Abstract
Bile acids (BAs) are known facilitators of nutrient absorption but recent paradigm shifts now recognize BAs as signaling molecules regulating both innate and adaptive immunity. Bile acids are synthesized from cholesterol in the liver with subsequent microbial modification and fermentation adding complexity to pool composition. Bile acids act on several receptors such as Farnesoid X Receptor and the G protein-coupled BA receptor 1 (TGR5). Interestingly, BA receptors (BARs) are expressed on immune cells and activation either by BAs or BAR agonists modulates innate and adaptive immune cell populations skewing their polarization toward a more tolerogenic anti-inflammatory phenotype. Intriguingly, recent evidence also suggests that BAs promote anti-tumor immune response through activation and recruitment of tumoricidal immune cells such as natural killer T cells. These exciting findings have redefined BA signaling in health and disease wherein they may suppress inflammation on the one hand, yet promote anti-tumor immunity on the other hand. In this review, we provide our readers with the most recent understanding of the interaction of BAs with the host microbiome, their effect on innate and adaptive immunity in health and disease with a special focus on obesity, bariatric surgery-induced weight loss, and immune checkpoint blockade in cancer.
Keywords: bariatric surgery, immunometabolism, microbiome, mitochondria, obesity, tumor microenvironment
1 |. INTRODUCTION
The human microbiome and diversity therein colonize niches on and throughout the human body, including the mammary gland and ducts, where it confers the host with non-mammalian genetic capacity to generate critical metabolites.1,2 While we are at the precipice of understanding their roles in disease, we believe the microbiota regulate metabolism through fundamental host-microbial interactions.1,2 Both the overall diversity of the microbiome and specific microbes regulate immune responses and this review will focus on the immune milieu in cancer. In this review, we discuss obesity, weight loss through bariatric surgery, and the potential impact of microbially modified bile acid (BA) composition and signaling. We pay most attention to breast cancer (BC), the most common cancer and the second cause of cancer-related deaths in US women, however touch upon other cancers. Several cancers are associated with changes in the gut microbiome including stomach, colon, liver, lung, and skin.3–8 Microbes appear to correlate with and mediate cancer onset. While the gut has been the main focus of microbiome research in the past, a largely underexplored yet impactful realm is the contribution of the extra-intestinal or local resident microbiome. We have published that certain microbes correlate with tumor stage, tumor subtype, and race in BC patients.9 Microbes also regulate immunological response to therapy and anti-tumor immunity with bona fide impacts on clinical outcomes. For example, Akkermansia muciniphila, which confers metabolic benefits despite colonizing the gut in low abundances, restored sensitivity to immune checkpoint blockade (ICB) therapy in murine models and correlated with ICB therapeutic response in BC patients.10,11 This field is in its infancy but it is believed that microbes may play a central role in cancer biology in several ways: (a) microbes and bacterial-derived proteins directly regulate immune cell content and activation12–15; (b) microbes regulate metabolism of our diet (ie, fiber16,17); (c) microbes alter estrogen bioavailability18–21; (d) bacterial-derived quorum-sensing proteins12–14 which are found in the mammary gland22,23; and (e) microbes modify local or systemic levels of their own metabolites and host metabolites, such as short-chain fatty acids or BAs.2 With this examination of the most recent literature and trials, we implore the reader to consider how obesity, microbes, and metabolites derived from gut or extra-intestinal depots impact tumor onset, progression, anti-tumor immunity, and response to therapy.
2 |. BILE ACID METABOLISM AND SIGNALING
Primary BAs, including cholic acid (cholate, CA), chenodeoxycholic acid (CDCA), and muricholic acid (MCA, in mice), are synthesized from cholesterol by hepatocytes via the classic and alternative pathways. Synthesis is regulated by negative feedback from BA through intracellular signaling pathways, including the small heterodimer partner (SHP). Primary BAs are conjugated with glycine (“G”-GCA and GCDCA) or taurine (“T” in TCA and TCDCA) in the liver before being stored in the gall bladder or immediately released into the intestine. In rodents, 95% of conjugates are T, while humans generally display a 3:1 ratio of G:T conjugation.24 Within the gut, microbial enzymes such as bile salt hydrolases deconjugate glycine and taurine salts, rendering the bile structure susceptible to further microbial fermentation. One common reaction is the conversion of primary BA into secondary BA by the microbial enzyme 7-alpha-hydroxylase, resulting in secondary BAs lithocholic acid (LCA) and deoxycholic acid (DCA). Deconjugated primary and newly formed secondary BA can be conjugated with taurine and glycine in the liver to provide a diverse composition of BA species present in the circulating BA pool.25 Collectively, BA comprises 80% of secreted bile salts, providing an amphipathic detergent solution that facilitates dietary fat absorption by emulsifying lipids and forming micelles for absorption in the mid-jejunum.24 The majority of bile salts are reabsorbed by the time they arrive in the ileum, mediated by sodium/bile salt cotransporters to shift BAs into portal blood circulation and back to the liver.26 Some deconjugated hydrophobic BAs, such as CA and DCA, are passively reabsorbed across the colonic mucosa, where no active BA transporters exist. The liver efficiently extracts bile salts from portal blood to maintain the hepatic BA pool. Total BA pool in circulation is approximately 2.5 grams with only minimal 5% losses daily in feces.27
Bile acids provide hormone-like receptor signaling functions on numerous pathways, including the enteroendocrine system, that regulate energy expenditure, inflammation, and diseases such as cancer in part through activation of transmembrane or nuclear hormone BA receptors. Bile acids activate BA receptors (BAR) primarily through G protein-coupled BA receptor 1 (GPBAR1 or TGR5) and the nuclear hormone receptor farnesoid X receptor (NR1H4 or FXR). Other nuclear hormone receptors targeted by BAs include pregnane X receptor (PXR), vitamin D receptor (VDR), constitutive androstane receptor (CAR), and membrane bound receptors such as sphingosine-1-phosphate receptor 2 (S1PR2) and cholinergic receptor muscarinic 2 (CHRM2).28 While most BAs have some activity against the various receptors throughout the body, the affinity of each BA species for each BA receptor varies. For instance, FXR is activated at lower EC50 by primary BAs whereas TGR5 is activated more potently by secondary BAs. FXR is the master regulator of BA metabolism including homeostasis through regulation of synthesis and transport.29 Thus, the specific composition of circulating BA fundamentally provides regulation of important homeostatic systems.
Bile acids mediate enteroendocrine hormone release to shape central and peripheral homeostasis. Within the gut, BAs also directly innervate the enteric nervous system through TGR5, which regulates many local physiological aspects the gut and mucosal associated immune tissues (20236244). One enteroendocrine molecule is cholecystokinin (CCK), which is secreted by intestinal I-cells when chyme reaches the duodenum (upper small bowel), triggering gallbladder contraction and relaxation of the sphincter of Oddi to cause a slow and sustained BA release into the duodenum. In contrast, upon reaching the ileum (lower small bowel), BA stimulation of glucagon-like peptide-2 (GLP-2) release from intestinal L-cells stimulates gallbladder refilling and may influence satiety.30,31 As such, BAs are emerging as a potential mechanism of improving metabolic homeostasis. Furthermore, recent studies reveal that BA regulated enteroendocrine hormones confer potent immunomodulatory functions. For instance, circulating GLP-2 levels are demonstrated to inhibit macrophage and microglial activation in response to bacterial lipopolysaccharide (LPS) stimulation.32,33 In addition to receptor-mediated functions, BAs can also influence cell membranes by physically inserting into the bilayer and participating in membrane dynamics, as they are cholesterol derivatives. While 20–40 μmol/L levels of BAs are found in circulation, supraphysiological levels of BA administration are demonstrated to lyse cell membranes and disrupt mitochondrial membrane structure.34,35 Another receptor-independent function is allosteric activities, where BAs can inactivate or stabilize enzymes.36
Since gut microbes harbor enzymes that modify BAs hydroxylation configurations, the gut microbiome composition and functions shape circulating BA pools and their signaling mediated affects throughout the body. For instance, the bacteria Clostridium cluster 14 and Bacteroides contain high levels of 7-alpha-hydroxylase, which convert primary BAs to secondary BAs by removing hydroxylation at the 7 alpha carbon position.24 In addition, many common commensal bacteria harbor the bile salt hydrolase (ie, remove glycine or taurine), which is posited to confer bacterial resistance to the highly antibacterial activity of BAs.37 On the other hand, deconjugation renders BAs more hydrophobic, decreasing their digestive activity and decreasing reabsorption which leads to increased fecal loss. Other fermentation reactions by the gut microbiome result in tertiary BAs, including ursodeoxycholic acid (UDCA), which results from the microbial epimerization of CDCA. Collectively, the array of enzymes contained within the gut microbiota fundamentally shapes the BA pool containing various conjugated primary, secondary, and tertiary BAs (Figure 1A). The microbiome and dietary factors also influence BA reabsorption, hepatic synthesis, and intestinal motility, which in part regulate the amount of BAs lost daily through feces. BA sequestrants (cholestyramine, colestipol) are negative charged compounds administered orally that bind BA within the intestinal lumen, preventing reabsorption, and leading to increased fecal BA losses. Since BAs are synthesized from cholesterol, sequestrants lower circulating cholesterol but also improve enteroendocrine signaling. The latter effect is thought to be mediated by diverting BAs along the distal bowel where they are still able to bind enteroendocrine cell surface receptors on their path out of the gut.
FIGURE 1.
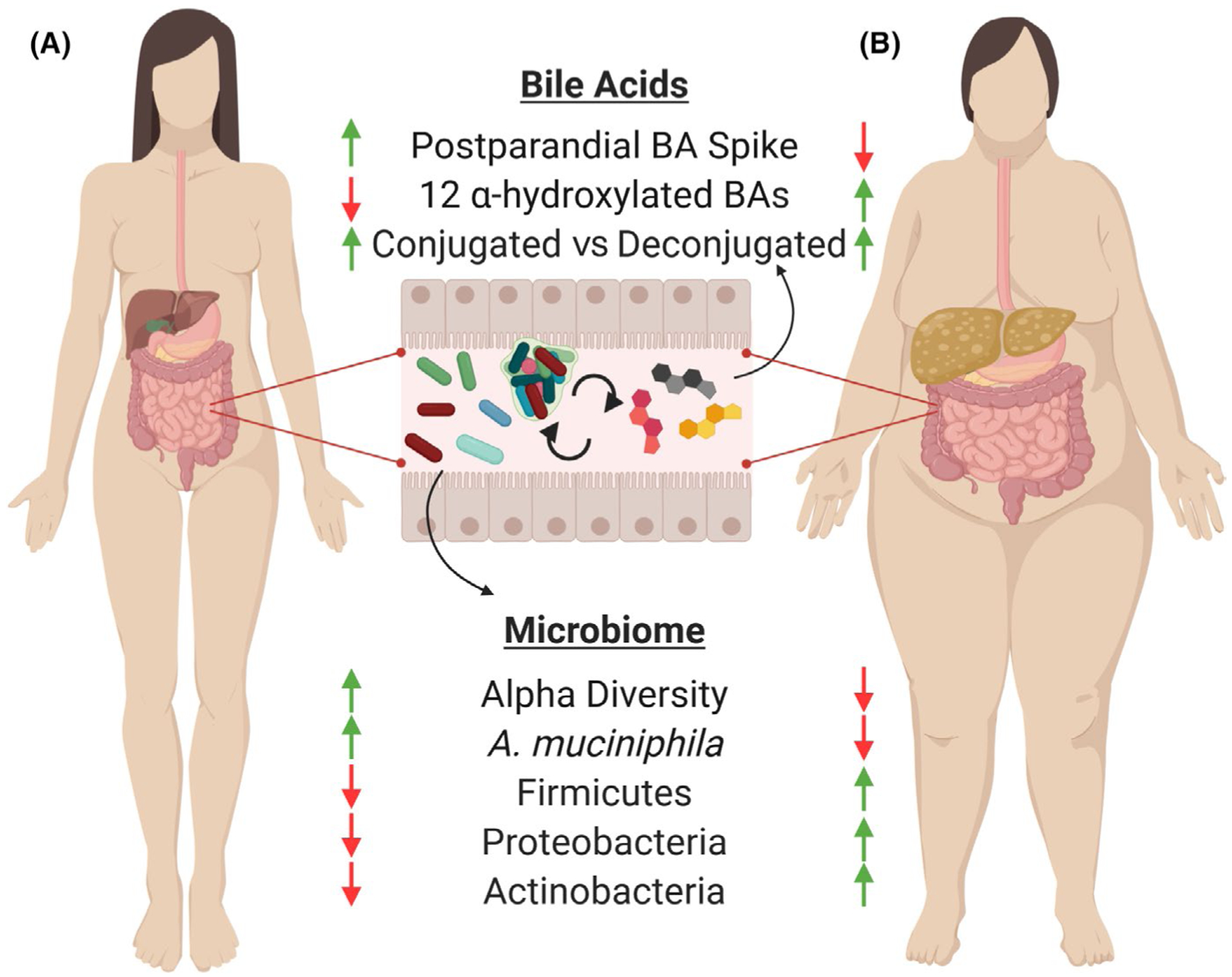
The gut microbiome, enterohepatic circulation, and obesity. The liver generates primary bile acids (BAs), usually conjugated with taurine and glycine, in part to aid with lipid and nutrient absorption immediately following meals. Within the gut, the microbiota deconjugate and ferment BAs into secondary BAs that are subsequently reabsorbed and may be further modified by the liver, a cycle known as “enterohepatic circulation.” This iterative process leads to diverse composition in the BA pools within the gut lumen, in circulation, and within various tissues. In addition to nutrient absorption, BAs serve as potent hormone-like signaling molecules through numerous receptors that confer a variety of metabolic and immunological effects throughout the body. Obesity disturbs this process, leading to blunted postprandial BA release, increased levels of the 12 alpha-hydroxylated BA forms, decreased intestinal microbiome diversity, and changes in taxonomic composition. Obesity is associated with a loss of the mucosal colonizing microbe, Akkermansia muciniphila, which has been shown to confer beneficial metabolic and immunotherapeutic anti-tumor benefits when administered to mouse and man
As BAs emerge as important immunometabolism mediators, pharmacologic agonists, antagonists, and sequestrants have been developed and used both clinically and experimentally. Pharmacological agonists are FDA approved including obeticholic acid, an FXR agonist. Given the numerous functions of FXR in various tissues, including the intestine, liver, muscle, and adipose, the effects of obeticholic acid provided mixed human clinical trial results in improving metabolic homeostasis. For instance, while improvements in glucose control have been observed in some diabetic patients,38 another clinical trial found increased fasting insulin following obeticholic acid administration.39 Intestinal FXR stimulates release of fibroblast growth factor (FGF) 19 in humans and FGF15 in mice, which suppresses hepatic BA synthesis through the rate-limiting enzyme Cyp7a1. Intestinal FXR also stimulates the release of ceramides, which stimulate lipogenesis and induce hepatic insulin resistance.40 Interestingly, the conjugated secondary BA, TUDCA, is a natural FXR antagonist in the intestine and improves metabolic outcomes. Consistent with this, experimental work with intestinal specific FXR knockouts demonstrate protection from diet-induced obesity, insulin resistance, and liver disease. On the other hand, hepatic specific FXR knockouts display susceptibility to metabolic syndrome when challenged with cholesterol diets. These contradictory phenotypes highlight the periphery vs intestinal role of FXR signaling in mediating metabolic homeostasis.
