Abstract
Numerous studies have examined the associations between air pollution and stroke. However, little is known about the associations between air pollution and transient ischemic attack (TIA). In this study, we aimed to conduct a time-series study to systematically examine the associations between hospital admissions for TIA and air pollutants. Admissions for TIA (ICD-10: G45) from 1 January 2014 to 31 December 2016 were identified based on the primary diagnosis from 134 hospitals in Beijing, China. Hourly measurements of air pollutants were obtained from the National Air Pollution Monitoring System. Generalized additive models with quasi-Poisson regression were used to determine the associations for each pollutant. Additionally, stratified analysis was implemented to examine whether age, gender, temperature, and season were the potential effect modifiers. Restricted cubic spline was applied to investigate the exposure-response curve. In total, 109,975 hospital admissions for TIA were included. The positive associations were detected between PM2.5, PM10, SO2, NO2, and CO and hospital admissions for TIA. The effects of PM2.5 and PM10 in men are stronger than in women. Additionally, the effects of PM2.5, PM10, SO2, and O3 are more pronounced on warm days than cool days. From exposure-response curves, we observe a nearly linear relationship for PM2.5, PM10, CO, and NO2. Further studies are needed to verify the association. This research contributes evidence on the association between air pollution and admissions for TIA in the low- and middle-income countries and may promote related public health policy development.
Keywords: Transient ischemic attack, Air pollution, Particulate matter, Time-series, Hospital admissions
Introduction
Air pollution is one of the leading causes of risk-attributable death, accounting for over 4 million worldwide deaths each year (Stanaway et al. 2018). As a potentially modifiable risk factor, the effects of air pollution on human health are a topic that has received much attention in recent decades. A large body of epidemiological and clinical studies supports the existence of positive associations between air pollution and the morbidity and mortality of ischemic stroke, as well as hemorrhagic stroke (Andersen et al. 2012; Huang et al. 2019; Sun et al. 2019; Zhang et al. 2018). According to the International Classification of Diseases (ICD), both transient ischemic attack (TIA) and stroke are categorized as cerebrovascular disorders (Bo Norrving et al. 2013; Sacco et al. 2013). Although clinical and pathological similarities exist between TIA and ischemic stroke, the criteria for clinical diagnosis are distinctive for each, and they are classified as different types of cerebrovascular disorders. Compared with stroke, there is little research on the effects of air pollution on TIA.
TIA is a focal neurologic dysfunction caused by focal brain or retinal ischemia. Typically, there is no evidence of infarction on imaging, and symptoms generally resolve within 24 h (Bo Norrving et al. 2013). TIA has been recognized as a strong predictor of subsequent stroke and is associated with poorer long-term survival (Clissold et al. 2020; Kleindorfer et al. 2005). The risk of subsequent stroke after experiencing a TIA was 17.3% at 3 months (Coull et al. 2004), and the mortality after experiencing a TIA was 12% in 1 year (Kleindorfer et al. 2005). Whether air pollution is the modifiable risk factor for TIA is uncertain. Moreover, the majority of previous studies (Bedada et al. 2012; Lisabeth et al. 2008) were conducted in high-income countries, with limited studies from low- and middle-income countries, where levels and components of various air pollutants were markedly different from high-income countries (Li et al. 2015).
Increased knowledge of risks associated with air pollution would allow for improved implementation of public health intervention strategies. In this study, we aimed to conduct a time-series analysis to systematically assess the association between short-term exposure to air pollution and hospital admissions for TIA. The air pollutants being explored include particulate matter with aerodynamic diameter < 2.5 microns (PM2.5), with aerodynamic diameter < 10 microns (PM10), carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), and ozone (O3).
Materials and methods
Data collection
We obtained data on daily hospital admissions from the Hospital Discharge Abstract Database, which is maintained by the Beijing Municipal Health Commission Information Center (HCIC). All hospitals were grade 2 or 3 and had the capability of diagnosing and treating patients with TIA. To improve the accuracy of disease diagnoses, the HCIC applies strict quality control procedures and organized validation programs annually. Using the International Classification of Diseases 10th Revision (ICD-10), all admissions for TIA (G45) were identified based on the primary diagnosis. The time period was 1 January 2014 to 31 December 2016. The daily counts of TIA admissions were also sorted by sex and age.
In addition, we obtained daily concentrations of air pollutants from the National Air Pollution Monitoring System, including PM2.5, PM10, CO, SO2, NO2, and O3. Hourly measurements of air pollutants were collected from all monitoring stations. Similar to the methods employed by several previous studies, we used average measurements as a proxy for exposure (Rodopoulou et al. 2015; Zhang et al. 2018). For PM2.5, PM10, CO, SO2, and NO2, 24-h average concentrations were used; for O3, maximum daily 8-h average concentrations were used (Tian et al. 2018, 2019). During the same study period, we also obtained meteorological data, including daily mean temperature and relative humidity.
