Abstract
Eosinophils are rare granulocytes that belong to the innate arm of the immune system. This cell population is traditionally defined as a destructive and cytotoxic mediator in asthma and helminth infection. Limited data in transplantation has suggested that eosinophils play a similar role in potentiating deleterious organ inflammation and immunologic rejection. Contrary to this long-held notion recent data has uncovered the possibility that eosinophils play an alternative role in immune homeostasis, defense against a wide range of pathogens, as well as downregulation of deleterious inflammation. Specifically, translational data from small animal models of lung transplantation has demonstrated a critical role for eosinophils in the downregulation of alloimmunity. These findings shed new light on the unique immunologic features of the lung allograft and demonstrate that environmental polarization may alter the phenotype and function of leukocyte populations previously thought to be static in nature. In this review we provide an update on eosinophils in the homeostasis of the lung as well as other solid organs.
Origin of the Eosinophil
Eosinophils are granulocytes that develop in the bone marrow from common myeloid progenitors (human1) or granulocytic/macrophage progenitors (mouse2) into eosinophil lineage-committed progenitors3. Transcription factors GATA-1 and Xbp1, along with several others, coordinate the differentiation of these CD34+IL-5Rα+progenitors into fully differentiated eosinophils (CD34−IL-5Rα+CCR3+Siglec-F+ or Siglec-8+ human) in the bone marrow4. Differentiation and survival of eosinophils in humans and mice is highly dependent IL-5 and signaling through the IL-5Rα while their migration to other tissues is orchestrated by a group of chemokines known as eotaxins that bind the chemokine receptor 3 (CCR3)5. At homeostasis, once eosinophils exit the bone marrow, they have a half-life of ~1.8 days in the blood and migrate into the thymus, secondary lymphatics, adipose tissue, gastrointestinal tract (except esophagus), lung, skin, and uterus in both mice and man. Albeit their function in these locations are not fully defined3,6 and their half-life varies greatly per tissue. Recent studies indicate that eosinophils contribute to developmental organ remodeling, metabolic homeostasis, microbiome homeostasis, and act as sentinels for infection and cancer6–10.
Eosinophils in Asthma
The potential role of eosinophils in solid organ transplants may be inferred from a deeper understanding of allergic asthma. As a disease allergic asthma is defined by reversible airways hyperreactivity that is often associated with an increased mobilization of eosinophils into the lungs11–13. Increased absolute blood eosinophil count, sputum count of eosinophils >2–3% or presence of extensive eosinophil degranulation in lung biopsies are measures to define eosinophilic asthma and associate with disease severity11,13. Various stimuli control the generation, differentiation, migration and activation of eosinophils in allergic inflammation and asthma, including the Th2 associated cytokines IL-3, IL-4, IL-5, IL-13, IL-33 and GM-CSF, eotaxins, IgA and IgG, PAMPs and DAMPs; secreted by a wide variety of cells. To reduce the numbers of lung eosinophils in asthma, and thus their classic role as destructive cells14,15, many newly approved biologics are aimed at inhibiting eosinophil survival and recruitment pathways (e.g., IL-5/IL-5Ra or CCR3)16,17. Although eosinophil degranulation is considered a paradigm of noxious response by these cells that leads to pathology in asthma, the secondary granule proteins from eosinophils, eosinophil peroxidase (EPX), eosinophil cationic protein (ECP), major basic protein-1(MBP-1), and eosinophil derived neurotoxin (EDN) are recently being found to have immune modulating functions18. Moreover, eosinophils have the capacity for specific and targeted release of immune mediators from their secondary granules, such as IL-4, through piecemeal degranulation or release of microvesicles6. In agreement with this, in mouse models of severe asthma with extensive eosinophil degranulation, deficiencies in EPX or MBP-1 was insufficient to reduce pathology, while deficiencies in eosinophil-derived IL-13 blocked mucus production in the airways and airways hyperresponsiveness to methacholine challenge19. These studies exemplify previously underappreciated roles for eosinophils as not just destructive mediators, but cells that participate in the immune responses in the lung.
Eosinophils in infectious disease
Eosinophils modulate immunity against various infectious pathogens. These include infection with helminth20, nematode microfilariae21,22, viral infection23–25, and infection with bacteria26–28. The protective impact of eosinophils against infectious agents varies depending on the stage of infection and the type of infectious agent to include either killing of the agent or limitation of growth/symbiotic relationship. For example, eosinophil exposure to Clostridium rodentium induced killing through formation of eosinophil extracellular DNA traps and yet eosinophil exposure to Helicobacter pylori triggered immune mechanisms that permitted bacterial survival in the host28. In general direct killing mechanisms include release of granule proteins, enzymes and release of DNA extracellular traps29. Eosinophils are thus critical for IL-25 and IL-33/ILC-2 mediated protection against Clostridium difficile infection26,30 and the virulence capacity of C. difficile was enhanced under conditions that suppressed colonic eosinophilia26,27. Destruction of viruses by eosinophils occurs due to a mixed immune response. For example, influenza A and parainfluenza are targeted by Th1 activated and eosinophil-dependent effector functions to mediate iNOS-mediated killing of viruses, MHC class I antigen presentation of viral proteins and recruitment of CD8+ T cells23,24. Strikingly the induced accumulation of eosinophils in allergic asthma models has been shown to dramatically improve viral titer loads and improve viral killing in mice31,32. Together, these studies demonstrate eosinophils have protective functions in complex immune responses and perform their protective activities through a wide variety of mechanisms.
