Abstract
Myocardial infarction (MI) and subsequent progressive heart failure pathology is the major cause of death worldwide; however, the mechanism of this pathology remains unclear. The present work aimed at testing the hypothesis whether the inflammatory response is superimposed with the formation of bioactive lipid resolving molecules at the site of the injured myocardium in acute heart failure pathology post-MI. In this view, we used a robust permanent coronary ligation model to induce MI, leading to decreased contractility index with marked wall thinning and necrosis of the infarcted left ventricle. Then, we applied mass spectrometry imaging (MSI) in positive and negative ionization modes to characterize the spatial distribution of left ventricle lipids in the infarcted myocardium post-MI. After micro-extraction, liquid chromatography coupled to tandem mass spectrometry was used to confirm the structures of the imaged lipids. Statistical tools such as principal component analysis were used to establish a comprehensive visualization of lipid profile changes in MI and no-MI hearts. Resolving bioactive molecules such as resolvin (Rv) D1, RvD5, RvE3, 17-HDHA, LXA4, and 18-HEPE were detected in negative ion mode MSI, whereas phosphatidyl cholines (PC) and oxidized derivatives thereof were detected in positive ion mode. MSI-based analysis demonstrated a significant increase in resolvin bioactive lipids with comprehensive lipid remodeling at the site of infarction. These results clearly indicate that infarcted myocardium is the primary location of inflammation-resolution pathomechanics which is critical for resolution of inflammation and heart failure pathophysiology.
Keywords: myocardial infarction, lipids, ischemic myocardium, resolution of inflammation, bioactive lipid molecules
Graphical abstract:
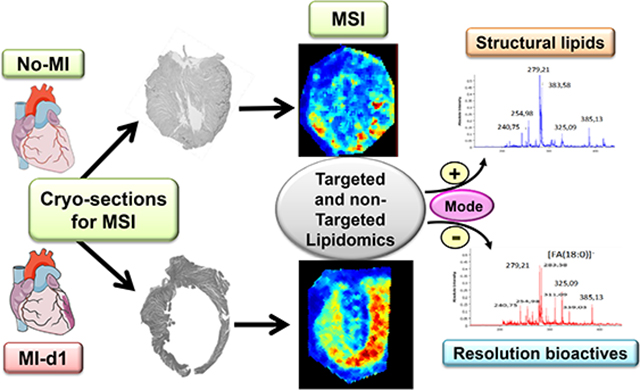
Applied scheme to determine comprehensive lipidomics in failing and non-failing heart.
Introduction
Coronary artery disease is the major cause of myocardial infarction (MI) event that progresses to subsequent heart failure-related morbidity and mortality. MI is the leading cause of acute and progressive chronic heart failure pathology worldwide [1]. Post-MI heart failure is a public health problem that is growing rapidly in developing and developed nations. More than 37.7 million patients suffer from heart failure worldwide, and in the USA alone the total cost of heart failure handling is expected to reach $53 billion by 2030 [2,1]. Therefore, a better understanding of this multidimensional heterogeneous pathology and identification of novel markers for clinical applications are of utmost importance. Concerning proteins, increased levels of markers such as creatine kinase and cardiac troponin I are currently used to determine the magnitude of the myocardial injury. Complementary prognostic information may be sought through the evidence of lipidomic measurements in the left ventricle (LV) or infarcted myocardium [3]. However, our knowledge of lipid composition and spatial lipid remodeling at the site of infarcted myocardium remains insufficiently documented so far. Therefore, we have combined a molecular imaging approach relying on matrix-assisted laser desorption ionization (MALDI) with a lipidomic approach to localize and identify lipid species in a m/z 200–900 mass range, t from fatty acids (FA) to phospholipids (PL) for post-MI and no-MI naïve control heart. For this, we used robust permanent coronary ligation model to indicate the change in acute heart failure pathology.
Molecular mass spectrometry imaging (MSI) allows identifying and localizing various classes of biomolecules at the surface of tissues. It is a qualitative and quantitative label-free imaging methodology that operates without a priori, delivering as many specific images as detected m/z species. For this purpose, tissue slices are cut around 10 μm thickness using a cryostat [4,5]. MALDI, the most widely used methodology for MSI, routinely offers a spatial resolution (pixel size) of 10–50 μm, although this value might be brought below 2 μm. MALDI- MSI opened a new age of discovery in protein and lipid identification and was successfully applied to multiple organs [6–9,5,10,11]. However, even at 50 μm pixel size, imaging of a mouse heart (a few mm2) requires the acquisition of tens of thousands of mass spectra. For data analysis, the multivariate has long been a staple of “omic” workflows but gained a growing interest in MSI technology [12,5,9,13]. Thus, common multivariate techniques, such as principal components analysis (PCA, unsupervised) and partial least-squares discriminant analysis (PLS-DA), are used for data reduction in multidimensional data sets.
In this report, MSI was linked to LC-MS-MS for structure confirmation and quantitation of lipid species imaged on mouse infarcted and non-infarcted left ventricle (LV). The complementary MSI and LC-MS/MS approach along with histology and immunohistochemistry has been used for two reasons: i) confident identification of lipid species by MALDI-MS/MS is often hampered by the rather low resolution of precursor ion selection (ion gates) in time of flight mass spectrometers, and ii) quantitative analysis of imaged species directly from tissue slices remains a delicate task. Therefore, we used a micro-extraction of lipids from cardiac tissues and applied a lipidomic approach for their structure confirmation and quantitation [14,4].
Materials and Methods
Mice and mice care compliance
Male C57Bl/6J mice (stock number 000664) of 8 to 12 weeks were purchased from The Jackson Laboratory, USA. All animal experiments were conducted according to the “Guide for the Care and Use of Laboratory Animals” (8th Edition. 2011), and AVMA Guidelines for the Euthanasia of Animals: (2013 Edition) and were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham, USA.
Coronary artery ligation to induce myocardial infarction (MI)
Mice were anesthetized using continuous isoflurane (2 %) mixed in 100 % oxygen through inhalation and then intubated and ventilated with air using a small-animal respirator (Harvard Apparatus, Holliston, MA, USA). Mice chest hairs were removed using a chemical depilatory (Nair™), and a thoracotomy was performed in the fourth left intercostal space. The left atrium and ventricle were observed; the thin pericardium layer was removed and then left anterior descending artery was permanently ligated (Figure 1A) with a 8–0 black polyamide monofilament suture (AROSurgical Instruments, T06A08N14–13) as previously described [15–17].
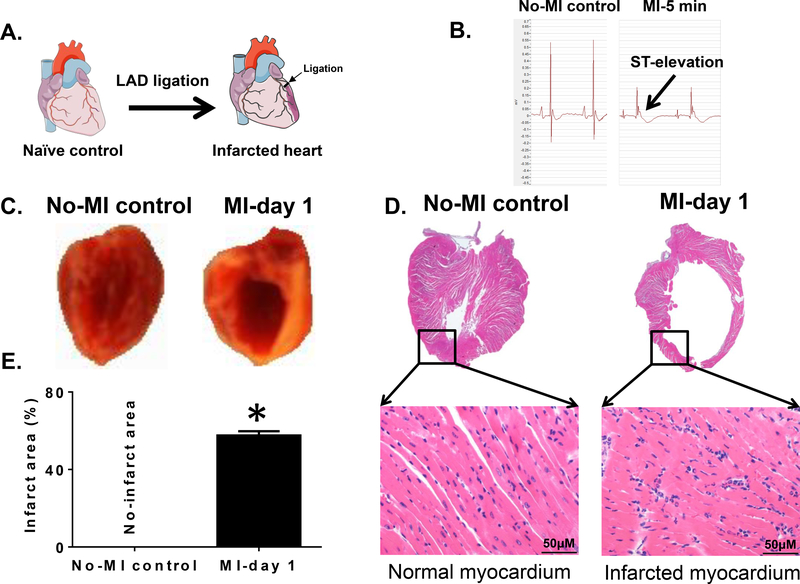
Figure 1. MI-induced ST-elevation and structural heart failure pathology in mice.