The study was approved by the ethics committee of the Beijing Tiantan Hospital. Since the data was analyzed at an aggregate level with no individual information involved, informed consent from the participants was waived for this study.
Statistical analysis
In order to investigate the association between air pollution and hospital admissions for TIA, we used the generalized additive model with quasi-Poisson distribution in a time-series analysis (Hastie 1990; Nitta et al. 2010). We controlled long-time and seasonal trends by using a smoothing spline with 7 degrees of freedom (df) per year in the regression model. For the daily mean temperature and relative humidity, we used a smoothing spline with 3 df to control their effects on TIA admissions (Tian et al. 2018). The details of the smoothing spline have been described in previous literature (Hastie 1990). It is understood that day of the week (DOW) and public holidays may have an effect on hospital admissions; therefore, we included the DOW and public holidays as dummy variables in the model. Consequently, the regression model was constructed:
where E(Yt) is the expected daily counts of hospital admission for TIA on day t, DOW is the day of the week, Holiday indicates a public holiday (0 = No, 1 = Yes), time is calendar time, temp is the daily mean temperature of the current day, hum is the relative humidity of the current day, β is the regression coefficient, and s indicates a cubic smoothing spline.
To investigate the lag effect associated with air pollutants, we used the following lag periods: single-day lag (the same day [lag 0], the previous day [lag 1], and the day before the previous day [lag 2]), multi-day lags (average concentration of the same day and previous day [lag 01], and average concentration of the same day and previous 2 days [day 02]). Additionally, we used a restricted cubic spline with 3 knots at the 5th, 50th, and 95th percentiles to explore the exposure-response relationship between air pollutants and hospital admissions for TIA.
We performed a subgroup analysis to evaluate whether the following subgroups were potential effect modifiers: gender (men or women), age (< 60 years or ≥ 60 years), temperature (cool days: < 15.7 °C, warm days: ≥ 15.7 °C; median temperature used as the cutoff,) and season (April to September, warm season; October to March, cool season). We used the Z test to compare differences in the association between subgroups (Altman and Bland 2003).
In the sensitivity analysis, we used the two pollutant models to estimate the association between the concentration of air pollutants and TIA admissions. Additionally, we used different df values for calendar time (5–10 per year), temperature (4–6), and relative moisture (4–6) to test the robustness of the results.
All results were presented as percentage changes and 95% confidence intervals (CIs) in daily hospital admissions for TIA per 10 μg/m3 increase in PM2.5, PM10, SO2, NO2, O3, and per 1 mg/m3 increase in CO. The analysis was conducted using the SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
In total, there were 109,975 hospital admissions for TIA from 134 hospitals in Beijing, China, from 1 January 2014 to 31 December 2016. Table 1 summarizes the characteristics of daily hospital admissions for TIA, meteorological variables, and concentrations of air pollutants. On average, there were 100 admissions for TIA per day over the study period. Of the patients, 49.5% were men, and 71% were aged ≥ 60 years. The mean temperature was 13.8 °C, and the relative humidity was 53.1%. The daily 24-h mean (standard deviation [SD]) concentration was 79.1 (68.3) μg/m3 for PM2.5, 103.8 (74.9) μg/m3 for PM10, 1.2 (1.0) mg/m3 for CO, 50.6 (24.3) μg/m3 for NO2, 14.4 (17.3) μg/m3 for SO2, and 110.4 (72.4) μg/m3 for O3, respectively. The concentrations of PM2.5, PM10, CO, NO2, and SO2 were positively correlated with each other, with Spearman correlation coefficients between 0.52 and 0.87 (P < 0.001). Meanwhile, O3 was negatively correlated with each of the other pollutants (Table 2).
Table 1.
Distribution of daily hospital admissions, meteorological factors, and air pollutants in Beijing, China, from 2014 to 2016
| Percentile | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | Standard deviation | Minimum | P25th | P50th | P75th | Maximum | IQR |
| TIA admission | 100.3 | 34.9 | 21 | 63 | 108 | 127 | 197 | 64 |
| Meteorological factors | ||||||||
| Temperature, °C | 13.9 | 11.0 | − 14.3 | 3.0 | 15.7 | 24.1 | 32.6 | 21.1 |
| Relative humidity, % | 52.7 | 20.0 | 8.0 | 37.0 | 53.0 | 69.0 | 99.0 | 32.0 |
| Air pollutants | ||||||||
| PM2.5, μg/m3 | 79.1 | 68.3 | 5.2 | 29.8 | 60 | 106.4 | 477.5 | 76.6 |
| PM10, μg/m3 | 103.8 | 74.9 | 1.7 | 47 | 86.8 | 135.9 | 480.8 | 88.9 |
| CO, mg/m3 | 1.2 | 1.0 | 0.2 | 0.6 | 1.0 | 1.5 | 8.1 | 0.9 |
| NO2, μg/m3 | 50.6 | 24.3 | 8.1 | 33.5 | 44.5 | 61.4 | 153.5 | 27.9 |
| SO2, μg/m3 | 14.4 | 17.3 | 1.8 | 3.6 | 7.9 | 17.4 | 133.1 | 13.8 |
| O3, μg/m3 | 110.4 | 72.4 | 3.0 | 57.0 | 92.0 | 158.5 | 343.0 | 101.5 |
IQR indicates inter-quartile range; PM2.5, particulate matter with aerodynamic diameter < 2.5 μm; PM10, particulate matter with aerodynamic diameter < 10 μm; CO, carbon monoxide; NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone
Table 2.