Eosinophils in Cancer
As in other immunological disease settings, the role of eosinophils in cancer is diverse and microenvironment dependent33–35. Eosinophils have been identified in several cancers of both epithelial and non-epithelial origin, including pancreatic cancer, glioblastoma, bladder, cervical, colorectal cancer, as well as melanoma, and lymphoma. The presence of tumor associated tissue eosinophils as a prognostic indicator of disease outcome varies significantly as eosinophils have been shown to have both pro- or anti-tumorigenic functions. In part this is likely due to the stage and type of cancer as well as the local microenvironment. For example in oral squamous cell carcinoma it has been proposed that early stage carcinoma, which is a Th1-type microenvironment, leads to anti-tumorigenic activation of eosinophils while later stages of carcinoma develop a Th2-type microenvironment and promote pro-tumorigenic potential of eosinophils36,37. In mouse models of colorectal cancer, where IFN-γ is increased, eosinophils directly kill tumors independent of CD8+ T cell functions38. This supports findings in colorectal patients where eosinophils correlate with better prognosis and fewer metastasis39. Alternatively in melanoma CD8+ T cell recruitment is enhanced by INF-γ and TNF-α activated eosinophils, resulting in melanoma tumor killing40. Thus in a complex disease such as cancer the role of the eosinophil varies based on the tumor microenvironment.
Eosinophils in immunosuppression
Despite their established role in cytotoxicity we, as well as others, have previously proposed that eosinophils have the capacity for broad dynamic differential activation and may vary their function drastically based on environmental cues9,41. The mechanisms of immune suppression by eosinophils that have been identified primarily in animal models are through either release of suppressive molecules and/or cell interactions with dendritic cells and lymphocytes. A homeostatic mechanism of immune suppression has been identified for tissue resident eosinophils in the lung that exhibit suppressive capacity to allergen induced Th2 immune responses42. Here, eosinophils mediate suppression through inhibition of dendritic cell activation, reducing activation of Th2 cells to allergen exposure in the airways. Conversely, we have shown that recruited eosinophils to the lung mediate suppression of Th1/Th17 cells through dendritic cells in allergen models of asthma10,43. The mechanistic diversity of eosinophil mediated immune suppression were further demonstrated in humans where a subset of CD16hi eosinophils were described to have suppressive capacity against T cells through a mechanism that is partially dependent on their upregulation of galectin-10 induced synapse formation44. Similar functions have been suggested in multiple myeloma45. In homeostatic conditions, eosinophil-T cell suppression has been demonstrated in the thymus, where eosinophils release indoleamine 2 3-dioxygenase to suppress Th1 polarization during thymic development46.
Eosinophils in solid organ transplantation
To date the role of eosinophils in organ transplantation remains poorly explored leading to confusion and controversy in the field. Early data generated in the 1980s suggested that eosinophils solely contribute to the rejection of most solid organs47–49. This was based on early studies associating peripheral blood eosinophilia to episodes of graft rejection50,51 as well as correlative studies linking tissue eosinophilia to graft rejection47,49,52,53. Recent studies continue these correlations, proposing eosinophil blood counts as a biomarker for acute liver rejection54,55. Yet a recent meta-analysis of >800 patients and more than 1000 sample points indicated that blood eosinophil counts are highly heterogenous and have only a 50% sensitivity for predicting acute liver rejection56. Despite the fact that limited mechanistic evidence was provided by any of these studies the destructive role of eosinophils was inferred from their increase at the time of rejection. In addition the predominance of eosinophil accumulation in the setting of irreversible graft dysfunction, compared with reversible graft damage, was used to further justify the role of eosinophils as mediators of graft rejection48,49. In many regards the view of eosinophils in graft rejection were similar to those of eosinophils in asthma; a sign and possible mediator of pathological inflammation and disease.
Eosinophils have been shown to influence Th2 polarization of the microenvironment57,58. Traditional data has suggested that Th1 polarization of the graft environment results in graft rejection while Th2 polarization results in tolerance and amelioration of the rejection response. For example pediatric liver allograft recipients with a predominantly a Th2 polarized cytokine profile have significantly reduced rates of rejection compared to patients with a Th1 cytokine profile59. Similarly, in renal transplant recipients it is clear that T cell clones from patients with chronic graft rejection produce higher levels of the Th1 associated cytokine IFN-γ, while T cells from accepting patients produce higher levels of IL-1060. Nevertheless such a seemingly clear cut notion is confounded by the demonstrations that adoptive transfer of Th2 polarized T cells can mediate graft rejection of both skin and heart grafts in Rag−/− or SCID mice even in the absence of classic cytotoxicity in vitro61–63. In addition Th2 polarized graft rejection demonstrates severe infiltration of eosinophils64. Taken together such data suggests that eosinophils, and their Th2 polarizing environmental influence, could play a role in the rejection of tissues and organs.
