Abstract
Colombia is one of the major producers and exporters of banana Musa paradisiaca. Its consumption is high then its agro-industrial wastes are important source to extract pectin. In the present study, inorganic acids (nitric acid and hydrochloride acid) and organic acids (citric acid and acetic acid) were evaluated to extract pectin from banana peels at pH 2 and 3, through acid hydrolysis during 1h at the temperature of 85 °C. The extraction yield, degree of esterification (DE) and intrinsic viscosity of the pectin were characterized. Nitric acid (NA) allowed to obtain higher extraction yield (54 ± 10%) and citric acid (CA) allowed to obtain higher DE (76.7 ± 2%). Additionally, the effect of polymer concentration and degree esterification, low degree of esterification (LDE, 33.1%) and high degree of esterification (HDE, 76.7%) were evaluated in the pectin nanoparticles formation. The pectin with HDE formed stable nanoparticles with a particle size of 255 ± 11 nm and the zeta potential value of -45 ± 3 mV, independently of the polymer concentration. Morphology analysis showed aggregated of the pectin nanoparticles.
Keywords: Banana peels, Degree esterification, Extraction, Nanoparticles, Pectin
Banana peels; degree esterification; extraction; nanoparticles; pectin
1. Introduction
According with the reports at 2018 of the Food and agriculture organization (FAO), Colombia is the third exporting country of organic bananas in the world, this implies that a large quantity of peels waste is generated (Chávez-Salazar et al., 2017); which is evidenced in the reports of the Ministry of Agriculture and Rural Development, a government agency, which inform that Colombia presented in 2002 a per capita consumption per year of 61.9 kg/person and in 2009 of 155 kg/person. According to the National Agricultural Census, in Colombia about 4.831.000 tons of banana are produced each year, and 39% of this production will be waste due to the peel obtained after the consumption of the pulp (Delgadillo and Vargas, 2019). Musa paradisiaca banana peels has been studied for pectin extraction using acid hydrolysis method (Castillo-Israel et al., 2015; Khamsucharit et al., 2018; Oliveira et al., 2016a, Oliveira et al., 2016b; Pagarra et al., 2019; Tanaid and Lauzon, 2018). Pectin is present in different food waste, thus, pectin extraction could be a solution to reduce the environmental impact of these waste at long term (Petkowicz et al., 2017). Pectin is an abundant polysaccharide presents in the plant cell walls, providing mechanical and functional properties in the cell wall during the different stages or process of harvest or store of the fruits and vegetables (Kyomugasho et al., 2015). The physicochemical properties of the pectin related with the extraction yield, degree of esterification (DE), solubility, gelling, pH and molecular weight, depend on different experimental variables such as the extraction method, temperature, medium pH, reaction time, acid type, and source and preparation method of the vegetable material (Garna et al., 2007; Yoo et al., 2012). In this way, pectin extracted from cocoa husks from pH 2.5 to 4.0 using hydrochloride acid showed a higher pectin yield (7.62%) than the one obtained using citric acid (Chan and Choo, 2013). Other studies carried out by using response surface methodology have showed that better pectin extraction yield from bananas was obtained at low pH values, temperature close to 85 °C and reaction time of 1–2 h (Qiu et al., 2010). On the other hand, the ultrasound assisted extraction of pectin from industrial waste of Musa balbisiana allowed to obtain yield of 8.99% at pH 3.2 using citric acid, and extraction time of 27 min (Maran et al., 2017). While the yield is an important variable to evaluate the economic potential of the process, the DE is an important property to determinate the application and commercial utility, so, pectin can be categorized according with the percentage of methyl-esterified carboxylic group as pectin with low degree of esterification (LDE<50%) and pectin with high degree of esterification (HDE≥50%) (Chaiwarit et al., 2020). Pectin with HDE presents good gelling property and its mainly use is in the food and pharmaceutical industries as stabilizer (Chaharbaghi et al., 2017); additionally, pectin with LDE is used as pharmaceutical excipients since its high content of carboxylic acid groups allow chemical modifications to improve the properties of bioadhesion (Villanova et al., 2015). Citric acid at pH 0.5 was used in the pectin extraction from pistachio green hull, under conditions of 90 °C of temperature during 30 min, which allowed to obtain a pectin with LDE (26–53%) and low molecular weight (12875.3 g.mol−1) (Chaharbaghi et al., 2017). DE value near to 54% was obtained for the pectin extraction from pomegranate peels with citric acid at pH 2.5, temperature 88 °C and time of 120 min (Pereira et al., 2016). Happi Emaga et al. (2008) also showed that pectin with low DE and higher yield was obtained by using harsher extraction conditions of temperature and pH.
Pectin is an anionic polymer biocompatible, biodegradable and safe for human consumption (Brouns et al., 2012; Hisyam et al., 2018). These properties are attractive for biomedical and pharmaceutical applications such as in the design of drug delivery system or in the food industry as carrier of molecules with biological activity for functional food (Hu et al., 2015; Krivorotova et al., 2016), and for the development of nanosensors for food safety (Kannan and Guo, 2020). Nanoparticles properties such as particle size and surface charge depend on polymer properties and preparation method, and these define the final use of them. Some studies have evaluated the pectin properties as molecular weight (Zainudin et al., 2018) and the electrostatic interaction with cationic polymers as chitosan to form nanoparticles (Kowalonek, 2017; Maciel et al., 2017; 2015; Rampino et al., 2016). However, a few studies have evaluated the effect of DE in the formation of nanoparticles from pectin extracted from banana peels. In the present study, pectin was extracted at different acid type (organic and inorganic) and pH (2 and 3) from Musa paradisiaca banana peels, where the variables such as yield, degree of esterification (DE), solubility and dynamic viscosity were evaluated. Pectin with LDE and HDE were selected to evaluate the effect of DE and polymer concentration in the preparation of nanoparticles by sonication in aqueous ethanol solution, through the study of the particle size, polydispersity index (PdI) and zeta potential. So, our study complements previous works through the investigation of the effect of extraction conditions on pectin properties and their application in the nanoparticle's formation under environmental conditions; getting a potential alternative for the use of agro-industrial waste such as banana peels, a waste generated by one of the most consumed products in Colombia.
2. Materials and methods
2.1. Materials
Musa paradisiaca banana peels were provided by local fruit market in Bogotá, Colombia. Acetic acid (AA), hydrochloric acid 37% (HA), citric acid (CA), nitric acid 65% (NA), ethanol methanol, acetone and sodium acetate were supplied by Merck KGaA, 64271 Darmstadt, Germany. Commercial sodium hypochlorite (NaOCl, 0,01%) was used.
