate, and the slope is strongly influenced by a relatively small number of patients with extreme values. B, Distribution of HbA1c at baseline in the range between 5% and 12%.
A similar pattern of response was observed for total (first and recurrent) hospitalizations for heart failure. When the placebo event rates were considered, there were 337 hospitalizations for heart failure in the 1856 patients with diabetes, but only 216 in the 1874 patients without diabetes, indicating an ≈50% to 60% higher rate in diabetic patients. The rate of heart failure hospitalization in the patients with prediabetes was similar to that of those with normoglycemia (Figure 5). Empagliflozin reduced the risk of a first or recurrent heart failure hospitalization to a similar degree in both diabetic and nondiabetic patients (HR, 0.65 [95% CI, 0.50–0.85] and 0.76 [95% CI, 0.57–1.01], respectively; Table 2 and Figure 3). Among the patients without diabetes, the HRs for the effect of empagliflozin on total hospitalizations for heart failure in patients with prediabetes and normoglycemia were 0.70 (95% CI, 0.50–0.99) and 0.90 (95% CI, 0.55–1.48), respectively; the latter estimate was imprecise because it was based on only 125 events (Figure 5).
Renal Outcomes
When only the placebo groups were considered, the rate of decline in eGFR in patients with diabetes was nearly twice that in patients without diabetes (–2.9 versus –1.7 mL/min/1.73 m2 per year, P=0.02; Table 2 and Figure I in the Data Supplement), but there was no difference in the rate of decline in eGFR between patients with prediabetes and those with normoglycemia (–1.7 versus –1.8 mL/min/1.73 m2 per year, respectively; Figure I in the Data Supplement). Furthermore, when the study medication was withdrawn to assess the effects of double-blind treatment unconfounded by the presence of an SGLT2 inhibitor, in those allocated to placebo, eGFR declined over a median of 16 months by 3.0 mL/min/1.73 m2 in patients with prediabetes (n=194) and by 3.5 mL/min/1.73 m2 in those with normoglycemia and by 5.4 mL/min/1.73 m2 in patients with diabetes. The incidence of the composite renal outcome was 4.2, 1.9, and 2.2 events per 100 patient-years of follow-up in patients with diabetes, prediabetes, and normoglycemia, respectively (Figure 5). For the latter 2 analyses, the decline in renal function and the incidence of adverse renal outcomes in the placebo group were greater in patients with diabetes, but were similar in patients with prediabetes and normoglycemia.
In light of the more rapid decline in glomerular function in diabetic patients, the magnitude of the effect of empagliflozin to slow the rate of decline in eGFR was somewhat greater in patients with diabetes than in those without diabetes (+2.2 versus +1.3 mL/min/1.73 m2), but the treatment-by-diabetes interaction was not significant (P=0.15). Furthermore, when the study medication was withdrawn to assess the unconfounded effects of double-blind treatment, empagliflozin, as compared with placebo, slowed decline in eGFR by 4.8 mL/min/1.73 m2 in patients with diabetes, by 1.3 mL/min/1.73 m2 in patients with prediabetes, and by 3.1 mL/min/1.73 m2 in patients with normoglycemia (P-trend=0.17), indicating no influence of baseline HbA1c on the ability of empagliflozin to mitigate the progressive decline in renal function during double-blind treatment.
Consistent with the lack of effect modification by baseline HbA1c on eGFR decline, glycemic status also did not modify the benefits of empagliflozin on clinically important renal events. Empagliflozin reduced the risk of the composite renal end point by 47% (HR, 0.53 [95% CI, 0.31–0.90]) in patients with diabetes and by 58% in patients without diabetes (HR, 0.42 [95% CI, 0.19–0.97]), with no significant treatment-by-diabetes interaction (P=0.65; Table 2 and Figure 4). The HRs of the risk reduction in patients with prediabetes and in patients with normoglycemia were 0.33 (95% CI, 0.11–1.03) and 0.59 (95% CI, 0.17–2.03), respectively; the latter estimate was based on only 11 events (Figure 5).
Other Efficacy Measures, Vital Signs, and Laboratory Tests
The effects of empagliflozin on first hospitalization for heart failure and cardiovascular mortality according to glycemic status are shown in Figure 5 and Figure II in the Data Supplement. The magnitude of the treatment effects in patients with or without diabetes was similar for these end points as well as for the change in KCCQ clinical summary score at 52 weeks in an on-treatment analysis (Figure III in the Data Supplement and Table 2).
