Abstract
Aims
Patients with left ventricular assist device (LVAD) suffer from persistent exercise limitation despite improvement of their heart failure syndrome. Exercise training (ET) programmes to improve aerobic capacity have shown modest efficacy. High‐intensity interval training (HIIT), as an alternative to moderate continuous training, has not been systematically tested in this population. We examine the feasibility of a short, personalized HIIT programme in patients with LVAD and describe its effects on aerobic capacity and left ventricular remodelling.
Methods and results
Patients on durable LVAD support were prospectively enrolled in a 15‐session, 5 week HIIT programme. Turndown echocardiogram, Kansas City Cardiomyopathy Questionnaire, and cardiopulmonary exercise test were performed before and after HIIT. Training workloads for each subject were based on pretraining peak cardiopulmonary exercise test work rate (W). Percentage of prescribed training workload completed and adverse events were recorded for each subject. Fifteen subjects were enrolled [10 men, age = 51 (29–71) years, HeartMate II = 12, HeartMate 3 = 3, and time on LVAD = 18 (3–64) months]. Twelve completed post‐training testing. HIIT was well tolerated, and 90% (inter‐quartile range: 78, 99%) of the prescribed workload (W) was completed with no major adverse events. Improvements were seen in aV̇O2 at ventilatory threshold [7.1 (6.5, 9.1) to 8.5 (7.7, 9.3) mL/kg/min, P = 0.04], work rate at ventilatory threshold [44 (14, 54) to 55 (21, 66) W, P = 0.05], and left ventricular end‐diastolic volume [168 (144, 216) to 159 (124, 212) mL, n = 7, P = 0.02]. HIIT had no effect on maximal oxygen consumption (V̇O2peak) or Kansas City Cardiomyopathy Questionnaire score.
Conclusions
Cardiopulmonary exercise test‐guided HIIT is feasible and can improve submaximal aerobic capacity in stable patients with chronic LVAD support. Further studies are needed on its effects on the myocardium and its potential role in cardiac rehabilitation programmes.
Keywords: Exercise capacity, Cardiopulmonary exercise test, High‐intensity interval training, Left ventricular assist device, Quality of life, Kansas City Cardiomyopathy Questionnaire
Introduction
Left ventricular assist devices (LVADs) are an established therapy for advanced heart failure (HF) with proven mortality benefit. 1 With 2 year survival now exceeding 80%, 2 the focus within the field has shifted towards optimization of quality of life for LVAD recipients. 3 Accordingly, it is notable that significant impairments in functional capacity with detrimental impacts on health status persist after implantation. 4 , 5
Available evidence consistently demonstrates the safety and benefits of therapeutic exercise training (ET) in this population, 6 , 7 , 8 but its implementation in clinical practice remains limited. 9 This has been partially attributed to insufficient knowledge in the field. 10 Currently available studies have employed a large variety of training modalities or have mostly enrolled patients shortly after LVAD implantation, when improvements in functional capacity are already expected because of amelioration of the HF syndrome. 11 , 12 , 13
The most consistently tested ET modality in LVAD patients is moderate continuous training (MCT), which leads to modest improvements in peak oxygen uptake (V̇O2peak) and quality of life scores compared with usual care or home‐based exercise. 7 , 14 More research is needed on the efficacy of alternative ET modalities, especially for patients on long‐term support. Ideally, a training modality with significant benefits and limited time commitment could be incorporated into outpatient cardiac rehabilitation programmes.
High‐intensity interval training (HIIT) is a type of training that uses very short intervals of very intense cardiovascular exercise followed by recovery periods of lower exercise intensity or complete rest. This allows for the achievement of overall high training workloads with low total exercise volume. 15
High‐intensity interval training is highly efficient at improving aerobic capacity compared with MCT in healthy individuals and in patients with HF. 16 , 17 However, adherence to the training schedule can limit its widespread use in HF patients, as demonstrated by larger studies. 18 This training modality has not been systematically applied to the LVAD population, with only rare reports available in the literature. 19 , 20
The effects of HIIT on skeletal muscle metabolism are well described and can be seen after training programmes as short as 2 weeks. 16 , 21 HIIT was also shown to induce reverse left ventricular (LV) remodelling in one study of patients with ischaemic cardiomyopathy. 17 Hence, this modality could improve aerobic capacity even at fixed LVAD speeds, when the rise in cardiac output is normally insufficient to sustain maximal effort levels, 22 by improving the function of the intrinsic LV 23 and peripheral muscle oxidative capacity. 24
In this pilot study, we hypothesized that a short, personalized HIIT programme could be completed without major adverse events by stable LVAD patients. We also examined the effects of HIIT on aerobic exercise capacity and LV function.
Methods
Study design
Patients with ongoing LVAD support at Montefiore Medical Center between 2017 and 2018 were screened for participation (Figure 1 ). To be considered, patients had to be in stable clinical condition, at least 3 months after LVAD implantation, and be of age 18 years or older. Exclusion criteria were recent frequent hospital readmissions (>1 over previous 3 months), inability to complete a cardiopulmonary exercise test (CPX), concomitant participation in cardiac rehabilitation, and inability to provide written informed consent. The study protocol was approved by the Einstein Institutional Review Board of the Albert Einstein College of Medicine.
Figure 1.
