Abstract
The objectives of the study were to investigate the functional role and potential mechanism of wild-type p53-induced phosphatase (Wip1) in cervical cancer cell line HeLa cells, along with the effect of knockdown of Wip1 in combination with γ-irradiation on the HeLa cells. Expression of Wip1 was silenced or overexpressed. After transfection, cell viability was determined. Moreover, γ-irradiation and SB203580 were performed to explore the effect of colony formation and cell apoptosis. Likewise, protein expression levels of p38, p-p38, p53, and p-p53 were assessed in the presence or not of SB203580 and overexpression of Wip1. Both the mRNA and protein levels of Wip1 were significantly decreased by transfection with Wip1-specific small interfering RNA (siRNA) but were significantly increased by transfection with pcDNA3.1-Wip1. Knockdown of Wip1 significantly decreased cell growth and colony formation ability and increased apoptotic rate. Additionally, better results were obtained by knockdown of Wip1 in combination with γ-irradiation. The protein expression levels of p-p38 (p < 0.05), p53 (p < 0.01), and p-p53 (p < 0.05) were all significantly increased by knockdown of Wip1. However, application of SB203580 reversed the effects. Our study confirms the important roles of Wip1 in cervical cancer. Knockdown of Wip1 enhances sensitivity to radiation in HeLa cells by inhibiting cell proliferation and inducing apoptosis through activation of p38 MAPK.
Key words: Cervical cancer, Wild-type p53-induced phosphatase (Wip1), p38 MAPK, γ-Irradiation, SB203580
INTRODUCTION
Cervical cancer is ranked as the second most frequent cancer worldwide, and it is the most common malignancy among women in many low-income countries (1). It has been estimated that approximately 200,000 (139,000–276,000) women with cervical cancer were killed in 2010, of whom 46,000 (33,000–64,000) aged 15–49 years were observed in developing countries (2). In our country, about 140,000 new cases are diagnosed each year (3). The persistent human papillomavirus (HPV) infection is mainly responsible for cervical cancer (4). The implementation of Pap smear screens and DNA testing contributes to the decline in morbidity and mortality. Although these are effective screening methods, it is still a major public health problem (5). Either as a preoperative adjuvant therapy or as a primary treatment, radiotherapy has remained the most excellent modality intervention for cervical cancer until now (6). However, about 30–50% of patients with cervical cancer in stages IIB–IV fail to obtain the expected results because of resistance to radiotherapy (7). Therefore, identification of a molecular mechanism involved in the pathogenesis of cervical cancer and enhancement of the radiation effect is important.
A growing body of evidence suggests that wild-type p53-induced phosphatase (Wip1), a newly discovered proto-oncogene (8), is involved in many human tumors, such as primary breast cancer (9), neuroblastoma (10), papillary thyroid cancer (11), ovarian clear cell adenocarcinomas (12), colorectal cancer (13), and medulloblastomas (14). In addition, it has been considered to be a novel prognostic marker for patient survival (15). Wip1 was originally described as a p53-regulated gene (16) and was later identified as a negative regulator of p53 because of its ability to attenuate the p38 mitogen-activated protein kinase (MAPK) activity (17). Wip1 is one of the members of the Ser/Thr PP2C family, which is encoded by the protein phosphatase magnesium-dependent 1 Δ (PPM1D) gene on chromosome 17q23–24. In spite of the functional role of Wip1 being investigated in many diseases, little information is available in respect to the association between Wip1 and cervical cancer.
Therefore, in our study, the functional role of Wip1 in cervical cancer cell line HeLa cells was explored. The expression of Wip1 was silenced or overexpressed by using transfection technology. Also, we observed the effect of silencing of Wip1 in combination with γ-irradiation on the HeLa cell viability, colony viability, and apoptotic rate, as well as the potential associated signaling pathway.
MATERIALS AND METHODS
Cell Lines
In the study, cervical cancer cell line HeLa cells were used, purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained in Dulbecco’s modified Eagle medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; BD Biosciences, San Diego, CA, USA), 100 U/ml penicillin, and 100 µg/ml streptomycin (Life Technologies) in a humidified 5% CO2 atmosphere at 37°C. The cells in the logarithmic phase of growth were used in the experiment.
Plasmids and siRNA Transfection
One day before transfection, the cells were trypsinized with 0.25% trypsin (Life Technologies), maintained in 2 ml DMEM containing 10% FBS at a density of 3 × 105/ml, and incubated in 5% CO2 incubator at 37°C. Transfection or other treatments could be performed when the cells reached 70% confluence. Wip1 was knocked down using the small interfering RNA (siRNA) approach with the target sequence for siWip1 that was designed and constructed by GenePharma Corporation (Shanghai, China). In addition, a Wip1 expression vector (pcDNA3.1-Wip1), namely overexpression Wip1, was constructed by inserting the full-length wild-type Wip1 DNA into pcDNA3.1(+) and confirmed by sequencing. The empty construct pcDNA3.1 was used as a control. HeLa cells were transfected using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instruction, and the interference efficiency was tested by real-time polymerase chain reaction (RT-PCR).