Taken together, the microbiome provides intricate control over circulating BAs, which result in various signaling cascades as well as regulation of energy expenditure, physiological responses, and immune cell phenotype and activation.
3 |. OBESITY, BARIATRIC SURGERY-INDUCED WEIGHT LOSS, AND BILE ACIDS
Obesity and metabolic syndrome are epidemics in the United States and many Westernized countries worldwide. The rise of obesity has correlated with numerous comorbidities, such as diabetes, heart disease, and cancers.41,42 Obesity is directly responsible for as many as three million deaths globally each year. As dietary weight loss is difficult to implement clinically with low adherence and a marked rebound effect, bariatric surgery has become more prevalent for sustained weight loss and long-lasting metabolic benefits in patients. While originally reserved for morbidly obese patients with body mass index (BMI) greater than 60, accumulating data demonstrate metabolic surgery also benefits type 2 diabetes and other comorbidities in lesser BMI patients. Therefore, metabolic surgeries have recently been expanded to less morbidly obese patients who also have significant health comorbidities. Metabolic surgeries, such as Roux-En-Y gastric bypass and vertical sleeve gastrectomy (VSG), are hypothesized to modify metabolic setpoints through alteration of endocrine and neuronal regulation. Evidence of this hypothesis is the observation that the beneficial metabolic effects of bariatric surgery are conferred upon patients within hours and days following surgery, long before weight loss outcomes are achieved.43 This implies that the fundamental mechanisms underlying metabolic surgery, including potentially the microbiome, BA homeostasis, endocrine regulation, and neuronal signals, are responsible for regulating metabolic homeostasis and providing key input into body weight set point. Metabolic surgery approaches, including VSG and Roux-En-Y gastric bypass, remain the most effective and sustainable treatments for obesity. VSG is typically the first-line approach for surgical weight loss. VSG serves as an attractive therapeutic approach to sustain weight loss because it causes little rearrangement of the intestinal tract with minimal nutrient malabsorption, yet so far has provided weight loss comparable to Roux-En-Y without the risk of surgical complications.44,45 VSG typically removes about 80% of the stomach, leaving a sleeve-like stomach which restricts intake. Roux-En-Y approaches include creating a small pouch by removing a portion of the stomach before connecting the gastric pouch directly to the small intestine, which lead to both restrictive food intake as well as malabsorption to reduce caloric intake. Gastric banding has become less common due to physical complications of the band and gastric organ.46 The various surgical strategies and mechanisms of action have been reviewed in detail elsewhere,47 but generally include some degree of gastric volume restriction, alteration to endocrine signaling, vagal stimulation, and BA metabolism, with changes in the microbiome.
While the molecular basis for the metabolic improvements from bariatric surgery is multifaceted and discoveries are ongoing, increasing evidence attributes these improvements to microbially influenced BAs and BAR signaling.44,47–49 Elevated BA pools are a mechanistic feature of improved outcomes after gastric bypass surgery that are proposed to be central to weight loss and other metabolic benefits in many, but not all, human and animal studies. In addition, elevated BAs after bariatric surgery may be one of the mechanisms that eliminate obesity-associated microbial dysbiosis due to anti-microbial effects of BA.25 While correlations in total circulating BA levels across metabolic phenotypes are not uniformly consistent in the literature, trends can be gleaned from large human studies. One such study examined plasma BAs from patients with normal weight (BMI 18–25), obesity (BMI ~ 48), or anorexia nervosa, and observed the greatest total circulating levels of BA in patients with obesity.50 However, other critical work demonstrated leanness is associated with large postprandial fluctuations in circulating BA levels, despite lower faster BA levels, suggesting that the timing and concentration of BAs may be important metabolic regulators.51 In this framework, consistently elevated BA levels in obesity may be the result of inefficient enterohepatic circulation and synonymous with the deleterious effects of consistently elevated insulin, cholesterol, and glucose in metabolic syndrome. In addition to total levels, changes in composition overall or specific BAs are observed in obese and diabetic patients. For instance, 12 alpha-hydroxylated BAs (CA, CDA and their salt conjugates) are elevated in individuals with insulin resistance by as much as twofold compared with healthy controls (Figure 1B).52 Experimental induction of obesity with high-fat diet in animals also leads to greater total levels of BAs, as well as elevated levels of CA, TCA, DCA, and TDCA compared with controls.44,53,54 High-fat diet feeding also reshapes the gut microbiome, and associations are found between total circulating BA levels, and the phyla Firmicutes, Proteobacteria, and Actinobacteria. Remarkably, when mice fed a low-fat diet are orally treated with the BAs elevated under a high-fat diet, the gut microbiota shifts to resemble a high-fat diet-fed mouse.54 This observation suggests that BAs may confer larger regulatory roles on microbiota selection than was previously realized.
Since BAs are proposed to confer many beneficial metabolic effects following various bariatric surgical strategies, Pierre and colleagues employed a novel metabolic surgery that specifically elevated BAs to determine their effects on systemic glucose tolerance in obesity. This bile diversion surgery (BD) elevates systemic BAs without any gastric volume restriction or other gross anatomical rearrangement of the intestinal tract.44,48 In BD surgery, the gallbladder is anastomosed to the ileum and the bile duct is ligated to divert BAs further down the intestinal tract, increasing BA reabsorption and shortening enterohepatic circulation time, which functionally results in elevated systemic BAs.44,48,49 In obese animals, BD surgery improved obesity-driven components of metabolic syndrome with no adverse malabsorptive effects on fecal lipid or BA excretion but this depends on location of anastomosis to the ileal cecal valve.44,49 Pierre et al reported that BD led to sustained weight loss, reduced adiposity to lean levels, and normalized glucose tolerance by 2 weeks after surgery.44,48 Strikingly, BD surgery resulted in sustained weight loss despite continued high-fat diet exposure. BD-associated browning of white adipose induced thermogenesis which was associated with weight loss, improved insulin sensitivity, and reduced insulin and leptin levels compared to lean or weight-matched controls. Notably, BD tripled systemic BA concentrations through increasing BA enterohepatic circulation.44,45 Critically, circulating BA changes were uniquely associated with BD—weight-matched sham controls who became as lean as BD-mice did not display elevated BAs. Therefore, these changes are not just the result of weight loss. After BD, primary BA levels increased including CA, TCA, TβMCA, and TCDCA, whereas BD significantly decreased the relative composition of secondary BAs DCA and LCA compared to sham. Pierre and others further reported that FXR is critical for BAR-mediated metabolic improvements after BD-induced weight loss using FXR deficient mice.48,49
Specific microbes may also mediate some beneficial effects of metabolic surgery. After BD, Firmicutes were decreased and Bacteroidetes were increased.44 Specifically, the mucin colonizer Akkermansia muciniphila was enriched in the gut microbiome in BD animals,44 which correlated with marked increases in both total circulating primary BAs and GLP-1 levels. This finding was consistent with Yan et al55 who observed that Roux-En-Y gastric bypass elevated A muciniphila and GLP-1 levels in diabetic rats. A significant and lasting increase in A muciniphila has also been observed in human patients following Roux-En-Y, but not in VSG, suggesting diversion of BAs conferred by BD or Roux-En-Y surgery may be necessary for alteration in the distal gut microbiome.56 Dietary interventions also modulate the gut microbiome.57 Consistent with observations in bariatric surgery and obesity, high-fat diet-induced obesity suppresses A muciniphila abundance in animal models.58 Numerous other human and animal studies also correlate A muciniphila levels with improvements in diabetes and obesity,59,60 and recent observations suggest extracellular vesicles released by A muciniphila may mediate these beneficial effects.61 Therefore, alteration of BA pools, specifically through modification of enterohepatic circulation, serves as an attractive method to modify Firmicutes, Bacteroidetes, and A muciniphila in the gut without the use of probiotics, which have heretofore proven variable and ineffective in large human trials.
In sum, weight loss through surgical-specific alterations in the microbiome and/or microbially derived metabolites, such as BAs as focused on in this review, may explain mechanistically driven improvements after weight loss and help pinpoint the critical inputs in set point theory of global energy homeostasis. These pathways should be the focus of future experimental models and human studies to definitively show surgery-specific mechanisms, since emerging evidence now supports the notion that BA pools may also influence obesity-associated comorbidities, such as cancer progression, through reprogramming of the immune microenvironment (as discussed further below).
4 |. BREAST CANCER, OBESITY, AND WEIGH LOSS
Obesity is currently associated with 13 cancers.62 Obesity leads to a higher risk of invasion, metastases, and recurrence, as well as impaired therapeutic response through various mechanisms, which together increases mortality.41,63–95 Triple-negative BC (TNBC) is an aggressive BC subtype that is more prevalent in young women. TNBCs also disproportionately affect African American and Hispanic women, paralleling the trends in obesity rates in minorities, which together create an urgent need to address these health disparities.77,84–85,96–104 TNBCs, primarily basal-like and claudin-low intrinsic subtypes, comprise ~15% of BC cases.11,105–108 Despite the lower prevalence of TNBC compared to other BC subtypes such as luminal A or B, poor TNBC survival rates emphasize a great unmet need for patients. TNBC is a subtype that is negative for estrogen receptor (ER), progesterone receptor (PR), and amplification of the human epidermal growth factor receptor 2 (HER2).18–110 As such, no targeted therapies exist for TNBC, resulting in elevated recurrence, metastasis rates, and poor overall survival relative to other BC subtypes.77,111 Thus, TNBC has generally eluded personalized medicine approaches, leaving only chemotherapy with few recent advances.
Makowski laboratory has taken advantage of a genetically engineered mouse model (GEMM) of TNBC which recapitulates human TNBC, called the C3(1)-T antigen (C3(1)-Tag) model, to address some of the questions regarding how obesity drives TNBC and to determine whether the effects of obesity can be reversed. They showed that obese mice display the greatest TNBC aggression compared to tumors in lean mice with early onset (reduced latency) or increased progression and activation of certain kinases and oncogenic signaling cascades in an obesity-specific manner.112–116 Formerly, obese mice that lost weight through diet alteration display reversal of that obesity-driven progression to lean levels in this spontaneous GEMM of basal-like TNBC,113,116 as well as ongoing studies using orthotopically transplanted syngenetic models (data not shown).
In patients, intentional weight loss and healthy lifestyle changes during cancer treatment are difficult to prioritize and implement. However, through retrospective studies of successful bariatric surgery weight loss studies, some conclusions can be drawn about risk and potential mechanisms to be targeted therapeutically. From a recent meta-analysis, bariatric surgery—and associated physiologic and metabolic changes—significantly reduced all-cause mortality from cancer (~33%−60%).117–131 Retrospective studies found bariatric surgery significantly reduced risk for pre- and postmenopausal BC as well as skin cancer (including melanoma).123,132 Of note for subtype-specific BC, bariatric surgery led to a 64% reduced risk in premenopausal ER tumors like TNBC.133 Importantly, it is not yet clear from this and other clinical studies if this benefit from surgery arises from weight loss per se or is a surgery-associated benefit.