Conventional methods of achieving eosinophils depletion, such as antibody mediated blockade of IL-5, failed to ameliorate the rejection of pig pancreatic proislets transplanted to mice, notwithstanding the effectiveness of the treatment in drastically reducing eosinophil infiltration into the xenografts65. This finding led the authors to question the clinical practice of using the infiltration of eosinophils into graft biopsies as a prognostic indicator for graft rejection and highlighted the need for a better definition and understanding of the role of eosinophils in graft tolerance and rejection. These findings led to the speculation that eosinophils might not primarily contribute to graft rejection but instead may play a secondary or accessory role in this process65. Alternatively it was proposed that eosinophils might essentially be innocent bystanders in some transplant models47. This notion was further supported in a model of allogeneic cardiac transplantation, where eosinophil depletion had a very minor impact on the survival of fully MHC mismatched heart allografts in the absence of CD8+ T-cells66. This data suggested no role or a CD8+ T cell-dependent role for eosinophil-mediated immunoregulation.
Additional confusion regarding the role of eosinophils in cardiac graft rejection arose from recent studies demonstrating that acute cellular rejection, as well as antibody mediated rejection, was associated with low level of blood eosinophils67. These authors put forth a possibility that an immunologic state of quiescence, manifested by a higher eosinophil counts, maybe involved in preventing immunologic graft rejection67. This study therefore opened the possibility that eosinophils might be playing a role in transplantation tolerance and may not unequivocally contribute to graft rejection. Nevertheless low numbers of eosinophils in most solid organs, such as hearts kidneys and livers, makes their role difficult to decipher.
Eosinophils in lung transplantation
Unlike most transplantable solid organs lung alloimmune responses occur within the graft independent of secondary lymphoid tissue68–70. Lungs also contain a large population of eosinophils, making them and ideal organ to investigate the role of this granulocyte in the alloimmune response. Observational data has been interpreted to suggest that accumulation of tissue eosinophils is associated with acute lung allograft rejection. Such a notion is based on their presence in bronchoalveolar lavage fluid (BAL) during acute rejection episodes. Nevertheless the significance of such eosinophilia in pathobiology of lung allograft rejection was not well defined and unclear52,53,71.
In order to establish clarity regarding the role of eosinophils in lung allograft homeostasis we utilized a clinically relevant murine model of left lung transplantation72,73. We initially depleted eosinophils from lung graft recipients through either neutralization of IL-5 or targeted deletion in transgenic mice, where the diphtheria-toxin receptor is expressed under the control of eosinophil peroxidase promoter (iPHIL mice)74,75. Interestingly eosinophil depletion did not ameliorate rejection but actually prevented co-stimulatory blockade (CSB)-mediated graft acceptance and accentuated rejection in the absence of immunosuppression (Figure 1). Unlike previous reports, that demonstrated an association of Th2 cytokines with the tolerance76–78, we demonstrated that both CSB-treated accepting as well non-immunosuppressed rejecting grafts demonstrate a Th1 polarized microenvironment74,75. This was marked by increased expression of IFN-γ and TNF-α without