Abstract
Mucosal healing (MH) has become a major target in the management of ulcerative colitis (UC). Because repeat endoscopy is expensive and invasive, we aimed to evaluate fecal calprotectin (FC) as an alternative marker to predict MH in UC.
Eighty patients with UC in clinical remission were consecutively included in a prospective observational study. FC was measured using a quantitative enzyme-linked immunosorbent assay. The colonic mucosa was assessed for endoscopic and histological measures of inflammatory status. Endoscopic and histological remission were defined according to the Mayo endoscopic subscore (MES) and Geboes score (GS), respectively. Deep remission was defined as a combination of the MES and GS. FC performance and cutoff values for identifying MH and deep remission were determined using contingency tables and receiver operator characteristic (ROC) and area under the curve (AUC) analysis.
The median FC concentration in patients who met the criteria for deep remission (MES ≤1 and GS < 3.1) was 65.5 μg/g, while that in patients with disease activity was 389.6 μg/g (P = .025). A FC cutoff value of 100 μg/g, determined by the ROC analysis, resulted in sensitivity and specificity of 91.7% and 57.1%, respectively, for histological remission, and 82.4% and 60.9%, respectively, for deep mucosal remission. Positive correlations were detected between FC concentrations with the histologic (CC: 0.435; P < .001) and the combined endoscopic and histologic (CC: 0.413; P < .001) scores.
FC can be used confidently as a noninvasive biomarker to predict deep remission in patients with UC in clinical remission when concentrations are below 100 μg/g.
Keywords: ulcerative colitis, mucosal healing, endoscopy, histology, fecal calprotectin, biomarker
1. Introduction
Ulcerative colitis (UC) represents one of the major forms of inflammatory bowel disease (IBD) and is characterized by chronic inflammation of the colonic mucosa associated with a set of symptoms, usually with a pattern of remission and recurrence. Given its chronic nature, long-term medical therapy and disease monitoring are required.[1,2] For decades, the resolution of clinical symptoms was the main goal of disease treatment, but it has been recognized that even asymptomatic patients often have evidence of active mucosal inflammation.[3] In addition, the introduction of new drugs for the treatment of UC has changed the endpoint of medical management from clinical remission to objective measures of complete resolution of mucosal inflammation.[4,5]
Although there is no validated definition of mucosal healing (MH), endoscopic healing is the objective target recommended by the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program promoted by The International Organization for the Study of Inflammatory Bowel Disease (IOBID).[6] Endoscopic healing has been associated with both an elevated clinical remission and corticosteroid-free period and reduced hospitalization and surgery rates in UC patients.[7–9]
Because of limited evidence of its utility in clinical practice, histological healing has not been recommended as a target in UC until present. However, as microscopic inflammation may persist in a subset of patients with endoscopically normal mucosa, some investigators have proposed the utilization of histological remission as a distinct endpoint for the management of IBD patients.[10–13] In fact, growing evidence suggests that histological remission is probably associated with better disease outcomes and lower colorectal cancer risk than endoscopic remission. This fact appears to support the idea that microscopic resolution of mucosal inflammation may be a more accurate predictor of disease outcomes.[10,14–16]
Whatever the target, identification of endoscopic and histological healing in UC patients requires an endoscopic procedure that is invasive and costly for repeat and frequent evaluations. As a result, a reliable, noninvasive biomarker of mucosal healing continues to be greatly needed. In the last decade, fecal calprotectin (FC) has been the most investigated and used biomarker in IBD. FC is a neutrophil-derived protein that is highly resistant to degradation by intestinal and bacterial enzymes and can be easily determined in stool samples.[17,18] FC levels have usually shown good correlation with disease activity in UC,[19,20] but the ability of FC to predict mucosal healing has not been sufficiently investigated. Furthermore, currently, there is no defined cutoff level of FC to predict inflammatory mucosal remission.