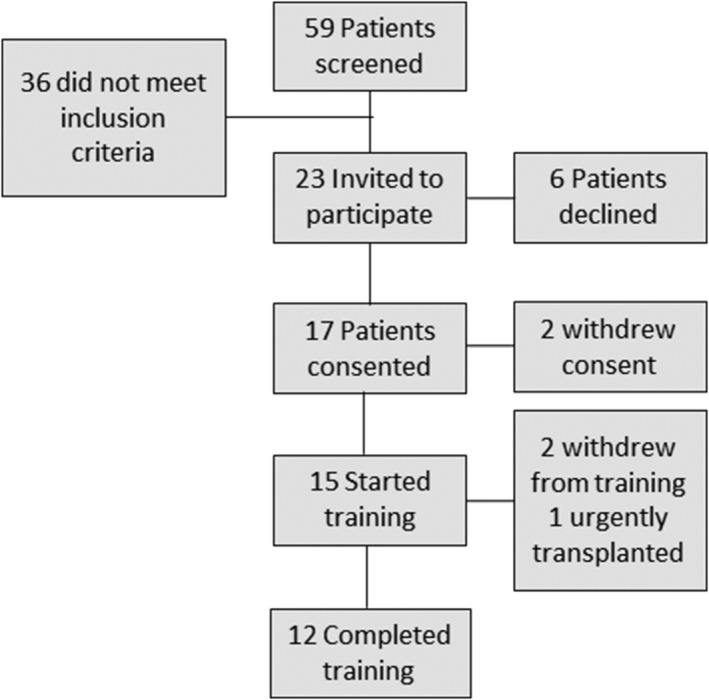
Patient flow during the study. Patients considered for the study had to be in stable clinical condition, at least 3 months after left ventricular assist device implantation and be of age 18 years or older.
After written informed consent, patients completed a Kansas City Cardiomyopathy Questionnaire (KCCQ), turndown echocardiogram (first 10 patients), and cycle ergometer‐based CPX. A 15‐session, supervised HIIT programme was then prescribed to be completed over 5 weeks (three sessions per week). All baseline testing was repeated in the same order after completion of training.
Subject testing
Turndown echocardiograms were planned for the first 10 subjects enrolled as an exploratory analysis of the LV remodelling effects of HIIT. Studies were performed only if the international normalized ratio was >2. Speed was progressively reduced to 6000 r.p.m. in HeartMate II (HM2) and 4000 r.p.m. in subjects with HeartMate 3 (HM3). 25 Images were obtained by a trained sonographer while on full LVAD support and 15 min after reaching lower speed target. Studies were interpreted by an experienced echocardiographer blinded to the testing sequence.
A KCCQ 23‐item form was completed by each subject independently before and after training.
A symptom‐limited CPX was performed using an upright cycle ergometer (Corival CPET, Lode B.V. Medical Technology, Groningen, the Netherlands) and an MGC metabolic cart (Ultima™ CardiO2 ®, St Paul, MN). A 15 W/min ramp protocol was applied. Continuous pulse oximetry and electrocardiogram monitoring were performed. Subjects were asked to fast for at least 4 h prior to testing, and all medications were continued before each test. Predicted V̇O2peak was calculated using the Wasserman/Hansen equation. Rate of perceived exertion (RPE) was recorded using the Borg scale (6–20 points) at each stage and at peak exercise. LVAD settings were not adjusted during testing. Device speed, power, and flow were recorded at baseline and at peak exercise.
To exclude a learning effect of repeated testing, the first 12 subjects enrolled completed two baseline CPX within 5 days. An interim analysis showed no learning effect on measures of gas exchange despite increased measures of effort on the second test (Supporting Information, Table S1 ). Repeated baseline testing was discontinued for subsequent subjects. For subjects with two baseline tests, the second test was used for comparison with post‐training values.
Gas exchange data were analysed independently by two experienced cardiologists who were blinded to the order of testing. Parameters were reported as 30 s averages. Peak oxygen consumption (V̇O2peak) was identified as the highest instantaneous value before exhaustion. Ventilatory threshold (VT) was determined using the V‐slope method. Ratio of minute ventilation to carbon dioxide was calculated at peak exercise. Peak power output (PPO) was the highest workload (W) achieved prior to exercise discontinuation.
Exercise training
Each subject was prescribed with a 15‐session HIIT programme to be completed over 5 weeks. This duration of training was chosen as a feasible protocol for ambulatory patients who continued to carry out normal daily activities. Prior studies have shown significant improvements in aerobic capacity and skeletal muscle adaptations after HIIT protocols as short as six sessions. 21
All sessions were supervised by a physician and could be interrupted upon subject's request or at the physician's discretion. Training was completed using the same cycle ergometer utilized during CPX testing. Each training session lasted 30 min: 3 min warm‐up and six 30 s high‐intensity intervals, each followed by a 4 min active recovery period. Similar protocols have proven to be efficacious in healthy individuals. 26
Workloads prescribed for each subject were individualized based on the peak work rate or PPO achieved during baseline CPX. For the first three sessions (‘induction phase’), prescribed workloads were 40% PPO warm‐up, 80% PPO intervals, and 30% PPO recovery periods. Workloads were increased on the fourth training session to 50% PPO warm‐up, 100% PPO high‐intensity intervals, and 40% PPO recovery periods. Reduction to the ‘induction phase’ workloads was allowed during any subsequent session where prescribed workloads were not tolerated. Watts were adjusted manually during each session on the cycle ergometer by the supervising staff. RPE and heart rate (HR) were recorded at each high‐intensity interval and 2 min into every other recovery period. LVAD settings were not adjusted during training. All medications were continued at previously prescribed doses throughout the training period.
