Abstract
Superchilling entails lowering the fish temperature to between the initial freezing point of the fish and about 1–2°C lower. The temperature of superchilled fresh fishery products (SFFP) in boxes without ice was compared to that of products subject to the currently authorised practice in boxes with ice (CFFP) under the same conditions of on‐land storage and/or transport. A heat transfer model was developed and made available as a tool to identify under which initial configurations of SFFP the fish temperature, at any time of storage/transport, is lower or equal to CFFP. A minimum degree of superchilling, corresponding to an ice fraction in the fish matrix of SFFP equal or higher than the proportion of ice added per mass of fish in CFFP, will ensure with 99–100% certainty (almost certain) that the fish temperature of SFFP and the consequent increase of relevant hazards will be lower or equal to that of CFFP. In practice, the degree of superchilling can be estimated using the fish temperature after superchilling and its initial freezing point, which are subject to uncertainties. The tool can be used as part of ‘safety‐by‐design’ approach, with the reliability of its outcome being dependent on the accuracy of the input data. An evaluation of methods capable of detecting whether a previously frozen fish is commercially presented as ‘superchilled’ was carried out based on, amongst others, their applicability for different fish species, ability to differentiate fresh fish from fish frozen at different temperatures, use as a stand‐alone method, ease of use and classification performance. The methods that were considered ‘fit for purpose’ are Hydroxyacyl‐coenzyme A dehydrogenase (HADH) test, α‐glucosidase test, histology, ultraviolet–visible–near–infrared (UV‐VIS/NIR) spectroscopy and hyperspectral imaging. These methods would benefit from standardisation, including the establishment of threshold values or classification algorithms to provide a practical routine test.
Keywords: superchilling, transport, storage, fresh fishery products, biological hazards, freezing, HADH enzymatic test
Summary
Following a request from the European Commission, the Scientific Panel on Biological Hazards (BIOHAZ) was asked to provide a scientific opinion on the use of the so‐called ‘superchilling’ technique for transporting fresh fishery products from the first on‐land establishment onwards.
In Term of Reference 1 (ToR1), EFSA was requested to compare the impact that the use of ‘superchilling’ could have on the survival and growth of biological hazards when compared with the current authorised practices (i.e. a temperature approaching that of melting ice). ToR2 was to evaluate the use of the HADH (Hydroxyacyl‐coenzyme A dehydrogenase) enzymatic test, or if possible, any other test that could be used by the competent authorities, to differentiate ‘superchilling’ from freezing (i.e. temperature of not more than –18°C).
The assessment focuses on domestic trade and import into the EU/EEA regarding the transport and storage of unpackaged, wrapped, prepared fresh fishery products (FFP) from the first on‐land establishment onwards, using the authorised practice ‘in boxes with ice’ (reference condition referred to as conventional FFP or ‘CFFP’), in comparison with FFP that, after being superchilled in the first on‐land establishment, are transported in ‘boxes without ice’ (alternative condition referred to as ‘SFFP’). Both CFFP and SFFP are considered until they are marketed or processed. The boxes used to store/transport CFFP and SFFP are generally made of expanded polystyrene (EPS), with other types of containers being outside the scope of the current mandate. Fish are expected to be kept in the boxes for a maximum duration of 5 days. The definition considered in the assessment was ‘superchilling includes temperatures between the initial freezing point of the fish (always below the temperature of melting ice, i.e. < 0°C) to about 1–2°C lower’.
Regarding ToR1, the requestor clarified that the purpose is to know if the SFFP stored/transported in boxes without ice is at least as safe (from a microbiological food safety perspective) as CFFP stored/transported in boxes with ice. In this context, it was agreed to follow a step‐wise approach: firstly, focusing the assessment on the reasonably foreseeable fish temperature during the storage/transport of SFFP in boxes without ice compared to CFFP in boxes with ice. Secondly, and only if higher temperatures can occur in SFFP compared to CFFP, to assess the impact on the growth of biological hazards. The assessment questions (AQ) were as follows.
AQ1a: Which SFFP configurations (i.e. initial degree of superchilling) ensure that the fish temperature, at any time of the storage/transport, is lower or equal to CFFP when exposed to the same conditions of on‐land storage and/or transport?
AQ1b: If the SFFP conditions allow fish temperatures to be higher than in CFFP during the on‐land storage and/or transport, what is the potential increase of relevant biological hazards (i.e. pathogen log 10 increase or change in histamine concentration) for on‐land SFFP compared to CFFP upon exposure to the same conditions of storage and/or transport?
A heat transfer modelling approach was developed to identify under which initial configurations the fish temperature of SFFP, at any time of the storage/transport, is lower or equal to CFPP, when exposed to the same conditions of on‐land storage and/or transport. This approach is feasible since the boxes used to transport the fish are the same (e.g. EPS boxes).
For simulation purposes, three types of fish were considered: salmon (as an example of fish with a high fat content), cod (as a lean fish) and Nile Perch (as a temperate freshwater fish). It was concluded that the capacity of the CFFP and SFFP to maintain the temperature when exposed to the same conditions of storage and/or transport depends on their capacity to absorb heat, which is determined by their initial configurations. These are:
for CFFP: the initial fish and ice temperatures and the proportion of ice:fish in the box; and
for SFFP: the degree of superchilling, i.e. the ice fraction in the fish matrix, which depends on the fish temperature after superchilling and the initial freezing point of the fish.
The identified assumptions and other sources of uncertainty were listed and, in some cases, the impact was quantified. The experts elicited the overall uncertainty associated with the final outcome of the AQs through expert group judgement taking into account the quantified and non‐quantified sources of uncertainty.
A degree of superchilling of SFFP equal to or higher than the proportion of ice:fish in the EPS box of CFFP properly adjusted to the envisaged conditions of transport and storage will ensure with 99–100% certainty (almost certain) that the fish temperature of SFFP and the consequent increase of the relevant hazards, including histamine will be lower or equal than those of CFFP (close to 0°C, i.e. the temperature of melting ice) at any time point during storage/transport.
When the degree of superchilling is unknown, which is normally the case, it can be estimated using the fish temperature after superchilling and its initial freezing point. These two parameters are subjected to uncertainties: (a) the fish temperature after superchilling is influenced by the application of the superchilling technology and the temperature measurement procedure; (b) the initial freezing point can be derived from the fish proximate composition, which is influenced by fish species and other factors such as seasonal variation and temperature of the catching waters.
An MS Excel spreadsheet tool, named the heat transfer model tool for the heat absorption capacity of superchilled fresh fishery products (HTM‐SFFP Tool), based on the developed model was built and made available through the Knowledge Junction under https://doi.org/10.5281/zenodo.4304283 that can be used as part of the ‘safety‐by‐design’ approach. More specifically, it allows the identification of scenarios of initial configuration under which the SFFP have an equivalent or higher capacity to absorb heat than CFFP. The reliability of the outcome provided by the tool depends on the accuracy of the input data introduced by the user.
Regarding ToR2, it was agreed to focus the assessment on analytical and instrumental methods, including the HADH enzymatic test and/or other methods, providing effective detection of the fraudulent use of previously frozen fish to market it as a superchilled product. The AQ was as follows:
AQ2: Which methods (including the HADH enzymatic test) are capable of detecting whether a previously frozen fish is commercially presented as ‘superchilled’?
A literature review was carried out to assess potential methods capable of differentiating between fresh/superchilled and frozen fish. The methods were listed, summarising key features, advantages and disadvantages for their use to assess whether a fish that has been previously frozen has been commercially presented as ‘superchilled’. An appraisal of the relevance of each study was carried out based on predefined criteria regarding the appropriateness to answer the ToR. Finally, an evaluation was carried out considering their applicability for different fish species; ability to differentiate fresh fish from fish frozen at various temperatures; use as a stand‐alone method; ease of use; classification performance in discriminating as either frozen/not frozen; evidence that superchilled fish will behave like fresh fish for the given method; and strength of evidence.
Following five out of 28 methods were considered ‘fit for purpose’:
HADH test proved suitable for almost all tested fish species. Superchilled Nile perch fillet has been proven to be distinguishable from deeply frozen fish based on the profile of the measured absorbance during the enzymatic test. The threshold value is generally species‐specific.
α‐glucosidase test proved suitable for almost all tested fish species but post‐harvest quality changes may interfere with the results. The threshold value is species‐specific.
histology proved suitable for all tested species, but it is laborious and requires specialised skills.
UV‐VIS/NIR spectroscopy and hyperspectral imaging proved suitable for all tested species and can be applied on‐line with handheld equipment or equipment mounted on the processing line. The threshold value may be species or group of species‐specific.
Some methods have the potential to be used, but the evidence is limited, or they currently require advanced instrumental infrastructure and user expertise, making them not suitable for routine applications, while for some methods, there is no evidence that they are suitable or there is evidence that they cannot be applied to all types of SFFP.
For the five ‘fit for purpose’ methods, based on the ice crystal formation and the limited structural changes, SFFP is considered to behave, under analytical procedures, with 90–95% certainty (very likely) like a fresh fishery product, rather than a frozen one.
Recommendations were formulated related to the need for reliable methods and data generation to accurately determine the initial freezing point (T*) and the ice fraction (Xice) of fish after superchilling and standardisation of methods aiming to differentiate fresh (never frozen) chilled and superchilled fish from frozen/thawed fish, including the establishment of threshold values or classification algorithms. The development of guidelines was recommended, covering key aspects of the superchilling technology, as well as procedures for the validation and verification of the degree of superchilling, thereby ensuring the safety of superchilled fish during the storage and transport on‐land.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Annex III to Regulation (EC) No 853/2004,1 Section VIII, Chapter VIII, point 1 reads as follows:
“1. During transport, fishery products must be maintained at the required temperature. In particular:
-
a)
fresh fishery products, thawed unprocessed fishery products, (…), must be maintained at a temperature approaching that of melting ice;
-
b)
frozen fishery products, (…), must be maintained during transport at an even temperature of not more than –18°C in all parts of the product (…)”.
Superchilling2 of fish is a method in which the temperature of fish is uniformly reduced to a point slightly below that obtained in melting ice. The method is also referred to as partial freezing, deep chilling or “sub‐chilling”. In current practice superchilling means reducing the fish temperature until 5–30% of the total water content of the fish is frozen, and keeping it at that temperature. Bacterial and enzymatic activity, and hence spoilage, are slowed somewhat at this lower temperature, so that the fish remains edible longer.
The slow freezing of fish flesh is undesirable because large ice crystals form which can damage the structure of the muscle, resulting in a thawed product that is less attractive and less palatable than the original fresh fish. The slower the water freezes, the larger the ice crystals and the greater the damage. For quality purposes, close control of the superchilling temperature is essential if the damaging effects of slow freezing are to be avoided.
According to a study3 coordinated by the Dutch Fish Importers Association using Nile perch (Lates niloticus) fillet (attached) “superchilling is a good alternative to maintain the cold chain of fresh fisheries products. Moreover, literature studies and microbiological analyses show that superchilling suppresses bacterial activity, maintains food freshness and preserves nutritional quality.”
The above‐referred study also included a HADH (Hydroxyacyl‐coenzyme A dehydrogenase) enzymatic test, carried out by a European Referential Laboratory, to measure the mitochondrial enzyme HADH in the Nile perch fillets. It showed that HADH could be a good indicator to assess whether the Nile perch fillets used had previously been deep frozen.
EFSA is asked to provide a scientific opinion on the use of the so called “superchilling technique” for transporting fresh fishery products from the first on‐land establishment onwards. In particular, EFSA is requested:
Terms of Reference (ToR) 1: To compare the impact that the use of ‘superchilling’ could have on the survival and growth of biological hazards when compared with the current authorised practices (i.e. temperature approaching that of melting ice);
ToR 2: To evaluate the use of the HADH (Hydroxyacyl‐coenzyme A dehydrogenase) enzymatic test, or if possible, any other test that could be used by the competent authorities, to differentiate ‘superchilling’ from freezing (i.e. temperature of not more than –18°C).
1.2. Interpretation of the Terms of Reference
The term ‘fishery products’ as defined by Regulation (EC) No 853/2004,1 Annex I, comprises all seawater and freshwater animals (except for live bivalve molluscs, live echinoderms, live tunicates and live marine gastropods, and all mammals, reptiles and frogs) whether wild or farmed and including all edible forms, parts and products of such animals. In Annex I, ‘fresh fishery products’ (FFP) are defined as unprocessed fishery products, whether whole or prepared, including products packaged under vacuum or in a modified atmosphere, that have not undergone any treatment to ensure preservation other than chilling. However, the current mandate is restricted to unpackaged FFP, with packaged FFP being beyond the scope of this assessment. It was clarified that the specific FFP to which the ToRs apply include ‘prepared fishery products’, in particular unprocessed fishery products that have undergone an operation affecting their anatomical wholeness, such as gutting, heading and filleting and are wrapped after this preparation. The FFP considered in the assessment are derived from the marine and land‐based environment, either wild or farmed.
The assessment focuses on domestic trade and import into the EU/EEA regarding the transport and storage of unpackaged, wrapped, prepared FFP from the first on‐land establishment onwards using the authorised practice ‘in boxes with ice’ (as the reference condition, referred to as conventional FFP or ‘CFFP’ throughout the rest of the document), in comparison with FFP that after being superchilled (referred to as ‘SFFP’ throughout the rest of the document) in the first on‐land establishment are transported in ‘boxes without ice’ (as alternative condition). Both CFFP and SFFP are considered until they are marketed or processed. The simple storage of FFP at superchilling temperatures without a preceding superchilling process is not considered in the present mandate (see Section 1.3.1.4 for further details about the superchilling technology for FFP).
The CFFP fillets are usually wrapped, with at least one layer of plastic being placed around the fresh fish fillets and either ice or ice/gel packs placed on the top. The fillets of SFFP are wrapped together (see Figure 1), while the headed and gutted SFFP are individually wrapped. The boxes used to store/transport CFFP and SFFP are generally made of expanded polystyrene (EPS), with other types of containers being outside the scope of the current mandate as agreed with the requestor. Fish are envisaged to be kept in the boxes for a maximum duration of 5 days.
Figure 1.
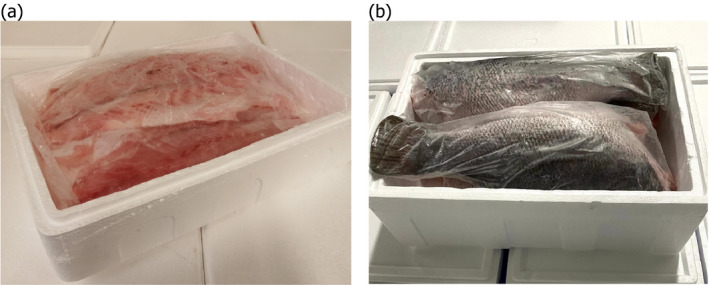
Superchilled Nile perch fillet (a) and headed and gutted Nile perch (b) after arrival at the European establishment (Source of picture: FIORITAL (Solimeo, 2020c))
It was agreed with the requestor to assume that the ice used to fill the boxes is made from potable or clean water (fresh water), according to their definitions in Regulation (EC) 852/20044 and Regulation (EC) No 853/2004,1 and thus should not be a source of microbiological contamination of the fish. Although ice made from salted or sea water is also allowed according to the current regulation, this is a practice used on‐board but not on‐land, and therefore, it is not to be considered in the present mandate. The growth and survival of spoilage microorganisms as well as quality traits of fish (e.g. sensory, commercial value etc.) are also beyond the scope of this mandate.
Regarding ToR1, the requestor clarified that the purpose is to know if the SFFP stored/transported in boxes without ice is at least as safe (from a microbiological food safety perspective) as CFFP stored/transported in boxes with ice. Temperature is known to be the major factor determining microbial growth in fresh fishery products during storage and transport, from catching to the end user (i.e. a processing plant or the consumer). The maintenance of the cold chain is necessary to control the growth of biological hazards. As detailed in the previous opinion (EFSA BIOHAZ Panel, 2020), the impact of survival of the biological hazards in chilled FFP is considered of no relevance from the safety point of view, either because it is negligible (equivalent to no change) or because the reduction of the levels of the biological hazards is not temperature dependent for the time span of the assessment. In this context, it was agreed with the requestor to follow a step‐wise approach: first focus the assessment on the reasonably foreseeable fish temperature during the storage/transport of SFFP in boxes without ice compared to CFFP in boxes with ice. Secondly, and only if higher temperatures can occur in SFFP compared to CFFP, to assess the impact on the growth of biological hazards.
The term ‘impact’ of a given condition on the growth of a biological hazard (including accumulation of histamine) was interpreted as the change in the concentration of the hazard, e.g. microorganism log10 increase or histamine concentration increase (EFSA BIOHAZ Panel, 2020). The requestor clarified that the associated public health impact was not to be assessed.
Regarding ToR2, the European Commission clarified that the differentiation of the use of fresh fish (not frozen) and superchilled fish from non‐conforming freezing of fish has a public health implication and is not just a quality issue. The legal basis used is Regulations (EC) No 853/20041 and Commission Delegated Regulation 2019/6255 and Commission Implementing Regulation (EU) 2019/6276 that cover public health requirements for food of animal origin. Those regulations consider that freezing at a temperature higher than –18°C could have public health implications. This is the reason why the distinction between the use of superchilling and illegal/fraudulent freezing is fundamental, particularly at the border control points. It was agreed to focus the assessment on analytical and instrumental methods, including the HADH enzymatic test and/or other methods, providing effective detection of the fraudulent use of previously frozen fish to market it as a superchilled product.
Based on the interpretations described above, the following assessment questions (AQs) were formulated in order to address the ToR:
AQ1a: Which SFFP configurations (i.e. initial degree of superchilling) ensure that the fish temperature, at any time of the storage/transport, is lower or equal to CFFP when exposed to the same conditions of on‐land storage and/or transport?
AQ1b: If the SFFP conditions allow fish temperatures to be higher than in CFFP during the on‐land storage and/or transport, what is the potential increase of relevant biological hazards (i.e. pathogen log10 increase or change in histamine concentration) for on‐land SFFP compared to CFFP upon exposure to the same conditions of storage and/or transport?
AQ2: Which methods (including the HADH enzymatic test) are capable of detecting whether a previously frozen fish is commercially presented as ‘superchilled’?
1.3. Additional information
1.3.1. Additional background information
1.3.1.1. Nile perch fillet study by the Dutch Fish Importers Association
The mandate included a study coordinated by the Dutch Fish Importers Association using Nile perch (Lates niloticus) fillets. The aim of the research was to give more insights into the superchilling technique used in the Nile perch value chain. According to the report, ‘The research describes how the cold chain for superchilled Nile perch works and what impact it has on quality and food safety of the fresh fish product’. Secondly, it provides the results of a study using the HADH enzymatic test to determine if fish products were (previously) frozen, to confirm that the raw material was fresh fish that had been superchilled, and that no deep‐frozen fish was used. This study will be referred to as the ‘Nile perch study’ in this scientific opinion.
Representatives of the study from FIORITAL (Vincenzo Di Leva, Antonio Solimeo) and from the Dutch Fish Importers Association (Mike Turenhout) attended the Working Group meeting on 26 June 2020 as hearing experts to provide information about the Nile perch study and to provide an overview of the superchilling technique used in the Nile perch value chain. The report of the Nile perch study is available in Annex A and additional information is available at Appendix A and Annex B as provided by Mike Turenhout (Manager Dutch Fish Importers Association) on behalf of FIORITAL and the members of the Dutch Fish Importers Association by e‐mail on 16 July 2019 (Turenhout, 2019).
1.3.1.2. Previous EFSA scientific opinions and reports
In 2015, EFSA published a report on the assessment of the temperature conditions, including a possible tolerance, to be applied for the storage and transport of packaged FFP, gutted or entire, including some parts of them, at retail level where icing is not possible. The main temperature‐dependent hazards identified were histamine as well as psychrotrophic bacteria such as Listeria monocytogenes,Clostridium botulinum and Yersinia enterocolitica. It was concluded that it is possible to store packaged FFP at refrigeration temperatures above 0°C (e.g. 3–5°C) and still be compliant with the current EU and international regulated microbiological criteria. For this, the storage time and the concentration of CO2 in the packaging headspace needed to be adjusted accordingly. The report provides several scenarios equivalent to storage at 0°C, consisting of combinations of storage temperature, shelf‐life and CO2 concentration in the packaged product (EFSA, 2015).
In 2017, EFSA assessed the incidents of histamine intoxication in some EU countries that were linked to consumption of tuna and were notified through the Rapid Alert System for Food and Feed (RASFF). All incidents of histamine intoxication were evaluated to highlight common factors in the food distribution chain that potentially contributed to the human cases, and to verify the possible correlation upstream in the food supply chain through the food business operators (FBO) involved. Due to the nature of histamine and the conditions that favour its production, it was concluded that it is likely that several concurrent factors had occurred at different stages along the food chain. It was recommended to maintain adequate chilling rates, carefully manage the cold chain and ensure hygienic conditions at each step of the supply chain of this product (EFSA, 2017).
Recently, EFSA evaluated the on‐land transport/storage of FFP for up to 3 days in the so‐called ‘tubs’ (made of three‐layered polyethylene; PE) filled with freshwater and ice in comparison with the currently authorised practice (fish boxes of high‐density polyethylene (HDPE) filled with ice). The impact on the survival and growth of biological hazards in fish and histamine production in fish species associated with a high amount of histidine was assessed considering that the FFP are stored on‐board in freshwater or seawater/ice (in tubs) and once on‐land they are ‘handled’ (i.e. sorted or gutted and/or filleted) and transferred to either tubs or boxes. The temperature of the FFP was assumed to be the most influential factor affecting relevant hazards. Under reasonably foreseeable ‘abusive’ scenarios and using a conservative modelling approach, the growth of the relevant hazards (i.e. L. monocytogenes,Aeromonas spp. and non‐proteolytic Cl. botulinum) was expected to be < 0.2 log10 units higher in tubs than in boxes after 3 days when the initial temperature of the fish is 0°C (‘keeping’ process). Starting at 7°C (‘cooling‐keeping’ process), the expected difference in the growth potential was higher (< 1 log10 for A. hydrophila and < 0.5 log10 for the other two hazards) due to the poorer cooling capacity of water and ice (tub) compared with ice (box). The survival of relevant hazards was not, or only negligibly, impacted. Histamine formation due to growth of Morganella psychrotolerans under the ‘keeping’ or ‘cooling‐keeping’ process was up to 0.4 ppm and 1.5 ppm higher, respectively, in tubs as compared to boxes after 3 days, without reaching the legal limit of 100 ppm. The water uptake associated with the storage of FFP in tubs (which may be up to 6%) was not found to make a relevant contribution to the differences in microbial growth potential compared to boxes (EFSA BIOHAZ Panel, 2020).
1.3.1.3. Legal background
According to the food safety requirements of Regulation (EC) No 178/2002,7 food shall not be placed on the marked if it is unsafe, i.e. if it is considered to be injurious to health and/or unfit for consumption (e.g. due to spoilage) taking into account the normal conditions of use at each stage of production, processing distribution and the consumer.
Regulation (EC) No 853/20041 lays down specific rules on the hygiene of food of animal origin for FBOs and supplements Regulation (EC) No 852/20044 on the hygiene of foodstuffs. Section VIII of Annex III of Regulation (EC) No 853/20041 deals with fishery products.
Relevant information from the Chapters in Annex III, Section VIII of the Regulation (EC) No 853/20041 is summarised here. Chapter I states that vessels designed and equipped to preserve fishery products for more than 24 h must be equipped with holds, tanks or containers for the storage of fishery products at the temperatures laid down in Chapter VII (see below).
Chapter II defines the requirements during and after landing. It specifies that, when it is not possible to refrigerate them on board vessels, FFPs shall be refrigerated as soon as possible after their landing and stored at a temperature close to that of melting ice. It also states that FBOs displaying FFPs for sale must ensure their refrigerated storage.
Chapter III defines the requirements for establishments, including vessels, which handle fishery products. It states that:
Where chilled, unpackaged products are not distributed, dispatched, prepared or processed immediately after reaching an establishment on land, they must be stored under ice in appropriate facilities. Re‐icing must be carried out as often as necessary. Packaged FFPs must be chilled to a temperature approaching that of melting ice.
Operations such as heading and gutting must be carried out hygienically and as quickly as possible after the products have been caught or landed. The products must be washed thoroughly immediately after these operations.
Operations such as filleting and cutting must be carried out so as to avoid contamination or spoilage of fillets and slices. Fillets and slices must not remain on the worktables beyond the time necessary for their preparation and must be wrapped and, where necessary, packaged and must be chilled as quickly as possible after their preparation.
Containers used for the dispatch or storage of unpackaged prepared FFPs stored under ice must ensure that water from melted ice does not remain in contact with the fish.
Establishment on land that freeze fishery products must have equipment satisfying the requirements for freezer vessels (in Section VIII, Chapter I, part I.C., points 1 and 2) in order to rapidly lower the fish core temperature to not more than –18°C and maintain frozen fishery product at not more than –18°C; recording the temperature of the storage holds in the area where the temperature in the hold is the highest.
Chapter VI states that containers in which FFPs are kept on ice must be water resistant. Chapter VII states that FFPs must be maintained at a temperature approaching that of melting ice during their storage, while Chapter VIII states that this also applies during their transport and reiterates that melt water must not remain in contact with the FFPs, when kept under ice. For frozen fishery products, Chapters VII (storage) and VIII (transport) state that they must be stored at a temperature of not more than –18°C in all parts of the products and maintained at this even temperature, possibly with short upward fluctuations of not more than 3°C; while whole fish initially frozen in brine intended for the manufacture of canned food may be kept at temperature of not more than –9°C. When frozen fishery products are transported from a cold store to an approved establishment to be thawed on arrival for the purposes of preparation and/or processing, if the journey is short and the competent authority so permits, there is no need to comply with the frozen temperature requirement during transport.
1.3.1.4. Superchilling of fresh fishery products
The primary purpose of superchilling of FFP is the extension of the shelf‐life of fresh fish as a result of the reduction of the growth rate of spoilage bacteria at superchilling temperatures in comparison with the conventional chilling temperature. The main principle behind the term ‘superchilling’ is that the product appears non‐frozen despite the presence of ice crystals. It is also referred to as ‘deep‐chilling’, ‘sub‐chilling’, ‘partial freezing’ or ‘shell freezing’ as superchilled products have a certain fraction of ice present inside the food matrix (Kaale et al., 2011; Bantle et al., 2016). Despite some papers considering superchilling to have occurred when the temperature is just below 0°C (i.e. melting ice temperature) without ice crystals being generated, this definition corresponds to the conditions for ‘supercooling’8 (Bantle et al., 2016). Thus, the term ‘supercooling’ refers to products that are also stored at temperatures between 0°C and the initial freezing point of the fish.
There is no commonly agreed definition of superchilling in the literature (Bantle et al., 2016). In the earliest times of the implementation of superchilling strategy, first described by Le Danois (1920) as a new method to refrigerate fish, the fish were immersed in salted water at –4°C and then quickly immersed in freshwater at 0°C to form a thin layer of ice on the fish surface. In 2001, the FAO defined superchilling as ‘reducing the temperature of fish uniformly to a point slightly below that obtained in melting ice’ and more specifically, ‘in present practice superchilling means reducing the fish temperature to about –2.2°C (28°F), at which point half the water is frozen, and keeping it there’ (FAO, 2001). ‘Superchilling’ is usually defined as a technological process causing the freezing of a certain part of the product's water content by quickly lowering the temperature of a food product below its initial freezing point. This temperature is variably described in literature depending on the authors: either just below the initial freezing point (Beaufort et al., 2009), between the initial freezing point of the products (including it) and 1–2°C below this (Duun and Rustad, 2007; Magnussen et al., 2008; Wu et al., 2014); to 1–2°C below its initial freezing point (Zhou et al., 2010; Kaale et al., 2011; Yu et al., 2019) and also between –1°C and –4°C without reference to the initial freezing point (Bahuaud et al., 2010; Pan et al., 2019). According to the definition of the International Institute of Refrigeration, superchilling of a product relates to conditions ‘very near its freezing point or even a few degrees below’.
In order to illustrate the range of temperatures associated with the different fish status and fish storage conditions included in the present opinion, a conceptual representation was developed (Figure 2), which relates the fish temperature to a range of fractions of frozen water within the fish matrix (i.e. ice fraction).
Figure 2.
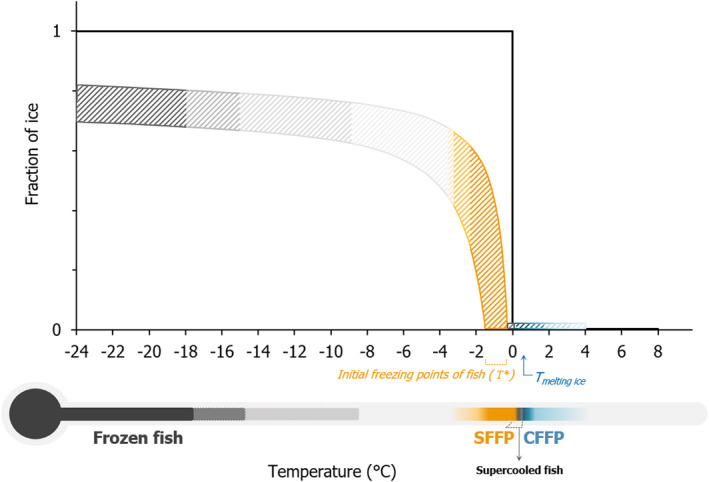
Conceptual representation of the fraction of frozen water (ice fraction) in fish (coloured pattern area) and in water (dark grey line) as a function of temperature
- CFFP: conventional fresh fishery products; SFFP: superchilled fresh fishery products.The initial freezing point of fish (T*) constitutes the boundary between the supercooling and superchilling status and varies depending on the fish composition.
Properly frozen fish must be kept at a temperature not higher than –18°C (with a maximum fluctuation of +3°C), although fish frozen in brine intended to be canned may be kept at ≤ –9°C. Fish frozen at higher temperatures are considered improperly frozen, thus not complying with the current legislation (see Section 1.3.1.3). Under the legally defined freezing temperature conditions, almost all the freezable water (which can be up to approximately 80% of the total water depending on the fish composition) of the fish matrix will be frozen. CFFP must be stored at a temperature close to that of melting ice, at which, no fraction of the fish water content is frozen. Although no upward tolerance limits are stated in the legislation, some competent authorities (CA) recommend CFFP not to exceed 4°C (e.g. in Belgium (Bekaert et al., 2016)). The superchilling of fish is not specifically covered by the legislation; therefore, the panel and working group relied on the most common definition found in the scientific literature, considering that superchilling includes fish temperatures between the initial freezing point of the fish (always below the temperature of melting ice, i.e. < 0°C) to about 1–2°C lower. This results in a variable fraction of frozen water within the fish matrix, which is lower than that occurring in deep‐frozen fish. The temperature of the supercooled fish ranges from 0°C (melting ice point) to the initial freezing point of the fish, and no ice is formed in the fish matrix. Supercooled fish is not considered CFFP and thus outside the scope of the present opinion. As the initial freezing point is product‐specific and varies depending on the fish composition, it is only possible to define from which temperature fish in general enters into the superchilling status if the initial freezing point of the given fish is known. As an example, a fish with an initial freezing point of –0.9°C would be considered supercooled at temperatures between 0°C and –0.9°C and superchilled at temperatures between –0.9°C and –1.9 to –2.9°C. Another fish with an initial freezing point of –0.5°C would be considered supercooled at temperatures between 0°C and –0.5°C and superchilled at temperatures between –0.5°C and –1.5 to –2.5°C.
The scientific publications dealing with SFFP generally consider that an ice fraction of 5–30% in fish is acceptable and low enough to keep its fresh appearance. Higher levels can cause an excessive drip loss with the consequent negative impact on the fish quality (Claussen, 2011; Margeirsson et al., 2012bb; Wu et al., 2014). However, the ice fraction was not measured in most of the published studies. Instead, the more easy and direct measurement of the superchilled fish temperature is reported.
The superchilling technology comprises two stages – the actual superchilling process and the subsequent superchilling storage. Additionally, in the superchilling technology for fish, the pre‐cooling (to lower the temperature difference between pre‐ and post‐superchilling) is also key for the quality of the superchilled fish as it influences the ice crystal formation during superchilling, together with the rate of the superchilling and the storage conditions (Kaale, 2014; Wu et al., 2014).
The superchilling process can be conducted in an impingement tunnel or cryogenic freezer at a temperature range from of –30°C to –40°C (air temperature) for 1.5–2 min (Duun and Rustad, 2007; Kaale et al., 2011; Cropotova et al., 2019), as well as in blast freezers usually taking longer time, e.g. up to 20–72 min (Bahuaud et al., 2008; Bantle et al., 2016) (Nile perch study), either alone or in combination with contact cooling (Valtýsdóttir et al., 2010; Erikson et al., 2011; Ólafsdóttir et al., 2012). The surface area/product weight ratio is a key parameter determining the rate of superchilling (Magnussen et al., 2008). On‐board practices, such as slurry ice or refrigerated salted water, could also be used for superchilling fish that will be subsequently transported/stored as superchilled on‐land (Thordarson et al., 2017).
After the first stage of initial surface freezing (shell freezing), the 1–3 mm layer of ice formed on the surface of the product (as a crust) absorbs heat from the inner parts eventually resulting in equalisation of the temperature (Figure 3). Once superchilled, the capacity of the fish to absorb heat is the same before and after the temperature equalisation, but with a more homogeneous spatial distribution after the equalisation. The superchilled product will have an internal ice (cold) reservoir capable of absorbing heat9 from the external environment during storage and distribution, without changing significantly the product temperature (Magnussen et al., 2008; Margeirsson et al., 2012b). This is mainly due to latent heat, which is the heat energy that when added (absorbed) or removed (released) causes a change in the state of a material with no change in temperature (e.g. from ice to water). Relatively large quantities of heat are needed for the change in state (Pham, 2016).
Figure 3.
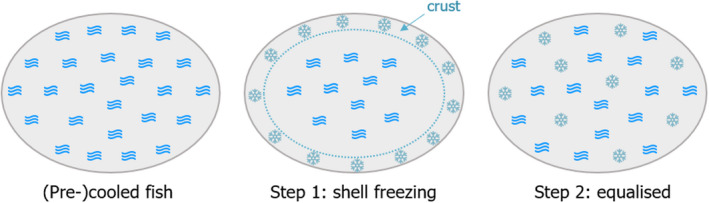
Steps in fish superchilling technology (adapted from Bantle et al. (2016)), where fish is represented as a section
The control of the superchilling process through the selection of optimal technological conditions depends on the equipment used as well as on the food product, dimensions (size and geometry) and the target degree of superchilling (as the ice fraction after the equalization step) depends on the intended use or processing after storage (Magnussen et al., 2008; Stevik et al., 2010). In practice, the key temperature measurement is the one performed after the temperature equalisation is complete (Magnussen et al., 2008; Wu et al., 2014).
According to Kaale et al. (2011), superchilling gives the food product an internal ice reservoir so that there is no need for external ice around the product during transportation or storage for short periods. However, for long‐term superchilled storage, refrigerated (chill environmental temperatures) storage keeping the fish at superchilled temperatures is required. To maintain the superchilled state, minimising the increase of the size of ice crystals inside the product, superchilled fish should not be exposed to temperature fluctuations (Kaale et al., 2011). Therefore, appropriate insulated packaging such as EPS boxes and strict control of temperature throughout the storage period should be used. This might be particularly important for fish supply chains including periods with no refrigeration e.g. loading and uploading for air transport (Mai et al., 2012). Temperature data loggers may be inserted into insulated packaging with the fish to monitor fluctuations of temperature during superchilled storage (Cropotova et al., 2019). Any disturbance in temperature or storage conditions may cause melting of small ice crystals inside the fish flesh and their further recrystallisation into large‐sized ice crystals (Wu et al., 2014), thus, reducing the fish quality. If the storage temperature melts all the ice in the fish matrix, the temperature of the fish will quickly increase, which can compromise the safety and quality of the product (Wu et al., 2014). This is due to sensible heat, which is the heat energy that is added or removed from a material that causes a change in temperature without a change of state, e.g. to warm up water (Pham, 2016).
Overall, superchilled storage and transport of fish allows not only the extension of shelf‐life but also a reduction in the amount of ice needed in the whole supply chain, with the consequent increase in the energy efficiency of the fish supply chain (Wang et al., 2008; Wu et al., 2014).
1.3.2. Approach to answer the ToR
A conceptual map of the conditions to be addressed in the current assessment is depicted in Figure 4. Upon arrival at the first on‐land establishment, the conditions to be compared in the assessment include:
Baseline or current condition: the CFFP are placed in expanded polystyrene (EPS) boxes with ice, where they are kept during transport and storage until the final establishment; and
Alternative condition: the SFFP are placed in EPS boxes without ice, where they are kept during transport and storage until the final establishment.
Figure 4.
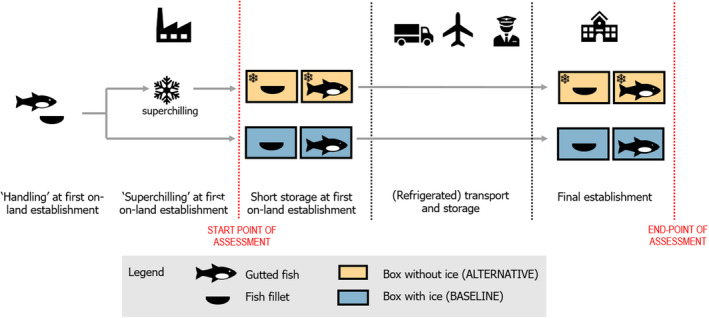
Conceptual map of the conditions to be addressed in the current assessment
For the present assessment, the FFP are handled (gutted and/or filleted) at the first establishment and may be chilled (baseline) or superchilled (alternative condition) for the subsequent storage and/or transport to the final establishment. Good practice when handling fish catches, as well as the use of sufficient ice, was assumed. It was also assumed that the factors contributing to the initial status of the fish (e.g. intrinsic characteristics, conditions on‐board, hygienic status) were equal for the two conditions, and thus, the relative impact during the on‐land transport and storage would be equivalent.
To address the AQs to answer the ToRs, the following steps were undertaken:
Step 1: The temperature range of melting ice made from freshwater, under baseline conditions (CFFP) was defined (0°C). The temperature of the fish after a superchilling process under the alternative conditions (SFFP) was assessed in relation to the percentage of frozen water in the fish matrix.
Step 2: A heat transfer modelling approach was developed to identify under which initial configurations the fish temperature of SFFP, at any time during the storage/transport, is lower or equal to CFPP, when exposed to the same conditions of on‐land storage and/or transport. This approach is feasible since the boxes used to transport the fish are the same (e.g. EPS boxes). A MS Excel spreadsheet tool based on this model was built to calculate the initial configurations of SFFP (i.e. ice fraction within the fish matrix associated with an initial superchilled fish temperature) that can be used by the FBO as part of the ‘safety‐by‐design’ approach. More specifically, this approach allows identification of scenarios under which initial configurations the SFFP will be equivalent or have a higher capacity to absorb heat than CFFP. For simulation purposes, three types of fish were considered: salmon (as an example of fish with a high fat content), cod (as a lean fish) and Nile Perch (as a temperate freshwater fish).
Step 3: A literature review was carried out to find data on the time/temperature profiles occurring during the storage and transport of SFFP, recording when available the critical parameters/factors determining the performance of the system to keep the fish temperature under control (e.g. initial fish temperature, outside temperature conditions). The search was particularly focused on the studies comparing SFFP with CFFP stored/transported under the same outside conditions. The data in the Nile perch study were also used.
Step 4: the temperature conditions of the alternative condition (SFFP) are more favourable for microbial growth compared to the baseline condition (CFFP), the relevant biological hazards for growth would be derived from the tubs opinion (EFSA BIOHAZ Panel, 2020) and the potential growth assessed by applying available predictive models for specific pathogens and histamine accumulation. Otherwise, the alternative condition would be considered as at least equivalent to the baseline condition (i.e. SFFP are considered at least equally efficient to control pathogen growth compared to CFFP based on their temperature).
Step 5: A literature review was carried out to assess potential methods (including the HADH enzymatic test as evaluated in the Nile perch study) capable of differentiating between fresh/superchilled and frozen fish. The methods were listed, summarising key features, advantages and disadvantages for their use to assess whether a fish that has been previously frozen has been commercially presented as ‘superchilled’.
Step 6: The methods were appraised based on predefined criteria, taking into account several criteria including their capability and performance to differentiate fresh fish from fish frozen at various temperatures and the strength of evidence.
2. Data and methodologies
2.1. Fish temperatures under both conditions
2.1.1. Heat transfer modelling
Temperatures of fish stored or transported in ice under the current authorised practices (CFFP) are close to the temperature of melting ice, i.e. 0°C, provided that ice is made of freshwater (EFSA BIOHAZ Panel, 2020). Fish is usually not pre‐cooled below 0°C before storage or transport and the minimum temperatures during storage are close to 0°C for fish in close contact with the melting ice. Re‐icing is necessary to avoid a rise in the temperature of the fish if the whole mass of ice is melted. The ratio of ice mass per fish mass (α) is a critical parameter in CFFP determining the amount of heat that the system can absorb before the whole mass of ice is melted. This ratio needs to be adjusted depending on factors such as initial fish temperature, temperature and duration of storage/transport, and the insulating properties of the box used (EFSA BIOHAZ Panel, 2020).
The superchilling process reduces the fish temperature below the initial fish freezing point (T*) before its storage or transport. The initial freezing point is the highest temperature at which ice crystals first appear in the fish. Below the initial freezing point, the freezable water inside the fish is in thermodynamic equilibrium with the ice. The ice fraction (Xice) expressed as kg‐ice/kg‐fish depends on the superchilled fish temperature (Ts) as follows (Pham, 2014b):
| (1) |
where (Xfree) is the free water content, i.e. freezable water content (kg‐water/kg‐fish), i.e. the total water content (XW) minus bound water content (Xb). The degree of superchilling is determined by the initial ice fraction in the fish matrix (Xice) after the superchilling process and depends on the fish temperature after the equalisation and the free water content as described by Equation 1).
This approximation holds for temperatures close to and below the initial freezing point, i.e. (Ts ≤ T*), and for non‐processed fish without added solutes (such as sodium chloride). The bound water and initial freezing point can be estimated from the proximate composition10 of the fish with the following empirical equations (Pham, 1996):
| (2) |
| (3) |
where Xash is the ash (mineral) mass fraction, Xprot is the protein fraction and Xother is the mass fraction of components (such as carbohydrates) other than ash, water, proteins or fat.
To assess under which conditions fish temperatures in SFFP may exceed temperatures in CFFP under equivalent storage conditions, a heat transfer balance was derived to know the heat absorbed by SFFP and CFFP. The following assumptions were made:
Before transport or storage, the initial fish temperature is homogeneous (assumption 1 in Table 4, with details in Appendix B.1) in the fish matrix (muscle, fat, bones, gut, tail) and denoted by for CFFP and for SFFP. The latter corresponds to the fish temperature after equalisation (once the initial layer of ice in the surface of the fish formed during superchilling has absorbed the heat from inner parts of the fish to reach an equilibrium), assuming that there will still be some ice in the matrix (Magnussen et al., 2008). Note that is higher than .
After transport and storage (end point of the assessment), ice becomes totally melted for both conditions (α = 0, Xice = 0) and fish temperatures are homogenous and equal to 0°C .
Table 4.
Potential sources of uncertainty identified in the assessment of the fish temperature and assessment of the impact that these uncertainties could have on the conclusion (i.e. over/underestimation of fish temperaturea and the extent of the over/underestimation)
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Heat transfer model. Assumption 1: no spatial distribution was considered |
The temperature distribution was assumed to be homogenous in both CFFP and SFFP. For CFFP, this assumption implies an ideal situation in which the ice in the box is in perfect contact with all of the fish. |
The impact of the assumption depends on the Biot number (representing the fish internal resistance compared with the surface of the fish resistance) and it would be high if the Biot number is much larger than 1 (Pham, 2014a). Estimations of the Biot number considering the parameters for a box made for HDPE with geometry and conductivities as defined in (EFSA BIOHAZ Panel, 2020), are always below 1 and therefore the impact is expected low (see Appendix B.1). The direction of the impact depends on the spatial location of the temperature inside the fish. For example, when the outside temperature is higher than the fish temperature, in general as the model estimates a temperature for the whole fish, it is an underestimation of the fish surface temperature and an overestimation of the temperature in the centre of the fish. In CFFP, this will be affected by the ice spatial distribution close to the fish, i.e. the underestimation of surface temperature would be only relevant for the fish surface far from the ice. In SFFP, this will have little influence as ice is inside the fish matrix. |
| Heat transfer model. Assumption 2: heat is absorbed at similar rate in SFFP and CFFP | Rate of absorption depends on the heat transfer coefficient (h), the box exposed area (A) and the differences between the outside‐of‐the‐box temperature and the fish temperature. Note that box and outside temperatures are equal for CFFP and SFFP, but not for the fish temperature (usually lower in SFFP) and therefore absorption rate might be faster in SFFP than in CFFP |
To assess the impact of this assumption, a model based on time‐to‐melt‐all‐ice, which does not rely on this assumption, was derived in Appendix B.2. In the worst case, when the capacity to absorb heat is equal in SFFP and CFFP and in a system without insulation, SFFP temperatures might be higher than CFFP only for the last 3% of the storage time. Therefore, the impact is low and results could slightly underestimate the temperature of SFFP, but only for a short period of time and always for less than 0.85°C in a configuration with a box without insulating material. As SFFP and CFFP are stored in insulating EPS boxes, and the temperature of the SFFP at the beginning of the storage will always be lower than CFFP, the impact will be very limited. |
| Heat transfer model. Assumption 3: specific heat capacities were considered constant |
Specific heat capacities (Cp parameter) used in the model are known to be temperature dependent, though mathematical functions are not easy to find/estimate. For the fish type assessed the quantitative relationship between Cp and temperature were not available. For the main assessment and the MS Excel tool developed, constant values at temperatures slightly above zero were used for fish, ice and water. As the Cp for Nile Perch is unknown, the Cp for cod (lean fish) has been used for the simulations. |
Cp values below 0°C are usually greater (see for example values for cod in (Margeirsson et al., 2012b) than those considered in the model. The values used were conservative and provided a slight overestimation of the temperature of SFFP. In any case, the impact of this approximation is low as it affects at sensible heat that was not as relevant as the latent heat in the current assessment. |
| Heat transfer model. Uncertainty in the estimation of the ice fraction in the fish matrix of SFFP X ice |
This uncertainty appears only when Xice is unknown and is to be estimated from the initial freezing point T*. Xice is calculated using a function of free water content: and initial freezing point (T*), as shown in the semi‐mechanistic Equation 1), based on thermodynamic equilibrium of freezable water and ice inside the fish). Xfree and T* are calculated with their relationship with the proximate composition. T* in raw muscle foods are often quoted as –2°C or lower, despite there is scientific evidence that according to measurements it is much nearer to –1°C (Miles et al., 1997). T* was calculated from an empirical equation using the fish composition (Equations 2 and 3). The food composition information is not always complete and subject to analytical and specific analysed sample uncertainty. Moreover, the composition provided in literature and databases is not always complete, particularly for the ash content. |
In Appendix B.3, the uncertainty on the capacity to absorb heat in SFFP was derived when there are uncertainties in Xfree and T* (that will impact the initial Xice in superchilling and therefore the degree of superchilling). The impact of this assumption is the largest of all the identified uncertainties, as it is proportional to the latent heat (λ), which is a large number. The direction depends on the direction of the errors in T* and Xfree as described in Appendix B.3. For instance, an overestimation of T* (assume T* = –0.3°C when it is T* = –0.4°C) will cause an overestimation of Xice, leading to consider that SFFP has a similar capacity to absorb heat compared to CFFP (QS = QC) when in reality it has lower capacity to absorb heat (QS < QC). Consequently, temperatures in SFFP will be actually higher than the estimated and could rise above 0°C before all ice is melted in CFFP. In this case, the temperatures of SFFP can be higher than those of CFFP at the end of storage/transport, but still lower than CFFP at the beginning. The impact of this uncertainty is particularly relevant when the degree of superchilling (as Xice) is monitored through the initial superchilled fish temperature ( ). In addition, the latter is subjected to uncertainties related to the superchilling technology (e.g. variability of the capacity to decrease the fish temperature within a batch of fish) and can also be subjected to measurement error (instrumental error) and variability depending on when and where (within the fish matrix) point it is measured. This additional uncertainty can cause both under‐ and overestimation of the initial superchilled temperature. In the current assessment, the capacity to absorb heat for CFFP and SFFP has been focused on the comparison of the initial configuration parameters α (proportion of ice:fish in CFFP) and Xice to minimise the impact of the uncertain estimation of Xice from T* and Xfree. However, the tool made available based on the developed heat transfer model, uses T* and Xfree to make the simulations. Therefore, the accuracy of the input data is key for obtaining reliable outputs. |
| Literature data. Search methodology. |
In the absence of a commonly agreed definition of superchilling in literature, data on time–temperature profiles of SFFP (particularly in grey literature) may have gone undetected due to variability of terminology used. A critical appraisal of the retrieved studies was not performed. |
Under‐ or overestimation of the performance of SFFP compared to CFFP. Given the use of an extensive literature search (and use of various terms related to superchilling such as partial freezing, deep chilling or sub‐chilling) and the criteria adopted for literature screening, uncertainty associated with missing evidence in this step was minimised. |
| Literature data. Number of retrieved data. | Limited availability of data directly comparing SFFP and CFFP during transport/storage. Particularly, given the few records addressing the topic, the effect on SFFP of factors such as different superchilling technologies and variability in the transport/storage chains was not investigated. | Under‐ or overestimation of the performance of SFFP compared to CFFP in relation to different superchilling technologies. |
HDPE: high‐density polyethylene
unless stated otherwise fish temperature refers both to CFFP and SFFP.
The capacity to absorb heat in CFFP during storage and transport relies on increasing the ice temperature from its initial value to 0°C and on melting ice and must compensate the energy needed to cool the fish from its initial temperature to 0°C and to keep it close to 0°C during storage and transport. Therefore, the capacity to absorb heat per kg of fish (QC) can be expressed through the next equation:
| (4) |
being λ the latent heat of ice, αα the mass of ice (mice) per mass of fish (mf) (kg‐ice/kg‐fish), Cpf the fish heat capacity and Cpice the ice heat capacity. Both Cpf and Cpice were assumed to be constant (see uncertainty Section 3.1.4).
The proportion of ice and fish (α) that is used to store and transport the CFFP varies notably as it is usually adjusted to ensure that enough ice is present to keep the fish temperature close to that of the melting ice, taking into account of the initial fish temperature and the storage and transport conditions, including the time (Alasavar and Quantick, 1997; EFSA BIOHAZ Panel, 2020). The following foreseeable scenarios were considered:
1 kg ice for 22 kg of fish (α = 0.045) of fish at an initial temperature of maximum 4°C (Lynum, 2007).
