Abstract
Purpose of Review
This review seeks to inform oncology clinicians and researchers about the development of novel immunotherapies for the treatment of glioblastoma. An enumeration of ongoing and recently completed clinical trials will be discussed with special attention given to current technologies implemented to overcome central nervous system–specific challenges including barriers to the peripheral immune system, impaired antigen presentation, and T cell dysfunction.
Recent Findings
The success of immunotherapy in other solid cancers has served as a catalyst to explore its application in glioblastoma, which has limited response to other treatments. Recent developments include multi-antigen vaccines that seek to overcome the heterogeneity of glioblastoma, as well as immune checkpoint inhibitors, which could amplify the adaptive immune response and may have promise in combinatorial approaches. Additionally, oncolytic and retroviruses have opened the door to a plethora of combinatorial approaches aiming to leverage their immunogenicity and/or ability to carry therapeutic transgenes.
Summary
Treatment of glioblastoma remains a serious challenge both with regard to immune-based as well as other therapeutic strategies. The disease has proven to be highly resistant to treatment due to a combination of tumor heterogeneity, adaptive expansion of resistant cellular subclones, evasion of immune surveillance, and manipulation of various signaling pathways involved in tumor progression and immune response. Immunotherapeutics that are efficacious in other cancer types have unfortunately not enjoyed the same success in glioblastoma, illustrating the challenging and complex nature of this disease and demonstrating the need for development of multimodal treatment regimens utilizing the synergistic qualities of immune-mediated therapies.
Keywords: Glioblastoma, Immunotherapy, Cancer vaccine, CAR-T, Oncolytic virus, Checkpoint inhibitor
Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumor, affecting approximately 3 out of 100,000 individuals in the USA [1]. Despite an aggressive standard of care regimen of maximal surgical resection followed by combination radiation and alkylating chemotherapy, prognosis remains poor with a 100% recurrence rate and a median overall survival (mOS) of approximately 20 months [2]. Interest in immunotherapy as an alternative approach has rapidly amplified in recent years, following the success of immune checkpoint inhibitors (ICIs) as well as oncolytic viral (OV) therapies in melanoma and other cancers [3–5]. In GBM, however, immune-based therapies have not enjoyed the same success, as demonstrated by the disappointing outcomes of recent randomized, advanced phase clinical trials [6, 7, 8•]. This review will discuss the current status of immunotherapy for GBM, covering inherent neuroanatomical and immunosuppressive challenges in GBM, as well as ongoing research including cell-based therapy, ICIs, vaccines, and viral therapy.
Neuroanatomical Barriers of Immunotherapy
The central nervous system (CNS) has long been believed to be an immune-privileged environment. However, recent live-imaging studies revealed a CNS lymphatic system with activated T cells crossing the blood-brain barrier (BBB), thus giving rise to new questions about the interaction of the CNS with the peripheral immune system [9–11].
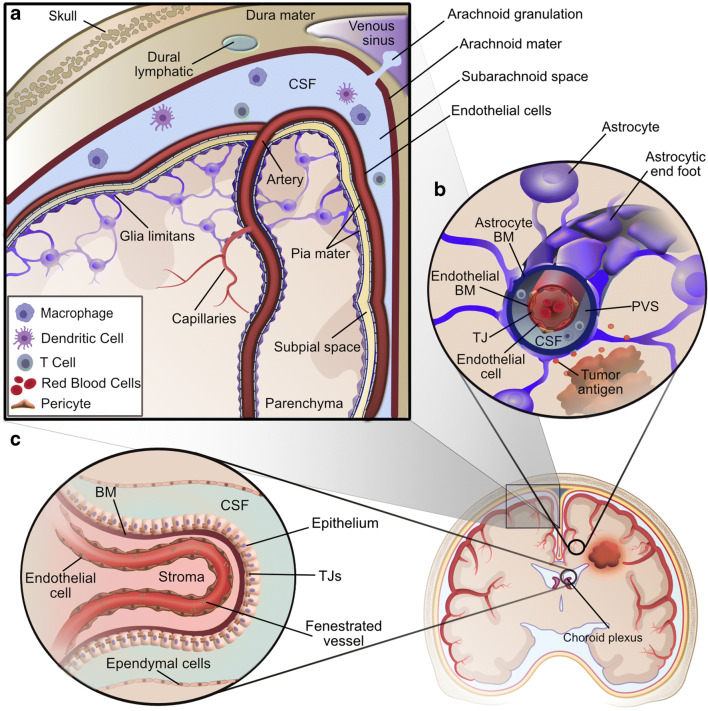
While evidence of CNS interaction with the immune system has been noted, three primary barriers deter entry of immune cells into the CNS: the blood-leptomeningeal barrier at the superficial surface, the BBB in the deep parenchyma-penetrating capillaries, and the choroid plexus epithelium (Fig. 1) [12••]. On the superficial surface of the brain, the pial meningeal layer coats arteries as they penetrate the brain surface, but does not coat veins exiting the parenchyma. A second layer of protection is provided by the glia limitans, composed of astrocyte foot processes, which re-enforces the basement membrane of arteries and veins as well as the superficial parenchyma itself. Low-molecular-weight molecules and fluid can pass through the glia limitans, but there is only limited exchange of T cells at this blood-leptomeningeal barrier [13–16]. Deeper in the brain, the BBB restricts the flow of systemic immune cells into the parenchyma. It is formed by specialized endothelial cells with tight junctions, the endothelial basement membrane enforced by pericytes, and the glia limitans. In the post-capillary venules, the endothelial basement membrane is separated from the glia limitans, creating a perivascular space in which immune cells may circulate for potential access to CNS antigens and entry into the CNS. Finally, the choroid plexus epithelium regulates passage of immune cells and solutes into the cerebrospinal fluid (CSF) through specialized tight junctions and efflux pumps [12••].
Fig. 1.
Central nervous system barriers to immunological communication. a The blood leptomeningeal barrier at the surface of the brain is composed of the glia limitans and pia mater covering the arteries, while veins exiting the parenchyma are protected only by the glia limitans. Additionally, the subarachnoid space is partitioned from the dural lymphatic system by the arachnoid mater, with the exception of arachnoid granulations allowing regulated drainage into the venous sinus. b The blood brain barrier deep in the brain parenchyma is composed of endothelial cells tightly linked together by tight junctions (TJ) and is further enforced by an endothelial basement membrane (BM). In the case of the post-capillary venule shown here, the endothelial BM is separated from the lining of glia limitans formed by astrocytic foot processes and the astrocytic BM, leaving a small perivascular space (PVS) filled with cerebral spinal fluid (CSF), in which immune cells can circulate and potentially interact with CNS antigens. c The choroid plexus tightly regulates parenchymal solute exchange with the CSF and inner stroma using a layer of epithelial cells, the epithelium, linked by tight junctions as well as a layer of ependymal cells (Adapted from Engelhardt, B et al. 2019) Created with BioRender.com and Affinity Designer software
Impaired Antigen Presentation Conceals Immune System Awareness of GBM
Antigen-presenting cells (APCs) have four routes from the CNS to reach lymph nodes where the immune response can be initiated. To reach deep cervical lymph nodes, CSF can either drain through the arachnoid villi to central venous sinuses or drain from the subarachnoid space to the lymphatics through the cribriform plate [10, 17]. CSF can also drain through dural lymphatic vessels in the skull base and through the perineurium of cranial and spinal nerve roots [9, 18]. These subarachnoid and dural lymphatic pathways allow some immune cell trafficking near the brain surface. By comparison, surveillance immune cells have limited access to the CNS parenchyma except in the post-capillary venules as described above. Interstitial fluid (ISF) flows though the basement membrane of arteries and capillaries, and this narrow pathway limits trafficking of large molecules as well as immune cells [19]. CSF and ISF exchange to a small degree; studies show low-molecular-weight tracers injected into the CSF enter the ISF through aquaporin-4 channels in the astrocyte end feet of the glia limitans [14, 20]. However, age-related protein deposition into blood vessel walls can limit ISF exchange over time [19, 21]. These forces collectively result in a more immune-privileged environment in the deep parenchyma where GBM often arises. Although the BBB may be partially disrupted in higher grade gliomas, there often remains a significant population of diffusely infiltrative tumor cells deep in the parenchyma that have an intact BBB and therefore may not be as readily accessed by circulating immune cells [22, 23].
The issue of restricted immune cell access is further compounded by the limited habitation of B and T cells in normal parenchyma, although CD4+ and CD8+ T cells can become activated by specific antigen either locally or systemically and then traverse the BBB, even in the absence of neuroinflammation [24, 25]. Entry of activated T cells into the brain parenchyma can occur through the dynamic interaction of adhesion and signaling molecules expressed by immune cells and endothelial cells lining post-capillary venules in parenchymal perivascular spaces [26•].
Tumor Heterogeneity Limits Degree and Durability of Response to Therapy
Tumor heterogeneity in GBM seriously undermines robust and durable responses to therapeutic interventions, driving poor survival outcomes. In addition to significant genetic diversity between different GBMs, there is substantial subclonal diversity within individual tumors, as defined by genetic and epigenetic profiling [27–31]. Furthermore, single-cell transcriptional profiling has demonstrated that individual GBM cells have temporal variation in gene expression regulating cellular function and the balance between stem-like and differentiation states [32]. In addition, exposure to chemotherapy and radiation, as well as microenvironmental differences in oxygen and other nutrients, can selectively pressure the expansion of certain subclones, or induce hypermutation [33].
Common genetic mutations in adult GBM frequently targeted by immunotherapy include the epidermal growth factor receptor variant III (EGFRvIII) mutation and the isocitrate dehydrogenase (IDH1) R132H mutation, while targets in pediatric gliomas include a conserved missense mutation in histone H3 from lysine (K) to methionine (M) at position 27 (H3 K27M mutation) [34–36]. While most tumor-specific antigens are expressed heterogeneously within tumor, the H3 K27M mutation is homogeneously distributed throughout the entire tumor [37, 38], suggesting that this may be a truncal mutation and less likely to lead to antigen loss–mediated escape of the tumor when targeted therapeutically.
Immunosuppression in Glioblastoma
Glioblastoma can impair both local CNS and systemic immune system functions, by hijacking major immunogenic signaling pathways and altering cellular immunity inside and outside the brain. In addition, there are immunosuppressive side effects of chemotherapy and radiation.
