Abstract
OBJECTIVES:
Continuation of aspirin for secondary prevention in persons with limited life expectancy (LLE) is controversial. We sought to determine the incidence and predictors of aspirin discontinuation in Veterans with LLE and/or advanced dementia (LLE/AD) who were taking aspirin for secondary prevention at nursing home (NH) admission, stratified by whether their limited prognosis (LP) was explicitly documented at admission.
DESIGN:
Retrospective cohort study using linked VA and Medicare clinical/administrative data and Minimum Data Set (MDS) resident assessments.
SETTING:
All Veterans Affairs nursing homes (referred to as Community Living Centers [CLCs]) in the United States.
PARTICIPANTS:
Older (≥65 years) CLC residents with LLE/AD, admitted for ≥7 days in fiscal years 2009–2015, who had history of CAD and/or stroke/TIA, and used aspirin within the first week of CLC admission (n=13,844).
MEASUREMENTS:
The primary dependent variable was aspirin discontinuation within the first 90 days after CLC admission, defined as 14 consecutive days of no aspirin receipt. Independent variables included an indicator for explicit documentation of LP, socio-demographics, environment of care characteristics, cardiovascular risk factors, bleeding risk factors, individual markers of poor prognosis (e.g., cancer, weight loss), and facility characteristics. Fine and Gray subdistribution hazard models with death as a competing risk were used to assess predictors of discontinuation.
RESULTS:
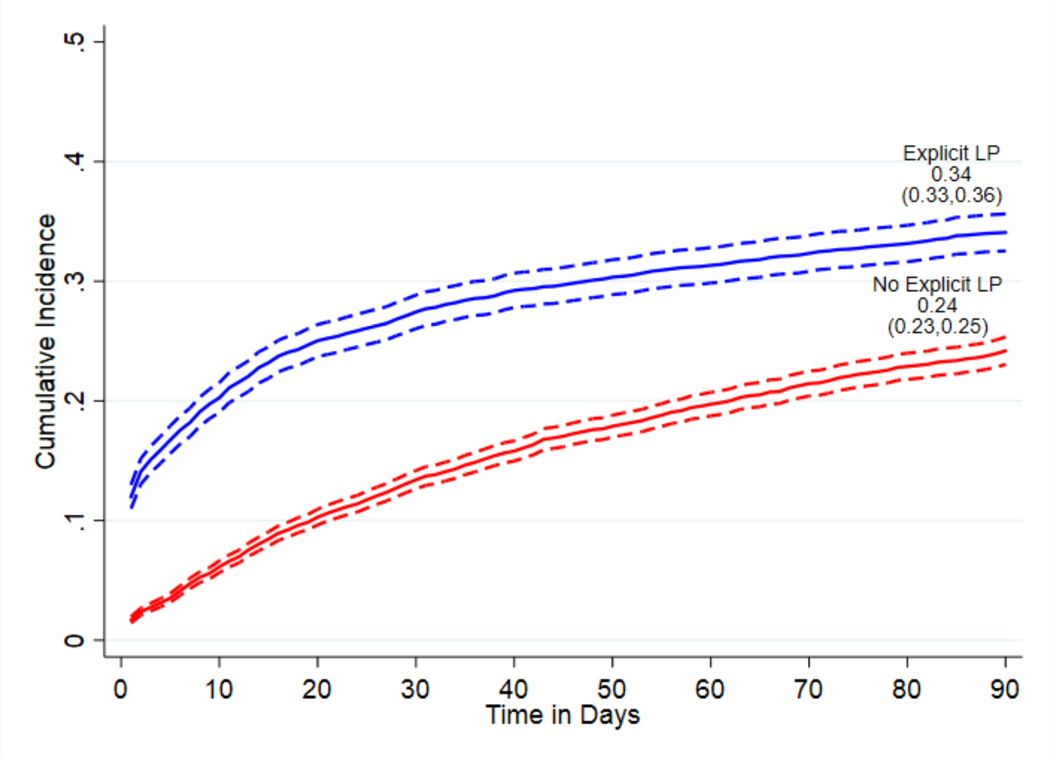
Cumulative incidence of aspirin discontinuation was 27% (95% CI 26%−28%) in the full sample, 34% (95% CI 33%−36%) in residents with explicit documentation of LP, and 24% (95% CI 23%−25%) in residents with no such documentation. The associations of independent variables with aspirin discontinuation differed in residents with versus without explicit LP documentation at admission.
CONCLUSION:
Just over a quarter of patients discontinued aspirin, which may reflect the unclear role of aspirin in end-of-life amongst prescribers. Future research should compare outcomes of aspirin deprescribing in this population.
Keywords: deprescribing, epidemiology, end-of-life, chronic disease management, Veterans
INTRODUCTION
Aspirin is a first-line agent for secondary prevention in cardiovascular disease (CVD), with guidelines strongly recommending initiating aspirin in those with CVD when benefit outweighs potential risks.1–3 However, guidelines do not address continued use of aspirin at end-of-life (EOL). Strong evidence for effectiveness of aspirin in secondary prevention combined with potential risk of a prothrombotic state upon discontinuation4–6 likely contribute to the continued use,7–9 despite a lack of direct evidence beyond 5 years.10
Changes in pharmacokinetics and pharmacodynamics associated with older age, and physiological changes as patients near EOL, place older adults at higher risk of adverse drug reactions.11,12 Although no trials have examined benefits and risks of aspirin for secondary prevention in older adults with limited life expectancy (LLE), two recent trials suggest decreased CVD benefits and increased bleeding risk associated with aspirin use in older adults.13,14 Further, a meta-analysis of 16 trials assessing risk of bleeding in adults receiving aspirin after a myocardial infarction (MI) or stroke found a 3-fold risk of major extracranial bleeding.15 A large cohort study reported similar findings in those presenting with MI or stroke, with those aged ≥75 years having highest risk for fatal or disabling gastrointestinal bleeding.16
There are no clinical practice guidelines regarding use of aspirin therapy at EOL. In a widely cited deprescribing consensus guideline for palliative care patients with advanced dementia, aspirin was one of 12 medications assessed with no consensus.17, This leaves providers with little guidance as to whether aspirin should be continued or discontinued in patients at EOL. Further, there have been no studies describing patterns of aspirin discontinuation in this population.
