Abstract
Objective
To systematically review evidence on the efficacy and safety of sleep deprivation (SD) as a treatment option for patients with unipolar or bipolar depression.
Methods
A systematic review according to PRISMA guidelines was conducted. The certainty of evidence was assessed using the GRADE approach. Controlled trials were included in efficacy analysis, case series for evaluating complications and qualitative studies for patients’ experiences.
Results
Eight controlled studies (368 patients), one qualitative study and seven case series (825 patients) were included. One week after treatment start, SD combined with standard treatment did not reduce depressive symptoms compared with standard treatment (standardized mean difference, SMD = −0.29, [95% confidence interval, CI: −0.84 to 0.25], p = 0.29). When excluding a study in elderly patients in a post hoc analysis, the difference was statistically significant (SMD = −0.54 ([95% CI: −0.86 to −0.22], p < 0.001)) but it diminished two weeks after treatment start. No superiority of SD was found compared with antidepressants, but SD may be superior to exercise in certain settings. It is uncertain whether SD affects quality of sleep, quality of life, everyday functioning or length of stay. Apart from switch to mania (ranging between 2.7% and 10.7%), no other serious complications were reported.
Conclusion
Sleep deprivation has been studied in a wide range of settings resulting in divergent results for the short‐term efficacy on depressive symptoms. Post hoc analyses indicated that there may be a significant but transient effect in certain populations. Further studies should focus on identifying subgroups of responders as well as examining feasibility in routine clinical care.
Keywords: bipolar syndrome, chronotherapy, depression, meta‐analysis, systematic review
Summations.
Sleep deprivation may have a transient effect on depressive symptoms in a subgroup of patients.
It is uncertain whether sleep deprivation affects the health‐related quality of life, everyday functioning, quality of sleep and length of hospital stay.
The transient effect of sleep deprivation limits its clinical relevance as an add‐on treatment to current antidepressants.
Limitations.
The meta‐analysis was based on post‐treatment assessments only, as information regarding mean change from baseline and the corresponding standard deviations was missing in almost all included studies.
Differences in several aspects of the included studies (mostly on study population, comparators, treatment protocols, and subsequent maintenance strategies) limited the certainty of evidence.
1. INTRODUCTION
Depression is a leading cause of disability worldwide, 1 causing a high burden of disease and substantial societal cost. 2 , 3 It is a major contributor to death by suicide 1 and is highly correlated with cardiovascular and other chronic disease‐related mortality. 4
Although antidepressant medications are more efficacious than placebo, a significant number of treatment‐seeking patients with depression do not respond sufficiently and even for responders several weeks may pass before an optimal therapeutic effect is reached. 5 , 6 , 7 This latency period (between start of medication to its full effect) is critical, as it has been found to be related to both increased risk for suicidal behaviour and poor treatment response. 8 , 9 Thus, identifying treatment options for alleviating depressive symptoms rapidly should be regarded as a prioritized goal in clinical psychiatric research.
A treatment method of interest is sleep deprivation (SD) or wake therapy, where a patient intentionally remains awake during one or more nights in order to regulate the diurnal rhythm and thereby alleviate depressive symptoms. Although instantaneous overnight remission of depressive symptoms after SD has been widely reported, relapse after recovery sleep is common. 10 In order to improve the effect of SD and to achieve maintained effect, several chronotherapeutic protocols have been developed. These protocols vary in several aspects: the type of SD (total, ie complete SD for a whole night or partial, ie parts of the night); the number of nights awake (single or repeated with intermittent nights with sleep); sleep management after SD (eg the length of recovery sleep, strategies for sleep phase advances and sleep time stabilization); maintenance strategies (eg concurrent light therapy or pharmacotherapy) and strategies for protocol adherence (eg hospital setting, various monitoring methods, availability of physical and social activities during the SD). When systematically evaluating the efficacy of SD as treatment of depression, it is important to take into account the heterogeneity of the treatment protocols as well as the instruments and timing of the clinical assessments. The effect of SD also needs to be evaluated in relation to the time period for which an additional treatment option is urgently needed (ie the time to response or remission of depression after start of treatment with the current antidepressants), a latency period of presumably more than two weeks. 8 A recent meta‐analysis suggests that SD may have an antidepressant effect in the first week of treatment in bipolar depression, 11 but a comprehensive review of SD for the whole spectrum of depressive disorders is warranted.
1.1. Aims of the review
The main objective of this review was to assess whether SD with or without subsequent light therapy is an effective treatment option by itself or in addition to standard treatment for patients with unipolar or bipolar depression compared with no SD or other treatment. In addition, the safety of SD was investigated.
2. METHODS
A systematic review was conducted as part of a health technology assessment (HTA) performed at HTA‐centrum, Sahlgrenska University Hospital in Gothenburg, Sweden. 12 The methods are based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA). 13 The PICO process (Population, Intervention, Comparator, Outcome) was used to define the research question and eligibility criteria for the literature search. The review was not registered in a prospective register prior to the literature search. However, the selection and analysis of the articles were based on the initially defined PICO and performed according to the current praxis at the HTA‐centrum.
2.1. Eligibility criteria
To be eligible, studies had to meet the following criteria:
Population: Study participants were adult patients (≥18 years old) with depression including bipolar depression (defined according to DSM criteria). 14
Intervention: SD for at least one night under supervision in an inpatient setting (with or without subsequent light therapy).
Comparator: (i) no SD with or without underlying standard treatment or (ii) other treatment (eg medication, exercise) than SD with or without standard treatment. Studies in which electroconvulsive therapy (ECT) was used as a standard treatment or comparator to SD were not eligible for inclusion.
Outcomes: The outcomes of primary interest were mortality (including suicide), self‐harm and depressive symptoms (assessed by validated instrument). Additional important outcomes were quality of sleep, health‐related quality of life measured with validated instruments, medication use, everyday functioning (activities of daily living, return to work) according to validated scales or administrative data, length of hospital stay, patients’ experience during treatment (based on qualitative studies), diurnal rhythm and complications. Eventual worsening in depressive symptoms was to be evaluated as part of effect measures rather than complications.
Types of study included the following: randomized controlled trials (RCTs) with at least five patients per group, cohort studies (with at least 10 patients per group), case series with at least 50 patients (for analysis of complications) and qualitative studies (for information on patients’ experience during treatment, with at least five patients). Studies had to be published in English or Scandinavian languages (Danish, Norwegian or Swedish). No restriction was applied to the date of publication.
2.2. Patient involvement
The PICO was reviewed by representatives from a local patient organization (Intresseförening Bipolär Sjukdom, IBIS) who confirmed the relevance of the outcomes at issue as well as emphasizing the importance of rapid relief of depressive symptoms from the patient's perspective.
2.3. Data sources and study selection
During March 2019, two authors (KM and IS) performed systematic searches in PubMed, EMBASE, the Cochrane Library, CINAHL, PsycInfo and a number of HTA databases. In June 2019, ClinicalTrials.gov was searched for relevant completed and ongoing trials. The details of the search strategy for each database are reported in Appendix S1. As a complement to this search, we also reviewed the reference lists of relevant articles. These two authors conducted the literature searches, selected studies, and independently of one another assessed the obtained abstracts and made a first selection of full‐text articles for inclusion or exclusion. Any disagreements were resolved in consensus. All remaining articles were sent to all authors who read the full‐text articles independently of one another and decided in a consensus meeting which articles should be included in the review. Excluded studies and reasons for exclusion are presented in the Appendix S2. The search was repeated in all the above databases in March 2020. Additional 139 abstracts were assessed by CW and MI without meeting the inclusion criteria.
