Abstract
Aims
Patients who survive acute myocardial infarction (AMI) are at risk of being rehospitalized owing to the occurrence of acute decompensated heart failure (HF). However, the clinical characteristics of HF after AMI, especially the frequency of each HF subtype, are unclear.
Methods and results
We retrospectively studied 1055 patients with AMI. We excluded 257 patients, who were admitted >48 h after the onset of AMI, died during hospitalization or after discharge, and whose echocardiogram data at index hospitalization and follow‐up data were missing. The remaining 798 patients (mean age: 66.5 ± 11.7 years) were investigated for a mean follow‐up period of 4.9 years. All patients underwent emergency coronary angiography. The mean maximum creatine kinase levels were 2898 ± 2627 IU/L, and mean left ventricular ejection fraction (LVEF) was 58.9 ± 10.2%. Eighty‐one patients (10.2%) were rehospitalized because of unexpected worsening of HF. Echocardiography data were available for 74 of the 81 patients during the acute phase of the second hospitalization, of which 30, 20, and 24 patients (41%, 27%, and 32%, respectively) were diagnosed as having HF with preserved LVEF (LVEF ≥ 50%), HF with mid‐range LVEF (40% ≤ LVEF < 50%), and HF with reduced LVEF (LVEF < 40%), respectively. The ejection fraction during index hospitalization was 58.3 ± 9.7% in the HF with preserved LVEF group, 53.3 ± 10.2% in the HF with mid‐range LVEF group, and 43.3 ± 10.5% in the HF with reduced LVEF group (P < 0.001).
Conclusions
The predominant subtypes of HF after AMI were HF with mid‐range ejection fraction and preserved ejection fraction, or HF with non‐reduced ejection fraction.
Keywords: Heart failure, Left ventricular ejection fraction, Myocardial infraction
Introduction
Recent advances in the management of acute myocardial infarction (AMI) have significantly reduced the probability of in‐hospital mortality. 1 , 2 , 3 , 4 However, the occurrence of heart failure (HF) in survivors of AMI, which requires readmission, has recently emerged as a critical clinical problem. 5 , 6 Moreover, HF is one of the most common causes of hospitalization with high mortality and an increasing prevalence. 7 , 8 , 9 Earlier studies, which investigated the frequency of HF and mortality following AMI using electronic health records, showed that approximately 16–24% of patients with a history of AMI were rehospitalized during the 3–4 year follow‐up after index hospitalization. 10 , 11 Conversely, the current registries of acute decompensated HF (ADHF) report that approximately 7.5–25% of patients have a history of AMI. 12 , 13
Heart failure has recently been classified into the following three subgroups based on the left ventricular ejection fraction (LVEF): HF with reduced LVEF (HFrEF) (LVEF < 40%), HF with mid‐range LVEF (HFmrEF) (40% ≤ LVEF < 50%), and HF with preserved LVEF (HFpEF) (LVEF ≥ 50%). Angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), beta‐blockers, and mineralocorticoid antagonists form the pillars of the current management of HFrEF. 7 However, no treatment has been shown to reduce mortality and morbidity in HFpEF. 14 Several cardiologists assume that most cases of HF that develop after AMI are classified as HFrEF because AMI affects the viable myocardium. A recent meta‐analysis that comprised four community‐based cohorts showed that although the history of MI was a significant predictor of both HFrEF and HFpEF, it was a more accurate predictor of HFrEF. 15 Nevertheless, no study has reported the most frequent subtype of post‐AMI HF. Therefore, we investigated the clinical profile of post‐AMI ADHF using the Nara Registry and Analysis for Myocardial Infarction (NARA‐MI) study to address this gap in the literature.
Methods
Study participants
Nara Registry and Analysis for Myocardial Infarction study was retrospective in design. NARA‐MI study enrolled 1055 patients with AMI (approximately 100 patients every year) who underwent emergency coronary angiography at Nara Medical University between 2007 and 2016. The definitive diagnosis of AMI was made on the basis of a history of chest pain, typical electrocardiography changes, and creatine kinase (CK) levels that were twice the upper limit. 16
We excluded 58 of the 1055 patients who were admitted >48 h after the onset of AMI, 43 patients who died during index hospitalization, 3 patients whose echocardiography data at index hospitalization were missing, and 18 patients who were lost to follow‐up. We excluded 135 of 933 patients, who died during the follow‐up period (mean: 4.9 years), from the final data analyses. Only seven of the 135 patients died of HF. Eighty‐one patients of 798 patients who survived were rehospitalized during the follow‐up period, because of unexpected worsening of HF. The echocardiography data were available for 74 of 81 patients at the second hospitalization but could not be obtained for seven patients. These 74 patients were divided into three groups according to the LVEF at rehospitalization due to ADHF (ADHF group). Thirty, 20, and 24 patients had HFpEF (LVEF ≥ 50%), HFmrEF (40% ≤ LVEF < 50%), and HFrEF (LVEF < 40%), respectively (Figure 1 ).
