Abstract
Purpose:
Alteration in mitochondrial membrane potential (ΔΨm) is an important feature of many pathologic processes, including heart failure, cardiotoxicity, ventricular arrhythmia, and myocardial hypertrophy. We present the first in vivo, noninvasive, assessment of regional ΔΨm in the myocardium of normal human subjects.
Methods:
Thirteen healthy subjects were imaged using [18F]-triphenylphosphonium ([18F]TPP+) on a PET/MR scanner. The imaging protocol consisted of a bolus injection of 300 MBq followed by a 120 min infusion of 0.6 MBq/min. A 60 min dynamic PET acquisition was started 1 hour after bolus injection. The extracellular space fraction (fECS) was simultaneously measured using MR T1-mapping images acquired at baseline and 15 minutes after gadolinium injection with correction for the subject’s hematocrit level. Serial venous blood samples were obtained to calculate the plasma tracer concentration. The tissue membrane potential (ΔΨT), a proxy of ΔΨm, was calculated from the myocardial tracer concentration at secular equilibrium, blood concentration, and fECS measurements using a model based on the Nernst equation.
Results:
In 13 healthy subjects, average tissue membrane potential (ΔΨT), representing the sum of cellular membrane potential (ΔΨc) and ΔΨm, was −160.7±3.7 mV, in excellent agreement with previous in vitro assessment.
Conclusion:
In vivo quantification of the mitochondrial function has the potential to provide new diagnostic and prognostic information for several cardiac diseases as well as allowing therapy monitoring. This feasibility study lays the foundation for further investigations to assess these potential roles.
ClinicalTrial Identifier: NCT03265431
Keywords: positron emission tomography, mitochondrial membrane potential, tissue membrane potential, triphenylphosphonium, mitochondria
Introduction
Myocardial mitochondrial dysfunction plays a key role in many pathologic processes, including heart failure [1], cardiotoxicity [2], ventricular arrhythmia [3], and reperfusion injury [4]. Alteration in mitochondrial membrane potential (ΔΨm) is a fundamental biomarker of mitochondrial and cellular dysfunction as under normal conditions, ΔΨm is maintained within narrow limits [5,6]. Currently, there is no technique enabling non-invasive measurement of ΔΨm. In vitro measurements of ΔΨm have been performed and validated for decades using fluorescent and radiolabeled lipophilic cation probes, including 3H-tetraphenylphosphonium (3H-TPP+) [7–9]. The in vitro assays rely on long-lived β emitters and are thus inappropriate for human in vivo investigations. Our group recently described the first successful method for in vivo mapping of the tissue membrane potential, ΔΨT, a proxy of ΔΨm, in pigs, using the fluorinated compound [18F]TPP+ [10]. Using PET/CT imaging, we were able to map the left ventricular ΔΨT in absolute units of millivolts (mV). The obtained results were consistent with previous in vitro bench-top methods and confirmed the basic feasibility of quantitative in vivo mapping of ΔΨT. Here, we extend this method to healthy volunteers and describe the first in vivo imaging of ΔΨT in humans.
Materials and Methods
Patient Population
Thirteen healthy subjects, 7 females and 6 males, aged between 41 and 74 years, without conditions or medication known to affect cellular membrane potential, such as diabetes, were studied. The study was conducted under the approval of the Partners Human Research Committee, the Institutional Review Board of Partners HealthCare.
Quantification of Membrane Potential
The method for in vivo quantification of ΔΨT has been described in detail previously [10]. Briefly, we divide the tissue distribution space of [18F]TPP+ into three compartments: the extracellular space comprised of the interstitial and plasma space, the cytosol and the mitochondria. Total activity in a voxel can be expressed as the sum of the activity in each compartment contained within that voxel. Therefore, at steady-state, the concentration of [18F]TPP+ measured by PET in a voxel can be written as:
| Eq. 1 |
Where and , represent the steady-state concentration of [18F]TPP+ in the mitochondria, cytosol, and extracellular space respectively, 𝑓𝐸𝐶𝑆 and 𝑓𝑚𝑖𝑡𝑜 represent the extracellular space fraction and mitochondrial fraction respectively. At equilibrium, the Nernst equation can be used to relate the concentration of tracer on each side of a membrane to its electric potential ΔΨ:
| Eq. 2 |
Where is the ratio of known physical parameters: z is the valence, F denotes Faraday’s constant, R is the universal gas constant and T is the temperature in Kelvin. Noting that at steady state, division of Eq. 1 by yields the following expression:
| Eq. 3 |
Where VT, ΔΨ𝑚, and ΔΨ𝑐 represent the volume of distribution of the tracer, and the mitochondrial and cellular membrane potential respectively. Because VT is >> 1, Eq. 3 can be approximated by
| Eq. 4 |
Where ΔΨ𝑇 is the total tissue membrane potential, defined as ΔΨ𝑚 + ΔΨ𝑐. The fmito parameter was assumed to be constant at 0.25 [11].