Experimental Design
The cells were randomly divided into eight groups with different treatments: control group; siWip1 group; siWip1 + overexpression Wip1 group; γ-irradiation group; siWip1 + γ-irradiation group; siWip1 + overexpression Wip1 + γ-irradiation group; siWip1 + SB203580 group; and siWip1 + SB203580 + γ-irradiation group. Each group consisted of three wells of cells under the same treatment. For irradiation, the cells were adjusted at a concentration of 5 × 105 cells/100-mm dish. The cells received 2–3 Gy of γ-irradiation in a GC 3000 Elan irradiator (MDS Nordion, Ottawa, ON, Canada). For inhibition of p38, SB203580 (10 µM; Calbiochem, San Diego, CA, USA) was pretreated 2 h in the cells that were intervened with Wip1-specific siRNA.
Cell Proliferation Assay
Cell viability was measured using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) colorimetric assay. Briefly, HeLa cells were harvested and washed with phosphate-buffered saline (PBS) and then were seeded in a 96-well plate at a final concentration of 5 × 104/ml. After incubation for 24 h, 48 h, 72 h, or 96 h, 10 µl MTT was added to each well and incubated at 37°C for another 4 h. The absorbance at 492 nm optical density (OD) was measured with a synergy 2 multimode microplate reader (BioTek Instruments, Winooski, VT, USA). Experiments were repeated three times.
Methylcellulose Colony Formation Assays
The cells were harvested and washed three times with Iscove’s modified Dulbecco’s medium (IMDM) (Sigma-Aldrich). The cell suspension (2 × 103 cells/ml) was maintained in semisolid methylcellulose medium (MethoCult H4100; StemCell Technologies, Vancouver, BC, Canada) supplemented with 10% FBS. Cells were then incubated in a humidified 5% CO2 atmosphere at 37°C. The total number of colony and colony radius (µm) was assessed after culture for 10 days.
Flow Cytometry (FCM) Detection
Cell apoptosis was determined by Annexin-V-fluorescein-5-isothiocyanate (Annexin-V-FITC) apoptosis detection kit (BD PharMingen, San Diego, CA, USA) according to the manufacturer’s instructions. The cells were harvested, washed with cold PBS, and incubated with 5 µl annexin and 5 µl propidium iodide (PI, 10 mg/L) in the dark for 15 min. Then the cells were read by a FCM (Becton Dickinson, San Jose, CA, USA), and the results were analyzed using CELLQuest 3.0 software (BD Biosciences, San Jose, CA, USA).
Quantitative RT-PCR (qRT-PCR)
Total mRNA was extracted with the TRIzol reagent (Invitrogen Corp., Carlsbad, CA, USA). First-strand complementary DNA (cDNA) was synthesized using the Reverse Transcription System (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s recommendation. The mRNA expression levels were determined using SYBR green-based qRT-PCR (SYBR Green PCR Master Mix; Applied Biosystems). Target gene expression was normalized against GAPDH expression.
Western Blotting Analysis
The cells in each group were harvested for protein extraction. Protein samples (20 µl) were resolved on a 10–12% sodium dodecyl sulfate (SDS)-polyacrylamide gel. Then the protein was transferred onto polyvinylidene difluoride (PVDF) or nitrocellulose membranes, blocked in 5% fresh nonfat dry milk for 2 h, and probed with the following primary antibodies overnight at 4°C: anti-Wip1 antibody(2380-MC-100; R&D Systems), anti-p38 antibody (Cell Signaling Technology), anti-p-p38 antibody (Cell Signaling Technology), anti-p53 antibody (Cell Signaling Technology), or anti-p-p53 antibody (Cell Signaling Technology). The membranes were then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature. Enhanced chemiluminescence and densitometric analysis were finally analyzed.
Statistical Analysis
The data of multiple experiments were expressed as the mean ± standard deviation (SD). Statistical analyses were performed using statistical package for the social sciences (SPSS, version 19.0; SPSS Inc., Chicago, IL, USA) statistical software. The p values were calculated using Student’s t-test (for two groups) or a one-way analysis of variance (ANOVA, for ≥three groups). A statistical significance was defined when p < 0.05.