significant impact on Th2-associated cytokines such as IL-4, GM-CSF and IL-3374. As suspected more pronounced Th1 polarization was evident in rejecting lungs that were not treated with immunosuppression but even in the presence of CSB, IFN-γ and TNF-α -mediated responses predominated74,75. Our observation supports the unique role of pro-inflammatory mediators in promoting lung allograft tolerance79 and reaffirms the uniqueness of lung-specific immune responses68,70,80–82. In line with Th1 polarization of the lung allograft microenvironment, eosinophils from both accepting and rejecting lung grafts were polarized to a Th1 (or E1)-like phenotype74,75. The Th1 signature of the lung allograft eosinophil differs from the Th2 (or E2)-type associated with asthma and allergic inflammation57.
Figure 1. Eosinophil mediated suppression of T cell alloimmune responses.
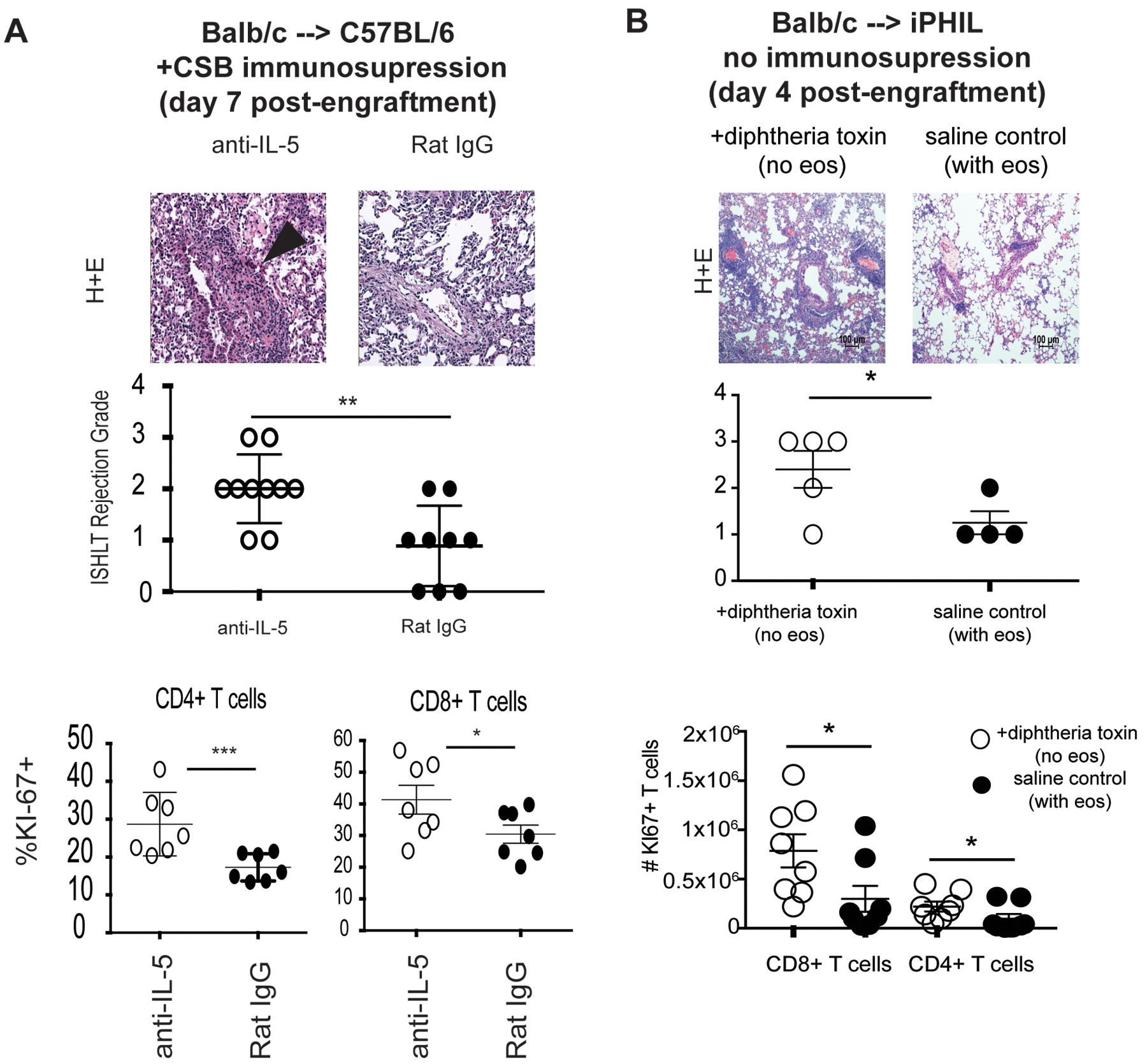
(A) ISHLT A rejection grade and KI67 (expressed as %) in CD8+ and CD4+ graft resident T cells seven days after engraftment of Balb/c lungs to C57BL/6 recipients with eosinophil depletion accomplished by IL-5 neutralization. All grafts were treated with co-stimulatory blockade immunosuppression affected by CTLA-4 Ig and anti-CD40L (clone MR1). (B) ISHLT A rejection grade and T cell proliferation (expressed as total number of KI67+ T cells) in Balb/c lungs engrafted to iPHIL mice on a C57BL/6 background with eosinophil depletion accomplished by diphtheria toxin administration. (reproduced from74,75 *** p<.001, **p<.01, *p<.05)
We explored the regulatory capacity of such Th1 polarized eosinophils. Indeed Th1 polarization was a key component to eosinophil regulatory capacity as amelioration of the Th1 phenotype blocked eosinophil-mediated suppression74. Interestingly we and others have previously described the expression of inducible nitric oxide synthase (iNOS) to be a key component of lung-specific immunoregulation74,83,84. Th1 polarization was characterized by upregulation of iNOS in lung resident eosinophils. In fact eosinophils were the dominant and sole producers of iNOS in the lung graft74. To this end we uncovered that the capacity of the Th1 polarized eosinophils to induce T cell mediated immunosuppression was dependent on their ability to upregulate iNOS and form a synapse with CD8+ T cells through PD-1/PD-L1 interactions75. Since PD-L1 expression is also upregulated by Th1 cytokines85 we therefore proposed a unique and possibly lung allograft-specific feedback loop whereby CD8+ T cells produce IFN-γ that drives eosinophils to express PD-L1 and iNOS which leads to synapse formation with CD8+ T cells to prevent effector differentiation (Figure 2). Depletion or neutralization of any component in this feedback loop prevents lung graft acceptance74,75,82. A similar mechanism for the downregulation of IFN-γ dependent Th1 immunity and eosinophil-T cell interactions has been reported in a gastrointestinal bacterial infection model28, as well as graft vs. host disease44, and in the downregulation of anti-tumor immunity against multiple myeloma45. Importantly, in the gastrointestinal bacterial infection model it was shown that the eosinophil dependent feedback inhibition of T cell mediated Th1 responses is only partially dependent on PD-L1. This reinforces our observation that in the lung PD-L1 is only necessary for the establishment of contact and immunological synapse between the eosinophil and CD8+ T cell while the amelioration of T cell activation and alloimmunity is iNOS dependent28,75.
Figure 2. Mechanism of eosinophil-CD8+ T cell tolerogenic feedback loops in the lung allograft.