[18F]TPP+ synthesis
[18F]TPP+ was synthesized and purified on a GE Tracerlab FXN synthesis unit using [18F]fluoride was produced onsite by a GE PETtrace cyclotron bombarding >98% enriched 18O-water. The [18F]TPP+ was sterile filtered into a sterile vial and tested to ensure it met all FDA and USP specifications for a PET sterile radiopharmaceutical prior to injection. The radiation dose exposure from [18F]TPP+ is estimated at 0.0178 mSv/MBq, corresponding to an effective dose of 6.6 mSv for a dose of 370 mBq.[12]
PET/MR imaging
A bolus of 300 MBq of [18F]TPP+ was injected intravenously using PTFE tubing immediately followed by an infusion of 0.6 MBq/min over 2 hours, so that the magnitude of the bolus (Kbol) corresponds to 500 min of infusate. This bolus with infusion protocol allows the system to reach secular tracer equilibrium in the myocardium and blood. Approximately 60 minutes after the bolus injection, a 60 minutes PET acquisition in list mode was performed centered on the thorax. During PET acquisition, MR sequences were acquired before and after administration of gadolinium (Dotarem 0.5 mM/mL, 0.1 mM/kg) to measure the myocardium extracellular space fraction. Modified Look-Locker Inversion recovery (MOLLI) sequences were used to acquire T1 maps before and 15 minutes after contrast administration [13]. Dynamic PET images were reconstructed using an iterative algorithm (OSEM 3 iterations, 21 subsets) with 344×344×127 pixels and 1 min frame length. Attenuation correction was performed using a segmentation method based on Dixon sequence with model-based bone estimation [14].
Extracellular Space Fraction Quantification
Region-of-interests (ROI) were drawn over the myocardium and left ventricular cavity on T1 images. The extracellular space (ECS) fraction was calculated using the following formula:
| Eq. 5 |
where 𝑇1 𝑚𝑦𝑜,𝑝𝑟𝑒 and 𝑇1 𝑚𝑦𝑜,𝑝𝑜𝑠𝑡 each denote the 𝑇1 of myocardium measured before and after gadolinium (Gd) injection, respectively, 𝑇1 𝑏𝑙𝑜𝑜𝑑,𝑝𝑟𝑒 and 𝑇1 𝑏𝑙𝑜𝑜𝑑,𝑝𝑜𝑠𝑡 each denote the 𝑇1 of blood measured before and after Gd injection, respectively, and 𝐻𝑐𝑡 denotes hematocrit in percentage measured from blood samples.
Results
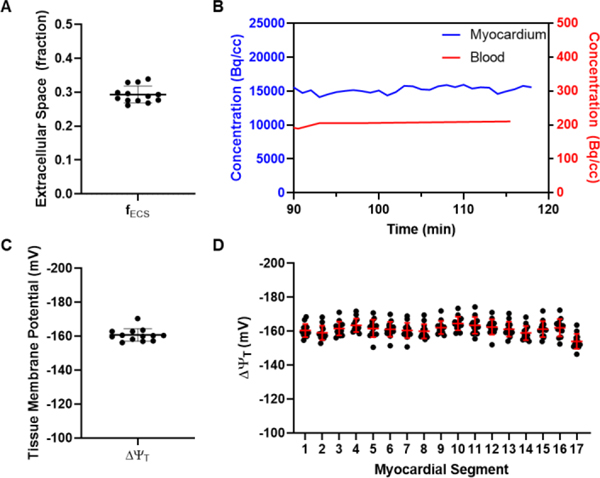
Characteristics of the studied population is presented in Table 1. For all 13 subjects, secular equilibrium was achieved after ≤ 90 minutes with relatively constant myocardial and blood tracer concentrations (Figure 1b). For one subject, motion introduced significant artifacts in the PET images and only the motion free portion of the acquisition was used for the analysis. Overall, the average fECS was 0.293 ± 0.025 and ranged from 0.261 to 0.339. Average VT was 73.3 ± 12.0 and ranged from 60.4 to 106.7. Average ΔΨT was −160.7 ± 3.7 mV and ranged from −170.4 to −156.2 mV (Figure 1c). Values of ΔΨT across the standard 17 left ventricular segments were homogeneous (Figure 1d, Table 2).