This study examined, in a national sample of Veterans admitted to Veterans Affairs (VA) nursing homes (known as Community Living Centers, or CLCs) with LLE and/or advanced dementia (AD) and a history of coronary artery disease (CAD) and/or stroke/transient ischemic attack (TIA), the cumulative incidence and predictors of aspirin discontinuation. We were interested in examining if residents whose limited prognosis (LP) was explicitly documented at admission would be more likely to have aspirin discontinued, and identifying clinical and environmental characteristics that predict discontinuation. The VA CLC setting offers a unique opportunity to study aspirin discontinuation in patients near EOL, due to the availability of medication records capturing detailed daily use of aspirin (an over-the-counter medication that is not reliably captured in prescription claims or pharmacy records) and the ability to link these data to extensive information on clinical and environmental characteristics.
METHODS
We conducted a retrospective cohort study using existing data from the Veterans Health Administration (VHA) from fiscal years (FY) 2009–2015. The VA Pittsburgh Healthcare System’s Institutional Review Board approved this study.
Data Sources
We used data from national VHA administrative and clinical databases. The VA Residential History File (RHF), which uses VA and Medicare utilization data to track location and timing of VA and non-VA healthcare utilization, was used to identify CLC episodes.18,19 VA Corporate Data Warehouse (CDW) provided information about VA healthcare utilization. Medicare claims were used to obtain information on non-VHA care for dually enrolled Veterans.20 The Minimum Dataset (MDS) provided comprehensive assessments of Veterans’ health status and care needs at CLC admission.21 We used variables that could be constructed using either version of the MDS (version 2.0 or 3.0). . We used bar-coded medication administration (BCMA) records to capture detailed information on all drugs and doses administered to patients while residing in the CLC. Data from VHA Support Service Center (VSSC) provided facility-level factors, and the VA Vital Status File provided death dates.
Sample
Figure S-1 (Supplemental Material) shows the sample construction process. We identified Veterans aged ≥65 years admitted to a CLC for ≥7 days over FY2009–2015 with LLE/AD at admission. CLC episodes from the RHF with a linked MDS admission assessment occurring within the first 30 days were included (n= 200,333). These episodes were limited to residents who met at least one criteria for LLE/AD at admission: 1) endorsement of the single MDS item asking whether the resident has end-stage disease or prognosis <6 months; 2) a score of ≥36 on the MDS Mortality Risk Index - Revised (MMRI-R), which has good specificity for 6-month mortality in nursing home (NH) residents;22,23 or 3) advanced dementia, identified by scoring ≥4 on the Cognitive Performance Scale or ≤7 on the Brief Interview for Mental Status in the MDS.24–26 We then limited the sample to Veterans with a history of CAD and/or stroke/TIA, using validated ICD-9-CM diagnosis algorithms27 applied to the VA CDW and Medicare claims, or endorsement of MDS indicators for these conditions.
Next, we limited the sample to episodes in which Veterans BCMA records showed receipt of preventive doses of aspirin (n= 17,973, 48%) for ≥1 day during the first 7 days of the CLC stay, with a total daily dose of 25–325 mg. Although the recommended preventive dose of aspirin is 50–325 mg in those with history of stroke/TIA, and 75–325 mg in those with CAD,7,8 we included the 4% (n=709) of residents who received only 25 mg of aspirin daily, to allow for missed or held doses. Finally, we restricted the sample to aspirin users with ≥14 days of follow-up after the first date of aspirin use, to allow enough follow-up time to observe discontinuation (n=13,844).
Measures
Aspirin Discontinuation
Aspirin discontinuation was defined as ≥14 consecutive days without receiving any doses of aspirin. Although 30 days is often used when measuring discontinuation of chronic medications with prescription refill records,28 we used a shorter gap length because daily BCMA records provides more precise information on whether medications were actually administered to patients each day. Further, it is unlikely that a sustained gap in use of ≥14 days would represent unintentionally missed doses in this setting. We followed Veterans until criteria for discontinuation was met, or until censoring due to death, CLC discharge, reaching 104 days of follow-up after admission, or end of available data (9/30/2015). Because the last 14 days of follow-up consisted of “immortal time” in which it was not possible to observe a gap in aspirin use of 14 days, the discontinuation date was defined as the first day of the 14-day gap and censoring dates were set to 14 days prior to the censoring event.29,30 Therefore, the maximum possible follow-up time was 90 days after CLC admission date. We ended follow-up at 90 days because a majority of the sample (n=9.896, 71%) had short-term stays (≤90 days) and we were particularly interested in understanding the extent to which CLC admission served as a cue for discontinuation.
Explicit Documentation of Limited Prognosis
Although all residents in the final sample were determined to have LLE and/or AD, only a subset were explicitly recognized at admission as having LP. A resident was considered to have explicit LP documentation if they met one of the following: 1) endorsement of the MDS item for end-stage disease/less than 6 months to live; 2) indication on the admission MDS of hospice use within the past 14 days, and/or 3) admission to a hospice treating specialty within the CLC, as indicated in the VA CDW. Explicit LP documentation was further used to stratify the sample, as our preliminary analyses confirmed our hypothesis that it was strongly associated with aspirin discontinuation.
Predictors of Discontinuation
We examined several types of clinical and environmental factors to examine as predictors of aspirin discontinuation, including socio-demographics, environment of care factors, facility characteristics, cardiovascular risk factors, individual markers of poor prognosis, and concomitant medications that may impact aspirin prescribing.
Socio-demographics were defined using the admission MDS and included age, sex, race/ethnicity, and marital status. Environment of care factors included admission source on the MDS (e.g., acute hospital, community), fiscal year of admission, and whether residents were hospitalized in the 90 days before admission, determined using Medicare claims and VA CDW. To capture caregiver factors, we abstracted next-of-kin information from CDW, including their relationship to the Veteran and distance from the centroid of their ZIP code to the CLC, categorized into quartiles. Facility characteristics included staff turnover rates, bed size, and facility complexity obtained from the VSSC for the VA parent station associated with the CLC. U.S census region, and rurality of the CLC U.S. census region and urban influence codes for the CLC were obtained by linking CLC ZIP code to data from the U.S. census and the Area Health Resource File, respectively.31-33
Cardiovascular risk factors included diabetes, congestive heart failure, hypertension, hyperlipidemia, recent stroke, recent MI, recent venous thromboembolism (VTE), and atrial fibrillation, identified using validated claims-based algorithms or endorsed MDS condition indicator.27,34 The MDS provided body mass index35 and smoking status.
Individual variables representing markers of poor prognosis were constructed using MDS items, including cancer diagnosis, swallowing problems, intravenous (IV) tube feeding, mechanical diet, poor appetite, recent weight loss, shortness of breath, recent changes in cognitive status, dehydration, renal failure, and pain. Having a fall in the 180 days before admission was identified using the MDS fall history item and/or meeting established claims-based criteria for serious fall injuries.36–38 Behavioral problems were assessed using the MDS Aggressive Behavior Scale.39 Dependency in activities of daily living (ADLs) was assessed using the ADL-short form.40 The Elixhuauser comorbidity index was used to count total number of comorbid conditions,41 excluding diabetes and hypertension, which were already captured.
