Abstract
Purpose
Complaints of auditory perceptual deficits, such as tinnitus and difficulty understanding speech in background noise, among individuals with clinically normal audiograms present a perplexing problem for audiologists. One potential explanation for these “hidden” auditory deficits is loss of the synaptic connections between the inner hair cells and their afferent auditory nerve fiber targets, a condition that has been termed cochlear synaptopathy. In animal models, cochlear synaptopathy can occur due to aging or exposure to noise or ototoxic drugs and is associated with reduced auditory brainstem response (ABR) wave I amplitudes. Decreased ABR wave I amplitudes have been demonstrated among young military Veterans and non-Veterans with a history of firearm use, suggesting that humans may also experience noise-induced synaptopathy. However, the downstream consequences of synaptopathy are unclear.
Method
To investigate how noise-induced reductions in wave I amplitude impact the central auditory system, the ABR, the middle latency response (MLR), and the late latency response (LLR) were measured in 65 young Veterans and non-Veterans with normal audiograms.
Results
In response to a click stimulus, the MLR was weaker for Veterans compared to non-Veterans, but the LLR was not reduced. In addition, low ABR wave I amplitudes were associated with a reduced MLR, but with an increased LLR. Notably, Veterans reporting tinnitus showed the largest mean LLRs.
Conclusions
These findings indicate that decreased peripheral auditory input leads to compensatory gain in the central auditory system, even among individuals with normal audiograms, and may impact auditory perception. This pattern of reduced MLR, but not LLR, was observed among Veterans even after statistical adjustment for sex and distortion product otoacoustic emission differences, suggesting that synaptic loss plays a role in the observed central gain.
Supplemental Material
It is becoming increasingly apparent that a normal audiogram does not necessarily represent a normal auditory system. An estimated 12% of individuals with clinically normal hearing report difficulty understanding speech in complex listening situations (Tremblay et al., 2015). Reported hearing difficulty is even more prevalent among noise-exposed populations such as military Veterans. In a sample of 99 Veterans who completed the Hearing Handicap Inventory for Adults, 27% indicated a mild-to-moderate hearing handicap even though the mean audiometric thresholds for this group were better than 20 dB HL throughout the standard audiometric range (Gordon et al., 2017). Likewise, an analysis of more than 3 million Veteran audiograms revealed that the prevalence of Veterans with clinically normal hearing (i.e., hearing thresholds from 250 to 8000 Hz of ≤ 25 dB HL) seeking hearing care is twice that of the civilian population (Billings et al., 2018). In addition, a recent retrospective analysis of Veterans Affairs health record data found that 6% of all Iraq and Afghanistan Veterans receiving VA care and 45% of Iraq and Afghanistan Veterans 26 years old and younger have a diagnosis code for tinnitus, but not for hearing loss (Swan et al., 2017). One explanation for auditory deficits “hidden” from the audiogram is partial loss of the synaptic connections between the inner hair cells (IHCs) and their afferent nerve fiber targets, a condition termed cochlear synaptopathy (see reviews by Bramhall, Beach, et al., 2019; Le Prell, 2019). Cochlear synaptopathy has been demonstrated in a variety of animal models (mouse, gerbil, rat, chinchilla, guinea pig, and nonhuman primates) in response to noise exposure or aging (Hickox et al., 2017; Kujawa & Liberman, 2009; Lin et al., 2011; Schmiedt et al., 1996; Sergeyenko et al., 2013; Valero et al., 2017). Data from human temporal bones suggest that cochlear synaptic loss can also precede hair cell loss in humans (Viana et al., 2015; Wu et al., 2018). Although the functional impacts of synaptopathy are predicted to include tinnitus, hyperacusis, and difficulty understanding speech in background noise (Kujawa & Liberman, 2015), these potential consequences are challenging to assess because confirmation of synaptopathy in humans requires postmortem temporal bone analysis. However, animal models indicate that the amplitude of wave I of the auditory brainstem response (ABR) is highly correlated with the degree of synaptic loss (Kujawa & Liberman, 2009; Lin et al., 2011; Sergeyenko et al., 2013). Several human studies have shown age-related (Bramhall et al., 2015; Konrad-Martin et al., 2012) or noise exposure–related (Bramhall et al., 2017; Grose et al., 2017; Stamper & Johnson, 2015; Valderrama et al., 2018) reductions in ABR wave I amplitude that are consistent with synaptopathy in animal models. Bramhall et al. (2017) observed a reduction in ABR wave I amplitude among Veterans with high levels of reported noise exposure during their military service and non-Veterans with a history of firearm use. In contrast, other studies have failed to observe a relationship between common recreational noise exposures, such as concerts, night clubs, and personnel music player use, and ABR wave I amplitude (e.g., Grinn et al., 2017; Prendergast et al., 2017). This suggests that noise-induced synaptopathy may not be a common consequence of these types of recreational noise exposures but may result from very high-intensity noise exposures, such as those experienced during military service or from firearm use.
Although no clear association has been demonstrated between ABR wave I amplitude and speech-in-noise perception (Bramhall et al., 2015, 2018; Fulbright et al., 2017; Yeend et al., 2017), several studies have suggested that tinnitus is associated with reduced ABR wave I amplitude (Bramhall, McMillan, et al., 2019; Bramhall et al., 2018; Gu et al., 2012; Schaette & McAlpine, 2011). These findings indicate that tinnitus may be a functional consequence of reduced auditory input at the level of the auditory nerve. One proposed mechanism for tinnitus generation is that compensation occurs in the central auditory system in response to reduced peripheral auditory input, and this compensatory hyperactivity gives rise to the perception of tinnitus (Jastreboff, 1990). A similar central gain mechanism has also been proposed for hyperacusis, a perceptual deficit that often co-occurs with tinnitus (reviewed in Eggermont & Roberts, 2015). Consistent with the central gain theory, Chambers, Resnik, et al. (2016) and Chambers, Salazar, et al. (2016) observed evidence of compensatory hyperactivity in the thalamus, inferior colliculus, and auditory cortex in response to reduced neuronal input in mice with near-complete cochlear deafferentation but normal hair cell function. The greatest degree of compensation was observed in the auditory cortex. Formby et al. (2007) showed evidence of central gain occurring in the human auditory system in response to decreased peripheral auditory input through use of earplugs. After wearing earplugs for 23 hr a day for 4 weeks, study participants had steeper loudness growth functions, suggestive of compensatory central gain.
Previous investigations of noise-induced synaptopathy in humans have focused on ABR measurements. However, it is difficult to determine the functional consequences of reduced ABR wave I amplitude without examining the central auditory system. This can be accomplished noninvasively using auditory evoked potentials with longer latencies than the ABR, such as the middle latency response (MLR) and the late latency response (LLR, also known as the P1–N1–P2). The MLR is an evoked potential generated by multiple sources in the auditory thalamocortical pathway and occurs approximately 10–70 ms after an auditory stimulus (reviewed in McGee & Kraus, 1996). The first negative and positive potentials in this time window are referred to as Na and Pa, respectively. In guinea pigs, pharmacologically induced auditory nerve degeneration is associated with reduced electrically evoked MLR amplitude (Jyung et al., 1989), suggesting that a reduction in MLR amplitude might be expected in individuals with synaptopathy. The LLR, generated by the primary and secondary auditory cortex and beyond, is sensitive to stimulus onsets and is made up of components that occur approximately 50 ms (P1), 100 ms (N1), and 200 ms (P2) after the stimulus onset (see reviews by Martin et al., 2008; Naatanen & Picton, 1987). Both the MLR and LLR are relatively exogenous potentials, meaning they are more dependent on external stimulus factors rather than cognitive processing.