2.2. Pectin extraction from Musa paradisiaca banana peels
To eliminate any impurities or microorganism, the Musa paradisiaca banana peels were treated with sodium hypochlorite 0,01% and water. Small peels piece (4500 g) were pretreated in 1L of distilled water at 90 °C during 10 min in order to inactive the pectic enzymes, which are responsible of the hydrolysis of methyl ester groups (Kyomugasho et al., 2015). After that, the banana peels were filtered with cloth canvas and dried at 50 °C until constant weight of raw material (900 g). The dried material was pulverized and stored in desiccator.
2.3. Effect of acid type and pH on the pectin properties: extraction yield, DE, solubility and dynamic viscosity
An experimental factorial design 22 was used to evaluate the effect of acid type and pH on the pectin properties, such as extraction yield, DE, solubility, and dynamic viscosity. The analyzed factors were pH (2 and 3) and acid type (organic: acetic acid (AA) and citric acid (CA); and inorganic: hydrochloric acid (HA) and nitric acid (NA)). The response variables evaluated were the effect on pectin yield extraction and degree of esterification (DE); the last one property was directly related to the solubility and viscosity.
Briefly, 20g of the pulverized material were treated with each acid solution AA, CA, HA, NA 0.1M, with different pH, with the final concentration of 0.05 g/mL. The mixture was heated at 85 °C for 1 h (Koubala et al., 2008; Ma et al., 2013). After this time, it was filtered with canvas cloth. The filtered was cooled in an ice bath for 15 min and then it was centrifuged for 10 min at 3000 rpm. The pectin was precipitated from supernatant with 95% ethanol, in a volume ratio 1:2 (supernatant: ethanol), with the objective to use less amount of solvent (Chaharbaghi et al., 2017). The precipitated was kept for 4 h in an ice bath and then the pectin was filtered with canvas cloth. The obtained pectin gel was washed with ethanol 70% until pH 6–7. Finally, the pectin gel was dried at 50 °C until constant weight and stored in desiccator.
The obtained pectin was characterized by FTIR analysis (Fourier Transform Infrared), and degree of esterification (DE) was determined by band deconvolution by FTIR. The pectin FTIR spectra were obtained in the range 4000–450 cm−1. The samples were dispersed in KBr and the transmittance recorded over 12 scans in a FTIR Perkin Elmer spectrum one (instrument distributed by PerkinElmer, USA). Degree of esterification (DE) was determined by the area's relation of the absorption bands at 1731 cm−1 (methyl esterified carboxylic group) and 1638 cm−1 (carboxylate group) according with previous reports (Kyomugasho et al., 2015; Oliveira et al., 2016a, Oliveira et al., 2016b; Pereira et al., 2016; Petkowicz et al., 2017). The extraction yield was calculated according to the method reported by some authors (Chan and Choo, 2013; Rodsamran and Sothornvit, 2019), Eq. (1); where Ws is the weight of the dry pectin and Wo is the weight of the pulverized material (20g). The rheological behavior and the solubility also were analyzed.
| (1) |
Since the polar or no polar nature of the pectin can vary due to its degree of esterification, it is important to determine the solubility of pectin in different solvents to carry out further studies such as viscosity and nanoparticles formation. The solubility was determined using methanol, ethanol, and acetone as solvents in volume ratio 1:7 solvent:water. 5mg of pectin sample were weighed and mixed with the different solvents; each sample was stirred for 5 h at the temperature of 45 °C (Chaharbaghi et al., 2017; Liu et al., 2006; Malviya and Kulkarni, 2012).
The rheological behavior was analyzed through the measure of the dynamic viscosity. To determine the dynamic viscosity of the pectin samples, solutions of known concentration 300–600 mg/mL were prepared in a solution of ethanol: water in ratio 1:7 (v/v). The samples were measured at room temperature on an Anton Paar Stabinger Viscometer SVM 3000.
2.4. Preparation and characterization of pectin nanoparticles
To prepare the nanoparticles, two samples were selected according to the results of the previous section, pectin with high degree of esterification (HDE ≥50%) and pectin with low degree of esterification (LDE <50%) (Chaiwarit et al., 2020). Pectin nanoparticles were prepared in a solution of ethanol: water in ratio 1:7 (v/v) as solvent by sonication. The mixture was sonicated with a SONICS VCX130 vibra-cell ultrasound probe, with a pulse function on 2 s and off 2 s, with 90% amplitude, 130 kW, for 5 min. The particle size, zeta potential, polydispersity index (PdI) and morphology were determined.
The hydrodynamic diameters of particles and polydispersity index were measured by dynamic light scattering (DLS, HORIBA LB- 550, 1-7-8 Higashi-Kanda Chiyoda-ku, Tokyo, Japan) and zeta potential (ζ) was measured by Malvern, Zetasizer Nano Z (instrument distributed by Cecoltec, Medellín, Colombia). The measurements were determined using freshly prepared samples and dispersed in MilliQ-water.
The morphology of the nanoparticles was determined by scanning electron microscopy (SEM, DENTON VACUUM Desk IV). The sample was prepared by adding a drop on the carbon tape, and then it was dried under vacuum for 24 h. The sample was covered with gold and then analyzed.
2.4.1. Effect of pectin concentration and DE on nanoparticle size, zeta potential and PdI
An experimental factorial design 22 was used, where the factors were pectin concentration (1 and 2 mg/mL) and the DE (low, below 50%; and high, above 50%) (Chaiwarit et al., 2020). To evaluate the effect of these two factors on the nanoparticle's formation, the response variables were: particle size, size distribution or PdI, and zeta potential. The measurements were developed according with previous description.
2.5. Statistics
All data are presented as mean ± standard deviation (±SD) from at least three measurements. Means are compared between groups by one-way analysis of variance (ANOVA) and multiple range test using the software Statgraphics Centurion XVI.I. A value p < 0.05 is considered statistically significant. The variables considered were yield, DE, particle size, PdI and zeta potential.
3. Results and discussion
3.1. Effect of acid type and pH on the pectin properties: extraction yield, DE, solubility and dynamic viscosity
3.1.1. Extraction yield
Twenty percentage of the initial raw material was obtained, using four thousand five hundred grams of banana peels; this means that 20% of these agro-industrial banana peel waste could be used as raw material, reducing the environmental impact.