We used BCMA records to capture concomintant prescribing of medications (on first or second day of CLC episode) which may impact aspirin prescribing, including those which increase (e.g., anti-thrombotic agents) or decrease (e.g., proton pump inhibitors [PPI]) bleeding risk.
Statistical analysis
Descriptive statistics were used to characterize the sample, overall and stratified by explicit LP documentation at admission. Hotdeck imputation was used to impute missing values.42
We calculated cumulative incidence of aspirin discontinuation by 90 days after admission in the overall sample and stratified by explicit LP documentation, treating death as a competing risk.43 We modeled the associations of all predictors described above with aspirin discontinuation using multivariable Fine and Gray competing risk subdistribution hazard models (reported as adjusted sub-distribution hazard ratios [aSDHR]), stratified by explicit documentation of LP.44 We used robust standard errors to account for intragroup correlation at the VA parent station level. Finally, we conducted sensitivity analyses to determine robustness of results to the length of the gap in aspirin used to define discontinuation; specifically, requiring a longer gap in therapy (≥30 days) and including Veterans who had ≥30 days of follow-up available after the index date (n=9,430).
RESULTS
Sample Characteristics
The sample consisted of 13,844 residents with LLE/AD and CAD/stroke/TIA who received aspirin in the first week of the CLC stay and had ≥14 days of follow-up. A majority (92%) were first observed to have aspirin use on days 1 or 2 of CLC admission. A majority did not have explicit LP documentation at admission (n=9,752; 70.4%). Most residents were ≥75 years old (72.3%), male (98.9%), married (52.0%), non-Hispanic white (80.3%), and were admitted from an acute hospital setting (72.1%). Those with LP documentation were more likely to be underweight (11.5% vs. 6.5%), and have cancer (42.5% vs. 29.7%), poor appetite (49.5% vs. 35.4%) and >5 Elixhauser comorbidities (43.0% vs. 38.7%) (Table 1, Table 2, Table S-3).
Table 1:
Demographic, Environment of Care, and Facility Characteristics of the Sample, Stratified by Explicit Documentation of Limited Prognosis.
| Full Sample (n=13,844) n (%) | Explicit Documentation of Limited Prognosis (n=4,092) n (%) | No Explicit Documentation of Limited Prognosis (n=9,752) n (%) | |
|---|---|---|---|
| Age at admission** | |||
| 65–74 | 3,839 (27.7) | 1,030 (25.2) | 2,809 (28.8) |
| 75–84 | 5,260 (38.0) | 1,557 (38.1) | 3,703 (38.0) |
| ≥85 | 4,745 (34.3) | 1,505 (36.8) | 3,240 (33.2) |
| Female sex** | 158 (1.1) | 72 (1.8) | 86 (0.9) |
| Race/ethnicity* | |||
| White | 11,121 (80.3) | 3,323 (81.2) | 7,798 (80.0) |
| Black | 1,926 (13.9) | 513 (12.5) | 1,413 (14.5) |
| Hispanic | 547 (4.0) | 178 (4.4) | 369 (3.8) |
| Other | 250 (1.8) | 78 (1.9) | 172 (1.8) |
| Married** | 7,199 (52.0) | 2,272 (55.5) | 4,927 (50.5) |
| Fiscal year of admission | |||
| 2009 | 1,864 (13.5) | 514 (12.6) | 1,350 (13.8) |
| 2010 | 1,852 (13.4) | 572 (14.0) | 1,280 (13.1) |
| 2011 | 1,960 (14.2) | 596 (14.6) | 1,364 (14.0) |
| 2012 | 1,986 (14.4) | 578 (14.1) | 1,408 (14.4) |
| 2013 | 2,124 (15.3) | 612 (15.0) | 1,512 (15.5) |
| 2014 | 2,118 (15.3) | 654 (16.0) | 1,464 (15.0) |
| 2015 | 1,940 (14.0) | 566 (13.8) | 1,374 (14.1) |
| Admission Source** | |||
| Acute hospital | 9,979 (72.1) | 2,889 (70.6) | 7,090 (72.7) |
| Community | 2,744 (19.8) | 826 (20.2) | 1,918 (19.7) |
| Nursing home | 788 (5.7) | 251 (6.1) | 537 (5.5) |
| Other | 333 (2.4) | 126 (3.1) | 207 (2.1) |
| Hospitalization in 90 days prior to admission** | 8,356 (60.4) | 2,584 (63.2) | 5,772 (59.2) |
| Next of kin relationship to the Veteran** | |||
| Spouse | 5,415 (39.1) | 1,506 (36.8) | 3,909 (40.1) |
| Child | 5,739 (41.5) | 1,799 (44.0) | 3,940 (40.4) |
| Sibling | 1,251 (9.0) | 362 (8.9) | 889 (9.1) |
| Other relative | 613 (4.4) | 192 (4.7) | 421 (4.3) |
| Friend or other person of unknown relation | 826 (6.0) | 233 (5.7) | 593 (6.1) |
| Distance from next of kin ZIP code to the CLC ** | |||
| Quartile 1 | 3,461 (25.0) | 1,176 (28.7) | 2,285 (23.4) |
| Quartile 2 | 3,461 (25.0) | 1,069 (26.1) | 2,392 (24.5) |
| Quartile 3 | 3,461 (25.0) | 997 (24.4) | 2,464 (25.3) |
| Quartile 4 | 3,461 (25.0) | 850 (20.8) | 2,611 (26.8) |
| US Census region of the CLC** | |||
| Northeast | 2,355 (17.0) | 821 (20.1) | 1,534 (15.7) |
| Midwest | 4,528 (32.7) | 1,070 (26.2) | 3,458 (35.5) |
| South | 4,604 (33.3) | 1,466 (35.8) | 3,138(32.2) |
| West | 2,357 (17.0) | 735 (18.0) | 1,622 (16.6) |
| Urban Influence Code for the CLC** | |||
| Large metro | 6,530 (47.2) | 1,891 (46.2) | 4,639 (47.6) |
| Small metro | 5,924 (42.8) | 1,871 (45.7) | 4,053 (41.6) |
| Micropolitan | 1,078 (7.8) | 267 (6.5) | 811 (8.3) |
| Noncore rural | 312 (2.3) | 63 (1.5) | 249 (2.6) |
| Complexity Level of the parent station** | |||
| 1a (Most Complex) | 5, 236 (37.8) | 1,430 (35.0) | 3,806 (39.0) |
| 1b | 1,583 (11.4) | 452 (11.1) | 1,131 (11.6) |
| 1c | 2,517 (18.2) | 829 (20.3) | 1,688 (17.3) |
| 2 | 1,949 (14.1) | 612 (15.0) | 1,337 (13.7) |
| 3 (Least Complex) | 2,559 (18.5) | 769 (18.8) | 1,790 (18.4) |
| Bed Size of CLC** | |||
| <60 beds | 2,034 (14.7) | 773 (18.9) | 1,261 (12.9) |
| 60–120 beds | 4,934 (35.6) | 1,399 (34.2) | 3,535 (36.3) |
| ≥ 120 beds | 6,876 (49.7) | 1,920 (46.9) | 4,956 (50.8) |
Table 2:
Cardiovascular Risk Factors, Markers of Poor Prognosis, and Medication Use for the Sample, Stratified by Explicit Documentation of Limited Prognosis.
