Abstract
Neuroblastoma (NB) is a deadly childhood disease that carries a 50% chance of relapse for anyone in remission and similar level of 5-year survival. We investigated the value of our proprietary approach – cell surface vimentin (CSV) positive circulating tumor cells (CTC) to monitor treatment response and predict relapse in NB patients under remission in a Phase II long-term preventative clinical trial. We longitudinally analyzed peripheral blood samples from 93 patients for 27 cycles (~25 months) and discovered that presence of CSV+ CTCs in the first two sequential samples (baseline, cycle 4 [month 3–4]) was a significant indicator of earlier relapse. We observed strong correlation between relapse-free survival (RFS) and lack of CSV+ CTCs in first 4 cycles of therapy (95%). There was sensitivity reaching 100% in predicting RFS in patients who had neither CSV+ CTCs nor MycN amplification. Of note, the low number of CSV+ CTCs seems equivalent to low tumor load because the prevention therapy difluoromethylornithine yields faster reduction of relapse risk when none or only 1–2 CSV+ CTCs (every 6 ml) are present in the blood samples compared to > 3 CSV+ CTCs. To the best of our knowledge, this is the first study that directly observes CTCs in under remission NB patients for relapse prediction and the first to gather sequential CSV+ CTC data in any study in a long-term longitudinal manner.
Keywords: Circulating tumor cell, neuroblastoma, relapse, difluoromethylornithine, liquid biopsies
Introduction
Neuroblastoma (NB) is an aggressive childhood tumor and high-risk NB patients have a 50% chance of suffering relapse post treatment1. The ability of NB cells to be targeted by anti-GD2 antibodies, which has become the standard of care, has improved survival and decreased likelihood of relapse2. However, tumor cells counteract these efforts by hiding in sanctuary sites (CNS, lymph nodes, and bone marrow) to cause relapse, making early detection of relapse an important task3–6.
Current means to screen for tumor relapse come with drawbacks. Imaging-based approaches such as the most commonly used iodine-123 meta-iodobenzylguanidine (MIBG) are very helpful in detecting NB metastasis, but the detection is late7. Alternatively, bone marrow biopsy-based metastasis or relapse screening assay is a relatively invasive procedure. An ideal approach will both minimize the drawbacks and detect early. Towards the early detection goal, circulating tumor cells (CTCs) have been demonstrated in multiple retrospective and prospective studies to be instrumental in predicting relapse/recurrence in various types of cancers8–12. Though there is an existing study into the role of CTCs in NB, there is otherwise a dearth of research in this field. In this study, Burchill et al. examined the role of circulating NB cells in children with NB at all stages. The investigators used tyrosine hydroxylase (TH) mRNA as an indirect metric for CTCs but did not directly observe these cells in the blood.13 They were able to use TH mRNA as it is a protein found only in dopaminergic neurons, which is not otherwise found in the blood of healthy patients. Our study seeks to prove a conclusive link between CTCs, specifically CSV+ CTCs, and NB relapse.
The role of cell surface vimentin (CSV) positive CTCs has not been investigated in NB. This CSV+ CTC analysis is important because CSV positive tumor cells were found to be associated with metastasis in other tumor models and CSV+ CTC number is positively correlated with metastasis (e.g. 70 to 100% metastatic colon tumor cells are CSV positive)12,14. The majority of circulating cell research in the context of NB has focused on immune cells’ (natural killer, and T-regulatory) surface expression of selected proteins or correlating levels with tumor status15,16. Current research has shown that difluoromethylornithine (DFMO) inhibits the metastatic process in NB by targeting ornithine decarboxylase, an important enzyme in the process by which bulk tumor cells become cancer stem cells, possibly preventing the development of CTCs, thereby presenting a potential therapeutic modality that targets relapse.17
The presence of vimentin on the surface of cells has been previously shown to be restricted to tumor cells, especially metastatic tumor cells14,18,19. Our internally-developed antibody is specific to CSV and has been demonstrated to be highly instrumental in detecting CTCs in tumors; CSV has demonstrated great utility in predicting chemoresistance in breast and prostate cancers18–20. Based on these published results, we hypothesized that CSV-based CTC detection can be a viable approach for detecting tumor relapse in NB using freshly-drawn patient blood. The results as presented in this report show the CSV method was highly sensitive in NB for predicting relapse. Absence of CSV+ CTCs at baseline or the first two sequential samples correlated with prolonged survival. A high CSV+ CTC count at baseline requires many more cycles of therapy to prevent relapse.
Materials and Methods:
Cell Culture.
Cell line CHP-134 (RRID:CVCL_1124) was obtained from the COG Cell Culture and Xenograft Repository and cultured as recommended. This cell line has been authenticated by DNA profiling within the last 3 years. All experiments were performed with mycoplasma-free cells.
Blood collection and processing.
Human NB patient samples were delivered via overnight shipments from hospitals and clinical institutions participating in the phase II clinical trial “Preventative Trial of DFMO in Patients with High Risk Neuroblastoma in Remission” - NCT01586260. Eligibility criteria included histologically confirmed diagnosis of neuroblastoma with high risk disease according to the International Neuroblastoma Risk Group Classification.21 MycN amplification and histological grade (F=favorable; UF=unfavorable) was derived from the patients’ medical record. MycN status was determined by cytogenetically using MYCN FISH analysis.22 Histological grading was performed by analysis of H&E stained slides in accordance with International Neuroblastoma Pathology Classification standards.23 Some patients were disqualified due to time restrictions associated with sample delivery and/or a patient’s inability to meet all the inclusion criteria.
CSV+ CTC Isolation and Immunofluorescence staining.
The method for CSV+ CTC isolation and immunofluorescence staining has been previously described18 and revised in a recent publication24. Briefly, whole blood was subject to gradient centrifugation. The buffy coat was then extracted. CD45+ cells were depleted using an EasySep human CD45 depletion kit (StemCell Technologies) according to the manufacturer’s recommendation. To minimize nonspecific binding, antibody against human Fc receptor (Miltenyi Biotec) was added to the cocktail. Second, the CD45− cell fraction was subjected to 84–1+ selection. Cells were labeled with the 84–1 anti-vimentin antibody (MD Anderson), and later mouse IgG-binding microbeads (Miltenyi Biotec) were added to the mixture. 84–1+ Cells were then extracted using the magnetic column according to the manufacturer’s recommendation (Miltenyi Biotec). The cells thus obtained were 84–1+ and CD45− and ready for further analysis. These cells were then fixed in 4% PFA, blocked with 0.5% FBS, and stained for 84–1 (MD Anderson) and CD45 at a concentration of 1:100 at 4° C overnight for both. Cells were then imaged on a Zeiss LSM 510 confocal microscope at a magnification of 63x using LSM5 image capture and analysis software (Zeiss, Oberkochen, Germany).
Statistical analysis.
All statistical analyses were performed using software GraphPad Prism 6 (San Diego, CA) and R v3.3.1 (Vienna, Austria) with packages lme4 v1.1–12, reshape v0.8.5, survival v2.39–4 and survminer v0.4.3. P values less than 0.05 were considered significant. The average spiking cell recovery data were expressed as the mean ± standard deviation and representative results were from at least three independent experiments. Relapse-free survival was defined as the time from disease-free (Start Date) to relapse, and was censored by death due to other cause or last follow-up, whichever occurred first. Kaplan-Meier estimates of relapse-free survival rate is plotted as a function of time. The CTC counts were categorized as 0 versus > 0 for baseline and for the first four months (baseline to 4 month). Log-rank test is applied to evaluate the difference of relapse-free survival between different groups. The correlation between spiking cell count and recovered cell count, as well as reproducibility of CTC measurements, was assessed using linear regression.