RESULTS
The Effect of Transfection on the Expression of Wip1
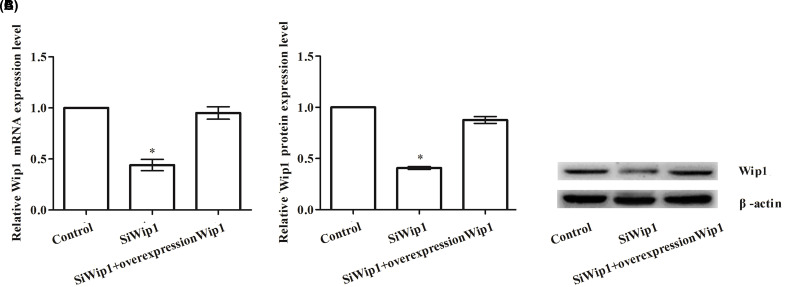
To explore the effect of transfection on the expression of Wip1 by transfection with pcDNA3.1-Wip1 and/or target sequence for Wip1-specific siRNA into HeLa cells, qRT-PCR and Western blotting were performed to confirm the mRNA and protein levels of Wip1, respectively. As shown in Figure 1A–C, both the mRNA and protein expression levels of Wip1 in the siWip1 group were significantly decreased by transfection with Wip1-specific siRNA but were significantly increased by transfection with pcDNA3.1-Wip1 compared to those in the control group (both values of p < 0.05) and siWip1 + overexpression Wip1 group (both p < 0.05). However, there were no significant differences between the control group and the siWip1 + overexpression Wip1 group.
Figure 1.
The influence on expression of Wip1 (wild-type p53-induced phosphatase) after transfection in HeLa cells. (A) Relative Wip1 mRNA level; (B) relative Wip1 protein level; (C) Western blotting pictures of Wip1. *p < 0.05.
Knockdown of Wip1 to HeLa Cells Decreased Cell Viability
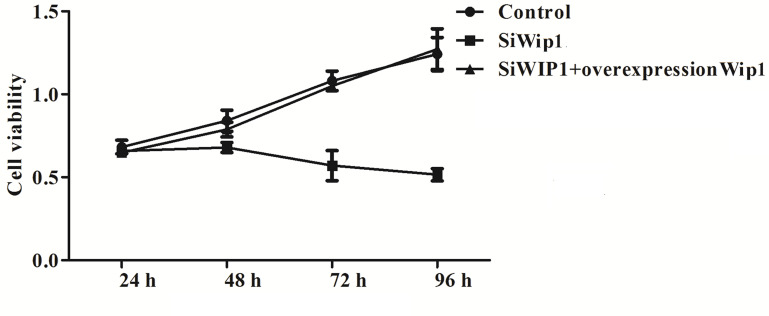
To investigate the effect of Wip1 on HeLa cells, cell viability was quantified by MTT assay after 24 h, 48 h, 72 h, and 96 h transfection with Wip1-specific siRNA or pcDNA3.1-Wip1. We found that knockdown of Wip1 could significantly reduce the cell growth (Fig. 2) compared with the control group (p < 0.05). However, no significant differences were found in cell growth between the control group and the siWip1 + overexpression Wip1 group.