2.4. Data extraction
Two reviewers (MI and LS) extracted data for each eligible study, and another author (CW or PS) verified the data extraction. We retrieved information on study design, location, clinical and demographic population data (including type of depression, gender and age distribution), treatment protocols, outcome measures and main findings. When needed, outcome values were retrieved from diagrams or calculated with help of online calculators. Additional study data were retrieved for three studies, after contacting the corresponding authors. 15 , 16 , 17
2.5. Assessment of quality
The risk of bias was evaluated by all authors using a checklist for assessment of RCTs from the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU). 18 This checklist, based on the Cochrane risk of bias tool, 19 assesses selection bias, performance bias, detection bias, attrition bias, reporting bias and conflicts of interest. Any discrepancies in assessments were resolved in consensus meetings. For qualitative studies, the tool of SBU for assessment of qualitative studies was used. 20
The certainty of evidence was assessed at outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. 21 The following factors were assessed: study limitations/risk of bias (including randomization, blinding, follow‐up, dropouts, compliance and intention‐to‐treat analysis); consistency (including direction and magnitude of effect across studies and overlap of confidence intervals); directness (including setting, population, intervention, control, outcome and comparison–in other words the generalizability); and precision (including sample size and width of confidence intervals). We initially assigned a high certainty level, but downgraded one or more levels to moderate, low or very low if issues with GRADE criteria regarding study quality, directness or precision were detected.
2.6. Publication bias
Potential publication bias was assessed by searching ClinicalTrials.gov and by visual inspection of funnel plots. The main strategy was to search for relevant studies that had been listed as completed on ClinicalTrials.gov but had not been published.
2.7. Statistics
When possible, data were combined in meta‐analysis for investigation of the aggregated effect. The meta‐analysis was performed with Review Manager (RevMan) [Computer program] version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. Notably, the meta‐analysis had to be based on post‐treatment assessments only, as information regarding mean change from baseline and the corresponding standard deviation is missing in almost all publications. This implies less statistical power to detect treatment effects, compared to the analysis in terms of change from baseline, which is used in the publications. Because different versions of Hamilton depression rating scale (HDRS) 22 were used for assessment of depression symptoms, we calculated standardized mean differences (SMD) and their 95% confidence intervals (CI). The level of statistical significance was set to p < 0.05. Effect sizes were pooled in a random‐effects model given expected heterogeneity between studies. Statistical heterogeneity was assessed with the χ 2 and I 2 statistics. When warranted, post hoc analyses were conducted for clarification, but not contributed in the overall conclusions of evidence synthesis (which were based on the PICO). No ethical approval was needed prior to the study as the analysed data were retrieved from previous published studies in which informed consent was obtained by primary investigators.
3. RESULTS
3.1. Search results
The literature search identified 2133 articles after removal of duplicates. After reading the abstracts, 2055 articles were excluded with additional 43 articles excluded after reading the articles in full text. The remaining 35 articles were sent to all participants of the project group to read in full text out of which 19 articles were finally included in the analysis. A flowchart of the study selection process is presented in Figure 1. No unpublished studies were found on our search on ‘ClinicalTrials.gov’.
Figure 1.
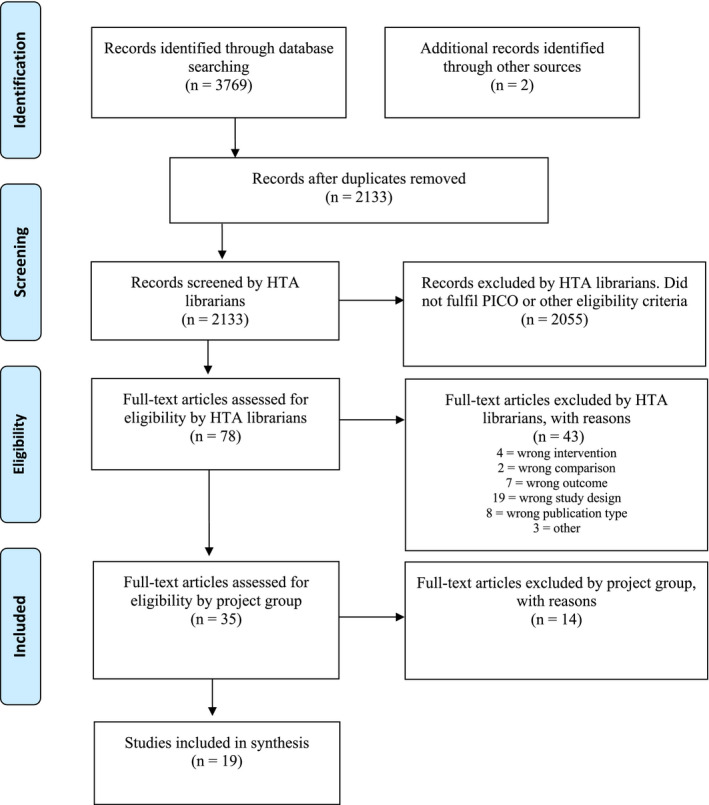
Flow diagram of selection process (PRISMA chart)
3.2. Characteristics of included studies
The included studies, their design and patient characteristics are presented in Table 1. In all, seven RCTs were included, 15 , 16 , 17 , 26 two of which had three treatment arms and contributed to both comparison of SD as add‐on to standard treatment and SD to other treatments. 14 , 22 The studies by Kundermann et al and by Martiny et al were reported in two 17 , 27 and three publications, 25 , 28 , 29 respectively. Six RCTs (n = 215 patients) and one cohort study (n = 41) investigated SD as add‐on compared with standard treatment. Three RCTs (n = 148 patients) compared SD with other treatment. 15 , 23 , 25 The characteristics of the included controlled trials and the results for each outcome are presented below separately for the comparison of i) SD as add‐on compared with standard treatment ii) for the comparison of SD versus other treatment. Apart from the studies above, seven case series 30 , 31 , 32 , 33 , 34 , 35 , 36 were included for the evaluation of rate of complications following SD and one qualitative study 37 contributed information regarding patients’ experience during treatment. Total rather than partial SD was used in all the included studies.
Table 1.
