Abstract
Aims
To investigate the effectiveness of sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors on the risk of progression to end‐stage renal disease (ESRD) and all‐cause mortality in a broad range of patients with type 2 diabetes (T2D) using a Korean nationwide cohort.
Materials and Methods
Using data from the Korean National Health Insurance Service database from January 2014 to December 2017, a total of 701 674 patients were identified with T2D. We divided these patients into new users of SGLT2 inhibitors and new users of other glucose‐lowering drugs (oGLDs). Using propensity scores, patients in the two groups were matched 1:1. We assessed the risk of ESRD and all‐cause death.
Results
There were 45 016 patients in each group, and baseline characteristics were well balanced between the groups. The patients' mean age was 58.1 ± 10.6 years and mean estimated glomerular filtration rate (eGFR) was 89.2 ± 27.4 mL/min/1.73m2, and 8% of patients had proteinuria. We identified 167 incident ESRD cases and 1070 all‐cause deaths during follow‐up. Use of SGLT2 inhibitors versus oGLDs was associated with a lower risk of ESRD (hazard ratio [HR] 0.47, 95% confidence interval [CI] 0.34 to 0.65) and all‐cause death (HR 0.82, 95% CI 0.73 to 0.93). In a subgroup analysis by eGFR, initiation of SGLT2 inhibitor treatment, compared with oGLD treatment, was associated with lower risk of progression to ESRD among patients with eGFR 60 to 90 mL/min/1.73m2 and those with eGFR < 60 mL/min/1.73m2, and a lower risk of all‐cause death was associated with SGLT2 inhibitors versus oGLDs in patients with eGFR ≥90 and 60 to 90 mL/min/1.73m2.
Conclusion
In this large nationwide study of Korean patients with T2D, initiation of SGLT2 inhibitors versus oGLDs was associated with lower risk of ESRD and all‐cause death.
Keywords: all‐cause death, ESRD, SGLT‐2 inhibitor
1. INTRODUCTION
Diabetic nephropathy ranks highest, both globally and nationwide in Korea, among causes of end‐stage renal disease (ESRD), and its incidence is growing exponentially. 1 However, no new medications had been approved for the treatment of diabetic kidney disease since the IDNT (Irbesartan in Diabetic Nephropathy Trial) and the RENAAL (Reduction of Endpoints in Non‐insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan) study, which showed beneficial effects of angiotensin II receptor blockers in patients with type 2 diabetes mellitus (T2D) and kidney disease. 2 Very recently, sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors have shown renoprotective effects in several large outcome trials. 3 , 4 , 5 , 6 SGLT2 inhibitor treatment inhibits the reabsorption of glucose and sodium in the proximal tubule of the kidney, resulting in glycosuria and the lowering of blood glucose independently of the action of insulin, and can also reduce body weight and blood pressure. 7
The effects of SGLT2 inhibitors on kidney outcomes as novel treatment options for diabetic kidney disease have not been well described in regions outside of North America and Europe. Although previous large randomized controlled trials including the EMPA‐REG OUTCOME trial (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes), the CANVAS programme (CANagliflozin cardioVascular Assessment Study), DECLARE‐TIMI 58 (Dapagliflozin Effect on CardiovascuLAR Events) and CREDENCE (Canagliflozin and Renal Event in Diabetes with Established Nephropathy Clinical Evaluation) included multiracial populations, 4 , 5 , 6 , 8 , 9 , 10 only a minority of patients (12.7%–21.0%) across these trials were recruited from Asian countries. Real‐world evidence on kidney outcomes and all‐cause death associated with SGLT2 inhibitors based on Asian data is also limited. The CVD‐REAL 2 (Cardiovascular Events Associated With SGLT‐2 Inhibitors Versus Other Glucose‐Lowering Drugs) study, a multinational retrospective observational study, included most of the data from Asian countries (the Republic of Korea, Japan and Singapore), but did not report on the effect of SGLT2 inhibitors on renal outcomes. 11 The CVD‐REAL 3 (Kidney outcomes associated with use of SGLT2 inhibitors in real‐world clinical practice) study recently published real‐world evidence from the European Union, Israel and Asia, including Japan and Taiwan. It demonstrated that the initiation of SGLT2 inhibitors was associated with a slower rate of kidney function decline and a lower risk of major kidney events. 12 However, only approximately 11% of the total study cohort were from Asian countries. As several distinctive features are apparent in pathogenetic factors for diabetes in Asian populations, 13 it is important to assess whether the benefits of SGLT2 inhibitors are applicable across different ethnicities. The so‐called “Asian phenotypes” in diabetes include low body mass index (BMI); greater amount of body fat, especially visceral adiposity; higher rate of central obesity and metabolic syndrome; insufficient β‐cell response in the setting of insulin resistance; and higher risk of developing renal complications among other inter‐ethnic clinical differences. 14 In the present study, we investigated the effects of SGLT2 inhibitors on the risk of progression to ESRD and all‐cause mortality in a broad range of patients with T2D using a Korean nationwide cohort.
2. MATERIALS AND METHODS
2.1. Data source and study population
This study used the National Health Insurance Service (NHIS) database, which is government managed and includes patients' sociodemographic information, use of inpatient and outpatient services, and pharmacy dispensing claims. The NHIS is the only insurer providing regular health checkup programmes to the public in the Republic of Korea. Those enrolled in the NHIS are recommended to undergo health checkups at least biannually. Patients with T2D who initiated an SGLT2 inhibitor or any other glucose‐lowering drug (oGLD) were identified, starting from the date of first prescription, in a time window selected from the date of the first SGLT2 inhibitor availability in this country until the last available data (from January 2014 to December 2017). Patients with known type 1 diabetes and gestational diabetes were excluded. No other a priori exclusion criteria were applied. We identified a total of 701 674 T2D patients who were new users of SGLT2 inhibitors or oGLDs and who had clinical and laboratory data recorded at baseline, within a time window of 30 days from the index date. Due to lack of data, we excluded 193 431 patients who had not undergone a health checkup within 4 years from the index date (Figure 1). Among the resulting 508 243 patients, those in the SGLT2 inhibitor category were defined as new users of SGLT2 inhibitors (n = 101 582), and those in the category of oGLD were defined as new users of oGLDs (n = 406 661). We then excluded 3823 patients who had already been diagnosed with ESRD and 9381 patients with missing data. Finally, 99 618 patients in the SGLT2 inhibitor category and 395 421 in the oGLD category were included for the final analysis. This study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of The Catholic University of Korea (No. SC18ZES10158). Because anonymized and deidentified information was used in the analyses, informed consent was not required.