(A).Schematic figure indicating coronary artery ligation point for surgery to induce myocardial infarction (MI). (B) Ligation-induced ST-elevation indicated successful MI surgery compared to no-MI controls. (C). Digital photograph of the vertical half-cut left ventricle (LV) stained with 2,3,5- triphenyltetrazolium chloride (TTC) following permanent coronary ligation indicating the infarcted area (white) and the non-infarcted remote area (red) compared to no-MI naïve controls. (D). Mice LV stained with hematoxylin (H) and eosin (E) post-MI. LV (1.25x) and amplified photographs (40x) of no-MI naive control indicate healthy myocardium and post-MI histology indicate signs of myocardial damage from the ligation-induced injury. (E). Graph is indicating the quantitative measurement of ligation-induced infarct area after coronary ligation versus non-ligated no-MI controls. *p<0.05 vs no-MI naïve controls (n=6 mice/group).
Transthoracic echocardiography
Cardiac structure and function were quantified by echocardiography with a 22–55 MHz transducer using MX-400 Vevo 3100, (VisualSonics Inc., Toronto, Canada) for post-MI hearts and naïve controls (no-MI). Mice were sedated (1–2% isoflurane) and four limb lead electrocardiograms were simultaneously recorded. LV fractional shortening (%) was defined as [(EDD-ESD)/EDD] X 100, where EDD is LV end-diastolic dimension and ESD is end-systolic dimension [15,18,16,17].
LV function, strain and strain rate analysis
LV strain analysis was based on combined speckle tracking algorithms applied on high-frequency ultrasound images. By definition, strain indicates how much the myocardial tissue has distorted, Strain (S) = ΔL/L0 = (L1-L0)/L0, and strain rate (SR) reflects how fast the myocardial LV tissue is deforming, SR = S/Δt = ΔL/L0/Δt. Thus, strain and strain rate can reflect the global systolic, diastolic and regional function. LV strain and strain rate were quantified in the longitudinal and circumferential axes by speckle tracking of 2D grayscale echocardiographic images acquired from the parasternal long- and short-axis views [17].
Necropsy to collect infarcted LV and infarct area analysis
No-MI naïve control and post-MI d1 mice were anesthetized using 2 % isoflurane in 100 % oxygen mix. Heparin (4 IU/g; I.P.) injection was used to collect plasma. The blood was collected from the carotid artery under isoflurane anesthesia after 5 minutes post-heparin administration, and was centrifuged for 5 min to collect plasma. The chest cavity was opened, and the LV was perfused with 2 ml cardioplegic solution to remove blood. The lungs, right ventricle, and LV were collected, weighed and processed as previously described. The LV further sectioned vertically into two halves and right ventricle was stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma Inc.) and photographed for infarct size determination. One set of LV was frozen in dry ice chilled isopentane (2-methyl butane) slurry and another set of LV (n=6) were processed in 5% formalin for hematoxylin and eosin staining procedure to determine and confirm obvious myocardial injury post-MI [19,15,20,17].
LV Immunohistochemistry for neutrophils
Formalin fixed LV mid-cavity sections were paraffin embedded and sectioned at 5 μm. Further sections were deparaffinized in citrisolv (Thermo Fisher Scientific) and used rat anti-mouse neutrophil antibody (CL 8993AP, clone 7 1:50, Cedarlane) for immunohistochemistry as described previously [16]. LV tissue matrix chemicals
α-cyano-4-hydroxycinnamic acid (HCCA), 9 aminoacridine (9AA), were supplied by Sigma-Aldrich. Trifluoroacetic acid (TFA), HPLC grade ethanol, acetonitrile and isopropanol were supplied by Fischer Scientific UK.
Extraction protocols and LC-MS and LC-MS-MS methods
The extensive two-step process was used for the extraction of PLs [21]. Briefly, heart tissues particularly LV homogenized with 200 μL of 180 mM ammonium acetate, pH 8 was extracted with 990 μL of chloroform/methanol 17/1 (v/v) to eliminate apolar lipids. Then, the aqueous phase was re-extracted with 990 μL of chloroform/methanol 2/1 (v/v) to isolate polar lipids in the organic phase; this PLs extract was dried under vacuum (Speedvac, Thermo). A Shimadzu gradient LC system (CBM-20A) interfaced to a QTrap mass spectrometer equipped with a TurboV electrospray source (SCIEX API 5500) was used for LC-ESI-MS/MS analysis of PLs. The separation of lipids was carried out at 40°C on a C8 Synergi Fusion-RP 150×1mm column, with 80 Å pore size, 4 μm particles (Phenomenex). Eluent A was isopropanol/methanol/H2O + 0.2 % formic acid + 0.028% NH4OH; Eluent B was isopropanol + 0.2% formic acid +0.028% NH4OH. The gradient elution program was : 0 min, 30% B; 5 min, 50% B; 30 min, 80% B; 31–41 min, 95% B; 42–47 min, 5% B; 52–62 min, 30% B. The flow rate was 40 μL/min. Phospholipid extracts were dissolved in 400 μL of solvent B used for the reversed phase liquid chromatography analysis; 5 μL sample volumes were injected. The mass spectrometer was operated over the m/z 250–1100 at a 1000 Da/s scan speed. The ion spray voltage was set at 5500 V in the positive ion mode and - 4500 V in the negative ion mode. The trap fill-time was set at 3 ms in the positive ion mode, and 5 ms in the negative ion mode. Individual PLs were identified from their retention time and m/z. In MRM mode, the combination with the detection of precursor ions and major fragment ions were used. Briefly, for the FAs detection and identification, infarcted and non-infarcted LV sections were suspended in 1.0 ml cold methanol and gently homogenized. Afterwards they were kept on ice for 1 h to allow for protein precipitation. Lipid mediators (LMs) were profiled as described in Colas et al. [22]. LMs were extracted using SPE on C18 columns, and methyl formate fractions were taken for LC-MS/MS-based lipidomics. The LV samples from the no-MI control and MI groups were analyzed on a LC–MS/MS system, QTRAP 6500 (ABSciex as described previously Colas et al. [22].
Tissue preparation for MSI
Mouse hearts perfused with water containing 0.9 % sodium chloride were frozen in a dry ice chilled isopentane and stored at − 80 °C. Frozen organs were transported from the −80 °C freezer to a cryostat on dry ice. For mounting, tissues were held atop a drop of HPLC grade water on the cutting stage and placed into the cryostat chamber, held at −19 °C to equilibrate. Frozen LV heart tissues were sliced into 12 μm sections. The heart sections were directly deposited on indium tin oxide (ITO) coated conductive glass slides for MALDI imaging and dried. Before matrix coating, tissue sections were preserved under vacuum.