Spearman correlation coefficients among ambient air pollutants
| PM2.5 | PM10 | CO | SO2 | NO2 | O3 | |
|---|---|---|---|---|---|---|
| PM2.5 | 1.00 | 0.87* | 0.83* | 0.52* | 0.79* | − 0.11* |
| PM10 | - | 1.00 | 0.73* | 0.53* | 0.78* | − 0.07† |
| CO | - | - | 1.00 | 0.61* | 0.82* | − 0.36* |
| SO2 | - | - | - | 1.00 | 0.63* | − 0.33* |
| NO2 | - | - | - | - | 1.00 | − 0.34* |
| O3 | - | - | - | - | - | 1.00 |
*P < 0.001
†P < 0.05
PM2.5 indicates particulate matter with aerodynamic diameter < 2.5 μm; PM10, particulate matter with aerodynamic diameter < 10 μm; CO, carbon monoxide; NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone
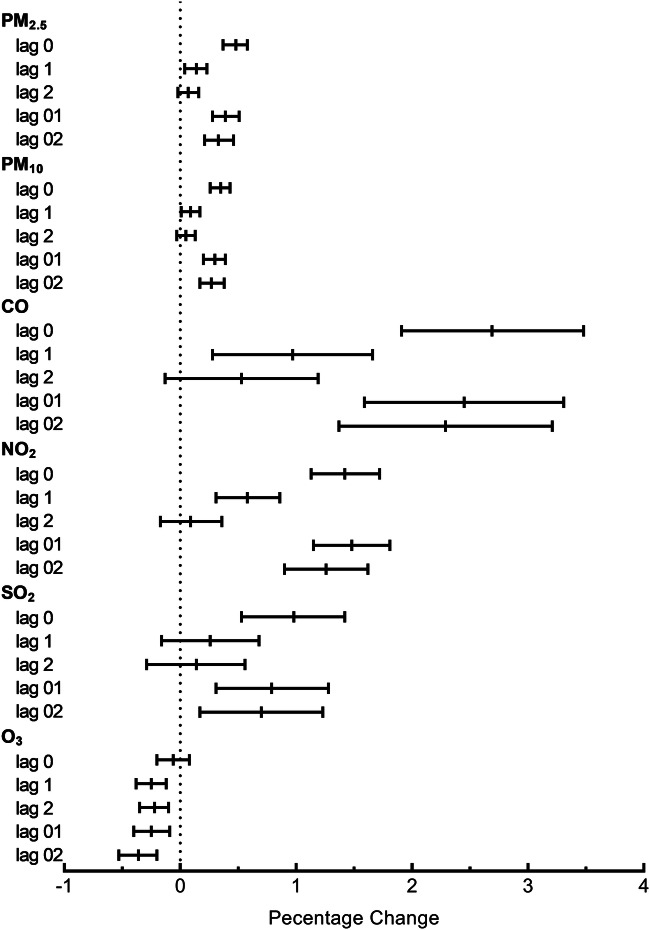
Figure 1 presents the percentage changes in hospital admissions for TIA on different lag days. With the exception of O3, the concentrations of PM2.5, PM10, CO, NO2, and SO2 on the same day (lag 0 days) showed the highest and significant associations with hospital admissions for TIA. The effects were attenuated at the other lag days. A 10 μg/m3 increase of PM2.5, PM10, NO2, SO2, and O3 in the same day and 1 mg/m3 increase in CO were associated with 0.48% (95% CI, 0.37–0.58%), 0.35% (95% CI, 0.26–0.43%), 1.42% (95% CI, 1.13–1.72%), 0.98% (95% CI, 0.53–1.42), − 0.06% (95% CI, − 0.2–0.08%), and 2.69% (95% CI 1.91–3.48%) increases in hospital admissions for TIA, respectively.
Fig. 1.
Percentage change and 95% confidence interval in daily hospital admissions for TIA per 10 μg/m3 increase in PM2.5, PM10, NO2, SO2, O3, and 1 mg/m3 increase in CO concentrations at different lag days
Table 3 shows the percentage change in hospital admissions for TIA per 10 μg/m3 increase in PM2.5, PM10, NO2, SO2, and O3 and 1 mg/m3 increase in CO concentrations on the same day, stratified by sex, age, temperature, and season. We observed that sex had a modifying effect on the association between PM2.5 and PM10 and hospital admissions for TIA; the association was stronger for men. For all pollutants, we did not find evidence of a modifying effect resulting from age. The associations between PM2.5, PM10, SO2, and O3 and admissions for TIA were more pronounced on warm days than cool days. However, we did not find significant effect modification by season for most pollutants, except for O3.