Major adverse events during training were defined as sustained ventricular tachycardia or supraventricular tachycardia with haemodynamic collapse, sustained reductions in LVAD speed due to ‘suck‐down’ effect, driveline exit‐site infection, syncope, and cardiac arrest. Subjects who opted to stop the training programme before its completion were invited to perform post‐training testing after their last training session.
Measures of interest
The primary measure of interest was the percentage of total prescribed workload (W) completed by each subject during the training programme. Exploratory measures of interest were as follows: (i) change in oxygen consumption at peak exercise (V̇O2peak) and at VT (V̇O2 at VT); (ii) left ventricular volumes at target low speed during turndown echocardiogram; (iii) KCCQ overall summary score and physical limitation subscale score; and (iv) workloads performed during CPX.
Statistical analysis
Nominal data are presented as percentages of total and continuous data as means (±standard deviations), medians and ranges (min–max), or inter‐quartile ranges (IQRs) (25th, 75th percentile) as appropriate. Wilcoxon signed‐rank test was used to compare within subject values before and after training. Alpha level was set at <0.05 for all analyses. All analyses were performed using JMP 14 software, SAS Institute Inc., Cary, NC.
Results
Study cohort
Fifty‐nine patients were screened for participation (Figure 1 ). Twenty‐three met inclusion criteria. Six declined to participate because of inability to commit to the training schedule. Two withdrew consent before baseline testing because of scheduling conflicts and were removed from data analyses.
Fifteen subjects underwent baseline testing (Table 1 ). Twelve completed post‐training testing. Two others withdrew early because of training intolerance, and one was urgently listed for transplant because of LVAD malfunction (short‐to‐shield phenomenon).
Table 1.
Baseline characteristics
| Age (years) | 51 (29–71) |
| BMI (kg/m2) | 30 (24–48) |
| Sex (male, %) | 10 (66%) |
| Race | |
| Black (%) | 9 (60%) |
| Hispanic (%) | 5 (33%) |
| Aetiology of CMP | |
| Non‐ischaemic (%) | 12 (80%) |
| Ischaemic (%) | 3 (20%) |
| Time with LVAD (months) | 18 (3–64) |
| LVAD type | |
| HM2 (%) | 12 (80%) |
| HM3 (%) | 3 (20%) |
| VAD strategy | |
| BTT (%) | 12 (80%) |
| DT (%) | 3 (20%) |
| ICD (%) | 13 (87%) |
| Atrial fibrillation (%) | 5 (33%) |
| Beta‐blockers (%) | 14 (93%) |
| Mineralocorticoid receptor antagonist (%) | 0 |
| ACE‐I/ARB (%) | 5 (33%) |
| Loop diuretics (%) | 6 (40%) |
| Sodium (mEq/L) | 141 ± 4 |
| Creatinine (mEq/L) | 1.2 ± 0.4 |
| Haemoglobin (g/dL) | 11.5 ± 2.3 |
| Lactate dehydrogenase (U/L) | 300 ± 75 |
| Age‐predicted maximal heart rate (b.p.m.) | 168 (157, 185) |
| Predicted V̇O2peak (mL/kg/min) | 26 (22, 28) |
| Baseline V̇O2peak (mL/kg/min) | 11.9 (9.5, 14.8) |
| Peak heart rate during HIIT (b.p.m.) | 118 (99, 136) |
| Recovery heart rate during HIIT (b.p.m.) | 110 (94, 127) |
| Total workload completed per session (W) | 1047 (745, 1438) |
| Percentage of total prescribed workload completed (total W in training period) | 90 (78, 99) |
| Training duration (days) | 35 (33, 40) |
| Time from end of training to testing (days) | 2 (1, 8) |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BTT, bridge to transplant; CMP, cardiomyopathy; DT, destination therapy; HM2, HeartMate II; HM3, HeartMate 3; ICD, implantable cardioverter device; LVAD, left ventricular assist device; VAD, ventricular assist device.
Values are counts (%), means ± standard deviation, median (range), or median (inter‐quartile range: 25th, 75th percentile).
Enrolled subjects were aged 51 (29–71) years; 66% were male; 93% were of non‐White race; 80% had non‐ischaemic cardiomyopathy; and 80% had HM2. Median time of LVAD support prior to training was 18 months (range 3–64 months). Prevalence of beta‐blocker therapy was 93%, 33% for angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, and 40% for loop diuretics. Medications were not changed during the study protocol. One‐third of the subjects had a history of atrial fibrillation.
Feasibility of high‐intensity interval training in left ventricular assist device patients
No major adverse events occurred during training including any driveline exit‐site infection. No sessions were discontinued by the supervising physician. One subject had a minor adverse event: recurrent asymptomatic supraventricular tachycardia that occurred after the planned increase in workloads during the fourth training session. This patient completed the training programme using the protocolized lower workloads intended for the first three sessions in all 15 sessions.