FIGURE 1.
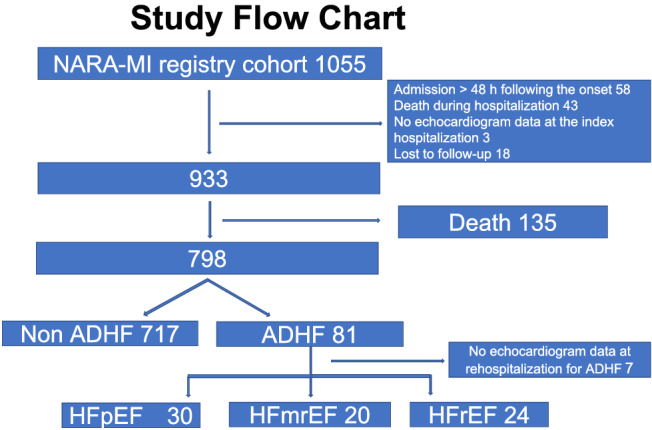
Flow chart of the study methodology. ADHF, acute decompensated heart failure; HFmrEF, heart failure with mid‐range left ventricular ejection fraction; HFpEF, heart failure with preserved left ventricular ejection fraction, HFrEF, heart failure with reduced left ventricular ejection fraction; NARA‐MI, Nara Registry and Analysis for Myocardial Infarction.
Data collection and endpoints
Coronary angiography and revascularization were performed using standard techniques. Revascularization procedures, such as thrombectomy, pre‐dilatation, stenting, and/or post‐dilatation, were performed at each operator's discretion. LVEF was measured using the biplane modified Simpson's method. Echocardiography was performed at index admission for AMI after shifting the patient from the coronary care unit to the general ward. Echocardiography was also performed in the acute period during readmission due to ADHF. The diagnosis of HF was based on the Framingham criteria. 17 NARA‐MI study was approved by the Ethics Committee of Nara Medical University (ID No. 2162) and was conducted in accordance with the 1975 Declaration of Helsinki guidelines for clinical research protocols. Informed consent was obtained from all patients. Research assistants recorded the data using individual chart review, and the patients' families were reached by phone if such information was unavailable.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation. The differences between the clinical characteristics and laboratory data of the ADHF and non‐ADHF groups were analysed using the unpaired t‐test or Wilcoxon rank‐sum test. Univariate and multivariate analyses of admission for HF were performed using Cox proportional hazard models. P < 0.05 was considered statistically significant for univariate analysis of the predictors of ADHF. We selected six variables with P values <0.01 for Cox multivariate analysis. Moreover, we used Bonferroni post hoc tests to determine the differences between HFrEF, HFmrEF, and HFpEF at rehospitalization for HF. All data were analysed using JMP Version 12.2 for Windows (SAS Institute, Cary, NC).
Results
Clinical characteristics
The final analysis included 798 patients after excluding 135 patients who died during the follow‐up period (mean 4.9 years), as shown in Figure 1 . The baseline clinical characteristics of the study population are shown in Table 1 . The mean age was 66.5 ± 11.7 years (23.8% women). Eighty‐one of the 798 patients developed ADHF (10.2%). The comparison of the clinical characteristics of the first hospitalization in patients with ADHF and non‐ADHF revealed that patients in the ADHF group were older than those in the non‐ADHF group. The percentage of women was higher in the ADHF group than that in the non‐ADHF group. The percentage of patients with diabetes mellitus in the ADHF group was higher than that in the non‐ADHF group. The incidence of Killip Class IV was higher in the ADHF group than that in the non‐ADHF group. Laboratory examination revealed that the levels of B‐type natriuretic peptide (BNP) were significantly higher in the ADHF group than those in the non‐ADHF group. Haemoglobin levels and estimated glomerular filtration rates (eGFRs) were significantly lower in the ADHF group than those in the non‐ADHF group. However, the maximum CK values were similar in both groups of patients. Moreover, the LVEF was significantly lower in patients in the ADHF group than that in the non‐ADHF group. Loop diuretics and mineralocorticoid receptor blockers were used more frequently in the ADHF group.
TABLE 1.