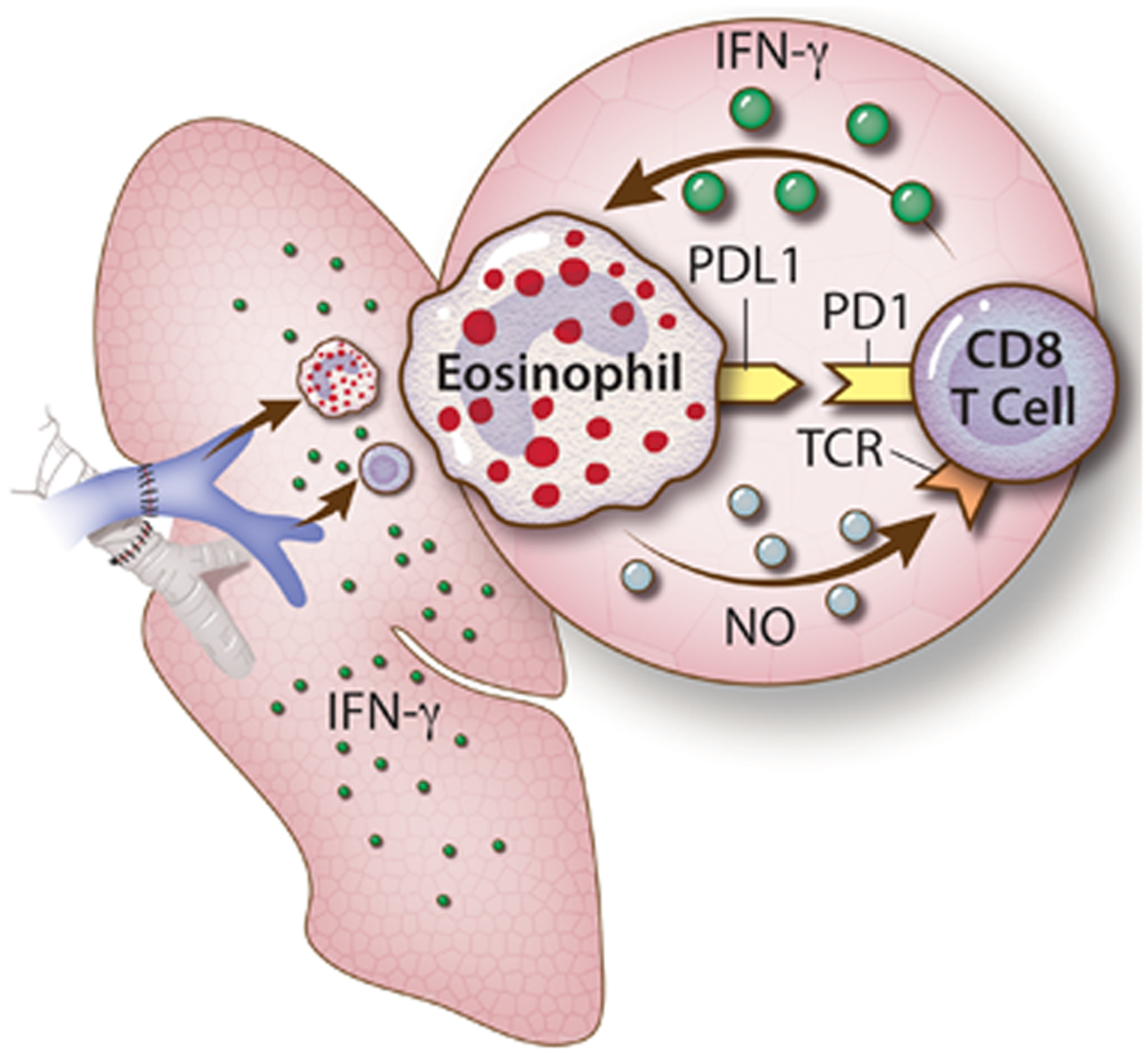
Effector CD8+ T cells are the major culprits in allogeneic lung graft rejection. Naïve CD8+ T cells become activated on experiencing alloantigen. This process is associated with TCR engagement and upregulation of differentiation associated molecules, such as PD-1, and the release of Th1 associated cytokines (IFN-γ and TNF-α). The Th1 cytokines released in the microenvironment milieu causes the polarization of eosinophils and their upregulation of PD-L1 and iNOS. PD-L1 binds to PD-1 to provide an immunologic synapse between eosinophil and CD8+ T cells while iNOS catalyzes the synthesis of nitric oxide (NO) that inhibits TCR signaling in a feedback loop (reproduced from data described in74,75,82).
Despite this many questions remain. While we have provided strong data regarding the supporting role of eosinophils for initial graft acceptance, their role in chronic graft fibrosis is unknown. Eosinophils were identified in 14 out of 15 examined allograft nephrectomies performed for obliterative arteriopathy. In the same report eosinophil conditioned media stimulated smooth muscle proliferation suggesting a direct link between eosinophil infiltration and chronic vascular rejection86. In lung allograft recipients increased BAL and blood eosinophilia were associated with worse graft outcome and chronic lung allograft dysfunction (CLAD). In fact BAL eosinophilia has been used to predict a form of graft fibrosis defined as restrictive CLAD (rCLAD)71,87. In murine models the role of eosinophil-mediated fibrosis has been linked to their promotion of collagen expression in epithelial cells through production of TGF-β after therapeutic radiation in the gut, another mucosal barrier organ88. In asthma models eosinophils were demonstrated as dispensable for airway hyperresponsiveness and mucous secretion, but critical for peri-bronchial collagen deposition89. Thus our recent proposal to facilitate lung allograft acceptance through increased eosinophil migration into the lung75 might come with a price of long-term CLAD. These and other aspects of eosinophil biology surrounding lung transplantation are thus prime areas for investigation.