The aims of this prospective study were to determine whether FC levels correlate with MH based on endoscopic and histological criteria and to establish an optimal cutoff level of FC to predict mucosal remission.
2. Methods
2.1. Ethical considerations
The Ethics Committee of the University Hospital of the State University of Rio de Janeiro approved the study protocol (CAAE: 49968215.7.0000.5259), which was implemented in agreement with the ethical standards described in the 1964 Declaration of Helsinki. All enrolled patients gave written informed consent before participating in the study.
2.2. Cohort of patients
This cross-sectional study was carried out in an IBD tertiary outpatient unit from the Department of Gastroenterology of the University Hospital of the State University of Rio de Janeiro. Adult patients over 18 years of age with a diagnosis of UC based on standard clinical, radiological, endoscopic and histologic criteria were consecutively enrolled. Demographics and clinical information, including age, sex, age at diagnosis, smoking status, presence of extraintestinal manifestations, extension of disease (Montreal classification),[21] and medical therapy at the time of enrollment, were obtained from hospital electronic medical records and patient interviews. Exclusion criteria consisted of patients receiving concomitant nonsteroidal antiinflammatory drugs, patients with a diagnosis of Crohn disease (CD) or an unclassified IBD, patients with a history of previous surgical resection, cancer or acute or chronic enteric infection (e.g., Clostridium difficile) and pregnant patients.
2.3. Clinical and endoscopic assessment of disease activity
Clinical disease activity was recorded at the time of initial enrollment by the Simple Clinical Colitis Activity Index (SCCAI).[22] Clinical remission was defined as an SCCAI ≤ 2. From March 2017 to May 2019, patients in clinical remission were consecutively selected to participate, at least 1 week prior to a scheduled routine endoscopic procedure. All patients underwent endoscopic examination (ileo-colonoscopy or flexible sigmoidoscopy according to individual clinical indications) with biopsies, within the maximum interval of 4 weeks from the clinical evaluation. Endoscopic evaluation was based on the Mayo endoscopic subscore (MES) classification: normal = 0; erythema, decreased vascular pattern, mild friability = 1; marked erythema, absent vascular pattern, friability, erosions = 2; and spontaneous bleeding, ulceration = 3.[23] Mucosal healing was defined as an MES of 0 or 1. Experts from the Department of Gastroenterology of the referral center, blinded to the clinical characteristics and to fecal calprotectin levels of the patients, performed all endoscopic procedures, evaluating the disease activity and collecting mucosal biopsies.
2.4. Histological score
Endoscopic biopsies were taken during endoscopic examination from inflamed or healed colonic mucosa. If no endoscopic abnormalities could be detected, biopsies were taken from random sites of the sigmoid colon or rectum. Biopsied tissues were fixed in 10% neutral formalin and embedded in paraffin, and sections were stained with hematoxylin and eosin. An expert gastrointestinal pathologist blinded to the clinical characteristics, endoscopic results and fecal calprotectin levels of the patients evaluated the histologic disease activity according to the Geboes score (GS), which comprises 7 different parameters: architectural distortion, density of chronic inflammatory infiltration, density of eosinophils in the lamina propria, density of neutrophils in the lamina propria, presence of neutrophils in the epithelium, crypt destruction and erosion, and ulceration.[24] The most severe grade of inflammation observed in any of the colonic segments was considered the maximal GS. Histological healing was defined as a GS < 3.1.
2.5. Measurement of fecal calprotectin
During the clinical evaluation, at the initial enrollment in the study, selected patients received the request for measuring fecal calprotectin. Stool samples were collected at home within 7 to 2 days before the preparation for the endoscopic procedure after an overnight fast, and kept in a refrigerator up to 48 hours prior to delivery to the study laboratory (Controllab, Rio de Janeiro, Brazil). FC was measured using a quantitative, commercially available enzyme-linked immune sorbent assay (ELISA) according to the manufacturer's instructions (Ridascreen Calprotectin, Darmstadt, Germany, kindly provided by Controllab).[17,19] The measurement range of the FC kit was between 19.5 and 1800 μg/g. The laboratory personnel were blinded to the patient's clinical history and endoscopic and histological findings.