Matrix application on LV tissue sections
ITO-coated slides bearing mouse heart sections were scanned (HP Scanjet G4050) prior to matrix deposition. α-Cyano-4-hydroxycinnamic acid (HCCA) was used at concentration of 7 mg/ml in acetone/water, 0.2 % TFA 1:1 (v:v) as a MALDI matrix for the analysis in positive ion mode. 9-Aminoacridine (9AA) was used at concentration of 10 mg/ml in acetonitrile/isopropanol 60/40 (v/v) for the negative ion mode. Matrix solutions were sonicated for 2 min before use.
Matrix was spray-coated by a vibrational sprayer system (Imageprep, Bruker Daltonics, Bremen, Germany). Matrix was applied in multiple cycles (spray generation, matrix incubation on the tissue section, and drying the sample using a soft nitrogen flow) to reach an adequate thickness without lateral delocalization of analytes (approximatively 4 ml matrix solution per slide).
LV tissue imaging
An Ultraflex III MALDI-time of flight (TOF)-TOF tandem mass spectrometer (Bruker Daltonics Inc., Bremen, Germany) was used for imaging. Briefly, mass spectra acquired for a m/z 140–1300 range were averaged from 300 consecutive laser shots. The raster spatial resolution was 90 μm. Depending on the image size, acquisition took between 40 min to 3 hours. MALDI-MSI images was produced from the corresponding spectra to generate a relative intensity ion map at a given m/z value, using FlexImaging 4.1 software (Bruker Daltonics Inc.).
Principal component analysis and statistical analysis
PCA using SCiLS Lab software (Bruker Daltonics-2016b) were conducted on MALDI data from both control and infarcted mouse hearts. A single tissue section of each heart was chosen to minimize any intersection variance that might occur. PCA has been used here to identify hidden variables between spectra taken from the regions of heart tissue. It is a statistical tool used to reduce multidimensional data sets to lower dimensions and to identify new, meaningful, hidden differences between the clusters [23]. The first step of analysis consisted in a series of pre-processing data on the loaded spectra: deconvolution on the mean spectrum to reduce the background noise and baseline subtraction; signal intensity normalization on total ion current of each spectrum. The software then selects the principal components which account for the greatest variation between each of the groups. The performance diagnostic of the test, to discriminate failing from non-failing hearts, was evaluated using receiver operating characteristic (ROC) curve analysis. A ROC curve is based on the notion of a “separator” scale, on which results for the diseased and non-diseased form a pair of overlapping distributions. The complete separation of the two underlying distributions implies a perfectly discriminating test while complete overlap implies no discrimination.
Results and discussion
Ischemic insult is responsible for diverse structural, molecular, cellular and lipid remodeling in a time-dependent manner to promote initiation, defective resolution, and progression towards heart failure [24,18,16]. By use of a robust MI model and molecular MSI combined to LC-MS-MS we were able to measure the distributions and types of lipid molecules localized in the infarcted myocardium at day 1 post-MI.
Coronary ligation induced electrophysiological, structural and functional cardiac pathology post-MI.
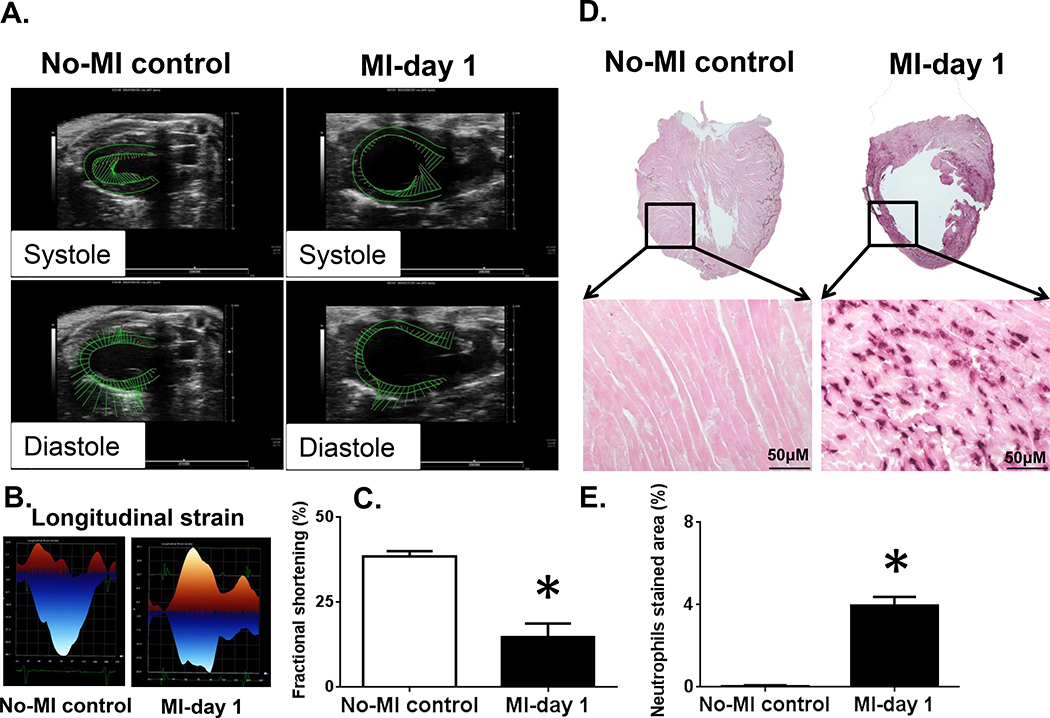
Permanent coronary ligation is an ideal method to surgically induce acute heart failure pathology maintaining naïve controls (Figure 1A). After coronary ligation, as indicated by snapshot ECG within 5 min post-MI, ST-elevation was immediately pronounced, indicating the success of MI surgery (Figure 1B). Post-MI LV dilative remodeling was marked with structural deformity of wall-thinning and myocardium necrosis, as revealed by hematoxylin (H) and eosin (E) stained myocardium. MI-induced LV dysfunction was confirmed using three approaches. First, LV was stained post-necropsy using 2,3,5-triphenyl tetrazolium chloride (TTC) for 5 min of incubation at 37° C, the infarcted area stained pale white, whereas the red colour indicated live myocardium tissue (Figure 1C). Second, evidence of significant LV structural dilation was confirmed by histology (H and E stain) and measurement of infarct area (Figure 1D–E). Third, echocardiography clearly indicated that MI-induced LV dysfunction with a marked reduction in systole and diastole thereby reduced fractional shortening and strain in MI-day 1 group compared with no-MI control mice that correlate with wall-thinning and necrosis (Figure 2A–C; Table 1). Gravimetric analysis indicated that LV showed oedematous milieu suggesting post-MI cardinal signs of inflammation (Table 1). MI-induced LV dysfunction and structural remodeling were marked by infiltration of leukocytes within 24 hr. Neutrophils density was measured using immunohistochemistry that showed higher neutrophils particularly in the border zone and infarcted area post-MI day 1 compared with no-MI controls (Figure 2D–E). Immunostained images of infarcted and non-infarcted LV confirmed that the neutrophils were primarily noted in the infarcted area (Figure 2D–E). Post-MI neutrophils presence in the infarcted LV indicated an active innate immune response. Therefore lipid composition at the site of the injured myocardium or in the infarcted area was examined by MSI. The results obtained in both positive and negative mode suggest robust active lipid remodeling within the infarcted area compared to naïve controls. According to Patterson and colleagues, the mass spectra from MSI should be interpreted with caution because of the probable degradation of tissue [25]. For this reason, before mass spectra interpretation, we ensure that the characteristic peaks of tissue degradation were absent in positive mode (oxidized PC with m/z +650.44) at the average spectra of the control and post-MI heart sections. Recent reports indicated that endogenous innate response overlap with resolving response with limited information on the localization of molecules in the infarcted myocardium. Apoptotic and necrotic inflamed myocardium is enriched with lipid molecules remodeling in post-MI healing [18]. This extensive lipid remodeling is identified using MSI in positive and negative mode and discussed in next sections.