Table 3.
The percentage change with 95% confidence interval in hospital admissions for TIA with 10 μg/m3 increases in PM2.5, PM10, NO2, SO2, O3, and 1 mg/m3 increase in CO on lag 0 days stratified by sex, age, temperature, and season
| Pollutants | Subgroups | Percentage change | 95% CI | P-value for effect modifications |
|---|---|---|---|---|
| PM2.5 | Men | 0.59 | 0.440.74 | 0.035 |
| Women | 0.36 | 0.21–0.51 | ||
| Age < 60 | 0.59 | 0.39–0.79 | 0.202 | |
| Age ≥ 60 | 0.44 | 0.31–0.56 | ||
| Cool days | 0.39 | 0.25–0.52 | 0.015 | |
| Warm days | 0.68 | 0.49–0.87 | ||
| Cool seasons | 0.53 | 0.30–0.76 | 0.268 | |
| Warm seasons | 0.38 | 0.25–0.52 | ||
| PM10 | Men | 0.47 | 0.35–0.59 | 0.004 |
| Women | 0.22 | 0.10–0.34 | ||
| Age < 60 | 0.37 | 0.21–0.53 | 0.764 | |
| Age ≥ 60 | 0.34 | 0.24–0.44 | ||
| Cool days | 0.28 | 0.17–0.39 | 0.019 | |
| Warm days | 0.5 | 0.35–0.66 | ||
| Cool seasons | 0.29 | 0.18–0.40 | 0.623 | |
| Warm seasons | 0.34 | 0.16–0.52 | ||
| CO | Men | 2.91 | 1.80–4.03 | 0.613 |
| Women | 2.50 | 1.40–3.61 | ||
| Age < 60 | 3.04 | 1.59–4.51 | 0.597 | |
| Age ≥ 60 | 2.57 | 1.64–3.51 | ||
| Cool days | 2.63 | 1.69–3.57 | 0.239 | |
| Warm days | 3.86 | 2.04–5.7 | ||
| Cool seasons | 2.60 | 1.65–3.56 | 0.700 | |
| Warm seasons | 3.02 | 1.13–4.94 | ||
| NO2 | Men | 1.50 | 1.08–1.92 | 0.613 |
| Women | 1.35 | 0.93–1.77 | ||
| Age < 60 | 1.51 | 0.96–2.06 | 0.714 | |
| Age ≥ 60 | 1.39 | 1.04–1.74 | ||
| Cool days | 1.37 | 1–1.74 | 0.638 | |
| Warm days | 1.54 | 0.93–2.16 | ||
| Cool seasons | 1.32 | 0.94–1.70 | 0.862 | |
| Warm seasons | 1.39 | 0.68–2.10 | ||
| SO2 | Men | 1.36 | 0.73–1.99 | 0.097 |
| Women | 0.61 | − 0.02–1.24 | ||
| Age < 60 | 0.88 | 0.06–1.70 | 0.769 | |
| Age ≥ 60 | 1.03 | 0.50–1.55 | ||
| Cool days | 0.77 | 0.26–1.28 | 0.027 | |
| Warm days | 2.44 | 1.05–3.85 | ||
| Cool seasons | 0.78 | 0.27–1.29 | 0.084 | |
| Warm seasons | 2.13 | 0.69–3.60 | ||
| O3 | Men | − 0.04 | − 0.23–0.16 | 0.755 |
| Women | − 0.08 | − 0.28–0.11 | ||
| Age < 60 | − 0.07 | − 0.32–0.19 | 0.962 | |
| Age ≥ 60 | − 0.06 | − 0.22–0.10 | ||
| Cool days | − 0.9 | − 1.26–− 0.54 | < 0.001 | |
| Warm days | 0.23 | 0.07–0.4 | ||
| Cool seasons | − 0.59 | −0.92–0.26 | < 0.001 | |
| Warm seasons | 0.13 | − 0.03–0.29 |
PM2.5 indicates particulate matter with aerodynamic diameter < 2.5 μm; PM10, particulate matter with aerodynamic diameter < 10 μm; CO, carbon monoxide; NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone
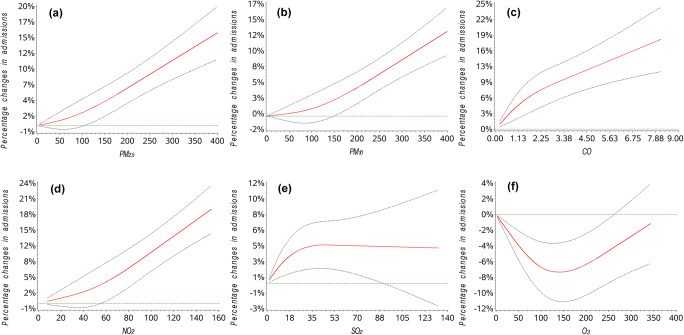
Figure 2 displays the exposure-response curves for the associations between air pollutants and hospital admissions for TIA, adjusted for time trend, week effect, holiday, and meteorological variables. We observed almost linear trends for PM2.5, PM10, CO, and NO2. The curve increased steeply when the concentration of SO2 fell below 35 μg/m3 and then leveled off when the concentration rose above 35 μg/m3. The exposure-response association curve between O3 and hospital admissions for TIA was U-shaped.