The goal of this study was to determine how previously reported noise-related reductions in ABR wave I amplitude among young Veterans with normal audiograms impact the central auditory system. The ABR, MLR, and LLR were measured in young Veterans and non-Veterans with normal audiograms and varying levels of noise exposure. To avoid the ambiguity associated with peak picking, particularly for the MLR, the rectified areas of the entire MLR and LLR waveforms were calculated for each participant. Distortion product otoacoustic emissions (DPOAEs) were measured to verify that all participants had relatively good outer hair cell (OHC) function and to account for variation in OHC status in the statistical analysis. Participants were divided into three groups to approximate three different levels of expected damage to the auditory system: non-Veterans (minimal damage), Veterans without tinnitus (moderate damage due to military noise exposure), and Veterans with tinnitus (significant damage based on military noise exposure and the perception of tinnitus). In response to a click stimulus, even after statistical adjustment for sex and average DPOAE levels, results indicate a reduction in MLR area associated with both Veteran groups, but no reduction in LLR area. However, a decrease in LLR area was observed among Veterans with tinnitus in response to 4- and 6-kHz pure-tone stimuli.
Method
Participants
Military Veterans and non-Veterans aged 19–35 years were recruited from previous studies conducted at the National Center for Rehabilitative Auditory Research, from a database of young Veterans seen by the VA Portland Healthcare System (VAPORHCS) and by posting fliers at the VAPORHCS and Portland area colleges and universities. Sixty-five young adults (aged 19–35 years) participated in this study and were divided into three study groups based on predicted auditory damage: non-Veterans (minimal auditory damage), Veterans without tinnitus (moderate auditory damage), and Veterans with tinnitus (significant auditory damage). Inclusion criteria for all participants included pure-tone air-conduction thresholds of ≤ 20 dB HL from 0.25 to 8 kHz, normal tympanogram (226-Hz tympanogram, compliance = 0.3–1.9 ml, and peak pressure between ± 50 dPa), normal DPOAEs (criteria described below), and no history of otologic or neurologic disorder (including traumatic brain injury or concussion). Only individuals meeting all audiometric criteria in at least one ear were invited to participate. While Veterans were eligible to participate in the study regardless of noise exposure history (all Veterans were assumed to have at minimum a history of firearm use during military basic training), non-Veteran controls were required to have minimal noise exposure, including no self-reported firearm usage (i.e., never discharged a firearm). If only one ear met the inclusion criteria for the study, the ABR, MLR, and LLR were measured in that ear. If both ears met the inclusion criteria, evoked potential measures were collected in the ear with the better DPOAEs to minimize OHC dysfunction. All participants provided written informed consent and were paid for their participation. All study procedures were approved by the VAPORHCS Institutional Review Board.
Procedure
Audiometry
Pure-tone air-conduction thresholds were assessed in all potential participants from 0.25 to 8 kHz as part of the screening evaluation. Bone conduction thresholds were measured at 0.5, 1, 2, and 4 kHz to rule out conductive hearing loss. Extended high-frequency thresholds from 9 to 16 kHz were also assessed using Sennheiser HDA 200 headphones, but were not used to determine study inclusion.
DPOAEs
DPOAE testing was conducted using a custom system that includes an ER-10 B+ probe microphone and EMAV software from Boys Town National Research Hospital (Neely & Liu, 1993). DPOAE stimuli were presented at a fixed primary frequency ratio f1/f2 = 1.2, and responses were obtained using a primary frequency sweep (DP-gram) from 1 to 8 kHz in 1/6-octave increments at stimulus frequency levels of L1 = 65 and L2 = 55 dB SPL. Responses were compared to the DPOAE levels from a distribution of individuals with abnormal pure-tone thresholds (Gorga et al., 1997, Table A1). Only individuals at or above the 90th percentile from 1.5 to 6 kHz were included in the study. These DPOAE criteria were somewhat relaxed in comparison to our previous study (Bramhall et al., 2018, 2017) to improve recruitment numbers. Measurement-based stopping rules were employed in which averaging continued until 30 s of artifact-free data were collected or until the noise floor was below −15 dB SPL. For two participants whose measured DPOAE levels at 8 kHz were below a conservative estimate of the system distortion level at that frequency (−20 dB SPL), DPOAE levels at 8 kHz were set to −20 dB SPL. Average DPOAE levels from 3 to 8 kHz were used to statistically adjust for differences in OHC function between study groups.
ABR
ABR testing was completed in 64 participants (21 non-Veterans, 26 Veterans without tinnitus, and 17 Veterans with tinnitus) using an Intelligent Hearing Systems SmartEP system and Etymotic Research gold foil ER3-26A tiptrode electrodes placed in the ear canal. The reference electrode was placed on the high forehead; and the ground electrode, on the low forehead. Waveforms were generated using alternating polarity 4-kHz tone burst stimuli presented at 90, 100, and 110 dB peSPL. Stimuli were 2 ms in duration with a rise/fall time of 0.5 ms and a Blackman envelope. A 4-kHz stimulus was chosen to target the cochlear frequency region most likely to show evidence of noise damage. The ABR response was band-pass filtered from 10 to 1500 Hz and averaged across 1,024–2,048 stimulus presentations. A stimulus repetition rate of 11.1/s was used, and at least two replications of each waveform were obtained. Electrode impedance was less than or equal to 5.0 kΩ in all but six participants; these participants had impedances less than or equal to 7.0 kΩ. The positive peak and the following negative trough for wave I were initially identified with an automated Python-based peak picking program (adapted from Buran, 2015). Peaks and troughs were then evaluated by two independent raters and reassigned if both were in agreement. In cases of disagreement, a third rater made a final assignment. Wave I amplitudes were defined as the difference between the voltage at the positive peak and the voltage at the following negative trough. Wave V amplitudes were calculated as the difference between the voltage at the peak and the average prestimulus baseline voltage calculated for the 1-ms period before the stimulus onset. One participant left the study before completing ABR testing.
MLR and LLR
MLR and LLR testing was completed while participants were seated in a comfortable chair and watched a closed-captioned movie. They were asked to ignore the auditory stimuli and minimize head/body movement during recording. For all participants, testing was performed in a double-walled sound-treated booth and occurred over one session lasting approximately 3 hr. Three different types of stimuli (described below) were presented in an order that was randomized across participants. Stimuli were presented monaurally to the test ear using an Etymotic ER-3A insert earphone. Stimulus presentation was controlled using the Compumedics Neuroscan Stim2 stimulus presentation system. Evoked potential activity was recorded using the Neuroscan SynampsRT amplifier and Curry Neuroimaging Suite 8.0XS software. Electroencephalography activity was recorded using a limited two-channel montage with individual electrodes placed at frontal and central midline locations (Cz and Fz). An off-line–generated linked mastoid reference was used in the analysis (M1 and M2). Eye movement was monitored using an electrode over the right supraorbital ridge of the frontal bone (Fz2). The ground electrode was located on the forehead (FPz), and the online reference electrode was located on the tip of the nose. All electrode impedances were ≤ 5.0 kΩ, with the exception of one participant with impedances ≤ 6.0 kΩ.
MLR stimuli consisted of a 100-μs click at four intensity levels (75, 85, 95, and 105 dB peSPL) presented in random order. During piloting, MLR testing was attempted using 4- and 6-kHz pure tones, but due to poor waveform morphology with the tones, a click was used instead. Stimuli were presented with 800 sweeps per condition using a random jittered interstimulus interval (onset to onset) of 866.67, 916.67, or 966.67 ms. The recording time window was 140 ms (−40 ms prestimulus, 100 ms poststimulus). MLR evoked responses were band-pass filtered online from 10 Hz (high-pass filter, 24 dB/octave) to 200 Hz (low-pass filter, 12 dB/octave). All channels were amplified with a gain of 10 and converted using an analog-to-digital sampling rate of 10 kHz. Trials with artifacts greater than ± 50 μV were rejected off-line using the Neuroscan software.
LLR responses to the click stimulus described above were extracted from the raw electroencephalography recordings detailed above using distinct filter settings. In a separate recording, the LLR was also generated in response to 4- and 6-kHz pure tones because these are the frequency regions expected to be most impacted by noise damage. The pure tones were 100 ms in duration and were randomly presented at four different intensity levels (50, 60, 70, and 80 dB SPL). Two hundred sweeps were completed for each pure-tone condition using a randomly jittered interstimulus interval (onset to onset) of 1550, 1600, or 1650 ms. The LLR recording time window for both clicks and pure tones was 800 ms (−200 ms prestimulus, 600 ms poststimulus). LLR recordings were band-pass filtered off-line from 1 to 30 Hz with online artifact rejection of ± 70 μV.