FIGURE 1.
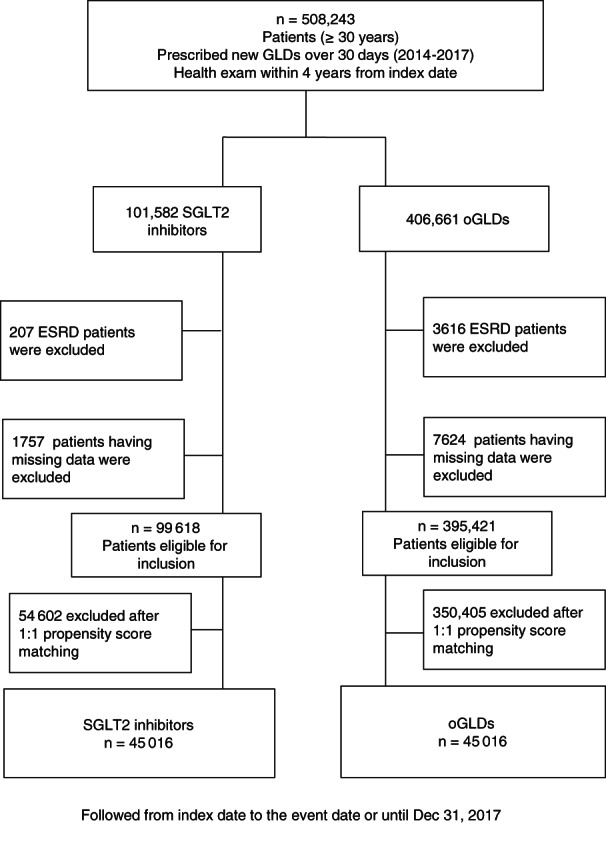
CONSORT study enrolment flow diagram. ESRD, end‐stage renal disease; GLD, glucose‐lowering drug; oGLD, other glucose‐lowering drug; SGLT2, sodium‐glucose co‐transporter‐2
2.2. Measurements and definitions
Comorbidities were defined by a combination of medical history (International Classification of Diseases [ICD]‐10 code and self‐reported during health checkup questionnaires) and use of medication history for the corresponding disease. The presence of hypertension was defined as at least one claim per year under ICD‐10 codes I10 or I11 and at least one claim per year for the prescription of an anti‐hypertensive agent, or systolic/diastolic blood pressure ≥140/90 mmHg. The presence of dyslipidaemia was defined as at least one claim per year for the prescription of antidyslipidaemic medication under ICD‐10 code E78. The presence of cardiovascular disease (CVD) was defined as at least one claim per year under ICD‐10 codes I20, I21, I22, I48, I50, G45, I60–I66, or I70–I79. Blood samples for the measurement of serum glucose, creatinine, total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol levels were drawn after fasting overnight. As the serum creatinine of NHIS health checkup was measured mainly by isotope dilution mass spectrometry traceable Jaffe methods, not enzyme methods, estimated GFR was calculated using the abbreviated Modification of Diet in Renal Disease formula: estimated glomerular filtration rate (eGFR) = 175 × serum creatinine (mg/dL) – 1.154 × age (years) – 0.203 × (0.742 if female). 15 The presence of proteinuria was defined as having urinary protein ≥1+ on dipstick testing in fasting morning urine. The presence of diabetic retinopathy was defined according to at least one claim per year under ICD‐10 code H36.0.
2.3. Study outcomes and follow‐up
The main outcomes for this study were newly diagnosed ESRD or all‐cause death. We defined incident ESRD using the combination of ICD‐10 codes (N18–N19, Z49, Z94.0, Z99.2) and initiation of renal replacement therapy for 30 days or more, and/or kidney transplantation during hospitalization. The Korean Health Insurance Review and Assessment Service reimburses all medical care expenses for dialysis. Patients with ESRD are also registered as special medical aid beneficiaries. Therefore, we could include each ESRD patient from the whole population and analyse the data for all ESRD patients who started dialysis or had an eGFR < 15 mL/min/1.73 m2. Patients without ESRD or all‐cause death during their follow‐up periods were considered to have completed the study at the date of their death or at the end of follow‐up, whichever came first. The study population was followed from baseline to the date of ESRD or all‐cause death, or until December 31, 2017, whichever came first.
2.4. Statistical methods
Baseline characteristics are presented as means ± SD or n (%). The incidence rates for the primary outcomes were calculated by dividing the number of incident cases by the total follow‐up duration (patient‐years). The disease‐free probability of primary outcomes was calculated using Kaplan–Meier curves, and a log‐rank test was performed to analyse differences between the groups. Hazard ratios (HRs) and 95% confidence intervals (CIs) for ESRD and all‐cause death were calculated using a Cox proportional hazards model for each category.
A non‐parsimonious propensity score for initiating an SGLT2 inhibitor was developed for each individual episode of a new treatment initiation. Variables that could potentially affect treatment assignment or outcomes were selected: eGFR, age, sex, BMI, waist circumference, height, weight, presence of proteinuria, T2D duration, fasting blood glucose, presence of diabetic retinopathy, prior CVD, hypertension, blood pressure, dyslipidaemia, smoking, drinking, exercise, income status, Charlson Comorbidity Index, insulin treatment, glucagon‐like peptide‐1 receptor agonists, number of oGLDs, and index date. The propensity matching was assessed by evaluating standardized differences of patient characteristics post‐match. A significant imbalance was considered to be present if a > 10% standardized difference was present between the two groups after propensity matching. An SAS (SAS Institute Inc., Cary, North Carolina) matching macro, “%OneToManyMTCH,” was used for this matching. It allows propensity‐score matching from 1‐to‐1 to 1‐to‐N. We set a caliper for nearest‐neighbour matching within the first four to eight digits; for example, two patients with propensity scores of 0.12345678 and 0.12347123 match on the first four digits (0.1234). The macro makes the “best” matches first and the “next‐best” matches next in a hierarchical sequence until no more matches can be made. If no patient in the oGLD group has a propensity score that lies within a four‐digit width of a propensity score of a patient in the SGLT2 inhibitor group, then that patient in the SGLT2 inhibitor group is left unmatched and is not used in subsequent analyses.