As the role of obesity in TNBC is established in some epidemiologic studies and experimental findings from our lab and others,79,99,101,112–116,134–144 the field continues to search for and identify novel underlying mechanisms. Systemic or local hormones and adipokines associated with obesity influence cancer growth, including insulin, IGF-1, hepatocyte growth factor (HGF), and leptin.145 Obesity also extensively mediates normal and tumor microenvironment dysfunction through components including immune cells and growth factors, which influence tumor onset and progression.79,146–150 Breast tumors frequently contain dense infiltrates of immune cells, especially TNBC subtypes.151 Work by our group and others demonstrated that obesity is associated with low-grade adipose inflammation and oxidative stress in the normal human breast, non-human primate, and murine mammary gland79,152–164 which could influence risk, tumor outcomes, and therapeutic efficacy in BC. These concepts have been reviewed in great detail elsewhere.165,166
Bariatric surgery may influence cancer outcomes though altering microbial and BA pools that are proposed to be central to weight loss and other metabolic benefits. Indeed, novel mediators of poor outcomes in obesity-associated TNBC are microbes and/or microbially derived metabolites, which are currently gaining great interest. In summary, bariatric surgery may inform researchers about biomarkers of immunosurveillance or novel approaches to improve treatment. If mechanisms associated with weight loss were better understood, then they could be leveraged therapeutically—independent of surgery or weight loss—to improve anti-tumor immunity potentially through targeting microbes or microbially mediated pathways.
5 |. MICROBES IN EXTRA-INTESTINAL TISSUE AND CANCER
The gut microbiome, mycobiome, and virome are associated with numerous cancers including stomach, colon, liver, lung, head and neck, cervical, salivary glands, pancreas, lymphoma, and skin.3–8 To date, only Helicobacter pylori has been accepted as a causative agent in stomach cancer. Aside from the gut, microbes are also found in extra-intestinal tissue. Bacteria are found in the breast with a Shannon diversity index similar to the gut.18 The microbiota of the breast may arise from translocation from the gut (the “enteromammary” route), through the nipple, or other routes.167,168 Alcohol, high-fat diet, corticosterone-induced stress, or obesity can induce barrier disruption (ie, a leaky gut), which exacerbates translocation of gut bacteria peripherally.146 Lactobacillus is commonly used in probiotic formulations. When consumed orally, it can lead to increased mammary gland or milk Lactobacillus content which was associated with decreased mastitis, suggesting a direct enteral to extra-hepatic tissue axis.169–171 In the obese breast, most studies show an increase in the Firmicutes/Bacteroidetes ratio compared to lean breast tissue, a broad biomarker of dysbiosis.167
Examination of the local microbiome in BC compared to normal tumor-adjacent or normal mammary gland has revealed interesting alterations.11,14,168,172–176 The Western diet is associated with obesity and poor tumor outcomes.177 Cook et al showed for the first time that Mediterranean vs Western diet could alter mammary gland microbes, specifically increasing Lactobacillus by 10-fold and reducing Ruminococcus in the mammary gland of non-human primates.164 The reduction of Ruminococcus and increase in Lactobacillus in monkey mammary gland is important because both genera are associated with BC. Elevations of Ruminococcus were found in the gut of BC patients,11 while tumors had lower Lactobacillus content compared to benign tissue.172 Of note, mice given oral consumption of Lactobacillus decreased 4T1 tumor growth through increased T-helper cell (Th1) activation, with open and intriguing underlying mechanisms.178,179 Together these findings suggest that an extra-intestinal microbiome exists and is modifiable by dietary and lifestyle factors which potentially may impact the immune milieu.
We identified a unique TNBC microbial signature, with high Streptococcaceae and Ruminococcus abundance in breast tumors and tumor-adjacent breast tissue, compared to normal mammary tissue from healthy subjects.9 We reported that microbes vary by tumor stage and subtype. Critically, we showed for the first time that abundance of microbes and microbial ratios vary by race, with African Americans displaying greater microbial dysbiosis compared to tumors from European American patients.9 Interestingly, race is one of the strongest associations for microbes, pathways, and clinical metadata according to the Human Microbiome Project Consortium study from 2012 but notably, breast was not studied.1 The largest study to examine microbes and BC was from The Cancer Genome Atlas (TCGA) wherein Proteobacteria, Actinobacteria, and Firmicutes were shown as the most abundant phyla in breast tumors, which is consistent with our work and others, and reminiscent of the gut.1,11,14,18,21,168,172–176,180,181 We detected dysbiosis in breast tumors and tumor-adjacent breast tissue compared to normal tissue from healthy subjects. Recall that Ruminococcus is high in the gut of BC patients11 and reduced by Mediterranean diet in the mammary glands of monkeys.164 These findings are important because health disparities associated with both obesity and BC exist, and are linked to poor cancer outcomes.
Finally, while functional effects are likely due to groups of microbes, evidence supports certain microbes that correlate with both obesity and therapeutic efficacy. For example, Akkermansia muciniphila, which as discussed above is decreased by obesity and elevated after bariatric surgery, restored sensitivity to ICB therapy in murine models and correlated with ICB therapeutic response in patients.8 These findings, conserved across species in both preclinical and clinical setting, are encouraging. Some of these microbial-cancer associations have only recently been described, and it is likely that other, as yet unappreciated associations remain to be discovered.
6 |. BILE ACIDS IN EXTRA-INTESTINAL TISSUE AND CANCER
Primary and microbially modified secondary BAs have been shown to regulate cancer progression or metastasis in certain cancers. Selective targeting of gram-positive vs gram-negative bacteria induced changes in BA profiles and tumor progression using narrow-spectrum antibiotics. This approach was used successfully by Ma et al182 to stably modify the microbiome to test role of primary vs secondary BAs in hepatocellular carcinoma. Ma from the Greten group took advantage of the fact that Vancomycin (Vanco) depletes gram-positive bacteria (primarily Clostridium cluster XIV183), which express 7-alpha-hydroxylase, the most important enzyme in the gut-mediated production of secondary BAs from primary BAs. Overall, Vanco depleted Clostridium, increased primary BAs, and reduced secondary BAs.182 Likewise, secondary (ω-MCA) vs primary CDCA BA feeding reduced or increased natural killer T cell (NKT) content in hepatocellular carcinoma.182 The proposed mechanism of action was through a BA responsive ligand-positive NKT population. Primary BAs drive expression of the CXCR6 ligand, CXCL16, whereas secondary BAs reduce CXCL16. Vanco increased anti-tumor CXCR6+ NKT cells in Ma’s hepatocellular carcinoma model as the driving mechanism of tumor reduction.182 In summary, the “BA→BAR→CXCR6+ NKT” pathway is protective in hepatocellular carcinoma.
Can BAs also influence BC? The presence of BAs in breast tissue was identified decades ago in humans—with evidence that oral BA administration preferentially accumulated in the breast.184,185 Cook et al reported that Mediterranean diet-consuming monkeys had significantly higher mammary gland levels of the primary BAs CA and CDCA, as well as bile salts GCA and TCA, compared with monkeys fed a Western diet.164 Strikingly, no difference in circulating BAs, except CA, was detected between dietary groups, emphasizing the potential for unique generation or accumulation of BAs in the local microenvironment of the mammary gland.164 Therefore, microbes associate with tumor subtype and stage, race, and diet, and microbes are altered by diet and BAs in the mammary gland.
BC patients had lower circulating primary BAs (CA, CDCA, GCA, TCA) which may imply a decreased activation of FXR.186 FXR is detected in benign and malignant breast tissue and associated with increased apoptosis in BC.187 Activation of FXR by BA endogenous ligand CDCA or synthetic FXR agonist GW4046 induced apoptosis in normal and malignant BC cells of various BC subtypes and reduced MCF-7 xenograft growth.188,189 High FXR associated with smaller tumor size, high proliferation rate, relapse-free survival, distant metastasis-free survival, and overall survival in BC patients.190,191 Indeed, FXR is an independent prognostic factor for overall and disease-free patients’ survival.191 Potential mechanisms of action include FXR-mediated inhibition of leptin-induced oncogenic cancer-associated fibroblasts (CAFs) in multiple BC cell lines by increasing suppressor of the cytokine signaling 3 (SOCS3) expression downstream of FXR-implicating an obesity-specific susceptibility.188 SOCS3 is downregulated in BC.192 FXR may also mediate tumor effects through destabilization of hypoxia-inducible factor-1 alpha (HIF1-α), a master regulator that reprograms cancer cell metabolism and increases growth factors.193–195 In addition, secondary BAs such as LCA also appear to be cytostatic through TGR5-dependent effects on cancer cell metabolism, lipogenesis, epithelial mesenchymal transition (EMT), pro- and anti-apoptotic signaling, and anti-tumor immunity in vitro and in vivo.186,196,197 Furthermore, FXR and TGR5 also inhibit COX2 and NFκB, which are both important to cancer biology.198,199 Finally, tumoricidal CXCR6+ NKT cell content is differentially regulated by primary and secondary BAs, discussed below.
7 |. ANTI-TUMOR IMMUNITY
Immune checkpoint blockade has transformed the treatment of many cancers, showing unprecedented durable anti-tumor responses. However, a limitation of ICB is that responders are a minority; most patients do not respond due in part to immunosuppression, a major problem in the clinic. The immune checkpoints programmed cell death protein-1 (PD-1), PD-ligand 1 (PD-L1, CD274), and cytotoxic CD8+ T-lymphocyte antigen 4 (CTLA-4) are immune-inhibitory (ie, immunosuppressive). Immune checkpoint signaling depends on crosstalk between the cancer cell, T cells, myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), dendritic cells (DCs), natural killer T cells (NKT), among others.20–205 PD-L1 is expressed on tumor epithelial cells, T cells, TAMs, DCs, MDSCs, other immune cells and stromal cells such as adipocytes, which implies a complex biology.22–207 For example, PD-L1 concentration correlates with FoxP3+ regulatory T cells (Tregs) which create an immunosuppressive tumor microenvironment, especially in basal-like TNBC and even in early lesions including basal-like ductal carcinoma in situ (DCIS).28,209 Higher PD-L1 expression is detected in TNBC vs non-TNBC samples and associates with poor prognosis.20,209–216 Immunotherapy non-responders also have high levels of circulating MDSCs.217–221 TNBC patients harbor higher levels of MDSC populations than non-TNBC patients. Consequently, TNBC patients who have high levels of circulating MDSCs respond poorly to ICB.222
The FDA recently approved ICB combination therapy for TNBC using anti-PD-L1 atezolizumab (Tecentriq) plus nab-paclitaxel (Abraxane) for the treatment of unresectable, locally advanced, or metastatic PD-L1+ tumors in March of 2019. However, this combination therapy is effective only in a subset of patients that highly expressed PD-L1.223 Due to these therapeutic limitations, patients with TNBC typically experience elevated recurrence and metastasis and poorer overall survival compared to patients with other BC subtypes. Anti-programmed death 1 (PD-1) monoclonal antibody pembrolizumab (Keytruda, Merck Sharp & Dohme) is effective with low-grade side effects when used as first-line treatment in patients with metastatic TNBC.211,224,225 Treatment with pembrolizumab plus neoadjuvant chemotherapy, with or without carboplatin, had shown efficacy with few major toxic side effects in patients with locally advanced TNBC in the phase 1b portion of the KEYNOTE-173 trial.226 Results from I-SPY2 phase 2 trial demonstrated that human epidermal growth factor receptor 2 (HER2)-negative BC patients had a higher pathological complete response compared to those who received neoadjuvant chemotherapy alone.227 Results from the KEYNOTE-522 phase 3 trial examining neoadjuvant pembrolizumab with chemotherapy or chemotherapy alone, followed by adjuvant pembrolizumab or placebo in TNBC patients with early disease were recently published in New England Journal of Medicine.228 Schmid et al report significantly greater pathological complete response in the neoadjuvant pembrolizumab + chemotherapy group compared to controls receiving just placebo and chemotherapy. While another trial reported efficacy of a PD-L1 inhibitor only in patients with PD-L1-positive metastatic TNBC in the IMpassion130,223 Schmid’s study using neoadjuvant plus adjuvant pembrolizumab was effective regardless of PD-L1 expression levels.228 Targeting solid tumors with ICB is difficult but the field continues to push forward to find novel ways to improve anti-tumor immunity.
8 |. OBESITY, THE MICROBIOME, AND THE OBESITY PARADOX IN IMMUNE CHECKPOINT INHIBITION
Obesity dramatically changes the immune milieu.165 Obesity’s effects on immunotherapy responses are a critical area of intense research.229,230 Interestingly, PD-1 and PD-L1 expression on ligand-positive immune cells are increased by obesity, such as on DCs in obese patients.24,231,232 Accumulation of PD-1+ T-cells is detected in visceral adipose of obese mice and human omental adipose of obese tumor-free patients.232 PD-1 and PD-L1 are increased in prostate tumors of obese mice.231 Furthermore, inflammatory cytokines present in the obese microenvironment stabilize cancer cell PD-L1 protein expression through de-ubiquitination.233 Thus, inflammation associated with the obese tumor microenvironment may synergistically increase PD-L1 or PD-1, enabling a more efficient immune checkpoint-mediated immunosuppression. Elevated immune checkpoint protein expression may be fortuitous for patients who generally anticipate less favorable outcomes.234 Indeed, clinical reports show that obese or overweight patients have improved immunotherapy efficacy in metastatic melanoma, non-small cell lung cancer (NSCLC), and other cancers leading to increased overall survival and/or progression-free survival with higher PD-L1 associated with better hazard ratios.235–240 Proposed mechanisms of action include inflammatory cytokines and mediators such as C-reactive protein or leptin signaling to modulate the T cell repertoire.238,241–245 Specifically, patients treated with ICB had reduced risk of death by 3.6% with every point increase in BMI.237,246 While those studies find an obesity-ICB efficacy link regardless of sex for most survival measures, one study found the obesity-associated boost in ICB efficacy for males only.236 Of special consideration is cytokine release syndrome or “cytokine storm” and immunotherapy-induced immune-related adverse events (irAEs) that may be potentially exacerbated in obesity, reported in one mouse obesity study in tumor-free mice.229,247 In our hands, however, immunotherapy does not induce cytokine release syndrome in obese mice with tumors (unpublished observations). Emerging data are exciting and clearly indicate that further study on the mechanisms underlying ICB and obesity is paramount.