Results
Neuroblastoma cells express cell surface vimentin (CSV) and can be specifically isolated from spiked blood samples.
To determine the feasibility of using CSV as a means of isolating CTCs, CSV expression in normal PBMCs, normal bone marrow cells, and endothelial cells as well as many types of tumor cells were analyzed previously; the results exclusively have confirmed the CSV-specific expression on tumor cells alone14,18,25,26. Of note, the CSV-based CTC capture from sarcoma patients found the expected genetic changes18. To further support our prior finding in the new context of NB, the representative NB cell line CHP-134 was stained for CSV. We observed definitive CSV expression in a subpopulation of NB cells (Fig. 1a, 1b). This result is consistent with previous discoveries in other tumor cell lines, in which 3–40% of cells in a given cell line express CSV14,19,27. These results, combined with the fact that CSV is highly abundant in relapsed and metastatic tumor cells in other cancers, demonstrate that CSV may be a reliable biomarker for predicting relapse/metastasis.14,18,19 Indeed, CSV+ CTC number is associated with metastasis status in sarcoma18. These pre-analytical results allowed us to analyze human NB patients’ peripheral blood samples for CTCs from a multiple center trial.
Figure 1.
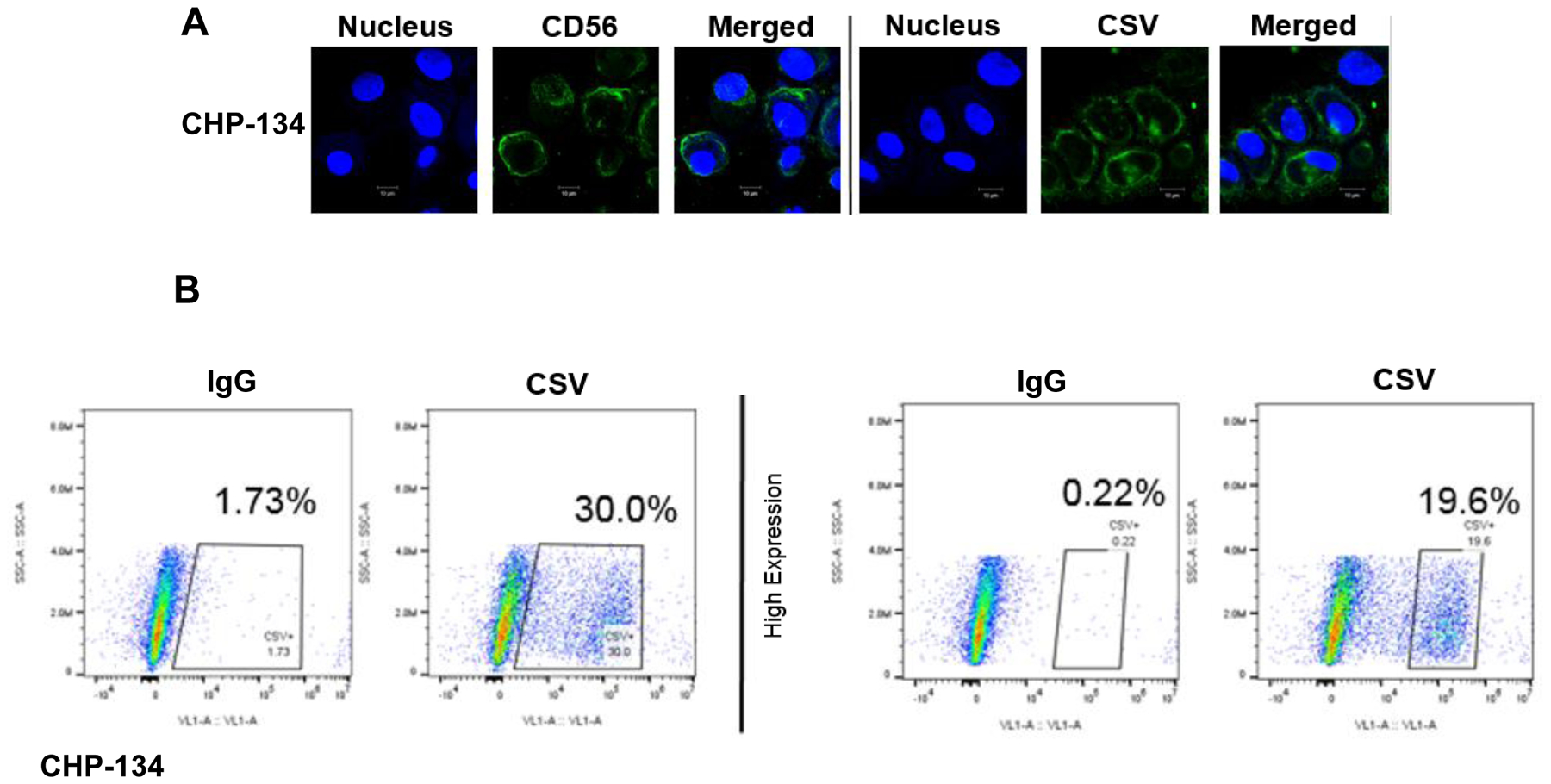
Cell line staining and CSV validation. a Neuroblastoma cell line CHP-134 stained for the neuronal marker CD56 (ThermoFisher Scientific, USA), and CSV (MD Anderson, USA) and nucleus (DRAQ5). b Flow cytometry analysis for CSV is shown in panel b. 50,000 events were captured in duplicate.
CSV+ CTC detection strategy in high-risk Neuroblastoma patients in remission.
The phase II study using Difluoromethylornithine (DFMO) aims to evaluate the preventative activity of DFMO in high risk NB patients that are in remission (NCT01586260). Patients received 27 cycles (28 days per cycle) of oral DFMO at a dose of 500 to 1000mg/m2. Treatments were given once a week for the first 3 weeks followed by rest for 1 week prior to initiation of next cycle. Patient dosage regime has been optimized based on previous research showing greatest efficacy with the current schedule28,29. Each patient stays on trial for 25 months unless they relapse, at which point the patient is removed from the trial. Freshly drawn blood from 93 patients at 3–4 cycle intervals (3–4 months) was processed using the CSV+ CTC capture method described in previous publication, as indicated by the heavy arrows in Fig. 2a18,19.
Figure 2.