4 kg ice for 22 kg of fish (α = 0.18) as a reasonable foreseeable condition (fish temperature not stated) (Margeirsson et al., 2017).
0.75 kg ice for 3 kg of fish (α = 0.25) (fish pre‐cooled but often rising in temperature of up to 7°C after being filleted and handled) as applied in Flemish fish auction as provided by Sven Van Acker (Director Operations at the Vlaamse Visveiling nv) by e‐mail on 2 December 2020 (Van Acker, 2020).
1 kg ice for 3 kg of fish (α = 0.33) with the fish temperature not stated (Alasavar and Quantick, 1997; Laguerre et al., 2018).
Capacity to absorb heat in SFFP during storage and transport relies on raising the fish temperature from its initial value to 0°C and keeping it close to 0°C, while frozen water inside the fish matrix melts. Mathematically, the capacity of absorbing heat per kg of fish (QS) can be expressed through:
| (5) |
being Xice the ice fraction (kg‐ice/kg‐fish) as described by Equation 1) and Cpf the heat capacity of superchilled fish, assumed constant.
For the main assessment, it was assumed that both conditions absorb heat at the same rate. The impact of this assumption was evaluated in Appendix B.2 and included in the uncertainty analysis (assumption 2 in Table 4). Therefore, only when the capacity to absorb heat of the SFFP system is lower than that of the CFFP (Qs < Qc), ice in SFFP will melt before ice in CFFP is melted and the temperatures of SFFP may be (but not necessarily be) higher than those of CFFP. In this respect, the ratio (R) between the capacity to absorb heat between both systems can be determined as:
For the particular case of Nile perch, a stochastic approach was applied in order to assess the impact of the variability distribution of key parameters, such as the initial freezing point of the fish, the free water content of the fish, the initial temperature of the ice and the fish in the CFFP and the initial fish temperature of the SFFP, on the probability that the capacity to absorb heat by the SFFP was at least equivalent to that of CFFP (i.e. R ≥ 1). This stochastic assessment was applied for four scenarios of the proportion of ice:fish (α) regarding the CFFP initial configuration considered in the deterministic approach.
Table 1 summarises the input parameters used for the deterministic simulations and stochastic assessment carried out with the heat transfer model together with the supporting references.
Table 1.
Input parameters for the simulations carried out with the heat transfer model using a deterministic approach for salmon, cod and Nile perch and using a stochastic approach for Nile perch
| Parameter [units] | Nomenclature | Input and parameter values for the deterministic approach | Input and parameter values or distribution functions for the stochastic approacha | Supporting information | |
|---|---|---|---|---|---|
| Specific heat capacity of ice [kJ/(K kg‐ice)] | Cpice | 2.052 | 2.052 | Comsol (example of ‘phase change’ in library)f and EFSA BIOHAZ Panel (2020) | |
| Latent heat of fusion (ice to water) [kJ/kg‐ice] | λ | 333.5 | 333.5 | Comsolf (example of ‘phase change’ in library) and EFSA BIOHAZ Panel (2020) | |
| Specific heat capacity of fish [kJ/(K kg‐fish)] | Cpf | 3.5 (for fat fish, salmon) | NA | (Rahman, 2009), (Tolstorebrov et al., 2019) and (Radhakrishnan, 1997) in EFSA BIOHAZ Panel (2020) | |
| 3.73 (for lean fish, cod and Nile perch) | 3.73 | (Margeirsson et al., 2012b) and EFSA BIOHAZ Panel (2020) | |||
| Initial freezing point of fish [°C] | T* |
–0.77 for salmon –0.67 for cod –0.33 for Nile perch |
NA NA Normal (–0.33; 0.14) |
Calculated from the proximate composition data found in literature (see Table 2) and Equation 3) | |
| Free water content in fish [kg water/kg fish] | Xfree |
0.6577 for salmon 0.7828 for cod 0.7279 for Nile perch |
NA NA Normal (0.7279; 0.0230) |
Calculated from proximate composition data found in literature (see Table 2) and Equation 2) | |
| Kg ice per kg of fish for CFFP [kg ice/kg fish] | α | 0.045; 0.18; 0.25; 0.33 | 0.045; 0.18; 0.25; 0.33 | Four different foreseeable scenarios of CFFP initial configuration (see Section 2.1.1) | |
| Initial ice temperature of CFFP [°C] |
|
–1°C | Triangular (–1.5; –1; 0) | Conservativeb assumption based on Laguerre et al. (2018); Jain et al. (2005) and EFSA BIOHAZ Panel (2020) | |
| Initial fish temperature of CFFP [°C] |
|
From 0 to 8°C | Triangular (0.25; 3.5 ;4.81) |
Deterministic approach: different foreseeable scenarios considering pre‐cooled fish (0°C) to fish showing a higher temperature after handling (gutting, filleting etc. up to 8°C) (Erikson et al., 2011; EFSA BIOHAZ Panel, 2020) Stochastic approach: distribution of temperature of Nile Perch before the superchilling process (‘Nile perch study’) |
|
| Initial ice fraction [kg ice/kg fish] | Xice | From ca. 0.03 to 0.42 | NA | Estimated with Equation 1) using different foreseeable scenarios of initial SFFP temperature ( )c | |
| Initial fish temperature of SFFP [°C]d |
|
NAe | Normal (–1.35; 0.32) | Distribution of temperature of Nile Perch after the superchilling process (‘Nile perch study’) |
NA: not applicable.
10,000 iterations made with @Risk (Palisade).
For the present comparative assessment framework, –1°C constitutes a conservative input value because compared to the slightly higher value usually considered (–0.5°C to 0°C), it favours the capacity to absorb heat by CFFP, and leads to a decrease in the R value.
The initial SFFP fish temperature (and the initial ice fraction, Xice in %) was: –0.88°C to –2.10°C (i.e. 3% to 42% ice fraction) for salmon; –0.70°C to –1.60°C (3% to 42% ice fraction) for cod; –0.34°C to –0.76°C (i.e. 3% to 42% ice fraction) for Nile perch. For each fish species, the initial ice fraction (Xice) in the SFFP matrix was calculated from Equation 1), using the T* and Xfree values described from the proximate composition of fish (Equations 2 and 3).
This parameter can be easily and routinely measured by the FBO. For the stochastic approach of the present assessment, this parameter was used to derive the initial ice fraction (Xice) used in the deterministic assessment.
In the deterministic approach the initial fish temperature of SFFP is derived from the initial ice fraction (degree of superchilling).
An MS Excel spreadsheet tool was built, based on the developed conservative heat transfer model, that can be used as part of ‘safety‐by‐design’ approach of the FBO to identify under which initial configurations the SFFP will be at least equivalent or better than CFFP in terms of total heat absorption capacity. The tool enables the setting of the degree of superchilling for SFFP (i.e. the ice fraction in the fish matrix, which depends on the fish temperature after superchilling and the initial freezing point of fish) that is considered able to maintain fish temperature below or equal to that of a given configuration of CFFP regarding the initial fish temperature and the proportion of ice added per mass of fish (ice:fish) in the box (R ≥ 1, corresponding to ≥). The tool facilitates identification of configurations under which the SFFP cannot be proved to be equivalent or better than CFFP (R < 1, corresponding to Qs < Qc). The tool has been made available through the Knowledge Junction under https://doi.org/10.5281/zenodo.4304283. It is named the heat transfer model tool for the heat absorption capacity of superchilled fresh fishery products (HTM‐SFFP Tool). It has following options and in each option there is the possibility to estimate the initial freezing point of fish and free water of fish from the proximate composition:
-
To derive if superchilling is able to maintain fish temperature below or equal to that of CFFP at any time of storage before all ice melts, based on
-
o
the target % of frozen free water in SFFP (option 1)
-
o
the initial superchilled fish temperature (option 2)
-
o
To derive the initial superchilled fish temperature and percentage of ice in superchilled fish needed to equal the absorbing heat capacity of CFFP before all ice melts (option 3)
-
To derive the proportion of ice in CFFP to equal the absorbing heat capacity of SFFP before all ice melts, based on
-
o
the target % of frozen free water in SFFP (option 4)
-
o
the initial superchilled fish temperature (option 5)
-
o
2.1.2. Literature
The strategy for conducting the literature searches and screening is provided in Appendix C. A general search was conducted in Web of ScienceTM Core Collection (1975–present) to retrieve various types of information on superchilling of FFP. The records were screened for information on the use of superchilling of FFP (with the exception of bivalve shellfish, as this was not relevant for this assessment) and on the temperatures of SFFP during subsequent storage. Further information on temperatures of SFFP during transport and/or storage was retrieved through the literature review, as detailed in Appendix C.1, and through expert consultation.
The information in the ‘Nile perch study’ was used to gather data observed in actual commercial superchilled fish from the Lake Victoria region to the EU (see Section 1.3.1.1).
2.2. Potential methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’
Two searches were conducted in Web of ScienceTM Core Collection (1975–present). The searches aimed to retrieve information on potential markers to distinguish fresh/superchilled fish meat from frozen/thawed fish meat. The strategy for conducting the literature searches and screening is provided in Appendix C.2.
Two tables were compiled with information at record level. The first one referred to as ‘record overview table’ was organised by method category, i.e. biochemical or morphological and physico‐chemical, with subgrouping within each group (e.g. enzymatic). Data were extracted about the methodology used, the conditions tested considering the fish species, the temperatures, duration and method used for fish freezing. The information extracted about the results included (if applicable and/or provided):
if a statistically significant difference in the measured parameter between fresh and frozen/thawed fish was found;
the analytical threshold values;
the influence of freezing temperatures, i.e. the minimum (tested) freezing temperature for which the method could detect the fish as frozen;
the influence of the freezing duration, i.e. the minimum (tested) freezing time for which the method could detect the reaction or the change that the method analyses;
the influence of freezing technology or process;
the influence of post‐harvest changes occurring in fish, either from biochemical or microbiological origin; and
the classification performance in discriminating fish as either frozen/not frozen through validation with additional/external samples.
An appraisal of the relevance of each study (appropriateness to answer the ToR) was carried out based on the following criteria to be answered by yes (score 1) or no (score 0). All criteria have the same weight:
Q1: Could different groups of samples (fresh and frozen/thawed) successfully be differentiated based on statistical tests?
-
Q2: Did the study include method validation?
-
o
For the biochemical methods, this refers to the analytical accuracy, precision, sensitivity, specificity and reproducibility.
-
o
For the physico‐chemical methods, this refers to testing the algorithm with samples (calibration/training set of samples) used for the developing the algorithm, i.e. cross‐validation describing the determination or correlation coefficient, accuracy (or overall rate of correct classification), sensitivity, specificity, etc.
-
o
Q3: Did the study consider different freezing temperatures?
Q4: Were freezing temperatures above –18°C included?
Q5: Did the study include superchilled samples or slightly frozen (e.g. ≥ –5°C) samples?
Q6: Did the study consider different frozen storage durations?
Q7: Did the study consider the influence of post‐harvest quality changes in fish?
Q8: Is there information available on the freezing technology or process (e.g. rate) in the study?
Q9: Was the method applied to different fish species?
Q10: Was a threshold or any differentiation tool/criteria proposed for each fish species in the study?
Q11: Was a classification performance (e.g. percentage of correct classification) included in the study using an independent set of samples (i.e. not part of the samples used for method validation in Q2 and/or fish coming from a different batch)?
The second table called ‘analytical capability table’ summarises the retrieved information related to the capability of the method to detect previously frozen fish, frozen at different temperatures and for different fish species.
Furthermore, two additional tables were compiled on method subgroup level. The third table called ‘advantages/disadvantages table’ describes the advantages and disadvantages by method subgroup based on the results of the different studies and other characteristics of the methods related to the ease of use and technical resources required (i.e. fast or slow execution, destructive or not, laborious or not, standard or more advanced (expensive or uncommon) laboratory equipment needed, simple or specialised skills needed, need of threshold/calibration setting by species or group of species; the technological readiness for commercial applications).
A fourth table called ‘evaluation table’ includes the evaluation of each method subgroup based on a scoring system for different evaluation criteria. The criteria are:
Applicability of the method towards species;
Ability of the method to differentiate fresh fish from fish frozen at various temperatures;
Use as a stand‐alone method;
Ease of use of the method (e.g. fast, not too laborious, not destructive);
The method was validated (e.g. determination of accuracy, precision, intra/interlaboratory‐reproducibility, sensitivity, specificity);
Classification performance in discriminating as either frozen/not frozen using an independent set of samples;
Evidence that superchilled fish will behave like fresh fish for the given method; and
Strength of evidence expressed as the number of records and the average appraisal score (from the 11 questions listed above), the latter being the division of the total appraisal score of all the records and the number of records; from the record overview tables.
The scoring system is defined in the following way:
0 = no information available;
– = poor performance;
+ = weak performance/weak evidence;
++ = good performance/good evidence; and
+++ = excellent performance/good evidence.
An overall score by method was given based on the information gathered from the literature. The scores were cross‐checked and validated by peers within the working group.
2.3. Uncertainty analysis
Based on the EFSA guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018a) and scientific opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment (EFSA Scientific Committee, 2018b), special attention was given to: (i) the interpretation of the ToRs, i.e. framing of the mandate and the AQs, (ii) identifying sources of uncertainty and (iii) their impact on the outcome of the assessment. The identified assumptions and other sources of uncertainty were listed and, in some cases, the impact was quantified. The experts elicited the overall uncertainty associated with the final outcome of the AQs through expert group judgement taking into account the quantified and non‐quantified sources of uncertainty.
3. Assessment
3.1. Temperature profile of transported/stored SFFP and CFFP and, if relevant, its impact on the increase of biological hazards
3.1.1. Modelling the temperature profile of transported/stored SFFP and CFFP
3.1.1.1. Temperature of melting ice and initial freezing point of fish
The temperature of melting ice is the freezing point of water at which the first ice crystal appears. If ice is made of freshwater, the melting ice temperature is 0°C at atmospheric pressure (Pham, 2016).
In fish, the temperature required to obtain the first crystals of ice in fish during the superchilling process is lower than in freshwater because free water in fish has solutes that makes the initial freezing point (T*) lower than the freezing point of freshwater. The soluble components such as ions, acids and soluble proteins contribute to freezing point depression, while insoluble components such as fat and insoluble proteins do not (Miles et al., 1997). Moreover, during superchilling and freezing of fish, when fish water freezes, it leaves increasing concentrations of solutes in the remaining non frozen water, which lowers its freezing point further (note that in water ‘initial freezing point’ and ‘freezing point’ are the same and equal to 0°C). In fish, there might be some water bound to the solid matrix (water associated with changed ionic groups of food components with restricted mobility) not participating in the freezing process (Rahman, 2009; Pham, 2016; Damodaran and Parkin, 2017), which needs to be taken into account when estimating the initial freezing point and the ice fraction in the fish matrix (see Section 2.1.1).
Therefore, T* in fish is below the temperature of melting ice. In fresh non‐dehydrated and non‐processed (added solutes) food, T* depends on several factors (Pham, 1987; Miles et al., 1997). The composition linked to the fish species in terms of free water content and solutes on free water explains why T* in freshwater fish is usually higher compared to seawater fish and that of pelagic fish is lower compared to demersal fish (Wu et al., 2014). The sea temperature where the fish lives before its capture influences the amount of solutes and thus the initial freezing point; e.g. in Atlantic cod (Gadus morhua), it was reported to vary between –0.8°C in July–August and –1.3°C in March (Simpson and Haard, 1987).
The T* values estimated from food proximate composition data are shown in Table 2. The lowest value of T* corresponds to salmon (mean –0.77°C), which has the highest fat content. Among the lean fish, similar T* values were estimated for cod (mean –0.67°C) and sea perch (mean –0.65°C). Very few data on the composition of the Nile perch were found in the literature, which was supplemented with data provided by Antonio Solimeo (quality manager FIORITAL) by e‐mail on 7 October 2020 and 6 November 2020 (Solimeo, 2020a,b). Available data show that this freshwater fish contains a lower content of ash compared to sea water fish, which results in the higher T* estimated for Nile perch (mean –0.33°C). The high T* of Nile perch could be related to the warmer environment compared to that of cod and salmon, as the mean water temperature of the Victoria lake ranges from 23.4 to 29.0°C.11
Table 2.
Values of initial freezing point estimated from the proximate composition data for salmon, cod, sea perch and Nile percha – see Section 2.1.1 for estimation details
| Fish | Water content (%) | Protein content (%) | Fat content (%) | Ash content (%) | T* | Source of proximate composition |
|---|---|---|---|---|---|---|
| Salmon | 76.35 | 19.94 | 3.45 | 1.22 | –0.74 | ASHRAE (2018) |
| 73.1 | 19.5 | 11.5 | 1.28 | –0.81 | Krzynowek and Murphy (1987) | |
| 74.1 | 21.3 | 3.86 | 1.18 | –0.74 | Krzynowek and Murphy (1987) | |
| 72.6 | 21.7 | 5.31 | 1.21 | –0.77 | Krzynowek and Murphy (1987) | |
| 75.6 | 19 | 4.76 | 1.2 | –0.74 | Krzynowek and Murphy (1987) | |
| 70 | 21.3 | 8.55 | 1.18 | –0.78 | Krzynowek and Murphy (1987) | |
| 71.9 | 22.8 | 6.3 | 1.2 | –0.77 | Krzynowek and Murphy (1987) | |
| 73.65 | 18.81 | 4.46 | 0.96 | –0.60 | Atanasoff et al. (2013) | |
| 61.07 | 20.28 | 17.23 | 1.23 | –0.93 | Şengör et al. (2013) | |
| 68.05 | 18.66 | 11.98 | 1.23 | –0.84 | Bastías et al. (2017) | |
| Cod | 81.22 | 17.81 | 0.67 | 1.16 | –0.66 | ASHRAE (2018) |
| 81.4 | 17.9 | 0.9 | 1.2 | –0.68 | Krzynowek and Murphy (1987) | |
| 80.9 | 18.42 | 0.42 | 0.92 | –0.53 | Krzynowek and Murphy (1987) | |
| 82.4 | 16 | 0.48 | 1.12 | –0.63 | Krzynowek and Murphy (1987) | |
| 81.1 | 17.6 | 0.96 | 1.2 | –0.69 | Krzynowek and Murphy (1987) | |
| 79.9 | 18.1 | 1.42 | 1.22 | –0.71 | Krzynowek and Murphy (1987) | |
| 79.73 | 14.93 | 4.28 | 1.3 | –0.76 | Krzynowek and Murphy (1987) | |
| 81.1 | 17.9 | 0.66 | 1.2 | –0.69 | Krzynowek and Murphy (1987) | |
| 81.8 | 17.1 | 0.78 | 1.06 | –0.60 | Krzynowek and Murphy (1987) | |
| 82 | 17 | 0.6 | 1.1 | –0.62 | Krzynowek and Murphy (1987) | |
| 82.9 | 4.06 | 1.9 | 1.52 | –0.85 | Krzynowek and Murphy (1987) | |
| Nile Perch | 78.5 | 19.8 | 0.63 | 0.63 | –0.37 | Okeyo et al. (2009) |
| 81.22 | 18.01 | 0.52 | 0.25 | –0.14 | Wen et al. (2016) | |
| 77.3 | 20.5 | 1.4 | 0.5 | –0.30 | Skin on fillet (Solimeo, 2020a) | |
| 77.7 | 20 | 1.5 | 0.5 | –0.30 | Skinless fillet (Solimeo, 2020a) | |
| 80.4 | 18.5 | 0.7 | 0.9 | –0.52 | Skinless fillet (Solimeo, 2020b) | |
| Perch | 83.07 | 15.31 | 1.54 | 1.2 | –0.67 | https://fdc.nal.usda.gov/fdc-app.html#/?query=perch |
| 79.29 | 18.93 | 1.79 | 1.07 | –0.63 | https://nutritiondata.self.com/facts/finfish-and-shellfish-products/4083/2 | |
| 79.17 | 19.33 | 1.00 | 1.17 | –0.68 | https://nutritiondata.self.com/facts/finfish-and-shellfish-products/4086/2 | |
| 83.07 | 15.31 | 1.54 | 1.2 | –0.67 | https://fdc.nal.usda.gov/fdc-app.html#/?query=perch | |
| 78.7 | 18.62 | 1.63 | 1.2 | –0.71 | (ASHRAE, 2018) | |
| 79.2 | 17.7 | 2 | 1 | –0.59 | Krzynowek and Murphy (1987) | |
| 79.2 | 19 | 1.43 | 1.16 | –0.68 | Krzynowek and Murphy (1987) | |
| 81.6 | 17 | 0.6 | 0.9 | –0.51 | Krzynowek and Murphy (1987) | |
| 77.55 | 19.8 | 2.5 | 1.2 | –0.72 | Krzynowek and Murphy (1987) | |
| 79.1 | 19.4 | 0.8 | 1.1 | –0.65 | Krzynowek and Murphy (1987) |
Cod species included Gadus morhua, Gadus macrocephalus, Ophiodon elongatus, Physiculus bacchus and unspecified species; Sea perch included Sebastes marinus, Sebastes alutus, Helicolenus papillosus, Morone americanus, Perca flavescens and unspecified species); Nile perch is Lates niloticus; and salmon included Salmo salar, Oncorhynchus tshawytscha, Oncorhynchus keta, Oncorhynchus kisutch, Oncorhynchus gorbuscha, Oncorhynchus nerka and unspecified species.
The T* values reported in the literature for cod, perch and salmon are shown in Appendix B.3. It is worth highlighting that in engineering texts (e.g. ASHARE) the T* of almost all fish types is –2.2°C without reporting the methodology for its determination, which led to the working group deciding to withdraw these values. From data retrieved with the other references, the mean T* of salmon, cod and sea perch are –1.37°C, –0.94°C and –0.88°C, respectively. These values are slightly lower than those estimated in the present work (i.e. –0.77°C, –0.67°C and –0.65°C, respectively).
3.1.1.2. Temperature range of fish using heat transfer balance
During the storage/transport of fish in a box, the temperatures of CFFP will tend to approach the temperature of melting ice (0°C, provided that ice is made of freshwater) as long as there is still ice in the box. The initial temperature of the CFFP can be as high as 4–8°C depending on the temperature before the handling during gutting, heading, filleting and the conditions of handling itself (Rotabakk et al., 2014; EFSA BIOHAZ Panel, 2020). The temperature will decrease due to the cooling capacity of the ice in the box, tending to approach the temperature of melting ice. In this process and during the subsequent storage, the ice will gradually melt due to the absorption of heat from the fish and the outside conditions. When the whole mass of ice is melted, the fish temperature will rise due the absorption of heat from the outside.
The initial temperatures of SFFP are up to 2°C below the initial freezing point of the fish, which is lower than the temperature of melting ice made from freshwater. During the subsequent storage in a box without ice, the fish temperature will gradually increase to 0°C, and therefore, the amount of ice inside the fish will decrease due to the absorption of heat from the outside.
Figure 5 conceptually represents the temperature dynamics of fish when stored/transported under the current authorised practices (CFFP) in comparison with the superchilling practices (SFFP) to the point in which all ice has melted. From a qualitative perspective:
under practices ensuring that there is ice in the box of CFFP (α > 0) and the SFFP keeps an ice fraction (Xice > 0) during the whole period of storage and transport, fish temperatures in SFFP are never higher, but usually lower, than fish temperatures in CFFP (Figure 5a); and
fish temperatures in SFFP can only be higher than in CFFP when the absorbed heat during storage/transport completely melts the ice inside the SFFP (Xice > 0) and raises the fish temperature above 0°C, while there is still ice in the box of CFFP (α > 0) (Figure 5b).
Figure 5.
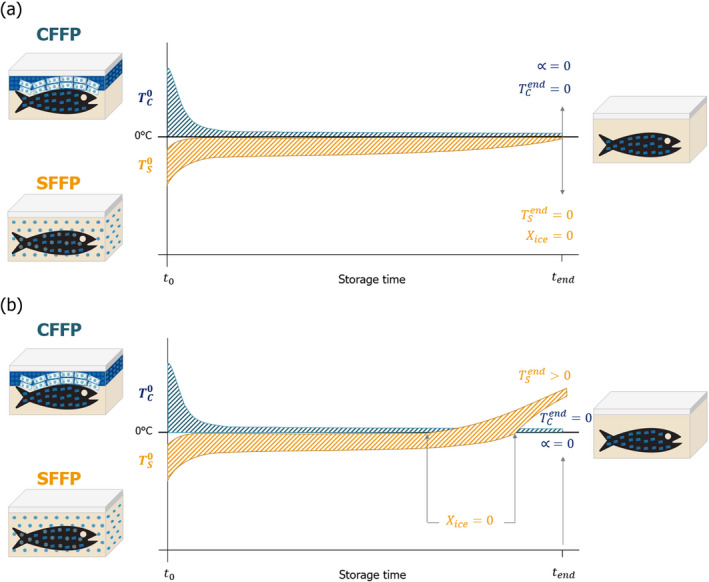
Conceptual representation of the temperature dynamics of the fresh fishery products when stored/transported under the currently authorised condition (CFFP) in comparison with the alternative condition (superchilled; SFFP) to the point in which all ice has melted (i.e. α = Xice = 0)
- The initial fish temperature in the fish matrix is denoted by for CFFP and for SFFP; the temperatures after storage and transport are and for CFFP and SFFP, respectively; the mass of ice in CFFP is mice, and the ice fraction in the SFFP is Xice. (a) under practices ensuring that there is ice in the box of CFFP and the SFFP maintains an ice fraction during the whole period of storage and transport; (b) when the absorbed heat during storage/transport completely melts the ice inside the SFFP and raises fish temperature above 0°C, while there is still ice in the box of CFFP.
From this qualitative reasoning, the questions that have been assessed using heat transfer balance models included (i) the initial configurations for both CFFP and SFFP that are equally capable of absorbing the same amount of heat before all ice melts, i.e. ice in the fish (Xice) for the SFFP and ice in the box (α) for the CFFP and (ii) the ratio between the quantity of heat that predefined initial conditions for SFFP and CFFP can absorb, before all ice melts.
Two representative cases of lean and fat sea fish species were used as examples in the assessment, i.e. cod and salmon. In addition, a species from temperate freshwater (e.g. Nile perch) was considered.
i) Initial proportion of ice in CFFP (α) needed to equal the absorbing heat capacity of SFFP
In Section 2.1.1, two heat balances were derived:
Equation 4: required heat by CFFP (Qc) to conclude the storage with a fish temperature with all ice melted (α = 0)
Equation 5: required heat in SFFP (Qs) to conclude the storage with a fish temperature without ice in the fish (Xice = 0)
Assuming that conditions of storage and transport are equivalent for both CFFP and SFFP (i.e. same outside temperature, storage time, box insulating capacity…) and rate of heat absorption is similar, they will absorb the same amount of heat (i.e. Qc = Qs) and the proportion of mass of ice per mass of fish in CFFP (α) can be calculated to simulate the behaviour of SFFP. Figure 6 provides an overview of the simulation process, i.e. the input data, the assumptions, equations and fixed parameters used in the model and the output. The calculations can be made using the option 5 of the HTM‐SFFP Tool developed, made available through the Knowledge Junction under https://doi.org/10.5281/zenodo.4304283).
Figure 6.
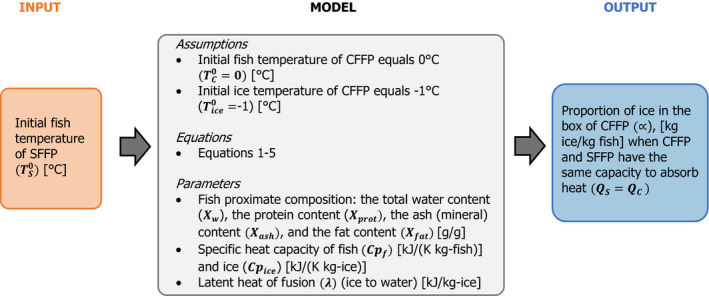
Overview of the model to derive the proportion of ice in CFFP (α) to equal the absorbing heat capacity of SFFP before all ice melts
The predictions for three different types of fish covering a high fat content fish (salmon), a lean fish (cod) and a temperate freshwater fish (Nile perch) are shown in Figure 7. For example, salmon that is superchilled to –1.04°C contains an ice fraction of Xice = 0.1704, being capable of absorbing the same amount of heat as conventionally chilled salmon placed in a box with α = 0.18 kg ice per kg of fish. Note that Xice = 0.1704 and α = 0.1800 have similar values, the small difference is due to the sensible heat, that is greater in SFFP than in CFFP and compensates for the difference in latent heat of 0.0096 kg ice per kg of fish. When the salmon is superchilled to lower temperatures, it will contain a higher ice fraction and be equivalent to a conventionally chilled salmon, with more ice per kg of fish.
Figure 7.
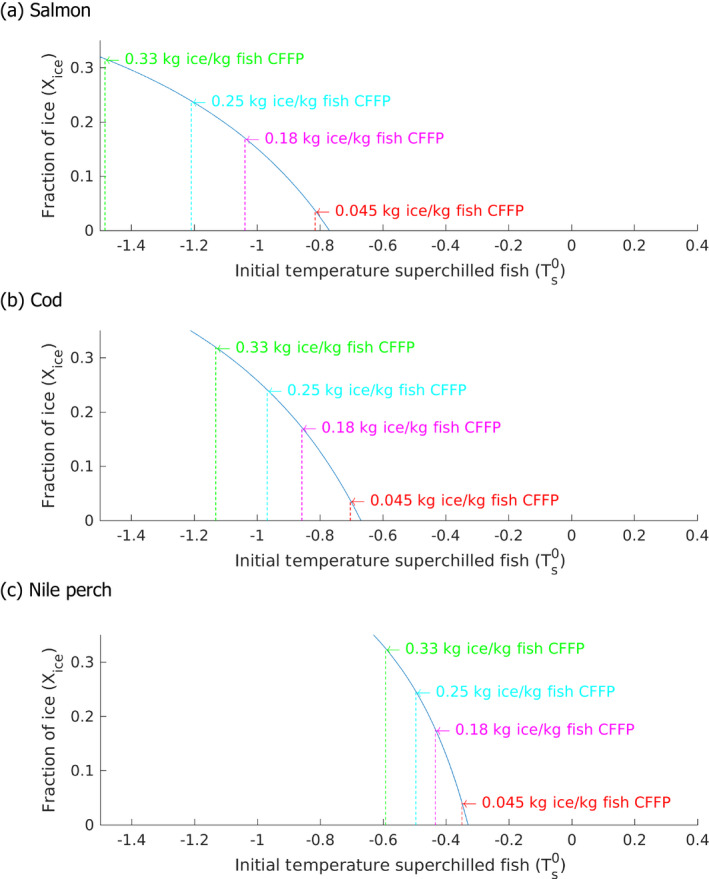
Proportion of ice in CFFP (α, kg ice/kg fish) needed to equal the absorbing heat capacity of SFFP as a function of the degree of superchilling (i.e. ice fraction or the associated initial temperature) of the SFFP
- (a) salmon, (b) cod, (c) Nile perch. The α values shown in the plots correspond to reasonably foreseeable scenarios described in Section 2.1.1.
For cod and Nile perch, the results are practically the same as for salmon considering the ice fraction in superchilled fish and the amount of ice added to the box. For example, superchilled cod with Xice = 0.1715 and superchilled Nile perch with Xice = 0.1733 correspond to α = 0.1800 in CFFP. Again, differences between species in superchilled ice are minor and due to differences in sensible heat. Their ice fraction would however correspond to a higher initial temperature of the superchilled cod (i.e. –0.85) and in particular for Nile perch (i.e. –0.34), compared to superchilled salmon, due to the higher initial freezing point (T*) of these two lean species.
ii) Ratio between the quantity of heat that SFFP can absorb compared to that of CFFP, before all ice melts
Using the heat transfer balances, for predefined initial configuration parameters of the SFFP and the CFFP, the estimated amount of heat needed to end up with fish at 0°C and ice completely melted when exposed to the same storage and transport configurations (i.e. same outside temperature, storage time, box insulating capacity…) is presented in Figure 5a. The initial configuration of SFFP considers the initial fish temperature , which determines the initial ice fraction inside the fish matrix (Xice). The initial conditions of CFFP include the proportion of kg of ice per kg of fish (α), initial temperature of fish and initial temperature of ice . Other parameters involved in the heat transfer balance are dependent on the fish species.
The SFFP temperatures may be higher (but not necessarily) than CFFP if the heat needed by SFFP to raise fish temperatures to 0°C without ice (QS) is lower than in CFFP (QC), i.e. R < 1.
The estimation of this ratio (R) allows assessment of which condition supports better the outside heat in order to maintain fish temperatures close to 0°C (for CFFP) or just below zero (for SFFP). Figure 8 provides an overview of the simulation process, i.e. the input data, the assumptions, equations and fixed parameters used in the model and the output. The calculations can be made using the option 2 of the HTM‐SFFP Tool developed, made available through the Knowledge Junction under https://doi.org/10.5281/zenodo.4304283).
Figure 8.
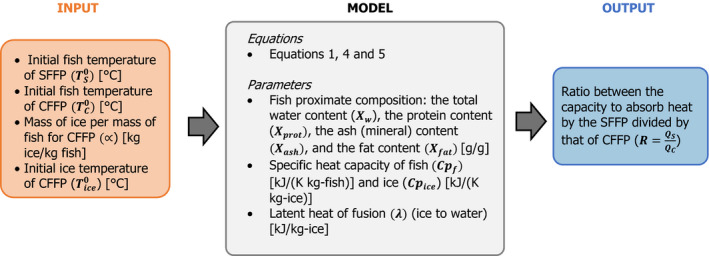
Overview of the model to derive the ratio between the quantity of heat that a predefined initial configuration for SFFP (i.e. degree of superchilling) can absorb compared to that of CFFP, before all ice
The ratio (R) varies with initial fish temperatures, both for CFFP and SFFP (corresponding to a given ice fraction) as well as the proportion of ice:fish in CFFP (α value). Superchilled initial conditions leading to R values above 1 indicate that the SFFP in boxes without ice have a higher capacity to absorb external heat compared to the CFFP in boxes with ice.
In Figure 9, the R values are depicted for different scenarios of initial configurations of CFFP (α values, as described in Section 2.1.1) in different plots and the initial fish temperature in the X‐axis of each plot. The degree of superchilling corresponding to different ice fraction targets is represented by different lines within each plot, which are associated with the initial superchilled fish temperature shown in parenthesis.
Figure 9.
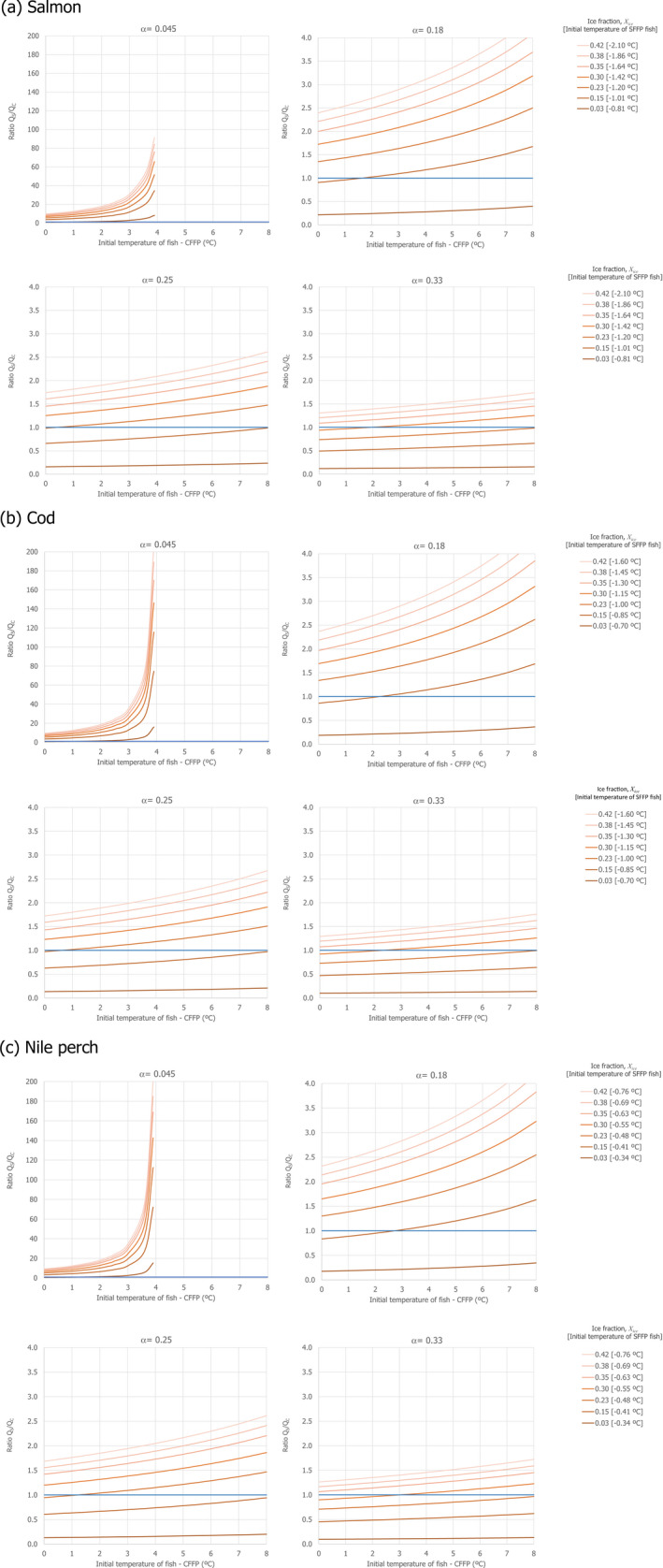
Ratio between the heat that the SFFP and the CFFP could absorb before all ice has melted (and fish would start increasing in temperature above 0°C) for (a) salmon, (b) cod and (c) Nile perch
- Each plot represents the chosen scenario for different proportions of ice:fish for CFFP (α value, as described in Section 2.1.1). Initial fish temperatures for CFFP are shown on the X axis of the plots. Different lines within each plot represent a target ice fraction of SFFP from 3% to 42%. For salmon, the ice fraction of 3% corresponds to a superchilled salmon with an estimated initial temperature of –0.81°C while an ice fraction of 42% corresponds to –2.10°C. For the simulations, the initial freezing point of salmon was T* = –0.77°C and the free water content was 65.77%. For cod, the ice fraction of 3% corresponds to a superchilled cod with an estimated initial temperature of –0.70°C while an ice fraction of 42% corresponds to –1.60°C. For the simulations, the initial freezing point of cod was T* = –0.68°C and the free water content was 71.75%. For Nile perch, the ice fraction of 3% corresponds to a superchilled Nile perch with an estimated initial temperature of –0.34°C while an ice fraction of 42% corresponds to –0.76°C. For the simulations, the initial freezing point of Nile perch was T* = –0.33°C and the free water content was 72.79%. The values of the initial freezing point and free water content were the mean values calculated from the proximate composition found in literature (Table 2).
For example, in salmon (Figure 9a), the R value is equal to or higher than 1 when α = 0.045 irrespectively of the degree of superchilling as long as it achieves at least 2% of ice fraction (corresponding to a temperature of –0.8°C). This proportion of 1 kg of ice for 22 kg of fish can be considered a worst‐case scenario for CFFP (i.e. with the lowest capacity to absorb heat) and is usually used when the fish is precooled to a maximum of 4°C. Also, a slightly superchilled cod (with at least 3% of ice fraction corresponding to temperature below –0.7°C) will have a higher capacity to absorb heat and keep the fish temperature under control during storage and transport.
For the other scenarios of CFFP (α values and initial fish temperature), a minimum degree of superchilling would be necessary to ensure that the SFFP will be able to absorb at least the same quantity of heat as the CFFP before all the ice has melted. The minimum degree of superchilling corresponding to an ice fraction in the SFFP (Xice value) would be similar to the α value for the CFFP.
When the degree of superchilling is higher than this minimum, the R value will always be higher than 1. For instance, a superchilled cod with an ice fraction of 30% (corresponding to an initial superchilling temperature of –1.4°C) could absorb:
nearly ninefold more heat than a conventionally chilled cod showing an initial temperature of 1°C (i.e. precooled) and stored in a box with 1 kg of ice per 22 kg of fish (α = 0.045).
about 1.8‐fold higher than that of a conventionally chilled cod showing an initial temperature of 2°C and stored in a box with 4 kg of ice per 22 kg of fish (α = 0.18).
about 1.3‐fold higher than that of a conventionally chilled cod showing an initial temperature of 3°C and stored in a box with 0.75 kg ice for 3 kg of fish (α = 0.25).
almost the same as that of a conventionally chilled cod showing an initial temperature of 1°C and stored in a box with 3.75 kg of ice per 15 kg of fish (α = 0.33). In fact, SFFP with an ice fraction of 30% represents practically the same proportion of ice considered with a CFFP of α = 0.33, i.e. 33% of ice with respect to the fish mass.
Similar figures can be derived from the simulations carried out for cod (Figure 9b) and Nile Perch (Figure 9c). However, as salmon has a lower T*, the initial temperature of the SFFP for a given ice fraction is lower compared to cod. In contrast, for Nile perch, with a higher T* than cod and salmon, initial SFFP temperatures just slightly below 0°C will correspond to a significant ice fraction in the fish matrix.
In cases where the degree of superchilling is below this minimum, the R value can still be higher than 1 depending on the initial temperature of the CFFP considered. The scenarios in which CFFP can absorb more heat than the SFFP (R < 1), correspond to worst‐case initial configurations of SFFP (due to low degree of superchilling) and a more optimal initial configuration for CFFP (i.e. high α value). Considering that the FBO needs to adjust the initial configuration of FFP to the envisaged conditions of storage and transport of the intended supply chain, the comparison of worst‐case vs. optimal configuration between the SFFP and CFFP would be meaningless from the technological and commercial point of view.
For Nile Perch, the distribution of temperatures reported in the Nile Perch study (see Section 3.1.2.1) was used to carry out a stochastic approach of the impact of the distribution of key parameters associated with both CFFP and the SFFP on the probability of an R value above 1, and thus, SFFP temperatures would be equal or lower than those of CFFP. More specifically, the temperature distribution before the superchilling (after filleting) was assumed to be a reasonably foreseeable distribution of the initial temperature of CFFP when transferred to the box with ice. The temperature distribution after the superchilling was used to describe the variability of the initial configuration of SFFP and to derive the initial ice fraction inside the fish, taking into account the mean and standard deviation of the initial freezing point estimated for Nile perch (Table 1).
When the superchilled Nile perch was compared with different CFFP scenarios (in terms of proportion of ice per mass of fish, α value), the probability of obtaining R ≥ 1 was 99.9%, 99.6%, 99.4%, 97.8% for the scenarios of α equal to 0.045, 0.18, 0.25 and 0.33, respectively (Figure 10).
Figure 10.
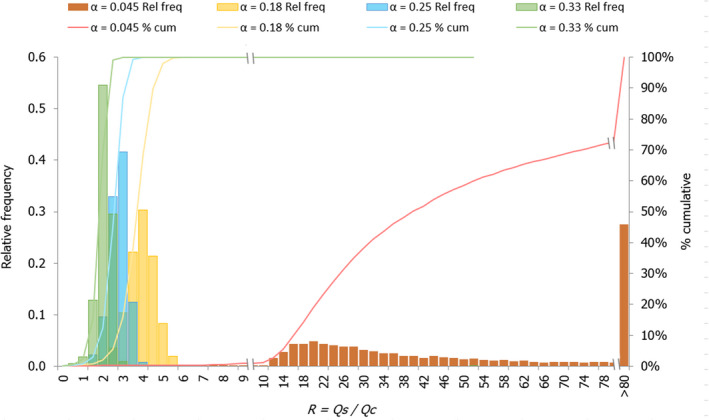
Probability distribution of the ratio (R) between Qs and Qc for Nile perch resulting from the stochastic approach through the heat transfer balance simulation (input values shown in Table 1)
- Relative frequency (Rel freq) and cumulative (% cum) distributions are shown.
In fact, the ice fraction (Xice), as calculated from the initial SFFP temperature for Nile perch, indicates that 90% of the Xice values would be within the range of 0.37–0.68 (Figure 11). These Xice values are considerably higher than the proportion of ice usually used in foreseeable scenarios of CFFP (α), making the capacity to absorb heat by the SFFP Nile perch higher than any of the considered scenarios of conventionally chilled Nile perch. Therefore, the usual practices of superchilling applied to Nile perch leads to a higher ice fraction than the target of 5–30%. These results can be mainly explained by the relatively high initial freezing point estimated for Nile perch (i.e. a T* close to 0°C) because it is a temperate freshwater fish, with a lower content of solutes in the water phase compared with colder seawater fish (Section 3.1.1.1).
Figure 11.
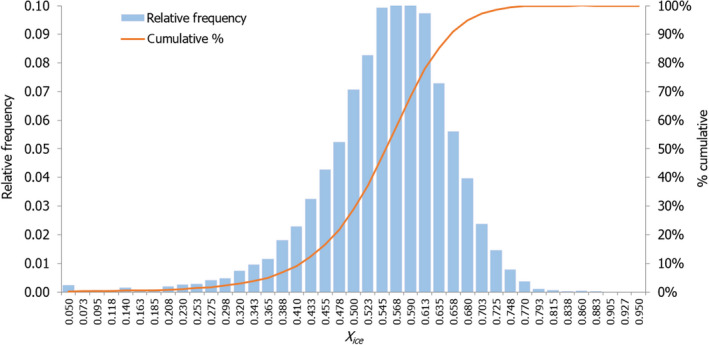
Probability distribution of the ice fraction (Xice) derived from the distribution of the initial temperature of superchilled Nile perch (data from Nile perch study) and the initial freezing point of Nile perch as described in Table 1
3.1.2. Observed data
3.1.2.1. Nile perch study by the Dutch Fish Importers Association
The ‘Nile perch study’ consisted of two field studies gathering temperature data associated with the superchilling process as well as the transport of the superchilled Nile perch fillets in EPS boxes (without ice) until arrival at the European establishment. The study was focused on measurements of samples of the commercial production and supply chain coming from several suppliers of Nile perch caught in the Lake Victoria. Specifically, the studies dealt with:
-
a)
Collection of data within the self‐monitoring programme of the six companies, suppliers of superchilled Nile perch during a period of 3 months (December 2017 to March 2018). Temperature data covered the superchilling process (n = 968 measured samples before and after the superchilling), the cold chain during air transport from Africa to Europe (using nine data loggers in the superchilled fish inside the box) and fish core temperature at the destination Europe establishment (n = 474).
-
b)
Collection of data from four companies carried out between 30 May and 5 June 2018; data were measured/verified by a third independent party (e.g. CA, quality bureaus and certified laboratories). Temperature data covered the superchilling process (n = 24 measured samples before and after the superchilling), the cold chain during air transport from Africa to Europe (using four data loggers in the superchilled fish inside the box) and fish core temperature at the destination Europe establishment (n = 24).
Figure 12 shows the distribution of the recorded temperature of the fish in each study and step. The descriptive statistics by supplier and overall are shown in Table 3. There were slight but statistically significant differences in the means of the recorded temperatures among the suppliers of superchilled Nile perch fillets.
Figure 12.
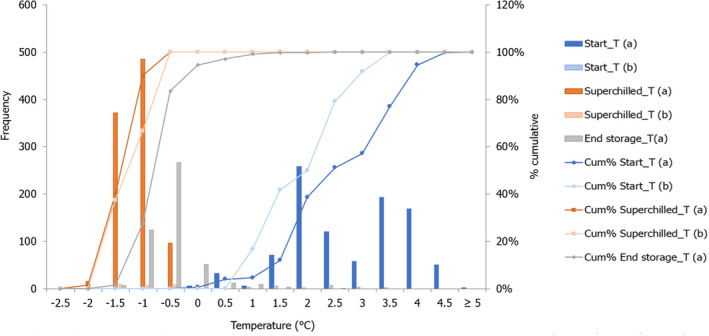
Frequency and cumulative (cum%) distribution of the temperature of Nile perch fillets before (Start_T) and after (Superchilled_T) the superchilling process at the origin (African supplier) and at arrival to the destination (European establishment, End storage_T)
- Data reported in the ‘Nile perch study’ were obtained from (a) the self‐monitoring programme of five companies and (b) measurements made by a third independent party. The data provided by the supplier were combined for this description. Descriptive statistics of the data by supplier of the self‐monitoring programme are summarised in Table 5.
Table 3.
Summary of the temperature of Nile perch fillets before and after the superchilling process at the origin (African supplier) and at arrival to the destination (European establishment)
| Company/Supplier of superchilled Nile perch fillets | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | all | |
| Start fish temperature (before superchilling) | ||||||
| Mean* | 1.9c | 3.6a | 2.0b | 1.7c | 0.3d | 2.6 |
| SD | 0.4 | 0.4 | 0.5 | 0.2 | 0.2 | 1.0 |
| max | 2.9 | 4.8 | 3.6 | 2.0 | 0.7 | 4.8 |
| P95 | 2.6 | 4.3 | 3.1 | 2.0 | 0.6 | 4.1 |
| P90 | 2.4 | 4.1 | 2.7 | 1.9 | 0.5 | 3.8 |
| P75 | 2.2 | 3.8 | 2.2 | 1.8 | 0.4 | 3.5 |
| P50 | 1.9 | 3.6 | 2.0 | 1.8 | 0.2 | 2.4 |
| P25 | 1.7 | 3.3 | 1.8 | 1.7 | 0.1 | 1.9 |
| min | 1.0 | 2.5 | 0.9 | 1.2 | 0.0 | 0.0 |
| (# samples) | (n = 290) | (n = 430) | (n = 177) | (n = 28) | (n = 43) | (n = 968) |
| Superchilled fish temperature (after superchilling) | ||||||
| Mean* | –1.1b | –1.6d | –1.2c | –0.9a | –1.6d | –1.3 |
| SD | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | 0.3 |
| max | –0.6 | –0.8 | –0.9 | –0.7 | –1.4 | –0.6 |
| P95 | –0.6 | –1.2 | –1.0 | –0.7 | –1.4 | –0.8 |
| P90 | –0.8 | –1.3 | –1.1 | –0.8 | –1.5 | –1.0 |
| P75 | –1.0 | –1.4 | –1.2 | –0.9 | –1.5 | –1.1 |
| P50 | –1.1 | –1.6 | –1.2 | –0.9 | –1.6 | –1.4 |
| P25 | –1.2 | –1.7 | –1.3 | –1.0 | –1.7 | –1.6 |
| min | –2.4 | –2.1 | –1.8 | –1.1 | –2.0 | –2.4 |
| (# samples) | (n = 290) | (n = 430) | (n = 177) | (n = 28) | (n = 43) | (n = 968) |
| Fish temperature at the end of transport/storage (at arrival to the EU establishment) | ||||||
| Mean* | –0.7 | –0.7 | –0.7 | –0.7 | –0.7 | –0.7 |
| SD | 0.5 | 0.6 | 0.3 | 0.4 | 0.4 | 0.4 |
| max | 1.2 | 2.4 | 0.7 | 1.4 | 0.9 | 2.4 |
| P95 | 0.3 | 0.4 | –0.2 | –0.1 | 0.4 | 0.3 |
| P90 | –0.3 | –0.2 | –0.5 | –0.3 | –0.3 | –0.3 |
| P75 | –0.5 | –0.6 | –0.6 | –0.5 | –0.6 | –0.6 |
| P50 | –0.8 | –0.8 | –0.8 | –0.8 | –0.8 | –0.8 |
| P25 | –1.0 | –1.0 | –0.9 | –0.9 | –0.9 | –1.0 |
| min | –1.6 | –1.5 | –1.4 | –1.6 | –1.6 | –1.6 |
| (# samples) | (n = 135) | (n = 91) | (n = 52) | (n = 137) | (n = 59) | (n = 474) |
SD: standard deviation; P: percentile.
