Abstract
Background
Although the United States is among the countries with the highest mortalities of COVID-19, inadequate geospatial studies have analyzed the disease mortalities across the nation.
Methods
In this county-level study, we investigated age-adjusted co-mortalities of 20 diseases, including cardiovascular, cancer, drug and alcohol disorder, respiratory and infectious diseases with COVID-19 over the first ten months of epidemic. One-way analysis of variance was applied to the Local Moran's I classes (High-High and Low-Low clusters, and non-significant counties of COVID-19) to examine whether the mean mortality measures of covariates that fall into the classes are significantly different. Moreover, a mixed-effects multinomial logistic regression model was employed to estimate the effects of mortalities on COVID-19 classes.
Results
Results showed that the distribution of COVID-19 case fatality ratio (CFR) and mortality rate co-occurrence of High-High clusters were mainly concentrated in Louisiana, Connecticut, and New Jersey. Also, positive associations were observed between High-High cluster of COVID-19 CFR and Asthma (OR = 4.584, 95 % Confidence Interval (CI): 2.583–8.137), Hepatitis (OR = 5.602, CI: 1.265–24.814) and Leukemia (OR = 2.172, CI: 1.518–3.106) mortality rates compared to the non-significant counties, respectively.
Conclusions
Our results indicated that counties with higher mortality of some cancers and respiratory diseases are more vulnerable to fall into clusters of HH COVID-19 CFR. Future vaccine allocation and more medical professionals and treatment equipment should be a priority to those High-High clusters.
Keywords: ANOVA, COVID-19 mortalities, Mixed effects model, Spatial analysis, United States
1. Introduction
SARS-CoV-2, the pathogen that causes coronavirus disease (COVID-19), has affected almost 191 countries and territories, leading to over 62 million infected confirmed cases and nearly 1.45 million deaths worldwide as of November 28, 2020 (Johns Hopkins University & Medicine, 2020). It is anticipated that the potential COVID-19 mortality of the pandemic would be approximately 6% of the worldwide population, accounting for nearly half a billion global deaths (Grech, 2020). Preliminary predictions indicate that individuals who contract COVID-19 may experience years of lost life of approximately 13.1 and 10.5 years for males and females, respectively (Hanlon et al., 2020).
The United States continues to lead in the number of cases and deaths globally, with almost 13.2 million positive cases and nearly 266,000 deaths as of November 28, 2020 (Kaiser Family Foundation, 2020). As of November 28, 2020, this country has reported a mortality rate of 80.95 cases per 100,000 population and the observed cases-fatality ratio (CFR) (i.e., the proportion of recorded death over the confirmed cases) of almost 2.0 %, ranking 8th country with highest mortality rates worldwide (Johns Hopkins University & Medicine, 2020).
Studying CFR is essential to assess the severity of COVID-19, for it might present variability associated with particular comorbidities (Rodriguez-Morales et al., 2020), geospatial distribution (Dahal, Mizumoto, Rothenberga, & Chowella, 2020), and disease transmission (Peeri et al., 2020). Rodriguez-Morales et al. (2020) performed a systematic review and meta-analysis of clinical, laboratory, and imaging pertaining to COVID-19 in China and Australia. They found that almost 20 % of individuals that were admitted to ICU presented with comorbidities (i.e., hypertension, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, malignancies, and chronic liver disease). Also, a CFR of 13 % was calculated for 632 hospitalized patients. In Spain, Dahal et al. (2020) analyzed the spatial variability of COVID-19 in 19 areas (2 autonomous cities and 17 independent communities) and estimated respective time-delay adjusted CFR. They found the highest CFR in Madrid (38.4 %) and lowest CFR for Murcia (4.0 %), and an average adjusted CFR of 23.9 % for Spain. Wilder-Smith, Chiew, and Lee (2020) compared COVID-19 to Severe Acute Respiratory Syndrome (SARS). In contrasts with the preliminary data from China that demonstrated low COVID-19 CFR (<2%) when compared to SARS CFR (10 %), their results suggest that the continual emergence of COVID-19 cases is expected as COVID-19 can result in more fatalities than SARS.
Several studies have reported mortality of COVID-19 concerning other diseases. Shi et al. (2020) examined the mortality among COVID-19 hospitalized patients and the association with cardiac injury. Their findings indicated that hospital mortality among the patients is positively associated with the cardiac injury (P < 0.001). Moreover, 82 out of 416 hospitalized patients (19.7 %) presented with both cardiac injury and COVID-19. Caramelo, Ferreira, and Oliveiros (2020) conducted a preliminary analysis of COVID-19 mortality in China. Their analysis demonstrated a relatively high CFR in patients with various comorbidities. Some of the explanatory variables were: cardiovascular disease (CFR: 8.97 %), cancer (CFR: 5.37 %), chronic respiratory disease (CFR: 5.97 %), hypertension (CFR: 5.74 %), and diabetes (CFR: 6.78 %). Ssentongo, Ssentongo, Heilbrunn, Ba, and Chinchilli (2020) conducted a systematic review and meta-analysis to examine the possible association of pre-existing conditions and COVID-19 mortality. Of 25 studies, they selected 11 comorbidities and concluded that cardiovascular disease, hypertension, diabetes, congestive heart failure, chronic kidney disease, and cancer are significantly associated with elevated COVID-19 mortalities. In a nested case-control study in Mainland China, Gu et al. (2020) collected information about 321 cases of COVID-19 (146 deaths and 175 survivors). Their findings suggested that having a history of comorbidity increased the risk of COVID-19 mortality by 29 % (P = 0.01). Also, cases with coronary heart disease (CHD) had a 92 % higher risk of mortality compared to cases without CHD. In a cohort study, Kuderer et al. (2020) collected and analyzed the data of 928 COVID-19 patients and found that 43 % had active cancer.