Because the MLR and LLR are typically maximal near the vertex, evoked potentials from the Cz channel were analyzed. Peak identification was often ambiguous, particularly for the MLR at low stimulus levels, and a preliminary analysis of peak picking results failed to show a clear increase in peak amplitudes with increase in stimulus intensity level. To provide a more objective measure of the MLR and LLR, total response waveform area was calculated. Area measurements were calculated in a constant time window encompassing the entire MLR or LLR complex (4–80 ms for the MLR and 30–330 ms for the LLR). A custom Neuroscan function was used to calculate the area under the curve within these time windows using the absolute value of each point and computing the integral. As a result, the areas for both positive-going and negative-going regions of the waveform were summed to compute a total rectified area.
Noise Exposure History Questionnaire
All potential participants completed the Lifetime Exposure to Noise and Solvents–Questionnaire (LENS-Q; Bramhall et al., 2017; Gordon et al., 2017). This in-depth questionnaire assesses the frequency and duration of noise exposure as well as the use of hearing protection for a large variety of possible sources of noise exposure across three categories: nonmilitary occupational, military occupational, and nonoccupational/recreational. Potential non-Veteran participants who reported ever using a firearm were excluded from the study. The LENS-Q was scored primarily as described in Bramhall et al. However, in the nonoccupational/recreational section, participants tended to report similar patterns of hearing protection use and frequency/duration of exposure for items within a particular category (e.g., Gunfire, Transportation, Music Attended, Music Played, Woodworking/Power Tools, Sports Games Attended, Motor Sports Events Attended, and Yard and Garden Power Equipment). To avoid overestimation of nonoccupational exposures, only the noise item with the highest exposure score within each category was included in the calculation of the final nonoccupational noise exposure score. Note also that the LENS-Q is scored on a log scale, so a difference of one between two scores indicates a 10-fold difference in lifetime noise exposure.
Hearing Questionnaire
All participants completed a hearing questionnaire including the question, “Do you have constant or frequent ringing in the ears?” If they responded “yes,” they were also asked which ear was affected. Participants who reported ringing in the test ear were rated as having tinnitus. No other information about the tinnitus was collected.
Statistical Analysis
Bayesian multilevel regression analysis was used to model the mean MLR and LLR area for each click stimulus level and study group, while adjusting for the possible confounders sex and OHC function (indicated by average DPOAE level from 3 to 8 kHz). In contrast to the conventional statistical approach using p values, Bayesian analysis allows for the calculation of the probability of a true difference in mean MLR (or LLR) area between study groups. We have described the benefits of this approach previously (Bramhall et al., 2018, 2017; McMillan & Cannon, 2019). MLR and LLR areas were modeled jointly as it was apparent in the raw data that these measures were correlated, with Pearson correlations ranging from .15 to .44. Each pair of MLR and LLR areas, indexed by i, were modeled as a vector of bivariate normal random variables, each with a mean μi and a variance matrix Σ consisting of the residual variance of each response area and their covariances. The mean vector μi was modeled as a function of stimulus level L, study group G, subject S, average DPOAE level D, and a female sex indicator variable F such that
| (1) |
Note the bracket [i] notation denotes the subject, group, or level to which the i th observation belongs. Level effects were modeled as linear (α+β⋅L i), but nonlinear level effects θL were included to account for variation among level effects around the line. Zero-mean vector, bivariate normal priors with identity covariance matrices were assigned to α, θL, δS, γG, vG,L, ω and ξ. The β prior, corresponding to the linear growth in the mean MLR and mean LLR area, was given bivariate normal priors and mean vectors reflecting the assumption that the mean area increases with stimulus level. Priors for each of the model coefficients are described in detail in Supplemental Material S1. Prior predicted data were generated from this model and closely matched the scale of the observed data.
The model was fit in PROC MCMC of the SAS software, Version 9.4. The model was run three times for 1 million iterations after a 1 million iteration burn-in period. Posterior samples were thinned to every 1,000th draw and combined across chains for inference. Gelman–Rubin diagnostics were below 1.1 for all parameters, and posterior predictive checks indicated no gross deviations of the fitted model from the data.
Results
Study Group Characteristics
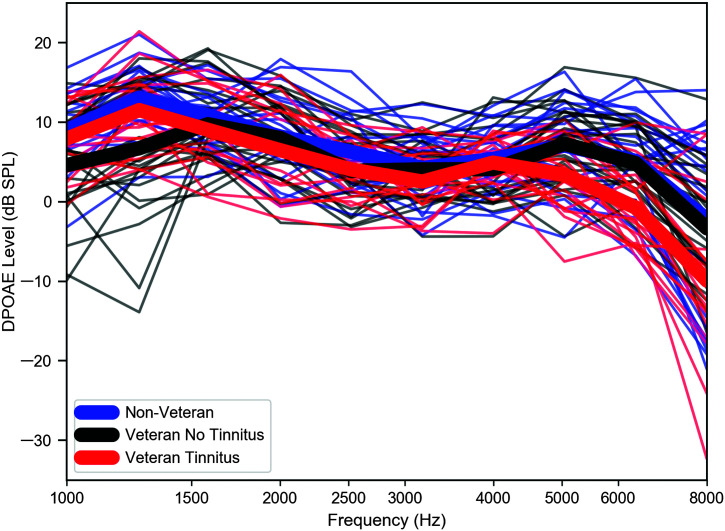
Characteristics of the three study groups (Non-Veteran, Veteran No Tinnitus, and Veteran Tinnitus) are summarized in Table 1. Mean age is similar across groups. The Non-Veteran and Veteran No Tinnitus groups are well balanced in terms of sex, whereas the Veteran Tinnitus group is dominated by males. Pure-tone averages, even in the extended high frequencies, differ by no more than 7 dB between groups, with the Veteran Tinnitus group having the poorest thresholds. Although participants were only required to meet the audiometric criteria in a single ear, only six participants had audiometric thresholds from 0.25 to 8 kHz in the nontest ear that were poorer than 20 dB HL. Four of these individuals (three Veterans with tinnitus and one non-Veteran) had no thresholds poorer than 25 dB HL. The remaining two (Veterans without tinnitus) each had a single threshold > 20 dB HL (at 30 and 35 dB HL). Mean LENS-Q scores are lowest for the non-Veteran group and highest for the Veteran groups. Of the 18 Veterans reporting tinnitus, 16 reported bilateral tinnitus and two reported tinnitus only in the test ear. DP-grams from 1 to 8 kHz, color-coded by study group, are plotted in Figure 1. Mean DPOAE levels are 3–8 dB lower for the Veteran Tinnitus group compared to the Non-Veteran group from 5 to 8 kHz.
Table 1.
Participant characteristics by study group.
| Variable | Non-Veteran | Veteran No Tinnitus |
Veteran Tinnitus |
|---|---|---|---|
| Age in years | 27.5 (4.2) | 29.7 (3.5) | 30.0 (2.7) |
| No. males | 11 | 12 | 15 |
| PTA (0.5, 1, and 2 kHz) | 6.2 (4.2) | 6.3 (4.5) | 8.3 (4.6) |
| High-frequency PTA (3, 4, and 6 kHz) | 3.3 (5.3) | 5.1 (4.0) | 7.1 (5.2) |
| EHF PTA (9–16 kHz) | 5.2 (9.3) | 4.7 (8.7) | 11.5 (15.3) |
| LENS-Q score | 4.1 (0.8) | 9.1 (0.8) | 9.3 (1.7) |
| Total participants | 21 | 26 | 18 |
Note. For mean values, standard deviations are shown in parentheses. PTA = pure-tone average; EHF = extended high frequency; LENS-Q = Lifetime Exposure to Noise and Solvents–Questionnaire.
Figure 1.
DPOAEs similar across study groups through 4 kHz. Group mean DPOAE levels for a DP-gram in response to L1/L2 levels of 65/55 dB SPL are shown in thick lines, and DPOAE levels for individual participants are indicated by thin lines. DPOAE = distortion product otoacoustic emission.
