Key Points
Question
What is the association of hyperthermic intraperitoneal chemotherapy (HIPEC) with prognosis and surgical outcomes of patients who undergo cytoreductive surgery for pseudomyxoma peritonei (PMP)?
Findings
In this propensity score–matched cohort study that included 1924 patients with PMP, HIPEC was associated with a better overall survival, generally without worsening the postoperative outcomes in terms of 30- and 90-day mortality, severe morbidity, and return to operating room.
Meaning
HIPEC should be considered in conjunction with cytoreductive surgery in patients with PMP.
This cohort study compares the outcomes of patients with pseudomyxoma peritonei who undergo hyperthermic intraperitoneal chemotherapy with cytoreductive surgery vs cytoreductive surgery alone.
Abstract
Importance
Studies on the prognostic role of hyperthermic intraperitoneal chemotherapy (HIPEC) in pseudomyxoma peritonei (PMP) are currently not available.
Objectives
To evaluate outcomes after cytoreductive surgery (CRS) and HIPEC compared with CRS alone in patients with PMP.
Design, Setting, and Participants
This cohort study analyzed data from the Peritoneal Surface Oncology Group International (PSOGI) registry, including 1924 patients with histologically confirmed PMP due to an appendiceal mucinous neoplasm. Eligible patients were treated with CRS with or without HIPEC from February 1, 1993, to December 31, 2017, and had complete information on the main prognostic factors and intraperitoneal treatments. Inverse probability treatment weights based on the propensity score for HIPEC treatment containing the main prognostic factors were applied to all models to balance comparisons between the CRS-HIPEC vs CRS-alone groups in the entire series and in the following subsets: optimal cytoreduction, suboptimal cytoreduction, high- and low-grade histologic findings, and different HIPEC drug regimens. Data were analyzed from March 1 to June 1, 2018.
Interventions
HIPEC including oxaliplatin plus combined fluorouracil-leucovorin, cisplatin plus mitomycin, mitomycin, and other oxaliplatin-based regimens.
Main Outcomes and Measures
Overall survival, severe morbidity (determined using the National Cancer Institute Common Terminology for Adverse Events, version 3.0), return to operating room, and 30- and 90-day mortality. Differences in overall survival were compared using weighted Kaplan-Meier curves, log-rank tests, and Cox proportional hazards multivariable models. A sensitivity analysis was based on the E-value from the results of the main Cox proportional hazards model. Differences in surgical outcomes were compared using weighted multivariable logistic models.
Results
Of the 1924 patients included in the analysis (997 [51.8%] men; median age, 56 [interquartile range extremes (IQRE), 45-65] years), 376 were in the CRS-alone group and 1548 in the CRS-HIPEC group. Patients with CRS alone were older (median age, 60 [IQRE, 48-70] vs 54 [IQRE, 44-63] years), had less lymph node involvement (14 [3.7%] vs 119 [7.7%]), received more preoperative systemic chemotherapy (198 [52.7%] vs 529 [34.2%]), and had higher proportions of high-grade disease (179 [47.6%] vs 492 [31.8%]) and suboptimal cytoreduction residual disease (grade 3, 175 [46.5%] vs 117 [7.6%]). HIPEC was not associated with a higher risk of worse surgical outcomes except with mitomycin, with higher odds of morbidity (1.99; 95% CI, 1.25-3.19; P = .004). HIPEC was associated with a significantly better overall survival in all subsets (adjusted hazard ratios [HRs], 0.60-0.68, with 95% CIs not crossing 1.00). The weighted 5-year overall survival was 57.8% (95% CI, 50.8%-65.7%) vs 46.2% (95% CI, 40.3%-52.8%) for CRS-HIPEC and CRS alone, respectively (weighted HR, 0.65; 95% CI, 0.50-0.83; P < .001; E-value, 2.03). Such prognostic advantage was associated with oxaliplatin plus fluorouracil-leucovorin (HR, 0.42; 95% CI, 0.19-0.93; P = .03) and cisplatin plus mitomycin (HR, 0.57; 95% CI, 0.42-0.78; P = .001) schedules.
Conclusions and Relevance
In this cohort study, HIPEC was associated with better overall survival when performed after CRS in PMP, generally without adverse effects on surgical outcomes.
Introduction
Pseudomyxoma peritonei (PMP) is an unusual clinical entity characterized by mucinous ascites, eventually leading to abdominal distension and bowel obstruction.1 The pathophysiology is a mucinous neoplasm, most commonly originating in the appendix.2 The combination of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has been regarded as standard of care for PMP.3 Cytoreductive surgery aims to remove all visible tumor and includes peritonectomy and resection of the involved organs.4 The surgical phase is completed with local-regional administration of hyperthermic chemotherapy to eliminate microscopic residual neoplastic disease. Data on the efficacy of HIPEC after CRS in the treatment of peritoneal malignant neoplasms are inconsistent according to the primary tumor type. It has been shown to be beneficial in epithelial ovarian cancer (when preceded by neoadjuvant systemic chemotherapy)5 and in peritoneal metastasis from gastric cancer,6 but not in peritoneal metastasis from colorectal cancer (PM-CRC).7 Moreover, HIPEC in the latter PRODIGE-7 (Partenariat de Recherche en Oncologie Digestive 7) study has been reported to increase 60-day surgical morbidity, although full details and publication of the results presented are awaited.
Thus, the primary end point in the present study aimed to evaluate the prognostic effect of HIPEC with CRS, compared with CRS alone, in patients with PMP. We analyzed an international registry of patients with PMP and performed multiple parallel weighted analyses to test whether HIPEC is associated with therapeutic benefit in various patient subsets. Secondary end points were the effects associated with different HIPEC drug schedules and whether HIPEC was associated with adverse perioperative outcomes.