| Full Sample (n=13,844) n (%) | Explicit Documentation of Limited Prognosis (n=4,092) n (%) | No Explicit Documentation of Limited Prognosis (n=9,752) n (%) | |
|---|---|---|---|
| Cardiovascular Risk Factors | |||
| Number of qualifying conditions ** | |||
| Coronary artery disease OR stroke (not both) | 10.451 (75.5) | 3,270 (79.9) | 7,181 (73.6) |
| Both coronary artery disease AND stroke | 3,393 (24.5) | 822 (20.1) | 2,571 (26.4) |
| Diabetes** | 7,466 (53.9) | 1,933 (47.2) | 5,533 (56.7) |
| Congestive heart failure* | 7,317 (52.9) | 2,228 (54.5) | 5,089 (52.2) |
| Hypertension** | 12,982 (93.8) | 3,777 (92.3) | 9,205 (94.4) |
| Hyperlipidemia* * | 9,742 (70.4) | 2,792 (68.2) | 6,950 (71.3) |
| Venous thromboembolism | 1,643 (11.9) | 509 (12.4) | 1,134 (11.6) |
| Atrial fibrillation | 2,648 (19.1) | 816 (19.9) | 1,832 (18.8) |
| Myocardial Infarction in the last year** | 1,161 (8.4) | 403 (9.9) | 758 (7.8) |
| Stroke in the last year** | 3,733 (27.0) | 897 (21.9) | 2,836 (29.1) |
| Current Smoker* | 1,233 (8.9) | 399 (9.8) | 834 (8.6) |
| Body Mass Index (kg/m2)** | |||
| Normal or healthy weight (18.5 to <25.0) | 5,968 (43.1) | 1,962 (48.0) | 4,006 (41.1) |
| Underweight (<18.5) | 1,107 (8.0) | 472 (11.5) | 635 (6.5) |
| Overweight (25.0 to <30.0) | 3,970 (28.7) | 1,084 (26.5) | 2,886 (29.6) |
| Obese (≥30) | 2,799 (20.2) | 574 (14.0) | 2,225 (22.8) |
| Markers of Poor Prognosis | |||
| Advanced dementia** | 4,211 (30.4) | 1,075 (26.3) | 3,136 (32.2) |
| Documentation of limited prognosis or hospice use | 4,094 (29.6) | 4,092 (100) | 0 (0) |
| Number of Elixhauser conditions** | |||
| 0–1 | 1,019 (7.4) | 221 (5.4) | 798 (8.2) |
| 2–3 | 3,144 (22.7) | 846 (20.7) | 2,298 (23.6) |
| 4–5 | 4,145 (29.9) | 1,264 (30.9) | 2,881 (29.5) |
| >5 | 5,536 (40.0) | 1,761 (43.0) | 3,775 (38.7) |
| Recent weight loss** | 5,555 (40.1) | 1,568 (38.3) | 3,987 (40.9) |
| Poor appetite** | 5,478 (39.6) | 2,025 (49.5) | 3,453 (35.4) |
| Renal failure** | 2,830 (20.4) | 681 (16.6) | 2,149 (22.0) |
| Dehydration | 138 (1.0) | 43 (1.1) | 95 (1.0) |
| Acute change in mental status** | 1,365 (9.9) | 496 (12.1) | 869 (8.9) |
| Shortness of breath** | 5,586 (40.4) | 1,883 (46.0) | 3,703 (38.0) |
| Cancer** | 4,633 (33.5) | 1,740 (42.5) | 2,893 (29.7) |
| Activities of Daily Living (ADL) score** | |||
| 0 - <1 | 1,438 (10.4) | 464 (11.3) | 974 (10.0) |
| 1 to <2 | 3,218 (23.2) | 733 (17.9) | 2,485 (25.5) |
| 2 to <3 | 4,714 (34.1) | 1,203 (29.4) | 3,511 (36.0) |
| 3 to <4 | 3,185 (23.0) | 1,127 (27.5) | 2,085 (21.1) |
| 4 | 1,289 (9.3) | 565 (13.8) | 724 (7.4) |
| Aggressive Behavior** | |||
| None | 11,405 (82.4) | 3,469 (84.8) | 7,936 (81.4) |
| Moderate | 1,552 (11.2) | 378 (9.2) | 1,174 (12.0) |
| Severe | 669 (4.8) | 193 (4.7) | 476 (4.9) |
| Very severe | 218 (1.6) | 52 (1.3) | 166 (1.7) |
| IV feeding tube in place** | 1,282 (9.3) | 275 (6.7) | 1,007 (10.3) |
| On Mechanical Diet** | 5,643 (40.8) | 1,869 (45.7) | 3,774 (38.7) |
| Swallowing Problems** | 2,538 (18.3) | 849 (20.8) | 1,689 (17.3) |
| Presence of any pain (n, % yes)** | 9,563 (69.1) | 3,005 (73.4) | 6,558 (67.3) |
| Fall or fracture in 180 days before admission** | 6,870 (49.6) | 1,929 (47.1) | 4,941 (50.7) |
| Medications Prescribed Wlich May Impact Aspirin Prescribing | |||
| Anti-platelet** | 2,558 (18.5) | 666 (16.3) | 1,892 (19.4) |
| Anti-thrombotic Agents** | 7,057 (51.0) | 1,707 (41.7) | 5,250 (54.9) |
| H2-Receptor Antagonists* | 1,547 (11.2) | 421 (10.3) | 1,126 (11.6) |
| Proton Pump Inhibitors | 7,290 (52.7) | 2,119 (51.8) | 5,171 (53.0) |
| NSAIDs** | 514 (3.7) | 124 (3.0) | 390 (4.0) |
Cumulative Incidence of Aspirin Discontinuation
The cumulative incidence of aspirin discontinuation by 90 days after admission in the full sample was 27% (95% CI, 26%−28%) (Figure S-2. Supplemental Material). In stratified analyses, the cumulative incidence was 34% (33%−36%) in those with LP documented, and 24% (95% CI 23%−25%) in those without LP documented (Figure 1).