To complicate matters, obesity also changes gut microbes, as discussed above. Emerging studies reported that the gut microbiome influences response to ICB,10,248–253 which obesity could modulate. Fecal microbial transplant, antibiotics, or feeding certain organisms to mice have been reported to alter ICB efficacy in cancers such as sarcoma and melanoma.10,251,254,255 Tumors may reflect a portion of gut microbes as in pancreatic cancer after transfaunation256 but this is not clear yet for all cancers. In sum, bacteria’s role in anti-tumor immunity is unclear. Clarifying mechanisms underlying clinical efficacy of ICB, the microbiome, locally relevant microbes and associated metabolites such as BAs, and potential modification by obesity are critical. Clinically, obesity as measured by BMI or waist to hip ratio (WHR) should be used to stratify patients in trials and in predicting risk or outcomes.
9 |. BILE ACID SIGNALING IN KEY IMMUNE CELLS
As the role of obesity in TNBC is established in epidemiologic studies11,140–144 and experimental findings are formed from our laboratory and others,79,99,112–116,134–139 it is essential to identify novel underlying mechanisms associated with obesity, weight gain, or weight loss that impact TNBC outcomes. Inflammation associated with obesity is one well-studied consequence that influences risk, tumor outcomes, and therapeutic efficacy.165,166 Breast and other tumors frequently contain dense infiltrates of immune cells.151 The novel link between obesity, BAs, and cancer outlined above coalesces in the interaction between systemic or local BAs and BAR in adaptive and innate immune cells and is summarized below (Figure 2).
FIGURE 2.
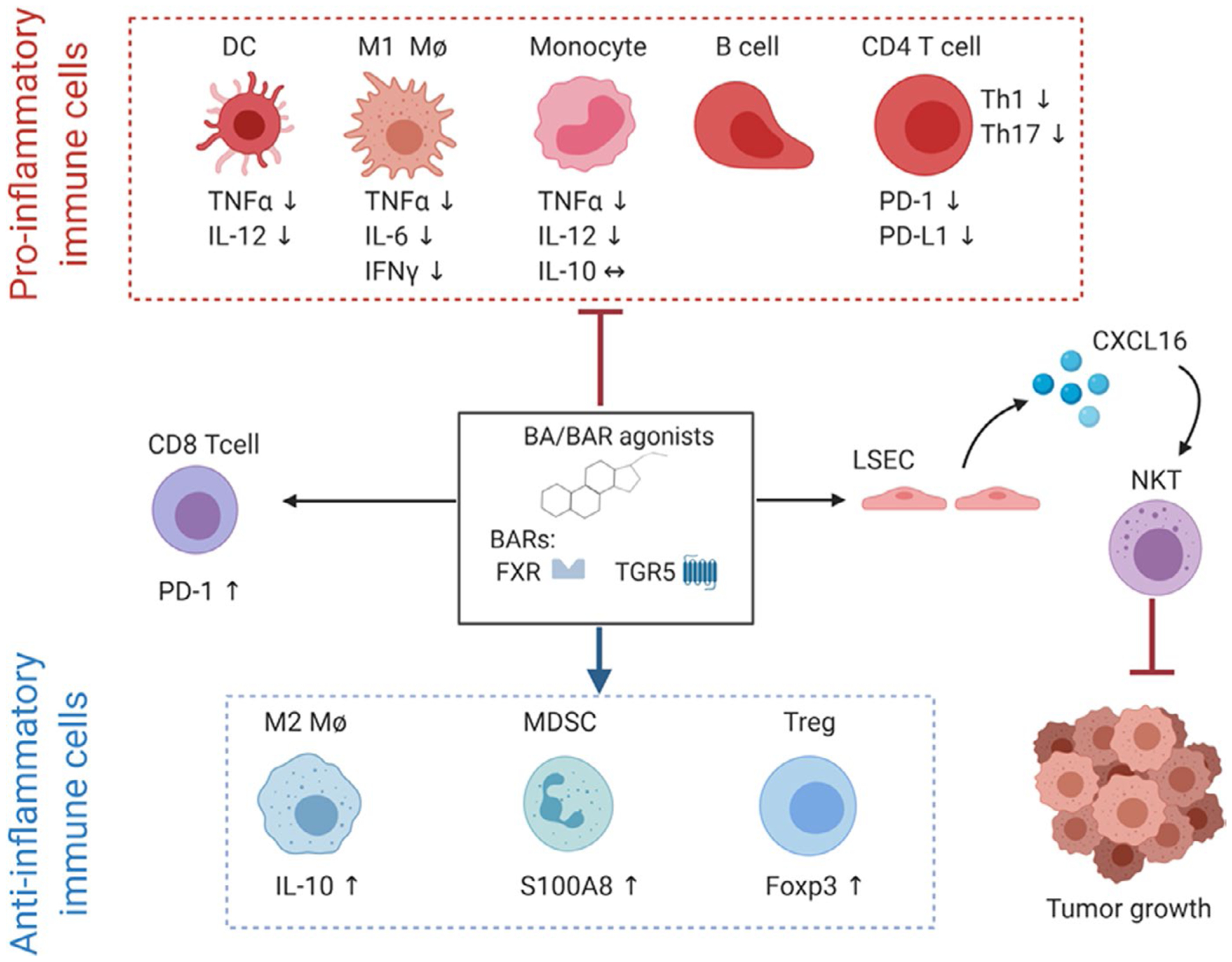
Bile acids or small molecule effects of bile acid receptors (BARs) Farnesoid X Receptor (FXR) and G Protein-coupled bile acid receptor (TGR5) agonism on immune cell populations. BAR agonism impairs pro-inflammatory functions of immune cells by downregulating tumor necrosis factor alpha (TNFα) and interleukin 12 (IL-12) on dendritic cells (DCs) and monocytes. BAR agonism also impairs M1-like pro-inflammatory polarization of macrophages (Mϕ) by downregulating TNFα, IL-6, and interferon gamma (IFNγ). Additionally, BAR agonism decreases the abundance of B cells and CD4 T cells, downregulates protein death receptor 1 (PD-1) and its ligand PD-L1 on CD4 T cells and impairs Th1 and Th17 polarization. On the other hand, BAR agonism favors anti-inflammatory cells such as myeloid-derived suppressor cells (MDSC) via upregulation of the calcium-binding protein S100A8, T regulatory cells (Treg), and immunoregulatory M2-like polarization of macrophages via upregulation of IL-10. BAR agonism also induces the expansion of CD8 T cells and their upregulation of PD-1; however, BA-induced expansion of CD8 T cells remains unknown. Finally, primary BAs induce liver sinusoidal endothelial cells (LSEC) to release the chemokine CXCL16 which will attract Natural Killer T cells (NKT) into the tumor site and inhibit cancer growth
9.1 |. Tumor-associated macrophages
Macrophages are a heterogeneous population of cells within the innate immune system that play critical roles in a myriad of processes including development, tissue homeostasis, host defense, and disease pathogenesis such as atherosclerosis, diabetes, and cancer.257–259 Macrophages exhibit a diverse spectrum of phenotypes from pro- to anti-inflammatory, phagocytic, to extracellular matrix remodeling, among others which are often overlapping and overall flexible depending on the unique characteristics of the microenvironment. Along with these phenotypes are typical metabolic characteristics of certain macrophage types.260–268 In vitro studies have informed early findings that classically activated (“M1”-like) macrophages utilize primarily glycolysis, which is linked to the pro-inflammatory phenotype characterized by the production of high levels of pro-inflammatory cytokines, reactive oxygen and nitrogen metabolites, microbicidal and phagocytic properties, and the generation of critical intermediates for anabolic pathways.269–271 On the other hand, alternatively activated (“M2”-like) macrophages predominantly rely on mitochondrial oxidative metabolism146,162,271–276 with a lesser dependence on glycolysis277–280 and are associated with tissue homeostasis and resolution of the inflammatory response. Yet in vivo and recent in vitro studies underscore that classical and alternative phenotypes are not dichotomous but rather present overlapping phenotypes and flexible metabolism.162,281–284
Work completed by our group and others demonstrated that obesity is associated with low-grade adipose inflammation and crown-like structures (CLS) in fat depots throughout the body including subcutaneous and visceral adipose162,165,283 as well as the lesser studied fat depot of the normal human breast in humans, non-human primates, and mammary gland in mice.79,152–164 In visceral fat, macrophages can comprise up to 50% of the cellularity of an adipose depot. Obesity-increased CLS are detected in obese patient breast adipose tissue compared to non-obese breast.160 Dannenberg et al reported that breast CLS (CLS-B) associate with poor patient survival, even when lean.285–287 In patients, TAMs impact every aspect of tumorigenesis, growth, and negatively influence response to anticancer therapies through establishing an immunosuppressive microenvironment, thus disallowing anti-tumor immune cells such as tumoricidal macrophages, cytotoxic (CD8+) T cell, and NKT cell activity.79,155,163,288–303 Elevated TAMs in breast tumors are independent prognostic indicators of reduced relapse-free survival and decreased overall survival.288,300,302,304–314 Therefore, microbially derived metabolites such as BAs may have a great impact on macrophages, such highly prevalent and dynamic cells.
In monocytes and macrophages, the BARs TGR5 and FXR are highly expressed. Activation of signaling via these receptors is mostly immunosuppressive or anti-inflammatory.315,316 BAs suppress LPS-induced cytokine expression (TNFα, IL-6) and phagocytosis in primary human macrophages, murine macrophages, and Kupffer cells through either the TGR5-cAMP-PKA pathway or impaired activation of NFκB.317–322 In monocytes, while BAs and TGR5 activation inhibited the secretion of the pro-inflammatory cytokines TNF-α and IL-12, it did not change the secretion of anti-inflammatory IL-10.315,318 These findings suggest a “mixed” phenotype macrophage in response to BAs, wherein the immunosuppressive M2-like phenotype predominates, as evidenced by an increase in the IL-10:IL-12 synthesis ratio.315,317–318,323,324 Similarly, FXR activation in macrophages by CDCA reduced NFκB-responsive cytokines after LPS-activation.325 While FXR activation in macrophages causes a robust downregulation of the expression of several IFNγ-regulated genes, FXR itself is reciprocally downregulated by IFNy-STAT1 signaling suggesting that strength and timing of stimuli are necessary to consider in FXR and inflammatory signaling.326
The anti-inflammatory effects of BAs may be mediated through the inflammasome. Inflammasome activation plays a key role in host defense against pathogens and inflammation and its over-activation is integral to several inflammatory disorders including obesity.327 Guo et al328 showed that BA signaling through TGR5 inhibits NLRP3 inflammasome caspase-1 activation and IL-1β secretion in several disease states including type 2 diabetes. High levels of circulating bile salts (in this case due to cholestasis or sepsis) activate the NLRP3 inflammasome in macrophages to cleave caspase-1 which induces maturation of pro-IL-1β.328–331 Thus, BARs may act as regulators of inflammation by preventing the activation of the inflammasome pathway or other signaling cascades. Taken together, these studies propose BAR agonism as a suitable therapeutic approach to prevent pro-inflammatory activation. How BAs effect macrophages in the obese adipose tissue including the mammary gland or breast fat pad are unknown. Likewise, the impact on TAMs is currently undefined.
9.2 |. Dendritic cells
Dendritic cells are a subset of innate immune cells which present antigens on major histocompatibility complex (MHC) molecules to T cells.332,333 DCs are a heterogenous population that comprises three major subsets: (a) conventional DCs which are further divided into two distinct subtypes: cDC1 and cDC2; (b) plasmacytoid DCs which are major producers of type 1 interferons; and (c) monocyte-derived DCs which play a substantial role in inflammation.334 BARs such as FXR and GPBAR1 are expressed by DCs in addition to their presence in other myeloid immune cells (macrophages and monocytes).321,335 Interestingly, two studies have reported that pharmacological activation of FXR by BAR agonist obeticholic acid reduced differentiation and activation of intestinal DCs, with downregulated pro-inflammatory factors such as TNFα in mouse models of colitis.335,336 Agonizing TGR5 induces differentiation of human CD14+ monocytes into IL-12 hypo-producing DCs via the TGR5-cAMP pathway.335 However, the role of BARs in DC subset differentiation and activation in pathological conditions such as cancer and inflammation remains unknown and requires further studies.
