Abstract
Klotho, an important anti-aging protein, may be related to elevated blood pressure (BP) and arterial stiffness. We aimed to investigate associations between the serum klotho concentration and peripheral/central BP and arterial stiffness based on the carotid–femoral pulse wave velocity (cfPWV) in a Chinese population. We invited all inhabitants aged ≥ 18 years in two Dali communities for participation. The SphygmoCor system was used to record radial arterial waveforms. Aortic waveforms were derived using a generalized transfer function. The central BP was assessed by calibrating the brachial BP, which was measured using an oscillometric device. The serum klotho concentration was measured using an enzyme-linked immunosorbent assay and logarithmically transformed. Of the 716 participants (mean age: 51.9 ± 12.6 years), 467 (65.2%) were women. The median serum klotho concentration was 381.8 pg/mL. The serum klotho concentration did not significantly differ between patients with and without hypertension (P > 0.05) and between those with and without arterial stiffness (cfPWV ≥ 10 m/s) (P > 0.05). After adjusting for confounders, the serum klotho concentration was not significantly associated with the peripheral or central BP (P > 0.05) and cfPWV (P > 0.05). Our data indicated that the serum klotho concentration was not associated with BP or cfPWV in the general Chinese population.
Subject terms: Biomarkers, Cardiology, Endocrinology
Introduction
Klotho is an anti-aging gene that shortens and extends the lifespan when disrupted and overexpressed, respectively1. Klotho-deficient mice exhibit signs of accelerated aging, such as arterial stiffness, hypertension, and chronic kidney disease (CKD)1. The α-klotho protein encoded by the klotho gene is a multifunctional protein that regulates the metabolism of calcium, phosphate, and vitamin D. The following three types of α-klotho protein with potentially different functions have been identified: full-length transmembrane α-klotho, truncated soluble α-klotho, and secreted α-klotho2. Previous studies have indicated that the prevalence of hypertension and arterial stiffness increases with age3,4, whereas the α-klotho concentrations decrease with age5.
A few studies have reported that klotho deficiency is associated with hypertension6, salt-sensitive hypertension7,8, CKD9, arterial stiffness10,11, and cardiomyopathy12. Therefore, evaluation of circulating klotho concentrations or klotho genotypes could help identify patients at higher risk of developing age-related cardiovascular morbidities. However, previous studies on the relationship between circulating klotho and blood pressure and arterial stiffness had small sample sizes13, used a case–control design14, performed analyses only in select patients13,15,16, measured peripheral blood pressure only8, or reported conflicting results15,17. In a recent study in young and middle-aged swine, elevated klotho secretion was associated with increased aortic stiffness and peripheral vascular resistance with aging17. In the present population-based study, we investigated the associations between the serum klotho (i.e., secreted α-klotho) concentrations and the peripheral/central blood pressure and arterial stiffness based on the carotid–femoral pulse wave velocity (cfPWV).
Results
Characteristics of the study population
Of the 716 participants, 467 (65.2%), 226 (31.6%), and 76 (10.6%) were women, had hypertension, and had diabetes, respectively. Table 1 presents the participant characteristics according to their sex. Men and women had similar characteristics, except for the rates of current smoking, alcohol consumption, hypertension, and diabetes mellitus, which were higher in men (P ≤ 0.01). Men also tended to be older and had a higher body weight, body height, body mass index, waist circumference, hip circumference, fasting levels of blood glucose, low-density lipoprotein cholesterol, triglyceride levels, peripheral systolic and diastolic blood pressures, central systolic and diastolic blood pressures, and cfPWV compared to women. The high-density lipoprotein cholesterol levels and glomerular filtration rate (P < 0.0001) were also lower in men than in women. Men and women had similar serum klotho concentrations (442 vs. 365 pg/mL, respectively, P = 0.17).
Table 1.
Characteristics of the study population.