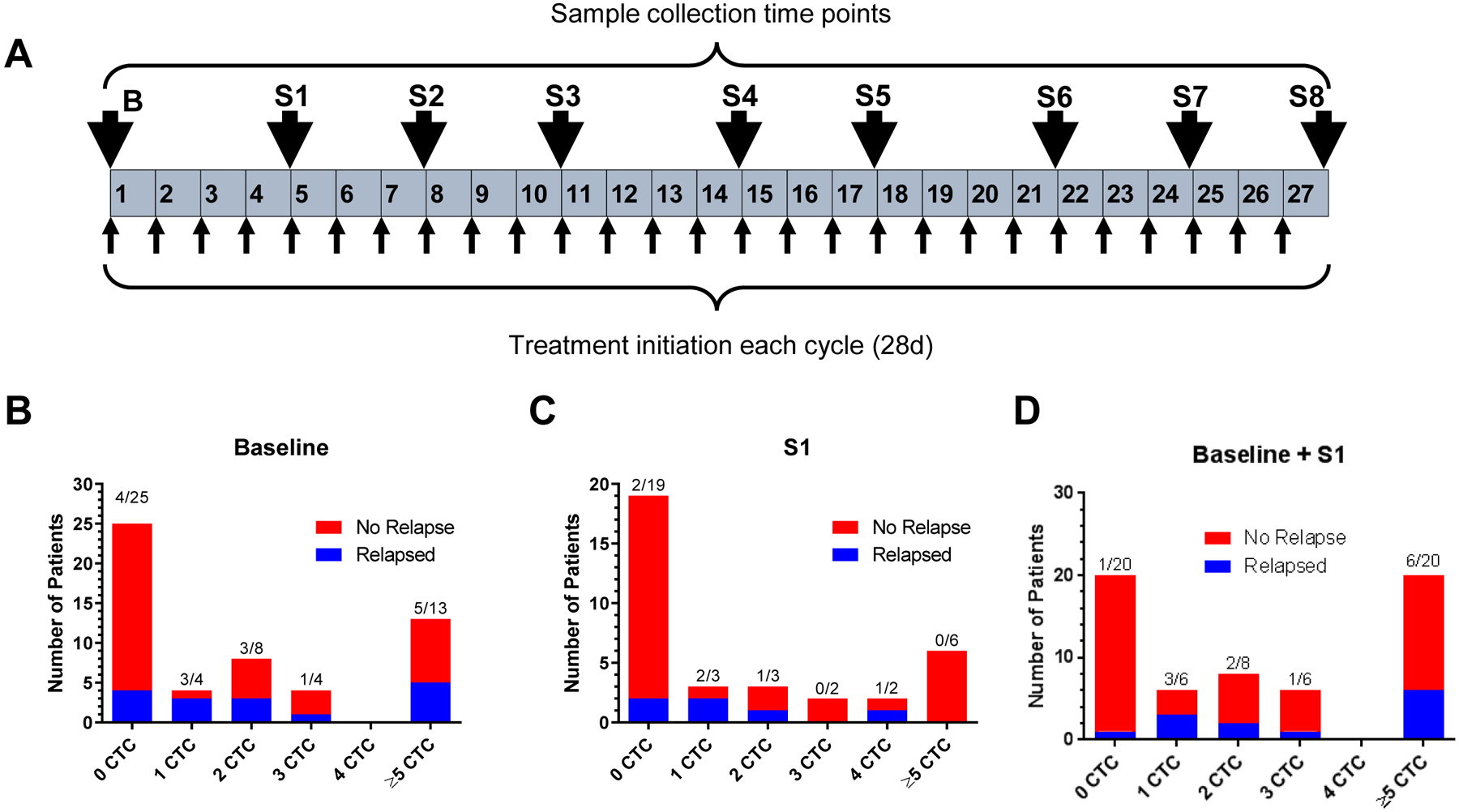
Study outline and early relapse. Panel a shows the study outline containing 27 cycles (28 days per cycle) of DFMO therapy. Blood samples were collected at 3–4 cycle intervals as shown by the arrows indicating the most frequent time points at which samples were received. In each cycle, treatments were given once a week for the first 3 weeks followed by rest for 1 week prior to initiation of next cycle. Panels b, c, and d illustrate the number of patients who relapsed within 25 months, respective to the number CTCs detected at baseline (b) based on the consented patients whose samples were available at baseline (n=54), S1 (c) based on the consented patients whose samples were available at S1 (n=34), or those who maintained the indicated CTC levels through first 2 samples (d) based on the patients whose samples were available at either baseline or S1 (n=60), respectively.
We chose this format of serial blood sample collection every 3–4 cycles (3–4 months) due to the long-term longitudinal nature of our project (> 2 years) as well as the multi-center collaboration, which would make the task of processing every sample after each cycle (1 month) too monumental. In contrast to other studies, we analyzed up to 9 samples from a single patient though there were some cases of disqualified samples, or patients under remission or missing clinical visits. As evident from the numbers provided, not all patients were able to have been consented for blood draw at baseline. The first sample (baseline) was received prior to the initiation of any treatment from patients who are in remission for < 2 months (labeled Baseline or “B”, Fig. 2a). Subsequent samples collected at indicated points in Fig. 2a are denoted as S1 starting at Cycle 4 (4 months) and progressively increase thereafter. As shown in Table 1, the majority of patients were under 10 years old. Similarly, all relapsed patients were also 10 years old or younger (Table 1).
Table 1.
Patient Characteristics and Relapse Information. Correlation between age and relapse status given by Fisher exact test.
| Characteristics | Number of patients (n=93) |
|---|---|
| Age (years) | |
| Median | 4 |
| Range | <1–17 |
| Age < 10 | 85 (91%) |
| Age ≥ 10 | 8 (9%) |
| Sex | |
| Male | 57 (61%) |
| Female | 36 (39%) |
| Race/ethnicity | |
| Non-Hispanic White | 66 (71%) |
| Non-Hispanic Black | 7 (7%) |
| Non-Hispanic Other | 11 (12%) |
| Hispanic | 9 (10%) |
| Relapse within 25 months | |
| By Age | p = .38 |
| Relapse-Free | 76 (82%) |
| Age ≤ 2 | 7 (9%) |
| Age > 2 | 69 (91%) |
| Relapsed | 17 (18%) |
| Age ≤ 2 | 3 (18%) |
| Age > 2 | 14 (82%) |
Early detection of CSV+ CTCs in NB patients is associated with relapse.
To determine the long-term longitudinal relevance of CSV-based CTC isolation, we investigated the rates of relapse in patients who did or did not have any detectable CSV+ CTCs across different treatment cycles, up to 9 sample collections once every 3–4 months; baseline samples were collected prior to initiation of therapy and all other samples were collected at the end of the labeled cycle number before starting the next cycle of therapy (Fig. 2a). As shown in Fig. 2b, and c, the vast majority of patients who had 0 CSV+ CTCs at baseline ‘B’ or the immediate subsequent sample ‘S1’ did not suffer relapse and are tumor free—88% (Fig. 2b and 2c). Of note, only 1 out of 20 patients who are absent CSV+ CTCs at both baseline and S1 relapsed and the rest of 19 are tumor free – 95% (Fig. 2d).
There was a statistically significant difference in relapse-free survival between patient population who are absent of CSV+ CTC at both baseline (B) and the immediate sequential sample (S1, 95% survival rate) and the population who are present of CSV+ CTC at either of these two sampling time point (61% survival rate, p=0.018, Fig. 3b). This p value was inferior (Fig. 3a, p=0.042, 83% relapse free survival rate vs. 55% relapse free survival rate), suggesting the combination of B and S1 are more reliable predictor. Of note, the well documented high relapse risk factor MycN amplification failed completely to predict relapse (Supplemental Fig. 1). Indeed, the two relapse free survival curves (MycN amplification positive, 82% relapse free survival rate, and negative, 73% relapse free survival rate) show highly similar outcomes. This may possibly be a result of the DFMO treatment, as inhibiting ornithine decarboxylase has been shown to impair development of MycN-amplified NB.30 Likewise, favorable or unfavorable histology readings also failed to make any substantive predictions as the survival probability was 80% and 82% for favorable as opposed to unfavorable histology readings at 25 months (Supplemental Fig. 2). Interestingly, a lack of both MycN amplification and CSV+ CTCs in the first two samples yields 100% prediction of relapse free survival, whereas presence of both CSV+ CTCs and MycN amplification yields a 74% prediction of relapse free survival, no CSV+ CTCs and presence of MycN amplification yields a 90% prediction of relapse free survival and presence of CSV+ CTCs and lack of MycN amplification yields a 54% prediction of relapse free survival. (Fig. 3c).