Superscript letters indicate statistically significant differences among mean values (Tukey HSD comparisons).
Note: Data reported in the ‘Nile perch study’ were obtained from the self‐monitoring programme of five companies.
The temperature of fish measured in the core of the product immediately after superchilling ranged from –2.4°C to –0.6°C, with differences in the mean SFFP temperature depending on the producer which would correspond to a degree of superchilling in terms of frozen water in the fish matrix (Xice) of 62.8%–32.8% according to estimation provided by Equation 1).
Overall, the core temperature of the fish at the destination establishment was below 0°C in the 96% of the measured samples (n = 474) (mean –0.7°C), only 0.5% of samples were between 1°C and 2.4°C (max). These data indicate that the initial configuration of SFFP was appropriate to keep the fish temperature under control throughout the air and truck transport of Nile perch fillets from Africa to Europe.
3.1.2.2. Literature data
The literature review provided six records with information on the temperature of SFFP (alternative condition) during real or simulated transport and/or storage. Five records also reported temperatures for CFFP (baseline condition) in experiments run in parallel with the alternative condition, though not always using the same outside temperature conditions of storage. One additional record was found dealing with Nile perch fillets from Lake Victoria imported to the European market, reporting temperatures of commercial SFFP and CFFP samples.
Three studies included information on SFFP and CFFP samples simultaneously stored under identical outside temperature conditions.
Rotabakk et al. (2014) analysed the effect of storage of CFFP (ice:fish ratio 1:4, α = 0.25α = 0.25) and SFFP (superchilled in a nitrogen freezer at –60°C for 4 min) in cod fillets obtained after two different blood removal procedures (initial temperature 6.5°C). Fillets were packed in EPS boxes, transported (2 days) and stored (further 5 days) under the same chilled conditions (0–0.5°C). Cod fillet temperatures were monitored using loggers placed in a fillet in the middle of the box. SFFP had an equilibrium temperature after processing of –0.7 ± 0.1°C and this temperature was held throughout the whole transport/storage period with only minimal fluctuations (–0.5 to –1°C). CFFP required the first 24 h to reach an equilibrium at 0°C, and then remained stable at this temperature up to the end of the experiment. Therefore, the SFFP temperature was overall 1.0–0.5°C lower than the CFFP throughout transport under a properly controlled chilling temperature.
In Margeirsson et al. (2017), gutted rainbow trout as CFFP (3–4 kg of ice for 21 kg of fish, ) or SFFP (superchilled in slurry ice to ca. –1.0 to –1.5°C) were packed in EPS boxes. The boxes were transported by truck (from Iceland), then transported by sea in containers (to Rotterdam) and then transported by truck to Poland, for an overall transport duration of 8 days. During transport, the temperature of the refrigerated trucks and containers was set at –1.0°C. Fish and outside temperatures were monitored. With the exception of brief temperature spikes principally during loading/unloading activities, outside air temperature was around 1–2°C in the first truck (on‐land transport in Iceland), close to –1°C in the container during sea transport and around 0°C in the truck during the last day of transport. In these conditions, SFFP in EPS displayed a slight increase of the temperature (from –1.5°C to approximately –0.7°C), while CFFP required approximately 24 h to reach the temperature of 0°C from the initial value of approximately 3°C, and then maintained a temperature between 0°C and –0.4°C during the remaining period. Therefore, the SFFP temperature was overall 1.5–0.3°C lower than the CFFP during the chilled transportation.
In Erikson et al. (2011), gutted Atlantic salmon (initial temperature of 8.1°C) were superchilled by immersion for 24 h into seawater slurry (salinity: 34 ppt, temperature –1.93 ± 0.27°C). Then, SFFP were moved to extruded polystyrene (Styrofoam) boxes and stored for 3 days in a cold room (4.2–4.5°C). As a control, gutted fishes were also stored under crushed ice (CFFP), ice constituting 40% of total fish weight (i.e. α = 0.4) in order to ensure proper chilled storage. This condition represented an excess of ice compared to the most extreme scenario assessed in this opinion (α = 0.33, Section 3.1.1.2). Both SFFP and CFFP boxes were stored in the same cold room. For both SFFP and CFFP, the temperature was recorded using loggers placed inside the fish body cavity and was also measured manually using a probe placed just under the skin. During storage, the core temperature of SFFP increased from ‐1.4°C to about –0.5°C on day 3 (end of transport phase), while the subcutaneous temperature reached approximately +0.5°C. On day 4 (representing the fish's arrival to the market), while the cold reservoir within the fish was being depleted, the temperature increased at greater speed, reaching about 2.5–3.0°C in the core and approximately 1°C higher on the fish surface. In contrast, between the first and the fourth day, the temperature in the CFFP increased, approximately from +0.5°C to 1.5°C (the surface temperature being around 1°C above the core temperature also in this case). In CFFP, approximately a 1°C difference between core and subcutaneous temperatures was recorded. By contrast, the temperature distribution in SFFP was more even and temperature variations between fish were smaller than those of CFFP, particularly during the first 72 h of storage. The study showed that the transport of salmon as SFFP without ice in the boxes seemed feasible under the assessed conditions (superchilling to –1.5 to –2.0°C, storage in closed Styrofoam boxes at a surrounding temperature of 4.5°C for maximum 3 days), keeping the temperature of the superchilled salmon approximately 1.5 to 1.0°C below that observed in the CFFP conditions. According to the authors’ conclusions, however, under less ideal storage conditions, SFFP would be vulnerable to a temperature rise that might considerably reduce fish quality.
Two more studies reported temperature profiles of SFFP and CFFP stored under slightly different external temperature conditions.
In Eliasson et al. (2019), gutted and bled cod processed on‐board (initial temperature of 6°C) were either iced or superchilled with chilled seawater in a screw conveyor with a two‐stage cooling process: first –0.8°C for 20 min and then –2.8°C for 8 min. Both CFFP and SFFP fish were then stored for 3 days in 460 L insulated tubs on the processing deck (iced cod; approximately 0°C) or in the ship hold (superchilled; –1°C). On day 3, fish were processed (filleting and packing). Fish temperature was monitored using loggers placed into the flesh (centre of the loin). After superchilling, SFFP reached –0.8°C and temperature remained stable throughout the 3‐day storage period. CFFP cod, instead, displayed a slower temperature decrease, reaching approximately 0.5°C within 24 h, then further decreasing to 0°C during the subsequent 48 h.
Tryggvason et al. (2020) evaluated the effect of traditional icing and superchilling on Atlantic salmon. Gutted fish were superchilled in brine to between –1 and –2°C and subsequently packed into either PE boxes of different size and volume (290, 460 or 660 L) or 40 L EPS boxes (79 × 39 × 23 cm and 17 cm depth). In parallel, the non precooled fish (~ 5°C) were packed in EPS boxes under ice (fish to ice ratio of 5:1). The fish in PE boxes were covered by a plastic layer, while the EPS boxes were closed by a lid. After packing, the boxes were transported by truck for approximately 560 km in a refrigerated container at around –1 to +5°C. Subsequently, they were stored for up to 14 days: for SFFP at around –1 to 0°C (mean temperature and standard deviation: –0.7 ± 0.7°C) and for CFFP at around 0 to +1°C (–0.3 ± 0.8°C for CFFP). The initial temperature of the superchilled salmon (measured in the abdominal cavity) ranged from just below –2°C to just above –1°C depending on the box. The average temperature of the superchilled salmon during the storage period in EPS boxes was –1.1 ± 0.2°C, while in insulated PE boxes was –1.0 ± 0.1°C, –1.4 ± 0.2°C and –1.2 ± 0.0°C in 290, 460 and 660 L boxes, respectively. The iced salmon in EPS boxes showed an initial temperature of up to 3°C and reached, after about 1 day, an average equilibrium temperature of +0.2 ± 0.3°C.
Another study (Margeirsson et al., 2012a) addressed the effect of transport and storage on SFFP products in boxes in which temperature control was further supported by the cooling effect of added gel‐packs. In this study, no comparison with the temperature of CFFP was undertaken. Fresh cod fillets (loins) were superchilled with a combined blast and contact (CBC) cooler, skinned, portioned and packed (5 kg) into two different types of EPS boxes (conventional type: 40 × 26.5 × 15.9 cm and 10.9 cm depth or new improved type with rounded corners: 40 × 26.5 × 13.5 cm and 9 cm depth). A frozen gel pack (around –18°C; 16 × 12.5 × 0.6 cm and 125 g) was put on top of the fillets in each box. Temperatures within the boxes and in the storage/transport environments were recorded using loggers. Following transport, the palletised boxes were stored under dynamic conditions (9°C for 9 h; then 0–4°C for 3 h and 16°C for 4 h) and then kept at around 2°C for up to 10 days. During the dynamic storage conditions, the temperature in the centre of the boxes containing the superchilled cod fillets increased from approximately –1°C to between 1 and 2°C, while the temperature in the corners and on top of the superchilled loins reached a maximum of 5°C and 3°C, respectively, reflecting the abusive temperatures (16°C for 4 h), before reaching – and maintaining – the outside temperature of 2°C 16 h post‐packaging.
Lastly, one article (Klapper et al., 2017) reported information on the temperature of fresh and superchilled Nile perch (Lates niloticus) fillets imported into the European market from Lake Victoria. Fresh fillets stored in ice (n = 210) were sampled between 2013 and 2014 at local retail markets in Hamburg (Germany), while superchilled fillets (n = 20) were purchased at a Dutch wholesaler and the product temperature was measured upon arrival at the laboratory. The average temperature (and standard deviation) for the Nile perch fillets was –0.7 ± 0.7°C for CFFP and –2.5 ± 0.6°C for SFFP.
3.1.3. Relevant biological hazards for growth
The temperature of the alternative condition (SFFP) is equal or usually less favourable for microbial growth compared to the baseline condition (CFFP), provided that the initial configuration of SFFP is properly adjusted to the envisaged reasonable foreseeable conditions of transport and storage as usually done for the CFFP. Under these circumstances, the increase of any relevant biological hazard (due to growth of vegetative pathogens and/or histamine accumulation) will be at most equal or usually lower in the SFFP compared to CFFP. Consequently, the SFFP will be at least as safe as CFFP from a microbiological point of view.
3.1.4. Uncertainties associated with fish temperature of transported/stored SFFP and CFFP
The uncertainties related to the outcome of the heat transfer model are described in Table 4 and consider the assumptions used in the model and the input data. Overall, the estimations of the heat transfer model are considered conservative in the sense that the model tends to underestimate the fish surface temperature for the CFFP (i.e. the estimated value is lower than the actual one), while it can slightly under‐ or overestimate the temperature of SFFP (i.e. the estimated value is slightly lower or higher than the actual one) depending on the assumption made. Consequently, the temperature difference between CFFP and SFFP will actually be, in general, higher than estimated using the model.
The uncertainty related to the observed data due to the limited number of comparative studies of SFFP and CFFP during transport/storage, may lead to either an over‐ or underestimation of the performance of the alternative condition (SFFP) compared to the reference one (CFFP).
The impact of the identified uncertainties on the final outcome is considered limited when the degree of superchilling, defined as the initial ice fraction (Xice) in the SFFP, is compared with the proportion of ice:fish (α) in CFFP. It was elicited that an initial ice fraction in SFFP equal or higher than the proportion of ice:fish in the EPS box of CFFP will ensure, with 99–100% certainty (almost certain), that the fish temperature of SFFP at any time point of storage/transport will be lower or equal to the fish temperature of CFFP (close to 0°C, i.e. the temperature of melting ice) when exposed to the same storage/transport conditions.
However, when the degree of superchilling is unknown, which is normally the case, it can be estimated from the fish temperature after superchilling and the initial freezing point (T*). The first is subject to uncertainties related to the application of the superchilling technology as well as the measurement procedure of the fish temperature after the superchilling. The latter (T*) can be derived from the fish proximate composition, which is influenced by fish species and other factors such as seasonal variation and temperature of the catching waters, which are not always known. When taken from literature, T* of fish is often underestimated (i.e. reported as lower than the actual values), thus leading to a higher degree of superchilling than required, particularly for lean fish from temperate waters. Though the fish proximate composition can be obtained from food composition databases, it is more accurate to determine it analytically for the specific fish intended to be superchilled, taking into consideration potential variability due to batch, catching area/season etc.
The HTM‐SFFP Tool based on the developed heat transfer model, made available to identify under which initial configuration the SFFP will be at least equivalent or better than CFFP in terms of total heat absorption capacity, requires that the user introduces the values for several inputs, including (T*) or the proximate composition of the fish to derive it. The accuracy of the input data introduced is key for obtaining reliable outputs.
3.1.5. Concluding remarks related to the temperature profile of transported/stored SFFP and CFFP and, if relevant, its impact on the growth of biological hazards
When good practice is applied, ensuring that there is ice in the box of CFFP and the SFFP maintains an ice fraction during the entire storage and transport, fish temperatures in SFFP will not be higher, and will usually be lower, than temperatures of the CFFP during exposure to the same storage and transport conditions. Temperatures of SFFP could only be higher than CFFP if the absorbed heat during storage/transport completely melts the ice inside the SFFP and raises the fish temperature above 0°C, while there is still ice in the box of CFFP.
The estimation through heat balance modelling of the ratio (R) between the capacity to absorb heat of SFFP (QS) and CFFP (QC) facilitates the comparison of the configurations of each condition. A minimum degree of superchilling is necessary to ensure that the SFFP will be able to absorb at least the same quantity of heat as the CFFP before all ice is melted (R < 1). The minimum requirement corresponds to an ice fraction in the fish matrix of SFFP (Xice value) similar to the proportion ofice:fish for CFFP (α value).
The ice fraction determining the degree of superchilling in SFFP depends on the initial freezing point (T*) and the free water content (Xfree), both parameters being dependent on the fish composition. Generally, as a fish with a higher fat content has a lower T* and Xfree compared to a lean fish, the ice fraction at a given superchilling temperature is lower in high fat content fish compared to lean fish.
The scenarios in which R < 1 correspond to worst‐case SFFP initial configurations compared to optimal ones for CFFP (high α value and low initial temperature of fish). Considering that the FBO needs to adjust the initial configuration of FFP to the envisaged conditions within the intended supply chain, such comparison would be not relevant from a technological and commercial point of view.
Measured data of fish temperatures before the superchilling (considered as initial temperatures of CFFP) and after the superchilling (considered as initial SFFP temperatures) from a field study on 968 samples, from several batches of commercial superchilled Nile perch fillet collected over 3 months by five producers, were used for a stochastic assessment. It indicated that superchilled Nile perch was able to absorb more heat than CFFP with a probability of 99.9% (when compared with an ice:fish proportion of 0.045) to 97.8% (when compared with an ice:fish proportion of 0.33), when exposed to the same conditions.
According to a study in which the core temperature of superchilled Nile perch fillets ranged from –2.4°C to 0.6°C (with differences depending on the producer), the fish temperature at the destination was below 0°C in 96% of the 474 samples (mean temperature of –0.7°C, irrespectively of the producer) and only 0.5% of the samples had a temperature ranging between 1°C and the maximum recorded temperature of 2.5°C.
With the limitations deriving from the scarcity of scientific literature dealing with the comparison of temperature trends in CFFP and SFFP during transport and/or storage, the available evidence indicates that the SFFP maintains a temperature from 1.5°C to 0.3°C lower than the CFFP counterpart when exposed to the same external temperature conditions (i.e. 7 days at 0–0.5°C, 8 days at temperatures fluctuating between –1.0 and +2.0°C, 3 days at 4.2–4.5°C).
A tool was developed named the HTM‐SFFP Tool, based on a conservative heat transfer model, that can be used as part of ‘safety‐by-design’ approach of the FBO to identify under which initial configurations the SFFP will be at least equivalent or better than CFFP in terms of total heat absorption capacity. The tool enables the setting of the degree of superchilling for SFFP (i.e. the ice fraction in the fish matrix, which depends on the fish temperature after superchilling and the initial freezing point of fish) that is considered able to maintain fish temperature below or equal to that of a given configuration of CFFP regarding the initial fish temperature and the proportion of ice added per mass of fish (ice:fish) in the box (R ≥ 1, corresponding to QS ≤ QC). The tool also facilitates identification of configurations under which the SFFP cannot be proved to be equivalent or better than CFFP (R < 1, corresponding to QS < QC). The reliability of the outcome provided by the tool depends on the accuracy of the input data introduced by the user.
In case the FBO wants to use an initial configuration with a lower degree of superchilling than that provided by the tool, there would be a need to document the safety e.g. by performing an experimental study for the specific supply chain and/or proving that the temperature of SFFP at arrival to the EU establishment is ≤ 0°C.
The temperature conditions of SFFP are equal or usually less favourable for microbial growth compared to CFFP, provided that the initial configuration of SFFP is properly adjusted to the envisaged outside conditions as usually done for the CFFP. Under these circumstances, the increase of relevant biological hazards (due to growth of vegetative pathogens and/or histamine accumulation) will be equal or usually lower in SFFP compared to CFFP.
3.2. Potential methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’
Freezing rate is critical for the size and shape of intracellular and extracellular ice crystals formed (Alizadeh et al., 2007). Small and regular ice crystals reduce mechanical damage, caused when the ice crystals physically rupture cell walls. Formation of extracellular ice crystals will cause diffusion of water outside the cells (and gradually the whole muscle) and thus tissue dehydration, unless the intracellular water freezes before it has time to diffuse outside the cell. Also, the freezing of water will gradually increase the concentration of enzymes and salt in the remaining unfrozen water fraction, causing protein denaturation and impaired protein functionality. Drip loss in frozen/thawed food is therefore often attributed to protein denaturation or to physical damage in the cells or to the cell membrane (Chevalier‐Lucia et al., 2000; Kaale et al., 2013). In the case of superchilling, the partial freezing process will also influence the size, distribution and shape of ice crystals. Compared to deep‐frozen fish, protein denaturation in superchilled fish may be minimal because not all freezable water is frozen, resulting in lower concentrations of enzymes and salt in the remaining water. Furthermore, the storage duration of superchilled fish is much shorter than that of frozen fish, limiting the action of proteases. In the study of Kaale et al. (2013), the crystals in the superchilled fish did not impact on the integrity of the muscle because the ice crystals were smaller or equal to the size of the muscle cells. The elasticity of the cellular structure in muscle helps to minimise the destructive effect of crystal formation. This supports the idea that the superchilling process and temperature range are low enough to reduce the bacterial growth and enzyme activity responsible for the spoilage of fish, but is unlikely to result in cellular damage (and the consequent release of enzymes) or structural changes to the same extent occurring by deep‐freezing. As such, the product would be assumed to appear and behave (under analytical procedures) more like a fresh product than a frozen one.
The potential methods that are capable of detecting whether a previously frozen fish is commercially presented as ‘superchilled’ are summarised in this section, mainly based on their suitability for differentiating frozen–thawed fish from fresh (not previously frozen) fish. The methods have been grouped into biochemical, histological and physico‐chemical methods. An overview is given in Table 5.
Table 5.
Overview of the methods considered to assess their capability to detect whether a previously frozen fish is commercially presented as ‘superchilled’
| Method category | Method group | Method subgroupa |
|---|---|---|
| Biochemical methods | Enzymatic activity | Mitochondrial enzymes
|
Lysosomal enzymes
| ||
Cytosolic enzyme
| ||
| Protein markers by electrophoresis |
|
|
| Profile analysis |
|
|
| Haematological methods |
|
|
| Other methods |
|
|
| Morphological methods | Eye lens analysis | |
| Histology | ||
| Physico‐chemical methods | Spectroscopy |
|
| Electrical parameters |
|
|
| Differential scanning calorimetry (DSC) |
The methods that were finally considered ‘fit for purpose’ are shown in bold.
Such as MIR = middle‐infrared; NIR = near‐infrared; UV‐VIS = ultraviolet–visible.
3.2.1. Biochemical methods
3.2.1.1. Enzymatic activity
Enzymatic methods for the detection of frozen/thawed fish are generally based on the release of specific intracellular enzymes from the muscle tissue to the extracellular matrix due to the damage caused by ice crystals during freezing. Thus, the measured enzymatic activity of frozen fish is higher than in fresh fish. Enzymatic activity is measured by adding the enzyme to a substrate and measuring the change in absorbance with a spectrophotometer. Enzymatic activity is often expressed as enzyme unit (U) = 1 μmol/min, as nanokatals (1 mol/s) or as specific activity (the activity of an enzyme per mg of total protein) which is expressed in μmol/min·mg. Other ways of expressing the results were also used in the records, for instance abs/min·mg or by using the slope of the measurement output along reaction time multiplied with a certain factor. Enzymatic differentiation between fresh and frozen–thawed fish products has been widely studied. Usually, mitochondrial, lysosomal and cytosolic enzymes in fish exudate, press juice or isotonic fish extract are used. Several research studies report on the use of the mitochondrial enzymes β‐hydroxyacyl coenzyme A dehydrogenase (HADH) (Garcia de Fernando et al., 1992; Hoz et al., 1992, 1993; Pavlov et al., 1994; Civera et al., 1996; Fernandez et al., 1999; Duflos et al., 2002; Bernardi et al., 2019) as a successful means of differentiation. Other enzyme assays involve mitochondrial citrate synthase (Skorpilova et al., 2019), succinate dehydrogenase (Civera et al., 1996) and the lysosomal enzymes β‐N-acetylglucosaminidase extracted from fish muscle (Rehbein et al., 1978; Rehbein, 1979; Nilsson and Ekstrand, 1993; Duflos et al., 2002; Alberio et al., 2014) or fish blood (Kitamikado et al, 1990; Yuan et al, 1988); α‐glucosidase (Rehbein et al., 1978; Rehbein, 1979; Nilsson and Ekstrand, 1993; Rehbein and Cakli, 2000; Duflos et al., 2002; Marlard et al., 2019); β‐galactosidase,β‐glucuronidase (Rehbein, 1979) and acid phosphatase (Rehbein, 1979; Nilsson and Ekstrand, 1993). The cytosolic enzyme lactate dehydrogenase has also been tested (Rehbein, 1979; Diop et al., 2016). Lactate dehydrogenase is deactivated by the freezing process in contrast to the other enzymes and the enzymatic activity is thus lower in frozen fish than in fresh fish.
3.2.1.2. Protein markers by electrophoresis
Cellulose acetate electrophoresis has been used to detect the presence of aspartate aminotransferase, an enzyme released due to mechanical cell damage in frozen–thawed fish (Salfi et al., 1985). The method is based on the extraction of muscle in isotonic and buffered medium, separation of the enzyme by electrophoresis (based on the electrical charge of the protein), staining followed by a fixing and drying process. Two‐dimensional gel electrophoresis has been carried out for the determination of protein markers by Ethuin et al. (2015) in sea bass and by Guglielmetti et al. (2018) in curled octopus (Eledone cirrhosa). The method relies on the fact that freezing and subsequent thawing causes protein denaturation, thus modifying the solubility of the proteins, which can be detected by means of electrophoresis as a separation technique. In these studies, electrophoresis profiles of fresh and frozen–thawed fish were compared, spots of differentiation were selected and finally identified with mass spectrometry. The proteins capable of differentiation were identified as parvalbumin in the case of sea bass and transgelin for curled octopus. Therefore, further research into the rapid detection of those markers would be useful as a next step.
3.2.1.3. Profile analysis
Formation of volatile compounds in fish is generated by enzymatic reactions, lipid autoxidation or microbial action. The action of enzymes does not cease during frozen storage; therefore, the profile of volatile compounds in frozen/thawed fish is different than the volatile profile of fresh fish. Volatile compound profiles can be determined by different methods such as determination by an ‘electronic nose’ (Di Natale et al., 2000) or separation and identification of the volatiles by solid phase micro extraction – gas chromatography – mass spectroscopy. The latter has been used for the identification of potential volatile markers capable of distinguishing between fresh and frozen–thawed fish. Iglesias et al. (2009) identified 1‐octen‐3‐ol, 1‐penten‐3‐ol, Z‐4‐heptenal and 2‐octen‐1‐ol, while Leduc et al. (2012) identified dimethyl sulfide, 3‐methylbutanal, ethyl acetate and 2‐methylbutanal as potential markers.
Phospholipid profile analysis as a means of differentiation has been reported in one research study (Losito et al., 2018). Compared to chilled fresh fish, freezing temperatures have been shown to influence phospholipid profiles of frozen fish through enzymatic hydrolysis. The study described the use of hydrophilic interaction liquid chromatography (HILIC) coupled with electrospray ionisation with high resolution/accuracy Fourier transform mass spectrometry (ESI‐FTMS) to evaluate the phospholipid profile. The ratios of LPE (lysoforms of phosphatidylethanolamines (PE))/PE and LPC (lysoforms of phosphatidylcholines (PC))/PC were used to discriminate between fresh and frozen/thawed fish.
3.2.1.4. Haematological methods
The haematocrit value in the blood of fish was determined as a marker of frozen storage, based on the principle that blood cells are destroyed when frozen. The haematocrit is determined by centrifugation of the blood, separating it into red blood cells and plasma, followed by measuring the volume percentage of red blood cells (Yoshioka and Kitamikado, 1983). Civera et al. (1996) studied the haemolysis of red blood cells in fresh and frozen/thawed fish by streaking the blood on microscope slide, observing microscopically and counting the percentage of haemolysed cells.
3.2.1.5. Other methods
Two other methods were tested for distinguishing fresh from frozen/thawed fish. The first one is based on determination of the free calcium concentration in the fish exudate. The release of free calcium in frozen/thawed fish could be caused by the disruption of the membrane integrity of the endoplasmic reticulum during defrosting, leading to the rise of free calcium in frozen–thawed fish, or could result from a conformational change of the tropomyosin during this denaturation. The test can be done as a rapid commercial assay (Marlard et al., 2019).
The second method is based on the measurement of nucleotides and related compounds (NRC) in fish exudate. This increase of NRC concentration in frozen–thawed fillets was probably due to the liberation of sarcoplasmic nucleotides linked to DNA damage. The analytical method is based on UV spectrophotometry (Marlard et al., 2019).
3.2.2. Morphological methods
3.2.2.1. Eye lens analysis
The method is based on the principle that the medulla of the fish eye becomes opaque during the freezing process. The medulla of the eye lens is therefore separated from the cortex (which can become turbid due to spoilage) by placing the lens in glycerol and simply pressing on the lens with the fingers. The opaque appearance of the medulla is then visually observed (Yoshioka and Kitamikado, 1983; Duflos et al., 2002).
3.2.2.2. Histology
The discrimination of fresh and frozen/thawed fish meat by histological methods is based on the recognition of structural changes in the fish muscle at microscopic level induced by freezing. Therefore, a thin slice of the muscle tissue is made, processed (such as staining) and examined under a light microscope. Some studies focus on classifying samples as positive (thawed) or negative (fresh) (Bozzetta et al., 2012), while other studies add criteria by counting and/or measuring the size of the vacuoles (morphometrical assessment) and work with a scoring system for the different parameters (Richelmi et al., 2013; Popelka et al., 2014; Meistro et al., 2016; Tinacci et al., 2018).
3.2.3. Physico‐chemical methods
3.2.3.1. Spectroscopy
Spectrophotometric and imaging methods can be used to distinguish fresh from frozen–thawed fish because of their ability to detect spatio‐temporal changes in molecular bonds of free water (O to H bonds) and components of fish muscle (e.g. proteins, amines, lipids), myoglobin oxidation and fatty acid composition (e.g. C to C bonds), caused by mechanical tissue damage (and leakage of intra‐cellular content) due to formation of ice crystals, as well as post‐harvest enzymatic and microbial activity during refrigerated storage or thawing (Karoui et al., 2007; Fasolato et al., 2012; Alamprese and Casiraghi, 2015; Hassoun et al., 2020). The molecular changes captured by these methods include changes in the conformation, structure and interactions between molecules. Relevant methods of this category are summarised below.
Ultraviolet–visible (UV‐VIS) and fluorescence spectroscopy rely on the absorption and emission, respectively, of a specific amount and type of radiation. UV‐VIS spectroscopy measures the amount of light (photons) retained by a molecule in a food sample, containing chromophore groups, e.g. myoglobin. Fluorescence spectroscopy measures the amount of light emitted by fluorophore groups (e.g. aromatic amino acids, such as tyrosine, tryptophan and phenylalanine, or vitamins and co‐factors, e.g. NADH), due to their excitation caused by absorption of photons (Karoui et al., 2006; Karoui et al., 2017; Ghidini et al., 2019; Hassoun et al., 2020). The unicity absorption or emission patterns of the entire UV‐VIS or fluorescence spectra, respectively, and the fluorescence intensity of certain compounds at single wavelengths, provide unique qualitative and quantitative information for the molecular composition of a food matrix.
Infrared (IR) spectroscopy, also called ‘vibrational’, involves the bending absorption of radiation in the range 13,000 to 10 cm−1 (Near IR‐NIR, Middle IR‐MIR and Far IR‐FIR), which triggers vibration of atoms in molecular bonds (Ghidini et al., 2019; Hassoun et al., 2020). Vibrations result in stretching and bending of bonds with variable outcomes depending on the spectral domain. NIR spectra are commonly associated with vibration of bonds with light atoms (e.g., O, H), leading to overlapping overtone and combination bands, whereas MIR spectra comprise of intense and delineated bands, forming fingerprints that are informative of the concentration of specific molecules in the samples. Extraction of useful information from IR spectra may be aided by Fourier Transform (FR) (Pink et al., 1998).
In studies relevant to the purpose of the current assessment, i.e. aiming to distinguish fresh from frozen/thawed fish, the visible–NIR (VIS/NIR) region is the most common target domain (see Table D.6).
Table D.6.
Analytical capability to distinguish fresh fish (chilled or superchilled) from frozen/thawed fish based on physico‐chemical methods subgroups electrical parameters and differential scanning calorimetry, spectroscopic methods, including (UV‐)VIS/NIR, hyperspectral imaging, NMR/MRI and Raman spectroscopy, and combination of physico‐chemical with biochemical methods
| Method subgroup | Speciesa | Reference groupb | –10°C | –10 to –12°C | –18°C | –20°C | –35°C | –40°C | –70°C | –80°C | –196°C | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectroscopy | ||||||||||||
| Combinations of UV‐VIS/NIR: NIR | Cuttle fish (Sepia officinalis) | C | – | – | – | YES | – | – | – | – | – | Sannia et al. (2019) |
| Combinations of UV‐VIS/NIR: VIS/NIR | Red sea bream (Pagrus major) | C | – | – | – | – | – | YES | – | – | – | Uddin et al. (2005) |
| Combinations of UV‐VIS/NIR: NIR | Horse mackerel (Trachurus japoncus) | C | – | – | – | – | – | YES | – | – | – | Uddin and Okazaki (2004) |
| Combinations of UV‐VIS/NIR: FT NIR, FT MIR | Atlantic mullet (Pseudupeneus prayensis) | C | – | – | YES | – | – | – | – | – | – | Alamprese and Casiraghi (2015) |
| Combinations of UV‐VIS/NIR: FT‐IR and DMA monitoring | Red hake (Urophycis chuss) | C | – | YES | – | – | – | – | – | – | – | Pink et al. (1998) |
| Combinations of UV‐VIS/NIR: VIS/NIR | Sword fish (Xiphias gladius L) | C | YES | – | YES | – | – | – | – | – | – | Fasolato et al. (2012) |
| Combinations of UV‐VIS/NIR: FT‐NIR | Tilapia (Oreochromis) | C | – | – | YES | – | – | – | – | – | – | Wang et al. (2018) |
| Combinations of UV‐VIS/NIR: UV‐VIS/NIR | Tuna (Thymnus thynnus) | C | – | – | – | – | – | – | – | YES | – | Reis et al. (2017) |
| Combinations of UV‐VIS/NIR: FT‐MIR | Whiting fillets (Merlangius merlangus) | C | – | – | – | YES | YES | – | – | – | – | Karoui et al. (2007) |
| Combinations of UV‐VIS/NIR | Gilthead sea bream (Sparus aurata), red mullet (Mallus barbatus), sole (Sole vulgaris), sword fish (Xiphias gladius L) | C | – | – | – | – | – | – | – | – | – | Ottavian et al. (2013)c |
| VIS/NIR with hyperspectral imaging | Atlantic cod (Gadus morhua) | C | – | – | – | – | – | YES | – | – | – | Sivertsen et al. (2011) |
| Hyperspectral imaging | Crucian carp (Carassius auratus) | C | – | – | – | YES | – | – | – | – | – | Shan et al. (2018) |
| Hyperspectral imaging | Atlantic cod | C | – | – | – | YES | – | YES | – | – | – | Washburn et al. (2017) |
| UV‐VIS/NIR with hyperspectral imaging | West African goat fish (Pseudupeneus prayensis) | C | – | – | YES | – | – | – | – | – | Ottavian et al. (2014) | |
| Hyperspectral imaging | Grass carp | C | – | – | – | YES | – | YES | – | – | – | Cheng et al. (2015) |
| VIS/NIR with hyperspectral imaging | Halibut (Psetta maxima) | C | – | – | – | YES | – | – | YES | – | – | Zhu et al. (2013) |
| VIS/NIR with hyperspectral imaging | Prawns (Metapenaeus ensis) | C | – | – | YES | – | – | – | – | – | – | Dai et al. (2015) |
| VIS/NIR with hyperspectral imaging | Atlantic salmon (Salmo salar L.) | C | – | – | – | – | – | YES | – | – | – | Kimiya et al. (2013) |
| Hyperspectral imaging | Shrimps (Metapenaeus ensis) | C | – | – | – | YES | – | – | – | – | – | Qu et al. (2015) |
| Hyperspectral imaging | Red snapper (Lutjanus campechanus), flounder, white bass, and tilapia | C | – | – | – | YES | – | – | – | – | – | Qin et al. (2020) |
| (Front‐face) fluorescence spectroscopy | Sea bass (Dicentrarchus labrax) | C | – | – | YES | – | – | – | – | – | – | Karoui et al. (2017) |
| (Front‐face) fluorescence spectroscopy | Whiting fillets (Merlangius merlangus) | C | – | – | – | YES | YES | – | – | – | – | Karoui et al. (2006) |
| NMR/MRI spectroscopy: NMR | Farmed Atlantic salmon | C | – | – | – | YES | – | YES | – | – | – | Shumilina et al. (2020) |
| NMR/MRI spectroscopy | Trout | C | – | – | YES | – | – | – | – | – | – | Nott et al. (1999b) |
| NMR/MRI spectroscopy | Cod and mackerel | C | – | – | – | YES | – | – | – | – | – | Nott et al. (1999a) |
| Raman spectroscopy | Horse mackerel (Trachurus trachurus), European anchovy (Engraulis encrasicolus), red mullet (Mullus surmuletus), Bluefish (Pomatamus saltatrix), Atlantic salmon (Salmo salar), flying gurnard (Trigla lucerna) | C | – | – | – | YES* | – | – | – | – | – | Velioglu et al. (2015) |
| Electrical parameters | ||||||||||||
| Electrical impedance | Atlantic salmon | C | – | – | YES | – | – | – | – | – | – | Fernandez‐Segovia et al. (2012) |
| Electrical impedance | Sea bass (Dicentrarchus labrax) | C | – | – | NO | – | – | – | – | – | – | Vidacek et al. (2012a) |
| Electrical impedance | Salmon fillets and rainbow trout | C | – | – | YES | – | – | – | – | – | – | Sun et al. (2020) |
| Electrical impedance | Sea bream (Sparus aurata) | C | – | – | NO | – | – | – | – | Fuentes et al. (2013) | ||
| Electrical impedance | Atlantic chub mackerel (Scomber colias) | C | – | – | – | YES | – | – | – | – | YES | Vidacek et al. (2012b) |
| Dielectrical properties: microwave | Salmon | C | – | – | – | YES* | – | – | – | – | – | Clerjon and Damez (2007) |
| Dielectrical properties: electrical (Torrymeter) and biochemical (k‐values) | Yellowtail (Seriola quinqueradiata) | C | – | – | – | YES | – | – | – | – | – | Kim et al. (1987) |
| DSC | ||||||||||||
| DSC | Seabream (Sparus aurata) | C | – | – | – | YES | – | – | – | – | – | Matos et al. (2011) |
– = not determined, YES = distinction between fresh or superchilled and frozen/thawed fish possible through statistical methods, NO = distinction not possible.
DMA = dimethylamine; DSC = differential scanning calorimetry; FR = Fourier Transform; MIR = middle‐infrared; MRI = magnetic resonance imaging; NIR = near‐infrared; NMR = Nuclear magnetic resonance; UV‐VIS = Ultraviolet–visible.
Classification performance was only graphically demonstrated.
With scientific name if provided.
Reference for comparison with frozen/thawed fish was a chilled (C) or superchilled (SC) fish.
Spectral data of this report derived from database of spectra from a variety of studies with different fish, stored at various freezing temperatures and durations. As such, it was difficult to trace and specify exact freezing temperatures, per the columns of this table.
NMR spectroscopy provides qualitative and quantitative information about the chemical composition of a food matrix and the regio/stereo‐chemistry of associated molecules, based on the magnetic properties of their atomic nuclei, exposed to a magnetic field. The concentration of responding nuclei in a sample can be estimated by the area of NMR spectral bands. NMR has been used to assess fish quality by correlating proton NMR relaxometry with the water content and water holding capacity of fish muscle (Hassoun et al., 2020; Shumilina et al., 2020); see Table D.6.
Raman spectroscopy is based on capturing the inelastically scattered radiation by molecules of a food sample, when irradiated with laser light typically in the VIS/NIR region (Ghidini et al., 2019). Scattered radiation is the result of molecular vibrations and changes in the polarisability of molecules. A typical Raman spectrum illustrates the intensity of scattered light at the wavelengths of Raman shift and provides information about the molecular structure and composition of the irradiated sample. The technique is sensitive to interference associated with the fluorescence and weak scattering. A promising alternative to overcome these potential limitations is Surface Enhanced Raman spectroscopy (SERS) (Ghidini et al., 2019).
Hyperspectral imaging is a special case of spectroscopy aided by high resolution cameras and advanced computational performance that provides the spatial distribution of absorption/reflectance, emission or light scattering spectra throughout a food sample. In this technique, spectra of a defined range, e.g. VIS/NIR, or RAMAN spectra, depending on the capacity of camera, are assigned to each pixel of a food image, thus, showing the spatial distribution of spectral properties of food components at different wavelengths (Sivertsen et al., 2011; Kimiya et al., 2013; Washburn et al., 2017; Shan et al., 2018; Hassoun et al., 2020; Qin et al., 2020). It is commonly implemented with ‘interactance’ imaging, to optimise signal detection and minimise adjacent surface reflectance noise (Sivertsen et al., 2011; Kimiya et al., 2013).
The application of the above methods in discriminating fresh from frozen/thawed fish is carried out through a classification algorithm, which correlates the output of each method with groups (classes) of samples (i.e. fresh or frozen/thawed) sharing common spectral characteristics. The classification of samples is based on spectral comparisons and is carried out via bioinformatic tools, particularly unsupervised and supervised multivariate statistical methods (Ghidini et al., 2019). Unsupervised methods include Principal Component Analysis (PCA) and Hierarchical Clustering (HC). PCA identifies the correlation among spectral variables (e.g. absorption or emission at different wavelengths) and reduces the representation of spectral data to a smaller number of uncorrelated variables accounting for the major variations common to all spectra, i.e. the major sources of variability in the spectral properties of different samples. HC classifies samples into groups (clusters) based on the degree of analogy between sample spectra, by calculating the Mahalanobis or Euclidean distance between samples. PCA is commonly applied as the first step in processing of spectral data, with the aim of identifying the PCs, which serve as inputs to the supervised classification methods. The latter correlate PCs to a classifier (e.g. fresh vs. frozen/thawed fish) through a mathematical expression (Ghidini et al., 2019; Hassoun et al., 2020); see Table D.6, that can be used in the future to also classify unidentified samples.
Supervised methods can be regression independent, e.g. Linear or Quadratic Discriminant Analysis (LDA and QDA) and k‐Nearest Neighbours (k‐NN) clustering, or regression‐dependent, including linear methods, such as Partial Least Square Regression (PLSR‐DA) or orthogonal PLSR‐DA, as well as nonlinear machine learning algorithms, relying on patter recognition, such as Support Vector Machines (SVM), Artificial Neural Networks (ANN) and Soft Independent Modelling of Class Analogy (SIMCA). LDA and SIMCA have been widely used to discriminate fresh from frozen/thawed fish based on spectroscopic methods (see Table D.6). Spectral data may be subjected to pre‐processing, before being used to develop the classification models. The most common data transformation techniques are signal correction methods, such as Standard Normal Variate, Multiplicative Scatter Correction or differentiation up to the 1st, 2nd or 3rd derivative, or filtering‐based methods, focusing on specific wavelengths (Ghidini et al., 2019).
3.2.3.2. Electrical parameters
Complex electrical properties of fish products have been intensively studied in the last two decades to differentiate between fresh and previously frozen–thawed fish (Kent et al., 2004, 2005; Vidacek et al., 2008; Zhang et al., 2010). These investigations showed some potential of electrical measurements for detecting structural changes of frozen fish as a result of freezing temperature or the number of freezing cycles (Vidacek et al., 2012b).
Dielectric properties. Studies on dielectric properties of fish were conducted in different frequency regions to differentiate between fresh and previously frozen fish (Kent et al., 2004, 2005; Kent and Oehlenschlager, 2009). The potential of dielectric properties to differentiate fresh from thawed, previously frozen fish achieve this differentiation as a result of the effect of cell membrane destruction during freezing and the changes in ionic mobility upon thawing (Vidacek et al., 2012b). It was suggested that ionic mobility is the main factor of alterations in the electrical properties of thawed fish when compared with fresh fish. During the measurement of dielectric response, the imaginary part12 is more sensitive to changes in ionic mobility than the real part. Ionic mobility is proportional to the concentration of ions which may be lost with drip loss during thawing (Vidacek et al., 2012b).
Electrical impedance. Electrical impedance is a frequency‐dependent property of material to resist electrical current flow. Fish tissues are built up mainly of protein and lipid‐based structures that have both resistive and capacitive properties. However, intracellular and extracellular spaces containing mainly water, solutes and ions contribute most to the impedance. At the same time, lipid bilayers of cell membranes behave as a capacitor that contributes to the imaginary part of the impedance.11 Electrical impedance changes after death or cell damage (e.g. during freezing), but also due to the changes in ionic mobility upon thawing, which provided the basis for application of this method for differentiation between fresh and thawed‐frozen fish (Vidacek et al., 2012b).
Others. Torrymeter is an instrument that can be used to assess freshness of fish in a scale from above 10 (fresh) to 0 (not fresh), based on electrical properties and changes in texture of fish, due to post‐harvest biochemical and microbial activity on fish. The readings of the instrument reduce, compared to those of fresh fish, not only with the loss of freshness, but also when the fish is frozen and then thawed (Kim et al., 1987).
3.2.3.3. Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a commonly used technique to analyse phase transition and thermo‐physical properties of food (Zaidul et al., 2008; Dahimi et al., 2014; Tolstorebrov et al., 2014; Karthikeyan et al., 2015). The analysed sample and the reference sample are scanned at a pre‐set cooling/heating rate and the difference in heat energy is recorded as a certain peak on DSC curve (Schubring, 1999; Zielbauer et al., 2016). In this way, it is possible to obtain data on the amount of energy released or received during phase transition (Tomaszewska‐Gras, 2013; Tolstorebrov et al., 2014). DSC can be used to differentiate fresh from previously frozen fish because melting of ice occurring during fish thawing is the most frequent phase transition which changes the peaks positions for certain proteins on DSC when compared to fresh fish (Tolstorebrov et al., 2014). It distinguishes fresh from previously frozen fish mainly based on the thermal properties of proteins, particularly in terms of protein denaturation taking place during freezing (Konieczny et al., 2016). The area of endothermic peaks corresponding to proteins on DSC curve can act as a measurement of the stability of the protein. This can be characterised by the following DSC parameters: the heat absorbed (ΔH), the temperature at which the peak starts to be deflected from the baseline (onset temperature, To) and the temperature at which the peak reaches a maximum (peak temperature, Tp). DSC allows differentiation of fresh from previously frozen fish based on protein behaviour in very small samples (Matos et al., 2011; Konieczny et al., 2016).
3.2.4. Comparative evaluation of the potential methods
Tables 6, 7 and 8 allow to compare the ‘fit for purpose’ of the potential methods. Detailed information of each relevant record retrieved from the literature search can be found in Tables D.1, D.2–D.3 (record overview table). Table 6 (analytical capability table) provides an overview of each on whether the different methods were capable to differentiate between chilled (C) or superchilled (SC) fish and frozen/thawed fish at different temperatures and for different fish species. The advantages and disadvantages of each method are presented in Table 7 based on predefined criteria. These criteria were: the execution speed being fast (< 0.5 days), medium (< 1 day) or slow (> 1 day), the destructiveness of the method, the effort required for each method being non‐laborious (no or easy sample preparation, few handling operations) or laborious (sample preparation necessary, many handling operations), the laboratory equipment needed (standard or advanced), the skills needed (simple or specialised), the need for threshold settings or calibration and if this is species‐specific or can be done for a group of species, and the technological readiness of the application (high, medium or low). The previously mentioned tables served as input to fill Table 8, in which a score is given for eight features of the potential methods. The information from the analytical capability table was used to score the ‘applicability of the method for different fish species’ and ‘the ability of the method to differentiate fresh fish from fish frozen at various temperatures’. The ‘ease of use’ of the method was deduced from the advantages/disadvantages table. The other features were scored based on the information retrieved directly from the record overview table.
Table 6.