| Variable | Men (n = 249) |
Women (n = 467) |
P |
|---|---|---|---|
| Current smoking, n (%) | 137 (55.0) | 8 (1.7) | < 0.0001 |
| Alcohol intake, n (%) | 73 (29.3) | 9 (1.9) | < 0.0001 |
| Hypertension, n (%) | 101 (40.6) | 126 (27.0) | 0.0002 |
| Diabetes mellitus, n (%) | 40 (16.0) | 36 (7.7) | 0.0005 |
| Age, years | 53.6 ± 13.5 | 51.0 ± 12.0 | 0.01 |
| Body height, cm | 168.3 ± 5.8 | 157.1 ± 5.9 | < 0.0001 |
| Body weight, kg | 69.5 ± 10.9 | 58.3 ± 8.8 | < 0.0001 |
| Body mass index, kg/m2 | 24.5 ± 3.4 | 23.6 ± 3.3 | 0.0005 |
| Waist circumference, cm | 89.0 ± 9.0 | 83.1 ± 8.9 | < 0.0001 |
| Hip circumference, cm | 96.8 ± 7.3 | 94.6 ± 7.3 | 0.0002 |
| Total protein, g/L | 76.8 ± 7.7 | 77.4 ± 4.6 | 0.26 |
| Fasting blood glucose, mmol/L | 5.9 ± 1.8 | 5.5 ± 1.4 | 0.001 |
| Total cholesterol, mmol/L | 4.8 ± 1.0 | 4.8 ± 0.9 | 0.86 |
| HDL-C, mmol/L | 1.3 ± 0.2 | 1.4 ± 0.2 | < 0.0001 |
| LDL-C, mmol/L | 2.7 ± 0.7 | 2.5 ± 0.7 | 0.002 |
| Triglycerides, mmol/L | 1.7 (1.2–2.4) | 1.4 (1.0–1.9) | 0.002 |
| GFR, mL/min/1.73 m2 | 87.0 ± 18.0 | 95.3 ± 18.7 | < 0.0001 |
| Serum klotho, pg/mL | 442 (199–921) | 365 (168–995) | 0.17 |
| Peripheral SBP, mmHg | 122.4 ± 17.5 | 117.0 ± 17.7 | 0.0001 |
| Peripheral DBP, mmHg | 79.7 ± 11.8 | 75.9 ± 10.8 | < 0.0001 |
| Peripheral PP, mmHg | 42.8 ± 12.0 | 41.2 ± 11.0 | 0.07 |
| Central SBP, mmHg | 118.9 ± 15.8 | 114.2 ± 17.2 | 0.0003 |
| Central DBP, mmHg | 81.7 ± 12.1 | 76.5 ± 11.4 | < 0.0001 |
| Central PP, mmHg | 36.7 ± 8.5 | 37.1 ± 9.5 | 0.55 |
| Pulse rate, beats/min | 71.2 ± 9.3 | 72.4 ± 8.5 | 0.09 |
| cfPWV, m/s | 11.2 ± 2.2 | 10.0 ± 1.8 | < 0.0001 |
Values are presented as means ± standard deviations, medians (interquartile ranges), or numbers (%).
cfPWV carotid–femoral pulse wave velocity, DBP diastolic blood pressure, GFR glomerular filtration rate, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, PP pulse pressure, SBP systolic blood pressure.
Table 2 summarizes the study population characteristics stratified by the presence or absence of hypertension. Participants with and without hypertension had similar serum klotho concentrations, body height, lipid profile, and pulse rate (P > 0.05). However, compared with normotensive participants, those with hypertension included fewer women and had lower glomerular filtration rates; higher rates of current smoking, alcohol intake, diabetes mellitus; older age; and higher body weight, body mass index, waist and hip circumference, total protein levels, peripheral and central blood pressures, and cfPWV (P ≤ 0.03).
Table 2.
Characteristics of the study population stratified by the hypertension status.
| Variable | Hypertensive (n = 227) |
Normotensive (n = 489) |
P |
|---|---|---|---|
| Women, n (%) | 126 (55.5) | 341 (69.7) | 0.0002 |
| Current smoking, n (%) | 57 (25.1) | 88 (18.0) | 0.03 |
| Alcohol intake, n (%) | 38 (16.7) | 44 (9.0) | 0.003 |
| Diabetes mellitus, n (%) | 39 (17.2) | 37 (7.6) | 0.0001 |
| Age, years | 58.3 ± 10.4 | 48.9 ± 12.4 | < 0.0001 |
| Body height, cm | 161.3 ± 8.2 | 160.1 ± 7.8 | 0.48 |
| Body weight, kg | 66.3 ± 11.4 | 60.3 ± 10.2 | < 0.0001 |
| Body mass index, kg/m2 | 25.4 ± 3.4 | 23.2 ± 3.1 | < 0.0001 |
| Waist circumference, cm | 89.7 ± 8.8 | 83.0 ± 8.9 | < 0.0001 |
| Hip circumference, cm | 98.6 ± 7.2 | 93.9 ± 7.0 | < 0.0001 |
| Total protein, g/L | 78.1 ± 7.7 | 76.8 ± 4.8 | 0.02 |
| Fasting blood glucose, mmol/L | 5.9 ± 1.8 | 5.5 ± 1.4 | 0.002 |
| Total cholesterol, mmol/L | 4.9 ± 1.0 | 4.8 ± 0.9 | 0.19 |
| HDL-C, mmol/L | 1.4 ± 0.2 | 1.4 ± 0.2 | 0.31 |
| LDL-C, mmol/L | 2.6 ± 0.7 | 2.6 ± 0.7 | 0.40 |
| Triglycerides, mmol/L | 1.8 (1.3–2.4) | 1.4 (1.0–1.9) | 0.002 |
| GFR, mL/min/1.73 m2 | 87.1 ± 20.5 | 94.8 ± 17.6 | 0.006 |
| Serum klotho, pg/mL | 382 (185–947) | 382 (178–952) | 0.52 |
| Peripheral SBP, mmHg | 135.5 ± 17.2 | 111.2 ± 12.0 | < 0.0001 |
| Peripheral DBP, mmHg | 86.4 ± 11.7 | 72.9 ± 8.2 | < 0.0001 |
| Peripheral PP, mmHg | 49.1 ± 12.9 | 38.3 ± 8.7 | < 0.0001 |
| Central SBP, mmHg | 128.8 ± 18.2 | 109.8 ± 12.1 | < 0.0001 |
| Central DBP, mmHg | 87.3 ± 12.7 | 74.2 ± 8.8 | < 0.0001 |
| Central PP, mmHg | 41.0 ± 12.1 | 35.1 ± 6.7 | < 0.0001 |
| Pulse rate, beats/min | 72.6 ± 9.1 | 71.7 ± 8.6 | 0.20 |
| cfPWV, m/s | 11.9 ± 2.1 | 9.7 ± 1.5 | < 0.0001 |
Values are presented as means ± standard deviations, medians (interquartile ranges), or numbers (%).