The Impact of Glioblastoma on Immune Cell Composition and Functionality
GBM alters physical and functional aspects of the brain immune system through production of immunosuppressive factors and modulation of cell surface receptors and immune cell subsets [39]. GBM induces systemic sequestration of naïve T cells in the bone marrow due to downregulation of sphingosine 1-phosphate receptor, contributing to low levels of effector T cells systemically and in the tumor micro-environment (TME) [40•]. The scarcity of effector T cells in the tumor is compounded by enrichment of regulatory T cells (Treg), a subpopulation of CD4+ T cells that suppress effector T cells through cytotoxic T lymphocyte–associated protein 4 (CTLA-4) signaling and secretion of cytokines TGF-β and interleukin-10 [41, 42]. Additionally, the immune cell composition of the GBM TME is characterized by a large population of macrophage and myeloid-derived cells, as compared to the meager presence of lymphocytes [43]. Tumor-associated macrophages (TAMs) support the progression of GBM through promoting angiogenesis and suppressing the adaptive immune response [44]. Collectively, solid tumor cancers, principally GBMs, that bear enrichment of macrophages are associated with shorter survival compared to tumors without such enrichment [43]. Myeloid-derived suppressor cells (MDSCs) further dampen the adaptive immune response by suppressing the proliferation and functionality of tumor-infiltrating T cells through the production of anti-inflammatory cytokines and T cell suppressive compounds as well as upregulation of the transmembrane protein, programmed death-ligand 1 (PD-L1) [45–47]. In addition to these complex cellular mechanisms, GBM-associated hypoxia results in over-production of angiogenic factors including vascular endothelial growth factor (VEGF) and hypoxia-inducible factor1-alpha (HIF-1a), creating an irregular vascular network that results in limited access to nutrients, immune cells, and therapeutic treatments [48].
Modulation of Centralized Signaling Pathways by Glioblastoma Promotes Immunosuppression
The ability of GBM to escape immune surveillance through deregulated signaling pathways enhances its ability to overcome many therapeutic strategies, including immunotherapies. For example, TGF-β, a major GBM-secreted factor, contributes to immunosuppression, angiogenesis, and maintenance of the glioma progenitor population through its involvement in multiple signaling pathways [49]. In addition, both GBM tumor cells and infiltrating immune cells induce constitutive activation of signal transducer and activator of transcription 3 (STAT3), which drives multiple pro-oncogenic pathways and dampens the anti-tumor immune response through recruitment and expansion of Treg cells and MDSCs, induction of T cell tolerance through STAT3 hyper-activation in APCs, and regulation of immunosuppressive cytokines [50]. However, while effective in preclinical settings of a variety of different cancers including GBM, inhibition of STAT3 fails to provide a viable therapeutic option as STAT3 regulates many other necessary biological processes, and inhibition results in unintended side effects such as thrombocytopenia [51]. In addition, the fibrinogen-like protein 2 (FGL2) pathway promotes immunosuppression through increasing expression of programmed cell death-1 (PD-1) on T cells and by expanding populations of TAMs, MDSCs, and Treg cells in the TME [52].
Suppression of the Immune Response by Standard Therapy and Corticosteroids
Patients with high-grade glioma that undergo standard-of-care (SOC) treatment with concurrent radiation and alkylating temozolomide (TMZ) may demonstrate CD4+ lymphopenia, which increases the patient’s risk for infections including Pneumocystis jiroveci pneumonia (PJP, formally known as PCP) [53]. In addition, patients are also often treated with dexamethasone, a synthetic glucocorticoid, for peri-operative control of intracerebral edema and consequent neurological symptoms (e.g., headaches, confusion, weakness, other focal neurological deficits, and seizures). However, dexamethasone and other glucocorticoids significantly dampen the overall immune response and can reduce therapeutic benefit of immunotherapies, resulting in consensus recommendations that their use be minimized prior to and during immunotherapy trials [54–56]. For example, it was recently reported that use of dexamethasone hampered vaccine-reactive T cell responses during neoantigen vaccine priming in patients with newly diagnosed malignant glioma [56, 57].
Recent Advances in Immunotherapy
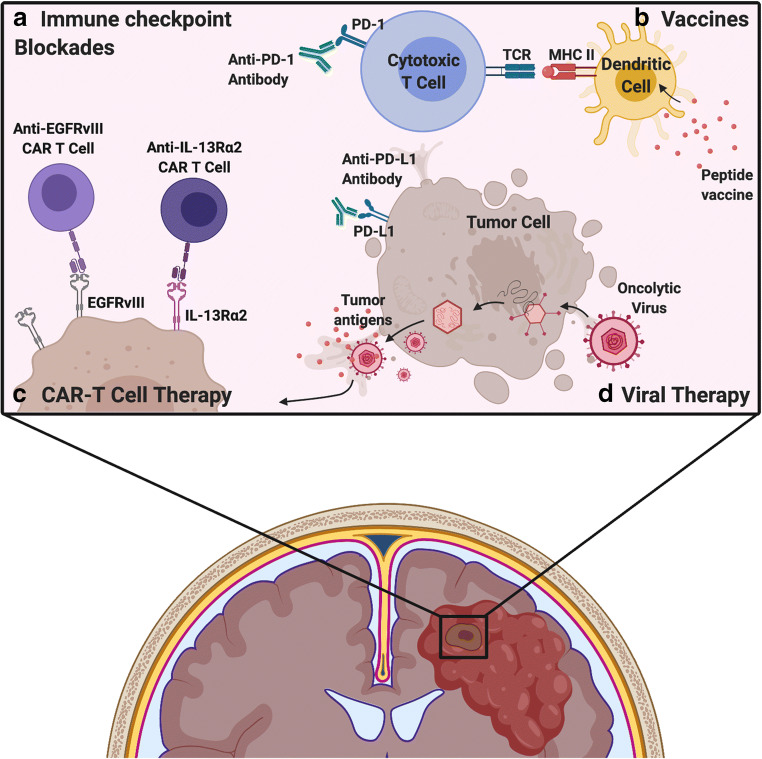
Over the past few decades, a multitude of immunotherapeutic strategies have attempted to eradicate and overcome GBM, including CAR-T cell, vaccine, immune checkpoint inhibitors (ICI), and OV therapies, discussed in detail below and summarized in Fig. 2.
Fig. 2.
Immunotherapeutic strategies for treatment of glioblastoma. a Immune checkpoint receptor/ligands such as PD-1 expressed on T cells and PD-L1 expressed on tissue cells downregulate the adaptive immune response in normal tissues. Tumors may express PD-L1 as well, thus inhibiting T cell activation in tumors. Immune checkpoint inhibitors are antibodies that block receptor-ligand interactions, such as between PD-1 and PD-L1, thus inhibiting the immunosuppressive effects of this interaction. b Vaccines introduce GBM-specific antigens to native APCs including dendritic cells and rely on MHC-dependent presentation to T cells to stimulate a GBM-targeted immune response. c CAR-T cell therapy uses autologous T cells, which are genetically modified to target GBM-specific surface antigens, such as EGFRvIII and IL-13Rα2. Unlike vaccines, CAR-T cells do not rely on MHC-dependent antigen presentation. d Viral therapy encompasses the use of oncolytic viruses and retroviruses to either initiate tumor cell lysis and release of tumor antigen or to integrate therapeutic transgenes for expression by the tumor cell
Cell-Based Therapy: Chimeric Antigen Receptor T Cell Therapy
The purpose of cell-based therapy is to enhance the anti-tumor activity of patient-derived T cells by engineering them to express either a chimeric antigen receptor (CAR) or a T cell receptor (TCR) targeting specific tumor antigens. However, this section will focus primarily on CAR-T cell therapy [58]. Unlike CAR-T cells, TCR-modified T cells rely on major histocompatibility class (MHC) peptide presentation, which GBM can suppress, thereby evading MHC-restricted T cell recognition and rendering TCR-modified T cells incapable of recognizing tumor-associated antigens (TAAs) [59]. To circumvent this issue, CAR-T cells are engineered to express a CAR, composed of a single-chain variable fragment specific to the target, a T cell activation domain (CD3ζ), and one or more co-stimulatory domains (such as 4-1BB, CD28, OX40), which redirects T cell specificity to an extracellular TAA [59, 60].
In a recent phase I study, autologous CAR-transduced T cells targeting the constitutively active EGFR variant EGFRvIII, which is present in 24–67% of GBM cases, were intravenously administered to 10 recurrent GBM patients. The mOS was 8.25 months, and 4 patients who underwent re-resection within 14 days of treatment demonstrated infiltration of CAR-T cells and increased activated lymphocytes within the resected tumor [61]. However, another phase I trial, which utilized a third-generation CAR construct of EGFRvIII, did not show therapeutic benefit [62]. The glioma-associated antigen interleukin-13 receptor alpha 2 (IL13Rα2), found in approximately 58% of adult GBM cases and 83% of pediatric brain tumors, has also been targeted by CAR-T cells in the clinical setting [63, 64]. Preliminary results for an ongoing phase I trial with IL-13α2 CAR-T cells demonstrated radiographic response of both intracranial and metastatic spinal tumors in one patient for 7.5 months [63, 65]. Interestingly, initial intracavitary CAR-T cell delivery resulted in localized tumor control with no effect on distant tumor sites, but there was significant regression of disseminated disease after adjunctive intraventricular delivery [65]. Other CAR-T clinical trials targeting single antigens in GBM have been largely unsuccessful; however, novel targets are currently under clinical investigation (Table 1).
Table 1.