Baseline clinical and lesion characteristics and medications
| Total (n = 798) | ADHF (n = 81) | Non‐ADHF (n = 717) | P value | |
|---|---|---|---|---|
| Age (years) | 66.5 ± 11.7 | 75.4 ± 11.9 | 65.5 ± 11.3 | <0.001 |
| Sex: female, n (%) | 190 (23.8) | 28 (34.6) | 162 (22.6) | 0.021 |
| Medical history | ||||
| Smoking, n (%) | 551 (69.1) | 52 (64.2) | 499 (69.6) | 0.325 |
| Diabetes mellitus, n (%) | 265 (33.2) | 35 (43.2) | 230 (32.1) | 0.048 |
| Hypertension, n (%) | 506 (63.4) | 53 (65.4) | 453 (63.2) | 0.689 |
| Dialysis, n (%) | 17 (2.1) | 6 (7.4) | 11 (1.5) | 0.005 |
| Killip class | ||||
| IV, n (%) | 49 (6.1) | 13 (16.1) | 36 (5.0) | <0.001 |
| STEMI, n (%) | 653 (81.8) | 66 (81.5) | 587 (81.9) | 0.932 |
| Laboratory data on admission | ||||
| Hb (g/dL) | 14.0 ± 2.0 | 12.8 ± 2.4 | 14.2 ± 1.9 | <0.001 |
| eGFR (mL/min/1.73 m2) | 68.8 ± 25.1 | 54.3 ± 32.0 | 70.5 ± 23.6 | <0.001 |
| LDL‐C (mg/dL) | 114.5 ± 38.1 | 100.7 ± 38.6 | 116.1 ± 37.7 | <0.001 |
| HDL‐C (mg/dL) | 46.8 ± 12.5 | 46.1 ± 12.8 | 46.9 ± 12.5 | 0.456 |
| BNP (pg/mL) | 126.6 (60.2–255.5) | 378.3 (171.3–853.1) | 116.3 (56.6–228) | <0.001 |
| Max CK (IU/L) | 2898 ± 2627 | 3621 ± 3181 | 2817 ± 2547 | 0.056 |
| Max CK (IU/L) ≥ 2000 | 423 (53.0) | 47 (58.0) | 376 (52.4) | 0.339 |
| EF (%) | 58.9 ± 10.2 | 52.4 ± 11.8 | 59.6 ± 9.8 | <0.001 |
| Culprit vessel | ||||
| RCA, n (%) | 287 (36.0) | 22 (27.2) | 265 (37.0) | 0.076 |
| LAD, n (%) | 386 (48.4) | 46 (56.8) | 340 (47.4) | 0.110 |
| LCX, n (%) | 101 (12.7) | 8 (9.9) | 93 (13.0) | 0.413 |
| LMT, n (%) | 8 (1.0) | 2 (2.5) | 6 (0.8) | 0.227 |
| Final TIMI flow grade | ||||
| 3, n (%) | 753 (94.4) | 76 (93.8) | 677 (94.4) | 0.828 |
| Medications at discharge | ||||
| Aspirin, n (%) | 778 (97.5) | 76 (93.8) | 702 (97.9) | 0.054 |
| ACEIs or ARBs, n (%) | 777 (97.4) | 76 (93.8) | 701 (97.8) | 0.067 |
| ACEIs, n (%) | 684 (85.7) | 69 (85.2) | 615 (85.8) | 0.886 |
| ARBs, n (%) | 109 (13.7) | 13 (16.1) | 96 (13.4) | 0.517 |
| Beta‐blockers, n (%) | 538 (67.4) | 55 (67.9) | 483 (67.4) | 0.922 |
| Loop diuretics, n (%) | 212 (26.6) | 56 (69.1) | 156 (21.8) | <0.001 |
| MR blockers, n (%) | 94 (11.8) | 18 (22.2) | 76 (10.6) | 0.005 |
| Statins, n (%) | 598 (74.9) | 41 (50.6) | 557 (77.7) | <0.001 |
ACEIs, angiotensin‐converting enzyme inhibitors; ADHF, acute decompensated heart failure; ARBs, angiotensin receptor blockers; BNP, B‐type natriuretic peptide; EF, ejection fraction; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LAD, left anterior descending artery; LCX, left circumflex artery; LDL‐C, low‐density lipoprotein cholesterol; LMT, left main trunk; Max CK, maximum creatinine kinase; MR, mineralocorticoid receptor; RCA, right coronary artery; STEMI, ST‐segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Data represented as n (%), mean ± standard deviation, or median (25th–75th percentile).
We stratified 798 patients with AMI using ejection fraction (EF) measured during the index AMI hospitalization. We found that 40 patients had reduced EF (rEF) (EF < 40%), 105 patients had mid‐range EF (mrEF) (40 ≤ EF < 50%), and 653 patients had preserved EF (pEF) (EF ≥ 50%). The baseline characteristics of these groups are summarized in Supporting Information, Table S1 . Patients with lower EF tend to be older. The peak CK and BNP levels in the pEF group were significantly lower than those in the mrEF and rEF groups, respectively. Fourteen patients (35%) out of 40 patients with rEF, 16 patients (15.2%) out of 105 patients with mrEF, and 51 patients (7.8%) out of 653 patients with pEF were rehospitalized because of ADHF (Figure 2 ).
