Summary
Post‐transplant diabetes mellitus (PTDM) shows a relationship with risk factors including obesity and tacrolimus‐based immunosuppression, which decreases pancreatic insulin secretion. Several of the sodium–glucose‐linked transporter 2 inhibitors (SGLT2is) and glucagon‐like peptide 1 receptor agonists (GLP1‐RAs) dramatically improve outcomes of individuals with type 2 diabetes with and without chronic kidney disease, which is, as heart failure and atherosclerotic cardiovascular disease, differentially affected by both drug classes (presumably). Here, we discuss SGLT2is and GLP1‐RAs in context with other PTDM management strategies, including modification of immunosuppression, active lifestyle intervention, and early postoperative insulin administration. We also review recent studies with SGLT2is in PTDM, reporting their safety and antihyperglycemic efficacy, which is moderate to low, depending on kidney function. Finally, we reference retrospective case reports with GLP1‐RAs that have not brought forth major concerns, likely indicating that GLP1‐RAs are ideal for PTDM patients suffering from obesity. Although our article encompasses PTDM after solid organ transplantation in general, data from kidney transplant recipients constitute the largest proportion. The PTDM research community still requires data that treating and preventing PTDM will improve clinical conditions beyond hyperglycemia. We therefore suggest that it is time to collaborate, in testing novel antidiabetics among patients of all transplant disciplines.
Keywords: diabetes mellitus, type 2, retrospective studies, prospective studies, glucagon‐like peptide‐1 receptor, glucose, hypoglycemic agents, immunosuppression, cardiovascular diseases, insulin, renal insufficiency, chronic, atherosclerosis
Introduction
PTDM incidence; major controversies embedded in previous consensus recommendations; outcomes associated with PTDM, as they relate to diagnosis
Post‐transplant diabetes mellitus (PTDM) [1, 2] is a common and important complication after solid organ transplantation. Although the majority of PTDM data are derived from kidney transplant recipients, PTDM incidence has been reported as being higher in other solid organs. Compared to kidney transplant recipients, whose PTDM incidence ranges between 10% and 20% [3, 4, 5], PTDM occurs in 20% to 30% of heart transplant recipients [6], 20% to 40% of liver transplant recipients [7, 8, 9, 10, 11], and 20% to 40% of lung transplant recipients [12, 13] (as reviewed in [14] and shown in Fig. 2). It may seem that PTDM is more common among liver rather than kidney transplant recipients, as one center, which reported virtually the same immunosuppressant use in both types of solid transplant recipients, found that the incidence of PTDM was much higher in individuals receiving a liver rather than those receiving a kidney transplant (30% vs. 19%, respectively [9]). Risk factors for development of PTDM in liver transplant recipients are most often reported to be age [8], higher BMI [8, 10, 11], and especially hepatitis C virus (HCV) infection [7, 8, 10, 11] as well as use of tacrolimus [7, 8, 15, 16]. In one study, the incidence of PTDM was 42% in HCV‐positive patients compared to 19% in HCV‐negative patients, and the risk was particularly enhanced in patients using tacrolimus instead of cyclosporine [11].
Figure 2.
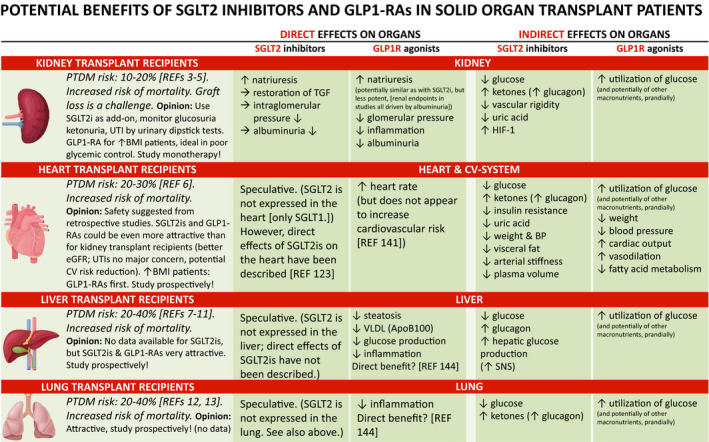
Potential benefits of SGLT2 inhibitors and GLP1R agonists in solid organ transplant patients. Abbreviations: PTDM = post‐transplantation diabetes mellitus, SGLT2i = sodium–glucose‐linked transporter 2 inhibitor, GLP1‐RA = glucagon‐like 1 receptor agonist, LDL = low‐density lipoprotein, TGF = tubuloglomerular feedback, SNS = sympathetic nervous system
The pathophysiology of PTDM is of intrinsic interest. Rather than being simply another form of type 2 diabetes, PTDM is a pathophysiological entity in its own right [17, 18, 19, 20]. The American Diabetes Association (ADA) previously classified PTDM in the category of “other specific types” of diabetes [21], but has recently described it as “diabetes after organ transplantation” [22]. At an expert meeting hosted in Vienna in 2013 [23], the objectives were to update previous consensus statements [17, 24], and secondly to debate deficiencies in the PTDM clinical evidence base. Discussions that ensued led to 7 recommendations (box):
Change terminology from New‐Onset Diabetes After Transplantation (NODAT) back to post‐transplantation diabetes mellitus (PTDM).
Exclude transient post‐transplantation hyperglycemia from PTDM diagnosis.
Expand screening tests for PTDM to incorporate postprandial (and evening) glucose monitoring and HbA1c to raise suspicion, while oral glucose tolerance tests remain the gold standard.
Identify patients at risk for PTDM.
Choose immunosuppression regimens so as to achieve the best outcome for patient and graft survival, irrespective of PTDM risk.
Adopt strategies for prevention and treatment beyond modification of immunosuppressive regimens.
Expand basic, translational, and clinical research in PTDM to address the areas of remaining controversy and speculation.
The previous recommendations 1, 2, and 7, in our understanding, have been well received and are widely accepted. The term PTDM, rather than “New‐Onset” Diabetes After Transplantation (NODAT), is an acknowledgment of the heterogeneity of dysglycemia within the group, with some patients having undiagnosed diabetes pretransplant and others destined to develop diabetes regardless of any effects associated with transplantation. Although not specifically mentioned in the paper [23], pretransplant identification of glucose disorders in patients on the transplant waitlist was also encouraged. Such screening efforts would ideally include an oral glucose tolerance test (OGTT) [25], but even an HbA1c measurement at time of transplantation, despite the well‐known pitfalls of the HbA1c arising, for example, from (changes in) erythropoiesis‐stimulating agents and the uremic state of patients with kidney disease [26], might be helpful for differentiating between PTDM and true NODAT, and also to clinically judge the course of hyperglycemia post‐transplantation.
However, there is still significant insecurity with respect to all other recommendations. Recommendations 3 and 4 deal with post‐transplant screening, diagnosis, and PTDM risk, and respective to these issues, it again has to be emphasized that PTDM and type 2 diabetes are not “one and the same.” Glycemic thresholds for diagnosis of type 2 diabetes have been thoroughly validated for the general population, based on microvascular complications (retinopathy) [27]. Only one study, from a single center, however, has specifically addressed retinopathy in transplanted individuals [28]. Therefore, it is currently unclear whether glycemic thresholds from the general population can be used in transplanted patients, due to their different pathophysiology for developing hyperglycemia. In the general population, diabetic complications may take years to manifest (for diabetic retinopathy, please refer to reference [29]), suggesting that studies in transplanted populations may require similarly long periods of time to be able to present diabetic secondary manifestations. Consistent with this assumption, an earlier registry study found only 8.3% ophthalmic complications among 4,105 patients with “new‐onset” diabetes over their 3‐year follow‐up (out of a total population of 21,489 primary kidney transplant recipients identified through the USRDS, 1995‐2001 [30]).
Based on the available evidence, the major concern with PTDM is not the risk of developing microvascular disease such as retinopathy (although data are scarce), but rather the increased risk of cardiovascular disease and death. For example, a study from the Oslo transplant center showed for the 10‐week post‐transplant time point that repeatedly elevated fasting plasma glucose ≥ 126 mg/dL and OGTT‐derived hyperglycemia at thresholds ≥ 200 mg/dL (diabetes) as well as ≥ 140 mg/dL (impaired glucose tolerance) were all associated with mortality in kidney transplant recipients, while HbA1c levels ≥ 6.5% were not associated with mortality [4]. In an earlier analysis, the same group has also shown a very similar mortality association for PTDM (“manifest” and diagnosed by OGTT‐derived 2‐hour plasma glucose) and impaired glucose tolerance [3]. The fact that the risk associated with hyperglycemia extends from PTDM to prediabetes was reaffirmed in a study from Spain where impaired glucose tolerance plus impaired fasting glucose, as well as PTDM itself, diagnosed at 12‐months post‐transplant, were associated with cardiovascular events in kidney transplanted patients [31]. How and when to screen for hyperglycemia during the unstable post‐transplant phase [32, 33], and the best way to identify patients at risk (previous recommendations 3 and 4) are therefore not trivial issues. However, the fact that there truly is an increased risk of mortality in kidney transplant recipients [34], especially if they have treated PTDM [35, 36], and that this mortality risk is most likely due to cardiovascular causes [3, 4, 5, 31, 37, 38], is by now well established.
Among solid organ transplant recipients other than kidney, analyzed together, PTDM based on HbA1c at 3 months post‐transplantation was recently reported to be prognostically relevant [39]. One study which involved nearly 1.000 liver transplant recipients found that PTDM persisting over 5‐10 years was associated with increased number of cardiovascular events [40], whereas 12‐yr survival was not affected in another study [15]. In a very detailed review from the year 2016 which also addressed this particular question, Shivaswamy et al. arrived at the conclusion that PTDM may only reduce short‐term survival after liver transplant, while the impact of PTDM on survival after lung transplant is unclear and PTDM after heart transplantation does not affect survival [1].
Understanding and further addressing the relationship between PTDM and outcomes will be important, as PTDM stands out as a potentially modifiable complication [41, 42, 43], but to date, there are no studies showing that treatment of PTDM actually positively modifies outcomes, other than glycemia [38]. One of the central debates in PTDM research related to this issue is the relative importance of the metabolic syndrome [20], and the question has been asked whether patients with morbid obesity should be transplanted at all, or whether the metabolic syndrome should come into control before transplant. Metabolic syndrome is prevalent among dialysis patients and, in the complex post‐transplant interplay between weight gain, exacerbation of underlying metabolic syndrome and increased diabetogenicity from immunosuppression can lead to increased risk for PTDM [44]. Pretransplantation weight is a risk factor for PTDM [45] and weight loss before kidney transplantation can successfully reduce the odds of developing PTDM [46]. This raises the question whether modifiable pretransplantation risk factors such as obesity should be targeted to reduce post‐transplant complications like PTDM. While no study has explored pharmacological interventions to target obesity pretransplantation, the strategy of surgical interventions has some limited evidence base. For example, in one published report bariatric surgery was shown to improve access to kidney transplantation while achieving measurable improvements in pretransplantation cardio‐metabolic profiles including weight loss and reduction in diabetes rates (albeit defined inadequately by need for glucose‐lowering therapy) [47]. In another small preliminary report, bariatric surgery was shown to improve pretransplant diabetes rates and PTDM was not observed in the 9/25 kidney transplant patients without pretransplant diabetes [48]. However, more definitive data are required concerning attenuation of post‐transplant cardio‐metabolic risks and long‐term safety profiles before surgical intervention can be advocated for eligible obese candidates.