2.6. Statistical analysis
Statistical analysis was performed using SPSS Statistical software for Windows (Version 20, SPSS, Inc., Chicago, IL). The distribution of individual characteristics was determined using simple descriptive statistics. Receiver operating characteristic (ROC) analysis was used to evaluate the sensitivity and specificity of FC in reference to
-
1.
endoscopic and histologic remission (defined as a MES ≤ 1 and a GS < 3.1, respectively); and
-
2.
deep remission (defined as a combination of MES ≤ 1 and GS < 3.1), and to determine optimal cutoff values for generating dichotomous variables.
Optimal cutoff values were determined by the maximum sum of sensitivity and specificity of FC values for screening intestinal inflammation/healing. The correlation between FC with endoscopic and histologic scores was assessed using Spearman rank correlation coefficient. All tests were two-tailed, and statistical significance was set at a P value of less than .05.
3. Results
One hundred fourteen patients with UC in clinical remission were initially considered for enrollment. Among them, 34 patients were excluded: 16 patients who did not return for colonoscopy or flexible sigmoidoscopy on the schedule date; 14 patients who did not provide fecal samples for FC measurement; 2 patients who had no biopsies taken during endoscopic examination; 1 patient for returning an inappropriate stool sample; and 1 patient due to a diagnosis of colorectal cancer during ileo-colonoscopy. A total of 80 patients were finally enrolled. The demographic and clinical characteristics of the final study cohort are given in Table 1.
Table 1.
Demographic and clinical characteristics of the patients.
| Variables | UC Cohort (N = 80) |
| Age (years), median (IQR) | 52 (38–58) |
| Male (%) | 26 (32.5) |
| Active smoker (%) | 21 (26) |
| Disease extent (Montreal classification) (%) | |
| Ulcerative proctitis | 15 (18.7) |
| Left-sided UC/distal UC | 34 (42.5) |
| Extensive UC/pancolitis | 31 (38.8) |
| Disease duration (years), median (IQR) | 10.5 (6–15.8) |
| UC related drugs at study entry | |
| Topical/systemic 5-ASA (%) | 74 (92.5) |
| Azathioprine (%) | 22 (27.5) |
| Anti-TNF alpha (%) | 4 (5) |
| Fecal calprotectin (μg/g), median (IQR) | 133.6 (31.7–518.6) |
3.1. Characteristics of the patients
Among the 80 patients included, 26 (32.5%) were male. The median age was 52 years (interquartile range (IQR), 38–58 years), and the median disease duration prior to FC level measurement was 10.5 years (IQR, 6–15.8 years). The proportions of proctitis, left-sided colitis, and extensive colitis in the cohort were 18.7%, 42.5%, and 38.8%, respectively. Regarding the history of UC-related drugs, 74 patients (92.5%) were using oral or topical 5-aminosalicylic acids, whereas 22 (27.5%) were on azathioprine, and 4 (5%) were on antitumor necrosis factor-α agents (Table 1).
3.2. Fecal calprotectin results
The overall median concentration of FC was 133.6 μg/g (IQR, 31.7–518.6 μg/g). No differences in FC levels were found among proctitis, left-sided colitis, and extensive colitis patients.
3.3. Correlation of FC with endoscopic evaluation
Ileo-colonoscopy or flexible sigmoidoscopy was performed in patients for clinical reasons within 1 month of study inclusion. Notably, 53 (66.2%) patients were in endoscopic remission (MES ≤ 1). Surprisingly, FC concentrations were not significantly different between patients in endoscopic remission and those with endoscopically active disease (P = .099) (Fig. 1A, B). A relatively weak but significant positive correlation was found between FC concentrations and the endoscopic Mayo subscores (CC: 0.298; P = .007).