Figure 2.
Silencing of Wip1 to HeLa cells decreases cell growth.
Knockdown of Wip1 Enhanced Sensitivity to Radiation in HeLa Cells by Decreasing Colony Formation Ability and Increasing Apoptotic Rate
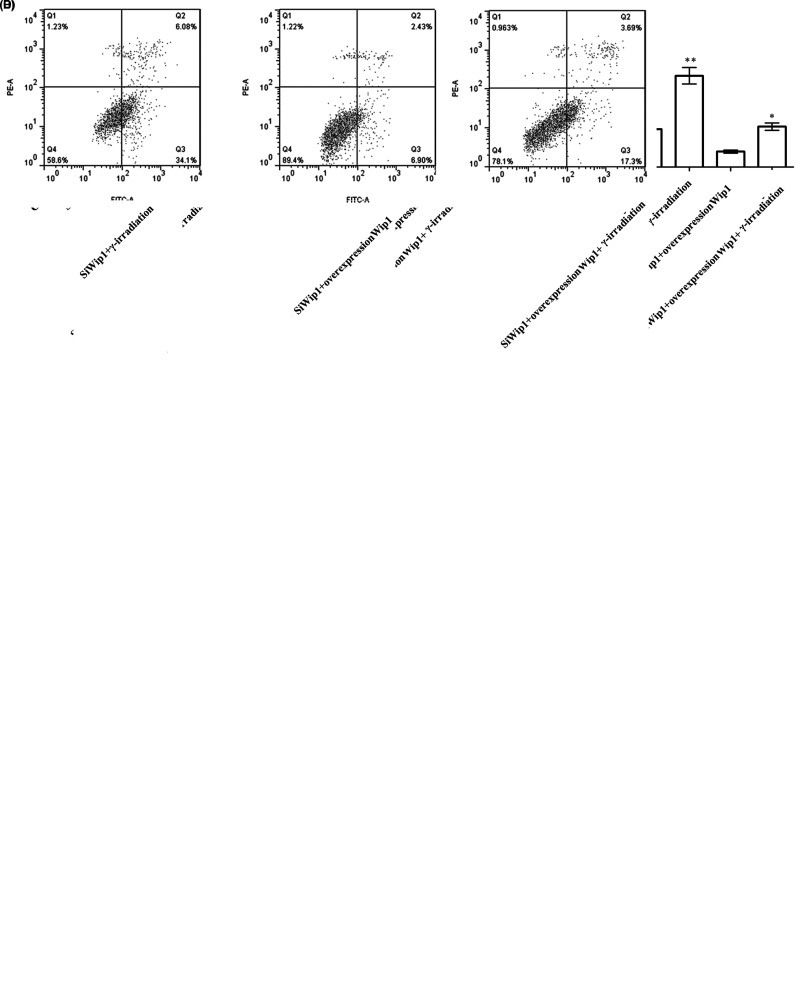
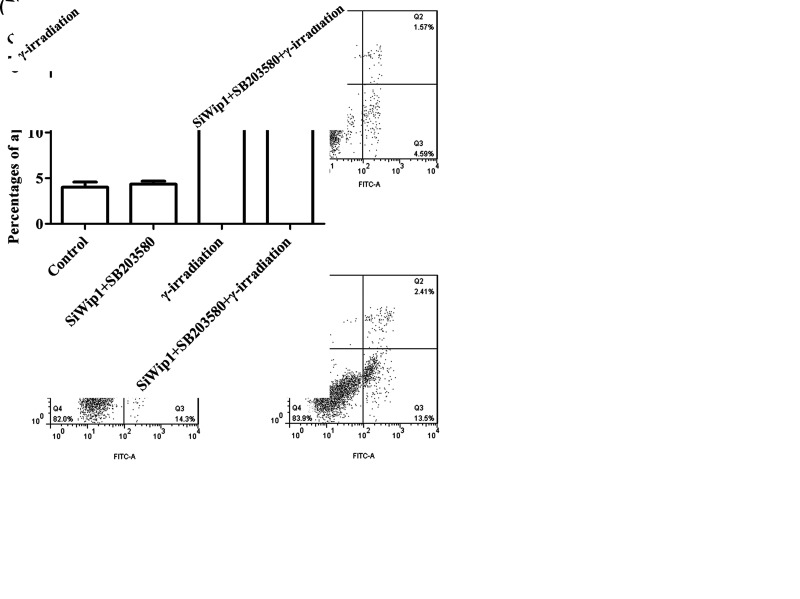
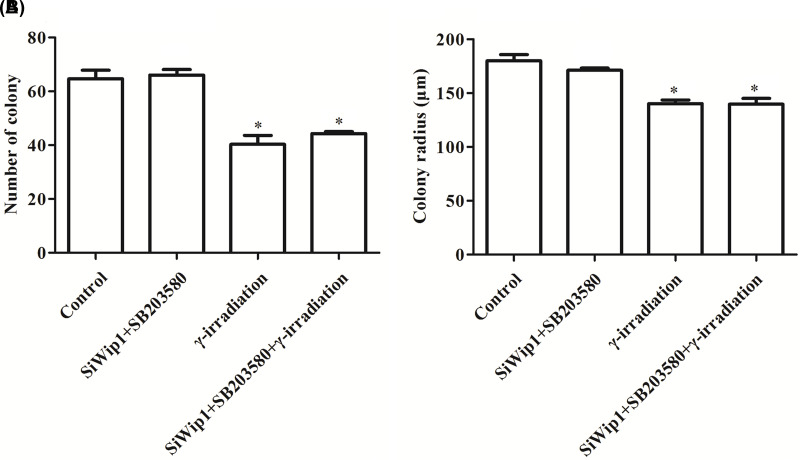
To investigate the effect of Wip1 in combination with γ-irradiation on HeLa cells, colony formation ability and apoptotic rate were examined after transfection with target sequence for Wip1-specific siRNA or pcDNA3.1-Wip1. The results showed that the colony formation ability that includes number of colony and colony radius was markedly reduced in the siWip1 group, γ-irradiation group, and siWip1 + overexpression Wip1 + γ-irradiation group compared with the control group (p < 0.05). Similarly, the colony formation ability was at the lowest level in the siWip1 + γ-irradiation group (p < 0.01). But there were no significant differences in the siWip1 + overexpression Wip1 group (Fig. 3A, B). Furthermore, the apoptotic rate presented with the opposite results (Fig. 3C, D).The results suggested that knockdown of Wip1 enhanced the sensitivity to radiation in HeLa cells by decreasing colony formation ability and increasing apoptotic rate.
Figure 3.
Silencing of Wip1 enhances the sensitivity to radiotherapy in HeLa cells by decreasing colony formation ability and increasing apoptotic rate. (A) Number of colonies in each group; (B) colony radius in each group; (C) percentages of apoptosis cell; (D) flow cytometry (FCM) pictures. *p < 0.05; **p < 0.01.