The effects of empagliflozin on body weight, hemoglobin, systolic blood pressure, and NT-proBNP according to glycemic status are shown in Figures IV–VII in the Data Supplement. The magnitude of the treatment effects in patients with or without diabetes was similar. Empagliflozin lowered HbA1c at 52 weeks only in patients with diabetes, but not in patients with prediabetes or normoglycemia; the treatment-by-diabetes interaction was significant (P-trend=0.033; Figure 6 and Figure VIII in the Data Supplement).
Figure 6.
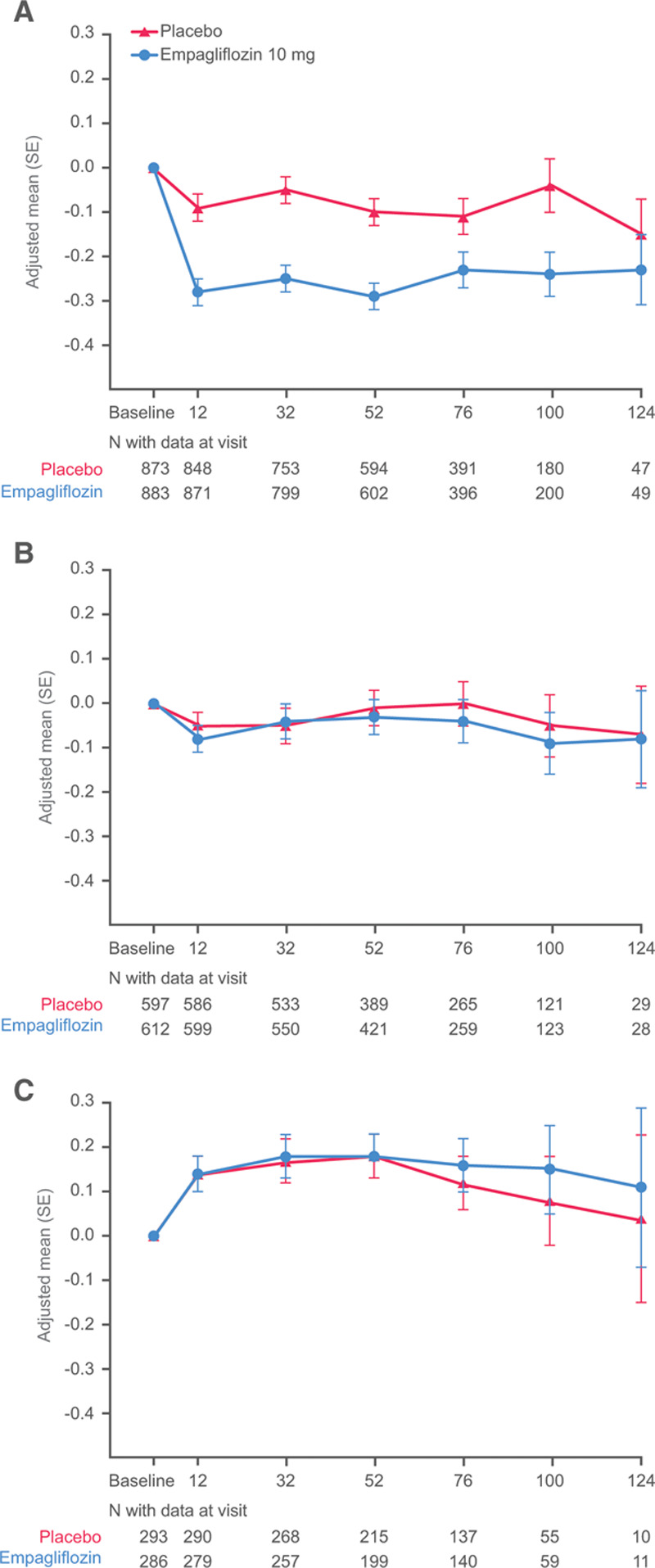
Glycohemoglobin changes from baseline by glycemic status. A, Diabetes. B, Prediabetes. C, Normoglycemia.