The present review will not answer the specific question of obesity, but addresses the issue of tackling obesity and PTDM by lifestyle modification and using novel antidiabetic drugs. Our work to a large extent represents the experience and reading of all co‐authors, which encompasses the limitation that a predefined literature search was not formally performed. The text was inspired by the realization that management of PTDM, as would be embedded in the previous consensus recommendations 5 and 6, is still among the most controversial issues, as of today, and that the reason is lack of evidence. Thus, we believe that there is a pressing need to discuss here and subsequently collaborate on PTDM management.
Management of PTDM: immunosuppression
Immunosuppression remains the major modifiable risk factor for development of PTDM [49]. The transplant community by now has a common understanding of the diabetogenicity of commonly used immunosuppressants, and again most studies have been conducted after kidney transplantation rather than other solid organs. Calcineurin inhibitors are the mainstay of immunosuppression and decrease insulin release from pancreatic beta cells [50, 51, 52, 53, 54]. A randomized controlled study (“DIRECT”) [55] confirmed an earlier meta‐analysis on the increased diabetogenicity of tacrolimus compared to cyclosporine post kidney transplantation [56]. However, this study had limitations including short follow‐up (6 months) and tacrolimus trough levels above those now targeted by many transplant centers. Indeed, strategies to minimize or avoid calcineurin inhibitor exposure have been shown to reduce the odds of developing PTDM in a meta‐analysis of 56 randomized controlled trials (with concomitant superior overall graft survival) [57]. Torres et al. randomized de novo kidney transplant recipients without previous diabetes history but high PTDM risk into a tacrolimus versus a cyclosporine group (with and without early corticosteroid withdrawal in the tacrolimus group). Participants in the cyclosporine group had a lower 1‐year PTDM incidence that failed to reach statistical significance, but also an increased probability of acute rejection [58].
Conversion of tacrolimus to cyclosporine for improvement of the glucose metabolism in stable kidney transplant recipients has been suggested, both from previous literature [59] and one randomized controlled trial [60], where replacement of tacrolimus by cyclosporine in patients with PTDM resulted in diabetes reversal. In this trial [60], concomitant changes in insulin secretion and/or sensitivity were unfortunately not reported, but a study from Vienna suggested that the balance between these two entities might be deranged in kidney transplant recipients, compared with general population subjects as controls [61]. Recently, Müller et al. analyzed belatacept‐treated kidney transplant recipients as yet another control group, in comparison to tacrolimus‐treated kidney transplant recipients [62]. The tacrolimus‐treated kidney transplant recipients had lower insulin release in the presence of higher insulin sensitivity, when compared to belatacept‐treated kidney transplant recipients. The study also confirmed a lower PTDM rate with belatacept, compared to tacrolimus [62], which has recently been observed in another retrospective study [63]. The tacrolimus‐belatacept comparison represents new information, as the available literature had previously only suggested superiority of belatacept, in terms of a reduced PTDM incidence [64, 65], when this immunosuppressive agent was compared to cyclosporine. Additional insight is provided by a study showing that patients receiving tacrolimus, but not those receiving cyclosporine after kidney transplantation had a higher risk of developing PTDM, if they also had high triglycerides levels (i.e., metabolic syndrome) pretransplant [66]. Furthermore, from a mechanistic point of view, tacrolimus seems to interact with free fatty acids to impair beta cell function [67]. The recent re‐classification of individuals from the general population with type 1 diabetes, type 2 diabetes, and late autoimmune diabetes in adults into 5 novel categories (clusters), by their degree of insulin deficiency, respective to insulin resistance [68] may help the PTDM community in solving the conflict whether it is impaired insulin secretion [61, 62, 69] or higher insulin resistance that dominates the pathophysiology of PTDM patients [70]. As in the general population, there may be individual differences in PTDM patients, which are further modified by the type of immunosuppression that these individuals are receiving after solid organ transplantation.
Present knowledge indicates that mammalian target of rapamycin (mTOR) inhibitors can no longer be considered inert for development of PTDM. For example, registry data demonstrated an increased risk of PTDM in association with use of sirolimus [71], though patients in this study may have been exposed to levels higher than currently used in some centers. In the Symphony study where target levels of sirolimus were 3 to 7 ng/mL, sirolimus‐treated patients developed PTDM at a rate in‐between cyclosporine and tacrolimus [72]. While at least one report has reported no increase in PTDM in patients converted to an mTOR inhibitor [73], and a retrospective study suggested potential benefit for patients with established PTDM converting to an mTOR inhibitor [74], there are insufficient data to recommend this practice, especially in view of increased noncancer mortality risk associated with sirolimus [75, 76]. Mechanistically, Teutonico et al. have shown that both a decrease in beta cell function and a decrease in insulin sensitivity occurs when patients are switched from either cyclosporine A or tacrolimus to sirolimus, suggesting that mTOR inhibitors actually do not ameliorate the glycometabolic profile of kidney transplant recipients [77].
In contrast to calcineurin inhibitors and mTOR inhibitors, no link has been established between use of anti‐proliferative agents such as mycophenolate mofetil or azathioprine and the risk of PTDM. However, controversy remains within the transplant community with regards to corticosteroid avoidance (defined as complete avoidance or withdrawal within the first days kidney transplantation [78]) or corticosteroid withdrawal (defined as discontinuation at a certain time point in the later post‐transplant phase [78]), as potential strategies to attenuate the risk of PTDM. Despite the known contribution of corticosteroids to hyperglycemia and diabetes in general [79], a clear long‐term benefit from corticosteroid‐sparing strategies has not been demonstrated [80]. A meta‐analysis of corticosteroid withdrawal between 3 and 6 months after transplantation found no meaningful effect on PTDM incidence [81]. Early corticosteroid withdrawal within the first‐week post‐transplant has shown decreased PTDM incidence, but only in the context of cyclosporine use [82]. The caveat to this approach is the higher risk of acute rejection, which may counteract the beneficial glycemic effects [81]. Withdrawal of corticosteroids to prevent PTDM is not supported in the study by Woodle et al. [80]. Furthermore, when insulin sensitivity was assessed by a state‐of‐the‐art euglycemic, hyperglycemic clamp technique in kidney transplant recipients, insulin action did not improve when prednisolone was withdrawn from a 5 mg daily dose [83]. In the most recent Cochrane review on the use of prednisolone in kidney transplanted patients, published in 2016, no significant difference in PTDM risk was found comparing corticosteroid withdrawal versus maintenance (relative risk = 0.77, 95% confidence interval 0.49 to 1.21), and there was also no evidence to suggest a difference in patient mortality or graft loss up to five years after transplantation [78], while the risk of acute rejection was significantly increased [78]. However, in the more recently published HARMONY trial (which was not included in the Cochrane review [78]), rapid corticosteroid withdrawal, again, was associated with reduced PTDM incidence, which in this study was defined as a secondary endpoint [84]. As a side note, with high doses of prednisolone (20 mg per day), split dosing can reduce glycemic variability and peak hyperglycemia [85], and may be an interesting, but understudied option to potentially reduce PTDM risk.
Finally, with respect to induction therapy, one study reported a reduction in PTDM incidence with alemtuzumab compared to IL2 receptor antagonists [86], which was suggested to be related to a reduction in calcineurin inhibitor and corticosteroid dosing with alemtuzumab use. Another single‐center retrospective study of 264 renal transplant recipients suggested that basiliximab provides an intrinsic diabetogenic risk [87], with PTDM occurring at 10‐week post‐transplantation in 51.5% of patients receiving basiliximab versus 36.9% who did not receive induction, P = 0.017) [87]. In the absence of other studies, the significance of these results remains unclear.
In summary, 6 years after the consensus recommendations [23], there is no clear evidence that risk of PTDM can be decreased (e.g., by use of cyclosporine instead of tacrolimus or steroid avoidance/withdrawal), without increasing the risk of rejection. Therefore, the previous recommendation, which the immunosuppressive regimen should maximize patient and allograft survival, regardless of whether this increases the risk of PTDM [23], still appears sensible. Importantly, this view still acknowledges a generally recognized reduction in cardiovascular mortality [88], primarily due to the restoration of renal function. The long‐term success of the graft is of paramount importance for the outcome, which is assured by effective immunosuppression. As a consequence, tacrolimus has been the recommended calcineurin inhibitor since the 2009 KDIGO guidelines [89], and it seems reasonable that immunosuppression should be given primarily according to recommended guidelines [89].
Management of PTDM: early prevention—lifestyle and early insulin
While some debate may continue with respect to immunosuppressant modification, there appears to be agreement that prevention strategies to reduce risk for PTDM should be more actively encouraged. Lifestyle modification (dietary advice, physical activity, and weight loss encouragement) in kidney allograft recipients was reported to facilitate reversal of impaired glucose tolerance to normal glucose tolerance within 6‐months in a nonrandomized study [90]. In support of this concept, central obesity rather than body mass index is associated with development of PTDM [91]. A randomized controlled trial (CAVIAR) comparing active versus passive lifestyle intervention postkidney transplantation [92] demonstrated improvements in secondary clinical endpoints including weight loss, reduced fat mass, and a suggestion of reduced PTDM incidence (7.6% versus 15.6% respectively, P = 0.123). A multi‐center study with reduction in PTDM as the primary outcome is being planned, powered by the empirical data obtained from the CAVIAR study.
A step‐wise approach to management of established PTDM has much earlier been advocated [17], but with hyperglycemia arising in the immediate post‐transplant period, the reverse was subsequently recommended [23]. Insulin is the safest, most effective agent in the early post‐transplant period with possibly enduring benefits: In a randomized controlled proof‐of‐concept study, institution of basal insulin following detection of early post‐transplant hyperglycemia (<3 weeks) reduced subsequent odds of persistent PTDM in the first‐year post‐transplantation by 73% [41]. The results of a larger randomized controlled clinical trial (ITP‐NODAT, NCT03507829) have recently been submitted. Although PTDM incidence in the subsequent trial was dramatically smaller than in the previous proof‐of‐concept study, early intervention against post‐transplant hyperglycaemia by capillary blood glucose monitoring and basal insulin treatment, again, was safe and prevented overt PTDM onset. However, the primary endpoint (incidence of PTDM at 1 year) was only reached in the per‐protocol analysis which excluded some severe protocol violations resulting in diabetes misclassification [93], and additional analyses showed that the results depend on protocol adherence [94]. Of note, data from the previous proof‐of‐concept trial showed that this strategy, albeit decreasing the incidence of PTDM, may not improve cardiovascular complications [38].
If management of post‐transplant hyperglycemia is placed in context with treatment of postoperative hyperglycemia, then insulin will remain first choice [95, 96]. However, there are also advantages for postoperative initiation of antihyperglycemic therapy other than insulin, especially if postoperative hyperglycemia is mild. Recently issued guidelines on the Detection and Management of Diabetes post‐Solid Organ Transplantation by the Association of British Clinical Diabetologists (ABCD) and by the British Renal Association (RA) aimed to stratify a hierarchy of pharmacological therapy and encouraged metformin as first‐line oral therapy for suitable kidney transplant recipients, defined as eGFR of ≥ 30 ml/min/1.73m2 and BMI ≥ 25 kg/m2 [97]. The writing group, however, emphasized additional ABCD/RA guidance, clearly suggesting the use of metformin “sick day rules” whereby metformin therapy should be temporarily stopped when there is a risk of hypovolemia [98]. Among oral agents, some therapies like sulfonylureas, meglitinides, and thiazolidinediones were suggested with significant caveats in mind due to lack of evidence regarding efficacy and potential for significant side effects [97]. Therefore, the use of novel anti‐diabetics for management of PTDM is of increased interest, while glucose‐lowering therapies in the context of PTDM treatment also include dipeptidylpeptidase‐4 inhibitors and have been summarized in several reviews prior to the year 2018, by us and others [1, 99, 100, 101].