Knockdown of Wip1 Activated p38 MAPK
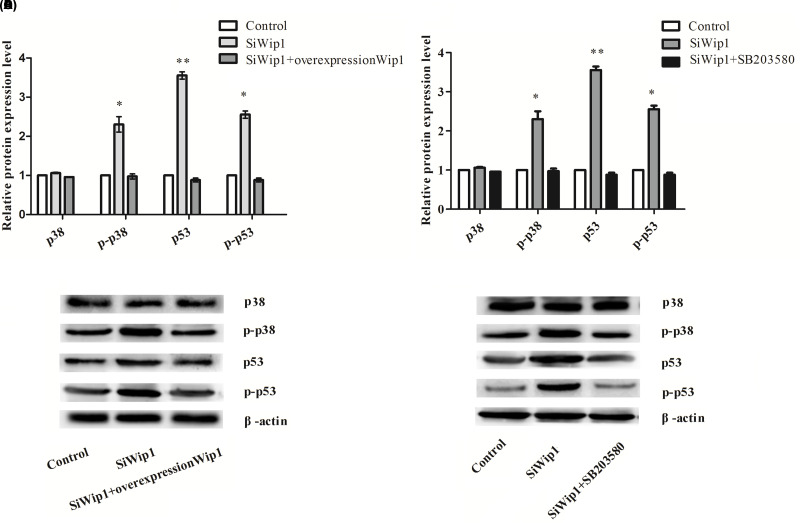
To demonstrate the possible associated signaling pathway in respect to the effect of Wip1 on HeLa cells, we determined the protein expression levels of p38, p-p38, p53, and p-p53 after transfection with target sequence for Wip1-specific siRNA or pcDNA3.1-Wip1. The results showed that protein expression levels of p-p38 (p < 0.05), p53 (p < 0.01), and p-p53 (p < 0.05) were all significantly increased by knockdown of Wip1 compared with the control group and the siWip1 + overexpression Wip1 group. However, there were no significant differences in the p38 protein level among the three groups (Fig. 4A, B). Knockdown of Wip1 could significantly activate the phosphorylation of p38 and enhance the transcriptional activity of p53, suggesting that the p38 MAPK signaling pathway was activated.
Figure 4.
Silencing of Wip1 activates p38 MAPK/p53 and p38 is regulated by Wip1. (A) Relative protein expression levels of p38, p-p38, p53, and p-p53 after interference with Wip1 expression; (B) Western blotting pictures; (C) relative protein expression levels of p38, p-p38, p53, and p-p53 after interference with Wip1 combined with SB203580; (D) Western blotting pictures. *p < 0.05; **p < 0.01.
p38 Was Regulated by Wip1
To further explore the relationship between Wip1 and p38, SB203580, an inhibitor of p38 MAPK, was pretreated into the cells that were intervened with Wip1-specific siRNA. The results demonstrated that the p-p38 (p < 0.05), p53 (p < 0.01), and p-p53 (p < 0.05) protein levels were significantly decreased in the siWip1 + SB203580 group compared with the siWip1 group (Fig. 4C, D). Moreover, we determined the colony formation ability and apoptotic rate in HeLa cells that were intervened with Wip1-specific siRNA in combination with SB203580. As shown in Figure 5A and B, there were no significant differences in the percentages of apoptosis cell between the siWip1 + SB203580 group and the control group. But the percentages of apoptosis cell in the γ-irradiation group and siWip1 + SB203580 + γ-irradiation group were significantly higher than the control group (p < 0.05). Moreover, the results of cell colony formation ability were similar as the percentages of apoptosis cell in each group (Fig. 6A, B). Application of SB203580 to the cells that were interfered with siWip1 could reverse the effects induced by siWip1 on HeLa cells, indicating that p38 may be a selective target regulated by Wip1.
Figure 5.
Apoptosis rate in the presence or not of SB203580. (A) Percentages of apoptosis cell in the presence or not of SB203580; (B) FCM pictures. *p < 0.05.
Figure 6.
Cell colony formation ability in the presence or not of SB203580. (A) Number of colony in each group; (B) colony radius in each group. *p < 0.05.
DISCUSSION
In the present study, we investigated the functional role and therapeutic implications of Wip1 in combination with radiotherapy (γ-irradiation) in cervical cancer cell line HeLa cells, as well as the potential mechanism. Our study suggests that Wip1 is involved in cervical cancer and may be a biomarker of cervical cancer. Knockdown of Wip1 could enhance the sensitivity to radiation in HeLa cells by inhibiting cell proliferation and inducing apoptosis through activation of p38 MAPK signaling pathway.