Table 1:
Population characteristics.
| Female, N (%) | 7 (53.8%) |
| Age (y), mean ± SD (range) | 56 ± 10 (41 − 74) |
| Ethnicity, N (%) | |
| Caucasian | 11 (84.6%) |
| African American | 1 (7.7%) |
| Hispanic | 0 (0.0%) |
| Asian | 1 (7.7%) |
| BMI (kg/m2), mean ± SD (range) | 25.4 ± 3.5 (18.8 − 31.5) |
| Hypertension, N (%) | |
| No | 11 (84.6%) |
| Yes | 2 (15.4%) |
| Diabetes, N (%) | |
| No | 13 (100.0%) |
| Yes | 0 (0.0%) |
| Smoking, N (%) | |
| Never | 12 (92.3%) |
| Current | 0 (0.0%) |
| Remote (>1 year) | 1 (7.7)% |
| Dyslipidemia, N (%) | |
| No | 13 (100.0%) |
| Yes | 0 (0.0%) |
| Heart Rate (min−1) | 66 ± 14 (46 − 96) |
| Systolic blood pressure (mmHg) | 127 ± 20 (101 − 173) |
| Diastolic blood pressure (mmHg) | 78 ± 7 (64 − 87) |
| Rate pressure product (mmHg/min) | 8297 ± 1949 (5757 − 12,032) |
Figure 1:
A, Average extracellular fraction (fECS) of the 13 study subjects. B, Time activity curves (TAC) of a representative subject showing that tracer concentrations in the blood and in the myocardium are at secular equilibrium between 90 and 120 minutes post tracer injection. C, Average tissue membrane potential (ΔΨT) of the 13 study subjects. D, ΔΨT of the 17 left ventricular segments of the 13 study subjects.
Table 2:
Average tissue membrane potential (ΔΨT) for the 17 left ventricular segments
| Segments | ΔΨT (mV) |
|---|---|
| 1 | −160.4 ± 3.7 |
| 2 | −159.0 ± 4.3 |
| 3 | −161.6 ± 4.1 |
| 4 | −163.5 ± 4.0 |
| 5 | −161.6 ± 5.2 |
| 6 | −161.1 ± 4.4 |
| 7 | −160.4 ± 4.5 |
| 8 | −159.9 ± 4.2 |
| 9 | −162.1 ± 3.9 |
| 10 | −164.5 ± 4.0 |
| 11 | −163.4 ± 4.9 |
| 12 | −162.5 ± 4.5 |
| 13 | −161.2 ± 4.4 |
| 14 | −158.9 ± 4.2 |
| 15 | −161.2 ± 4.7 |
| 16 | −162.1 ± 5.2 |
| 17 | −154.0 ± 4.4 |
| Left Ventricle | −160.7 ± 3.7 |
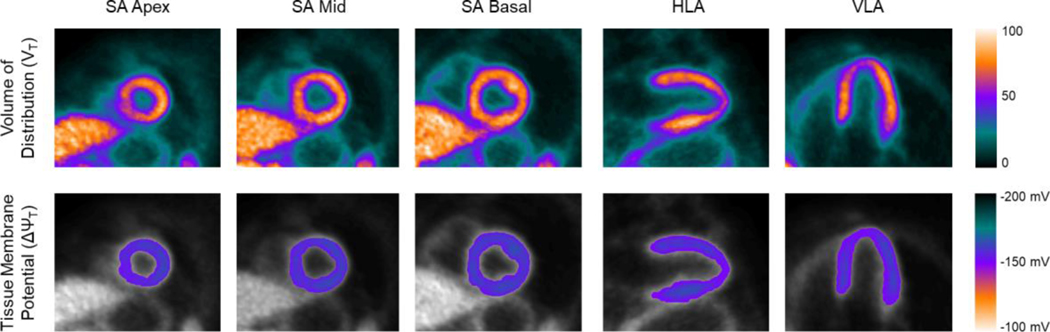
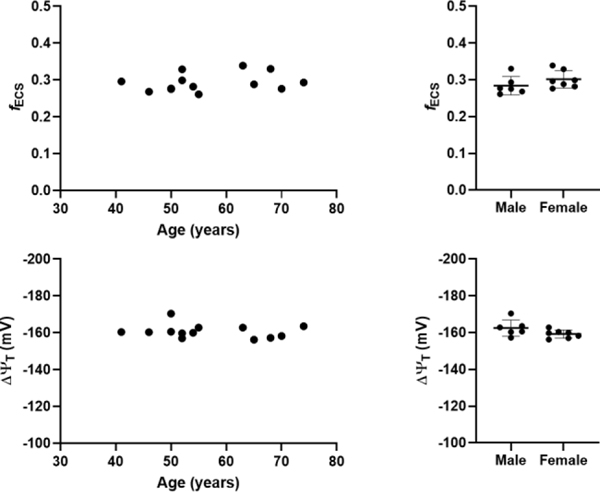
With this method, high quality parametric images of ΔΨT can be generated, allowing for regional assessment of ΔΨT (Figure 2). In our sample, no significant correlations between age and fECS (R2=0.06579, p=0.40) or between age and ΔΨT (R2 = 0.0340, p=0.55) was observed (Figure 3). In addition, there was no significant difference between male and female in fECS (0.284±0.025 vs 0.301±0.024, p=0.23) and ΔΨT (−162.4±4.4 vs −159.2±2.2 mV, p=0.11).