Figure 1.
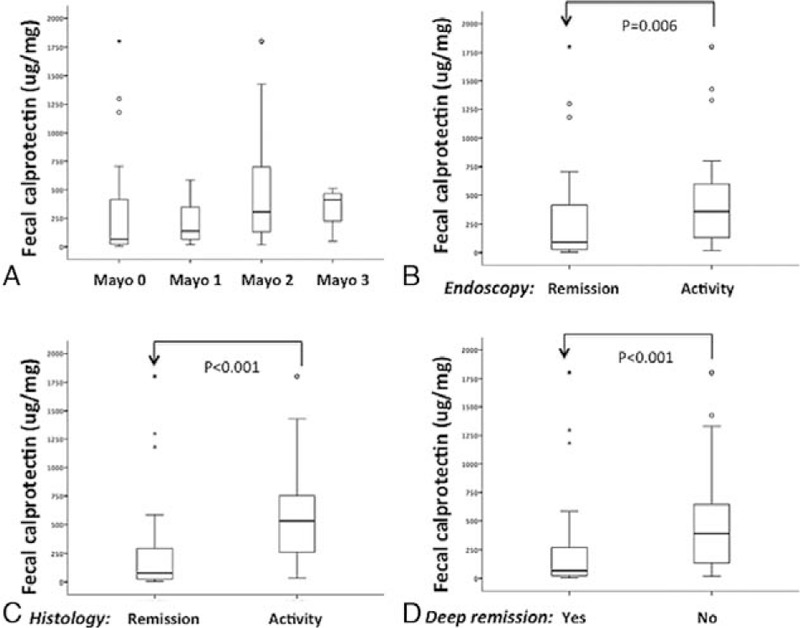
Fecal calprotectin concentrations are stratified according to the Mayo endoscopic subscore (A); the simplified Mayo endoscopic subscore, combining 0-1 (remission) and 2-3 (activity) (B); the simplified Geboes histologic score (C); and the combined endoscopic and histologic criteria (deep remission) (D). The analysis was performed by Kruskal–Wallis ANOVA on ranks, in which multiple comparisons were carried out using Dunnett test (A), or by the Mann–Whitney rank-sum test (B, C, D). The horizontal bars represent the medians, and the boxes represent the 25th and 75th percentiles. Significant results are depicted.
3.4. Correlation of FC with histological analysis
Fifty six (70%) patients had a Geboes score <3.1, which was considered the threshold for histological remission based on previous studies.[24,25] Median FC concentrations were significantly lower for patients with histological remission than for those with active histological disease (78 μg/g vs 531 μg/g, P < .001) (Fig. 1C). A significant positive correlation was detected between FC concentrations and the histologic Geboes score (CC: 0.435; P < .001).
3.5. Correlation of FC with deep remission criteria
The median FC concentration for patients who met the criteria of deep remission, defined as the combination of MES ≤ 1 and GS < 3.1, was 65.5 μg/g (N = 46), while those with histologically or endoscopically active disease had a median of 389.6 μg/g (P = .025) (Fig. 1D). A significant positive correlation was identified between FC concentrations and the combined endoscopic and histologic scores (CC: 0.413; P < .001).
3.6. Determination of optimal FC cutoff value
The concentrations of FC were analyzed against predefined endoscopic, histological and deep remission, as well as endoscopic and histological activity, to determine an estimated optimal cutoff value. To define the state variable in the ROC analysis, we categorized all participants as either in remission or with activity according to the predefined criteria. ROC analysis yielded an area under the curve (AUC) of FC for distinguishing MH from activity of 0.687, with a standard error of 0.061 and a 95% confidence interval (CI) of 0.568 to 0.807; for distinguishing histological remission from activity of 0.806, with a standard error of 0.049 and 95% confidence interval (CI) of 0.709 to 0.903; and for distinguishing deep remission from activity of 0.741, with a standard error of 0.056 and 95% confidence interval (CI) of 0.630 to 0.851 (Fig. 2).