Characteristics of included studies
| First author, year, country | Study design | Length of follow‐up | Study group; intervention vs control | Patients (n) |
Age (mean ± SD) [range] |
Men (%) |
Outcome variables Comments |
|---|---|---|---|---|---|---|---|
| Benedetti, 2005, Italy 30 | Case series | 9 months | I: 3 TSD cycles + LT + standard treatment | Bipolar depression (60) | I: 46 ± 11 | Complications | |
| Benedetti, 1997, Italy 24 | RCT | 28 days |
I: 3 TSD cycles + fluoxetine C: fluoxetine |
Hospitalized patients with bipolar depression (10) |
I: 40 ± 12 C: 42 ± 10 |
33 |
HDRS |
| Colombo, 1999, Italy 31 | Case series |
I: 3 TSD cycles + standard treatment |
Bipolar depression (206) | I:46 ± 12 | 34 | Complications | |
| Colombo, 2000, Italy 32 | Case series | 1 week |
I: 3 TSD cycles + standard treatment |
Bipolar depression (115) | 33 | Complications | |
| Elsenga, 1982 Netherlands 23 | RCT | 15 days |
Ia: 4 TSD cycles + clomipramine Ib: 4 TSD cycles + placebo C: clomipramine |
Hospitalized patients with depression (30) |
Ia; 49 ± 14 Ib: 51 ± 18 C:51 ± 13 |
20 | HDRS |
|
Fähndrich, 1981, Germany 35 |
Case series | 4 days | I: TSD + standard treatment | Unipolar or bipolar depression (80) | 49 [20–78] | 41 | Complications |
| Gorgulu, 2009, Turkey 38 | Cohort study | 42 days |
I: 3 TSD cycles + sertraline C: sertraline |
Patients with major depression (41) |
I; 40 ± 12 C:33 ± 11 |
37 | HDRS, complications |
| Kragh, 2017a, Denmark 16 | RCT | 9 weeks |
I: 3 TSD cycles + LT +STS + standard treatment C: Standard treatment |
Hospitalized patients with depression (64) |
I: 38 (± 12) C: 40 (± 12) |
57 | HDRS, HrQoL, length of stay, level of functioning, quality of sleep, complications |
| Kragh, 2017b, Denmark 37 | Qualitative study | ‐ |
I: 3 TSD cycles + LT +STS + standard treatment |
Hospitalized patients with depression (13) | 37 [18‐66] | 62 | Comment: Overlap of study population with Kragh et al 2017 |
|
Kundermann, 2008, Germany 17 |
RCT | 3 weeks |
I: 6 TSD cycles + CBT C: CBT |
Hospitalized patients with depression (19) | 37 ± 8 | 57 | HDRS |
| Kundermann, 2009, Germany 27 | RCT | 3 weeks |
I: 6 TSD cycles + CBT C: CBT |
Hospitalized patients with depression (18) |
I: 37 ± 8 C: 37 ± 8 |
62 | Comment: Overlap of study population with Kundermann 2008 |
| Martiny, 2012, Denmark 25 | RCT | 9 weeks |
I: 3 TSD cycles + LT +STS + duloxetine C: Daily exercise + duloxetine |
Hospitalized patients with depression (75) |
I:47 ± 13 C:49 ± 11 |
41 | HDRS, level of functioning, HrQoL, quality of sleep, complications |
| Martiny, 2013, Denmark 28 | RCT | 1 week |
I: 3 TSD cycles + LT +STS + duloxetine C: Daily exercise + duloxetine |
Hospitalized patients with depression (75) |
I:47 ± 13 C:49 ± 11 |
41 |
Complications Comment: same study population as Martiny 2012 |
| Martiny, 2015, Denmark 29 | RCT | 20 weeks |
I: 3 TSD cycles + LT +STS + duloxetine C: Daily exercise + duloxetine |
Hospitalized patients with depression (75) |
I:47 ± 13 C:49 ± 11 |
41 |
HDRS, level of functioning, quality of sleep, complications Comment: same study population as Martiny 2012 |
| Reynolds, 2005, USA 15 | RCT | 2 weeks |
Ia: 1TSD cycle + placebo Ib: 1TSD cycle + paroxetine C: Paroxetine |
Outpatients with late‐life depression (80) |
Ia:71 ± 8 Ib:71 ± 7 C:70 ± 7 |
32 | HDRS |
| Rudolf, 1978, Germany 34 |
Case series Mixed method |
Night with TSD | I: 1 TSD cycle | Patients with depression, the majority hospitalized (67) | I: 48 | 39 | Complications |
| Suzuki, 2018, Japan 33 | Case series | 6 days | I: 3 TSD cycles + LT |
Hospitalized patients with bipolar depression (220) |
I: 47 ± 11 | 35 | Complications |
| Svendsen, 1976, Denmark 36 | Case series | TSD until discharge | I: 1 to 6 TSD cycles |
Hospitalized or patients or outpatients with unipolar or bipolar depression (77) |
I:[20 ‐ 72] | 19 | Complications |
| Wu, 2009, USA 26 | RCT | 7 weeks |
I: 1 TSD cycle + LT +SPA + medication C: medication |
Outpatients with bipolar major depressive episode (49) |
I: 39 ± 13 C:40 ± 14 |
74 | HDRS, complications |
Abbreviations: C, control group; ca, circa (approximately); CBT, cognitive behavioural therapy; HDRS, Hamilton depression rating scale; HrQoL, health‐related quality of life; I, intervention group; LT, light therapy; RCT, randomized controlled trial; SD, standard deviation; SPA, sleep phase advance; STS, sleep time stabilization; TSD, total sleep deprivation.
3.2.1. SD as add‐on compared to standard treatment
Six RCTs with a total of 215 patients 15 , 16 , 17 , 23 , 24 , 26 , 27 and one cohort study 38 in 49 patients compared SD as add‐on to standard treatment. Antidepressant medication was used as standard treatment in all studies except in one RCT where CBT was used. 17 , 27 Only one of these studies had no limitations regarding directness, precision and risk of bias. 16 All other studies had minor or major risk of bias—mainly due to limitations in blinding, and high or incompletely described dropout rates. The directness was limited in four of the studies, for example due to differing SD protocols (1 up to 6 wake nights) and patient populations (eg one study in elderly patients with late‐onset depression). 15 , 17 , 24 , 38 Furthermore, two studies had small sample sizes limiting the precision. 17 , 24 Two studies combined SD with chronotherapeutic interventions (light therapy, sleep time stabilization). 16 , 26
3.2.2. SD compared with other treatment
Three RCTs were included with a total of 148 patients comparing SD with other active treatment. Two studies compared SD with medication. 15 , 23 The third study compared SD combined with subsequent chronotherapeutic maintenance (light therapy and sleep time stabilization) with exercise as active comparator. 25 , 28 , 29 The risk of bias was judged to be minor in all three studies (some limitations in blinding, and some questions regarding the control treatments). Questions regarding directness were raised for two studies (one study only included elderly patients, and for the study comparing SD with exercise, the latter was of limited duration and intensity). Furthermore, one of these three studies had a small sample size limiting the precision. 23 The quality assessment of RCTs and the cohort study is presented in the outcome tables.
3.3. Outcomes
A summary of key findings is presented in Table 2.
Table 2.