9.3 |. Myeloid-derived suppressor cells
Myeloid-derived suppressor cells are a poorly understood innate immature immunosuppressive cell population that emerges from the bone marrow and drastically expands in pathological conditions such as cancer and chronic inflammation.337 MDSCs are heterogenous and comprise two distinct subtypes: polymorphonuclear (PMN-MDSCs) which resemble neutrophils and monocytic (M-MDSC) which are monocyte-like.338 Both MDSC subtypes suppress innate and adaptive immunity in the tumor microenvironment. The main function of MDSCs limits T cell-mediated immune responses through production of immunosuppressive factors such as arginase 1 which depletes L-arginine, an amino acid which is essential for T cell proliferation and activation.339 In a study published by Zhang et al340, FXR activation in MDSCs enhanced their suppressor function which attenuated immune-mediated liver injury. Mechanistically, FXR-mediated activation of MDSCs was dependent on FXR binding to the paired Ig-like receptor B (PIR-B) promoter which resulted in upregulation of S100 calcium-binding protein A8 (S100A8). This is the only study reporting the role of BARs in MDSCs and more studies are needed in order to understand how BARs affect MDSCs in both autoimmune diseases and cancer.
T cells including regulatory T cells (Treg), Th17, mucosal-associated invariant T (MAIT), natural killer T cells (NKT), and B cells.
Although most work has focused on BA regulation of innate immune cells, exciting investigation of the adaptive immune cell compartment in various diseases has been forthcoming. In congruence with the immunosuppressive effects of BA, LCA impedes Th1 activation in human and mouse CD4+ T cells through decreasing production of Th1 cytokines IFNγ and TNFα.341 Additionally, FXR agonists obeticholic acid and CDCA treatment ameliorated experimental autoimmune encephalomyelitis (EAE) in mice by suppressing lymphocyte activation and pro-inflammatory cytokine production. Interestingly, obeticholic acid treatment of EAE mouse model reduced CD4+ T cells and CD19 B cells, as well as their expression of PD-1 and PD-L1. However, obeticholic acid treatment increased CD8+ T cells and their expression of PD-1. Taken together, these findings highlight a positive role of BAs and FXR signaling in regulating neuroinflammation and propose FXR agonism through obeticholic acid as a potential therapeutic approach for multiple sclerosis (MS).342
In a recent study, Hang et al343 screened BA metabolites and found two LCA metabolites (namely 3-oxo-LCA and isoallo-LCA) that regulated T cell differentiation. 3-Oxo-LCA blunted differentiation of Th17 cells through retinoid-related orphan receptor-γt (RORγt). Interestingly, isoallo-LCA functioned through a different mechanism to increase Treg (Foxp3+) differentiation in a mitochondrial reactive oxygen species (mitoROS)-dependent manner. Combined treatment of mice with these LCA metabolites therefore skewed T cells in the intestinal lamina propria from Th17 toward Treg.343 Further analysis has shown genetic ablation of BA metabolic pathways in individual gut symbionts significantly decreased Treg populations.344 These data suggest that BA and microbes regulate Tregs and Th17 cells in the gut and possibly other tissues which could have potent impacts on anti-tumor immunity.
As introduced above, Ma and colleagues showed a direct link between the microbiome, BA, and hepatocellular carcinoma through the recruitment of natural killer T-cells (NKT). Primary BAs increased NKT cell accumulation via liver sinusoidal endothelial cell (LSEC) CXCL16 expression which effectively reduced liver tumor growth. When microbiota was present and conversion of primary BAs into secondary BAs occurred, NKT cells failed to be recruited and the tumor growth was elevated.182 In sum, Greten’s group demonstrated a direct link between microbiota, BAs, and the immune tumor microenvironment through the “BA→BAR→CXCR6+ NKT” pathway.
Lastly, crosstalk between macrophages and T cells may be through the FXR downstream mediator SHP which is found in macrophages and blunts NFκB activation.345 The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. High SHP in macrophages led to reduced activation and proliferation of T cell expansion with reduction especially in Tregs through reduced IL-2 and TGFβ, while SHP knockdown led to Treg expansion. SHP enrichment was found in the promoters of IL-2, CD86, MHCII, and NOS2. Under normal conditions, SHP likely limits indiscriminate inflammation (ie, macrophage cytokine driven pro-inflammatory activation) but promotes a targeted T cell response (ie, skews T cells toward Teffector vs Tregs).346
Mucosal-associated invariant T (MAIT) cells are a relatively new subset of innate-like T cells which are highly abundant in the human liver. MAITs comprise from 10% to 50% of total liver T cells.347 MAITs are also localized to the gut mucosa.347,348 Importantly, the development of MAITs is absent in germ-free mice, indicating that microbiota are essential for the differentiation of MAIT cells.347 In children from the Lifestyle Immune System Allergy (LISA) cohort, Mendler and colleagues showed that BAs positively associated with pro-inflammatory markers such as C-reactive protein, IL-8, and MIP-1α. In contrast, a negative association between conjugated-BA concentrations and the number of activated MAIT cells was found.349 Furthermore, BA-mediated inhibition of MAIT cell activation in vitro was shown. Interestingly, overweight children in the LISA study had reduced MAIT activation349 in agreement with obesity-associated alterations in MAIT cell function and distribution in children and adults.350 Recent studies indicate that MAIT cells display tumor promoting functions by suppressing T cells and NK cells in a murine model of melanoma lung metastasis.351 The role of BA and MAITs in cancer is completely unexplored.
In summary, most publications to date on BA and BAR in immune cells focus on gut-associated disease, autoimmune disease, or acute phenomena such as sepsis, with little work on the tumor microenvironment, demonstrating a large gap in our knowledge which we intend to fill with this review. It is apparent that the role of BARs in innate and adaptive immunity implies that BAs or agonism of BARs leads to a general downregulation of inflammation in some cell types but an activation of other cells such as NKT. This will continue to be an area of active investigation.
10 |. CONCLUSIONS AND FUTURE DIRECTIONS
Compelling evidence suggests a protective role of BAs and BAR agonists involved in metabolic and immune -linked pathologies which with further study could be exploited in the treatment of cancer and other autoimmune diseases. To attempt to address the lack of evidence for BARs in cancer, using online expression data from tumors, we examined BAR TGR5 expression in correlation with immune cell infiltrate and overall survival. Correlation of TGR5 expression with immune infiltration level in BC overall and skin cutaneous melanoma showed positive and significant associations for all cell types examined. TGR5 expression has significantly positive correlations with infiltrating levels of CD8+ T cells and DCs as determined by gene expression signatures for immune cells using the Tumor IMmune Estimation Resource (TIMER) database352 (Figure 3A). Cumulative survival was also examined for high and low expressers of TGR5 in tumors from BC and melanoma patients and specific immune signatures. CD8+ T cells and DCs predicted significantly improved overall survival in patient tumor’s that highly expressed TGR5 compared to patient tumors with low expression (Figure 3B). In melanoma, B cells and neutrophil population levels also associated with better survival in TGR5 high expressing tumors compared to low expressing tumors (data not shown). Our novel findings support BARs as a promising target for additional preclinical and clinical studies.
FIGURE 3.
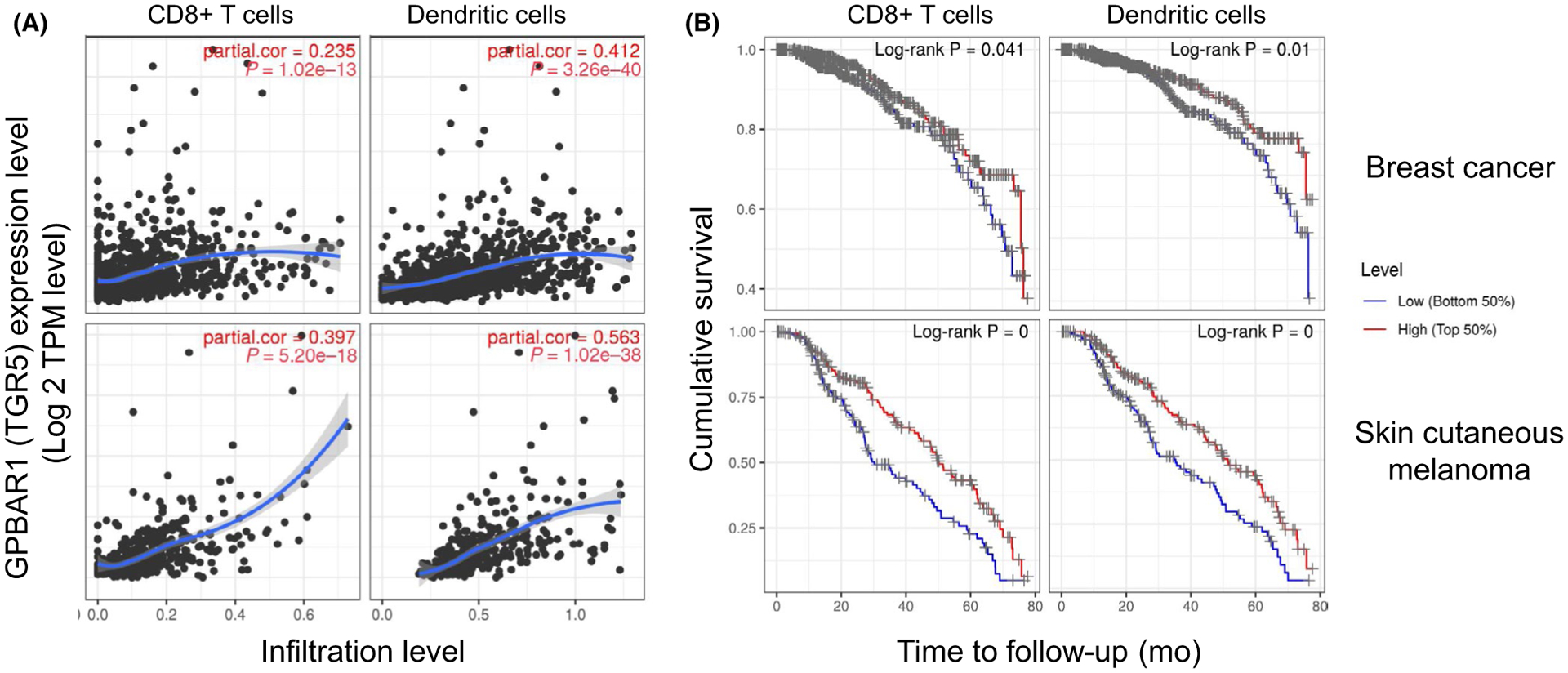
Correlation of GPBAR1 (TGR5) expression with immune cell infiltration levels in breast cancer and skin cutaneous melanoma. Breast cancer is depicted in top graphs and skin cutaneous melanoma in bottom graphs. A, GPBAR1 expression has significant positive correlations with infiltrating levels of CD8+ T cells and dendritic cells determined by using TIMER database.352 B, High expression of GPBAR1 has a significant favorable overall survival when correlated with high infiltration of CD8+ T cells and dendritic cells as compared to low expression of GPBAR1 in both breast cancer and skin cutaneous melanoma (TIMER database). Cumulative survival is shown for 80 mo
Our lack of understanding of how the gut and/or extra-intestinal microbiome affect the molecular mechanisms and signaling pathways mediating effective anti-tumor outcomes poses a substantial obstacle to advancement in cancer therapy, as well as a potential therapeutic opportunity. It is possible that immune perturbations resulting from microbiome composition and microbially modified metabolites can be targeted for therapeutic benefit. To date, several current clinical trials are testing probiotics or examining gut microbiome in BC, with some analyzing the mammary microbiome. Probiotics, which have heretofore proven variable and somewhat ineffective in large human trials, may not be effective in restoring tissue dysbiosis. Only one clinical trial to date is examining the role of bariatric surgery intervention on biomarkers of BC in high-risk patients (NCT02681120). The National Cancer Institute (NCI) and National Institute on Dental and Craniofacial Research (NIDCR) are participating in a recent program announcement entitled “Microbial-based Cancer Therapy-Bugs as Drugs” and another with NCI participating entitled “Modulating Intestinal Microbiota to Enhance Protective Immune Responses against Cancer.” Thus, these recent NIH program announcements show increased evidence of commitment toward understanding the contribution of microbes in regulation of the immune response and anti-tumor therapy.
ACKNOWLEDGEMENTS
We thank Emily Korba and Mary Camille Lovely for review of the manuscript. We acknowledge the use of BioRender for figure generation. LM acknowledges the University of Tennessee Health Science Center (UTHSC) Methodist Mission Fund. JFP acknowledges the Tennessee Governor Pediatric Recruitment Grant. Laura M Sipe acknowledges The Obesity Society/The Komen Foundation funding and NCI Transdisciplinary Research on Energetics and Cancer (TREC) fellowship.
Footnotes
CONFLICT OF INTEREST
No authors have conflict.