Spatial statistics have been exploited to analyze and illustrate the geographic distribution of diseases, including recently emerged COVID-19 (Mollalo, Alimohammadi, Shirzadi, & Malek, 2015; Mollalo, Rivera, & Vahedi, 2020; Mollalo, Vahedi et al., 2020). A study in the early stages of COVID-19 in China assessed the spread of the pandemic using Moran’s I spatial statistic and found a spatial clustering of the disease (Kang, Choi, Kim, & Choi, 2020). In New York City, Cordes and Castro (2020) utilized spatial scan statistics to identify clusters of testing rates and the proportion of positive rates of COVID-19 at the zip code level. Their results indicated higher test rates and higher proportion of positive tests mostly in areas of black population and without health insurance compared to areas of white population and higher income. Guliyev (2020) used a spatial panel data model to assess the influences of deaths rate and recovered cases due to treatment and their spatial effects on the rate of confirmed cases of COVID-19 in Mainland China. In Iran, Pourghasemi et al. (2020) conducted spatial modeling and risk mapping of COVID-19 using random forest machine learning techniques and identified high-risk provinces. In the United States, Mollalo, Vahedi, & Rivera (2020) utilized multiscale geographically weighted regression (MGWR) to locally model variations of COVID-19 incidence rates. The MGWR could explain 68.1 % of disease variations, and income inequality was found as an influential variable, particularly in the tri-state area.
Several individual-level studies from different parts of the world have examined the comorbidity/co-mortality of COVID-19 and other diseases (Asmundson et al., 2020; Freeman et al., 2020; Shekerdemian et al., 2020; Singh & Khan, 2020). However, to our knowledge, there are inadequate population-based studies that analyzed the geographic variability of mortalities of COVID-19 (Mizumoto, Dahal, & Chowell, 2020; Zhang et al., 2020) across the United States, in particular, in relation to other infectious and chronic illnesses. Understanding the comorbidity spectrum of pre-existing conditions influencing COVID-19 mortality may be helpful for the control and management of the disease outbreak. Future nationwide vaccine allocation, especially if produced in limited supply, should be targeted to the unsustainable areas with an abnormal rate of pre-existing conditions. Thus, this study's primary purpose is to apply spatial and statistical analysis to better understand the geospatial distributions of the COVID-19 mortality rate (MR) and CFR in the United States. Also, the co-occurrence of COVID-19 mortality with other diseases is examined.
2. Materials and methods
2.1. Data collection and preparation
COVID-19 spread is tracked daily through dashboards generated by Kaiser Family Foundation, Johns Hopkins University & Medicine Coronavirus Resource Center, and public health agencies such as the Centers for Disease Control and Prevention. We collected cumulative COVID-19 cases and deaths from USAFacts (https://usafacts.org/) at the county-level across the continental United States. Using the total number of cases from January 22, 2020, to November 22, we computed CFR and MR. To avoid unbiased estimations of CFR and mortality, counties with less than 16 reported deaths were excluded from subsequent analyses. In total, only 1544 counties had at least 16 cases of deaths.
Moreover, we collected age-adjusted mortality rates of 20 infectious and non-infectious illnesses as covariates. The covariates were retrieved from the University of Washington Global Health Data Exchange (http://ghdx.healthdata.org/us-data). The covariates were under the following categories: cardiovascular (i.e., Cardiovascular disease, Cerebrovascular disease, Cardiomyopathy and myocarditis, Peripheral vascular disease, Hypertensive heart, and Atrial fibrillation), behavioral disorders (i.e., Alcohol and drug use), chronic respiratory diseases (i.e., Asthma, Pulmonary sarcoidosis & interstitial lung disease, and Chronic obstructive pulmonary disorder (COPD)), cancer (i.e., “Tracheal, bronchus, and lung cancer”, Pancreatic, Leukemia, Hodgkin lymphoma, and Mesothelioma), and infectious diseases (i.e., Hepatitis, Lower respiratory infection (LRI), HIV/AIDS, and Tuberculosis (TB)). All data used in this study were publicly available. The descriptive statistics of covariates used in this study are provided in Supplementary Table S1.
2.2. Spatial analysis
We evaluated the geographic patterns of COVID-19 mortalities (CFR and MR, separately) using Global Moran’s statistic (Moran, 1950). The statistic is defined as follows:
| (1) |
Where and are mortality values for county and county ; is the mean mortality measure; is the inverse distance weight matrix; and is the total number of counties. The coefficient of ranges between -1 and +1. The value approaching +1 indicates a clustered pattern (positive autocorrelation), the value approaching -1 suggests dispersed distribution (negative autocorrelation), and the value close to 0 denote the random distribution of mortality measure (Anselin, 2003; Mollalo, Mao, Rashidi, & Glass, 2019).
Moreover, Anselin Local Moran’s was utilized to identify the concentrations of low and high mortality measures at a 95 % confidence level. Using the same notation as Eq (1), the statistic is computed as:
| (2) |
| (3) |
A positive value for either shows a county with high mortality is surrounded by counties with high mortalities (i.e., High-High (HH) cluster) or a county with low mortality is surrounded by counties with low mortalities (i.e., Low-Low (LL) cluster). A negative value for suggests that a county with high mortality is surrounded by counties with low mortalities and vice versa (i.e., Outlier) (Anselin, 2003; Mollalo, Blackburn, Morris, & Glass, 2017). We conducted both Global and Local Moran’s I statistics of mortality measures using ArcGIS 10.7 (ESRI, Redlands, CA). Moreover, Z-score and P < 0.05 were used to evaluate the statistical significance of the indices.