Figure 2.
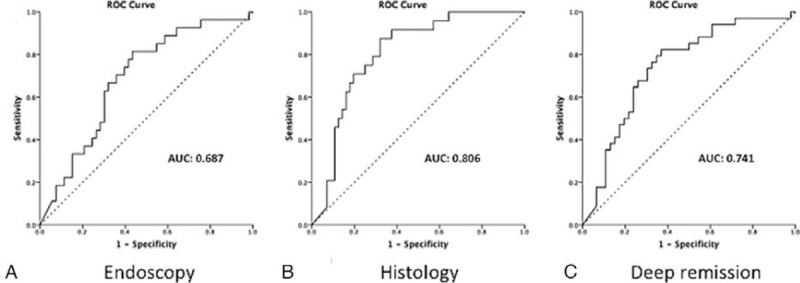
Receiver operating characteristic (ROC) curves illustrating the diagnostic ability of fecal calprotectin in relation to the endoscopic (A), histologic (B), and combined endoscopic and histologic (deep remission) (C) criteria. Diagonal segments are produced by ties. The area under the curve (AUC) is shown in each plot.
To identify patients in endoscopic, histological, and deep remission, the ROC analysis suggested that an FC level <100 μg/g would be the best range to identify, with high sensitivity, endoscopic remission (81.5%), histological remission (91.7%), and deep remission (82.4%). However, in regard to specificity, the level of FC < 100 μg/g resulted in relatively low values (54.7%, 57.1%, and 60.9%, respectively). Nonetheless, the determination of endoscopic, histological, and deep remission for FC < 100 μg/g reached negative predictive values (NPVs) of 85.3% (95% CI = 69.9-93.6), 94.1% (95% CI = 80.9–98.4), and 82.4% (95% CI = 66.5–91.6), respectively. Additional higher cutoff values of 200 and 400 μg/g were used in an attempt to improve the specificity of FC for identifying mucosal inflammation, accommodating both histologic and deep remission criteria. As expected, higher levels of FC did correlate with a higher risk of mucosal inflammation, particularly related to the histological analysis. FC cutoff data and analyses are shown in Table 2.
Table 2.
Performance analysis of different cutoff values of fecal calprotectin in relation to endoscopic and histological criteria.
| Criteria | Cutoff (μg/g) | Sensitivity | Specificity | PPV | NPV | Accuracy |
| Endoscopic | 400 | 44.4 (27.6–62.7) | 73.6 (60.4–83.6) | 46.2 (28.8–64.5) | 72.2 (59.1–82.4) | 63.8 (52.8-73.4) |
| 200 | 66.7 (47.8–81.4) | 66.0 (52.6–77.3) | 50.0 (34.5–65.6) | 79.6 (65.5–88.9) | 66.2 (55.4-75.7) | |
| 100 | 81.5 (63.3–91.8) | 54.7 (41.4–67.3) | 47.8 (34.1–61.7) | 85.3 (69.9–93.6) | 63.8 (52.8-73.4) | |
| Histological | 400 | 66.7 (46.7–82.0) | 82.1 (70.2–90.0) | 61.5 (42.5–77.6) | 85.2 (73.4–92.3) | 77.5 (67.2-85.3) |
| 200 | 79.2 (59.5–90.8) | 69.6 (56.7–80.1) | 52.8 (37.0–68.0) | 88.6 (76.0–95.0) | 72.5 (61.9-81.1) | |
| 100 | 91.7 (74.1–97.7) | 57.1 (44.1–69.2) | 47.8 (34.1–61.9) | 94.1 (80.9–98.4) | 63.8 (52.8-73.4) | |
| Deep remission (combined endoscopic-histological) | 400 | 50.0 (34.1–65.9) | 80.4 (66.8–89.4) | 65.4 (46.2–80.6) | 68.5 (55.3–79.3) | 67.5 (56.6-76.8) |
| 200 | 67.6 (50.8–80.8) | 71.7 (57.4–82.7) | 63.9 (47.6–77.5) | 75.0 (60.6–85.4) | 70.0 (59.2-78.9) | |
| 100 | 82.4 (66.5–91.6) | 60.9 (46.5–73.6) | 60.9 (46.5–73.6) | 82.4 (66.5–91.6) | 70.0 (59.2-78.9) |
4. Discussion
In this study, we performed a prospective observational investigation examining the potential role of FC as a biomarker to predict MH in patients with UC in clinical remission. Overall, the results obtained indicate a favorable performance of FC in the context of clinical remission and reinforce the critical importance of routine histological examination in the follow-up of patients with UC.