Management of PTDM: novel antidiabetics
The landscape of diabetes therapy in the general population has changed dramatically since the success of novel antidiabetic agents inhibiting sodium–glucose linked transporter 2 (SGLT2i), and acting as agonists of the glucagon‐like peptide 1 receptor (GLP1‐RA). Among several medical societies that have by now endorsed the SGLT2is and GLP1‐RAs in their guidelines are the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Specifically, an ADA/EASD Consensus Report from the year 2018 recommended GLP1‐RAs or SGLT2is for type 2 diabetics with established atherosclerotic cardiovascular disease (ASCVD) or chronic kidney disease (CKD), if their glycated hemoglobin (HbA1c) levels under therapy with metformin and lifestyle modification were above 7%, the recommended target for nonpregnant adults [102]. The choice of using either SGLT2i or a GLP1‐RA should depend on the predominance of CKD, ASCVD, or heart failure: Patients with a predominance of ASCVD should receive a GLP1‐RA, while patients with a predominance of heart failure patients or CKD should receive an SGLT2i with proven benefit in the respective conditions.
Only a few months after the publication of the ADA/EASD recommendations, the European Renal Association—European Dialysis and Transplant Association (ERA‐EDTA) working groups “DIABESITY” and “EUropean REnal and CArdiovascular Medicine” (EURECA‐m) published their consensus statement on nephroprotection and cardioprotection with SGLT2is and GLP1‐RAs in patients with diabetes mellitus and CKD [103]. An important update to the ADA/EASD Consensus Report [104] rendered the treatment less glucose‐centered, as was also stated in the most recent ADA document [105]: “Among patients with type 2 diabetes who have established atherosclerotic cardiovascular disease or indicators of high risk, established kidney disease, or heart failure, a sodium–glucose cotransporter 2 inhibitor or glucagon‐like peptide 1 receptor agonist with demonstrated cardiovascular disease benefit is recommended as part of the glucose‐lowering regimen independent of A1C and in consideration of patient‐specific factors.” Thus, metformin is still first choice when the patient does not have ASCVD, heart failure, or CKD. However, if one or more of the two comorbidities are present, metformin, and an SGLT2i, alternatively a GLP‐1RA (always with proven benefit) should be started together with metformin. And if a patient is on metformin and develops one such comorbidity, an SGLT2i or GLP‐1RA should be started regardless of HbA1c.
Recapitulating the highlights of the previous ADA/EASD guidelines [102, 104] and of the subsequent consensus statement by DIABESITY and EURECA‐m [103] is beyond the scope of this review, as they both focus on the general population with type 2 diabetes. However, we have summarized the results of the recent, major trials using SGLT2is and GLP1‐RAs in Table 1 (in the first row and third row, for the general population with T2DM). One trial that is not mentioned in Table 1 is Vertis (studying the SLGT2i ertugliflozin), which was presented at the ADA in June 2020. This trial found no significant superiority of the treatment versus placebo group, with respect to the primary outcome, although the numerical signal was in the same favorable direction as seen for the other SGLT2is. Among the explanations for this puzzling result, seemingly subtle differences in eligibility criteria have been put forth, as Vertis, when compared to EMPA‐REG‐OUTCOME, recruited patients above 40 years (versus any age in EMPA‐REG‐OUTCOME) [106]. Vertis moreover included only 6.4% and 8.4% participants of Asian and South American origin, respectively (versus 19% and 15% in EMPA‐REG‐OUTCOME, respectively) [106]. Despite the fact that both study populations had established cardiovascular disease, the existence of a class effect among the SGLT2is might not have to be questioned altogether.
Table 1.
Recent studies with novel antidiabeticss in the general population with type 2 diabetes (T2DM) and in solid organ transplant patients with post‐transplant diabetes mellitus (PTDM)
| Drug Class | Population | Study/Author | Agent | N patients | Organ | Result | Endpoint |
|---|---|---|---|---|---|---|---|
| SGLT2is | T2DM | EMPA‐REG OUTCOME [148] | Empagliflozin | n = 4687 versus n = 2333 (placebo) | HR = 0.86 (0.74‐0.99) | Death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. [Primary Endpoint.] | |
| EMPA‐REG OUTCOME [149] | Empagliflozin | n = 4687 versus n = 2333 (placebo) | HR = 0.61 (0.53‐0.70) | Incident or worsening nephropathy (progression to macroalbuminuria, doubling of serum creatinine, initiation of renal replacement therapy, or death from renal disease). [Prespecified renal endpoint.] | |||
| CANVAS [150] | Canagliflozin | n = 5795 versus n = 4347 (placebo) | HR = 0.86 (0.75‐0.97) | Death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. [Primary Endpoint.] | |||
| DECLARE‐TIMI [151] | Dapagliflozin | n = 8582 versus n = 8578 (placebo) | HR = 0.93 (0.84‐1.03) | Major adverse cardiovascular events, defined as cardiovascular death, myocardial infarction, or ischemic stroke. [Primary Endpoint.] | |||
| DECLARE‐TIMI [151] | Dapagliflozin | n = 8582 versus n = 8578 (placebo) | HR = 0.83 (0.73‐0.95) | Composite of cardiovascular death or hospitalization for heart failure. [Primary Endpoint.] | |||
| CREDENCE [152] | Canagliflozin | n = 2202 versus n = 2199 (placebo) | HR = 0.70 (0.59‐0.82) | End‐stage kidney disease (dialysis, transplantation, and sustained eGFR < 15 ml/min/ 1.73 m2), doubling of serum creatinine, or death from renal or cardiovascular causes. [Primary Endpoint.] | |||
| PTDM | Schwaiger et al. [111] | Empagliflozin | n = 14 | Kidney | no sign. | OGTT‐derived 2‐hour plasma glucose before versus 4 weeks after therapy initiation (replacement). | |
| Halden et al. [112] | Empagliflozin | n = 22 versus n = 22 (placebo) | Kidney | not possible to report | Change in weighted mean glucose by cont gluc monitoring, baseline to week 24 compared with placebo. | ||
| Mahling et al. [113] | Empagliflozin | n = 10 | Kidney | n.a. | Case series. | ||
| Shah et al. [114] | Canagliflozin | n = 24 | Kidney | n.a. | Not prespecified. | ||
| Attallah et al. [115] | Empagliflozin | n = 8 | Kidney | n.a. | Case series. | ||
| Cehic et al. [116] | Empagliflozin | n = 22 | Heart | n.a. | Retrospective study. | ||
| Muir et al. [117] | Empagliflozin | n = 19 | Heart | n.a. | Retrospective study. | ||
| GLP1‐RAs | T2DM | ELIXA [153] | Lixisenatide | n = 3034 versus n = 3034 (placebo) | HR = 1.02 (0.89‐1.17) | Cardiovascular death, myocardial infarction, stroke, or hospitalization for unstable angina. [Primary Endpoint.] | |
| LEADER [154] | Liraglutide | n = 4668 versus n = 4672 (placebo) | HR = 0.87 (0.78‐0.97) | First occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. [Primary Endpoint.] | |||
| LEADER [155] | Liraglutide | n = 4668 versus n = 4672 (placebo) | HR = 0.78 (0.67‐0.92) | New‐onset persistent macroalbuminuria, persistent doubling of serum creatinine, end‐stage kidney disease, or death due to renal disease. [Prespecified secondary renal outcomes.] | |||
| SUSTAIN‐6 [135] | Semaglutide | n = 1648 versus n = 1649 (placebo) | HR = 0.74 (0.58‐0.95) | First occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. [Primary Endpoint.] | |||
| EXSCEL [156] | Exenatide | n = 7356 versus n = 7396 (placebo) | HR = 0.91 (0.83‐1.00) | First occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. [Primary Endpoint.] | |||
| Harmony [157] | Albiglutide | n = 4731 versus n = 4732 (placebo) | HR = 0.78 (0.68‐0.90) | First occurrence of cardiovascular death, myocardial infarction, or stroke. [Primary Endpoint.] | |||
| REWIND [134] | Dulaglutide | n = 4949 versus n = 4952 (placebo) | HR = 0.88 (0.79‐0.99) | First occurrence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. [Primary Endpoint.] | |||
| PTDM | Halden et al. [130] | GLP‐1 | n = 12 (PTDM) vs n = 12 (no‐PTDM) | Kidney | Sign. lower in PTDM | Sample size calculation was based on GLP‐1 induced suppression of glucagon. | |
| Pinelli et al. [124] | Liraglutide | n = 5 | Kidney | n.a. | Case series. | ||
| Liou et al. [125] | Liraglutide | n = 7 | Kidney | n.a. | Retrospective study. | ||
| Singh et al. [126] | Dulaglutide | n = 63 | Kidney, liver, heart | n.a. | Retrospective study. | ||
| Singh et al. [127] | Dulaglutide., Liraglutide | n = 63, n = 25, respectively | Kidney, liver, heart | n.a. | Retrospective study. | ||
| Kukla et al. [128] | Lira, Exena, Dulaglutide | n = 17 | Kidney | n.a. | Retrospective study. | ||
| Thangavelu et al. [129] | Exena, Lira, Dula, Semaglutide | n = 19 | Kidney, liver, heart | n.a. | Retrospective study. |
Besides Vertis, the most recent developments (DAPA‐heart failure; DAPA‐CKD and EMPEROR) were also beyond the scope of this review. However, it is worth mentioning here that the 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease by the Kidney Disease: Improving Global Outcomes (KDIGO) initiative provides perhaps the most detailed overview for the general population, now emphasizing comprehensive care with risk factor management and antihyperglycemic therapy individualization in individuals with CKD and diabetes mellitus [107]. We strongly recommend this excellent and timely KDIGO publication as an important source of information and guidance, acknowledging, however, that the guideline is not specific in the setting of solid organ transplantation, where the target population does not consist 100% of individuals with CKD, and where patients also receive immunosuppressive therapy.
Mechanistic considerations
Although the mechanisms of action differ completely between SGLT2is that lead to excretion of glucose into the urine [108] and GLP1‐RAs which enhance the incretin effect [109], their effects on the primary endpoint (usually cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) shown in most trials seem amazingly similar between both drug classes (Table 1). However, when both drug classes are considered at detail, their impact on the kidney and heart is meaningfully different (e.g., GLP1‐RAs affect proteinuria but do not slow CKD progression and do not improve heart failure [110]). Some of the postulated mechanisms known form the general population are summarized with respective references in Table 2. These mechanisms of nephroprotection and cardioprotection have been thoroughly discussed and reviewed in the EURECA‐m and DIABESITY consensus statements [103].
Table 2.