Figure 3.
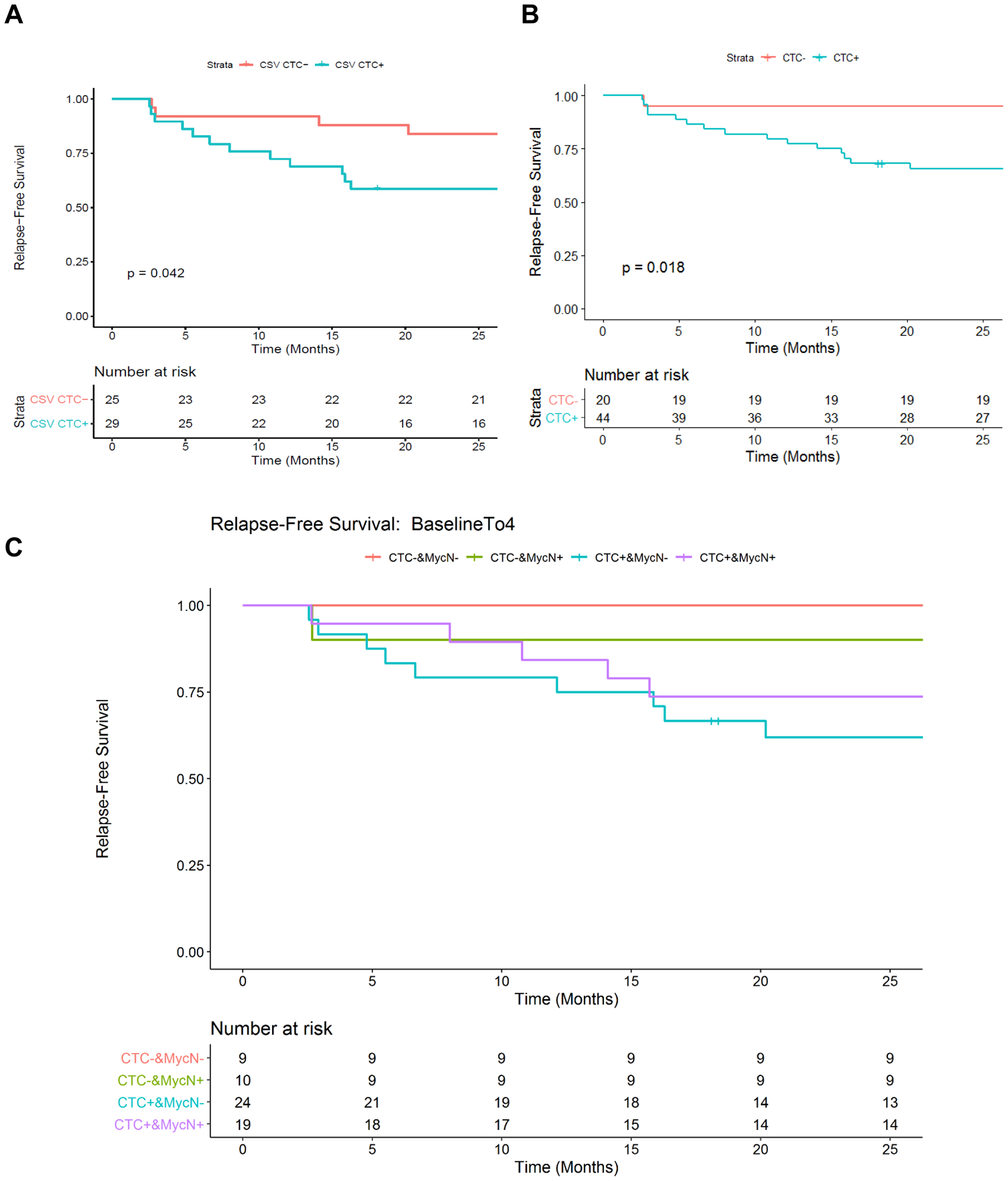
Relapse-free survival prediction based on CTC and MycN amplification status. Panels a and b show the relapse-free survival difference between patients with or without detectable CTCs at baseline (a) or between patients who were or were not CTC from baseline through first 4 months (b). Pairwise comparison between different groups was conducted. Significant difference in relapse-free survival was observed between CTC-&MycNAmp- and CTC+&MycNAmp- groups (p = 0.041) without adjusting for multiple testing correction (c).
Longitudinal CSV+ CTCs analysis for monitoring treatment response.
To monitor treatment response using CSV+ CTCs, we compared the CSV+ CTC detection rates at each time point and their relevance to the number of relapses observed whenever samples are available from the enrolled 93 patients. In that regard, this study was designed to analyze periodic samples that total 9 per patient throughout the course of therapy. This enabled us to glean a greater resolution of CTC status to better interpret therapeutic response to maintenance therapy and tumor status. The lack of CSV+ CTCs strongly correlated with the absence of relapse at every sampling time point (Fig. 4a).
Figure 4.
