Abstract
Aims
White matter lesions (WML) are common structural alterations in the white matter of the brain and their prevalence increases with age. They are associated with cerebral ischaemia and cognitive dysfunction. Patients with heart failure (HF) are at risk for cognitive decline. We hypothesized that the presence and duration of HF are associated with WML.
Methods and results
The LIFE‐Adult Study is a population‐based study of 10 000 residents of Leipzig, Germany. WML were quantitated in 2490 participants who additionally underwent cerebral MRI using the Fazekas score. Mean age was 64 years, and 46% were female; 2156 (86.6%) subjects had Fazekas score of 0–1, and 334 (13.4%) had Fazekas score of 2–3. Thirty participants had a medical history of HF, 1019 had hypertension, and 51 had a history of stroke. Median left ventricular ejection fraction of the participants with HF was 57% (interquartile ranges 54–62). Age, troponin T, NT‐proBNP, body mass index, history of acute myocardial infarction, stroke, HF, and diabetes were positively associated with WML in univariate analysis. On multivariate analysis, age, hypertension, stroke, and HF were independently associated with WML. The odd's ratio for the association of WML (Fazekas 2–3) with HF was 2.8 (95% CI 1.2–6.5; P = 0.019). WML increased with longer duration of HF (P = 0.036 for trend).
Conclusions
In addition to age, hypertension, and stroke, the prevalence and duration of HF are independently associated with WML. This observation sets the stage to investigate the prognostic value of WML in HF and the impact of HF therapies on WML.
Keywords: Heart failure, White matter lesions, Cognitive impairment, Magnetic resonance imaging, Population‐based study
Introduction
White matter lesions (WML), also called white matter hyperintensities, are common in the elderly with or without neurological symptoms. 1 , 2 They appear hyperintense on T2‐weighted magnetic resonance imaging (MRI) and are considered as a type of sporadic small vessel diseases, typically affecting periventricular regions and deep white matter sparing U‐fibres. 3 , 4
While age and arterial hypertension are strong predictors of WML, they are linked to various vascular risk factors and cerebrovascular diseases and are related to a three‐fold increase in risk for stroke. 5 , 6 , 7 A high burden of WMLs was found to be negatively associated with cognitive function and to the physical function. 8 , 9 , 10 , 11 , 12 , 13 Cross‐sectional and prospective studies further indicated a relation between WML and the onset of dementia. 7 , 14 Importantly, the prevalence of cognitive impairment is increased in patients with cardiovascular diseases. There is consistent evidence that cognitive impairment is highly prevalent in patients with heart failure (HF). 15 , 16 , 17 In previous reports and meta‐analyses, the prevalence for cognitive deficits among patients with HF varies from 25% to 80%, depending on the study design and the study population. 17 , 18 The cognitive deficits in patients with HF are characterized by increased alterations in the domains of attention, verbal memory, and emotional processing. 19 , 20 As structural correlates, medial temporal lobe atrophy (MTA), grey matter loss (GM), and WML have been reported in patients with HF using cerebral MRI (cMRI) scans. 1 , 19 , 20
To date, there are few studies on HF and the risk for structural brain damage; however, the findings are limited due to the study designs, which included mostly a high‐risk population group compared with a selected control group. The association of WML with cardiovascular risk factors and with HF in an unselected population remains elusive.
Therefore, the purpose of the present study was to analyse the association between cardiovascular risk factors, HF, and WML in a population‐based cohort study, the LIFE‐Adult Study (The Leipzig Research Centre for Civilization Diseases).
Methods
Study population
The LIFE‐Adult Study, as part of the ‘Leipzig Research Center for Civilization Diseases’ (LIFE), is a population‐based cohort study with 10 000 randomly selected residents from Leipzig (age ranged from 18–80 years), a city in the eastern part of Germany (approximately 600 000 inhabitants). 21 The study constituted an age and gender stratified sample as described previously. 22
All subjects signed an informed consent form, and the study protocol was in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Leipzig.
The study was initiated to evaluate the prevalence, early onset markers, genetic predispositions, and the role of lifestyle factors of major civilization diseases. Metabolic and vascular diseases, heart function, and cognitive impairment are also in the central focus of the LIFE project. 22
All subjects underwent an extensive core assessment program (5–6 h) including structured medical interviews, medical and psychological questionnaires, physical examination, and bio specimen collection. 22
Heart function
Heart failure was defined by the self‐reported diagnosis from the medical questionnaires, or rather, participants were asked if they have ever been diagnosed with HF by a physician.