REFERENCES
- 1.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez KB, Pierre JF, Chang EB. The gut microbiota: the gateway to improved metabolism. Gastroenterol Clin North Am. 2016;45:601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lofgren JL, Whary MT, Ge Z, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couturier-Maillard A, Secher T, Rehman A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep. 2014;16:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan X, Yang M, Liu J, et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5:3111–3122. [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y, Champer J, Beynet D, Kim J, Friedman AJ. The role of the cutaneous microbiome in skin cancer: lessons learned from the gut. J Drugs Dermatol. 2015;14:461–465. [PubMed] [Google Scholar]
- 9.Smith A, Pierre JF, Makowski L, Tolley E, Lyn-Cook B, Lu L, Vidal G, Starlard-Davenport A. Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic Black and non-Hispanic White women. Sci Rep. 2019;9:11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. [DOI] [PubMed] [Google Scholar]
- 11.Goedert JJ, Jones G, Hua X, et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst. 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakritz JR, Poutahidis T, Mirabal S, et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 2015;6:9387–9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaga C, Takagi A, Kano M, et al. Lactobacillus casei Shirota enhances the preventive efficacy of soymilk in chemically induced breast cancer. Cancer Sci. 2013;104:1508–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xuan C, Shamonki JM, Chung A, et al. Microbial dysbiosis is associated with human breast cancer. PLoS ONE. 2014;9:e83744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao C, Ganesh BP, Shi Z, et al. Gut microbe-mediated suppression of inflammation-associated colon carcinogenesis by luminal histamine production. Am J Pathol. 2017;187:2323–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donohoe DR, Holley D, Collins LB, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastian C, Mostoslavsky R. Untangling the fiber yarn: butyrate feeds Warburg to suppress colorectal cancer. Cancer Discov. 2014;4:1368–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani S. Microbiota and breast cancer. Prog Mol Biol Transl Sci. 2017;151:217–229. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrman BJ, Schairer C, Gail MH, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill MJ, Goddard P, Williams RE. Gut bacteria and aetiology of cancer of the breast. Lancet. 1971;2:472–473. [DOI] [PubMed] [Google Scholar]
- 21.Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Spiegeleer B, Verbeke F, D’Hondt M, et al. The quorum sensing peptides PhrG, CSP and EDF promote angiogenesis and invasion of breast cancer cells in vitro. PLoS ONE. 2015;10:e0119471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balhouse BN, Patterson L, Schmelz EM, Slade DJ, Verbridge SS. N-(3-oxododecanoyl)-L-homoserine lactone interactions in the breast tumor microenvironment: Implications for breast cancer viability and proliferation in vitro. PLoS ONE. 2017;12:e0180372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176–193. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann AF. The enterohepatic circulation of bile acids in man. Adv Intern Med. 1976;21:501–534. [PubMed] [Google Scholar]
- 28.Yu-Jui YL. Regulation of bile acid receptor activity. Liver Research. 2018;2:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. [DOI] [PubMed] [Google Scholar]
- 30.Cazzo E, Pareja JC, Chaim EA, Geloneze B, Barreto MR, Magro DO. GLP-1 and GLP-2 levels are correlated with satiety regulation after Roux-en-Y gastric bypass: results of an exploratory prospective study. Obes Surg. 2017;27:703–708. [DOI] [PubMed] [Google Scholar]
- 31.Yusta B, Matthews D, Flock GB, et al. Glucagon-like peptide-2 promotes gallbladder refilling via a TGR5-independent, GLP-2R-dependent pathway. Mol Metab. 2017;6:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, Liu BW, Ren WZ, et al. GLP-2 attenuates LPS-induced inflammation in BV-2 cells by inhibiting ERK1/2, JNK1/2 and NF-kappaB signaling pathways. Int J Mol Sci. 2016;17:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie S, Liu B, Fu S, et al. GLP-2 suppresses LPS-induced inflammation in macrophages by inhibiting ERK phosphorylation and NF-kappaB activation. Cell Physiol Biochem. 2014;34:590–602. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo P, Melchiorre E, La Torre C, et al. Effects of phosphatidylcholine and sodium deoxycholate on human primary adipocytes and fresh human adipose tissue. Int J Immunopathol Pharmacol. 2010;23:481–489. [DOI] [PubMed] [Google Scholar]
- 35.Sousa T, Castro RE, Pinto SN, et al. Deoxycholic acid modulates cell death signaling through changes in mitochondrial membrane properties. J Lipid Res. 2015;56:2158–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margheritis E, Castellani B, Magotti P, et al. Bile acid recognition by NAPE-PLD. ACS Chem Biol. 2016;11:2908–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung JY, Shaffer EA, Costerton JW. Antibacterial activity of bile salts against common biliary pathogens. Effects of hydrophobicity of the molecule and in the presence of phospholipids. Dig Dis Sci. 1993;38:2104–2112. [DOI] [PubMed] [Google Scholar]
- 38.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(574–582):e571. [DOI] [PubMed] [Google Scholar]
- 39.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdel-Salam E, Peters PA, Abdel Meguid AE, Abdel Meguid AA, Mahmoud AA. Discrepancies in outcome of a control program for schistosomiasis haematobia in Fayoum governorate. Egypt. Am J Trop Med Hyg. 1986;35:786–790. [DOI] [PubMed] [Google Scholar]
- 41.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. [DOI] [PubMed] [Google Scholar]
- 44.Pierre JF, Martinez KB, Ye H, et al. Activation of bile acid signaling improves metabolic phenotypes in high-fat diet-induced obese mice. Am J Physiol Gastrointest Liver Physiol. 2016;311: G286–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierre JF, Li Y, Gomes CK, Rao P, Chang EB, Yin DP. Bile Diversion improves metabolic phenotype dependent on Farnesoid X Receptor (FXR). Obesity (Silver Spring). 2019;27:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eid I, Birch DW, Sharma AM, Sherman V, Karmali S. Complications associated with adjustable gastric banding for morbid obesity: a surgeon’s guides. Can J Surg. 2011;54:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulla CM, Middelbeek RJW, Patti ME. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann N Y Acad Sci. 2018;1411:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierre JF, Li Y, Gomes CK, Rao P, Chang EB, Bile YDP. Diversion improves metabolic phenotype dependent on Farnesoid X Receptor (FXR). Obesity (Silver Spring). 2019;27:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albaugh VL, Banan B, Antoun J, et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2019;156(4):1041–1051.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prinz P, Hofmann T, Ahnis A, et al. Plasma bile acids show a positive correlation with body mass index and are negatively associated with cognitive restraint of eating in obese patients. Front Neurosci. 2015;9:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haeusler RA, Camastra S, Nannipieri M, et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. J Clin Endocrinol Metab. 2016;101:1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin H, An Y, Tang H, Wang Y. Alterations of bile acids and gut microbiota in obesity induced by high fat diet in rat model. J Agric Food Chem. 2019;67:3624–3632. [DOI] [PubMed] [Google Scholar]
- 54.Zheng X, Huang F, Zhao A, et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017;15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan M, Song MM, Bai RX, Cheng S, Yan WM. Effect of Roux-en-Y gastric bypass surgery on intestinal Akkermansia muciniphila. World J Gastrointest Surg. 2016;8:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmisano S, Campisciano G, Silvestri M, et al. Changes in gut microbiota composition after bariatric surgery: a new balance to decode. J Gastrointest Surg. 2019. 10.1007/s11605-019-04321-x [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 57.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneeberger M, Everard A, Gomez-Valades AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derrien M, Van Baarlen P, Hooiveld G, Norin E, Muller M, de Vos WM. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the Mucin-Degrader Akkermansia muciniphila. Front Microbiol. 2011;2:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chelakkot C, Choi Y, Kim DK, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15(3):139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer-viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 65.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- 66.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;2011(61):409–418. [DOI] [PubMed] [Google Scholar]
- 67.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 68.Bliss JM, Gray R. Breast cancer and hormone-replacement therapy: the Million Women Study. Lancet. 2003;362(9392):1328–1329; author reply 1330–1321. [DOI] [PubMed] [Google Scholar]
- 69.Marsden J, A’Hern R. The Million Women Study and breast cancer. J Br Menopause Soc. 2003;9(95):97. [DOI] [PubMed] [Google Scholar]
- 70.Beral V Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. [DOI] [PubMed] [Google Scholar]
- 71.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 72.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 73.AMA.AMA adopts new policies on second day of voting at annual meeting. Press release, 2013. http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-18-new-amapolicies-annual-meeting.page. Accessed September 1, 2013.
- 74.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gillespie EF, Sorbero ME, Hanauer DA, et al. Obesity and angiolymphatic invasion in primary breast cancer. Ann Surg Oncol. 2010;17:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anders CK, Abramson V, Tan T, Dent R. The evolution of triple-negative breast cancer: from biology to novel therapeutics. Am Soc Clin Oncol Educ Book. 2016;35:34–42. [DOI] [PubMed] [Google Scholar]
- 78.Lashinger LM, Rossi EL, Hursting SD. Obesity and resistance to cancer chemotherapy: interacting roles of inflammation and metabolic dysregulation. Clin Pharmacol Ther. 2014;96:458–463. [DOI] [PubMed] [Google Scholar]
- 79.Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. J Carcinog. 2013;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ewertz M, Jensen M-B, Gunnarsdóttir KÁ, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. [DOI] [PubMed] [Google Scholar]
- 81.Osman MA, Hennessy BT. Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clinical Medicine Insights Oncology. 2015;9:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazzarella L, Disalvatore D, Bagnardi V, et al. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur J Cancer. 2013;49:3588–3597. [DOI] [PubMed] [Google Scholar]
- 83.Staiano AE, Harrington DM, Johannsen NM, et al. Uncovering physiological mechanisms for health disparities in type 2 diabetes. Ethn Dis. 2015;25:31–37. [PMC free article] [PubMed] [Google Scholar]
- 84.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Plank-Bazinet JL, Kornstein SG, Clayton JA, et al. A Report of the Women’s Health Congress Workshop on the health of women of color: a critical intersection at the corner of sex/gender and race/ethnicity. J Womens Health (Larchmt). 2016;25:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14:1718–1725. [DOI] [PubMed] [Google Scholar]
- 87.Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: a review of the recent literature. Curr Nutr Rep. 2014;3:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Widschwendter P, Friedl TW, Schwentner L, et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. Breast Cancer Res. 2015;17:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Drygalski A, Tran TB, Messer K, et al. Obesity is an independent predictor of poor survival in metastatic breast cancer: retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Breast Cancer. 2011;2011:523276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loi S, Milne RL, Friedlander ML, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686–1691. [DOI] [PubMed] [Google Scholar]
- 92.Abrahamson PE, Gammon MD, Lund MJ, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1871–1877. [DOI] [PubMed] [Google Scholar]
- 93.Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–3415. [DOI] [PubMed] [Google Scholar]
- 94.Biglia N, Peano E, Sgandurra P, et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013;29:263–267. [DOI] [PubMed] [Google Scholar]
- 95.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson GL, Neuhouser ML. Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res. 2012;5:515–521. [DOI] [PubMed] [Google Scholar]
- 97.Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obesity Rev. 2013;14:665–678. [DOI] [PubMed] [Google Scholar]
- 98.Chen L, Cook LS, Tang MT, et al. Body mass index and risk of luminal, HER2-overexpressing, and triple negative breast cancer. Breast Cancer Res Treat. 2016;157:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:307–314. [DOI] [PubMed] [Google Scholar]
- 100.Turkoz FP, Solak M, Petekkaya I, et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON. 2013;18:335–341. [PubMed] [Google Scholar]
- 101.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342–1349. [DOI] [PubMed] [Google Scholar]
- 103.Zhang S, Chung WC, Wu G, Egan SE, Miele L, Xu K. Manic fringe promotes a claudin-low breast cancer phenotype through notch-mediated PIK3CG induction. Cancer Res. 2015;75:1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DeSantis CE, Fedewa SA, Goding Sauer A, et al. Convergence of incidence rates between black and white women. CA Cancer J Clin. 2015;2016(66):31–42. [DOI] [PubMed] [Google Scholar]
- 105.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kohler BA, Sherman RL, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US Adults, 1999–2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 108.Carey LA, Perou CM, Livasy CA, et al. Breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. [DOI] [PubMed] [Google Scholar]
- 109.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. [DOI] [PubMed] [Google Scholar]
- 110.CGAN. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–428. [DOI] [PubMed] [Google Scholar]
- 112.Cozzo AJ, Sundaram S, Zattra O, et al. cMET inhibitor crizotinib impairs angiogenesis and reduces tumor burden in the C3(1)-Tag model of basal-like breast cancer. Springerplus. 2016;5:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qin Y, Sundaram S, Essaid L, et al. Weight loss reduces basal-like breast cancer through kinome reprogramming. Cancer Cell Int. 2016;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sundaram S, Freemerman AJ, Galanko JA, et al. Obesity-mediated regulation of HGF/c-Met is associated with reduced basal-like breast cancer latency in parous mice. PLoS ONE. 2014;9:e111394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sundaram S, Freemerman AJ, Johnson AR, et al. Role of HGF in obesity-associated tumorigenesis: C3(1)-TAg mice as a model for human basal-like breast cancer. Breast Cancer Res Treat. 2013;142:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sundaram S, Le TL, Essaid L, et al. Weight loss reversed obesity-induced HGF/c-met pathway and basal-like breast cancer progression. Front Oncol. 2014;4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. [DOI] [PubMed] [Google Scholar]