Methods
Study Design
We performed a secondary analysis of the Peritoneal Surface Oncology Group International (PSOGI) registry data. This cohort study received institutional review board approval by all participating centers, and patients were exempted from providing informed consent, according to European Union General Data Protection Regulation, owing to the observational nature of the study, pseudoanonymized data, and the fact that participation did not imply any further risk to the patients. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Setting
The PSOGI multicenter registry was set up in 2010 and represented a collaborative effort of main peritoneal surface malignancy international centers to prospectively collect data. Data were collected from 3495 patients with PMP who underwent CRS with or without HIPEC from February 1, 1993, to December 31, 2017.
Participants
Inclusion criteria consisted of histologically confirmed PMP from an appendiceal mucinous neoplasm and a CRS procedure alone or in combination with HIPEC. Data for 1924 of the 3495 registry patients with complete data regarding the preoperative and postoperative parameters and tumor characteristics established in the literature as main prognostic factors were retrieved (eFigure 1 in the Supplement).
Variables
The exposure variable was HIPEC (yes or no). The variables investigated as effect modifiers were histological subtype (high or low grade according to World Health Organization criteria),8 prior systemic chemotherapy (yes or no), prior surgical score (0 indicates no surgery or biopsy; 1, surgery in 1 abdominal region only; 2, surgery in 2 to 5 regions; and 3, surgery in ≥6 regions),9 peritoneal cancer index,9 lymph node status (positive or negative), and completeness of cytoreduction (complete [CC-0/1], defined as residual disease of <2.5 mm; residual disease of 2.5 mm to 2.5 cm [CC-2]; and residual disease of >2.5 cm [CC-3]).9 Age at surgery and early postoperative intraperitoneal chemotherapy (EPIC) (yes or no) were considered as possible confounders. The outcomes compared between exposure groups were overall survival (calculated from the time of the surgery of the primary tumor to death due to any cause or last follow-up for living patients), severe morbidity (grades 3-5 determined by the National Cancer Institute Common Terminology for Adverse Events, version 3.0), return to the operating room, and 30- and 90-day mortality. There were no differences in the assessment of variables between the groups exposed to HIPEC (CRS-HIPEC) and those unexposed (CRS-alone).
Treatments
The surgical approach involved peritonectomy procedures as described by Sugarbaker.9 HIPEC was administered after CRS using an open coliseum or closed technique, depending on the individual unit’s preference. The perfusate was heated to achieve a temperature ranging from 40 °C to 43 °C. The most frequent HIPEC drug regimens included mitomycin, 35 mg/m2, or a fixed dose of 40 mg; oxaliplatin, 360 to 460 mg/m2 plus combined fluorouracil and leucovorin, 400 mg/m2; oxaliplatin, 200 mg/m2; and cisplatin, 25 mg/m2 per liter of perfusate associated with mitomycin, 3.3 mg/m2 per liter of pefusate (eTable 1 in the Supplement). Some units administered EPIC consisting of intraperitoneal fluorouracil, 650 mg/m2, on days 1 to 5 at room temperature. We classified perioperative morbidity according to National Cancer Institute Common Terminology for Adverse Events, version 3.0.10 Patients were followed up by clinical examination, computed tomographic scan of the chest and abdomen, and measurement of serum tumor marker levels every 4 to 6 months for the first 2 years and every 6 to 12 months thereafter.
Statistical Analysis
Data were analyzed from March 1 to June 1, 2018. We handled missing data with complete case analysis because the proportions of incomplete information in some key variables were too high to be managed with missing-imputation technique. Patients in the CRS-alone group were compared with those in the CRS-HIPEC group. We estimated propensity scores as balancing scores to account for the bias consistent with a nonrandom assignment of treatment in the study groups for each comparison.11 For this purpose, we used multivariable logistic models with a binary response (CRS-HIPEC or CRS alone) and the retrieved above-mentioned prognostic factors. We used the method of stabilized weights.12 To account for the bias introduced from the inverse probability treatment weight procedure in the estimation of the standard error of the β statistic in regression models, we used a bootstrap estimator to calculate the 95% CIs.13
Differences in means (numerical variables) and proportions (categorical variables) between baseline patient characteristics of the excluded and included patients and treatment groups were compared using the standardized mean difference.14 Standardized mean differences of at least 0.300 were considered indicative of a relevant between-group imbalance. We calculated the standardized mean difference before and after inverse probability treatment weighted adjustment in the study groups.
Overall survival differences were assessed between the CRS-alone and CRS-HIPEC groups overall and in the following subsets: optimal cytoreduction (CC-0/1), suboptimal cytoreduction (CC-2/3), low-grade PMP, and high-grade PMP. We decided not to distinguish between CC-2 and CC-3 because the former group was numerically small. We also tested the differences in overall survival between CRS alone and CRS-HIPEC according to different HIPEC drug regimens. We assessed differences in overall survival using weighted log-rank tests and multivariable Cox proportional hazards models including all the retrieved above-mentioned prognostic factors. The center factor was assessed as random effect in univariable and multivariable Cox models for the whole cohort and dropped as not statistically significant (likelihood ratio test P = .99 and P = .49, respectively). The median follow-up was estimated with the reverse Kaplan-Meier method on weighted data.15
We assessed differences in surgical outcomes between CRS-alone and CRS-HIPEC groups according to different HIPEC drug regimen subgroups, as previously described, using weighted multivariable logistic models. We performed penalized logistic models to obtain evaluable results for 90- and 30-day mortality in the oxaliplatin plus fluorouracil-leucovorin subgroup. We did not assess any interaction terms. We modeled age and peritoneal cancer index as continuous variables by using 3-knot restricted cubic splines to obtain flexible fit.16
We evaluated selection bias by means of difference in overall survival calculated by log-rank tests between included vs excluded patients and between CRS-alone vs CRS-HIPEC groups in the excluded patients group. We conducted a sensitivity analysis on hazard ratios (HRs) and the 95% CI limit (CL) closer to the CL of the main Cox proportional hazards model by means of the E-value. The latter is the minimal strength at which a confounder would need to be associated with both the outcome and the exposure of interest to fully explain away the observed association between treatment and outcome.17
Results
For the main analysis of the 1924 included patients (927 [48.2%] women and 997 [51.8%] men; median age, 56 [interquartile range extremes (IQRE), 45-65] years), we considered clinical-pathological and treatment data from 376 patients who underwent CRS alone and 1548 patients who underwent CRS-HIPEC (eFigure 1 in the Supplement). Patients who received CRS alone were older (median age, 60 [IQRE, 48-70] vs 54 [IQRE, 44-63] years), had less lymph node involvement (14 [3.7%] vs 119 [7.7%]), received more preoperative systemic chemotherapy (198 [52.7%] vs 529 [34.2%]), had a higher proportion of high-grade disease (179 [47.6%] vs 492 [31.8%]), and had a higher proportion of incomplete cytoreductions (CC-3, 175 [46.5%] vs 117 [7.6%]). After the weighting, we obtained a good balance in the distribution of prognostic factors (Table 1). Similarly, we obtained a good balance for all factors in every subset analysis (eTables 2-9 in the Supplement).