To further describe potential arrhythmias or to specify the heart function, a 12‐lead electrocardiogram (ECG, Page‐Writer TC50® ECG system, Philips Medical Systems GmbH, Hamburg, Germany) was used to investigate cardiac arrhythmias, and echocardiography was used to obtain a phenotyping of the heart function to investigate the different types of HF (systolic vs. diastolic dysfunction).
The ECGs were separately evaluated for atrial fibrillation (AF) by two independent evaluators. Echocardiography was one of the routine examinations in LIFE Adult and was performed using the GE Vivid 7 dimension BTO8 echocardiography station (GE Healthcare, Chicago, Illinois, USA), according to the recommendations of the American Society of Echocardiography (ASE) and the European Society of Cardiology (ESC). 22 For this analysis, left ventricular ejection fraction (LVEF, Simpson's biplane method was used and computed by biplane 2D measurements) as systolic functional parameter and E/E′ as diastolic function parameter was used for evaluation.
Magnetic resonance imaging and Fazekas score
A total of 2490 participants additionally underwent MRI of the head. The cMRI scans were performed at 3‐Tesla on a MAGNETOM Verio Scanner (Siemens, Erlangen, Germany). T1‐weighted MPRAGE and FLAIR images were acquired as part of a standardized protocol: MPRAGE [flip angle (FA) = 9°, relaxation time (TR) = 2300 ms, inversion time (TI) = 900 ms, echo time (TE) = 2.98 ms, 1 mm isotropic resolution, acquisition time (AT) = 5.10 min]; FLAIR (TR = 5,000 ms, TI = 1800 ms, TE = 395 ms, 1 × 0.49 × 0.49 mm resolution, AT = 7.02 min). Structural MRI parameters were used for identifying neurodegenerative diseases, and WML have been analysed/acquired by an automated assessment such that higher score reflects the increased overall burden of cerebral WML. 23 WML were rated by using the Fazekas score (the scale ranges from 0 to 3), which reflects the overall burden of WML. 24 , 25 The Fazekas score is a visual rating of WML and is used in every day clinical practice. 26 Rating score 0 is defined by no lesions and represents a ‘healthy brain’. Score 1 describes focal lesions. Fazekas 0 and 1 are common in the elderly and are in most cases asymptomatic. The beginning of confluence lesions is defined by rating score 2 and diffuse involvement of the entire region is rated by score 3. Fazekas scores 2 and 3 are associated with cognitive dysfunction. 26 The volume of the white matter was adjusted to the volume of the head.
Neurocognitive testing
Participants older age (>60 years) underwent additionally neurocognitive testing at baseline examination. The Trail‐Making‐Test (TMT) A and B, as subtests of the CERADplus test, have been evaluated for this analysis. These are part from an extended neuropsychological test battery of the Consortium to Establish a Registry for Alzheimer's Disease. 27 , 28 The TMT A and B are used to identify deficits in attention, perceptual speed, cognitive flexibility, and executive function. TMT measures speed and mental flexibility. 29
Basically, in the first part of the test (TMT‐A), the targets are all numbers, while in the second part (TMT‐B), participants need to alternate between numbers and letters. The TMT was evaluated according to the execution times in seconds for adults specified in the manual. 30 , 31 Importantly, in both TMT‐A and B, the time to complete the task quantifies the performance; therefore, lower scores indicate better performance. 30 , 31 In this study, the measured time required to perform the tests was analysed in relation to the individual factors.
Statistical analysis
Data analysis was performed using SPSS version 25 for Windows (IBM, Chicago, USA).
Continuous variables are given as mean ± standard deviation (SD) or as median with interquartile ranges (25th–75th). Student's t‐test or non‐parametric tests (Mann–Whitney U‐test) were used for continuous variables. Categorical data are given as numbers of patients (%) and were compared using χ 2 test or Fishers' exact test (if necessary).
Binary regression model with WML Fazekas score 0–1 vs. 2–3 as the dependent variables was used for univariate and multivariate analyses. The natural logarithm of NT‐proBNP values was used in univariate and multivariate analyses. Variables that showed a significant correlation in univariate analysis and may be clinically important were entered into multiple binary regression model. Linear regression models were used for the cognitive tests. 32 The two‐sided P‐value <0.05 was considered as statistically significant. For selected comparisons between groups, the odds ratio (OR) or regression coefficient (RC) with 95% confidence interval (CI) are presented.