- 118.Christou NV, Lieberman M, Sampalis F, Sampalis JS. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4:691–695. [DOI] [PubMed] [Google Scholar]
- 119.Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. [DOI] [PubMed] [Google Scholar]
- 120.Moulin CM, Marguti I, Peron JP, Halpern A, Rizzo LV. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes Surg. 2011;21:112–118. [DOI] [PubMed] [Google Scholar]
- 121.Casagrande DS, Rosa DD, Umpierre D, Sarmento RA, Rodrigues CG, Schaan BD. Incidence of cancer following bariatric surgery: systematic review and meta-analysis. Obes Surg. 2014;24:1499–1509. [DOI] [PubMed] [Google Scholar]
- 122.Kim A, Scharf K, Senthil M, Solomon N, Garberoglio C, Lum SS. The prevalence of overweight and obesity in a breast clinic population: consideration for weight loss as a therapeutic intervention. Surg Obes Relat Dis. 2014;10:348–353. [DOI] [PubMed] [Google Scholar]
- 123.Anveden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hassinger TE, Mehaffey JH, Hawkins RB, et al. Overall and Estrogen Receptor-Positive Breast Cancer Incidences Are Decreased Following Bariatric Surgery. Obes Surg. 2019;29:776–781. [DOI] [PubMed] [Google Scholar]
- 125.Kauppila JH, Tao W, Santoni G, et al. Effects of obesity surgery on overall and disease-specific mortality in a 5-country, population-based study. Gastroenterology. 2019;157(1):119–127.e1. [DOI] [PubMed] [Google Scholar]
- 126.Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang K, Luo Y, Dai H, Deng Z. Effects of bariatric surgery on cancer risk: evidence from meta-analysis. Obes Surg. 2019. 10.1007/s11695-019-04368-4 [DOI] [PubMed] [Google Scholar]
- 128.Peltonen M, Taube M, Carlsson LM. Letter to the Editor: effects of bariatric surgery on cancer risk. Obes Surg. 2020. 10.1007/s11695-020-04481-9 [DOI] [PubMed] [Google Scholar]
- 129.Zhang S, Ikramuddin S, Beckwith HC, Sheka AC, Wirth KM, Blaes AH. The impact of bariatric surgery on breast cancer recurrence: case series and review of literature. Obes Surg. 2020;30:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seganfredo FB, Blume CA, Moehlecke M, et al. Weight-loss interventions and gut microbiota changes in overweight and obese patients: a systematic review. Obes Rev. 2017;18:832–851. [DOI] [PubMed] [Google Scholar]
- 131.Wiggins T, Antonowicz SS, Markar SR. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obes Surg. 2019;29:1031–1039. [DOI] [PubMed] [Google Scholar]
- 132.Taube M, Peltonen M, Sjoholm K, et al. Association of bariatric surgery with skin cancer incidence in adults with obesity. A nonrandomized controlled trial. JAMA Dermatol. 2020;156(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feigelson HS, Caan B, Weinmann S, et al. Bariatric surgery is associated with reduced risk of breast cancer in both premenopausal and postmenopausal women. Ann Surg. 2019. 10.1097/SLA.0000000000003331 [DOI] [PubMed] [Google Scholar]
- 134.Dunlap SM, Chiao LJ, Nogueira L, et al. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev Res (Phila). 2012;5:930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee E, McKean-Cowdin R, Ma H, et al. Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: results from a population-based study of young women. J Clin Oncol. 2011;29:4373–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31 10.1186/bcr2261. Epub 2009 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Usary J, Darr DB, Pfefferle AD, Perou CM. Overview of genetically engineered mouse models of distinct breast cancer subtypes. Curr Protoc Pharmacol. 2016;72:14.38.1–14.38.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci U S A. 1994;91:11236–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20(7):1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stead LA, Lash TL, Sobieraj JE, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–443. [DOI] [PubMed] [Google Scholar]
- 145.Bowers LW, Rossi EL, O’Flanagan CH, deGraffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol (Lausanne). 2015;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gatenby RAG, Brown RJ, Joel S. Of cancer and cave fish. Nat Rev Cancer. 2011;11:237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hanahan D, Weinberg RA. Hallmarks of Cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 151.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15:846–847. [DOI] [PubMed] [Google Scholar]
- 153.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila). 2011;4:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Subbaramaiah K, Howe LR, Bhardwaj P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila). 2011;4:329–346. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 157.Johnson AR, Justin Milner J, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sampey BP, Freemerman AJ, Zhang J, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS ONE. 2012;7:e38812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sampey BP, Vanhoose AM, Winfield HM, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring). 2011;19:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun X, Casbas-Hernandez P, Bigelow C, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johnson AR, Wilkerson MD, Sampey BP, Troester MA, Hayes DN, Makowski L. Cafeteria diet-induced obesity results in increased oxidative damage in white adipose. Biochem Biophys Res Commun. 2016;473(2):545–550. 10.1016/j.bbrc.2016.03.113. Epub 2016 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johnson AR, Qin Y, Cozzo AJ, et al. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol Metab. 2016;5:506–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Subbaramaiah K, Morris PG, Zhou XK, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 164.Shively CA, Register TC, Appt SE, et al. Consumption of mediterranean versus western diet leads to distinct mammary gland microbiome populations. Cell Rep. 2018;25(47–56):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cozzo AJ, Fuller AM, Makowski L. Contribution of adipose tissue to development of cancer. Compr Physiol. 2017;8:237–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fernandez MF, Reina-Perez I, Astorga JM, Rodriguez-Carrillo A, Plaza-Diaz J, Fontana L. Breast cancer and its relationship with the microbiota. Int J Environ Res Public Health. 2018;15:1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Urbaniak C, Cummins J, Brackstone M, et al. Microbiota of human breast tissue. Appl Environ Microbiol. 2014;80:3007–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.de Moreno de LeBlanc A, Matar C, LeBlanc N, Perdigón G. Effects of milk fermented by Lactobacillus helveticus R389 on a murine breast cancer model. Breast Cancer Res. 2005;7:R477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jimenez E, Fernandez L, Maldonado A, et al. Oral administration of Lactobacillus strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl Environ Microbiol. 2008;74:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fernandez L, Cardenas N, Arroyo R, et al. Prevention of infectious mastitis by oral administration of Lactobacillus salivarius PS2 during late pregnancy. Clin Infect Dis. 2016;62:568–573. [DOI] [PubMed] [Google Scholar]
- 172.Hieken TJ, Chen J, Hoskin TL, et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. 2016;6:30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82:5039–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang H, Altemus J, Niazi F, et al. Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget. 2017;8:88122–88138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yazdi HR, Movafagh A, Fallah F, et al. Evaluation of Methylobacterium radiotolerance and Sphyngomonas yanoikoaie in sentinel lymph nodes of breast cancer cases. Asian Pac J Cancer Prev. 2016;17:279–285. [DOI] [PubMed] [Google Scholar]
- 176.Banerjee S, Tian T, Wei Z, et al. Distinct microbial signatures associated with different breast cancer types. Front Microbiol. 2018;9:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maroof H, Hassan ZM, Mobarez AM, Mohamadabadi MA. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J Clin Immunol. 2012;32:1353–1359. [DOI] [PubMed] [Google Scholar]
- 179.Aragon F, Carino S, Perdigon G, de Moreno de LeBlanc A. The administration of milk fermented by the probiotic Lactobacillus casei CRL 431 exerts an immunomodulatory effect against a breast tumour in a mouse model. Immunobiology. 2014;219:457–464. [DOI] [PubMed] [Google Scholar]
- 180.Thompson KJ, Ingle JN, Tang X, et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS ONE. 2017;12:e0188873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yao J, Rock CO. How bacterial pathogens eat host lipids: implications for the development of fatty acid synthesis therapeutics. J Biol Chem. 2015;290:5940–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. [DOI] [PubMed] [Google Scholar]
- 184.Javitt NB, Budai K, Miller DG, Cahan AC, Raju U, Levitz M. Breast-gut connection: origin of chenodeoxycholic acid in breast cyst fluid. Lancet. 1994;343:633–635. [DOI] [PubMed] [Google Scholar]
- 185.Raju U, Levitz M, Javitt NB. Bile acids in human breast cyst fluid: the identification of lithocholic acid. J Clin Endocrinol Metab. 1990;70:1030–1034. [DOI] [PubMed] [Google Scholar]
- 186.Miko E, Vida A, Kovacs T, et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim Biophys Acta. 2018;1859:958–974. [DOI] [PubMed] [Google Scholar]
- 187.Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66:10120–10126. [DOI] [PubMed] [Google Scholar]
- 188.Giordano C, Barone I, Vircillo V, et al. Activated FXR inhibits leptin signaling and counteracts tumor-promoting activities of cancer-associated fibroblasts in breast malignancy. Sci Rep. 2016;6:21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Giordano C, Catalano S, Panza S, et al. Farnesoid X receptor inhibits tamoxifen-resistant MCF-7 breast cancer cell growth through downregulation of HER2 expression. Oncogene. 2011;30:4129–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Barone I, Vircillo V, Giordano C, et al. Activation of Farnesoid X Receptor impairs the tumor-promoting function of breast cancer-associated fibroblasts. Cancer Lett. 2018;437:89–99. [DOI] [PubMed] [Google Scholar]
- 191.Giaginis C, Karandrea D, Alexandrou P, et al. High Farnesoid X Receptor (FXR) expression is a strong and independent prognosticator in invasive breast carcinoma. Neoplasma. 2017;64:633–639. [DOI] [PubMed] [Google Scholar]
- 192.Ghafouri-Fard S, Oskooei VK, Azari I, Taheri M. Suppressor of cytokine signaling (SOCS) genes are downregulated in breast cancer. World J Surg Oncol. 2018;16:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moon Y, Choi SM, Chang S, et al. Chenodeoxycholic acid reduces hypoxia inducible factor-1alpha protein and its target genes. PLoS ONE. 2015;10:e0130911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Phelan JP, Reen FJ, Caparros-Martin JA, O’Connor R, O’Gara F. Rethinking the bile acid/gut microbiome axis in cancer. Oncotarget. 2017;8:115736–115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Phelan JP, Reen FJ, Dunphy N, O’Connor R, O’Gara F. Bile acids destabilise HIF-1alpha and promote anti-tumour phenotypes in cancer cell models. BMC Cancer. 2016;16:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu X, Chen B, You W, Xue S, Qin H, Jiang H. The membrane bile acid receptor TGR5 drives cell growth and migration via activation of the JAK2/STAT3 signaling pathway in non-small cell lung cancer. Cancer Lett. 2018;412:194–207. [DOI] [PubMed] [Google Scholar]
- 197.Luu TH, Michel C, Bard JM, Dravet F, Nazih H., Bobin-Dubigeon C. Intestinal proportion of blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr Cancer. 2017;69:267–275. [DOI] [PubMed] [Google Scholar]
- 198.Singh B, Cook KR, Vincent L, et al. Cyclooxygenase-2 induces genomic instability, BCL2 expression, doxorubicin resistance, and altered cancer-initiating cell phenotype in MCF7 breast cancer cells. J Surg Res. 2008;147:240–246. [DOI] [PubMed] [Google Scholar]
- 199.Singh B, Cook KR, Vincent L, Hall CS, Martin C, Lucci A. Role of COX-2 in tumorospheres derived from a breast cancer cell line. J Surg Res. 2011;168:e39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dawood S, Rugo HS. Targeting the host immune system: PD-1 and PD-L1 antibodies and breast cancer. Curr Opin Support Palliat Care. 2016;10:336–342. [DOI] [PubMed] [Google Scholar]
- 201.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015;125:3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Josefsson A, Nedrow JR, Park S, et al. Imaging, biodistribution, and dosimetry of radionuclide-labeled PD-L1 antibody in an immunocompetent mouse model of breast cancer. Cancer Res. 2016;76:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Beatson R, Tajadura-Ortega V, Achkova D, et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol. 2016;17:1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Del Corno M, D’Archivio M, Conti L, et al. Visceral fat adipocytes from obese and colorectal cancer subjects exhibit distinct secretory and omega6 polyunsaturated fatty acid profiles and deliver immunosuppressive signals to innate immunity cells. Oncotarget. 2016;7:63093–63105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu B, Sun X, Gupta HB, et al. Adipose PD-L1 modulates PD-1/PD-L1 checkpoint blockade immunotherapy efficacy in breast cancer. Oncoimmunology. 2018;7:e1500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kumar S, Wilkes DW, Samuel N, et al. DeltaNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J Clin Invest. 2018;128:5095–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Papaspyridonos M, Matei I, Huang Y, et al. Id1 suppresses anti-tumour immune responses and promotes tumour progression by impairing myeloid cell maturation. Nat Commun. 2015;6:6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Abba MC, Gong T, Lu Y, et al. A Molecular portrait of high-grade ductal carcinoma in situ. Cancer Res. 2015;75:3980–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li Z, Dong P, Ren M, et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer. 2016;7:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Atlas CG N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Guo L, Li W, Zhu X, et al. PD-L1 expression and CD274 gene alteration in triple-negative breast cancer: implication for prognostic biomarker. Springerplus. 2016;5:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488–1493. [DOI] [PubMed] [Google Scholar]
- 215.Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weber J, Gibney G, Kudchadkar R, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res. 2016;4:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Meyer C, Cagnon L, Costa-Nunes CM, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. [DOI] [PubMed] [Google Scholar]
- 222.Weber R, Fleming V, Hu X, et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol. 2018;9:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 224.Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:405–411. [DOI] [PubMed] [Google Scholar]
- 225.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397–404. [DOI] [PubMed] [Google Scholar]