Table 1. Prognostic Factors in CRS-HIPEC and CRS-Alone Groups Before and After IPTW .
| Factor | Study group, unweighted | Study group, IPTW weighted | ||||
|---|---|---|---|---|---|---|
| CRS alone (n = 376) | CRS-HIPEC (n = 1548) | SMD | CRS alone (n = 305) | CRS-HIPEC (n = 300) | SMD | |
| Age, median (IQRE), y | 60 (48-70) | 54 (44-63) | 0.381 | 59 (46-67) | 59 (46-68) | 0.010 |
| Prior systemic chemotherapy, No. (%) | ||||||
| Yes | 198 (52.7) | 529 (34.2) | 0.380 | 149.4 (48.9) | 149.0 (49.5) | 0.013 |
| No | 178 (47.3) | 1019 (65.8) | 156.2 (51.1) | 151.8 (50.5) | ||
| Lymph node involvement, No. (%) | ||||||
| Yes | 14 (3.7) | 119 (7.7) | 0.172 | 12.9 (4.2) | 12.9 (4.3) | 0.003 |
| No | 362 (96.3) | 1429 (92.3) | 292.7 (95.8) | 287.9 (95.7) | ||
| Peritoneal cancer index, median (IQRE)a | 25 (13-35) | 19 (10-28) | 0.314 | 24 (11-33) | 23 (11-34) | 0.002 |
| Completeness of cytoreduction score, No. (%)b | ||||||
| 0/1 | 164 (43.6) | 1352 (87.3) | 1.065 | 163.9 (53.6) | 167 (55.5) | 0.099 |
| 2 | 37 (9.8) | 79 (5.1) | 35.4 (11.6) | 25.9 (8.6) | ||
| 3 | 175 (46.5) | 117 (7.6) | 106.3 (34.8) | 108.0 (35.9) | ||
| Prior surgical score, No. (%)c | ||||||
| 0 | 118 (31.4) | 355 (22.9) | 0.335 | 86.7 (28.4) | 89.0 (29.6) | 0.039 |
| 1 | 99 (26.3) | 467 (30.2) | 86.9 (28.4) | 87.4 (29.0) | ||
| 2 | 129 (34.3) | 456 (29.5) | 103.8 (33.9) | 97.1 (32.3) | ||
| 3 | 30 (8.0) | 270 (17.4) | 28.3 (9.3) | 27.4 (9.1) | ||
| EPIC | ||||||
| Yes | 53 (14.1) | 250 (16.1) | 0.057 | 47.0 (15.4) | 252.0 (83.8) | 0.024 |
| No | 323 (85.9) | 1298 (83.9) | 258.7 (84.6) | 48.8 (16.2) | ||
| Histologic finding, No. (%) | ||||||
| Low grade | 197 (52.4) | 1056 (68.2) | 0.328 | 172.8 (56.5) | 168.1 (55.9) | 0.014 |
| High grade | 179 (47.6) | 492 (31.8) | 132.8 (43.5) | 132.7 (44.1) | ||
Abbreviations: CC-0/1, complete cytoreduction; CC-2 and CC-3, suboptimal cytoreduction; CRS, cytoreductive surgery; EPIC, early postoperative intraperitoneal chemotherapy; HIPEC, hyperthermic intraperitoneal chemotherapy; IPTW, inverse probability treatment weighting; IQRE, interquartile range extremes; SMD, standardized mean difference.
Scores range from 0 to 39, with higher scores indicating worse prognosis.
CC-0/1 indicates residual disease of less than 2.5 mm; CC-2, residual disease of 2.5 mm to 2.5 cm; and CC-3, residual disease of greater than 2.5 cm.
Zero indicates no surgery or biopsy; 1, surgery in 1 abdominal region only; 2, surgery in 2 to 5 regions; and 3, surgery in 6 or more regions.
Survival Analysis in the Overall Series
During a median follow-up of 52 (IQRE, 23-83) months, we estimated a 5-year overall survival of 46.2% (95% CI, 40.3%-52.8%) for the CRS-alone group and 57.8% (95% CI, 50.8%-65.7%) for the CRS-HIPEC group (P < .001) (Figure 1A). After applying the multivariable Cox proportional hazards model, the HR was 0.65 (95% CI, 0.50-0.83; P = .001) (Figure 2 and Table 2). The HRs for influential prognostic factors included in the study ranged from 1.14 (95% CI, 0.82-1.58) for EPIC to 4.12 (95% CI, 2.75-6.18) for peritoneal cancer index (Table 2).
Figure 1. Kaplan-Meier Overall Survival Curves According to Treatment.