Fig. 2.
Adjusted exposure-response association curves between the concentrations of PM2.5, PM10, CO, SO2, NO2, and O3 and percentage changes in hospital admissions for TIA
In the two pollutant models, the associations were still significant for PM2.5 and NO2 after adjusting for other pollutants. In contrast, associations were diminished for PM10 and CO after adjusting for PM2.5 and NO2. The effect of SO2 became insignificant after adjusting for PM2.5, PM10, and CO. The relationships were insignificant for O3 after adjusting for PM10, CO, NO2, and SO2 (Table 4). When changing the df value for time trend (5–10 per year), the associations between air pollutants and hospital admissions for TIA were not changed.
Table 4.
Percentage change with 95% confidence interval in transient ischemic attack admission with air pollutants in two pollutant models
| Variable | PM2.5 | PM10 | CO | NO2 | SO2 | O3 |
|---|---|---|---|---|---|---|
| Adjusted for PM2.5 | - | 0.05 (− 0.13–0.23) | 0.49 (− 0.73–1.73) | 0.99 (0.55–1.43) * | − 0.29 (− 0.82–0.23) | − 0.15 (− 0.29–− 0.01) * |
| Adjusted for PM10 | 0.42 (0.19–0.65) * | - | 1.23 (0.11–2.36) * | 1.21 (0.75–1.67) * | − 0.06 (− 0.57–0.46) | − 0.1 (− 0.24–0.04) |
| Adjusted for CO | 0.42 (0.25–0.59) * | 0.24 (0.12–0.37) * | - | 1.4 (0.97–1.83) * | − 0.08 (− 0.62–0.46) | − 0.02 (− 0.16–0.12) |
| Adjusted for NO2 | 0.21 (0.06–0.37) * | 0.09 (− 0.05–0.22) | 0.06 (− 1.06–1.2) | - | − 0.78 (− 1.33–− 0.24) * | − 0.01 (− 0.15–0.13) |
| Adjusted for SO2 | 0.51 (0.39–0.64) * | 0.35 (0.25–0.45) * | 2.77 (1.81–3.73) * | 1.71 (1.35–2.08)* | - | − 0.04 (− 0.18–0.09) |
| Adjusted for O3 | 0.49 (0.38–0.60)* | 0.35 (0.26–0.44)* | 2.69 (1.90–3.48)* | 1.42 (1.13–1.72)* | 0.97 (0.52–1.41)* | - |
*P < 0.05
PM2.5 indicates particulate matter with aerodynamic diameter < 2.5 μm; PM10, particulate matter with aerodynamic diameter < 10 μm; CO, carbon monoxide; NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone
Discussion
To the best of our knowledge, this is the first study that systematically assessed the effects of short-term exposure to ambient air pollution on hospital admission for TIA in low- and middle-income countries. Our findings suggest that the concentrations of all analyzed air pollutants, except for O3, were positively associated with daily hospital admissions for TIA. Among different lag days, it was on the same day (lag 0) that the highest effects of PM2.5, PM10, CO, NO2, and SO2 on hospital admissions for TIA were observed. PM2.5 and PM10 had more pronounced effects in men, and the associations for PM2.5, PM10, SO2, and O3 on warm days were significantly stronger than on cool days.
In the past 20 years, the effects of air pollution on stroke have been explored in detail. However, there are few studies on TIA; this may due to the difficulty in accurately identifying TIA cases (Nadarajan et al. 2014; Sacco 2004). TIA has transience of symptoms and various clinical symptoms, and its diagnosis also relies heavily on an accurate interpretation of the patient’s clinical history (Schrock et al. 2012). The association between TIA and air pollution is not yet certain. A study from Canada found that exposure to SO2 during the summer months may increase the risk of TIA; however, no statistically significant associations between the other air pollutants and TIA were found (Villeneuve et al. 2006). From the Dijon Stroke Registry, a population-based study in France, a significant association was observed between O3 and TIA, but no associations were found for PM10, SO2, NO2, and CO (Henrotin et al. 2007). Another study was performed using the data from two UK cities; in Manchester, it was found that short-term change in concentration of NO on 3-lag day was positively associated with the occurrence of TIA and minor stroke. And, in Liverpool, PM10 on the same day, and NO on 3-lag day, was negatively associated with TIA and minor stroke (Bedada et al. 2012). However, the sample sizes of aforementioned studies were limited, and the data used were from developed countries with low-level air pollution. A recent study from 248 Chinese cities found that a 10 μg/m3 increase in PM2.5 was significantly associated with a 0.26% increase in hospital admissions for TIA (Gu et al. 2020). In our study, we found a much stronger effect: 0.48% changes on TIA admissions for a 10 μg/m3 increase in PM2.5. This difference may be due to the fact that the former study used data from a variety of locations, where the effects of PM2.5 may display different patterns (Chen et al. 2017; Liu et al. 2017). The former study represented the mean effect of 248 cities. Our study, which used the data from Beijing, on the other hand, represented an area with relatively severe air pollution, with a daily mean concentration of PM2.5 79.1 μg/m3.