In the analysis of percentage of prescribed workload completed, all enrolled subjects who began the training programme were included to reflect overall tolerance to HIIT (n = 15). Median workload completed per session was 1047 W (745, 1438 W), which represented 90% (IQR: 78, 99%) of the total prescribed workload (Table 1 ). The median number of sessions completed was 13 (range: 6–15). Eight subjects (53%) completed all 15 sessions, three (20%) completed 13 sessions, one completed 12 sessions, one completed 11 sessions, one completed 10 sessions, and one completed 6 sessions. Subjects who withdrew before completion were invited to return for final testing.
Resting HR before the first training session was 80 (IQR: 74, 88) b.p.m. Median age‐predicted maximal HR was 168 (IQR: 157, 185) b.p.m. Training HR was 118 (IQR: 99, 136) b.p.m. during high‐intensity intervals and 110 (IQR: 94, 127) b.p.m. during recovery periods (Figure 2 ). RPE during high‐intensity intervals was 13 (IQR: 11, 15). A consistent increment was noted on Training Session 4 with the protocolized increase in workloads, with no change in subsequent training sessions (Figure 3 , P = 0.7, for comparison of median RPE across training sessions).
Figure 2.

Heart rate (HR) during training. HR was measured for every subject at rest and during each high‐intensity interval (peak) and 2 min into every other recovery period (recovery). APMHR, age‐predicted maximal HR (220 − age). Columns and error bars are medians and inter‐quartile range.
Figure 3.
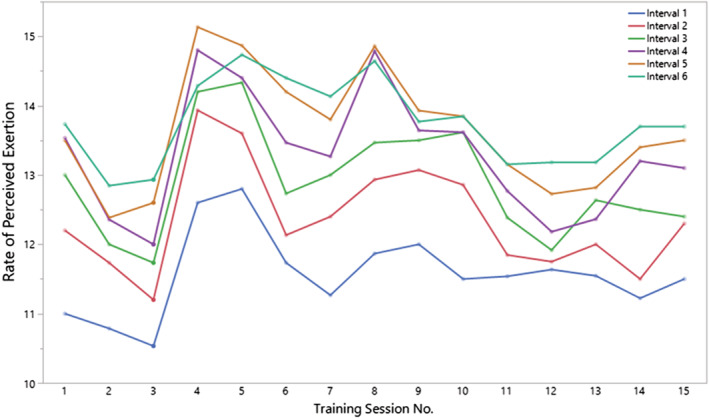
Rate of perceived exertion during high‐intensity intervals over time. Lines represent progression of median score on Borg scale (6–20 points) for each subject during each high‐intensity interval across all training sessions.
No difference was noted in LVAD flow increase from rest to peak exercise before and after training in subjects with either LVAD type.
Physiological response to high‐intensity interval training
High‐intensity interval training led to a significant improvement in V̇O2 at VT [7.1 (6.5, 9.1) to 8.5 (7.7, 9.3) mL/kg/min, P = 0.04] with small improvements in submaximal work rates [44 (14, 54) to 55 (21, 66) W, P = 0.05]. There was no improvement in V̇O2peak [11.9 (9.5, 14.8) to 12 (10.0, 15.0) mL/kg/min, P = 0.6] (Table 2 and Figure 4 ). Improvements in oxygen uptake were larger in subjects with the lowest baseline aerobic capacity. Subject's baseline submaximal and maximal oxygen uptake was strongly correlated with improvements in V̇O2 at VT (R 2 = 0.54, P = 0.006) and modestly correlated with improvements in V̇O2peak (R 2 = 0.32, P = 0.05), respectively (Supporting Information, Figure S2 ).
Table 2.
Cardiopulmonary exercise parameters (N = 12)
| Baseline | Post‐HIIT | P‐value | |
|---|---|---|---|
| V̇O2peak (mL/kg/min) | 11.9 (9.5, 14.8) | 12 (10, 15) | 0.6 |
| V̇O2 at VT (mL/kg/min) | 7.1 (6.5, 9.1) | 8.5 (7.7, 9.3) | 0.04* |
| Heart rate at VT (b.p.m.) | 100 (86, 109) | 96 (86, 109) | 0.9 |
| Peak heart rate (b.p.m.) | 120 (103, 145) | 109 (97, 141) | 0.3 |
| Respiratory exchange ratio (RER) | 1.3 (1.2, 1.4) | 1.3 (1.16, 1.3) | 0.16 |
| Ventilatory efficiency (VE/VCO2) | 40 (35, 44) | 42 (37, 43) | 0.88 |
| FEV1 (L) | 2.3 (2.1, 2.4) | 2.4 (2.1, 2.6) | 0.7 |
| Work rate at VT (W) | 44 (14, 54) | 55 (21, 66) | 0.054 |
| Peak work rate (W) | 99 (25, 141) | 100 (67, 124) | 0.18 |
| Exercise time (s) | 540 (439, 595) | 528 (391, 633) | 0.33 |
| Peak rate of perceived exertion (RPE) | 16 (14, 17) | 15 (14, 17) | 0.7 |
| HM2 pump speed (r.p.m.) | 9110 ± 363 | 9111 ± 363 | 0.8 |
| HM3 pump speed (r.p.m.) | 5300 ± 216 | 5350 ± 70 | 0.7 |
| HM2 flow increment rest to peak (L/min) | 1.0 (1.0, 1.5) | 1.2 (0.7, 1.8) | 0.8 |
| HM3 flow increment rest to peak (L/min) | 0.7 (0.1, 0.9) | 0.5 (0.2, 0.7) | 0.5 |
| KCCQ overall summary score | 72 (60, 79) | 64 (53, 84) | 0.5 |
| KCCQ physical limitation subscale score | 75 (62, 88) | 75 (62, 92) | 0.51 |
HIIT, high‐intensity interval training; HM2, HeartMate II; HM3, HeartMate 3; KCCQ, Kansas City Cardiomyopathy Questionnaire; RER, respiratory exchange ratio; VCO2, volume of exhaled carbon dioxide; VE, ventilation; VT, ventilatory threshold.