Summary of findings, by comparison
| Outcomes | Number and type of studies (participants) | Absolute effect estimates | Certainty of evidence GRADE | |
|---|---|---|---|---|
| Sleep deprivation vs no sleep deprivation as add‐on treatment | ||||
| Depressive symptoms | ||||
| Within 1 week | 6 RCTs (215) & 1 cohort study d (49) |
SMD: −0.29 (95% CI −0.84 to 0.25), n.s. Subgroup analysis excluding a study in elderly patients with late‐onset depression: SMD: −0.54 (95% CI −0.86 to −0.22), p < 0.001 in favour of TSD. |
⊕⊕◯◯ a | |
| After more than 1 week | 6 RCTs (215) | SMD: −0.04 (95% CI −0.33 to 0.24), n.s. | ⊕⊕◯◯ a | |
| Quality of Sleep | 1 RCT (64) | Between‐group difference in weeks 2‐9, n.s. | ⊕◯◯◯ b | |
| HRQL | 1 RCT (64) | Between‐group difference in WHO‐5, n.s. | ⊕◯◯◯ b | |
| Everyday functioning | 1 RCT (64) | Between‐group difference in GAF, n.s. | ⊕◯◯◯ b | |
| Sleep deprivation vs other treatment | ||||
| Depressive symptoms | Sleep deprivation vs medication | ⊕◯◯◯ b | ||
| 2 RCTs (73) | Between‐group difference in HDRS n.s. in both studies | |||
| Sleep deprivation + chronotherapeutic maintenance vs exercise | ⊕⊕◯◯ c | |||
| 1 RCT (75) | Between‐group difference in HDRS, sign. advantage for TSD up to week 29. | |||
| Quality of Sleep | 1 RCT (75) |
Sleep deprivation + light therapy vs exercise: Sign. more patients with increased quality of sleep days 1‐8 after TSD than in control F1 = 10.5, p < 0.001 |
⊕◯◯◯ b | |
| HRQL | 1 RCT (75) |
Sleep deprivation + light therapy vs exercise: Between‐group difference in WHO‐5 Week 2 sign. in favour of TSD, week 8, n.s. |
⊕◯◯◯ b | |
| Everyday functioning | 1 RCT (75) |
Sleep deprivation + light therapy vs exercise Between‐group difference in GAF, n.s. |
⊕◯◯◯ b | |
| Certainty of evidence (GRADE): | ||||
| High certainty⊕⊕⊕⊕ | We are very confident that the true effect lies close to that of the estimate of the effect. | |||
| Moderate certainty ⊕⊕⊕◯ | We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. | |||
| Low certainty⊕⊕◯ ◯ | Confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. | |||
| Very low certainty⊕ ◯◯◯ | We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
Abbreviations: CI, confidence interval; GAF, global assessment of functioning; GRADE, grading of recommendations, assessment, development and evaluations; HDRS, Hamilton depression rating scale; HRQL, health‐related quality of life; n.s., not significant; RCT, randomized controlled trials; sign., significant; SMD, standardized mean difference; TSD, total sleep deprivation; vs, versus.
Downgraded two steps for some imprecision, some inconsistencies, some indirectness and serious study limitations (eg unclear randomization, high dropout, limitations in blinding).
Downgraded three steps for serious imprecision, some indirectness and serious study limitations (eg unclear randomization, lack of information on procedures in control group, high dropout, limitations in blinding).
Downgraded two steps for single study with some indirectness and some study limitations (eg limitations in blinding, questions about comparator of exercise).
The cohort study did not contribute to the GRADE rating.
3.4. Mortality (including suicide) and self‐harm
None of the included studies reported data regarding mortality or self‐harm.
3.5. Depressive symptoms
In all the included studies, depressive symptoms were assessed using the HDRS at baseline and several subsequent times during the studies. The HDRS ratings were assessed by clinicians/raters in all studies, but different versions of the scale have been used.
3.5.1. SD as add‐on compared with standard treatment
Six RCTs 15 , 16 , 17 , 23 , 24 , 26 , 27 and one cohort study 38 investigated the effect of SD as add‐on to standard treatment on depressive symptoms. Depressive symptoms were assessed for a study duration of 2–9 weeks (Appendix S3).
Effects of SD during the first week after treatment start
Four RCTs reported statistically significant differences between the treatment groups regarding depressive symptoms during the first week. In three studies, results were in favour of SD 16 , 24 , 26 and in one study in favour of the comparator. 15 A meta‐analysis of the post‐treatment HDRS data during the first week was statistically non‐significant for SD combined with standard treatment compared with standard treatment only (SMD = −0.29 [95% CI: −0.84 to 0.25], p = 0.29), with substantial heterogeneity in the analysis (I 2 = 70%) (Figure 2).
Figure 2.
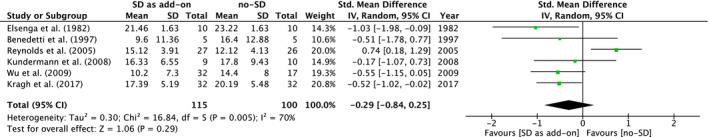
Meta‐analysis of the Hamilton depression rating scale (HDRS) scores during the first week after start of treatment with sleep deprivation as add‐on to standard treatment compared with standard treatment only
The RCT by Reynolds et al 15 was found to be the main source of the statistical heterogeneity. In a post hoc sensitivity analysis excluding Reynolds et al 15 , and thereby the only study specifically conducted in elderly patients, the heterogeneity resolved (I 2 = 0%) and the overall effect was statistically significantly different with a standardized mean difference of SMD = ‐0.54 [95% CI: −0.86 to −0.22], p = 0.0009 in favour of SD.
Given the idea to offer SD as an add‐on treatment to psychopharmacological treatment alone, an additional post hoc analysis of the five RCTs (n = 196 patients) with this design was conducted. The overall effect was not statistically significant one week after the start of treatment (SMD = −0.32 [95% CI −0.97 to 0.32]; p = 0.32) but showed statistically significant difference when excluding Reynolds et al 15 as above (SMD = −0.59 [95% CI: −0.94 to −0.25], p = 0.0007).
Further post hoc analyses were conducted by separating bipolar and unipolar depression. The RCT by Kragh et al 16 was excluded from these analyses because of mixed population. Thus, three studies were included in the meta‐analysis for unipolar depression 15 , 17 , 23 and two for bipolar depression. 24 , 26 One week after treatment start, the effects of SD were not statistically significant in studies on unipolar depression (SMD = −0.10 [95% CI −1.16 to 0.96], p = 0.85, I 2 = 81%). For studies on bipolar depression, there was a tendency towards significance at the same time point (SMD = −0.54 [95% CI −1.08 to 0.00], p = 0.05, I 2 = 0%).
When excluding the RCT on late‐life depression by Reynolds et al 15 , the effect size of SD in unipolar depression was numerically similar to bipolar depression but still not statistically significant (SMD = −0.59 [95% CI −1.44 to 0.25], p = 0.17, I 2 = 40%). Note, these analyses regarding subgroups of patients were conducted post hoc and are thus merely explorative.
Based on the GRADE assessment (Table 2), we conclude that SD given in addition to standard treatment, in patients with depression, may result in little or no difference in depressive symptoms compared with no add‐on treatment during the first week after treatment start (low certainty of evidence).
Effects of SD more than one week after treatment start
In five out of the six RCTs, no statistically significant effect of SD was observed in the subsequent weeks after SD. 15 , 16 , 17 , 23 , 24 One RCT reported a maintained effect of SD. 26 Meta‐analysis of the HDRS scores two to three weeks after first SD did not show any statistically significant differences (SMD = 0.13 [95% CI −0.38 to 0.64]; p = 0.61), I 2 = 63%. No reliable variability data were available for one of the studies 23 which therefore does not contribute to the meta‐analysis on the effect of SD more than one week after treatment start (Appendix S4). When excluding the study by Reynolds et al 15 , as above, the heterogeneity resolved (I 2 = 0%) but the comparison was not statistically different (SMD = −0.07 [95% CI −0.40 to 0.27], p = 0.70, I 2 = 0%). Stratified post hoc analyses did not reveal notable differences for bipolar depression (SMD = −0.33 [95% CI −0.87 to 0.20], p = 0.22, I 2 = 0%) or unipolar depression (SMD = 0.43 [95% CI −0.66 to 1.51], p = 0.44, I 2 = 76%). Inspection of the funnel plots based on the meta‐analyses of the post‐treatment HDRS data revealed no evidence of publication bias. Based on our GRADE assessment (Table 2), we conclude that SD given in addition to standard treatment, in patients with depression or bipolar depression, may have little or no persisting effect on depressive symptoms, after more than one week, compared with no add‐on treatment (low certainty of evidence).