Figure 1:
Cumulative incidence of aspirin discontinuation, using primary definition requiring a 14-day gap in use, stratified by explicit documentation of limited prognosis (LP) at admission.
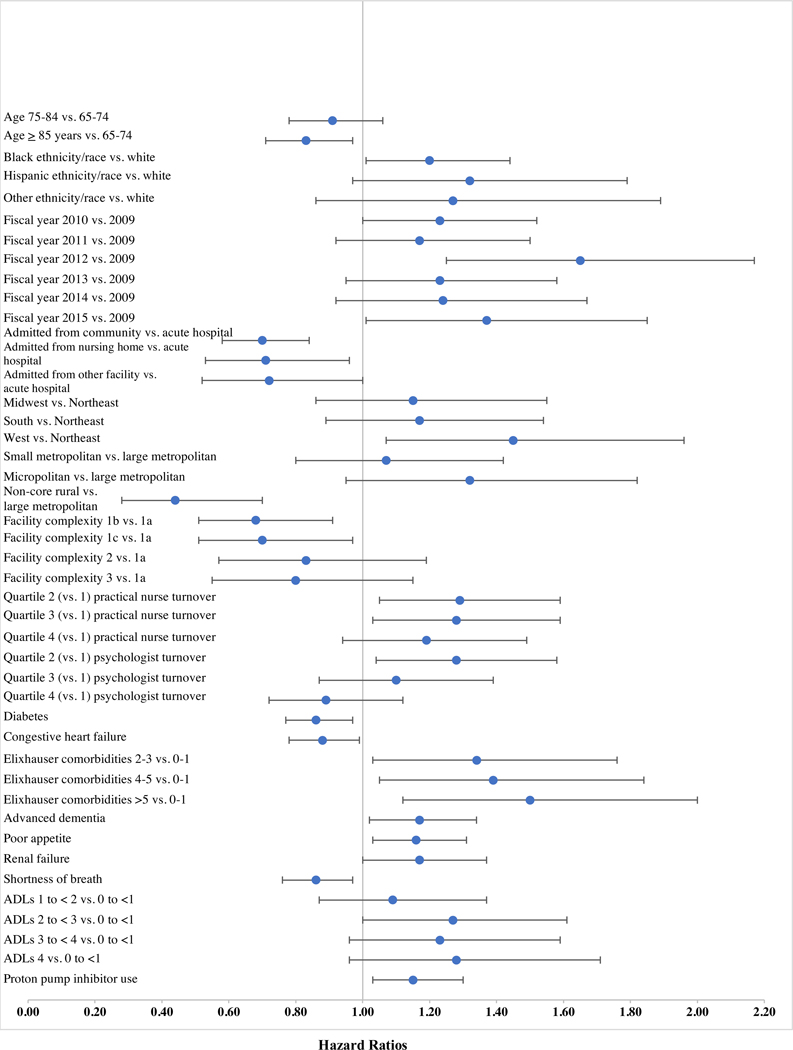
Factors Associated with Aspirin Discontinuation - Veterans with Explicit LP Documentation
Figure 2 presents factors significantly associated with aspirin discontinuation in Veterans with explicit LP documentation (n=4,092). Supplemental Table S-1 shows complete results of the competing risk models.
Figure 2:
Hazard ratios (HR) and 95% confidence intervals for factors with statistically significant (p<.05) associations with aspirin discontinuation in residents with explicit documentation of limited prognosis (LP) at admission (n=4,092).
Veterans aged ≥85 years were less likely to discontinue aspirin compared to those 65–74 years (aSDHR 0.83, 95% CI 0.71–0.97), while Black, non-Hispanic Veterans were more likely than White, non-Hispanic Veterans to discontinue (aSDHR 1.20, 95% CI 1.01–1.44). Among environment of care factors, admission in FY2012 (aSDHR 1.65, 95% CI 1.25–2.17) and FY2015 (aSDHR 1.37, 95% CI 1.01–1.85) versus FY2009 was associated with increased likelihood of discontinuation. Admission from the community (aSDHR 0.70, 95% CI 0.58–0.84) or a NH (aSDHR 0.71, 95% CI 0.53–0.96) versus an acute hospital was associated with lower likelihood of discontinuation. Several facility characteristics were associated with increased likelihood of discontinuation. Veterans in the Western versus Northeastern United States or with higher levels of turnover of practical nurses and psychologists tended to discontinue at a higher rate (see Figure 1 and Table S-1). However, residing in a rural CLC was associated with a lower likelihood of discontinuation compared to those in large metropolitan areas (aSDHR 0.44, 95% CI 0.28–0.70).
Of the cardiovascular risk factors, only diabetes (aSDHR 0.86, 95% CI 0.77–0.97) or congestive heart failure (aSDHR 0.88, 95% CI 0.78–0.99) were associated with decreased likelihood of discontinuation. However, several markers of poor prognosis were associated with increased likelihood of discontinuation, including greater number of comorbidities (see Figure 1 and Table S-1), advanced dementia (aSDHR 1.17, 95% CI 1.02–1.34), poor appetite (aSDHR 1.16, 95% CI 1.03–1.31), renal failure (aSDHR 1.17, 95% CI 1.00–1.37) and higher ADL dependency (score 2 to <3 vs. <1; aSDHR 1.27, 95% CI 1.00–1.61). Shortness of breath was associated with lower likelihood of discontinuation (aSDHR 0.86, 95% CI 0.76–0.97). Use of PPIs was the only bleeding risk factor associated with increased likelihood of discontinuation (aSDHR 1.15, 95% CI 1.03–1.30).
