Abstract
Aim
To evaluate the impact of sodium glucose cotransporter 2 inhibitors (SGLT-2i) on risk of heart failure hospitalization in patients with type 2 diabetes.
Methods
We searched the PubMed, Embase, The Cochrane Library, CNKI, Wanfang, CBM, and other web knowledge databases for data from randomized controlled trials. We performed statistical analyses by using review Manager (RevMan) 5.3 and STATA 12.0 for meta-analysis.
Results
Eight randomized controlled trials that compared SGLT-2i versus placebo met our inclusion criteria and were included in the study. The final meta-analysis included a total of 55,763 type 2 diabetes patients. Compared with placebo, SGLT-2i reduced the risk of heart failure hospitalization (RR, 0.63; 95% CI, 0.53 to 0.74; P < 0.00001), MACE (defined as cardiovascular death, myocardial infarction, or ischemic stroke) (RR, 0.92; 95% CI, 0.86 to 0.98; P < 0.007), cardiovascular death (RR, 0.78; 95%CI, 0.62 to 0.99; P = 0.04) in type 2 diabetes patients. SGLT-2i could reduce the risk of death from any cause (RR, 0.77; 95% CI, 0.59 to 1.01; P = 0.06) without statistical significance in type 2 diabetes patients.
Conclusion
Compared with placebo, SGLT-2i may reduce the risk of heart failure hospitalization, MACE, and cardiovascular death. Therefore, SGLT-2i may be an ideal choice for type 2 diabetes mellitus patient with heart failure. These results will help inform practitioners, patients, and authorities making appropriate choices in hypoglycemic therapy clinical practice.
Keywords: sodium glucose cotransporter 2 inhibitors, type 2 diabetes mellitus, heart failure hospitalization, systematic review, meta-analysis
Introduction
Diabetes mellitus is one of the most important independent risk factor for cardiovascular diseases (1). Compared with non-diabetic individuals, diabetes confers about a two-fold excess risk for a wide range of cardiovascular diseases, independently from other conventional risk factors (2). Recent evidence suggests that heart failure is another predominant cardiovascular disease, which is as common as ischemic events in type 2 diabetes patients (3). Besides, heart failure hospitalization is a significant burden of cardiovascular death among type 2 diabetes mellitus patients (4). Patients with type 2 diabetes mellitus and concomitant heart failure require complex medication regimens; what’s more, glucose-lowering medications such as thiazolidinedions (5), dipeptidyl peptidase-4 (DPP-4) inhibitors (6, 7) (saxagliptin and alogliptin) also can increase risk for heart failure hospitalization. Therefore, the cardiovascular safety benefits of glucose-lowering agents could be an ideal choice for type 2 diabetes patients with heart failure.
Recent clinical trials involving the SGLT-2i, such as empagliflozin (8), canagliflozin (9), dapagliflozin (10), and ertugliflozin (11) have surprisingly demonstrated a reduction in the risk of heart failure outcomes among patients with type 2 diabetes who have established cardiovascular disease (CVD) or are at high cardiovascular failure risk. Although three published meta-analyses have reported the discovery of SGLT-2i reducing heart failure hospitalization with only three RCTs; five other RCTs have been published after these meta-analyses published. Therefore, it is necessary to update the meta-analysis. So we collated the most recent evidence into the prior information, and aim to identify the impact of SGLT-2i on heart failure hospitalization in patients with type 2 diabetes.
Methods
Data Sources and Search Strategy
We conducted a computerized literature search of PubMed, EMbase, the Cochrane Library, CNKI, Wanfang, CBM, and other data resources (we search for articles published up to November 19, 2020) by using the Boolean combinations of the key terms “Sodium-Glucose Transporter 2 Inhibitors” AND “Diabetes Mellitus” AND “Heart Failure.” The Medical Subject Headings (MeSH) or keywords were used when the searching database with option was available. Language of the published papers was restricted to English and Chinese, and only human studies were included. Besides, references of the included articles were examined to identify additional studies.
Study Selection
Two authors independently screened the studies and extracted data by using a standardized form. All disagreements were resolved with group consensus. To conclude, the inclusion criteria were as follows: (1) Type 2 diabetes patients were treated with SGLT-2i; (2) Data on re-hospitalization due to heart failure were well documented in the literature; (3) The research follow-up ≥1 year with complete data records; (4) The research was a randomized controlled trail; (5) Language of literature were English or Chinese. Studies were excluded from the primary analysis if any of the following was met: (1) Letters, case reports, review articles, conference abstracts, editorials, insufficient information researches, and duplicate studies. (2) The mechanism of SGLT2 for heart failure, animal experiment, test protocol was reported without relevant study results.