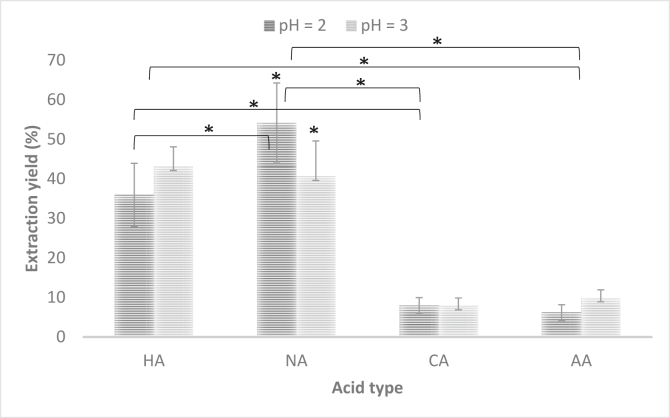
The extraction yield of the pectin was obtained through the calculation between the initial quantity of raw material used (20 g) and the grams of obtained pectin; according with the method of Rodsamran & Sothornvit, (2019); the Figure 1 shows these results. It is observed that inorganic acids (HA, NA) allowed to obtain a higher extraction yield, so the pectin extracted with nitric acid at pH 3 (NApH3) presented a yield of 54 ± 10%. The lowest percentage of extraction yield was obtained with organic acids, where the extractant of acetic acid at pH 2 (AApH2) allowed a yield of 6 ± 2%. These results are coherent with recent studies for the pectin extraction from lime peels where used inorganic acid (HA) and organic acid (CA). The results evidenced that hydrochloric acid (HA) as the extractant allowed to obtain a higher yield of pectin (23%) in comparison to CA (16%) (Rodsamran and Sothornvit, 2019). Pectin extracted from cacao pod husks (Theobroma cacao L.) using responde surface methodology with NA as extractant showed a yield of 9.5% (Vriesmann et al., 2011). Colodel & Petkowicz, (2019) also demonstrated that pectin extracted from Cubiu (Solanum sessiliflorum Dunal) fruit peels using NA and CA at pH 1.5 or 2.0; the NA allowed to obtain the highest yield (14.2%). This effect is due to the fact that stronger acidic conditions allow a greater disruption of the cell wall of the vegetal material, which leads to a greater release of pectin from the wall structure and it can be dissolved in the acid medium of the extractant solution (Ma et al., 2013). So, it was evident that inorganic acids (HA and NA) allowed to obtain higher extraction yield; additionally, the yield values differ with the reported, but it is important to note that the properties of pectin also depend on the extraction source and variables such as the temperature (Srivastava and Malviya, 2011).
Figure 1.
Extraction yield of the pectin extracted from Musa paradisiaca banana peels under different pH values (2 and 3) and acid type (organic and inorganic acids). HA: hydrochloride acid, NA: nitric acid, CA: citric acid, AA: acetic acid. Data represents the mean ± SD (n = 3). Statistically significant: ∗p = 0.001.
On the other hand, the pH variable did not show a significant difference in the yield values, possibly because both pH (2.0 and 3.0) are acidic enough to disrupt the wall, so the effect is not appreciable, as was demonstrated in the study of Chan & Choo, (2013); where pectin was extracted from cocoa husks with HA and CA, at pH 2.5 and 4.5, the yield extraction (4–5%) was similar independent of pH value. In a previous work, it was observed that in the pectin extraction from banana peels with HA and CA at pH 1.0 and 2.0, the pH variable did not show an effect in extraction yield (8–23 %) (Méndez and López, 2020). However, pH variable affects yield extraction when are considered other variables such as temperature and reaction time; as it was studied by Qiu et al. (2010), where pectin was extracted from banana with sodium hexametaphosphate at pH 1.5, 2.0 and 2.5, temperatures of 80, 85 and 90 °C, and reaction times of 1.0, 1.5 and 2.0 h. The results showed that low pH value got higher yields of banana pectin at the temperature of 85 °C and reaction time of 1.5 h; possibly these conditions improved the solubility of the extracted pectin and increased the amount of pectin in the acidic solution; but in our study were not considered these type of varibles.
3.1.2. FTIR analysis – degree esterification (DE)
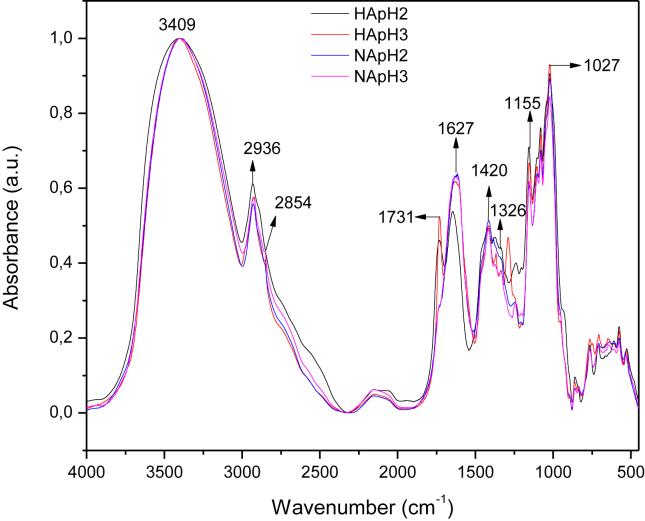
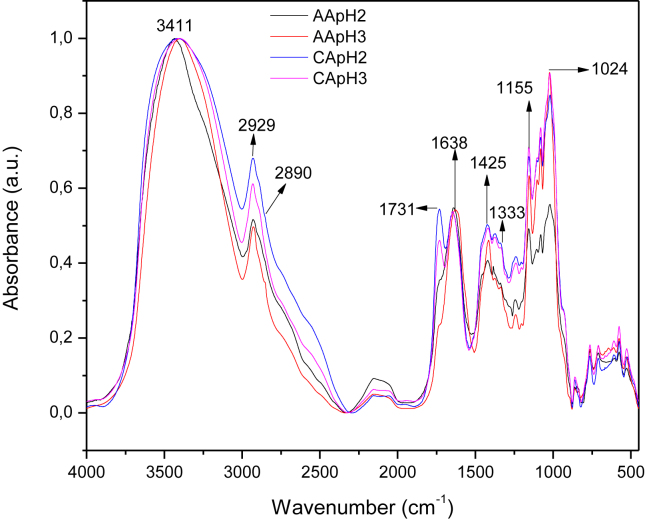
Generally, the pectin can be identified by the degree of esterification (DE) as pectin with low degree of esterification (LDE<50%) and high degree of esterification (HDE≥50%) (Chaiwarit et al., 2020). According to the Figures 2 and 3, the characteristic bands of the pectin obtained by acid hydrolysis with inorganic and organic acids, respectively, were identified. The extension signal observed at 3409 cm−1 was attributed to the hydroxyl groups (-OH) of the pectin skeleton, which may overlap with the hydroxyl group of the carboxylic acid group (-COOH). The 2936 and 2854 cm−1 bands are the extension of C–H bond of –CH2 alkyl groups. The band at 1731 cm−1 is characteristic of the –C=O bond ester carbonyl group (-COOCH3). At 1627 cm−1, the carboxylate group signal (-COO-) was observed. The band at 1420 cm−1 is a flexion of the C–H bond, and signals associated with primary amide groups. Characteristic stretch bands of the C–O–C bond are found in the 1155-1027 cm−1 region (Ogutu and Mu, 2017; Rodsamran and Sothornvit, 2019). The bands were identified according with previous studies, Tanaid & Lauzon, (2018) obtained pectin from unripe banana peel (Musa acuminata × balbisiana var. Cardaba), and the signals observed are consistent with those found in our work. Pectin extracted from pomegranate peels (Pereira et al., 2016), and from banana peels of different varieties (Kluai Khai and Kluai Leb Mu Nang from Musa (AA); Kluai Hom Thong from Musa (AAA); Kluai Nam Wa and Kluai Hin from Musa (ABB)) evidenced a coherency with the FTIR profile analyzed previously.