The primary analysis used an intention‐to‐treat approach, in which patients were followed from the start of an index treatment until either occurrence of the first outcome event or the censoring date (whichever was earlier), regardless of whether the index treatment was discontinued. A sensitivity analysis was performed with restriction of the follow‐up period to 3 years because of the short follow‐up duration of the SGLT2 inhibitor group. Also, the analyses for each outcome were conducted using an on‐treatment approach, in which follow‐up was censored at discontinuation of the index treatment. Statistical analyses were performed using SAS version 9.4, and a P value <0.05 was considered to indicate significance.
3. RESULTS
3.1. Study population
Following propensity matching with 508 243 new SGLT2 inhibitor or oGLD initiation episodes, a total of 90 032 new SGLT2 inhibitor or oGLD users were identified with 45 016 in each treatment group (Table 1). The distribution of specific SGLT2 inhibitors were dapagliflozin 73.3%, empagliflozin 20.8% and ipragliflozin 6.0%, respectively, and the mean follow‐up period was 1.49 ± 0.85 years. Patient characteristics were well balanced between the two groups. The mean age was 58 years and 43% were women. The mean BMI was 26 kg/m2, and the mean duration of diabetes was 5.64 years and 5.87 years in the SGLT2 inhibitor and oGLD groups, respectively. Among the study population, 30% of patients had diabetic retinopathy. The mean eGFR values of the patients in the SGLT2 inhibitor and oGLD groups were 89.02 ± 25.6 mL/min/1.73 m2 and 89.44 ±29.18 mL/min/1.73 m2, respectively, and 8% of patients had proteinuria.
TABLE 1.
Baseline characteristic of patients using sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs in the total study population
| SGLT2 inhibitors | oGLDs | ||
|---|---|---|---|
| (n = 45 016) | (n = 45 016) | ASD | |
| Age, years | 58.25 ± 10.87 | 57.88 ± 10.38 | 0.0343 |
| Sex, male, n (%) | 25 832 (57.38) | 25 949 (57.64) | 0.0053 |
| BMI, kg/m2 | 26.38 ± 3.76 | 26.59 ± 3.96 | 0.0534 |
| Waist circumference, cm | 88.12 ± 9.31 | 88.55 ± 9.67 | 0.0453 |
| Height, cm | 163.45 ± 9.1 | 163.62 ± 9.43 | 0.0185 |
| Weight, kg | 70.77 ± 13.24 | 71.45 ± 13.7 | 0.0507 |
| eGFR, mL/min/1.73m2 | 89.02 ± 25.6 | 89.44 ± 29.18 | 0.0152 |
| eGFR, n (%) | |||
| <60 mL/min/1.73m2 | 3261 (7.24) | 3583 (7.96) | 0.0277 |
| 60–90 mL/min/1.73m2 | 21 527 (47.82) | 20 905 (46.44) | |
| ≥90 mL/min/1.73m2 | 20 228 (44.94) | 20 528 (45.6) | |
| Presence of proteinuria, n (%) | 3719 (8.26) | 3827 (8.5) | 0.0087 |
| eGFR/proteinuria, n (%) | 0.0400 | ||
| <60 mL/min/1.73m2/absent | 2751 (6.11) | 2864 (6.36) | |
| <60 mL/min/1.73m2/present | 510 (1.13) | 719 (1.6) | |
| ≥60 mL/min/1.73m2/absent | 38 546 (85.63) | 38 325 (85.14) | |
| ≥60 mL/min/1.73m2/present | 3209 (7.13) | 3108 (6.9) | |
| T2D duration, years | 6 (1–10) | 6 (2–10) | 0.0574 |
| Glucose, mmol/L | 8.76 ± 3.13 | 8.85 ± 3.44 | 0.0299 |
| Diabetic retinopathy, n (%) | 13 933 (30.95) | 13 494 (29.98) | 0.0212 |
| Prior cardiovascular diease, n (%) | |||
| Myocardial infarction | 1527 (3.39) | 1534 (3.41) | 0.0009 |
| Unstable angina | 2482 (5.51) | 2519 (5.6) | 0.0036 |
| Angina pectoris | 7838 (17.41) | 7885 (17.52) | 0.0028 |
| Heart failure | 3284 (7.3) | 3477 (7.72) | 0.0163 |
| Atrial fibrilation | 1171 (2.6) | 1246 (2.77) | 0.0103 |
| Stroke | 5292 (11.76) | 5167 (11.48) | 0.0087 |
| Hypertension, n (%) | 25 706 (57.1) | 25 545 (56.75) | 0.0072 |
| Systolic blood pressure, mmHg | 127.86 ± 14.93 | 127.94 ± 14.99 | 0.0057 |
| Diastolic blood pressure, mmHg | 78.53 ± 9.94 | 78.65 ± 10.07 | 0.0118 |
| Dyslipidaemia, n (%) | 31 455 (69.88) | 31 619 (70.24) | 0.0080 |
| Total cholesterol, mmol/L | 4.84 ± 1.36 | 4.87 ± 1.26 | 0.0190 |
| Triglycerides, mmol/L | 1.68 (1.68–1.69) | 1.71 (1.70–1.72) | 0.0215 |
| HDL cholesterol, mmol/L | 1.28 ± 0.35 | 1.28 ± 0.33 | 0.0000 |
| LDL cholesterol, mmol/L | 2.68 ± 1.2 | 2.7 ± 1.15 | 0.0140 |
| Smoking status, n (%) | 0.0050 | ||
| Non‐smoker | 24 426 (54.26) | 24 313 (54.01) | |
| Ex‐smoker | 9363 (20.8) | 9374 (20.82) | |
| Current smoker | 11 227 (24.94) | 11 329 (25.17) | |
| Heavy drinker, n (%) | 4137 (9.19) | 4268 (9.48) | 0.0100 |
| Regular exercise, n (%) | 9135 (20.29) | 8884 (19.74) | 0.0139 |
| Income: low 25%, n (%) | 10 470 (23.26) | 10 546 (23.43) | 0.0040 |
| ACE inhibitors, n (%) | 1300 (2.89) | 1564 (3.47) | 0.0334 |
| ARBs, n (%) | 20 863 (46.35) | 21 775 (48.37) | 0.0406 |
| Loop diuretics, n (%) | 2333 (5.18) | 2539 (5.64) | 0.0202 |
| Thiazide diuretics, n (%) | 3095 (6.88) | 3374 (7.5) | 0.0240 |
| Statins, n (%) | 29 042 (64.51) | 30 404 (67.54) | 0.0639 |
| Beta blockers, n (%) | 6960 (15.46) | 7478 (16.61) | 0.0313 |
| Aldosterone, n (%) | 914 (2.03) | 1160 (2.58) | 0.0364 |
| Index year, n (%) | 0.0000 | ||
| 2014 | 1621 (3.6) | 1621 (3.6) | |
| 2015 | 9621 (21.37) | 9621 (21.37) | |
| 2016 | 14 159 (31.45) | 14 159 (31.45) | |
| 2017 | 19 615 (43.57) | 19 615 (43.57) | |
| Charlson comorbidity index | 2.7 ± 1.95 | 2.68 ± 2.09 | 0.0074 |
Note: Values are mean ± SD or median value (interquartile range), unless otherwise indicated. Presence of proteinuria was defined as having urinary protein ≥1+ dipstick testing in fasting morning urine.