2.3. Univariate statistical analysis
Using the results obtained from the Local Moran’s I, the counties were divided into three main categories: Non-significant (NN) counties, HH cluster, and LL cluster of COVID-19 mortalities. Analyzing the spatial distribution of outliers (i.e., High-Low and Low-High clusters) was not the purpose of our study and thus excluded from the computations. We calculated the mean mortality measures of each covariate that fall into the categories. Shapiro-Wilk test and Levene’s test were used to examine normality and homogeneity of variance for each covariate, respectively. A one-way analysis of variance (ANOVA) test was performed to investigate the significant difference of mean age-adjusted mortalities of covariates classified by Local Moran’s I categories of COVID-19 mortalities. While, for non-normally distributed covariates, Kruskal-Wallis non-parametric test was used. Tukey's honestly significant difference (HSD) post-hoc test was applied to the significant differences to compare all pairs of means/medians at a 95 % confidence level.
2.4. Multivariate statistical analysis
The correlation coefficients between covariates were computed, and the most uncorrelated covariates were selected as inputs of the model to reduce potential multi-collinearity. A mixed-effects multinomial logistic regression (MMLR) model was further employed to estimate the effects of covariates on COVID-19 mortality categories as follows:
| (4) |
| (5) |
Where is the number of counties, is the Local Moran’s I cluster for the county with = 3 categories (i.e., code 1 for HH cluster, code 2 for LL cluster, and code 3 for NN counties); indicates a vector of covariates for the county; is a vector of coefficients related to the covariates. Moreover, indicates a normal random-variable to incorporate the county's initial mortality before the outbreak effect in the prediction model (Agresti, 2018; Hedeker, 2003). This random term explains a portion of the COVID-19 MR variance due to the different initial stages of COVID-19 mortality in different counties. Moreover, explains the variability due to the other risk factors which are not being considered in the model. Covariates were estimated compared to the reference using the maximum marginal likelihood method. All univariate and multivariate statistical analyses were implemented in R3.6.2 statistical software (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
During the study period, the mean CFR of COVID-19 was 2.36 %, with a minimum of 0.17 %, a maximum of 11.94 %, and a standard deviation of 1.50 %. Also, the mean COVID-19 MR per 100,000 individuals was 97.87 individuals with a minimum of 8.27, a maximum of 720.789, and a standard deviation of 71.34.
3.1. Spatial analysis
The global Moran’s I for both CFR and MR were positive and significant, suggesting a clustered pattern of COVID-19 mortalities across the continental United States (CFR: Morans’I = 0.43, P < 0.001, Z-score = 57.60; MR: Morans’I = 0.31, P < 0.001, Z-score = 40.82).
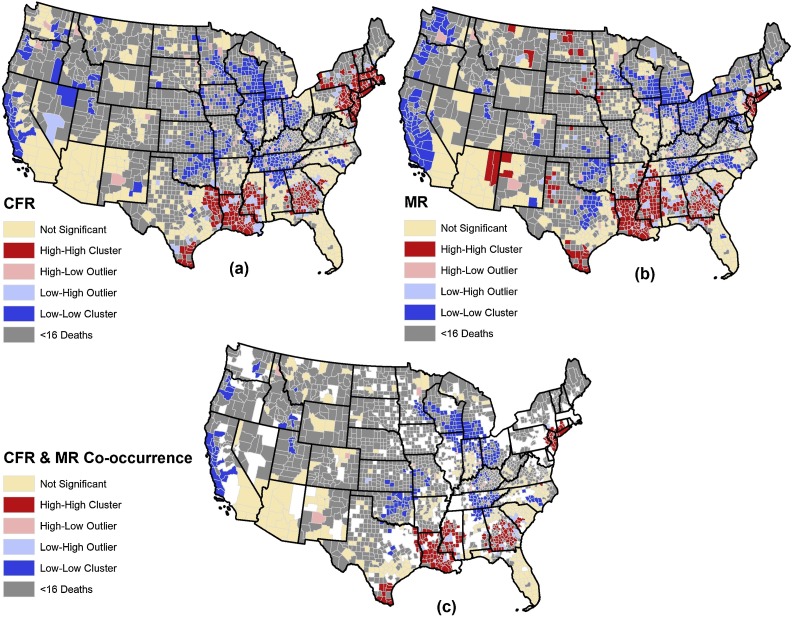
Local Moran’s I classified the counties as HH clusters (n = 296, 19.17 %), LL clusters (n = 441, 28.56 %), NN counties (n = 654, 42.36 %), HL outlier (n = 69, 4.47 %) and LH outlier (n = 84, 5.44 %) of COVID-19 CFR. The counties that were identified as LL clusters of CFR were mostly concentrated in the Central, Western, and Midwestern parts of the United States. Table 1 presents the top states with the highest number of HH and LL clusters of CFR. Fig. 1 (a) illustrates the distribution of HH and LL clusters, HL and LH outliers, and NN counties of CFR.
Table 1.
States with the highest number of HH and LL clusters of CFR, MR, and Co-occurrence of CFR and MR.
| Measure | High-High State (count, %) * | Low-Low State (count, %) |
|---|---|---|
| CFR | New Jersey (n = 21, 100 %) | Tennessee (55, 57.9 %) |
| Connecticut (n = 7, 87.5 %) | Wisconsin (38, 52.8 %) | |
| Massachusetts (n = 12, 85.7 %) | Illinois (49, 48.0 %) | |
| Rhode Island (n = 4, 80 %) | Ohio (32, 36.4 %) | |
| MR | Louisiana (53, 82.8 %) | Ohio (54, 61.4 %) |
| New Jersey (n = 17, 80.95 %) | Maryland (14, 60.7 %) | |
| Mississippi (58, 70.7 %) | California (33, 56.9 %) | |
| Connecticut (n = 4, 50 %) | Washington (14, 39 %) | |
| CFR & MR (Co-occurrence) | New Jersey (n = 17, 80.95 %) | Wisconsin (28, 38.9 %) |
| Connecticut (n = 4, 50 %) | Ohio (32, 36.4 %) | |
| Louisiana (45, 70.3 %) | Tennessee (31, 32.6 %) | |
| Mississippi (32, 39.0 %) | California (20, 34.5 %) |
The first number in the parentheses represents the number of counties, and the second number represents % of counties in that state.