ABR Wave I Amplitudes, But Not Wave V Amplitudes, Are Smaller for Groups With More Predicted Auditory Damage
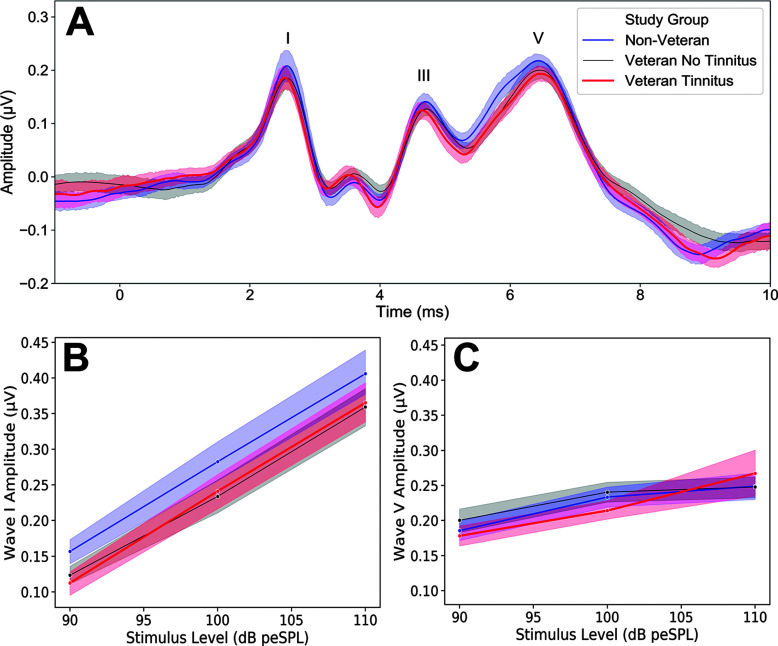
When the study participants are separated into three groups based on their expected auditory damage (Non-Veteran, Veteran No Tinnitus, and Veteran Tinnitus), the group predicted to have the least damage, the Non-Veteran group, shows the highest mean ABR wave I amplitude in response to a 4-kHz tone burst, whereas wave I amplitudes show a modest reduction for the two Veteran groups (see Figures 2A and 2B). Mean ABR wave V amplitudes for the 4-kHz tone burst are similar across groups (see Figure 2C).
Figure 2.
Auditory brainstem response wave I, but not wave V, amplitude reduced among groups with more expected auditory damage. (A) Grand-average auditory brainstem response waveforms are plotted by study group for a 100–dB peSPL, 4-kHz tone burst. Waves I, III, and V are indicated on the plot. (B) ABR wave I and (C) wave V amplitude input/output functions are plotted according to study group for a 4-kHz tone burst. Shaded regions indicate standard error of the mean.
Mean MLR Areas Are Reduced for Groups With Greater Expected Auditory Damage
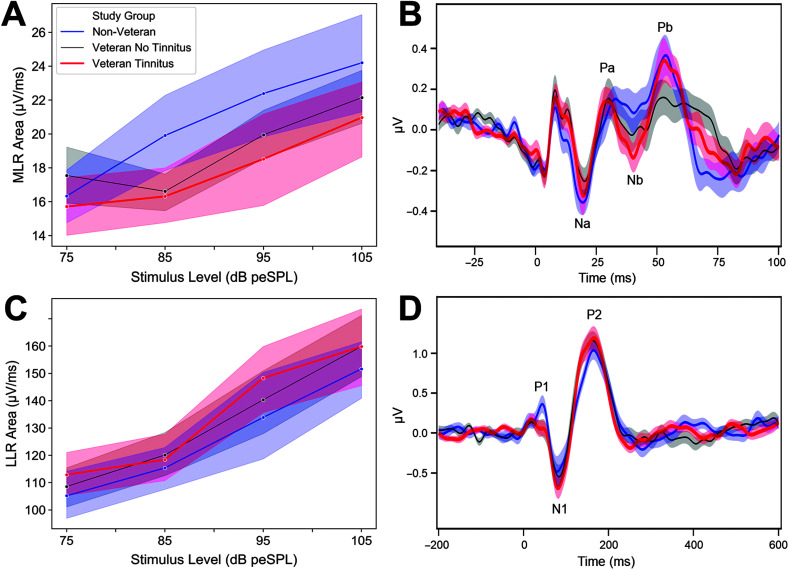
Mean MLR areas are largest for the non-Veterans (the group with the least expected auditory damage) in response to a click stimulus and smallest for the Veteran Tinnitus group (the group with the most expected damage; see Figure 3A). The MLR area reduction among the Veterans is most apparent at 85 dB peSPL and above. A similar comparison between the Non-Veteran group and the Veteran No Tinnitus group can be observed in the grand-average MLR waveforms, particularly in the Na and Pb peaks (see Figure 3B). The decreased response for the Veteran Tinnitus group compared to the Non-Veteran group is less apparent in the average waveforms than the mean areas, potentially because two Veterans with tinnitus had very large Pb peaks that were not representative of the group as a whole, but had a big impact on the average waveform peaks. Five participants (one non-Veteran, two Veterans without tinnitus, and two Veterans with tinnitus) had very large biphasic peaks in the 12- to 18-ms range for the MLR condition, consistent with postauricular muscle artifact (O'Beirne & Patuzzi, 1999). These participants were excluded from the grand-average waveforms but were included in all other analyses.
Figure 3.
Middle latency response (MLR) area reduced for groups with more expected auditory damage, but not late latency response (LLR) area. (A) Mean MLR areas are plotted by study group. (B) Grand-average MLR waveforms are plotted by study group. Na, Pa, Nb, and Pb peaks are indicated on the plot. Five participants (one non-Veteran, two Veterans without tinnitus, and two Veterans with tinnitus) had large biphasic peaks in the MLR in the 12- to 18-ms range, consistent with postauricular muscle artifact, and were excluded from the grand-average waveforms. (C) Mean LLR areas are plotted by study group. (D) LLR grand-average waveforms are plotted by study group. P1, N1, and P2 peaks are labeled on the plot. Shaded regions indicate standard error of the mean.
Mean LLR Areas (for a Click Stimulus) Are Not Reduced for the Groups With More Expected Auditory Damage
In contrast to the ABR and MLR, mean LLR area in response to the click stimulus was not reduced in the Veteran groups as compared to the Non-Veteran group (see Figure 3C). In fact, particularly for Veterans with tinnitus, mean LLR area was somewhat increased compared to non-Veterans. An increase in LLR strength for Veterans as compared with non-Veterans can also be observed in both N1 and P2 of the LLR -average waveforms (see Figure 3D).
Model-Based MLR and LLR Means Show Similar Group Effects After Statistical Adjustment for Sex and DPOAEs
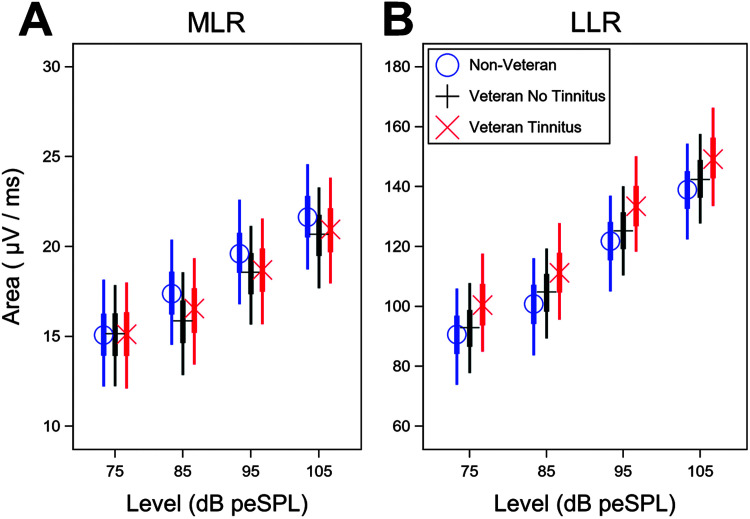
A Bayesian statistical model was used to adjust for sex and average DPOAE (from 3 to 8 kHz) differences between groups that could confound the evoked potential measurements. In response to a click, the model results indicate reduced mean MLR area for the two Veteran groups compared to the Non-Veteran group, particularly for stimulus levels of 85–105 dB peSPL (see Figure 4A). When the modeled differences in group mean MLR area are averaged across level, the mean area for the Non-Veteran group is 0.5 μV/ms greater than the area for the Veteran Tinnitus group (90% confidence interval [−2.7, 4.4 μV/ms]). The mean area for the Non-Veteran group is 0.8 μV/ms greater than the area for the Veteran No Tinnitus group (90% confidence interval [−2.1, 4.6 μV/ms]). Over the time course of 4–80 ms over which the MLR was measured, this is a mean reduction in amplitude of 38–61 μV for the two Veteran groups compared to the Non-Veteran group (a 2.8%–4.3% decrease).