Figure 2.
Infrared analysis of the samples of pectin extracted with the inorganic acids at pH 2 and 3: hydrochloride acid (HApH2  , HApH3
, HApH3  ) and nitric acid (NApH2
) and nitric acid (NApH2  , NApH3
, NApH3  ).
).
Figure 3.
Infrared analysis of the samples of pectin extracted with the organic acids at pH 2 and 3: acetic acid (AApH2  , AApH3
, AApH3  ) and citric acid (CApH2
) and citric acid (CApH2  , CApH3
, CApH3  ).
).
The deconvolution of the bands of the region 1800-1510 cm−1 was carried out through the Origin program in order to calculate of DE according with the method of Oliveira et al., 2016b, Oliveira et al., 2016a. The statistical analysis showed that the DE is affected by the acid type but not by the pH. Pectin extracted with the organic acid CA pH2 and pH3 showed the HDE values (78.7 ± 2.4% and 76.7 ± 2.3), Table 1. Similar HDE values were obtained using citric acid to extract pectin from passion fruit peels (59.8%) (Liew et al., 2014), lime peel (70.81%) (Rodsamran and Sothornvit, 2019), pistachio green hull (53%) (Chaharbaghi et al., 2017), banana peles (51%) (Oliveira et al., 2016b, Oliveira et al., 2016a), pomegranate peles (54%) (Pereira et al., 2016), and mango peel (85.43%) (Wang et al., 2016). By the contrary, pectin with LDE was obtained using inorganic acids as sulfuric acid, hydrochloride acid and nitric acid, as extractant solution. Some studies are according with the results; Oliveira et al., 2016b, Oliveira et al., 2016a extracted pectin from cocoa husks at different pH, temperature and reaction times, using CA and HA; their results got DE values of 15.06%–57.86% and 7.17%–37.36%, respectively. However, Petkowicz et al. (2017) used NA at pH 1.5 for 2 h at 100 °C to extract pectin from cubiu (Solanum sessiliflorum D.) fruit peel, obtaining a DE of 62%. Muñoz-Almagro et al., (2018) reported the pectin extraction from sunflower heads, using sodium citrate and nitric acid at different temperatures (40–90 °C), reaction times (25.5–294.5 min) and extractant concentration (0.01–0.2 %), which got to obtain DE values in the range 18–44% with NA. It means, the pectin properties depend on the extraction conditions and the vegetable material source. So, organic acids allowed to obtain pectin with HDE and inorganic acids pectin with LDE; this behavior is due to the inorganic acids present stronger acid conditions and hydrolyzing capacity (Chan and Choo, 2013; Colodel and Petkowicz, 2019; Oliveira et al., 2016a, Oliveira et al., 2016b), favoring the acid hydrolysis process during the pectin extraction, where the esterified groups of the pectin could also be hydrolyzed and form carboxylate groups; then, LDE values are favored.
Table 1.
Results of DE calculated by FTIR and dynamic viscosity values for pectin extracted from Musa paradisiaca banana peels.
| Pectin sample | DE (%) ± SD | Dynamic viscosity (mPa.s)a |
|---|---|---|
| HApH2 | 33.1 ± 0.9 ∗ | 137.5 |
| HApH3 | 38.6 ± 1.1 ∗ | 47.1 |
| NApH2 | 8.0 ± 0.2 | 61.2 |
| NApH3 | 7.1 ± 0.2 | 29.7 |
| CApH2 | 78.7 ± 2.4 | 71.6 |
| CApH3 | 76.7 ± 2.3 ∗ | 363.3 |
| AApH2 | 21.7 ± 0.6 ∗ | 57.3 |
| AApH3 | 8.7 ± 0.2 ∗ | 185.8 |
Blank sample presented a dynamic viscosity value of 1.90 mPa.s. DE: degree of esterification. SD: standard deviation. Data represents the mean ± SD (n = 3). Statistically significant: ∗p = 0.001.
3.1.3. Solubility
The solubility of the pectin depends on degree of esterification, molecular weight, pH, temperature and ions present in the solution. In our study, DE was analyzed; the results showed that the mixture of 95% ethanol: water in a 1:7 volume ratio allowed to obtain a homogeneous solution in each sample studied. So, all samples with HDE (CApH2 and CApH3) and LDE (HApH2, HApH3, NApH2, NApH3, AApH2, AApH3) were soluble in the ethanolic solution at 12.5% after 5h of stirring and warming at 45 °C. This behavior is due to the interactions as hydrogen bonds between the hydroxyl groups of the ethanol solvent and those of pectin, in addition, the ethyl carbon chain (-CH2CH3) of the ethanol solvent favors the interaction by London forces with the carbon chains of pectin; important forces in the solubility and gelling properties of pectin (Willats et al., 2006). Low solubility was found in acetone solvent due to it is an aprotic polar solvent, so hydrogen bonds are not favored. On the other hand, as defined in most texts, pectin is a complex heteropolysaccharide, which implies that it contains a variety of monosaccharides and polysaccharides in its structure, such as glucose, mannose, fructose, others, which are also responsible of the solubility behavior of the pectin; and most of these neutral sugars have a higher solubility in ethanolic solution (Yapo, 2009), which could also explain the solubility in our prepared mixture. The most studies have reported that the solubility of the pectin depends on the pH of the medium. The solubility of jackfruit waste pectin was evaluated under the pH range of 2–10; the results showed that the solubility increases when the pH increases, due to the negatively charged carboxyl groups increase allowing an electrostatic repulsion, which avoid the gel formation (Begum et al., 2017). However, Begum et al. (2014) demonstrated that the extractant also affects this property; the pectin extraction with salts such as ammonium oxalate and sodium hexametaphosphate affected the solubility due to the presence of electrolytes and cations which can favors cross-links between ions-pectin to form gels (Gawkowska et al., 2018). In the present work inorganic and organic acids were used, and the pectin gels obtained were washed with ethanol 70% until pH 6–7; for that reason, the presence of ions or changes in the pH did not have influence.