Fig. 1.
Spatial distribution of COVID-19 HH and LL clusters, HL and LH outliers, and NN counties of (a) CFR, (b) MR, and (c) co-occurrence of CFR and MR.
Likewise, 297 counties (19.24 %) were identified as HH clusters, 479 counties (31.02 %) as LL clusters, 605 counties (39.18 %) as NN counties, and 73 counties (4.73 %) as HL outlier, and 90 counties (5.83 %) as LH outlier of COVID-19 MR. An almost similar pattern to HH clusters of CFR is observed for HH clusters of MR. Counties that were identified as HH clusters of MR were mostly concentrated in the northeastern and southern United States. Moreover, an approximately similar pattern was observed for the LL cluster of MR compared to the LL cluster of CFR. Both were mostly concentrated in the central and western United States. Table 1 presents the top states with the highest number of HH and LL clusters of MR. Fig. 1 (b) illustrates the distribution of HH and LL clusters, HL and LH outliers, and NN counties of COVID-19 MR. The intersection (co-occurrence) of HH clusters of CFR and MR identified 193 counties (Table 1). Also, the intersection of LL clusters of CFR and MR identified 274 counties. Fig. 1 (c) illustrates the distribution of HH and LL co-occurrence clusters of MR and CFR of COVID-19.
3.2. Univariate statistical analysis
Results of the Shapiro-Wilk test indicated that none of the covariates followed normal distribution (P < 0.01). Thus, we used the non-parametric Kruskal-Wallis ANOVA test to investigate the significant difference of mean age-adjusted mortalities of covariates classified by Local Moran’s I categories. Results of the ANOVA test indicated significant (P < 0.05) differences of mean mortality of covariates that fall into CFR categories, excluding “Cerebrovascular disease”, “Alcohol use disorder, and “Drug use disorder” mortality rates (P > 0.05). Among the covariates, “Pancreatic cancer” had the largest mean differences than expected by chance (F-value = 52.99), followed by “Cardiomyopathy and myocarditis” (F-value = 51.96) and “Tuberculosis” (F-value = 47.29).
Tukey's HSD test on the significant covariates indicated that for ten covariates, the mean difference between each covariate that fall into HH clusters and NN counties of CFR were significant. The covariates that their mean mortalities in HH clusters were significantly different than mean mortalities in NN counties of CFR were “Pancreatic”, and “Hodgkin lymphoma” cancers; “Cardiovascular disease”, “Hypertensive heart disease”, “Cardiomyopathy and myocarditis”, “Atrial fibrillation”, and “Peripheral vascular disease”; “TB” and “HIV/AIDS”; and “Asthma”. Table 2, Table 3 present the mean of covariates that fall into the HH and LL clusters and NN counties of CFR and MR, respectively.
Table 2.
Mean age-adjusted mortality rates of covariates that fall into HH and LL clusters and NN counties of COVID-19 CFR.
| Theme | Covariate | Mean CFR |
||
|---|---|---|---|---|
| HH | LL | NN | ||
| Cardiovascular | Cardiovascular disease | 305.29 | 274.53 | 276.53 |
| Cerebrovascular disease | 55.91 | 53.94 | 54.31 | |
| Hypertensive heart disease | 14.74 | 10.15 | 11.22 | |
| Cardiomyopathy and myocarditis | 10.22 | 7.84 | 8.87 | |
| Atrial fibrillation | 6.83 | 7.53 | 7.24 | |
| Peripheral vascular disease | 2.79 | 2.69 | 2.67 | |
| Cancer | Pancreatic | 14.06 | 12.77 | 12.91 |
| Mesothelioma | 0.95 | 1.00 | 0.95 | |
| Hodgkin lymphoma | 0.43 | 0.39 | 0.40 | |
| Leukemia | 9.32 | 9.73 | 9.32 | |
| Tracheal, bronchus, and lung | 64.84 | 64.22 | 62.21 | |
| Disorder | Drug use disorder | 9.93 | 11.06 | 10.57 |
| Alcohol use disorder | 2.61 | 2.79 | 3.05 | |
| Respiratory | COPD | 51.77 | 55.92 | 54.60 |
| Asthma | 1.45 | 1.17 | 1.27 | |
| Interstitial lung disease & pulmonary sarcoidosis | 5.63 | 5.85 | 5.85 | |
| Infectious | TB | 0.34 | 0.20 | 0.26 |
| HIV/AIDS | 3.29 | 0.93 | 1.95 | |
| Hepatitis | 0.27 | 0.22 | 0.27 | |
| LRI | 37.15 | 28.81 | 30.63 | |
Table 3.