cfPWV carotid–femoral pulse wave velocity, DBP diastolic blood pressure, GFR glomerular filtration rate, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, PP pulse pressure, SBP systolic blood pressure.
Univariate analyses
The median serum klotho concentration was 382 pg/mL. The serum klotho concentration was not significantly associated with the peripheral blood pressure, central blood pressure, pulse rate, or cfPWV (P > 0.05).
Peripheral and central blood pressure in relation to the serum klotho concentration
In the unadjusted analyses, no significant differences were found in the peripheral or central blood pressure levels across the quartiles of the serum klotho concentration (P > 0.05). Further adjustments for conventional cardiovascular risk factors including sex, age, body mass index, glomerular filtration rate, hypertension, diabetes mellitus, antihypertensive treatment, current smoking, and alcohol intake did not affect these results (P > 0.05) (Table 3). There was no significant difference in the serum klotho concentration between participants with and without hypertension (Fig. 1).
Table 3.
Peripheral and central blood pressure in relation to the serum klotho concentration.
| Characteristics | Klotho groups | P | |||
|---|---|---|---|---|---|
| 1st quartile (n = 179) (37–178 pg/mL) |
2nd quartile (n = 179) (181–382 pg/mL) |
3rd quartile (n = 179) (382–952 pg/mL) |
4th quartile (n = 179) (952–10,957 pg/mL) |
||
| Unadjusted | |||||
| Peripheral SBP, mmHg | 118.0 ± 1.3 | 119.0 ± 1.3 | 120.6 ± 1.3 | 118.1 ± 1.3 | 0.49 |
| Peripheral DBP, mmHg | 76.1 ± 0.8 | 78.3 ± 0.8 | 78.0 ± 0.8 | 76.5 ± 0.8 | 0.18 |
| Peripheral PP, mmHg | 41.9 ± 0.8 | 40.7 ± 0.8 | 42.6 ± 0.8 | 41.6 ± 0.8 | 0.44 |
| Central SBP, mmHg | 115.1 ± 1.3 | 115.9 ± 1.3 | 115.5 ± 1.3 | 116.8 ± 1.3 | 0.82 |
| Central DBP, mmHg | 77.2 ± 0.9 | 79.4 ± 0.9 | 78.0 ± 0.9 | 78.77 ± 0.9 | 0.33 |
| Central PP, mmHg | 37.4 ± 0.7 | 36.0 ± 0.7 | 37.0 ± 0.7 | 37.5 ± 0.7 | 0.36 |
| Adjusted* | |||||
| Peripheral SBP, mmHg | 118.6 ± 1.0 | 118.9 ± 1.0 | 120.2 ± 1.0 | 118.0 ± 1.0 | 0.40 |
| Peripheral DBP, mmHg | 76.7 ± 0.7 | 77.8 ± 0.7 | 77.8 ± 0.7 | 76.5 ± 0.7 | 0.37 |
| Peripheral PP, mmHg | 41.9 ± 0.7 | 41.1 ± 0.7 | 42.4 ± 0.7 | 41.4 ± 0.7 | 0.57 |
| Central SBP, mmHg | 115.8 ± 1.0 | 115.8 ± 1.0 | 115.2 ± 1.0 | 116.5 ± 1.0 | 0.84 |
| Central DBP, mmHg | 77.8 ± 0.7 | 79.1 ± 0.7 | 77.8 ± 0.7 | 78.7 ± 0.7 | 0.49 |
| Central PP, mmHg | 37.4 ± 0.6 | 36.2 ± 0.6 | 36.9 ± 0.6 | 37.3 ± 0.6 | 0.52 |
Values are presented as means ± standard errors. *Values are adjusted for age, sex, body mass index, glomerular filtration rate, hypertension, diabetes status, antihypertensive treatment, current smoking, and alcohol intake.
DBP diastolic blood pressure, PP pulse pressure, SBP systolic blood pressure.
Figure 1.