Previous studies have demonstrated that MH is closely related to a favorable clinical course of IBD in terms of relapse, hospitalization, and surgery.[4] However, the definition of MH is still controversial. The STRIDE identifies endoscopic healing as the therapeutic target, but some researchers suggest that MH should also include the histological absence of mucosal inflammation, since there have been an increasing number of studies that show an association of histological remission with improved clinical outcomes.[6,10–16] Endoscopic evaluation of the mucosa and the collection of biopsies for histological analysis continue to represent the gold standard in the follow-up of patients with UC. However, histological assessment of the mucosa depends on endoscopic examination, which is invasive, time consuming and uncomfortable for patients. This appears to justify the continued search for a noninvasive biomarker of mucosal inflammation with comparable performance to colonoscopy and sigmoidoscopy.
Some investigators have claimed that FC correlates closely with the degree of histological inflammation, particularly in UC patients.[13] In the current study, we confirm that FC levels are associated with the overall histological assessment of the colonic mucosa of patients with UC. Specifically, we observed that lower levels of FC are strongly associated with histological remission. Similar to this study, the results from a previous investigation identified a correlation between FC and the severity of inflammation in IBD.[26] Moreover, they also found a better correlation of FC concentration with histological rather than the endoscopic findings. Taken together, the results of these studies appear to corroborate the need for confirmation of histological remission with routine biopsies in this group of patients, which is currently not established in the literature.
In addition, we analyzed the association of FC with the combination of the MES and GS, which we defined as deep remission, applying the same cutoff values used for the isolated scores. This combination of endoscopic and histological scores was used in a previous study by Carlsen and colleagues, who found a significant correlation of the combined scores with FC level.[27] In this study, the median FC concentration of patients who met the criteria for deep remission was 6 times lower than that of patients with actively inflamed mucosa, probably highlighting the excellent correlation between FC and the histological component of the deep remission criteria.
Available evidence indicates that even low levels of persistent mucosal inflammation are detrimental to the future course of the disease.[10,11,16,28] For this reason, identifying patients with inflammation at the microscopic level should urgently become the new treatment goal in UC. Nevertheless, while such information per se may not justify immediate changes in medical management, it should at least alert the medical team for the need to reassess the patient at shorter intervals due to the increased risk of clinical relapse or disease complications. In this respect, FC can constitute the ideal noninvasive biomarker: easy to assess and with an excellent association with microscopic disease.
Our study also aimed to determine an ideal cutoff level of FC to predict remission and inflammatory activity in the context of UC. Previous studies have suggested a variety of cutoff levels for the ideal interpretation of test results, but there is still no consensus in the literature about appropriate FC cutoff values for the prediction of activity or remission of mucosal inflammation.[13,29,30] In fact, cutoff values for FC have shown a considerably high variability among studies and also inter-individual variability. In this study, we attempted to identify the highest combined sensitivity and specificity for screening intestinal inflammation. The maximum sum of sensitivity and specificity occurred exactly in the range between 100 and 200 μg/g. Given a cutoff value of less than 100 μg/g for FC, we reached an excellent sensitivity for histological remission (97.7%) and for deep remission (82.4%); but also obtained low specificity, similar to previous observations by Carlsen et al.[27] This cutoff level of FC also showed an excellent negative predictive value for histological remission.