Potential mechanisms of nephroprotection and cardioprotection exerted by novel antidiabetics (data from the general population)
| Drug Class | Benefit | Observations | First shown for … in | Mechanism | Details |
|---|---|---|---|---|---|
| SGLT2is | Nephroprotection | Estimated GFR dropped but then stabilized; Patients were less likely to double their serum creatinine and to start kidney replacement therapy. | Empagliflozin, EMPA‐REG OUTCOME[149, 158] | Hemodynamic effect | Amelioration of hyperfiltration in animal models of diabetic nephropathy [159, 160, 161]; according to a study measuring GFR and effective renal plasma flow in type 1 diabetics [162]: in hyperfiltration, empagliflozin attenuated GFR (hyperglycemia> euglycemia) by reducing increased Na and gluc reabsorption in proximal tubule, leading to more Na reaching lumen of distal tubule, being measured in macula densa, restoring tubuloglomerular feedback, increasing resistance in the vas afferens, reducing vasodilation (hallmark of the hyperfiltrating, and diabetic kidney). |
| Progression to macroalbuminuria was less frequent. | Empagliflozin, EMPA‐REG OUTCOME [149] | A consequence of the hemodynamic effect | Reduction in vascular rigidity [163, 164]; decrease in serum uric acid [165, 166]; natriuresis [167]; role of glucagon [168]; activation of HIF‐1 (hypoxia‐inducible factor) [169]. | ||
| Cardioprotection | CV events were reduced and hospitalization due to heart failure was less frequent. | Empagliflozin, EMPA‐REG OUTCOME [148] | Unknown; Could be several mechanisms [103, 170] | Reduction in SBP and DBP [171] (per se benefits diabetics [172]); diuretic effect [173, 174]); reduction of interstitial volume [175]; reduction in waist circumference [148] and vascular stiffness [164, 170]; changes in hematocrit mediating 50% of CV benefit [176]; reduction in renal blood flow leading to heart relief [103]; increased glucagon levels and associated ketone body formation may be beneficial [103, 120, 177]. | |
| GLP1‐RAs | Nephroprotection | Estimated GFR decreased (a small decrease of 2%). | Liraglutide, LEADER [155] | Complex | Mediated directly by GLP1; mediated by nitrogen monoxide (NO); various indirect effects, involving tubuloglomerular feedback, RAS, and (reviewed in [139] and [140]); intestine–kidney axis may be involved; GLP1RAs trigger natriuresis and diuresis [178, 179]. |
| New onset of persistent macroalbuminuria was reduced; Albuminuria was reduced. | Liraglutide, LEADER [155] | Anti‐inflammatory | Anti‐inflammatory in the kidney (animal model [180]); inhibition of T‐cell proliferation in experimental glomerulonephritis animal model [143]; lower inflammatory biomarkers in RCT [181]; suggested benefit for a variety of inflammatory diseases (reviewed in [144]). | ||
| Cardioprotection | CV events including MCIs were reduced. | Liraglutide, LEADER [154]; simultaneously for Semaglutide, SUSTAIN‐6 [135] | General improvement in CV risk profile | Reduction of body weight and blood lipids [182, 183]; even for albiglutide (unlikely to cross the blood‐brain barrier), a reduction in CV events has been shown [157], suggesting that GLP1‐RAs exert important peripheral effects on the cardiovascular system (summarized here: [184]). | |
| Anti‐atherosclerotic | Shown in ApoE deficient mice [185]; reduced intake of oxidized LDL cholesterol into foam cells [186]; GLP1‐RAs were shown to be anti‐inflammatory [186, 187]. |
SGLT2is
Metabolic and cardiovascular effects of empagliflozin shown in published PTDM studies
Based on the rationale that novel antidiabetic agents have been shown to be immensely beneficial in the general population with T2DM, the SGLT2i empagliflozin was used at the Vienna transplant center in patients with PTDM after kidney transplantation, in a study which resulted in the formally first publication on this subject (the EMpagliflozin in Post‐TRAnsplant Diabetes Mellitus [EMPTRA‐DM] pilot study [111]). Only a few months later, the transplant center in Oslo also published a randomized controlled study on empagliflozin administration after kidney transplantation (EMPA‐Renal Tx [112]), and several other studies have followed on kidney [113, 114, 115] and heart [116, 117] transplant patients.
EMPTRA‐DM: The Vienna study [111] aimed at obtaining data on glycemic control in post‐transplant diabetics under empagliflozin. Therefore, participants with pre‐existing insulin therapy were initially given empagliflozin (10 mg) as monotherapy over a period of 4 weeks. Oral glucose tolerance tests (OGTTs) before and after the 4‐week empagliflozin monotherapy, as well as the evaluated glucose profiles showed that empagliflozin monotherapy yielded inferior glucose control, in comparison to the pre‐existing insulin therapy [111]. The average 2‐hour plasma glucose value from the OGTT (primary endpoint), however, barely missed the limit of significance to show inferiority of empagliflozin, compared to previous insulin therapy (P = 0.06) [111]. To include the relatively small number of participants studied in EMPTRA‐DM (n = 14, per prespecified sample size calculation), markedly more patients had to be screened from 1120 outpatients. After the 4‐week empagliflozin monotherapy, the EMPTRA‐DM study was continued for 11 months further, but 6 participants withdrew, due to poor glycemic control, urinary tract infections, and worsened eGFR. Of the 8 participants remaining in the study, 3 had to be placed back on insulin therapy.
EMPA‐Renal Tx: The study in Oslo by Halden et al. was a randomized controlled double‐blind study [112]. Only patients with stable PTDM and transplant duration of at least 1 year were included. These patients were identified during their extensive post‐transplant visit at year 1, where all patients receive an OGTT. The EMPA‐Renal Tx study examined the efficacy and safety of empagliflozin (10 mg) as monotherapy or add‐on therapy in a total of 24 patients, against 25 placebo patients. Two participants did not complete the entire 24‐week study period. Two participants were able to reduce their pre‐existing insulin therapy, but no patient was able to completely omit the pre‐existing antidiabetic medication. An OGTT, a 24‐hour blood pressure measurement, and a body composition measurement (dual X‐ray) were also carried out at the beginning of the study and after 24 weeks. In addition, the study included a 24‐hour urine collection to evaluate glucosuria.
Considering the hierarchy inherent in the different study designs, the randomized, placebo‐controlled study from Oslo is clearly to be preferred over the Vienna study, which had only one study arm, so that each study participant served as his own control. Despite these differences in design, both studies arrived at remarkably similar conclusions:
i) Weak antihyperglycemic action of empagliflozin: The antihyperglycemic effect of empagliflozin was weak in both studies (HbA1c decrease of 0.2% at week 24 in Oslo compared to a slight HbA1c increase [+ 0.1%] in the placebo group; HbA1c increase of 0.4% in Vienna at the end of the study [with fewer cases]). The Oslo results also showed that HbA1c decrease depended on kidney function, based on the fact that the increase in renal glucose excretion by SGLT2 inhibition is strongly GFR‐dependent: at an eGFR < 50 mL/min/1.73m2 empagliflozin‐induced glucosuria and HbA1c reduction were virtually absent (Fig. 1a) [112].
ii) Kidney function under empagliflozin: In both studies (from Vienna and Oslo), the eGFR initially dropped, but then stabilized, indicating perhaps that one of the principal mechanisms of action of the SGLT2is (the hemodynamic effect [see Table 2]) took place in the study patients.
iii) Decrease in body weight but unaltered body composition: Empagliflozin led to a decrease in body weight in the Oslo study (3.5 kg compared to placebo in week 24; −5 kg in Vienna in month 12), but neither study noted a significant change in body composition (examined by bioimpedance spectroscopy [Vienna] or DXA [Oslo]) at study termination.
iv) Metabolism: Empagliflozin led to a decrease in uric acid concentration in both studies. In Oslo, the OGTTs (in contrast to Vienna, where glucose control became significantly worse) showed no group difference in insulin secretion or insulin sensitivity.
v) Urinary tract infections: Symptomatic bacterial urinary tract infections (UTIs) occurred in 2 dropouts and in 3 participants who completed the EMPTRA‐DM study [111]. UTIs were initially perceived as an important side effect of the empagliflozin therapy. However, the analysis of a suitable reference population (n = 24), matched at a 2:1 ratio to the EMPTRA‐DM participants over the same treatment period showed that 9 matched patients also suffered from a symptomatic UTI (P = 0.81, compared to empagliflozin patients, Fig. 1b). Not only 5 study participants in Vienna, but also 3 patients from the Oslo empagliflozin group and 3 patients from the Oslo placebo group suffered from urinary tract infections during the course of the respective studies. However, 2 participants of the Oslo empagliflozin group with urinary tract infections had to prematurely drop out of the EMPA‐Renal Tx study (due to urosepsis and recurrent UTI, which was already known in the latter case [112]).
Figure 1.
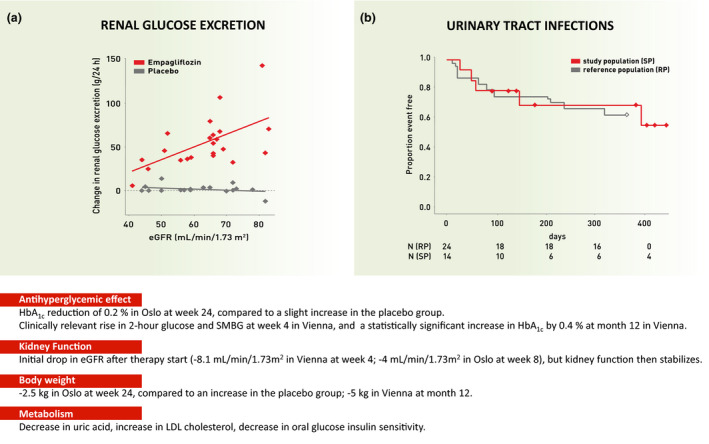
Messages derived from two prospective SGLT2 inhibitor studies in PTDM patients. EMPA‐Renal Tx study (Oslo, a = left panel [112]) and EMPTRA‐DM study (Vienna, b = right panel [111]). Abbreviations: LDL = low‐density lipoprotein, SMBG = self‐monitored blood glucose
Mechanistic considerations
The high number of dropouts in the EMPTRA‐DM study [111] and also the need for close monitoring, which was a primary finding from both the EMPTRA‐DM and the EMPA‐Renal Tx studies [112], demonstrate clearly that the antidiabetic agent empagliflozin and presumably also other SGLT2is can only be given with caution in PTDM patients. Mechanistically, kidney function is the primary limiting obstacle for glucose lowering. As the drug loses its glucose‐lowering effects [112, 118, 119], it might be reasonable to argue that prescribing SGLT2is in PTDM should be limited by eGFR, at least as long as a long‐term protective effect on the kidney graft (or cardiovascular outcomes) has not been proven for PTDM patients (in contrast to the general population with type 2 diabetes).
Another mechanistic aspect with SGLT2i that may often be overlooked (which is positive) and therefore deserves mentioning is the indirect organ protection (including cardioprotection), mediated by a mild increase of ketones observed under SGLT2 inhibition. With excess glucose leaving the human body through the urine, the ketone β‐hydroxybutyrate is freely taken up by various organs and oxidized to fatty acids. Such a fuel selection improves the transduction of oxygen into work efficiency at the mitochondrial level, through increased energy supply, and may thus also improve the metabolic status and function of heart, kidney, and possibly also other organs [120] (Fig. 2). Furthermore, this change in metabolism might also directly influence immune cells since these cells especially T‐cell subpopulations have a different metabolic profile [121, 122]. Thereby, ketosis might limit immune activation, which stops the development of fibrosis. Still, these effects could not only have a beneficial effect in CKD, but also in the post‐transplant situation. An additional, fascinating question is whether SGLT2is may exert a direct effect on the human heart (reviewed in [123]).