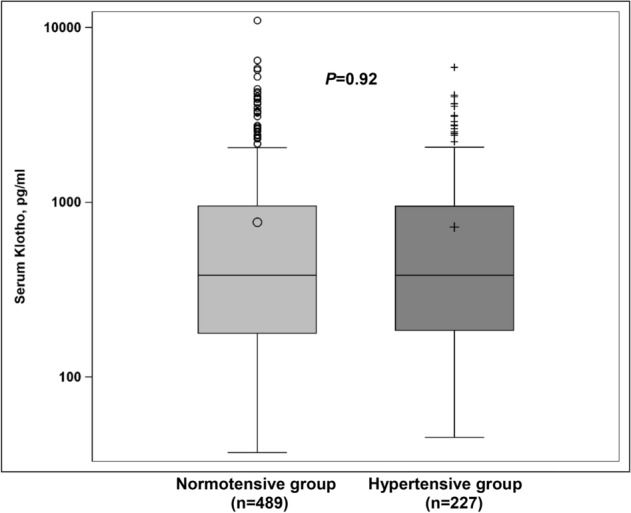
Differences in the serum klotho concentration between patients with and without hypertension.
cfPWV in relation to the serum klotho concentration
Irrespective of the adjustments for conventional cardiovascular risk factors, there were no significant differences in cfPWV across the quartiles of the serum klotho concentration (P > 0.05) (Table 4). There was no significant difference in the serum klotho concentration between the participants with and without arterial stiffness defined by cfPWV (Fig. 2).
Table 4.
Pulse wave velocity in relation to the serum klotho concentration.
| Characteristics | Klotho groups | P | |||
|---|---|---|---|---|---|
| 1st quartile (n = 179) (37–178 pg/mL) |
2nd quartile (n = 179) (181–382 pg/mL) |
3rd quartile (n = 179) (382–952 pg/mL) |
4th quartile (n = 179) (952–10,957 pg/mL) |
||
| Unadjusted | |||||
| cfPWV, m/s | 10.4 ± 0.2 | 10.4 ± 0.2 | 10.4 ± 0.2 | 10.5 ± 0.2 | 0.93 |
| Adjusted* | |||||
| cfPWV, m/s | 10.4 ± 0.1 | 10.4 ± 0.1 | 10.2 ± 0.1 | 10.5 ± 0.1 | 0.38 |
Values are presented as means ± standard errors. *Values are adjusted for age, sex, body height, pulse rate, glomerular filtration rate, hypertension, diabetes status, antihypertensive treatment, current smoking, and alcohol intake.
cfPWV carotid–femoral pulse wave velocity.
Figure 2.
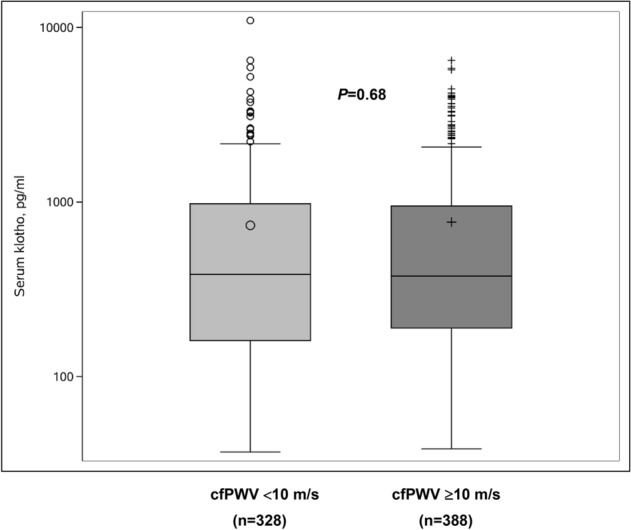
Differences in the serum klotho concentration between participants with cfPWV ≥ 10 m/s and those with cfPWV < 10 m/s. cfPWV carotid–femoral pulse wave velocity.
Discussion
To the best of our knowledge, this is the first study that systematically investigated the relationship between the serum klotho concentration and blood pressure/arterial stiffness using a population-based design. Our results indicated that the serum klotho concentration is not associated with peripheral or central blood pressure or indices of arterial stiffness.
Few previous studies have indicated that a lower circulating klotho concentration was associated with higher blood pressure or was a risk factor of hypertension. Especially, in an analysis of 79 and 30 older adults with and without hypertension, respectively, Su et al. reported that klotho protein absorbance (0.303 ± 0.096) was lower in the hypertensive than in the non-hypertensive group (0.489 ± 0.216)13. In a 1:1 matched case–control study (mean age: 51.7 years) of 197 Chinese patients with hypertension and 197 without hypertension, Zhou et al.14 reported that the median serum klotho concentrations were 269.67 and 313.95 ng/L (P = 0.004) in the hypertensive and normotensive groups, respectively. After adjustments for covariates including age, lifestyle, family history of hypertension, body mass index, fasting blood glucose, serum uric acid, lipid levels, and microalbumin, the risks (odds ratio, OR [95% confidence interval, CI]) of hypertension in the lowest three quartile groups were 2.01 (1.08–3.71), 2.29 (1.20–4.39), and 1.55 (0.82–2.92) compared with those in the highest quartile group, respectively14.