Figure 2. MI-triggered cardiac dysfunction and neutrophil-mediated inflamed pathology.
Mice LV images pre, and post-MI acquired using Vevo 3100 ultrasound following left anterior descending coronary artery ligation. (A). No-MI control indicates normal systolic (inward green vector long line) and diastolic function (outward green vector long line) with preserved wall-thickness and post-MI day 1 systole and diastole dysfunction with LV wall-thinning from ligation-induced injury (inward and outward green vector- short line). (B) LV longitudinal strain indicating strain loss post-MI compared with no-MI naïve controls. (C) Fractional shortening indicating dysfunction at day 1 post-MI compared with normal function in naïve controls. *p<0.05 vs. no-MI control. (D). Immunohistochemistry 1.25X photographs of the vertical half-cut of LV stained with anti-neutrophil antibody following permanent coronary artery ligation compared to naïve no-MI controls. Amplified immunohistochemistry photographs 40X showed infiltration of neutrophils in the infarcted LV compared to no-MI naïve controls. (E). Quantitative density measurement of the neutrophil stained area in LV from d1 post-MI and non-MI controls (n=4/ mice, 4–5 images per slide were used to quantitate density). *p<0.05 vs. no-MI naïve controls.
Table 1.
Surgically-induced infarction showed LV edematous milieu and cardiac dysfunction in mice.
| Parameters | No-MI controls | MI day 1 |
|---|---|---|
| C57BL/6 mice (n) | 6 | 6 |
| Body weight (g) | 23.3 ± 1.0 | 22.6 ± 1.5 |
| LV weight (mg) | 73 ± 3.0 | 87 ± 5* |
| LV/tibia | 4.2 ± 0.2 | 5.0 ± 0.4* |
| Lung weight (mg) | 156 ± 15 | 145 ± 15 |
| Fractional shortening (%) | 35 ± 3 | 10 ± 2* |
| Longitudinal strain | −22 ± 4 | −5 ± 2* |
| Circumferencial strain | −30 ± 5 | −8 ± 4* |
LV; left ventricle, MI; myocardial infarction
P<0.05 vs no-MI naïve controls by student t-test, n= 6 sample size/group
MSI identified resolvin precursors and resolvin species in the infarcted LV post-MI
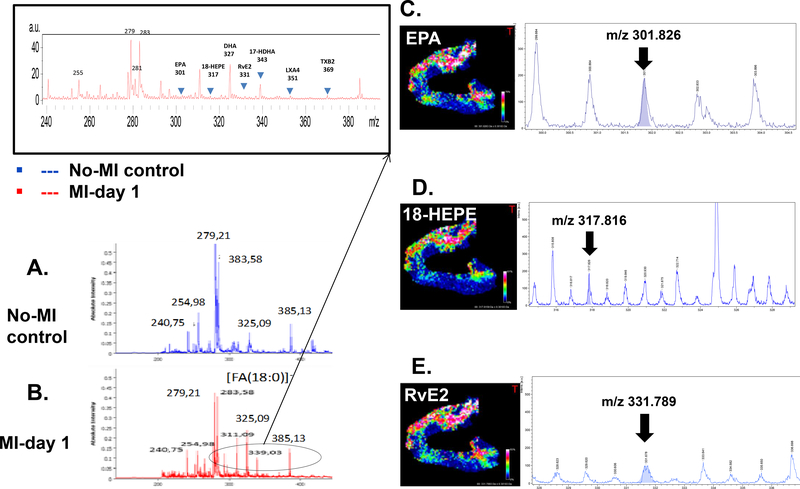
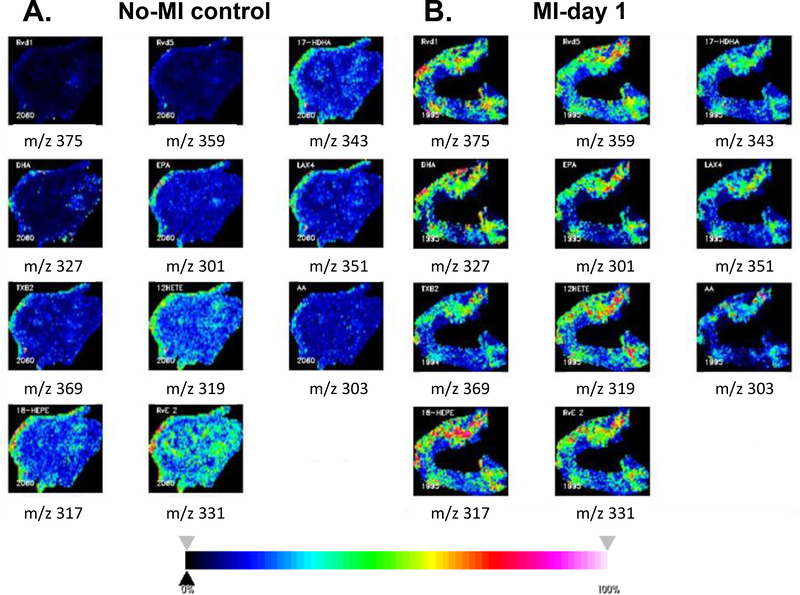
The quality and the quantity of the fatty acids determine the bioactive lipid species levels, particularly DHA and EPA-derived D- and E-series resolvins respectively to promote healing, while lipoxins are generated from AA along with prostanoids and leukotrienes [18,26,27]. A number of single cell in-vitro cardiomyocyte preparation and metabolomics approaches can provide qualitative and quantitative information on the lipid species as a function of external stimuli as injury or hypoxia, but that requires extensive controlled environment and multiple steps that increase the risk of artificial variable that are unrelated to myocardial injury and healing mechanism. In clinical setting such as obesity, diabetes, and hypertension or aging-related microenvironment add variable factors to the reparative mechanism of LV remodeling [18,27]. Therefore classical histological approach coupled with MSI of post-MI LV samples with spatial resolutions defines a mechanism in inflammation and resolution of inflammation pathway to focus on future endogenic bioactive lipids. Precise measurement and identification of bioactive lipids in pre-clinical models provides localization without great constraints regarding microenvironment or sample preparation steps. Our recent study using a quantitative approach to determine the resolvin molecules indicated that spleen and infarcted LV coordinate the specialized proresolving mediators (SPMs) generation at the site of injury using lipoxygenase enzymes and FAs substrates. Differential lipoxygenase such as 5-,12-, 15-LOX uses differential FAs such as AA, DHA and EPA and other FAs to form differential eicosanoids, docosanoids, and prostanoids [26,18,28]. Here, we used negative mode MSI and confirmed the localization of DHA, 17-HDHA, RvD1, RvD5, AA, 12-HETE, LXA4, TXB2, EPA, 18-HEPE, RvE2 in failing heart (Figure 3A–E). The negative ionization mode was used for the localization of fatty acids by MSI in post-MI and non-infarcted LVs. Compared to non-infarcted heart, average MALDI mass spectra recorded from the infarcted area in a m/z 200–400 mass range, reveal a higher complexity. This complexity is explained by the presence of hydroxylated forms of fatty acids, such as 12-hydroxyeicosatetraenoic acid (HETE) at m/z 319.3, 18-hydroxyeicosapentaenoic acid (HEPE) at m/z 317.8 and 17-hydroxy docosahexaenoic Acid (HDHA) at m/z 343.3, besides common fatty acids that serve as precursors for resolvin molecules (Figure 3A–E). RvD1, RvD5, and RvE3 were identified at m/z 375.3, m/z 359.3, and m/z 331.7 respectively. Other eicosanoids such as lipoxin A4 (LXA4, m/z 351.3) and thromboxane B2 (TXB2, m/z 369.3) were also detected. Structure assignments of these ions were confirmed after extraction of lipids from LV tissue and LC-MS analysis, as presented in Table 2. A comprehensive set of representative MSI images of FAs, resolvin precursor, and resolvin molecules are presented in Figure 4 that showed clearly the presence of resolvin molecules in the infarcted LV compared with no-MI controls.