Figure 4.
Model-based middle latency response (MLR) and late latency response (LLR) areas by study group show same trends after adjusting for sex and average distortion product otoacoustic emission (DPOAE) level. After statistical adjustment for sex and average DPOAE level from 3 to 8 kHz, the Veteran groups continue to show a reduction in the MLR area, but not in the LLR area, compared to the non-Veteran control group. (A) Model-based mean MLR and (B) LLR areas by stimulus level are indicated by symbol and color for each study group. MLR and LLR mean areas are plotted given male sex and an average DPOAE level from 3 to 8 kHz equal to the sample mean. Thin lines show the 90% Bayesian confidence interval, and thick lines represent the interquartile range.
In contrast, the Veteran Tinnitus group shows larger model-based mean LLR areas for a click stimulus than the other two groups (see Figure 4B). When averaged across level, the modeled mean LLR area for the Non-Veteran group is 9.5 μV/ms (90% confidence interval [32.7 μV/ms smaller, 6.2 μV/ms bigger]) smaller than the Veteran Tinnitus group and 2.9 μV/ms smaller than the Veteran No Tinnitus group (90% confidence interval [20.5 μV/ms smaller, 12.0 μV/ms bigger]). Over the 30- to 330-ms time course of the LLR, this is a mean increase in amplitude of 2,850 μV for the Veteran Tinnitus group compared to the Non-Veteran group (an 8.3% increase).
Using Bayesian analysis, rather than determining if an effect is conventionally statistically significant, we can quantify our certainty that the mean MLR or LLR area for one group is greater than the mean area for another group by evaluating the posterior probability distribution for the difference between the two groups and then taking the integral of the portion of the resulting probability distribution that is greater than zero. Table 2 shows the posterior probabilities for all group comparisons for the modeled MLR and LLR areas. The posterior probability that the MLR area is larger for the Non-Veteran group than for the Veteran No Tinnitus and Veteran Tinnitus groups is 68% and 63%, respectively. In contrast, there is an 82% posterior probability that the LLR area is smaller for the Non-Veteran group than for the Veteran Tinnitus group.
Table 2.
Posterior probabilities for modeled middle latency response (MLR) and late latency response (LLR) group contrasts.
| Variable | MLR | LLR |
|---|---|---|
| Non-Veteran – Veteran No Tinnitus | 68% | 37% |
| Non-Veteran – Veteran Tinnitus | 63% | 18% |
| Veteran No Tinnitus – Veteran Tinnitus | 44% | 26% |
| Veteran No Tinnitus – Non-Veteran | 32% | 63% |
| Veteran Tinnitus – Non-Veteran | 37% | 82% |
| Veteran Tinnitus – Veteran No Tinnitus | 56% | 74% |
Note. This table shows modeled posterior probabilities that the first study group has a greater MLR or LLR area in response to a click stimulus than the second study group, averaged across stimulus levels. Posterior probabilities are calculated by evaluating the posterior probability distribution for the difference between two groups and then taking the integral of the portion of the resulting probability distribution that is greater than zero.
The coefficient of the average DPOAE parameter in the model, ω, represents the effect size of DPOAE level on the MLR and LLR for any given study group, sex, or stimulus level. The value of ω is 0.20 μV/ms for the MLR (90% confidence interval [−0.17, 0.60 μV/ms]) and −0.66 μV/ms for the LLR (90% confidence interval [−2.51, 1.78 μV/ms]). In this data set, there is an average DPOAE level (from 3 to 8 kHz) difference of 3 dB between the Non-Veteran group and the Veteran Tinnitus group. To better conceptualize the DPOAE level effect size, we can calculate the impact of a 3-dB decrease in average DPOAE level on the MLR and LLR areas. When average DPOAE level decreases by 3 dB, the predicted mean MLR area decreases 0.6 μV/ms and the predicted mean LLR area increases 7.53 μV/ms, assuming sex and study group remain constant.
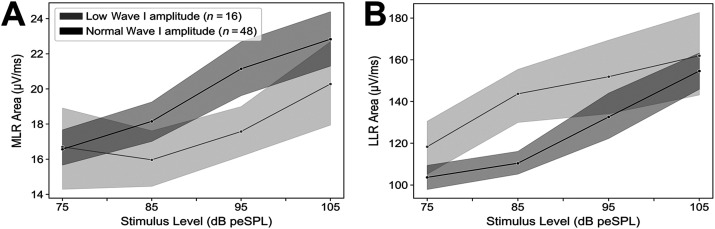
MLR Areas Are Reduced and LLR Areas Are Increased Among Participants With Low ABR Wave I Amplitudes
The ABR wave I amplitude in response to a 100–dB peSPL, 4-kHz tone burst for each participant was compared to the distribution of wave I amplitudes for the whole sample. Individuals with wave I amplitudes below the 25th percentile were labeled as having low wave I amplitudes, and participants with a wave I amplitude at or above this cutoff were classified as having a normal wave I amplitude. MLR area was reduced among individuals with low ABR wave I amplitudes (see Figure 5A), suggesting a relationship between the two measures. However, LLR area was increased for individuals with low ABR wave I amplitudes compared to those with normal wave I amplitudes (see Figure 5B).
Figure 5.
Middle latency response (MLR) area, but not late latency response (LLR) area, decreased in individuals with low ABR wave I amplitudes. (A) Mean MLR and (B) LLR areas are plotted by stimulus level for individuals with ABR wave I amplitudes (in response to a 100–dB peSPL, 4-kHz tone burst) above (gray) and below (black) the 25th percentile for the whole sample. Shaded regions indicate standard error of the mean.
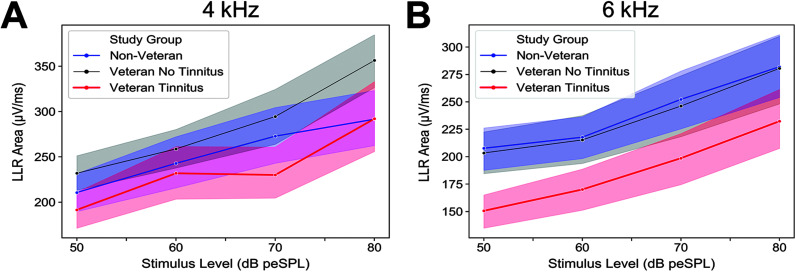
LLR Areas in Response to 4- and 6-kHz Pure Tones Are Smallest in Veterans With Tinnitus
In contrast to the click stimulus, mean LLR area was reduced in the Veteran Tinnitus group compared to the Non-Veteran and Veteran No Tinnitus groups for 4- and 6-kHz pure tones (see Figures 6A and 6B). This was evident across all four intensity levels. Mean LLR areas for the Non-Veteran and Veteran No Tinnitus groups were similar in response to 6-kHz pure tones and slightly larger in the Veteran No Tinnitus group for 4-kHz pure tones, particularly at 80 dB SPL.
Figure 6.
Veterans with tinnitus show reduced late latency response (LLR) area for 4- and 6-kHz tone bursts. Mean LLR area for (A) 4-kHz and (B) 6-kHz tone bursts are plotted by study group. Shaded regions indicate standard error of the mean.