In contrast to the results of some previous studies, our findings did not support an association between the serum klotho concentration and blood pressure in the general population13,14. However, our results were consistent with those of a larger population-based study18. In the InCHIANTI study of 1,203 Italian adults, the median (25th–75th percentile) plasma klotho concentration was 676 (530–819) pg/mL. Plasma klotho was correlated with age but not with systolic blood pressure18. Despite convincing experimental evidence regarding the role of klotho deficiency in the pathogenesis of hypertension and salt hypertension6,19–21, the roles of klotho in human blood pressure regulation and pathogenesis of hypertension remain largely unknown. The discrepancies between the present and previous results may be attributable to differences in the study population age13 or small sample sizes of previous studies (≤ 394 participants)13,14. Furthermore, it is also possible that klotho is important in regulating blood pressure only in those processes or pathologies that are associated with a very drastic reduction in its serum concentrations. Further large-scale prospective studies are required to verify the relationships between the circulating klotho levels and blood pressure changes and the risk of hypertension in different age groups and disease conditions.
Few previous studies have investigated the relationship between circulating klotho and arterial stiffness based on baPWV or cfPWV measurements. Our finding that the serum klotho concentration was not associated with arterial stiffness in the general Chinese population is consistent with the results of most, but not all, previous studies15,16,22,23. A previous study in 2101 participants from the KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) cohort reported no significant association between the serum klotho concentration and baPWV after adjustments (β = 0.003; 95% CI: − 0.04–0.05; P = 0.876)16. In 109 patients with diabetic nephropathy, pulse wave velocity (PWV) was reported to increase in those with CKD but was not related to serum klotho concentration22. However, our results contradicted those reported in some small-scale studies15,23 and in studies that included patients undergoing hemodialysis23 or those with CKD15. In a case–control study of 130 age- and sex-matched patients undergoing hemodialysis, α-klotho concentrations were inversely associated with the aortic–brachial PWV ratio (β = − 0.070; 95% CI: − 0.133 to − 0.006)23. In another study that included 114 patients with CKD, there were significant decreases in the serum klotho concentration in those with baPWV ≥ 1400 cm/s15.
A growing body of evidence has suggested that some single nucleotide polymorphisms in the klotho gene were correlated with the circulating klotho levels and the susceptibility to hypertension24, salt hypertension8, and coronary artery disease25 as well as the onset of stroke26. Though we did not find a significant relationship between the serum klotho concentrations, blood pressure, and PWV, we cannot exclude the possibility that genetic polymorphisms of the klotho gene may be associated with blood pressure and arterial stiffness.
Although our study did not support a relationship between the serum klotho and blood pressure and arterial stiffness in middle-aged Chinese individuals, previous experimental studies have indicated that klotho deficiency affected the regulation of blood pressure and pathogenesis of arterial stiffness via promoting oxidative stress, inflammation, apoptosis, and fibroblast growth factor receptor (FGFR)/FGF23 resistance11,27. Klotho can function as a humoral factor and regulate nitric oxide production in the endothelium, thus, preserving endothelial permeability, calcium homeostasis in the kidneys, and inhibiting insulin-like growth factor-1 signaling28. It can also serve as a mediator for the actions of FGF23, namely urinary phosphate excretion, inhibition of calcitriol secretion, and inhibition of parathyroid hormone synthesis and secretion29. Klotho deficiency increases NADPH oxidase activity and superoxide production, collagen expression, and elastin fragmentation in the aortic media30. Furthermore, klotho deficiency causes salt-sensitive hypertension and renal damage in mice by CC chemokine receptor 2-mediated inflammation7.
Our findings clarified and complicated the understanding of the relationship between klotho and blood pressure/arterial stiffness. We measured the serum klotho concentrations, peripheral and central blood pressures, and cfPWV in a much larger population-based sample compared with previous studies, thus, increasing the possibility of identifying any significant associations. However, klotho concentration, blood pressure, and arterial stiffness were highly dependent on age and other cardiovascular risk factors, such as diabetes mellitus, cigarette smoking, CKD, and metabolic syndrome31–34. When compared with aging, cigarette smoking, diabetes mellitus, CKD, and other typical cardiovascular risk factors, klotho deficiency may be a relatively weaker risk factor for cardiovascular disease in humans. Further prospective follow-up studies with larger sample sizes are required to elucidate the relationships between circulating klotho, blood pressure, and arterial stiffness.