Figure 3:
Mass spectra of no-MI control (A) and MI day 1 (B) LV with the m/z values in the mass range of 200–1200 in negative ionization mode. Two different lipid profiles are distinct with a relative intensity and abundance in MI day 1 compared to no-MI naïve control. Representative selected MALDI-MSI images in negative ionization mode. Spatial distribution of m/z ions in the MI-Day 1 (C) EPA m/z 301.826±0.3 Da with S/N=10; (D) 18-HEPE m/z 317.816±0.3 Da with S/N=9; (E) RvE2 m/z 331.789±0.3 Da with S/N=4; For figure 3C-E white pixels represent a high abundance whereas blue-dark pixels correspond to regions with low abundance or absence of the corresponding m/z.
Table 2:
MSI identified specific lipids in the infarcted LV post-MI by MALDI-MSI and confirmed by LC-MS-MS, and area under curves (AUC) of receiver operating characteristic (ROC).
| Fatty acid substrate, precursor, bioactive lipid mediators | Centroid [m/z] | MALDI [M-H]-(m/z) (±0.301Da) | Calc. [M-H]-(m/z) | AUC at centroid | Significance (T test) | |
|---|---|---|---|---|---|---|
| AA | AA | 303 | 303.3 | 303.232 | 0.753896 | <0.001 |
| LXA4 | 351 | 351.3 | 351.217 | 0.890697 | <0.001 | |
| TXB2 | 369 | 369.3 | 369.228 | 0.909575 | <0.001 | |
| 12HETE | 319 | 319.3 | 319.227 | 0.842002 | <0.001 | |
| EPA | EPA | 301 | 301.4 | 301.217 | 0.719712 | <0.001 |
| RvE2 | 331 | 331.78 | 331.45 | 0.33325 | <0.001 | |
| 18HEPE | 317 | 317.3 | 317.212 | 0.638968 | <0.001 | |
| DHA | DHA | 327 | 327.3 | 327.232 | 0.870729 | <0.001 |
| RvD5 | 359 | 359.3 | 359.222 | 0.90556 | <0.001 | |
| 17HDHA | 343 | 343.3 | 343.227 | 0.8778 | <0.001 | |
| RvD1 | 375 | 375.3 | 375.217 | 0.919774 | <0.001 | |
AA; arachidonic acid, DHA; docosahexaenoic acid, EPA; eicosapentaenoic acid, HDHA; hydroxy-docosahexaenoic Acid, HEPE; Hydroxyeicosapentaenoic acid, HETE; Hydroxyeicosatetraenoic acid, Rv; Resolvin, TX; Thromboxane
Figure 4:
Representative MALDI-MSI images in negative ionization mode. Spatial distribution of m/z ions in the no-MI control (A) and MI day 1 (B) in negative ionization mode using fleximaging. The spatial distribution of the m/z lipids shows a difference between the no-MI control and MI-day 1 LV, with a preferential localization in the heart tissue section of each m/z. Red-pink pixels represent a high abundance whereas blue-dark pixels correspond to regions with low abundance or absence of the corresponding m/z.
MSI determined the diversity of PC in the infarcted LV post-MI
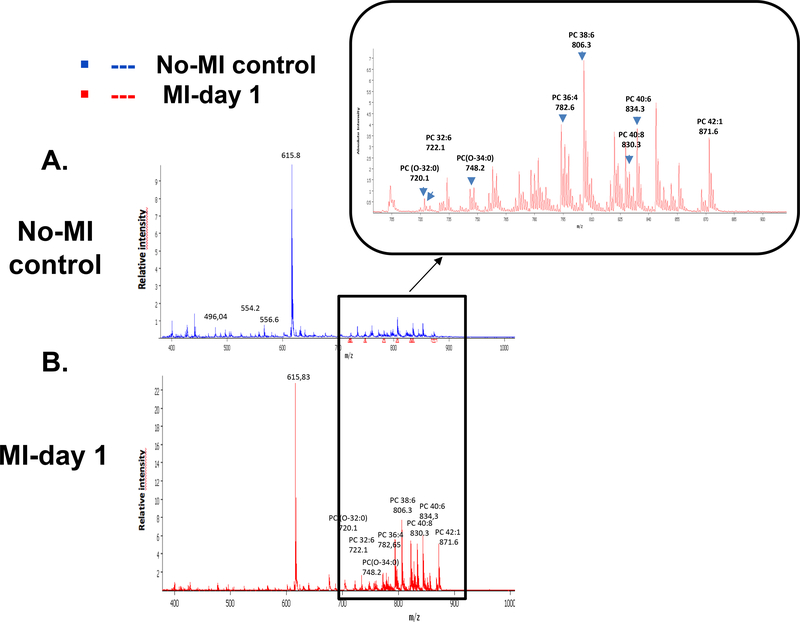
Lipids are the most diversified complex bioactive signaling molecules involved in the atherogenesis, atheroprogression, atherosclerosis, obesity, inflammation, autoimmune diseases and cardiovascular diseases [29,18,30,27]. A strong increase of signal intensity in the PL region (m/z 700–900) was observed for post-MI LV tissue (Figure 5A and 5B) indeed more than 90 individual mass were found to be more expressed in the infarcted heart (see Electronic Supplementary Material Figure 1 and Table 1). In contrast to Manger et al., we did not observe a strong increase of lysophospholipids in the post-MI tissue [31]. Major species were identified as phosphatidylcholines, but post-MI tissue images revealed the occurrence of additional signals attributed to oxidized phosphatidylcholines (OxPCs). The absence in control sample tissues of representative oxidized phospholipids such as m/z +650.44 (PC 16:0/9:0(CHO)) known as markers of artefactual tissue degradation was checked systematically, thus establishing the link between MI and the occurrence of OxPCs [25]. Structure assignments of PC and OxPC species were obtained by LC-MS/MS analysis, through precursor ion scanning based on ions representative of their polar heads and fatty acyl chains. Major OxPCs were found to be long-chain products: PC (O-32:0) (m/z 720.11), PC (O-34:0) (m/z 748.23) and PC (O-42:2) (m/z 855.88). Multiple investigations have suggested the important role of oxidized phospholipids (PLs-Ox) as markers of various pathologies associated with inflammation, such as atherosclerosis and diabetes [32–36]. Bioactive phospholipid oxidation products may also present anti-inflammatory properties, playing an important role in modulation of the inflammatory process [37,38]. The unsaturated fatty acid chains present in phospholipids are the main targets of oxidation [39,34]. In biological systems, these changes may occur through radical and non-radical reactions involving enzymatic (lipoxygenase; LOX, myeloperoxidase; MPO) or non-enzymatic systems (•OOH, •OH, Fe2+, Cu+, radiation) [32]. Interestingly, the comparison of post-MI and control LV MSI spectra revealed differences in the ratios of polyunsaturated and saturated lipids (Figure 6A and 6B): PC (32:6) (m/z 722.18)/PC (32:0), PC (36:4) (m/z 782.04)/ PC (36:0), PC 40:8 (m/z 830.60), PC 40:6 (m/z 834.60)/ PC (40:0). It was found in most aggressive cancers that the increase in the degree of saturation of acyl chains could render cell membrane less fluid [40]. In the post-MI heart, PC and PL containing lipids were increased considerably; we assume that they can be at the origin of the fluidity of the fluid cell membrane, thus initiating and then promoting inflammatory and resolution reactions. It is also known that polyunsaturated acyl chains are more susceptible to peroxidation, and oxidized lipid species may be ultimately degraded into smaller reactive products such as 4-hydroxyalkenal and malondialdehyde, which can cause cell damage [41,42].