Figure 2:
Parametric image of the volume of distribution (top) and tissue membrane potential (bottom). SA, short axis. HLA, horizontal long axis. VLA, vertical long axis.
Figure 3:
Extracellular fraction (fECS) and tissue membrane potential (ΔΨT) versus age (left) and according to sex (right).
Discussion
Quantification of ΔΨm has been performed in vitro by measuring the concentration of different lipophilic cations in mitochondria. These molecules cross the cellular and mitochondrial phospholipid membranes without significant interaction and their concentration on either side of a membrane at equilibrium abide by the Nernst equation. In this study, we demonstrated for the first time the feasibility of in vivo quantification of ΔΨT, a proxy of ΔΨm, in humans. The method presented in this work relies on the exact same principle on which in vitro techniques are founded but uses PET imaging to measure tissue concentration of the lipophilic cation [18F]TPP+. The total tissue membrane potential, ΔΨT, is often used instead of ΔΨm for in vitro studies, including explanted hearts, as isolation of the different cellular compartment is not easily feasible [9,15].
As normal mitochondrial function depends on the maintenance of membrane potential in a narrow range, pathologies associated with mitochondrial dysfunction could potentially be studied with membrane potential tracers such as [18F]TPP+. Indeed, several pathologies have been associated with mitochondrial dysfunction, including cancers [18], diabetes [19], and cardiotoxicity [16]. The first attempt to measure ΔΨT in vivo has been reported over 30 years ago by Fukuda et al. [15] who studied the distribution of the lipophilic cation triphenylmethylphosphonium labeled with 11C (11C-TPMP).[15] Using primitive PET systems, they reported ΔΨT in dogs (−148.1±6.0 mV), rats (−146.7±3.8 mV), and mice (−139.3 ± 5.8 mV) hearts, with results comparable to those obtained for humans in this study (−160.7 ± 3.7 mV). However, the resolution of the PET scanner was very low and quantitation methods are questionable by today’s standards. More recently, Gurm et al reported an attempt to measure myocardial ΔΨm in vivo with [18F]TPP+ in a swine model of ischemic heart disease [17]. Their results were however discordant with previous in vitro and in vivo studies, with a reported average ΔΨm of −91 mV. This is likely related to two critical methodological issues: first, the Nernst equation was applied while tracer concentrations in the blood and myocardium were changing, whereas both the tracer and subject must be in steady state for concentrations to exhibit Nernstian behavior [18,19]. Second, they did not account for extracellular volume, which would lead to underestimation of ΔΨm. Our group reported the first successful technique to image and quantify ΔΨT in swine [10]. The ΔΨT values obtained in that study, which is based on similar methodology, were slightly different (−129 mV) than those obtained in the present study [10]. We hypothesize that two main factors could explain these differences. First, species variability could account for part of this difference, and second, the use of isoflurane for anesthesia in pigs could lead to partial mitochondrial depolarization [20,21].