Results
Baseline characteristics
A total of 2490 individuals with cMRI were included in the full analysis. Nine hundred sixty‐five participants (38.8%) had Fazekas score 0, 1191 (47.8%) had Fazekas score 1, 290 (11.6%) had Fazekas score 2, and 44 (1.8%) had Fazekas score 3, respectively.
For further analysis, patients were stratified into two groups: none or mild WML [Fazekas 0–1, 2156 (86.6%) of the participants] vs. moderate or severe WML [Fazekas 2–3, 334 (13.4%) of the participants].
The baseline characteristics subdivided into the two groups Fazekas 0–1 vs. Fazekas 2–3 are presented in Table 1 . Individuals with more WML (Fazekas 2–3) were older, had more comorbidities (hypertension, diabetes, and atrial fibrillation), more often a history of stroke, myocardial infarction, or HF and higher plasma levels of troponin T and NT‐proBNP.
Table 1.
Baseline characteristics of the study participants with cerebral magnetic resonance imaging (cMRI) stratified by the Fazekas score 0–1 and 2–3, respectively
| Factors | FAZEKAS | ||
|---|---|---|---|
| Grade 0 and 1 (n = 2156) | Grade 2 and 3 (n = 334) | P‐value | |
| Median age, year (quantiles) | 63 (46;70) | 71 (66;75) | ≤0.001 |
| Female sex, no. (%) | 994 (46.6%) | 142 (43.0%) | 0.221 |
| BMI ≥ 25 no. (%) | |||
| Normal | 750 (35.2%) | 81 (24.6%) | ≤0.001 |
| Pre‐obesity | 920 (43.2%) | 139 (42.2%) | |
| Obesity | 458 (21.5%) | 109 (33.1%) | |
| Smoking, no. (%) | 322 (15.7%) | 40 (13.1%) | 0.247 |
| Myocardial infarction, no. (%) | 17 (0.8%) | 9 (2.8%) | 0.003 |
| Stroke, no. (%) | 32 (1.5%) | 19 (5.8%) | ≤0.001 |
| Heart failure, no. (%) | 18 (0.9%) | 12 (4.0%) | ≤0.001 |
| Diabetes, no. (%) | 216 (10.2%) | 58 (17.7%) | ≤0.001 |
| Hypertension, no. (%) | 799 (41.6%) | 220 (72.8%) | ≤0.001 |
| Troponin T (in pg/mL), median (quantiles) | 5 (3;7) | 7 (4.8;10.3) | ≤0.001 |
| NT‐proBNP (in pg/mL), median (quantiles) | 64.7 (34.4;113.9) | 105.4 (55.6;178.2) | ≤0.001 |
| AF in 12‐lead ECG, no. (%) | 27 (1.2%) | 11 (3.3%) | 0.005 |
AF, Atrial fibrillation; BMI, Body Mass Index; ECG, electrocardiogram; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Predictors of white matter lesions
In the univariate binary regression analysis, age, sex, troponin T, NT‐proBNP, body mass index (BMI), history of acute myocardial infarction, stroke, HF, AF, hypertension, and diabetes were positively associated with WML (data not shown).
In multivariate analysis, older age (OR 1.099, 95% CI 1.078–1.120, P ≤ 0.001), stroke almost reached statistical significance (OR 2.0, 95% CI 1.0–4.1, P = 0.05), hypertension (OR 2.0, 95% CI 1.5–2.8, P < 0.001), smoking (OR 1.9, 95% CI 1.2; 2.9, P = 0.003), and HF (OR 2.8, CI: 1.2–6.5, P = 0.019) were independently associated with severe WML (Fazekas 2–3), Figure 1 .
Figure 1.
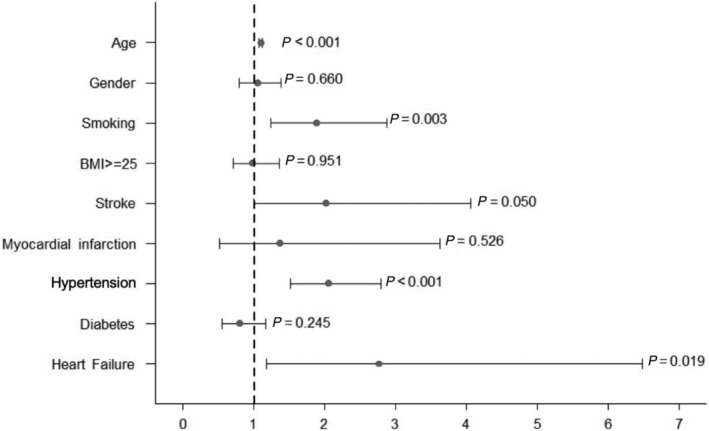
Multivariate analysis of factors associated with WML (Fazekas 2–3). Odd's ratio with 95% confidence interval and the respective P‐value are shown.