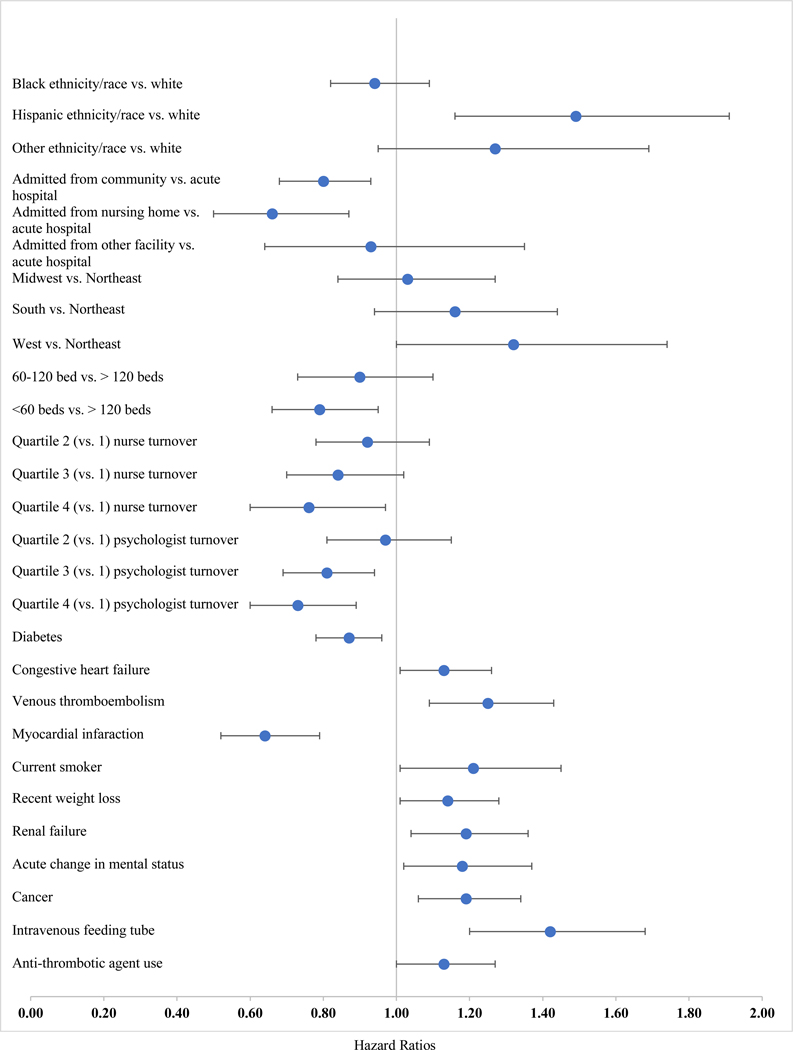
Factors Associated with Aspirin Discontinuation - Veterans without Explicit LP Documentation
Figure 3 presents factors significantly associated with aspirin discontinuation in Veterans without explicit LP documentation; see Table S-1 for full model results. The pattern of significant associations in those without explicit LP documentation was for the most part different from those observed in those with explicit LP documentation, with a few exceptions. As with the documented LP group, those without documented LP were less likely to be discontinued if they were admitted from the community (aSDHR 0.80, 95% CI 0.68–0.93) or another NH (aSDHR 0.66, 95% CI 0.50–0.87) or had diabetes (aSDHR 0.87, 95% CI 0.78–0.96), and were more likely to be discontinued if they had renal failure (aSDHR 1.19, 95% CI 1.04–1.36,p<0.05), or were cared for in a facility located in the West versus (aSDHR 1.32, 95% CI 1.00–1.74).
Figure 3.
Hazard ratios (HR) and 95% confidence intervals for factors with statistically significant (p<.05) associations with aspirin discontinuation in residents with no explicit documentation of limited prognosis (LP) at admission (n=9,752).
In addition, several additional predictors of discontinuation were observed in those without LP documentation. Hispanic Veterans had a significantly higher likelihood of discontinuation compared to non-Hispanic Veterans (aSDHR 1.49, 95% CI 1.16–1.91). Among the facility factors, receiving care in a facility with <60 beds versus >120 beds (aSDHR 0.79, 95% CI 0.66–0.95) and higher nurse and psychologist turnover rates were associated with decreased likelihood of discontinuation (Figure 3; Supplemental Table S-1). The relationship of cardiovascular risk factors to aspirin discontinuation in those without LP documentation was mixed. In addition to diabetes, having had a recent MI (aSDHR 0.64, 95% CI 0.52–0.79) was associated with decreased discontinuation, while congestive heart failure (aSDHR 1.13, 95% CI 1.01–1.26), VTE (aSDHR 1.25, 95% CI 1.09–1.43), and being a current smoker (aSDHR 1.21, 95% CI 1.01–1.45) wereassociated with increased discontinuation. Several markers of poor prognosis were associated with discontinuation in this subgroup, in addition to renal failure. These included recent weight loss (aSDHR 1.14, 95% CI 1.01–1.28), acute change in mental status (aSDHR 1.18, 95% CI 1.02–1.37), cancer (aSDHR 1.19, 95% CI 1.06–1.34), and requiring an IV feeding tube (aSDHR 1.42, 95% CI 1.20–1.68). Finally, concomitant antithrombotic prescribing was associated with higher likelihood of discontinuation in this subgroup (aSDHR 1.13, 95% CI 1.00–1.27).
Sensitivity analysis
In sensitivity analyses using a 30-day gap to define discontinuation (n=9,430), the overall cumulative incidence of discontinuation within 90 days was 22% (95% CI 21%−23%) (Figure S-3, Supplemental Material). Stratified analyses revealed a cumulative incidence of discontinuation of 29% (95% CI 28%−31%) in residents with explicit LP documentation, and 19% (95% CI 18%−20%) in residents without explicit documentation (Figure S-4, Supplemental Material).
Results of the stratified competing risk models using the alternative discontinuation definition are shown in Table S-2. In those with LP documentation, the substantive pattern of results remained very similar. In the subgroup with no LP documentation, the pattern of results did change to some extent. Of clinical importance, having a diagnosis of AD became significantly associated with a lower likelihood of aspirin discontinuation. The associations of several factors, including many markers of poor prognosis, were no longer statistically significant, including admission from another NH, recent weight loss, renal failure, shortness of breath, and cancer.
DISCUSSION
Continuation of aspirin for secondary prevention at EOL is controversial, given increased risks and unclear benefits. To our knowledge, this is the first national study providing real-world data on incidence and predictors of aspirin discontinuation in an EOL NH population. Using robust data on daily medication use in the NH, we found that 27% of initial users discontinued aspirin by 90 days after admission, and that incidence of aspirin discontinuation was considerably higher in those with explicit documentation of LP at admission (34% versus 24%). Although the entire sample included individuals with LP, only 30% of the sample had their LP explicitly documented. In both subgroups, multiple specific markers of poor prognosis were associated with greater likelihood of discontinuation, although the nature of these prognostic factors differed. Such patterns may indicate that even without explicit documentation, clinical factors related to poor prognosis prompt providers to consider deprescribing of aspirin.