Figure 5:
Mass spectra of no-MI control (A) and MI- day 1 (B) LV scanned in positive ionization mode with the m/z values in the mass range of 150–1000. The differences are highlighted using the overview spectra.
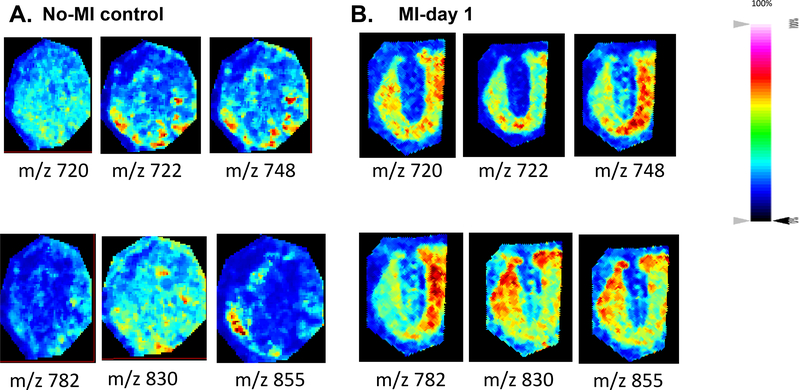
Figure 6:
Spatial distribution of ions discriminating no-MI control (A) and MI day 1 (B) infarcted LV of the MALDI-MSI data in positive ionization mode using two no-MI controls and MI day 1 infarcted hearts.
AUC and PCA differentiated MSI signals in the infarcted and non-infarcted LV post-MI
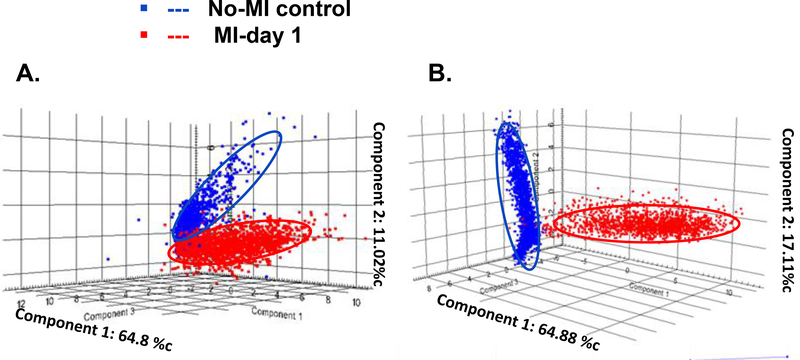
To assess significant differences between control (no-MI) and infarcted (MI) peak intensities were compared by a t-test, and the area under the curve (AUC) of each m/z peak were obtained using SCilS lab software. The AUC values of ions from the PL range (m/z 700–900) confirmed that acquired data allow discriminating infarcted heart (MI-day 1) from the no-MI control (Table 3). Using Flex imaging software, we selected the 6 first relevant m/z values (with the highest AUC) (m/z 720, m/z 722, m/z 748, m/z 782, m/z 830, m/z 855) and subsequently obtained their ion distribution map to visualize their position along the whole heart sections. These peaks occurring in both heart types revealed differences in their relative abundance and distribution, with an increase in relative intensity in the MI-day 1 heart compared to the no-MI heart (Figure 6A and 6B) (4 other lipids in positive mode and 4 others in negative mode with significant difference between the control and infarcted hearts are showed in the Electronic Supplementary Material Figure 2 and 3). Complementary analysis using LC-MS/MS in the MRM mode allowed the identification of those compounds. The result of principal component analysis (PCA) is presented as a three-dimensional plot (Figure 7) that suggests diverse post-MI lipid remodeling. Post-MI day 1 LV samples with similar peak patterns are clustered closely on the PCA plot. Likewise no-MI LV peaks are grouped together. The X and Y axis in both positive and negative ionization modes in Figures 7A and 7B describe the cumulative % data in the two first principal components. On the basis of negative mode PCA that indicates a cumulative proportion rate of 81.99% (PC1 represents 64.8% of data and PC2 11.02%, Figure 7A), and positive mode PCA that indicates a cumulative proportion rate of 81.8% (PC1 represents 64.88% of data and PC2 17.11%, Figure 7B), data were divided into two groups. Clear differences appeared in the spatial distribution and relative intensities of the selected m/z values for the control versus post-MI LV. Nevertheless, a small zone of superposition between the two clusters (control vs. MI) indicates the presence of some common metabolites or lipid species (<5%). These results indicate that spectra can be used to differentiate the post-MI day 1 LV from the No-MI control LV. Indeed, we identified a total of 13 m/z values in negative mode and 93 m/z in positive mode (see Electronic Supplementary Material Table 1) characterized by an important increase of relative intensities for post-MI LV (p < 0.0001).
Table 3.
MSI identified left ventricle specific lipids after MI, by MALDI MSI and confirmed by LC-MS-MS and area under curves (AUC) of receiver operating characterestic (ROC).
| PC | Centroid [m/z] | AUC at centroid | Significance (T-test) |
|---|---|---|---|
| PC (0–32:0) | 720.107 | 0.173 | < 0.001 |
| PC (32:6) | 722.178 | 0.345 | < 0.001 |
| PC (O-34:0) | 748.23 | 0.345 | < 0.001 |
| PC (36:4) | 782.04 | 0.345 | < 0.001 |
| PC 40:8 | 830.6 | 0.998 | < 0.001 |
| PC 40:6 | 834.6 | 0.998 | < 0.001 |
| PC P-42 :2 | 855.88 | 1.000 | < 0.001 |
PC; phospholipids, m/z; mass-to-charge ratio
Figure 7:
Tri-dimensional PCA results using SCiLS Lab software in negative mode (A) and positive one (B) for the no-MI control (Blue) and MI-day 1 (red) left ventricle. A plot of principal component 1 (PC1-MI day 1) versus principal component 2 (PC2-no-MI control) was chosen since these components resulted in the highest overall degree of separation of the spectra within the PCA scores plots. At the top of each axis from the PCA, the percentage of explained data for each PC was reported.