- 226.Schmid PYHP, Muñoz-Couselo E, Kim S-B, et al. Pembrolizumab (pembro) + chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): preliminary results from KEYNOTE-173. J Clin Oncol. 2017;35:556–556. [Google Scholar]
- 227.Nanda RMCL, Yau C, Asare S, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J Clin Oncol. 2017;35:506–506.28029304 [Google Scholar]
- 228.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. [DOI] [PubMed] [Google Scholar]
- 229.Canter RJ, Le CT, Beerthuijzen JMT, Murphy WJ. Obesity as an immune-modifying factor in cancer immunotherapy. J Leukoc Biol. 2018;104:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rassy EE, Ghosn M, Rassy NA, Assi T, Robert C. Do immune checkpoint inhibitors perform identically in patients with weight extremes? Immunotherapy. 2018;10:733–736. [DOI] [PubMed] [Google Scholar]
- 231.Yang S, Zhang Q, Liu S, Wang AR, You Z. PD-1, PD-L1 and PD-L2 expression in mouse prostate cancer. Am J Clin Exp Urol. 2016;4:1–8. [PMC free article] [PubMed] [Google Scholar]
- 232.Shirakawa K, Shinmura K, Endo J, et al. Abstract 11105: obesity accelerates T cell senescence in visceral adipose tissue. Circulation. 2015;132:A11105–A11105. [Google Scholar]
- 233.Lim SO, Li CW, Xia W, et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Murphy WJ, Longo DL. The surprisingly positive association between obesity and cancer immunotherapy efficacy. JAMA. 2019;321:1247–1248. [DOI] [PubMed] [Google Scholar]
- 235.Richtig G, Hoeller C, Wolf M, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi-centre study. PLoS ONE. 2018;13:e0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang Z, Monjazeb AM, Murphy WJ. The complicated effects of obesity on cancer and immunotherapy. Immunotherapy. 2019;11:11–14. [DOI] [PubMed] [Google Scholar]
- 240.Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019: e195241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gerriets VA, Danzaki K, Kishton RJ, et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol. 2016;46:1970–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol. 2014;5:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sanchez-Jimenez F, Perez-Perez A, de la Cruz-Merino L, Sanchez-Margalet V. Obesity and breast cancer: role of leptin. Front Oncol. 2019;9:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fang SY, Wang YL, Dang YF, et al. Association between body mass index, C-reactive protein levels, and melanoma patient outcomes. J Invest Dermatol. 2017;137:1792–1795. [DOI] [PubMed] [Google Scholar]
- 246.Xu H, Cao D, He A, Ge W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: A pooled analysis of 4090 cancer patients. Int Immunopharmacol. 2019;74:105745. [DOI] [PubMed] [Google Scholar]
- 247.Naik A, Monjazeb AM, Decock J. The obesity paradox in cancer, tumor immunology, and immunotherapy: potential therapeutic implications in triple negative breast cancer. Front Immunol. 2019;10:1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Langan EA, Gratz V, Billmann F, Zillikens D, Terheyden P. Does the gastrointestinal microbiome contribute to the ‘obesity paradox’ in melanoma survival? Br J Dermatol. 2018;179:225–226. [DOI] [PubMed] [Google Scholar]
- 249.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Frankel AE, Coughlin LA, Kim J, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Delaune V, Orci LA, Lacotte S, et al. Fecal microbiota transplantation: a promising strategy in preventing the progression of non-alcoholic steatohepatitis and improving the anti-cancer immune response. Expert Opin Biol Ther. 2018;18:1061–1071. [DOI] [PubMed] [Google Scholar]
- 256.Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178(4):795–806.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cozzo AJ, Fuller AJ, Makowski L. Contribution of adipose tissue to development of cancer. Compr Physiol. 2017;8:237–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gordon S, Pluddemann A, Martinez EF. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262:36–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kratz M, Coats BR, Hisert KB, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Huang SC, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cader MZ, Boroviak K, Zhang Q, et al. C13orf31 (FAMIN) is a central regulator of immunometabolic function. Nat Immunol. 2016;17:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Olenchock BA, Rathmell JC, Vander Heiden MG. Biochemical underpinnings of immune cell metabolic phenotypes. Immunity. 2017;46:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Van den Bossche J, O’Neill LA, Menon D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017;38:395–406. [DOI] [PubMed] [Google Scholar]
- 268.Ryan DG, O’Neill LAJ. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. 2017;38:395–406. [DOI] [PubMed] [Google Scholar]
- 269.Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a pro-inflammatory phenotype. J Biol Chem. 2014;289:7884–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Newsholme P, Curi R, Gordon S, Newsholme EA. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986;239:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Salh B, Wagey R, Marotta A, Tao JS, Pelech S. Activation of phosphatidylinositol 3-kinase, protein kinase B, and p70 S6 kinases in lipopolysaccharide-stimulated Raw 264.7 cells: differential effects of rapamycin, Ly294002, and wortmannin on nitric oxide production. J Immunol. 1998;161:6947–6954. [PubMed] [Google Scholar]
- 275.Spolarics Z, Wu JX. Tumor necrosis factor alpha augments the expression of glucose-6-phosphate dehydrogenase in rat hepatic endothelial and Kupffer cells. Life Sci. 1997;60:565–571. [DOI] [PubMed] [Google Scholar]
- 276.Aouadi M, Vangala P, Yawe JC, et al. Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. Am J Physiol Endocrinol Metab. 2014;307:E374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Van den Bossche J, Baardman J, Otto NA, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–696. [DOI] [PubMed] [Google Scholar]
- 278.Huang SC, Smith AM, Everts B, et al. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45:817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tan Z, Xie N, Cui H, et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol. 2015;194:6082–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Covarrubias AJ, Aksoylar HI, Yu J, et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. eLife. 2016;5:e11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Langston PK, Shibata M, Horng T. Metabolism supports macrophage activation. Front Immunol. 2017;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Geeraerts X, Bolli E, Fendt SM, Van Ginderachter JA. Macrophage metabolism as therapeutic target for cancer, atherosclerosis, and obesity. Front Immunol. 2017;8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Freemerman AJ, Zhao L, Pingili AK, et al. Myeloid Slc2a1-deficient murine model revealed macrophage activation and metabolic phenotype are fueled by GLUT1. J Immunol. 2019;202:1265–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhao L, Cozzo AJ, Johnson AR, et al. Lack of myeloid Fatp1 increases atherosclerotic lesion size in Ldlr(−/−) mice. Atherosclerosis. 2017;266:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Iyengar NM, Brown KA, Zhou XK, et al. Adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev Res (Phila). 2017;10:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Greenlee H, Shi Z, Hibshoosh H, et al. Obesity-associated breast inflammation among hispanic/latina breast cancer patients. Cancer Prev Res (Phila). 2019;12:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Iyengar NM, Zhou XK, Gucalp A, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2016;22:2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. [DOI] [PubMed] [Google Scholar]
- 289.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. [DOI] [PubMed] [Google Scholar]
- 290.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. [DOI] [PubMed] [Google Scholar]
- 291.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522–2525. [DOI] [PubMed] [Google Scholar]
- 292.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. [DOI] [PubMed] [Google Scholar]
- 295.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. [DOI] [PubMed] [Google Scholar]
- 297.Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Krijgsman D, Hokland M, Kuppen PJK. The role of natural killer T cells in cancer - a phenotypical and functional approach. Front Immunol. 2018;9:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Arendt LM, McCready J, Keller PJ, et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73:6080–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Williams CB, Yeh ES. Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lin EY, Li JF, Gnatovskiy L, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. [DOI] [PubMed] [Google Scholar]
- 303.Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Can Res. 2004;64:7022–7029. [DOI] [PubMed] [Google Scholar]
- 304.Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Can Res. 1996;56: 4625–4629. [PubMed] [Google Scholar]
- 306.Campbell MJ, Tonlaar NY, Garwood ER, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. [DOI] [PubMed] [Google Scholar]
- 309.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Shree T, Olson OC, Elie BT, et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Escamilla J, Schokrpur S, Liu C, et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 2015;75:950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Quail D, Joyce J. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376. [PMC free article] [PubMed] [Google Scholar]
- 315.Hogenauer K, Arista L, Schmiedeberg N, et al. G-protein-coupled bile acid receptor 1 (GPBAR1, TGR5) agonists reduce the production of proinflammatory cytokines and stabilize the alternative macrophage phenotype. J Med Chem. 2014;57:10343–10354. [DOI] [PubMed] [Google Scholar]
- 316.Schote AB, Turner JD, Schiltz J, Muller CP. Nuclear receptors in human immune cells: expression and correlations. Mol Immunol. 2007;44:1436–1445. [DOI] [PubMed] [Google Scholar]
- 317.Calmus Y, Poupon R. Shaping macrophages function and innate immunity by bile acids: mechanisms and implication in cholestatic liver diseases. Clin Res Hepatol Gastroenterol. 2014;38:550–556. [DOI] [PubMed] [Google Scholar]
- 318.Haselow K, Bode JG, Wammers M, et al. Bile acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and reduce proinflammatory capability of human macrophages. J Leukoc Biol. 2013;94:1253–1264. [DOI] [PubMed] [Google Scholar]
- 319.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. [DOI] [PubMed] [Google Scholar]
- 320.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology. 2011;54:1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ichikawa R, Takayama T, Yoneno K, et al. Bile acids induce monocyte differentiation toward interleukin-12 hypo-producing dendritic cells via a TGR5-dependent pathway. Immunology. 2012;136:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Keitel V, Kubitz R, Haussinger D. Endocrine and paracrine role of bile acids. World J Gastroenterol. 2008;14:5620–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.McMahan RH, Wang XX, Cheng LL, et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J Biol Chem. 2013;288:11761–11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. [DOI] [PubMed] [Google Scholar]
- 326.Renga B, Migliorati M, Mencarelli A, Fiorucci S. Reciprocal regulation of the bile acid-activated receptor FXR and the interferon-gamma-STAT-1 pathway in macrophages. Biochim Biophys Acta. 2009;1792:564–573. [DOI] [PubMed] [Google Scholar]
- 327.Horng T, Hotamisligil GS. Linking the inflammasome to obesity-related disease. Nat Med. 2011;17:164–165. [DOI] [PubMed] [Google Scholar]
- 328.Guo C, Xie S, Chi Z, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:802–816. [DOI] [PubMed] [Google Scholar]
- 329.Gong Z, Zhou J, Zhao S, et al. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget. 2016;7:83951–83963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Hao H, Cao L, Jiang C, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 2017;25:856–867.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schubert K, Olde Damink SWM, von Bergen M, Schaap FG. Interactions between bile salts, gut microbiota, and hepatic innate immunity. Immunol Rev. 2017;279:23–35. [DOI] [PubMed] [Google Scholar]
- 332.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. [DOI] [PubMed] [Google Scholar]
- 333.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Du X, Chapman NM, Chi H. Emerging roles of cellular metabolism in regulating dendritic cell subsets and function. Front Cell Deve Biol. 2018;6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Massafra V, Ijssennagger N, Plantinga M, et al. Splenic dendritic cell involvement in FXR-mediated amelioration of DSS colitis. Biochem Biophys Acta. 2016;1862:166–173. [DOI] [PubMed] [Google Scholar]
- 336.Gadaleta RM, van Erpecum KJ, Oldenburg B, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. [DOI] [PubMed] [Google Scholar]
- 337.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Investig. 2015;125:3365–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Investig. 2015;125:3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zhang H, Liu Y, Bian Z, et al. The critical role of myeloid-derived suppressor cells and FXR activation in immune-mediated liver injury. J Autoimmun. 2014;53:55–66. [DOI] [PubMed] [Google Scholar]
- 341.Pols TWH, Puchner T, Korkmaz HI, Vos M, Soeters MR, de Vries CJM. Lithocholic acid controls adaptive immune responses by inhibition of Th1 activation through the Vitamin D receptor. PLoS ONE. 2017;12:e0176715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ho PP, Steinman L. Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2016;113:1600–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hang S, Paik D, Yao L, et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Song X, Sun X, Oh SF, et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature. 2020;577:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Prestin K, Olbert M, Hussner J, Völzke H, Meyer zu Schwabedissen HE. Functional assessment of genetic variants located in the promoter of SHP1 (NR0B2). Pharmacogenet Genomics. 2017;27:410–415. [DOI] [PubMed] [Google Scholar]
- 346.Shahoei SH, Kim YC, Cler SJ, et al. Small heterodimer partner regulates dichotomous T cell expansion by macrophages. Endocrinology. 2019;160:1573–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. [DOI] [PubMed] [Google Scholar]
- 348.Tang XZ, Jo J, Tan AT, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190:3142–3152. [DOI] [PubMed] [Google Scholar]
- 349.Mendler A, Pierzchalski A, Bauer M, et al. MAIT cell activation in adolescents is impacted by bile acid concentrations and body weight. Clin Exp Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Carolan E, Tobin LM, Mangan BA, et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol. 2015;194:5775–5780. [DOI] [PubMed] [Google Scholar]
- 351.Yan J, Allen S, McDonald E, et al. MAIT cells promote tumor initiation, growth, and metastases via tumor MR1. Cancer Discov. 2020;10:124–141. [DOI] [PubMed] [Google Scholar]
- 352.Li B, Severson E, Pignon JC, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174. [DOI] [PMC free article] [PubMed] [Google Scholar]