From the exposure-response curve, a nearly linear relationship can be observed between PM2.5, PM10, CO, and NO2 and hospital admissions for TIA. Few studies have examined the exposure-response relationship between air pollutants and TIA. In the previously mentioned study from 248 cities in China, the curve increased steeply at low concentrations of PM2.5 and reached a plateau at high concentrations (Gu et al. 2020). Differences in the shape of exposure-response curves may be a result of differences in geographic regions and estimation methods. In regard to the other pollutants, to our knowledge, this is the first study to explore exposure-response relationships between these pollutants and TIA. We should note that the number of observations for high levels of pollutants was small. Moreover, the restricted cubic spline is assumed to be linear beyond the extreme knots; we may observe an almost linear curve when assessing the whole range of pollutant levels. Therefore, we should use caution when deciphering the exposure-response curve. Future studies are needed to further estimate exposure-response associations, as well as explore the safety level if applicable.
In the subgroup analysis, we found that associations for PM2.5 and PM10 were more pronounced in women than in men. However, it was inconclusive whether or not gender is a modifier for the association between PM2.5 and PM10 and TIA. Regarding ischemic stroke, some studies have indicated that women are more vulnerable to air pollution (Franklin et al. 2007; Kan et al. 2008). The modifiable effect of sex for ischemic stroke was also unclear. More studies are warranted to further investigate this issue. Additionally, in our study, we found no evidence of age-specific differences in associations between air pollutants and admissions for TIA. This is consistent with the findings of the previous study (Gu et al. 2020) though the classification of age groups differs. Temperature may be another potential modifying factor. Previous studies have suggested that the association between particulate matters and stroke was higher on warm days than cold days (Huang et al. 2016; Tian et al. 2018). Similarly, we observed a stronger effect of PM2.5, PM10, SO2, and O3 on admissions for TIA on warm days than cold days. There were several potential explanations proposed for this increased association on warm days. First, the ambient temperature may influence the emission, transportation, dilution, chemical transformation, and deposition of air pollutants (Macdonald et al. 2005). Second, on warm days, people tend to spend more time outdoors (Tian et al. 2019), resulting in more exposure to ambient air pollution. However, when stratified by season, we observed no significant difference between associations in cool seasons and warm seasons. This was supported by the prior study (Gu et al. 2020).
In our study, we found a positive relationship between ambient air pollution and hospital admissions for TIA. We observed a 0.48% increase in hospital admissions for TIA per 10 μg/m3 increase in PM2.5. Although the magnitude of association appears to be relatively small, the public health burden derived from the risk could be highly significant. If the average concentration of PM2.5 was reduced from the current concentration of 79 μg/m3 to 10 μg/m3, which is the level proposed by the WHO air quality guidelines (Krzyzanowski and Cohen 2008), it was estimated that about 3.3 hospital admissions for TIA per day could be avoided in Beijing, China. The study strengthens the rationale for reducing concentrations of air pollutants in low- and middle-income countries.
Several limitations should be considered in our study. First, unlike ischemic stroke or hemorrhagic stroke, the diagnosis of TIA is difficult, because the symptoms and signs usually have resolved by the time of assessment (Nadarajan et al. 2014; Sheehan et al. 2009). Additionally, TIA mimics rate is higher (Nadarajan et al. 2014). The diagnosis for TIA may be less reliable. This is especially true when it comes to the administrative database; this may result in misclassification of the disease. In order to improve the accuracy of disease diagnosis, the Beijing Municipal Health Commission launches quality monitoring projects every year. However, misclassification is likely unrelated to air pollution and may reduce the precision of association or cause associations to be underestimated (Wellenius et al. 2005). Thus, our results should be explored with caution. Further research is needed to confirm the observed associations between air pollution and TIA admissions by examining these associations using a negative control cohort. Additionally, due to data limitations, we could not assess some potential modifiers, such as smoking, drinking, and individual disease history. We recommend that future research takes these potential modifiers into consideration in order to acquire more precise associations. Finally, exposure measurement errors may be present, since we used average exposure to air pollution as a proxy for true personal exposure. Measurement errors tend to lead the association to null (Zeger et al. 2000) and the conclusion to be more conservative.
Conclusions
In conclusion, the study suggests that short-term exposure to air pollution is associated with hospital admission for TIA. Elevated levels of PM2.5, PM10, CO, NO2, and SO2 can result in an increase in hospital admissions for TIA. Gender and temperature may be the two potential effect modifiers on the association between air pollution and admission for TIA. Further research is needed to verify the expose-response between air pollution and admissions for TIA. This research contributes evidence on the association between air pollution and admissions for TIA in the low- and middle-income countries and may promote related public health policy development.