Data Extraction and Quality Assessment
The following information was extracted from each included study: the first author’s last name, year of publication, geographic origin, study design, age and gender of patients, type 2 diabetes duration, glycosylated hemoglobin level, follow-up period, sodium-glucose transporter 2 inhibitors regimens, total number of participants in clinical trials. The primary outcome was heart failure hospitalization and the key secondary outcomes were MACE, cardiovascular death, and death from any cause.
The methodological quality of the included studies was evaluated separately by two authors using Cochrane risk of bias tool (12), with consensus reached when different opinions exist on the results. The Cochrane risk of bias tool was used to assess the quality of the RCTs based on five domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). Two reviewers assessed the quality of the evidence independently using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (13, 14). For evidence body of RCT, the quality can be downgraded for five reasons (study limitations, consistency of effect, imprecision, indirectness, and publication bias). The quality of the evidence will be graded as high, moderate, low, and very low.
Statistical Analysis
Rigorous statistical analysis was performed using Review Manager (RevMan) program version 5.3 (Cochrane Collaboration, Oxford, UK) for meta-analysis and STATA statistical software (Version 12.0, Stata Corporation, College Station, TX, USA) for sensitivity analyses and Egger’s linear regression test. The Risk Ratio (RR) with corresponding 95% confidence interval (95% CI) were calculated to evaluate the association between sodium-glucose transporter 2 inhibitors and heart failure hospitalization, cardiovascular death, and death from any cause. The Z test was used to estimate the statistical significance of pooled RRs. Between-study heterogeneity was assessed by Cochran’s Q-statistic and I 2 test (15). If the Q-test exhibited a P > 0.05 or the I 2 test showed <50%, which means that these studies were homogeneous, the fixed-effects model was conducted; otherwise, the random-effect model was used. We also use sensitivity analyses to explore sources of heterogeneity and P < 0.05 was considered as the signal of existence of the statistical significance heterogeneity. Funnel plots and Egger’s linear regression test was used to investigate the potential publication bias (16).
Results
Study Characteristics and Selection
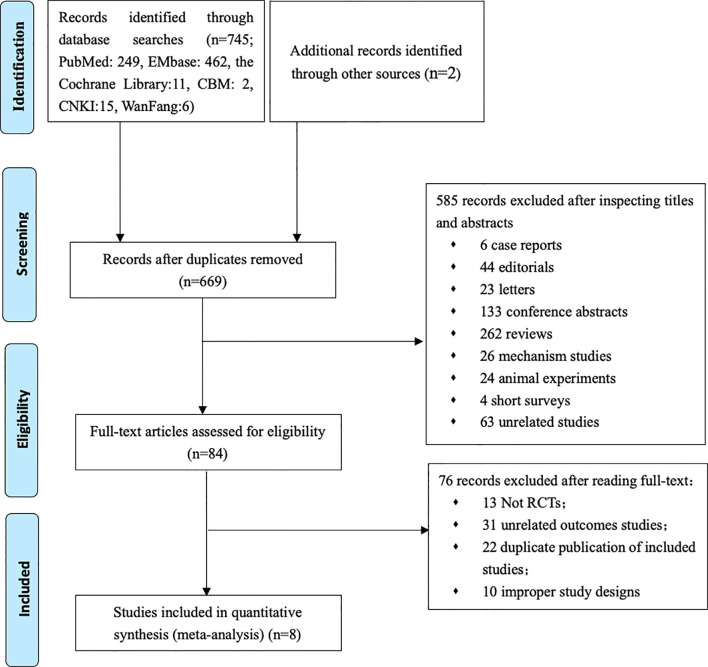
Our primary searches originally yielded 747 articles, 669 papers remaining after the removal of duplicates. After reading through titles and abstracts, 6 case reports, 44 editorials, 23 letters, 133 conference papers, 262 reviews, 26 mechanism studies, 24 animal experiments, 4 short surveys, 63 unrelated studies were excluded; 84 studies remained. We reviewed the full texts of the remaining articles, and furtherly excluded 13 non-RCT studies, 31 literatures with irrelevant outcomes, 22 overlapping data with the included study, 10 literatures on pilot programed design. Finally, 8 remaining studies were included in the meta-analysis ( Figure 1 ) (8–11, 17–20). Characteristics of the included trials are summarized in Table 1 . In total, 55,763 type 2 diabetes mellitus patients were randomly assigned to receive different SGLT-2i (empagliflozin, canagliflozin, dapagliflozin) and placebo. Participants were generally middle-aged (mean age 63.0–66.4). Follow-up duration ranged from 1 to 4.2 years.