The independent association of HF was confirmed when AF was incorporated into the model. Gender, BMI, myocardial infarction, and diabetes were not associated with WML.
Duration of heart failure and hypertension
Twelve out of 30 participants (40.0%) with HF had WML Fazekas 2–3 as compared with 322 out of 2138 participants without HF (15.1%), P < 0.001. In the end model, age, gender, stroke, hypertension, and HF were included. Longer duration of HF was associated with an increased risk for WML (OR 0.8 for HF‐duration <3 years, 95% CI 0.1–7.5; OR 1.6 for HF 4–6 years duration, 95% CI 0.3–9.6 and OR 2.9 for HF diagnosis >6 years, 95% CI 1.1–7.9), P = 0.036 for trend, Table 2 .
Table 2.
Multivariate analysis of heart failure duration and other factors independently associated with white matter lesions (Fazekas 2 and 3)
| Factors | Odds ratio | P‐value | Confidence interval (95%) |
|---|---|---|---|
| Gender (female) | 1.1 | 0.660 | (0.8–1.4) |
| Age | 1.094 | ≤0.001 | (1.074–1.114) |
| Stroke | 2.0 | 0.034 | (1.0–3.9) |
| Hypertension | 2.0 | ≤0.001 | (1.5–2.7) |
| Heart failure (no vs. ≤3 years after diagnosis) | 0.8 | 0.811 | (0.1–7.5) |
| Heart failure (no vs. 4–6 years after diagnosis) | 1.6 | 0.580 | (0.3–9.6) |
| Heart failure (no vs. >6 years after diagnosis) | 2.9 | 0.036 | (1.1–7.9) |
BMI, body mass index.
A longer duration of heart failure ≥6 years was associated with severe white matter lesions.
Hypertension was also correlated with increased risk of WML, but the risk was independent from the duration of hypertension [Table 3 , OR 2.0 for hypertension duration <3 years (vs. no hypertension), OR 2.2 for hypertension duration 4–6 years, and OR 1.9 for hypertension duration >6 years].
Table 3.
Multivariate analysis of hypertension duration and other factors independently associated with white matter lesions (Fazekas 2 and 3)
| Factors | Odds ratio | P‐value | Confidence interval (95%) |
|---|---|---|---|
| Gender (female) | 1.1 | 0.603 | 0.8;1.4 |
| Age | 1.1 | ≤0.00 | 1.08;1.12 |
| Stroke | 2.2 | 0.018 | 1.1;4.1 |
| Hypertension (no vs. ≤3 years after diagnosis) | 2.0 | 0.002 | 1.3;3.2 |
| Hypertension (no vs. 4–6 years after diagnosis) | 2.2 | ≤0.001 | 1.4;3.4 |
| Hypertension (no vs. > 6 years after diagnosis) | 1.9 | ≤0.001 | 1.4;2.6 |
There was no relevant difference between the duration of hypertension and the occurrence of white matter lesions.
Atrial fibrillation and white matter lesions
Thirty‐eight out of 2490 participants had AF (1.5%). In univariate analysis, participants with more WML suffered more often from AF. After adjustment for age and gender, AF was no longer independently associated with WML (Figure 2 ). When including HF in the model, AF remained not statistical significant, while HF remained independently associated with WML (AF: P = 0.701, OR 1.2, 95% CI 0.5–2.8; HF: P = 0.024, OR 2.5, 95% CI 1.1–5.4).
Figure 2.
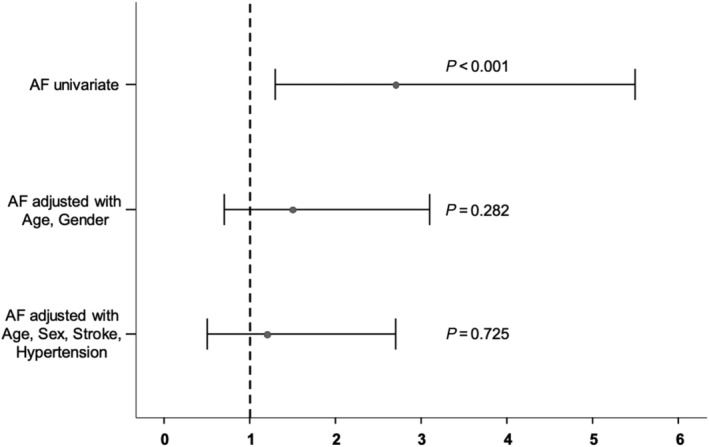
Univariate analysis of atrial fibrillation associated with WML and adjustment for age/gender and age/gender/history of stroke/history of hypertension. Odd's ratio with 95% confidence interval and the respective P‐value are shown.