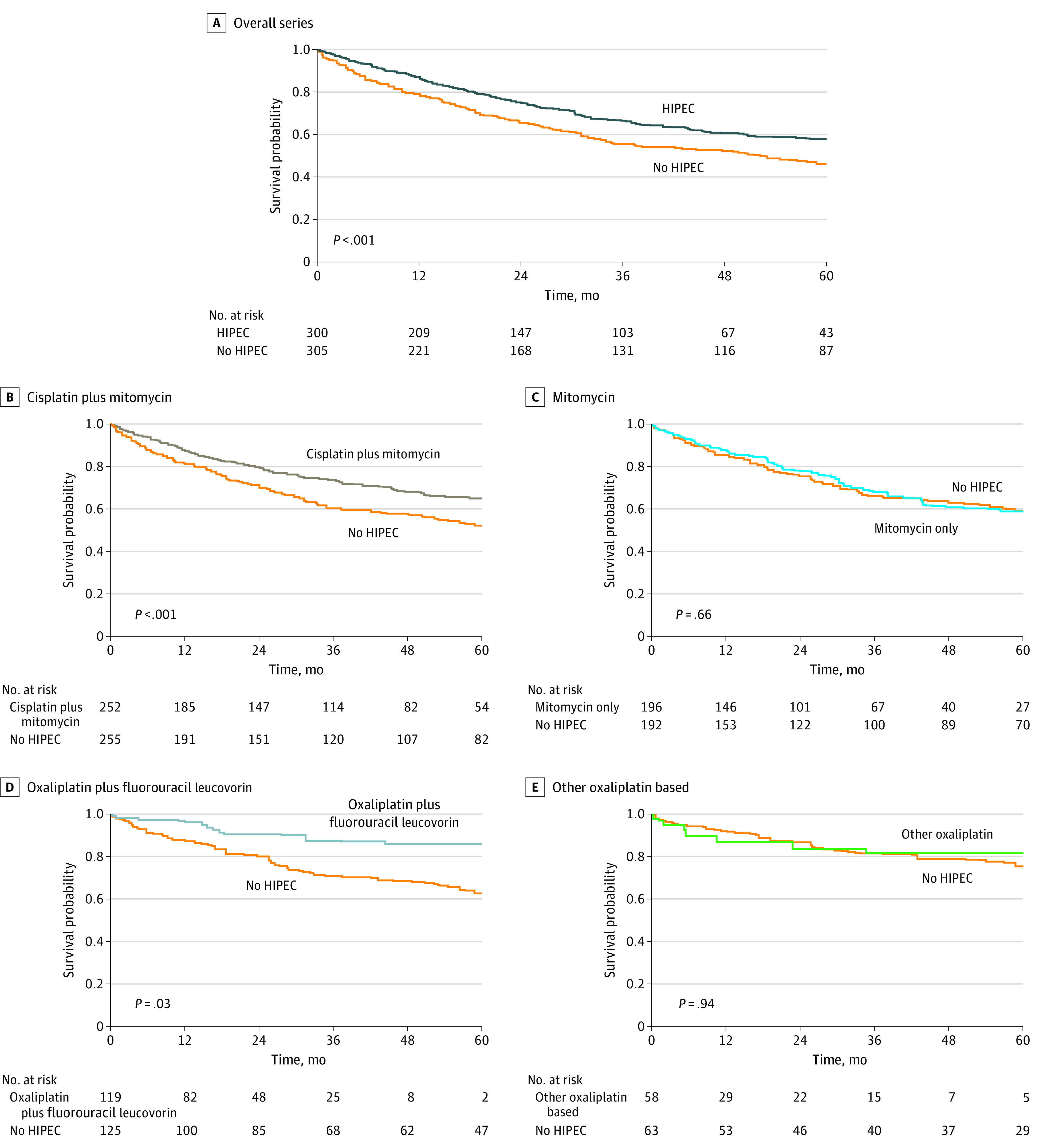
Data in the overall series (A) include patients undergoing cytoreductive surgery (CRS) alone and those undergoing CRS with hyperthermic intraperitoneal chemotherapy (HIPEC). Remaining data are stratified by the HIPEC regimen compared with CRS alone (B-E). Data are shown as weighted comparisons.
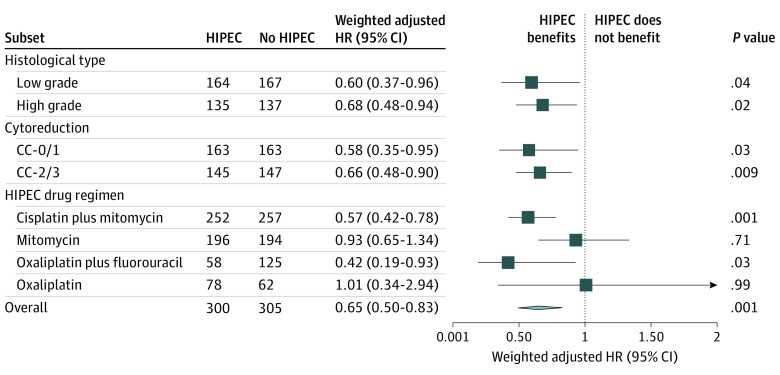
Figure 2. Effect Size of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) on Overall Survival According to Subsets and HIPEC Regimens.
Data are presented as weighted hazard ratios (HRs) of death using multivariable Cox proportional hazards models and bootstrap 95% CI for HIPEC treatment. Arrow indicates 95% CI extends beyond boundary of the graph; diamond center, overall HR; ends of diamond, overall 95% CI. CC-0/1 indicates optimal cytoreduction (residual disease of <2.5 mm); CC-2/3, suboptimal cytoreduction (residual disease of ≥2.5 mm).
Table 2. Results of the Weighted Multivariable Cox Proportional Hazards Model Including HIPEC and the Main Prognostic Factors for Overall Survival.
| Factor | HR (95% CI) | P value |
|---|---|---|
| HIPEC, yes vs no | 0.65 (0.50-0.83) | .001 |
| Age, 65 vs 45 ya | 1.24 (1.04-1.48) | .007 |
| Prior systemic chemotherapy, yes vs no | 1.58 (1.23-2.03) | <.001 |
| Lymph node involvement, yes vs no | 1.69 (0.95-3.01) | .08 |
| Peritoneal cancer index, 29 vs 10a | 4.12 (2.75-6.18) | <.001 |
| Prior surgical score | ||
| 1 vs 0 | 1.41 (1.02-1.95) | .09 |
| 2 vs 0 | 1.41 (1.05-1.91) | |
| 3 vs 0 | 1.44 (0.90-2.30) | |
| EPIC, yes vs no | 1.14 (0.82-1.58) | .44 |
| Higher histologic grade, yes vs no | 2.36 (1.83-3.04) | <.001 |
Abbreviations: EPIC, early postoperative intraperitoneal chemotherapy; HIPEC, hyperthermic intraperitoneal chemotherapy; HR, hazard ratio.