Analytical capability to distinguish fresh fish (chilled or superchilled) from frozen/thawed fish for the methods that were finally considered ‘fit for purpose’ HADH and α‐glucosidase (enzymatic methods), histology (morphological methods); and UV‐VIS/NIR spectroscopy and hyperspectral imaging (physico‐chemical methods) – see Tables D.4, D.5–D.6
| Speciesa | Reference group | –5°C | –10°C | –10°C to –12°C | –18°C | –20°C | –24°C | –29°C | –35°C | –40°C | –70°C | –80°C | –196°C | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HADH | ||||||||||||||
| Crawfish (Procambarus clarkia) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Hoz et al. (1992) |
| Hake (Merluccius merluccius) | C | – | – | – | NO | – | – | – | – | – | – | – | NO | Fernandez et al. (1999) |
| Hake (Merluccius merluccius) | C | – | YES | – | YES | – | – | – | YES | – | – | YES | YES | Pavlov et al. (1994) |
| Small hake (Merluccius merluccius) | C | – | NO | – | NO | – | – | – | NO | – | – | NO | NO | Pavlov et al. (1994) |
| Norwegian lobster (Nephrops norvegicus) | C | – | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Mackerel (Scomber scombrus) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Mackerel (Scomber scombrus) | C | – | YES | – | YES | – | – | – | YES | – | – | YES | YES | Pavlov et al. (1994) |
| Nile perch | SC | – | – | – | YES* | – | – | – | – | – | – | – | – | Nile perch study |
| Plaice (Pleuronectes platessa) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Prawn (Penaeus japonicus) | C | – | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Kuruma prawn (Penaeus japonicas) | C | – | YES | – | YES | – | – | – | YES | – | – | YES | YES | Hoz et al. (1993) |
| Salmon (Salmo salar) | C | – | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Seabass (Dicentrarchus labrax) | C | – | – | – | NO | – | – | – | – | – | – | – | NO | Fernandez et al. (1999) |
| Gilthead sea bream (Sparus aurata) | C | – | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Sea bream (Pagellus centrodontus) | C | – | YES | – | YES | – | – | – | YES | – | – | YES | YES | Pavlov et al. (1994) |
| Sea bream (Pagellus centrodontus) | C | – | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Sole (Solea solea) | C | – | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Sole (Solea solea) | C | – | YES | – | YES | – | – | – | YES | – | – | YES | YES | Pavlov et al. (1994) |
| Squid (Loligo vulgaris) | C | – | YES | – | YES | – | – | – | YES | – | – | YES | YES | Pavlov et al. (1994) |
| Swordfish (Xyphius gladius) | C | – | YES | YES | – | Civera et al. (1996) | ||||||||
| Trout (Salmo gairdneri) | C | YES | – | – | YES | – | YES | – | YES | – | – | YES | YES | Garcia de Fernando et al. (1992) |
| Trout (Salmo gairdneri) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Hoz et al. (1992) |
| Yellowfin tuna (Thunnus albacares) | C | –a | – | – | – | – | YES | – | – | – | – | – | – | Bernardi et al. (2019) |
| Tuna (Thunnus alalunga) | C | – | YES | – | YES | – | – | – | YES | – | – | YES | YES | Pavlov et al. (1994) |
| Whiting (Merlangus merlangus) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| α‐glucosidase | ||||||||||||||
| Anchovy (Engraulis encrasicolus) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Cod (Gadus morhua) | C | – | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Cod (Gadus morhua) | C | – | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein (1979) |
| Haddock (Gadus aeglefinus) | C | – | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Chub mackerel (Scomber japonicus) | C | – | – | – | NO | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Horse mackerel (Trachurus trachurus) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Mackerel (Scomber scombrus) | C | – | – | – | – | NO | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Plaice (Pleuronectes platessa) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Saithe (Gadus virens) | C | – | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Salmon (Salmo salar) | C | – | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein and Cakli (2000) |
| Sardines (Sardina pilchardus) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Seabass (Dicentrarchus labrax) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Marlard et al. (2019) |
| Rainbow trout (Oncorhynchus mykiss) | C | – | – | – | – | – | – | – | – | YES* | – | – | – | Nilsson and Ekstrand (1993) |
| Red fish (Sebastes marinus) | C | – | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Whiting (Merlangus merlangus) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Histology | ||||||||||||||
| Anchovy (Engraulis encrasicolus) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Meistro et al. (2016) |
| Atlantic bonito (Sarda sarda) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Richelmi et al. (2013) |
| European hake (Merluccius merluccius) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Tinacci et al. (2018) |
| Red mullet (Mullus barbatus) | C | – | – | – | – | – | – | – | – | – | – | YES | – | Bozzetta et al. (2012) |
| Salmon (Salmo salar) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Richelmi et al. (2013) |
| Gilthead seabream (Sparus aurata) | C | – | – | – | – | – | – | – | – | – | – | YES | – | Bozzetta et al. (2012) |
| Swordfish (Xiphias gladius) | C | – | – | – | – | – | – | – | – | – | – | YES | – | Bozzetta et al. (2012) |
| Rainbow trout (Oncorhynchus mykiss) | C | – | – | – | YES* | – | – | – | – | – | – | – | Popelka et al. (2014) | |
| Albacore tuna (thunnus alalunga) | C | – | – | – | – | YES* | – | – | – | – | – | – | – | Richelmi et al. (2013) |
| Little tunny (Euthynnus alletteratus) | C | – | – | – | – | YES* | – | – | – | – | – | – | – | Richelmi et al. (2013) |
| Turbot (Psetta maxima) | C | – | – | – | – | YES* | – | – | – | – | – | – | – | Richelmi et al. (2013) |
| 35 species differing in protein and fat content | C | – | – | – | – | – | YES | – | – | – | – | YES | – | Bozzetta et al. (2012) |
| Combinations of UV-VIS/NIR | ||||||||||||||
| Cuttle fish (Sepia officinalis) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Sannia et al. (2019) |
| Red sea bream (Pagrus major) | C | – | – | – | – | – | – | – | – | YES | – | – | – | Uddin et al. (2005) |
| Horse mackerel (Trachurus japoncus) | C | – | – | – | – | – | – | – | – | YES | – | – | – | Uddin and Okazaki (2004) |
| Atlantic mullet (Pseudupeneus prayensis) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Alamprese and Casiraghi (2015) |
| Red hake (Urophycis chuss) | C | – | – | YES | – | – | – | – | – | – | – | – | – | Pink et al. (1998) |
| Sword fish (Xiphias gladius L) | C | – | YES | – | YES | – | – | – | – | – | – | – | – | Fasolato et al. (2012) |
| Tilapia (Oreochromis) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Wang et al. (2018) |
| Tuna (Thymnus thynnus) | C | – | – | – | – | – | – | – | – | – | – | YES | – | Reis et al. (2017) |
| Whiting fillets (Merlangius merlangus) | C | – | – | – | – | YES | – | – | YES | – | – | – | – | Karoui et al. (2007) |
| Gilthead sea bream (Sparus aurata), red mullet (Mallus barbatus), sole (Sole vulgaris), sword fish (Xiphias gladius L) | C | – | – | – | – | – | – | – | – | – | – | – | – | Ottavian et al. (2013)b |
| Hyperspectral imaging | ||||||||||||||
| Atlantic cod (Gadus morhua) | C | – | – | – | – | – | – | – | – | YES | – | – | – | Sivertsen et al. (2011) |
| Crucian carp (Carassius auratus) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Shan et al. (2018) |
| Atlantic cod | C | – | – | – | – | YES | – | – | – | YES | – | – | – | Washburn et al. (2017) |
| West African goat fish (Pseudupeneus prayensis) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Ottavian et al. (2014) |
| Grass carp | C | – | – | – | – | YES | – | – | – | YES | – | – | – | Cheng et al. (2015) |
| Halibut (Psetta maxima) | C | – | – | – | – | YES | – | – | – | – | YES | – | – | Zhu et al. (2013) |
| Prawns (Metapenaeus ensis) | C | – | – | – | YES | – | – | – | – | – | – | – | – | Dai et al. (2015) |
| Atlantic salmon (Salmo salar L.) | C | – | – | – | – | – | – | – | – | YES | – | – | – | Kimiya et al. (2013) |
| Shrimps (Metapenaeus ensis) | C | – | – | – | – | YES | – | – | – | – | – | – | – | Qu et al. (2015) |
| Red snapper (Lutjanus campechanus), flounder, white bass, and tilapia | C | – | – | – | – | YES | – | – | – | – | – | – | – | Qin et al. (2020) |
–: not determined, YES: distinction between fresh or superchilled and frozen/thawed fish possible through statistical methods, NO: distinction not possible; HADH: β‐hydroxyacyl coenzyme A dehydrogenase; NIR: near‐infrared; UV‐VIS: Ultraviolet–visible.
No statistical test performed in the study.
With scientific name if provided.
Spectral data of this report derived from database of spectra from a variety of studies with different fish, stored at various freezing temperatures and durations. As such, it was difficult to trace and specify exact freezing temperatures, per the columns of this table.
Table 7.
Advantages/disadvantages related to the ease of use and technical resources required for each method to detect whether a previously frozen fish is commercially presented as ‘superchilled’
| Method category | Method group | Method subgroup | Execution speed | Destructiveness | Laboriousness | Laboratory equipment | Skills needed | Threshold/calibration setting | Technological readiness |
|---|---|---|---|---|---|---|---|---|---|
| Biochemical | Enzymatic | HADH | M | D | L | ST | SI | T | H |
| α‐glucosidase | M | D | L | ST | SI | T | H | ||
| Histology | Histology | S | D | L | AD | SP | NT | H | |
| Physico‐chemical | Imaging/spectroscopy | Combinations of Ultraviolet–visible/NIR (UV‐VIS/NIR) | F | ND | NL | AD | SP | NT | H |
| Hyperspectral imaging | F | ND | NL | AD | SP | T | H |
F = fast execution speed (< 0.5 days); M = medium execution speed (< 1 day); S = slow execution speed (> 1 day).
D = destructive method; ND = non‐destructive method.
L = laborious method (sample preparation necessary, many handling operations); NL = non‐laborious method (no or easy sample preparation, few handling operations).
ST = standard laboratory equipment; AD = advanced laboratory equipment.
SI = simple skills needed; SP = specialised skills needed.
T = need of threshold/calibration setting by species or group of species; NT = no need of threshold/calibration setting by species or group of species.
H = high technological readiness for commercial applications.
Note: Only the methods that were finally considered ‘fit for purpose’ are shown, i.e. HADH and α‐glucosidase (enzymatic methods), histology (morphological methods); and UV‐VIS/NIR spectroscopy and hyperspectral imaging (physico‐chemical methods); while all methods can be found in Table D.7.
Table 8.
Evaluation of the methods that were finally considered ‘fit for purpose’
| Evaluation criteria | HADH | α‐glucosidase | Histology | UV‐VIS/NIR | Hyperspectral imaging |
|---|---|---|---|---|---|
| Applicability of the method for different fish species | ++ | ++ | +++ | +++ | ++ |
| Ability of the method to differentiate fresh fish from fish frozen at various temperatures | ++ | ++ | ++ | +++ | ++ |
| Use of a single method possible | +++ | ++ | +++ | +++ | +++ |
| Ease of use of the method | ++ | ++ | + | ++ | ++ |
| Validation of the method | + | + | ++ | +++ | +++ |
| Classification performance in discriminating as either frozen/not frozen | ++ | 0 | +++ | +++ | +++ |
| Evidence that superchilled fish will behave like fresh fish | + | 0a | 0a | 0a | 0a |
| Strength of overall evidence (number of records; average appraisal scoreb) | 9; 4.9 | 7; 2.9 | 5; 3.8 | 10; 5.6 | 10c; 4.9 |
0 = no information available; − = poor performance; + = weak performance/weak evidence; ++ = good performance/good evidence; +++ = excellent performance/good evidence.
There is no evidence, but it has been assumed that superchilled fish would behave in the same way as fresh fish.
The division of the total appraisal score of all the records and the number of records.
Studies with hyperspectral spectroscopy involve measurements in the domain of the VIS/NIR spectra. As such, the number of papers under UV‐VIS/NIR refers only to those studies that did not use hyperspectral camera.
Table D.1.
Record overview table of the studies dealing with biochemical methods to distinguish fresh fish (chilled or superchilled) from frozen/thawed fish
| Method subgroup | Short description of method | Conditions tested: fish species | Conditions tested: temperatures and duration | Conditions tested: freezing methods | Short description of resultsa | Performance classification | Appraisalb | Reference |
|---|---|---|---|---|---|---|---|---|
| Enzymatic Methods | ||||||||
| HADH | Juice was obtained from fresh and frozen samples using a press machine and diluted (1:100) in phosphate buffer. Then 100 μL diluted juice was brought into a cuvette with 200 μL EDTA (34.4 mmol/L), 2.6 mL phosphate buffer (0.1 mol/L, pH 6.0), 50 μL HADH (7.5 mmol/L), and mixed. Finally, 50 μL acetoacetyl‐CoA (5.9 mmol/L) was added and the reaction started. Absorbance at 340 nm was measured spectrophotometrically immediately and after 3 min. HADH activity was expressed in IU per mL. | Yellowfin tuna (Thunnus albacares) |
16 samples analysed fresh and after 20–25 days of frozen storage at –24°C (pilot study). 5 samples analysed fresh and after 1, 2, 7, 12 and 28 days of frozen storage (freezing temperature not provided). Analysis of unknown market samples. |
Not available |
Significant difference between fresh and frozen/thawed samples. Thresholds were set at 3.7 U/mL and 1.8 U/mL (1% and 5% probability, respectively, of being unfrozen/thawed). Four market samples did not match with labelling. |
No classification performance available |
10000110010 4 |
Bernardi et al. (2019) |
| HADH | Extracts were obtained from fresh and thawed samples (2 g) by immersion in phosphate buffer, pH 5.6, at 25°C for 30 min. 0.1 mL of extract was filtrated on paper S&S 595 and put in a cuvette with 0.2 mL of EDTA (34.4 mmol/L), 2.6 mL of phosphate buffer 0.1 mol/L, pH 5.6 and 0.1 mL HADH (1.5 mmol/L). Everything was kept at 25°C. Then 0.1 mL of diacetoacetyl CoA (5.9 mmol/L) was added. The absorbance was measured every 30 s during at least 3 min. Results were expressed as U/g. | Swordfish (Xyphius gladius) (10 samples) | Certified fresh samples were purchased from the market and analysed fresh. Samples were frozen at –20°C and –40°C and tested after 1, 2, 7, 14, 21 and 28 days of frozen storage. | Not available |
No significant difference after up to 2 days of frozen storage between fresh and frozen/thawed fish. Significant difference (p < 0.05) between fresh fish and 1‐week frozen/thawed fish and extremely significant (p < 0.001) differences between fresh and frozen/thawed fish after 21 and 28 days of frozen storage. Used in combination with other tests: analysis together with haemolysis of the erythrocytes may provide information on the freezing history of the product. |
No classification performance available |
10100100000 3 |
Civera et al. (1996) |
| HADH | 2 g of dorsomedial flesh was suspended in 4 mL of 0.1 mol/L phosphate buffer at pH 6 for 15 min at room temperature. The suspension was filtered and 34 μL of the filtrate was mixed with 70 μL of EDTA (34.4 mmol/L) and 880 μL of phosphate buffer (0.1 mol/L, pH 6) in a spectrometer microcuvette, followed by addition of 25 μL of HADH and 25 μL of acetyl‐CoA. After 3 min at room temperature the OD was read at 340 nm every 30 s for 3 min and the HADH activity was calculated using the formula: activity = –OD × 104. | Mackerel (Scomber scombrus), plaice (Pleuronectes platessa), whiting (Merlangus merlangus) | Analysis of 30 samples: fresh and after 1 day at –20°C (fast and slow freezing conditions) | Two freezing conditions: slow freezing in a cold room and fast freezing in a cell. |
Significant difference between fresh and frozen/thawed HADH activities (p < 0.05) for all species. Large standard deviations, so difficult to set cut‐off values. No significant difference between the two freezing methods. |
No classification performance available |
10000001100 3 |
Duflos et al. (2002) |
| HADH | Idem as the paper of (Garcia de Fernando et al., 1992): extracts were obtained from fresh and thawed samples (2 g) by immersion in two volumes (4 mL) of 0.1 mol/L phosphate buffer, pH 6.0, at room temperature for 15 min. The HADH released in the extracts was assayed by mixing in a spectrophotometer cell (10 mm light path length, nominal working volume 1.5 mL), 34 μL extract, 70 μL EDTA (34.4 mmol/L) and 880 μL phosphate buffer (0.1 mol/L, pH 6.0). The mixture was kept at room T for 3 min and, finally, 20 μL HADH (1.5 mmol/L) and 20 μL acetoacetyl‐CoA (5.9 mmol/L) were added. The HADH activity was determined by measuring immediately the absorbance of the mixtures at 340 nm. The absorbance was recorded every 30 s during 3 min and the HADH value was established as –104 × slope of the line resulting from the periodic spectrophotometric measures. | Hake (Merluccius merluccius), Norwegian lobster (Nephrops norvegicus), prawn (Penaeus japonicus), salmon (Salmo salar), seabass (Dicentrarchus labrax), gilthead sea bream (Sparus aurata), sea bream (Pagellus centrodontus), sole (Solea solea) |
Analysis of fresh samples stored at 2°C after 0, 3 and 6 days. Analysis of frozen (–18°C and –196°C) and thawed samples after 0, 3 and 6 days of storage. Analysis of frozen (–18°C) thawed and refrozen (–196°C, 15 min)/thawed samples after 0, 3 and 6 days of storage. Threshold calculated measuring ratio between frozen (–18°C)/thawed/refrozen (–196°C) activity and frozen activity (–18°C) or ratio between frozen (–196°C)/thawed and fresh activity. |
Two freezing conditions: –196°C in liquid nitrogen, –18°C (no information). No info on the duration of frozen storage before thawing. |
Significant differences in HADH activity between fresh and frozen/thawed samples except for hake and sea bass. No consistent effect of storage time on HADH activity for fresh and frozen/thawed samples (no influence of spoilage). No effect of a second freezing process on HADH activity. Similar HADH activity between samples frozen at –196°C and –18°C. Threshold (as ratio) set at 2. If < 2: sample is previously frozen, if > 2: sample is fresh. |
90% of frozen/thawed samples well classified 87.3% of fresh samples well classified |
10100011110 6 |
Fernandez et al. (1999) |
| HADH | Portions (2 g) of trout muscle were immersed in two volumes of phosphate buffer at 25°C for 15 min. The juice was filtered and HADH activity was measured by mixing 34 μL of juice, 70 μL of EDTA (34.4 mmol/L) and 880 μL of phosphate buffer (0.1 mol/L, pH 6.0) in a spectrophotometric cell. The mix was maintained at room temperature for 3 min and finally, 20 μL of HADH (1.5 mmol/L) and 20 μL of acetoacetyl‐CoA (5.9 mmol/L) added. The absorbance at 340 nm was measured every 30 s up to 3 min. Then, the regression equations of the resultant lines were calculated. HADH activity was expressed as –104 × slope. | Trout (Salmo gairdneri) |
Samples were analysed fresh and frozen at –5, –18, –35, –80 or –196°C during one week. Fresh and frozen/thawed (1 week at –18°C) samples analysed after 0, 3, 6 and 10 days of fresh storage. |
Liquid nitrogen for –196°C samples. No information for the other freezing methods. |
HADH activity at all freezing temperatures was significantly higher than that of refrigerated fillets. HADH activity of fresh trout muscle was not affected by spoilage (long storage time at refrigeration temperature). Threshold was set as follows: HADH value higher than 70 considered as freezing material, lower than 32 as unfrozen, grey zone between upper and lower value. |
No classification performance available |
10111011010 7 |
(Garcia de Fernando et al., 1992) |
| HADH | Extracts were obtained from fresh and thawed samples (2 g) by immersion in two volumes (4 mL) of 0.1 mol/L phosphate buffer, pH 6.0, at 25°C for 15 min. The decanted juice was diluted (1:2, v/v) in the same phosphate buffer and was assayed by mixing in a quartz cuvette: 0.1 mL of juice, 2.6 mL phosphate buffer (0.1 mol/L, pH 6.0), 0.2 mL EDTA (34.4 mmol/L), 0.05 mL HADH (1.5 mmol/L) and 0.05 mL acetoacetyl‐CoA (5.9 mmol/L). Absorbances at 340 nm were measured every 30 s up to 6 min. The simple regression equations of the resulting data were calculated and the slope of each line, multiplied by –104, was used as a measure of the HADH activity. | Crawfish (Procambarus clarkia), trout (Salmo gairdneri) |
Fresh and frozen/thawed samples were stored at 0°C and analysed after 0, 2 and 3 days of storage. Fresh and frozen/thawed samples were stored at 8°C and analysed after 0, 2 and 3 days of storage. Freezing of samples took place at –18°C during 48 h and thawing was done at 4°C. |
Not available |
Significant differences in HADH activity between fresh and thawed for both species but there was some overlap in range for crawfish between fresh and frozen/thawed samples. Significant increase in HADH activity during storage at 8°C for fresh and frozen/thawed crawfish and at 0°C for the thawed crawfish samples. For trout there was significant increase in the samples stored at 8°C (fresh and thawed samples). The increase was negligible in relation to differences between unfrozen and frozen/thawed Threshold value trout: < 19 is fresh; > 19 is frozen/thawed based on 95% CI or threshold value of 30 not chosen on the basis of statistical analysis Threshold value crawfish: < 14 is fresh; > 27 is frozen/thawed, in between: uncertain. |
For trout: 5% of fresh was classified as frozen/thawed and 0% of frozen/thawed was classified as fresh based on threshold value of 19 but all were well classified with threshold value of 30 |
10000010111 5 |
(Hoz et al., 1992) |
| HADH | Idem as the paper of (Garcia de Fernando et al., 1992): extracts were obtained from fresh and thawed samples (2 g) by immersion in two volumes (4 mL) of 0.1 mol/L phosphate buffer, pH 6.0, at room temperature for 15 min. The HADH released in the extracts was assayed by mixing in a spectrophotometer cell (10 mm light path length, nominal working volume 1.5 mL), 34 μL extract, 70 μL EDTA (34.4 mmol/L) and 880 μL phosphate buffer (0.1 mol/L, pH 6.0). The mixture was kept at room T for 3 min and, finally, 20 μL HADH (1.5 mmol/L) and 20 μL acetoacetyl‐CoA (5.9 mmol/L) were added. The HADH activity was determined by measuring immediately the absorbance of the mixtures at 340 nm. The absorbance was recorded every 30 s during 3 min and the HADH value was established as –104 × slope of the line resulting from the periodic spectrophotometric measures. | Kuruma prawn (Penaeus japonicas) |
Fresh samples were analysed after 0, 1, 2, 4 and 6 days of storage in ice. Samples were frozen at –10, –18°C, –35, –80 or –196°C during one week and thawed (4°C). Samples frozen at –18 °C were analysed after 0, 1, 2, 4, and 6 days of iced storage. Samples frozen at –10 °C, –35 °C, –80°C or –196°C were analysed immediately after thawing |
Liquid nitrogen for –196°C samples. No information for the other freezing methods. |
Significant differences in HADH activity between fresh and thawed prawn. No significant difference between freezing temperatures. Significant increase in HADH activity during storage in ice (influence of spoilage) for the thawed samples (not for the fresh samples). Threshold value: < 70 is fresh; > 80 is frozen/thawed, in between: uncertain. |
83% of samples is well classified, 9% uncertain and 8% misclassified |
10110011011 7 |
(Hoz et al., 1993) |
| HADH | Idem as the paper of (Garcia de Fernando et al., 1992): extracts were obtained from fresh and thawed samples (2 g) by immersion in two volumes (4 mL) of 0.1 mol/L phosphate buffer, pH 6.0, at room temperature for 15 min. The HADH released in the extracts was assayed by mixing in a spectrophotometer cell (10 mm light path length, nominal working volume 1.5 mL), 34 μL extract, 70 μL EDTA (34.4 mmol/L) and 880 μL phosphate buffer (0.1 mol/L, pH 6.0). The mixture was kept at room T for 3 min and, finally, 20 μL HADH (1.5 mmol/L) and 20 μL acetoacetyl‐CoA (5.9 mmol/L) were added. The HADH activity was determined by measuring immediately the absorbance of the mixtures at 340 nm. The absorbance was recorded every 30 s during 3 min and the HADH value was established as –104 × slope of the line resulting from the periodic spectrophotometric measures. | Hake (Merluccius merluccius), small hake (Merluccius merluccius), mackerel (Scomber scombrus), sea bream (Pagellus centrodontus), sole (Solea solea), squid (Loligo vulgaris), tuna (Thunnus alalunga) |
Fresh samples were analysed after 0, 1, 2, 4 and 6 days of storage in ice. Samples were frozen at –10, –18°C, –35, –80 or –196°C during one week and thawed (4°C). Samples frozen at –18 °C were analysed after 0, 1, 2, 4, and 6 days of iced storage. Samples frozen at –10, –35, –80 or –196°C were analysed immediately after thawing. |
Liquid nitrogen for –196°C samples. No information for the other freezing methods. |
Significant differences in HADH activity between fresh and thawed fish for all storage conditions combined, except for small hake. Significant increase in HADH activity during storage in ice (influence of spoilage) except for sole and tuna, but the increase was negligible in relation to differences between unfrozen and frozen/thawed. No significant difference between freezing temperatures except for squid and hake. No threshold was set. |
No classification performance available |
10111011100 7 |
(Pavlov et al., 1994) |
| HADH | The conversion rate of HADH to HAD+ was monitored by measuring the decrease in absorption at 340 nm. | Nile perch | Four superchilled samples of Nile perch fillets were analysed. One frozen sample was analysed as a reference. Sample was frozen for 48 h at –24°C | Not available | Based on the visual comparison of the slopes of the enzymatic reactions of the superchilled samples and the frozen sample, it was concluded that the superchilled samples had not been frozen before. | No classification performance available. |
00011000000 2 |
‘Nile perch study’ (see Annex A) |
| β‐N‐acetylgluco‐saminidase | Homogenised fish (10 g) was added to 30 mL of 0.2 mol/L citrate–phosphate buffer at pH 6.3 and agitated for 24 h at 4 ± 0.5°C. The assay was performed by combining 0.2 mL of p‐nitrophenyl‐N‐acetyl‐β‐D‐glucosamide (0.2 mg/mL), (0.3 mL of potassium citrate (0.1 mol/L, pH 4.5), 0.2 mL of distilled water and 0.3 mL of fish extract at 37°C. The reaction was stopped after 30 min by adding 1 mL of KOH (0.3 mol/L). The enzyme activity was calculated using a molar extinction coefficient of ε = (18,500 mol/L−1 cm−1) (p‐nitrophenol at 405 nm) and 1 U was defined as the hydrolysis of 1 μmol substrate per min at 37°C and pH 4.5. | Anchovy (Engraulis encrasicolus), chub mackerel (Scomber japonicas), horse mackerel (Trachurus trachurus), sardines (Sardina pilchardus) |
Fresh samples were retrieved from commercial fisheries. Fresh samples were stored at 4°C. Enzyme analysis was conducted within one day. Frozen samples were stored at –18°C and analysed on day 0, 1, 2, 7, 14, 21. |
Not available | No significant increase in enzyme activity in none of the fish species. | No classification performance available |
00000100100 2 |
(Alberio et al., 2014) |
| β‐N‐acetylgluco‐saminidase |
Juice was extracted by centrifugation (12,500 rpm at 5°C for 30 min) of 40 g of dorsomedial muscle. A mixture of 0.2 mL of p‐nitrophenyl‐N‐acetyl‐b‐D‐glucosaminide (0.2 mg/mL), 0.3 mL of potassium citrate (0.1 mol/L at pH 4.5), 0.2 mL of distilled water and 0.3 mL of pressed juice was incubated in a haemolysis tube at 37°C. The reaction was stopped after 30 min by the addition of 1 mL of potassium hydroxide (0.3 mol/L). The enzymatic activity was calculated as activity (mg‐1 protein h‐1) = (ODtest − ODcontrol)/[protein] × 0.3 × 2. |
Mackerel (Scomber scombrus), plaice (Pleuronectes platessa), whiting (Merlangus merlangus) | Analysis of 30 samples: fresh and after 1 day at –20°C (fast and slow freezing conditions). | Two freezing conditions: slow freezing in a cold room and fast freezing in a cell. | No significant difference between fresh and frozen/thawed samples | No classification performance available |
00000001100 2 |
(Duflos et al., 2002) |
| β‐N‐acetylgluco‐saminidase | 50 μL of blood was mixed with 0.1 mL of isotonic solution (10 mmol/L phosphate buffer, pH 7.0, containing 0.1 mol/L NaCl, 2 mmol/L EDTA, 10 mmol/L 2‐mercaptoethanol and 20 mmol/L N‐acetyl‐D‐galactosamine) and with 50 μL of substrate solution (0.8 mmol/L 4‐methylumbellifelyl‐2‐acetamido‐2‐deoxy‐β‐D‐glucopyranoside in 2‐methoxyethanol).The mixture was incubated in a test tube at 20°C for 10 min, then 3.3 mL of GBES solution (50 mmol/L glycine buffer, pH 10.4, with 5 mmol/L EDTA and 0.1 mol/L NaCl) was added to inactivate the enzyme. The released 4‐methylumbelliferone was determined by a spectrofluorometer at excited wavelength 365 nm and emission wavelength 460 nm. | Carp (Cyprinus carpio), red sea bream (Chrysophrys major) |
Fresh samples were stored at 4°C and analysed daily during one week. Frozen samples were analysed after being frozen for 1 day, 1 week, 1, 2, 6 months, 1 and 2 years. Freezing of samples took place at –20°C and were thawed in tap water of around 15°C. |
Not available |
Enzyme activity was more than a 100‐fold higher in frozen–thawed than in fresh fish. Differentiation was already possible after 1 day of freezing. Enzyme activity of fresh fish increased during storage (influence of spoilage); after 6 days of storage in ice, enzyme activity increased, but fish quality was no longer acceptable. No threshold value was set. |
No classification performance available |
00000110100 3 |
(Kitamikado et al., 1990) |
| β‐N‐acetylgluco‐saminidase | Slices (5–7 g) were centrifuged for 30 min at 28,000 × g and the fluid was collected. Extracts were obtained by homogenising fish tissue in Tris‐HCl buffer. After standing for 30 min at 4°C, the mixture was centrifuged. β‐N‐Acetyl‐glycosaminidase was measured with p‐nitrophenyl‐N‐acetyl‐β‐D‐glucose amide as substrate. The reaction mixture contained 0.3 mL 0.1 mol Na‐citrate buffer, pH 4.5, 0.2 mL of 0.6 mol KCl 0.2 mL of 1.0 mmol solution of the substrate and 0.5 mL of appropriately diluted sample (protein content in the final mixture about 2 mg). The reaction mixture was incubated at 37°C for 30 min, and the reaction was stopped by addition of 1 mL of 0.3 mol KOH. The absorbance was measured immediately (λ = 405 nm, ε = 19,500 mol/L−1 cm−1). | Rainbow trout (Oncorhynchus mykiss) |
Fresh fish was kept on ice and analysed after 0, 3, 6, 10 and 14 days of storage. Fish previously stored on ice for 3, 6, 10 and 14 days, was frozen and stored at –40°C during 6–8 weeks, thawed and analysed. Separate tests were done for freezing in a freezing tunnel at –20, –25, –30, and –35°C and storage during 4–6 weeks. |
Freezing tunnel, four different freezing speeds tested |
Enzymatic activity increased between three and four times between fresh and frozen/thawed fish Small increase of enzyme activity during storage on ice (influence of spoilage). Increase in enzymatic activity when freezing rate was slower. No threshold value was set. |
No classification performance available |
00100111000 4 |
(Nilsson and Ekstrand, 1993) |
| β‐N‐acetylgluco‐saminidase | Press juice was obtained by centrifugation at 5°C at 18,000 rpm for 30 min. The assay contained 1 mMp‐nitrophenyl‐N‐acetyl‐8‐D‐glucosaminide, 0.1M K‐citrate pH 4.5 and enzyme solution. After 30 min at 37°C the reaction was stopped by addition of an equal volume of 0.1 mol/L KOH. The alkaline solution was centrifuged, and the absorbance measured at 405 nm. Enzyme activity was expressed as ∆E405 × 10‐2 h‐1 × mg protein. | Cod (Gadus morhua), saithe (Gadus virens), red fish (Sebastes marinus), haddock (Gadus aeglefinus) |
Fresh fish was analysed after 0, 4, 7, 11, 14, 18 and 20 days of storage. Fresh fish was frozen immediately in a freezer chest for 1 days at –29°C. Frozen blocks of fish were also obtained for commercial trawlers and stored at –25 to –30°C in a freezing room. |
Domestic freezer chest |
Marked enzymatic activity increase between fresh and frozen/thawed fish for all species. (no statistical analysis performed). Marked increase in enzymatic activity during fresh storage (influence of spoilage). No threshold value was set. |
No classification performance available |
00100111100 5 |
(Rehbein et al., 1978) |
| β‐N‐acetylgluco‐saminidase | 40 g of fillet was cut into pieces and centrifuged at 5°C and 18,000 rpm for 30 min. The clear supernatant was decanted. Enzymatic assay was performed. | Cod (Gadus morhua) |
Fresh fish was analysed immediately. Fresh fish was frozen in a freezer chest for 1 day at –29°C, thawed and analysed. |
Domestic freezer chest | Enzymatic activity increased three times between fresh and frozen/thawed fish. No threshold value was set. | No classification performance available |
00000001000 1 |
(Rehbein, 1979) |
| β‐N‐acetylgluco‐saminidase | Blood was collected from the dorsal aorta. To 0.1 mL of the isotonic solution (10 mmol/L phosphate buffer, pH 7.0, containing 0.11 mol/L NaCl, 2 mmol/L EDTA, 10 mmol/L 2‐mercaptoethanol, and 20 mmol/L N‐acetyl‐D‐galactosamine) were added 50 μL of the blood and 50 μL of the substrate solution of 0.8 mmol/L 4‐methylumbelliferyl‐2‐acetamido‐2‐deoxy‐β‐D‐glucopyranoside which had been dissolved in 2‐methoxyethanol in 10 mmol/L concentration as stock solution and diluted with the isotonic solution when used. The above mixture in a test tube was incubated at 20°C for 10 min, then 3.3 mL of 50 mmol/L glycine buffer, pH 10.4, with 5 mmol/L EDTA and 0.1 mol/L NaCl was added. The released 4‐methylumbelliferone was determined by a spectrofluorometer (at Ex 365 nm and Em 460 nm). One unit of the enzyme was the activity which released one μmol of 4‐methylumbelliferone per min, under these conditions. |
Carp (Cyrpinus carpio) Red sea bream (Chrysophrys major) |
Fresh carp was stored at 4°C and frozen carp at –1°C, –20°C and –40°C. Fresh seabream was stored at 4°C and frozen seabream at –20°C. Applicability tested to 25 common edible species (analysed fresh and frozen/thawed at –40°C for 1 day). |
Not available |
Low enzyme activity (close to 0) in blood of fresh carp and carp at –1°C; enzyme activity remained low up to 5 days at 4°C and 12 days at –1°C; high enzyme activity in blood of frozen/thawed carp. Low enzyme activity (close to 0) in blood of fresh seabream; high enzyme activity in blood of frozen/thawed seabream (100‐fold higher). Increase of enzyme activity during fresh storage (method not applicable on spoiled fish). Applicability to common edible species: though the enzyme activity varied among the species, there were clear‐out differences in the activity between the unfrozen and frozen–thawed fish of every species. |
No classification performance available |
001110110100 6 |
(Yuan et al., 1988) |
| α‐glucosidase | 10 g of homogenised fish were added to 30 mL of 0.2 mol/L citrate–phosphate buffer at pH 6.3 and agitated for 24 h at 4 ± 0.5°C. The sample was centrifuged at 10,000 × g for 30 min. Then, the pellet was separated from the supernatant and used for the test. The reagents used included 0.2 mL of p‐nitrophenyl‐α‐D‐glucopyranoside (7.53 mg mol−1), 0.3 mL of potassium citrate (0.2 mol/L, pH 4.5), 0.2 mL of NaCl (1 mol/L), 0.1 mL of distilled water and 0.4 mL of enzyme extract of the fish at 37°C. The reaction was incubated at 37°C and stopped by the addition of 1 mL of sodium carbonate (2%). α‐glucosidase activity was calculated using a molar extinction coefficient of ε = (18,500 mol/L−1 cm−1) (p‐nitrophenol at 405 nm) and 1 U was defined as the hydrolysis of 1 μmol substrate per min at 37°C and pH 4.5. | Anchovy (Engraulis encrasicolus), chub mackerel (Scomber japonicas), horse mackerel (Trachurus trachurus), sardines (Sardina pilchardus) |
Fresh samples were retrieved from commercial fisheries. Fresh samples were stored at 4°C. Enzyme analysis was conducted within one day. Frozen samples were frozen and stored at –18°C and analysed on days 0, 1, 2, 7, 14, 21. |
Not available |
Significant increase in anchovy, sardines and horse mackerel even after 1 day of frozen storage. No significant difference in chub mackerel. |
No classification performance available |
10000100100 3 |
(Alberio et al., 2014) |
| α‐glucosidase | Juice was extracted by centrifugation (12,500 rpm at 5°C for 30 min) of 40 g of dorsomedial muscle. A mixture of 0.2 mL of p‐nitrophenyl‐α‐D‐glucopyranoside (7.53 mg/mL), 0.3 mL of potassium citrate (0.2 mol/L at pH 4.5), 0.2 mL of sodium chloride (1 mol/L), 0.2 mL of distilled water and 0.3 mL of pressed juice was incubated in a haemolysis tube at 37°C. The reaction was stopped after 2 h by the addition of 1 mL of potassium hydroxide (0.2 mol/L) with vigorous stirring. The absorbance was read in a spectrometer at 405 nm. Controls were prepared in the same way, but the pressed juice was added after stoppage of the reaction with potassium hydroxide. The enzymatic activity was calculated as activity (mg‐1 protein h‐1) = (ODtest − ODcontrol)/[protein] × 0.3 × 2. | Mackerel (Scomber scombrus), plaice (Pleuronectes platessa), whiting (Merlangus merlangus) | Analysis of 30 samples: fresh and after 1 day at –20°C (fast and slow freezing conditions). | Two freezing conditions: slow freezing in a cold room and fast freezing in a cell. |
Significant difference between fresh and frozen/thawed samples, except for mackerel. Small increase in α‐glucosidase activity due to spoilage. Increase in α‐glucosidase activity during frozen storage. Threshold values were set for plaice (0.5) and whiting (0.15). |
No classification performance available |
10000001110 4 |
(Duflos et al., 2002) |
| α‐glucosidase | Fish exudates were obtained from flesh juice after centrifugation (12,500 rpm at 5°C). Each exudate was prepared from a single fillet. Protein concentration in exudate was determined with the Bradford method using the Bio‐Rad reagent and bovine serum albumin as a standard. | Sea bass (Dicentrarchus labrax) | 10 samples were analysed fresh and 10 samples were analysed after freezing at –30°C and storage at –20°C for 40 days. | Not available |
Significant difference between fresh and frozen/thawed samples. No possibility to set threshold value due to large standard deviations. |
No classification performance available |
10100000000 2 |
(Marlard et al., 2019) |
| α‐glucosidase | Slices (5–7 g) were centrifuged for 30 min at 28,000 ×g and the fluid was collected. Extracts were obtained by homogenising fish tissue in Tris‐HCl buffer. After standing for 30 min at 4°C, the mixture was centrifuged. The α‐glucosidase activity was measured spectrophotometrically: 0.3 mL of 0.05 mol Na citrate buffer, pH 4.0 was mixed with 0.2 mL of 1.0 mol NaCl, 0.2 mL of 4.2 mmol p‐nitrophenyl‐α‐glucopyranoside solution and 0.8 mL sample solution adjusted with distilled water to a total protein content of about 5 mg. This mixture was incubated at 37°C for 60 min, whereafter the reaction was stopped by addition of 1 mL of 0.2 mL KOH and the absorbance measured immediately (λ = 405 nm, ε = 19,500 mol/L−1 cm−1). | Rainbow trout (Oncorhynchus mykiss) |
Fresh fish was kept on ice and analysed after 0, 3, 6, 10 and 14 days of storage. Fish previously stored on ice for 3, 6, 10 and 14 days, was frozen and stored at –40°C during 6–8 weeks, thawed and analysed. Separate tests were done for freezing in a freezing tunnel at –20°C, –25°C, –30°C, –35°C and storage for 4–6 weeks at –40°C. |
Freezing tunnel, four different freezing speeds tested |
Enzymatic activity increased between 2.5 and 1.5 times between fresh and frozen/thawed fish. No marked increase of enzyme activity during storage on ice (influence of spoilage). Increase in enzymatic activity with slower freezing rate. No threshold value was set. |
No classification performance available |
00100111000 4 |
(Nilsson and Ekstrand, 1993) |
| α‐glucosidase | Press juice was obtained by centrifugation at 5°C at 18,000 rpm for 30 min. α‐glucosidase activity was determined spectrophotometrically. The assay mixture contained 0.20 mL p‐nitrophenyl‐α‐glucopyranoside solution (7.53 mg/mL distilled water), 0.30 mL 0.2 mol/L K‐citrate pH 4.5, 0.20 mL 1 mol/L NaCl, and enzyme solution (press juice); the mixture was adjusted with distilled water to 1.2 mL test volume. The reaction was started by the addition of enzyme and allowed to proceed for 2 h at 37°C. It was stopped by adding 1.0 mL 0.2 mol/L KOH with vigorous stirring. Immediately after centrifugation for 1.5 min (8,000 × g) the absorbance was read against a blank at 405 nm. Enzyme activity was expressed as ∆E405 × 10‐2 h−1 × mg protein. | Cod (Gadus morhua), haddock (Gadus aeglefinus), saithe (Gadus virens), red fish (Sebastes marinus) |
Fresh fish was analysed after 0, 4, 7, 11, 14, 18 and 20 days of storage. Fresh fish was frozen in a freezer chest for 1 day at –26°C to –29°C. Frozen blocks of fish were also obtained for commercial trawlers and stored at –25 to –30°C in a freezing room. |
Domestic freezer chest |
Marked enzymatic activity increase between fresh and frozen/thawed fish for all species. Marked increase in enzymatic activity during fresh storage (influence of spoilage). No threshold value was set. |
No classification performance available |
00100111100 5 |
(Rehbein et al., 1978) |
| α‐glucosidase | 40 g of fillet was cut into pieces and centrifuged at 5°C and 18,000 rpm for 30 min. The clear supernatant was decanted. Enzymatic assay was performed. | Cod (Gadus morhua) |
Fresh fish was analysed immediately. Fresh fish was frozen in a freezer chest for 1 day at –29°C, thawed and analysed. |
Domestic freezer chest |
Enzymatic activity increased between three and eight times between fresh and frozen/thawed fish. No threshold value was set. |
No classification performance available |
00000001000 1 |
(Rehbein, 1979) |
| α‐glucosidase | Press juice was obtained by centrifuging 40 g of fish muscle. Extracts were obtained by homogenising an aliquot of the sediment with Triton x‐100. After standing for 30 min at 4°C, the mixture was centrifuged as for the press juice. Alfa‐glucosidase activity was determined: the assay mixture contained 0.20 mL p‐nitrophenyl‐α‐D‐glucopyranoside solution, 0,30 mL 0.2 mol/L K‐citrate pH 4.5, 0.2 mL 1 mol/L mol/L NaCl, and enzyme solution; the mixture was adjusted with distilled water 1.20 mL test volume. The reaction was started by the addition of enzyme and allowed to incubate for 2 h at 37°C. It was stopped by adding 1.0 mL 0.2 mol/L KOH with vigorous stirring. Absorbance at 405 nm was measured after centrifugation for 1.5 min at 8000 × g. | Salmon (Salmo salar) | Fresh and sea‐frozen samples which were stored at –25°C to –30°C. | Not available |
Alfa‐glucosidase activity is higher is frozen/thawed fish than in fresh fish. No threshold value was set. |
No classification performance available. |
00000100000 1 |
(Rehbein and Cakli, 2000) |
| Acid phosphatase | Slices (5–7 g) were centrifuged for 30 min at 28,000 × g and the fluid was collected. Extracts were obtained by homogenising fish tissue in Tris‐HCl buffer. After standing for 30 min at 4°C, the mixture was centrifugated. Acid phosphatase was measured with p‐nitrophenylphosphate as substrate in a reaction mixture containing 1 mL sample (0.2 mol Na‐acetate buffer, pH 5.0, and 1 mg protein) and 0.5 mL of 34 mmol/L substrate solution, which was incubated at 37°C for 15 min. The reaction was stopped by adding 2 mL ice‐cold 0.4 mol/L TRIS‐HC1‐K2HPO4 buffer, pH 8.0. The samples were then centrifuged in a Sorvall bench centrifuge at 300 ×g for 5 min. The absorbance was measured immediately (λ = 405 nm, ε = 19,500 mol/L –1 cm‐1). Enzyme activity was calculated as katals per gram of muscle tissue. | Rainbow trout (Oncorhynchus mykiss) |
Fresh fish was kept on ice and analysed after 0, 3, 6, 10 and 14 days of storage. Fish previously stored on ice for 3, 6, 10 and 14 d, was frozen and stored at –40°C during 6–8 weeks, thawed and analysed. Separate test was done with freezing in freezing tunnel at –20°C, –25°C, –30°C, –35°C and stored during 4–6 weeks. |
Freezing tunnel, four different freezing speeds tested |
Enzymatic activity increased two times between fresh and frozen/thawed fish. Marked increase of enzyme activity during storage on ice (influence of spoilage). Increase in enzymatic activity with slower freezing rate. No threshold value was set. |
No classification performance available |
00100111000 4 |
(Nilsson and Ekstrand, 1993) |
| Acid phosphatase | 40 g of fillet was cut into pieces and centrifuged at 5°C and 18,000 rpm for 30 min. The clear supernatant was decanted. Enzymatic assay was performed. | Cod (Gadus morhua) |
Fresh fish was analysed immediately. Fresh fish was frozen in a freezer chest for 1 day at –29°C, thawed and analysed. |
Domestic freezer chest |
No increase in enzymatic activity was detected. No threshold value was set. |
No classification performance available |
00000001000 1 |
(Rehbein, 1979) |
| β‐glucuronidase | 40 g of fillet was cut into pieces and centrifuged at 5°C and 18,000 rpm for 30 min. The clear supernatant was decanted. Enzymatic assay was performed. | Cod (Gadus morhua) |
Fresh fish was analysed immediately. Fresh fish was frozen in a freezer chest for 1 day at –29°C, thawed and analysed. |
Domestic freezer chest |
Enzymatic activity increased four times between fresh and frozen/thawed fish. No threshold value was set. |
No classification performance available |
00000001000 1 |
(Rehbein, 1979) |
| β‐galactosidase | 10 g of homogenised fish was mixed with 40 mL of 0.2 mol/L citrate–phosphate buffer at pH 4,1 mol/L mol/L mol/L NaCl and 1 mmol/L DL‐dithiothreitol (DTT). Each of these mixtures was homogenised for 2 h at 40°C, centrifuged at 4,000 × g for 10 min, filtered and ultrafiltered with a cut‐off of 50 kDa. The assay reagents included 0.45 mL of substrate 0.0055 mol/L, 10 mL of C–P buffer 0.1 mol/L at pH 4, and 0.25 mL of enzyme extract. After 30 min at 30°C, the reaction was stopped by adding 1 mL of 1M Na2CO3. The free p‐nitrophenol was measured at 400 nm and activities were assessed in relation to the internal standard, p‐nitrophenol. Protein concentration was determined according to the dye‐binding method of Bradford, with bovine serum albumin as the standard. | Anchovy (Engraulis encrasicolus), chub mackerel (Scomber japonicas), horse mackerel (Trachurus trachurus), sardines (Sardina pilchardus) |
Fresh samples were retrieved from commercial fisheries. Fresh samples were stored at 4°C. Enzyme analysis was conducted within one day. Frozen samples were stored at –18°C and analysed on days 0, 1, 2, 7, 14, 21. |
Not available |
Significant increase in horse mackerel and chub mackerel even after one day of frozen storage. No significant difference in anchovy and sardines. |
No classification performance available |
10000100100 3 |
(Alberio et al., 2014) |
| β‐galactosidase | 40 g of fillet was cut into pieces and centrifuged at 5°C and 18,000 rpm for 30 min. The clear supernatant was decanted. Enzymatic assay was performed. | Cod (Gadus morhua) |
Fresh fish was analysed immediately. Fresh fish was frozen in a freezer chest for 1 day at –29°C, thawed and analysed. |
Domestic freezer chest |
Enzymatic activity increased four times between fresh and frozen/thawed fish. No threshold value was set. |
No classification performance available |
00000001000 1 |
(Rehbein, 1979) |
| Lactate dehydrogenase | Each tube contained a muscle cube (about 1 cm3) and 15 mL of phosphate‐buffered saline (30 mmol/L of NaCl). The tubes were shaken at 200 rpm for 1 h at 4°C. They were centrifuged after at 1,500 rpm for 5 min at +4°C. 1 mL of the supernatant was removed and stored at +4°C until measurement of the activity of free α‐glucosidase and free LDH. The remainder of the tube was crushed by Ultra Turrax with T‐10 basic disperser of 10 mm, 1/cs (1 min, 24,000 rpm) and then sonicated by ultrasound (1 min 100 W, 24 kHz) to release all the enzymes contained in the cells. The tubes were centrifuged at 3,400 rpm for 5 min at +4°C. One mL of supernatant was taken for measurement of the activity of total α‐glucosidase and/or total LDH. The relative activity of the LDH corresponded to the ratio between the free LDH activity (released LDH) and the total LDH activity (intra‐ and extracellular LDH obtained by crushing and muscle sonication). The activity of LDH (free and total LDH) was measured as described in the kit ‘Cytotoxicity Detection Kit (LDH)’ (Roche). For sea bream muscle, activities were expressed by gram of muscle. | Sea bream (Sparus aurata) | Fish was stored at 4°C and was quickly frozen and then stored at –20°C for 24 h. Thawing at room temperature. | Quick freezing (0.3°C/min) |
Activity of LDH released from frozen‐thawed fillets was lower (20%) than the activity from fresh fillets. LDH could be used in combination with α‐glucosidase to permit the detection of a difference between fresh and frozen–thawed fillets. |
No classification performance available |
00000001000 1 |
(Diop et al., 2016) |
| Lactate dehydrogenase | 40 g of fillet was cut into pieces and centrifuged at 5°C and 18,000 rpm for 30 min. The clear supernatant was decanted. Enzymatic assay was performed. | Cod (Gadus morhua) |
Fresh fish was analysed immediately. Fresh fish was frozen in a freezer chest for 1 day at –29°C, thawed and analysed. |
Domestic freezer chest |
No increase in enzymatic activity of LDH could be observed. No threshold value was set. |
No classification performance available |
00000001000 1 |
(Rehbein, 1979) |
| Citrate synthase | Citrate activity was detected in the exudate after tempering the meat at room temperature and diluting 0.1 mL with demineralised water (1:9). Sigma Aldrich enzyme test (Sigma‐Aldrich, Germany) was used for the measurement. | Salmon (Salmo salar) | Fresh portions were placed in a refrigerator at 2 ± 1°C during 10 days. Samples portions were frozen at –28 ± 2°C during 5 months and analysed after 4, 15, 28, 58 and 162 days. | Not available |
Significant statistical difference between chilled and frozen/thawed salmon. Enzyme activity in chilled samples was relatively stable, but the enzyme activity in frozen/thawed samples rose with storage time A threshold value of 1.85 micro‐mol mL−1 min−1 is proposed. |
No classification performance available |
10001110010 5 |
(Skorpilova et al., 2019) |
| Succinate dehydrogenase | Method from (Parisi et al., 1978). A portion of muscle without connective tissue, blood, skin was incubated with a mixture containing 1:1 3‐triphenyltetrazolium, sodium succinate and phosphate buffer 0.1 mol/L mol/L pH 7.6 during 90 min at 37°C. The results were expressed in μg triphenylformazan/g fish meat. | Anglerfish (Lophius spp.), Chondrichthyes, cod (Gadus morhua), pleuronectidae, salmon (Salmo salar), swordfish (Xiphias gladius) |
Samples were taken from the market (presented and sold as fresh). Samples were analysed immediately and after freezing in the lab at –20°C during 24 and 48 h. Only very fresh fish was included in the test (percentage of intact red blood cells above 70% as determined with the haemolysis test. |
Not available |
No differentiation possible for cod and anglerfish due to overall low enzyme activity. No differentiation possible for salmon and chondrichthyes due to overall high enzyme activity. Enzyme highly active in fresh, whole pleuronectidae and low in frozen fillets, however, overlap in values between fresh and frozen‐thawed fish. Enzyme is active in refrigerated product and activity is retained also in frozen fish (swordfish); overlap in values between fresh and frozen‐thawed fish. Low discrimination ability of the test due to slow deactivation of this enzyme during freezing. |
No classification performance available |
00000100100 2 |
(Civera et al., 1996) |
| Protein markers by electrophoresis | ||||||||
| Aspartate aminotransferase | Fish extract was prepared. Electrophoresis was carried out on 57x140 mm cellulose acetate membrane with a cold Tris (75 mmol/L)‐glycine (200 mmol/L)‐NaCl (22 mmol/L) pH 8.9 buffer system. Samples were placed on the cathodic end of the membrane with a multi‐microapplicator. Electrophoresis was performed followed by a staining method for presence of aspartate aminotransferase. | 16 marine species and 6 freshwater species | Samples of fresh or frozen muscles were taken. | Not available | The electrophoretic‐colorimetric response was unambiguous and general, irrespective of species, and depended on the fresh or frozen state of the specimens. | No classification performance available |
00000100110 3 |
(Salfi et al., 1985) |
| Parvalbuminc | Two‐dimensional gel electrophoresis (2‐DE). Soluble proteins were obtained from 20 g of diced white flesh (cubes of approximately 1 cm on each side) of fresh or frozen–thawed fillets placed in one volume (w/v) of phosphate buffer (10 mmol/L, pH 7.4) containing anti‐proteases: 1% phenylmethylsulfonyl fluoride (PMSF) (10 mg/mL isopropanol), 0.2% pepstatin (0.35 mg/mL ethanol) and 0.1% leupeptin–aprotinin (combination of leupeptin 0.5 mg/mL and aprotinin 1 mg/mL). After gentle stirring, suspensions were centrifuged at 34,000 ×g, for 30 min at +4°C to obtain exudates. The supernatant was filtered twice on a 0.45 μm membrane. First dimension isoelectric electrophoresis focusing (IEF) was performed with ReadyStrip immobilised pH gradient (IPG) strips. Equilibrated strips were loaded on 12.5% SDS gels (19.5 × 19.5 × 0.05 cm) and underwent Sodium Dodecyl Sulfate – Poly Acrylamide Gel Electrophoresis (SDS‐PAGE), as described in (Hochstrasser et al., 1988). | Sea bass (Dicentrarchus labrax) |
Fresh sea bass was stored between 0 and 4°C and analysed after 0, 3, 6, 9 and 15 days of storage. Sea bass was frozen at –30°C after 0, 3, 6, 9 and 15 days of fresh storage and stored at –20°C for less than 15 days. Fillets were thawed and analysed. |
Frozen at processing plant. |
The 2‐DE patterns from the exudates revealed areas of differentiation between fresh and frozen–thawed fish. The protein present in exudates of frozen/thawed fish was identified as parvalbumin. The 2‐DE analyses could differentiate between fresh and frozen–thawed fish fillets up until day 13. The 2‐DE method is therefore valuable for testing fish fillets up until their use‐by date. |
No classification performance available |
00000011010 3 |
(Ethuin et al., 2015) |
| Transgelinc | Two‐dimensional gel electrophoresis. Soluble proteins were obtained from 0.2–0.3 g of fresh or frozen–thawed skinless tentacles placed in 10 volumes (w/v) of buffer containing 7 mol/L urea, 2 mol/L thiourea, 4% 3‐[(3‐cholamidopropyl) dimethylammonium]‐1‐propanesulfonate (CHAPS), 30 mmol/L Tris(hydroxymethyl)aminomethane (Tris), 100 mmol/L DTT. After homogenisation, the samples were incubated for 1 h at +4°C and then centrifuged at 12,000 ×g for 15 min at +4°C. The lipids were discarded by pipetting, and the supernatant was collected and stored at −20°C until analysis. IEF was performed with ReadyStrip IPG strips. Equilibrated strips were loaded on 15% SDS gels and underwent SDS–PAGE. | Curled octopus (Eledone cirrhosa) | Fresh samples (skinless tentacles) were analysed immediately. Frozen/thawed samples were stored at – 20°C for 72 h and thawed before analysis. | Not available | The 2‐DE analysis revealed a quantitative variation of two protein spots that were decreased in the frozen‐thawed samples as compared to the fresh sample. The proteins were identified as transgelin. | No classification performance available |
00000000010 1 |
(Guglielmetti et al., 2018) |
| Profile analysis | ||||||||
| Volatile profile | The measurements were performed with an electronic nose based on eight thickness shear mode resonator sensors each coated with a different film of pyrrolic macrocycles. For each of the two experiments al linear discriminant analysis has been performed. As sensor feature for each measurement the steady state variation of frequency has been considered. Discriminant analysis has been calculated as a partial least squares problem where the classes, defined as a one‐of‐many encoding, were the y‐block and the electronic nose data were the x‐block. A leave‐one‐out validation procedure has been adopted in model calibration. | Trout |
Fresh fish were stored at 4°C during 5 days of storage. Fish was frozen at –20°C for one week, thawed and stored at 4°C during 5 days. |
Not available | Differentiation of fresh and frozen/thawed samples based on discriminant analysis only possible for the first 2 days of storage with 84% of samples being well classified. | No classification performance on new samples available |
10000010000 2 |
(Di Natale et al., 2000) |
| Volatile profilec | Analyses were carried out on a Thermo Finnigan thermoQuest gas chromatograph equipped with a split/splitless injector and coupled with a Trace quadrupole mass detector. Compounds were separated on a 30 mol/L × 0.32 mm × 1 μm film thickness, fused silica DB‐1701 capillary column. The identification of the volatile compounds was achieved by comparing their mass spectra with those stored in the National Institute of Standards and Technology (NIST) US Government library. Pure standards were also injected to confirm misidentifications. | Gilthead seabream (Sparus aurata) |
Fresh fish samples were immediately analysed. Frozen fish was maintained at –20°C during 266 days. Samples were analysed after 0 (fresh), 18, 28, 39, 60, 120 and 266 days. |
Not available | 1‐octen‐3‐ol, 1‐penten‐3‐ol, Z‐4‐heptenal and 2‐octen‐1‐ol can possibly be used as markers to differentiate between fresh and frozen‐thawed fish. | No classification performance available |
00000100000 1 |
Iglesias et al, 2009 |
| Volatile profilec | The GC was equipped with an SLB‐5 ms capillary column (60 mol/L × 0.25 mm × 0.25 μm) (Supelco/Sigma Aldrich). Volatile compounds were identified using mass spectral databases (NIST 2008), relative retention indices, linear retention indices (LRIs) and chemical standards. | Cod (Gadus morhua), salmon (Salmo salar), European sea bass (Dicentrarchus labrax), gilthead seabream (Sparus aurata), |
Fresh fish samples were analysed immediately. Fish were stored under vacuum at –20°C for 90 days. Frozen fish was analysed after 1 and 3 months. |
Not available | Dimethyl sulfide, 3‐methylbutanal, ethyl acetate and 2‐methylbutanal can be considered as potential markers of differentiation between fresh and frozen–thawed fish. | No classification performance available |
00000100100 2 |
(Leduc et al., 2012) |
| Phospholipid profile | Lysophospholipid analysis was performed by hydrophilic Interaction Liquid Chromatography‐Electrospray Ionisation Fourier Transform Mass Spectroscopy. Determination of phospholipids (PL), lysophospholipids (LPL), phosphatidylcholines (PC) and lysoforms of PC (LPC) phophatidylethanolamines (PE) and lysoforms of PE (LPE). | Shrimp (parapenaeus longirostris), octopus (Octopus vulagaris) |
Fish was obtained commercially. Fresh and thawed octopus was analysed immediately and after 6 days of storage at 0–4°C. Frozen vacuum‐sealed octopuses were thawed, analysed immediately and after 4 and 7 days of storage at 0–4°C. Fresh and thawed shrimp was analysed immediately and after 6 days of storage at 0–4°C. |
Not available |
Octopus: significant difference in LPE/PE and LPC/PC ratios only between fresh and frozen/thawed samples after a refrigerated storage of 4 and 7 days. Shrimps: significant difference between fresh and frozen/thawed samples already after immediate analysis. No threshold values were set. |
No classification performance available |
10000010100 3 |
(Losito et al., 2018) |
| Haematological methods | ||||||||
| Haematocrit value | Blood was drawn from the bulbus arteriosus and the ventricle with a syringe coated with heparin sodium solution and divided into blood cells and blood plasma in a haematocrit capillary by centrifugation at 11,000 rpm for 5 min. Haematocrit values for frozen carp were determined after they were thawed in running water. | Carp |
Fish was stored at 5°C, 0°C, –5°C and –20°C for 10 days. Some samples were frozen at –70°C in dry ice‐ethyl alcohol and stored at –20°C for 1 day. |
Not available | Haematocrit values of frozen fish was 0% even after 1 day. Refrigerated carp reached 0% only after 10 day. Those were not acceptable any more for human consumption. | No classification performance available |
00111110010 6 |
(Yoshioka, 1983) |
| Haemolysis of red blood cells | The grade of haemolysed red blood was analysed by streaking the blood on a microscope slide, staining it with May–Grunwald–Giemsa. The percentage of haemolysed cells were counted on more than one visual field. | Anglerfish (Lophius spp.) (10 samples), Chondrichthyes (8 samples), cod (Gadus morhua) (10 samples), salmon (Salmo salar) (10 samples), swordfish (55 samples) (Xyphius gladius), pleuronectidis (27 samples) |
Samples were purchased from the market (presented and sold as fresh). Samples were analysed immediately and after freezing in the lab at –20°C and –40°C during 24 h and 48 h. |
Not available |
No haemolysis of red blood cells in fresh fish from freshness category A was observed. A certain level of haemolysis was detected also during prolonged iced storage of fish (quality category B). After 24 h of freezing, haemolysis was 70–75%; after 48 h of freezing, haemolysis was above 95%; after more than 48 h of freezing, the percentage of red blood cells still intact was less than 1–2%. Method was not affected by species, freezing temperature, nor seasonal variation. As total lysis of red blood cells is not reached in 48 h, assessment of product should be coupled with organoleptic evaluation. This is particularly relevant when intact erythrocytes are around 30% as this value can be achieved both following a short freezing of high‐quality fish Extra/A) and following long storage under ice. |
No classification performance available |
00100100110 4 |
(Civera et al., 1996) |
| Other methods | ||||||||
| Free calcium concentration | Free calcium concentration in the exudate was estimated with the Calcium Detection Kit (Abcam Company) according to the manufacturer's protocol. | Sea bass (Dicentrarchus labrax) | 10 samples were analysed fresh and 10 samples were analysed after freezing at –30°C and storage at –20°C for 40 days and thawing. | Not available |
Significant difference between fresh and frozen/thawed samples (twofold higher in frozen–thawed fillets). No threshold value was set. |
No classification performance available |
10000000000 1 |
(Marlard et al., 2019) |
| Nucleotides and related compounds (NRC) | The concentration of NRCs in exudates was assessed with UV spectrophotometry at 260 nm according to the method of (Barcelo et al., 1986). Calibration curve was generated with denatured herring sperm DNA. | Sea bass (Dicentrarchus labrax) | 10 samples were analysed fresh and 10 samples were analysed after freezing at –30°C and storage at –20°C for 40 days and thawing. | Not available |
Significant difference between fresh and frozen/thawed samples (twofold higher in frozen–thawed fillets). No threshold value was set. |
No classification performance available |
10000000000 1 |
(Marlard et al., 2019) |
2‐DE = two‐dimensional gel electrophoresis; CHAPS = 3‐[(3‐cholamidopropyl) dimethylammonium]‐1‐propanesulfonate; CI = confidence interval; DTT = DL‐dithiothreitol; EDTA = Ethylenediaminetetraacetic acid; ε = molar extinction coefficient; HADH = β‐hydroxyacyl coenzyme A dehydrogenase; IEF = isoelectric electrophoresis focusing; IPG = immobilised pH gradient; IU = International Units; LDH = lactate dehydrogenase; LPC = lysoforms of phosphatidylcholines; LPE = lysoforms of phophatidylethanolamines; LPL = lysophospholipids; LRI = linear retention index; NIST = National Institute of Standards and Technology; NRC = Nucleotides and related compounds; OD = optical density; PC = phosphatidylcholines; PE = phophatidylethanolamines; PL = hospholipids; PMSF = phenylmethylsulfonyl fluoride; rpm = rounds per minute; SDS‐PAGE = Sodium Dodecyl Sulfate – Poly Acrylamide Gel Electrophoresis; U = unit;.