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; ASD, absolute standardized difference; eGFR, estimated glomerular filtration rate; oGLD, other glucose‐lowering drug; SGLT2, sodium‐glucose co‐transporter‐2; T2D, type 2 diabetes.
3.2. Renal outcomes: SGLT2 inhibitors versus oGLDs
During 67 133 person‐years of follow‐up, there were 167 cases of incident ESRD, of which 53 occurred in the SGLT2 inhibitor group (incidence rate 0.79 per 1000 patient‐years) and 114 in the oGLD group (incidence rate 1.70 per 1000 patient‐years). Initiation of SGLT2 inhibitors versus oGLDs was associated with a lower risk of incident ESRD (HR 0.47, 95% CI 0.34–0.65; Table 2). The incidence rate of ESRD showed a substantial decrease in the SGLT2 inhibitor group compared with the oGLD group in the Kaplan–Meier curve (P values by log‐rank <0.0001; Figure 2A). In a subgroup analysis by eGFR, initiation of SGLT2 inhibitors versus oGLDs was associated with a lower risk of progression to ESRD among patients with eGFR 60 to 90 mL/min/1.73 m2 or less than 60 mL/min/1.73 m2 (HR 0.39, 95% CI 0.21–0.75 and HR 0.39, 95% CI 0.25–0.63, respectively). The results were consistent regardless of the presence or absence of proteinuria. Furthermore, a sensitivity analysis was performed to adjust for the differences in the person‐years of follow‐up and periods between the two groups; HR trends for ESRD were similar to the 3‐year follow‐up results (Table S1 and Figure S1) and those of the on‐treatment analysis (Table S2 and Figure S2).
TABLE 2.
Risk of end‐stage renal disease and all‐cause deaths associated with sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs
| N | ESRD | Person‐years of follow up | Incidence rate (per 1000) | HR (95% CI) | P for interaction | Death | Person‐years of follow up | Incidence rate (per 1000) | HR (95% CI) | P for interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | oGLDs | 45 016 | 114 | 67 061 | 1.70 | 1 (ref.) | 586 | 67 119 | 8.73 | 1 (ref.) | ||
| SGLT2 inhibitors | 45 016 | 53 | 67 204 | 0.79 | 0.47 (0.34, 0.65) | 484 | 67 247 | 7.20 | 0.82 (0.73, 0.93) | |||
| eGFR < 60 mL/min/1.73m2 | oGLDs | 3583 | 70 | 5538 | 12.64 | 1 (ref.) | 0.01 | 96 | 5576 | 17.22 | 1 (ref.) | 0.10 |
| SGLT2 inhibitors | 3261 | 24 | 4812 | 4.99 | 0.39 (0.25, 0.63) | 91 | 4832 | 18.83 | 1.1 (0.83, 1.47) | |||
| eGFR 60–90 mL/min/1.73m2 | oGLDs | 20 905 | 33 | 31 430 | 1.05 | 1 (ref.) | 284 | 31 443 | 9.03 | 1 (ref.) | ||
| SGLT2 inhibitors | 21 527 | 13 | 32 117 | 0.40 | 0.39 (0.21, 0.75) | 239 | 32 127 | 7.44 | 0.82 (0.69, 0.98) | |||
| eGFR ≥ 90 mL/min/1.73m2 | oGLDs | 20 528 | 11 | 30 093 | 0.37 | 1 (ref.) | 206 | 30 100 | 6.84 | 1 (ref.) | ||
| SGLT2 inhibitors | 20 228 | 16 | 30 276 | 0.53 | 1.45 (0.67, 3.11) | 154 | 30 288 | 5.08 | 0.74 (0.6, 0.91) | |||
| Without proteinuria | oGLDs | 41 189 | 57 | 61 426 | 0.93 | 1 (ref.) | 0.12 | 523 | 61 453 | 8.51 | 1 (ref.) | 0.96 |
| SGLT2 inhibitors | 41 297 | 34 | 61 819 | 0.55 | 0.60 (0.39, 0.92) | 435 | 61 847 | 7.03 | 0.83 (0.73, 0.94) | |||
| With proteinuria | oGLDs | 3827 | 57 | 5635 | 10.12 | 1 (ref.) | 63 | 5666 | 11.12 | 1 (ref.) | ||
| SGLT2 inhibitors | 3719 | 19 | 5386 | 3.53 | 0.35 (0.21, 0.59) | 49 | 5400 | 9.07 | 0.81 (0.56, 1.18) | |||
| eGFR/proteinuria | 0.43 | 0.14 | ||||||||||
| <60 mL/min/1.73m2/absent | oGLDs | 2864 | 28 | 4469 | 6.27 | 1 (ref.) | 77 | 4484 | 17.17 | 1 (ref.) | ||
| SGLT2 inhibitors | 2751 | 11 | 4109 | 2.68 | 0.43 (0.21, 0.87) | 73 | 4120 | 17.72 | 1.04 (0.76, 1.43) | |||
| <60 mL/min/1.73m2/present | oGLDs | 719 | 42 | 1068 | 39.31 | 1 (ref.) | 19 | 1092 | 17.40 | 1 (ref.) | ||
| SGLT2 inhibitors | 510 | 13 | 703 | 18.50 | 0.47 (0.25, 0.87) | 18 | 712 | 25.28 | 1.44 (0.76, 2.75) | |||
| ≥60 mL/min/1.73m2/absent | oGLDs | 38 325 | 29 | 56 957 | 0.51 | 1 (ref.) | 446 | 56 969 | 7.83 | 1 (ref.) | ||
| SGLT2 inhibitors | 38 546 | 23 | 57 710 | 0.40 | 0.79 (0.46, 1.37) | 362 | 57 728 | 6.27 | 0.80 (0.70, 0.92) | |||
| ≥60 mL/min/1.73m2/present | oGLDs | 3108 | 15 | 4566 | 3.28 | 1 (ref.) | 44 | 4574 | 9.62 | 1 (ref.) | ||
| SGLT2 inhibitors | 3209 | 6 | 4683 | 1.28 | 0.40 (0.15, 1.02) | 31 | 4687 | 6.61 | 0.69 (0.43, 1.09) |
Note: Presence of proteinuria was defined as having urinary protein ≥1+ dipstick testing in fasting morning urine.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; HR, hazard ratio; oGLD, other glucose‐lowering drug; SGLT2, sodium‐glucose co‐transporter‐2 inhibitors.