The 2 values are the third and first quartiles of the variable distribution, respectively.
Survival Analysis in the Subsets
Compared with the CRS-alone group, the CRS-HIPEC group had better 5-year overall survival in all subsets, including CC-0/1 (71.4% [95% CI, 64.2%-79.3%] vs 77.4% [95% CI, 69.2%-86.6%]; P = .009), CC-2/3 (16.1% [95% CI, 10.4%-24.8%] vs 28.4% [95% CI, 19.6%-41.1%]; P = .007), low-grade PMP (64.1% [95% CI, 56.3%-72.9%] vs 73.8% [95% CI, 65.0%-83.8%]; P < .001), and high-grade PMP (26.8% [95% CI, 20.0%-36.0%] vs 39.3% [95% CI, 30.0%-51.6%]; P = .002) (eFigure 2 in the Supplement). With multivariable Cox proportional hazards models, the HRs were 0.58 (95% CI, 0.35-0.95; P = .03) for CC-0/1, 0.66 (95% CI, 0.48-0.90; P = .009) for CC-2/3, 0.60 (95% CI, 0.37-0.96; P = .04) for low-grade PMP, and 0.68 (95% CI, 0.48-0.94; P = .02) for high-grade PMP (Figure 2).
Overall Survival According to Drug Schedules
Treatment with CRS-HIPEC was superior to CRS alone when the drug schedules were oxaliplatin plus fluorouracil-leucovorin (5-year overall survival, 86.1% [95% CI, 0.76%-0.97%] vs 62.6% [95% CI, 0.54%-0.73%]; P = .03) or cisplatin plus mitomycin (5-year overall survival, 64.9% [95% CI, 58.0%-72.7%] vs 52.2% [95% CI, 45.8%-59.4%]; P < .001) (Figure 1B and D). In multivariable Cox proportional hazards models, the HRs were 0.42 (95% CI, 0.19-0.93; P = .03) for oxaliplatin plus fluorouracil-leucovorin and 0.57 (95% CI, 0.42-0.78; P = .001) for cisplatin plus mitomycin subgroups (Figure 2).
No prognostic advantage was observed in subgroups receiving mitomycin (5-year overall survival, 58.8% [95% CI, 50.1%-69.0%] vs 59.1% [51.9%-67.2%]; P = .68) and other oxaliplatin-based HIPEC (5-year overall survival, 81.8% [95% CI, 69.7%-96.0%] vs 75.2% [95% CI, 64.4%-88.0%]; P = .95) (Figure 1C and E). After applying multivariable Cox proportional hazards models, the HRs were 0.93 (95% CI, 0.65-1.34; P = .71) for mitomycin and 1.01 (95% CI, 0.34-2.94; P = .99) for other oxaliplatin-based subgroups (Figure 2).
Postoperative Outcomes
Within the entire series (n = 1924), incidence of 90-day mortality was 4.2% (95% CI, 3.6%-5.2%); 30-day mortality, 2.1% (95% CI, 1.5%-2.8%); return to the operating room, 9.3% (95% CI, 8.0%-10.6%); and severe morbidity, 32.0% (95% CI, 30.0%-34.1%). The details of the incidence of complications in the entire series are outlined in eTable 10 in the Supplement. After applying multivariable logistic models, no significant differences between the CRS-HIPEC and CRS-alone groups were observed overall or according to HIPEC drug regimens except the mitomycin regimen (odds ratio for morbidity, 1.99; 95% CI, 1.25-3.19; P = .004) (Figure 3).
Figure 3. Effect Size of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) on Short-term Surgical Outcomes According to Subsets and Drug Combinations.
Data are presented as weighted odds ratios (ORs) of unfavorable surgical outcomes from multivariable logistic models and bootstrap 95% CI for patients treated with HIPEC. Morbidity is determined by grades 3 to 5 in the National Cancer Institute Common Terminology for Adverse Events, version 3.0. Arrow indicates width of 95% CI extends beyond boundary of the graph. CRS indicates cytoreductive surgery.
Sensitivity Analysis and Assessment of Selection Bias
Comparing the prognostic factors between included and excluded patients (1924 vs 1571), we observed that only EPIC was more frequently performed in the excluded cases (525 of 1490 [35.2%] vs 303 of 1924 [15.7%]). The excluded cases had a better 10-year overall survival (66.9% [95% CI, 62.9%-71.0%] vs 51.3% [95% CI, 46.5%-56.7%]; P < .001) and substantially lower severe morbidity (283 of 1521 [18.6%] vs 615 of 1924 [32.0%]; P = .31) (eTable 11 in the Supplement). Within excluded patients (233 CRS alone vs 1342 CRS-HIPEC, with 6 missing), the CRS-HIPEC group was more frequently treated with EPIC (503 of 1304 [38.6%] vs 22 of 183 [12.0%]; P = .64) and showed better 10-year overall survival (71.7% [95% CI, 67.5%-76.0%] vs 30.7% [95% CI, 20.1%-46.7%]; P < .001). Incidence of severe morbidity was 19.0% (251 of 1323) in the CRS-HIPEC group and 16.2% (31 of 191) in the CRS group (eTable 12 in the Supplement).
The E-value for the HR was 2.03, so that the main result could be explained by an unmeasured confounder that was associated with both the treatment and the outcome by a risk ratio of 2-fold each, above and beyond the measured confounders. The E-value for the CL was 1.53, so that it could be moved to include 1 by an unmeasured confounder that was associated with both the treatment and the outcome by a risk ratio of 1.5-fold each, above and beyond the measured confounders.