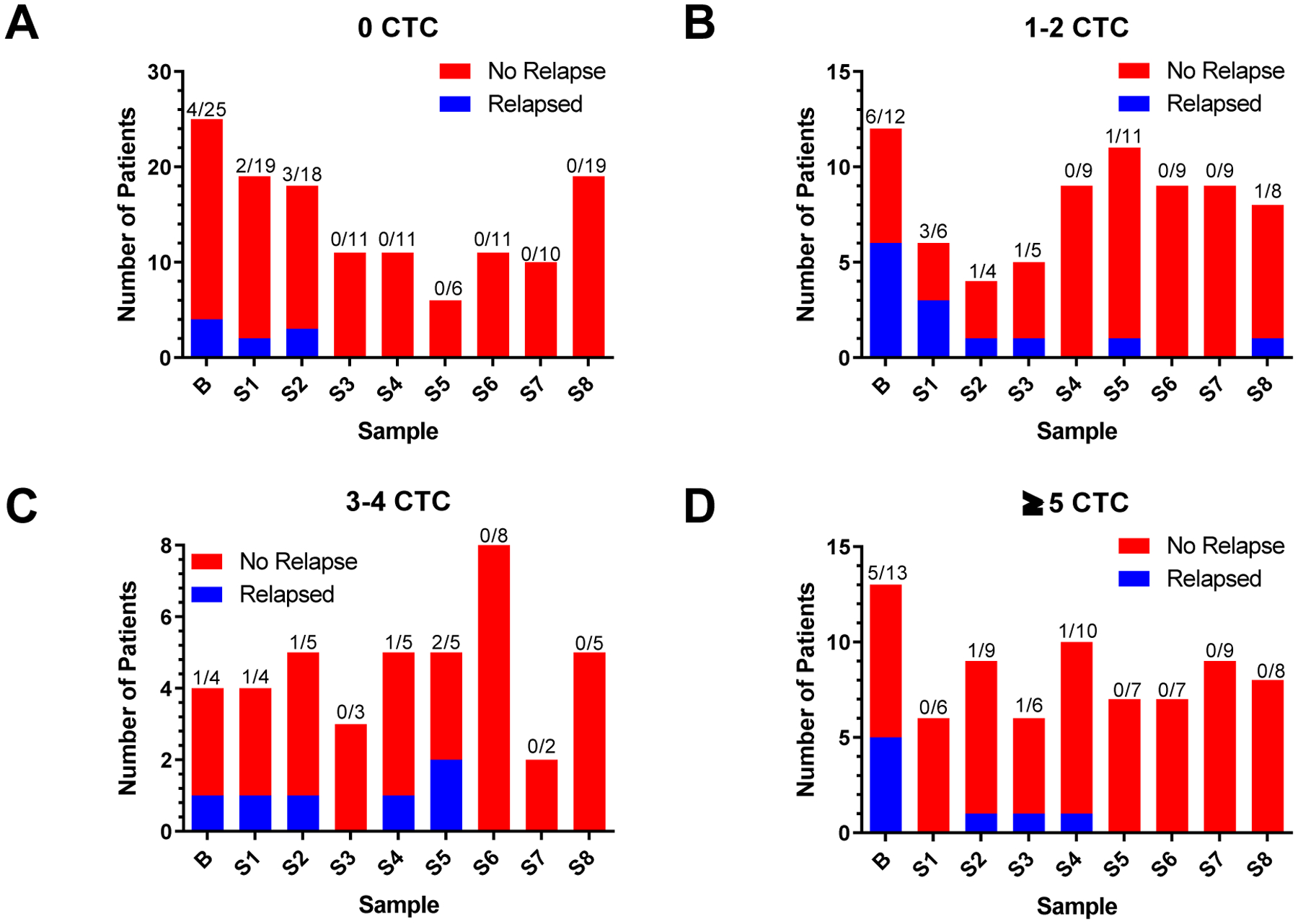
Treatment monitoring in pool of patients. Each panel represents the number of patients that relapsed or not. Panels differ based on number of detected CTCs in each pool as indicated in the title of each graph. a Number of patients with 0 CTC who did or did not relapse at the indicated cycles. b Number of patients with 1 to 2 CTCs who did or did not relapse at the indicated cycles. c Number of patients with 3 to 4 CTCs who did or did not relapse at the indicated cycles. d Number of patients with ≥5 CTCs who did or did not relapse at the indicated cycles. B = Baseline. A Fisher exact test was performed on the B + S1 populations, p = 0.0278
It is especially interesting that our data in Fig. 4 shows that in patients with 0 CSV+ CTCs there was no relapse after 7 cycles of therapy (S2) (Fig. 4a). Similarly, in patients with 1–2 CSV+ CTCs after S3 were 96% relapse-free (Fig. 4b). This apparent threshold for no relapse is delayed in patients with 3–4 CTCs to S5 and later (Fig. 4c). Similar prolonging of this effect is observed in those with ≥5 CTCs to cycles 21 onwards (Fig. 4d). This result suggests that a low number of CSV+ CTCs (0–2) seems equivalent to low tumor load, so this group of patients may be responding to DFMO treatment faster, resulting in complete response. Overall, a lower CSV+ CTC count correlates with decreased chances of suffering relapse (Fig. 4a, 4b).
CSV-guided CTC detection may contribute to deeper personalization of therapy.
While the data presented thus far indicates that early stage CTC detection may be key to predicting relapse, we also noticed long-term longitudinal trends in CTC detection profiles of some patients. As shown in Fig. 5a, 5b, and 5c, three relapse-free survival patients showed a consistent low CTC count. Whereas in CTC profiles of patients in Fig. 5d, and 5e there is an inability to maintain a long-term suppression of CTC counts after initial decrease (Fig. 5d) or an ongoing struggle with an oscillating pattern observed in CTC trends (Fig. 5e). Both of these patients (Fig. 5d, 5e) eventually relapsed at a later date after the end of maintenance therapy as indicated in red. Noticeably in Fig. 5c, a patient who was CTC-free until cycle 14 did not relapse, while in contrast a patient who showed a steady decline in CTC numbers through the course of therapy still suffered relapse (Fig. 5f) indicating the importance of baseline and early cycle CTC count in predicting relapse and treatment response.
Figure 5.
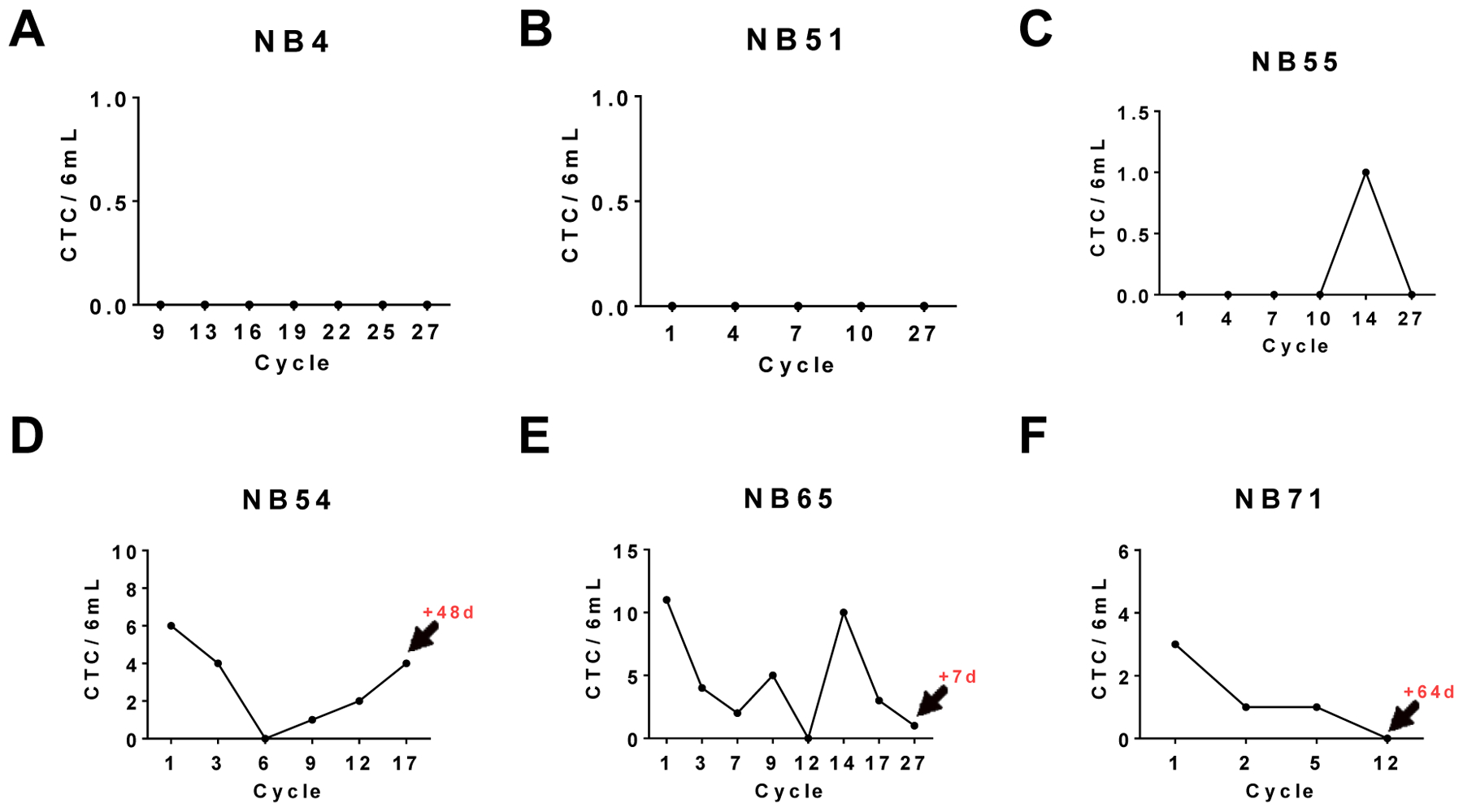
Overall CTC profiles of six individual patients. Three patients who maintained relapse-free survival are shown in panels a, b, and c; three relapsed patients are shown in panels c, d, and e. Graph depicts CTC count per 6ml of blood at treatment cycle (total 27 cycles; 25 months). Arrow and red number indicates days after sample collection at indicated cycle when the patient relapsed.