3.5.2. SD compared with other treatment
Two RCTs with minor study limitations including 73 patients compared SD and concurrent administration of placebo with initiation of antidepressant medication. 15 , 23 No difference was found between SD compared with antidepressant medication during the two‐week follow‐up in the studies (Appendix S5). Based on our GRADE assessment (Table 2), we conclude that it is uncertain whether SD compared with medication affects depressive symptoms in patients with depression (very low certainty of evidence).
One study with minor study limitations (described in three publications focusing on different length of follow‐up) 25 , 28 , 29 compared SD followed by chronotherapeutic maintenance with exercise of limited duration and intensity. Patients in the SD group showed a rapid, statistically significant and larger reduction in depression scores than patients in the exercise group one week after start of treatment with SD (SMD = −0.83 [95% CI −1.30 to −0.36]; p = 0.06). The between‐group difference diminished over the 29 weeks of follow‐up (Appendix S5). Based on our GRADE assessment (Table 2), we conclude that SD with subsequent chronotherapeutic maintenance may result in reduced depressive symptoms compared with exercise in patients with depression starting antidepressant medication (low certainty of evidence).
Taken altogether, a meta‐analysis of the overall post‐treatment HDRS data during the first week showed no statistically significant superiority of SD compared with other treatment (antidepressants or exercise) (SMD = −0.18 ([95% CI: −0.92 to 0.55], p = 0.63). However, the heterogeneity in the analysis was substantial (I 2 = 77%) (Appendix S6).
3.6. Quality of sleep
The outcome quality of sleep was investigated in one RCT comparing SD as add‐on versus no add‐on treatment 16 and in one RCT comparing SD with exercise 25 , 28 , 29 (Appendix S7). In both studies, quality of sleep was self‐reported using non‐validated instruments. Both studies reported positive effects of the combination of SD, light therapy and sleep time stabilization on patients’ sleep duration, sleep maintenance and self‐reported sleep quality. A statistically significant advance of the sleep‐wake cycle was observed in one study, 29 indicating less problems falling asleep. Kragh et al 16 report a decrease in awakenings during the night and less day time sleeping in the first weeks after SD. Based on our GRADE assessment (Table 2), we conclude that it is uncertain whether SD affects the quality of sleep in patients with depression compared with no or other treatment (very low certainty evidence).
3.7. Health‐related quality of life (HRQL)
HRQL was measured with validated instruments in one RCT 16 comparing SD as add‐on versus no add‐on treatment and in one RCT 29 comparing SD to exercise (Appendix S8). Both studies 16 , 29 evaluated similar chronotherapeutic interventions (combination of SD, light therapy and sleep time stabilization) and measured HRQL with the WHO‐5 scale. Only one study 29 showed statistically significantly better self‐reported HRQL in the SD treatment group than in the control group. Based on our GRADE assessment (Table 2), we conclude that it is uncertain SD affects the health‐related quality of life measured in patients with depression compared with no or other treatment (very low certainty evidence).
3.8. Everyday functioning
Everyday functioning was investigated by using GAF assessments in one RCT 16 comparing SD as add‐on to medication with no add‐on treatment and in one RCT 29 comparing SD to exercise (Appendix S9). The two studies had similar intervention protocols for the SD groups, but they report GAF scores in different post‐treatment time periods (9 weeks and 29 weeks after SD, respectively). 16 , 29 No statistically significant effect on everyday functioning was found. Based on our GRADE assessment (Table 2), we conclude that it is uncertain whether SD affects the everyday functioning in patients with depression compared with no or other treatment (very low certainty evidence).
3.9. Length of hospital stay
One RCT 16 investigated the length of hospital stay in patients treated with SD as add‐on compared with no add‐on treatment (Appendix S10). No statistically significant difference was found between groups. Noticeably, the median length of hospital stay was numerically longer for the SD group. No studies investigated this outcome in comparison of SD to other treatment. Based on our GRADE assessment (Table 2), we conclude that it is uncertain whether SD in addition to standard treatment affects the length of hospital stay compared with no add‐on treatment in patients with depression (very low certainty of evidence).
3.10. Medication use
None of the included studies investigated the need for or changes in medication use before and after intervention. Data on psychotropic medication were reported in two studies 16 , 29 mainly serving as control information for a possible cofounder. No statistically significant differences between intervention and control groups were reported (very low certainty evidence).
3.11. Patient‐reported experience
Only one qualitative study was found to focus on patients’ experiences of SD when taking part in an RCT. 37 The quality of the study was evaluated as moderate because of lack of information on ethical rational (ie power imbalances during interviewing) and theoretical foundation (ie insufficient presentation of manifest analysis). The participants’ overall experiences were reported to be positive. A rapid but transient antidepressant effect was experienced by some patients whereas others described long‐term benefits, such as improved sleep and diurnal rhythms. Negative experiences were limited, and mostly related to disappointment surrounding inadequate or transient responses.
3.12. Complications
The systematic documentation of complications is limited in the included studies. Data are provided in three RCTs, 16 , 26 , 29 one cohort study 38 and seven case series 30 , 31 , 32 , 33 , 34 , 35 , 36 (Appendix S11).
The switch rate to manic state in patients with depression was reported in eight studies. 16 , 25 , 26 , 30 , 31 , 32 , 33 , 36 Summarized over all included studies above, the average switch rate in patients with bipolar disorder during SD treatment (650 patients with bipolar disorder) was 5.5% (ranging between 2.7% and 10.7%). The publications do not provide any information as to when the switch to mania occurred in relation to the SD. No conclusive data could be retrieved on mood switching in SD‐treated patients with unipolar depression.
Regarding the tolerability and feasibility of the treatment, relevant data were retrieved from three RCTs 26 , 29 , 38 and one cohort study. 38 Of the 152 patients who were treated with SD, 17 (11.2%) were reported as dropouts. The reasons for dropout were not specified in all cases, but ECT treatment and failure to adhere to study protocol were mentioned. A comparison with the control groups is not possible, since information on dropouts in the control groups is very limited. One patient in the control group developed polarity switch. 29 Two studies 16 , 29 described development or worsening of anxiety in a small number of patients following SD.
4. DISCUSSION
The primary aim of the systematic review was to assess the efficacy and safety of SD with or without subsequent chronotherapeutic maintenance in patients with depressive symptoms including bipolar depression. In summary, the meta‐analysis showed no statistically significant difference one week following start of intervention. However, in post hoc analyses excluding a study focusing on elderly patients, the effect size was moderate and statistically significant. Given the limited data available, treatment effect on other relevant outcomes is uncertain. Furthermore, no superiority of SD was found compared with antidepressants. Finally, one study suggested that SD with subsequent chronotherapeutic maintenance may be superior to exercise in patients with depression starting antidepressant medication and the superiority could be maintained for several weeks. 29 However, these findings based on a single study need replication for a thorough evaluation.