Structural and functional echocardiographic parameters and white matter lesions
Seven hundred forty‐one participants had additional transthoracic echocardiography, including 23 of the participants with HF. Mean ejection fraction in HF subjects was 57% (54–62), and only two had a reduced LVEF (<50%). Neither LVEF (OR 0.99, 95% CI 0.83–1.18, P = 0.89) nor left ventricular filling index (E/E′) (OR 0.88, 95% CI 0.63–1.23, P = 0.46), nor left atrial volume indexed to body surface area (OR 1.01, 95% CI 0.96–1.07, P = 0.63) were associated with WML.
Heart failure and cognitive testing
When LIFE participants without cMRI (n = 6817) were included into the analysis, a long‐standing diagnosis of HF was associated with worse results in TMT A (Supporting Information, Table S1 , regression coefficient for TMT A: 3.65, 95% CI 0.56–6.74, P = 0.021). Not significant, but relevant association with regression coefficient 3.81 (95% CI –5.70–13.31, P = 0.433) could be evaluated for the TMT B (Supporting Information, Table S1 ).
Discussion
In this population‐based cross‐sectional analysis, we found that age, hypertension, stroke, and HF were independently associated with WML. Our results further indicated that a higher Fazekas score of WML was correlated with longer duration of HF, while no significant association was found regarding the duration of hypertension. However, echocardiographic parameters of left ventricular structure and function were not associated with WML.
Consistent with our findings, previous studies reported associations of increased systolic blood pressure and age with WML. 2 , 33 , 34 For instance, a previous study reported an increased risk for WML with longer duration of hypertension while we did not observe such an association. 35 It is important to mention that the patients in the LIFE Adult Study may had well‐diagnosed and well‐controlled hypertension, while poor control of blood pressure was associated with WML. 35 Corroborating our data, the history of stroke has previously been associated with WML. 7
Heart failure, atrial fibrillation, and correlating brain damage
The association between HF and WML has not been analysed in population‐based cohorts before. There are only a few studies reporting about HF and correlating structural brain measures in selected HF patients compared with age‐matched and gender‐matched controls, which lead to strong selection bias. 19 , 20 , 36 The relation of LVEF with underlying brain pathology remains controversial because some studies found an independent association between low LVEF and WML, while others did not. 1 , 37 While the exact role of WML for patients with HF with reduced LVEF (HFrEF) remains to be determined, our results indicated a clear association of WML with HF and preserved LVEF (HFpEF). In line with a previous study, we showed that participants with a long‐standing (>6 years) diagnosis of HF had more severe WML on cMRI compared with participants without HF or a diagnosis less than 6 years. 1
Previous findings reported an association between AF and WML. 38 , 39 For instance, Mayasi et al. retrospectively analysed a cohort with embolic stroke in their medical history (n = 234) and found a significant presence of anterior subcortical WML associated with AF.
Contrary to our initial expectations, our results showed no association between AF and WML after adjustment for age, gender, stroke, and hypertension. WML may develop in AF by silent brain infarcts, mostly in patients not on anticoagulation.
Heart failure and cognition
Considering the cognitive function in patients with HF, common findings are deficits in attention, working memory, and a reduced psychomotor speed. 40 , 41 We observed a significant association between the medical history of HF and worse results in the TMT A, but no significant association with the results of the TMT B. Other studies found similar results where patients with HF had increased task completion time in TMT A as compared with healthy control subjects. 16 However, older age was the strongest predictor for worse outcome in cognitive function.
Heart failure and cognitive decline are often coexisting in an aging population. However, the exact interaction is challenging, and information about the underlying mechanism is lacking. Several proposed pathophysiological pathways, such as reduced cerebral blood flow, 42 , 43 impaired cerebral autoregulation, and multiple embolic lesions have been considered for the development of WML. 1 , 44 Future studies are needed to investigate the impact of HF treatment on structural brain changings including WML alterations.