Figure 1.
Flow chart of study selection.
Table 1.
Demographic and clinical characteristics of studies included in the meta-analysis.
| Author | Year | Location | Study design | Patients (female%) | Age(mean) | T2DM duration(year) | Complication | HbA1c(%) | Follow-up (year) | Interventions | Control measure | Dose(mg/d) | Sample size(Interventions/control) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zinman (8) | 2016 | United States, Argentina, Australia, Belgium, etc. 615 centers of 40 countries | RCT | 2,036 (29%) |
63.1 | >10 | Myocardial infarction, coronary artery disease, coronary revascularization coronary artery | 7.0–10 | 3.1 | empagliflozin | placebo | 10/25 | 7,020 (4,687/2,333) |
| Radholm (9) | 2018 | United States, Argentina, Australia, Belgium, etc. 313 centers of 26 countries | RCT | 3,631 (35.8%) |
63.3 | 13.5 | Hypertension, atrial fibrillation | 7.0–10.5 | 3.6 | canagliflozin | placebo | 100/300 | 10,142 (5,795/4,347) |
| McMurray (10) | 2019 | UK., United States, Denmark, Germany, etc. 417 centers of 19 countries | RCT | 1,109 (23.4%) |
66.4 | — | Ischemic, nonischemic, atrial fibrillation, | >6.5 | 1.5 | dapagliflozin | placebo | 10 | 4,744 (2,373/2,371) |
| Cannon (11) | 2020 | United States | RCT | 2,471 (30%) | 64.4 | 12.9 | Coronary artery disease, heart failure, myocardial infarction, coronary revascularization | 7.0–10.5 | 3.5 | Ertugliflozin | placebo | 5/15 | 8,238(5,499/2,747) |
| Wiviott (17) | 2019 | United States, Argentina, Australia, Belgium, etc. 804 centers of 33 countries | RCT | 6,422 (37.4%) |
64 | 11 | Coronary artery disease, peripheral artery disease | 6.5–12 | 4.2 | dapagliflozin | placebo | 10 | 17,160 (8,582/8,578) |
| Kosiborod (18) | 2017 | United States | RCT | 119 (37.2%) |
64.3 | 13.5–14 | Coronary artery disease, atrial fibrillation | 7.3–9.1 | 1 | dapagliflozin | placebo | 10 | 320 (171/149) |
| Isreb (19) | 2019 | United States, Argentina, Australia, Brazil, etc. 593 centers of 34 countries. | RCT | 1,492 (33.9%) |
63 | — | Hypertension, cardiovascular disease | 6.5–10.5 | 2.62 | canagliflozin | placebo | 100 | 4,401 (2,202/2,199) |
| Packer (20) | 2020 | United States, Argentina, Australia, Belgium, etc. 520 centers of 20 countries. | RCT | 893 (23.9%) |
66.9 | — | Heart failure, atrial fibrillation, hypertension | — | 1.33 | empagliflozin | placebo | 10 | 3,730 (1,863/1,867) |