FIGURE 2.
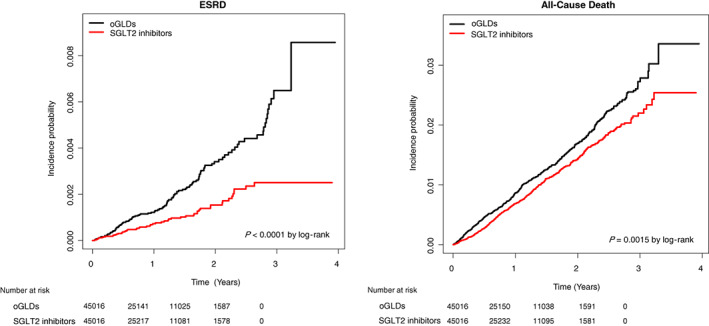
Kaplan–Meier estimates of the incidence of end‐stage renal disease (ESRD; left) and all‐cause death (right) in patients using sodium‐glucose co‐transporter‐2 inhibitors (SGLT2 inhibitors; red) versus other glucose lowering drugs (oGLDs; black)
3.3. All‐cause death: SGLT2 inhibitors versus oGLDs
During 67 183 person‐years of follow‐up, there were 1070 all‐cause deaths, of which 484 occurred in the SGLT2 inhibitor group (incidence rate 7.20 per 1000 patient‐years) and 586 in the oGLD group (incidence rate 8.73 per 1000 patient‐years). Initiation of SGLT2 inhibitors versus oGLDs was associated with a lower risk of all‐cause death (HR 0.82, 95% CI 0.73–0.93; Table 2). The incidence rate of all‐cause death showed a substantial decrease in the SGLT2 inhibitor group compared with the oGLD group in the Kaplan–Meier curve (P values by log‐rank = 0.0016; Figure 2B). The results were consistent across the baseline eGFR and regardless of the presence or absence of proteinuria (Table 2). In addition, a sensitivity analysis was performed to adjust for the differences in the person‐years of follow‐up and periods between the two groups; HR trends for all‐cause death were similar to the 3‐year follow‐up results (Table S1 and Figure S1) and those of the on‐treatment analysis (Table S2 and Figure S2).
3.4. Subgroup analysis
To evaluate the effect of modifiers on the associations between SGLT2 inhibitors and outcomes, we conducted a stratified analysis using several factors including age, sex, BMI, abdominal obesity, diabetic retinopathy, hypertension and prior CVD (Table 3). The association of SGLT2 inhibitors versus oGLDs with lowering incident ESRD risk was stronger in obese patients (HR 0.27, 95% CI 0.16–0.44) than in non‐obese patients (HR 0.80, 95% CI 0.51–1.25; P for interaction = 0.002). For all‐cause death, the association was stronger in younger, female and obese patients and subgroups without hypertension or prior CVD. For example, the association of SGLT2 inhibitors versus oGLDs with lower all‐cause deaths risk was stronger in female patients (HR 0.64, 95% CI 0.52–0.79) than in male patients (HR 0.94, 95% CI 0.81–1.09; P for interaction = 0.001).
TABLE 3.
Hazard ratios of end‐stage renal disease and all‐cause deaths for sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs in various subgroups
| Subgroups | N | ESRD | P for interaction | All cause deaths | P for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Person‐years of follow up | Incidence rate (per 1000) | HR (95% CI) | Events | Person‐years of follow up | Incidence rate (per 1000) | HR (95% CI) | |||||
| Age, year | ||||||||||||
| <65 | oGLDs | 33 123 | 74 | 49 213 | 1.50 | 1 (ref.) | 0.06 | 247 | 49 255 | 5.01 | 1 (ref.) | 0.001 |
| SGLT2 inhibitors | 32 364 | 26 | 49 007 | 0.53 | 0.35 (0.23, 0.55) | 154 | 49 027 | 3.14 | 0.62 (0.51, 0.76) | |||
| ≥65 | oGLDs | 11 893 | 40 | 17 847 | 2.24 | 1 (ref.) | 339 | 17 863 | 18.98 | 1 (ref.) | ||
| SGLT2 inhibitors | 12 652 | 27 | 18 197 | 1.48 | 0.67 (0.41, 1.09) | 330 | 18 219 | 18.11 | 0.96 (0.82, 1.11) | |||
| Sex | ||||||||||||
| Male | oGLDs | 25 949 | 67 | 37 204 | 1.80 | 1 (ref.) | 0.21 | 353 | 37 241 | 9.48 | 1 (ref.) | 0.003 |
| SGLT2 inhibitors | 25 832 | 37 | 37 711 | 0.98 | 0.55 (0.37, 0.82) | 337 | 37 739 | 8.93 | 0.94 (0.81, 1.09) | |||
| Female | oGLDs | 19 067 | 47 | 29 856 | 1.57 | 1 (ref.) | 233 | 29 878 | 7.80 | 1 (ref.) | ||
| SGLT2 inhibitors | 19 184 | 16 | 29 492 | 0.54 | 0.35 (0.20, 0.61) | 147 | 29 507 | 4.98 | 0.64 (0.52, 0.79) | |||
| BMI | ||||||||||||
| <25 kg/m2 | oGLDs | 16 276 | 42 | 23 851 | 1.76 | 1 (ref.) | 0.002 | 309 | 23 872 | 12.94 | 1 (ref.) | 0.02 |
| SGLT2 inhibitors | 16 641 | 34 | 24 349 | 1.40 | 0.80 (0.51, 1.25) | 294 | 24 375 | 12.06 | 0.93 (0.79, 1.09) | |||
| ≥25 kg/m2 | oGLDs | 28 740 | 72 | 43 208 | 1.67 | 1 (ref.) | 277 | 43 247 | 6.41 | 1 (ref.) | ||
| SGLT2 inhibitors | 28 375 | 19 | 42 855 | 0.44 | 0.27 (0.16, 0.44) | 190 | 42 872 | 4.43 | 0.69 (0.57, 0.83) | |||
| Abdominal obesity | ||||||||||||
| No | oGLDs | 21 742 | 45 | 31 909 | 1.41 | 1 (ref.) | 0.003 | 316 | 31 936 | 9.89 | 1 (ref.) | 0.06 |
| SGLT2 inhibitors | 22 205 | 35 | 32 814 | 1.07 | 0.76 (0.49, 1.19) | 295 | 32 842 | 8.98 | 0.90 (0.77, 1.06) | |||
| Yes | oGLDs | 23 274 | 69 | 35 151 | 1.96 | 1 (ref.) | 270 | 35 183 | 7.67 | 1 (ref.) | ||
| SGLT2 inhibitors | 22 811 | 18 | 34 390 | 0.52 | 0.27 (0.16, 0.45) | 189 | 34 405 | 5.49 | 0.72 (0.59, 0.86) | |||
| Diabetic retinopathy | ||||||||||||
| No | oGLDs | 31 522 | 42 | 45 688 | 0.92 | 1 (ref.) | 0.38 | 408 | 45 706 | 8.93 | 1 (ref.) | 0.12 |
| SGLT2 inhibitors | 31 083 | 24 | 46 414 | 0.52 | 0.56 (0.34, 0.93) | 320 | 46 431 | 6.89 | 0.77 (0.67, 0.89) | |||
| Yes | oGLDs | 13 494 | 72 | 21 372 | 3.37 | 1 (ref.) | 178 | 21 413 | 8.31 | 1 (ref.) | ||
| SGLT2 inhibitors | 13 933 | 29 | 20 790 | 1.39 | 0.42 (0.27, 0.65) | 164 | 20 815 | 7.88 | 0.95 (0.77, 1.18) | |||