Limitations
There are some limitations to consider. The present data only represent a cross‐sectional design. Longitudinal follow‐up of study participants is underway. Baseline characteristics are strictly based on the medical history reported by the participants. There was no indication to differentiate between systolic or diastolic HF in the questionnaires. Therefore, we may lack data on potentially more individuals with HF, and we were not able to distinguish between participants with HFpEF and participants with previously HFrEF who have now recovered with HF therapy. Moreover, the majority of participants had a preserved LVEF, and our results may be different in subjects with reduced LVEF. The number of patients with HF and the number of patients with stroke was low, limiting the power of our analysis. This is the most likely explanation why only heart failure duration >6 years was independently associated with WML (P = 0.036), while hypertension (which was much more prevalent) showed a consistent odd's ratio between 1.9 and 2.2 with a very low P‐value.
Conclusions
In a population‐based sample, we found an independent association between a history of heart failure, a history of stroke, hypertension, and age with WML on cerebral MRI. More pronounced WML were associated with a longer duration of heart failure. Further studies are needed to investigate if WML can be used as a surrogate marker of cognitive decline in patients with heart failure under heart failure therapy.
Conflict of interest
None declared.
Funding
LIFE (Leipzig Research Centre for Civilization Diseases) is funded by means of the European Union, by the European Regional Development Fund (ERDF) and by funds of the Free State of Saxony within the framework of the excellence initiative (project numbers 713‐241202, 713‐241202, 14505/2470, and 14575/2470).
Supporting information
Table S1. Multivariate analysis of Trail‐Making‐Test A and B and other factors independently associated with white matter lesions (Fazekas 2 and 3).
Acknowledgements
This work is supported by LIFE–Leipzig Research Centre for Civilization Diseases, an organizational unit affiliated to the Medical Faculty of the University of Leipzig. LIFE was supported by a research grant by means of the European Union, by the European Regional Development Fund (ERDF), and by funds of the Free State of Saxony within the framework of the excellence initiative. We thank the LIFE Adult study team (‘Data Cluster’, ‘Ambulance Department’, ‘Data Quality Management’, and IT teams) for organizing the course of the study. We thank also the MRI team, especially to Leonie Lampe.
Open access funding enabled and organized by Projekt DEAL.
Stegmann, T. , Chu, M. L. , Witte, V. A. , Villringer, A. , Kumral, D. , Riedel‐Heller, S. G. , Roehr, S. , Hagendorff, A. , Laufs, U. , Loeffler, M. , Wachter, R. , and Zeynalova, S. (2021) Heart failure is independently associated with white matter lesions: insights from the population‐based LIFE‐Adult Study. ESC Heart Failure, 8: 697–704. 10.1002/ehf2.13166.
Contributor Information
Tina Stegmann, Email: Tina.Stegmann@medizin.uni-leipzig.de.
Mai L. Chu, Email: iamlinh9223@icloud.com
Veronica A. Witte, Email: witte@cbs.mpg.de.
Arno Villringer, Email: Arno.Villringer@medizin.uni-leipzig.de.
Deniz Kumral, Email: denizkumral89@gmail.com.
Steffi G. Riedel‐Heller, Email: Steffi.Riedel-Heller@medizin.uni-leipzig.de.
Susanne Roehr, Email: Susanne.Roehr@medizin.uni-leipzig.de.
Andreas Hagendorff, Email: Andreas.hagendorff@medizin.uni-leipzig.de.
Ulrich Laufs, Email: Ulrich.Laufs@medizin.uni-leipzig.de.
Markus Loeffler, Email: markus.loeffler@imise.uni-leipzig.de.
Rolf Wachter, Email: Rolf.Wachter@medizin.uni-leipzig.de.
Samira Zeynalova, Email: Samira.Zeynalova@imise.uni-leipzig.de.