Our findings should be interpreted within the context of the strengths and limitations of this study. Our study included 716 individuals who underwent comprehensive assessments of central blood pressure and cardiovascular function. However, it presented the following limitations. First, the cross-sectional design did not allow us to make any inferences regarding causality. The current design of our study did not allow us to test the association of klotho deficiency with microalbuminuria and the predictive value for renal function decline35,36. Second, the serum klotho concentration was estimated only once, which can be influenced by several factors and may fluctuate with time. Third, the serum was stored at – 30 ℃, and we could not exclude the possibility that the stability of serum klotho decreases with longer storing time37. Fourth, although we had very detailed demographic information of each individual, the data regarding antihypertensive medications, which might alter the serum klotho levels38, were not available. Nonetheless, the self-reported antihypertensive treatment rate was 19.6%, suggesting that the antihypertensive treatment rate was very low in this study. Further adjustment for antihypertensive treatment did not significantly change our results. Similar results were found in the sensitivity analysis among those not receiving antihypertensive treatment. Fifth, we did not measure the vitamin D and FGF23 levels. The molecular interactions of FGF23, klotho, and vitamin D coordinate to regulate the delicate phosphate levels of human body. Vitamin D can induce FGF23 and klotho synthesis to influence renal phosphate balance39. Thus, we could not exclude the possibility that vitamin D and FGF23 could affect the blood pressure regulation and pathogenesis of arterial stiffness. Sixth, the reproducibility of cfPWV measurement was not tested. Nonetheless, the cfPWV measurement was well standardized, and the same technician performed all the cfPWV measurements in this study. Thus, this limitation did not affect our results. Finally, our participants had a relatively lower profile of cardiovascular risk than those included in previous clinical studies. Future large-scale prospective studies should include participants at higher risk to explore the relationships between the serum klotho concentration, blood pressure, hypertension, and arterial stiffness.
In conclusion, our findings demonstrated that the serum klotho concentration was not associated with peripheral/central blood pressure or arterial stiffness in the general Chinese population. Nonetheless, future prospective studies should investigate the importance of circulating klotho measurements in cardiovascular risk stratification.
Methods
Study participants
This cross-sectional analysis was based on data collected as part of an ongoing population study of multiple cardiovascular risk factors in Dali, Yunnan Province, China. The study participants were recruited from two communities in Dali. Between October and December 2018, we cooperated with the medical staff of the local community health service center to invite all inhabitants aged ≥ 18 years to participate in the study through notice and telephone calls. Of those invited, 764 (70%) participated. We excluded 48 individuals from our analysis because they did not have blood samples (n = 4) or arterial (n = 39) and data (n = 5). Therefore, a total of 716 participants were included in the present analysis.
The Ethics Committee of Dali University approved the study protocol, and all participants provided written informed consent. All procedures were performed in accordance with the Declaration of Helsinki.
Field work
After each participant had rested for at least 5 min in the sitting position, two experienced physicians measured the blood pressure five consecutive times using a mercury-based sphygmomanometer. These five readings were averaged for the final analysis. The same physicians also administered a standardized questionnaire to collect information related to the medical history, smoking habits, alcohol intake, and medications. Hypertension was defined as a peripheral systolic or diastolic blood pressure of at least 140 and 90 mmHg, respectively, while seated40. Patients who were prescribed antihypertensive drugs were also considered to have hypertension40. A trained physician performed the anthropometric measurements. The body mass index was calculated as the body weight in kilograms divided by the body height in meters squared.
Venous blood samples were drawn after overnight fasting for the measurement of plasma glucose and serum total cholesterol and other biochemical analyses. The glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease Study equation41. Diabetes mellitus was defined as a fasting plasma glucose level of at least 7.0 mmol/L, hemoglobin A1c level of at least 6.5%, or the use of antidiabetic agents42.
Central blood pressure and cfPWV measurement
To ensure a steady state, a trained physician performed all arterial measurements via applanation tonometry after participants had rested for 15 min in the supine position. The participants were instructed to refrain from smoking, vigorous exercise, and drinking alcohol or caffeinated beverages for at least 2 h before the examination. We used a high-fidelity SPC-301 micromanometer (Millar Instruments, Houston, TX, USA) interfaced with a laptop computer running SphygmoCor software v7.1 (AtCor Medical, West Tyde, New South Wales, Australia) to record the arterial waveforms. The recordings were discarded when the variability in consecutive waveforms exceeded 5% or when the amplitude of the pulse wave signal was < 80 mV. We calibrated the pulse wave based on the average of two consecutive brachial blood pressure readings obtained with a participant in the supine position immediately prior to SphygmoCor recordings using a validated Omron HEM-7051 oscillometric blood pressure monitor (Omron, Kyoto, Japan). The SphygmoCor software calculates the aortic pulse wave from the radial signal using a validated generalized transfer function. The central systolic and diastolic blood pressures were derived from the aortic pulse wave43,44.
For the cfPWV measurement, the physician recorded the right carotid and femoral waveforms (12 s each) in succession. Based on simultaneously recorded electrocardiogram data (lead 2), the time delay between the feet of the two pressure waveforms was taken as the transit time between the carotid and femoral pressure waves. The distance traveled by the pressure wave was determined based on the difference between the distances from the sternal notch to the femoral location and from the sternal notch to the carotid location. PWV was calculated as the distance traveled divided by the transit time43,44. Arterial stiffness was defined as a cfPWV ≥ 10 m/s45.
Serum klotho measurements
Serum samples were stored at − 30 °C prior to measurements. The serum klotho concentration was measured using the enzyme-linked immunosorbent assay method in accordance with manufacturer instructions (DY5334-05, R&D Systems, Inc., Minneapolis, MN, USA). The detection range was 78.10–5000 pg/mL. The limit of the kit’s sensitivity was 50 pg/mL.