| Hypertension | ||||||||||||
| No | oGLDs | 19 471 | 12 | 27 926 | 0.43 | 1 (ref.) | 0.11 | 217 | 27 930 | 7.77 | 1 (ref.) | 0.0001 |
| SGLT2 inhibitors | 19 310 | 11 | 28 887 | 0.38 | 0.86 (0.38, 1.96) | 131 | 28 896 | 4.53 | 0.58 (0.47, 0.72) | |||
| Yes | oGLDs | 25 545 | 102 | 39 134 | 2.61 | 1 (ref.) | 369 | 39 189 | 9.42 | 1 (ref.) | ||
| SGLT2 inhibitors | 25 706 | 42 | 38 317 | 1.10 | 0.43 (0.30, 0.61) | 353 | 38 350 | 9.20 | 0.98 (0.84, 1.13) | |||
| Prior CVD | ||||||||||||
| No | oGLDs | 23 166 | 26 | 33 561 | 0.77 | 1 (ref.) | 0.70 | 193 | 33 572 | 5.75 | 1 (ref.) | 0.004 |
| SGLT2 inhibitors | 22 683 | 14 | 34 168 | 0.41 | 0.54 (0.28, 1.04) | 124 | 34 178 | 3.63 | 0.63 (0.50, 0.79) | |||
| Yes | oGLDs | 21 850 | 88 | 33 500 | 2.63 | 1 (ref.) | 393 | 33 547 | 11.71 | 1 (ref.) | ||
| SGLT2 inhibitors | 22 333 | 39 | 33 036 | 1.18 | 0.45 (0.31, 0.66) | 360 | 33 069 | 10.89 | 0.93 (0.80, 1.07) | |||
Abbreviations: BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; ESRD, end‐stage renal disease; HR, hazard ratio; oGLD, other glucose‐lowering drug; SGLT2, sodium‐glucose co‐transporter‐2 inhibitors.
4. DISCUSSION
The results of the present study suggest that initiation of SGLT2 inhibition as compared with oGLD treatment had a statistically significant and clinically relevant association with lower risk of ESRD and all‐cause death in patients with T2D in a general population of patients with T2D in Korea, evidence that is complementary to and consistent with what has been already observed in previous randomized controlled trials for this class of compounds. T2D patients in the SGLT2 inhibitor group had a significantly lower risk of incident ESRD and all‐cause death compared with patients in the oGLD group. Although ESRD and all‐cause death are rare events in the general population and would be unexpected in those with near‐normal baseline eGFR, due to the large sample size, we were able to accrue a substantial number of these events to demonstrate that initiation of SGLT2 inhibitors was significantly associated with a lower risk of these outcomes compared with oGLDs. Recent randomized clinical trials demonstrated a lower risk of renal composite events and all‐cause death with SGLT2 inhibitors in T2D patients; however, results showing the beneficial effect of SGLT2 inhibitors on ESRD and all‐cause death in routine clinical care are scarce. 3 , 4 , 6 The present report complements the results from the CVD REAL 3 study, 12 providing further evidence from an Asian patient population from the nationwide Korean registry including ~80 000 patients with T2D and with ~67 000 patient‐years of follow‐up.
It is noteworthy that the present study assessed the effect of SGLT2 inhibition on risk of incident ESRD and all‐cause death stratified by renal function including eGFR and presence of proteinuria, and the overall results were consistent across these subgroups. The patients with eGFR 60 to 90 mL/min/1.73 m2 or <60 mL/min/1.73 m2 were observed to have a lower risk of progression to ESRD with SGLT2 inhibitors versus oGLDs, whereas those with eGFR ≥90 mL/min/1.73 m2 were not. We interpret the results with caution because the discordance may be dependent on how renal events were defined and because of the relatively short follow‐up period for incident ESRD in these real‐world data. There were few ESRD events in the subgroup with eGFR ≥90 mL/min/1.73 m2, which had a relatively low risk for ESRD development considering the clinical natural course of chronic kidney disease progression. CREDENCE is the only published randomized controlled trial to examine a population with diabetic kidney disease, especially in terms of the outcomes of eGFR subgroups. The CREDENCE study population had more advanced chronic kidney disease; their mean eGFR was 56.2 mL/min/1.73 m2 and 99% of the patients had an microalbuminuria level > 30 mg/g and a mean T2D duration of 15.8 years, while the population in our real‐world study had an eGFR level of 89.2 mL/min/1.73m2, an 8% rate of proteinuria, and a mean T2D duration of 6 years. In CREDENCE, patients in the eGFR 45 to 60 mL/min/1.73 m2 subgroup had an HR of 0.47 (95% CI 0.31–0.72) for the risk of a renal composite outcome. Interestingly, the results were comparable with our real‐world data, in which the HR for incident ESRD was 0.39 (95% CI 0.25–0.63) in the subgroup with an eGFR level < 60 mL/min/1.73 m2. As for all‐cause death, the patients with eGFR 60 to 90 and ≥ 90 mL/min/1.73 m2 were observed to have a lower risk of all‐cause deaths with SGLT2 inhibitors versus oGLDs, whereas those with eGFR <60 mL/min/1.73 m2 were not. However, it is difficult to draw any firm conclusions around this segment because there were relatively low numbers of patients and events in the subgroup with eGFR < 60 mL/min/1.73 m2, and the P for interaction was not statistically significant.