Discussion
The combination CRS-HIPEC treatment has been regarded as a standard of care in PMP despite the lack of randomized data. In this cohort study, HIPEC was associated with a better overall survival in the entire series and in all subsets. Notably, patients in the CC-2/3 subgroup represent a particular circumstance in which HIPEC should not be effective owing to the limited capacity of the intraperitoneal drugs to penetrate in gross residual disease.18 Moreover, in the entire series, HIPEC was not associated with increased risks of severe morbidity, return to operating room, or 30- and 90-day mortality.
Concern is long-standing among some surgeons using HIPEC in addition to CRS in PMP. Some have suggested that HIPEC efficacy is limited owing to the poor response of PMP to chemotherapy. In fact, PMP is characterized by low cell proliferative activity,19 mucin overproduction,8 and high frequency of KRAS mutation.20 All these properties are related to chemoresistance in colorectal cancer.21 Likewise, the PMP response percentages to systemic chemotherapy are low, ranging from 4% to 24%.22,23,24,25,26 Nevertheless, HIPEC conferred a survival advantage in PMP of similar magnitude to the recently published randomized clinical trial of secondary debulking surgery with or without HIPEC in epithelial ovarian cancer,5 with a 35% reduction of risk of mortality.
Of note, a higher prognostic advantage between the HIPEC regimens was observed in the cisplatin plus mitomycin and oxaliplatin plus fluorouracil-leucovorin HIPEC subgroups. Since its development in the early 2000s,27 the oxaliplatin plus fluorouracil-leucovorin combination for HIPEC gained popularity until the recent preliminary results of the PRODIGE-7 trial.7 The short duration of the oxaliplatin regimen has raised concern because it is uncertain whether 30 minutes of delivery time could overcome de novo platinum resistance in PM-CRC.28
The success of HIPEC with oxaliplatin plus fluorouracil-leucovorin in the present study, in contrast to the failure in PM-CRC, might be connected to differences in the biology of these clinical entities. Although PM-CRC is a locoregional disease, consistent evidence suggests that the mechanism of peritoneal spread occurs not only transcoelomically but also hematogeneously.29,30
Pseudomyxoma peritonei progresses transcoelomically with a superficial invasion of the peritoneal lymphatics.31 Pseudomyxoma peritonei generally is confined to the peritoneal cavity for most of its natural history with a markedly low chance of distant metastasis. The negative results of initial reports of the PRODIGE-7 trial have shifted the investigator’s attention to mitomycin, which is the drug of choice for HIPEC use in PM-CRC according to the American Society of Peritoneal Surface Malignancies.32 In the present study, however, HIPEC with mitomycin did not seem to be associated as much with the prognosis as other regimens and was associated with an increased frequency of severe morbidity. These data contrast with some centers’ reports using a much lower dose (10 mg/m2) with low toxic effects and good outcomes, so that the dosage may also be an issue that should be cleared by future studies.33
Also, the cisplatin plus mitomycin subgroup was associated with a significant reduction in the risk of death. This combination was developed in the late 1980s for the treatment of gastric cancer with peritoneal metastases. In particular, cisplatin is supported by level I evidence for HIPEC use in advanced epithelial ovarian cancer,5 whereas its combination with mitomycin is supported only by experimental data showing that mitomycin enhances the intracellular accumulation of cisplatin, thus favoring the production of platin DNA adducts.34,35
Regarding short-term outcomes, we only observed the association of mitomycin with increased severe morbidity. This finding is interesting because it is well known that all intraperitoneal chemotherapies after surgery hamper the tissue-healing process and increase the chances of surgical complications.7,36,37,38,39 In PRODIGE-7, it has been reported that HIPEC with oxaliplatin plus fluorouracil-leucovorin was associated with a significantly higher 60-day complication rate.7
Considering these experimental and clinical data, an increased risk of HIPEC-associated adverse effects might have been expected for all drug regimens, although only mitomycin was associated with an increased risk of severe complications. Although the potential contribution of HIPEC in increasing postoperative morbidity is consistent, the primary determinant of surgical complications remains the extent of the CRS. In contrast to the PRODIGE-7 experience, the hazard produced by intraperitoneal chemotherapy in the present study might have been eclipsed by the effects of a generally more extensive surgical procedure required for PMP compared with PM-CRC. Therefore, in the present study, differences in surgical outcomes between CRS-HIPEC and CRS-alone groups are subsumed in the high incidences.
Limitations
The limitations of the present study are related to its retrospective nature, the adoption of outdated World Health Organization pathological classification for appendiceal tumors, the lack of information regarding the site of recurrence, the adoption of overall survival instead of disease-specific survival as the primary outcome, potential selection bias, and the potential bias due to unmeasured confounders that were not controlled by the inverse probability treatment weighted propensity score. The outdated World Health Organization pathological classification for appendiceal tumors could imply a potential imbalance between the study groups. However, in the present analysis, the HRs associated with World Health Organization categories (high- vs low-grade vs overall series) ranged from 0.58 to 0.66, with overlapping 95% CIs. We speculate that the HR associated with HIPEC, at least in the overall series, would not have changed, should the PSOGI pathological grading system be adopted, because definition of PMP syndrome did not change in the new system.8
Data on distant metastasis were not recorded because PMP is a disease that biologically harbors the nearly null potential for hematogeneous dissemination. Virtually every recurrence occurs locoregionally inside the abdominal cavity.8 Data on the site of recurrence would not have been very informative.
Data on disease-specific survival would have provided a more accurate estimation of the efficacy of HIPEC, allowing us to exclude concurrent causes of death unrelated to PMP. However, patient candidates for CRS-HIPEC are relatively young (median age, 56 [IQRE, 45-65] years) and not affected by severe comorbidities; otherwise, they may not withstand a major procedure. We do not expect disease-specific survival to be much different from overall survival, because chances of dying of causes other than PMP would not be clinically relevant. The Charlson comorbidity index is likely to be unbalanced, considering its noninclusion in the inverse probability treatment weighted propensity score analysis. However, this parameter might not have biased our main result, because it has not been associated with overall survival.40
We did not find any relevant unbalanced factor between included and excluded patients of our initial cohort. Anyway, the possibility of selection bias is plausible, owing to the differences observed in overall survival and incidence of postoperative outcomes. However, such bias should not be large enough to explain away our main result in favor of HIPEC, because the CRS-HIPEC group had a better overall survival compared with the CRS-alone group among the excluded patients.