In an attempt to determine the cutoff value that would most accurately reflect disease activity, we also tested different levels of FC, such as 400, 250, 200, and 150 μg/g. The higher cutoff values of 200 and 400 μg/g of FC were then used in an attempt to improve the specificity of the test and they did correlate with a higher risk of mucosal inflammation. However, the sensitivity for these different levels ranged from 50 to 70.6%, and the specificity ranged from 80 to 60.9% when considering the combined endoscopic and histological criteria. Thus, it was not possible to adopt any of these values as a safe cutoff to determine disease activity, making it necessary to carry out other diagnostic procedures, such as endoscopy with mucosal biopsies and histopathologic analysis, for example, to assess the presence and severity of inflammation.
Our study had limitations, including the number of patients and its cross-sectional nature. A larger sample would have allowed an assessment of a more precise estimation of the cutoff value for the prediction of histologic activity. In addition, although there was a great concern in synchronizing the collection of data and samples from the patients, it is possible that the timing of the different evaluations, including clinical, endoscopic and laboratory could affect the final analysis, and still generate a bias. Follow-up data from the patients and serial FC measurements could have allowed an assessment of whether changes in FC level would be able to indicate early recurrence. Despite the aforementioned limitations, we highlight the fact that there are few studies in the literature on FC aimed only at patients with UC in clinical remission. We also believe that our study contributes positively to assist in the management of this group of patients, aiming at reducing invasive exams to assess mucosal healing, particularly in those who remain asymptomatic. In conclusion, our study indicates that FC is a good noninvasive method for the assessment of mucosal healing in patients with UC in clinical remission when the concentrations are below 100 μg/g and can be used as an isolated predictive test for patients.
Acknowledgments
The authors thank the Brazilian research foundations CNPq and FAPERJ for their financial support, and Prof. Ronir R. Luiz (Institute of Public Health Studies, Federal University of Rio de Janeiro) for his technical assistance with the statistical analysis.
Author contributions
Malvao LR, Esberard BC and Amorim RF participated in the conception and design of the study, the acquisition, analysis, and interpretation of data, and the drafting of the manuscript. Silva KS, Farias e Silva K, Madi K, participated in the acquisition, analysis, and interpretation of data. Carvalho ATP and de Souza HSP participated in the conception and design of the study, obtained funding, analyzed and interpreted data, and critically revised the manuscript for important intellectual content. All authors gave final approval of the submitted version of the manuscript.
Conceptualization: Ludimilla dos Reis Malvão, Heitor S. de Souza, Ana Teresa P. Carvalho.
Data curation: Kalil Madi, Barbara C. Esberard, Renata Fernandes de Amorim, Kelly dos Santos Silva, Katia Farias e Silva.
Formal analysis: Heitor S. de Souza, Ana Teresa P. Carvalho.
Funding acquisition: Heitor S. de Souza.
Investigation: Ludimilla dos Reis Malvão.
Methodology: Ludimilla dos Reis Malvão, Barbara C. Esberard, Renata Fernandes de Amorim, Kelly dos Santos Silva, Katia Farias e Silva.
Resources: Kalil Madi, Barbara C. Esberard, Renata Fernandes de Amorim, Kelly dos Santos Silva.
Supervision: Heitor S. de Souza, Ana Teresa P. Carvalho.
Validation: Barbara C. Esberard, Renata Fernandes de Amorim, Katia Farias e Silva.
Visualization: Kalil Madi, Kelly dos Santos Silva.
Writing – original draft: Ludimilla dos Reis Malvão.
Writing – review & editing: Heitor S. de Souza, Ana Teresa P. Carvalho.