FIGURE 2.
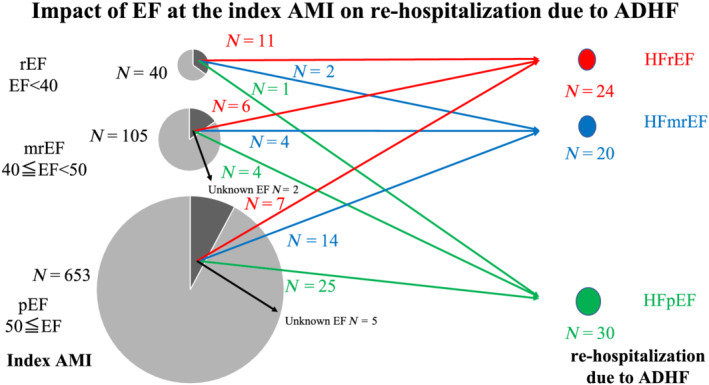
Effect of ejection fraction (EF) on the index acute myocardial infarction (AMI) on rehospitalization due to acute decompensated heart failure (ADHF) [red line: heart failure with reduced left ventricular ejection fraction (HFrEF); blue line: heart failure with mid‐range left ventricular ejection fraction (HFmrEF); and green line: heart failure with preserved left ventricular ejection fraction (HFpEF)]. mrEF, mid‐range ejection fraction; pEF, preserved ejection fraction; rEF, reduced ejection fraction.
Predictors of rehospitalization
We subsequently investigated the predictors of rehospitalization using univariate and multivariate analyses (Table 2 ). Univariate analysis revealed that age, haemoglobin, eGFR, BNP, LVEF, and use of loop diuretics were significant predictors of ADHF. Multivariate analysis found that age [hazard ratio (HR) 1.047; 95% confidence interval (CI) 1.020–1.077, P = 0.0005], BNP (HR 1.076; 95% CI 1.031–1.120, P = 0.001), LVEF (HR 0.959; 95% CI 0.938–0.980, P = 0.0001), and use of loop diuretics (HR 3.284; 95% CI 1.980–5.595, P < 0.0001) were independent predictors of ADHF.
TABLE 2.
Univariate and multivariate analyses of predictors for ADHF
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Age (years) | 1.094 (1.070–1.121) | <0.0001 | 1.047 (1.020–1.077) | 0.0005 |
| Sex: male | 0.572 (0.365–0.916) | 0.0207 | ||
| Diabetes mellitus | 1.651 (1.057–2.557) | 0.0279 | ||
| Hypertension | 1.081 (0.690–1.732) | 0.7369 | ||
| Hb (g/dL) | 0.727 (0.661–0.802) | <0.0001 | 0.950 (0.838–1.082) | 0.4368 |
| eGFR (mL/min/1.73 m2) | 0.972 (0.963–0.980) | <0.0001 | 0.992 (0.980–1.003) | 0.1540 |
| BNP (100 pg/mL) | 1.168 (1.134–1.201) | <0.0001 | 1.076 (1.031–1.120) | 0.0010 |
| Max CK (100 IU/L) | 1.009 (1.002–1.014) | 0.0176 | ||
| EF (%) | 0.938 (0.919–0.957) | <0.0001 | 0.959 (0.938–0.980) | 0.0001 |
| Final TIMI flow Grade 3 | 0.841 (0.377–2.395) | 0.7146 | ||
| Loop diuretics | 7.140 (4.507–11.64) | <0.0001 | 3.284 (1.980–5.595) | <0.0001 |
CI, confidence interval. Other abbreviations as in Table 1 .
Classification by ejection fraction at rehospitalization due to acute decompensated heart failure
We analysed EF in 74 patients whose echocardiography data were collected during the second hospitalization. Consequently, 30, 20, and 24 patients (41%, 27%, and 32%, respectively) were classified into the HFpEF, HFmrEF, and HFrEF groups, respectively (Figure 1 and Supporting Information, Figure S1 ). Clinical characteristics, such as age, sex, and medical history, were similar for patients with ADHF from the three groups for during the index AMI admission (as shown in Table 3 ). Although the maximum CK levels were the highest in patients with HFrEF, followed by those with HFmrEF and HFpEF, their differences were not statistically significant. The EF in the HFrEF group was significantly lower than that in the HFpEF and HFmrEF groups (43.3 ± 10.5% vs. 58.3 ± 9.7%, P < 0.001, and 43.3 ± 10.5% vs. 53.3 ± 10.2%, P = 0.004), but there was no significant difference between the EF of the HFpEF and HFmrEF groups (58.3 ± 9.7% vs. 53.3 ± 10.2%, P = 0.081). The prescription rates of ACEIs, ARBs, beta‐blockers, and loop diuretics were similar for all three groups.