Mean age-adjusted mortality rates of covariates that fall into HH and LL clusters and NN counties of COVID-19 MR.
| Theme | Covariate | Mean MR |
||
|---|---|---|---|---|
| HH | LL | NN | ||
| Cardiovascular | Cardiovascular disease | 319.49 | 265.13 | 276.65 |
| Cerebrovascular disease | 58.25 | 52.32 | 54.30 | |
| Hypertensive heart disease | 18.09 | 10.10 | 9.79 | |
| Cardiomyopathy and myocarditis | 10.17 | 8.28 | 8.62 | |
| Atrial fibrillation | 6.52 | 7.77 | 7.24 | |
| Peripheral vascular disease | 2.82 | 2.63 | 2.69 | |
| Cancer | Pancreatic | 14.20 | 12.79 | 12.85 |
| Mesothelioma | 0.82 | 1.04 | 0.99 | |
| Hodgkin lymphoma | 0.44 | 0.40 | 0.39 | |
| Leukemia | 9.48 | 9.48 | 9.43 | |
| Tracheal, bronchus, and lung | 66.73 | 61.07 | 63.59 | |
| Disorder | Drug use disorder | 9.02 | 11.32 | 10.67 |
| Alcohol use disorder | 2.90 | 2.62 | 3.06 | |
| Respiratory | COPD | 54.75 | 53.40 | 54.69 |
| Asthma | 1.62 | 1.12 | 1.24 | |
| Interstitial lung disease & pulmonary sarcoidosis | 5.74 | 5.84 | 5.84 | |
| Infectious | TB | 0.39 | 0.20 | 0.25 |
| HIV/AIDS | 3.41 | 1.15 | 1.81 | |
| Hepatitis | 0.27 | 0.25 | 0.26 | |
| LRI | 38.95 | 28.17 | 30.43 | |
3.3. Multivariate statistical analysis
Results of MMLR showed positive associations between the HH cluster of CFR and Asthma (OR = 4.584, 95 % Confidence Interval (CI): 2.583–8.137), Atrial fibrillation (OR = 1.324, CI: 1.130–1.551), Cardiomyopathy & myocarditis (OR = 1.233, CI: 1.113–1.366), Cerebrovascular (OR = 1.267, CI: 1.169–1.374), Hepatitis (OR = 5.602, CI: 1.265–24.814), Hypertensive heart disease (OR = 1.214, CI: 1.126–1.309), Interstitial lung disease (OR = 1.218, CI: 1.012–1.466), Tracheal, bronchus & lung (OR = 1.043, CI: 1.016–1.070), and Leukemia (OR = 2.172, CI: 1.518–3.106) mortality rates. In other words, for a one-percent increase in the mortality rate of Asthma, Atrial fibrillation, Cardiomyopathy & myocarditis, Cerebrovascular, Hepatitis, Hypertensive heart disease, Interstitial lung disease, Tracheal, bronchus & lung, and Leukemia, the odds of the CFR being a hotspot significantly increased by 4.584, 1.324, 1.233, 1.267, 5.602, 1.214, 1.218, 1.043 and 2.172 compared to the non-significant counties, respectively.
On the contrary, negative associations were observed between HH cluster of CFR and Cardiovascular (OR = 0.817, CI: 0.759–0.880), HIV/AIDS (OR = 0.850, CI: 0.753–0.960), LRI (OR = 0.943, CI: 0.921–0.966) and TB (OR = 0.094, CI: 0.012–0.761) mortalities. In other words, for a one-percent increase in the mortality rate of Cardiovascular, HIV/AIDS, LRI, and TB and it is expected to observe 18.3 %, 15.0 %, 5.7 %, and 90.6 % significant decrease in the odds of CFR being a hotspot compared to the non-significant counties, respectively. The associations of other covariates such as Alcohol use disorder, COPD, Drug use disorder, Hodgkin lymphoma, Mesothelioma, and Peripheral vascular disease with HH cluster of CFR were insignificant (P > 0.05). The OR estimates for HH and LL clusters of CFR are presented in Table 4 . The bold categories of covariates show statistical significance at a 95 % confidence level.
Table 4.
Associations between multiple classifications of COVID-19 CFR and mortalities of other diseases.
| Covariate | CFR Category* | Odds Ratio** | SE | P-value | 95 % CI |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Alcohol use disorder | HH | 1.088 | 0.061 | 0.168 | 0.965 | 1.227 |
| LL | 1.149 | 0.049 | 0.005 | 1.044 | 1.266 | |
| Asthma | HH | 4.584 | 0.293 | <0.001 | 2.583 | 8.137 |
| LL | 0.818 | 0.293 | 0.492 | 0.461 | 1.452 | |
| Atrial fibrillation | HH | 1.324 | 0.081 | 0.001 | 1.130 | 1.551 |
| LL | 0.992 | 0.066 | 0.902 | 0.872 | 1.128 | |
| Cardiomyopathy & myocarditis | HH | 1.233 | 0.052 | <0.001 | 1.113 | 1.366 |
| LL | 1.130 | 0.050 | 0.014 | 1.024 | 1.246 | |
| Cardiovascular | HH | 0.817 | 0.038 | <0.001 | 0.759 | 0.880 |
| LL | 0.949 | 0.036 | 0.142 | 0.884 | 1.018 | |
| Cerebrovascular | HH | 1.267 | 0.041 | <0.001 | 1.169 | 1.374 |
| LL | 1.053 | 0.038 | 0.180 | 0.977 | 1.135 | |
| COPD | HH | 0.996 | 0.010 | 0.705 | 0.976 | 1.016 |
| LL | 1.028 | 0.009 | 0.002 | 1.010 | 1.046 | |
| Drug use disorder | HH | 1.016 | 0.022 | 0.491 | 0.972 | 1.061 |
| LL | 0.960 | 0.017 | 0.016 | 0.928 | 0.992 | |
| Hepatitis | HH | 5.602 | 0.759 | 0.023 | 1.265 | 24.814 |
| LL | 0.808 | 0.746 | 0.774 | 0.187 | 3.483 | |
| HIV/AIDS | HH | 0.850 | 0.062 | 0.009 | 0.753 | 0.960 |
| LL | 2.061 | 0.116 | <0.001 | 1.641 | 2.588 | |
| Hodgkin lymphoma | HH | 0.008 | 2.529 | 0.059 | 0.000 | 1.196 |
| LL | 48.361 | 2.204 | 0.078 | 0.644 | 3633.023 | |
| Hypertensive heart disease | HH | 1.214 | 0.038 | <0.001 | 1.126 | 1.309 |
| LL | 1.034 | 0.036 | 0.361 | 0.963 | 1.109 | |
| Interstitial lung disease | HH | 1.218 | 0.095 | 0.037 | 1.012 | 1.466 |
| LL | 0.826 | 0.083 | 0.021 | 0.702 | 0.972 | |
| Leukemia | HH | 2.172 | 0.183 | <0.001 | 1.518 | 3.106 |