3.1.4. Rheological characterization: dynamic viscosity
Rheological properties of the materials allow to analyze the behavior of how the stress or force applied in that material is related to the flow and deformation of the material; which depend on the interaction with the solvent and molecular size (Rasidek et al., 2018). Table 1 shows the results obtained for the dynamic viscosity of the pectin extracted at pH 2 and 3 with organic and inorganic acids. The viscosity values did not present a direct relationship with the two factors evaluated, the pH and acid type; so, it was not possible analyze a tendency of the results. Possibly, this behavior is due to the pH of the final solution of pectin, which varies according with the number of carboxylic acid groups present in the pectin structure. In our study, different DE values were obtained and the behavior of the pectin in solution could be different due to the pH was not controlled after pectin solubilization. Some studies have evaluated the effect of the pH on the dynamic viscosity of the pectin solutions, founding that acidic pH favors higher values of viscosity due to that the dissociation of carboxylic acid groups allows rearrangements of pectic networks, which increases the hydrophobic interactions forming a gel structure (Guimarães et al., 2009). Another study showed that lowest values of dynamic viscosity are observed in solutions of pectin with HDE at pH values close to their pKa (Torkova et al., 2018). Lopes da Silva et al. (1994) developed a study with aqueous solutions of pectin with HDE and LDE with 3.5% of concentration, pH 3 and different temperatures (5–65 °C); the study concluded that the different density of charged groups in the polymer is determinant in the viscoelastic behavior; a wide range of viscosity values could be observed due to the ionic strength of the pectin solutions. The above indicates that the final pH of the pectin solution must be controlled before carrying out the viscosity measurement; so, the effect of the extraction variables (pH and acid type) could be analyzed in the rheological properties of the pectin extracted in this investigation. According with the results, the sample CApH3 (363.3 mPa.s) presented a higher viscosity value and the sample NApH3 (29.7 mPa.s) the lower value; which could be due to the different acid conditions in the extraction. Mineral acids could promote polymer degradation due to the drastic conditions (Colodel and Lúcia De Oliveira Petkowicz, 2018), achieving smaller molecular sizes of the pectin; for the contrary, organic acids do not favor a drastic hydrolysis process (Colodel and Petkowicz, 2019); and the molecular size is not affected, allowing possibly a major molecular weight, which is related with the higher viscosity value. Maneerat et al. (2017) presented the extraction of pectin from Nam Wa banana peels using 0.05 HA and water, at pH 1.5 and 6.0, respectively; and the reaction was carried out for 30, 60 or 120 min, at the temperature of 90 °C. The results evidenced that intrinsic viscosity (cm3/g), molecular weight (Da) and apparent viscosity (mPa.s) of the pectin decreased with increasing reaction time, due to the prolonged acid hydrolysis process.
Based on the previous results, the pectin samples selected to evaluate the effect of the DE on nanoparticles formation were CApH3 with HDE (76.7 ± 2.3%) and high viscosity (363.3 mPa.s), and HApH2 with LDE (33.1 ± 0.9%) and moderate viscosity (137.5 mPa.s).
3.2. Effect of pectin concentration and DE on nanoparticle size, zeta potential and PdI
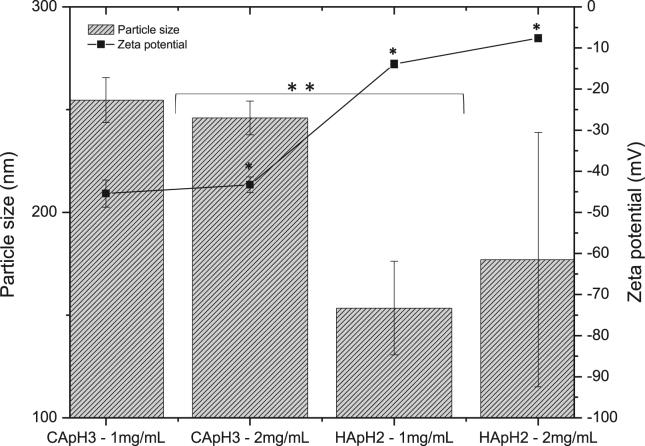
DE (low-33.1% and high-76.7%) (Chaiwarit et al., 2020) and polymer concentration were evaluated in the nanoparticle properties: particle size, zeta potential and PdI. The statistical analysis showed a significant different in particle size and zeta potential values due to the DE, but pectin concentration did not present an effect in particle size. DE and pectin concentration presented an effect in PdI. It was observed that CApH3 pectin with HDE (76.7 ± 2.3%) allowed to obtain particle sizes of 255 ± 11 and 246±8nm, and particle size distribution more homogeneous (PdI, 0.10 ± 0.02 and 0.26 ± 0.16) at the concentration of 1 and 2 mg/mL, respectively. Pectin with LDE (HApH2) formed nanoparticles with particle sizes of 153 ± 23 and 177 ± 62nm, and PdI (0.33 ± 0.13 and 0.50 ± 0.15), at the concentration 1 and 2 mg/mL, respectively. This is possibly due to the hydrophobic interactions of the methoxyl groups present in the structure of pectin with HDE and to the electrostatic repulsions between the carboxylate groups charged negatively, which favor the formation of stable colloidal systems (Jonassen et al., 2013). Hydrophobic-hydrophilic balance could get the self-assembled of the pectin, in this case esterified groups and neutral sections of the pectin polymer favor the hydrophobic nature, and the carboxylate and hydroxyl groups favor the hydrophilic nature; getting to form nanoparticles with a hydrophobic core and the hydrophilic groups exposed in the shell; behavior observed for chitosan modified with oleic acid (300 nm), where acetylated groups and oleic acid favor the hydrophobic interactions and the protonated amine groups favor the repulsion and hydrophilic interactions. So, HDE pectin favors hydrophobic interactions, which avoids the water flow inside the particle and the swelling of them, allowing a low PdI (Méndez et al., 2017).
Zeta potential values showed more negative values in nanoparticles prepared with pectin HDE (-45.4 ± 3.3 and -43.3 ± 1.9 mV); meanwhile, the sample with pectin LDE presented a less negative zeta potential (-13.9 ± 1.0 and (-7.6 ± 0.9 mV), indicating that it has fewer carboxylic acid groups in its structure, which could be contradictory, Figure 4. The higher viscosity (363.3 mPa.s) of the pectin HDE can be an indicative of higher molecular weight and longer chain length, which implies major number of carboxylate groups; also, the carboxylate groups (-COO-) can be more exposed and distributed throughout the chain, evidencing a more negative zeta potential, independent of the concentration. On the other hand, pectin with LDE and viscosity of 137.5 mPa.s possibly has shorter chains due to its low molecular weight, which could explain this behavior.