Statistical analysis
We used SAS v9.4 (SAS Institute, Cary, NC, USA) for database management and statistical analyses. Normality of data was assessed using the Shapiro–Wilk test. The serum klotho concentration exhibited a non-normal distribution and was, therefore, logarithmically transformed for statistical analysis. The means and proportions were compared using Student’s t-tests and Fisher’s exact tests, respectively. We performed unadjusted and multivariate-adjusted variance analyses to investigate the associations of serum klotho concentration with the central and peripheral blood pressures and arterial stiffness. P values < 0.05 were considered statistically significant.
Acknowledgments
The authors gratefully acknowledge the voluntary involvement of all study participants. We would like to thank Editage for English language editing.
Author contributions
L.H.L. was involved in the study design. W.Y.L., L.H.W., J.H.W., Q.L.L., Q.Y.L., N.Q.H., and Q.L. were involved in data collection. L.H.L. analyzed the data and drafted the manuscript.
Funding
This study was financially supported by Grants from the National Natural Science Foundation of China (Grants 81460084, 81660072, and 81860084), the Young and Middle-aged Academic Leader Training Foundation of Yunnan Province (2015HB056), the Medical Academic Leader Foundation of Yunnan Provincial Bureau of Health (D-201672), the Innovation Team of Hypertension Prevention and Treatment of Dali University (ZKPY2019304), and the Ten-thousand Talents Program of Yunnan Province.
Data availability
No datasets were generated or analyzed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wan-Ying Liang, Li-Hong Wang and Jian-Hang Wei.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Sun Z. Molecular basis of klotho: From gene to function in aging. Endocr. Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao J, et al. The risk of hypertension doubles every 10 years in China: Age, period, and birth cohort effects on the prevalence of hypertension from 2004 to 2013. Am. J. Hypertens. 2019;32:492–502. doi: 10.1093/ajh/hpz003. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, et al. Trajectories of age-related arterial stiffness in Chinese men and women. J. Am. Coll. Cardiol. 2020;75:870–880. doi: 10.1016/j.jacc.2019.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Takumida M, Ishibashi T, Hamamoto T, Hirakawa K, Anniko M. Age-dependent changes in the expression of klotho protein, TRPV5 and TRPV6 in mouse inner ear. Acta Otolaryngol. 2009;129:1340–1350. doi: 10.3109/00016480902725254. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Chen K, Lei H, Sun Z. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J. Am. Soc. Nephrol. 2015;26:121–132. doi: 10.1681/ASN.2013101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citterio L, et al. Klotho gene in human salt-sensitive hypertension. Clin. J. Am. Soc. Nephrol. 2020;15:375–383. doi: 10.2215/CJN.08620719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y, Kuro-o M, Sun Z. Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology. 2013;154:3855–3863. doi: 10.1210/en.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y, Chen J, Sun Z. Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension. 2016;67:564–573. doi: 10.1161/HYPERTENSIONAHA.115.06825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamal T, Liang WY, Li LH. Klotho, hypertension and arterial stiffness: A review. Austin J. Nephrol. Hypertens. 2019;6:1082. [Google Scholar]
- 12.Xie J, Wu YL, Huang CL. Deficiency of soluble alpha-klotho as an independent cause of uremic cardiomyopathy. Vitam. Horm. 2016;101:311–330. doi: 10.1016/bs.vh.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Su XM, Yang W. Klotho protein lowered in elderly hypertension. Int. J. Clin. Exp. Med. 2014;7:2347–2350. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y-T, et al. The relationship between serum klotho protein and hypertension. Chin. J. Hypertens. 2018;26:265–270. doi: 10.1097/01.hjh.0000549079.68044.2a. [DOI] [Google Scholar]
- 15.Kitagawa M, et al. A decreased level of serum soluble klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE. 2013;8:e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, et al. The association between soluble klotho and cardiovascular parameters in chronic kidney disease: results from the KNOW-CKD study. BMC. Nephrol. 2018;19:51. doi: 10.1186/s12882-018-0851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Kassab GS. Increased serum klotho with age-related aortic stiffness and peripheral vascular resistance in young and middle-aged swine. Front. Physiol. 2020;11:591. doi: 10.3389/fphys.2020.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semba RD, et al. Plasma klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 2011;59:1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Sun Z. RNAi silencing of brain klotho potentiates cold-induced elevation of blood pressure via the endothelin pathway. Physiol. Genomics. 2010;41:120–126. doi: 10.1152/physiolgenomics.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamari Y, et al. The effect of klotho treatment on atherogenesis, blood pressure, and metabolic parameters in experimental rodent models. Horm. Metab. Res. 2016;48:196–200. doi: 10.1055/s-0035-1549879. [DOI] [PubMed] [Google Scholar]