Several retrospective studies have confirmed the satisfactory outcomes for patients with cervical cancer in early stage or advanced stage, who are treated with definitive radiotherapy (18–20). One randomized clinical trial (RCT) reported that no significant difference was found in the overall survival of cervical cancer patients between those who received surgery and those who received definitive radiotherapy (21). In addition to surgery and chemotherapy, radiotherapy has been considered as an effective treatment strategy. Despite the most careful supervision of the dosage, however, radiation complications, such as gastrointestinal toxicity and urocystitis, do occur at a variable rate (22,23). Moreover, the increased resistance to radiotherapy and recurrence are main causes of treatment failure (7). Recently, gene therapy has been paid great attention to in the treatment of cervical cancer (24,25); however, few genes could be reasonably applied to clinical practice, and novel therapeutic targets have not been identified. Therefore, novel and effective treatment is thought necessary for patients with cervical cancer. In recent years, the Wip1 oncogene, a human protein phosphatase, has been well reported to be associated with many human tumors. However, the exact tumorigenic mechanism of Wip1 remains undefined (26).
Wip1 is an evolutionarily conserved protein phosphatase encoded by PPM1D, belonging to the PP2C family. Previous studies showed that deficiency of Wip1 activated ataxia-telangiectasia mutated (ATM) kinase, while overexpression of Wip1 inhibited the ATM-dependent signaling cascade after DNA damage. Therefore, it may foster tumorigenesis by promoting cell cycle progression (27,28). Recently, studies have suggested that Wip1 may bind to checkpoint kinases2 (CHK2) phosphorylated by ATM, and dephosphorylates and inactivates CHK2, and ultimately result in tumorigenesis (29,30). In addition, it has been reported that Wip1 could directly inhibit the activation of p38 MAPK, an important negative regulator of cell cycle progression, by dephosphorylating a threonine residue that is necessary for activation of full enzyme (17). p38 MAPK downregulates the expression levels of D-type cyclins at transcriptional and posttranslational cell cycle regulation (31). It phosphorylates and degrades the cell division cycle 25A (Cdc25A) phosphatase (32), suppresses the activation of Cdc25B phosphatase (33), and phosphorylates the p53, which plays important roles in cancer biology (17). In turn, inactivation of p38 MAPK may disturb the progression of the cell cycle, leading to tumorigenesis (34).
In an effort to demonstrate more potential functions of Wip1, we first silenced and overexpressed the expressions of Wip1 in human cervical cancer cell line HeLa cells by transfection techniques using a target sequence for Wip1-specific siRNA and the recombinant expression vector pcDNA3.1-Wip1, respectively. Our results showed that the expression levels of Wip1, either mRNA or protein, were significantly downregulated by transfection with Wip1-specific siRNA but upregulated by transfection with pcDNA3.1-Wip1. Subsequently, the effect of Wip1 on cell viability was investigated after transfection. We found that knockdown of Wip1 significantly reduced the cell growth compared with the control group. To further confirm the treatment of Wip1 on cervical cancer, we performed the colony formation assay and apoptotic rate in the presence or not of γ-irradiation. The results indicated that the colony formation ability was markedly decreased, while the apoptotic rate was significantly increased by transfection with Wip1-specific siRNA, which were similar to the results by only application of γ-irradiation. Moreover, better results were achieved in the siWip1 group combined with γ-irradiation, suggesting that Wip1 may enhance the sensitivity to radiation in HeLa cells. These results were inconsistent with the previous studies, in which the results suggested that Wip1 inactivation or depletion in PPM1D amplified human neuroblastoma cells, and breast cancer inhibited cell proliferation and induced apoptosis (10,35). Similarly, our study confirmed the important role of Wip1 in tumorigenesis. Additionally, the possible associated signaling pathway was investigated. The results demonstrated that inhibition of Wip1 might activate the p38 MAPK signaling pathway. In addition, we inactivated p38 MAPK with SB203580 to explore the exact control points. Our results revealed that it was the p38 that was directly regulated by Wip1.
Taken together, our study demonstrates the important role of Wip1 in cervical cancer. Knockdown of Wip1 inhibits positive regulators of cell proliferation, activates tumor-suppressor pathways, and induces apoptosis. In addition, knockdown of Wip1 gene expression could enhance the sensitivity to radiation in HeLa cells, in which the p38 MAPK signaling pathway is activated. Our results provide a novel target for cervical cancer therapy. However, further investigation would be merited to confirm the results.