Summary
While traditional dogma suggests that eosinophils are uniquely destructive in their actions, and contribute solely to allograft rejection, our recently expanded understanding of their biology puts this notion into question. It is now evident that eosinophils are uniquely suited to exert immunomodulatory functions largely influenced by their microenvironment. While their role in lung organ transplantation is still unclear, recent work from our laboratory has demonstrated that under Th1 pro-inflammatory conditions eosinophil polarization plays a critical part in a feedback loop that controls excessive inflammation and tissue damage as well as tolerance. In certain Th1 environments eosinophils maintain the moderate inflammatory state required for immunosuppression-mediated tolerance, but may also enhance the survival of some pathogens and aggravate infections. Conversely in other contexts eosinophil Th1 polarization may induce pathogen killing. This recent understanding of the immunomodulatory role of eosinophils in the lung and gastrointestinal track situates eosinophils as potential guardians whose efforts to protect their host tissues could sometimes be excessive and lead to detrimental side effects. Nevertheless the potential for manipulating the eosinophil to facilitate organ specific tolerance offers exciting and novel avenues of translational investigation and deserves further work.
Acknowledgments
ASK Supported by PO1 AI116501, R01 AI145108-01, 1 I01 IBX004588A
Abbreviations:
- iPHIL mice
mice expressing diphtheria-toxin receptor under the control of eosinophil peroxidase promoter
- CLAD
chronic lung allograft dysfunction
- iNOS
inducible nitric oxide synthase
- CSB
co-stimulatory blockade
- PAMPs
pathogen associated molecular patterns
- DAMPs
danger associated molecular patterns
- BAL
bronchioalveolar lavage
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Mori Y, Iwasaki H, Kohno K, et al. Identification of the human eosinophil lineage-committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J Exp Med. 2009;206(1):183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki H, Mizuno S, Mayfield R, et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med. 2005;201(12):1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JJ, Jacobsen EA, Ochkur SI, et al. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130(3):572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouffi C, Kartashov AV, Schollaert KL, et al. Transcription Factor Repertoire of Homeostatic Eosinophilopoiesis. J Immunol. 2015;195(6):2683–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. [DOI] [PubMed] [Google Scholar]
- 6.Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17(12):746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochner BS. The eosinophil: For better or worse, in sickness and in health. Ann Allergy Asthma Immunol. 2018;121(2):150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40(4):563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120(19):3882–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr TF, Zeki AA, Kraft M. Eosinophilic and Noneosinophilic Asthma. American journal of respiratory and critical care medicine. 2018;197(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119(6):1303–1310; quiz 1311–1302. [DOI] [PubMed] [Google Scholar]
- 13.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med. 2001;344(5):350–362. [DOI] [PubMed] [Google Scholar]
- 14.Walsh GM, Al-Rabia M, Blaylock MG, Sexton DW, Duncan CJ, Lawrie A. Control of eosinophil toxicity in the lung. Curr Drug Targets Inflamm Allergy. 2005;4(4):481–486. [DOI] [PubMed] [Google Scholar]
- 15.Kariyawasam HH, Robinson DS. The eosinophil: the cell and its weapons, the cytokines, its locations. Semin Respir Crit Care Med. 2006;27(2):117–127. [DOI] [PubMed] [Google Scholar]
- 16.Grozdanovic M, Laffey KG, Abdelkarim H, et al. Novel peptide nanoparticle-biased antagonist of CCR3 blocks eosinophil recruitment and airway hyperresponsiveness. J Allergy Clin Immunol. 2019;143(2):669–680 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh GM. Biologics targeting IL-5, IL-4 or IL-13 for the treatment of asthma - an update. Expert Rev Clin Immunol. 2017;13(2):143–149. [DOI] [PubMed] [Google Scholar]
- 18.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289(25):17406–17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen EA, Ochkur SI, Doyle AD, et al. Lung Pathologies in a Chronic Inflammation Mouse Model Are Independent of Eosinophil Degranulation. American journal of respiratory and critical care medicine. 2017;195(10):1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Appleton JA. Eosinophils in Helminth Infection: Defenders and Dupes. Trends in parasitology. 2016;32(10):798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadman ET, Thysse KA, Bearder S, et al. Eosinophils are important for protection, immunoregulation and pathology during infection with nematode microfilariae. PLoS Pathog. 2014;10(3):e1003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons JE, Rothenberg ME, Lawrence RA. Eotaxin-1-regulated eosinophils have a critical role in innate immunity against experimental Brugia malayi infection. Eur J Immunol. 2005;35(1):189–197. [DOI] [PubMed] [Google Scholar]
- 23.Drake MG, Bivins-Smith ER, Proskocil BJ, et al. Human and Mouse Eosinophils Have Antiviral Activity against Parainfluenza Virus. Am J Respir Cell Mol Biol. 2016;55(3):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeMessurier KS, Samarasinghe AE. Eosinophils: Nemeses of Pulmonary Pathogens? Curr Allergy Asthma Rep. 2019;19(8):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravin KA, Loy M. The Eosinophil in Infection. Clin Rev Allergy Immunol. 2016;50(2):214–227. [DOI] [PubMed] [Google Scholar]