- 21.Chen K, Sun Z. Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension. J. Mol. Med. 2019;97:1615–1625. doi: 10.1007/s00109-019-01841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inci A, et al. Soluble klotho levels in diabetic nephropathy: Relationship with arterial stiffness. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3230–3237. [PubMed] [Google Scholar]
- 23.Desbiens LC, et al. FGF23-klotho axis, bone fractures, and arterial stiffness in dialysis: A case-control study. Osteoporos. Int. 2018;29:2345–2353. doi: 10.1007/s00198-018-4598-2. [DOI] [PubMed] [Google Scholar]
- 24.Gao LL, Ding X, Xie DM, Yang M, Dong BR. G-395A polymorphism in the promoter region of the KLOTHO gene and hypertension among elderly (90 years and older) Chinese individuals. Genet. Mol. Res. 2015;14:15444–15452. doi: 10.4238/2015.November.30.22. [DOI] [PubMed] [Google Scholar]
- 25.Akbari H, et al. Association of klotho gene polymorphism with hypertension and coronary artery disease in an Iranian population. BMC. Cardiovasc. Disord. 2018;18:237. doi: 10.1186/s12872-018-0971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama S, et al. A klotho gene single nucleotide polymorphism is associated with the onset of stroke and plasma klotho concentration. Aging (Albany NY) 2018;11:104–114. doi: 10.18632/aging.101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olejnik A, Franczak A, Krzywonos-Zawadzka A, Kałużna-Oleksy M, Bil-Lula I. The biological role of klotho protein in the development of cardiovascular diseases. BioMed. Res. Int. 2018;2018:5171945. doi: 10.1155/2018/5171945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung CP, et al. α-Klotho expression determines nitric oxide synthesis in response to FGF-23 in human aortic endothelial cells. PLoS ONE. 2017;12:e0176817–e0176817. doi: 10.1371/journal.pone.0176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G, et al. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553:461–466. doi: 10.1038/nature25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao D, et al. Activation of SIRT1 attenuates Klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension. 2016;68:1191–1199. doi: 10.1161/HYPERTENSIONAHA.116.07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amaro-Gahete FJ, et al. Relationship between plasma S-Klotho and cardiometabolic risk in sedentary adults. Aging (Albany NY) 2020;12:2698–2710. doi: 10.18632/aging.102771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamizono Y, et al. Impact of cigarette smoking cessation on plasma α-klotho levels. Medicine (Baltimore) 2018;97:e11947. doi: 10.1097/MD.0000000000011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keles N, et al. Is serum klotho protective against atherosclerosis in patients with type 1 diabetes mellitus? J. Diabetes Complicat. 2016;30:126–132. doi: 10.1016/j.jdiacomp.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, et al. Serum klotho is inversely associated with metabolic syndrome in chronic kidney disease: Results from the KNOW-CKD study. BMC. Nephrol. 2019;20:119. doi: 10.1186/s12882-019-1297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maltese G, Fountoulakis N, Siow RC, Gnudi L, Karalliedde J. Perturbations of the anti-ageing hormone Klotho in patients with type 1 diabetes and microalbuminuria. Diabetologia. 2017;60:911–914. doi: 10.1007/s00125-017-4219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fountoulakis N, Maltese G, Gnudi L, Karalliedde J. Reduced levels of anti-ageing hormone klotho predict renal function decline in type 2 diabetes. J. Clin. Endocrinol. Metab. 2018;103:2026–2032. doi: 10.1210/jc.2018-00004. [DOI] [PubMed] [Google Scholar]
- 37.Adema AY, Vervloet MG, Blankenstein MA, Heijboer AC. α-Klotho is unstable in human urine. Kidney Int. 2015;88:1442–1444. doi: 10.1038/ki.2015.238. [DOI] [PubMed] [Google Scholar]
- 38.Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin. J. Am. Soc. Nephrol. 2013;8:1899–1905. doi: 10.2215/CJN.02700313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razzaque MS. FGF23, klotho and vitamin D interactions: What have we learned from in vivo mouse genetics studies? Adv. Exp. Med. Biol. 2012;728:84–91. doi: 10.1007/978-1-4614-0887-1_5. [DOI] [PubMed] [Google Scholar]
- 40.Joint Committee for Guideline Revision 2018 Chinese guidelines for prevention and treatment of hypertension—a report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J. Geriatr. Cardiol. 2019;16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levey AS, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 42.Expert committee on the diagnosis and classification of diabetes mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care26, S5–S20 (2003). [DOI] [PubMed]
- 43.Huang QF, et al. Arterial stiffness and wave reflections in relation to plasma advanced glycation end products in a Chinese population. Am. J. Hypertens. 2013;26:754–761. doi: 10.1093/ajh/hpt014. [DOI] [PubMed] [Google Scholar]
- 44.Huang QF, et al. Central and peripheral blood pressures in relation to plasma advanced glycation end products in a Chinese population. J. Hum. Hypertens. 2016;30:430–435. doi: 10.1038/jhh.2015.60. [DOI] [PubMed] [Google Scholar]
- 45.Van Bortel LM, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.