Values are means ± standard deviation or medians (inter‐quartile range: 25th, 75th percentile).
Difference of baseline vs. post‐HIIT, P < 0.05.
Figure 4.

Change in oxygen consumption after high‐intensity interval training. Maximal (V̇O2peak) and submaximal [V̇O2 at ventilatory threshold (VT)] oxygen consumption during cardiopulmonary exercise test before and after 5 weeks of high‐intensity interval training (HIIT). Columns and bars represent medians and inter‐quartile range.
Left ventricular remodelling after high‐intensity interval training
Of the initial 10 subjects with turndown echocardiograms, pre‐imaging and post‐imaging were available in only seven: two were deferred because of subtherapeutic international normalized ratio at the time of planned post‐training imaging, and one had suboptimal windows. These seven subjects were all supported with HM2 = 7; 57% (n = 4) were male; median age was 53 (IQR: 30, 64) years; and duration of LVAD support was 29 (IQR: 26, 31) months. All were on a beta‐blocker, while only one was on angiotensin‐converting enzyme inhibitor. HIIT resulted in a significant reduction in LV end‐diastolic volume [168 (144, 216) to 159 (124, 212) mL, P = 0.02] but no improvements in LV end‐systolic volume [118 (94, 152) to 99 (90, 147) mL, P = 0.08] or ejection fraction [30 (23, 31) to 28 (27, 30), P = 0.3] (Table 3 and Supporting Information, Figure S1 ). Importantly, all seven subjects showed some reduction in LV end‐diastolic volume. Other parameters such as mitral regurgitation severity (mild 62% vs. 67%, P = 0.9) and right ventricular systolic pressure (31, IQR: 21, 40 mmHg vs. 26, IQR: 18, 45 mmHg, P = 0.7) remained unchanged.
Table 3.
Turndown echocardiogram (N = 8)
| Baseline | Post‐HIIT | P‐value | |
|---|---|---|---|
| Left ventricular end‐diastolic diameter (cm) | 6.5 (5.7, 6.9) | 6.5 (5.7, 7.2) | 0.9 |
| Left ventricular end‐diastolic volume (mL) | 168 (144, 216) | 159 (124, 212) | 0.02* |
| Left ventricular end‐systolic volume (mL) | 118 (94, 152) | 99 (90, 147) | 0.08 |
| Left ventricular ejection fraction (%) | 30 (23, 31) | 28 (27, 30) | 0.3 |
HIIT, high‐intensity interval training.
Values are medians (inter‐quartile range: 25th, 75th percentile).
Difference of baseline vs. post‐HIIT, P < 0.05.
Quality of life
Kansas City Cardiomyopathy Questionnaire overall summary and physical limitation subscale scores did not show significant improvements after this 5 week HIIT programme. Other subscales in the questionnaire did not change significantly after training (Table 2 ).
Discussion
The main finding of this study is that a personalized, CPX‐guided HIIT protocol is feasible in stable LVAD patients on prolonged support. After 204 supervised sessions, there were no major adverse events.
Although only 25% of subjects initially screened for inclusion were enrolled in this study, 80% of those enrolled completed at least 80% of the training sessions. This suggests that HIIT can be tolerated by a subset of LVAD patients. Although, the applicability of HIIT in this population may be limited, it cannot be estimated accurately from this study given that our exclusion criteria were largely arbitrary because of the lack of pre‐existing data in this field.
Our HIIT protocol used each subject's baseline peak work rate (W) during CPX to guide training intensity. The customary use of training HR for this purpose would have been inadequate given the high prevalence of chronotropic incompetence and impaired HR recovery in this patient group 27 , 28 (Figure 2 ).
We increased workloads after the first three sessions, and RPE increased accordingly in all subjects on Training Session 4. In subsequent sessions, RPE did not vary significantly but showed a downward trajectory reflecting adaptation to training (Figure 3 , P = 0.7).
After only fifteen 30 min sessions, HIIT led to a significant improvement in submaximal exercise capacity (V̇O2 at VT). Despite the absence of a control group, prior data would suggest that these improvements can be attributed to the effects of HIIT, given the prolonged time on LVAD support and clinical stability of this cohort. Improvements in functional capacity are unlikely at this stage without a directed intervention. 8
The greatest improvements in submaximal capacity were seen in the most deconditioned subjects. Those with the lowest baseline V̇O2peak values [<12 mL/kg/min (N = 6)] saw the greatest (15%) improvement in V̇O2 at VT (P = 0.006) (Supporting Information, Figure S2 ).