Compilation of active or completed clinical trials investigating immunotherapeutic modalities in GBM, IDH mutant glioma, and pediatric DIPG/DMG diseases (2015–2020)
| Trial name | Treatment | Phase | Diagnosis | Outcomes |
|---|---|---|---|---|
| GBM: VACCINES | ||||
| ReACT NCT01498328 |
1) Rindopepimut/GM-CSF 2) Bevacizumab + KLH |
II (n = 73) | Recurrent EGFRvIII + GBM |
1) PFS-6: 28% 2) PFS-6: 16% (P = 0.12, one-sided) [66] |
| ACT IV NCT01480479 |
1) Rindopepimut/GM-CSF + TMZ 2) KLH + TMZ |
III (n = 745) | Newly diagnosed EGFRvIII + GBM |
1) mOS: 20.1 mos. 2) mOS: 20.0 mos. (HR = 1.01, 95% CI 0.79–1.30, P = 0.93) [7] |
|
IMA-950 |
IMA950 multi-peptide vaccine + poly-ICLC | I/II (n = 16) | Newly diagnosed, HLA-A2 + GBM | mOS: 19 mos. (95% CI: 17.25–27.87) [67] |
|
ICT-107 |
1) ICT-107 (peptide-pulsed DC vaccine) 2) Un-pulsed DCs |
II (n = 124) | Newly diagnosed GBM |
1) mOS: 17.0 mos. 2) mOS: 15.0 mos. (HR = 0.87, P = 0.58) [68] |
|
GAPVAC-101 |
1) APVAC1 or APVAC2 (multi-peptide vaccines)/GM-CSF + poly-ICLC + TMZ | I (n = 16) | Newly diagnosed, HLA-A*02:01 or HLA-A*24:02 + GBM |
mPFS: 14.2 mos. mOS: 29 mos. (for n = 15) (P = N/A) [69] |
|
DCVax-L |
1) DCVax-L (tumor lysate-pulsed DC vaccine) 2) PBMC (control) |
III (n = 348) | Newly diagnosed GBM |
mOS: 23.1 mos. (95% CI 21.2–25.4; P = N/A) [70] |
| NCT02078648 | SL701/GM-CSF + poly-ICLC and bevacizumab | I/II (n = 74) | HLA-A2 + recurrent GBM |
Stage 1 OS-12: 37% Stage 2 OS-12: 43% (P = N/A) [71] |
|
I-ATTAC |
CMV pp65-LAMP mRNA-pulsed DCs/GM-CSF/Td + TMZ | II (n = 48) | Newly diagnosed, CMV + MGMT unmethylated GBM | Recruiting |
|
ACTION |
TTRNA (tumor RNA) pulsed DCs/GM-CSF/Td ± HSCs | I (n = 8) | HGG | Recruiting |
| NCT01204684 | Tumor lysate-pulsed DCs ± resiquimod or poly-ICLC | II (n = 60) | New or recurrent HGG | Active |
| NCT03382977 | VBI-1901/GM-CSF | I/II (n = 38) | Recurrent, IDH-1/2 wildtype GBM | Recruiting |
| GBM: Checkpoint inhibitors | ||||
|
CheckMate 143 |
1) Nivolumab 2) Bevacizumab |
III (n = 626) | Newly diagnosed, first recurrence, or MGMT unmethylated GBM |
1) mOS: 9.8 mos. 2) mOS: 10.0 mos. (HR = 1.04; P = 0.76)[8•] |
|
CheckMate 498 |
1) Nivolumab + RT 2) TMZ + RT |
III (n = 550) | Newly diagnosed, MGMT Unmethylated GBM | Failure to reach primary objective of OS |
|
CheckMate 548 |
1) Nivolumab + TMZ + RT 2) TMZ + RT |
III (n = 693) | Newly diagnosed, MGMT Methylated GBM | Ongoing; failed to meet PFS primary endpoint; OS under investigation |
| NCT02337491 |
1) Pembrolizumab + bevacizumab 2) Pembrolizumab |
II (n = 80) | Recurrent GBM |
1) PFS-6: 26% mOS: 8.8 mos. 2) PFS-6: 6.7% mOS: 11.3 mos. [72] |
| NCT02313272 | Pembrolizumab + bevacizumab + HFSRT | I (n = 32) | Recurrent HGG |
OS-6: 94% OS-12: 64% [73] |
| NCT02794883 |
Durvalumab (anti-PD-L1) ± tremelimumab (anti-CTLA4) |
II (n = 36) | Recurrent HGG | Active |
| NCT02968940 | Avelumab (anti-PD-L1) + HFSRT | II (n = 43) | HGG | Completed |
| NCT02311920 | Ipilimumab + nivolumab + TMZ | I (n = 32) | Newly diagnosed GBM or gliosarcoma | Active |
| GBM: Adoptive cell therapy | ||||
| NCT02208362 | IL13Rα2-specific CAR T cells | I (n = 92) | Refractory/recurrent GBM |
Ongoing; interval analysis PFS (n = 1): 7.5 mos. [65] |
| NCT02209376 | EGFRvIII CAR T cells | I (n = 11) | Recurrent, EGFRvIII + GBM |
mOS: ~ 8 mos. PFS: not evaluable [61] |
| NCT01454596 | EGFRvIII CAR T cells, aldesleukin, fludarabine, and cyclophosphamide | I (n = 18) | New or recurrent, EGFRvIII + GBM |
mOS: 6.9 mos. PFS: 1.3 mos. Outlier: 12.5 mos. [62] |
|
HERT-GBM |
HER.CAR CMV-specific CTLs | I (n = 16) | Recurrent, HER2 + CMV seropositive GBM |
Ongoing; interim ORR (PR + SD): 33% [74] |
| NCT04077866 | B7-H3 CAR-T + TMZ | I/II (n = 40) | Recurrent or refractory GBM | Recruiting |
| NCT04045847 | CD147 CAR T cells | I (n = 31) | Recurrent CD147 + GBM | Recruiting |
| NCT04214392 | Chlorotoxin CAR T cells | I (n = 36) | Recurrent MPP2 + GBM | Recruiting |
| GBM: Viral therapy | ||||
|
DNX-2401 (Delta-24-RGD) |
DNX-2401 (adenovirus) 1) IT injection 2) IT infusion, resection |
I (n = 37) | Recurrent HGG |
1) mOS: 9.5 mos. 2) mOS: 13.0 mos. (P = N/A) [75] |
|
D24GBM |
DNX-2401 + TMZ | I (n = 31) | Recurrent GBM |
Ongoing; 3 objective responses (30, 19, and 27 mos.) [76] |
|
PVSRIPO |
1) Recombinant poliovirus 2) Historical controls |
I (n = 61) | Recurrent GBM |
1) mOS: 12.5 mos. (95% CI, 9.9 to 15.2) 2) mOS: 11.3 mos. (95% CI, 9.8 to 12.5) (P = N/A) [77] |
| NCT02986178 | PVSRIPO + lomustine | II (n = 122) | Recurrent GBM | Active |
| NCT01156584 |
1) Toca 511 + Toca FC 2) External control |
I (n = 54) | Recurrent, unresectable HGG |
1) mOS: 13.6 mos. 2) mOS: 7.1 mos. |
| NCT01470794 | Toca 511 + Toca FC | I (n = 56) | Recurrent HGG | mOS: 11.9 mos. (95% CI, 10.7 to 15.1) [80] |
| NCT02414165 |
1) Toca 511 + Toca FC 2) SOC |
II/III (n = 403) |
Recurrent HGG; IDH-mut stratified |
1) mOS: 11.1 mos. 2) mOS: 12.2 mos. (HR = 1.06; P = 0.6154) [6] |
|
rQNestin |
rQNestin34.5v.2 HSV-1 ± cyclophosphamide | I (n = 108) | Recurrent HGG | Ongoing |
| UMIN000002661 | G47delta (2nd-gen. oncolytic HSV-1) | I-II (n = 21) | Recurrent GBM |
Completed No results reported yet |
| UMIN000015995 |
1) G47delta 2) Historical control |
II (n = 30) | Recurrent GBM |
Ongoing 1) OS-12: 92.3% 2) OS-12: 15% [81] |
| NCT00390299 |
MV-CEA (measles virus) 1) IC injection 2) IT infusion + resection + IC injection |
I (n = 23) | Recurrent GBM |
1) mOS: 11.8 mos. PFS-6: 22.2% 2) mOS: 11.4 mos. PFS-6: 23.1% [82] |
|
ParvOryx01 |
H-1PV (H-1 parvovirus) | I/IIa (n = 18) | Progressive primary or recurrent GBM patients |
mOS: ~ 15.5 mos. PFS: ~ 4 mos. (P = N/A) [83] |
| GBM: Combination therapies (not including SOC) | ||||
|
Ad5-DNX-2401 |
Ad5-DNX-2401 (adenovirus delivered in MSCs) | I (n = 36) | Recurrent, IDH-1 wildtype HGG/AAs | Ongoing |
| NCT03726515 | EGFRvIII-targeted CAR-T + pembrolizumab | I (n = 7) | Newly diagnosed MGMT Unmethylated, EGFRvIII + GBM | Ongoing |
| NCT04003649 | IL13Rα2 TN/MEM cells, ipilimumab and nivolumab (neoadjuvant, adjuvant) | I (n = 60) | Recurrent or refractory GBM | Recruiting |
|
NeoVax |
NeoAntigen (multi-peptide vaccine), RT, pembrolizumab | I (n = 46) | Newly diagnosed MGMT Unmethylated GBM | Ongoing |
|
CAPTIVE/KEYNOTE-192 |
DNX-2401, pembrolizumab | II (n = 49) | Recurrent GBM patients |
Ongoing; interim analysis: OS-9: 100% (first 7 patients) [84] |
| NCT04013672 | Pembrolizumab, SurVaxM (survivin vaccine)/GM-CSF, Montanide ISA 51 | II (n = 51) | Recurrent GBM | Recruiting |
|
IMA950-106 |
Pembrolizumab + IMA950/poly-ICLC | I (n = 24) | Recurrent GBM | Ongoing |
| NCT03743662 | Bevacizumab, nivolumab, RT, re-resection | II (n = 94) | Recurrent, IDH wildtype GBM | Recruiting |
|
MEDI4736 |
Durvalumab ± RT or bevacizumab | II (n = 159) | Newly diagnosed or recurrent MGMT Unmethylated GBM | Active |
| NCT03425292 | Nivolumab ± ipilimumab ± bevacizumab ± TMZ | I (n = 90) | Newly diagnosed HGG | Recruiting |
| NCT04225039 | SRS + GITR agonist (INCAGN01876) + anti-PD1 (INCMGA00012) | II (n = 32) | Recurrent, IDH wildtype GBM | Recruiting |
| NCT01811992 |
Ad-hCMV-tk and Ad-hCMV-Flt3L (adenoviral vectors) |
I (n = 19) | Newly diagnosed GBM | Ongoing |
| NCT03576612 | AdV-tk + valacyclovir + nivolumab + RT + TMZ | I (n = 36) | Newly diagnosed HGG | Recruiting |
| GLOBE NCT02511405 |
1) VB-111, bevacizumab 2) Bevacizumab |
III (n = 256) | Recurrent GBM |
1) mOS: 6.8 mos. 2) mOS: 7.9 mos. (HR, 1.20; 95% CI: 0.91–1.59; P = 0.19) [85] |
|
DNX-2440 |
DNX-2440 (OX40L adenoviral vector) | I (n = 24) | Recurrent GBM | Ongoing |
|
M032-HSV-1 |
M032-HSV-1 (2nd-gen. HSV) with IL-12 | I (n = 36) | Recurrent GBM | Ongoing |
| IDH mutant glioma: Vaccine | ||||
| RESIST NCT02193347 | IDH peptide vaccine (PEPIDH1M) + TMZ | I (n = 24) | Recurrent Grade II glioma, IDH mutant | Ongoing |
|
NOA-16 |
IDH1R132H peptide vaccine, montanide, imiquimod |
I (n = 39) | Newly diagnosed AA and GBM; IDH1R132H mutant | 80% specific T cell response; 87% with specific humoral response [86] |
| IDH mutant glioma: Combination therapy | ||||
| NCT03532295 | INCMGA00012 (anti-PD1) + epacadostat + bevacizumab + RT | II (n = 55) |
Recurrent GBM IDH wildtype or mutant |
Recruiting |
| NCT03422094 | NeoVax, ipilimumab, nivolumab | I (n = 3) | Newly diagnosed MGMT Unmethylated, IDH1/IDH2 mutant GBM | Ongoing |
| Pediatric: Adoptive cell therapy | ||||
| NCT04196413 | GD2-CAR T cells | I (n = 54) | DMG with H3.3 K27M mutation | Recruiting |
| NCT04185038 | B7H3-CAR T cells | I (n = 70) | DMG, or recurrent CNS tumor | Recruiting |
| NCT03389230 | HER2(EQ)BBζ/CD19t+ T cells | I (n = 42) | Recurrent, HER2 + HGG | Recruiting |
| NCT03500991 | I (n = 48) | Refractory or recurrent, HER2+ pediatric CNS tumor | Recruiting | |
| NCT03638167 | EGFR806-CAR T cells | I (n = 36) | EGFR + refractory or recurrent CNS tumor | Recruiting |
| Pediatric: Vaccine | ||||
| NCT02960230 | H3.3 K27M peptide vaccine ± nivolumab | I (n = 29) | New glioma with H3.3 K27M mutation | Ongoing [56] |
|
BRAVO |
TTRNA (tumor RNA) pulsed DCs/GM-CSF/Td + HSCs + TMZ or cyclophosphamide/fludarabine | I (n = 21) | DMG | Recruiting |
| Pediatric: Immune checkpoint inhibition | ||||
| NCT04323046 | Neoadjuvant ipilimumab ± nivolumab | I (n = 45) | Recurrent HGG | Not yet recruiting |
| Pediatric: Viral therapy | ||||
| NCT03178032 | DNX-2401 + SOC | I (n = 12) | Naïve DIPG |
Completed No results reported yet |
| NCT03043391 | PVSRIPO | I (n = 12) | Recurrent HGG | Recruiting |
| NCT03911388 | HSV-G207 | I (n = 15) | Recurrent GBM and CNS tumors | Recruiting |
| Pediatric: Combination therapy | ||||
| NCT00634231 | AdV-tk plus valacyclovir + RT | I (n = 12) | Malignant glioma or recurrent ependymoma |
Active OS (n = 3): 24+ mos. PFS (n = 2): 37.3 and 47.7 mos. [87] |
Clinical trial information was found on www.clinicaltrials.gov accessed August 5, 2020. IV intravenous, mOS median overall survival, PFS-# progression free survival-number of months, OS-# overall survival-number of months, CI confidence interval, HR hazard ratio, ORR overall response rate, PR partial response, SD stable disease, SOC standard of care, TMZ temozolomide, KLH keyhole limpet hemocyanin, DC dendritic cell, IT intratumoral, IC intracavitary, RT radiotherapy, HFSRT hypofractionated radiation therapy, Td tetanus diphtheria toxoid, HSCs hematopoietic stem cells, MSCs mesenchymal stem cells, SRS stereotactic radiosurgery, DIPG diffuse intrinsic pontine glioma