REFERENCES
- 1. Farley J.; Shin H.; Bray F.; Forman D.; Mathers C.; Parkin D. GLOBOCAN 2008: Cancer incidence and mortality worldwide. Lyon France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 2. Forouzanfar M. H.; Foreman K. J.; Delossantos A. M.; Lozano R.; Lopez A. D.; Murray C. J.; Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet 378:1461–1484; 2011. [DOI] [PubMed] [Google Scholar]
- 3. Li J.; Jia H.; Xie L.; Wang X.; He H.; Lin Y.; Hu L. Correlation of inhibitor of differentiation 1 expression to tumor progression, poor differentiation and aggressive behaviors in cervical carcinoma. Gynecol. Oncol. 114:89–93; 2009. [DOI] [PubMed] [Google Scholar]
- 4. Sushma M.; Vamsikrishna B.; Babu M.; Mohanraj R. A review on role of human papilloma virus (HPV) in cervical cancer. Pharma Tutor 2:21–30; 2014. [Google Scholar]
- 5. Arbyn M.; Castellsague X.; de Sanjose S.; Bruni L.; Saraiya M.; Bray F.; Ferlay J. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 22:2675–2686; 2011. [DOI] [PubMed] [Google Scholar]
- 6. Dutta S.; Nguyen N. P.; Vock J.; Kerr C.; Godinez J.; Bose S.; Jang S.; Chi A.; Almeida F.; Woods W. Image-guided radiotherapy and-brachytherapy for cervical cancer. Front. Oncol. 5:64; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waggoner S. E. Cervical cancer. Lancet 361:2217–2225; 2003. [DOI] [PubMed] [Google Scholar]
- 8. Le Guezennec X.; Bulavin D. V. WIP1 phosphatase at the crossroads of cancer and aging. Trends Biochem. Sci. 35:109–114; 2010. [DOI] [PubMed] [Google Scholar]
- 9. Li J.; Yang Y.; Peng Y.; Austin R. J.; van Eyndhoven W. G.; Nguyen K.; Gabriele T.; McCurrach M. E.; Marks J. R.; Hoey T. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat. Genet. 31:133–134; 2002. [DOI] [PubMed] [Google Scholar]
- 10. Saito-Ohara F.; Imoto I.; Inoue J.; Hosoi H.; Nakagawara A.; Sugimoto T.; Inazawa J. PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res. 63:1876–1883; 2003. [PubMed] [Google Scholar]
- 11. Yang D.; Zhang H.; Hu X.; Xin S.; Duan Z. Abnormality of p16/p38MAPK/p53/Wip1 pathway in papillary thyroid cancer and its significance. Chin. J. Gen. Surg. 20:1199–1202; 2011. [Google Scholar]
- 12. Hirasawa A.; Saito-Ohara F.; Inoue J.; Aoki D.; Susumu N.; Yokoyama T.; Nozawa S.; Inazawa J.; Imoto I. Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas with poor prognosis and identification of PPM1D and APPBP2 as likely amplification targets. Clin. Cancer Res. 9:1995–2004; 2003. [PubMed] [Google Scholar]
- 13. Li Z.-T.; Zhang L.; Gao X.-Z.; Jiang X.-H.; Sun L.-Q. Expression and significance of the Wip1 proto-oncogene in colorectal cancer. Asian Pac. J. Cancer Prev. 14:1975–1979; 2013. [DOI] [PubMed] [Google Scholar]
- 14. Castellino R. C.; De Bortoli M.; Lu X.; Moon S.-H.; Nguyen T.-A.; Shepard M. A.; Rao P. H.; Donehower L. A.; Kim J. Y. Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J. Neurooncol. 86:245–256; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satoh N.; Maniwa Y.; Bermudez V. P.; Nishimura K.; Nishio W.; Yoshimura M.; Okita Y.; Ohbayashi C.; Hurwitz J.; Hayashi Y. Oncogenic phosphatase Wip1 is a novel prognostic marker for lung adenocarcinoma patient survival. Cancer Sci. 102:1101–1106; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fiscella M.; Zhang H.; Fan S.; Sakaguchi K.; Shen S.; Mercer W. E.; Woude G.F.V.; O’Connor P. M.; Appella E. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc. Natl. Acad. Sci. USA 94:6048–6053; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takekawa M.; Adachi M.; Nakahata A.; Nakayama I.; Itoh F.; Tsukuda H.; Taya Y.; Imai K. p53-inducible Wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 19:6517–6526; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anker C. J.; Cachoeira C. V.; Boucher K. M.; Rankin J.; Gaffney D. K. Does the entire uterus need to be treated in cancer of the cervix? Role of adaptive brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 76:704–712; 2010. [DOI] [PubMed] [Google Scholar]
- 19. Hareyama M.; Sakata K. I.; Oouchi A.; Nagakura H.; Shido M.; Someya M.; Koito K. High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix. Cancer 94:117–124; 2002. [DOI] [PubMed] [Google Scholar]