In summary, these data from Vienna and Oslo suggest that empagliflozin should primarily be used as add‐on therapy in PTDM due to its weak antihyperglycemic effect. Patients receiving empagliflozin should be closely monitored for kidney function, ketoacidosis, and urinary tract infections [111, 112]. Urinary dipstick tests, preferably performed by the patients themselves, while taking empagliflozin, may be particularly advisable. In support of these data from Vienna and Oslo, 5 additional publications, all case series and retrospective studies [113, 114, 115, 116, 117], have been subsequently published for the SGLT2is so far (summarized in Table 1, second row). Two of these publications reported on empagliflozin in heart transplant patients [116, 117].
GLP1‐RAs
Metabolic and cardiovascular effects of GLP1‐RAs shown in published studies
Compared to SGLT2is, the clinical experience of GLP1‐RAs in PTDM is entirely limited to case series and retrospective studies [124, 125, 126, 127, 128, 129]. The largest study, from Singh and colleagues, investigated the use of dulaglutide in 63 solid organ transplant recipients with diabetes (of whom 20 had PTDM compared to 43 with pre‐existing type 2 diabetes) [126]. At 12 months, a significant decrease was observed in weight and insulin requirement but not in HbA1c or survival outcome measures. Due to the retrospective nature of this and all other studies on GLP1‐RAs, changes in HbA1c could be a consequence of concomitant adoptions of the additional antihypergylcemic therapy (mostly insulin dosage). HbA1c reduction with GLP1‐RAs, when compared to the SGLT2is, does not depend on kidney function and has been clinically meaningful (from 10.0% to 8.1% in average) in 7 diabetic patients with poor glycemic control [125] (this study unfortunately did not explicitly state that these were PTDM patients, as opposed to T2DM patients after kidney transplantation). However, weight reduction was observed in all of these studies [124, 125, 126, 127, 128, 129] (Kukla et al. reporting a nonsignificant P‐value of 0.2 [128]).
Mechanistic considerations
From a mechanistic perspective, Halden and colleagues, using the hyperglycemic clamp, have shown PTDM patients in general have impaired insulin release and high glucagon levels which could partly be normalized with GLP‐1 infusion [130]. This study demonstrating the potential benefit of GLP1‐RAs in correcting pathophysiological defects in glucose metabolism suggests that further investigation of the benefits of GLP1‐RAs for PTDM is warranted.
Among GLP1‐RAs, the dosing of liraglutide does not require to be adjusted to impaired kidney function. However, liraglutide administration bares the risk of gastrointestinal side effects with reduced volume intake and consequently worsening eGFR [131]. Liraglutide should therefore no longer be used for the indication “weight regulation,” if the eGFR < 30 mL/min/1.73m2. For the indication diabetes mellitus, the only contraindication exists for actual end‐stage kidney failure [131], although liraglutide has been safely used in hemodialysis patients without increments in plasma levels of the drug [132]. Dulaglutide and semaglutide need to be injected only once weekly and can also be used in patients with impaired kidney function, down to an eGFR of 15 mL/min/1.73m2 (dulaglutide), and without contraindication except terminal kidney failure (semaglutide) [133]. The positive results from REWIND [134] and SUSTAIN‐6 [135] with regard to the primary endpoint (see Table 1) render dulaglutide and semaglutide attractive agents for patients with diabetes after kidney transplantation. The significant HbA1c reduction observed with dulaglutide [136] argues in favor of its use in patients with “derailed” type 2 diabetes after kidney transplantation and patients with “severe” PTDM, especially if obese [137]. Due to the gastrointestinal side effects, it may be necessary to monitor the calcineurin inhibitor trough levels (tacrolimus and cyclosporine). Although a recently published retrospective analysis on the use of dulaglutide after solid organ transplantation does not provide details about calcineurin inhibitor levels [126], a very recent study showed that endogenous incretin levels vary according to the type of calcineurin inhibitor used [138]. One of the primary tasks while performing outcome studies with GLP1‐RAs would therefore be to confirm the data on immunosuppressant blood levels, for example, tacrolimus area under the curve, during its use, which were previously published for a case series [124].
Several mechanisms have been proposed how GLP1‐RAs exert their cardio‐ but also nephroprotective effects (partly summarized in Table 2, illustrated in Fig. 2 and reviewed in [139] and [140]). Direct effects of GLP1‐RAs on the heart may to our knowledge not play a major role and include a small but significant rise in heart rate [141]. Indirect effects might be more important. Specifically, vasodilation through mechanisms that include blockade of atrial natriuretic peptide, endothelin‐1, the renin–angiotensin–aldosterone system, and sodium reabsorption in the proximal tubular system may play a central role [139]. Interestingly, a gut–renal axis may also be of note and involves both neural and non‐neural pathways [140]. One hypothesis that may not be well known is that by reducing prandial intraglomerular pressure, macronutrient loss into the glomerular filtrate may be prohibited [140]. Reduction in intraglomerular pressure and a subsequent antiproteinuric affect in the diabetic kidney, however, are also a consequence of GLP1‐RA induced natriuresis and subsequent increase in tubuloglomerular feedback, which most likely originates from GLP1‐RA‐induced inhibition of the sodium hydrogen exchanger 3 [140]. Besides inhibition of reactive oxygen species, GLP1‐RAs have also been shown to exert immunomodulatory properties [142]. The latter seem to be of utmost interest in the setting of organ transplantation, since recent murine data point toward a T‐cell anti‐proliferative capacity of GLP1‐RAs by changing glucose metabolism in T cells [143]. Based on previous literature, an earlier review also suggested a role for GLP1‐RAs in diseases of liver and lung, such as nonalcoholic steatohepatitis, asthma, and psoriasis [144]. Future studies, especially in humans, are needed to evaluate the effect of GLP1‐RAs on T‐cell proliferation and their potential role in the transplantation of these solid organs.
Summary and outlook
Management of PTDM remains the most controversial issue since the 2013 consensus recommendations [23]. In the most recent ADA document [105] following the 2018 ADA/EASD consensus report [102] and its update [104] on management of hyperglycemia in type 2 diabetes, an SGLT2i or GLP1‐RA was recommended for individuals with type 2 diabetes and established ASCVD, CKD, or heart failure as part of the glucose‐lowering regimen. The choice of using either SGLT2i or a GLP1‐RA should depend on the predominance of CKD, ASCVD, or heart failure: Patients with a predominance of ASCVD should receive a GLP1‐RA, while patients with a predominance of heart failure patients or CKD should receive an SGLT2i with proven benefit in the respective conditions. The mechanisms by which these novel antidiabetics may protect the kidney and the cardiovascular system are both complex and interesting, and they are currently being researched intensively. Individuals with PTDM after kidney and other solid organ transplantation, and even more so those with type 2 diabetes who have a known pretransplant history of hyperglycemia, are particularly affected by CKD and ASCVD. In consequence, it is sensible to implement recommendations for novel antidiabetics in the solid organ post‐transplant setting, as has already been proposed for liver [145] and heart transplant patients [146]. To date, there are, however, no studies showing that treatment of PTDM actually positively modifies outcomes, other than glycemia. It would therefore be trend‐setting to be able to demonstrate for SGLT2is and/or GLP1‐RAs in PTDM patients after solid organ transplantation that they modify progression of kidney disease, cardiovascular disease, and heart failure, perhaps translating into improved clinical outcomes, including mortality. In the case of the SGLT2is, several small studies have been conducted after transplantation, two of them prospective [111, 112, 113, 114, 115, 116, 117]. These studies show that SGLT2is can potentially be used in post‐transplant diabetes, but the patients should undergo close monitoring. In the case of the GLP1‐RAs, only retrospective case reports for PTDM patients are available [124, 125, 126, 127, 128, 130], wherein no serious safety concerns have arisen so far. Eight years after the initial call for clinical trials on PTDM treatment, it is therefore once again “time to collaborate” [147].
Conflict of interest
The authors have declared no conflict of interest.
Funding
The authors have declared no funding.
Author contribution
Author roles for each author: MH, AS, KE, and TJ wrote the paper.
Acknowledgements
We thank Elisabeth Schwaiger and Greisa Vila for critically revising the present manuscript.