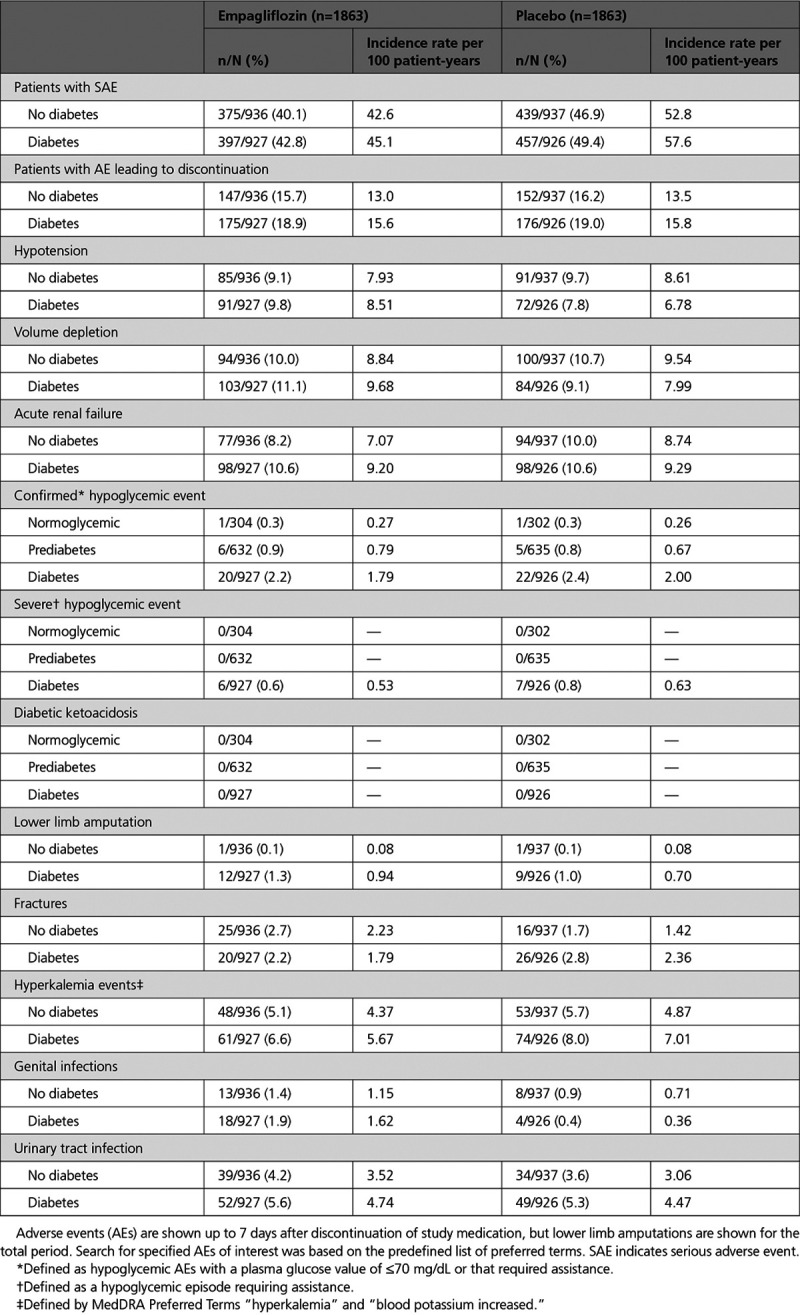
Adverse Events
The study medication was stopped because of adverse events in 147 patients (15.7%) in the empagliflozin group and 152 patients (16.2%) in the placebo group among patients without diabetes and in 175 patients (18.9%) in the empagliflozin group and 176 patients (19.0%) in the placebo group among patients with diabetes (Table 3). For adverse events other than genital tract infections, there were no meaningful increases in the empagliflozin group and the pattern of between-group differences was not influenced by the presence or absence of diabetes. Although confirmed hypoglycemia occurred more frequently in diabetic than nondiabetic patients, no imbalance between treatment groups was seen. There were no episodes of severe hypoglycemia requiring assistance in patients without diabetes.
Table 3.