Discussion
According to the International Neuroblastoma Risk Group (INRG) classification, the MS risk group stage considers eligibility for children younger than 18 months who have metastatic disease to skin, liver, and/or bone marrow31. This group of patients is highly vulnerable to metastatic spread of the disease. In these patients, bone marrow aspirates/biopsies are used as prognostic indicators to plan treatment regimens. Bone marrow aspirates and biopsies are relatively invasive procedures when compared with liquid biopsy. Despite the physical obstacles, these procedures are necessary due to the fact that 5-year survival of high-risk NB patients in remission is 50%1,32. For this group, it is imperative to detect relapse early and find the resistance to maintenance therapy early. Significant progress has been made in improving survival using GD2 targeting antibodies as a treatment for NB. However, in terms of preventing relapse, this strategy has met limited success with only 20% improvement over a 2 year span in high-risk NB patients under remission when anti-GD2 antibody (14G2a) was used in combination with isotretinoin along with either granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin-2 in a 5 month treatment33,34. In another trial using the anti-GD2 ch14.18 alone in infants or children with stage 4 NB showed no difference in event free survival when compared to maintenance therapy35,36. We have shown that DFMO is well tolerated by NB patients. Furthermore, we have shown that by using DFMO to inhibit ornithine synthesis and conversion of bulk tumor cells to cancer stem cells, we are able to more than double two year relapse-free survival rates in NB patients in remission.29 The effectiveness of this treatment is further borne out by the fact that we observed no discernible differences in rates of relapse between the most vulnerable cohort of patients, those under 2 years old, and other patients (Table 1).
GD2 is documented as an NB biomarker, but two well-documented NB cell lines showed either no positive staining or a portion positive GD2 cells (data not shown), even though the GD2 antibody used is the same as for clinical NB assay. This fact clearly indicated that NB, especially relapsed NB, may no longer have GD2 expression post GD2 therapy. CSV+ CTC may serve as an alternative for early detection of relapse and indicator of the response to maintenance therapy, though the literature discussing CTCs in NB for this type of application is missing37. Much of the CTC-focused literature regarding tumor relapse/recurrence has been confined to the study of epithelial tumors such as breast, prostate, and colon cancers where CTCs are confirmed to have a predictive value8–11. Some investigations of cell free or circulating tumor RNA – ctRNA have also been conducted in NB patients38. The potential candidate genes for ctRNA could be mutated RAS-MAPK, amplified MYCN, and other associated genes such as ALK and ATRK2,39. Indeed, there is evidence of new mutations in ALK and MYCN present in cerebrospinal fluid (CSF) during NB relapse40,41. While these studies are instrumental in monitoring tumor burden, they have recently also shown the ability to predict relapse by measuring transcripts of selected target genes42,43. However, our analysis based on data from a large cohort clearly shows no difference in terms of relapse between MYCN amplified and non-amplified patients (Fig. 3c). The fact that the MYCN analysis data is not from our laboratory but from an independent CLIA lab lends further credence to our results. It is most likely that our treatment may be effective on patients with MYCN amplification, which impairs the prognostic power of MYCN. Previous research has shown that targeting ornithine decarboxylase is especially effective at impairing development of MYCN amplified NB in an NB mouse model.30
At the outset of this study our goal was to gather data towards validating our hypothesis that CSV+ CTCs can be used as means of monitoring treatment response and tumor status using a longitudinal CSV+ CTC analysis method. Surprisingly, we discovered that any positive CSV+ CTC count at the baseline was an 88% significant indicator of future relapse. Furthermore, CSV+ CTC negative patients additionally had a detectable decrease in likelihood of relapse (Figure 4, Fisher exact test). To boost this sensitivity, additional CSV+ CTC assays could be added to patients who are CSV+ CTC negative because combined analysis of baseline through 4 months showed a 95% sensitivity for predicting relapse. It should be noted that since this is a multi-center trial, there is an inherent absence of clinical or investigational bias. This is a unique observation since there is no available means of predicting relapse at such an early stage of therapy. In practical terms, validation of our work can mean that patients who are CSV+ CTC-negative at the beginning or early cycles of prevention therapy may be allowed to come off therapy earlier which can prevent personal costs and the often harsh side effects of therapy. Meanwhile, a low count of 1–2 CTCs/6ml blood can serve as a baseline from where the patient can be further monitored for an increase or decrease; high levels of CSV+ CTC at an early time may suggest the possibility of relapse also. One limitation in the current study is that a portion of samples were not analyzed due to delays in delivery to the lab. This research can be further expanded by genetic analysis of the captured CSV+ CTCs. Indeed, others found increased copy number variations in CTCs is associated with chemoresistance, which could lead to relapse44.
The current state of CTC research has largely been driven by the FDA-approved, EpCAM-based CellSearch platform for detection purposes11,45–53. Publications in recent years have explored the value of CTCs as a marker of therapeutic efficacy, most notably in breast cancer11,46–54. Most research in the CTC field has been done with baseline while a few notable studies in other tumor types have used 1 to 2 follow-up sample collection55,56. While some studies have done long term follow-up of patients, to the best of our knowledge there have not been any to study patients who are under remission in the long-term longitudinal method as ours48. The goal of our study is to extend this analysis to NB and determine the feasibility of CSV+ CTC analysis in monitoring and guiding therapy. Our CSV-based CTC targeting approach has previously been demonstrated to accurately isolate cells from liquid biopsies in breast, prostate, sarcoma and other tumors18–20,27. Here, we took this approach to the next step by attempting to monitor therapy response and retrospectively determine the CSV-guided technology’s ability to predict patient relapse. As shown in the presented data, we found a significantly sensitive means of doing so at the earliest possible point of therapy: baseline. Notably, our CSV+ CTC detection method predicted relapse from 2 months to nearly 2 years early, based on the baseline count. While these novel results highlight the value of CTCs and liquid biopsies in general, we must take notice of our imperfect sensitivity. The sensitivity can be improved by taking more than 1st cycle readings. For example, a combination of baseline-S1 yielded >95% sensitivity vs. 88% via baseline alone. This sensitivity can be further improved by including MYCN amplification data (100%) though caution needs to be made because no significance was found between the 95% and 100% prediction rate (Fig. 3c). Perhaps, this is because the MYCN amplification is based on measurement of tumor biopsy or bone marrow. A more reliable method will be to analyze MYCN amplification in the captured CSV+ CTC cells. This hypothesis needs to be validated in the future study.