TABLE 3.
Baseline clinical and lesion characteristics and medications in the index AMI
| Total (n = 74) | HFpEF (n = 30) | HFmrEF (n = 20) | HFrEF (n = 24) | HFmrEF vs. HFpEF | HFrEF vs. HFpEF | HFmrEF vs. HFrEF | ||
|---|---|---|---|---|---|---|---|---|
| Age (years) | 74.7 ± 12.1 | 75.6 ± 10.3 | 74.8 ± 11.9 | 73.4 ± 14.7 | 0.953 | |||
| Sex: female, n (%) | 24 (32.4) | 8 (26.7) | 8 (40.0) | 8 (33.3) | 0.611 | |||
| Medical history | ||||||||
| Smoking, n (%) | 51 (68.9) | 23 (76.7) | 13 (65.0) | 15 (62.5) | 0.479 | |||
| Diabetes mellitus, n (%) | 30 (40.5) | 11 (36.7) | 8 (40.0) | 11 (45.8) | 0.792 | |||
| Hypertension, n (%) | 49 (66.2) | 23 (76.7) | 14 (70.0) | 12 (50.0) | 0.113 | |||
| Dialysis, n (%) | 5 (6.8) | 1 (3.3) | 3 (15.0) | 1 (4.2) | 0.271 | |||
| Killip class | ||||||||
| IV, n (%) | 12 (16.2) | 5 (16.7) | 3 (15.0) | 4 (16.7) | 0.985 | |||
| STEMI, n (%) | 60 (81.1) | 22 (73.3) | 16 (80.0) | 22 (91.7) | 0.201 | |||
| Laboratory data in the index AMI admission | ||||||||
| Hb (g/dL) | 12.9 ± 2.4 | 12.6 ± 2.8 | 12.9 ± 2.1 | 13.4 ± 2.0 | 0.432 | |||
| eGFR (mL/min/1.73 m2) | 55.7 ± 32.9 | 52.2 ± 27.1 | 54.6 ± 44.9 | 61.1 ± 28.0 | 0.489 | |||
| LDL‐C (mg/dL) | 102.8 ± 39.2 | 98.8 ± 39.4 | 105.2 ± 47.0 | 106.0 ± 33.0 | 0.516 | |||
| HDL‐C (mg/dL) | 46.0 ± 13.0 | 46.2 ± 14.0 | 45.5 ± 12.7 | 46.0 ± 12.6 | 0.960 | |||
| BNP (pg/mL) | 395.6 (141.5–841.1) | 501.1 (196.0–1095.7) | 301.0 (233.3–736.8) | 289.2 (100.5–853.1) | 0.459 | |||
| Max CK (IU/L) | 2604 (1215–4675) | 2111 (927–4193) | 2964 (1333–5957) | 4001 (1470–4921) | 0.384 | |||
| Max CK (IU/L) ≥ 2000 | 43 (58.1) | 15 (50.0) | 13 (65.0) | 15 (62.5) | 0.499 | |||
| EF (%) | 52.0 ± 11.9 | 58.3 ± 9.7 | 53.3 ± 10.2 | 43.3 ± 10.5 | <0.001 | 0.081 | <0.001 | 0.004 |
| Culprit vessel | ||||||||
| RCA, n (%) | 19 (25.7) | 9 (30.0) | 5 (25.0) | 5 (20.8) | 0.742 | |||
| LAD, n (%) | 43 (58.1) | 18 (60.0) | 8 (40.0) | 17 (70.8) | 0.113 | |||
| LCX, n (%) | 7 (9.5) | 2 (6.7) | 4 (20.0) | 1 (4.2) | 0.192 | |||
| LMT, n (%) | 2 (2.7) | 0 | 2 (10.0) | 0 | 0.068 | |||
| Final TIMI flow grade | ||||||||
| 3, n (%) | 69 (93.2) | 29 (96.7) | 18 (90.0) | 22 (91.7) | 0.589 | |||
| Medications at discharge in the index AMI admission | ||||||||
| Aspirin, n (%) | 70 (94.6) | 28 (93.3) | 18 (90.0) | 24 (100.0) | 0.181 | |||
| ACEIs or ARBs, n (%) | 69 (93.2) | 28 (93.3) | 19 (95.0) | 22 (91.7) | 0.907 | |||
| ACEIs, n (%) | 62 (83.8) | 23 (76.7) | 18 (90.0) | 21 (87.5) | 0.384 | |||
| ARBs, n (%) | 13 (17.6) | 8 (26.7) | 3 (15.0) | 2 (8.3) | 0.191 | |||
| Beta‐blockers, n (%) | 51 (68.9) | 20 (66.7) | 14 (70.0) | 17 (70.8) | 0.941 | |||
| Loop diuretics, n (%) | 51 (68.9) | 21 (70.0) | 12 (60.0) | 18 (75.0) | 0.560 | |||
| MR blockers | 18 (24.3) | 8 (26.7) | 3 (15.0) | 7 (29.2) | 0.489 | |||
| Statins, n (%) | 37 (50.0) | 13 (43.3) | 13 (65.0) | 11 (45.8) | 0.282 | |||
AMI, acute myocardial infarction; HFmrEF, heart failure with mid‐range left ventricular ejection fraction; HFpEF, heart failure with preserved left ventricular ejection fraction; HFrEF, heart failure with reduced left ventricular ejection fraction. Other abbreviations as in Table 1 .