Adverse Events of Interest by Diabetes Status
Discussion
Disorders of glycemic control are exceptionally common in patients with chronic heart failure,9,10 perhaps because heart failure itself represents a state of insulin resistance,11,12 which is partially driven by the severity of the hemodynamic abnormality.13 More than 80% of our patients had either diabetes or prediabetes, a prevalence similar to that in previous reports on patients with heart failure and a reduced ejection fraction.9,10 In the current trial, compared with patients with heart failure and normoglycemia, only patients with diabetes showed a marked increase in the risk of heart failure and renal events in the absence of treatment with an SGLT2 inhibitor. Diabetes increased the risk of hospitalizations for heart failure by >50% and it nearly doubled the rate of decline in eGFR as well as the risk of a major renal event. In contrast, the rate of evolution and progression of heart failure and kidney disease did not differ among patients with prediabetes as compared with those with normoglycemia. Our observation regarding the risk of heart failure events is consistent with earlier reports that when compared with normoglycemia, nondiabetic dysglycemia (ie, prediabetes) is associated with little or a modest increase in the risk of cardiovascular death and heart failure hospitalization in patients with heart failure and reduced ejection fraction.9,10 Furthermore, our finding regarding the risk of renal disease progression is consistent with the lack of an increased risk of nephropathy in patients with prediabetes who do not have heart failure.14,15 Taken collectively, these observations highlight the importance of distinguishing between diabetes and prediabetes in the prediction of end-organ injury, even in patients with heart failure and a reduced ejection fraction.
Empagliflozin reduced the risk of the primary outcome variables as well as total hospitalizations for heart failure by 25% to 30% and treatment with the drug slowed the rate of decline in eGFR during double-blind therapy and reduced the risk of serious adverse events by 50%. The benefits were seen in patients with or without diabetes and the magnitude of these favorable effects was not influenced by the baseline HbA1c level. The effect of empagliflozin on heart failure and renal outcomes was such that treatment with the drug effectively negated the deleterious effect of diabetes on the risk of heart failure and renal events. For the primary outcome of cardiovascular death or hospitalization for heart failure, the event rate per 100 patient-years of follow-up among patients with diabetes was reduced from 24.6 in the placebo group to 17.7 in the empagliflozin group, a risk similar to that seen in patients with normoglycemia who received placebo (ie, 16.5). For total hospitalizations for heart failure, the event rate per 100 patient-years among those with diabetes was reduced from 27.2 in the placebo group to 18.0 in the empagliflozin group, a risk similar to that seen in patients with normoglycemia who received placebo (ie, 16.9). For the composite renal end point, the event rate per 100 patient-years of follow-up among patients with diabetes was reduced from 4.2 to 2.3 by empagliflozin; the latter risk was similar to that seen in patents with normoglycemia who were treated with placebo (ie, 2.2).
Beyond lowering excess risk associated with diabetes, treatment with empagliflozin also reduced the risk of heart failure and renal events in patients with prediabetes and normoglycemia. The magnitude of the benefit in nondiabetic patients was similar to that in patients with diabetes and the heart failure and renal benefits of empagliflozin were consistent across a broad range of baseline values for HbA1c. Consistent with its known mechanism of action to lower blood glucose only in the setting of hyperglycemia, empagliflozin only reduced HbA1c in patients with diabetes and not in patients with prediabetes or normoglycemia. These findings strongly underscore the conclusion that the benefits of empagliflozin on the heart and the kidneys are not related to the level of dysglycemia or to changes in glycohemoglobin. The precise mechanism of action of SGLT2 inhibitors to lower the risk of heart failure events remains to be defined, but given our findings, the pathophysiologic abnormalities that are ameliorated by empagliflozin are not dependent on or are unlikely to be meaningfully influenced by abnormalities in blood glucose.4 It is therefore noteworthy that various biomarkers of the effect of empagliflozin in heart failure (ie, changes in body weight, natriuretic peptides, and hemoglobin) were influenced to a similar degree in patients with or without diabetes.
There were no meaningful imbalances in tolerability or safety events between the empagliflozin and placebo groups among patients with heart failure with and without diabetes. Adverse effects that are typical of other heart failure medications (such as hyperkalemia and hypotension) were observed similarly in the placebo and empagliflozin groups. No reports of diabetic ketoacidosis or severe hypoglycemic episode occurred with empagliflozin in patients with prediabetes or normoglycemia. This shows the favorable benefit–risk profile of empagliflozin and provides reassurance to clinicians in initiating SGLT2 inhibitors in patients with heart failure who do not have diabetes.