Overall, our data shows that even in a high-risk population of NB patients, a “one size fits all” approach towards therapeutic treatment may not be ideal. The CSV+ CTC data indicates a stratification of patients into low, medium, and high risk groups that could be treated with a reduced dose, continuing as is, or be switched to alternative treatment due to a lack of response, respectively. This next step in therapeutic efficiency represents further personalization of patient care and will undoubtedly improve relapse-free, and overall survival.
Supplementary Material
Novelty and Impact.
Neuroblastoma is a deadly childhood disease that carries a 50% chance of relapse for anyone in remission and similar level of 5-year survival. We show that CSV can be used as a marker for CTCs in NB and these CSV+ CTCs can be used alongside analysis of MycN amplification to predict relapse. These two factors, when combined, have 100% sensitivity in predicting relapse free survival in patients.
Funding
This work was supported by the National Institutes of Health Grant No. R01EB026291 02.
Ethics Approval and Consent to Participate: All samples for CTC analysis were approved by the MD Anderson Cancer Center’s Institutional Review Board (protocol PA13–0353). Normal human blood used for spiking assays was purchased from the Gulf Coast Blood Center in Houston, TX or the MD Anderson Cancer Center Blood Bank. Written informed consent from the subject (if 18 or over), or from a parent and/or legal guardian (if under 18) for study participation was obtained for all subjects. All methods were performed in accordance with relevant guidelines and regulations. This study was performed in accordance with the Declaration of Helsinki. ClinicalTrials.gov Identifiers: NCT01586260 Unique ID: NMTRC 003 Released 4/24/2012 as well as NCT02395666 Unique ID: NMTRC003B Released 3/5/2015.
Abbreviations:
- CSV
Cell Surface Vimentin
- CTC
Circulating Tumor Cell
- DFMO
Difluoromethylornithine
- NB
Neuroblastoma
- RFS
Relapse-Free Survival
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Data Accessibility
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- 1.Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F, Schleiermacher G, Park JR, Valteau-Couanet D, Pearson ADJ, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol 2015;33:3008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung N-KV, Dyer M a. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 2013;13:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair RR, Tolentino J, Hazlehurst LA. The bone marrow microenvironment as a sanctuary for minimal residual disease in CML. Biochem Pharmacol 2010;80:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gainor JF, Ou S-HI, Logan J, Borges LF, Shaw AT. The Central Nervous System as a Sanctuary Site in ALK-Positive Non–Small-Cell Lung Cancer. J Thorac Oncol 2013;8:1570–3. [DOI] [PubMed] [Google Scholar]
- 5.Kordbacheh T, Law WY, Smith IE. Sanctuary site leptomeningeal metastases in HER-2 positive breast cancer: A review in the era of trastuzumab. Breast 2016;26:54–8. [DOI] [PubMed] [Google Scholar]
- 6.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, Luong R, Rosenblum MD, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 2013;123:2447–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraal KC, van Dalen EC, Tytgat GA, Van Eck-Smit BL. Iodine-131-meta-iodobenzylguanidine therapy for patients with newly diagnosed high-risk neuroblastoma. Cochrane database Syst Rev 2017;4:CD010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iinuma H, Watanabe T, Mimori K, Adachi M, Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M, Mori M. Clinical Significance of Circulating Tumor Cells, Including Cancer Stem-Like Cells, in Peripheral Blood for Recurrence and Prognosis in Patients With Dukes’ Stage B and C Colorectal Cancer. J Clin Oncol 2011;29:1547–55. [DOI] [PubMed] [Google Scholar]
- 9.Hall CS, Karhade M, Laubacher BA, Kuerer HM, Krishnamurthy S, DeSnyder S, Anderson AE, Valero V, Ueno NT, Li Y, Su X, Lucci A. Circulating Tumor Cells and Recurrence After Primary Systemic Therapy in Stage III Inflammatory Breast Cancer. J Natl Cancer Inst 2015;107:djv250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S. Circulating tumour cells in non-metastatic breast cancer: A prospective study. Lancet Oncol 2012;13:688–95. [DOI] [PubMed] [Google Scholar]
- 11.Ma X, Xiao Z, Li X, Wang F, Zhang J, Zhou R, Wang J, Liu L. Prognostic role of circulating tumor cells and disseminated tumor cells in patients with prostate cancer: a systematic review and meta-analysis. Tumour Biol 2014;35:5551–60. [DOI] [PubMed] [Google Scholar]
- 12.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015;6:10697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burchill SA, Lewis IJ, Abrams KR, Riley R, Imeson J, Pearson ADJ, Pinkerton R, Selby P. Circulating neuroblastoma cells detected by reverse transcriptase polymerase chain reaction for tyrosine hydroxylase mRNA are an independent poor prognostic indicator in stage 4 neuroblastoma in children over 1 year. J Clin Oncol 2001;19:1795–801. [DOI] [PubMed] [Google Scholar]
- 14.Satelli A, Mitra A, Brownlee Z, Xia X, Bellister S, Overman MJ, Kopetz S, Ellis LM, Meng QH, Li S. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin Cancer Res 2015;21:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilak T, Sherawat S, Agarwala S, Gupta R, Vishnubhatla S, Bakhshi S. Circulating T-Regulatory Cells in Neuroblastoma: A Pilot Prospective Study. Pediatr Hematol Oncol 2014;31:717–22. [DOI] [PubMed] [Google Scholar]
- 16.Semeraro M, Rusakiewicz S, Zitvogel L, Kroemer G. Natural killer cell mediated immunosurveillance of pediatric neuroblastoma. Oncoimmunology 2015;4:e1042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozier AM, Rich ME, Grawe AP, Peck AS, Zhao P, Chang ATT, Bond JP, Sholler GS. Targeting ornithine decarboxylase reverses the LIN28/Let-7 axis and inhibits glycolytic metabolism in neuroblastoma. Oncotarget 2015;6:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satelli A, Mitra A, Cutrera JJ, Devarie M, Xia X, Ingram DR, Dibra D, Somaiah N, Torres KE, Ravi V, Ludwig J a, Kleinerman ES, et al. Universal marker and detection tool for human sarcoma circulating tumor cells. Cancer Res 2014;74:1645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satelli A, Brownlee Z, Mitra A, Meng QH, Li S. Circulating Tumor Cell Enumeration with a Combination of Epithelial Cell Adhesion Molecule- and Cell-Surface Vimentin-Based Methods for Monitoring Breast Cancer Therapeutic Response. Clin Chem 2015;266:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satelli A, Batth IS, Brownlee Z, Rojas C, Meng QH, Kopetz S, Li S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci Rep 2016;6:28910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohn SL, Pearson ADJ, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG task force report. J Clin Oncol 2009;27:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew P, Valentine MB, Bowman LC, Rowe ST, Nash MB, Valentine VA, Cohn SL, Castleberry RP, Brodeur GM, Look AT. Detection of MYCN gene amplification in neuroblastoma by fluorescence in situ hybridization: A pediatric oncology group study. Neoplasia 2001;3:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimada H International neuroblastoma pathology classification. Pathology 2012;44:S11–2. [Google Scholar]