Discussion
ABR Wave I Amplitudes Are Reduced in Groups With More Predicted Auditory Damage
A decrease in ABR wave I amplitude for a 4-kHz tone burst was observed for the two Veteran groups in comparison to the Non-Veteran group. This suggests a noise-related reduction in synaptic/neuronal function among the Veterans and is in agreement with our previous findings (Bramhall et al., 2018, 2017), although group differences are more modest than previously reported, potentially due to more relaxed DPOAE inclusion criteria in this study. In contrast, in agreement with previous findings (Bramhall et al., 2018), ABR wave V amplitudes are similar across groups. This suggests that reduced synaptic/neuronal function is associated with subcortical compensatory gain. This is consistent with mouse models of both age-related and noise-induced synaptopathy where ABR wave I amplitudes are reduced for mice with synaptopathy compared to control animals, but ABR wave V amplitudes are similar across groups (Hickox & Liberman, 2014; Sergeyenko et al., 2013).
MLR Areas Are Reduced in Groups With More Predicted Auditory Damage
The reduction in MLR area among Veterans compared to non-Veterans, given that the Veteran groups have higher self-reported lifetime noise exposure, suggests a noise exposure-related decrease in MLR area. OHCs and cochlear synapses are particularly vulnerable elements of the peripheral auditory system (Wu et al., 2018); therefore, this MLR area decrease is most likely a consequence of OHC and/or synaptic loss or dysfunction. Consistent with an impact of OHC damage, the mean model coefficients for average DPOAE level indicate that better DPOAEs are associated with increased MLR areas. However, the fact that the two Veteran groups still display decreased MLR areas compared to the Non-Veteran group even after statistical adjustment for differences in sex and high-frequency (3–8 kHz) DPOAE levels between the study groups suggests that cochlear synaptic loss also contributes to the reduction in MLR area. The observed reduction in MLR area for the two Veteran groups compared to the non-Veteran controls is somewhat surprising given that ABR wave V amplitude is similar across the three groups and animal models of selective IHC or neuronal loss show a progressive increase in the amount of compensatory central gain as a stimulus travels from the cochlear nucleus up through the auditory cortex (Chambers, Resnik, et al., 2016; Jiang et al., 2017; Salvi et al., 2016). However, the MLR results are consistent with the work of Jyung et al. (1989), which showed reductions in the slope of the electrically evoked MLR input/output function among guinea pigs with drug-induced spiral ganglion cell loss. This suggests that the MLR may be more sensitive to cochlear synaptic or neuronal loss than ABR wave V, even though it occurs at a later latency. This is easier to conceptualize by viewing the auditory system as a series of parallel pathways rather than a simple transmission line. It is important to remember that the MLR is a far-field recording of the response of multiple generators to an auditory stimulus and that these generators receive both excitatory and inhibitory inputs from a variety of sources, so it is difficult to directly compare the MLR to multi-unit recordings from the inferior colliculus or the auditory cortex in animals. In addition, although MLR area was reduced for the Veteran groups compared to the controls, this does not necessarily indicate the absence of central gain; it just demonstrates that not enough central gain has occurred to bring the MLR areas up to the level seen in controls. Without pre-exposure MLR data from the study participants, it cannot be determined whether their MLR areas have increased over time.
LLR Areas (in Response to a Click) Are Increased for the Group With the Most Predicted Auditory Damage
The fact that a reduction in MLR area was observed in both Veteran groups but no reduction was seen in the LLR area suggests that the Veteran groups both experienced compensatory central gain in response to the reduced peripheral input. In fact, the LLR area in the Veteran Tinnitus group appears to overshoot the normal level of activity seen in the Non-Veteran control group. This is similar to what was observed by Chambers, Resnik, et al. (2016) in a mouse model of near-complete cochlear synaptic loss induced by ouabain treatment, a Na/K pump inhibitor that specifically damages Type I spiral ganglion neurons. The pattern of pure-tone evoked neuronal activity they observed in the inferior colliculus for control mice versus mice 30 days post–drug treatment (see their Figure 2H) is very similar to the MLR results shown here for the non-Veterans versus Veterans, while firing in the auditory cortex (see their Figure 2I) resembles the LLR results by study group for this study. This suggests that subclinical noise-related peripheral auditory damage in the Veteran groups is associated with increased central gain at the level of the auditory cortex. A number of previous studies have provided evidence of an association between central gain and the perception of tinnitus (reviewed in Sedley, 2019). However, because increased gain was observed in this study among Veterans both with and without tinnitus, it is unlikely that this overactive response by itself is sufficient to induce tinnitus.
MLR Area and ABR Wave I Amplitude Appear to Be Related
The positive relationship observed between MLR area and ABR wave I amplitude suggests that both measures are sensitive to reductions in auditory nerve input. This is consistent with an observed reduction in electrically evoked MLR in a guinea pig model where spiral ganglion cells were damaged by treatment with a combination of kanamycin and ethacrynic acid (Jyung et al., 1989). In that model, the MLR reduction was proportional to the degree of spiral ganglion cell loss. This suggests that reductions in auditory nerve input persist at later stages of the auditory pathway. However, it is important to acknowledge that reductions in ABR wave I amplitude (and the MLR) could be caused by a number of factors including OHC loss, IHC loss, synaptic loss, neuronal loss or measurement error due to differences in electrode impedance, head size and anatomy, or sex. However, factors inducing measurement error in the MLR would be expected to have a similar impact on the LLR. For example, if males have smaller MLRs than females, they would also be expected to have smaller LLRs. Given that a similar reduction was not observed in the LLR, the reduced MLR area for the Veteran groups is most likely related to OHC loss, IHC loss, or synaptic/neuronal loss. However, given that the mean DPOAEs for the Veteran No Tinnitus group are nearly identical to those for the Non-Veteran control group, OHC loss alone is unlikely to explain the observed data.
LLR Area for 4- and 6-kHz Pure Tones Are Reduced in Veterans With Tinnitus
The reduction in LLR area for 4- and 6-kHz pure tones observed only in the Veteran Tinnitus group is surprising given that no reduction was seen for a click stimulus. One possible explanation for this finding is that the 4- and 6-kHz pure tones were less salient for participants with tinnitus because these frequencies are close to the perceived pitch of their tinnitus. Although no pitch matching was performed in this study, the majority of patients with tinnitus pitch match their tinnitus at or above 3 kHz (Meikle & Taylor-Walsh, 1984).
A number of studies have compared LLR amplitudes in individuals with and without tinnitus, with mixed results (reviewed in Sedley, 2019). The results of these studies are difficult to compare to the current study because of the inclusion of participants with hearing loss and because many of the studies used low-frequency (0.5 or 1 kHz) stimuli to avoid the region of hearing loss. It is also possible that other differences in the click and pure-tone stimuli, such as the rate, led to the observed differences in the LLR area, although it is unclear why this would impact the two Veteran groups differently.
Perceptual Consequences of Noise-Induced Peripheral Damage
In the Veteran No Tinnitus group, where a reduction in MLR area compared to the Non-Veteran group without a reduction LLR area indicates central gain, it is unclear whether there are any perceptual consequences of this hyperactivity. In this group, coding of a simple stimulus, such as a click, is not reduced at the level of the auditory cortex, suggesting that the central auditory system may be capable of compensating for the peripheral damage observed in the MLR response. However, the results of Chambers, Resnik, et al. (2016) suggest that this type of compensatory central gain may impede temporal coding. A previous study of a similar cohort of young Veterans and non-Veterans with normal audiograms did not find a correlation between ABR wave I amplitude and speech perception in noise as measured by the Words in Noise Test (Bramhall et al., 2018). However, this does not rule out the possibility that central gain resulting from synaptic or neuronal loss has negative consequences for complex speech perception in individuals with pure-tone thresholds outside the normal range, as suggested by the findings of Bramhall et al. (2015).
In the Veteran Tinnitus group, the LLR click results suggest that, in individuals with greater degrees of peripheral auditory damage, there are coding changes in the auditory cortex even in response to simple stimuli. The perceptual impact of this type of hyperactivity, particularly on speech perception, is unclear, although tinnitus is a likely consequence. Given that these observations were made in young people with clinically normal audiograms, this highlights the fact that subclinical noise-induced peripheral auditory damage can impact the central auditory system and auditory perception.