Figure 4.
Particle size (bars) and zeta potential (line) of pectin nanoparticles prepared with HDE (CApH3) and LDE (HApH2) samples, at the polymer concentrations 1 and 2 mg/mL. Data represents the mean ± SD (n = 3). Statistically significant: ∗∗p = 0.03, ∗p = 0.001.
The most studies have reported pectin hybrid nanoparticles where other material such as chitosan, silver (Shankar et al., 2016), cations, zein/caisenate (Chang et al., 2017) and chitosan/tripolyphosphate have been used. Rashidipour et al. (2019) reported nanoparticles prepared by ionotropic gelation with pectin HDE/chitosan/TPP with particle size of 249.85 ± 2.43 nm, zeta potential of -21.5 mV and PdI of 0.41. V. B. V. Maciel et al. (2017) obtained microparticles with particle size of 2000 nm and zeta potential of -22.5 mV from chitosan-pectin HDE. Zainudin et al. (2018) prepared nanoparticles from pectin HDE in the presence of inorganic salts, obtaining particle size smaller than 600 nm. Rampino et al. (2016) reported nanoparticles prepared by ionotropic gelation with pectin HDE/chitosan and pectin LDE/chitosan with particle size below 300 nm and zeta potential value in the range of -25 to -15 mV. According with our results, these agree with previous studies due to got particle size below 300 nm and zeta potential values in the range -10 to -45 mV.
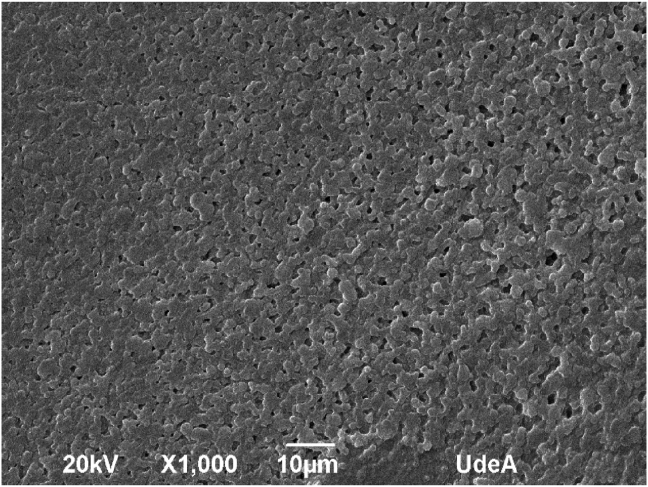
SEM micrography showed aggregated of the pectin nanoparticles, it is due to the dried process and gelling property of the pectin, behavior observed in the study of Chang et al. (2017). In general, the nanoparticles presented a particle size close to 400 nm by SEM (see Figure 5).
Figure 5.
SEM image of pectin nanoparticles prepared with pectin HDE at the concentration 1 mg/mL.
4. Conclusion
Acid type is an important factor to define the pectin properties as yield and DE, so, inorganic acids as nitric acid (NA) allowed to obtain higher yield (54 ± 10%) and organic acids as citric acid extracted pectin with HDE (78.7 ± 2.4%). The pH of the pectin solution is an important variable to evaluate the rheological properties for the future studies. The pectin with HDE (CApH3) allowed to form stable nanoparticles with particle size below 300 nm and the zeta potential value of -45 mV, under environmental conditions by sonication in ethanol: water 1:7 (v/v). Pectin properties such as viscosity and DE affect the nanoparticle formation; it is suggested that pectin extracted from banana peels with CA is a more environmentally friendly alternative, and that future studies could include the encapsulation of molecules as a controlled delivery system or reservoir of molecules with biological activity.
Declarations
Author contribution statement
David Arias: Performed the experiments.
Paula Méndez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Johny Rodríguez: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Betty López: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank to the research group CIENMATE of the University of Antioquia for the research cooperation.
References
- Begum R., Aziz M.G., Uddin M.B., Yusof Y.A. Characterization of jackfruit (Artocarpus heterophyllus) waste pectin as influenced by various extraction conditions. Agric. Agric. Sci. Proc. 2014;2:244–251. [Google Scholar]
- Begum Rokeya, Yusof Y.A., Aziz M.G., Uddin M.B. Structural and functional properties of pectin extracted from jackfruit (Artocarpus heterophyllus) waste: effects of drying. Int. J. Food Prop. 2017;20(1):S190–S201. [Google Scholar]
- Brouns F., Theuwissen E., Adam A., Bell M., Berger A., Mensink R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012;66(5):591–599. doi: 10.1038/ejcn.2011.208. [DOI] [PubMed] [Google Scholar]
- Castillo-Israel K.A.T., Baguio S.F., Diasanta M.D.B., Lizardo R.C.M., Dizon E.I., Mejico M.I.F. Extraction and characterization of pectin from Saba banana [Musa ’saba’(Musa acuminata x Musa balbisiana)] peel wastes: a preliminary study. Int. Food Res. J. 2015;22(1):202–207. [Google Scholar]
- Chaharbaghi E., Khodaiyan F., Hosseini S.S. Optimization of pectin extraction from pistachio green hull as a new source. Carbohydr. Polym. 2017;173:107–113. doi: 10.1016/j.carbpol.2017.05.047. [DOI] [PubMed] [Google Scholar]
- Chaiwarit T., Rachtanapun P., Kantrong N., Jantrawut P. Preparation of clindamycin hydrochloride loaded de-esterified low-methoxyl mango peel pectin film used as a topical drug delivery system. Polymers. 2020;12(5):1–18. doi: 10.3390/polym12051006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.Y., Choo W.S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013;141(4):3752–3758. doi: 10.1016/j.foodchem.2013.06.097. [DOI] [PubMed] [Google Scholar]
- Chang C., Wang T., Hu Q., Luo Y. Zein/caseinate/pectin complex nanoparticles: formation and characterization. Int. J. Biol. Macromol. 2017;104:117–124. doi: 10.1016/j.ijbiomac.2017.05.178. [DOI] [PubMed] [Google Scholar]
- Chávez-Salazar A., Bello-Pérez L.A., Agama-Acevedo E., Castellanos-Galeano F.J., Álvarez-Barreto C.I., Pacheco-Vargas G. Isolation and partial characterization of starch from banana cultivars grown in Colombia. Int. J. Biol. Macromol. 2017;98:240–246. doi: 10.1016/j.ijbiomac.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Colodel C., Lúcia De Oliveira Petkowicz C. Acid extraction and physicochemical characterization of pectin from cubiu (Solanum sessiliflorum D.) fruit peel. Food Hydrocolloids. 2018;(June):1. [Google Scholar]
- Colodel C., Petkowicz C.L. de O. Acid extraction and physicochemical characterization of pectin from cubiu (Solanum sessiliflorum D.) fruit peel. Food Hydrocolloids. 2019;86:193–200. [Google Scholar]