We conducted a sensitivity analysis to further scrutinize potential bias derived from selection and unmeasured confounders possibly related to patient, institution, or surgeon. The E-values assessing the robustness of the observed HR and CL were 2.03 and 1.53, respectively. The HRs for the known, influential prognostic factors included in the study ranged from 1.14 to 4.12. Even if the E-value we obtained seems to leave a strict margin, we are unaware of any other potential confounder of this strength that is not already included in the model.
Conclusions
This cohort study found that HIPEC was associated with better survival without worsening the postoperative outcomes in patients with PMP. Despite the relative robustness of our results to both selection bias and unmeasured confounders, confirmation by a large randomized clinical trial is warranted.
eFigure 1. Flowchart of Patients Selected for the Analysis (Unweighted Numbers)
eFigure 2. Kaplan-Meier Overall Survival Curves According to Treatment
eTable 1. HIPEC Schedules
eTable 2. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment in CC-0/1 Cases
eTable 3. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment in CC-2/3 Cases
eTable 4. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment in Low-Grade Disease
eTable 5. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment in High-Grade Disease
eTable 6. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment for HIPEC With Cisplatin Plus Mitomycin
eTable 7. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment for HIPEC With Mitomycin
eTable 8. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment for HIPEC With Oxaliplatin Plus Fluorouracil/Leucovorin
eTable 9. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment for HIPEC With Other Oxaliplatin-Based Combinations
eTable 10. Surgical Outcomes of Patients With PMP Undergoing CRS-HIPEC (n = 1548) and CRS Alone (n = 376)
eTable 11. Unweighted Comparison of the Distribution of the Prognostic Factors and Outcomes Between Included and Excluded Cases
eTable 12. Unweighted Distribution of Prognostic Factors Between the Study Groups in the Cases That Were Excluded Due to Incomplete Data
References
- 1.Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12(3):585-603. doi: 10.1016/S1055-3207(03)00026-7 [DOI] [PubMed] [Google Scholar]
- 2.Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor-like lesions of the appendix: a clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer. 1995;75:757-768. doi: [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7(1):69-76. doi: 10.1016/S1470-2045(05)70539-8 [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker PH. Comprehensive management of peritoneal surface malignancy using cytoreductive surgery and perioperative intraperitoneal chemotherapy: the Washington Cancer Institute approach. Expert Opin Pharmacother. 2009;10(12):1965-1977. doi: 10.1517/14656560903044974 [DOI] [PubMed] [Google Scholar]
- 5.van Driel WJ, Koole SN, Sirkorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230-240. doi: 10.1056/NEJMoa1708618 [DOI] [PubMed] [Google Scholar]
- 6.Bonnot PE, Piessen G, Kepenekian V, et al. ; FREGAT and BIG-RENAPE Networks . Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol. 2019;37(23):2028-2040. doi: 10.1200/JCO.18.01688 [DOI] [PubMed] [Google Scholar]
- 7.Quenet F, Elias D, Roca L, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol. 2018;36(18 suppl). doi: 10.1200/JCO.2018.36.18_suppl.LBA3503 [DOI] [Google Scholar]
- 8.Carr N, Sobin L: Tumors of the appendix. In: Bosman FT, Carneiro F, Hruban RH, et al, eds. WHO Classification of Tumours of the Digestive System. 4th ed. IARC Press; 2010:122–125. [Google Scholar]
- 9.Sugarbaker PH. Peritonectomy procedures. Cancer Treat Res. 2007;134:247-264. doi: 10.1007/978-0-387-48993-3_15 [DOI] [PubMed] [Google Scholar]
- 10.Younan R, Kusamura S, Baratti D, Cloutier AS, Deraco M. Morbidity, toxicity, and mortality classification systems in the local regional treatment of peritoneal surface malignancy. J Surg Oncol. 2008;98(4):253-257. doi: 10.1002/jso.21057 [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516-524. doi: 10.1080/01621459.1984.10478078 [DOI] [Google Scholar]
- 12.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642-5655. doi: 10.1002/sim.7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flury BK, Reidwyl H. Standard distance in univariate and multivariate analysis. Am Stat. 1986;40(3):249-251. [Google Scholar]
- 15.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343-346. doi: 10.1016/0197-2456(96)00075-X [DOI] [PubMed] [Google Scholar]
- 16.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551-561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 17.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 18.Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol. 1990;25(6):389-394. doi: 10.1007/BF00686048 [DOI] [PubMed] [Google Scholar]
- 19.Carr NJ, Emory TS, Sobin LH. Epithelial neoplasms of the appendix and colorectum: an analysis of cell proliferation, apoptosis, and expression of p53, CD44, bcl-2. Arch Pathol Lab Med. 2002;126(7):837-841. doi: [DOI] [PubMed] [Google Scholar]
- 20.Pietrantonio F, Perrone F, Mennitto A, et al. Toward the molecular dissection of peritoneal pseudomyxoma. Ann Oncol. 2016;27(11):2097-2103. doi: 10.1093/annonc/mdw314 [DOI] [PubMed] [Google Scholar]
- 21.Reynolds IS, Furney SJ, Kay EW, McNamara DA, Prehn JHM, Burke JP. Meta-analysis of the molecular associations of mucinous colorectal cancer. Br J Surg. 2019;106(6):682-691. doi: 10.1002/bjs.11142 [DOI] [PubMed] [Google Scholar]
- 22.Pietrantonio F, Berenato R, Maggi C, et al. GNAS mutations as prognostic biomarker in patients with relapsed peritoneal pseudomyxoma receiving metronomic capecitabine and bevacizumab: a clinical and translational study. J Transl Med. 2016;14(1):125. doi: 10.1186/s12967-016-0877-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro JF, Chase JL, Wolff RA, et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: a single-institution experience. Cancer. 2010;116(2):316-322. doi: 10.1002/cncr.24715 [DOI] [PubMed] [Google Scholar]