Intriguingly, we also observed LV reverse remodelling at a time when remodelling due to unloading is expected to plateau. 29 The observed magnitude of decrease in diastolic LV volumes was small, 5% over a 5 week period, but provides important insights into the effects of HIIT.
Prior to this study, HIIT had rarely been reported in the LVAD population. In one case report, a severely deconditioned patient, unable to tolerate MCT, improved after a short heart rate‐guided HIIT programme. 20 In another study, 12 patients completed an unspecified HIIT protocol as part of a multimodal cardiac rehabilitation programme with improvement in cerebral blood flow during CPX. 30
Our findings open a pathway for the systematic application of this training modality in LVAD patients. Future studies should directly compare HIIT with MCT in patients with the lowest aerobic capacity and investigate its effects on peripheral and central determinants of oxygen consumption. We suggest that baseline CPX peak work rate, and not HR, should serve to guide training intensity, and protocolized adaptations in workloads should be contemplated to preserve high RPEs throughout the training programme.
One important question that remains is why V̇O2peak values did not follow the improvements seen in V̇O2 at VT. Without interventions to increase CO, such as increasing the LVAD speed during exercise 31 or providing rate response pacing in patients with chronotropic incompetence, 28 LVADs provide sufficient flow to support submaximal efforts but not peak exercise. 22 Hence, HIIT may have increased V̇O2 at VT through improvements in peripheral determinants of oxygen consumption, such as mitochondrial oxidative capacity 21 and capillary density of skeletal muscle. 32
Other reasons for the lack of improvement in V̇O2peak may include the small size and heterogeneity of our sample (n = 12 HM2 and n = 3 HM3) or the short duration of our training protocol (5 weeks).
However, improvements in V̇O2peak may not be a paramount goal in this patient population. V̇O2peak has a weak correlation with 6 min walk test distance in patients with LVAD compared with unsupported HF patients. 33 Therefore, improvements in submaximal exercise capacity, reflected by V̇O2 at VT, may be more relevant for improvements in health status. 4
Another important question is why we saw no consistent improvements in KCCQ scores. This may be explained by insensitivity of this questionnaire to detect short‐term changes in clinically stable LVAD. 3 On the other hand, our intervention was limited to physical training, and multimodal interventions affecting different subscales of the questionnaire may be needed to drive perceptible improvements in the overall score. 7
Study limitations
The main limitation of our study is the lack of a control group to compare the effect of HIIT with standard of care; however, improvements in exercise capacity without ET are concentrated during the first 3 to 6 months after LVAD implantation, and most patients in our cohort had passed this period.
A second limitation is the lack of progressive increments in training workloads after the fourth training session. Although the stability of RPE scores confirms a consistent effort intensity throughout training, some patients were training at suboptimal effort levels (RPE < 13), and a further increase in workloads was not contemplated in our protocol.
A third limitation is the short duration of our training protocol. This duration was chosen based on prior studies where HIIT showed significant benefits after even shorter training periods 21 and because patients in our study were asked to coordinate the training session schedule with their usual activities and other medical care. This would have complicated compliance with a longer duration protocol. However, patients on long‐term support may require longer periods of ET to see greater improvements. 8
And lastly, White patients were largely under‐represented in our sample, which could limit the generalizability of our results.
Conclusions
In conclusion, a personalized, CPX‐guided HIIT protocol is feasible in stable patients on LVAD support. More studies are needed to evaluate its effects on central and peripheral determinants of aerobic capacity.
Conflict of interest
U.P.J. serves as a consultant for Abbott Inc. but receives no honoraria. No other relevant conflicts of interest are reported.
Funding
None.
Supporting information
Figure S1. Left Ventricular End‐Diastolic Volume (LVEDV) before and after HIIT (n = 7)– LVEDV was measured during device turndown before and after training. HIIT resulted in a significant reduction in LVEDV: 168 (144, 216) mL to 159 (124, 212) mL.
Figure S2. Association between improvement in oxygen consumption and baseline level of fitness. Subject's baseline submaximal and maximal oxygen uptake was strongly correlated with improvements in V̇O2 at VT (R2 = 0.54, P = 0.006) and modestly correlated with improvements in V̇O2peak (R2 = 0.32, P = 0.05) respectively
Table S1. Reproducibility of CPX parameters.
Alvarez Villela, M. , Chinnadurai, T. , Salkey, K. , Furlani, A. , Yanamandala, M. , Vukelic, S. , Sims, D. B. , Shin, J. J. , Saeed, O. , Jorde, U. P. , and Patel, S. R. (2021) Feasibility of high‐intensity interval training in patients with left ventricular assist devices: a pilot study. ESC Heart Failure, 8: 498–507. 10.1002/ehf2.13106.