- Delgadillo L.P., Vargas H.J. Universitaria Agustiniana - Repositorio Institucional. Universitaria Agustiniana; 2019. Estudio de prefactibilidad para el aprovechamiento del plátano no comercializado en un cultivo ubicado en el municipio de Puerto López Meta.http://repositorio.uniagustiniana.edu.co/handle/123456789/894 [Google Scholar]
- Garna H., Mabon N., Robert C., Cornet C., Nott K., Legros H., Wathelet B., Paquot M. Effect of extraction conditions on the yield and purity of apple pomace pectin precipitated but not washed by alcohol. J. Food Sci. 2007;72(1):C001–C009. doi: 10.1111/j.1750-3841.2006.00227.x. [DOI] [PubMed] [Google Scholar]
- Gawkowska D., Cybulska J., Zdunek A. Structure-related gelling of pectins and linking with other natural compounds: a review. Polymers. 2018;10(7):1–25. doi: 10.3390/polym10070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães G.C., Júnior M.C.C., Rojas E.E.G. Density and kinematic viscosity of pectin aqueous solution. J. Chem. Eng. Data. 2009;54(2):662–667. [Google Scholar]
- Happi Emaga T., Ronkart S.N., Robert C., Wathelet B., Paquot M. Characterisation of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chem. 2008;108(2):463–471. doi: 10.1016/j.foodchem.2007.10.078. [DOI] [PubMed] [Google Scholar]
- Hisyam B., Wui T., Hamdan H. Design of low molecular weight pectin and its nanoparticles through combination treatment of pectin by microwave and inorganic salts. Polym. Degrad. Stabil. 2018;147(October 2017):35–40. [Google Scholar]
- Hu K., Huang X., Gao Y., Huang X., Xiao H., McClements D.J. Core-shell biopolymer nanoparticle delivery systems: synthesis and characterization of curcumin fortified zein-pectin nanoparticles. Food Chem. 2015;182:275–281. doi: 10.1016/j.foodchem.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Jonassen H., Treves A., Kjøniksen A.L., Smistad G., Hiorth M. Preparation of ionically cross-linked pectin nanoparticles in the presence of chlorides of divalent and monovalent cations. Biomacromolecules. 2013 doi: 10.1021/bm4008474. [DOI] [PubMed] [Google Scholar]
- Kannan P., Guo L. Nanosensors for Smart Cities. INC; 2020. Nanosensors for food safety. [Google Scholar]
- Khamsucharit P., Laohaphatanalert K., Gavinlertvatana P., Sriroth K., Sangseethong K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018;27(3):623–629. doi: 10.1007/s10068-017-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubala B.B., Kansci G., Mbome L.I., Crépeau M.J., Thibault J.F., Ralet M.C. Effect of extraction conditions on some physicochemical characteristics of pectins from “Améliorée” and “Mango” mango peels. Food Hydrocolloids. 2008;22(7):1345–1351. [Google Scholar]
- Kowalonek J. Studies of chitosan/pectin complexes exposed to UV radiation. Int. J. Biol. Macromol. 2017;103:515–524. doi: 10.1016/j.ijbiomac.2017.05.081. [DOI] [PubMed] [Google Scholar]
- Krivorotova T., Cirkovas A., Maciulyte S., Staneviciene R., Budriene S., Serviene E., Sereikaite J. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocolloids. 2016;54:49–56. [Google Scholar]
- Kyomugasho C., Christiaens S., Shpigelman A., Van Loey A.M., Hendrickx M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015;176:82–90. doi: 10.1016/j.foodchem.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Liew S.Q., Chin N.L., Yusof Y.A. Extraction and characterization of pectin from passion fruit peels. Agric. Agric. Sci. Proc. 2014;2:231–236. [Google Scholar]
- Liu Y., Shi J., Langrish T.A.G. Water-based extraction of pectin from flavedo and albedo of orange peels. Chem. Eng. J. 2006;120(3):203–209. [Google Scholar]
- Lopes da Silva J.A., Gonçalves M.P., Rao M.A. Influence of temperature on the dynamic and steady-shear rheology of pectin dispersions. Carbohydr. Polym. 1994;23(2):77–87. [Google Scholar]
- Ma S., Yu S.J., Zheng X.L., Wang X.X., Bao Q.D., Guo X.M. Extraction, characterization and spontaneous emulsifying properties of pectin from sugar beet pulp. Carbohydr. Polym. 2013;98(1):750–753. doi: 10.1016/j.carbpol.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Maciel V.B.V., Yoshida C.M.P., Franco T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015;132:537–545. doi: 10.1016/j.carbpol.2015.06.047. [DOI] [PubMed] [Google Scholar]
- Maciel V.B.V., Yoshida C.M.P., Pereira S.M.S.S., Goycoolea F.M., Franco T.T. Electrostatic self-assembled chitosan-pectin nano- and microparticles for insulin delivery. Molecules. 2017;22(10):1–21. doi: 10.3390/molecules22101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya R., Kulkarni G.T. Extraction and characterization of mango peel pectin as pharmaceutical excipient. Polim. Med. 2012;42(3–4):185–190. [PubMed] [Google Scholar]
- Maneerat N., Tangsuphoom N., Nitithamyong A. Effect of extraction condition on properties of pectin from banana peels and its function as fat replacer in salad cream. J. Food Sci. Technol. 2017;54(2):386–397. doi: 10.1007/s13197-016-2475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran J.P., Priya B., Al-Dhabi N.A., Ponmurugan K., Moorthy I.G., Sivarajasekar N. Ultrasound assisted citric acid mediated pectin extraction from industrial waste of Musa balbisiana. Ultrason. Sonochem. 2017;35:204–209. doi: 10.1016/j.ultsonch.2016.09.019. [DOI] [PubMed] [Google Scholar]
- Méndez Paula A., López Betty L. Polyelectrolyte nanoparticles of Amphiphilic chitosan/pectin from banana peel as potential carrier system of hydrophobic molecules. Polymers. 2020;12(9):1–12. doi: 10.3390/polym12092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez P.A., Vásquez G.M., Gartner C., López B.L. Chitosan/OA nanoparticle as delivery system for celecoxib: parameters affecting the particle size, encapsulation, and release. J. Appl. Polym. Sci. 2017;134(7):44472. (1-11) [Google Scholar]