References
- 1. Vogels RLC, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder‐Tanka JM, Henry CW. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail 2007; 9: 1003–1009. [DOI] [PubMed] [Google Scholar]
- 2. Vernooij MW. Incidental findings on brain MRI in the general population. N Engl J Med 2007; 357: 1821–1828. [DOI] [PubMed] [Google Scholar]
- 3. Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 2008; 64: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge RV, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M. STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Leeuw F‐E. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verhaaren BJF, Vernooij MW, de Boer R, Hofman A, Niessen WJ, van der Lugt A, Ikram MA. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension 2013; 61: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 7. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ 2010; 341: c3666–c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akisaki T, Sakurai T, Takata T, Umegaki H, Araki A, Mizuno S, Tanaka S, Oshashi Y, Iguchi A, Yokono K, Itos H. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J‐EDIT). Diabetes Metab Res Rev 2006; 22: 376–384. [DOI] [PubMed] [Google Scholar]
- 9. de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MMB. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000; 47: 145–151. [DOI] [PubMed] [Google Scholar]
- 10. Sachdev P, Wen W, Christensen H, Jorm A. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry 2005; 76: 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prins ND, van DEJ, den HT, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofmann A, Breteler MMB. Cerebral white matter lesions and the risk of dementia. Arch Neurol 2004; 61: 1531–1534. [DOI] [PubMed] [Google Scholar]
- 12. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015; 11: 157–165. [DOI] [PubMed] [Google Scholar]
- 13. Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, Provenzano FA, Schupf N, Manly JJ, Stern Y, Luchsinger JA, Mayeux R. Reconsidering harbingers of dementia: Progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging 2015; 36: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, van der Flier WM, Scheltens P, Barkhof F, Visser MC, Fazekas F, Schmidt R, O'Brien J, Waldemar G, Wallin A, Chabriat H, Pantoni L, Inzitari D, Erkinjuntti T. Longitudinal cognitive decline in subcortical ischemic vascular disease—the LADIS Study. Cerebrovasc Dis Basel Switz 2009; 27: 384–391. [DOI] [PubMed] [Google Scholar]
- 15. Gure TR, Blaum CS, Giordani B, Koelling TM, Galecki A, Pressler SJ, Hummel SL, Langa KM. The prevalence of cognitive impairment in older adults with heart failure. J Am Geriatr Soc 2012; 60: 1724–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vogels RLC, Scheltens P, Schroeder‐Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. Eur J Heart Fail 2007; 9: 440–449. [DOI] [PubMed] [Google Scholar]
- 17. Sterling MR, Jannat‐Khah D, Bryan J, Banerjee S, McClure LA, Wadley VG, Unverzagt FW, Levitan EB, Goyal P, Peterson JC, Manky JJ, Levine DA, Safford M. The prevalence of cognitive impairment among adults with incident heart failure: the “Reasons for geographic and racial fifferences in stroke” (REGARDS) Study. J Card Fail 2019; 25: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hajduk AM, Kiefe CI, Person SD, Gore JG, Saczynski JS. Cognitive change in heart failure: a systematic review. Circ Cardiovasc Qual Outcomes 2013; 6: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Almeida OP, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Flicker L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J 2012; 33: 1769–1776. [DOI] [PubMed] [Google Scholar]
- 20. Frey A, Sell R, Homola GA, Malsch C, Kraft P, Gunreben I, Morbach C, Alkonyi B, Schmid E, Colonna I, Hofer E, Müllges W, Ertl G, Heuschmann P, Solymosi L, Schmidt R, Störck S, Stoll G. Cognitive deficits and related brain lesions in patients with chronic heart failure. JACC Heart Fail 2018; 6: 583–592. [DOI] [PubMed] [Google Scholar]
- 21. Statistisches Landesamt des Freistaates Sachsen , Ordnungsamt Leipzig (Einwohnerregister).
- 22. Loeffler M, Engel C, Ahnert P, Alfermann D, Arelin K, Baber R, Beutner F, Binder H, Brähler E, Burkhardt R, Ceglarek C, Enzenbach C, Fuchs M, Glaesmer H, Girlich F, Hagendorff A, Häntzsch M, Hegerl U, Henger S, Hensch T, Hinz A, Holzendorf V, Husser D, Kersting A, Kiel A, Kirsten T, Kratzsch J, Krohn K, Luck T, Melzer S, Netto J, Nüchter M, Raschpichler M, Rauscher FG, Riedel‐Heller SG, Sander C, Scholz M, Schönknecht P, Schroeter ML, Simon JC, Speer R, Stäker J, Stein R, Stöbel‐Richter Y, Stumvoll M, Tarnok A, Teren A, Teupser D, Then FS, Tönjes A, Treudler R, Villringer A, Weissgerber A, Wiedemann P, Zachariae S, Wirkner K, Thiery J. The LIFE‐Adult‐Study: objectives and design of a population‐based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015; 15: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lampe L, Zhang R, Beyer F, Huhn S, Masouleh SK, Preusser S, Bazin PL, Schroeter ML, Villringer A, Witte V. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol 2019; 85: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mariß J, Maurer CJ. Neuroradiologische Messverfahren, Klassifikationen und Zeichen. Stuttgart/New York: Georg Thieme Verlag; 2018; 119–133. [Google Scholar]