We analysed the relationship between EF and HF during the index AMI and that during rehospitalization. Eleven of the 24 patients with HFrEF at rehospitalization had rEF, 6 patients had mrEF, and 7 patients had pEF at index AMI. On the contrary, 25 of the 30 patients with HFpEF at rehospitalization were classified as pEF, 4 patients were classified as mrEF, and only 1 patient was classified as rEF (Figure 2 ). The average EF decreased significantly from index hospitalization for AMI to rehospitalization in patients with HFrEF and HFmrEF (from 43.3 ± 10.5% to 29.4 ± 6.7% P < 0.01, and from 53.3 ± 10.2% to 45.0 ± 2.8%, P < 0.01, respectively) (Figure 3 ). However, the EF of the HFpEF group did not show significant change between index hospitalization and rehospitalization (from 58.3 ± 9.7% to 59.3 ± 6.1%, P = 0.69).
FIGURE 3.
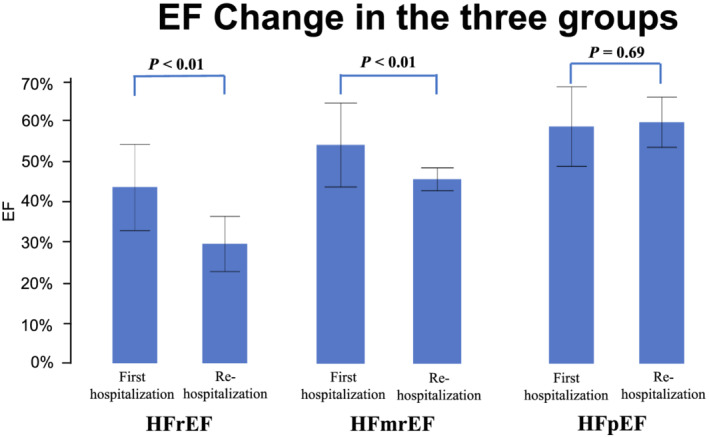
Change in the ejection fraction (EF) between the first hospitalization for acute myocardial infarction and rehospitalization for acute decompensated heart failure in the three groups. HFmrEF, heart failure with mid‐range left ventricular ejection fraction; HFpEF, heart failure with preserved left ventricular ejection fraction; HFrEF, heart failure with reduced left ventricular ejection fraction.
Timing of rehospitalization due to acute decompensated heart failure
Forty‐one (55%) of the 74 patients analysed were readmitted because of ADHF within 1 year of discharge from index hospitalization (Figure 4 A ). The LVEF was significantly lower, while BNP levels were higher in these 41 patients than their respective values in patients who were readmitted after 1 year (Supporting Information, Table S2 ). The frequency of readmission within 1 year of the first hospitalization was higher in each subgroup of HF (Figure 4 A ). The percentage of patients with HFrEF to the total number of patients seemed to be higher in patients rehospitalized within 1 year (of index hospitalization), in contrast to those who were rehospitalized after 1 year, although the difference was not statistically significant (Figure 4 B and 4 C ).
FIGURE 4.