- Muñoz-Almagro Nerea, Rico-Rodriguez Fabián, Peter J., Wilde A.M., M.V Structural and technological characterisation of pectin extracted with sodium citrate and nitric acid from sunflower heads. Electrophoresis. 2018;39:1984–1992. doi: 10.1002/elps.201800130. [DOI] [PubMed] [Google Scholar]
- Ogutu F.O., Mu T.H. Ultrasonic degradation of sweet potato pectin and its antioxidant activity. Ultrason. Sonochem. 2017;38:726–734. doi: 10.1016/j.ultsonch.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Oliveira T.Í.S., Rosa M.F., Cavalcante F.L., Pereira P.H.F., Moates G.K., Wellner N., Mazzetto S.E., Waldron K.W., Azeredo H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016;198:113–118. doi: 10.1016/j.foodchem.2015.08.080. [DOI] [PubMed] [Google Scholar]
- Oliveira T.Í.S., Rosa M.F., Cavalcante F.L., Pereira P.H.F., Moates G.K., Wellner N., Mazzetto S.E., Waldron K.W., Azeredo H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016;198:113–118. doi: 10.1016/j.foodchem.2015.08.080. [DOI] [PubMed] [Google Scholar]
- Pagarra H., Hartati, Purnamasari A.B., Rachmawaty, Rahman R.A. Optimization of pectin extraction from kepok banana peels (musa paradisiaca) using surface response methodology. J. Phys. Conf. 2019;1317(1):1–6. [Google Scholar]
- Pereira P.H.F., Oliveira T.Í.S., Rosa M.F., Cavalcante F.L., Moates G.K., Wellner N., Waldron K.W., Azeredo H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016;88:373–379. doi: 10.1016/j.ijbiomac.2016.03.074. [DOI] [PubMed] [Google Scholar]
- Petkowicz C.L.O., Vriesmann L.C., Williams P.A. Pectins from food waste: extraction, characterization and properties of watermelon rind pectin. Food Hydrocolloids. 2017;65:57–67. [Google Scholar]
- Qiu L.P., Zhao G.L., Wu H., Jiang L., Li X.F., Liu J.J. Investigation of combined effects of independent variables on extraction of pectin from banana peel using response surface methodology. Carbohydr. Polym. 2010;80(2):326–331. [Google Scholar]
- Rampino A., Borgogna M., Bellich B., Blasi P., Virgilio F., Cesàro A. Chitosan-pectin hybrid nanoparticles prepared by coating and blending techniques. Eur. J. Pharmaceut. Sci. 2016;84:37–45. doi: 10.1016/j.ejps.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Rashidipour M., Maleki A., Kordi S., Birjandi M., Pajouhi N., Mohammadi E., Heydari R., Rezaee R., Rasoulian B., Davari B. Pectin/Chitosan/tripolyphosphate nanoparticles: efficient carriers for reducing soil sorption, cytotoxicity, and mutagenicity of paraquat and enhancing its herbicide activity [Research-article] J. Agric. Food Chem. 2019;67:5736–5745. doi: 10.1021/acs.jafc.9b01106. [DOI] [PubMed] [Google Scholar]
- Rasidek N.A.M., Nordin M.F.M., Iwamoto K., Rahman N.A., Nagatsu Y., Tokuyama H. Rheological flow models of banana peel pectin jellies as affected by sugar concentration. Int. J. Food Prop. 2018;21(1):2087–2099. [Google Scholar]
- Rodsamran P., Sothornvit R. Microwave heating extraction of pectin from lime peel: characterization and properties compared with the conventional heating method. Food Chem. 2019;278:364–372. doi: 10.1016/j.foodchem.2018.11.067. [DOI] [PubMed] [Google Scholar]
- Shankar S., Tanomrod N., Rawdkuen S., Rhim J.W. Preparation of pectin/silver nanoparticles composite films with UV-light barrier and properties. Int. J. Biol. Macromol. 2016;92:842–849. doi: 10.1016/j.ijbiomac.2016.07.107. [DOI] [PubMed] [Google Scholar]
- Srivastava P., Malviya R. Sources of pectin, extraction and its applications in pharmaceutical industry - an overview. Indian J. Natl. Prod. Res. 2011;2(1):10–18. [Google Scholar]
- Tanaid R.A.B., Lauzon R.D. Extraction optimization of pectin from unripe banana (musa acuminata × balbisiana var. Cardaba) peel. ETP Int. J. Food Eng. 2018;4(4):308–315. [Google Scholar]
- Torkova A.A., Lisitskaya K.V., Filimonov I.S., Glazunova O.A., Kachalova G.S., Golubev V.N., Fedorova T.V. Physicochemical and functional properties of Cucurbita maxima pumpkin pectin and commercial citrus and apple pectins: a comparative evaluation. PloS One. 2018;13(9):1–24. doi: 10.1371/journal.pone.0204261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanova J.C.O., Ayres E., Oréfice R.L. Design, characterization and preliminary in vitro evaluation of a mucoadhesive polymer based on modified pectin and acrylic monomers with potential use as a pharmaceutical excipient. Carbohydr. Polym. 2015;121:372–381. doi: 10.1016/j.carbpol.2014.12.052. [DOI] [PubMed] [Google Scholar]
- Vriesmann L.C., Teófilo R.F., Petkowicz C.L.D.O. Optimization of nitric acid-mediated extraction of pectin from cacao pod husks (Theobroma cacao L.) using response surface methodology. Carbohydr. Polym. 2011;84(4):1230–1236. [Google Scholar]
- Wang M., Huang B., Fan C., Zhao K., Hu H., Xu X., Pan S., Liu F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016;91:794–803. doi: 10.1016/j.ijbiomac.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Willats W.G.T., Knox J.P., Mikkelsen J.D. Pectin: new insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006;17(3):97–104. [Google Scholar]
- Yapo B.M. Pectin quantity, composition and physicochemical behaviour as influenced by the purification process. Food Res. Int. 2009;42(8):1197–1202. [Google Scholar]
- Yoo S.H., Lee B.H., Lee H., Lee S., Bae I.Y., Lee H.G., Fishman M.L., Chau H.K., Savary B.J., Hotchkiss A.T. Structural characteristics of pumpkin pectin extracted by microwave heating. J. Food Sci. 2012;77(11):1169–1173. doi: 10.1111/j.1750-3841.2012.02960.x. [DOI] [PubMed] [Google Scholar]
- Zainudin B.H., Wong T.W., Hamdan H. Design of low molecular weight pectin and its nanoparticles through combination treatment of pectin by microwave and inorganic salts. Polym. Degrad. Stabil. 2018;147(October 2017):35–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.