Limitations
Although cochlear synaptopathy has received a lot of attention as a source of auditory deficits “hidden” from the audiogram, noise-related OHC damage, as indicated by otoacoustic emissions, can also coexist with normal pure-tone thresholds (Desai et al., 1999; Hamdan et al., 2008; Marshall et al., 2009; Seixas et al., 2005). Subclinical OHC damage may alter ABR wave I amplitude (Verhulst et al., 2016) and can have functional consequences such as impacting speech-in-noise perception (Badri et al., 2011) or leading to the perception of tinnitus (Bramhall, McMillan, et al., 2019). This makes it difficult to separate out the relative impacts of OHC dysfunction and synaptic/neuronal loss. In this study, statistical adjustment for average DPOAE level from 3 to 8 kHz was used to illustrate that the observed effects of noise exposure were not simply a result of OHC damage, although OHC dysfunction does have an impact. The MLR/LLR model results suggest the presence of auditory dysfunction beyond the OHCs. Confirmation of where this dysfunction is occurring (i.e., IHCs, cochlear synapses, auditory nerve) is not currently possible without histological analysis, but human temporal bone studies suggest the IHCs are relatively resistant to age-related loss and the cochlear synapses are particularly vulnerable (Wu et al., 2018). This makes noise-related synaptic loss the more compelling explanation for the results presented here. However, as suggested by the modeled impact of average DPOAE level on MLR and LLR area, it is important to remember that the noise-related MLR and LLR differences observed in this study are likely not unique to synaptic loss but may generalize to any type of peripheral auditory damage.
Supplementary Material
Acknowledgments
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service Awards C2104-W (awarded to N. F. B.) and C9230-C (awarded to the National Center for Rehabilitative Auditory Research) and by National Institute on Deafness and Other Communication Disorders Grant R01DC015240 (awarded to C. J. B.). Research audiologist support was also provided by the Department of Defense Hearing Center of Excellence and zCore Business Solutions, Inc. The opinions and assertions presented are private views of the authors and are not to be construed as official or as necessarily reflecting the views of the Department of Veterans Affairs or the Department of Defense.
Funding Statement
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service Awards C2104-W (awarded to N. F. B.) and C9230-C (awarded to the National Center for Rehabilitative Auditory Research) and by National Institute on Deafness and Other Communication Disorders Grant R01DC015240 (awarded to C. J. B.).
References
- Badri R., Siegel J. H., & Wright B. A. (2011). Auditory filter shapes and high-frequency hearing in adults who have impaired speech in noise performance despite clinically normal audiograms. The Journal of the Acoustical Society of America, 129(2), 852–863. https://doi.org/10.1121/1.3523476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings C. J., Dillard L. K., Hoskins Z. B., Penman T. M., & Reavis K. M. (2018). A large-scale examination of veterans with normal pure-tone hearing thresholds within the Department of Veterans Affairs. Journal of the American Academy of Audiology, 29(10), 928–935. https://doi.org/10.3766/jaaa.17091 [DOI] [PubMed] [Google Scholar]
- Bramhall N., Beach E. F., Epp B., Le Prell C. G., Lopez-Poveda E. A., Plack C. J., Schaette R., Verhulst S., & Canlon B. (2019). The search for noise-induced cochlear synaptopathy in humans: Mission impossible? Hearing Research, 377, 88–103. https://doi.org/10.1016/j.heares.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Bramhall N. F., Konrad-Martin D., & McMillan G. P. (2018). Tinnitus and auditory perception after a history of noise exposure: Relationship to auditory brainstem response measures. Ear and Hearing, 39(5), 881–894. https://doi.org/10.1097/AUD.0000000000000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall N. F., Konrad-Martin D., McMillan G. P., & Griest S. E. (2017). Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function. Ear and Hearing, 38(1), e1–e12. https://doi.org/10.1097/AUD.0000000000000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall N., McMillan G., Gallun F., & Konrad-Martin D. (2019). Auditory brainstem response demonstrates that reduced peripheral auditory input is associated with self-report of tinnitus. The Journal of the Acoustical Society of America, 146(5), 3849 https://doi.org/10.1121/1.5132708 [DOI] [PubMed] [Google Scholar]
- Bramhall N., Ong B., Ko J., & Parker M. (2015). Speech perception ability in noise is correlated with auditory brainstem response wave I amplitude. Journal of the American Academy of Audiology, 26(5), 509–517. https://doi.org/10.3766/jaaa.14100 [DOI] [PubMed] [Google Scholar]
- Buran B. (2015, May. May). Auditory-wave-analysis: v1.1. Retrieved August 28, 2019, from https://zenodo.org/record/17365
- Chambers A. R., Resnik J., Yuan Y., Whitton J. P., Edge A. S., Liberman M. C., & Polley D. B. (2016). Central gain restores auditory processing following near-complete cochlear denervation. Neuron, 89(4), 867–879. https://doi.org/10.1016/j.neuron.2015.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A. R., Salazar J. J., & Polley D. B. (2016). Persistent thalamic sound processing despite profound cochlear denervation. Frontiers in Neural Circuits, 10, 72 https://doi.org/10.3389/fncir.2016.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Reed D., Cheyne A., Richards S., & Prasher D. (1999). Absence of otoacoustic emissions in subjects with normal audiometric thresholds implies exposure to noise. Noise Health, 1(2), 58–65. [PubMed] [Google Scholar]
- Eggermont J. J., & Roberts L. E. (2015). Tinnitus: Animal models and findings in humans. Cell and Tissue Research, 361(1), 311–336. https://doi.org/10.1007/s00441-014-1992-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formby C., La Guinn P. S., Gold S. L., & Hawley M. L. (2007). Adaptive recalibration of chronic auditory gain. Seminars in Hearing, 28(4), 295–302. https://doi.org/10.1055/s-2007-990716 [Google Scholar]
- Fulbright A. N. C., Le Prell C. G., Griffiths S. K., & Lobarinas E. (2017). Effects of recreational noise on threshold and suprathreshold measures of auditory function. Seminars in Hearing, 38(4), 298–318. https://doi.org/10.1055/s-0037-1606325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. S., Griest S. E., Thielman E. J., Carlson K. F., Helt W. J., Lewis M. S., Blankenship C., Austin D., Theodoroff S. M., & Henry J. A. (2017). Audiologic characteristics in a sample of recently-separated military veterans: The noise outcomes in service members epidemiology study (NOISE Study). Hearing Research, 349, 21–30. https://doi.org/10.1016/j.heares.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Gorga M. P., Neely S. T., Ohlrich B., Hoover B., Redner J., & Peters J. (1997). From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear and Hearing, 18(6), 440–455. https://doi.org/10.1097/00003446-199712000-00003 [DOI] [PubMed] [Google Scholar]
- Grinn S. K., Wiseman K. B., Baker J. A., & Le Prell C. G. (2017). Hidden hearing loss? No effect of common recreational noise exposure on cochlear nerve response amplitude in humans. Frontiers in Neuroscience, 11, 465 https://doi.org/10.3389/fnins.2017.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose J. H., Buss E., & Hall J. W. III (2017). Loud music exposure and cochlear synaptopathy in young adults: Isolated auditory brainstem response effects but no perceptual consequences. Trends in Hearing, 21 2331216517737417 https://doi.org/10.1177/2331216517737417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J. W., Herrmann B. S., Levine R. A., & Melcher J. R. (2012). Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. Journal of the Association for Research in Otolaryngology, 13(6), 819–833. https://doi.org/10.1007/s10162-012-0344-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan A. L., Abouchacra K. S., Zeki Al Hazzouri A. G., & Zaytoun G. (2008). Transient-evoked otoacoustic emissions in a group of professional singers who have normal pure-tone hearing thresholds. Ear and Hearing, 29(3), 360–377. https://doi.org/10.1097/AUD.0b013e31816a0d1e [DOI] [PubMed] [Google Scholar]