While hematologic cancers have been successfully treated with CAR-T cell therapy, its efficacy against CNS and other solid tumors has been limited by a host of barriers including heterogeneously expressed tumor antigens, limited persistence and homing to distant tumor sites, and TME-mediated immunosuppression [88]. To address GBM heterogeneity, there are several exploratory CAR-T systems in development that target multiple antigens, either in parallel or in tandem [89–93]. Additionally, improving CAR-T persistence within the CNS requires refinement of delivery, timing, and frequency and has been limited by incomplete understanding of CAR-T cell viability after CNS entry. In a phase I clinical trial with EGFRvIII CAR-T cells delivered through a single intravenous infusion, peak peripheral expansion of CAR-Ts was noted ~ 3–10 days after infusion, and detected up to 30 days after infusion, as defined by flow cytometry of peripheral blood samples [61]. Although this study looked at in situ tumor engraftment of CAR-T cells in tumors that were later resected, sample size was small (n = 7) and engraftment variable, so it is difficult to determine if tumor engraftment is related to amount and duration of CAR-T cells in the periphery or other factors [61]. Intratumoral, intracavitary, and/or intraventricular delivery may further improve durability of response. In comparison to intravenous therapy, preclinical studies have suggested that intraventricular (intrathecal) delivery may lead to faster tumor infiltration, increased penetration into the tumor core, and longer persistence within the tumor [94, 95]. However, preliminary evidence in one patient who received intrathecally delivered CAR-Ts targeting IL13Rα2 showed that CAR-T cells may persist in the cerebrospinal fluid for only 7 days, although this study did not look at CAR-T infiltration into the tumor itself [65]. Delivery issues including method and frequency need further investigation and would benefit from improved, non-invasive monitoring techniques, as discussed later.
Immune Checkpoint Inhibitors
Despite success in other cancers, ICI blockade of PD-1/PD-L1 and CTLA-4 has been grossly unsuccessful thus far in GBM. A phase III trial comparing the PD-1 inhibitor nivolumab to VEGF-A inhibitor bevacizumab showed no improvement in survival in patients with recurrent GBM (CheckMate-143) [8•]. The Checkmate-498 trial, comparing nivolumab plus radiation (RT) versus standard-of-care temozolomide plus RT in patients with newly diagnosed GBM without MGMT promoter methylation (a poor prognostic factor), was stopped after nivolumab with radiation did not show improved survival compared to temozolomide with radiation (NCT02617589, publication pending). There is a complementary ongoing trial in patients with newly diagnosed MGMT promoter methylated GBM (CheckMate-548, NCT02667587). Preliminary results have shown that there is no improvement in progression-free survival (PFS) with the addition of nivolumab to temozolomide and RT, but final results regarding OS are pending. Several ongoing trials are also exploring the combination of nivolumab with the CTLA-4 inhibitor, ipilimumab (Table 1).
Interestingly, while checkpoint inhibition has been mostly ineffective in GBM, there has been some efficacy demonstrated in brain metastases (BMs) from systemic cancers including melanoma and non-small cell lung cancer [96–98]. The discrepancy in efficacy of ICI therapy between BMs and GBM may be partially explained by the variation in immune cell phenotype, function, and spatial organization of each tumor type’s respective TME [99]. Two recent, comprehensive analyses of the TME in both primary and metastatic brain malignancies found that BMs, especially melanomas, were characterized by higher proportions of lymphocytes, including CD8+ T cells, unlike gliomas, which instead exhibited an abundance of tissue-resident microglia and myeloid cells [99, 100••]. The efficacy of ICIs in metastases may also relate to neuroanatomical differences; metastases have BBB remodeling that may allow for more efficient immune cell intravasation [101, 102]. In addition, metastases are often well circumscribed, and a higher percent volume of each metastasis has BBB disruption, compared to diffusely infiltrating astrocytomas that have large areas of tumor with an intact BBB [103].
Other factors contributing to the failure of ICIs in GBM may include decreased passage through the BBB of ICI and activated T cells, as well as low expression of neoantigens by GBM compared to other systemic tumors [104]. As there are few tumor-infiltrating lymphocytes (TILs) in GBM [105], maximal efficacy of checkpoint inhibitors may require initial peripheral activation of circulating T cells [106•]. There is an ongoing study to evaluate if there is utility in combining intravenous/intrathecal delivery of PD-1 inhibitors in brain metastases of melanoma (NCT03025256), but no such trial has yet been undertaken in primary brain tumors. Compounding the lack of access of CTLA4-expressing APCs and PD-1-expressing T cells to the CNS, not all GBMs express PD-L1, and those that do show PD-L1 expression in fewer than 5% of cells [107–110]. In addition, the tumor mutational burden and neoantigen expression are lower compared to systemic cancers [107–110]. Furthermore, patients with GBM often have impaired systemic immunity, limiting the potential response to checkpoint inhibition [40•]. With ICI exposure, there may be compensatory upregulation of the tumor-driven immunosuppressive pathways, including upregulation of other checkpoint pathway ligands that could cause T cell exhaustion. Finally, there may be some relevance to ICI timing, as a recent small trial demonstrated improved survival in GBM with treatment with the anti-PD-1 antibody, pembrolizumab, prior to surgery, suggesting possible utility to immune system priming prior to antigen exposure during surgery [111•].
Vaccine Therapy
Given the immunologically “cold” environment of GBM, efforts have been made to enhance the adaptive arm of the immune system to target GBM cells through cell-mediated immunity [112]. Three peptide or dendritic cell (DC)-based vaccines recently reached phase III clinical trial testing. In the phase III trial Act IV, rindopepimut, an immunogenic peptide vaccine targeting EGFRvIII, elicited a strong humoral response but conferred no significant improvement in PFS or mOS [7]. Durability of response may have been hampered by acquired loss of EGFRvIII expression, illustrating one of the greatest shortcomings of single-antigen vaccines [7]. On the other hand, a multi-peptide DC vaccine, ICT-107, targeting six GBM-specific HLA-A1 and A2 antigens was tested in a randomized phase II trial and prolonged patient PFS to 11.4 months, a significant increase compared to the control group’s PFS of 10.1 months (HR = 0.64, p = 0.033) [68]. ICT-107 was most effective in extending the mOS and PFS in HLA-A2+ patients or patients with MGMT-methylated GBM [68]. A follow-up phase III trial testing ICT-107 was attempted, but was discontinued due to inadequate funding. The third vaccine to reach phase III testing is DCVax-L, derived by educating autologous DCs with whole tumor lysate extracted from individual patient resections. The interim results of the placebo-controlled phase III DCVax-L trial showed an mOS of 23.1 months; however, the analysis included all patients and was not broken down by treatment group [69, 70].
Other single- and multi-antigen vaccines are under investigation in early phase trials, including (IDH1) R132H and H3.3K27M peptide vaccines. The (IDH1) R132H peptide vaccine targets a frequently occurring mutation in the IDH1 gene found primarily in lower grade gliomas, which is responsible for aberrant neural signaling and glioma progression [35]. In the phase I NOA-16 trial, this vaccine was administered with SOC in newly diagnosed patients with (IDH1) R132H-positive WHO Grade III and IV astrocytomas. Preliminary results demonstrated induction of a humoral immune response with a reasonable toxicity profile [86]. In a recent multicenter pilot study, vaccine targeting H3.3K27M, along with Toll-like receptor 3 agonist, polyinosinic-polycytidylic acid stabilized with polylysine and carboxy-methylcellulose (poly-ICLC), was administered to HLA-A02.201+ pediatric patients with H3.3K27M+ diffuse midline glioma (DMG). A subset of patients (n = 18) were selected for available multi-timepoint blood draws and 39% exhibited an expansion of H3.3K27M-reactive CD8+ T cells (mOS 16.3 months) and showed significantly prolonged OS compared to tumors who failed to demonstrate a T cell response (mOS 9.9 months) [56]. Furthermore, dexamethasone administration is inversely associated with H3.3K27M-reactive CD8+ T cell responses [56].
Multi-peptide vaccines are designed to elicit a strong T cell response to multiple tumor antigens. For example, IMA-950 (multi-peptide vaccine composed of 9 MHC class I and 2 MHC class II peptides), with poly-ICLC, was recently tested in a phase I clinical trial and resulted in patients developing tumor peptide-specific CD4+ and CD8+ T cell responses, with the IMA-950 antigens remaining stably expressed on the tumor throughout the course of the disease [67]. Another multi-peptide vaccine was tested in the GAPVAC-101 trial, in which personalized cocktails of pre-manufactured peptide vaccines utilized non-mutated GBM antigens (APVAC1) and neoepitopes (APVAC2) to elicit an immunological response. This trial demonstrated sustained memory CD8+ T cell responses induced by APVAC1, while a TH1 CD4+ T cell response was elicited by APVAC2 [113]. Another recent phase I/Ib study tested the efficacy of a personalized neoantigen vaccine in newly diagnosed GBM patients and found the vaccination promoted neoantigen-specific CD4+ and CD8+ T cell responses and additionally increased the number of tumor-infiltrating T cells [57].