| LL | 0.432 | 0.145 | <0.001 | 0.325 | 0.573 | |
| LRI | HH | 0.943 | 0.012 | <0.001 | 0.921 | 0.966 |
| LL | 1.047 | 0.012 | <0.001 | 1.022 | 1.071 | |
| Mesothelioma | HH | 0.847 | 0.247 | 0.502 | 0.522 | 1.375 |
| LL | 1.954 | 0.280 | 0.017 | 1.130 | 3.380 | |
| Pancreatic | HH | 0.474 | 0.117 | <0.001 | 0.377 | 0.596 |
| LL | 1.343 | 0.110 | 0.007 | 1.082 | 1.666 | |
| Peripheral vascular disease | HH | 1.034 | 0.179 | 0.851 | 0.729 | 1.468 |
| LL | 0.953 | 0.149 | 0.745 | 0.711 | 1.276 | |
| Tracheal, bronchus & lung | HH | 1.043 | 0.013 | 0.002 | 1.016 | 1.070 |
| LL | 0.967 | 0.010 | 0.001 | 0.947 | 0.986 | |
| Tuberculosis | HH | 0.094 | 1.068 | 0.027 | 0.012 | 0.761 |
| LL | 0.142 | 0.872 | 0.025 | 0.026 | 0.784 | |
* Non-significant is considered as the reference category.
**ORs were calculated by taking exponent of coefficient for each covariate.
4. Discussion
In this study, confirmed cases of COVID-19 death at the county level during the first ten months of the outbreak in the United States were used to assess the geographic pattern of disease mortality. We mostly concentrated on CFR to represent the status of disease fatality. However, it should be noted that CFR varies widely as mortality data become more available every day, and a considerable number of infected people never get tested due to lack or mild flu-like symptoms. Therefore, the estimation of CFR is susceptible to bias. Since the COVID-19 pandemic began, there has not been much understanding of the factors that can contribute to the mortality of the disease. Once the pandemic settles down, it will be more beneficial for researchers to evaluate the COVID-19 mortality in relation to many more diseases to better control re-emerging or even a similar pandemic.
Our findings implied that public health policymakers should pay attention to the counties with sustainable elevated pre-existing mortality rates when combating the COVID-19 outbreak. Although policy interventions are normally given at the state level, county-level mandates are a more targeted approach. Enacting swift change through county-level intervention is necessary as some counties have their policies such as wearing masks in public and limiting restaurants and bars operational hours. Time is of the essence during the pandemic, and slower legislation may potentially lead to virus sustainability leading to a faster spread and higher mortality. Resources to combat against the disease should be targeted to areas with a higher risk of infection for frontline healthcare workers, such as personal protective equipment and vaccine allocation.
Global and Local Moran’s I statistics were used to statistically evaluate the distribution of the disease mortality patterns. Results showed that the distribution of disease MR/CFR was clustered (P < 0.05), and mainly was concentrated in northeast and southern states. However, CFR had a more focused distribution (higher Z-score) compared to MR. Several international studies have analyzed the spatial distribution of COVID-19 CFR/MR. Islam et al. (2020) examined Spatio-temporal variations of COVID-19 mortality rate among different age and gender groups in India using Getis-Ord Gi* as of May 15, 2020. They identified a hotspot with a 99 % confidence interval, located at the central portions of this country. However, they couldn't locate any cold spots in this country. Sorci, Faivre, and Morand (2020) examined the spatial and temporal variability of COVID-19 CFR across countries. They found several factors positively associated with temporal variations in CFR, particularly in countries with the highest values of disability-adjusted life years lost of cardiovascular, cancer, and chronic respiratory diseases.
Co-occurrence of MR and CFR hot spots indicate some counties, predominantly in Louisiana, New Jersey, and Connecticut, suffer from a high severity of COVID-19 and at the same time an increased risk of mortality. This suggests that these counties require more careful attention, such as personnel and budget allocations, to combat the disease. On the other hand, identifying the counties that were identified as coldspots of CFR and MR, mainly in the Midwest, such as Ohio and Wisconsin, can be followed up to learn effective preventive actions. In some countries such as Singapore, due to the massive allocation of resources to tracing suspected cases, the CFR was reported as low as 0.3 % (Rajgor, Lee, Archuleta, Bagdasarian, & Quek, 2020). Although the highest reported cases of death are observed in areas such as southern Florida, Washington, and southern California, no HH cluster of MR/CFR was identified. This is most likely either due to a large population of counties in these areas or non-uniform screening programs such as testing and tracing. In turn, it may not end up with an accurate estimation of CFR and MR and, subsequently, the hotspots of CFR and MR.