Average ΔΨc can be estimated from typical ventricular action potential. Assuming a QT of 400 ms, using the average subjects’ heart rate of 65 bpm, a polarized (phase 4) ΔΨc of −90 mV, and an average depolarized (phase 0–3) ΔΨc of +30 mV (peak +50mV), the average ΔΨc is approximately −38 mV. Therefore, we can estimate ΔΨm (ΔΨm = ΔΨT-ΔΨc) to be −123 mV. This value is in excellent agreement with the measurements of −118 mV obtained in explanted intact perfused rat hearts using the Langendorff method and a physiological glucose infusion [9]. It is important to acknowledge that the mitochondrial environment significantly affects ΔΨm and it must be taken into consideration when comparing in vivo and in vitro measurements. Indeed, it has been shown that in situ assessment of ΔΨm typically yields smaller values compared to isolated mitochondrion (−180 to −190 mV) [22–24]. This can be accounted for by variable substrate availability which alters ΔΨm [9,25].
A main limitation of this study is the lack of an in vivo gold standard for the measurement of ΔΨm. However, this technique simply represents the extension of well-established chemistry principles and in vitro methods. Importantly, membrane potential measurements of this study agreed well with those of previous and comparable experiments, supporting the validity of the presented methodology. Another limitation is the fact that the cellular mitochondrial content (fmito) was not quantified and assumed the same for all subjects. However, myocardial mitochondrial morphology and volume is known to be affected in several conditions such as diabetes [26,27] and heart failure [28]. Unfortunately, in vivo measurement of fmito is not currently feasible. Using Eq. 1, we can estimate the variation induced by not accounting for fmito: a change of 10% in fmito will lead to a change of 2–3 mV in ΔΨT. In several pathologies of interest, it is expected that fmito will decrease, leading to overestimation of depolarization (less negative ΔΨT). It should also be noted that fmito enters Eq. 1 as constant multiplier, meaning that for normal human subjects systematic errors in fmito do not affect the method’s ability to compare subjects or groups. Another limitation of this study is its small sample size. Nonetheless, this study demonstrated the feasibility of in vivo measurements of membrane potential in humans. Finally, 2 subjects had a medical history of hypertension and 3 subjects had a systolic blood pressure >130 mmHg at time of imaging, which could affect the measured ΔΨT.
Conclusion
We demonstrated for the first time the feasibility of noninvasive, in vivo, quantitative assessment of cardiac ΔΨT in humans, with very low variability of ΔΨT between subjects meaning that relatively small sample size would be sufficient for hypotheses testing. Furthermore, true quantitation provided by this method allows comparison between subjects and enables the investigation of several pathologies of interest such as diabetes and chemotherapy-induced cardiotoxicity, which are typically associated with diffuse myocardial involvement. ΔΨT has the potential to provide early diagnosis for various pathologies, including cardiotoxicity. In addition, given the new therapies targeting the mitochondria, ΔΨT could serve as a surrogate endpoint in clinical trials and be used for the assessment of response to therapy. Finally, the method is generalizable to other organs and tissue, and could be used for characterization of tumors.[29] More studies are required to assess these potential roles.
Acknowledgments
Funding: This study was funded by the NIH (grant numbers: P41EB022544 R01HL137230 T32EB013180).
Funding: P41EB022544 R01HL137230 T32EB013180
Informed consent: Informed consent was obtained from all individual participants included in the study.
Footnotes
Disclosures: none
Compliance with Ethical Standards:
Conflict of Interest:
Matthieu Pelletier-Galarneau declares that he has no conflict of interest. Yoann Petibon declares that he has no conflict of interest. Chao Ma declares that he has no conflict of interest. Paul Han declares that he has no conflict of interest. Sally Ji Who Kim declares that she has no conflict of interest. Felicitas J Detmer declares that she has no conflict of interest. Daniel Yokell declares that he has no conflict of interest. Nicolas Guehl declares that he has no conflict of interest. Marc Normandin declares that he has no conflict of interest. Georges El Fakhri declares that he has no conflict of interest. Nathaniel M Alpert declares that he has no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (The Partners Human Research Committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Lin L, Sharma VK, Sheu S-S. Mechanisms of reduced mitochondrial Ca2+ accumulation in failing hamster heart. Pflügers Archiv - European Journal of Physiology. 2007;454:395–402. [DOI] [PubMed] [Google Scholar]