Viral Therapy
Aberrant cellular pathways in GBM provide a favorable environment for specially modified viruses to selectively replicate [114]. Following successful replication within target tumor cells, oncolytic viruses (OVs) elicit lytic cell death, provoking a strong inflammatory response followed by a tumor-targeted immune response, initiated by the liberation of damage-associated molecular patterns (DAMPs) and TAAs from tumor cells [115, 116]. A variety of viruses have been reengineered to target GBM and have been critically reviewed by Chiocca et al. (2019) [117••].
Adenoviruses can be readily manipulated to drive cell death, facilitate an immunogenic anti-tumor response, or carry therapeutic transgenes [118]. For example, conditionally replicative adenoviruses have been modified to selectively replicate in tumor cells with aberrant p16/Rb/E2F signaling (which controls the activity of the retinoblastoma tumor suppressor protein (Rb), a regulator of the E2F transcription factor) [119]. DNX-2401 (previously delta-24-RGD) selectively replicates within brain tumor stem cells with an abnormal p16INK4/Rb pathway [120]. Recently, a phase I clinical trial demonstrated that replication of DNX-2401 in recurrent GBM tumors facilitated tumor cell death and some immunogenic cell death by infiltrating CD8+ T cells and TH cells, lengthening mOS to 13.0 months [75]. Another phase I trial in newly diagnosed GBM seeks to capitalize on putative synergy between DNX-2401 and temozolomide, improving tumor recognition by CD8+ T cells [121, 122]. Interim results reported several objective radiological responses up to 30 months [76]. G47delta, a conditionally replicating, double mutant derivative of herpes simplex virus-1 (HSV-1), is another oncolytic virus undergoing current investigation. In a phase II trial in recurrent/residual glioblastoma, G47delta was injected intracranially in combination with adjuvant TMZ, resulting in an influx of lymphocytes, and interim results have reported a 1-year survival of 92.3% [123].
Unlike OVs, replicating retroviruses (RRVs) are non-lytic, require an actively dividing host cell to support viral replication, and can act as selective carriers of exogenous pro-cytotoxic transgenes [124]. Toca 511 (vocimagene amiretrorepvec) is an RRV carrying a modified yeast-derived cytosine deaminase gene (CD) that integrates into the tumor cell genome and converts systemically delivered pro-drug, 5-fluorocytosine (5-FC) into cytotoxic 5-fluorouracil (5-FU) [124]. Despite promising phase I results, the phase II/III trial failed to improve OS as compared to a SOC cohort, with mOS of 11.1 and 12.2 months, respectively, leading to premature termination [6]. It should be noted that certain factors predicted improved survival, namely diagnosis of anaplastic astrocytoma, presence of IDH1 R132H mutation, and second disease recurrence.
Moving Forward
Thus far, immunotherapy has had minimal impact on the survival of GBM patients, challenged by limitations with antigen presentation, immune cell trafficking into the CNS, tumor infiltration, and immunosuppression from the tumor and its treatments. Additionally, accurate assessment of the efficacy of immunological therapies is challenged by the limited functionality of non-invasive monitoring tools, resulting in cases of premature treatment discontinuation and a limited understanding of treatment impact.
Overcoming Adaptive Immune Resistance and Immune Escape
Novel therapies need to address mechanisms of immune escape, including T cell exhaustion and adaptive resistance [125]. One strategy addressing T cell exhaustion includes co-administration of a glycogen synthase kinase 3 (GSK3) inhibitor with CAR-T cell therapy as there is some evidence that this may reduce CAR-T exhaustion and help establish an effector memory T cell population [126]. Due to some evidence that the PD-L1 ligand may be upregulated after CAR-T therapy [61, 127], one ongoing trial has incorporated checkpoint inhibition in conjunction with CAR-T (Table 1). Another similar strategy involved modification of Delta-2401 (previously delta-24-RGD) adenovirus to deliver the gene for the OX40 ligand T cell stimulatory gene to glioma tumor cells. An exploratory study administered this modified virus (Delta-24-RGDOX or DNX2440) with a PD-L1 inhibitor and noted an increase in infiltrative T cells in vivo in a preclinical tumor model, leading to the initiation of a phase I clinical trial (see Table 1) [128]. Finally, anti-CTLA-4 (ipilimumab) and anti-PD1 (pembrolizumab) have been used in clinical trials and are currently undergoing investigation in combination with a vaccine therapy (Table 1).
Advances in gene therapy offer a multimodal approach to targeting GBM. In an ongoing phase I trial, patients were given intratumoral injections of an adenovirus encoding HSV type I thymidine kinase (Ad-TK), which converts systemically delivered valacyclovir to cytotoxic ganciclovir within infected tumor cells, in addition to a second adenovirus delivering the dendritic cell (DC)–recruiting Fms-like tyrosine kinase 3 ligand (Ad-Flt3L) to transform the tumors into DC-attracting hot zones. In mouse xenografts, this gene therapy was combined with a combinatorial PD-1/CTLA-4 blockade, which reduced the MDSC population and enhanced survival [129•].
Other combinatorial approaches have attempted to simultaneously target the lifeblood of the tumor while forcing tumor cells into apoptosis by using VEGF inhibitors and gene therapy. For example, in the phase III randomized GLOBE trial, VEGF inhibitor bevacizumab was combined with the VB-111 adenovirus, which delivers a transgene activating the Fas pro-apoptotic pathway in tumor endothelial cells; however, this combination did not extend mOS compared to bevacizumab monotherapy (Table 1) [85]. Of note, early phase trials with VB-111 had a median OS of 13.6 months, but VB-111 was administered concurrently with bevacizumab, rather then prior to bevacizumab as was the case in later trials [85]. Other ongoing combinatorial therapies are summarized in Table 1.
Current Diagnostic Shortcomings and Solutions
Advancement in the use of immunotherapy in GBM will also require improved methods to assess immune response in the tumor. Unfortunately, this assessment currently relies on invasive biopsy, with limited functionality of non-invasive monitoring tools.
Magnetic resonance imaging (MRI) is limited in its ability to distinguish between tumor, treatment effect, and inflammatory infiltration [130]. The Immune Response Assessment in Neuro-Oncology (iRANO) guidelines for interpreting MRIs while on immunotherapy were released in 2015 [54]. These recommend deferring final interpretation of MRI changes for 3 months, a relatively long period for a disease with mOS of 20–22 months from initial diagnosis and approximately 9 months from recurrence. Other advanced imaging techniques, including MRI with delayed or dynamic susceptibility contrast, dynamic MRI perfusion, arterial spin labeling, diffusor tensor imaging, MR spectroscopy, and position emission tomography (PET), all lack the sensitivity, specificity, and/or resolution to reliably predict which areas of imaging correlate to tumor progression versus immune infiltrate [130]. There has been some effort to label lymphocytes ex vivo with a radionuclide or MRI probe at the cell surface, or to engineer cytolytic CD8+ T cells to express a radionuclide, PET reporter gene, or MRI reporter gene [131]. In other neuro-inflammatory conditions including multiple sclerosis (MS) and cerebral infarct, radioisomer C11-PK11195-labeled translocator protein can identify activated astrocytes, microglia, astrocytes, and macrophages imaged using PET [130, 132]. In MS, ultra-small paramagnetic iron oxide nanoparticles have also been used to image infiltrative immune cells where the BBB is intact [133]. These technologies could potentially be extended to immunotherapy in GBM.
Conclusion
Therapeutic success with immunotherapy in GBM has been challenged by immune surveillance evasion through multiple deregulated signaling pathways, limited antigen presentation, and production of immunosuppressive cytokines and regulatory immune cells. These failures have emphasized that successful treatment of GBM may rely on combination therapies, with cell-based therapies targeting multiple specific antigens and therapies that indiscriminately target a broad population of GBM cells, such as oncolytic viruses. Furthermore, thoughtful design of dosing schedules and delivery methods of cell-based therapies, oncolytic viruses, immune checkpoint inhibitors, and vaccines is essential in affirming their best possible performance when used in the combination therapy setting.
Compliance with Ethical Standards
Conflict of Interest
Abigail L. Mende and Jessica D. Schulte declare no conflict of interest. Hideho Okada has received research funding from Agios Pharmaceuticals, has received compensation for service as a consultant from Bristol-Myers Squibb and Agios Pharmaceuticals, and receives royalties on three issued patents: inventor of H3.3k27M TCR (licensed to Tmunity, Inc.), IL-13Ra2 (345-353:1A9V) peptide (licensed to Stemline, Inc.), and EGFRvIII-CAR (licensed to Novartis). Jennifer L. Clarke has received clinical trial funding from Agios Pharmaceuticals, Merck, and Novartis and has received compensation from Agios Pharmaceuticals for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Neuro-oncology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abigail L. Mende and Jessica D. Schulte contributed equally to this work.
Contributor Information
Abigail L. Mende, Email: abigail.mende@ucsf.edu
Jessica D. Schulte, Email: jessica.schulte@ucsf.edu
Hideho Okada, Email: hideho.okada@ucsf.edu.