References
- 1. Goldstein DJ, Meyns B, Xie R, Cowger J, Pettit S, Nakatani T, Netuka I, Shaw S, Yanase M, Kirklin JK. Third annual report from the ISHLT mechanically assisted circulatory support registry: a comparison of centrifugal and axial continuous‐flow left ventricular assist devices. J Heart Lung Transplant 2019; 38: 352–363. [DOI] [PubMed] [Google Scholar]
- 2. Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno C, Walsh MN, Milano CA, Patel CB, Ewald GA, Itoh A. Two‐year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018; 378: 1386–1395. [DOI] [PubMed] [Google Scholar]
- 3. Cowger JA, Naka Y, Aaronson KD, Horstmanshof D, Gulati S, Rinde‐Hoffman D, Pinney S, Adatya S, Farrar DJ, Jorde UP, MOMENTUM 3 Investigators . Quality of life and functional capacity outcomes in the MOMENTUM 3 trial at 6months: a call for new metrics for left ventricular assist device patients. J Heart Lung Transplant 2018; 37: 15–24. [DOI] [PubMed] [Google Scholar]
- 4. Kerrigan DJ, Williams CT, Ehrman JK, Bronsteen K, Saval MA, Schairer JR, Swaffer M, Keteyian SJ. Muscular strength and cardiorespiratory fitness are associated with health status in patients with recently implanted continuous‐flow LVADs. J Cardiopulm Rehabil Prev 2013; 33: 396–400. [DOI] [PubMed] [Google Scholar]
- 5. Dunlay SM, Allison TG, Pereira NL. Changes in cardiopulmonary exercise testing parameters following continuous flow left ventricular assist device implantation and heart transplantation. J Card Fail 2014; 20: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marko C, Danzinger G, Käferbäck M, Lackner T, Müller R, Zimpfer D, Schima H, Moscato F. Safety and efficacy of cardiac rehabilitation for patients with continuous flow left ventricular assist devices. Eur J Prev Cardiol 2015; 22: 1378–1384. [DOI] [PubMed] [Google Scholar]
- 7. Kerrigan DJ, Williams CT, Ehrman JK, Saval MA, Bronsteen K, Schairer JR, Swaffer M, Brawner CA, Lanfear DE, Selektor Y, Velez M, Tita C, Keteyian SJ. Cardiac rehabilitation improves functional capacity and patient‐reported health status in patients with continuous‐flow left ventricular assist devices: the Rehab‐VAD randomized controlled trial. JACC: Heart Fail 2014; 2: 653–659. [DOI] [PubMed] [Google Scholar]
- 8. Laoutaris ID, Dritsas A, Adamopoulos S, Manginas A, Gouziouta A, Kallistratos MS, Koulopoulou M, Voudris V, Cokkinos DV, Sfirakis P. Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long‐term postimplantation. Eur J Cardiovasc Prev Rehabil 2011; 18: 33–40. [DOI] [PubMed] [Google Scholar]
- 9. Gal TB, Piepoli MF, Corrà U, Conraads V, Adamopoulos S, Agostoni P, Piotrowicz E, Schmid JP, Seferovic PM, Ponikowski P, Filippatos G. Exercise programs for LVAD supported patients: a snapshot from the ESC affiliated countries. Int J Cardiol 2015; 201: 215–219. [DOI] [PubMed] [Google Scholar]
- 10. Adamopoulos S, Corrà U, Laoutaris ID, Pistono M, Agostoni PG, Coats AJ, Crespo Leiro MG, Cornelis J, Davos CH, Filippatos G, Lund LH, Jaarsma T, Ruschitzka F, Seferovic PM, Schmid JP, Volterrani M, Piepoli MF. Exercise training in patients with ventricular assist devices: a review of the evidence and practical advice. A position paper from the Committee on Exercise Physiology and Training and the Committee of Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 3–13. [DOI] [PubMed] [Google Scholar]
- 11. Hayes K, Leet AS, Bradley SJ, Holland AE. Effects of exercise training on exercise capacity and quality of life in patients with a left ventricular assist device: a preliminary randomized controlled trial. J Heart Lung Transplant 2012; 31: 729–734. [DOI] [PubMed] [Google Scholar]
- 12. Kugler C, Malehsa D, Schrader E, Tegtbur U, Guetzlaff E, Haverich A, Strueber M. A multi‐modal intervention in management of left ventricular assist device outpatients: dietary counselling, controlled exercise and psychosocial support. Eur J Cardiothorac Surg 2012; 42: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 13. Karapolat H., Engin C., Eroglu M., Yagdi T., Zoghi M., Nalbantgil S., Durmaz B., Kirazlı Y., Özbaran M., eds. Efficacy of the cardiac rehabilitation program in patients with end‐stage heart failure, heart transplant patients, and left ventricular assist device recipients. Transplantation Proceedings 2013; 45: 3381–3385. [DOI] [PubMed] [Google Scholar]
- 14. Loyaga‐Rendon RY, Plaisance EP, Arena R, Shah K. Exercise physiology, testing, and training in patients supported by a left ventricular assist device. J Heart Lung Transplant 2015; 34: 1005–1016. [DOI] [PubMed] [Google Scholar]
- 15. Tabata I, Nishimura K, Kouzaki M, Hirai Y, Ogita F, Miyachi M, Yamamoto K. Effects of moderate‐intensity endurance and high‐intensity intermittent training on anaerobic capacity and ·VO2max . Med Sci Sports Exerc 1996; 28: 1327–1330. [DOI] [PubMed] [Google Scholar]
- 16. Gibala MJ, Little JP, MacDonald MJ, Hawley JA. Physiological adaptations to low‐volume, high‐intensity interval training in health and disease. J Physiol 2012; 590: 1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients a randomized study. Circulation 2007; 115: 3086–3094. [DOI] [PubMed] [Google Scholar]