- 24.Li H, Meng QH, Noh H, Batth IS, Somaiah N, Torres KE, Xia X, Wang R, Li S. Detection of circulating tumor cells from cryopreserved human sarcoma peripheral blood mononuclear cells. Cancer Lett 2017;403:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed SD, Li S. Pre-clinical toxicity assessment of tumor-targeted interleukin-12 low-intensity electrogenetherapy. Cancer Gene Ther 2011;18:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S. Discovery of a linear peptide for improving tumor targeting of gene products and treatment of distal tumors by IL-12 gene therapy. Mol Ther 2011;19:1468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noh H, Yan J, Hong S, Kong L-Y, Gabrusiewicz K, Xia X, Heimberger AB, Li S. Discovery of cell surface vimentin targeting mAb for direct disruption of GBM tumor initiating cells. Oncotarget 2016;7:72021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sholler GLS, Gerner EW, Bergendahl G, MacArthur RB, VanderWerff A, Ashikaga T, Bond JP, Ferguson W, Roberts W, Wada RK, Eslin D, Kraveka JM, et al. A phase I trial of DFMO targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PLoS One 2015;10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sholler GLSS, Ferguson W, Bergendahl, Bond JP, Neville K, Eslin D, Brown V, Roberts W, Wada RK, Oesterheld J, Mitchell D, Foley J, et al. Maintenance DFMO Increases Survival in High Risk Neuroblastoma. Sci Rep 2018;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rounbehler RJ, Li W, Hall MA, Yang C, Fallahi M, Cleveland JL. Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma. Cancer Res 2009;69:547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK, Nuchtern JG, Von Schweinitz D, Simon T, et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J Clin Oncol 2009;27:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Cancer Society. Cancer Facts and Figures 2018. Am Cancer Soc 2018;1–71. [Google Scholar]
- 33.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang RK, Sondel PM. Anti-GD2 Strategy in the Treatment of Neuroblastoma. Drugs Future 2010;35:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, Berthold F. Infants with stage 4 neuroblastoma: the impact of the chimeric anti-GD2-antibody ch14.18 consolidation therapy. Klin Padiatr 2005;217:147–52. [DOI] [PubMed] [Google Scholar]
- 36.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, Berthold F. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol 2004;22:3549–57. [DOI] [PubMed] [Google Scholar]
- 37.Kuroda T, Morikawa N, Matsuoka K, Fujino A, Honna T, Nakagawa A, Kumagai M, Masaki H, Saeki M. Prognostic significance of circulating tumor cells and bone marrow micrometastasis in advanced neuroblastoma. J Pediatr Surg 2008;43:2182–5. [DOI] [PubMed] [Google Scholar]
- 38.Brownhill SC, Burchill SA. PCR-based amplification of circulating RNAs as prognostic and predictive biomarkers – Focus on neuroblastoma. Pract Lab Med 2016;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luksch R, Castellani MR, Collini P, De Bernardi B, Conte M, Gambini C, Gandola L, Garaventa A, Biasoni D, Podda M, Sementa AR, Gatta G, et al. Neuroblastoma (Peripheral neuroblastic tumours). Crit Rev Oncol Hematol 2016;107:163–81. [DOI] [PubMed] [Google Scholar]
- 40.Schleiermacher G, Javanmardi N, Bernard V, Leroy Q, Cappo J, Rio Frio T, Pierron G, Lapouble E, Combaret V, Speleman F, de Wilde B, Djos A, et al. Emergence of new ALK mutations at relapse of neuroblastoma. J Clin Oncol 2014;32:2727–34. [DOI] [PubMed] [Google Scholar]
- 41.Kimoto T, Inoue M, Tokimasa S, Yagyu S, Iehara T, Hosoi H, Kawa K. Detection of MYCN DNA in the cerebrospinal fluid for diagnosing isolated central nervous system relapse in neuroblastoma. Pediatr Blood Cancer 2011;56:865–7. [DOI] [PubMed] [Google Scholar]
- 42.Hirase S, Saitoh A, Hartomo TB, Kozaki A, Yanai T, Hasegawa D, Kawasaki K, Kosaka Y, Matsuo M, Yamamoto N, Mori T, Hayakawa A, et al. Early detection of tumor relapse/regrowth by consecutive minimal residual disease monitoring in high-risk neuroblastoma patients. Oncol Lett 2016;12:1119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yáñez Y, Hervás D, Grau E, Oltra S, Pérez G, Palanca S, Bermúdez M, Márquez C, Cañete A, Castel V. TH and DCX mRNAs in peripheral blood and bone marrow predict outcome in metastatic neuroblastoma patients. J Cancer Res Clin Oncol 2016;142:573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter L, Rothwell DG, Mesquita B, Smowton C, Leong HS, Fernandez-Gutierrez F, Li Y, Burt DJ, Antonello J, Morrow CJ, Hodgkinson CL, Morris K, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med 2016;23. [DOI] [PubMed] [Google Scholar]
- 45.Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat Rev 2012;38:68–75. [DOI] [PubMed] [Google Scholar]
- 46.Pierga J-Y, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, Dieras V, Rolland E, Mignot L, Mathiot C, Bidard F-C. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol 2012;23:618–24. [DOI] [PubMed] [Google Scholar]
- 47.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM, Hayes DF. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N Engl J Med 2004;351:781–91. [DOI] [PubMed] [Google Scholar]
- 48.Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, Zwingers T, Friedl TWP, Lorenz R, Tesch H, Fasching P a., Fehm T, Schneeweiss A, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014;106:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LWWM, Pienta KJ, Raghavan D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 2008;14:6302–9. [DOI] [PubMed] [Google Scholar]
- 50.Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med 2010;2:25ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krebs MG, Sloane R, Priest L, Lancashire L, Hou J-M, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, et al. Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 2011;29:1556–63. [DOI] [PubMed] [Google Scholar]
- 52.Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, Doyle GV, Tissing H, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 2009;20:1223–9. [DOI] [PubMed] [Google Scholar]
- 53.Scher HI, Heller G, Molina A, Attard G, Danila DC, Jia X, Peng W, Sandhu SK, Olmos D, Riisnaes R, McCormack R, Burzykowski T, et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol 2015;33:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller V, Riethdorf S, Rack B, Janni W, Fasching P a, Solomayer E, Aktas B, Kasimir-Bauer S, Pantel K, Fehm T. Prognostic impact of circulating tumor cells assessed with the CellSearch System™ and AdnaTest Breast™ in metastatic breast cancer patients: the DETECT study. Breast Cancer Res 2012;14:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan MS, Kirkwood AA, Tsigani T, Lowe H, Goldstein R, Hartley J a., Caplin ME, Meyer T. Early Changes in Circulating Tumor Cells Are Associated with Response and Survival Following Treatment of Metastatic Neuroendocrine Neoplasms. Clin Cancer Res 2015;1–8. [DOI] [PubMed] [Google Scholar]
- 56.Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF, O’Rourke M a, Lew DL, Doyle GV, Gralow JR, et al. Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer: SWOG S0500. J Clin Oncol 2014;32:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