Authors’ contributions
Conceptualization: Runhua Zhang, Yongjun Wang, and Gaifen Liu
Data curation: Guitao Zhang and Miaoxin Yu
Funding acquisition: Yongjun Wang and Gaifen Liu
Methodology: Runhua Zhang
Software and supervision: Yong Jiang and Yongjun Wang
Validation: Guitao Zhang
Writing—original draft: Runhua Zhang
Writing—review and editing: Yongjun Wang and Gaifen Liu
Funding
This work was partially funded by the National Key Research and Development Program of the Ministry of Science and Technology of the People’s Republic of China (grant number 2017YFC1307702) and Capital’s Funds for Health Improvement and Research (grant number 2020-1-2041).
Data availability
The data on air pollution and meteorological factors can be obtained from the National Air Pollution Monitoring System (http://www.cnemc.cn) and the China Meteorological Data Sharing Service System (https://data.cma.cn). The data on admissions for TIA are available from the corresponding author upon reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yongjun Wang, Email: yongjunwang@ncrcnd.org.cn.
Gaifen Liu, Email: liugaifen@ncrcnd.org.cn.
References
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ (Clin Res Ed) 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ZJ, Kristiansen LC, Andersen KK, Olsen TS, Hvidberg M, Jensen SS, Ketzel M, Loft S, Sorensen M, Tjonneland A, Overvad K, Raaschou-Nielsen O. Stroke and long-term exposure to outdoor air pollution from nitrogen dioxide: a cohort study. Stroke. 2012;43:320–325. doi: 10.1161/strokeaha.111.629246. [DOI] [PubMed] [Google Scholar]
- Bedada GB, Smith CJ, Tyrrell PJ, Hirst AA, Agius R. Short-term effects of ambient particulates and gaseous pollutants on the incidence of transient ischaemic attack and minor stroke: a case-crossover study. Environ Health Glob Access Sci Source. 2012;11:77. doi: 10.1186/1476-069X-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo Norrving DL, Brainin M, Davis S (2013) Stroke definition in the ICD-11 at the WHO. https://worldneurologyonline.com/article/stroke-definition-in-the-icd-11-at-the-who/. Accessed 23 July 2020
- Chen R, Yin P, Meng X, Liu C, Wang L, Xu X, Ross JA, Tse LA, Zhao Z, Kan H, Zhou M. Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med. 2017;196:73–81. doi: 10.1164/rccm.201609-1862OC. [DOI] [PubMed] [Google Scholar]
- Clissold B, Phan TG, Ly J, Singhal S, Srikanth V, Ma H. Current aspects of TIA management. J Clin Neurosci. 2020;72:20–25. doi: 10.1016/j.jocn.2019.12.032. [DOI] [PubMed] [Google Scholar]
- Coull AJ, Lovett JK, Rothwell PM, Oxford Vascular S. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ (Clin Res Ed) 2004;328:326. doi: 10.1136/bmj.37991.635266.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17:279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- Gu J, Shi Y, Chen N, Wang H, Chen T. Ambient fine particulate matter and hospital admissions for ischemic and hemorrhagic strokes and transient ischemic attack in 248 Chinese cities. Sci Total Environ. 2020;715:136896. doi: 10.1016/j.scitotenv.2020.136896. [DOI] [PubMed] [Google Scholar]
- Hastie TJTRJ. Generalized Additive Models. New York: Chapman and Hall; 1990. [Google Scholar]
- Henrotin JB, Besancenot JP, Bejot Y, Giroud M. Short-term effects of ozone air pollution on ischaemic stroke occurrence: a case-crossover analysis from a 10-year population-based study in Dijon, France. Occup Environ Med. 2007;64:439–445. doi: 10.1136/oem.2006.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Luo Y, Guo Y, Tao L, Xu Q, Wang C, Wang A, Li X, Guo J, Yan A, Guo X (2016) Particulate matter and hospital admissions for stroke in Beijing, China: modification effects by ambient temperature. J Am Heart Assoc 5. 10.1161/JAHA.116.003437 [DOI] [PMC free article] [PubMed]
- Huang K, Liang F, Yang X, Liu F, Li J, Xiao Q, Chen J, Liu X, Cao J, Shen C, Yu L, Lu F, Wu X, Zhao L, Wu X, Li Y, Hu D, Huang J, Liu Y, Lu X, et al. Long-term exposure to ambient fine particulate matter and incidence of stroke: prospective cohort study from the China-PAR project. BMJ (Clin Res Ed) 2019;367:l6720. doi: 10.1136/bmj.l6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, London SJ, Chen G, Zhang Y, Song G, Zhao N, Jiang L, Chen B. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: the Public Health and Air Pollution in Asia (PAPA) Study. Environ Health Perspect. 2008;116:1183–1188. doi: 10.1289/ehp.10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, Schneider A, Alwell K, Jauch E, Miller R, Moomaw C, Shukla R, Broderick JP. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–723. doi: 10.1161/01.STR.0000158917.59233.b7. [DOI] [PubMed] [Google Scholar]