Effect of SGLT-2i on Heart Failure Hospitalization in Patients With Type 2 Diabetes
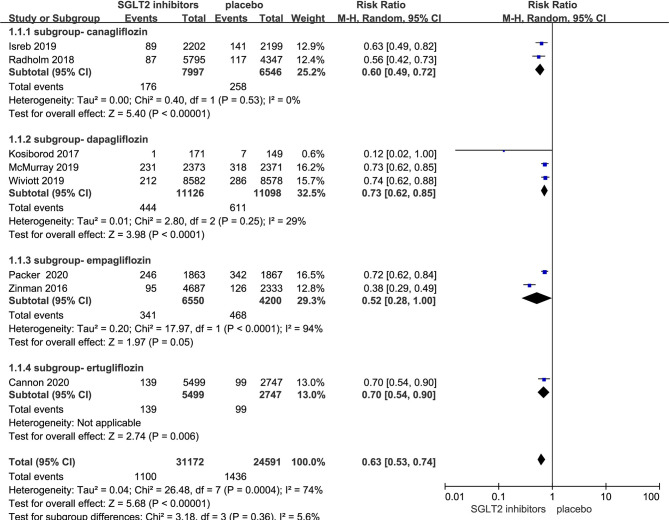
Eight RCTs evaluating SGLT-2i effect on heart failure hospitalization were identified (8–11, 17–20), 55,763 type 2 diabetes mellitus patients were randomly assigned to 31,172 receive SGLT-2i and 24,591 receive placebo, 1,100 and 1,436 patients were re-hospitalized due to heart failure, respectively. There was significant heterogeneity among eight studies (P<0.05, I 2 = 74%). The meta-analysis using the random-effects model showed that SGLT-2i significantly decreased heart failure hospitalization occurrence in patients with type 2 diabetes compared with control group (RR, 0.63; 95% CI, 0.53 to 0.74; P < 0.00001) ( Figure 2 ). Subgroup analyses by different agents of SGLT-2i on heart failure hospitalization showed that compared with controls, canagliflozin (RR, 0.60; 95% CI, 0.49 to 0.72; P < 0.00001), dapagliflozin (RR, 0.73; 95% CI 0.62 to 0.85; P < 0.0001), empagliflozin (RR, 0.52; 95% CI, 0.28 to 1.00; P = 0.05) and ertugliflozin (RR, 0.70; 95% CI, 0.54 to 0.90; P = 0.006) all significantly decreased the occurrence of heart failure hospitalization in patients with type 2 diabetes. Therefore, the quality of evidence will be graded as high.
Figure 2.
Heart failure hospitalization in type 2 diabetes patients receiving SGLT2 inhibitors versus control.
Effect of SGLT-2i on MACE in Patients With Type 2 Diabetes
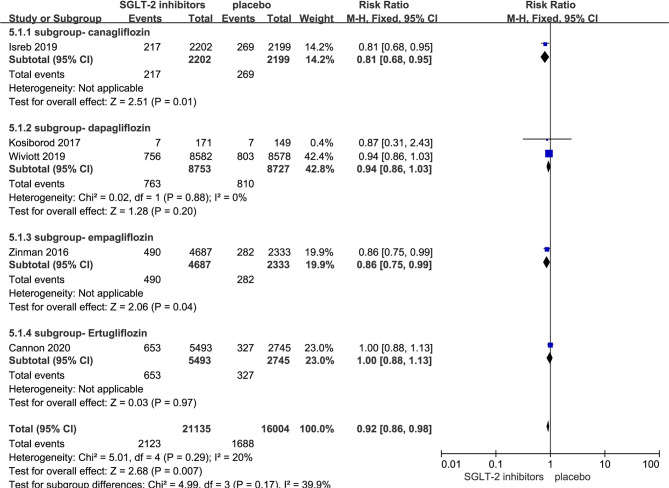
Five RCTs evaluating SGLT-2i on MACE were identified (8, 11, 17–19), 37,139 type 2 diabetes mellitus patients were randomly assigned to 21,135 receive different SGLT-2i and 16,004 receive placebo; 2,123 and 1,688 patients incurred MACE, respectively. There was no significant heterogeneity among five studies (P < 0.05, I 2 = 20%). The meta-analysis using the fixed-effects model showed that SGLT-2i resulted in a lower rate of MACE (RR, 0.92; 95% CI, 0.86 to 0.98; P < 0.007) ( Figure 3 ). Besides, Subgroup analyses by different agents of SGLT-2i on MACE showed that compared with controls, only canagliflozin (RR, 0.81; 95% CI, 0.68 to 0.95; P < 0.01) and empagliflozin (RR, 0.86; 95% CI, 0.75 to 0.99; P < 0.04) significantly decreased MACE occurrence in patients with type 2 diabetes. Because of subgroup analyses result, the quality of evidence will be graded as moderate.
Figure 3.
MACE in type 2 diabetes patients receiving SGLT2 inhibitors versus control.