- 2.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–33. [DOI] [PubMed] [Google Scholar]
- 3.Rutledge C, Dudley S. Mitochondria and arrhythmias. Expert Rev Cardiovasc Ther.2013;11:799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turer AT, Hill JA. Pathogenesis of Myocardial Ischemia-Reperfusion Injury and Rationale for Therapy. The American Journal of Cardiology. 2010;106:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda). 2005;20:303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–56. [DOI] [PubMed] [Google Scholar]
- 7.Kauppinen R. Proton electrochemical potential of the inner mitochondrial membrane in isolated perfused rat hearts, as measured by exogenous probes. Biochim Biophys Acta. 1983;725:131–7. [DOI] [PubMed] [Google Scholar]
- 8.Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol. 1984;81:127–38. [DOI] [PubMed] [Google Scholar]
- 9.Wan B, Doumen C, Duszynski J, Salama G, Vary TC, LaNoue KF. Effects of cardiac work on electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am J Physiol. 1993;265:H453–460. [DOI] [PubMed] [Google Scholar]
- 10.Alpert NM, Guehl N, Ptaszek L, Pelletier-Galarneau M, Ruskin J, Mansour MC, et al. Quantitative in vivo mapping of myocardial mitochondrial membrane potential. PLoS ONE. 2018;13:e0190968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barth E, Stämmler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–81. [DOI] [PubMed] [Google Scholar]
- 12.Elmaleh D, Kardan A, Barrow S, Dragotakes S, Correia J, Weise S, et al. A phase I study evaluating dosimetry and myocardial pharmacokinetic behavior of BFPET, a new F-18 labeled tracer for myocardial perfusion imaging. J Nucl Med. Society of Nuclear Medicine; 2009;50:420–420. [Google Scholar]
- 13.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–6. [DOI] [PubMed] [Google Scholar]
- 14.Paulus DH, Quick HH, Geppert C, Fenchel M, Zhan Y, Hermosillo G, et al. Whole-Body PET/MR Imaging: Quantitative Evaluation of a Novel Model-Based MR Attenuation Correction Method Including Bone. J Nucl Med. 2015;56:1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda H, Syrota A, Charbonneau P, Vallois J, Crouzel M, Prenant C, et al. Use of 11C-triphenylmethylphosphonium for the evaluation of membrane potential in the heart by positron-emission tomography. Eur J Nucl Med. 1986;11:478–83. [DOI] [PubMed] [Google Scholar]
- 16.McCluskey S, Haslop A, Coello C, Gunn R, Tate E, Southworth R, et al. Imaging chemotherapy induced acute cardiotoxicity with 18F-labelled lipophilic cations. J Nucl Med. 2019;jnumed.119.226787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurm GS, Danik SB, Shoup TM, Weise S, Takahashi K, Laferrier S, et al. 4-[18F]-Tetraphenylphosphonium as a PET Tracer for Myocardial Mitochondrial Membrane Potential. JACC: Cardiovascular Imaging. 2012;5:285–92. [DOI] [PubMed] [Google Scholar]
- 18.Dedkova EN, Blatter LA. Measuring mitochondrial function in intact cardiac myocytes. J Mol Cell Cardiol. 2012;52:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Meth Enzymol. 2003;361:353–89. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, et al. The Mitochondrial Pathway of Anesthetic Isoflurane-induced Apoptosis. J Biol Chem. 2010;285:4025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–57. [DOI] [PubMed] [Google Scholar]
- 22.Gerencser AA, Chinopoulos C, Birket MJ, Jastroch M, Vitelli C, Nicholls DG, et al. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium induced de and hyperpolarization of neuronal mitochondria. The Journal of Physiology. 2012;590:2845–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamo N, Muratsugu M, Hongoh R, Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979;49:105–21. [DOI] [PubMed] [Google Scholar]
- 24.Hafner RP, Brown GC, Brand MD. Analysis of the control of respiration rate, phosphorylation rate, proton leak rate and protonmotive force in isolated mitochondria using the “top-down” approach of metabolic control theory. Eur J Biochem. 1990;188:313–9. [DOI] [PubMed] [Google Scholar]
- 25.Ainscow EK, Brand MD. Internal regulation of ATP turnover, glycolysis and oxidative phosphorylation in rat hepatocytes. Eur J Biochem. 1999;266:737–49. [DOI] [PubMed] [Google Scholar]
- 26.Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, et al. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu T, Sheu S-S, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta A, Gupta S, Young D, Das B, McMahon J, Sen S. Impairment of ultrastructure and cytoskeleton during progression of cardiac hypertrophy to heart failure. Lab Invest. 2010;90:520–30. [DOI] [PubMed] [Google Scholar]
- 29.Momcilovic M, Jones A, Bailey ST, Waldmann CM, Li R, Lee JT, et al. In vivo imaging of mitochondrial membrane potential in non-small-cell lung cancer. Nature. 2019;575:380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]