- 26.Buonomo EL, Cowardin CA, Wilson MG, Saleh MM, Pramoonjago P, Petri WA Jr., Microbiota-Regulated IL-25 Increases Eosinophil Number to Provide Protection during Clostridium difficile Infection. Cell Rep. 2016;16(2):432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowardin CA, Buonomo EL, Saleh MM, et al. The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nature microbiology. 2016;1(8):16108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold IC, Artola-Boran M, Tallon de Lara P, et al. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J Exp Med. 2018;215(8):2055–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee M, Lacy P, Ueki S. Eosinophil Extracellular Traps and Inflammatory Pathologies-Untangling the Web! Front Immunol. 2018;9:2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frisbee AL, Saleh MM, Young MK, et al. IL-33 drives group 2 innate lymphoid cell-mediated protection during Clostridium difficile infection. Nature Communications. 2019;10(1):2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Percopo CM, Dyer KD, Ochkur SI, et al. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123(5):743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samarasinghe AE, Melo RC, Duan S, et al. Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. J Immunol. 2017;198(8):3214–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon SCS, Utikal J, Umansky V. Opposing roles of eosinophils in cancer. Cancer Immunol Immunother. 2019;68(5):823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatault S, Legrand F, Delbeke M, Loiseau S, Capron M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61(9):1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichman H, Karo-Atar D, Munitz A. Emerging Roles for Eosinophils in the Tumor Microenvironment. Trends Cancer. 2016;2(11):664–675. [DOI] [PubMed] [Google Scholar]
- 36.Looi LM. Tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. A pathologic study of 422 primary and 138 metastatic tumors. Cancer. 1987;59(3):466–470. [DOI] [PubMed] [Google Scholar]
- 37.Yellapurkar S, Natarajan S, Boaz K, et al. Tumour-Associated Tissue Eosinophilia in Oral Squamous Cell Carcinoma- A Boon or a Bane? J Clin Diagn Res. 2016;10(4):ZC65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichman H, Itan M, Rozenberg P, et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol Res. 2019;7(3):388–400. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88(7):1544–1548. [PubMed] [Google Scholar]
- 40.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16(6):609–617. [DOI] [PubMed] [Google Scholar]
- 41.Abdala-Valencia H, Coden ME, Chiarella SE, et al. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J Leukoc Biol. 2018;104(1):95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126(9):3279–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187(11):6059–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lingblom C, Andersson J, Andersson K, Wenneras C. Regulatory Eosinophils Suppress T Cells Partly through Galectin-10. J Immunol. 2017;198(12):4672–4681. [DOI] [PubMed] [Google Scholar]
- 45.Driscoll J, Aslam I, Malek E. Eosinophils Upregulate PD-L1 and PD-L2 Expression to Enhance the Immunosuppressive Microenvironment in Multiple Myeloma. Blood. 2017;130(Suppl 1):4417–4417. [Google Scholar]
- 46.Odemuyiwa SO, Ghahary A, Li Y, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173(10):5909–5913. [DOI] [PubMed] [Google Scholar]
- 47.Goldman M, Le Moine A, Braun M, Flamand V, Abramowicz D. A role for eosinophils in transplant rejection. Trends Immunol. 2001;22(5):247–251. [DOI] [PubMed] [Google Scholar]
- 48.Kormendi F, Amend WJ Jr., The importance of eosinophil cells in kidney allograft rejection. Transplantation. 1988;45(3):537–539. [DOI] [PubMed] [Google Scholar]
- 49.Nagral A, Ben-Ari Z, Dhillon AP, Burroughs AK. Eosinophils in acute cellular rejection in liver allografts. Liver Transpl Surg. 1998;4(5):355–362. [DOI] [PubMed] [Google Scholar]
- 50.Lautenschlager I, von Willebrand E, Hayry P. Blood eosinophilia, steroids, and rejection. Transplantation. 1985;40(4):354–357. [DOI] [PubMed] [Google Scholar]
- 51.Shalev O, Rubinger D, Barlatzky Y, Kopolovic J, Drukker A. Eosinophilia associated with acute allograft kidney rejection. Nephron. 1982;31(2):182–183. [DOI] [PubMed] [Google Scholar]
- 52.Dosanjh AK, Elashoff D, Kawalek A, Moss RB, Esrig S. Activation of eosinophils in the airways of lung transplantation patients. Chest. 1997;112(5):1180–1183. [DOI] [PubMed] [Google Scholar]
- 53.Mogayzel PJ Jr., Yang SC, Wise BV, Colombani PM. Eosinophilic infiltrates in a pulmonary allograft: a case and review of the literature. J Heart Lung Transplant. 2001;20(6):692–695. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Peralvarez M, Germani G, Tsochatzis E, et al. Predicting severity and clinical course of acute rejection after liver transplantation using blood eosinophil count. Transpl Int. 2012;25(5):555–563. [DOI] [PubMed] [Google Scholar]
- 55.Wang GY, Li H, Liu W, et al. Elevated blood eosinophil count is a valuable biomarker for predicting late acute cellular rejection after liver transplantation. Transplant Proc. 2013;45(3):1198–1200. [DOI] [PubMed] [Google Scholar]
- 56.Krenzien F, Keshi E, Splith K, et al. Diagnostic Biomarkers to Diagnose Acute Allograft Rejection After Liver Transplantation: Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. Front Immunol. 2019;10:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobsen EA, Doyle AD, Colbert DC, et al. Differential activation of airway eosinophils induces IL-13-mediated allergic Th2 pulmonary responses in mice. Allergy. 2015;70(9):1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobsen EA, Ochkur SI, Pero RS, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205(3):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ganschow R, Nolkemper D, Hoffmann T, et al. Influence of Th1 and Th2 cytokine patterns on graft acceptance in pediatric liver transplantation. Transplant Proc. 1999;31(1–2):465–466. [DOI] [PubMed] [Google Scholar]