Our findings should be considered in light of both strengths and limitations of the current trial. Each of our major end points was prespecified and the analysis of the effect of empagliflozin in patients with and without diabetes was designated as our most important subgroup analysis. The patients without diabetes comprised a substantial proportion of our population (50%) and we were also able to study the effect of empagliflozin in a considerable number of patients with prediabetes. Because insulin resistance is exceptionally common in heart failure and a reduced ejection fraction, we expected that the proportion of patients with normoglycemia would be comparatively small, leading to estimates of a treatment effect in patients with normoglycemia that were necessarily less precise. However, when comparing the magnitude of the treatment effect across the 3 glycemia subgroups, there was a remarkable consistency of the benefit of empagliflozin with no evidence for heterogeneity.
In this secondary analysis of EMPEROR-Reduced, empagliflozin significantly reduced the risk of cardiovascular death or heart failure hospitalizations, decreased total hospitalizations for heart failure, slowed decline in renal function, prevented serious renal events, and improved measures of health status to a similar degree in patients with and without diabetes. Our findings reinforce those recently reported in a similar trial with dapagliflozin in heart failure with reduced ejection fraction, but we extend those findings to include benefits on the evolution of renal disease as well as defined benefits in patients with prediabetes and no glycemic disorder. The combined results of the 2 trials reinforce a new role for SGLT2 inhibitors in patients with heart failure and reduced ejection fraction independent of diabetes or the baseline level of glycohemoglobin.
Acknowledgments
The authors thank Tomoko Iwata for statistical support and Ivana Ritter for the compilation and management of safety-related aspects. Graphical assistance, supported financially by Boehringer Ingelheim, was provided by Jonathon Gibbs of Elevate Scientific Solutions.
Sources of Funding
EMPEROR-Reduced was funded by the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance.
Disclosures
Dr Anker reports grants and personal fees from Vifor International and Abbott Vascular and personal fees from Astra-Zeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Servier, and Vifor International. Dr Butler reports consulting fees from BI, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave Ltd, and Vifor. Dr Filippatos reports committee member contributions in trials sponsored by Medtronic, Vifor, Servier, Novartis, and Boehringer Ingelheim. Dr Khan reports no relevant disclosures. Dr Marx is funded by the German Research Foundation SFB TRR 219 (projects M-03 and M-05); reports giving lectures for and receiving honoraria from Amgen, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Lilly, and Novo Nordisk; receiving unrestricted research grants from Boehringer Ingelheim; serving as an advisor for Amgen, Bayer, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, and Novo Nordisk; serving in trial leadership for Boehringer Ingelheim and Novo Nordisk; and declining all personal compensation from pharmaceutical and device companies. Dr Lam reports research grants from Bayer, Boston Scientific, Roche Diagnostic, Medtronic, Vifor Pharma, and AstraZeneca; consulting fees from Merck, Bayer, Boston Scientific, Roche Diagnostic, Vifor Pharma, AstraZeneca, Novartis, Amgen, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, Novo Nordisk, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, Cytokinetics, WebMD Global LLC, Radcliffe Group Ltd, and Corpus; and serves as cofounder and nonexecutive director of eKo.ai. S. Schnaidt, Dr Ofstad, Dr Brueckmann, and Dr Jamal are employees of Boehringer Ingelheim. Dr Bocchi reports receiving consulting fees from Servier and Astra-Zeneca; subsidized travel/hotel/registration fees from Servier and Baldacci; membership in steering committees for Servier and Novartis; contracted research from Jansen, Bayer/Merck, Astra-Zeneca, Boehringer, and Ingelheim; and honoraria from Servier, Novartis, and Astra-Zeneca. Dr Ponikowski reports personal fees from Boehringer Ingelheim, Astra Zeneca, Servier, BMS, Amgen, Novartis, Merck, Pfizer, and Berlin Chemie, and grants and personal fees from Vifor Pharma. Dr Perrone serves on advisory boards and receives personal fees from Laboratorios Ferrer, Abbott–St Jude, Novartis, and Laboratorios Bago. Dr Januzzi reports grant support, consulting income, and participation in clinical end point committees/data safety monitoring boards from Janssen; participation in clinical end point committees/data safety monitoring boards from Boehringer Ingelheim; grant support from Novartis, Innolife, Applied Therapeutics, and Siemens Diagnostics; and consultancy fees from Novartis, Roche Diagnostics, and Abbott Diagnostics. Dr Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Sun Pharmaceuticals, PhaseBio, and the Toronto Knowledge Translation Working Group; is a member of the scientific excellence committee of EMPEROR-Reduced and served as a national lead investigator of the DAPA-HF trial and EMPEROR-Reduced; and is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. Dr Böhm reports fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Servier, Medtronic, ReCor, Vifor, Novartis, and Abbott; and is supported by the Deutsche Forschungsgemeinschaft (grant TTR 219, S-01). Dr Ferreira is a consultant for Boehringer Ingelheim. Dr Pocock is a consultant for Boehringer Ingelheim. Dr Zannad has recently received steering committee or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cardior, CVRx, Janssen, Livanova, Merck, Mundipharma, Novartis, Novo Nordisk, and Vifor Fresenius. Dr Packer reports personal fees from Boehringer Ingelheim during the conduct of the study and personal fees from AbbVie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk, outside the submitted work.