References
- 1. Shivaswamy V, Boerner B, Larsen J. Post‐Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes. Endocr Rev 2016; 37: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharif A, Cohney S. Post‐transplantation diabetes‐state of the art. Lancet Diabetes Endocrinol 2016; 4: 337. [DOI] [PubMed] [Google Scholar]
- 3. Valderhaug TG, Hjelmesaeth J, Hartmann A, et al The association of early post‐transplant glucose levels with long‐term mortality. Diabetologia 2011; 54: 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eide IA, Halden TA, Hartmann A, et al Mortality risk in post‐transplantation diabetes mellitus based on glucose and HbA1c diagnostic criteria. Transpl Int 2016; 29: 568. [DOI] [PubMed] [Google Scholar]
- 5. Cosio FG, Kudva Y, van der Velde M, et al New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int 2005; 67: 2415. [DOI] [PubMed] [Google Scholar]
- 6. Kim HJ, Jung SH, Kim JJ, et al New‐Onset Diabetes Mellitus After Heart Transplantation‐ Incidence, Risk Factors and Impact on Clinical Outcome. Circ J 2017; 81: 806. [DOI] [PubMed] [Google Scholar]
- 7. AlDosary AA, Ramji AS, Elliott TG, et al Post‐liver transplantation diabetes mellitus: an association with hepatitis C. Liver Transpl 2002; 8: 356. [DOI] [PubMed] [Google Scholar]
- 8. Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. Risk factors for new‐onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing database. Transplantation 2010; 89: 1134. [DOI] [PubMed] [Google Scholar]
- 9. Munshi VN, Saghafian S, Cook CB, Werner KT, Chakkera HA. Comparison of post‐transplantation diabetes mellitus incidence and risk factors between kidney and liver transplantation patients. PLoS One 2020; 15: e0226873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parolin MB, Zaina FE, Araujo MV, Kupka E, Coelho JC. Prevalence of new‐onset diabetes mellitus in Brazilian liver transplant recipients: association with HCV infection. Transplant Proc 2004; 36: 2776. [DOI] [PubMed] [Google Scholar]
- 11. Saliba F, Lakehal M, Pageaux GP, et al Risk factors for new‐onset diabetes mellitus following liver transplantation and impact of hepatitis C infection : an observational multicenter study. Liver Transpl 2007; 13: 136. [DOI] [PubMed] [Google Scholar]
- 12. Hackman KL, Snell GI, Bach LA. Poor Glycemic Control Is Associated With Decreased Survival in Lung Transplant Recipients. Transplantation 2017; 101: 2200. [DOI] [PubMed] [Google Scholar]
- 13. Hackman KL, Bailey MJ, Snell GI, Bach LA. Diabetes is a major risk factor for mortality after lung transplantation. Am J Transplant 2014; 14: 438. [DOI] [PubMed] [Google Scholar]
- 14. Jenssen T, Hartmann A. Post‐transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol 2019; 15: 172. [DOI] [PubMed] [Google Scholar]
- 15. Aravinthan AD, Fateen W, Doyle AC, et al The Impact of Preexisting and Post‐transplant Diabetes Mellitus on Outcomes Following Liver Transplantation. Transplantation 2019; 103: 2523. [DOI] [PubMed] [Google Scholar]
- 16. Yoshida EM, Lilly LB, Marotta PJ, Mason AL, Bilodeau M, Vaillancourt M. Canadian national retrospective chart review comparing the long term effect of cyclosporine vs. tacrolimus on clinical outcomes in patients with post‐liver transplantation hepatitis C virus infection. Ann Hepatol. 2013; 12: 282. [PubMed] [Google Scholar]
- 17. Davidson J, Wilkinson A, Dantal J, et al New‐onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003; 75: SS3. [DOI] [PubMed] [Google Scholar]
- 18. Sarno G, Muscogiuri G, De Rosa P. New‐Onset Diabetes After Kidney Transplantation: Prevalence, Risk Factors, and Management. Transplantation 2012; 93: 1189. [DOI] [PubMed] [Google Scholar]
- 19. Hecking M, Kainz A, Werzowa J, et al Glucose Metabolism After Renal Transplantation. Diabetes Care 2013; 36: 2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hecking M, Sharif A, Port FK, et al Can new‐onset diabetes after kidney transplant be prevented? Diabetes Care 2013;36:1406–1412. Diabetes Care 2013; 36: e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diagnosis and classification of diabetes mellitus . Diabetes Care 2014; 37(Suppl 1): S81. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes‐2018. Diabetes Care 2018; 41: S13. [DOI] [PubMed] [Google Scholar]
- 23. Sharif A, Hecking M, de Vries AP, et al Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014; 14: 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilkinson A, Davidson J, Dotta F, et al Guidelines for the treatment and management of new‐onset diabetes after transplantation. Clin Transplant 2005; 19: 291. [DOI] [PubMed] [Google Scholar]
- 25. Guthoff M, Vosseler D, Langanke J, et al Diabetes Mellitus and Prediabetes on Kidney Transplant Waiting List‐ Prevalence, Metabolic Phenotyping and Risk Stratification Approach. PLoS One 2015; 10: e0134971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharif A, Baboolal K. Diagnostic application of the A(1c) assay in renal disease. J Am Soc Nephrol 2010; 21: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. International Expert Committee Diabetes Care International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. 2009; 32: 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Londero TM, Giaretta LS, Farenzena LP, et al Microvascular Complications of Posttransplant Diabetes Mellitus in Kidney Transplant Recipients: A Longitudinal Study. J Clin Endocrinol Metab 2019; 104: 557. [DOI] [PubMed] [Google Scholar]
- 29. Solomon SD, Chew E, Duh EJ, et al Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017; 40: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burroughs TE, Swindle J, Takemoto S, et al Diabetic complications associated with new‐onset diabetes mellitus in renal transplant recipients. Transplantation 2007; 83: 1027. [DOI] [PubMed] [Google Scholar]
- 31. Porrini E, Diaz JM, Moreso F, et al Prediabetes is a risk factor for cardiovascular disease following renal transplantation. Kidney Int 2019; 96: 1374. [DOI] [PubMed] [Google Scholar]
- 32. Porrini EL, Diaz JM, Moreso F, et al Clinical evolution of post‐transplant diabetes mellitus. Nephrol Dial Transplant 2016; 31: 495. [DOI] [PubMed] [Google Scholar]
- 33. Guthoff M, Wagner R, Weichbrodt K, et al Dynamics of Glucose Metabolism After Kidney Transplantation. Kidney Blood Press Res 2017; 42: 598. [DOI] [PubMed] [Google Scholar]
- 34. Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post‐transplant diabetes. Kidney Int 2002; 62: 1440. [DOI] [PubMed] [Google Scholar]
- 35. Dienemann T, Fujii N, Li Y, et al Long‐term patient survival and kidney allograft survival in post‐transplant diabetes mellitus: a single‐center retrospective study. Transpl Int 2016; 29: 1017. [DOI] [PubMed] [Google Scholar]
- 36. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003; 3: 178. [DOI] [PubMed] [Google Scholar]
- 37. Wauters RP, Cosio FG, Suarez Fernandez ML, Kudva Y, Shah P, Torres VE. Cardiovascular Consequences of New‐Onset Hyperglycemia After Kidney Transplantation. Transplantation 2012; 94: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Topitz D, Schwaiger E, Frommlet F, Werzowa J, Hecking M. Cardiovascular events associate with diabetes status rather than with early basal insulin treatment for the prevention of post‐transplantation diabetes mellitus. Nephrol Dial Transplant 2020; 35: 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mizrahi N, Braun M, Ben Gal T, Rosengarten D, Kramer MR, Grossman A. Post‐transplant diabetes mellitus: incidence, predicting factors and outcomes. Endocrine 2020; 69: 303. [DOI] [PubMed] [Google Scholar]
- 40. Roccaro GA, Goldberg DS, Hwang WT, et al Sustained Posttransplantation Diabetes Is Associated With Long‐Term Major Cardiovascular Events Following Liver Transplantation. Am J Transplant 2018; 18: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hecking M, Haidinger M, Doller D, et al Early basal insulin therapy decreases new‐onset diabetes after renal transplantation. J Am Soc Nephrol 2012; 23: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hecking M, Werzowa J, Haidinger M, et al Novel views on new‐onset diabetes after transplantation: development, prevention and treatment. Nephrol Dial Transplant 2013; 28: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chakkera HA, Weil EJ, Pham PT, Pomeroy J, Knowler WC. Can new‐onset diabetes after kidney transplant be prevented? Diabetes Care 2013; 36: 1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wissing KM, Pipeleers L. Obesity, metabolic syndrome and diabetes mellitus after renal transplantation: prevention and treatment. Transplant Rev (Orlando) 2014; 28: 37. [DOI] [PubMed] [Google Scholar]
- 45. Marrero D, Hernandez D, Tamajon LP, et al Pre‐transplant weight but not weight gain is associated with new‐onset diabetes after transplantation: a multi‐centre cohort Spanish study. NDT Plus 2010; 3: ii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang E, Abdalla B, Andre M, Bunnapradist S. Pre‐Transplant Weight Change and New‐Onset Diabetes After Kidney Transplantation [abstract]. Am J Transplant 2015; 15(suppl 3): 408. [Google Scholar]
- 47. Kassam AF, Mirza A, Kim Y, et al Long‐term outcomes in patients with obesity and renal disease after sleeve gastrectomy. Am J Transplant 2020; 20: 422. [DOI] [PubMed] [Google Scholar]
- 48. Bailey A, Kim Y, Woodle S, et al Prevention of New Onset Diabetes after Transplantation Using Bariatric Surgery [abstract]. Am J Transplant 2017; 17(suppl 3): 478. [Google Scholar]
- 49. Sharif A, Baboolal K. Risk factors for new‐onset diabetes after kidney transplantation. Nat Rev Nephrol 2010; 6: 415. [DOI] [PubMed] [Google Scholar]
- 50. Ozbay LA, Smidt K, Mortensen DM, Carstens J, Jorgensen KA, Rungby J. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS‐1E beta‐cells. Br J Pharmacol 2011; 162: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heit JJ, Apelqvist AA, Gu X, et al Calcineurin/NFAT signalling regulates pancreatic beta‐cell growth and function. Nature 2006; 443: 345. [DOI] [PubMed] [Google Scholar]
- 52. van Hooff JP, Christiaans MH, van Duijnhoven EM. Evaluating mechanisms of post‐transplant diabetes mellitus. Nephrol Dial Transplant 2004; 19: vi8. [DOI] [PubMed] [Google Scholar]
- 53. Polastri L, Galbiati F, Bertuzzi F, et al Secretory defects induced by immunosuppressive agents on human pancreatic beta‐cells. Acta Diabetol 2002; 39: 229. [DOI] [PubMed] [Google Scholar]
- 54. Redmon JB, Olson LK, Armstrong MB, Greene MJ, Robertson RP. Effects of tacrolimus (FK506) on human insulin gene expression, insulin mRNA levels, and insulin secretion in HIT‐T15 cells. J Clin Invest 1996; 98: 2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vincenti F, Friman S, Scheuermann E, et al Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 2007; 7: 1506. [DOI] [PubMed] [Google Scholar]
- 56. Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2005; 4: CD003961. [DOI] [PubMed] [Google Scholar]
- 57. Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R. Meta‐analysis of calcineurin‐inhibitor‐sparing regimens in kidney transplantation. J Am Soc Nephrol 2011; 22: 2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Torres A, Hernandez D, Moreso F, et al Randomized Controlled Trial Assessing the Impact of Tacrolimus Versus Cyclosporine on the Incidence of Posttransplant Diabetes Mellitus. Kidney Int Rep 2018; 3: 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ghisdal L, Bouchta NB, Broeders N, et al Conversion from tacrolimus to cyclosporine A for new‐onset diabetes after transplantation: a single‐centre experience in renal transplanted patients and review of the literature. Transpl Int 2008; 21: 146. [DOI] [PubMed] [Google Scholar]
- 60. Wissing KM, Abramowicz D, Weekers L, et al Prospective randomized study of conversion from tacrolimus to cyclosporine A to improve glucose metabolism in patients with posttransplant diabetes mellitus after renal transplantation. Am J Transplant 2018; 18: 1726. [DOI] [PubMed] [Google Scholar]
- 61. Hecking M, Kainz A, Werzowa J, et al Glucose metabolism after renal transplantation. Diabetes Care 2013; 36: 2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Muller MM, Schwaiger E, Kurnikowski A, et al Glucose Metabolism After Kidney Transplantation: Insulin Release and Sensitivity With Tacrolimus‐ Versus Belatacept‐Based Immunosuppression. Am J Kidney Dis 2020; 6: 30932. [DOI] [PubMed] [Google Scholar]
- 63. Terrec F, Jouve T, Naciri‐Bennani H, et al Late Conversion From Calcineurin Inhibitors to Belatacept in Kidney‐Transplant Recipients Has a Significant Beneficial Impact on Glycemic Parameters. Transplant Direct 2020; 6: e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vanrenterghem Y, Bresnahan B, Campistol J, et al Belatacept‐based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT‐EXT studies). Transplantation 2011; 91: 976. [DOI] [PubMed] [Google Scholar]
- 65. Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev. 2014; 3: CD010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Porrini E, Delgado P, Alvarez A, et al The combined effect of pre‐transplant triglyceride levels and the type of calcineurin inhibitor in predicting the risk of new onset diabetes after renal transplantation. Nephrol Dial Transplant 2008; 23: 1436. [DOI] [PubMed] [Google Scholar]