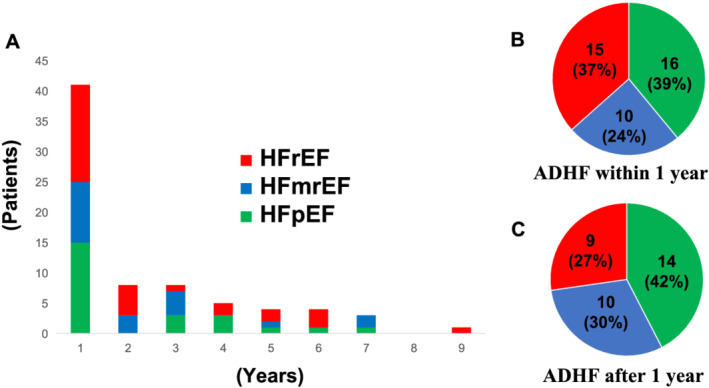
(A) Distribution of heart failure‐free time. Distribution of heart failure subgroups for (B) patients rehospitalized within 1 year after index hospitalization and (C) patients rehospitalized 1 year after the index hospitalization. ADHF, acute decompensated heart failure; HFmrEF, heart failure with mid‐range left ventricular ejection fraction; HFpEF, heart failure with preserved left ventricular ejection fraction; HFrEF, heart failure with reduced left ventricular ejection fraction.
Discussion
The principal finding of the present study was that approximately 10% of patients with AMI were rehospitalized because of ADHF during the follow‐up period of 4.9 years, and older patients and those with lower EF and higher BNP levels showed a greater probability of being rehospitalized. We classified the rehospitalized patients into three groups depending on the subtype of HF (i.e. HFrEF, HFmrEF, and HFpEF) based on the EF measured at rehospitalization. We found that 41% was HFpEF, 27% was HFmrEF, and 32% was HFrEF, and more than half of the patients were rehospitalized within 1 year after index hospitalization in each subgroup. To the best of our knowledge, this was the first study to report the frequency of occurrence of the three subtypes of HF after AMI. Importantly, the frequency of post‐AMI HFpEF was higher than speculated.
The frequency of post‐AMI HFpEF has not been studied well, although the concept of HFpEF has been widely accepted during the last two decades. 18 , 19 , 20 Similarly, the reason for the high prevalence of HFpEF following AMI (approximately 40%) remains unclear. One possible explanation is that the size of the infarct is smaller in contemporary AMI, which is supported by our result that the average EF during index AMI was approximately 60% (Table 1 ). All patients underwent emergency coronary angiography in the present study, and approximately 50% had peak CK levels of less than 2000 IU/L; that is, the size of the infarction was small. Moreover, patients with HFpEF showed lower peak CK values compared with the other two subgroups, although the peak CK levels during the first hospitalization did not differ significantly among the three subgroups. In fact, 25 of 30 patients with HFpEF were categorized in the pEF group during the index AMI (Figure 2 ). More than 90% of patients were prescribed with ACEIs or ARBs, and beta‐blockers were prescribed in 64.6% of patients in the pEF group during discharge after index AMI hospitalization. However, the lower prescription rate of beta‐blockers at discharge was not associated with the development of ADHF (HR 1.235; 95% CI 0.702–2.234, P = 0.468). New treatment strategies need to be developed in patients with AMI with EF > 40%, because no drug can improve cardiac outcomes in patients with HFpEF or HFmrEF. The use of sodium–glucose co‐transporter 2 inhibitors would be possible for patients with AMI and diabetes mellitus, or sacubitril/valsartan would also be possible for patients with EF < 50%.
Another reason is that the prevalence of AMI is higher in older patients. Patients with HFpEF are generally older than those with HFmrEF or HFrEF. In the present study, patients with post‐AMI ADHF were approximately 10 years older than those without ADHF. Therefore, it is possible that patients with HFpEF would have developed AMI. Therefore, further studies are required to investigate the mechanism by which HFpEF develops in patients with AMI.
We also conducted another study in patients with ADHF, known as the Nara Registry and Analyses for Heart Failure cohort study (NARA‐HF study). NARA‐HF study found that 31% of patients with a history of AMI were classified as HFpEF, 13 supporting the present findings on the frequency of HFpEF after AMI. In contrast, only 18% of patients with HFpEF presented with a history of AMI in the NARA‐HF study. Thus, AMI is an important in the aetiology of HFpEF but not the most common aetiology. Moreover, HFpEF, HFrEF, and HFmrEF occurred frequently within 1 year of discharge after index AMI hospitalization, indicating that special care should be taken during 1 year after discharge, irrespective of the type of HF.
Moreover, we compared the prognosis in the HFpEF, HFmrEF, and HFrEF groups and found no significant differences in survival rate after rehospitalization of the three groups, although the number of patients was small (log rank P = 0.6823, data not shown).
In this study, the frequency of ADHF was approximately 10% during the follow‐up period (mean 4.9 years) and considerably lower than that found in the electronic health records of Western‐based population databases. We do not know if these differences are the result of variations in race, size, and/or database characteristics.