The glucose‐lowering effects of SGLT2 inhibitors decrease as renal function declines, which raises the possibility that the magnitude of benefit on glycaemia might be somewhat attenuated in patients with lower baseline eGFR levels mediated by reduced available nephron mass and diminished glucose reabsorption capacity. 16 , 17 , 18 However, SGLT2 inhibitors consistently decrease the risk of incident ESRD regardless of baseline eGFR, which indicates that the effects of SGLT2 inhibitors are partly mediated via non glucosuric‐dependent mechanisms. 19 , 20 , 21 Although pleiotropic effects of SGLT2 inhibitors have been inferred, improvement of glycaemic control, lowering of systemic blood pressure and intraglomerular pressure, reduction in albuminuria, and amelioration of volume overload are all plausible protective mechanisms with the possibility of a more comprehensive interactive effect. 5 , 22 , 23 SGLT2 inhibitors induce natriuresis, which activates tubulo‐glomerular feedback, reducing glomerular hypertension and hyperfiltration to limit kidney damage. The proposal of the haemodynamic mechanism of SGLT2 inhibitors is supported by the transient decline of eGFR after initiation of SGLT2 inhibitors followed by spontaneous recovery. 8 In this regard, the renoprotective mechanism of SGLT2 inhibitors can be linked in part to the renin‐angiotensin system blockade‐induced improvement of diabetic nephropathy, mainly by lowering intraglomerular pressure. 24 Furthermore, the impact of SGLT2 inhibitors on the risk of ESRD differed according to BMI in the present study, although a few cases of incident ESRD developed. Thus, obese patients (BMI ≥25 kg/m2) experienced a greater benefit of lowering the risk of ESRD compared to those with BMI <25 kg/m2. These findings suggest that the renal benefits of SGLT2 inhibitors may be amplified especially in obesity‐associated glomerular hyperfiltration in patients with T2D, although this issue warrants further investigation. 25 Another proposed mechanism of SGLT2 inhibitors is mediated by metabolic effects. SGLT2 inhibitors appear to decrease energy demand by reducing sodium transport and ameliorate cellular stress by enhancing AMPK/SIRT1, eventually leading to protection from functional and structural tubular injury by diabetes. 26 In addition, SGLT2 inhibitors could limit the activity of transforming growth factor β1, a known intrarenal cytokine associated with progressive kidney failure and lowering of the expression of inflammatory molecules. 27 , 28
The findings of the present study should be interpreted in the context of several potential limitations. First, due to the observational nature of the study, we cannot exclude the possibility of unmeasured confounding factors, which cannot be overcome by propensity‐score matching. Confounders such as socio‐economic factors, or metabolic variables that were not measured after baseline in this study, could have affected both the choice of glucose‐lowering medication and the outcomes. A novel drug may also be more readily prescribed to healthier patients, and those with relatively uncontrolled diabetes in the early post‐marketing period in the real world. 29 Second, an immortal time bias can arise if some proportion of the follow‐up time is excluded or misclassified. Third, renal function, including eGFR and the presence of proteinuria, was measured only at enrolment; therefore, subtle changes in renal function could not be included in the analyses. The presence of proteinuria was evaluated using a semi‐quantitative dipstick test, which has an approximately 30% false‐negative rate for microalbuminuria. Fourth, the follow‐up period was relatively short considering the clinical course of renal outcomes in patients with T2D, and there was no information about the precise cause of ESRD or mortality, or uric acid levels (which can be reduced by SGLT2 inhibitors). 30 Finally, these results have a limited generalizability to other (non‐Korean) populations.
The strengths of the present study include a large sample size, which encompassed the entire South Korean population, and the inclusion of several types of subgroup analyses, especially focused on the stratified renal function with eGFR and presence of proteinuria. These observational findings can be considered as complementary to the other large renal outcome trials of SGLT2 inhibitors including the CREDENCE trial, 4 the DAPA‐CKD 31 , 32 and ongoing EMPA‐Kidney trials. 33 Nevertheless, the vast majority of participants in these trials were recruited in the United States and Europe, thereby restricting the generalizability of the results. The present study reflects the utilization of SGLT2 inhibitors in clinical practice in an East Asian population. Thus, our findings suggest that the renal benefits of SGLT2 inhibitors may extend across different ethnic backgrounds.
In conclusion, the results of the present study suggest that SGLT2 inhibition had a statistically significant and clinically relevant association with lower risk of ESRD and all‐cause death in patients with T2D in a general Korean population of patients with T2D, evidence that is complementary to and consistent with what has been already observed in previous randomized controlled trials for this class of compounds. This benefit was consistent across the spectrum of eGFR at baseline. Findings from ongoing SGLT2 inhibitor trials will provide further evidence regarding how best to integrate these therapies into the care of patients with chronic kidney disease to improve outcomes. Moreover, longer‐duration studies in patients with renal dysfunction will ultimately inform the long‐term safety of SGLT2 inhibitor use.
CONFLICTS OF INTEREST
E.S.K., K.D.H., S.J.Y., H.S.K. received research grant from AstraZeneca. Y.S.N., E.T.W., P.F. are AstraZeneca employees. M.K. has served on advisory boards for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Glytec, Janssen, Merck (Diabetes), Novartis, Novo Nordisk, and Sanofi; served as a consultant for Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Intarcia, Novo Nordisk, and Sanofi, and received research grants from AstraZeneca and Boehringer Ingelheim. H.J.L.H. has consultancy agreements with Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Janssen, Fresenius, Gilead and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, and has a policy of honoraria going to his employer. He has also received grant support from Boehringer Ingelheim, AstraZeneca and Janssen (funding to his employer).