Jennifer L. Clarke, Email: jennifer.clarke@ucsf.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro-Oncology. 2019;21(Suppl 5):v1–v100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5(1):e1115641. doi: 10.1080/2162402X.2015.1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6(1):35. doi: 10.1186/s40425-018-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634–642. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloughesy T, Petrecca K, Walbert T, Butowski N, Salacz M, Perry J, et al. LTBK-08. Toca 511 & Toca FC versus standard of care in patients with recurrent high grade glioma. Neuro-Oncology. 2019;21(Supplement_6):vi284-vi. doi: 10.1093/neuonc/noz219.1199. [DOI] [Google Scholar]
- 7.Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, Ashby L, Mechtler L, Goldlust SA, Iwamoto F, Drappatz J, O'Rourke DM, Wong M, Hamilton MG, Finocchiaro G, Perry J, Wick W, Green J, He Y, Turner CD, Yellin MJ, Keler T, Davis TA, Stupp R, Sampson JH, Butowski N, Campian J, Recht L, Lim M, Ashby L, Drappatz J, Hirte H, Iwamoto F, Mechtler L, Goldlust S, Becker K, Barnett G, Nicholas G, Desjardins A, Benkers T, Wagle N, Groves M, Kesari S, Horvath Z, Merrell R, Curry R, O'Rourke J, Schuster D, Wong M, Mrugala M, Jensen R, Trusheim J, Lesser G, Belanger K, Sloan A, Purow B, Fink K, Raizer J, Schulder M, Nair S, Peak S, Perry J, Brandes A, Weller M, Mohile N, Landolfi J, Olson J, Finocchiaro G, Jennens R, DeSouza P, Robinson B, Crittenden M, Shih K, Flowers A, Ong S, Connelly J, Hadjipanayis C, Giglio P, Mott F, Mathieu D, Lessard N, Sepulveda SJ, Lövey J, Wheeler H, Inglis PL, Hardie C, Bota D, Lesniak M, Portnow J, Frankel B, Junck L, Thompson R, Berk L, McGhie J, Macdonald D, Saran F, Soffietti R, Blumenthal D, André de SBCM, Nowak A, Singhal N, Hottinger A, Schmid A, Srkalovic G, Baskin D, Fadul C, Nabors L, LaRocca R, Villano J, Paleologos N, Kavan P, Pitz M, Thiessen B, Idbaih A, Frenel JS, Domont J, Grauer O, Hau P, Marosi C, Sroubek J, Hovey E, Sridhar PS, Cher L, Dunbar E, Coyle T, Raymond J, Barton K, Guarino M, Raval S, Stea B, Dietrich J, Hopkins K, Erridge S, Steinbach JP, Pineda LE, Balana QC, Sonia del BB, Wenczl M, Molnár K, Hideghéty K, Lossos A, Myra van L, Levy A, Harrup R, Patterson W, Lwin Z, Sathornsumetee S, Lee EJ, Ho JT, Emmons S, Duic JP, Shao S, Ashamalla H, Weaver M, Lutzky J, Avgeropoulos N, Hanna W, Nadipuram M, Cecchi G, O'Donnell R, Pannullo S, Carney J, Hamilton M, MacNeil M, Beaney R, Fabbro M, Schnell O, Fietkau R, Stockhammer G, Malinova B, Odrazka K, Sames M, Miguel Gil G, Razis E, Lavrenkov K, Castro G, Ramirez F, Baldotto C, Viola F, Malheiros S, Lickliter J, Gauden S, Dechaphunkul A, Thaipisuttikul I, Thotathil Z, Ma HI, Cheng WY, Chang CH, Salas F, Dietrich PY, Mamot C, Nayak L, Nag S. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 8.• Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma. JAMA Oncol. 2020. 10.1001/jamaoncol.2020.1024A recent phase III clinical using checkpoint inhibition that failed to show improvement over bevacizumab treatment. [DOI] [PMC free article] [PubMed]
- 9.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 13.Carrithers MD, Visintin I, Kang SJ, Janeway CA., Jr Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123:1092–1101. doi: 10.1093/brain/123.6.1092. [DOI] [PubMed] [Google Scholar]
- 14.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra11. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris AWJ, Sharp MMG, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, Weller RO, Carare RO. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016;131:725–736. doi: 10.1007/s00401-016-1555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer F, Schäfer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–614. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 18.Hatterer E, Davoust N, Didier-Bazes M, Vuaillat C, Malcus C, Belin MF, et al. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood. 2006;107:806–812. doi: 10.1182/blood-2005-01-0154. [DOI] [PubMed] [Google Scholar]
- 19.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JAR, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 20.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 21.Hawkes CA, Härtig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- 22.Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical Trials. Neurotherapeutics. 2017;14:307–320. doi: 10.1007/s13311-016-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, DeGroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 24.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Galea I, Bernardes-Silva M, Forse PA, Van Rooijen N, Liblau RS, Perry VH. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204:2023–2030. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok D, Okada H. T-Cell based therapies for overcoming neuroanatomical and immunosuppressive challenges within the glioma microenvironment. J Neurooncol. 2020;147(2):281–295. doi: 10.1007/s11060-020-03450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Benz C, Barnholtz-Sloan J, Barrett W, Ostrom Q, Wolinsky Y, Black KL, Bose B, Boulos PT, Boulos M, Brown J, Czerinski C, Eppley M, Iacocca M, Kempista T, Kitko T, Koyfman Y, Rabeno B, Rastogi P, Sugarman M, Swanson P, Yalamanchii K, Otey IP, Liu YS, Xiao Y, Auman JT, Chen PC, Hadjipanayis A, Lee E, Lee S, Park PJ, Seidman J, Yang L, Kucherlapati R, Kalkanis S, Mikkelsen T, Poisson LM, Raghunathan A, Scarpace L, Bernard B, Bressler R, Eakin A, Iype L, Kreisberg RB, Leinonen K, Reynolds S, Rovira H, Thorsson V, Shmulevich I, Annala MJ, Penny R, Paulauskis J, Curley E, Hatfield M, Mallery D, Morris S, Shelton T, Shelton C, Sherman M, Yena P, Cuppini L, DiMeco F, Eoli M, Finocchiaro G, Maderna E, Pollo B, Saini M, Balu S, Hoadley KA, Li L, Miller CR, Shi Y, Topal MD, Wu J, Dunn G, Giannini C, O'Neill BP, Aksoy BA, Antipin Y, Borsu L, Berman SH, Brennan CW, Cerami E, Chakravarty D, Ciriello G, Gao J, Gross B, Jacobsen A, Ladanyi M, Lash A, Liang Y, Reva B, Sander C, Schultz N, Shen R, Socci ND, Viale A, Ferguson ML, Chen QR, Demchok JA, Dillon LAL, Shaw KRM, Sheth M, Tarnuzzer R, Wang Z, Yang L, Davidsen T, Guyer MS, Ozenberger BA, Sofia HJ, Bergsten J, Eckman J, Harr J, Myers J, Smith C, Tucker K, Winemiller C, Zach LA, Ljubimova JY, Eley G, Ayala B, Jensen MA, Kahn A, Pihl TD, Pot DA, Wan Y, Eschbacher J, Foltz G, Hansen N, Hothi P, Lin B, Shah N, Yoon JG, Lau C, Berens M, Ardlie K, Beroukhim R, Carter SL, Cherniack AD, Noble M, Cho J, Cibulskis K, DiCara D, Frazer S, Gabriel SB, Gehlenborg N, Gentry J, Heiman D, Kim J, Jing R, Lander ES, Lawrence M, Lin P, Mallard W, Meyerson M, Onofrio RC, Saksena G, Schumacher S, Sougnez C, Stojanov P, Tabak B, Voet D, Zhang H, Zou L, Getz G, Dees NN, Ding L, Fulton LL, Fulton RS, Kanchi KL, Mardis ER, Wilson RK, Baylin SB, Andrews DW, Harshyne L, Cohen ML, Devine K, Sloan AE, VandenBerg SR, Berger MS, Prados M, Carlin D, Craft B, Ellrott K, Goldman M, Goldstein T, Grifford M, Haussler D, Ma S, Ng S, Salama SR, Sanborn JZ, Stuart J, Swatloski T, Waltman P, Zhu J, Foss R, Frentzen B, Friedman W, McTiernan R, Yachnis A, Hayes DN, Perou CM, Zheng S, Vegesna R, Mao Y, Akbani R, Aldape K, Bogler O, Fuller GN, Liu W, Liu Y, Lu Y, Mills G, Protopopov A, Ren X, Sun Y, Wu CJ, Yung WKA, Zhang W, Zhang J, Chen K, Weinstein JN, Chin L, Verhaak RGW, Noushmehr H, Weisenberger DJ, Bootwalla MS, Lai PH, Triche TJ, Jr, van den Berg DJ, Laird PW, Gutmann DH, Lehman NL, VanMeir EG, Brat D, Olson JJ, Mastrogianakis GM, Devi NS, Zhang Z, Bigner D, Lipp E, McLendon R. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadieux B, Ching TT, VandenBerg SR, Costello JF. Genome-wide hypomethylation in human glioblastomas associated with specific copy number alteration, methylenetetrahydrofolate reductase allele status, and increased proliferation. Cancer Res. 2006;66:8469–8476. doi: 10.1158/0008-5472.CAN-06-1547. [DOI] [PubMed] [Google Scholar]
- 30.Ma H, Zhao C, Zhao Z, Hu L, Ye F, Wang H, Fang Z, Wu Y, Chen X. Specific glioblastoma multiforme prognostic-subtype distinctions based on DNA methylation patterns. Cancer Gene Ther. 2019;27:1–13. doi: 10.1038/s41417-019-0142-6. [DOI] [PubMed] [Google Scholar]
- 31.Martinez R, Martin-Subero JI, Rohde V, Kirsch M, Alaminos M, Fernandez AF, et al. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics. 2009;4:255–264. doi: 10.4161/epi.9130. [DOI] [PubMed] [Google Scholar]
- 32.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suva ML, Regev A, Bernstein BE. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muscat AM, Wong NC, Drummond KJ, Algar EM, Khasraw M, Verhaak R, et al. The evolutionary pattern of mutations in glioblastoma reveals therapy-mediated selection. Oncotarget. 2018;9:7844–7858. doi: 10.18632/oncotarget.23541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Wong AJ. EGF receptor variant III as a target antigen for tumor immunotherapy. Expert Rev Vaccines. 2008;7(7):977–985. doi: 10.1586/14760584.7.7.977. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Yu J, Tu L, Huang N, Li H, Luo Y. Isocitrate dehydrogenase mutations in glioma: from basic discovery to therapeutics development. Front Oncol. 2019;9:506. doi: 10.3389/fonc.2019.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chheda ZS, Kohanbash G, Okada K, Jahan N, Sidney J, Pecoraro M, Yang X, Carrera DA, Downey KM, Shrivastav S, Liu S, Lin Y, Lagisetti C, Chuntova P, Watchmaker PB, Mueller S, Pollack IF, Rajalingam R, Carcaboso AM, Mann M, Sette A, Garcia KC, Hou Y, Okada H. Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med. 2018;215(1):141–157. doi: 10.1084/jem.20171046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez GY, Oberheim Bush NA, Phillips JJ, Bouffard JP, Moshel YA, Jaeckle K, Kleinschmidt-DeMasters BK, Rosenblum MK, Perry A, Solomon DA. Diffuse midline gliomas with subclonal H3F3A K27M mutation and mosaic H3.3 K27M mutant protein expression. Acta Neuropathol. 2017;134(6):961–963. doi: 10.1007/s00401-017-1780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016;26(5):569–580. doi: 10.1111/bpa.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015;17(Suppl 7):vii9–vii14. doi: 10.1093/neuonc/nov151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, II, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 42.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 43.Iglesia MD, Parker JS, Hoadley KA, Serody JS, Perou CM, Vincent BG. Genomic analysis of immune cell infiltrates across 11 tumor types. J Natl Cancer Inst. 2016;108(11). 10.1093/jnci/djw144. [DOI] [PMC free article] [PubMed]