- Hickox A. E., Larsen E., Heinz M. G., Shinobu L., & Whitton J. P. (2017). Translational issues in cochlear synaptopathy. Hearing Research, 349, 164–171. https://doi.org/10.1016/j.heares.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox A. E., & Liberman M. C. (2014). Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? Journal of Neurophysiology, 111(3), 552–564. https://doi.org/10.1152/jn.00184.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff P. J. (1990). Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neuroscience Research, 8(4), 221–254. https://doi.org/10.1016/0168-0102(90)90031-9 [DOI] [PubMed] [Google Scholar]
- Jiang C., Luo B., Manohar S., Chen G. D., & Salvi R. (2017). Plastic changes along auditory pathway during salicylate-induced ototoxicity: Hyperactivity and CF shifts. Hearing Research, 347, 28–40. https://doi.org/10.1016/j.heares.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyung R. W., Miller J. M., & Cannon S. C. (1989). Evaluation of eighth nerve integrity by the electrically evoked middle latency response. Otolaryngology—Head & Neck Surgery, 101(6), 670–682. https://doi.org/10.1177/019459988910100610 [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D., Dille M. F., McMillan G., Griest S., McDermott D., Fausti S. A., & Austin D. F. (2012). Age-related changes in the auditory brainstem response. Journal of the American Academy of Audiology, 23(1), 18–35. https://doi.org/10.3766/jaaa.23.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G., & Liberman M. C. (2009). Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. Journal of Neuroscience, 29(45), 14077–14085. https://doi.org/10.1523/JNEUROSCI.2845-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G., & Liberman M. C. (2015). Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Research, 330(Pt. B), 191–199. https://doi.org/10.1016/j.heares.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell C. G. (2019). Effects of noise exposure on auditory brainstem response and speech-in-noise tasks: A review of the literature. International Journal of Audiology, 58(Suppl. 1), S3–S32. https://doi.org/10.1080/14992027.2018.1534010 [DOI] [PubMed] [Google Scholar]
- Lin H. W., Furman A. C., Kujawa S. G., & Liberman M. C. (2011). Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. Journal of the Association for Research in Otolaryngology, 12(5), 605–616. https://doi.org/10.1007/s10162-011-0277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L., Lapsley Miller J. A., Heller L. M., Wolgemuth K. S., Hughes L. M., Smith S. D., & Kopke R. D. (2009). Detecting incipient inner-ear damage from impulse noise with otoacoustic emissions. The Journal of the Acoustical Society of America, 125(2), 995–1013. https://doi.org/10.1121/1.3050304 [DOI] [PubMed] [Google Scholar]
- Martin B. A., Tremblay K. L., & Korczak P. (2008). Speech evoked potentials: From the laboratory to the clinic. Ear and Hearing, 29(3), 285–313. https://doi.org/10.1097/AUD.0b013e3181662c0e [DOI] [PubMed] [Google Scholar]
- McGee T., & Kraus N. (1996). Auditory development reflected by middle latency response. Ear and Hearing, 17(5), 419–429. https://doi.org/10.1097/00003446-199610000-00008 [DOI] [PubMed] [Google Scholar]
- McMillan G. P., & Cannon J. B. (2019). Bayesian applications in auditory research. Journal of Speech, Language, and Hearing Research, 62(3), 577–586. https://doi.org/10.1044/2018_JSLHR-H-ASTM-18-0228 [DOI] [PubMed] [Google Scholar]
- Meikle M., & Taylor-Walsh E. (1984). Characteristics of tinnitus and related observations in over 1800 tinnitus clinic patients. The Journal of Laryngology & Otology, 98(S9), 17–21. https://doi.org/10.1017/S1755146300090053 [DOI] [PubMed] [Google Scholar]
- Naatanen R., & Picton T. (1987). The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology, 24(4), 375–425. https://doi.org/10.1111/j.1469-8986.1987.tb00311.x [DOI] [PubMed] [Google Scholar]
- Neely S., & Liu Z. (1993). EMAV: Otoacoustic emission averager (Tech Memo No. 17). Boys Town National Research Hospital. [Google Scholar]
- O'Beirne G. A., & Patuzzi R. B. (1999). Basic properties of the sound-evoked post-auricular muscle response (PAMR). Hearing Research, 138(1–2), 115–132. https://doi.org/10.1016/S0378-5955(99)00159-8 [DOI] [PubMed] [Google Scholar]
- Prendergast G., Guest H., Munro K. J., Kluk K., Leger A., Hall D. A., Heinz M. G., & Plack C. J. (2017). Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hearing Research, 344, 68–81. https://doi.org/10.1016/j.heares.2016.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R., Sun W., Ding D., Chen G. D., Lobarinas E., Wang J., Radziwon K., & Auerbach B. D. (2016). Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Frontiers in Neuroscience, 10, 621 https://doi.org/10.3389/fnins.2016.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R., & McAlpine D. (2011). Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. Journal of Neuroscience, 31(38), 13452–13457. https://doi.org/10.1523/JNEUROSCI.2156-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt R. A., Mills J. H., & Boettcher F. A. (1996). Age-related loss of activity of auditory-nerve fibers. Journal of Neurophysiology, 76(4), 2799–2803. https://doi.org/10.1152/jn.1996.76.4.2799 [DOI] [PubMed] [Google Scholar]
- Sedley W. (2019). Tinnitus: Does gain explain? Neuroscience, 407, 213–228. https://doi.org/10.1016/j.neuroscience.2019.01.027 [DOI] [PubMed] [Google Scholar]
- Seixas N., Goldman B., Sheppard L., Neitzel R., Norton S., & Kujawa S. (2005). Prospective noise induced changes to hearing among construction industry apprentices. Occupational and Environmental Medicine, 62(5), 309–317. https://doi.org/10.1136/oem.2004.018143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y., Lall K., Liberman M. C., & Kujawa S. G. (2013). Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. The Journal of Neuroscience, 33(34), 13686–13694. https://doi.org/10.1523/JNEUROSCI.1783-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper G. C., & Johnson T. A. (2015). Auditory function in normal-hearing, noise-exposed human ears. Ear and Hearing, 36(2), 172–184. https://doi.org/10.1097/AUD.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A. A., Nelson J. T., Swiger B., Jaramillo C. A., Eapen B. C., Packer M., & Pugh M. J. (2017). Prevalence of hearing loss and tinnitus in Iraq and Afghanistan Veterans: A chronic effects of neurotrauma consortium study. Hearing Research, 349, 4–12. https://doi.org/10.1016/j.heares.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Tremblay K. L., Pinto A., Fischer M. E., Klein B. E., Klein R., Levy S., Tweed T. S., & Cruickshanks K. J. (2015). Self-reported hearing difficulties among adults with normal audiograms: The beaver dam offspring study. Ear and Hearing, 36(6), e290–e299. https://doi.org/10.1097/AUD.0000000000000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderrama J. T., Beach E. F., Yeend I., Sharma M., Van Dun B., & Dillon H. (2018). Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility. Hearing Research, 365, 36–48. https://doi.org/10.1016/j.heares.2018.06.003 [DOI] [PubMed] [Google Scholar]
- Valero M. D., Burton J. A., Hauser S. N., Hackett T. A., Ramachandran R., & Liberman M. C. (2017). Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hearing Research, 353, 213–223. https://doi.org/10.1016/j.heares.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S., Jagadeesh A., Mauermann M., & Ernst F. (2016). Individual differences in auditory brainstem response wave characteristics: Relations to different aspects of peripheral hearing loss. Trends in Hearing, 20, 2331216516672186 https://doi.org/10.1177/2331216516672186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana L. M., O'Malley J. T., Burgess B. J., Jones D. D., Oliveira C. A., Santos F., Merchant S. N., Liberman L. D., & Liberman M. C. (2015). Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hearing Research, 327, 78–88. https://doi.org/10.1016/j.heares.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. Z., Liberman L. D., Bennett K., de Gruttola V., O'Malley J. T., & Liberman M. C. (2018). Primary neural degeneration in the human cochlea: Evidence for hidden hearing loss in the aging ear. Neuroscience, 407, 8–20. https://doi.org/10.1016/j.neuroscience.2018.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeend I., Beach E. F., Sharma M., & Dillon H. (2017). The effects of noise exposure and musical training on suprathreshold auditory processing and speech perception in noise. Hearing Research, 353, 224–236. https://doi.org/10.1016/j.heares.2017.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.