Boland et al 39 reported a meta‐analysis of the antidepressant effects of SD with focus on short‐term response rates and correlations of response to factors such as medication status, type of SD, age and gender. That review has a methodological approach that does not meet PRISMA guidelines. 13 A major limitation of that review article is the lack of comparison to a control group. Boland et al 39 observe that the response to SD was not correlated with the type of SD, medication status, diagnosis, age or gender of the study population. A more recent meta‐analysis supported that chronotherapy (SD combined with other interventions) has a rapid effect on depression. 40 However, our review had more stringent inclusion criteria focused on SD and we included RCTs that are not included in the meta‐analysis by Humptson et al 40 Moreover, the present analysis distinguished between different comparators (add‐on vs no add‐on, SD vs medication and SD vs exercise), include several outcomes and followed a different statistical approach. Ramirez‐Mahaluf et al 11 conducted a meta‐analysis on SD effect in bipolar depression, including exclusively studies on patients with bipolar disorder. In their efficacy analysis of SD as an add‐on treatment, two studies were included, both of which are covered in our analysis. 24 , 26 Ramirez‐Mahaluf et al 11 argue for a statistically significant effect of SD after one week. However, in our post hoc analyses, we found only a tendency towards significance (p = 0.05) for the same time period. Namely, Ramirez‐Mahaluf et al 11 measured time from the initiation of the study treatment protocol (including drug titration periods) and not specifically from the treatment start with SD. Thus, different baseline time points were used, leading to different conclusions on the short‐term effects of SD.
The effects of SD on unipolar compared with bipolar depression are worth further discussion. Circadian rhythm disruptions are common both in unipolar and bipolar depression. 41 Despite common mechanism‐of‐action targets for unipolar and bipolar depression, it has been debated whether the polarity of depression affects the response to SD, eventually in favour of bipolar depression. 42 In our post hoc analyses, we found similar numerical yet not statistically significant effect sizes for patients with bipolar depression and non‐elderly patients with unipolar depression. However, these considerations are merely explorative as they build on post hoc analyses of studies which in addition have methodological limitations (see below).
Total SD was used in all the included studies. Although total SD is the most established method in research and clinical praxis, different types of SD, such as late partial SD and selective Rapid Eye Movement‐SD (REM‐SD), have been presumed to have antidepressant effects. 43 , 44 However, a single study did not show any advantages of late partial compared to total SD regarding efficacy or adherence. 45 Moreover, Grözinger et al 46 compared REM‐SD to non‐REM‐SD without finding any significant difference on the alleviation of depressive symptoms.
4.1. Limitations
A key limitation of the present review was that the meta‐analysis was based on post‐treatment assessments only, as information regarding mean change from baseline and the corresponding standard deviation was missing in almost all publications. This approach is less powerful than the statistical analyses used in the individual publications, which consider repeated measures at different time points. Further limitations were the heterogeneity between studies in the study population (eg either or both unipolar and bipolar depressions), SD protocols (eg number of wake nights, use of other subsequent chronotherapeutic interventions) concurrent treatment (ongoing or starting antidepressant medication, other standard treatments) and outcome measures (eg different versions of the HDRS). In order to take both heterogeneity and differences in the included studies into account, we analysed the data using a random‐effects model, which is more conservative.
The study population varied across the included studies—mainly in terms of the diagnoses and suicidality of the included patients. Regarding diagnoses: two studies included patients with bipolar disorder 24 , 26 ; three studies recruited only patients with unipolar depression 15 , 17 , 38 ; two studies included patients with either unipolar or bipolar depression 16 , 25 ; and in one study, no exact information regarding the kind of depression was available. 23 All but two studies listed suicidality as an exclusion criterion. 24 , 38 It should be noted that the variety in sleep disturbances in patients with depression—ranging from insomnia to hypersomnia—has not been considered explicitly in the included studies. Moreover, anxiety is a common, agonizing symptom of depression and Martiny et al 25 commented that a high level of anxiety may be a contraindication for SD. For the other studies, it is unclear how many patients suffered from anxiety.
The treatment protocol varied from a single wake night 15 up to six wake nights within three weeks. 17 Subsequent maintenance strategies varied the following: some studies combined SD with medication only, whilst three trials 16 , 26 , 29 provided additional chronotherapeutic interventions (light therapy, sleep phase advance and/or sleep time stabilization). Overall, the most favourable results were reported after SD for three wake nights within one week in combination with medication and other chronotherapeutic interventions. It should be emphasized that the support offered to patients during wake nights differed considerably—in some studies various activities (requiring room and personnel) were offered, whereas patients in other studies merely were instructed to stay awake with very limited further support.
The limitation of using HDRS as depression rating scale is worth consideration—especially when investigating the effect of SD. The scale has been criticized, as changes in HDRS score may be observed even if a clinically relevant change in cardinal symptoms of depression is lacking. 47 , 48 , 49 Namely, the HDRS score may decrease due to changes in a subset of items related to sleep or appetite without corresponding changes in core symptoms such as depressed mood, and anhedonia. Moreover, a modified version of HDRS has been used in three of the included studies 17 , 23 , 26 and the comparability of these results may be affected.
4.1.1. Implication to practice
The paramount question is whether SD has a clinically relevant effect. In evaluating placebo‐controlled clinical trials of antidepressant medication, the American Food and Drug Administration (FDA) and European Medicines Agency (EMA) considered an average two‐point difference in HDRS‐17 score as a minimal clinically significant difference (comparing an active substance to placebo). 50 , 51 Meta‐analyses of currently used antidepressant medication compared with placebo regarding HDRS reported an SMD of −0.35 to −0.30 in patients with mild‐to‐moderate depression. 52 In this context, the effect size in the post hoc meta‐analysis of SD as add‐on treatment in non‐elderly psychiatric population would qualify as clinically relevant. However, the confidence interval is wide and the overall confidence in this finding from a post hoc analysis is low. Furthermore, the included studies reported transient effects that lasted for some days after SD—this duration needs to be evaluated in relation to the need for additional treatment options during the first weeks it takes until antidepressant medication gains effect. Still, even if SD only reduces depression symptoms for a limited duration, this may be of clinical value in the absence of other treatment options. Also, it remains to be seen, if SD may be repeated for renewed effect in patients who respond to this treatment.
Another important question regarding the clinical practice is the risk of complications because of the treatment. Apart from the risk of switch to mania, no other serious complication has been reported in the included studies. Switch from depression to mania is regarded as a fundamental and defining feature of bipolar disorder. 53 It may occur spontaneously or precipitated by stress or concurrent treatment. 54 The switch rate to mania during treatment with placebo has been estimated to 4.2% for patients with bipolar disorder. 55 According to Benedetti 56 , the switch rate to mania may rise to 15‐40% during treatment with antidepressants. There is also evidence that the study design, the type of antidepressants and the age of the participants may explain the variance in switch rate. 57 In the studies included in this review, a switch rate around 5.5% was reported. Yet, this observation is limited by the heterogeneity of treatment modalities and insufficient reporting of complications in most of the publications. Moreover, no study was specifically designed to assess the risk of manic switch meaning that a meta‐analysis of risk for patients with bipolar disorder was not possible. With respect to the clinical relevance, the risk of switch to mania should not be considered as an absolute contraindication for inpatient SD treatment of patients with bipolar disorder.