- 44.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohanbash G, Okada H. Myeloid-derived suppressor cells (MDSCs) in gliomas and glioma-development. Immunol Investig. 2012;41(6–7):658–679. doi: 10.3109/08820139.2012.689591. [DOI] [PubMed] [Google Scholar]
- 47.Umansky V, Blattner C, Gebhardt C, Utikal J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines (Basel). 2016;4(4). 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed]
- 48.Anthony C, Mladkova-Suchy N, Adamson DC. The evolving role of antiangiogenic therapies in glioblastoma multiforme: current clinical significance and future potential. Expert Opin Investig Drugs. 2019;28(9):787–797. doi: 10.1080/13543784.2019.1650019. [DOI] [PubMed] [Google Scholar]
- 49.Han J, Alvarez-Breckenridge CA, Wang QE, Yu J. TGF-β signaling and its targeting for glioma treatment. Am J Cancer Res. 2015;5(3):945–955. [PMC free article] [PubMed] [Google Scholar]
- 50.Chang N, Ahn SH, Kong DS, Lee HW, Nam DH. The role of STAT3 in glioblastoma progression through dual influences on tumor cells and the immune microenvironment. Mol Cell Endocrinol. 2017;451:53–65. doi: 10.1016/j.mce.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Furtek SL, Backos DS, Matheson CJ, Reigan P. Strategies and approaches of targeting STAT3 for cancer treatment. ACS Chem Biol. 2016;11(2):308–318. doi: 10.1021/acschembio.5b00945. [DOI] [PubMed] [Google Scholar]
- 52.Yan J, Kong LY, Hu J, Gabrusiewicz K, Dibra D, Xia X, et al. FGL2 as a multimodality regulator of tumor-mediated immune suppression and therapeutic target in gliomas. J Natl Cancer Inst. 2015;107(8). 10.1093/jnci/djv137. [DOI] [PMC free article] [PubMed]
- 53.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, NABTT CNS Consortium Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–ee42. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desjardins A, Herndon JE, McSherry F, Ravelo A, Lipp ES, Healy P, et al. Single-institution retrospective review of patients with recurrent glioblastoma treated with bevacizumab in clinical practice. Health Sci Rep. 2019;2(4):e114. doi: 10.1002/hsr2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller S, Taitt JM, Villanueva-Meyer JE, Bonner ER, Nejo T, Lulla RR et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J Clin Invest. 2020;In press. [DOI] [PMC free article] [PubMed]
- 57.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E, Shukla SA, Hu Z, Li L, le PM, Allesøe RL, Richman AR, Kowalczyk MS, Abdelrahman S, Geduldig JE, Charbonneau S, Pelton K, Iorgulescu JB, Elagina L, Zhang W, Olive O, McCluskey C, Olsen LR, Stevens J, Lane WJ, Salazar AM, Daley H, Wen PY, Chiocca EA, Harden M, Lennon NJ, Gabriel S, Getz G, Lander ES, Regev A, Ritz J, Neuberg D, Rodig SJ, Ligon KL, Suvà ML, Wucherpfennig KW, Hacohen N, Fritsch EF, Livak KJ, Ott PA, Wu CJ, Reardon DA. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Shen F, Yao Y, Wang LL, Zhu Y, Hu J. Adoptive cell therapy: a novel and potential immunotherapy for glioblastoma. Front Oncol. 2020;10:59. doi: 10.3389/fonc.2020.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 61.O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goff SL, Morgan RA, Yang JC, Sherry RM, Robbins PF, Restifo NP, Feldman SA, Lu YC, Lu L, Zheng Z, Xi L, Epstein M, McIntyre LS, Malekzadeh P, Raffeld M, Fine HA, Rosenberg SA. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J Immunother. 2019;42(4):126–135. doi: 10.1097/CJI.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thaci B, Brown CE, Binello E, Werbaneth K, Sampath P, Sengupta S. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro-Oncology. 2014;16(10):1304–1312. doi: 10.1093/neuonc/nou045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, Zhai Y, Bading JR, Ressler JA, Portnow J, D'Apuzzo M, Forman SJ, Jensen MC. Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D’Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reardon DA, Desjardins A, Vredenburgh JJ, O'Rourke DM, Tran DD, Fink KL, Nabors LB, Li G, Bota DA, Lukas RV, Ashby LS, Duic JP, Mrugala MM, Cruickshank S, Vitale L, He Y, Green JA, Yellin MJ, Turner CD, Keler T, Davis TA, Sampson JH, ReACT trial investigators Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (ReACT): results of a double-blind randomized phase II trial. Clin Cancer Res. 2020;26(7):1586–1594. doi: 10.1158/1078-0432.CCR-18-1140. [DOI] [PubMed] [Google Scholar]
- 67.Migliorini D, Dutoit V, Allard M, Grandjean Hallez N, Marinari E, Widmer V, Philippin G, Corlazzoli F, Gustave R, Kreutzfeldt M, Blazek N, Wasem J, Hottinger A, Koka A, Momjian S, Lobrinus A, Merkler D, Vargas MI, Walker PR, Patrikidou A, Dietrich PY. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro-Oncology. 2019;21(7):923–933. doi: 10.1093/neuonc/noz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen PY, Reardon DA, Armstrong TS, Phuphanich S, Aiken RD, Landolfi JC, Curry WT, Zhu JJ, Glantz M, Peereboom DM, Markert JM, LaRocca R, O'Rourke DM, Fink K, Kim L, Gruber M, Lesser GJ, Pan E, Kesari S, Muzikansky A, Pinilla C, Santos RG, Yu JS. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;25(19):5799–5807. doi: 10.1158/1078-0432.CCR-19-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wick W, van den Bent MJ. First results on the DCVax phase III trial: raising more questions than providing answers. Neuro-Oncology. 2018;20(10):1283–1284. doi: 10.1093/neuonc/noy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, Heth JA, Salacz M, Taylor S, D’Andre SD, Iwamoto FM, Dropcho EJ, Moshel YA, Walter KA, Pillainayagam CP, Aiken R, Chaudhary R, Goldlust SA, Bota DA, Duic P, Grewal J, Elinzano H, Toms SA, Lillehei KO, Mikkelsen T, Walbert T, Abram SR, Brenner AJ, Brem S, Ewend MG, Khagi S, Portnow J, Kim LJ, Loudon WG, Thompson RC, Avigan DE, Fink KL, Geoffroy FJ, Lindhorst S, Lutzky J, Sloan AE, Schackert G, Krex D, Meisel HJ, Wu J, Davis RP, Duma C, Etame AB, Mathieu D, Kesari S, Piccioni D, Westphal M, Baskin DS, New PZ, Lacroix M, May SA, Pluard TJ, Tse V, Green RM, Villano JL, Pearlman M, Petrecca K, Schulder M, Taylor LP, Maida AE, Prins RM, Cloughesy TF, Mulholland P, Bosch ML. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):142. doi: 10.1186/s12967-018-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peereboom DM, Nabors LB, Kumthekar P, Badruddoja MA, Fink KL, Lieberman FS, et al. Phase 2 trial of SL-701 in relapsed/refractory (r/r) glioblastoma (GBM): correlation of immune response with longer-term survival. J Clin Oncol. 2018;36(15_suppl):2058. doi: 10.1200/JCO.2018.36.15_suppl.2058. [DOI] [Google Scholar]
- 72.Reardon DA, Nayak L, Peters KB, Clarke JL, Jordan JT, Groot JFD, et al. Phase II study of pembrolizumab or pembrolizumab plus bevacizumab for recurrent glioblastoma (rGBM) patients. J Clin Oncol. 2018;36(15_suppl):2006. doi: 10.1200/JCO.2018.36.15_suppl.2006. [DOI] [Google Scholar]
- 73.Sahebjam S, Forsyth P, Arrington J, Jaglal M, Tran ND, Etame AB, et al. ATIM-18. A phase I trial of hypofractionated stereotactic irradiation (HFSRT) with pembrolizumab and bevacizumab in patients with recurrent high grade glioma ( NCT02313272) Neuro-Oncol. 2017;19(suppl_6):vi30-vi. doi: 10.1093/neuonc/nox168.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmed NM, Brawley VS, Diouf O, Ghazi A, Yi J, Liu H, et al. Autologous HER2 CMV bispecific CAR T cells for progressive glioblastoma: results from a phase I clinical trial. J Clin Oncol. 2015;33(15_suppl):3008. doi: 10.1200/jco.2015.33.15_suppl.3008. [DOI] [Google Scholar]
- 75.Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD, Gumin J, Vence LM, Wistuba I, Rodriguez-Canales J, Villalobos PA, Dirven CMF, Tejada S, Valle RD, Alonso MM, Ewald B, Peterkin JJ, Tufaro F, Fueyo J. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alonso MM, García-Moure M, Gonzalez-Huarriz M, Marigil M, Hernandez-Alcoceba R, Buñales M, et al. Abstract CT027: oncolytic virus DNX-2401 with a short course of temozolomide for glioblastoma at first recurrence: clinical data and prognostic biomarkers. Cancer Res. 2017;77(13 Supplement):CT027-CT. doi: 10.1158/1538-7445.am2017-ct027. [DOI] [Google Scholar]
- 77.Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalkanis SN, Aghi MK, Cloughsy TF, Kaptain G, Portnow J, Vogelbaum MA, et al. DDEL-06: Preliminary safety of Toca 511, a retroviral replicating vector, in patients with recurrent high grade glioma across three separate phase 1 studies. Neuro-Oncology. 2015;17(suppl_5):v74-v. doi: 10.1093/neuonc/nov212.06. [DOI] [Google Scholar]
- 79.Cloughesy TF, Landolfi J, Hogan DJ, Bloomfield S, Carter B, Chen CC, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra75. doi: 10.1126/scitranslmed.aad9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cloughesy TF, Landolfi J, Vogelbaum MA, Ostertag D, Elder JB, Bloomfield S, Carter B, Chen CC, Kalkanis SN, Kesari S, Lai A, Lee IY, Liau LM, Mikkelsen T, Nghiemphu P, Piccioni D, Accomando W, Diago OR, Hogan DJ, Gammon D, Kasahara N, Kheoh T, Jolly DJ, Gruber HE, Das A, Walbert T. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology. 2018;20(10):1383–1392. doi: 10.1093/neuonc/noy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Todo T. ATIM-14. Results of phase II clinical trial of oncolytic herpes virus G47delta in patients with glioblastoma. Neuro-Oncology. 2019;21(Supplement_6):vi4-vi. doi: 10.1093/neuonc/noz175.014. [DOI] [Google Scholar]
- 82.Clinical Trials. Viral therapy in treating patients with recurrent glioblastoma multiforme. 2020, January 2. https://clinicaltrials.gov/ct2/show/results/NCT00390299.