When the significance is stated, statistical analysis was performed. In all other cases, no statistical analysis was done.
An appraisal of the relevance of each study (appropriateness to answer the ToR) was carried out based on the following criteria to be answered by yes (score 1) or no (score 0). Q1: Could different groups of samples (fresh and frozen/thawed) successfully be differentiated based on statistical tests?; Q2: Did the study include method validation? For the biochemical methods, this refers to the analytical accuracy, precision, sensitivity, specificity, and reproducibility. For the physico‐chemical methods, this refers to testing the algorithm with samples (calibration/training set of samples) already used for the developing the algorithm, i.e. cross‐validation describing the determination or correlation coefficient, accuracy (or overall rate of correct classification), sensitivity, specificity etc.); Q3: Did the study consider different freezing temperatures?; Q4: Were freezing temperatures above ‐ 18°C included?; Q5: Did the study include superchilled samples or slightly frozen (e.g. ≥ ‐ 5°C) samples?; Q6: Did the study consider different frozen storage durations?; Q7: Did the study consider the influence of post‐harvest quality changes in fish?; Q8: Is there information available on the freezing technology or process (e.g. rate) in the study?; Q9: Was the method applied to different fish species?; Q10: Was a threshold or any differentiation tool/criteria proposed for each fish species in the study?; Q11: Was a classification performance (e.g. percentage of correct classification) included in the study using an independent set of samples (i.e. not part of the samples used for method validation in Q2 and/or fish coming from a different batch)? The answer to each of the questions is provided in the order of the questions and, finally, the total score is given in bold.
study focused on finding markers to distinguish.
Table D.2.
Record overview table of the studies dealing with morphological methods to distinguish fresh fish (chilled or superchilled) from frozen/thawed fish
| Method subgroup | Short description of method | Conditions tested: fish species | Conditions tested: temperatures and duration | Conditions tested: freezing methods | Short description of results | Performance classification | Appraisal | Reference |
|---|---|---|---|---|---|---|---|---|
| Eye lens analysis | ||||||||
| Eye lens | The medulla of the crystalline lens was observed by dissection of the eye of the same fish before and after freezing. | Mackerel (Scomber scombrus), plaice (Pleuronectes platessa), whiting (Merlangus merlangus) | Analysis of 30 samples: fresh and after 1 day at –20°C (fast and slow freezing conditions). | Two freezing conditions: slow freezing in a cold room and fast freezing in a cell | Visual differentiation possible only for whiting and mackerel, not for plaice | No classification performance available |
00000001110 3 |
(Duflos et al., 2002) |
| Eye lens | The medulla was separated from the cortex by placing the lens in glycerol and the cortex was crushed with the thumb and first finger of examiner. Then the medulla was examined with the naked eye. | Carp (Cyprinus carpio), black cod (Anoplopoma fimbria), eel (Anguilla japonica), flat fish (Limanda herzensteini), hoki, kingklip, common horse mackerel (Trachurus japonicus), pacific mackerel (Pneumatophorus japonicus japonicas), porgy (Chrysophrys major), yellow sea bream (Tainus tumifrons), stockfish (Merluccius capensis), southern blue whiting, yellowtail (Seriola quinqueradiata) |
Immediate analysis of fresh fish and after fresh storage for 2 to 4 days at 0°C. Analysis of frozen fish: frozen at –20°C and stored during 10 days. The method was tested separately on commercially obtained fish, frozen at –30°C and stored at –25°C for more than 3 months |
No information available on freezing speed |
Visual differentiation possible for all fish species except for flat fish and eel (possibly, the small medullae of these fishes experience almost no effect from freezing). For the commercially obtained fish: all specimens except only 3 flat fish species revealed an evident opacity of the medulla in one eye or both. |
No classification performance available |
00100110110 5 |
(Yoshioka and Kitamikado, 1983) |
| Histology | ||||||||
| Histology | Muscle tissue was cut into slices (0.5 cm thick) and fixed in formalin. After fixation, samples were routinely processed, embedded in paraffin, cut at 3 ± 2 μm, and stained with haematoxylin and eosin. The slide preparations were examined microscopically (× 10 magnification) by an expert histopathologist in three different labs and classified as positive (thawed) or negative (fresh). |
Red mullet (Mullus barbatus), gilthead seabream (Sparus aurata), swordfish (Xiphias gladius) 35 species differing in protein and fat content for the classification performance of the method. |
Fresh muscle (0.5 cm thick) was processed immediately for analysis (fixed in formalin). Samples (0.5 cm thick) were frozen for 12 h at –80°C and subsequently stored at –20°C for different time periods. Samples (35 species) for validation were frozen for 24 h at –24°C or at –80°C before processing. |
No information available on freezing speed |
Very high sensitivity (97.18%) and specificity (98.59%) were obtained by all three laboratories. Precision (agreement between readers) was very high (total combined kappa of 0.93). |
Mean sensitivity was 94.57% and mean specificity was 95.53% for the 35 species. Precision was high (total combined kappa of 0.66) |
01100100111 6 |
(Bozzetta et al., 2012) |
| Histology | All samples were fixed in 10% neutral buffered formalin for 24 h. Paraffin‐embedded blocks were cut on a microtome into 3 μm (± 2 μm) sections and stained with haematoxylin and eosin. Three expert histopathologists independently examined the slide preparations by optical microscopy at increasing magnification (x 100, × 200, and × 400) and classified them as frozen–thawed or fresh according to the standard operating procedure criteria in use at the laboratory. Frozen–thawed samples were identified when vacuoles of various dimensions, optically empty or filled with eosinophilic material, caused by ice crystals, were observed in the cytoplasm of muscle cells. Fresh samples were identified when these microscopic changes were absent. A ‘non conclusive’ classification, owing to unspecific microscopic findings according to the standard operating procedure criteria in use, was also considered as a result. | Anchovy (Engraulis encrasicolus) (marinated) | 30 fish were marinated at 4°C for 24 h, and 30 were frozen at –20°C for 24 h before being marinated for 24 h | No information available |
All 60 fresh and frozen samples were correctly identified. No samples were classified as ‘non‐conclusive’ Statistical analysis showed 100% sensitivity, 100% specificity and perfect agreement between the readers. |
11000000010 3 |
(Meistro et al., 2016) | |
| Histology | Blocks of muscles (5 × 5 × 5 mm) of three rainbow trouts from each group were taken from the upper layer of the right dorsal section for light microscopy. Histological examinations included the preparation of paraffin sections (longitudinal and transversal) and their staining using the haematoxylin‐eosin method. The samples were photographed under a light microscope (× 400 magnification). | Rainbow trout (Oncorhynchus mykiss) |
Group 1: Fresh fish was stored in a cold room (0–2°C) for 3 day. Group 2: Samples were frozen at –18°C during 8 day. Group 3: Samples were frozen at –18°C and stored at –18°C during 3 day. After that, they were thawed in cold conditions and after re‐freezing, stored again at –18°C for 3 day. |
Samples were individually quick frozen and reached –18°C in 20 min. |
Muscle cells of fresh fish demonstrated only minimal variation of microstructural changes caused by sample processing. No remarkable histological changes were observed in both longitudinal and transversal sections. In sections obtained from frozen fish, mild damages of muscle fibres were demonstrated. Intense damage of muscle fibre structure and total disruption of muscle fibres is visible in sections from double – frozen fish. |
No classification performance available |
00000001010 3 |
(Popelka et al., 2014) |
| Histology | 40 g of muscle was collected, fixed in formaldehyde (4%) buffered with phosphate (0.05 mol/L). From each sample, four sections were taken. Sections were stained with haemotoxylin and eosin. The observation of microvacuoles (size less than half of the muscular fibre cell) and macrovacuoles (size higher than half of the muscular fibre cell). If vacuoles are observed in 1 to 4 out of 12 optical fields, then score ‘rare (focal)’ is given. If vacuoles are observed in 5 to 8 out of 12 optical fields, then score ‘dispersed’ (multi‐focal) is given. If vacuoles are observed in 9 to 12 out of 12 optical fields, then score ‘diffused’ is given. | Atlantic bonito (Sarda sarda), salmon (Salmo salar), albacore tuna (thunnus alalunga), turbot (Psetta maxima), little tunny (Euthynnus alletteratus), |
Samples were purchased fresh and analysed immediately. Samples were frozen at –20°C during 12 h and 24 h, and then analysed. |
No information on freezing method |
No vacuoles observed in fresh fish samples. Always either micro‐or macrovacuoles present after freezing for 12 and 24 h. Microvacuoles appear diffuse after 12 h and multi‐focal after 24 h. Macrovacuoles appear rare after 12 h and diffuse after 24 h. |
No classification performance available |
00000100110 3 |
(Richelmi et al., 2013) |
| Histology | Samples were either promptly fixed in a 10% buffered formalin solution (pH 7.4) for paraffin embedding or cryo‐protected with 30% sucrose for cryo‐sectioning. Tissue processing of formalin fixed samples was performed in a controlled automatic processor (Shandon TP 1020, Leika) and paraffin embedding was accomplished to obtain transversal sections of the muscle fibres. Five μm thick sections were stained with haematoxylin and eosin under standard protocol. Cryo‐sectioned samples were stained with Oil Red O to evaluate the lipid distribution within samples. Morphological and morphometrical assessment was performed (scoring system with evaluation grid of different parameters). | European hake (Merluccius merluccius) |
Fresh samples were immediately processed or after 24 h, 72 h and 120 h of fresh storage at 4°C. Samples were frozen in a conventional laboratory freezer at –20°C for 15 days. 13 additional commercially obtained fresh and 17 individually quick‐frozen samples for final validation. |
No information available on freezing speed. |
No significant difference in the size of lytic areas during cold storage. Significant difference between fresh fish and conventionally frozen fish. Significant difference between fresh fish and individually quick‐frozen fish. High sensitivity and specificity (all > 94%); high agreement between readers 91%). |
100% of external samples were correctly classified |
10000010011 4 |
(Tinacci et al., 2018) |
Table D.3.
Record overview table of the studies dealing with physico‐chemical methods to distinguish fresh fish (chilled or superchilled) from frozen/thawed fish
| Method subgroup | Short description of method | Conditions tested: fish species | Conditions tested: temperatures and duration | Conditions tested: freezing methods | Short description of results | Performance classification | Appraisal | Reference |
|---|---|---|---|---|---|---|---|---|
| Spectroscopy | ||||||||
| Combinations of UV‐VIS/NIR: FT‐NIR, FT‐MIR |
FT‐NIR spectra were recorded at 12 cm−1 resolution (64 scans) from flesh of whole fillets at room temperature with FT‐NIR MPA spectrometer, fitted with integrating sphere (12,500–3,750 cm−1) and optical fibre (11,000–3,750 cm−1). FT‐MIR spectra (or else simply FT‐IR) were recorded with VERTEX 70, at 4 cm−1 resolution (16 scans) from minced skinless samples at room temperature, in the range 4,000 to 700 cm−1. Classification algorithm: soft independent modelling of Class Analogy (SIMCA) on 30 smoothed (standard normal variate or SNV, multiplicative scatter correction (MSC, second derivatives, etc.) spectral features selected by principal component analysis (PCA). Validation set was generated by algorithm that randomly selected 30% of the spectra composing the original data. The remaining spectra were used for calibrating the model. |
Atlantic mullet (Pseudupeneus prayensis) |
Fresh fish (4°C) for max 48 h. Frozen fish (–18°C) for up to 2 months, thawed at 4°C for 48 h. |
Not specified | The spectral differences among fresh and frozen samples were due to O‐H bond of water in the FT‐NIR data and to amines and carboxylic acids in FT‐MIR data. |
SIMCA model gave high classification performance as follows: ‐ FT‐NIR integrated sphere data, pretreated with MSC: 93–95% classification ability (i.e. correct classification), 88–89% prediction ability (i.e. ability to classify in one of the two classes), 68–73% sensitivity and 75–77% specificity. ‐ FT‐NIR optical fibre smoothed data: 87–92% classification ability, 82–87% prediction ability, 85–88% sensitivity and 57–60% specificity. ‐ FT‐MIR data, pretreated with MSC: 98% classification ability, 88–98% prediction ability, 60–70% sensitivity and 95–100% specificity. |
11000100111 6 |
(Alamprese and Casiraghi, 2015) |
| Combinations of UV‐VIS/NIR: NIR, VIS/NIR |
NIR spectra for whole and minced sample, were acquired with NIR spectrophotometer (FOSS Nirsystems 5000 Instrument) from 1,100 to 2,500 nm and 2 nm resolution in reflectance mode. VIS/NIR spectra, from 380 to 1080 nm, for whole fish were acquired with portable MMS1 spectrophotometer (Zeiss) equipped with fibre‐optic accessory at incidence angle of 45° in reflectance mode. Classification algorithms: (a) PCA on pre=treated data with SNV, smoothing and first/second derivative; (b) partial least squares regression discriminant analysis (PLS‐DA); and (c) Multivariate Logistic Regression. |
Swordfish (Xiphias gladius |
Fresh vacuum packaged (polyethylene) white muscle cutlets (male and female) at 2°C for variable storage durations up to 10 days. Rapidly frozen at –18°C and slowly frozen at –10°C, both for 30 days. |
Slow freezing at –10°C, within 3.5 to 5 h. Fast freezing within 110 to 180 min at –18°C. Equipment not specified. |
NIR: spectral differences were detected in the regions 1,388–1,594 nm and 1,700–2,498 nm, associated with free water, O‐H functional groups and water overtones. Cross‐validation showed the highest classification accuracy (96.7%) for the whole fish samples that were rapidly frozen. VIS/NIR: Principal bands at 430 and 550–570 nm accounted for spectral differences due to changes in myoglobin. Cross‐validation accuracy was 100%. |
No classification performance with additional independent samples was performed |
11110001010 6 |
(Fasolato et al., 2012) |
| Combinations of UV‐VIS/NIR: FT‐MIR |
MIR spectra were recorded between 3,000 and 900 cm−1, at a 4 cm−1 resolution with FTIR spectrometer OMNIC software, mounted with an Attenuated Reflectance accessory (ATR: with ZNSe crystal, at incidence angle of 45°). Classification algorithm: normalisation of MIR spectral data to a value of 1. Normalised data were analysed with PCA separately on regions 3,000–2,800 cm−1, 1,700–1,500 cm−1 and 1,500–900 cm−1. Then, Factorial Discriminant Analysis (FDA) was applied to the first 5 PCs. |
Whiting fillets (Merlangius merlangus) |
Fresh (not specified). Frozen slow at –20°C or fast at –35°C and each thawed slowly at 0–3°C in cold room (7–10 h) or rapidly in microwave (7 min). |
Slow freezing in cold room. Fast freezing in cooling cell. |
The spectral region 3,000–2,800 cm−1 corresponds to C‐H bound of methyl and methylene groups of fatty acids. The 1,700–1,500 cm−1 region corresponds to the amide I and II bands and the 1,500–900 cm−1 region is the fingerprint region referring to C‐O and C‐C stretching modes. |
FDA applied on the first 5 PCs gave overall correct classification rate of 75, 37.5 and 87.5% for the 1,500–900, 1,700–1,500 and 3,000–2,800 cm−1 spectral regions, respectively. Concatenation of the 5 PCs of all three regions into a single matrix resulted in 87.5% correct classification rate, based on cross‐validation, i.e. using random spectra from the calibration set, as opposed to using spectra from independent samples, i.e. not included in the original calibration set. |
11000001010 4 |
(Karoui et al., 2007) |
| Combinations of UV‐VIS/NIR: VIS/NIR |
The method was based on capturing NIR spectra of minced epaxial white muscle of 4 fish species (calibration data set), with two different instruments: (a) FOSS NIRSystem 5000, at 2 nm intervals from 1,100 to 2,500 nm and (b) a UNITY Scientific SpectraStar 2500TW at 1 nm intervals from 680 to 2,500 nm. Classification by PCA (capturing the main components) followed by PLS‐DA multivariate model. |
Gilthead sea bream (Sparus aurata) Red mullet (Mallus barbatus) Sole (Sole vulgaris) Sword fish (Xiphias gladius) |
Not specified, but referred to samples and their spectra from databases, from published studies, so a wide range of temperatures and freezing duration must have been considered. | Not specified | Three PLS‐DA‐based strategies were applied to untreated or SNV pre‐treated spectral data to differentiate fresh from frozen/thawed fish (with one calibration and one validation set): one strategy considered all fish together, while the other two could also classify samples according to fish species. |
The study presents a NIR‐based method for differentiating fresh from frozen–thawed fish also with potential to and explicitly separate fish species. It has been validated against several fish species with classification accuracy between 78 and 92%, depending on the modelling strategy (i.e. dependent or independent of the fish species) and the fish species. |
11100100101 6 |
(Ottavian et al., 2013) |
| Combinations of UV‐VIS/NIR: FT‐IR in combination with dimethylamine (DMA) estimation during storage |
FT‐IR spectra were acquired with a Nicolet Magna 750 FT‐IR spectrophotometer equipped with a 40° incidence angle ZnSe crystal (referred to as ATR crystal), at 4,000 to 800 cm−1 with 4 cm−1 resolution at room temperature. Classification algorithm: discriminant analysis (DA) based on PCA scores and partial Least Square Regression correlating spectral data with changes in DMA during frozen storage. |
Red hake (Urophycis chuss) |
Samples comprised vacuum packaged ground white muscle of: ‐ refrigerated (fresh) samples ‐ Samples frozen for unknown duration in a domestic refrigerator at –10 to –12°C or in a Westinghouse upright freezer at –14 to –15°C. The conditions of thawing are not described. |
Domestic freezer or Westinghouse upright freezer | Spectral differences caused by frozen storage, were evident in the regions 3,800 to 2,800 cm−1, associated with increase in free water, and from 1,700 to 800 cm−1, representing DMA and trimethylamine oxide (TMAO). |
Randomly selected samples from the same batch (not used for calibration) were used for testing the classification performance (N=114). Percentage of correct classification was 86.0% for fresh fish, and 81.3–93.8% for frozen fish groups (i.e. with different DMA content) |
11110110011 8 |
(Pink et al., 1998) |
| Combinations of UV‐VIS/NIR: UV‐VIS/NIR |
Reflectance spectra were collected using a LabSpec 5000 (ASD Inc.) with a customised probe. The spectral range of the spectrophotometer was 350–2,500 nm, with spectral resolution of 3 nm at 700 nm, 10 nm at 1,400 nm and 2,100 nm. The scanning time was 10 ms and a sampling interval of 1.4 nm at the 350–1,000 nm range and 2 nm in the 1,000–2,500 nm range (wavelength accuracy ± 1 nm). Scanning took place before and after freezing/thawing. Classification by PCA (blooming detection) and double cross validation, using two calibration and 1 validation subs‐sets, coupled with PLS‐DA (classification of fresh vs. frozen/thawed). |
Tuna (Thymnus thynnus) |
Fresh samples: equilibrated from 4 to 16–18°C (1 h) and then scanned. Scanned samples kept at 4°C (2 h), wrapped in film and frozen at –80°C for 5, 21 and 35 day. Thawing took place at 4°C for 24 h. |
Not specified |
The method was capable in differentiating fresh fish from frozen for varying durations and then thawed. Discrimination was also based on the detection of (instrumentally detectable) blooming caused by myoglobin oxygenation due to initial exposure to the air. Fillets frozen below –60°C did not show detectable changes in visual characteristics when thawed, hindering discrimination from not frozen (fresh) samples. |
The classification performance in the two categories, namely fresh and frozen/thawed samples were as follows: 92% probability that a fresh sample is predicted correctly as fresh and 82% that frozen/thawed is a correctly classified. The above are based on the validation subset that was not used to calibrate the model (i.e. calibration data set included two original subsets). |
11000100011 5 |
(Reis et al., 2017) |
| Combinations of UV‐VIS/NIR: NIR |
NIR spectra acquired by portable spectrophotometer poliSPECNIR (ITPhotonics), operated in a scanning range of 900–1,650 nm at 2 nm intervals, by direct contact of sample with the probe surface. Classification by PLS‐DA multivariate method on scaled (autoscale, SNV) variables. Model accuracy was assessed with cross‐validation and classification performance was assessed with a random validation set of independent samples. |
Cuttlefish (Sepia officinalis) |
Freezing at –25°C (3 h) then storage at –20°C for 5 ± 1 months. Thawing overnight (16–18 h) at temperature not specified. Fresh samples stored at 2°C for unspecified duration (but probably till end of shelf life because total viable count (TVC)/total volatile basic nitrogen (TVB‐N) were also monitored). |
Not specified |
20% randomly selected samples from the original data set of spectra, were left out to be used for model validation. The rest composed the calibration data set of the PLS model, which was cross‐validated. Spectral differences between fresh and frozen–thawed samples were detected in the region 960–980 nm which is associated with O‐H bonds, mainly related to the moisture content in the muscle. The discrimination between fresh and frozen was not affected by the duration of freezing. |
75 to 90% of fresh samples of the fish species tested, were correctly classified as fresh. |
11000110011 6 |
(Sannia et al., 2019) |
| Combinations of UV‐VIS/NIR: VIS/NIR |
Scanning with NIRSystems 6500 spectrophotometer with a surface interactance fibre‐optic accessory. Spectra (optical density units in log 1/T, with T=the % of energy transmitted) recorded at wavelength range of 400–1100 nm at 2 nm intervals. Sample classification: SIMCA and LDA, using PCA. SIMCA relies on independent PCA per group, LDA uses PCA scores as input variables. |
Red sea bream (Pagrus major) |
Fresh fish kept in ice‐cold water for 30 min. Frozen fish (–40°C) for 30 days, thawed overnight at 5°C. |
Not specified |
Reflectance of fish samples was affected by freezing/thawing due to alterations in the physical structure of surface. Only one PC was enough to separate fresh from frozen–thawed samples. Original (untreated) absorbance data led to better classification than data treated with multiplicative scatter correction LDA had 100% classification efficiency as compared to 7% of SIMCA. |
LDA models based on direct absorbance data may distinguish frozen–thawed sea bream from fresh with 100% accuracy. Classification accuracy was based on independent unknown samples, i.e. belonging to the prediction set, and not the calibration data set. |
11000000011 4 |
(Uddin et al., 2005) |
| Combinations of UV‐VIS/NIR: NIR |
Dry extract spectroscopy by infrared reflection (DESIR), was performed on the meat juice or natural drip from 15 g of fresh/frozen fish or fillets. Tissue was centrifuged and aliquots (0.6 mL) of extract/drip were collected via micro‐perforated discs on glass‐fibre filter paper, that was dried (30°C, 30 min) and scanned in diffuse reflectance mode in the 1,100 to 2,500 nm range, on the deposit side in a NIR spectrophotometer (model 6500, NIRSystems Inc.). Scanning was performed on a Standard Sample Cup, manually rotated in 5 steps. NIR reflectance spectra were recorded in 2 nm steps. Sample classification: (a) PCA and (b) a dummy regression by stepwise multiple linear regression (MLR) with dummy scores: 1 = fresh; 2 = frozen/thawed. |
Horse mackerel (Trachurus japoncus) |
Fresh fish, soon after killing, equilibrated at 20°C. Whole fish frozen at –40°C for 10 days. Frozen fillets at –40°C for 10 days. |
Not specified |
NIR spectra of fresh fish clearly differed from that of frozen/thawed and wavelength‐specific changes, associated with protein denaturation, were evident in the 2nd derivative spectra. Correlation coefficient of calibration > 0.95, with both multivariate methods. |
Spectra were randomly assigned to calibration set and validation set. Classification performance was estimated merging spectra used for calibration (n=54) and validation (n=54). 100% of fresh (n=54) and frozen/thawed (n=54) fish samples were correctly classified. |
11000000011 4 |
(Uddin and Okazaki, 2004) |
| Combinations of UV‐VIS/NIR: FT‐NIR |
Scanning with NIRFlex N500 FT‐IR, equipped with a NIRFlex solid sample cell and indium gallium arsenide detector and spinning sampling module. Spectra recorded with 32 scans in the range from 10,000 to 4,000 cm−1 with 4 cm−1 resolution. Sample classification: PCA and Mahalanobis distance discrimination analysis. |
Tilapia (Oreochromis) |
Samples were dorsal and belly fillets. The objective was to discriminate samples subjected to consecutive freezing‐thawing cycles: ‐ one frozen cycle: frozen (–18°C) for 12 h and thawed for 12 h. ‐ two to seven frozen cycles as above. |
Not specified |
Differences between the NIR reflectance of fresh and frozen/thawed samples were attributed to stretching of the OH‐NH bonds at 5,100 and 6,825 cm−1. Samples were randomly divided to calibration and validation data sets. Better discrimination was achieved among the once and repeated freezing/thawed samples in the frozen state (without or with MSC pretreatment of spectral data) than in the thawed state. Accuracy in samples classification was better in dorsal (without pre‐treatment of spectral data) than in belly samples. |
The observed maximum classification accuracy ranged from 80 to 93.3%, for samples, respectively, frozen once or repeatedly, and from 86.67 to 93.33% particularly for dorsal (frozen) samples. |
11100110011 7 |
(Wang et al., 2018) |
| Hyperspectral imaging | The HSI system consisted of a line‐scanning imaging spectrograph (ImSpector V10E) covering the spectral range of 308–1,105 nm with the spectroscopic resolution of 2.8 nm; a charge‐coupled device (CCD) camera (DL‐604M) with the effective resolution of 1,004 × 1,002 pixels by 12 bits and its corresponding camera lens (OLE23) with the focal length of 23 mm; an illumination source including two 150 W halogen lamps (3900‐ER). | Grass carp |
Group 1: fresh fish, group. Group 2: fish cold‐stored at 4°C for 7 days. Group 3: fish frozen at –20°C for 30 day and thawed at 4°C for 12 h. Group 4: fish frozen at –40°C for 30 day and thawed at 4°C for 12 h. |
Not mentioned | Visible and near infrared hyperspectral imaging in the spectral range of 400–1,000 nm in tandem with classifiers and spectral preprocessing techniques showed good discrimination of fresh, cold‐stored (4°C) and frozen (–20°C and –40°C) thawed grass carp fish fillets. | Classification performance was 92.34% |
11100100001 4 |
(Cheng et al., 2015) |
| VIS/NIR with hyperspectral imaging | A line‐scanning hyperspectral imaging system in reflectance mode was employed to capture the hyperspectral images for each prawn in the wavelength range of 300–1,100 nm. The main components of the system included a spectrograph (ImSpector V10E), a 12‐bit CCD camera (DL‐604 mol/L), two 500 W halogen lamps (3900‐ER), a conveying stage operated by a stepper motor (ST‐1212‐300), and a computer supported with spectracube data. | Prawns (Metapenaeus ensis) | Four groups (N=70) of prawns with different processing were included in this study, namely unfrozen–fresh, unfrozen–soaked, frozen–fresh and frozen–soaked groups. ‘Fresh’ refers to the prawns being treated with crashed ice to a sudden death, while the ‘soaked’ ones were subsequently soaked into 10 L of sea water (25 C) for 2 h. Peeled prawns were used ‘unfrozen’ or stored at –18°C for 2 months (‘frozen’) | Not specified | The results had Charnes, Cooper, and Rhodes (1978) (CCR) of 98.33% for least‐squares support‐vector machines (LS‐SVM) model for distinguishing fresh and frozen–thawed prawns based on spectral variables. However, the average CCR only reached 73.33% for differentiating unfrozen–fresh group from unfrozen–soaked group. | The remaining (independent) 60 samples of 30 fresh and 30 soaked prawns formed the prediction set. Classification performance was 98.33%. |
11000100001 4 |
(Dai et al., 2015) |
|
VIS/NIR with hyperspectral imaging VIS/NIR with portable equipment |
Two spectral scans: 1) Interactance imaging (hyperspectral) with imaging spectrometer (VNIR‐640) with a field of view 1 mm × 300 mm and spatial resolution 1 mm × 0.5 mm. Each pixel represents radiation in the region of 400–1,000 nm with 10 nm resolution. Scanning speed (on white diffuse conveyer) of 400 nm. 2) Interactance spectroscopy with handheld probe (XDS Optiprobe Analyser), composed of transmitting and receiving fibre bundles. Spectral data recorded as absorbance units at 400–2,500 nm and resolution of 0.5 nm. Classification algorithm: PCA applied on SNV and Savitzky–Golay second derivative pretreated spectral data. K‐nearest neighbour classifier with leave‐one‐out cross validation was used for classification. Separation line between classes was calculated using the Rosenblatt's perceptron. |
Atlantic salmon (Salmo salar) |
Fresh fillets (2–4°C), covered with ice. Fresh, stored in ice for 0 and 2 days, then frozen at –40°C for 3 weeks, thawed overnight at 2–4°C (fillets) or under running water (whole fish, which was then gutted and filleted). |
Not specified | The spectral region 605–735 nm, represented on the 1st PC, is appropriate for discrimination of fresh from frozen–thawed fillets, based on 2nd derivative pretreated data. Differences are associated with spectral changes due to oxidation of haem proteins during freezing–thawing and chilled storage in ice. |
Classification rate varied on the fillets, from 60 to the maximum of 100%, the latter in the tick loin region. Better discrimination of frozen/thawed samples from fresh samples was obtained for fillets compared to whole fish. Segmented cross validation was applied, by leaving spectral data of one day out for validation and calibrating the model with the remaining one. The procedure was applied to all days. |
11000110011 6 |
(Kimiya et al., 2013) |
|
UV‐VIS/NIR with hyperspectral imaging VIS/NIR hyperspectral imaging with digital camera (RGB imaging) |
VIS/NIR spectra were collected without sample pretreatment using a portable instrument (VIS/NIR diode array, Hamamatsu S3904) scanning wavelength from 300 to 1,100 nm in transflection mode at 2 nm intervals. RGB images (red, green and blue colour space) were collected using a compact digital camera Kodak EasyShare M530 (4,000×3,000 pixels) within a 60×60×60 cm3 photographic box screened from the environmental light with opaque walls and equipped with four fluorescent tubes to control the illumination. Classification by PCA (capturing the main components), followed by PLS‐DA multivariate model on scaled variables |
West African goatfish fillets (Pseudupeneus prayensis) |
Freezing at –32°C, then storage at –18°C for up to 48 h. Thawing overnight (18 h) at 4°C, sampling immediately or after another 24 h at 4°C. Fresh samples were kept at 2°C. |
Not specified | The NIR and RGB imaging differentiated (correctly classified) frozen/thawed from fresh fish with 98–100% sensitivity (Se) and 100% specificity (Sp) immediately after thawing. Se of the two methods reduced to 60–80% after 24 h of chilled storage. Sp of NIR remained high (100%) after additional storage, but Sp of RGB imaging reduced to 80%. Combining the two methods, increased Se and Sp to almost 100% even after 24 h of storage. |
A combination of NIR with RGB could differentiate frozen/thawed from fresh fish both immediately after thawing and after 24 h. The method was tested against a single species and the results could be affected by seasonality. 3 data sets were used, split in 1 for calibration and 2 for validation. Calibration of model against data sets with spectra from different seasons is recommended to increase the robustness of classification. |
11000010011 5 |
(Ottavian et al., 2014) |
| Hyperspectral imaging |
Three in‐house developed line‐scan hyperspectral imaging systems were used to collect four types of image data from fish fillet samples: (1) reflectance images in VIS/NIR region, (2) fluorescence images by 365 nm UV excitation, (3) reflectance images in short‐wave infrared (SWIR) region, (4) Raman images by 785 nm laser excitation. |
Red snapper (Lutjanus campechanus), |
14 fillets including: ‐ fresh (imaged immediately at 20°C). ‐ 1st freezing cycle (the fillets were frozen in a −20°C for 24 h and then thawed in a 4°C for 24 h). ‐ 2nd freezing cycle (same freezing and thawing process). |
Ordinary kitchen freezer | For VIS/NIR, Raman, and fluorescence, the fresh fillets were more easily misclassified as frozen/thawed fillets in the first cycle rather than those in the second cycle. This suggests there is a progressive change in the fish tissue associated with the freeze‐thaw process. |
Independent data sets and fish samples were used for performance classification. The highest accuracies were achieved at 100% using full VNIR reflectance spectra for the species classification and 99.9% using full SWIR reflectance spectra for the freshness classification. |
11000101111 7 |
(Qin et al., 2020) |
| Hyperspectral imaging | The hyperspectral images were captured in reflectance mode, which involved a spectrograph (ImSpector V10E), a high‐performance camera (DL‐604M) in a high resolution of 1,004x1,002 (spatial × spectral) pixels coupled with a camera lens, an illumination unit involving two 150 W halogen lamps (2900‐ER), a conveyer belt motivated by a stepper motor and a computer. The working spectral range was 328–1,115 nm. | Shrimps (Metapenaeus ensis) |
Fresh: 79 shrimps. Chilled: 80 shrimps stored at 4°C for 80 h. Frozen–thawed: 65 shrimps frozen at –20°C for 90 h and thawed at 25°C for 2 h. |
Not specified | The results indicated that the selected optimal wavelengths had a strong discriminative ability for fresh, chilled and frozen–thawed shelled shrimps. The most effective wavelengths (783, 689, 435, 416, 813, 639, 452 and 478 nm) were decided by UVE (PLS)‐SPA to be utilised for model establishment. Principal components (PCs) images explained more than 99% of variances of all spectral bands. | No performance classification |
11001100000 4 |
(Qu et al., 2015) |
| Hyperspectral imaging | A Micro‐Hyperspec VNIR camera with 17 mm lens was used to acquire hyperspectral images. Spectral data was collected from 400 to 1,000 nm, with spectral resolution of 5 nm. Firstly, hyperspectral images were obtained from both sides of intact fish (with scales). Then, fish scales were removed totally, and hyperspectral imaging measurement was applied on both sides of the scaled intact fish. After that, hyperspectral images were measured on both sides of each fish fillet, including flesh and skin sides. | Crucian carp (Carassius auratus) |
1st group: 6 fresh fish. 2nd group: 18 fish were stored at 4 °C for 1, 2, and 3 days. 3rd group: 16 fish kept at −20°C for 7, 14, and 21 days. |
Ordinary freezer | The PLS‐DA classification results for fresh and frozen/thawed fish showed that PLS‐DA models can identify fresh and frozen/thawed intact fish well. Cross‐validation of the models developed showed an accuracy of: 100% for intact scaled fish; 92.5% for intact fish with scales; 97.5% for fish fillets (flesh side); and 97.5% for fish fillets (skin side). | No performance classification |
11000101000 4 |
(Shan et al., 2018) |
| VIS/NIR with hyperspectral imaging |
Interactance imaging: using an imaging spectrometer (VNIR‐640), two custom made fibre optic light lines (200 mm long, powered by 150 W aluminium coated halogen lamps), with focusing acrylic rod lenses and two black painted aluminium screens for light baffling. Illumination adjacent and parallel to the detectors field of view. Focal distance 1,000 mm and field depth 25 mm. Handheld interactance probe: single beam spectrometer XDS Optiprobe analyser (FOSS) with transmitting and receiving fibre bundles. Measurements on middle of the loin and in the tail region. Data were expressed as absorbance units from 400 to 2,500 nm with 0.5 nm resolution. Classification algorithm: spectral data (32 pixels/spectra) were pre‐treated with SNV and 2nd derivative using Savitzky–Golay with a 2nd order polynomial and 50 nm wide smoothing window. Classification was made by PCA and Rosen‐blatts perceptron coupled with a sigmoid response function was used to separate classes in the PCA space. K‐Nearest neighbour classifier using the Euclidean distance was applied for discrimination of fresh from frozen–thawed samples. |
Atlantic cod (Gadus morhua) |
Fresh gutted iced fish sent by plane and stored in ice for up to 13 days. Fillets with skin, without skin and whole fish were frozen at –40°C on day 0 and after 2 days of storage. Frozen samples were kept for 2 weeks and then thawed overnight at 4°C. |
Not specified |
Spectral differences between fresh and frozen/thawed samples were evident around 507 and 636 nm, as well as the PC loading of wavelengths 487 and 646 nm, all associated with the absorption by the oxidised haem pigments (e.g. methaemoglobin and metmyoglobin). The average correct classification rate was 87.8, maximum classification (96–100%) obtained in the loin and close to the centre line of the fillet. |
Classification algorithm was cross‐validated, i.e. without independent samples |
11010010010 5 |
(Sivertsen et al., 2011) |
| Hyperspectral imaging | The illumination source was a pair of fibre‐optic line lights, each 200 mm wide and powered by three 150 W halogen lamps. Cylindrical lenses mounted in front of the line lights focused the light into two 10 mm thick parallel lines, 40 mm apart. The fibre optic line lights and detector were mounted at heights of 150 mm and 1,030 mm, respectively, above the conveyor belt. The hyperspectral camera operated in the VIS‐NIR range from 430 to 1,000 nm. Imaging of the samples was performed on a conveyor belt travelling at 40 cm/s. | Atlantic cod | 200 loin pieces with two modes of sample freezing and sample thawing: fast freezing at –40°C and slow freezing at –20°C followed by thawing in 4°C circulating water (fast) and at 4°C by gently circulating air in a climate controlled cabinet (slow). | Blast freezing at –40°C (fast) and freezing in still air at –20°C (slow). | The two freezing methods showed clear separation in the first principal component. The two groups could be classified with 100% accuracy. Using the 450–600 nm region, the –40°C samples were predicted with 98% accuracy and the –20°C samples with 99% accuracy. The samples could be classified into once‐frozen and twice‐frozen with good accuracy, with success rates ranging from 97% to 100%. | The performance classification for fresh samples achieved 100%, for once‐thawed 98% and for twice‐thawed 93% for full spectrum. |
11100001001 5 |
(Washburn et al., 2017) |
| VIS/NIR with hyperspectral imaging | The VIS/NIR hyperspectral imaging system was employed to capture hyperspectral images of fillets in reflectance mode. The system consists of a spectrograph, a 12‐bit CCD camera with a C‐mount 23‐mm lens having 672×512 (spatial × spectral) pixels, two 150 W tungsten halogen lamps for illumination, a conveyer belt driven by a stepping motor, and a computer with data acquisition and preprocessing software. The spectral resolution is 2.8 nm in 380–1,030 nm. | Halibut (Psetta maxima) | 108 fillets, of which: 48 fresh and frozen fillets; 30 fillets stored at the constant temperature of −70 and −20°C for fast freezing (FF) and slow freezing (SF); 72 samples of 32 fresh and 40 frozen–thawed after 9 days (20 FF‐T and 20 SF‐T). | Slow and fast freezing | The general trends of the spectral curves for three categories of fish were similar. However, the overall absorbance level was found to decrease in FF‐T samples. Spectral characteristics of FF‐T were more similar with fresh fish, and the total absorbance level of FF‐T was higher than SF‐T fish. The spectral changes at 970, 729, 836, 928, 552, 512 and 620 nm among fresh, FF‐T, and SF‐T fish made the differentiation more well founded. |
From the same batch of fish samples, 16 fresh samples and 20 frozen–thawed samples (10 FF‐T and 10 SF‐T) formed the prediction set. Correct classification rate was 93.75% for fresh and 90% for frozen–thawed fish (average 91.637%). |
10100101001 5 |
(Zhu et al., 2013) |
| (Front‐face) fluorescence spectroscopy |
Fluorescence spectra acquired with FluoroMax2 spectrofluorimeter, with a variable angle front‐surface accessory and incidence angle of the excitation radiation at 56°. Emission spectra of tryptophan residues (305–400 nm with 191 measurements at 0.5 nm increment) was recorded at excitation wavelength 290 nm. Emission spectra of nicotinamide adenine dinucleotide (NADH) (360–570 nm with 211 measurements at 1 nm increment) was recorded at excitation wavelengths 340 nm. Classification algorithm: PCA followed by FDA on the first 5 PCs. |
Whiting fillets (Merlangius merlangus) |
Fresh fillets (unspecified storage temperature and duration). Frozen at slow or fast rate to –20°C, kept for 8 days, then thawed fast (0°C in the centre after 7 min) in microwave, or slowly in cold room at 0–3°C for 7–10 h. |
Slow freezing in cold room at –20°C Fast freezing in cooling cell at –35°C |
Classification rate was calculated based on both calibration and independent validation spectra Tryptophane fluorescence spectra had a maximum for fresh fish at 326 nm and at 330 nm for frozen–thawed. NADH emission spectra of fresh had a maximum at 455 nm and a shoulder at 403 nm. The corresponding maximum emissions for frozen–thawed fish were observed at 379 and 455 nm. The correct classification rate based on NADH was 100%. |
Correct classification rate between fresh and frozen fish was 70.8% based on tryptophane, and 100% based on NADH. |
11010011011 7 |
(Karoui et al., 2006) |
| (Front‐face) fluorescence spectroscopy |
Fluorescence spectra acquired directly on the fish muscle using a Fluoromax‐4 spectrofluorometer (Jobin Yvon). The incidence angle of the excitation radiation was set at 60° to ensure that reflected light, scattered radiation, and depolarisation phenomena were minimised at 20°C. The emission spectra of tryptophan residues (305–450 nm), NADH (360–600 nm) and riboflavin and other unknown fluorescent compounds (405–650 nm) were recorded with the excitation wavelengths sets at 290, 340 and 380 nm, respectively. The excitation spectra of vitamin A (250–390 nm) were obtained after emission wavelength set at 410 nm. Classification by canonical correlation analysis, coupled with FDA. |
Sea bass (Dicentrarchus labrax) |
Fresh fillets: up to 13 days at 4°C. Sequence of freezing, thawing and refrigerated storage: i) frozen–thawed (3 months at –18°C), then refrigerated up to 9 days at 4°C, and ii) refrigerated (up to 9 days at 4°C), then frozen–thawed (3 months at –18°C) fillets. |
Not specified | The discrimination of fresh from frozen samples was primarily based on changes in the emission of tryptophane and NADH at ~368 and 468 nm, respectively, although the choice of multiple wavelengths was overall shown to affect classification performance. | Depending on the excitation set (290, 340, 380, 410 nm), correct classification of groups corresponding to different sequences of freezing/thawing refrigerated storage duration ranged from 83.33 to 94.87%. |
10000110011 5 |
(Karoui et al., 2017) |
| NMR/MRI spectroscopy | All 1H MRI images were acquired using a Bruker BMT imaging console connected to a 2.35 Tesla, 31 cm horizontal bore super‐conducting magnet. A cylindrical eight strut bird‐cage radio‐frequency (RF) probe, internal diameter 9.4 cm, was used in quadrature mode to transmit and receive the magnetic resonance (MR) signal from the probe. | Cod and mackerel |
Fresh fish. Frozen at –18°C in a domestic freezer for periods of 2, 4, 8 or 12 weeks and then thawed to room temperature 4 h. |
Not specified | The MR parameters of both cod and mackerel were sensitive towards freeze‐thawing and it was possible to differentiate between fresh and frozen–thawed fish for both species. There was a significant difference at the 1% level (p < 0.01) between the fresh sample and that after freeze–thaw treatment. | No performance classification |
10000100100 3 |
(Nott et al., 1999a) |
| NMR/MRI spectroscopy | All 1H MRI images were acquired using a Bruker BMT imaging console connected to a 2.35 Tesla, 31 cm horizontal bore super‐conducting magnet. A 20 cm gradient set built ‘in‐house’ with each axis powered by a Techron gradient amplifier provided gradient strengths up to 100 mT m21. An ‘in‐house’ built, cylindrical, eight strut, bird‐cage RF probe, internal diameter 9.4 cm, was used in the quadrature mode to transmit and receive the MR signal. | Trout |
Fast frozen fish (4) at –18°C for 4 weeks and then thawed. Slow frozen fish (4) at –18°C for 4 weeks and then thawed. |
Fast freezing in liquid nitrogen followed by storage in a domestic freezer at –18°C | Quantitative MRI provided parameters that were sensitive to the effects of freeze/thawing and to the method of freezing and duration of frozen‐storage. For fish which had been previously frozen, the change in the MR parameters after repeat freeze–thawing were smaller than those observed for the initial 2 day freeze–thaw of fresh fish, indicating a method which could be used to allow for the variability observed for fresh trout. | No performance classification |
10000101000 3 |
(Nott et al., 1999b) |
| NMR/MRI spectroscopy (NMR) | TCA extracts of the salmon fillets and the NMR samples were analysed by NMR. 1D 1H NMR spectra of all TCA extracts were acquired at 300 K on a Bruker Avance 600‐MHz spectrometer equipped with a 5‐mm z‐gradient TXI (H/C/N) cryoprobe. The NMR data were acquired with the Bruker pulse sequences noesygppr 1d, NS = 48 and RG = 144. | (Farmed) Atlantic salmon |
Fresh fish kept at 4°C and sampled on day 5, 6, 7, 9, 11, 14, 18. Frozen at −40°C for 16 h and thawed before sampling on day 5, 6, 7, 9, 11, 14 and 18. Fish frozen at −20°C and kept chilled at 4°C and both stored in sealed plastic bags at 4°C for 14 days. Sampling on day 10, 11, 12 and 14. |
Not specified |
The score plot showed a distinct fresh – thawed samples grouping according to NMR data upon performing PCA (74% PC1). Based on spectral regions, inferences can be made about changes in certain metabolites/compounds, e.g. aspartate, fumartate and phenylalanine. Thawing induces the formation of aspartase (new metabolite), due to mitochondrial enzymatic activity. Identification of aspartase could be a reliable indicator of thawing. |
No performance classification Extended incubation at 4°C (e.g. > 3 day) seems to enhance differentiation between thawed and never frozen (fresh) sample. Study with a single species and under unspecified freezing conditions. |
11100100000 4 |
(Shumilina et al., 2020) |
| Raman spectroscopy |
Raman spectra of fat samples (preheated at 50°C in waterbath) were obtained with a DeltaNu Examiner Raman Microscopy with a 785 nm laser source and charge‐coupled device (0°C). Integration time of 15 s and 100 mW laser power. Spectra ranged from 200 to 2,000 cm−1 with 2 cm−1 resolution. Gas Chromatography (GC) was applied for estimating changes in fatty acid compositions. Classification model: Chemometrics with PCA. |
Horse mackerel (Trachurus trachurus) European anchovy (Engraulis encrasicolus) Red mullet (Mullus surmuletus) Bluefish (Pomatamus saltatrix) Atlantic salmon (Salmo salar) Flying gurnard (Trigla lucerna) |
Separate models for discrimination of fish species and differentiating fresh from once or twice frozen–thawed fish. Fish were washed and filleted. Fresh fish, kept refrigerated at 4°C for 12 h. Frozen fish at –18°C for 24 h, thawed for 12 h at 4°C. Frozen–thawed at –18°C for 24 h and thawed for 12 h at 4°C then re‐frozen/thawed again. |
Deep freezer |
PCA was efficient in discriminating fish species and fresh from once or twice frozen–thawed fish of either species, based on Raman spectra data. Changes in of spectra data were attributed to differences in the content of fatty acids and the structure of lipids. |
Classification and discrimination efficiency of the proposed method was evidently high, but illustrated only graphically, without providing performance indicators. Accurate quantitative expression of classification performance is hindered, or not feasible. |
10000110110 5 |
(Velioglu et al., 2015) |
| Electrical parameters | ||||||||
| Electrical impedance | The system and sensor for measuring impedance in fish samples consisted of a software application an electronic equipment. For each one of the frequencies the electronic equipment generated the corresponding sinusoidal voltage waveform and applied it to the electrode. | Atlantic salmon |
3 fillets on each day: ‐ Fresh (day 0). ‐ F1 (frozen group 1) Frozen at –18°C and thawed at 4°C for 24 h: on day 15, 30, and 60. ‐ F2 (frozen group 2) Fish samples submitted to 2 freezing cycles. |
Shelf‐freezer equipped with T‐type thermocouples (0.1 mm diameter). |
Four discriminant functions were obtained, the two first functions (F1 and F2) explaining more than 97% variance (F1 90.80% and F2 6.51%). 71.93% of cases were correctly classified. F1 determined the separation of fresh (day 0) from frozen–thawed samples. No separation between the frozen–thawed samples depending on the storage time or freezing cycles was observed. |
No performance classification. Just a PCA, followed by a DA were conducted with existing samples. |
10000111000 4 |
(Fernandez‐Segovia et al., 2012) |
| Electrical impedance | The impedance system consisted of an electronic equipment and a software application that runs on a computer. The software application performed a frequency sweep, obtaining the impedance modulus and the phase of the sample for a configured range of frequencies (50 frequencies between 1 Hz and 1 MHz). The impedance measurements were carried out by inserting the sensors into the sample perpendicular to the muscular fibres of the fish. | Sea bream (Sparus aurata) |
Fresh samples (3). Frozen samples at −18°C and analysed on day 15, 30, and 60 after thawing at 4°C for 24 h (27). Samples subjected to a 2nd freeze/thaw cycle: on day 15, 30 and 60 after repeated freezing thawing at 4°C for 24 h (3). |
Ordinary freezer | The module and phase impedance spectra of fresh samples and frozen/thawed sea bream obtained with the electrode AH were similar, which means that this electrode could not differentiate the different types of samples. No differences in the spectra of samples submitted to 2 freezing cycles compared with only 1 cycle were observed. |
No performance classification. Just principal component analysis (PCA), followed by a discriminant analysis (DA), was conducted with data obtained with the electrodes used (internal samples). |
00000111000 3 |
(Fuentes et al., 2013) |
| Electrical impedance | The impedance equipment consisted of a computer, the impedance instrument (CHI660E), and an electrode (10 mm apart, 1 mm in diameter) composed of four gold‐plated copper needles. The sinusoidal voltage of 10–1–105 Hz is released by the workstation as the excitation signal. The impedance measurements were made by inserting the electrodes into the fish muscle fibres at an angle of 90° and ensuring that the electrodes were fully introduced into the sample. Fish fillets were placed in small refrigerators and connected to external instruments by wires. | Salmon fillets and rainbow trout |
Fresh salmon and trout to distinguish the fish species. Chilled salmon stored at 4 ± 0.5°C during 12 days and taken each 3 days. Frozen–thawed salmon frozen at –18 ± 0.5°C for 3 days and thawed at 4 ± 0.5°C. |
Not specified | Atlantic salmon/rainbow trout, chilled/frozen–thawed salmon and fresh/stale salmon could be distinguished quickly with a 100% recognition accuracy achieved in training set and prediction set. | 60 salmon fillets were selected for freshness detection and the performance classification displayed 100% accuracy for the discrimination between fresh and frozen–thawed fish samples. |
10000000111 4 |
(Sun et al., 2020) |
| Electrical impedance | The HP 4284A Precise LCR meter was used to measure the electrical impedance of fish samples. Impedance magnitude (|Z|) and impedance phase (u) were measured at twenty‐seven frequencies from 100 to 1 MHz (0.1, 0.2, 0.5, 0.8, 1, 2, 5, 8, 10, 15, 20, 30, 50, 60, 71.4, 80, 85.7, 100, 150, 200, 300, 500, 600, 666.66, 800, 960 and 1,000 kHz) under constant current of 0.2 mA. | Sea bass (Dicentrarchus labrax) |