- 67. Trinanes J, Rodriguez‐Rodriguez AE, Brito‐Casillas Y, et al Deciphering Tacrolimus‐Induced Toxicity in Pancreatic beta Cells. Am J Transplant 2017; 17: 2829. [DOI] [PubMed] [Google Scholar]
- 68. Ahlqvist E, Storm P, Karajamaki A, et al Novel subgroups of adult‐onset diabetes and their association with outcomes: a data‐driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018; 6: 361. [DOI] [PubMed] [Google Scholar]
- 69. Langsford D, Obeyesekere V, Vogrin S, et al A Prospective Study of Renal Transplant Recipients: A Fall in Insulin Secretion Underpins Dysglycemia After Renal Transplantation. Transplant Direct 2016; 2: e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bergfeld L, Werzowa J, Saemann M, Hecking M. Correspondence regarding the impact of kidney transplantation on insulin sensitivity. Transpl Int 2018; 31: 456. [DOI] [PubMed] [Google Scholar]
- 71. Johnston O, Rose CL, Webster AC, Gill JS. Sirolimus is associated with new‐onset diabetes in kidney transplant recipients. J Am Soc Nephrol 2008; 19: 1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ekberg H, Tedesco‐Silva H, Demirbas A, et al Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562. [DOI] [PubMed] [Google Scholar]
- 73. Murakami N, Riella LV, Funakoshi T. Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: a systematic review and meta‐analysis. Am J Transplant 2014; 14: 2317. [DOI] [PubMed] [Google Scholar]
- 74. Veroux M, Tallarita T, Corona D, et al Conversion to sirolimus therapy in kidney transplant recipients with new onset diabetes mellitus after transplantation. Clin Dev Immunol 2013; 2013: 496974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Knoll GA, Kokolo MB, Mallick R, et al Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta‐analysis of individual patient data. BMJ 2014; 349: g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sharif A. Sirolimus after kidney transplantation. BMJ 2014; 349: g6808. [DOI] [PubMed] [Google Scholar]
- 77. Teutonico A, Schena PF, Di Paolo S. Glucose metabolism in renal transplant recipients: effect of calcineurin inhibitor withdrawal and conversion to sirolimus. J Am Soc Nephrol 2005; 16: 3128. [DOI] [PubMed] [Google Scholar]
- 78. Haller MC, Royuela A, Nagler EV, Pascual J, Webster AC. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev 2016; 4: CD005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu D, Ahmet A, Ward L, et al A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 2013; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P. A prospective, randomized, double‐blind, placebo‐controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long‐term, low‐dose corticosteroid therapy. Ann Surg 2008; 248: 564. [DOI] [PubMed] [Google Scholar]
- 81. Pascual J, Galeano C, Royuela A, Zamora J. A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation 2010; 90: 343. [DOI] [PubMed] [Google Scholar]
- 82. Pascual J, Royuela A, Galeano C, Crespo M, Zamora J. Very early steroid withdrawal or complete avoidance for kidney transplant recipients: a systematic review. Nephrol Dial Transplant 2012; 27: 825. [DOI] [PubMed] [Google Scholar]
- 83. Midtvedt K, Hjelmesaeth J, Hartmann A, et al Insulin resistance after renal transplantation: the effect of steroid dose reduction and withdrawal. J Am Soc Nephrol 2004; 15: 3233. [DOI] [PubMed] [Google Scholar]
- 84. Thomusch O, Wiesener M, Opgenoorth M, et al Rabbit‐ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open‐label, multicentre, randomised controlled trial. Lancet 2016; 388: 3006. [DOI] [PubMed] [Google Scholar]
- 85. Yates CJ, Fourlanos S, Colman PG, Cohney SJ. Divided dosing reduces prednisolone‐induced hyperglycaemia and glycaemic variability: a randomized trial after kidney transplantation. Nephrol Dial Transplant 2014; 29: 698. [DOI] [PubMed] [Google Scholar]
- 86. Morgan RD, O'Callaghan JM, Knight SR, Morris PJ. Alemtuzumab induction therapy in kidney transplantation: a systematic review and meta‐analysis. Transplantation 2012; 93: 1179. [DOI] [PubMed] [Google Scholar]
- 87. Aasebo W, Midtvedt K, Valderhaug TG, et al Impaired glucose homeostasis in renal transplant recipients receiving basiliximab. Nephrol Dial Transplant 2010; 25: 1289. [DOI] [PubMed] [Google Scholar]
- 88. Pilmore H, Dent H, Chang S, McDonald SP, Chadban SJ. Reduction in cardiovascular death after kidney transplantation. Transplantation 2010; 89: 851. [DOI] [PubMed] [Google Scholar]
- 89. KDIGO KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl 3): S1. [DOI] [PubMed] [Google Scholar]
- 90. Sharif A, Moore R, Baboolal K. Influence of lifestyle modification in renal transplant recipients with postprandial hyperglycemia. Transplantation 2008; 85: 353. [DOI] [PubMed] [Google Scholar]
- 91. von During ME, Jenssen T, Bollerslev J, et al Visceral fat is better related to impaired glucose metabolism than body mass index after kidney transplantation. Transpl Int 2015; 28: 1162. [DOI] [PubMed] [Google Scholar]
- 92. Kuningas K, Driscoll J, Mair R, et al Comparing Glycaemic Benefits of Active Versus Passive Lifestyle Intervention in Kidney Allograft Recipients: A Randomized Controlled Trial. Transplantation 2020; 104: 1491. [DOI] [PubMed] [Google Scholar]
- 93. Schwaiger E, Krenn S, Kurnikowski A, Jenssen T, Hecking M. Early postoperative basal insulin therapy for the prevention of post‐transplant diabetes onset after kidney transplantation (ITP‐NODAT) [abstract], presented at EASD virtual meeting. https://www.easd.org/virtualmeeting2020/#!resources/early‐postoperative‐basal‐insulin‐therapy‐for‐the‐prevention‐of‐post‐transplant‐diabetes‐onset‐after‐kidney‐transplantation‐itp‐nodat‐bb2c171d‐d2ed‐48aa‐8942‐600fe3ddf0a5. 2020.
- 94. Krenn S, Schwaiger E, Kurnikowski A, Nordheim E, Jenssen T, Hecking M.Missing glucose measurements and failure to initiate early postoperative insulin therapy associate with PTDM and dropout‐rate in the ITP‐NODAT study [abstract], presented at EASD virtual meeting, https://www.easd.org/virtualmeeting2020/#!resources/missing‐glucose‐measurements‐and‐failure‐to‐initiate‐early‐postoperative‐insulin‐therapy‐associate‐with‐ptdm‐and‐dropout‐rate‐in‐the‐itp‐nodat‐study‐8eca0e7d‐494e‐4fdd‐91a5‐98ee2a6ecf07. 2020.
- 95. Houlden R, Capes S, Clement M, Miller D. In‐hospital management of diabetes. Can J Diabet 2013; 37(Suppl 1): S77. [DOI] [PubMed] [Google Scholar]
- 96. American Diabetes A . Diabetes Care in the Hospital: Standards of Medical Care in Diabetes‐2020. Diabetes Care 2020; 43: S193. [DOI] [PubMed] [Google Scholar]
- 97. Renal https://renal.org/wp‐content/uploads/2020/01/ABCD‐RA‐PTDM‐v8.pdf.
- 98. Lea‐Henry TN, Baird‐Gunning J, Petzel E, Roberts DM. Medication management on sick days. Aust Prescr 2017; 40: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sharif A. Treatment options for post‐transplantation diabetes mellitus. Curr Diabet Rev 2015; 11: 155. [DOI] [PubMed] [Google Scholar]
- 100. Werzowa J, Saemann M, Haidinger M, Krebs M, Hecking M. Antidiabetic therapy in post kidney transplantation diabetes mellitus. Transplant Rev (Orlando) 2015; 29: 145. Epub 2015 Jan 14. [DOI] [PubMed] [Google Scholar]
- 101. Jenssen T, Hartmann A. Emerging treatments for post‐transplantation diabetes mellitus. Nat Rev Nephrol 2015; 11: 465. [DOI] [PubMed] [Google Scholar]
- 102. Davies MJ, D'Alessio DA, Fradkin J, et al A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 2018: 2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sarafidis P, Ferro CJ, Morales E, et al SGLT‐2 inhibitors and GLP‐1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA‐m and the DIABESITY working groups of the ERA‐EDTA. Nephrol Dial Transplant 2019; 34: 208. [DOI] [PubMed] [Google Scholar]
- 104. Buse JB, Wexler DJ, Tsapas A, et al A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 2020: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. American Diabetes A. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes‐2020. Diabetes Care 2020; 43: S98. [DOI] [PubMed] [Google Scholar]
- 106. Koufakis T, Papanas N, Dimitriadis G, Zebekakis P, Kotsa K. Interpreting the results of the VERTIS‐CV trial: Is this the end of the "class effect" perspective? J Diabetes 2020; 12: 942. [DOI] [PubMed] [Google Scholar]
- 107. Kidney Disease: Improving Global Outcomes Diabetes Work G . KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020; 2020: S1. [DOI] [PubMed] [Google Scholar]
- 108. Merovci A, Solis‐Herrera C, Daniele G, et al Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014; 124: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58: 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zelniker TA, Wiviott SD, Raz I, et al Comparison of the Effects of Glucagon‐Like Peptide Receptor Agonists and Sodium‐Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 2019; 139: 2022. [DOI] [PubMed] [Google Scholar]
- 111. Schwaiger E, Burghart L, Signorini L, et al Empagliflozin in posttransplantation diabetes mellitus: A prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant. 2019; 19: 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Halden TAS, Kvitne KE, Midtvedt K, et al Efficacy and Safety of Empagliflozin in Renal Transplant Recipients With Posttransplant Diabetes Mellitus. Diabetes Care 2019; 42: 1067. [DOI] [PubMed] [Google Scholar]
- 113. Mahling M, Schork A, Nadalin S, Fritsche A, Heyne N, Guthoff M. Sodium‐Glucose Cotransporter 2 (SGLT2) Inhibition in Kidney Transplant Recipients with Diabetes Mellitus. Kidney Blood Press Res 2019; 44: 984. [DOI] [PubMed] [Google Scholar]
- 114. Shah M, Virani Z, Rajput P, Shah B. Efficacy and Safety of Canagliflozin in Kidney Transplant Patients. Indian J Nephrol. 2019; 29: 278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Attallah N, Yassine L. Use of Empagliflozin in Recipients of Kidney Transplant: A Report of 8 Cases. Transplant Proc 2019; 51: 3275. [DOI] [PubMed] [Google Scholar]
- 116. Cehic MG, Muir CA, Greenfield JR, et al Efficacy and Safety of Empagliflozin in the Management of Diabetes Mellitus in Heart Transplant Recipients. Transplant Direct 2019; 5: e450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Muir CA, Greenfield JR, MacDonald PS. Empagliflozin in the management of diabetes mellitus after cardiac transplantationResearch Correspondenceretain. J Heart Lung Transplant 2017; 36: 914. [DOI] [PubMed] [Google Scholar]
- 118. Barnett AH, Mithal A, Manassie J, et al Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369. [DOI] [PubMed] [Google Scholar]
- 119. Cherney DZI, Cooper ME, Tikkanen I, et al Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int 2018; 93: 231. [DOI] [PubMed] [Google Scholar]
- 120. Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA‐REG OUTCOME Trial: A "Thrifty Substrate" Hypothesis. Diabetes Care 2016; 39: 1108. [DOI] [PubMed] [Google Scholar]
- 121. Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol 2015; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wu H, Estrella V, Beatty M, et al T‐cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat Commun 2020; 11: 4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Uthman L, Baartscheer A, Schumacher CA, et al Direct Cardiac Actions of Sodium Glucose Cotransporter 2 Inhibitors Target Pathogenic Mechanisms Underlying Heart Failure in Diabetic Patients. Front Physiol 2018; 9: 1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pinelli NR, Patel A, Salinitri FD. Coadministration of liraglutide with tacrolimus in kidney transplant recipients: a case series. Diabetes Care 2013; 36: e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liou JH, Liu YM, Chen CH. Management of Diabetes Mellitus With Glucagonlike Peptide‐1 Agonist Liraglutide in Renal Transplant Recipients: A Retrospective Study. Transplant Proc 2018; 50: 2502. [DOI] [PubMed] [Google Scholar]
- 126. Singh P, Pesavento TE, Washburn K, Walsh D, Meng S. Largest single‐centre experience of dulaglutide for management of diabetes mellitus in solid organ transplant recipients. Diabetes Obes Metab 2019; 21: 1061. [DOI] [PubMed] [Google Scholar]
- 127. Singh P, Taufeeq M, Pesavento TE, Washburn K, Walsh D, Meng S. Comparison of the glucagon‐like‐peptide‐1 receptor agonists dulaglutide and liraglutide for the management of diabetes in solid organ transplant: A retrospective study. Diabetes Obes Metab 2020; 22: 879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kukla A, Hill J, Merzkani M, et al The Use of GLP1R Agonists for the Treatment of Type 2 Diabetes in Kidney Transplant Recipients. Transplant Direct 2020; 6: e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Thangavelu T, Lyden E, Shivaswamy V. A Retrospective Study of Glucagon‐Like Peptide 1 Receptor Agonists for the Management of Diabetes After Transplantation. Diabetes Ther 2020; 11: 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Halden TA, Egeland EJ, Asberg A, et al GLP‐1 Restores Altered Insulin and Glucagon Secretion in Posttransplantation Diabetes. Diabetes Care 2016; 39: 617. [DOI] [PubMed] [Google Scholar]
- 131. Dosing https://dosing.de/nierelst.php.