Given inadequate guidance in current practice guidelines and direct evidence regarding benefits and risks associated with discontinuing aspirin in patients near the EOL, it is difficult to determine whether the incidence of discontinuation of aspirin we observed is reflective of “good” or “bad” care. However, the predomiant model of care that is used in geriatrics and palliative care for deprescribing would support discontinuation in this population, after shared decision-making conversations.12 Indeed, a survey of 134 physicians found that aspirin was the most common medication recommended for discontinuation.45 That we found aspirin to be discontinued in only 27% of our national sample of Veterans with LLE/AD suggests that there may be substantial barriers to discussing or implementing aspirin discontinuation in real-world practice. Higher rates of discontinuation amongst those with explicit documentation of LP may indicate that discussions about aligning chronic medications with goals of care occur more often in this subgroup. In a sample where all would be considered to have LP, the additional step of documenting LP may trigger discussions about discontinuation of potentially unnecessary or harmful medications. Further qualitative studies may be useful in evaluating these barriers in NH residents with LLE.
Our results also highlight the need for, and inform the design of, future comparative effectiveness and safety studies of aspirin withdrawal in this population. While over 25% of this sample experienced aspirin discontinuation, we have no outcomes data to evaluate the safety of this decision. However, future observational comparative effectiveness studies with this cohort are feasible, given large numbers of residents who discontinued aspirin, and we identified key predictors of aspirin discontinuation that should be included as potential confounders in future analyses.
Several limitations of this research should be noted. First, generalizability of findings to non-VHA NHs (with predominantly female residents) and non-NH settings is unknown. Relatedly, the incidence and predictors of discontinuation reported in this study may apply only to patients near the EOL who are still taking aspirin at NH admission, which in this study represented about half (48%) of those diagnosed with CAD and/or stroke/TIA. Second, the optimal period for identifying aspirin discontinuation using daily medication administration records is unknown, and we found that estimates of the cumulative incidence of discontinuation were somewhat sensitive to the gap length chosen, falling from 27% to 22% when using a 30-day gap rather than 14-day gap. However, using daily medication administration records rather than pharmacy claims or dispensing records theoretically allows for the use of a shorter gap to identify discontinuation, because of the greater degree of certainty that the patient did not take the medication during the gap period. Further, the use of a 14-day gap period to define discontinuation may reduce selection bias due to excluding or censoring potentially sicker patients who do not have longer follow-up time available. Third, because we did not have data on medication use prior to CLC admission, it is possible that the sample of aspirin users included both Veterans taking aspirin prior to admission and new users. Finally, we cannot determine conclusively from administrative medications records that all identified discontinuations represent intentional deprescribing, although it is unlikely that continuous 14-day or 30-day gaps in aspirin use would be unintentional in this care setting.
CONCLUSION
Among Veteran NH residents with LLE/AD receiving aspirin for secondary cardiovascaulr prevention at admission, the overall incidence of discontinuation was low (27%), although somewhat higher in residents whose LP was explicitly recognized and documented at admission (34% vs. 24%). There is a critical need for rigorously designed studies examining patient-centered outcomes of continuing versus discontinuing aspirin for secondary prevention in the context of LP, including cardiovascular events, mortality, bleeding, and patient quality of life, to inform more evidence-based decisions for this patient population.
Supplementary Material
Figure S-1. Construction of study design.
Figure S-2. Cumulative incidence of aspirin discontinuation, using primary discontinuation definition requiring a 14-day gap in use (N=13,844).
Figure S-3. Cumulative incidence of aspirin discontinuation, using alternative definition requiring a 30-day gap in use (n=9,430).
Figure S-4. Cumulative incidence of aspirin discontinuation, using alternative discontinuation variable requiring a 30-day gap in use, stratified by explicit documentation of limited prognosis (LP) at admission (N=9,430).
Impact statement:
We certify that this work is novel clinical epidemiological research examining patterns of discontinuation of aspirin for secondary prevention in patients near the end-of-life. To our knowledge, this is the first study of the incidence and predictors of aspirin discontinuation in nursing home residents with limited life expectancy and/or advanced dementia. Although a few prior studies have examined the prevalence of aspirin use in other populations near the end of life, our study is novel in its focus on identifying factors that may contribute to the decision to discontinue aspirin in previous users - both in those explicitly identified at admission as having limited prognosis (LP), as well as those not explicitly identified as such. It is also novel in its national scope, large sample size, and comprehensiveness of the range of clinical and facility-level factors examined in relation to aspirin deprescribing. This study found that discontinuation of aspirin for secondary prevention of cardiovascular disease occurred in 27% of those with limited life expectancy and/or advanced dementia in the first 90 days of their nursing home stay, including 34% of those explicitly documented as having LP and 24% of those not explicitly documented as LP. We also found a different pattern of factors associated with aspirin discontinuation in residents with versus without explicit documentation of LP. This study highlights the variability in real-world practice patterns regarding the discontinuation of aspirin in patients with limited life expectancy and underscores the need for future studies examining the benefits and harms of deprescribing aspirin for secondary prevention in patients near the end-of-life.
ACKNOWLEDGEMENTS
The research was supported by the Department of Veterans Affairs (IIR 14–306, PI C. Thorpe; Office of Academic Affairs Fellowship in Medication Safety & Pharmacy Outcomes, S. Springer) and the National Institute on Aging (T32AG021885, J. Niznik). Support for VA/CMS data provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02–237 and 98–004). One co-author (JH) is a full-time employee of GlaxoSmithKline. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02–237 and 98–004).
Funding Sources and related paper presentations: The research was supported by the Department of Veterans Affairs (IIR 14–306, PI C. Thorpe; Office of Academic Affairs Fellowship in Medication Safety & Pharmacy Outcomes, S. Springer) and the National Institute on Aging (T32AG021885, J. Niznik). The investigators retained full independence in the conduct of this research. The views expressed are those of the authors and do not represent the views of the Department of Veterans Affairs. Portions of this research were presented as a poster at the 2019 Annual Scientific Meeting of the American Geriatrics Society in Portland, Oregon (abstract #C132).
Sponsor’s role: the contents of this publication are solely the responsibility of the authors and do not represent the views of the Department of Veterans Affairs.