- 25. Fazekas F, Chawluk J, Alavi A, Hurtig H, Zimmerman R. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol 1987; 149: 351–356. [DOI] [PubMed] [Google Scholar]
- 26. Kynast J, Lampe L, Luck T, Frisch S, Arelin K, Hoffmann KT, Loeffler M, Riedel‐Heller SG, Villringer A, Schroeter ML. White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory. J Cereb Blood Flow Metab 2018; 38: 996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moms JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The consortium to establish a registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39: 1159–1159. [DOI] [PubMed] [Google Scholar]
- 28. Thalmann B, Monsch AU, Schneitter M, Ermini‐Fünfschilling D, Spiegel R, Stählin HB. Die CERAD Neuropsychologische Testbatterie ‐ Ein gemeinsames minimales Instrumentarium zur Demenzabklärung. Memory Clinic, Geriatrische Universitätsklinik Basel; 1998. http://www.memoryclinic.ch/index.php%3Foption%3Dcom%5Fcontent%26task%3Dblogcategory%26id%3D11%26Itemid%3D16 [Google Scholar]
- 29. Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol 1955; 19: 393–394. [DOI] [PubMed] [Google Scholar]
- 30. Tischler L, Petermann F. Trail making test (TMT). Z Für Psychiatr Psychol Psycho 2010; 58: 79–81. [Google Scholar]
- 31. Reitan RM, Wolfson D. Traumatic brain injury. Vol. II. Recovery and rehabilitation. Neuropsychology Press, Tucson, AZ; 1988. [Google Scholar]
- 32. Collett D. Modelling survival data in medical research. 3rd New ed., Boca Raton: Chapman & Hall/CRC; 2015. [Google Scholar]
- 33. Launer LJ. Epidemiology of white matter lesions. Int Psychogeriatr 2003; 15: 99–103. [DOI] [PubMed] [Google Scholar]
- 34. de Leeuw F, de Groot JC, Oudkerk M, Witteman JCM, Hofman A, van Gijn J, Breteler MM. A follow‐up study of blood pressure and cerebral white matter lesions. Ann Neurol 1999; 46: 827–833. [DOI] [PubMed] [Google Scholar]
- 35. de Leeuw F‐E, de Groot JC, Oudkerk M, Witteman JCM, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 2002; 125: 765–772. [DOI] [PubMed] [Google Scholar]
- 36. Almeida OP, Garrido GJ, Etherton‐Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Brain and mood changes over 2 years in healthy controls and adults with heart failure and ischaemic heart disease. Eur J Heart Fail 2013; 15: 850–858. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt R, Fazekas F, Offenbacher H, Dusleag J, Lechner H. Brain magnetic resonance imaging and neuropsychologic evaluation of patients with idiopathic dilated cardiomyopathy. Stroke 1991; 22: 195–199. [DOI] [PubMed] [Google Scholar]
- 38. de Leeuw FE, de Groot JC, Oudkerk M, Kors JA, Hofman A, van Gijn J, Breteler MMB. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology 2000; 54: 1795–1801. [DOI] [PubMed] [Google Scholar]
- 39. Mayasi Y, Helenius J, McManus DD, Goddeau RP, Jun‐O'Connell AH, Moonis M, Henninger N. Atrial fibrillation is associated with anterior predominant white matter lesions in patients presenting with embolic stroke. J Neurol Neurosurg Psychiatry 2018; 89: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alagiakrishnan K, Mah D, Ahmed A, Ezekowitz J. Cognitive decline in heart failure. Heart Fail Rev 2016; 21: 661–673. [DOI] [PubMed] [Google Scholar]
- 41. Almeida OP, Flicker L. The mind of a failing heart: a systematic review of the association between congestive heart failure and cognitive functioning. Intern Med J 2001; 31: 290–295. [DOI] [PubMed] [Google Scholar]
- 42. Wardlaw JM, Valdés Hernández MC, Muñoz‐Maniega S. What are white matter hyperintensities made of? J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2015; 4: 001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alves TCTF, Rays J, Fráguas R, Wajngarten M, Meneghetti JC, Prando S, Busatto GF. Localized cerebral blood flow reductions in patients with heart failure: a study using 99mTc‐HMPAO SPECT. J Neuroimaging Off J Am Soc Neuroim 2005; 15: 150–156. [DOI] [PubMed] [Google Scholar]
- 44. Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and alzheimer disease: a population‐based cohort study. Arch Intern Med 2006; 166: 1003–1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multivariate analysis of Trail‐Making‐Test A and B and other factors independently associated with white matter lesions (Fazekas 2 and 3).