Supplemental Materials
Data Supplement Table I
Data Supplement Figures I–VIII
Supplementary Material
Footnotes
Sources of Funding, see page 348
The Guest Editor for this article was Jeffrey M. Testani, MD, MTR.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.051824.
References
- 1.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39. doi: 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Handelsman Y, Bakris G, Verma S. Use of sodium-glucose co-transporter-2 inhibitors in patients with and without type 2 diabetes: implications for incident and prevalent heart failure. Eur J Heart Fail. 2020; 22:604–617. doi: 10.1002/ejhf.1708 [DOI] [PubMed] [Google Scholar]
- 3.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019; 7:845–854. doi: 10.1016/S2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
- 4.Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care. 2020; 43:508–511. doi: 10.2337/dci19-0074 [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 6.Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020; 323:1353–1368. doi: 10.1001/jama.2020.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, et al. EMPEROR-Reduced Trial Committees and Investigators. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail. 2019; 21:1270–1278. doi: 10.1002/ejhf.1536 [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. EMPEROR-Reduced Trial Investigators. cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 9.Kristensen SL, Jhund PS, Lee MMY, Køber L, Solomon SD, Granger CB, Yusuf S, Pfeffer MA, Swedberg K, McMurray JJVCHARM Investigators and Committees. Prevalence of prediabetes and undiagnosed diabetes in patients with HFpEF and HFrEF and associated clinical outcomes. Cardiovasc Drugs Ther. 2017; 31:545–549. doi: 10.1007/s10557-017-6754-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, Martinez F, Starling RC, Desai AS, Lefkowitz MP, et al. PARADIGM-HF Investigators and Committees. Risk related to pre-diabetes and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. 2016; 9:e002560 doi: 10.1161/CIRCHEARTFAILURE.115.002560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008; 51:93–102. doi: 10.1016/j.jacc.2007.10.021 [DOI] [PubMed] [Google Scholar]
- 12.Yang CD, Shen Y, Lu L, Ding FH, Yang ZK, Zhang RY, Shen WF, Jin W, Wang XQ. Insulin resistance and dysglycemia are associated with left ventricular remodeling after myocardial infarction in non-diabetic patients. Cardiovasc Diabetol. 2019; 18:100 doi: 10.1186/s12933-019-0904-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uriel N, Naka Y, Colombo PC, Farr M, Pak SW, Cotarlan V, Albu JB, Gallagher D, Mancini D, Ginsberg HN, et al. Improved diabetic control in advanced heart failure patients treated with left ventricular assist devices. Eur J Heart Fail. 2011; 13:195–199. doi: 10.1093/eurjhf/hfq204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, Park S, Kwon SH, Jeon JS, Han DC, Noh H. Impaired fasting glucose and development of chronic kidney disease in non-diabetic population: a Mendelian randomization study. BMJ Open Diabetes Res Care. 2020; 8:e001395 doi: 10.1136/bmjdrc-2020-001395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathi N, Whelton PK, Chertow GM, Cushman WC, Cheung AK, Wei G, Boucher R, Kimmel PL, Bress AP, Kramer HJ, et al. Influence of prediabetes on the effects of intensive systolic blood pressure control on kidney events. Am J Hypertens. 2019; 32:1170–1177. doi: 10.1093/ajh/hpz105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