Effect of SGLT-2i on Cardiovascular Death in Patients With Type 2 Diabetes
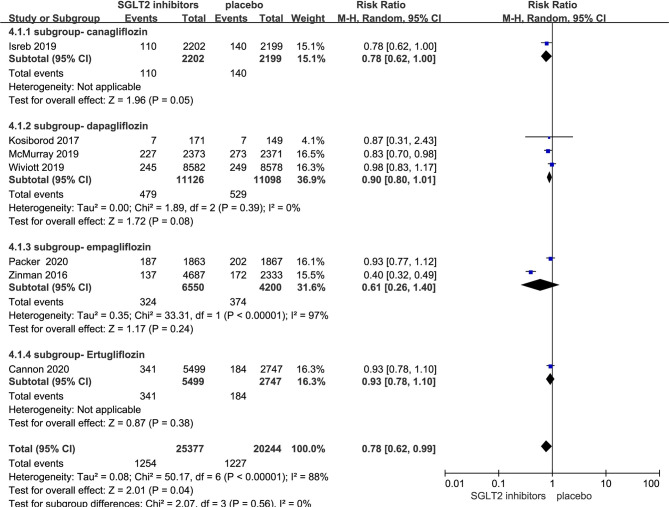
Seven RCTs evaluating SGLT-2i on cardiovascular death were identified (8, 10, 11, 17–20), 45,621 type 2 diabetes mellitus patients were randomly assigned to 25,377 receive SGLT-2i and 20,244 receive placebo; 1,254 and 1,227 patients incurred cardiovascular death, respectively. There was significant heterogeneity among seven studies (P < 0.05, I 2 = 88%). The meta-analysis using the random-effects model showed that SGLT-2i could decrease the occurrence of cardiovascular death (RR, 0.78; 95% CI, 0.62 to 0.99; P = 0.04) ( Figure 4 ). Subgroup analyses by different agents of SGLT-2i on cardiovascular death showed that compared with controls, only canagliflozin decreased cardiovascular death occurrence but without statistical significance in patients with type 2 diabetes (RR, 0.78; 95% CI, 0.62 to 1.00; P = 0.05). Because of the significant heterogeneity among seven studies and subgroup analyses result, the quality of evidence will be graded as low.
Figure 4.
Cardiovascular death in type 2 diabetes patients receiving SGLT2 inhibitors versus control.
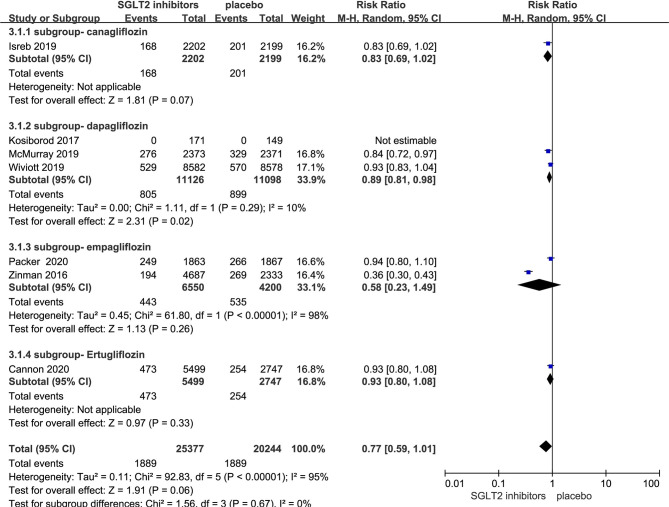
Effect of SGLT-2i on Death From Any Cause in Patients With Type 2 Diabetes
Seven RCTs evaluating SGLT-2i on death from any cause were identified (8, 10, 11, 17–20), 45,621 type 2 diabetes mellitus patients were randomly assigned to 25,377 receive different SGLT-2i and 20,244 receive placebo; 1,889 and 1,889 patients incurred death from any cause, respectively. There was significant heterogeneity among seven studies (P < 0.05, I 2 = 95%). The meta-analysis using the random-effects model showed that SGLT-2i could decrease death occurrence from any cause but without statistical significance (RR, 0.77; 95% CI, 0.59 to 1.01; P = 0.06) ( Figure 5 ). Subgroup analyses by different agents of SGLT-2i on death from any cause showed that compared with controls, only dapagliflozin (RR, 0.89; 95% CI, 0.81 to 0.98; P = 0.02) significantly decreased the occurrence death from any cause in patients with type 2 diabetes. Because of the significant heterogeneity among seven studies and subgroup analyses result, the quality of evidence will be graded as moderate.
Figure 5.
Death from any cause in type 2 diabetes patients receiving SGLT2 inhibitors versus control.