Limitations
First, this was a retrospective, single‐centre study; therefore, there is the possibility of selection bias. Second, the study was conducted in Japan and only included Japanese patients. Therefore, other populations were not assessed. Third, the numbers of documented patients with HFpEF, HFmrEF, and HFrEF were too small to further conduct Cox multivariate analysis. Fourth, we were not able to explain that the loss of cardiac function was caused by AMI and not due to exacerbation of pre‐existing HF before the index AMI, because we had little information on the echocardiographic findings before AMI.
Conclusions
Following AMI, 41% of patients who were rehospitalized because of ADHF were classified as HFpEF, 27% were HFmrEF, and 32% were HFrEF, which should be a consideration in the management of patients with AMI.
Conflict of interest
None declared.
Funding
This study did not receive any funding from private, public, or not‐for‐profit sectors.
Supporting information
Figure S1. Distribution of heart failure subgroups
Table S1. Baseline clinical characteristics and medications in the index AMI
Table S2. Baseline clinical and lesion characteristics and medications
Acknowledgements
We wish to thank Yoko Wada, Yuki Kamada, and Ikuyo Yoshida for their support with the data collection process.
Kamon, D. , Sugawara, Y. , Soeda, T. , Okamura, A. , Nakada, Y. , Hashimoto, Y. , Ueda, T. , Nishida, T. , Onoue, K. , Okayama, S. , Watanabe, M. , Kawakami, R. , and Saito, Y. (2021) Predominant subtype of heart failure after acute myocardial infarction is heart failure with non‐reduced ejection fraction. ESC Heart Failure, 8: 317–325. 10.1002/ehf2.13070.
References
- 1. Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA Jr, Granger CB, Flather MD, Budaj A, Quill A, Gore JM, GRACE Investigators . Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA 2007; 297: 1892–1900. [DOI] [PubMed] [Google Scholar]
- 2. Dégano IR, Salomaa V, Veronesi G, Ferriéres J, Kirchberger I, Laks T, Havulinna AS, Ruidavets JB, Ferrario MM, Meisinger C, Elosua R, Marrugat J, Acute Myocardial Infarction Trends in Europe (AMITIE) Study Investigators . Twenty‐five‐year trends in myocardial infarction attack and mortality rates, and case‐fatality, in six European populations. Heart 2015; 101: 1413–1421. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 2012; 344: e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cui Y, Hao K, Takahashi J, Miyata S, Shindo T, Nishimiya K, Kikuchi Y, Tsuburaya R, Matsumoto Y, Ito K, Sakata Y, Shimokawa H. Age‐specific trends in the incidence and in‐hospital mortality of acute myocardial infarction over 30 years in Japan—report from the Miyagi AMI Registry Study. Circ J 2017; 81: 520–528. [DOI] [PubMed] [Google Scholar]
- 5. Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997; 337: 1360–1369. [DOI] [PubMed] [Google Scholar]
- 6. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 8. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018; 137: e67–e492. [DOI] [PubMed] [Google Scholar]
- 9. Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015; 385: 812–824. [DOI] [PubMed] [Google Scholar]
- 10. Hung J, Teng TH, Finn J, Knuiman M, Briffa T, Stewart S, Sanfilippo FM, Ridout S, Hobbs M. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: a population‐based study of 20 812 patients with first acute myocardial infarction in Western Australia. J Am Heart Assoc 2013; 2: e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gho JMIH, Schmidt AF, Pasea L, Koudstaal S, Pujades‐Rodriguez M, Denaxas S, Shah AD, Patel RS, Gale CP, Hoes AW, Cleland JG, Hemingway H, Asselbergs FW. An electronic health records cohort study on heart failure following myocardial infarction in England: incidence and predictors. BMJ Open 2018; 8: e018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA 2011; 306: 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ueda T, Kawakami R, Nakada Y, Nakano T, Nakagawa H, Matsui M, Nishida T, Onoue K, Soeda T, Okayama S, Watanabe M, Okura H, Saito Y. Differences in blood pressure riser pattern in patients with acute heart failure with reduced mid‐range and preserved ejection fraction. ESC Heart Fail 2019; 6: 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tomasoni D, Adamo M, Lombardi CM, Metra M. Highlights in heart failure. ESC Heart Fail 2019; 6: 1105–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail 2016; 9: e003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nomenclature and criteria for diagnosis of ischemic heart disease: report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 1979; 59: 607–609. [DOI] [PubMed] [Google Scholar]
- 17. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993; 22: 6A–13A. [DOI] [PubMed] [Google Scholar]
- 18. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC, Get With the Guidelines Scientific Advisory Committee and Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 19. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 20. Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta‐analysis. Eur Heart J 2012; 33: 1750–1757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of heart failure subgroups
Table S1. Baseline clinical characteristics and medications in the index AMI
Table S2. Baseline clinical and lesion characteristics and medications