It is essential to understand what other diseases may be associated with the presence of the HH cluster of COVID-19 CFR. With the exception of " Hodgkin lymphoma" mortality, our findings demonstrated that cancers (Pancreatic, Mesothelioma, Tracheal, bronchus & lung, and Leukemia) mortalities were significantly associated with HH clusters of COVID-19 CFR. In other words, for a one-percent increase in the mortality rate of the cancers, it is expected to observe a significant change in the odds of CFR being a hotspot/coldspot compared to the non-significant counties. At the individual level in China, Zhang et al. (2020) performed a retrospective cohort study to investigate cancer mortality among 28 patients diagnosed with COVID-19. They found a CFR of 28.6 %, and the most common type of cancer was lung cancer. In Italy, Onder, Rezza, and Brusaferro (2020) found that among a subsample of 355 dead patients of COVID-19, 72 patients (20.3 %) had active cancer. The high rate of mortality among cancer patients can be attributed to underlying malignancy, treatment-related immunosuppression, or increased comorbidities (Mehta et al., 2020). Although county-level studies should not be directly compared with individual-level researches due to the ecological fallacy, the significant association is most likely due to the compromised immune of patients due to cancer itself or cancer therapies such as steroids that may make them more susceptible to viruses.
As suggested by the Chinese Center for Disease Control and Prevention, patients with chronic respiratory diseases and COVID-19 may present a case fatality rate of almost 6.30 % (Wu & McGoogan, 2020), which demonstrates to be higher than overall COVID-19 CFR. In both univariate and multivariate analysis, the mean mortality of COPD was not significantly different in HH clusters and NN counties. In contrast, the positive association between HH cluster and asthma was observed. The significant association might be a result of inactivation of the patients' defense system, and underexpression of pulmonary interferon-β, a cytokine involved in the immunity against the virus (Pal & Bhadada, 2020). The results are in consistent with the findings of Yang et al. (2020) who conducted a systematic review and meta-analysis to investigate the association of COVID-19 with comorbidities with COPD. They found that COPD is a significant risk factor of COVID-19. According to Wang, Li, Lu, and Huang (2020), COPD may increase the risk of COVID-19 progression as patients with COPD had a 5.9-fold higher risk of aggravation than other patients.
A major limitation in this study is attributed to the presence of potential confounders such as race and socio-economic factors. Since the only available COVID-19 mortality data were the total number of deaths for each county, we could not adjust the mortality rates to control their effects, which may mask the actual associations. Concerning the limitation, this study's findings suggest that COVID-19 CFR and MR continue to be a challenge, especially in the northeast and the southern United States, with the higher HH cluster of both CFR and MR. Our findings imply that further budget and resources such as testing and tracing patients, more medical doctors, nurses, and other healthcare workers should be allocated for those HH clusters. Additionally, essential personal protective equipment such as N95 masks, ventilators, and intensive care unit beds is a priority to these areas. Furthermore, our results indicated that counties with higher mortality of some cancers and respiratory diseases are more vulnerable to fall into clusters of HH COVID-19 CFR.
In conclusion, comprehensive strategies should be designed to safeguard the susceptible areas amid a pandemic and post-pandemic. A multidisciplinary approach is necessary to design a sustainable and healthy environment once the pandemic subsides (Megahed & Ghoneim, 2020). Moreover, as suggested by Hu, Roberts, Azevedo, and Milner (2020), geographic variabilities, social determinants of health, and environmental factors pertaining to COVID-19 mortalities should be carefully investigated in future studies.
Funding
This study received no financial supports.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank anonymous reviewers for taking the time and effort to review the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.scs.2021.102738.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Agresti A. John Wiley & Sons; 2018. An introduction to categorical data analysis. [Google Scholar]
- Anselin L. Spatial Analysis Laboratory, University of Illinois; Champagne-Urbana, Illinois: 2003. An introduction to spatial autocorrelation analysis with GeoDa. (2003, June 16) [Google Scholar]
- Asmundson G.J., Paluszek M.M., Landry C.A., Rachor G.S., McKay D., Taylor S. Do pre-existing anxiety-related and mood disorders differentially impact COVID-19 stress responses and coping? Journal of Anxiety Disorders. 2020;74 doi: 10.1016/j.janxdis.2020.102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramelo F., Ferreira N., Oliveiros B. Estimation of risk factors for COVID-19 mortality-preliminary results. medRxiv. 2020 doi: 10.1101/2020.02.24.20027268. [DOI] [Google Scholar]
- Cordes J., Castro M.C. Spatial analysis of COVID-19 clusters and contextual factors in New York City. Spatial and Spatio-temporal Epidemiology. 2020;34 doi: 10.1016/j.sste.2020.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal S., Mizumoto K., Rothenberga R., Chowella G. Investigating spatial variability in COVID-19 pandemic severity across 19 geographic areas, Spain. medRxiv. 2020 doi: 10.1101/2020.04.14.20065524. [DOI] [Google Scholar]
- Freeman E.E., McMahon D.E., Fitzgerald M.E., Fox L.P., Rosenbach M., Takeshita J.…Hruza G.J. The american academy of dermatology COVID-19 registry: Crowdsourcing dermatology in the age of COVID-19. Journal of the American Academy of Dermatology. 2020;83(2):509–510. doi: 10.1016/j.jaad.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grech V. Unknown unknowns–COVID-19 and potential global mortality. Early Human Development. 2020;105026 doi: 10.1016/j.earlhumdev.2020.105026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gu T., Chu Q., Yu Z., Fa B., Li A., Xu L.…He Y. History of coronary heart disease increases the mortality rate of coronavirus disease 2019 (COVID-19) patients: A nested case-control study based on publicly reported confirmed cases in Mainland China. medRxiv. 2020 doi: 10.1101/2020.03.23.20041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guliyev H. Determining the spatial effects of COVID-19 using the spatial panel data model. Spatial Statistics. 2020;100443 doi: 10.1016/j.spasta.2020.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon P., Chadwick F., Shah A., Wood R., Minton J., McCartney G.…McAllister D.A. COVID-19–Exploring the implications of long-term condition type and extent of multimorbidity on years of life lost: A modelling study. Wellcome Open Research. 2020;5 doi: 10.12688/wellcomeopenres.15849.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D. A mixed‐effects multinomial logistic regression model. Statistics in Medicine. 2003;22(9):1433–1446. doi: 10.1002/sim.1522. [DOI] [PubMed] [Google Scholar]