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.American Diabetes A. 9. Cardiovascular Disease and Risk Management. Diabetes Care. 2017;40(Suppl 1):S75–S87. [DOI] [PubMed] [Google Scholar]
- 2.Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. [DOI] [PubMed] [Google Scholar]
- 4.Lordkipanidze M, Diodati JG, Pharand C. Possibility of a rebound phenomenon following antiplatelet therapy withdrawal: a look at the clinical and pharmacological evidence. Pharmacol Ther. 2009;123(2):178–186. [DOI] [PubMed] [Google Scholar]
- 5.Sibon I, Orgogozo JM. Antiplatelet drug discontinuation is a risk factor for ischemic stroke. Neurology. 2004;62(7):1187–1189. [DOI] [PubMed] [Google Scholar]
- 6.Burger W, Chemnitius JM, Kneissl GD, Rucker G. Low-dose aspirin for secondary cardiovascular prevention - cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation - review and meta-analysis. J Intern Med. 2005;257(5):399–414. [DOI] [PubMed] [Google Scholar]
- 7.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(1):227–276. [DOI] [PubMed] [Google Scholar]
- 8.Smith SC Jr., Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58(23):2432–2446. [DOI] [PubMed] [Google Scholar]
- 9.Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e601S-e636S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossello X, Pocock SJ, Julian DG. Long-Term Use of Cardiovascular Drugs: Challenges for Research and for Patient Care. J Am Coll Cardiol. 2015;66(11):1273–1285. [DOI] [PubMed] [Google Scholar]
- 11.Sera LC, McPherson ML. Pharmacokinetics and pharmacodynamic changes associated with aging and implications for drug therapy. Clin Geriatr Med. 2012;28(2):273–286. [DOI] [PubMed] [Google Scholar]
- 12.Holmes HM. Rational prescribing for patients with a reduced life expectancy. Clin Pharmacol Ther. 2009;85(1):103–107. [DOI] [PubMed] [Google Scholar]
- 13.McNeil JJ, Wolfe R, Woods RL, et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med. 2018;379(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowman L, Mafham M, Stevens W, et al. ASCEND: A Study of Cardiovascular Events iN Diabetes: Characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am Heart J. 2018;198:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antithrombotic Trialists C, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Geraghty OC, Mehta Z, Rothwell PM, Oxford Vascular S. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390(10093):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166(6):605–609. [DOI] [PubMed] [Google Scholar]
- 18.Intrator OM R; Cai S; Miller SC. The Veterans Health Adminstration Residential History File: A Resource for Research and Operations. In. 2015 HSR&D/QUERI National Conference2015. [Google Scholar]
- 19.Intrator O, Hiris J, Berg K, Miller SC, Mor V . The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res. 2011;46(1 Pt 1):120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes DM, Koelling K, Stroupe K, et al. Veterans’ access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45(3):214–223. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Medicare & Medicaid Services. Long-Term Care Facility Resident Assessment Instrument 3.0 User’s Manual Version 1.16 In:2018. [Google Scholar]
- 22.Niznik JD, Zhang S, Mor MK, et al. Adaptation and Initial Validation of Minimum Data Set (MDS) Mortality Risk Index to MDS Version 3.0. J Am Geriatr Soc. 2018;66(12):2353–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porock D, Parker-Oliver D, Petroski GF, Rantz M. The MDS Mortality Risk Index: The evolution of a method for predicting 6-month mortality in nursing home residents. BMC Res Notes. 2010;3:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chodosh J, Edelen MO, Buchanan JL, et al. Nursing home assessment of cognitive impairment: development and testing of a brief instrument of mental status. J Am Geriatr Soc. 2008;56(11):2069–2075. [DOI] [PubMed] [Google Scholar]
- 25.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49(4):M174–182. [DOI] [PubMed] [Google Scholar]
- 26.Saliba D, Buchanan J, Edelen MO, et al. MDS 3.0: brief interview for mental status. J Am Med Dir Assoc. 2012;13(7):611–617. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Medicare and Medicaid Services. Chronic Conditions Warehouse Conditions Categories. https://www.ccwdata.org/web/guest/condition-categories. Published 2019. Accessed.
- 28.Vouri SM, Schootman M, Strope SA, Xian H, Olsen MA. Antimuscarinic use and discontinuation in an older adult population. Arch Gerontol Geriatr. 2019;80:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niznik JD, Zhao X, He M, et al. Factors Associated With Deprescribing Acetylcholinesterase Inhibitors in Older Nursing Home Residents With Severe Dementia. J Am Geriatr Soc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–499. [DOI] [PubMed] [Google Scholar]
- 31.Bureau USC. Census Bureau Regions and Divisions with State FIPS Codes. https://www2.census.gov/geo/docs/maps-data/maps/regdiv.txt. Published 2017. Accessed 7/21/2017, 2017.
- 32.Area Health Resource File (AHRF). 2012-2013. http://ahrf.hrsa.gov/. Accessed July 27, 2015.
- 33.Urban Influence Codes. 2013. http://www.ers.usda.gov/data-products/urban-influence-codes.aspx. Accessed July 27, 2015.
- 34.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 1:154–162. [DOI] [PubMed] [Google Scholar]
- 35.WHO. World Health Organization BMI Classification. http://apps.who.int/bmi/index.jsp?introPage=intro3.html&. Published2018. Accessed2019.
- 36.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703–714. [DOI] [PubMed] [Google Scholar]
- 37.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53(2):183–194. [DOI] [PubMed] [Google Scholar]
- 38.Aspinall SL, Springer SP, Zhao X, et al. Central Nervous System Medication Burden and Risk of Recurrent Serious Falls and Hip Fractures in Veterans Affairs Nursing Home Residents. J Am Geriatr Soc. 2019;67(1):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perlman CM, Hirdes JP. The aggressive behavior scale: a new scale to measure aggression based on the minimum data set. J Am Geriatr Soc. 2008;56(12):2298–2303. [DOI] [PubMed] [Google Scholar]
- 40.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546–553. [DOI] [PubMed] [Google Scholar]
- 41.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 42.Creating imputed variables through single hotdeck imputation. StataCorp; 2017. [Google Scholar]
- 43.Noordzij M, Leffondre K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28(11):2670–2677. [DOI] [PubMed] [Google Scholar]
- 44.Fine JP GR. A Proportional Hazard Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1997;94:496–509. [Google Scholar]
- 45.Ni Chroinin D, Ni Chroinin C, Beveridge A. Factors influencing deprescribing habits among geriatricians. Age Ageing. 2015;44(4):704–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S-1. Construction of study design.
Figure S-2. Cumulative incidence of aspirin discontinuation, using primary discontinuation definition requiring a 14-day gap in use (N=13,844).
Figure S-3. Cumulative incidence of aspirin discontinuation, using alternative definition requiring a 30-day gap in use (n=9,430).
Figure S-4. Cumulative incidence of aspirin discontinuation, using alternative discontinuation variable requiring a 30-day gap in use, stratified by explicit documentation of limited prognosis (LP) at admission (N=9,430).