50 fish samples: ‐ 1st group – fresh (control) after 48 h after the catch. ‐ chilled and stored for 3 and 6 days at 8°C. ‐ frozen 1 month at –18°C with/without temperature fluctuations and thawed at +4 °C. ‐ frozen 4 months at –18°C with/without temperature fluctuations and thawed at +4°C. ‐ 2nd freeze‐thawed cycle. |
Freezing chamber | The results showed that there was a difference between the control and frozen–thawed groups in |Z| measured at low and medium frequencies, but there was no difference among the frozen–thawed groups in |Z| at none of the frequencies measured. | No performance classification |
00000100000 1 |
(Vidacek et al., 2012a) |
| Electrical impedance | The HP LCR‐Meter‐4284A was used to measure resistance and reactance. The electronic device operated on 19 frequencies from 1 Hz to 1 MHz (0.1, 0.2, 0.5, 1, 2, 5, 10, 15, 20, 50, 80, 100, 200, 300, 400, 500, 800 and 1,000 kHz), measuring electrical properties by the constant current method (0.2 mA). Measurement was configured in a two‐electrode format. The fish samples were placed on a wooden isolator, and the electrodes were inserted in the muscle tissue. | Atlantic chub mackerel (Scomber colias) |
Fresh fish samples. Samples on first freeze‐thaw cycle: one group was frozen at –20°C (slow freezing) and the other group by immersion in liquid nitrogen at temperature –196°C (fast freezing). Both groups were stored for 14 days at –20°C and then thawed by air at 4°C. Samples also underwent a second freeze‐thaw cycle using the same parameters as above. |
Freezer with a natural airflow at –20°C | Thawed Atlantic chub mackerel samples previously frozen with different freezing rates had different reactance at frequencies higher than 150 kHz. Thus, the different reactance allowed discrimination of fresh/frozen samples. | No performance classification |
10100101000 4 |
(Vidacek et al., 2012b) |
| Dielectric properties (Microwave) |
Measuring average muscle anisotropy and electrical impedance anisotropy (microwave reflection coefficient) of the muscle surface with a 10 GHz microwave probe (active area 2 cm), placed parallel and perpendicularly to the muscle at 9 points Classification method: based on the average and variance of anisotropy distribution muscle fibres. |
Salmon |
Fresh fillets stored for 3, 4 and 10 days at 5°C. Fresh samples after storage as above subjected to fast (in alcohol) and slow (in air) freezing at –20°C, then thawed at 5°C for 12 h. |
In alcohol (fast) In air (slow) |
Fresh muscle is more anisotropic than frozen and lean more than fat tissue. Freezing and fat tissue reduce the average muscle anisotropy and increase the variation of anisotropy along the muscle surface, shifting the overall anisotropy distribution to the left of the fresh muscle anisotropy distribution. |
No classification performance data are presented. Differentiation of samples is illustrated only visually though the partially (and not entire) overlapping anisotropy distributions. As such, accurate (and reproducible) quantitative classification performance indicators cannot be deduced, neither a classification threshold for extent of distribution overlapping, e.g. specific percentile, or else. |
00000111010 4 |
(Clerjon and Damez, 2007) |
| Dielectric properties (Torry meter and k‐values) |
Torry meter readings taken with GR Torry meter above the lateral line (skin side) and on the dorsal muscle (bone side) in the individual mode. K‐values determined for 5 g of dorsal muscle extracted with 19% perchloric acid, targeting ATP and its degradation products in perchloric acid. Classification algorithm: no statistical analysis, but only threshold values for the output of the two methodologies. |
Yellowtail (Seriola quinqueradiata) |
Fresh fish in ice for up to 18 days. Frozen at –20°C for 18 h, in freezer or dipped in liquid nitrogen for 30–40 min followed or not by storage in freezer. |
Not specified |
Fresh samples had higher total mixed ratio (TMR) (10–11 on skin and approx. 15 on bone side) and very low (close to 0) K‐values. Chill or frozen storage reduced TMR and increases K‐values. Liquid‐Nitrogen frozen samples behaved similarly to frozen samples kept in the freezer. Freezing affects the dielectric properties of the muscle. |
Accurate classification performance cannot be deduced from graphs or table of the paper. |
10001011010 5 |
(Kim et al., 1987) |
| Differential Scanning Calorimetry (DSC) | ||||||||
| DSC | A heat‐flux differential scanning calorimeter (DSC‐60, Shimadzu) was used to perform Differential Scanning Calorimetry (DSC) analysis. Muscle samples (~30 mg) were sealed in an aluminium capsule and scanned from 30 to 90°C at a rate of 5°C per min, against an empty reference capsule. After baseline subtraction in each thermogram, total denaturation enthalpy, myosin and actin peak denaturation enthalpy, were estimated. | Seabream (Sparus aurata) |
Samples covered with ice and stored on ice for 7 days at 4–5°C. Single‐frozen samples at –20°C and stored for 40 days. Double‐frozen samples at –20°C for an additional 7 days after thawing for 8 h. |
Not specified | PCA explained 53.7% of the total observed variation and clearly separated the samples in three clusters without overlap: the first group contained the fresh fish samples, the second referred to the single‐frozen fillets and the third to the double‐frozen treatment. In this work, seabream single frozen fillets had significantly higher total denaturation enthalpy than double frozen fillets. | No classification performance |
00000100000 1 |
(Matos et al., 2011) |
CCD = charge‐coupled device; CCR = Charnes, Cooper, and Rhodes; DA = Discriminant Analysis; DESIR = dry extract spectroscopy by infrared reflection; DMA = dimethylamine; DSC = differential scanning calorimetry; FDA = Factorial Discriminant Analysis; FF = fast freezing; FT = Fourier transform; GC = Gas Chromatography; IR = infrared; LDA = Linear Discriminant Analysis; LS‐SVM = least‐squares support‐vector machines; MLR = multiple linear regression; NADH = nicotinamide adenine dinucleotide; PC = principal component; PCA = principal component analysis; PLS‐DA = partial least squares regression discriminant analysis; Se = sensitivity; SIMCA = Soft independent modelling of Class Analogy; Sp = specificity; SWIR = short‐wave infrared; SNV = standard normal variate; MSC = multiplicative scatter correction; MIR = middle‐infrared; MR = magnetic resonance; MRI = magnetic resonance imaging; NIR = near‐infrared; NMR = Nuclear magnetic resonance; RGB = red, green, and blue colour space; RF = radio‐frequency; SF = slow freezing; TMAO = trimethylamine oxide; TMR = total mixed ratio; TVB‐N = total volatile basic nitrogen; TVC = total viable count; u = impedance phase; UV = ultraviolet; UV‐VIS = ultraviolet–visible; UV‐VIS/NIR = ultraviolet–visible/near‐infrared; VIS/NIR = visible/near‐infrared; (|Z|) = impedance magnitude.
The overview tables for all evaluated methods for the analytical capability, advantages and disadvantages and evaluation for all methods can be found in Appendix D and are discussed below. A selection of these tables included below focus only on the methods that were finally considered ‘fit for purpose’: HADH and α‐glucosidase (enzymatic methods), histology (morphological methods); and UV‐VIS/NIR spectroscopy and hyperspectral imaging (physico‐chemical methods).
It has been demonstrated that HADH shows significant differences in enzyme activity between fresh and frozen/thawed fish in most fish species (except for hake and sea bass, for which results were less consistent) (see Table 6). Some increase of HADH‐activity during prolonged storage on ice of fresh or frozen/thawed fish was noted, but in general this increase was much lower than the sharp increase due to the freezing/thawing before the subsequent storage at chill temperature. When evaluating the independence of the method towards different freezing temperatures, it seems that the HADH method is applicable to many temperatures (varying between –5°C and –196°C) (Table 6). The work of Garcia de Fernando et al. (1992) using –5°C as a freezing temperature, showed that HADH activity did not differ significantly between fish frozen at –5°C and at lower freezing temperatures, meaning that even fish frozen at higher temperatures than those required by the current regulation (i.e. at > –18°C) could be detected as frozen fish. Moreover, in the case of improperly frozen fish, ice crystals may be larger and more irregular due to slow freezing or temperature fluctuations, leading to an increase in crystal size, cell damage and leakage of the enzyme. The studies including different freezing durations, did not observe an effect of freezing time, except in (Civera et al., 1996) where fish had to be frozen for 1 week before being able to discriminate. The HADH method is relatively easy to use, being moderately fast and laborious (sample preparation and setting a calibration line is needed) and requiring simple skills and equipment (UV‐Vis spectrophotometer). However, the method is destructive and a threshold value is needed to be able to classify the samples. To assess the robustness and efficiency of HADH as a stand‐alone method for the differentiation of fresh from frozen/thawed fish, its classification capacity needs to be statistically tested against independent samples. In limited studies, the classification performance was determined using external samples based on the setting of a threshold value, the results being satisfactory in general (see separate documents). However, the setting of a valid threshold value was in some cases complicated due to an overlap in enzymatic activity between fresh and frozen/thawed samples or large standard deviation (Hoz et al., 1992; Duflos et al., 2002) leading to an inconclusive zone in which it is not possible to classify the measured samples. The study of Fernandez et al. (1999) presents a methodology to set a unique relative threshold for several species, which can be of interest in the practical application at laboratory scale. To our knowledge, no validation of the method was carried out. Indeed, different methods for extracting the enzyme, measuring and calculating the enzyme activity were used between studies. When evaluating the evidence that superchilled fish will behave like fresh fish in the HADH analysis, the results of the Nile perch study were taken into account. In this study, compared to superchilled Nile perch, the enzymatic activity in frozen Nile perch samples was much higher based on the visual comparison of the trend of the absorbance monitored during the enzymatic reaction. However, fresh Nile perch (without superchilling) was not included in the study (‘Nile Perch study’ in Annex A).
Seven studies reported on the use of α‐glucosidase as a means to distinguish fresh from frozen/thawed fish. At all temperatures (all ≤ –18°C) and in all species tested, except from mackerel and chub mackerel, a significant increase in enzymatic activity could be noticed after the freeze/thawing sample (Alberio et al., 2014; Marlard et al., 2019). A marked increase (ca. 2‐fold higher) was also noticed by Nilsson and Ekstrand (1993) and Rehbein (1979) (3‐ to 8‐fold higher), but no statistics were performed in these studies. No information on freezing temperatures above –18°C was available, however, due to cell damage caused by large and irregular ice crystals formation in slowly frozen fish, enzyme leakage is expected to be similar to that observed with the HADH assay. Moreover, Nilsson and Ekstrand (1993) reported on an increase in enzyme activity with slower freezing rates. Some considerations should be taken into account to apply α‐glucosidase as a stand‐alone method. An increase of enzyme activity during fresh storage of fish could be observed (Rehbein et al., 1978; Duflos et al., 2002). This was, however, not always the case (Nilsson and Ekstrand, 1993). Therefore, in some species, the method might need to be combined with freshness analysis of the fish. Only one study set a threshold value for the examined species, but the classification performance was not evaluated in that case (Duflos et al., 2002). As for HADH, the α‐glucosidase method is relatively easy to use, being moderately fast and laborious (sample preparation and setting a calibration line is needed) and requiring simple skills and equipment (UV‐VIS spectrophotometer). However, the method is destructive, and a threshold value is needed to be able to classify the samples. The method is relatively easy to use, being rather fast and requiring simple skills and equipment. No information was available on validation or standardisation of the α‐glucosidase methodology. As for HADH activity, variation in the tested analytical protocol exists between studies.
Several studies reported the use of tests based on β‐N-acetylglucosaminidase; however, the capability of the method for differentiating fresh from frozen/thawed fish was variable and often not supported by statistical analysis. Moreover, the method was applied both on blood samples and muscle cells. The disadvantage of the use of blood is that it is only applicable for whole fish. Therefore, it was not considered to be a suitable method for differentiating all types of fresh and frozen/thawed fish.
The enzymatic assays based on the determination of the enzymatic activity of acid phosphatase, β‐glucuronidase, β‐galactosidase, lactate dehydrogenase, citrate synthase lack information on the applicability of the method for different fish species and different freezing temperatures or statistical information to be conclusive on the potential of these methods to discriminate between fresh and frozen/thawed fish. Succinate dehydrogenase was not able to discriminate between fresh and frozen/thawed fish (Civera et al., 1996).
Regarding the electrophoretic methods and the methods for volatile and phospholipid profiles, information is also lacking on the applicability for various fish species and freezing temperatures. Moreover, the methods are less convenient (slower and requiring many manual operations in comparison to other methods), making them less attractive as a routine control test. Research for a method for the rapid detection of the identified biomarkers would be required.
Two papers were available describing haematological methods either based on the determination of the haematocrit value (Yoshioka, 1983) or haemolysis of red blood cells (Civera et al., 1996). The methods seemed to discriminate well between fresh and frozen/thawed fish for different species and different freezing temperatures. However, the use of the method is limited to the whole fish as blood has to be retrieved from the sample.
One study was available (Marlard et al., 2019) on the use of free calcium concentration and nucleotides and related compounds in sea bass. Information is lacking on the applicability of the methods to different fish species and freezing temperatures.
Eye lens and histology. Two studies were available on visual observation of the opacity of the eye lens. The method presents a rapid means to distinguish between fresh from frozen/thawed fish However, the method is not applicable for small fish or for fish fillets. No information is available on the validation of the method nor on the classification performance. Histology of fresh and frozen/thawed samples was examined in five records. The method showed applicable for all studied species and temperatures (all lower or equal to –18°C) (Bozzetta et al., 2012; Richelmi et al., 2013; Popelka et al., 2014; Meistro et al., 2016; Tinacci et al., 2018). Moreover, when studied, the validation parameters based on sensitivity and specificity were very satisfactory (always > 94%) (Bozzetta et al., 2012; Meistro et al., 2016; Tinacci et al., 2018) and the precision was very high. The classification performance of samples not previously used for the validation procedure was also very high (always > 94%) (Bozzetta et al., 2012; Tinacci et al., 2018) and therefore histology is very suitable as a stand‐alone method. Histological examination of samples is, however, quite laborious with several steps in the sample preparation. The analysis is destructive and requires specialised and trained personnel, but has the advantage that no threshold values nor classification algorithms are needed No evidence was available that superchilled fish will show the same morphological characteristics as chilled fish, which is necessary for the discrimination from frozen/thawed fish. But as for the enzymatic assays, it is assumed that cell damage and structural changes are minimal during superchilling (Kaale et al., 2013), thus allowing the discrimination between superchilled and frozen/thawed fish.
Measurement of complex electrical and dielectric impedance was performed in five studies to differentiate fresh from frozen–thawed fish. Studies on dielectric⁄impedance properties of fish are were performed in different frequency regions – from 1 Hz to 1 MHz (Vidacek et al., 2008, 2012a; Fuentes et al., 2013). However, most of the published studies described serious drawbacks in the electrical methods applied, which limit their application for differentiation between fresh and previously frozen fish. In the paper of Vidacek et al. (2012a), the values of impedance phase for thawed fillets tend to approach zero which, when combined with the problems of anisotropy of the muscle fibres, limit the usage of the method for practical applications. In addition, statistical analysis showed that there was no difference among the frozen–thawed groups in impedance magnitude |Z| at none of the 27 frequencies measured from 0.1 to 1,000 kHz and even higher (Vidacek et al., 2012a). However, in the study of Sun et al. (2020), the differentiation between chilled and previously frozen fish (salmon and trout) based on impedance characteristics achieved 100% recognition accuracy in both training and prediction set. In addition, one of the main challenges for the reliable differentiation between fresh and previously frozen fish can be different structures and compositions of fish species with different fat content, which may affect the electrical properties of the fish to a great extent (Fuentes et al., 2013).
Regarding the spectroscopic methods, Ultraviolet–visible–near–infrared (UV‐VIS/NIR) and hyperspectral imaging (HI), the latter practically referring to domains of the VIS‐NIR spectra have been most widely studied to differentiate fresh from frozen/thawed fish. According to the available evidence, this has been successfully applied for 15 different fish species, targeting different infrared (IR) domains, including Fourier transformation (Pink et al., 1998; Alamprese and Casiraghi, 2015; Karoui et al., 2017), with classification performance above 85%, most commonly between 90 and 100%. Based on the available evidence (see Table D.6), the discrimination of frozen from fresh fish by UV‐VIS/NIR spectroscopy is unaffected by the duration of frozen storage (Reis et al., 2017), or the temperature and rate of freezing, or by the number of freezing/thawing cycles (Pink et al., 1998; Karoui et al., 2007; Sivertsen et al., 2011; Fasolato et al., 2012; Alamprese and Casiraghi, 2015; Reis et al., 2017; Wang et al., 2018). Visible spectra are sensitive to spatial colour variability, e.g. local (spot) discolorations, and roughness of muscle surface. As such, it requires capturing the spatial distribution of spectra throughout sample surface to ensure accurate classification (Kimiya et al., 2013). Sample seasonality, especially associated with the redness of the muscle, may impact spectra in the VIS‐NIR domain (Ottavian et al., 2014). An effective practice to maintain the robustness of a classification model for fresh vs. frozen/thawed fish, encompassing such variations in spectral data, is to calibrate the classification (multivariate) model with data sets composed of spectral data from samples from different seasons, or batches (Ottavian et al., 2014). UV‐VIS/NIR‐based spectroscopy methods. They are rapidly executable, non‐invasive (non‐destructive), non‐laborious, without the need for chemical reagents. They require minimal to no sample preparation and are carried out with benchtop or handheld equipment that may be mounted online, depending on the industrial setting and sample size. No threshold value is needed, as it relies on machine‐learning pattern recognition. As with hyperspectral imaging, it is well‐documented and extensively studied and thus, is of high technological readiness for commercial applications. Advanced bioinformatic expertise is however needed for data (pre‐) processing and sample classification. Data vary with fish species and composition (fat, water, proteins etc.) and as such, species‐specific (or group of species) algorithms may be needed. Even though the equipment is easy to handle, it requires a marked initial capital investment for meeting technological specifications. Hyperspectral imaging shares the advantages of the aforementioned UV‐VIS/NIR‐based spectroscopy methods regarding the execution speed, sample preparation, instrument handling, technological readiness and strength of evidence. It also has common disadvantages associated with the high expertise requirements in data processing and limitations related to potential fish specificity. The equipment can be mounted on‐line and images can be captured during processing, with whole fish or fillets passing below the camera, while placed on a conveyor belt. Nonetheless, it requires advanced technical specifications, coupled with high computational capacity computer.
Of note is the potential for using the same NIR spectral data in two steps, first for identifying fish species and then for distinguishing fresh from frozen/thawed samples (Ottavian et al., 2013; Alamprese and Casiraghi, 2015). A similar capability has been demonstrated for RAMAN spectroscopy (Velioglu et al., 2015), but the overall evidence for the applicability of RAMAN technique for the purpose of the current assessment is markedly limited compared to NIR and HI. The same accounts for NMR/MRI and Front‐face fluorescence spectroscopy, which, even though they are considered highly potent for chemical fingerprinting of various foods, they are still at low technological readiness levels for commercial applications in the direction of the current mandate. Possible reasons include the requirements for advanced equipment, high expert knowledge, statistical skills and the sensitivity of methods to sample interferences, a fact that requires extensive optimisation (Ghidini et al., 2019; Hassoun et al., 2020).
Differential scanning calorimetry (DSC) has the potential to be used for discriminating fresh and chilled fish from previously frozen fish based on the changes in thermal properties of proteins, particularly protein denaturation taking place during freezing (Matos et al., 2011; Tolstorebrov et al., 2014; Konieczny et al., 2016). However, only one study (Matos et al., 2011) was available on the application of DSC to differentiate between frozen and fresh/chilled fish and the method has a low technological readiness level and requires high expert knowledge and statistical skills.
3.2.5. Uncertainties associated with the potential methods
The uncertainties related to the assessment of methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’ are described in Table 9.
Table 9.
Potential sources of uncertainty identified in the assessment of the potential methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’ and assessment of the impact that these uncertainties could have on the conclusion (i.e. over/underestimation of the method performance and the extent of the over/underestimation)
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Ice crystals formation, consequent tissue damage and structural changes in SFFP |
As the temperature and % of frozen water, along with the superchilling methods applied, are variable, it is not clear whether SFFP and CFFP are similar so that they can be both (equally and) clearly distinguished from frozen fish. The experimental comparisons assessed in the available evidence are limited to fresh vs. deeply (< –10°C) frozen fish. As such, it is uncertain whether the method performs equally well in detecting frozen/thawed fish as compared to fresh fish and in differentiating a frozen/thawed fish from a super‐chilled one (i.e. not (deeply) frozen). |
Possibly overestimation of method performance, when it is solely based on the ability to differentiate frozen/thawed from fresh fish. |
| Applicability of the method in variety of fish species | There could be limitations in the classification performance of methods in certain fish species (fat/lean, cold/temperate water, etc.). | Overestimation of method performance. |
| Batch variability within the same fish species | Variability in the targeted parameters of biochemical and morphological methods, or spectral data of fish samples at a certain temperature may mask the difference between frozen/thawed and fresh fish and lead to misclassification or poor discrimination | Overestimation of method performance |
| Applicability of the method for a variety of freezing temperatures | Not all the temperature ranges have been evaluated for all the species. There was a need for extrapolation. | Overestimation of method performance. |
| Impact of more recent freezing techniques | Relatively little information in records on the used freezing method and freezing rate. There is a lack of information on behaviour of samples frozen with more recent freezing techniques such as pressure shift freezing. | Overestimation of method performance |
| Impact of post‐harvest quality changes | Lack of comparison between long stored samples and spoiled vs. fresh and frozen in the same study. If there is no impact and the method is not used, there is an under‐estimation of the performance. | Under‐ or overestimation of method performance |
| Impact of information not retrieved from the literature | Not all the information may be retrieved using the search strategy. | Under‐ or overestimation of method performance |
There is a lack of data on the similarities between superchilled and fresh fish so that they can be both (equally and) clearly distinguished from frozen fish. The uncertainties are primarily depended on fish species (fat/lean, cold/temperate water etc.), freezing conditions and equipment, range of freezing temperatures, impact of post‐harvest quality changes and lack of similar information in the literature resulting in possible overestimation of method performance. Overall, it is expected that there could be an overestimation of the method performance, as it is not known whether the methods (incl. the threshold and classification algorithm) would be valid for non‐tested species and (super)chilling and freezing conditions. Based on the ice crystal formation and the limited structural changes, the experts judged that SFFP is considered to behave under the above analytical procedures, with 90–95% certainty (very likely) as a fresh fishery product, rather than a frozen one.
3.2.6. Concluding remarks related to the potential methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’
Based on the ice crystal formation and the limited structural changes, SFFP is expected to behave like a fresh fishery product rather than a frozen one when the samples are analysed. The methods were evaluated for being ‘fit for purpose’ (i.e. to detect whether a previously frozen fish is commercially presented as ‘superchilled’). This assessment was based on their applicability for different fish species, ability to differentiate fresh fish from fish frozen at various temperatures, use as a stand‐alone method, ease of use; classification performance in discriminating as either frozen or not frozen, evidence that superchilled fish will behave like fresh fish; and strength of evidence.
-
The methods that were considered ‘fit for purpose’ are HADH and α‐glucosidase (enzymatic methods), histology (morphological methods), and UV‐VIS/NIR spectroscopy and hyperspectral imaging (physico‐chemical methods). All methods would benefit from standardisation and setting of threshold values or classification algorithms, to make them practical routine applications, possibly requiring updates for different fish species, or encompassing batch variability. They have the following specific characteristics:
-
o
HADH test proved suitable for almost all tested fish species. Superchilled Nile perch fillet has been proven to be distinguishable from deeply frozen fish based on the profile of the measured absorbance during the enzymatic test. The threshold value is generally species‐specific.
-
o
α‐glucosidase test proved suitable for almost all tested fish species but post‐harvest quality changes may interfere with the results. The threshold value is species specific.
-
o
histology proved suitable for all tested species, but is laborious and requires specialised skills.
-
o
UV-VIS/NIR spectroscopy and hyperspectral imaging proved suitable for all tested species and can be applied on‐line with hand‐held equipment or equipment mounted on the processing line. The classification algorithm may be specific for certain species or group of species.
-
o
-
Some methods have the potential to be used but the evidence is limited, or they currently require advance instrumental infrastructure and user expertise, making them not suitable for routine applications:
-
o
Front‐Face fluorescence has potential to be applied, on a similar spectroscopic base as UV‐VIS/NIR, but the current evidence is based on only two studies.
-
o
NMR/MRI and Raman spectroscopy have potential to be applied, but the current evidence is based on three and one studies, respectively. Moreover, they require advance instrumental infrastructure and user expertise.
-
o
DSC has potential to be applied, but the evidence is based on a single study.
-
o
-
For some methods, there is no evidence that they are suitable or there is evidence they cannot be applied to all types of SFFP, e.g. fillets:
-
o
β‐N-acetylglucosaminidase showed variable suitability, which was often not supported by statistical analysis. When the method is applied on blood, it is not suitable for fillets.
-
o
Acid phosphatase, β‐glucuronidase, β‐galactosidase, lactate dehydrogenase, citrate synthase tests lack information on the applicability for different fish species, freezing temperatures or because evidence provided is not statistically supported.
-
o
Succinate dehydrogenase was proved unsuitable in a single available study.
-
o
Haematological methods were reported by one study and are limited to whole fish.
-
o
The remaining biochemical methods (electrophoresis, profiles and other methods) were studied in an exploratory approach to identify markers potentially useful for development of rapid detection methods.
-
o
The eye lens method is limited to large whole fish.
-
o
Electrical methods based on dielectric properties and impedance require expertise for assembling and using the equipment, and displayed poor accuracy.
-
o
4. Conclusions
ToR 1: To compare the impact that the use of ‘superchilling’ could have on the survival and growth of biological hazards when compared with the current authorised practices (i.e. temperature approaching that of melting ice);
AQ1a: Which SFFP configurations (i.e. initial degree of superchilling) ensure that the fish temperature, at any time of the storage/transport, is lower or equal to CFFP when exposed to the same conditions of on‐land storage and/or transport?
AQ1b: If the SFFP conditions allow fish temperatures to be higher than in CFFP during the on‐land storage and/or transport, what is the potential increase of relevant biological hazards (i.e. pathogen log 10 increase or change in histamine concentration) for on‐land SFFP compared to CFFP upon exposure to the same conditions of storage and/or transport?
-
The capacity of the CFFP and SFFP to maintain their temperature when exposed to the same conditions of storage and/or transport depends on their capacity to absorb heat, which is determined by their initial configurations. These are:
-
o
for CFFP: the initial fish and ice temperatures and the proportion of ice:fish in the box; and
-
o
for SFFP: the degree of superchilling, i.e. the ice fraction in the fish matrix, which depends on the fish temperature after superchilling and the initial freezing point of the fish.
-
o
A degree of superchilling of SFFP equal to or higher than the proportion of ice:fish in the EPS box of CFFP properly adjusted to the envisaged conditions of transport and storage will ensure with 99–100% certainty (almost certain) that the fish temperature of SFFP and the consequent increase of the relevant hazards, including histamine, will be lower or equal than those of CFFP at any time point during storage/transport.
When the degree of superchilling is unknown, which is normally the case, it can be estimated using the fish temperature after superchilling and its initial freezing point. These two parameters are subjected to uncertainties: a) the fish temperature after superchilling is influenced by the application of the superchilling technology and the temperature measurement procedure; b) the initial freezing point can be derived from the fish proximate composition, which is influenced by fish species and other factors such as seasonal variations and temperature of the catching waters.
A tool was made available, named the HTM‐SFFP Tool, based on a conservative heat transfer model developed, that can be used as part of ‘safety‐by-design’ approach to identify under which initial configuration, the SFFP will be at least equivalent or better than CFFP in terms of total heat absorption capacity. The reliability of the outcome provided by the tool depends on the accuracy of the input data introduced by the user.
ToR 2: To evaluate the use of the HADH (Hydroxyacyl‐coenzyme A dehydrogenase) enzymatic test, or if possible any other test that could be used by the competent authorities, to differentiate ‘superchilling’ from freezing (i.e. temperature of not more than –18°C)
AQ 2: Which methods (including the HADH enzymatic test) are capable of detecting whether a previously frozen fish is commercially presented as ‘superchilled’?
-
Five out of 28 methods were, based on the available information, considered ‘fit for purpose’ based on their applicability for different fish species; ability to differentiate fresh fish from fish previously frozen at various temperatures; use as a stand‐alone method; ease of use; classification performance in discriminating FFP as either frozen/not frozen; evidence that superchilled fish will behave like fresh fish in the test; and strength of evidence. These methods are:
-
o
HADH test proved suitable for almost all tested fish species. Superchilled Nile perch fillet has been proven to be distinguishable from deeply frozen fish based on the profile of the measured absorbance during the enzymatic test. The threshold value is generally species specific.
-
o
α‐glucosidase test proved suitable for almost all tested fish species but post‐harvest quality changes may interfere with the results. The threshold value is species‐specific.
-
o
histology proved suitable for all tested species, but it is laborious and requires specialised skills.
-
o
UV‐VIS/NIR spectroscopy and hyperspectral imaging proved suitable for all tested species and can be applied on‐line with hand‐held equipment or equipment mounted on the processing line. The classification algorithm may be specific for certain species or groups of species.
-
o
For these five methods, based on the ice crystal formation and the limited structural changes, SFFP is considered to behave, under the above analytical procedures, with 90–95% certainty (very likely) like a fresh fishery product, rather than a frozen one.
5. Recommendations
Reliable methods and data generation are needed to accurately determine the initial freezing point (T*) and the ice fraction (Xice) of fish after superchilling. This will decrease the uncertainty in the conversion of Xice to the initial superchilling temperature of fish as an easy‐to-monitor indicator of the achievement of the target degree of superchilling.
Guidelines should be developed covering key aspects of the superchilling technology, as well as procedures for the validation and verification of the degree of superchilling ensuring the safety of superchilled fish during storage and transport on‐land.
All methods aiming to differentiate fresh (never frozen) chilled and superchilled fish from frozen/thawed fish should be standardised, including the establishment of threshold values or classification algorithms that may also need updating to account for multiple fish species and/or to encompass batch variability within species.
Evaluation of combinations of methods could be evaluated a means to improve the classification performance of currently appraised methods.
Abbreviations
- AQ
assessment question
- CA
competent authorities
- CFFP
conventional fresh fishery products
- DMA
dimethylamine
- DSC
differential scanning calorimetry
- EPS
expanded polystyrene
- FBO
food business operator
- FT
Fourier Transform
- FFP
fresh fishery products
- HADH
Hydroxyacyl‐coenzyme A dehydrogenase
- HDPE
high density polyethylene
- MIR
middle‐infrared
- MRI
magnetic resonance imaging
- NMR
nuclear magnetic resonance
- NIR
near‐infrared
- NRC
Nucleotides and related compounds
- PE
polyethylene
- RASFF
Rapid Alert System for Food and Feed
- SFFP
superchilled fresh fishery products
- Superchilling of fish
process leading to a decrease of fish temperature between the initial freezing point of the fish (always below the temperature of melting ice, i.e. < 0°C) to about 1–2°C lower
- UV‐VIS
Ultraviolet–visible
- VIS
visible
Appendix A – Additional information related to the Nile perch study
1.
The report of the Nile perch study is available in the Annex A and additional information as provided by Mike Turenhout (Manager Dutch Fish Importers Association) on behalf of FIORITAL and the members of the Dutch Fish Importers Association by e‐mail on 16 July 2019 (Turenhout, 2019) is available in below table and in Annex B.
| Question | Answer |
|---|---|
| Questions related to the superchilling process | |
| 1/ What is the target fish temperature set by the processor (e.g. in the HACCP) for the superchilling? How is the superchilling process managed/controlled, e.g. by time (depending on the load of the blast equipment) or by monitoring the fish temperature real‐time? |
The target fish temperature set by the processor for the superchilling lies between –0.5°C and –2°C. The temperature is measured (as regular temperature measurement requires) in the core of the product. Different variables are important to manage/control the superchilling process. In general, these are the size of the fillets, chiller/blast temperature, fillet shape, quantity of fillets in the chiller and the starting temperature of the product. Temperature is measured before and after chilling. Please see Annex B – part I (‘Homogenisation of fish temperature after superchilling in closed packaging’) & Annex B – part II (‘Superchilling temperatures a different establishments origin’). |
| 2/ Is there data available on the freezing point of Nile perch and the proportion of ice within the fish matrix just after the superchilling process (or after equalisation)? | There is no data available on the freezing point of Nile perch and the proportion of ice within the fish matrix just after the superchilling process. Modelled ice content for salmon gives an idea of the freezing point. |
| 3/ In Figure 3.1.2 and Figure 3.1.3, it seems that there is an inversely proportional correlation between the treatment duration and the fish core temperature distribution. What are the factors impacting on the fish core temperature distribution (e.g. fish size, starting temperature before superchilling)? | Treatment duration depends on different variables like chiller temperature, load, type of chiller (blast, spiral, deep, etc.), size of the fish, starting temperature of the fish and method (e.g. using stands, plastic crates, metallic cans, etc). See also Annex B – part II (‘Superchilling temperatures a different establishments origin’). |
| 4/ Which are the factors explaining the treatment duration in Figure 3.2.3 | See above. The procedure (in addition to depending on physical factors) is not the same for the different companies but is adopted according to its own self‐control plan. In any case, the output is common, i.e. superchilling with a product at a temperature between –0.5°C to –2.0°C (Attached some process procedures). See also Annex B – part II (‘Superchilling temperatures a different establishments origin’). |
| Questions related to the transport of the fish | |
| 1/ What was the size of the EPS boxes used in the study? |
Boxes used in the report were of conventional sizes used in daily supply chain. Depending on volume of the products 3 kg or 6 kg boxes were used. In general, the sizes of the boxes are: o 1 box of 3 kg → 388 × 290 × 95 mm (L × W × H) → Nile perch fillet o 1 box of 6 kg → 400 × 300 × 147 mm (L × W × H) → Nile perch fillet o 1 box by 15 kg → 595 × 395 × 220 mm (L × W × H) → Nile because headed and gutted (H & G) OR o 3 kg – 40 × 12 × 30 cm (L × W × H) → Nile perch fillet o 6 kg – 40 × 14 × 30 cm (L × W × H) → Nile perch fillet o 15 kg – 65 × 22 × 40 cm (L × W × H) → Nile because H&G |
| 2/ During the (validated?) study, the boxes were filled with six fillets per box. It is indicated in the report that usually those boxes are full of fillets? What would have been the usual number of fillets? Were the boxes full of fish in the self‐monitoring program? |
See above. The procedure (in addition to depending on physical factors) is not the same for the different companies but is adopted according to its own self‐control plan. In any case, the output is common, i.e. superchilling with a product at a temperature between –0.5°C and –2.0°C (Attached some process procedures). In self‐monitoring program boxes are full of fish (number of fillets depending on size of fillet and weight in the box). Size of the boxes is adjusted to the content. In the validated research in two situations, 6 kg size boxes were used instead of 3 kg size boxes with around 3 kg content. See Annex B – part III (‘Dataloggers’) for more information. |
| 3/ Can it be confirmed that no ice pack has been placed inside the boxes with superchilled fish? | We can confirm there are no ice‐packs inside the boxes with superchilled fish. The boxes were directly sent to the EU reference lab to check. |
| Questions related to the data loggers | |
| 1/ What was the position of the data loggers inside the box? This is important to understand what is the temperature that the loggers represent (e.g. surface fish temperature of a fish in the upper part close to the lid or of a fish located in the inner/central part of the box). | The loggers were located in the middle of the box. See attachment ‘datalogger’ for more information about the position of the dataloggers in the validated research. Also see extra validation in Annex B – part IV (‘Temperatures cold store and transport’). |
| 2/ Is there a difference in location of the data loggers for the self‐monitoring programme and the validated study? | The loggers are placed in the middle of the box. In the box, the temperature will be fully homogenised. The position on the pallet could influence the temperature slightly. Product temperature will be measured and documented at production plant before arrival and at destination. For the self‐monitoring programme loggers and temperature measurements were done in the same way as in the validated research. In both cases, boxes were filled like in a commercial supply chain (full boxes) except of two boxes in the validated research (see Annex B – part III (‘Dataloggers’)). |
| 3/ Can an actual profile be explained? In some profiles, two lines are shown. What does this represent? | We do think it is the temperature loss through the box to the outside when the box is outside the cold chain. See Annex B ‐ part III (‘Dataloggers’). In this attachment you can read that the blue line represents the logger temperature (air temperature) and the green line represents the probe temperature (core temperature product). |
| 4/ In two profiles, a temperature increase was noticed (FRE, page 65; EAS, page 67). What is the reason for this and how can the temperature drop after this increase? | The temperature increase was realised by exposure to higher outside temperatures. The temperature drops again due to the temperature of the product that homogenise again. Annex B ‐ part III (‘Dataloggers’). In the attachment you can read that in these examples the used boxes were too big for the amount of samples. |
| 5/ Only the temperature increase during transport is reported. Can the temperature of the data logger at the African establishment (just before the transport) and at the arrival be obtained? | Yes. On both sides accredited cold stores are used, HACCP accredited by local authorities. See attachment ‘temperatures cold store and transport’. |
| 6/ Can the raw data of these temperature profiles be obtained? | Information is available on request at cold stores. See Annex B ‐ part IV (‘Temperatures cold store and transport’). |
| 7/ Are data available of the temperature profiles outside the fish boxes? | There is no data available about the temperature profiles outside the fish boxes. See Annex B ‐ part IV (‘Temperatures cold store and transport’). The holds of aircraft are pressurised at controlled temperatures, as other fresh products (e.g. flowers, vegetables) are stowed together with super chilled fish. The transportation from the EU airport to the consignee is at controlled temperature (0–4°C), normally with other fresh fish products. At receiving the air temperature of the truck is occasionally checked and the carrier provides recordings of the transportation temperatures on demand. |
| Questions related to the fish temperatures | |
| 1/ For measuring the fish temperatures at the EU establishment (end of transport): where was the fish located inside the box (close to the lid or more internally?). Is there variation in the temperature depending on the location of the fish inside the box? | Location does not matter, because product temperature is homogenised throughout the box. See Annex B – part I (‘Homogenisation of fish temperature after superchilling in closed packaging’) |
| 2/ The report included the self‐monitoring data. Is the temperature verification at different points of the supply chain an implemented practice (e.g. within the HACCP plan). If so, what is being verified? | Yes, it is an implemented practice. At the processing plant the product temperature and chilling time are verified. As well in the African establishment as in the EU establishment temperatures are measured and registered. For African establishment the temperatures are measured for every batch. At receiving at the establishment in EU the temperatures are measured based on the EU establishments HACCP plan (HACCP plan of the establishment). |
| 3/ In the Nile perch study report data (although very few) included temperature from 1 to 2.5°C. Which is the maximum temperature tolerated by the EU establishment at arrival? Is this a critical limit set in the HACCP plan, thus measured and recorded in every batch | Temperature to be accepted at the establishment are legally required temperatures for fresh fish products. |
| Questions related to the supply chain | |
| 1/ How are the fish kept before the superchilling process? | The whole fish are continuously stored on ice before getting into the factory and between processing steps (during filleting). |
| 2/ How is the gutting/filleting done (manual/equipment)? How long does it take? | Gutting/filleting is done manually and takes around 2–3 min per whole fish. The entire process before chilling (washing, filleting, skinning, trimming, fillet washing, grading, arranging on trays and stands) takes around 30 min. Between the different processing steps the fish is iced. |
| 3/ Are the superchilled fish wrapped together in plastic before placing them in the fish box? | The products are not individually wrapped packed (IWP). There is a food grade cover sheet at top of box to protect the fish from potential contamination after opening the box. |
| 4/ In case of conventional transport (in boxes with ice), what would have been the practice and how much ice would be added? |
For 25 years, the fresh Nile perch supply chain products are superchilled and no ice is used during transport by air. So, this needs to be redesigned. In the current boxes probably 30–50% of the weight of the fish ice is needed to keep the products chilled. However, you will have to change box‐type (more isolation value) and size etc. if you export with ice. NOTE: boxes need to be free of leaking (insulated and sealed boxes). Melted ice will stay in the box if transporting in a conventional way. In the flow of the conventional product to share some data of the imported fish refrigerated with ice pack, we observed: By way of example, other chilled product delivered by air freight refrigerated with ice pack or gel pack:
|
| 5/ Can it be confirmed that the type of boxes used for that chain is expanded polystyrene (EPS)? Are these boxes also used for other chains transporting superchilled fish? | We can confirm that the type of boxes used for that chain is EPS. We have no experience with other chains. |
| 6/ What are the usual sizes of fish boxes used for transporting superchilled fish? | For fillets in general 3–6 kg. For other (whole and H&G), bigger sizes are used. See above. |
| 7/ Once the superchilled fish arrives at the EU establishment, how is it stored – transported to subsequent establishment (retail) and displayed on the market? | Fish is stored and transported in a refrigerated chilled environment. Majority of the fish are sold via fresh fish counters on ice or are provided directly to the end user (restaurant/foodservice). |
| 8/ Are you aware of the commercialisation of any other superchilled fish and/or other supply chain of the superchilled fish? In this case, which would be the similarities and the differences from the Nile perch supply chain? | Iceland cod supply to the EU |
| Questions related to the HADH enzymatic test | |
| 1/ HADH is supposed to be released extracellularly upon cell damage due to crystal formation. Is this dependent on the size of ice crystals, that is affected by the freezing method and thus, the freezing speed? | The HADH enzymatic test is based on https://link.springer.com/article/10.1007/s002170050481 operational working with the mitochondrial enzyme HADH that is released in cell damage from ice crystal formation. A faster freezing process will form smaller ice crystals. The smaller the ice crystals will be the smaller the resulting cell damage will be and thereby the amount of HADH. In conclusion: Yes, it depends on the conditions of the freezing process. |
| 2/ Is there a sensitivity threshold, i.e. a value of slope below/above, which a fish may be considered positive (i.e. previously frozen)? | The threshold is determined experimentally per animal species in the study. A sample with a known history (i.e. fresh and not frozen) is used for this research. |
| 3/ It is mentioned in the report that ‘an EU reference laboratory’ carried out the HADH analysis’. Can it be confirmed that this laboratory is not a reference laboratory for HADH? | RIKILT Wageningen University is not an EU reference laboratory for HADH. |
| 4/ In the report, it is said that ‘the calculations of the ratios tell us that the sample was not previously frozen’. Which ratio was calculated? Is this the absorbance between time 0 and end of absorbance measurement? Or between the one frozen sample and the superchilled samples? | A received sample is split into two parts with one part measured directly for 3 min (Xo) and 1 part stored at –18°C for 2 days and then measured for 3 min (X1). The ratio (R1) is calculated using Xo/X1. |
| 5/ What were these ratios (the values) and was there a threshold value? | For the positive reference sample the value 0.1113, for the superchilled samples this ratio was on average a factor of 10 smaller. The rejection or approval limit value has not been determined. |
Appendix B – Effect of the assumptions and estimations made on the heat transfer modelling
B.1. No spatial distribution was assumed: analysis of the Biot number
The Biot number (Bi) defines the ratio of the heat transfer resistances inside the solid (fish in this case) and at the surface of the body, being
where k is the conductivity of the fish (0.43 W/m°C for lean fish and 0.41 W/m°C for fat fish (EFSA BIOHAZ Panel, 2020)), R is the fraction of volume (V) and external area (A) of the box (following EFSA BIOHAZ Panel (2020); being L = 0.8 m, W = 0.45 m, H = 0.27 m, then R = 0.0697 m), and h is the effective heat transfer coefficient described with the equation
where hsurf is the heat transfer coefficient of the box with the air (assuming no wind 5 W/m2°C (EFSA BIOHAZ Panel, 2020)), Lbox and Lair are the thickness of the box and the air between the fish and the box, respectively, and kbox and kair the conductivity of the box and the air. Assuming Lbox = 0.005 m and kbox = 0.44 W/m°C as in the previous EFSA opinion (EFSA BIOHAZ Panel, 2020) and no air in the box (Lair = 0), the heat transfer coefficient is h = 4.73 W/m2°C. In case Lair > 0, the values of h and Biot number will decrease and the spatial distribution would be even less relevant.