Conclusion
To explore the categories of biomarkers involved in the infarcted heart (post-MI) and better understand the kinetics of the immune reaction implied in cardiac remodeling following a heart attack, we have combined immunohistochemistry, MALDI-MSI and classic MS/MS analyses. The comparison of infarcted heart with control heart tissue revealed that distinct lipid biomarkers were detected at elevated levels within failing LV tissue at day 1 post-MI. MALDI-MSI images clearly highlight the inherent heterogeneity of the two kinds of heart sections. PCA and the ROC curves showed an excellent discrimination profile based on lipids of interest. The negative ionization mode identified and localized lipid molecules that are critical for resolution of inflammation and the positive ionization mode revealed species that are important for structural integrity in the context of heart failure pathology. LC-MS/MS analysis confirmed the structure assignments of these lipids. Application of PCA and ROC confirmed the variability of the answers between the two experimental sets, which is correlated with the biological or pathological status of the organs, and confirms the reliability of the tests. The presence or absence of bioactive lipids is key to regulate resolving or non-resolving inflammation in heart failure pathology [43,44,28]. Thus, our MSI approach is offering an innovative platform for localization of bioactive lipids in infarcted heart samples.
Supplementary Material
Table 1: List of 93 ions discriminating no-MI control and MI day 1 infarcted left ventricle from the MALDI IMS imaging data in positive ionization mode with ROC values obtained from SCilLs software 2016b
S. Figure 1: Spatial distribution of 93 ions discriminating no-MI control and MI day 1 infarcted left ventricle of the MALDI IMS imaging data in positive ionization mode using two no-MI controls and two MI day 1 infarcted hearts.
S. Figure 2: Area under operating characteristic (ROC) curve (AUC) of spatial distribution and relative intensity of 4 bioactive lipids in negative ionization mode: (3A) m/z 375 AUC=0.9 – (3B) m/z 369 AUC=0.9 – (3C) m/z 351 AUC=0.89 – (3D) m/z 359 AUC=0.9. The maximum AUC=1 means that the diagnostic test is perfect in the differentiation between the no-MI control and MI day 1 left ventricle samples.
S. Figure 3: Area under operating characteristic (ROC) curve (AUC) of spatial distribution and relative intensity of 4 bioactive lipids in positive ionization mode: (4A) m/z 871.9 AUC=1,000 - (4B) m/z 834 AUC=0.998 – (4C) m/z 806.37 AUC=1.000 – (4D) m/z 830 AUC=0.998.
Acknowledgement
We acknowledge the support from National Institutes of Health [AT006704 and HL132989] and The University of Alabama at Birmingham (UAB) Pittman scholar award to GVH; American Heart Association postdoctoral fellowship POST31000008 to VK and Idex program from University of Bordeaux, France to BF.
List of abbreviations
- AA
Arachidonic acid
- AUC
Area under curve
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FA
Fatty acids
- HDHA
Hydroxydocosahexaenoic acid
- HEPE
Hydroxyeicosapentaenoic acid
- HETEs
Hydroperoxyeicosatetraenoic acid
- LC-MS/MS
Liquid chromatography-mass spectrometry
- LM
Lipid mediators
- LT
Leukotrienes
- LX
Lipoxins
- LV
Left ventricle
- MALDI
Matrix-assisted laser desorption ionization
- MI
Myocardial infarction
- MSI
Molecular mass spectrometry imaging
- MRM
Multiple reaction monitoring
- PCA
Principal component analysis
- PL
phospholipids
- Rv
Resolvins
- TX
Thromboxane
Footnotes
Conflict of Interest
Authors have no conflict of interest.
References
- 1.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G (2014) Heart failure: preventing disease and death worldwide. ESC Heart Failure 1 (1):4–25. [DOI] [PubMed] [Google Scholar]
- 2.Ziaeian B, Fonarow GC (2016) Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 13 (6):368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ertl G, Frantz S (2005) Healing after myocardial infarction. Cardiovasc Res 66 (1):22–32 [DOI] [PubMed] [Google Scholar]
- 4.Grey AC, Gelasco AK, Section J, Moreno-Rodriguez RA, Krug EL, Schey KL (2010) Molecular morphology of the chick heart visualized by MALDI imaging mass spectrometry. Anat Rec (Hoboken, NJ : 2007) 293 (5):821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trim PJ, Henson CM, Avery JL, McEwen A, Snel MF, Claude E, Marshall PS, West A, Princivalle AP, Clench MR (2008) Matrix-assisted laser desorption/ionization-ion mobility separation-mass spectrometry imaging of vinblastine in whole body tissue sections. Anal. Chem. 80 (22):8628–8634. [DOI] [PubMed] [Google Scholar]
- 6.Gessel MM, Norris JL, Caprioli RM (2014) MALDI imaging mass spectrometry: spatial molecular analysis to enable a new age of discovery. J. Proteom. 107:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones EE, Powers TW, Neely BA, Cazares LH, Troyer DA, Parker AS, Drake RR (2014) MALDI imaging mass spectrometry profiling of proteins and lipids in clear cell renal cell carcinoma. Proteomics 14 (7–8):924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Sio G, Smith AJ, Galli M, Garancini M, Chinello C, Bono F, Pagni F, Magni F (2015) A MALDI-Mass Spectrometry Imaging method applicable to different formalin-fixed paraffin-embedded human tissues. Mol. BioSyst. 11 (6):1507–1514. [DOI] [PubMed] [Google Scholar]
- 9.Diehl HC, Beine B, Elm J, Trede D, Ahrens M, Eisenacher M, Marcus K, Meyer HE, Henkel C (2015) The challenge of on-tissue digestion for MALDI MSI- a comparison of different protocols to improve imaging experiments. Anal. Bioanal. Chem. 407 (8):2223–2243. [DOI] [PubMed] [Google Scholar]
- 10.Angel PM, Spraggins JM, Baldwin HS, Caprioli R (2012) Enhanced sensitivity for high spatial resolution lipid analysis by negative ion mode matrix assisted laser desorption ionization imaging mass spectrometry. Anal. Chem. 84 (3):1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angel PM, Bayoumi AS, Hinton RB, Ru Su Y, Bichell D, Mayer JE, Scott Baldwin H, Caprioli RM (2016) MALDI Imaging Mass Spectrometry as a Lipidomic Approach to Heart Valve Research. J Heart Valve Dis 25 (2):240–252 [PubMed] [Google Scholar]
- 12.Steurer S, Singer JM, Rink M, Chun F, Dahlem R, Simon R, Burandt E, Stahl P, Terracciano L, Schlomm T, Wagner W, Hoppner W, Omidi M, Kraus O, Kwiatkowski M, Doh O, Fisch M, Soave A, Sauter G, Wurlitzer M, Schluter H, Minner S (2014) MALDI imaging-based identification of prognostically relevant signals in bladder cancer using large-scale tissue microarrays. Urol Oncol 32 (8):1225–1233. [DOI] [PubMed] [Google Scholar]