- 24.Pietrantonio F, Maggi C, Fanetti G, et al. FOLFOX-4 chemotherapy for patients with unresectable or relapsed peritoneal pseudomyxoma. Oncologist. 2014;19(8):845-850. doi: 10.1634/theoncologist.2014-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farquharson AL, Pranesh N, Witham G, et al. A phase II study evaluating the use of concurrent mitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br J Cancer. 2008;99(4):591-596. doi: 10.1038/sj.bjc.6604522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raimondi A, Corallo S, Niger M, et al. Metronomic capecitabine with cyclophosphamide regimen in unresectable or relapsed pseudomyxoma peritonei. Clin Colorectal Cancer. 2019;18(2):e179-e190. doi: 10.1016/j.clcc.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Elias D, Sideris L, Pocard M, et al. Efficacy of intraperitoneal chemohyperthermia with oxaliplatin in colorectal peritoneal carcinomatosis: preliminary results in 24 patients. Ann Oncol. 2004;15(5):781-785. doi: 10.1093/annonc/mdh186 [DOI] [PubMed] [Google Scholar]
- 28.Ceelen W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: the end of the road? Eur J Surg Oncol. 2019;45(3):400-402. doi: 10.1016/j.ejso.2018.10.542 [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner JM, Raymond VM, Lanman RB, et al. Preoperative circulating tumor DNA in patients with peritoneal carcinomatosis is an independent predictor of progression-free survival. Ann Surg Oncol. 2018;25(8):2400-2408. doi: 10.1245/s10434-018-6561-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pradeep S, Kim SW, Wu SY, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26(1):77-91. doi: 10.1016/j.ccr.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonemura Y, Canbay E, Endou Y, et al. Mechanisms of the formation of peritoneal surface malignancy on omental milky spots from low grade appendiceal mucinous carcinoma. J Clin Exp Oncol. 2015;3(4):1-6. doi: 10.4172/2324-9110.1000130 [DOI] [Google Scholar]
- 32.Turaga K, Levine E, Barone R, et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014;21(5):1501-1505. doi: 10.1245/s10434-013-3061-z [DOI] [PubMed] [Google Scholar]
- 33.Govaerts K, Chandrakumaran K, Carr NJ, et al. Single centre guidelines for radiological follow-up based on 775 patients treated by cytoreductive surgery and HIPEC for appendiceal pseudomyxoma peritonei. Eur J Surg Oncol. 2018;44(9):1371-1377. doi: 10.1016/j.ejso.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 34.Saikawa Y, Kubota T, Kuo TH, et al. Synergistic antitumor activity of mitomycin C and cisplatin against gastric cancer cells in vitro. J Surg Oncol. 1993;54(2):98-102. doi: 10.1002/jso.2930540209 [DOI] [PubMed] [Google Scholar]
- 35.Saikawa Y, Kubota T, Kuo TH, et al. Synergistic antitumor activity of combination chemotherapy with mitomycin C and cisplatin against human gastric cancer xenografts in nude mice. J Surg Oncol. 1994;56(4):242-245. doi: 10.1002/jso.2930560408 [DOI] [PubMed] [Google Scholar]
- 36.Kusamura S, Younan R, Baratti D, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106(5):1144-1153. doi: 10.1002/cncr.21708 [DOI] [PubMed] [Google Scholar]
- 37.Pelz JO, Doerfer J, Decker M, Dimmler A, Hohenberger W, Meyer T. Hyperthermic intraperitoneal chemoperfusion (HIPEC) decrease wound strength of colonic anastomosis in a rat model. Int J Colorectal Dis. 2007;22(8):941-947. doi: 10.1007/s00384-006-0246-y [DOI] [PubMed] [Google Scholar]
- 38.Makrin V, Lev-Chelouche D, Even Sapir E, Paran H, Rabau M, Gutman M. Intraperitoneal heated chemotherapy affects healing of experimental colonic anastomosis: an animal study. J Surg Oncol. 2005;89(1):18-22. doi: 10.1002/jso.20161 [DOI] [PubMed] [Google Scholar]
- 39.Kanellos D, Pramateftakis MG, Mantzoros I, et al. The effects of the intraperitoneal administration of oxaliplatin and 5-FU on the healing of colonic anastomoses: an experimental study. Tech Coloproctol. 2011;15(suppl 1):S111-S115. doi: 10.1007/s10151-011-0754-9 [DOI] [PubMed] [Google Scholar]
- 40.Kusamura S, Baratti D, Hutanu I, et al. The role of baseline inflammatory-based scores and serum tumor markers to risk stratify pseudomyxoma peritonei patients treated with cytoreduction (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol. 2015;41(8):1097-1105. doi: 10.1016/j.ejso.2015.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of Patients Selected for the Analysis (Unweighted Numbers)
eFigure 2. Kaplan-Meier Overall Survival Curves According to Treatment
eTable 1. HIPEC Schedules
eTable 2. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment in CC-0/1 Cases
eTable 3. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment in CC-2/3 Cases
eTable 4. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment in Low-Grade Disease
eTable 5. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment in High-Grade Disease
eTable 6. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment for HIPEC With Cisplatin Plus Mitomycin
eTable 7. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment for HIPEC With Mitomycin
eTable 8. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment for HIPEC With Oxaliplatin Plus Fluorouracil/Leucovorin
eTable 9. Distribution of Prognostic Factors Between the Study Groups Before and After IPTW Weighting Adjustment for HIPEC With Other Oxaliplatin-Based Combinations
eTable 10. Surgical Outcomes of Patients With PMP Undergoing CRS-HIPEC (n = 1548) and CRS Alone (n = 376)
eTable 11. Unweighted Comparison of the Distribution of the Prognostic Factors and Outcomes Between Included and Excluded Cases
eTable 12. Unweighted Distribution of Prognostic Factors Between the Study Groups in the Cases That Were Excluded Due to Incomplete Data