Therefore, the Biot number is 0.77 for lean fish and 0.80 for fat fish.
B.2. Absorption heat rate was similar in SFFP and CFFP
Assessment was carried out assuming that heat is absorbed at similar rate in SFFP and CFFP. To assess the impact of this assumption, both conditions are compared using the time‐to‐melt‐all‐ice instead of the capacity to absorb heat. Note that in this alternative approach, absorbed rate is not assumed equivalent for SFFP and SFFP. On the other hand, this implies a more complex model with more parameters that are usually poorly known.
Comparing the time‐to-melt‐all-ice in both conditions
Note that in both cases ice is made from water without solutes and the melting point is 0°C. Even in SFFP ice is formed from pure water and solutes remain in the liquid fraction of free water. For this reason, there is not a unique freezing point, but there are different freezing temperatures depending on the concentration of solutes in the liquid free water.
Therefore, the following steps are consecutive and can be distinguished:
Ice temperature (whole fish for SFFP, as ice and fish muscle are considered as a mixture) increases until reaching 0°C (sensible heat);
Ice melts at 0°C without increasing temperature (latent heat); and
When ice melts completely, SFFP and CFFP temperatures start to rise above 0°C (sensible heat).
SFFP is best if time to‐melt‐all‐ice for superchilling is greater than in CFFP ( ).
Step 1 ‐ from time 0 to time t1temperature increases
For SFFP (considered as a whole mixture of water ice and fish), the temperature increases to 0°C following the equation
with the integral
and where T represents temperature TS is the superchilled fish temperature, is the initial superchilled fish temperature and Tout is the outside‐of‐the‐box temperature), t is time, mf and Cpf are the fish total mass in the box and its specific heat capacity and h and AA are the heat transfer coefficient and the outside area of the box, respectively.
The time for increasing the fish temperature to 0°C is therefore
For CFFP, we assume that ice is surrounding the fish and that fish is at 0°C without change, therefore
with integral
where Tice, mice and Cpice are the temperature, mass and specific heat capacity of the ice.
The time for increasing the ice temperature to 0°C is therefore
Step 2 – from time t1to tendwhere all the ice is melted
For SFFP, since all the outside heat is used to melt the ice, therefore
where Xice is the ice fraction and λ is the latent heat of ice.
During this process, TS is at 0°C and the time to melt all the ice (tend) can be calculated using
Note how tend is the sum of the time to rise the temperatures to 0°C and the subsequent the time to melt the ice.
For CFFP, the time‐to‐melt‐all‐ice is calculated using a similar reasoning with equations:
SFFP is better than CFFP if . If, in addition, the fish temperature when all ice is melted can be also calculated as described in next point.
Step 3 – larger times than tend
The temperatures are similar to the ones in step 1 but with if and fish temperatures increasing exponentially as follows:
for SFFP and CFFP, respectively
Analysis of the discrepancies between models based on capability to absorb heat and time‐to-melt‐all-ice
Two different approaches were derived to determine the initial configurations of SFFP and CFFP ensuring that the temperatures of SFFP are equal or lower than CFFP at any point of the storage/transport before all ice in the fish (SFFP) and in the box (CFFP) melts:
Qs ≥ QC in the case of the approach was based of capability to absorb heat, which assumes that heat is absorbed at the same rate in the conditions (this is the approach applied in the assessment described in Sections 2.1.1 and 3.1.1)
in the case of the approach based on time‐to-melt‐all-ice, it is assessed in the frame of the analysis of the uncertainty associated with the assumption that SFFP and CFFP absorb heat at the same rate.
Second approach does not need to assume same rate to absorb heat in SFFP and CFFP, but depends on unknown or poorly known parameters. Therefore, an optimisation problem is the calculation of the parameters ( ) that maximises the temperature in SFFP at the end of the storage ( ) if we use the condition QS ≥ QC and assuming that fish in CFFP was previously pre‐cooled ( , best case for CFFP). The optimisation problem reads:
The worse temperature for is 0.8502 and the parameters solution of the problem are as follows:
Any value of hAhA result in the same and therefore it does not affect the worse temperature. It only affects the time to reach this temperature. Results are shown for a fish that is not insulated, in case of insulation the time to reach this temperature is longer, beyond the usual storage/transport times. Therefore, this worse case of = 0.8502, cannot be observed for insulated boxes as commonly used in practice.
of CFFP is 0 (maximum differences between velocities of absorbed heat)
α goes to maximum of 0.33 kg of ice/kg of fish (i.e. best‐case of CFFP scenarios considered)
is calculated to get Xice slightly less than α plus some sensible heat such as we get the restriction QS > QC
The value of outside temperature that maximises the difference between CFFP and SFFP is 1.588°C
With these configurations, the temperatures profiles shown in Figure B.1 are obtained.
Figure B.1.
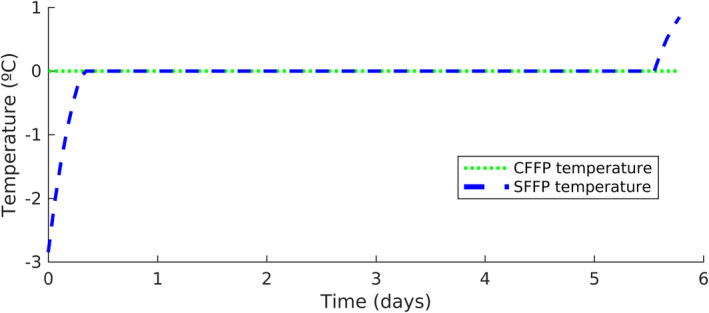
Temperature profiles resulting from the modelling approach based on the ‘time‐to‐melt‐all‐ice’ for which the rate to absorb heat in SFFP and CFFP might not be equal
It should be noted that, therefore, if QS ≫ QC SFFP fish temperatures are always below CFFP fish temperatures. If QS > QC, SFFP fish temperatures might, in the worst case without any insulation, increase to 0.85°C at the very end of the storage time considered while the temperature of CFFP will still be 0°C.
B.3. Impact of the uncertainty associated with the estimation of the ice fraction (Xice)
There is a lack of standardised procedures to determine the ice fraction (Xice) as a function of the fish temperature as well as for the initial freezing point (T*) of fish.
In the present opinion Xice was approximated as described in Section 2.1.1 (Equation 1) using thermodynamic properties of the food (semi‐mechanistic approach), being proved to be accurate for fresh meat, fish, fruit and non‐fat dairy products (Pham, 1996). This approach relies on the proximate composition of the food to calculate both the fraction of freezable water (from bound water, Equation 2) and the initial freezing point (Equation 3) through empirical equations (Pham, 2014a). The estimated values of T* using these calculations (Table 2) were slightly higher than those reported in the literature (Table B.1) determined through other approximations, instrumental methods or not specifying the methodology.
Table B.1.
Values of initial freezing point (T*) reported in the literature for cod, perch and salmon, together with the water content – the method used for T* determination and the references reported are included
| Fish | T* (°C) | Water content (%) | Method for determining T* | Reference |
|---|---|---|---|---|
| Salmon | –2.2 | 76.35 | NR | ASHRAE (2006) |
| –1.5 | 70.6 | a | Tolstorebrov et al. (2014) | |
| –2.2 | 67 | NR | Rahman et al. (2009) citing (Murakami and Okos, 1989) citing (Lentz, 1961) | |
| –2.2 | 67 | NR | Rahman et al. (2009) citing (Murakami and Okos, 1989) citing Lenz (1961) | |
| –2.2 | 64 | NR | Rahman et al. (2009) citing (ASHRAE, 1981) | |
| –1.5 | NR | d | Wu et al. (2014) citing (Alizadeh et al., 2007) | |
| –1.1 | NR | d | Kaale et al. (2014) | |
| Cod | –2.2 | 81.22 | NR | ASHRAE (2018) |
| –1.1 | 85 | a | Tolstorebrov et al. (2014) | |
| –0.8 | 80.3 | b | Boonsupthip and Heldman (2007) | |
| –0.78 | 80.3 | b | Chang and Tao (1981) | |
| –0.91 | 80.3 | b | Fikiin (1998) | |
| –1.00 | 80.3 | NR | Heldman (1974) citing (Long, 1955) | |
| –1.11 | 80.3 | b | Miki and Hayakawa (1996) citing (Lacey and Payne, 1991) | |
| –0.879 | 82 | b | Pham (1987) | |
| –0.907 | 80.3 | b | Pham (1987) | |
| –0.9 | 82 | NR | Rahman et al. (2009) citing (Chen, 1986) | |
| –1 | 81.2 | NR | Rahman et al. (2009) citing (Mannapperuma and Singh, 1989) | |
| –1.08 | 81.2 | NR | Rahman et al. (2009) citing (Mellor, 1983) | |
| –0.63 | 80.3 | NR | Rahman et al. (2009) citing (Dickerson, 1969) | |
| –2.2 | 78 | NR | Rahman et al. (2009) citing (Murakami and Okos, 1989) citing Lenz (1961) | |
| –1 | 74.5 | NR | Rahman et al. (2009) citing (Long, 1955) | |
| –2.2 | 70 | NR | Rahman et al. (2009) citing (ASHRAE, 1967) | |
| –0.8 | NR | c | Simpson and Haard (1987) | |
| –1.3 | NR | c | Simpson and Haard (1987) | |
| –1.02 | NR | c | Simpson and Haard (1987) | |
| –0.9 | NR | c | Simpson and Haard (1987) | |
| –2.2 | 78 | NR | Wu et al. (2014) citing (Murakami and Okos, 1989) citing Lenz (1961) | |
| Perch | –2.2 | 78.7 | NR | ASHRAE (2006) |
| –0.86 | 79.1 | b | Boonsupthip and Heldman (2007) | |
| –0.83 | 79.1 | b | Fikiin (1998) | |
| –0.8 | 79.1 | b | Murakami and Okos (1996) citing (Lusk et al., 1964) | |
| –0.76 | 79.1 | NR | Murakami and Okos (1996) citing (Chang and Tao, 1981) | |
| –0.861 | 79.1 | b | Pham (1987) | |
| –1 | 80.3 | NR | Rahman et al. (2009) citing Murakami and Okos (1989) citing Lusck et al (1964) | |
| –1.03 | 79.1 | b | Wu et al. (2014) citing (Miki and Hayakawa, 1996) citing (Lacey and Payne, 1991) |
NR: not reported.
DSC (differential scanning calorimetry).
From food composition (different empirical approximations depending on the article).
Freezing point osmometer.
Observation at the beginning of the freezing plateau at the centre of the sample.
The ice fraction in the fish matrix can also be determined either from a relationship obtained by fitting experimental data about Xice at different temperatures. This option is dependent on the availability of data as well as the experimental conditions and methods providing the results. Some data could be found for salmon and cod (Ottestad et al., 2009; Stevik et al., 2010; Bantle et al., 2016), and though the shape of the relationship was similar in all cases, the absolute value of Xice was considerably different among the experiments, mainly due to the use of different T* values. In addition, no data was found for Nile perch.
Though the T* values are reported for certain fish species (Table B.1) the methodology for its determination is usually not reported. The characteristics of the fish and the key factors determining the T* (e.g. amount of bound water or the molar concentration of solutes in the aqueous face of the fish) is unknown. The lack of this relevant information makes it difficult to compare the available data and to assess its representativeness for the current assessment.
The impact of the uncertainty of the initial freezing point of fish (T* on the fraction of ice of the superchilled fish and the corresponding ratio between the capacity to absorb heat of SFFP and CFFP (R value) was assessed though the stochastic approach for Nile perch. The Monte Carlo simulation presented in Section 3.1.1.2 (Figures 10 and 11) was run only changing the T* distribution (defined in Table 1: Normal (–0.33; 0.14)) by a fixed value at –0.52°C (i.e. the maximum value from the available data gathered in Table 2). When the superchilled Nile perch was compared with different CFFP scenarios (in terms of proportion of ice per mass of fish, αα value), the probability of obtaining R ≥ 1 was 99.6%, 98.8%, 97.3%, 93.3% for the scenarios of α 0.045, 0.18, 0.25 and 0.33, respectively (Figure B.2a). The ice fraction (Xice), as calculated from the initial SFFP temperature for Nile perch and the new value for T*, indicates that 90% of the Xice values would be within the range of 0.26–0.53 (Figure B.2a).
Figure B.2.
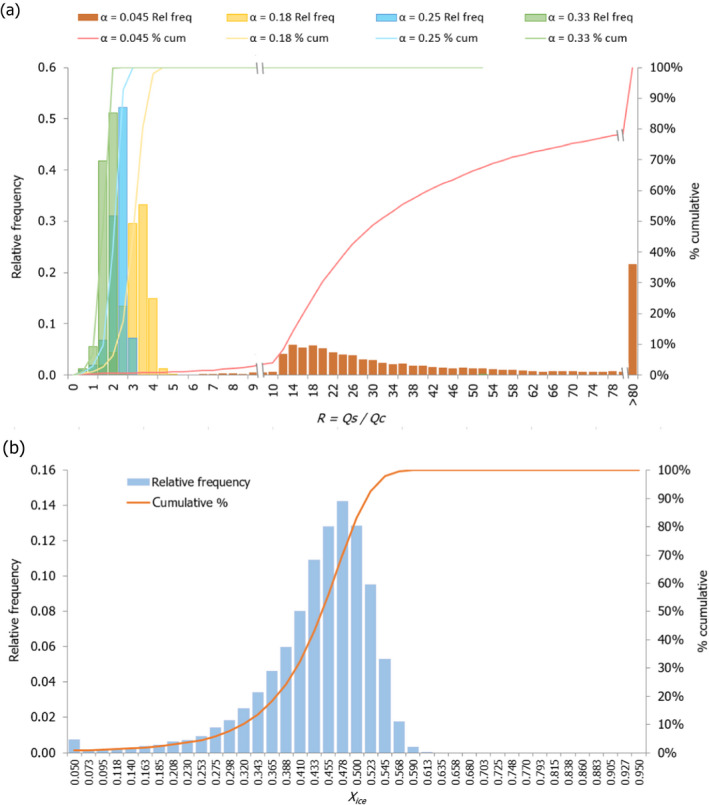
Probability distributions of (a) the ratio (R) between Qs and Qc for Nile Perch resulting from the stochastic approach through the heat transfer model simulation and (b) of the ice fraction (Xice) derived from the distribution of the initial temperature of superchilled Nile Perch (data from Nile perch study)
- Input values shown in Table 1, except for T* which was fixed at –0.52°C. Relative frequency (Rel freq) and cumulative (% cum) distributions are shown.
Analytical derivation of a formula to calculate the uncertainty on capacity to absorb heat of SFFP due to uncertainties in free water content and initial freezing point.
Assuming correctness of Equation 1) (valid for close to T*), the impact in the capacity of absorbing heat in SFFP due to uncertainties in the estimation of Xice might be important. The direction of impact of the uncertainty depends on the direction of the uncertainty on the free water content and the initial freezing point as follows:
Note that the impact on the uncertainty on absorbed heat might be important as this uncertainty is proportional to the latent heat (λ), which is a large quantity.
The uncertainty on capacity to absorb heat in SFFP is derived by
being QS as described in Equation 5, and considering that by Equation 11 the uncertainty on ice fraction reads:
where uncertainties on the free water content are due to uncertainties on the bound and total water content ( .
Appendix C – Search strategies and outcome of the literature searches
C.1. Literature searches supporting ToR 1
A literature search supporting ToR 1 (the fish temperatures) was conducted in the Web of ScienceTM Core Collection (1975–present) on 4 May 2020. The search strategy is reported in Table C.1.
Table C.1.
Details of search strings used for literature searches on fish temperatures using superchilling technology using Web of ScienceTM Core Collection (1975–present)
| Set number | Search | No of records |
|---|---|---|
| #1 | TOPIC: (fish OR fishes OR “fishery product” OR “fishery products” OR seafood OR seafoods OR albacore OR amberjack OR anchovy OR angler OR argentine OR bacha OR barbel OR barracuda OR basa OR bass OR “sea bass” OR beluga OR bib OR bigeye OR blackfish OR bleak OR blenny OR bluefish OR “blue runner” OR “blue shark” OR bonito OR branzino OR bream OR seabream OR “sea bream” OR brill OR burbot OR butterfish OR carp OR catfish OR catshark OR chub OR cod OR comber OR conger OR corb OR cutlassfish OR dab OR “danubian wels” OR dentex OR dogfish OR eel OR emperor OR flathead OR flounder OR “flying fish” OR forkbeard OR garfish OR garrick OR goby OR goldline OR grouper OR guitarfish OR gurnard OR haddock OR hake OR halibut OR hammerhead OR herring OR hoki OR huss OR icefish OR “John dory” OR lamprey OR lanternfish OR leerfish OR ling OR “little tunny” OR lythe OR mackerel OR “mahi mahi” OR marlin OR megrim OR melva OR monkfish OR moonfish OR mullet OR needlefish OR oreo OR pacu OR pandoras OR panga OR pangasius OR parrotfish OR “parrot fish” OR perch OR picarel OR pike OR pilchard OR pilotfish OR “pilot fish” OR plaice OR pollan OR Pollack OR Pollock OR ponyfish OR porbeagle OR pout OR ray OR ribbonfish OR rigg OR rockfish OR rosefish OR sablefish OR sailfish OR salmon OR sandeel OR sardine OR sardinella OR scabbardfish OR scorpionfish OR sheatfish OR “shi drum” OR sild OR sillago OR skipjack OR smelt OR smooth hound OR “smooth‐hound” OR snapper OR snook OR sole OR sparling OR spearfish OR “St Peter's fish” OR stargazer OR stingray OR sturgeon OR “surgeon fish” OR swordfish OR tailor OR tench OR tilapia OR threadfin OR triggerfish OR trout OR tubefish OR tuna OR turbot OR tusk OR walleye OR weever OR whitebait OR whiting OR wrasse OR yellowtail OR octopus OR squid OR crab OR lobster OR prawn OR shrimp OR cuttlefish OR crayfish OR langoustine OR scampi OR urchin) | 2,590,525a |
| #2 | TOPIC: (superchill* OR super‐chill* OR “super chill*” OR partial‐frozen OR “partial frozen” OR partial‐freezing OR “partial freezing” OR deep‐chill* OR “deep chill*” OR subchill* OR sub‐chill* OR “sub chill*”) | 303a |
| #3 | TOPIC: #1 AND #2 | 143a |
| #4 | TOPIC: #1 AND #2 | 141b |
DocType = All document types; Language = All languages; Time span = All years.
DocType = All document types; Language = English or German (two Japanese records excluded); Timespan = All years.
Screening at title and abstract level:
Question 1: does the record contain info about the use of superchilling of an FFP (with the exception of bivalve shellfish)?
Reply to question 1:
Yes: go to full text screening
No: exclude
Unclear: go to full text screening
Screening at full text level:
Question 1: does the record contain info about the use of superchilling of an FFP (with the exception of bivalve shellfish)?
Reply to question 1:
Yes: go to next question
No: exclude
Question 2: does the record contain info about the temperatures of FFP (with the exception of bivalve shellfish) after superchilling?
Reply to question 2:
Yes: include
No: exclude
If another reference seems relevant in the obtained records, it will be retrieved and screened.
Further information on temperatures of superchilled products during transport and/or storage were retrieved through a non‐systematic literature review conducted on 11 April 2020 by Google search with the following search strings:
-
a)
Transport AND (superchilling OR subchilling OR ‘deep chilling’)
-
b)
Storage AND (superchilling OR subchilling OR ‘deep chilling’)
Two additional records were retrieved through this search.
C.2. Literature searches supporting ToR 2
Two literature searches supporting ToR 2 (markers) were conducted in the Web of ScienceTM Core Collection (1975–present). The search strategies are reported in Table C.2 (search done on 20 May 2020) and Table C.3 (search done on 28 May 2020).
Table C.2.
Details of search strings used for literature searches on the HADH enzymatic test to distinguish fresh/superchilled fish meat from frozen/thawed fish meat using Web of ScienceTM Core Collection (1975–present)
| Set number | Search | No of records |
|---|---|---|
| #1 | TOPIC: (fish OR fishes OR “fishery product” OR “fishery products” OR seafood OR seafoods OR albacore OR amberjack OR anchovy OR angler OR argentine OR bacha OR barbel OR barracuda OR basa OR bass OR “sea bass” OR beluga OR bib OR bigeye OR blackfish OR bleak OR blenny OR bluefish OR “blue runner” OR “blue shark” OR bonito OR branzino OR bream OR seabream OR “sea bream” OR brill OR burbot OR butterfish OR carp OR catfish OR catshark OR chub OR cod OR comber OR conger OR corb OR cutlassfish OR dab OR “danubian wels” OR dentex OR dogfish OR eel OR emperor OR flathead OR flounder OR “flying fish” OR forkbeard OR garfish OR garrick OR goby OR goldline OR grouper OR guitarfish OR gurnard OR haddock OR hake OR halibut OR hammerhead OR herring OR hoki OR huss OR icefish OR “John dory” OR lamprey OR lanternfish OR leerfish OR ling OR “little tunny” OR lythe OR mackerel OR “mahi mahi” OR marlin OR megrim OR melva OR monkfish OR moonfish OR mullet OR needlefish OR oreo OR pacu OR pandoras OR panga OR pangasius OR parrotfish OR “parrot fish” OR perch OR picarel OR pike OR pilchard OR pilotfish OR “pilot fish” OR plaice OR pollan OR Pollack OR Pollock OR ponyfish OR porbeagle OR pout OR ray OR ribbonfish OR rigg OR rockfish OR rosefish OR sablefish OR sailfish OR salmon OR sandeel OR sardine OR sardinella OR scabbardfish OR scorpionfish OR sheatfish OR “shi drum” OR sild OR sillago OR skipjack OR smelt OR smooth hound OR “smooth‐hound” OR snapper OR snook OR sole OR sparling OR spearfish OR “St Peter's fish” OR stargazer OR stingray OR sturgeon OR “surgeon fish” OR swordfish OR tailor OR tench OR tilapia OR threadfin OR triggerfish OR trout OR tubefish OR tuna OR turbot OR tusk OR walleye OR weever OR whitebait OR whiting OR wrasse OR yellowtail OR octopus OR squid OR crab OR lobster OR prawn OR shrimp OR cuttlefish OR crayfish OR langoustine OR scampi OR urchin) | 2,597,472(a) |
| #2 | TOPIC: (HADH OR “Hydroxyacyl‐coenzyme‐A‐dehydrogenase” OR “Hydroxyacyl‐coenzyme A dehydrogenase” OR “hydroxyacyl coenzyme A dehydrogenase” OR “hydroxyacyl‐COA‐dehydrogenase” OR “hydroxyacyl COA dehydrogenase” OR “hydroxyacyl‐COA dehydrogenase”) | 1,670(a) |
| #3 | TOPIC: #1 AND #2 | 129(a) |
DocType = All document types; Language = All languages; Time span = All years.
Table C.3.
Details of search strings used for literature searches on markers to distinguish fresh/superchilled fish meat from frozen/thawed fish meat using Web of ScienceTM Core Collection (1975–present)
| Set number | Search | No of records |
|---|---|---|
| #1 | TOPIC: (fish OR fishes OR “fishery product” OR “fishery products” OR seafood OR seafoods OR albacore OR amberjack OR anchovy OR angler OR argentine OR bacha OR barbel OR barracuda OR basa OR bass OR “sea bass” OR beluga OR bib OR bigeye OR blackfish OR bleak OR blenny OR bluefish OR “blue runner” OR “blue shark” OR bonito OR branzino OR bream OR seabream OR “sea bream” OR brill OR burbot OR butterfish OR carp OR catfish OR catshark OR chub OR cod OR comber OR conger OR corb OR cutlassfish OR dab OR “danubian wels” OR dentex OR dogfish OR eel OR emperor OR flathead OR flounder OR “flying fish” OR forkbeard OR garfish OR garrick OR goby OR goldline OR grouper OR guitarfish OR gurnard OR haddock OR hake OR halibut OR hammerhead OR herring OR hoki OR huss OR icefish OR “John dory” OR lamprey OR lanternfish OR leerfish OR ling OR “little tunny” OR lythe OR mackerel OR “mahi mahi” OR marlin OR megrim OR melva OR monkfish OR moonfish OR mullet OR needlefish OR oreo OR pacu OR pandoras OR panga OR pangasius OR parrotfish OR “parrot fish” OR perch OR picarel OR pike OR pilchard OR pilotfish OR “pilot fish” OR plaice OR pollan OR Pollack OR Pollock OR ponyfish OR porbeagle OR pout OR ray OR ribbonfish OR rigg OR rockfish OR rosefish OR sablefish OR sailfish OR salmon OR sandeel OR sardine OR sardinella OR scabbardfish OR scorpionfish OR sheatfish OR “shi drum” OR sild OR sillago OR skipjack OR smelt OR smooth hound OR “smooth‐hound” OR snapper OR snook OR sole OR sparling OR spearfish OR “St Peter's fish” OR stargazer OR stingray OR sturgeon OR “surgeon fish” OR swordfish OR tailor OR tench OR tilapia OR threadfin OR triggerfish OR trout OR tubefish OR tuna OR turbot OR tusk OR walleye OR weever OR whitebait OR whiting OR wrasse OR yellowtail OR octopus OR squid OR crab OR lobster OR prawn OR shrimp OR cuttlefish OR crayfish OR langoustine OR scampi OR urchin) | 2,601,154a |
| #2 | TOPIC: (frozen OR freezing OR freeze OR thawed OR thawing OR thaw* OR frost* OR defrost* OR iced) | 417,868a |
| #3 | TOPIC: (fresh OR superchill* OR super‐chill* OR “super chill*” OR partial‐frozen OR “partial frozen” OR partial‐freezing OR “partial freezing” OR deep‐chill* OR “deep chill*” OR subchill* OR sub‐chill* OR “sub chill*” OR unfrozen) | 274,078a |
| #4 | TOPIC: (distinct* OR differentiat* OR distinguish* OR authenticat* OR adulterat* OR separat* OR discriminat*) | 4,328,232a |
| #5 | TOPIC: #1 AND #2 AND #3 AND #4 | 396a |
DocType = All document types; Language = All languages; Time span = All years.
Screening at title and abstract level:
Question 1: does the record describe a method to distinguish fresh/superchilled fish meat from frozen/thawed fish meat?
Reply to question 1:
Yes: go to full text screening
No: exclude
Unclear: go to full text screening
Screening at full text level:
Question 1: does the record describe a method to distinguish fresh/superchilled fish meat from frozen/thawed fish meat?
Reply to question 1:
Yes: include
No: exclude
If another reference seems relevant in the obtained records, it will be retrieved and screened.
Appendix D – Overview tables of the potential methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’
1.
Table D.4.
Analytical capability to distinguish fresh fish (chilled or superchilled) from frozen/thawed fish using the biochemical methods grouped by species within the method subgroups
| Speciesa | Reference groupb | –5°C | –10°C | –18°C | –20°C | –24°C | –29°C | –30°C | –35°C | –40°C | –80°C | –196°C | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENZYMATIC METHODS | |||||||||||||
| HADH | |||||||||||||
| Crawfish (Procambarus clarkia) | C | – | – | YES | – | – | – | – | – | – | – | – | Hoz et al. (1992) |
| Hake (Merluccius merluccius) | C | – | – | NO | – | – | – | – | – | – | – | NO | Fernandez et al. (1999) |
| Hake (Merluccius merluccius) | C | – | YES | YES | – | – | – | – | YES | – | YES | YES | Pavlov et al. (1994) |
| Small hake (Merluccius merluccius) | C | – | NO | NO | – | – | – | – | NO | – | NO | NO | Pavlov et al. (1994) |
| Norwegian lobster (Nephrops norvegicus) | C | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Mackerel (Scomber scombrus) | C | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Mackerel (Scomber scombrus) | C | – | YES | YES | – | – | – | – | YES | – | YES | YES | Pavlov et al. (1994) |
| Nile perch | SC | – | – | YES* | – | – | – | – | – | – | – | – | Nile perch study |
| Plaice (Pleuronectes platessa) | C | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Prawn (Penaeus japonicus) | C | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Kuruma prawn (Penaeus japonicas) | C | – | YES | YES | – | – | – | – | YES | – | YES | YES | Hoz et al. (1993) |
| Salmon (Salmo salar) | C | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Seabass (Dicentrarchus labrax) | C | – | – | NO | – | – | – | – | – | – | – | NO | Fernandez et al. (1999) |
| Gilthead sea bream (Sparus aurata) | C | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Sea bream (Pagellus centrodontus) | C | – | YES | YES | – | – | – | – | YES | – | YES | YES | Pavlov et al. (1994) |
| Sea bream (Pagellus centrodontus) | C | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Sole (Solea solea) | C | – | – | YES | – | – | – | – | – | – | – | YES | Fernandez et al. (1999) |
| Sole (Solea solea) | C | – | YES | YES | – | – | – | – | YES | – | YES | YES | Pavlov et al. (1994) |
| Squid (Loligo vulgaris) | C | – | YES | YES | – | – | – | – | YES | – | YES | YES | Pavlov et al. (1994) |
| Swordfish (Xyphius gladius) | C | YES | YES | Civera et al. (1996) | |||||||||
| Trout (Salmo gairdneri) | C | YES | – | YES | – | YES | – | – | YES | – | YES | YES | Garcia de Fernando et al. (1992) |
| Trout (Salmo gairdneri) | C | – | – | YES | – | – | – | – | – | – | – | – | Hoz et al. (1992) |
| Yellowfin tuna (Thunnus albacares) | C | – | – | – | – | YES | – | – | – | – | – | – | Bernardi et al. (2019) |
| Tuna (Thunnus alalunga) | C | – | YES | YES | – | – | – | – | YES | – | YES | YES | Pavlov et al. (1994) |
| Whiting (Merlangus merlangus) | C | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| β‐N-acetylglucosaminidase | |||||||||||||
| Anchovy (Engraulis encrasicolus) | C | – | – | NO | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Carp (blood) (Cyrpinus carpio) | C | – | – | – | YES* | – | – | – | – | YES* | – | – | Yuan et al. (1988) |
| Carp (blood) (Cyrpinus carpio) | SC | – | – | – | YES* | – | – | – | – | YES* | – | – | Yuan et al. (1988) |
| Carp (blood) (Cyprinus carpio) | C | – | – | – | YES* | – | – | – | – | – | – | – | Kitamikado et al. (1990) |
| Cod (Gadus morhua) | C | – | – | – | – | – | YES | – | – | – | – | – | Rehbein et al. (1978) |
| Cod (Gadus morhua) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein (1979) |
| Haddock (Gadus aeglefinus) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Chub mackerel (Scomber japonicas) | C | – | – | NO | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Horse mackerel (Trachurus trachurus) | C | – | – | NO | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Mackerel (Scomber scombrus) | C | – | – | – | NO | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Saithe (Gadus virens) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Sardines (Sardina pilchardus) | C | – | – | NO | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Red seabream (blood) (Chrysophrys major) | C | – | – | – | YES* | – | – | – | – | – | – | – | Yuan et al. (1988) |
| Red seabream (blood) (Chrysophrys major) | C | – | – | – | YES* | – | – | – | – | – | – | – | Kitamikado et al. (1990) |
| Plaice (Pleuronectes platessa) | C | – | – | – | NO | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Red fish (Sebastes marinus) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Rainbow trout (Oncorhynchus mykiss) | C | – | – | – | – | – | – | – | – | YES* | – | – | Nilsson and Ekstrand (1993) |
| Whiting (Merlangus merlangus) | C | – | – | – | NO | – | – | – | – | – | – | – | Duflos et al. (2002) |
| α‐glucosidase | |||||||||||||
| Anchovy (Engraulis encrasicolus) | C | – | – | YES | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Cod (Gadus morhua) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Cod (Gadus morhua) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein (1979) |
| Haddock (Gadus aeglefinus) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Chub mackerel (Scomber japonicas) | C | – | – | NO | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Horse mackerel (Trachurus trachurus) | C | – | – | YES | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Mackerel (Scomber scombrus) | C | – | – | – | NO | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Plaice (Pleuronectes platessa) | C | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Saithe (Gadus virens) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Salmon (Salmo salar) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein and Cakli (2000) |
| Sardines (Sardina pilchardus) | C | – | – | YES | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Seabass (Dicentrarchus labrax) | C | – | – | – | YES | – | – | – | – | – | – | – | Marlard et al. (2019) |
| Rainbow trout (Oncorhynchus mykiss) | C | – | – | – | – | – | – | – | – | YES* | – | – | Nilsson and Ekstrand (1993) |
| Red fish (Sebastes marinus) | C | – | – | – | – | – | YES* | – | – | – | – | – | Rehbein et al. (1978) |
| Whiting (Merlangus merlangus) | C | – | – | – | YES | – | – | – | – | – | – | – | Duflos et al. (2002) |
| Acid phosphatase | |||||||||||||
| Cod (Gadus morhua) | C | – | – | – | – | – | NO* | – | – | – | – | – | Rehbein (1979) |
| Rainbow trout (Oncorhynchus mykiss) | C | – | – | – | – | – | – | – | – | YES* | – | – | Nilsson and Ekstrand (1993) |
| β‐glucuronidase | |||||||||||||
| Cod (Gadus morhua) | C | – | – | – | – | – | – | – | – | YES* | – | – | Rehbein (1979) |
| β‐galactosidase | |||||||||||||
| Anchovy (Engraulis encrasicolus) | C | – | – | NO | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Cod (Gadus morhua) | C | – | – | – | – | – | – | – | – | YES* | – | – | (Rehbein (1979) |
| Chub mackerel (Scomber japonicas) | C | – | – | YES | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Horse mackerel (Trachurus trachurus) | C | – | – | YES | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Sardines (Sardina pilchardus) | C | – | – | NO | – | – | – | – | – | – | – | – | Alberio et al. (2014) |
| Lactate dehydrogenase | |||||||||||||
| Cod (Gadus morhua) | C | – | – | – | – | – | – | – | – | NO* | – | – | Rehbein (1979) |
| Sea bream (Sparus aurata) | C | – | – | YES* | – | – | – | – | – | – | – | – | Diop et al. (2016) |
| Citrate synthase | |||||||||||||
| Salmon (Salmo salar) | C | – | – | – | – | YES | – | – | – | – | – | – | Skorpilova et al. (2019) |
| Succinate dehydrogenase | |||||||||||||
| Anglerfish (Lophius spp.) | C | – | – | – | NO* | – | – | – | – | NO* | – | – | Civera et al. (1996) |
| Chondrichthyes | C | – | – | – | NO* | – | – | – | – | NO* | – | – | Civera et al. (1996) |
| Cod (Gadus morhua) | C | – | – | – | NO* | – | – | – | – | NO* | – | – | Civera et al. (1996) |
| Pleuronectidae | C | – | – | – | NO* | – | – | – | – | NO* | – | – | Civera et al. (1996) |
| Salmon (Salmo salar) | C | – | – | – | NO* | – | – | – | – | NO* | – | – | Civera et al. (. (1996) |
| Swordfish (Xyphius gladius) | C | – | – | – | NO* | – | – | – | – | NO* | – | – | Civera et al. (1996) |
| Protein markers by electrophoresis | |||||||||||||
| Aspartate aminotransferase | |||||||||||||
| 16 marine species and 6 freshwater species | C | – | – | YES*, c | – | – | – | – | – | – | – | – | Salfi et al. (1985) |
| Parvalbumin | |||||||||||||
| Sea bass (Dicentrarchus labrax) | C | – | – | – | – | – | – | YES* | – | – | – | – | Ethuin et al. (2015) |
| Transgelin | |||||||||||||
| Curled octopus (Eledone cirrhosa) | C | – | – | – | YES* | – | – | – | – | – | – | – | Guglielmetti et al. (2018) |
| Profile analysis | |||||||||||||
| Volatile profiles | |||||||||||||
| Cod (Gadus morhua) | C | – | – | – | YES | – | – | – | – | – | – | – | Leduc et al. (2012) |
| Salmon (Salmo salar) | C | – | – | – | YES | – | – | – | – | – | – | – | Leduc et al. (2012) |
| European sea bass (Dicentrarchus labrax) | C | – | – | – | YES | – | – | – | – | – | – | – | Leduc et al. (2012) |
| Gilthead seabream (Sparus aurata) | C | – | – | – | YES | – | – | – | – | – | – | – | Iglesias et al. (2009) |
| Gilthead seabream (Sparus aurata), | C | – | – | – | YES | – | – | – | – | – | – | – | Leduc et al. (2012) |
| Trout | C | – | – | – | YES | – | – | – | – | – | – | – | Di Natale et al. (2000) |
| Phospholipid profile | |||||||||||||
| Octopus (Octopus vulagaris) | C | – | – | NOc | – | – | – | – | – | – | – | – | Losito et al. (2018) |
| Shrimp (parapenaeus longirostris) | C | – | – | YESc | – | – | – | – | – | – | – | – | Losito et al. (2018) |
| Haematological methods | |||||||||||||
| Haematocrit value | |||||||||||||
| Carp | C | YES* | – | – | YES* | – | – | – | – | – | – | – | Yoshioka (1983) |
| Haemolysis of red blood cells | |||||||||||||
| Anglerfish (Lophius spp.) | C | – | – | – | YES* | – | – | – | – | YES* | – | – | Civera et al. (1996) |
| Chondrichthyes | C | – | – | – | YES* | – | – | – | – | YES* | – | – | Civera et al. (1996) |
| Cod (Gadus morhua) | C | – | – | – | YES* | – | – | – | – | YES* | – | – | Civera et al. (1996) |
| Salmon (Salmo salar) | C | – | – | – | YES* | – | – | – | – | YES* | – | – | Civera et al. (1996) |
| Swordfish (Xyphius gladius) | C | – | – | – | YES* | – | – | – | – | YES* | – | – | Civera et al. (1996) |
| Pleuronectidae | C | – | – | – | YES* | – | – | – | – | YES* | – | – | Civera et al. (1996) |
| Other methods | |||||||||||||
| Free calcium concentration | |||||||||||||
| Sea bass (Dicentrarchus labrax) | C | – | – | – | YES | – | – | – | – | – | – | – | Marlard et al. (2019) |
| Nucleotides and related compounds | |||||||||||||
| Sea bass (Dicentrarchus labrax) | C | – | – | – | YES | – | – | – | – | – | – | – | Marlard et al. (2019) |
– = not determined, YES = distinction between fresh or superchilled and frozen/thawed fish possible through statistical methods, NO = distinction not possible. HADH: β‐hydroxyacyl coenzyme A dehydrogenase.
No statistical test performed in the study.
With scientific name if provided.
Reference for comparison with frozen/thawed fish was a chilled (C) or superchilled (SC) fish.
No temperature specified in the study, –18°C has been assumed.
Table D.5.
Analytical capability to distinguish fresh fish (chilled or superchilled) from frozen/thawed fish based on morphological methods grouped by species within the subgroups
| Speciesa | Reference groupb | –18°C | –20°C | –24°C | –30°C | –80°C | Reference |
|---|---|---|---|---|---|---|---|
| Eye lens analysis | |||||||
| Carp (Cyprinus carpio) | C | – | YES* | – | – | – | Yoshioka and Kitamikado (1983) |
| Black cod (Anoplopoma fimbria) | C | – | – | – | YES* | – | Yoshioka and Kitamikado (1983) |
| Eel (Anguilla japonica) | C | – | NO* | – | – | – | Yoshioka and Kitamikado (1983) |
| Flat fish (Limanda herzensteini) | C | – | NO* | – | NO* | – | Yoshioka and Kitamikado (1983) |
| Hoki | C | – | – | – | YES* | – | Yoshioka and Kitamikado (1983) |
| Kingklip | C | – | – | – | YES* | – | Yoshioka and Kitamikado (1983) |
| Common horse mackerel (Trachurus japonicus) | C | – | YES* | – | – | – | Yoshioka and Kitamikado (1983) |
| Mackerel (Scomber scombrus) | C | – | YES* | – | – | – | Duflos et al. (2002) |
| Pacific mackerel (Pneumatophorus japonicus japonicas) | C | – | YES* | – | YES* | – | Yoshioka and Kitamikado (1983) |
| Plaice (Pleuronectes platessa) | C | – | NO* | – | – | – | Duflos et al. (2002) |
| Porgy (Chrysophrys major) | C | – | YES* | – | – | – | Yoshioka and Kitamikado (1983) |
| Yellow sea bream (Tainus tumifrons) | C | – | – | – | YES* | – | Yoshioka and Kitamikado (1983) |
| Stockfish (Merluccius capensis) | C | – | – | – | YES* | – | Yoshioka and Kitamikado (1983) |
| Southern blue whiting | C | – | – | – | YES* | – | Yoshioka and Kitamikado (1983) |
| Whiting (Merlangus merlangus) | C | – | YES | – | – | – | Duflos et al. (2002) |
| Yellowtail (Seriola quinqueradiata) | C | – | YES | – | YES | – | Yoshioka and Kitamikado (1983) |
| Histology | |||||||
| Anchovy (Engraulis encrasicolus) | C | – | YES | – | – | – | Meistro et al. (2016) |
| Atlantic bonito (Sarda sarda) | C | – | YES | – | – | – | Richelmi et al. (2013) |
| European hake (Merluccius merluccius) | C | – | YES | – | – | – | Tinacci et al. (2018) |
| Red mullet (Mullus barbatus) | C | – | – | – | – | YES | Bozzetta et al. (2012) |
| Salmon (Salmo salar) | C | – | YES | – | – | – | Richelmi et al. (2013) |
| Gilthead seabream (Sparus aurata) | C | – | – | – | – | YES | Bozzetta et al. (2012) |
| Swordfish (Xiphias gladius) | C | – | – | – | – | YES | Bozzetta et al. (2012) |
| Rainbow trout (Oncorhynchus mykiss) | C | YES* | – | – | – | Popelka et al. (2014) | |
| Albacore tuna (thunnus alalunga) | C | – | YES* | – | – | – | Richelmi et al. (2013) |
| Little tunny (Euthynnus alletteratus) | C | – | YES* | – | – | – | Richelmi et al. (2013) |
| Turbot (Psetta maxima) | C | – | YES* | – | – | – | Richelmi et al. (2013) |
| 35 species differing in protein and fat content | C | – | – | YES | – | YES | Bozzetta et al. (2012) |
– = not determined, YES = distinction between fresh or superchilled and frozen/thawed fish possible through statistical methods, NO = distinction not possible;
No statistical test performed in the study.
With scientific name if provided.
Reference for comparison with frozen/thawed fish was a chilled (C) or superchilled (SC) fish.
Table D.7.
Advantages/disadvantages related to the ease of use and technical resources required for each method of the methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’
| Method category | Method group | Method subgroup | Execution speed | Destructiveness | Laboriousness | Laboratory equipment | Skills needed | Threshold/calibration setting | Technological readiness |
|---|---|---|---|---|---|---|---|---|---|
| Biochemical | Enzymatic | HADH | M | D | L | ST | SI | T | H |
| β‐N‐acetylglucosaminidase | M | D | L | ST | SI | T | H | ||
| α‐glucosidase | M | D | L | ST | SI | T | H | ||
| Acid phosphatase | M | D | L | ST | SI | T | H | ||
| β‐glucuronidase | M | D | L | ST | SI | T | H | ||
| β‐galactosidase | M | D | L | ST | SI | T | H | ||
| Lactate dehydrogenase | M | D | L | ST | SI | T | H | ||
| Citrate synthase | M | D | L | ST | SI | T | H | ||
| Succinate dehydrogenase | M | D | L | ST | SI | T | H | ||
| Protein markers by electrophoresis | Aspartate aminotransferase | S | D | L | ST | SP | NT | H | |
| Parvalbumin | S | D | L | ST | SP | NT | M | ||
| Transgellin | S | D | L | ST | SP | NT | M | ||
| Profile analysis | Volatile profile | S | D | L | AD | SP | T | M | |
| Phospholipid profile | S | D | L | AD | SP | T | M | ||
| Haematological method | Haematocrit value | F | D | NL | ST | SI | NT | H | |
| Haemolysis of red blood cells | F | D | NL | ST | SI | NT | H | ||
| Others | Free calcium concentration | M | D | L | ST | SI | T | H | |
| Nucleotides and related compounds | M | D | L | ST | SI | T | H | ||
| Morphological | Eye lens | Eye lens | F | D | NL | ST | SI | NT | H |
| Histology | Histology | S | D | L | AD | SP | NT | H | |
| Physico‐chemical | Imaging/spectroscopy | Combinations of Ultraviolet–visible/NIR (UV‐VIS/NIR) | F | ND | NL | AD | SP | NT | H |
| (Front‐face) fluorescence spectroscopy | F | ND | NL | AD | SP | NT | L | ||
| Nuclear magnetic resonance (NMR)/MRI | F | ND | NL | AD | SP | NT | L | ||
| Raman spectroscopy | F | ND | NL | AD | SP | NT | L | ||
| Hyperspectral imaging | F | ND | NL | AD | SP | T | H | ||
| Electrical parameters | Electrical and dielectric impedance | F | ND | NL | AD | SP | NT | L | |
| DSC | DSC | F | ND | NL | AD | SI | T | H |
F = fast execution speed (< 0.5 days); M = medium execution speed (< 1 day); S = slow execution speed (> 1 day).
D = destructive method; ND = non‐destructive method.
L = laborious method (sample preparation necessary, many handling operations); NL = non‐laborious method (no or easy sample preparation, few handling operations).
ST = standard laboratory equipment; AD = advanced laboratory equipment.
SI = simple skills needed; SP = specialised skills needed.
T = need of threshold/calibration setting by species or group of species; NT = no need of threshold/calibration setting by species or group of species.
L = low technological readiness for commercial applications; M = medium technological readiness for commercial applications; H = high technological readiness for commercial applications.
Table D.8.
Evaluation table of the biochemical methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’
| Method evaluated | Enzymatic | Electrophoresis | Profiles | Haemological methods | Other methods | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evaluation criteria | HADH | \ralpha‐N‐acetyl glucosaminidase | \ralpha‐glucosidase | Acid phosphatase | \rbeta‐glucuronidase | \rbeta‐galactosidase | Lactate dehydrogenase | Citrate synthase | Succinatedehydrogenase | Aspartateaminotransferase | Parvalbumin | Transgelin | Volatile profile | Phopholipid profile | Haematocrit value | Haemolysis ofred blood cells | Free calcium concentration | Nucleotides and related compounds |
| Applicability of the method to different fish species | ++ | + | ++ | 0 | 0 | 0 | 0 | 0 | – | + | 0 | 0 | ++ | 0 | 0 | + | 0 | 0 |
| Ability of the method to differentiate fresh fish from fish frozen at various temperatures | ++ | ++ | ++ | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | + | + | 0 | 0 |
| Use of a single method possible | +++ | + | ++ | + | + | + | – | + | – | + | + | + | ++ | ++ | ++ | +++ | ++ | + |
| Ease of use of the method | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | + | + | + | + | ++ | ++ | ++ | ++ |
| Validation of the method | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Classification performance in discriminating as either frozen/not frozen | ++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Evidence that superchilled fish will behave like fresh fish in the given analysis | + | + | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| Strength of overall evidence (number of records; average appraisal scoreb) | 9; 4.9 | 7; 3.3 | 7; 2.9 | 2; 2.5 | 1; 1 | 2; 2 | 2; 1 | 1; 5 | 1; 2 | 1; 3 | 1; 3 | 1; 1 | 3; 1.7 | 1; 3 | 1; 6 | 1; 4 | 1; 1 | 1; 1 |
0 = no information available; – = poor performance; + = weak performance/weak evidence; ++ = good performance/good evidence; +++ = excellent performance/good evidence.