- 18. Ellingsen Ø, Halle M, Conraads VM, Støylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V. High intensity interval training in heart failure patients with reduced ejection fraction. Circulation 2017; 135: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith KJ, Suarez IM, Scheer A, Chasland LC, Thomas HJ, Correia MA, Dembo LG, Naylor LH, Maiorana AJ, Green DJ. Cerebral blood flow during exercise in heart failure: effect of ventricular assist devices. Med Sci Sports Exerc 2019; 51: 1372–1379. [DOI] [PubMed] [Google Scholar]
- 20. Ugata Y, Wada H, Sakakura K, Ibe T, Ito M, Ikeda N, Fujita H, Shin‐ichi M. High‐intensity interval training for severe left ventricular dysfunction treated with left ventricular assist device. Int Heart J 2018; 59: 216–219. [DOI] [PubMed] [Google Scholar]
- 21. Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low‐volume high‐intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 2010; 588: 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brassard P, Jensen AS, Nordsborg N, Gustafsson F, Møller JE, Hassager C, Boesgaard S, Hansen PB, Olsen PS, Sander K, Secher NH, Madsen PL. Central and peripheral blood flow during exercise with a continuous‐flow left ventricular assist device: constant versus increasing pump speed: a pilot study. Circ Heart Fail 2011; 4: 554–560. [DOI] [PubMed] [Google Scholar]
- 23. Martina J, Jonge N, Rutten M, Kirkels JH, Klöpping C, Rodermans B, Sukkel E, Hulstein N, Mol B, Lahpor J. Exercise hemodynamics during extended continuous flow left ventricular assist device support: the response of systemic cardiovascular parameters and pump performance. Artif Organs 2013; 37: 754–762. [DOI] [PubMed] [Google Scholar]
- 24. Vincent G, Lamon S, Gant N, Vincent P, MacDonald J, Markworth J, Edge J, Hickey A. Changes in mitochondrial function and mitochondria associated protein expression in response to 2‐weeks of high intensity interval training. Front Physiol 2015; 6: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. George RS, Sabharwal NK, Webb C, Yacoub MH, Bowles CT, Hedger M, Khaghani A, Birks EJ. Echocardiographic assessment of flow across continuous‐flow ventricular assist devices at low speeds. J Heart Lung Transplant 2010; 29: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 26. Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol 2005; 98: 1985–1990. [DOI] [PubMed] [Google Scholar]
- 27. Grosman‐Rimon L, McDonald MA, Bar‐Ziv SP, Jacobs I, Tumiati LC, Cherney DZ, Rao V. Chronotropic incompetence, impaired exercise capacity, and inflammation in recipients of continuous‐flow left ventricular assist devices. J Heart Lung Transplant 2013; 32: 930–932. [DOI] [PubMed] [Google Scholar]
- 28. Villela MA, Guerrero‐Miranda CY, Chinnadurai T, Patel SR, Jorde UP. Rate response pacing in left ventricular assist device patients. ASAIO J 2019; 66: e29–e30. [DOI] [PubMed] [Google Scholar]
- 29. Drakos SG, Wever‐Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, Saidi A, Diakos NA, Stoker S, Davis ES, Movsesian M, Li DY, Stehlik J, Kfoury AG, UCAR (Utah Cardiac Recovery Program) Investigators . Magnitude and time course of changes induced by continuous‐flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol 2013; 61: 1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith KJ, Moreno‐Suarez I, Scheer A, Dembo L, Naylor LH, Maiorana AJ, Green DJ. Cerebral blood flow responses to exercise are enhanced in left ventricular assist device patients after an exercise rehabilitation program. J Appl Physiol 2020; 128: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vignati C, Apostolo A, Cattadori G, Farina S, Del Torto A, Scuri S, Gerosa G, Bottio T, Tarzia V, Bejko J, Sisillo E. Lvad pump speed increase is associated with increased peak exercise cardiac output and vo2, postponed anaerobic threshold and improved ventilatory efficiency. Int J Cardiol 2017; 230: 28–32. [DOI] [PubMed] [Google Scholar]
- 32. Tzanis G, Philippou A, Karatzanos E, Dimopoulos S, Kaldara E, Nana E, Pitsolis T, Rontogianni D, Koutsilieris M, Nanas S. Effects of high‐intensity interval exercise training on skeletal myopathy of chronic heart failure. J Card Fail 2017; 23: 36–46. [DOI] [PubMed] [Google Scholar]
- 33. Nahumi N, Morrison KA, Garan AR, Uriel N, Jorde UP. Peak exercise capacity is a poor indicator of functional capacity for patients supported by a continuous‐flow left ventricular assist device. J Heart Lung Transplant 2014; 33: 213–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Left Ventricular End‐Diastolic Volume (LVEDV) before and after HIIT (n = 7)– LVEDV was measured during device turndown before and after training. HIIT resulted in a significant reduction in LVEDV: 168 (144, 216) mL to 159 (124, 212) mL.
Figure S2. Association between improvement in oxygen consumption and baseline level of fitness. Subject's baseline submaximal and maximal oxygen uptake was strongly correlated with improvements in V̇O2 at VT (R2 = 0.54, P = 0.006) and modestly correlated with improvements in V̇O2peak (R2 = 0.32, P = 0.05) respectively
Table S1. Reproducibility of CPX parameters.