- 13.Kofeler HC, Fauland A, Rechberger GN, Trotzmuller M (2012) Mass spectrometry based lipidomics: an overview of technological platforms. Metabolites 2 (1):19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bure C, Ayciriex S, Testet E, Schmitter JM (2013) A single run LC-MS/MS method for phospholipidomics. Anal. Bioanal. Chem. 405 (1):203–213. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML (2013) Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Cir Res 112 (4):675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV (2015) Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am J Physiol Heart Circ Physiol. 308 (4):H269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halade GV, Kain V, Ingle KA (2017) Heart Functional and Structural Compendium of Cardiosplenic and Cardiorenal Networks in Acute and Chronic Heart Failure Pathology. Am J Physiol Heart Circ Physiol. :ajpheart 00528 02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA (2016) Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging 8 (11):2611–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halade GV, Ma Y, Ramirez TA, Zhang J, Dai Q, Hensler JG, Lopez EF, Ghasemi O, Jin YF, Lindsey ML (2013) Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 305 (12):H1830–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heaberlin JR, Ma Y, Zhang J, Ahuja SS, Lindsey ML, Halade GV (2013) Obese and diabetic KKAy mice show increased mortality but improved cardiac function following myocardial infarction. Cardiovasc Pathol. : the official journal of the Society for Cardiovascular Pathology 22 (6):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ejsing CS, Sampaio JL, Surendranath V, Duchoslav E, Ekroos K, Klemm RW, Simons K, Shevchenko A (2009) Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 106 (7):2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN (2014) Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 307 (1):C39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiele H, Heldmann S, Trede D, Strehlow J, Wirtz S, Dreher W, Berger J, Oetjen J, Kobarg JH, Fischer B, Maass P (2014) 2D and 3D MALDI-imaging: conceptual strategies for visualization and data mining. Biochim. Biophys. Acta. 1844 (1 Pt A):117–137. [DOI] [PubMed] [Google Scholar]
- 24.Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8 (5):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson NH, Doonan RJ, Daskalopoulou SS, Dufresne M, Lenglet S, Montecucco F, Thomas A, Chaurand P (2016) Three-dimensional imaging MS of lipids in atherosclerotic plaques: Open-source methods for reconstruction and analysis. Proteomics 16 (11–12):1642–1651. [DOI] [PubMed] [Google Scholar]
- 26.Halade GV, Kain V, Ingle KA, Prabhu SD (2017) Interaction of 12/15-lipoxygenase with fatty acids alters the leukocyte kinetics leading to improved postmyocardial infarction healing. Am J Physiol Heart Circ Physiol. 313 (1):H89–H102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halade GV, Kain V (2017) Obesity and Cardiometabolic Defects in Heart Failure Pathology. Compr. Physiol. 7 (4):1463–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510 (7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G (2010) Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J. Immunol. 184 (9):5280–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halade GV, Jin YF, Lindsey ML (2012) Roles of saturated vs. polyunsaturated fat in heart failure survival: not all fats are created equal. Cardiovasc Res 93 (1):4–5. doi: 10.1093/cvr/cvr298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menger RF, Stutts WL, Anbukumar DS, Bowden JA, Ford DA, Yost RA (2012) MALDI mass spectrometric imaging of cardiac tissue following myocardial infarction in a rat coronary artery ligation model. Anal. Chem. 84 (2):1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fruhwirth GO, Loidl A, Hermetter A (2007) Oxidized phospholipids: from molecular properties to disease. Biochim. Biophys. Acta. 1772 (7):718–736. [DOI] [PubMed] [Google Scholar]
- 33.Leitinger N (2005) Oxidized phospholipids as triggers of inflammation in atherosclerosis. Molecular nutrition & food research 49 (11):1063–1071. [DOI] [PubMed] [Google Scholar]
- 34.Niki E, Yoshida Y, Saito Y, Noguchi N (2005) Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 338 (1):668–676. [DOI] [PubMed] [Google Scholar]
- 35.Spickett CM, Dever G (2005) Studies of phospholipid oxidation by electrospray mass spectrometry: from analysis in cells to biological effects. BioFactors (Oxford, England) 24 (1–4):17–31 [DOI] [PubMed] [Google Scholar]
- 36.Subbanagounder G, Deng Y, Borromeo C, Dooley AN, Berliner JA, Salomon RG (2002) Hydroxy alkenal phospholipids regulate inflammatory functions of endothelial cells. Vasc. Pharmacol. 38 (4):201–209 [DOI] [PubMed] [Google Scholar]
- 37.Erridge C, Spickett CM (2007) Oxidised phospholipid regulation of Toll-like receptor signalling. Redox Rep. : communications in free radical research 12 (1):76–80. [DOI] [PubMed] [Google Scholar]
- 38.Bochkov VN, Leitinger N (2003) Anti-inflammatory properties of lipid oxidation products. J Mol Med (Berlin, Germany) 81 (10):613–626. [DOI] [PubMed] [Google Scholar]
- 39.Spiteller G (2006) Peroxyl radicals: inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic. Biol. Med. 41 (3):362–387. [DOI] [PubMed] [Google Scholar]
- 40.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniels VW, Machiels J, Vanderhoydonc F, Smans K, Waelkens E, Verhoeven G, Swinnen JV (2010) De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 70 (20):8117–8126. [DOI] [PubMed] [Google Scholar]
- 41.Deigner HP, Hermetter A (2008) Oxidized phospholipids: emerging lipid mediators in pathophysiology. Curr Opin Lipidol. 19 (3):289–294. [DOI] [PubMed] [Google Scholar]
- 42.Schneider C, Porter NA, Brash AR (2008) Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J. Biol. Chem. 283 (23):15539–15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tourki B, Halade G (2017) Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J. : official publication of the Federation of American Societies for Experimental Biology 31 (10):4226–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kain V, Prabhu SD, Halade GV (2014) Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol. 109 (6):444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: List of 93 ions discriminating no-MI control and MI day 1 infarcted left ventricle from the MALDI IMS imaging data in positive ionization mode with ROC values obtained from SCilLs software 2016b
S. Figure 1: Spatial distribution of 93 ions discriminating no-MI control and MI day 1 infarcted left ventricle of the MALDI IMS imaging data in positive ionization mode using two no-MI controls and two MI day 1 infarcted hearts.
S. Figure 2: Area under operating characteristic (ROC) curve (AUC) of spatial distribution and relative intensity of 4 bioactive lipids in negative ionization mode: (3A) m/z 375 AUC=0.9 – (3B) m/z 369 AUC=0.9 – (3C) m/z 351 AUC=0.89 – (3D) m/z 359 AUC=0.9. The maximum AUC=1 means that the diagnostic test is perfect in the differentiation between the no-MI control and MI day 1 left ventricle samples.
S. Figure 3: Area under operating characteristic (ROC) curve (AUC) of spatial distribution and relative intensity of 4 bioactive lipids in positive ionization mode: (4A) m/z 871.9 AUC=1,000 - (4B) m/z 834 AUC=0.998 – (4C) m/z 806.37 AUC=1.000 – (4D) m/z 830 AUC=0.998.