There is no evidence, but it has been assumed that superchilled fish would behave as fresh fish.
The division of the total appraisal score of all the records and the number of records.
Table D.9.
Evaluation table of the morphological methods to detect whether a previously frozen fish is commercially presented as ‘superchilled’
| Evaluation criteria | Method evaluated | |
|---|---|---|
| Eye lens | Histology | |
| Applicability of the method for different fish species | + | +++ |
| Ability of the method to differentiate fresh fish from fish frozen at various temperatures | + | ++ |
| Use of a single method possible | + | +++ |
| Ease of use of the method | +++ | + |
| Validation of the method | 0 | ++ |
| Classification performance in discriminating as either frozen/not frozen | 0 | +++ |
| Evidence that superchilled fish will behave like fresh fish | 0a | 0a |
| Strength of overall evidence (number of records; average appraisal scoreb) | 2; 4 | 5; 3.8 |
0 = no information available; – = poor performance; + = weak performance/weak evidence; ++ = good performance/good evidence; +++ = excellent performance/good evidence.
There is no evidence, but it has been assumed that superchilled fish would behave as fresh fish.
The division of the total appraisal score of all the records and the number of records.
Table D.10.
Evaluation table of the physico‐chemical methods subgroup spectroscopy to detect whether a previously frozen fish is commercially presented as ‘superchilled’
| Evaluation criteria | Method evaluated | ||||
|---|---|---|---|---|---|
| UV‐VIS/NIR | Hyperspectral imaging | (Front‐face) fluorescence | NMR/MRI | Raman | |
| Applicability of the method for different fish species | +++ | ++ | + | ++ | ++ |
| Ability of the method to differentiate fresh fish from fish frozen at various temperatures | +++ | ++ | ++ | ++ | + |
| Use of a single method possible | +++ | +++ | +++ | +++ | +++ |
| Ease of use of the method | ++ | ++ | + | + | + |
| Validation of the method | +++ | +++ | +++ | +++ | +++ |
| Classification performance in discriminating as either frozen/not frozen | +++ | +++ | ++ | ++ | ++ |
| Evidence that superchilled fish will behave like fresh fish | 0a | 0a | 0a | 0a | 0a |
| Strength of overall evidence (number of records; average appraisal scoreb) | 10; 5.6 | 10(c); 4.9 | 2; 6.0 | 3; 3.3 | 1; 5 |
0 = no information available; – = poor performance; + = weak performance/weak evidence; ++ = good performance/good evidence; +++ = excellent performance/good evidence.
There is no evidence, but it has been assumed that superchilled fish would behave as fresh fish.
The division of the total appraisal score of all the records and the number of records.
Studies with hyperspectral spectroscopy involve measurements in the domain of the VIS/NIR spectra. As such, the number of papers under UV‐VIS/NIR refers only to those studies that did not use hyperspectral camera.
Table D.11.
Evaluation table of the physico‐chemical methods subgroups electrical parameters and differential scanning calorimetry to detect whether a previously frozen fish is commercially presented as ‘superchilled’
| Evaluation criteria | Method evaluated | ||
|---|---|---|---|
| Electrical parameters | Differential scanning calorimetry | ||
| Dielectric properties | Electrical impedance | ||
| Applicability of the method for different fish species | – | + | – |
| Ability of the method to differentiate fresh fish from fish frozen at various temperatures | + | + | + |
| Use of a single method possible | + | ++ | + |
| Ease of use of the method | ++ | ++ | ++ |
| Validation of the method | + | + | + |
| Classification performance in discriminating as either frozen/not frozen | + | + | 0 |
| Evidence that superchilled fish will behave like fresh fish | 0a | 0a | 0a |
| Strength of overall evidence (number of records; average appraisal score(b)) | 2; 4.5 | 5; 3.2 | 1; 1 |
0 = no information available; – = poor performance; + = weak performance/weak evidence; ++ = good performance/good evidence; +++ = excellent performance/good evidence.
There is no evidence, but it has been assumed that superchilled fish would behave as fresh fish.
The division of the total appraisal score of all the records and the number of records.
Annex A – Report Superchilling Fish Products – Nile perch (Lates niloticus) fillet (14‐09‐2018; coordinated by the Dutch Fish Importers Association). This study has been referred to as ‘Nile perch study’ in this scientific opinion
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2021.6378
Appendix B – Additional information as referred to in Appendix A as provided by Mike Turenhout (Manager Dutch Fish Importers Association) on behalf of FIORITAL and the members of the Dutch Fish Importers Association by e‐mail on 16 July 2019 (Turenhout, 2019)
1.
Annex B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2021.6378
Supporting information
Report Superchilling Fish Products – Nile perch (Lates niloticus) fillet (14‐09‐2018; coordinated by the Dutch Fish Importers Association).This study has been referred to as ‘Nile perch study’ in this scientific opinion
Additional information as referred to in Appendix A as provided by Mike Turenhout (Manager Dutch Fish Importers Association) on behalf of FIORITAL and the members of the Dutch Fish Importers Association by e‐mail on 16 July 2019 (Turenhout, 2019)
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Bekaert K, Cropotova J, García MR, Messens W and Bover‐Cid S, 2021. Scientific Opinion on the use of the so‐called ‘superchilling’ technique for the transport of fresh fishery products. EFSA Journal 2021;19(1):6378, 156 pp. 10.2903/j.efsa.2021.6378
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00437
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis, and Elisabetta Suffredini.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © FIORITAL
Acknowledgements: The Panel wishes to acknowledge the hearing experts Vincenzo Di Leva, Antonio Solimeo (representing FIORITAL), and Mike Turenhout (representing the Dutch Fish Importers Association) and Sigurjon Arason.
Adopted: 10 December 2020
Model: Heat transfer model tool for the heat absorption capacity of superchilled fresh fishery products (HTM‐SFFP Tool) is available on the EFSA Knowledge Junction at: https://doi.org/10.5281/zenodo.4304283
Notes
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. OJ L 139, 30.4.2004, p. 1–55.
FAO Job Number: X5910; Author: J. J. WATERMAN and D. H. TAYLOR; Serial Title: Torry Advisory Notes; Series number: No. 32.
Dutch Fish Importers Association (2018). Superchilling Fish Products ‐ Nile perch (Lates niloticus) fillet. 106 pp.
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. OJ L 139, 30.4.2004, p. 1–54.
Commission Delegated Regulation (EU) 2019/625 of 4 March 2019 supplementing Regulation (EU) 2017/625 of the European Parliament and of the Council with regard to requirements for the entry into the Union of consignments of certain animals and goods intended for human consumption. OJ L 131, 17.5.2019, p. 18–30.
Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls. OJ L 131, 17.5.2019, p. 51–100.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Along the document, capacity to absorb heat will be used, but other terms can be found in the literature such as “heat load” and “internal cold reservoir”.
Proximate composition, also referred to compositional data, describes the composition of the food (fish in this case) in terms of groups or classes of substances, i.e. water, proteins, fat carbohydrates and ash (http://www.fao.org/3/x5957e01.htm and http://www.fao.org/3/y4705e/y4705e12.htm).
The real part of impedance is called resistance and the imaginary part is called reactance. The imaginary component of the impedance provides a measure of the reactive part of the impedance (e.g. capacitance). The real part of this impedance gives the factor between the input current and output voltage which are in direct proportion.
References
- Alamprese C and Casiraghi E, 2015. Application of FT‐NIR and FT‐IR spectroscopy to fish fillet authentication. Lwt‐Food Science and Technology, 63, 720–725. [Google Scholar]
- Alasavar C and Quantick PC, 1997. Temperature modelling and relationships in fish transportation In: Hall GM. (ed.). Fisg Processing Technology. Chapmann & Hall; pp. 249–288. [Google Scholar]
- Alberio GRA, Barbagallo RN, Todaro A, Bono G and Spagna G, 2014. Effect of freezing/thawing process in different sizes of blue fish in the Mediterranean through lysosomal enzymatic tests. Food Chemistry, 148, 47–53. [DOI] [PubMed] [Google Scholar]
- Alizadeh E, Chapleau N, de Lamballerie M and Le‐Bail A, 2007. Effect of different freezing processes on the microstructure of Atlantic salmon (Salmo salar) fillets. Innovative Food Science & Emerging Technologies, 8, 493–499. [Google Scholar]
- ASHRAE , 1967. Thermal properties of foods. In Fundamentals Handbook. American Society of Heating, Refrigeration and Air conditioning Engineers, New York.
- ASHRAE , 1981. Thermal properties of foods. American Society of Heating, Refrigeration and Air conditioning Engineers, New York, In Fundamentals Handbook. [Google Scholar]
- ASHRAE , 2006. ASHRAE Handbook ‐ Refrigeration. Chapter 9: Thermal Properties of Foods.
- ASHRAE , 2018. ASHRAE Handbook ‐ Refrigeration. Chapter 19: Thermal Properties of Foods. Available online: https://www.ashrae.org/technical-resources/ashrae-handbook/description-2018-ashrae-handbook-refrigeration.
- Atanasoff A, Nikolov G, Staykov Y, Zhelyazkov G and Sirakov I, 2013. Proximate and mineral analysis of Atlantic salmon (Salmo Salar) cultivated in Bulgaria, Scientific Works. Series C. Veterinary Medicine, 1, 87–91. [Google Scholar]
- Bahuaud D, Morkore T, Langsrud O, Sinnes K, Veiseth E, Ofstad R and Thomassen MS, 2008. Effects of −1.5°C Super‐chilling on quality of Atlantic salmon (Salmo salar) pre‐rigor Fillets: Cathepsin activity, muscle histology, texture and liquid leakage. Food Chemistry, 111, 329–339. [DOI] [PubMed] [Google Scholar]
- Bahuaud D, Morkore T, Ostbye TK, Torstensen BE, Veiseth‐Kent E, Ruyter B, Ofstad R and Thomassen MS, 2010. Atlantic salmon (salmo salar L.) Flesh quality role of lysosomal cathepsins b and l in muscle degradation. Lacopo P and Riemma M (eds.).
- Bantle M, Claussen IC and Tolstorebrov I (SINTEF Energy Research Efficient Energy usage 2016‐08-01), 2016. Superchilling of organic food: Part 1: Concept, State‐of-the‐Art and Potential for small scale implementation. SINTEF Energi. Rapport;TR A7583, TR A7583.
- Barcelo F, Barcelo I, Gavilanes F, Ferragut JA, Yanovich S and Gonzalezros JM, 1986. Interaction of anthracyclines with nucleotides and related‐compounds studied by spectroscopy. Biochimica Et Biophysica Acta, 884, 172–181. [DOI] [PubMed] [Google Scholar]
- Bastías JM, Balladares P, Acuña S, Quevedo R and Muñoz O, 2017. Determining the effect of different cooking methods on the nutritional composition of salmon (Salmo salar) and chilean jack mackerel (Trachurus murphyi) fillets. PLoS ONE, 12, e0180993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufort A, Cardinal M, Le‐Bail A and Midelet‐Bourdin G, 2009. The effects of superchilled storage at −2°C on the microbiological and organoleptic properties of cold‐smoked salmon before retail Display. International Journal of Refrigeration‐Revue Internationale Du Froid, 32, 1850–1857. [Google Scholar]
- Bekaert K, Deloof D, Vandermeersch G, De Witte B, Vlaemynck G and De Reu K (Institute for Agricultural and Fisheries Research (ILVO)), 2016. Project Qualitubfish. Opvolging van de kwaliteitsveranderingen van pladijs gedurende de opslag in tubs. 35 pp.
- Bernardi C, Tirloni E, Stella S, Anastasio A, Cattaneo P and Colombo F, 2019. beta‐hydroxyacyl‐CoA‐dehydrogenase activity differentiates unfrozen from frozen‐thawed Yellowfin tuna (Thunnus albacares). Italian Journal of Food Safety, 8, 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonsupthip W and Heldman DR, 2007. Prediction of frozen food properties during freezing using product composition. Journal of Food Science, 72, E254–E263. [DOI] [PubMed] [Google Scholar]
- Bozzetta E, Pezzolato M, Cencetti E, Varello K, Abramo F, Mutinelli F, Ingravalle F and Teneggi E, 2012. Histology as a valid and reliable tool to differentiate fresh from frozen‐thawed fish. Journal of Food Protection, 75, 1536–1541. [DOI] [PubMed] [Google Scholar]
- Chang HD and Tao LC, 1981. Correlations of enthalpies of food systems. Journal of Food Science, 46, 1493–1497. [Google Scholar]
- Chen CS, 1986. Effective molecular‐weight of aqueous‐solutions and liquid foods calculated from the freezing‐point depression. Journal of Food Science, 51, 1537–1539. [Google Scholar]
- Cheng JH, Sun DW, Pu HB, Chen XH, Liu YL, Zhang H and Li JL, 2015. Integration of classifiers analysis and hyperspectral imaging for rapid discrimination of fresh from cold‐stored and frozen‐thawed fish fillets. Journal of Food Engineering, 161, 33–39. [Google Scholar]
- Chevalier‐Lucia D, Sequeira‐Munoz A, Le‐Bail A, Simpson B and Ghoul M, 2000. Effect of freezing conditions and storage on ice crystal and drip volume in turbot (Scophthal‐mus maximus) evaluation of pressure shift Freezing vs. Airblast freezing. Innovative Food Science & Emerging Technologies, 1, 193–201. [Google Scholar]
- Civera T, Rebufatti P and Parisi E, 1996. Thawed fish: validity and limits of some analytical technique and suggestions for their employment. Industrie Alimentari, 35, 813–818. [Google Scholar]
- Claussen IC, 2011. Superchilling concepts enabling safe, high quality and long term storage of foods In: Saravacos G, Taoukis P, Krokida M, Karathanos V, Stoforos N, Tzia C. and Yanniotis S. (eds.). 11th International Congress on Engineering and Food. pp. 1907–1909. [Google Scholar]
- Clerjon S and Damez JL, 2007. Microwave sensing for meat and fish structure evaluation. Measurement Science and Technology, 18, 1038–1045. [Google Scholar]
- Cropotova J, Mozuraityte R, Standal IB, Grovlen MS and Rustad T, 2019. Superchilled, chilled and frozen storage of Atlantic mackerel (Scomber scombrus) fillets ‐ changes in texture, drip loss, protein solubility and oxidation. International Journal of Food Science and Technology, 54, 2228–2235. [Google Scholar]
- Dahimi O, Rahim AA, Abdulkarim SM, Hassan MS, Hashari S, Mashitoh AS and Saadi S, 2014. Multivariate statistical analysis treatment of DSC thermal properties for animal fat adulteration. Food Chemistry, 158, 132–138. [DOI] [PubMed] [Google Scholar]
- Dai Q, Cheng JH, Sun DW, Pu HB, Zeng XA and Xiong Z, 2015. Potential of visible/near‐infrared hyperspectral imaging for rapid detection of freshness in unfrozen and frozen prawns. Journal of Food Engineering, 149, 97–104. [Google Scholar]
- Damodaran S and Parkin K, 2017. Fennema's Food Chemistry. CRC Press, Boca Raton: 10.1201/9781315372914. pp 1123. [DOI] [Google Scholar]
- Di Natale C, Macagnano A, Nardis S, Paolesse R, Mantini A, Martinelli E and D'Amico A, 2000. Electronic nose evaluation of storage days of fresh and thawed trout fishes. In: DiNatale C, Damico A and Siciliano P (eds.).
- Dickerson RW, 1969. Thermal properties of foods In: Tressler DK, Van Arsdel WB. and Copley MR. (eds.). The Freezing Preservation of Food. AVI Publishing, Westport, CT: pp. 26–51. [Google Scholar]
- Diop M, Watier D, Masson PY, Diouf A, Amara R, Grard T and Lencel P, 2016. Assessment of freshness and freeze‐thawing of sea bream fillets (Sparus aurata) by a cytosolic enzyme: lactate dehydrogenase. Food Chemistry, 210, 428–434. [DOI] [PubMed] [Google Scholar]
- Duflos G, Le Fur B, Mulak V, Becel P and Malle P, 2002. Comparison of methods of differentiating between fresh and frozen‐thawed fish or fillets. Journal of the Science of Food and Agriculture, 82, 1341–1345. [Google Scholar]
- Duun AS and Rustad T, 2007. Quality changes during superchilled storage of cod (Gadus morhua) fillets. Food Chemistry, 105, 1067–1075. [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Scientific and technical assistance on the evaluation of the temperature to be applied to pre‐packed fishery products at retail level. EFSA Journal 2015;13(7):4162, 48 pp. 10.2903/j.efsa.2015.4162 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Assessment of the incidents of histamine intoxication in some EU countries. EFSA supporting publication 2017;EN‐1301, 37 pp. 10.2903/sp.efsa.2017.EN-1301 [DOI]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2020. Scientific Opinion on the use of the so‐called ‘tubs’ for transporting and storing fresh fishery products. EFSA Journal 2020;18(4):6091, 123 pp. 10.2903/j.efsa.2020.6091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018a. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson S, Arason S, Margeirsson B, Bergsson AB and Palsson OP, 2019. The effects of superchilling on shelf‐life and quality indicators of whole Atlantic cod and fillets. Lwt‐Food Science and Technology, 100, 426–434. [Google Scholar]
- Erikson U, Misimi E and Gallart‐Jornet L, 2011. Superchilling of rested Atlantic salmon: different chilling strategies and effects on fish and fillet quality. Food Chemistry, 127, 1427–1437. [Google Scholar]
- Ethuin P, Marlard S, Delosiere M, Carapito C, Delalande F, Van Dorsselaer A, Dehaut A, Lencel V, Duflos G and Grard T, 2015. Differentiation between fresh and frozen‐thawed sea bass (Dicentrarchus labrax) fillets using two‐dimensional gel electrophoresis. Food Chemistry, 176, 294–301. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations in partnership with Support unit for International Fisheries and Aquatic Research), 2001. Superchilling. Torry advisory note No. 32.
- Fasolato L, Balzan S, Riovanto R, Berzaghi P, Mirisola M, Ferlito JC, Serva L, Benozzo F, Passera R, Tepedino V and Novelli E, 2012. Comparison of visible and near‐infrared reflectance spectroscopy to authenticate fresh and frozen‐thawed swordfish (Xiphias gladius L). Journal of Aquatic Food Product Technology, 21, 493–507. [Google Scholar]
- Fernandez M, Mano S, de Fernando DG, Ordonez JA and Hoz L, 1999. Use of beta‐hydroxyacyl‐CoA‐dehydrogenase (HADH) activity to differentiate frozen from unfrozen fish and shellfish. European Food Research and Technology, 209, 205–208. [Google Scholar]
- Fernandez‐Segovia I, Fuentes A, Alino M, Masot R, Alcaniz M and Barat JM, 2012. Detection of frozen‐thawed salmon (Salmo salar) by a rapid low‐cost method. Journal of Food Engineering, 113, 210–216. [Google Scholar]
- Fikiin KA, 1998. Ice content prediction methods during food freezing: a survey of the Eastern European literature. Journal of Food Engineering, 38, 331–339. [Google Scholar]
- Fuentes A, Masot R, Fernandez‐Segovia I, Ruiz‐Rico M, Alcaniz M and Barat JM, 2013. Differentiation between fresh and frozen‐thawed sea bream (Sparus aurata) using impedance spectroscopy techniques. Innovative Food Science & Emerging Technologies, 19, 210–217. [Google Scholar]
- Garcia de Fernando GDG, Fernandez M, Diaz O, Ordonez JA and Hoz L, 1992. An objective method to differentiate between fresh and thawed trout meat (Salmo‐Gairdneri). Archiv Fur Lebensmittelhygiene, 43, 13–14. [Google Scholar]
- Ghidini S, Varra MO and Zanardi E, 2019. Approaching authenticity issues in fish and seafood products by qualitative spectroscopy and chemometrics. Molecules, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmetti C, Manfredi M, Brusadore S, Sciuto S, Esposito G, Ubaldi PG, Magnani L, Gili S, Marengo E, Acutis PL and Mazza M, 2018. Two‐dimensional gel and shotgun proteomics approaches to distinguish fresh and frozen‐thawed curled octopus (Eledone cirrhosa). Journal of Proteomics, 186, 1–7. [DOI] [PubMed] [Google Scholar]
- Hassoun A, Shumilina E, Di Donato F, Foschi M, Simal‐Gandara J and Biancolillo A, 2020. Emerging techniques for differentiation of fresh and frozen‐thawed seafoods: highlighting the potential of spectroscopic techniques. Molecules, 25, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman DR, 1974. Predicting the relationship between unfrozen water fraction and temperature during food freezing using freezing point depression. Transactions of the ASAE, 17, 63‐0066. [Google Scholar]
- Hochstrasser DF, Harrington MG, Hochstrasser AC, Miller MJ and Merril CR, 1988. Methods for increasing the resolution of two‐dimensional protein electrophoresis. Analytical Biochemistry, 173, 424–435. [DOI] [PubMed] [Google Scholar]
- Hoz L, Yustes C, Camara JM, Ramos MA and Defernando GDG, 1992. Beta‐hydroxyacyl‐coa‐dehydrogenase (HADH) differentiates unfrozen from frozen‐thawed crawfish (procambarus‐clarkii) and trout (salmo‐gairdneri) meat. International Journal of Food Science and Technology, 27, 133–136. [Google Scholar]
- Hoz L, Fernandez M, Diaz O, Ordonez JA, Pavlov A and Defernando GDG, 1993. Differentiation of unfrozen and frozen‐thawed kuruma prawn (Penaeus‐Japonicus) from the activity of beta‐hydroxyacyl‐coa‐dehydrogenase (hadh) in aqueous extracts. Food Chemistry, 48, 127–129. [Google Scholar]
- Iglesias J, Medina I, Bianchi F, Careri M, Mangia A and Musci M, 2009. Study of the volatile compounds useful for the characterisation of fresh and frozen‐thawed cultured gilthead sea bream fish by solid‐phase microextraction gas chromatography‐mass spectrometry. Food Chemistry, 115, 1473–1478. [Google Scholar]
- Jain D, Ilyas SM, Pathare P, Prasad S and Singh H, 2005. Development of mathematical model for cooling the fish with ice. Journal of Food Engineering, 71, 324–329. [Google Scholar]
- Kaale LD, 2014. Modelling and ice crystallization/recrystallization of foods in superchilling technology ‐ Superchilling of Atlantic salmon (Salmo salar) Thesis for the degree of Philosophiae Doctor. Norwegian University of Science and Technology (NTNU). [Google Scholar]
- Kaale LD, Eikevik TM, Rustad T and Kolsaker K, 2011. Superchilling of food: a review. Journal of Food Engineering, 107, 141–146. [Google Scholar]
- Kaale LD, Eikevik TM, Rustad T, Nordtvedt TS, Bardal T and Kjorsvik E, 2013. Ice crystal development in pre‐rigor Atlantic salmon fillets during superchilling process and following storage. Food Control, 31, 491–498. [Google Scholar]
- Kaale LD, Eikevik TM, Kolsaker K and Stevik AM, 2014. Modeling and simulation of food products in superchilling technology. Journal of Aquatic Food Product Technology, 23, 409–420. [Google Scholar]
- Karoui R, Thomas E and Dufour E, 2006. Utilisation of a rapid technique based on front‐face fluorescence spectroscopy for differentiating between fresh and frozen‐thawed fish fillets. Food Research International, 39, 349–355. [Google Scholar]
- Karoui R, Lefur B, Grondin C, Thomas E, Demeulemester C, De Baerdemaeker J and Guillard AS, 2007. Mid‐infrared spectroscopy as a new tool for the evaluation of fish freshness. International Journal of Food Science and Technology, 42, 57–64. [Google Scholar]
- Karoui R, Hassoun A and Ethuin P, 2017. Front face fluorescence spectroscopy enables rapid differentiation of fresh and frozen‐thawed sea bass (Dicentrarchus labrax) fillets. Journal of Food Engineering, 202, 89–98. [Google Scholar]
- Karthikeyan JS, Desai KM, Salvi D, Bruins R and Karwe MV, 2015. Effect of temperature abuse on frozen army rations. Part 1: Developing a heat transfer numerical model based on thermo‐physical properties of food. Food Research International, 76, 595–604. [DOI] [PubMed] [Google Scholar]
- Kent M and Oehlenschlager J, 2009. Measuring electrical properties In: Rehbein H. and Oehlenschlager J. (eds.). Fishery products: Quality, safety and Authenticity. Blackwell publishing Ltd., Chichester, UK: pp. 286–297. [Google Scholar]
- Kent M, Oehlenschlager J, Mierke‐Klemeyer S, Knochel R, Daschner F and Schimmer O, 2004. Estimation of the quality of frozen cod using a new instrumental method. European Food Research and Technology, 219, 540–544. [Google Scholar]
- Kent M, Knochel R, Daschner F, Schimmer O, Tejada M, Huidobro A, Nunes L, Batista I and Martins A, 2005. Determination of the quality of frozen hake using its microwave dielectric properties. International Journal of Food Science and Technology, 40, 55–65. [Google Scholar]
- Kim JB, Murata M and Sakaguchi M, 1987. A method for the differentiation of frozen‐thawed from unfrozen fish fillets by a combination of torrymeter readings and k‐values. Bulletin of the Japanese Society of Scientific Fisheries, 53, 159–164. [Google Scholar]
- Kimiya T, Sivertsen AH and Heia K, 2013. VIS/NIR spectroscopy for non‐destructive freshness assessment of Atlantic salmon (Salmo salar L.) fillets. Journal of Food Engineering, 116, 758–764. [Google Scholar]
- Kitamikado M, Yuan CS and Ueno R, 1990. An enzymatic method designed to differentiate between fresh and frozen‐thawed fish. Journal of Food Science, 55, 74–76. [Google Scholar]
- Klapper R, Meyer C, Kuhn T and Karl H, 2017. Food safety aspects of fresh Nile perch (Lates niloticus) fillets from Lake Victoria imported to the European market: Helminth parasites and microbiological status. Food Control, 78, 311–316. [Google Scholar]
- Konieczny P, Tomaszewska‐Gras J, Andrzejewski W, Mikolajczak B, Urbanska M, Mazurkiewicz J and Stangierski J, 2016. DSC and electrophoretic studies on protein denaturation of Anodonta woodiana (Lea, 1834). Journal of Thermal Analysis and Calorimetry, 126, 69–75. [Google Scholar]
- Krzynowek J and Murphy J, 1987. Proximate composition, energy, fatty acid, sodium, and cholesterol content of finfish, shellfish, and their products. NOAA Technical Report NMFS 55. [Google Scholar]
- Lacey RE and Payne FA, 1991. A model to estimate thermodynamic properties of biological materials during freezing. Transactions of the ASAE, 34, 1836–1841. [Google Scholar]
- Laguerre O, Derens E and Flick D, 2018. Modelling of fish refrigeration using flake ice. International Journal of Refrigeration‐Revue Internationale Du Froid, 85, 97–108. [Google Scholar]
- Le Danois E, 1920. Nouvelle methode de frigorification du poisson. France Patent, Application No. 506. 296. Available online: https://patents.google.com/patent/FR506296A/fr?q=Nouvelle+methode+de+frigorification+du+poisson.&q=A23B4%2f08&q=A23B4%2f08
- Leduc F, Krzewinski F, Le Fur B, N'Guessan A, Malle P, Kol O and Duflos G, 2012. Differentiation of fresh and frozen/thawed fish, European sea bass (Dicentrarchus labrax), gilthead seabream (Sparus aurata), cod (Gadus morhua) and salmon (Salmo salar), using volatile compounds by SPME/GC/MS. Journal of the Science of Food and Agriculture, 92, 2560–2568. [DOI] [PubMed] [Google Scholar]
- Lentz CP, 1961. Thermal conductivity of meats, fats, gelatin gels and ice. Food Technology, 15, 243–247. [Google Scholar]
- Long AK, 1955. Some thermodynamic properties of fish and their effect on the rate of freezing. Journal of the Science and Food Agriculture, 6, 621–632. [Google Scholar]
- Losito I, Facchini L, Catucci R, Calvano CD, Cataldi TRI and Palmisano F, 2018. Tracing the thermal history of seafood products through lysophospholipid analysis by hydrophilic interaction liquid chromatography‐electrospray ionization fourier transform mass spectrometry. Molecules, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk G, Karel M and Goldblith SA, 1964. Thermal conductivity of some freeze‐dried fish. Food Technology, 18, 1625–1628. [Google Scholar]
- Lynum L, 2007. Videreforedling av fisk. Tapir Akademisk Forlag, Trondheim. [Google Scholar]
- Magnussen OM, Haugland A, Torstveit Hemmingsen AK, Johansen S and Nordtvedt TS, 2008. Advances in superchilling of food – process characteristics and product quality. Trends in Food Science & Technology, 19, 418–424. [Google Scholar]
- Mai NTT, Margeirsson B, Margeirsson S, Bogason SG, SigurgÍSladÓTtir S and Arason S, 2012. Temperature mapping of fresh fish supply chains – air and sea transport. Journal of Food Process Engineering, 35, 622–656. [Google Scholar]
- Mannapperuma JD and Singh RP, 1989. A computer‐aided method for the prediction of properties and freezing/thawing times of foods. Journal of Food Engineering, 9, 275–304. [Google Scholar]
- Margeirsson B, Palsson H, Popov V, Gospavic R, Arason S, Sveinsdottir K and Jonsson MP, 2012a. Numerical modelling of temperature fluctuations in superchilled fish loins packaged in expanded polystyrene and stored at dynamic temperature, conditions. International Journal of Refrigeration, 35, 1318–1326. [Google Scholar]
- Margeirsson B, Lauzon HL, Palsson H, Popov V, Gospavic R, Jonsson MP, Sigurgisladottir S and Arason S, 2012b. Temperature fluctuations and quality deterioration of chilled cod (Gadus morhua) fillets packaged in different boxes stored on pallets under dynamic temperature conditions. International Journal of Refrigeration, 35, 187–201. [Google Scholar]
- Margeirsson B, Thordarson G and Gudjonsson A, 2017. Transport of chilled and superchilled rainbow trout in insulated containers and expanded polystyrene boxes. 4 pp.
- Marlard S, Doyen P and Grard T, 2019. Rapid multiparameters approach to differentiate fresh skinless sea bass (dicentrarchus labrax) fillets from frozen‐thawed ones. Journal of Aquatic Food Product Technology, 28, 253–262. [Google Scholar]
- Matos E, Silva TS, Tiago T, Aureliano M, Dinis MT and Dias J, 2011. Effect of harvesting stress and storage conditions on protein degradation in fillets of farmed gilthead seabream (Sparus aurata): a differential scanning calorimetry study. Food Chemistry, 126, 270–276. [Google Scholar]
- Meistro S, Pezzolato M, Muscolino D, Giarratana F, Baioni E, Panebianco A and Bozzetta E, 2016. Histology as a valid tool to differentiate fresh from frozen‐thawed marinated fish. Journal of Food Protection, 79, 1457–1459. [DOI] [PubMed] [Google Scholar]
- Mellor JD, 1983. Critical evaluation of thermophysical properties of foodstuffs and outline of future development In: Jowitt R, Escher F, Hallstrom B, Meffert HFT, Spiess WEL. and Vos G. (eds.). Physical Properties of Foods. Applied Science Publishers, New York. [Google Scholar]
- Miki H and Hayakawa K, 1996. An empirical equation for estimating food enthalpy in a freezing temperature range. Food Science and Technology‐Lebensmittel‐Wissenschaft & Technologie, 29, 659–663. [Google Scholar]
- Miles CA, Mayer Z, Morley MJ and HousÆka M, 1997. Estimating the initial freezing point of foods from composition data. International Journal of Food Science & Technology, 32, 389–400. [Google Scholar]
- Murakami EG and Okos MR, 1989. Measurement and prediction of thermal properties of foods In: Singh RP, Medina AG. (eds.). Food Properties and Computer Aided Engineering of Food Processing Systems. Kluwer Academic Publishers, New York. [Google Scholar]
- Murakami EG and Okos MR, 1996. Calculation of initial freezing point, effective molecular weight and unfreezable water of food materials from composition and thermal conductivity data. Journal of Food Process Engineering, 19, 301–320. [Google Scholar]
- Nilsson K and Ekstrand B, 1993. The effect of storage on ice and various freezing treatments on enzyme leakage in muscle‐tissue of rainbow‐trout (Oncorhynchus‐Mykiss). Zeitschrift Fur Lebensmittel‐Untersuchung Und‐Forschung, 197, 3–7. [DOI] [PubMed] [Google Scholar]
- Nott KP, Evans SD and Hall LD, 1999a. Quantitative magnetic resonance imaging of fresh and frozen‐thawed trout. Magnetic Resonance Imaging, 17, 445–455. [DOI] [PubMed] [Google Scholar]
- Nott KP, Evans SD and Hall LD, 1999b. The effect of freeze‐thawing on the magnetic resonance imaging parameters of cod and mackerel. Food Science and Technology‐Lebensmittel‐Wissenschaft & Technologie, 32, 261–268. [Google Scholar]
- Okeyo GO, Lokuruka MNI and Matofari J, 2009. Nutritional composition and shelflife of the lake victoria nile perch (lates niloticus) stored in ice. African Journal of Food, Agriculture, Nutrition and Development, (ISSN: 1684‐5358), 9, 9 pp. [Google Scholar]
- Ólafsdóttir A, Margeirsson B, Sveinsdóttir K, Arason S, Reynisson E and Martinsdóttir E (MATIS), 2012. Effect of superchilled processing of whole whitefish – pre‐rigor Skýrsla Matís 22‐12, ISSN 1670‐7192.
- Ottavian M, Fasolato L, Facco P and Barolo M, 2013. Foodstuff authentication from spectral data: toward a species‐independent discrimination between fresh and frozen‐thawed fish samples. Journal of Food Engineering, 119, 765–775. [Google Scholar]
- Ottavian M, Fasolato L, Serva L, Facco P and Barolo M, 2014. Data fusion for food authentication: fresh/frozen‐thawed discrimination in West African Goatfish (Pseudupeneus prayensis) Fillets. Food and Bioprocess Technology, 7, 1025–1036. [Google Scholar]
- Ottestad S, Høy M, Stevik A and Wold J, 2009. Prediction of ice fraction and fat content in superchilled salmon by non‐contact interactance near infrared imaging. Journal of Near Infrared Spectroscopy, 17, 77–87. [Google Scholar]
- Pan C, Chen SJ, Hao SX and Yang XQ, 2019. Effect of low‐temperature preservation on quality changes in Pacific white shrimp, Litopenaeus vannamei: a review. Journal of the Science of Food and Agriculture, 99, 6121–6128. [DOI] [PubMed] [Google Scholar]
- Parisi E, Salati F and Ceretto F, 1978. Ricerche qualitive e quantitative della succinico‐deidrogenasi in alcuni camioni di pesci freschi prelevati dal commercio e congelati. Annali della Facolta di Medicina Veterinaria di Torino XXV, 366–377. [Google Scholar]
- Pavlov A, Defernando GDG, Diaz O, Fernandez M, Lopez D, Ordonez JA and Delahoz I, 1994. Effect of freezing on the beta‐hydroxyacyl‐coa‐dehydrogenase (HADH) activity of fish meat. Zeitschrift Fur Lebensmittel‐Untersuchung Und‐Forschung, 198, 465–468. [Google Scholar]
- Pham QT, 1987. Calculation of bound water in frozen food. Journal of Food Science, 52, 210–212. [Google Scholar]
- Pham QT, 1996. Prediction of calorimetric properties and freezing time of foods from composition data. Journal of Food Engineering, 30, 95–107. [Google Scholar]
- Pham QT, 2014a. Heat transfer coefficient and physical properties. In: Food Freezing and Thawing Calculations; Springer Briefs in Food, Health, and Nutrition. 10.1007/978-1-4939-0557-7_2 [DOI]
- Pham QT, 2014b. Food Freezing and Thawing Calculations. In: SpringerBriefs in Food, Health, and Nutrition Series. 10.1007/978-1-4939-0557-7 [DOI]
- Pham QT, 2016. Freezing theory. Encyclopedia of Food and Health. 10.1016/B978-0-12-384947-2.00329-9 [DOI] [Google Scholar]
- Pink J, Naczk M and Pink D, 1998. Evaluation of the quality of frozen minced red hake: use of fourier transform infrared spectroscopy. Journal of Agricultural and Food Chemistry, 46, 3667–3672. [DOI] [PubMed] [Google Scholar]
- Popelka P, Nagy J, Pipova M, Marcincak S and Lenhardt L, 2014. Comparison of chemical, microbiological and histological changes in fresh, frozen and double frozen rainbow trout (Oncorhynchus mykiss). Acta Veterinaria Brno, 83, 157–161. [Google Scholar]
- Qin JW, Vasefi F, Hellberg RS, Akhbardeh A, Isaacs RB, Yilmaz AG, Hwang CS, Baek I, Schmidt WF and Kim MS, 2020. Detection of fish fillet substitution and mislabeling using multimode hyperspectral imaging techniques. Food Control, 114. [Google Scholar]
- Qu JH, Cheng JH, Sun DW, Pu HB, Wang QJ and Ma J, 2015. Discrimination of shelled shrimp (Metapenaeus ensis) among fresh, frozen‐thawed and cold‐stored by hyperspectral imaging technique. Lwt‐Food Science and Technology, 62, 202–209. [Google Scholar]
- Radhakrishnan S, 1997. Measurement of the thermal properties of seafood. Masters of Science in Biological Systems Engineering, Faculty of the Virginia Polytechnic. Vol 92 Institute and State University Blacksburg, Virginia. [Google Scholar]
- Rahman S, 2009. Food Properties Handbook, 2nd Edition CRC Press, Publisher, Boca Raton, FL: 859 pp. [Google Scholar]
- Rahman MS, Machado‐Velasco KM, Sosa‐Morales ME and Velez‐Ruiz JF, 2009. Freezing Point: Measurement, Data, and Prediction In: Rahman MS. (ed.). Food Properties Handbook. CRC Press; Taylor & Francis Group, LLC. [Google Scholar]
- Rehbein H, 1979. Development of an enzymatic method to differentiate fresh and sea‐frozen and thawed fish fillets.1. Comparison of the applicability of some enzymes of fish muscle. Zeitschrift Fur Lebensmittel‐Untersuchung Und‐Forschung, 169, 263–265. [DOI] [PubMed] [Google Scholar]
- Rehbein H and Cakli S, 2000. The lysosomal enzyme activities of fresh, cooled, frozen and smoked salmon fish species (Onchorhyncus keta and Salmo salar). Turkish Journal of Veterinary & Animal Sciences, 24, 103–108. [Google Scholar]
- Rehbein H, Kress G and Schreiber W, 1978. Enzymic method for differentiating thawed and fresh fish fillets. Journal of the Science of Food and Agriculture, 29, 1076–1082. [Google Scholar]
- Reis MM, Martinez E, Saitua E, Rodriguez R, Perez I and Olabarrieta I, 2017. Non‐invasive differentiation between fresh and frozen/thawed tuna fillets using near infrared spectroscopy (Vis‐NIRS). Lwt‐Food Science and Technology, 78, 129–137. [Google Scholar]
- Richelmi GB, Pezzolato M, Gib S, Gallina S, Decastelli L, Tarasco R, Abete MC, Ingravalle F, Serracca L, Pavino D, Vivaldi B, Riina MV, Acutis PL, Prearo M, Caramelli M and Bozzetta E, 2013. Pilot project to set up a control programme on fishery products. Italian Journal of Food Safety, 2, 86–90. [Google Scholar]
- Rotabakk BT, Bleie H, Stien LH and Roth B, 2014. Effect of blood removal protocol and superchilling on quality parameters of prerigor filleted farmed atlantic cod (Gadus morhua). Journal of Food Science, 79, E881–E886. [DOI] [PubMed] [Google Scholar]
- Salfi V, Fucetola F and Arata P, 1985. Optimized procedures of biochemical‐analysis for the differentiation between fresh and frozen thawed fish products.1 Test of mitochondrial aspartate‐aminotransferase. Industrie Alimentari, 24, 701–703. [Google Scholar]
- Sannia M, Serva L, Balzan S, Segato S, Novelli E and Fasolato L, 2019. Application of near‐infrared spectroscopy for frozen‐thawed characterization of cuttlefish (Sepia officinalis). Journal of Food Science and Technology‐Mysore, 56, 4437–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubring R, 1999. DSC studies on deep frozen fishery products. Thermochimica Acta, 337, 89–95. [Google Scholar]
- Şengör GFÜ, Alakavuk DÜ and Tosun ŞY, 2013. Effect of cooking methods on proximate composition, fatty acid composition, and cholesterol content of atlantic salmon (salmo salar). Journal of Aquatic Food Product Technology, 22, 160–167. [Google Scholar]
- Shan JJ, Wang X, Russel M, Zhao JB and Zhang YT, 2018. Comparisons of fish morphology for fresh and frozen‐thawed crucian carp quality assessment by hyperspectral imaging technology. Food Analytical Methods, 11, 1701–1710. [Google Scholar]
- Shumilina E, Moller IA and Dikiy A, 2020. Differentiation of fresh and thawed Atlantic salmon using NMR metabolomics. Food Chemistry, 314. [DOI] [PubMed] [Google Scholar]
- Simpson MV and Haard NF, 1987. Temperature acclimation of atlantic cod (gadus morhua) and its influence on freezing point and biochemical damage of postmortem muscle during storage at 0° and −3°C. Journal of Food Biochemistry, 11, 69–93. [Google Scholar]
- Sivertsen AH, Kimiya T and Heia K, 2011. Automatic freshness assessment of cod (Gadus morhua) fillets by Vis/Nir spectroscopy. Journal of Food Engineering, 103, 317–323. [Google Scholar]
- Skorpilova T, Sistkova I, Adamcova M, Pohunek V, Kruzik V and Sevcik R, 2019. Measuring citrate synthase activity as an enzymatic approach to the differentiation of chilled and frozen/thawed meat. Meat Science, 158. [DOI] [PubMed] [Google Scholar]
- Solimeo A, 2020a. Re: Data on the proximal composition of Nile perch fillet. Message to Winy Messens. 7 October 2020. E‐mail.
- Solimeo A, 2020b. Re: Data on the proximal composition of Nile perch fillet. Message to Winy Messens. 6 November 2020.
- Solimeo A, 2020c. Re: Use of picture from “Superchilling Fish Products ‐ Nile perch fillet” report in EFSA opinion. Message to Winy Messens. 4 December 2020.
- Stevik AM, Duun AS, Rustad T, O'Farrell M, Schulerud H and Ottestad S, 2010. Ice fraction assessment by near‐infrared spectroscopy enhancing automated superchilling process lines. Journal of Food Engineering, 100, 169–177. [Google Scholar]
- Sun ZB, Liang LM, Li JK, Liu XY, Sun J, Zou XB, Zuo M and Guo ZM, 2020. Rapid detection of Atlantic salmon multi‐quality based on impedance properties. Food Science & Nutrition, 8, 862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordarson G, Arason S and Karlsdóttir M (MATIS), 2017. Sub chilling of fish. Skýrsla Matís 06‐17, ISSN 1670‐7192, 65 pp.
- Tinacci L, Armani A, Guidi A, Nucera D, Shvartzman D, Miragliotta V, Coli A, Giannessi E, Stornelli MR, Fronte B, Di Iacovo F and Abramo F, 2018. Histological discrimination of fresh and frozen/thawed fish meat: European hake (Merluccius merluccius) as a possible model for white meat fish species. Food Control, 92, 154–161. [Google Scholar]
- Tolstorebrov I, Eikevik TM and Bantle M, 2014. A DSC study of phase transition in muscle and oil of the main commercial fish species from the North‐Atlantic. Food Research International, 55, 303–310. [Google Scholar]
- Tolstorebrov I, Eikevik T, Widell K and Nordtvedt T, 2019. Study of convective heat transfer and chilling kinetics of Atlantic salmon in refrigerated sea water: experiments and modelling. 25th International Congress of Refrigeration, Montreal, Canada: p. 8 pp. [Google Scholar]
- Tomaszewska‐Gras J, 2013. Melting and crystallization DSC profiles of milk fat depending on selected factors. Journal of Thermal Analysis and Calorimetry, 113, 199–208. [Google Scholar]
- Tryggvason RI, Margeirsson B, Karlsdóttir M, Ólafsdóttir A, Gudjónsdóttir M and Arason S, 2020. Effects of food container depth on the quality and yield of superchilled and iced Atlantic salmon. Packaging Technology and Science, 33, 289–302. [Google Scholar]
- Turenhout M, 2019. RE: Questions related to the study entitled “Superchilling Fish Products ‐ Nile perch fillet”. Message to Winy Messens. 16 July 2019.
- Uddin M and Okazaki E, 2004. Classification of fresh and frozen‐thawed fish by near‐infrared spectroscopy. Journal of Food Science, 69, C665–C668. [Google Scholar]
- Uddin M, Okazaki E, Turza S, Yumiko Y, Tanaka M and Fukuda Y, 2005. Non‐destructive visible/NIR spectroscopy for differentiation of fresh and frozen‐thawed fish. Journal of Food Science, 70, C506–C510. [Google Scholar]
- Valtýsdóttir K, Margeirsson B, Arason S, Lauzon H and Martinsdóttir E (MATIS), 2010. Guidelines for precooling of fresh fish during processing and choice of packaging with respect to temperature control in cold chains. Skýrsla Matís 40‐10, ISSN 1670‐7192.
- Van Acker S, 2020. RE: Amount of ice and fish packaged in expanded polystyrene boxes. Message to Winy Messens. 2 December 2020.
- Velioglu HM, Temiz HT and Boyaci IH, 2015. Differentiation of fresh and frozen‐thawed fish samples using Raman spectroscopy coupled with chemometric analysis. Food Chemistry, 172, 283–290. [DOI] [PubMed] [Google Scholar]
- Vidacek S, Medic H, Botka‐Petrak K, Nezak J and Petrak T, 2008. Bioelectrical impedance analysis of frozen sea bass (Dicentrarchus labrax). Journal of Food Engineering, 88, 263–271. [Google Scholar]
- Vidacek S, Janci T, Brdek Z, Udovicic D, Marusic N, Medic H, Petrak T and Lackovic I, 2012a. Differencing sea bass (Dicentrarchus labrax) fillets frozen in different conditions by impedance measurements. International Journal of Food Science and Technology, 47, 1757–1764. [Google Scholar]
- Vidacek S, Medic H, Marusic N, Tonkovic S and Petrak T, 2012b. Influence of different freezing regimes on bioelectrical properties of atlantic chub mackerel (scomber colias). Journal of Food Process Engineering, 35, 735–741. [Google Scholar]
- Wang T, Sveinsdottir K, Magnusson H and Martinsdottir E, 2008. Combined application of modified atmosphere packaging and superchilled storage to extend the shelf life of fresh cod (Gadus morhua) loins. Journal of Food Science, 73, S11–S19. [DOI] [PubMed] [Google Scholar]
- Wang WL, Chen WH, Tian HY and Liu Y, 2018. Detection of frozen‐thawed cycles for frozen tilapia (oreochromis) fillets using near infrared spectroscopy. Journal of Aquatic Food Product Technology, 27, 609–618. [Google Scholar]
- Washburn KE, Stormo SK, Skjelvareid MH and Heia K, 2017. Non‐invasive assessment of packaged cod freeze‐thaw history by hyperspectral imaging. Journal of Food Engineering, 205, 64–73. [Google Scholar]
- Wen J, Zeng L, Chen Z and Xu Y, 2016. Comparison of nutritional quality in fish maw product of croaker Protonibea diacanthus and perch Lates niloticus. Journal of Ocean University of China, 15, 726–730. [Google Scholar]
- Wu CH, Yuan CH, Ye XQ, Hu YQ, Chen SG and Liu DH, 2014. A critical review on superchilling preservation technology in aquatic product. Journal of Integrative Agriculture, 13, 2788–2806. [Google Scholar]
- Yoshioka K, 1983. Differentiation of freeze‐thawed fish from fresh fish by the determination of hematocrit value. Bulletin of the Japanese Society of Scientific Fisheries, 49, 149 pp. [Google Scholar]
- Yoshioka K and Kitamikado M, 1983. Differentiation of freeze‐thawed fish from fresh fish by the examination of medulla of crystalline lens. Bulletin of the Japanese Society of Scientific Fisheries, 49, 151 pp. [Google Scholar]
- Yu DW, Wu LY, Regenstein JM, Jiang QX, Yang F, Xu YS and Xia WS, 2019. Recent advances in quality retention of non‐frozen fish and fishery products: a review. Critical Reviews in Food Science and Nutrition. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Yoshioka K, Ueno R and Kitamikado M, 1988. Differentiation of frozen‐thawed fish from unfrozen fish by determination of neutral beta‐N‐acetylglucosaminidase activity in the blood. Nippon Suisan Gakkaishi, 54, 2143–2148. [Google Scholar]
- Zaidul ISM, Absar N, Kim SJ, Suzuki T, Karim AA, Yamauchi H and Noda T, 2008. DSC study of mixtures of wheat flour and potato, sweet potato, cassava, and yam starches. Journal of Food Engineering, 86, 68–73. [Google Scholar]
- Zhang LN, Shen HX and Luo YK, 2010. Study on the electric conduction properties of fresh and frozen‐thawed grass carp (Ctenopharyngodon idellus) and tilapia(Oreochromis niloticus). International Journal of Food Science and Technology, 45, 2560–2564. [Google Scholar]
- Zhou GH, Xu XL and Liu Y, 2010. Preservation technologies for fresh meat ‐ a review. Meat Science, 86, 119–128. [DOI] [PubMed] [Google Scholar]
- Zhu FL, Zhang DR, He Y, Liu F and Sun DW, 2013. Application of visible and near infrared hyperspectral imaging to differentiate between fresh and frozen‐thawed fish fillets. Food and Bioprocess Technology, 6, 2931–2937. [Google Scholar]
- Zielbauer BI, Franz J, Viezens B and Vilgis TA, 2016. Physical aspects of meat cooking: time dependent thermal protein denaturation and water loss. Food Biophysics, 11, 34–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Report Superchilling Fish Products – Nile perch (Lates niloticus) fillet (14‐09‐2018; coordinated by the Dutch Fish Importers Association).This study has been referred to as ‘Nile perch study’ in this scientific opinion
Additional information as referred to in Appendix A as provided by Mike Turenhout (Manager Dutch Fish Importers Association) on behalf of FIORITAL and the members of the Dutch Fish Importers Association by e‐mail on 16 July 2019 (Turenhout, 2019)