- 132. Idorn T, Knop FK, Jorgensen MB, et al Safety and Efficacy of Liraglutide in Patients With Type 2 Diabetes and End‐Stage Renal Disease: An Investigator‐Initiated, Placebo‐Controlled, Double‐Blind, Parallel‐Group. Randomized Trial. Diabetes Care 2016; 39: 206. [DOI] [PubMed] [Google Scholar]
- 133. Nauck MA, Meier JJ. MANAGEMENT OF ENDOCRINE DISEASE: Are all GLP‐1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol 2019; 181: R211. [DOI] [PubMed] [Google Scholar]
- 134. Gerstein HC, Colhoun HM, Dagenais GR, et al Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet 2019; 394: 121. [DOI] [PubMed] [Google Scholar]
- 135. Marso SP, Bain SC, Consoli A, et al Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2016; 375: 1834. [DOI] [PubMed] [Google Scholar]
- 136. Gallwitz B, Dagogo‐Jack S, Thieu V, et al Effect of once‐weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab 2018; 20: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Fuechtenbusch M, Aberle J, Heitmann E, Nicolay C, Jung H. Weight loss in patients with type 2 diabetes receiving once‐weekly dulaglutide plus insulin lispro or insulin glargine plus insulin lispro: A post‐hoc analysis of the AWARD‐4 study across baseline body mass index subgroups. Diabetes Obes Metab 2019; 21: 1340. [DOI] [PubMed] [Google Scholar]
- 138. Yilmaz N, Sari R, Suleymanlar G, Ozdem S. Effects of Different Immunosuppressive Drugs on Incretins in Renal Transplant Patients. J Natl Med Assoc 2020; 112: 250. [DOI] [PubMed] [Google Scholar]
- 139. Muskiet MHA, Tonneijck L, Smits MM, et al GLP‐1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017; 13: 605. [DOI] [PubMed] [Google Scholar]
- 140. Sloan LA. Review of glucagon‐like peptide‐1 receptor agonists for the treatment of type 2 diabetes mellitus in patients with chronic kidney disease and their renal effects. J Diabetes. 2019; 11: 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lorenz M, Lawson F, Owens D, et al Differential effects of glucagon‐like peptide‐1 receptor agonists on heart rate. Cardiovasc Diabetol 2017; 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Alonso N, Julian MT, Puig‐Domingo M, Vives‐Pi M. Incretin hormones as immunomodulators of atherosclerosis. Front Endocrinol (Lausanne). 2012; 3: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Moschovaki Filippidou F, Kirsch AH, Thelen M, et al Glucagon‐Like Peptide‐1 Receptor Agonism Improves Nephrotoxic Serum Nephritis by Inhibiting T‐Cell Proliferation. Am J Pathol. 2020; 190: 400. [DOI] [PubMed] [Google Scholar]
- 144. Lee YS, Jun HS. Anti‐Inflammatory Effects of GLP‐1‐Based Therapies beyond Glucose Control. Mediators Inflamm 2016; 2016: 3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Patoulias D, Katsimardou A, Kalogirou MS, Karagiannis A, Doumas M. Is there any place for sodium‐glucose co‐transporter‐2 inhibitors in post‐liver transplantation patients? Dig Liver Dis 2020; 52: 239. [DOI] [PubMed] [Google Scholar]
- 146. Grubic Rotkvic P, Cigrovski Berkovic M, Rotkvic L, Bulj N. Prevention of cardiac allograft vasculopathy ‐ A new possible indication for SGLT‐2 inhibitors? Med Hypotheses 2020; 137: 109594. [DOI] [PubMed] [Google Scholar]
- 147. Sharif A, de Vries AP, Porrini E, Hecking M, Saemann M. Clinical Trials for Treatment of NODAT: Time to Collaborate. Transplantation 2012; 94: e23. [DOI] [PubMed] [Google Scholar]
- 148. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373: 2117. [DOI] [PubMed] [Google Scholar]
- 149. Wanner C, Inzucchi SE, Lachin JM, et al Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016; 375: 323. [DOI] [PubMed] [Google Scholar]
- 150. Neal B, Perkovic V, Mahaffey KW, et al Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017; 377: 644. [DOI] [PubMed] [Google Scholar]
- 151. Wiviott SD, Raz I, Bonaca MP, et al Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2019; 380: 347. [DOI] [PubMed] [Google Scholar]
- 152. Perkovic V, Jardine MJ, Neal B, et al Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019; 380: 2295. [DOI] [PubMed] [Google Scholar]
- 153. Pfeffer MA, Claggett B, Diaz R, et al Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med 2015; 373: 2247. [DOI] [PubMed] [Google Scholar]
- 154. Marso SP, Daniels GH, Brown‐Frandsen K, et al Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016; 375: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mann JFE, Orsted DD, Brown‐Frandsen K, et al Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med 2017; 377: 839. [DOI] [PubMed] [Google Scholar]
- 156. Holman RR, Bethel MA, Mentz RJ, et al Effects of Once‐Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2017; 377: 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hernandez AF, Green JB, Janmohamed S, et al Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet 2018; 392: 1519. [DOI] [PubMed] [Google Scholar]
- 158. Wanner C, Heerspink HJL, Zinman B, et al Empagliflozin and Kidney Function Decline in Patients with Type 2 Diabetes: A Slope Analysis from the EMPA‐REG OUTCOME Trial. J Am Soc Nephrol 2018; 29: 2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Malatiali S, Francis I, Barac‐Nieto M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp Diabetes Res 2008; 2008: 305403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Arakawa K, Ishihara T, Oku A, et al Improved diabetic syndrome in C57BL/KsJ‐db/db mice by oral administration of the Na(+)‐glucose cotransporter inhibitor T‐1095. Br J Pharmacol 2001; 132: 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 2013; 345: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Cherney DZ, Perkins BA, Soleymanlou N, et al Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587. [DOI] [PubMed] [Google Scholar]
- 163. Chilton R, Tikkanen I, Cannon CP, et al Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2015; 17: 1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Cherney DZ, Perkins BA, Soleymanlou N, et al The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016; 134: 752. [DOI] [PubMed] [Google Scholar]
- 166. Lytvyn Y, Skrtic M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria‐mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol 2015; 308: F77. [DOI] [PubMed] [Google Scholar]
- 167. de Boer IH, Kahn SE. SGLT2 Inhibitors‐Sweet Success for Diabetic Kidney Disease? J Am Soc Nephrol 2017; 28: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia 2018; 61: 2108. [DOI] [PubMed] [Google Scholar]
- 169. Chang YK, Choi H, Jeong JY, et al Dapagliflozin, SGLT2 Inhibitor, Attenuates Renal Ischemia‐Reperfusion Injury. PLoS One 2016; 11: e0158810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Pham SV, Chilton RJ. EMPA‐REG OUTCOME: The Cardiologist's Point of View. Am J Cardiol 2017; 120: S53. [DOI] [PubMed] [Google Scholar]
- 171. Abdul‐Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 Inhibitors and Cardiovascular Risk: Lessons Learned From the EMPA‐REG OUTCOME Study. Diabetes Care 2016; 39: 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703. [PMC free article] [PubMed] [Google Scholar]
- 173. Fitchett D, Zinman B, Wanner C, et al Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME(R) trial. Eur Heart J 2016; 37: 1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kimura G. Diuretic Action of Sodium‐Glucose Cotransporter 2 Inhibitors and Its Importance in the Management of Heart Failure. Circ J 2016; 80: 2277. [DOI] [PubMed] [Google Scholar]
- 175. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018; 20: 479. [DOI] [PubMed] [Google Scholar]
- 176. Inzucchi SE, Zinman B, Fitchett D, et al How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA‐REG OUTCOME Trial. Diabetes Care 2018; 41: 356. [DOI] [PubMed] [Google Scholar]
- 177. Bonner C, Kerr‐Conte J, Gmyr V, et al Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015; 21: 512. [DOI] [PubMed] [Google Scholar]
- 178. Crajoinas RO, Oricchio FT, Pessoa TD, et al Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon‐like peptide‐1. Am J Physiol Renal Physiol 2011; 301: F355. [DOI] [PubMed] [Google Scholar]
- 179. Gutzwiller JP, Tschopp S, Bock A, et al Glucagon‐like peptide 1 induces natriuresis in healthy subjects and in insulin‐resistant obese men. J Clin Endocrinol Metab 2004; 89: 3055. [DOI] [PubMed] [Google Scholar]
- 180. Kodera R, Shikata K, Kataoka HU, et al Glucagon‐like peptide‐1 receptor agonist ameliorates renal injury through its anti‐inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011; 54: 965. [DOI] [PubMed] [Google Scholar]
- 181. von Scholten BJ, Persson F, Rosenlund S, et al Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: A sub‐analysis of a randomized, placebo‐controlled, double‐blind, crossover trial. Diabetes Obes Metab 2017; 19: 901. [DOI] [PubMed] [Google Scholar]
- 182. Newman JD, Vani AK, Aleman JO, Weintraub HS, Berger JS, Schwartzbard AZ. The Changing Landscape of Diabetes Therapy for Cardiovascular Risk Reduction: JACC State‐of‐the‐Art Review. J Am Coll Cardiol 2018; 72: 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. McHugh KR, DeVore AD, Mentz RJ, Edmonston D, Green JB, Hernandez AF. The emerging role of novel antihyperglycemic agents in the treatment of heart failure and diabetes: A focus on cardiorenal outcomes. Clin Cardiol 2018; 41: 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Drucker DJ. The Ascending GLP‐1 Road From Clinical Safety to Reduction of Cardiovascular Complications. Diabetes 2018; 67: 1710. [DOI] [PubMed] [Google Scholar]
- 185. Gaspari T, Welungoda I, Widdop RE, Simpson RW, Dear AE. The GLP‐1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(‐/‐) mouse model. Diab Vasc Dis Res 2013; 10: 353. [DOI] [PubMed] [Google Scholar]
- 186. Rakipovski G, Rolin B, Nohr J, et al The GLP‐1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE(‐/‐) and LDLr(‐/‐) Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl Sci 2018; 3: 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Liu H, Dear AE, Knudsen LB, Simpson RW. A long‐acting glucagon‐like peptide‐1 analogue attenuates induction of plasminogen activator inhibitor type‐1 and vascular adhesion molecules. J Endocrinol 2009; 201: 59. [DOI] [PubMed] [Google Scholar]