Footnotes
Abbreviations: FC = fecal calprotectin, IBD = inflammatory bowel disease, MH = mucosal healing, UC = ulcerative colitis.
How to cite this article: Malvão Ld, Madi K, Esberard BC, Amorim RF, Silva Kd, Farias e Silva K, Souza HS, Carvalho AT. Fecal calprotectin as a noninvasive test to predict deep remission in patients with ulcerative colitis. Medicine. 2021;100:3(e24058).
This work was supported by grants from the Brazilian Research Council (CNPq) (306634/2019-8) and the FAPERJ (Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro) (E26/202.781/2017).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Values are presented as numbers (%) or medians (interquartile ranges).
UC = ulcerative colitis, N = number, IQR = interquartile range, 5-ASA = 5-aminosalicylic acid, TNF = tumor necrosis factor.
Endoscopic criteria based on the Mayo endoscopic sub score; histological criteria based on the Geboes score.
PPV = positive predictive value, NPV = negative predictive value; parentheses show lower-upper 95% CI.
References
- [1].Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- [2].Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018;16: 343-356 e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baars JE, Nuij VJ, Oldenburg B, et al. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflam Bowel Dis 2012;18:1634–40. [DOI] [PubMed] [Google Scholar]
- [4].Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- [5].Molander P, Sipponen T, Kemppainen H, et al. Achievement of deep remission during scheduled maintenance therapy with TNFalpha-blocking agents in IBD. J Crohn's Colitis 2013;7:730–5. [DOI] [PubMed] [Google Scholar]
- [6].Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- [7].Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- [8].Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflam Bowel Dis 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- [9].Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012;61:1619–35. [DOI] [PubMed] [Google Scholar]
- [10].Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016;65:408–14. [DOI] [PubMed] [Google Scholar]
- [11].Narang V, Kaur R, Garg B, et al. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intest Res 2018;16:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Patel A, Panchal H, Dubinsky MC. Fecal calprotectin levels predict histological healing in ulcerative colitis. Inflam Bowel Dis 2017;23:1600–4. [DOI] [PubMed] [Google Scholar]
- [13].Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn's disease. Inflam Bowel Dis 2016;22:623–30. [DOI] [PubMed] [Google Scholar]
- [14].Christensen B, Hanauer SB, Erlich J, et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol 2017;15: 1557-1564 e1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Riley SA, Mani V, Goodman MJ, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut 1991;32:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bessissow T, Lemmens B, Ferrante M, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol 2012;107:1684–92. [DOI] [PubMed] [Google Scholar]
- [17].Roseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992;27:793–8. [DOI] [PubMed] [Google Scholar]
- [18].Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn's disease. Gut 2000;47:506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burri E, Beglinger C, von Felten S, et al. Fecal calprotectin and the clinical activity index are both useful to monitor medical treatment in patients with ulcerative colitis. Digest Dis Sci 2015;60:485–91. [DOI] [PubMed] [Google Scholar]
- [20].D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflam Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- [21].Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. NEngl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- [24].Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol 2014;12: 929-934 e922. [DOI] [PubMed] [Google Scholar]
- [26].Bunn SK, Bisset WM, Main MJ, et al. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2001;33:14–22. [DOI] [PubMed] [Google Scholar]
- [27].Carlsen K, Riis LB, Elsberg H, et al. The sensitivity of fecal calprotectin in predicting deep remission in ulcerative colitis. Scand J Gastroenterol 2018;53:825–30. [DOI] [PubMed] [Google Scholar]
- [28].van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guardiola J, Lobaton T, Rodriguez-Alonso L, et al. Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol 2014;12:1865–70. [DOI] [PubMed] [Google Scholar]
- [30].Urushikubo J, Yanai S, Nakamura S, et al. Practical fecal calprotectin cut-off value for Japanese patients with ulcerative colitis. World J Gastroenterol 2018;24:4384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