- 60.Kist-van Holthe JE, Gasser M, Womer K, et al. Regulatory functions of alloreactive Th2 clones in human renal transplant recipients. Kidney international. 2002;62(2):627–631. [DOI] [PubMed] [Google Scholar]
- 61.VanBuskirk AM, Wakely ME, Orosz CG. Transfusion of polarized TH2-like cell populations into SCID mouse cardiac allograft recipients results in acute allograft rejection. Transplantation. 1996;62(2):229–238. [DOI] [PubMed] [Google Scholar]
- 62.VanBuskirk AM, Wakely ME, Orosz CG. Acute rejection of cardiac allografts by noncytolytic CD4(+) T cell populations. Transplantation. 1996;62(2):300–302. [DOI] [PubMed] [Google Scholar]
- 63.Zelenika D, Adams E, Mellor A, et al. Rejection of H-Y disparate skin grafts by monospecific CD4+ Th1 and Th2 cells: no requirement for CD8+ T cells or B cells. J Immunol. 1998;161(4):1868–1874. [PubMed] [Google Scholar]
- 64.Matesic D, Valujskikh A, Pearlman E, Higgins AW, Gilliam AC, Heeger PS. Type 2 immune deviation has differential effects on alloreactive CD4+ and CD8+ T cells. J Immunol. 1998;161(10):5236–5244. [PubMed] [Google Scholar]
- 65.Simeonovic CJ, Townsend MJ, Wilson JD, et al. Eosinophils are not required for the rejection of neovascularized fetal pig proislet xenografts in mice. J Immunol. 1997;158(5):2490–2499. [PubMed] [Google Scholar]
- 66.Braun MY, Desalle F, Le Moine A, et al. IL-5 and eosinophils mediate the rejection of fully histoincompatible vascularized cardiac allografts: regulatory role of alloreactive CD8(+) T lymphocytes and IFN-gamma. Eur J Immunol. 2000;30(5):1290–1296. [DOI] [PubMed] [Google Scholar]
- 67.Arbon KS, Albers E, Kemna M, Law S, Law Y. Eosinophil count, allergies, and rejection in pediatric heart transplant recipients. J Heart Lung Transplant. 2015;34(8):1103–1111. [DOI] [PubMed] [Google Scholar]
- 68.Li W, Bribriesco AC, Nava RG, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5(5):544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi T, Hsiao HM, Tanaka S, et al. PD-1 expression on CD8(+) T cells regulates their differentiation within lung allografts and is critical for tolerance induction. Am J Transplant. 2018;18(1):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gelman AE, Li W, Richardson SB, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verleden SE, Ruttens D, Vandermeulen E, et al. Elevated bronchoalveolar lavage eosinophilia correlates with poor outcome after lung transplantation. Transplantation. 2014;97(1):83–89. [DOI] [PubMed] [Google Scholar]
- 72.Okazaki M, Krupnick AS, Kornfeld CG, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7(6):1672–1679. [DOI] [PubMed] [Google Scholar]
- 73.Krupnick AS, Lin X, Li W, et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Onyema OO, Guo Y, Wang Q, et al. Eosinophils promote inducible NOS-mediated lung allograft acceptance. JCI Insight. 2017;2(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Onyema OO, Guo Y, Mahgoub B, et al. Eosinophils downregulate lung alloimmunity by decreasing TCR signal transduction. JCI Insight. 2019;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donckier V, Wissing M, Bruyns C, et al. Critical role of interleukin 4 in the induction of neonatal transplantation tolerance. Transplantation. 1995;59(11):1571–1576. [PubMed] [Google Scholar]
- 77.Gao Q, Chen N, Rouse TM, Field EH. The role of interleukin-4 in the induction phase of allogeneic neonatal tolerance. Transplantation. 1996;62(12):1847–1854. [DOI] [PubMed] [Google Scholar]
- 78.Chen N, Gao Q, Field EH. Prevention of Th1 response is critical for tolerance. Transplantation. 1996;61(7):1076–1083. [DOI] [PubMed] [Google Scholar]
- 79.Sanchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gelman AE, Okazaki M, Lai J, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180(7):4754–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gelman AE, Okazaki M, Sugimoto S, et al. CCR2 regulates monocyte recruitment as well as CD4 T1 allorecognition after lung transplantation. Am J Transplant. 2010;10(5):1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krupnick AS, Lin X, Li W, et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest. 2014;124(3):1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aldridge JR Jr., Moseley CE, Boltz DA, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A. 2009;106(13):5306–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Speyer CL, Neff TA, Warner RL, et al. Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am J Pathol. 2003;163(6):2319–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100(9):5336–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nolan CR, Saenz KP, Thomas CA 3rd, Murphy KD. Role of the eosinophil in chronic vascular rejection of renal allografts. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1995;26(4):634–642. [DOI] [PubMed] [Google Scholar]
- 87.Verleden SE, Ruttens D, Vandermeulen E, et al. Predictors of survival in restrictive chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2016;35(9):1078–1084. [DOI] [PubMed] [Google Scholar]
- 88.Takemura N, Kurashima Y, Mori Y, et al. Eosinophil depletion suppresses radiation-induced small intestinal fibrosis. Sci Transl Med. 2018;10(429). [DOI] [PubMed] [Google Scholar]
- 89.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305(5691):1776–1779. [DOI] [PubMed] [Google Scholar]