- Hu M., Roberts J., Azevedo G.P., Milner D. The role of built and social environmental factors in Covid-19 transmission: A look at America’s capital city. Sustainable Cities and Society. 2020;102580 doi: 10.1016/j.scs.2020.102580. [DOI] [Google Scholar]
- Islam A., Sayeed M.A., Rahman M.K., Ferdous J., Shano S., Choudhury S.D.…Hassan M.M. Spatiotemporal patterns and trends of community transmission of the pandemic COVID-19 in South Asia: Bangladesh as a case study. Journal of Biosafety & Health Education. 2020 doi: 10.1016/j.bsheal.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University, Medicine . 2020. COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU)https://coronavirus.jhu.edu/map.html Retrieved November 28, 2020 from. [Google Scholar]
- Kaiser Family Foundation . 2020. COVID-19 coronavirus tracker.https://www.kff.org/coronavirus-covid-19/fact-sheet/coronavirus-tracker/ Retrieved November 28, 2020 from. [Google Scholar]
- Kang D., Choi H., Kim J.H., Choi J. Spatial epidemic dynamics of the COVID-19 outbreak in China. International Journal of Infectious Diseases. 2020;94:96–102. doi: 10.1016/j.ijid.2020.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R.…Grivas P. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megahed N.A., Ghoneim E.M. Antivirus-built environment: Lessons learned from Covid-19 pandemic. Sustainable Cities and Society. 2020;61 doi: 10.1016/j.scs.2020.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A.…Verma A. Case fatality rate of Cancer patients with COVID-19 in a New York Hospital System. Cancer Discovery. 2020 doi: 10.1158/2159-8290.CD-20-0516. CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Dahal S., Chowell G. Spatial variability in the risk of death from COVID-19 in Italy, 2020. medRxiv. 2020 doi: 10.1101/2020.04.01.20049668. [DOI] [PubMed] [Google Scholar]
- Mollalo A., Alimohammadi A., Shirzadi M.R., Malek M.R. Geographic information system‐based analysis of the spatial and spatio‐temporal distribution of zoonotic cutaneous leishmaniasis in Golestan Province, north‐east of Iran. Zoonoses and Public Health. 2015;62(1):18–28. doi: 10.1111/zph.12109. [DOI] [PubMed] [Google Scholar]
- Mollalo A., Blackburn J.K., Morris L.R., Glass G.E. A 24-year exploratory spatial data analysis of Lyme disease incidence rate in Connecticut, USA. Geospatial Health. 2017;12:588. doi: 10.4081/gh.2017.588. [DOI] [PubMed] [Google Scholar]
- Mollalo A., Mao L., Rashidi P., Glass G.E. A GIS-based artificial neural network model for spatial distribution of tuberculosis across the continental United States. International Journal of Environmental Research and Public Health. 2019;16(1):157. doi: 10.3390/ijerph16010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollalo A., Rivera K.M., Vahedi B. Artificial neural network modeling of novel coronavirus (COVID-19) incidence rates across the Continental United States. International Journal of Environmental Research and Public Health. 2020;17(12):4204. doi: 10.3390/ijerph17124204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollalo A., Vahedi B., Bhattarai S., Hopkins L.C., Banik S., Vahedi B. Predicting the hotspots of age-adjusted mortality rates of lower respiratory infection across the continental United States: Integration of GIS, spatial statistics and machine learning algorithms. International Journal of Medical Informatics. 2020;142 doi: 10.1016/j.ijmedinf.2020.104248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollalo A., Vahedi B., Rivera K.M. GIS-based spatial modeling of COVID-19 incidence rate in the continental United States. The Science of the Total Environment. 2020;728 doi: 10.1016/j.scitotenv.2020.138884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P.A. Notes on continuous stochastic phenomena. Biometrika. 1950;37(1/2):17–23. [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Pal R., Bhadada S.K. COVID-19 and non-communicable diseases. Postgraduate Medical Journal. 2020 doi: 10.1136/postgradmedj-2020-137742. postgradmedj-2020-137742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S.…Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? International Journal of Epidemiology. 2020;49:717–726. doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourghasemi H.R., Pouyan S., Heidari B., Farajzadeh Z., Shamsi S.R.F., Babaei S.…Safaeian R. Spatial modeling, risk mapping, change detection, and outbreak trend analysis of coronavirus (COVID-19) in Iran (days between February 19 and June 14, 2020) International Journal of Infectious Diseases. 2020;98:90–108. doi: 10.1016/j.ijid.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgor D.D., Lee M.H., Archuleta S., Bagdasarian N., Quek S.C. The many estimates of the COVID-19 case fatality rate. The Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P.…Paniz-Mondolfi A. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Medicine and Infectious Disease. 2020:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A.…Priestley M.A. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatrics. 2020;174(9):868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F.…Huang H. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiology. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Khan A. Clinical characteristics and outcomes of COVID-19 among patients with pre-existing liver disease in United States: A multi-center research network study. Gastroenterology. 2020;159(2):768–771. doi: 10.1053/j.gastro.2020.04.064. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci G., Faivre B., Morand S. Explaining among-country variation in COVID-19 case fatality rate. Scientific Reports. 2020;10(1):1–11. doi: 10.1038/s41598-020-75848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PloS One. 2020;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging. 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Chiew C.J., Lee V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? The Lancet Infectious Diseases. 2020;20(5):e102–e107. doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the 367 coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 368 72314 cases from the chinese center for disease control and prevention. Jama. 2020:369. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q.…Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. International Journal of Infectious Diseases. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R.…Peng P. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Annals of Oncology. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.