4.1.2. Implication for research
As conclusions are limited by the heterogeneity of treatment modalities and study population, further well‐designed RCTs are required to investigate the optimal treatment protocol and patient subgroups who could benefit from the treatment. A major issue in investigating the effects of SD is the impossibility of double‐blinded studies—thus, head‐to‐head to other treatment methods may be preferable.
The heterogeneity in the clinical response to SD may partly reflect the heterogeneous nature of depression 58 , 59 , 60 . The differentiation of unipolar and bipolar depression should as in most previous studies be considered in future research. Moreover, neuroimaging may improve the selection of patients who respond to SD although further research is required. 61 , 62 , 63
The age of the participants may play role in the heterogeneity in the clinical response to SD. Ageing affects namely the circadian rhythms as changes occur in seminal parts of the circadian system such as the retina and the suprachiasmatic nucleus in hypothalamus. 10 , 64 Moreover, late‐life depression may differ from early‐life depression in aetiology and response to treatment. 65 Thus, elderly depressed patients may respond differently to chronotherapies as also indicated by the not replicated study on elderly depressed patients. 15 Further studies need to evaluate the impact of ageing on sleep and the circadian system and on the efficacy of SD.
In conclusion, SD may have a role in the rapid relief of depression; however, the certainty of evidence is low. Furthermore, it is uncertain whether SD affects quality of sleep, health‐related quality of life, everyday functioning or length of hospital stay. Generally, the method is well‐tolerated, although the risk of switch to mania exists. Albeit the low grade of evidence, the treatment method of SD should be considered an important part of the future research in rapid relief of depression.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare in relation to the present work.
PEER REVIEW
The peer review history for this article is available at https://publo ns.com/publo n/10.1111/acps.13253.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Appendix S9
Appendix S10
Appendix S11
ACKNOWLEDGEMENTS
The authors wish to acknowledge Ulla Wide and Ludger Grote for valuable comments on the HTA report upon which this systematic review is based.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article.
REFERENCES
- 1. WHO . Depression and Other Common Mental Disorders: Global Health Estimates. Geneva, Switzerland: World Health Organization; 2017. Report No.: WHO/MSD/MER/2017.2. [Google Scholar]
- 2. Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the Global Burden of Disease study. J Psychiatr Res. 2020;2017:134‐140. [DOI] [PubMed] [Google Scholar]
- 3. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta‐analysis. JAMA Psychiatry. 2015;72(4):334‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Machado MO, Veronese N, Sanches M, et al. The association of depression and all‐cause and cause‐specific mortality: an umbrella review of systematic reviews and meta‐analyses. BMC Med. 2018;16(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905‐1917. [DOI] [PubMed] [Google Scholar]
- 6. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta‐analysis. Lancet. 2018;391(10128):1357‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hieronymus F, Emilsson JF, Nilsson S, Eriksson E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol Psychiatry. 2016;21(4):523‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Machado‐Vieira R, Baumann J, Wheeler‐Castillo C, et al. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals. 2010;3(1):19‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28‐40. 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 10. Wirz‐Justice A, Benedetti F, Terman M. Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light and Wake Therapy. 2nd revised ed Basel, New York: S. Karger; 2013:124 p. [Google Scholar]
- 11. Ramirez‐Mahaluf JP, Rozas‐Serri E, Ivanovic‐Zuvic F, Risco L, Vöhringer PA. Effectiveness of sleep deprivation in treating acute bipolar depression as Augmentation strategy: a systematic review and meta‐analysis. Front Psychiatry. 2020;11 10.3389/fpsyt.2020.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ioannou M, Greenbrook JTV, Larson T et al. Efficacy of sleep deprivation in patients with depression including bipolar depression. Gothenburg; 2019. Report No.: HTA report 2019:110.
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. APA . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 15. Reynolds CF, Smith GS, Dew MA, et al. Accelerating symptom‐reduction in late‐life depression: a double‐blind, randomized, placebo‐controlled trial of sleep deprivation. Am J Geriatr Psychiatry. 2005;13(5):353‐358. [DOI] [PubMed] [Google Scholar]
- 16. Kragh M, Martiny K, Videbech P, et al. Wake and light therapy for moderate‐to‐severe depression ‐ a randomized controlled trial. Acta Psychiatr Scand. 2017;136(6):559‐570. [DOI] [PubMed] [Google Scholar]
- 17. Kundermann B, Hemmeter‐Spernal J, Huber MT, Krieg J‐C, Lautenbacher S. Effects of total sleep deprivation in major depression: overnight improvement of mood is accompanied by increased pain sensitivity and augmented pain complaints. Psychosom Med. 2008;70(1):92‐101. [DOI] [PubMed] [Google Scholar]
- 18. SBU ‐ Swedish Agency for Health Technology Assessment and Assessment of Social Services . Checklist for quality assessment of randomised trials. https://www.sbu.se/globalassets/ebm/bedomning_randomiserade_studier_fullfolja.pdf Accessed May 25, 2019
- 19. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. SBU ‐ Swedish Agency for Health Technology Assessment and Assessment of Social Services . Tool to assess methodological limitations of qualitative evidence synthesis. https://www.sbu.se/globalassets/ebm/bedomning_studier_kvalitativ_metodik.pdf Accessed May 25, 2009
- 21. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elsenga S, van den Hoofdakker RH. Clinical effects of sleep deprivation and clomipramine in endogenous depression. J Psychiatr Res. 1982 1983;17(4):361‐374. [DOI] [PubMed] [Google Scholar]
- 24. Benedetti F, Barbini B, Lucca A, Campori E, Colombo C, Smeraldi E. Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci. 1997;247(2):100‐103. [DOI] [PubMed] [Google Scholar]
- 25. Martiny K, Refsgaard E, Lund V, et al. A 9‐week randomized trial comparing a chronotherapeutic intervention (wake and light therapy) to exercise in major depressive disorder patients treated with duloxetine. J Clin Psychiatry. 2012;73(9):1234‐1242. [DOI] [PubMed] [Google Scholar]
- 26. Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66(3):298‐301. [DOI] [PubMed] [Google Scholar]
- 27. Kundermann B, Strate P, Hemmeter‐Spernal J, Huber MT, Krieg J‐C, Lautenbacher S. Mid‐term effects of serial sleep deprivation therapy implemented in cognitive‐behavioral treatment on the neuroendocrine response to clomipramine in patients with major depression. J Psychiatr Res. 2009;43(7):711‐720. [DOI] [PubMed] [Google Scholar]