- 83.Geletneky K, Hajda J, Angelova AL, Leuchs B, Capper D, Bartsch AJ, Neumann JO, Schöning T, Hüsing J, Beelte B, Kiprianova I, Roscher M, Bhat R, von Deimling A, Brück W, Just A, Frehtman V, Löbhard S, Terletskaia-Ladwig E, Fry J, Jochims K, Daniel V, Krebs O, Dahm M, Huber B, Unterberg A, Rommelaere J. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol Ther. 2017;25(12):2620–2634. doi: 10.1016/j.ymthe.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zadeh G, Lang F, Daras M, Cloughesy T, Colman H, Ong S, et al. ATIM-24. Interim results of a phase II multicenter study of the conditionally replicative oncolytic adenovirus DNX-2401 with pembrolizumab (Keytruda) for recurrent glioblastoma; CAPTIVE study (KEYNOTE-192) Neuro-Oncology. 2018;20(suppl_6):vi6-vi. doi: 10.1093/neuonc/noy148.019. [DOI] [Google Scholar]
- 85.Cloughesy TF, Brenner A, de Groot JF, Butowski NA, Zach L, Campian JL, Ellingson BM, Freedman LS, Cohen YC, Lowenton-Spier N, Rachmilewitz Minei T, Fain Shmueli S, GLOBE Study Investigators. Avgeropoulos N, Beck J, Benkers T, Bokstein F, Brenner A, Burton E, Butowski N, Campian J, Carrillo J, Cloughesy T, de Groot J, de Robles P, Drappatz J, Dunbar I, Fink K, Groves M, Han X, Adila H, Jensen R, Kowalska A, Kumthekar P, Lee M, Lesser G, Lossos A, Lukas R, Macdonald D, Mammoser A, Mechtler L, Mohile N, Nagpal S, Nicholas G, Kreisl T, Pan E, Peak S, Pearlman M, Perry J, Peterson R, Piccioni D, Robins H, Ronan L, Salacz M, Schiff D, Tran D, Zach L, Tzuk-Shina T, Walbert T, Wen P, Youst S, Wen PY. A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE) Neuro-Oncology. 2020;22(5):705–717. doi: 10.1093/neuonc/noz232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Platten M, Schilling D, Bunse L, Wick A, Bunse T, Riehl D, et al. ATIM-33. NOA-16: A first-in-man multicenter phase I clinical trial of the German Neurooncology Working Group evaluating a mutation-specific peptide vaccine targeting IDH1R132H in patients with newly diagnosed malignant astrocytomas. Neuro-Oncol. 2018;20(suppl_6):vi8–vi9. doi: 10.1093/neuonc/noy148.028. [DOI] [Google Scholar]
- 87.Kieran MW, Goumnerova L, Manley P, Chi SN, Marcus KJ, Manzanera AG, Polanco MLS, Guzik BW, Aguilar-Cordova E, Diaz-Montero CM, DiPatri AJ, Tomita T, Lulla R, Greenspan L, Aguilar LK, Goldman S. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro-Oncology. 2019;21(4):537–546. doi: 10.1093/neuonc/noy202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H, Ye ZL, Yuan ZG, Luo ZQ, Jin HJ, Qian QJ. New strategies for the treatment of solid tumors with CAR-T cells. Int J Biol Sci. 2016;12(6):718–729. doi: 10.7150/ijbs.14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, Joseph SK, Hegde M, Ahmed N. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro-Oncology. 2017;20:506–518. doi: 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, Heslop HE, Gottschalk S, Wels WS, Baker ML, Ahmed N. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KKH, Brawley VS, Byrd TT, Krebs S, Gottschalk S, Wels WS, Baker ML, Dotti G, Mamonkin M, Brenner MK, Orange JS, Ahmed N. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Investig. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, Lim WA. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164:780–791. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mulazzani M, Fräßle SP, von Mücke-Heim I, Langer S, Zhou X, Ishikawa-Ankerhold H, Leube J, Zhang W, Dötsch S, Svec M, Rudelius M, Dreyling M, von Bergwelt-Baildon M, Straube A, Buchholz VR, Busch DH, von Baumgarten L. Long-term in vivo microscopy of CAR T cell dynamics during eradication of CNS lymphoma in mice. Proc Natl Acad Sci U S A. 2019;116:24275–24284. doi: 10.1073/pnas.1903854116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nellan A, Rota C, Majzner R, Lester-McCully CM, Griesinger AM, Mulcahy Levy JM, et al. Durable regression of medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J ImmunoTher Cancer. 2018;6:30. doi: 10.1186/s40425-018-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, Yu J, Hegde U, Speaker S, Madura M, Ralabate A, Rivera A, Rowen E, Gerrish H, Yao X, Chiang V, Kluger HM. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA, Menzies AM, McArthur GA. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 98.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, Atkins MB, Ernstoff MS, Reardon DA, Puzanov I, Kudchadkar RR, Thomas RP, Tarhini A, Pavlick AC, Jiang J, Avila A, Demelo S, Margolin K. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, Brouland JP, Iacobuzio-Donahue CA, Brennan C, Tabar V, Gutin PH, Daniel RT, Hegi ME, Joyce JA. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181(7):1643–1660. doi: 10.1016/j.cell.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friebel E, Kapolou K, Unger S, Nunez NG, Utz S, Rushing EJ, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020;181(7):1626–42 e20. doi: 10.1016/j.cell.2020.04.055. [DOI] [PubMed] [Google Scholar]
- 101.Lyle LT, Lockman PR, Adkins CE, Mohammad AS, Sechrest E, Hua E, Palmieri D, Liewehr DJ, Steinberg SM, Kloc W, Izycka-Swieszewska E, Duchnowska R, Nayyar N, Brastianos PK, Steeg PS, Gril B. Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin Cancer Res. 2016;22(21):5287–5299. doi: 10.1158/1078-0432.CCR-15-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barajas RF, Jr, Cha S. Metastasis in adult brain tumors. Neuroimaging Clin N Am. 2016;26(4):601–620. doi: 10.1016/j.nic.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brahm CG, van Linde ME, Enting RH, Schuur M, Otten RHJ, Heymans MW, et al. The current status of immune checkpoint inhibitors in neuro-oncology: a systematic review. Cancers (Basel). 2020;12(3). 10.3390/cancers12030586. [DOI] [PMC free article] [PubMed]
- 105.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. 2013;14:1014–22. 10.1038/ni.2703. [DOI] [PMC free article] [PubMed]
- 106.Taggart D, Andreou T, Scott KJ, Williams J, Rippaus N, Brownlie RJ, et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8(+) T cell trafficking. Proc Natl Acad Sci U S A. 2018;115(7):E1540–E15E9. doi: 10.1073/pnas.1714089115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nduom EK, Wei J, Yaghi NK, Huang N, Kong L-Y, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, Burks JK, Fuller GN, Calin GA, Conrad CA, Creasy C, Ritthipichai K, Radvanyi L, Heimberger AB. PD-L1 expression and prognostic impact in glioblastoma. Neuro-Oncol. 2015;18:195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551:517–520. doi: 10.1038/nature24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TBK, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25:477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Swartz AM, Batich KA, Fecci PE, Sampson JH. Peptide vaccines for the treatment of glioblastoma. J Neuro-Oncol. 2015;123(3):433–440. doi: 10.1007/s11060-014-1676-y. [DOI] [PubMed] [Google Scholar]
- 113.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, Platten M, Tabatabai G, Dutoit V, van der Burg SH, thor Straten P, Martínez-Ricarte F, Ponsati B, Okada H, Lassen U, Admon A, Ottensmeier CH, Ulges A, Kreiter S, von Deimling A, Skardelly M, Migliorini D, Kroep JR, Idorn M, Rodon J, Piró J, Poulsen HS, Shraibman B, McCann K, Mendrzyk R, Löwer M, Stieglbauer M, Britten CM, Capper D, Welters MJP, Sahuquillo J, Kiesel K, Derhovanessian E, Rusch E, Bunse L, Song C, Heesch S, Wagner C, Kemmer-Brück A, Ludwig J, Castle JC, Schoor O, Tadmor AD, Green E, Fritsche J, Meyer M, Pawlowski N, Dorner S, Hoffgaard F, Rössler B, Maurer D, Weinschenk T, Reinhardt C, Huber C, Rammensee HG, Singh-Jasuja H, Sahin U, Dietrich PY, Wick W. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 114.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 115.Martikainen M, Essand M. Virus-based immunotherapy of glioblastoma. Cancers (Basel). 2019;11(2). 10.3390/cancers11020186. [DOI] [PMC free article] [PubMed]
- 116.Brown MC, Dobrikova EY, Dobrikov MI, Walton RW, Gemberling SL, Nair SK, Desjardins A, Sampson JH, Friedman HS, Friedman AH, Tyler DS, Bigner DD, Gromeier M. Oncolytic polio virotherapy of cancer. Cancer. 2014;120(21):3277–3286. doi: 10.1002/cncr.28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chiocca EA, Nassiri F, Wang J, Peruzzi P, Zadeh G. Viral and other therapies for recurrent glioblastoma: is a 24-month durable response unusual? Neuro Oncol. 2019;21(1):14–25. doi: 10.1093/neuonc/noy170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baker AT, Aguirre-Hernández C, Halldén G, Parker AL. Designer oncolytic adenovirus: coming of age. Cancers (Basel). 2018;10(6). 10.3390/cancers10060201. [DOI] [PMC free article] [PubMed]
- 119.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 120.Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, Xu J, Kondo Y, Bekele BN, Colman H, Lang FF, Fueyo J. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99(18):1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 121.Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 2007;67(24):11499–11504. doi: 10.1158/0008-5472.CAN-07-5312. [DOI] [PubMed] [Google Scholar]
- 122.Kleijn A, van den Bossche W, Haefner ES, Belcaid Z, Burghoorn-Maas C, Kloezeman JJ, Pas SD, Leenstra S, Debets R, de Vrij J, Dirven CMF, Lamfers MLM. The sequence of Delta24-RGD and TMZ administration in malignant glioma affects the role of CD8. Mol Ther Oncolyt. 2017;5:11–19. doi: 10.1016/j.omto.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Todo T. ATIM-14. Results of phase II clinical trial of oncolytic herpes virus g47δ in patients with glioblastoma. Neuro-Oncol. 2019;21(Supplement_6):vi4-vi. doi: 10.1093/neuonc/noz175.014. [DOI] [Google Scholar]
- 124.Perez OD, Logg CR, Hiraoka K, Diago O, Burnett R, Inagaki A, Jolson D, Amundson K, Buckley T, Lohse D, Lin A, Burrascano C, Ibanez C, Kasahara N, Gruber HE, Jolly DJ. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20(9):1689–1698. doi: 10.1038/mt.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghosh D, Nandi S, Bhattacharjee S. Combination therapy to checkmate glioblastoma: clinical challenges and advances. Clin Transl Med. 2018;7(1):33. doi: 10.1186/s40169-018-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sengupta S, Katz SC, Sengupta S, Sampath P. Glycogen synthase kinase 3 inhibition lowers PD-1 expression, promotes long-term survival and memory generation in antigen-specific CAR-T cells. Cancer Lett. 2018;433:131–139. doi: 10.1016/j.canlet.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 127.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Investig. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, Yuan Y, Lang FF, Toniatti C, Hossain MB, Fueyo J. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 2017;77(14):3894–3907. doi: 10.1158/0008-5472.CAN-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kamran N, Kadiyala P, Saxena M, Candolfi M, Li Y, Moreno-Ayala MA, et al. Immunosuppressive myeloid cells’ blockade in the glioma microenvironment enhances the efficacy of immune-stimulatory gene therapy. Mol Ther. 2017;25(1):232–248. doi: 10.1016/j.ymthe.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aquino D, Gioppo A, Finocchiaro G, Bruzzone MG, Cuccarini V. MRI in glioma immunotherapy: evidence, pitfalls, and perspectives. J Immunol Res. 2017;2017:5813951–5813916. doi: 10.1155/2017/5813951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, Gambhir SS. Noninvasive detection of therapeutic cytolytic T cells with 18 F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. GLIA. 2013;61:10–23. doi: 10.1002/glia.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tourdias T, Roggerone S, Filippi M, Kanagaki M, Rovaris M, Miller DH, Petry KG, Brochet B, Pruvo JP, Radüe EW, Dousset V. Assessment of disease activity in multiple sclerosis phenotypes with combined gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology. 2012;264:225–233. doi: 10.1148/radiol.12111416. [DOI] [PubMed] [Google Scholar]