- Krzyzanowski M, Cohen A. Update of WHO air quality guidelines. Air Qual Atmos Health. 2008;1:7–13. doi: 10.1007/s11869-008-0008-9. [DOI] [Google Scholar]
- Li P, Xin J, Wang Y, Li G, Pan X, Wang S, Cheng M, Wen T, Wang G, Liu Z. Association between particulate matter and its chemical constituents of urban air pollution and daily mortality or morbidity in Beijing City. Environ Sci Pollut Res Int. 2015;22:358–368. doi: 10.1007/s11356-014-3301-1. [DOI] [PubMed] [Google Scholar]
- Lisabeth LD, Escobar JD, Dvonch JT, Sanchez BN, Majersik JJ, Brown DL, Smith MA, Morgenstern LB. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol. 2008;64:53–59. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Tian Y, Xu Y, Zhang J. Ambient particulate matter concentrations and hospitalization for stroke in 26 Chinese cities: a case-crossover study. Stroke. 2017;48:2052–2059. doi: 10.1161/STROKEAHA.116.016482. [DOI] [PubMed] [Google Scholar]
- Macdonald RW, Harner T, Fyfe J. Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Sci Total Environ. 2005;342:5–86. doi: 10.1016/j.scitotenv.2004.12.059. [DOI] [PubMed] [Google Scholar]
- Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol. 2014;14:23–31. doi: 10.1136/practneurol-2013-000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta H, Yamazaki S, Omori T, Sato T. An introduction to epidemiologic and statistical methods useful in environmental epidemiology. J Epidemiol. 2010;20:177–184. doi: 10.2188/jea.JE20100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodopoulou S, Samoli E, Chalbot MC, Kavouras IG. Air pollution and cardiovascular and respiratory emergency visits in Central Arkansas: a time-series analysis. Sci Total Environ. 2015;536:872–879. doi: 10.1016/j.scitotenv.2015.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL (2004) Risk factors for TIA and TIA as a risk factor for stroke. Neurology 62:S7–S11. 10.1212/wnl.62.8_suppl_6.s7 [DOI] [PubMed]
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrock JW, Glasenapp M, Victor A, Losey T, Cydulka RK. Variables associated with discordance between emergency physician and neurologist diagnoses of transient ischemic attacks in the emergency department. Ann Emerg Med. 2012;59:19–26. doi: 10.1016/j.annemergmed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Sheehan OC, Merwick A, Kelly LA, Hannon N, Marnane M, Kyne L, McCormack PM, Duggan J, Moore A, Moroney J, Daly L, Harris D, Horgan G, Kelly PJ. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke. 2009;40:3449–3454. doi: 10.1161/STROKEAHA.109.557074. [DOI] [PubMed] [Google Scholar]
- Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I, Abdulkader RS, Abebe M, Abebe Z, Abera SF, Abil OZ, Abraha HN, Abrham AR, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Stewart JD, Eliot MN, Yanosky JD, Liao D, Tinker LF, Eaton CB, Whitsel EA, Wellenius GA. Short-term exposure to air pollution and incidence of stroke in the Women's Health Initiative. Environ Int. 2019;132:105065. doi: 10.1016/j.envint.2019.105065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Liu H, Zhao Z, Xiang X, Li M, Juan J, Song J, Cao Y, Wang X, Chen L, Wei C, Hu Y, Gao P. Association between ambient air pollution and daily hospital admissions for ischemic stroke: a nationwide time-series analysis. PLoS Med. 2018;15:e1002668. doi: 10.1371/journal.pmed.1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Liu H, Wu Y, Si Y, Song J, Cao Y, Li M, Wu Y, Wang X, Chen L, Wei C, Gao P, Hu Y. Association between ambient fine particulate pollution and hospital admissions for cause specific cardiovascular disease: time series study in 184 major Chinese cities. BMJ (Clin Res Ed) 2019;367:l6572. doi: 10.1136/bmj.l6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve PJ, Chen L, Stieb D, Rowe BH. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur J Epidemiol. 2006;21:689–700. doi: 10.1007/s10654-006-9050-9. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke. 2005;36:2549–2553. doi: 10.1161/01.str.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, Cohen A. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Liu G, Jiang Y, Li G, Pan Y, Wang Y, Wei Z, Wang J, Wang Y. Acute effects of particulate air pollution on ischemic stroke and hemorrhagic stroke mortality. Front Neurol. 2018;9:827. doi: 10.3389/fneur.2018.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on air pollution and meteorological factors can be obtained from the National Air Pollution Monitoring System (http://www.cnemc.cn) and the China Meteorological Data Sharing Service System (https://data.cma.cn). The data on admissions for TIA are available from the corresponding author upon reasonable request.