- 28. Martiny K, Refsgaard E, Lund V, et al. The day‐to‐day acute effect of wake therapy in patients with major depression using the HAM‐D6 as primary outcome measure: results from a randomised controlled trial. PLoS One. 2013;8(6):e67264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martiny K, Refsgaard E, Lund V, et al. Maintained superiority of chronotherapeutics vs. exercise in a 20‐week randomized follow‐up trial in major depression. Acta Psychiatr Scand. 2015;131(6):446‐457. [DOI] [PubMed] [Google Scholar]
- 30. Benedetti F, Barbini B, Fulgosi MC, et al. Combined total sleep deprivation and light therapy in the treatment of drug‐resistant bipolar depression: acute response and long‐term remission rates. J Clin Psychiatry. 2005;66(12):1535‐1540. [DOI] [PubMed] [Google Scholar]
- 31. Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Res. 1999;86(3):267‐270. [DOI] [PubMed] [Google Scholar]
- 32. Colombo C, Lucca A, Benedetti F, Barbini B, Campori E, Smeraldi E. Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry Res. 2000;95(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 33. Suzuki M, Dallaspezia S, Locatelli C, Uchiyama M, Colombo C, Benedetti F. Does early response predict subsequent remission in bipolar depression treated with repeated sleep deprivation combined with light therapy and lithium? J Affect Disord. 2018;15(229):371‐376. [DOI] [PubMed] [Google Scholar]
- 34. Rudolf GA, Tölle R. The course of the night with total sleep deprivation as antidepressant therapy. Waking Sleeping. 1978;2(2):83‐91. [Google Scholar]
- 35. Fähndrich E. Effects of sleep deprivation on depressed patients of different nosological groups. Psychiatry Res. 1981;5(3):277‐285. [DOI] [PubMed] [Google Scholar]
- 36. Svendsen K. Sleep deprivation therapy in depression. Acta Psychiatr Scand. 1976;54(3):184‐192. [DOI] [PubMed] [Google Scholar]
- 37. Kragh M, Møller DN, Wihlborg CS, et al. Experiences of wake and light therapy in patients with depression: a qualitative study. Int J Ment Health Nurs. 2017;26(2):170‐180. [DOI] [PubMed] [Google Scholar]
- 38. Gorgulu Y, Caliyurt O. Rapid antidepressant effects of sleep deprivation therapy correlates with serum BDNF changes in major depression. Brain Res Bull. 2009;80(3):158‐162. [DOI] [PubMed] [Google Scholar]
- 39. Boland EM, Rao H, Dinges DF, et al. Meta‐Analysis of the Antidepressant Effects of Acute Sleep Deprivation. J Clin Psychiatry. 2017;78(8):e1020‐e1034. [DOI] [PubMed] [Google Scholar]
- 40. Humpston C, Benedetti F, Serfaty M, et al. Chronotherapy for the rapid treatment of depression: A meta‐analysis. J Affect Disord. 2020;261:91‐102. [DOI] [PubMed] [Google Scholar]
- 41. Walker WH, Walton JC, DeVries AC, Nelson RJ. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barbini B, Colombo C, Benedetti F, Campori E, Bellodi L, Smeraldi E. The unipolar–bipolar dichotomy and the response to sleep deprivation. Psychiatry Res. 1998;79(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 43. Vogel GW, Vogel F, McAbee RS, Thurmond AJ. Improvement of depression by REM sleep deprivation: new findings and a theory. Arch Gen Psychiatry. 1980;37(3):247‐253. [DOI] [PubMed] [Google Scholar]
- 44. Schilgen B, Tölle R. Partial sleep deprivation as therapy for depression. Arch Gen Psychiatry. 1980;37(3):267‐271. [DOI] [PubMed] [Google Scholar]
- 45. Giedke H, Klingberg S, Schwärzler F, Schweinsberg M. Direct comparison of total sleep deprivation and late partial sleep deprivation in the treatment of major depression. J Affect Disord. 2003;76(1):85‐93. [DOI] [PubMed] [Google Scholar]
- 46. Grözinger M, Kögel P, Röschke J. Effects of REM sleep awakenings and related wakening paradigms on the ultradian sleep cycle and the symptoms in depression. J Psychiatr Res. 2002;36(5):299‐308. [DOI] [PubMed] [Google Scholar]
- 47. Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton depression rating scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161(12):2163‐2177. [DOI] [PubMed] [Google Scholar]
- 48. Hieronymus F, Nilsson S, Eriksson E. A mega‐analysis of fixed‐dose trials reveals dose‐dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pettersson A, Boström KB, Gustavsson P, Ekselius L. Which instruments to support diagnosis of depression have sufficient accuracy? A systematic review. Nord J Psychiatry. 2015;69(7):497‐508. [DOI] [PubMed] [Google Scholar]
- 50. Melander H, Salmonson T, Abadie E, van Zwieten‐Boot B. A regulatory Apologia–a review of placebo‐controlled studies in regulatory submissions of new‐generation antidepressants. Eur Neuropsychopharmacol. 2008;18(9):623‐627. [DOI] [PubMed] [Google Scholar]
- 51. Montgomery SA, Möller H‐J. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24(3):111‐118. [DOI] [PubMed] [Google Scholar]
- 52. Socialstyrelsen . Nationella riktlinjer: Vård vid depression och ångestsyndrom (National quidelines for depression and anxiety) [Internet]. Stockholm; 2017.
- 53. Goodwin FK, Jamison KR. Manic‐Depressive Illness. 2nd ed. New York: Oxford University Press; 2007. [Google Scholar]
- 54. Salvadore G, Quiroz JA, Machado‐Vieira R, Henter ID, Manji HK, Zarate CA. The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010;71(11):1488‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peet M. Induction of mania with selective serotonin re‐uptake inhibitors and tricyclic antidepressants. Br J Psychiatry J Ment Sci. 1994;164(4):549‐550. [DOI] [PubMed] [Google Scholar]
- 56. Benedetti F. Rate of switch from bipolar depression into mania after morning light therapy: a historical review. Psychiatry Res. 2018;261:351‐356. [DOI] [PubMed] [Google Scholar]
- 57. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106‐116. [DOI] [PubMed] [Google Scholar]
- 58. Østergaard SD, Jensen SOW, Bech P. The heterogeneity of the depressive syndrome: when numbers get serious. Acta Psychiatr Scand. 2011;124(6):495‐496. [DOI] [PubMed] [Google Scholar]
- 59. Ghaemi SN, Vöhringer PA. The heterogeneity of depression: an old debate renewed. Acta Psychiatr Scand. 2011;124(6):497. [DOI] [PubMed] [Google Scholar]
- 60. Merikangas KR, Wicki W, Angst J. Heterogeneity of depression: classification of depressive subtypes by longitudinal course. Br J Psychiatry. 1994;164(3):342‐348. [DOI] [PubMed] [Google Scholar]
- 61. Wu JC, Gillin JC, Buchsbaum MS, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(1–3):181‐186. [DOI] [PubMed] [Google Scholar]
- 62. Wu J, Buchsbaum MS, Gillin JC, et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry. 1999;156(8):1149‐1158. [DOI] [PubMed] [Google Scholar]
- 63. Clark CP, Brown GG, Archibald SL, et al. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Res. 2006;146(1):43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Campos Costa I, Nogueira Carvalho H, Fernandes L. Aging, circadian rhythms and depressive disorders: a review. Am J Neurodegener Dis. 2013;2(4):228‐246. [PMC free article] [PubMed] [Google Scholar]
- 65. Kok RM, Reynolds CF. Management of depression in older adults: a review. JAMA. 2017;317(20):2114‐2122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Appendix S9
Appendix S10
Appendix S11
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.