CONTRIBUTION STATEMENT
E.S.K., K.D.H., P.F., M.K., H.J.L.H. and H.S.K. contributed to the development of the study concept and design. E.S.K., K.D.H. and H.S.K. contributed to data collection and analysis, interpretation of the data, and writing of the article. Y.S.N., E.W. and S.J.Y. contributed to the interpretation of the data and critical review and revision of the article. P.F., M.K. and H.J.L.H. contributed to interpretation of the data, data report finalization, and critical review and revision of the article.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14239.
Supporting information
Figure S1 Kaplan–Meier estimates of the incidence of ESRD (left) and all‐cause death (right) between SGLT2 inhibitors (SGLT‐2i) versus other glucose‐lowering drugs (oGLDs) from sensitivity analysis restricting the follow‐up period to 3 years
Figure S2 Kaplan–Meier estimates of the incidence of ESRD (left) and all‐cause death (right) between SGLT2 inhibitors (SGLT‐2i) versus other glucose‐lowering drugs (oGLDs) from on‐treatment analysis
Table S1 Sensitivity analysis restricting the follow‐up period to 3 years
Table S2 Risk of ESRD and all‐cause deaths associated with SGLT2 inhibitors versus other glucose‐lowering drugs from on‐treatment analysis
Appendix S1 STROBE Statement—checklist of items that should be included in reports of observational studies
ACKNOWLEDGMENTS
This study was performed using the database from the NHIS (NHIS‐2019‐1‐230), and the results do not necessarily represent the opinion of the National Health Insurance Corporation. This study was funded by AstraZeneca.
Koh ES, Han K, Nam Y‐S, et al. Renal outcomes and all‐cause death associated with sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL 3 Korea). Diabetes Obes Metab. 2021;23:455–466. 10.1111/dom.14239
DATA AVAILABILITY STATEMENT
Data are available through the Korean National Health Insurance Sharing Service (NHISS). Researchers who wish to access the data can apply at https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do.
REFERENCES
- 1. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ravera M, Re M, Vettoretti S. Economic evaluation of angiotensin receptor blockers in type 2 diabetes, hypertension, and nephropathy. J Am Soc Nephrol. 2006;17(4 Suppl 2):S44‐S48. [DOI] [PubMed] [Google Scholar]
- 3. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 4. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. [DOI] [PubMed] [Google Scholar]
- 5. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 6. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606‐617. [DOI] [PubMed] [Google Scholar]
- 7. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587‐597. [DOI] [PubMed] [Google Scholar]
- 8. Pollock C, Stefansson B, Reyner D, et al. Albuminuria‐lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7(6):429‐441. [DOI] [PubMed] [Google Scholar]
- 9. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 10. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 11. Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628‐2639. [DOI] [PubMed] [Google Scholar]
- 12. Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real‐world clinical practice (CVD‐REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 13. Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375(9712):408‐418. [DOI] [PubMed] [Google Scholar]
- 14. Chan JCN, Yeung R, Luk A. The Asian diabetes phenotypes: challenges and opportunities. Diabetes Res Clin Pract. 2014;105(1):135‐139. [DOI] [PubMed] [Google Scholar]
- 15. Lamb EJ, Tomson CRV, Roderick PJ. Clinical Sciences Reviews Committee of the Association for Clinical B. Estimating kidney function in adults using formulae. Ann Clin Biochem. 2005;42(Pt 5):321‐345. [DOI] [PubMed] [Google Scholar]
- 16. Mima A. Renal protection by sodium‐glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J Diabetes Complications. 2018;32:720‐725. [DOI] [PubMed] [Google Scholar]
- 17. van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, RG IJ, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41(8):1543‐1556. [DOI] [PubMed] [Google Scholar]
- 18. Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752‐772. [DOI] [PubMed] [Google Scholar]
- 19. Petrykiv S, Sjostrom CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12(5):751‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138(15):1537‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium‐glucose cotransporter‐2 inhibitors. Kidney Int. 2018;94(1):26‐39. [DOI] [PubMed] [Google Scholar]
- 22. Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium‐glucose cotransporter 2 inhibitors: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(4):422‐434. [DOI] [PubMed] [Google Scholar]
- 23. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20(3):479‐487. [DOI] [PubMed] [Google Scholar]
- 24. Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45(2):281‐287. [DOI] [PubMed] [Google Scholar]
- 25. Helal I, Fick‐Brosnahan GM, Reed‐Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8(5):293‐300. [DOI] [PubMed] [Google Scholar]
- 26. Packer M. Interplay of adenosine monophosphate‐activated protein kinase/sirtuin‐1 activation and sodium influx inhibition mediates the renal benefits of sodium‐glucose co‐transporter‐2 inhibitors in type 2 diabetes: a novel conceptual framework. Diabetes Obes Metab. 2020;22(5):734‐742. [DOI] [PubMed] [Google Scholar]
- 27. Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low‐grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44(6):457‐464. [DOI] [PubMed] [Google Scholar]
- 29. Han E, Kim A, Lee SJ, et al. Characteristics of dapagliflozin responders: a longitudinal, prospective, nationwide dapagliflozin surveillance study in Korea. Diabetes Ther. 2018;9(4):1689‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bailey CJ. Uric acid and the cardio‐renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21(6):1291‐1298. [DOI] [PubMed] [Google Scholar]
- 31. Heerspink HJL, Stefansson BV, Chertow GM, et al. Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA‐CKD) randomized controlled trial. Nephrol Dial Transplant. 2020;35(2):274‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436‐1446. [DOI] [PubMed] [Google Scholar]
- 33. Zelniker TA, Braunwald E. Clinical benefit of cardiorenal effects of sodium‐glucose cotransporter 2 inhibitors: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(4):435‐447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Kaplan–Meier estimates of the incidence of ESRD (left) and all‐cause death (right) between SGLT2 inhibitors (SGLT‐2i) versus other glucose‐lowering drugs (oGLDs) from sensitivity analysis restricting the follow‐up period to 3 years
Figure S2 Kaplan–Meier estimates of the incidence of ESRD (left) and all‐cause death (right) between SGLT2 inhibitors (SGLT‐2i) versus other glucose‐lowering drugs (oGLDs) from on‐treatment analysis
Table S1 Sensitivity analysis restricting the follow‐up period to 3 years
Table S2 Risk of ESRD and all‐cause deaths associated with SGLT2 inhibitors versus other glucose‐lowering drugs from on‐treatment analysis
Appendix S1 STROBE Statement—checklist of items that should be included in reports of observational studies
Data Availability Statement
Data are available through the Korean National Health Insurance Sharing Service (NHISS). Researchers who wish to access the data can apply at https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do.