- 20. Nakano T.; Kato S.; Ohno T.; Tsujii H.; Sato S.; Fukuhisa K.; Arai T. Long-term results of high-dose rate intracavitary brachytherapy for squamous cell carcinoma of the uterine cervix. Cancer 103:92–101; 2005. [DOI] [PubMed] [Google Scholar]
- 21. Landoni F.; Maneo A.; Colombo A.; Placa F.; Milani R.; Perego P.; Favini G.; Ferri L.; Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 350:535–540; 1997. [DOI] [PubMed] [Google Scholar]
- 22. Salama J. K.; Mundt A. J.; Roeske J.; Mehta N. Preliminary outcome and toxicity report of extended-field, intensity-modulated radiation therapy for gynecologic malignancies. Int. J. Radiat. Oncol. Biol. Phys. 65:1170–1176; 2006. [DOI] [PubMed] [Google Scholar]
- 23. Ma Y.; Huang L.; Song C.; Zeng X.; Liu G.; Mei L. Nanoparticle formulation of poly (ε-caprolactone-co-lactide)-d-α-tocopheryl polyethylene glycol 1000 succinate random copolymer for cervical cancer treatment. Polymer 51:5952–5959; 2010. [Google Scholar]
- 24. Zheng Y.; Chen H.; Zeng X.; Liu Z.; Xiao X.; Zhu Y.; Gu D.; Mei L. Surface modification of TPGS-b-(PCL-ran-PGA) nanoparticles with polyethyleneimine as a co-delivery system of TRAIL and endostatin for cervical cancer gene therapy. Nanoscale Res. Lett. 8:1–12; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu B.; Han S.-M.; Tang X.-Y.; Han L.; Li C.-Z. Cervical cancer gene therapy by gene loaded PEG-PLA nanomedicine. Asian Pac. J. Cancer Prev. 15:4915–4918; 2013. [DOI] [PubMed] [Google Scholar]
- 26. Doucette T. A.; Yang Y.; Pedone C.; Kim J. Y.; Dubuc A.; Northcott P. D.; Taylor M. D.; Fults D. W.; Rao G. WIP1 enhances tumor formation in a sonic hedgehog-dependent model of medulloblastoma. Neurosurgery 70:1003; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shreeram S.; Demidov O. N.; Hee W. K.; Yamaguchi H.; Onishi N.; Kek C.; Timofeev O. N.; Dudgeon C.; Fornace A. J.; Anderson C. W.; Minami Y.; Appella E.; Bulavin D. V. Wip1 phosphatase modulates ATM-dependent signaling pathways. Mol. Cell 23:757–764; 2006. [DOI] [PubMed] [Google Scholar]
- 28. Lu X.; Bocangel D.; Nannenga B.; Yamaguchi H.; Appella E.; Donehower L. A. The p53-induced oncogenic phosphatase PPM1D interacts with uracil DNA glycosylase and suppresses base excision repair. Mol. Cell 15:621–634; 2004. [DOI] [PubMed] [Google Scholar]
- 29. Spinnler C.; Hedstrom E.; Li H.; de Lange J.; Nikulenkov F.; Teunisse A. F.; Verlaan-de Vries M.; Grinkevich V.; Jochemsen A. G.; Selivanova G. Abrogation of Wip1 expression by RITA-activated p53 potentiates apoptosis induction via activation of ATM and inhibition of HdmX. Cell Death Differ. 18:1736–1745; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park J. Y.; Song J. Y.; Kim H. M.; Han H. S.; Seol H. S.; Jang S. J.; Choi J. p53-Independent expression of wild-type p53-induced phosphatase 1 (Wip1) in methyl methanesulfonate-treated cancer cell lines and human tumors. Int. J. Biochem. Cell Biol. 44:896–904; 2012. [DOI] [PubMed] [Google Scholar]
- 31. Casanovas O.; Miro F.; Estanyol J. M.; Itarte E.; Agell N.; Bachs O. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2-dependent manner. J. Biol. Chem. 275:35091–35097; 2000. [DOI] [PubMed] [Google Scholar]
- 32. Goloudina A.; Yamaguchi H.; Chervyakova D. B.; Appella E.; Fornace A. J. Jr.; Bulavin D. V. Regulation of human Cdc25A stability by Serine 75 phosphorylation is not sufficient to activate a S phase checkpoint. Cell Cycle 2:473–478; 2003. [PubMed] [Google Scholar]
- 33. Bulavin D. V.; Higashimoto Y.; Popoff I. J.; Gaarde W. A.; Basrur V.; Potapova O.; Appella E.; Fornace A. J. Jr. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411:102–107; 2001. [DOI] [PubMed] [Google Scholar]
- 34. Brancho D.; Tanaka N.; Jaeschke A.; Ventura J. J.; Kelkar N.; Tanaka Y.; Kyuuma M.; Takeshita T.; Flavell R. A.; Davis R. J. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 17:1969–1978; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Natrajan R.; Lambros M. B.; Rodriguez-Pinilla S. M.; Moreno-Bueno G.; Tan D. S.; Marchio C.; Vatcheva R.; Rayter S.; Mahler-Araujo B.; Fulford L. G.; Hungermann D.; Mackay A.; Grigoriadis A.; Fenwick K.; Tamber N.; Hardisson D.; Tutt A.; Palacios J.; Lord C. J.; Buerger H.; Ashworth A.; Reis-Filho J. S. Tiling path genomic profiling of grade 3 invasive ductal breast cancers. Clin. Cancer Res. 15:2711–2722; 2009. [DOI] [PubMed] [Google Scholar]