Sensitivity Analysis and Risk of Bias
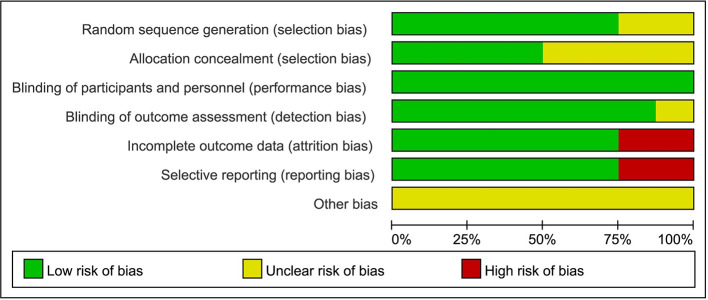
Generally, the studies were of high methodologic quality. The overall quality assessment indicated that about one-third had unclear reporting of randomization sequence, one-second had unclear reporting of allocation concealment, one-fourth had unclear reporting of blinding of outcome assessment, and one-third had high risk bias of incomplete outcome data or selective reporting ( Figure 6 ). No indication of publication bias was detected with visual assessment of Egger’s test (t = −2.05, p>|t| = 0.087>0.05) in the meta-analysis.
Figure 6.
Quality of including studies in the meta-analysis.
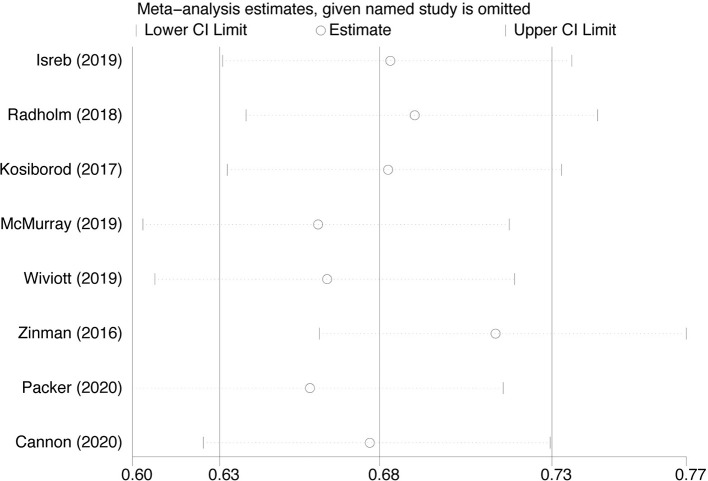
Because of significant heterogeneity among the SGLT-2i on heart failure hospitalization studies, we conducted leave-one-out sensitivity analyses. Individual studies were consecutively excluded from the sensitivity analysis to investigate whether the obtained results were robust. The analysis showed that the results obtained in the meta-analysis were statistically robust because the corresponding combined RRs in all of the separate subgroup analyses were relatively stable if any individual study is deleted ( Figure 7 ).
Figure 7.
Sensitivity analysis for Heart failure hospitalization in type 2 diabetes patients receiving SGLT2 inhibitors versus control.
Discussion
Our meta-analysis has shown that SGLT-2i could reduce the risk of heart failure hospitalization and subgroup analyses showed the same result in cases using different agents of SGLT-2i, suggesting that SGLT-2i will be an ideal choice for type 2 diabetes mellitus patient with heart failure. However, SGLT-2i did not result in a lower rate on death from any cause; what’s more, the results were different from the current researches (21, 22). Therefore, these data will help inform practitioners, patients, and authorities regarding the heart failure hospitalization of SGLT-2i in routine clinical practice.
SGLT-2i have gaining more attention, not only for the glycemic benefits but also for favorable effects on cardiovascular outcomes. As we all know, SGLT-2i have been recommended as a first choice in add-on treatment for type 2 diabetes mellitus patient with cardiovascular disease in ADA guidelines (23). The reduction in heart failure hospitalization was also observed in all agents of SGLT-2i in our research. Each study of different agents of SGLT-2i was performed in the different study design with varied backgrounds; so there is significant heterogeneity among the four agents of SGLT-2i on heart failure hospitalization studies. Therefore, we conducted leave-one-out sensitivity analyses and the results were relatively stable if any individual study is deleted. What’s more, the result was largely influenced by dapagliflozin and Empagliflozin, which accounted for 61.8% of the total weight in the analysis. Regardless of the presence or absence of diabetes, dapagliflozin and Empagliflozin reduces the risk of worsening heart failure (10, 20). Dapagliflozin could also prevent hospitalization due to heart failure, regardless of a history of atherosclerotic cardiovascular disease or failure (18). Besides, SGLT-2i also could reduce the risk of MACE and cardiovascular death. Therefore, SGLT-2i will be an ideal choice for type 2 diabetes mellitus patient with heart failure. However, in our meta-analysis, there is concern that SGLT-2i did not result in a lower rate of death from any cause than placebo. Among the four agents of SGLT-2i, only dapagliflozin resulted in a significantly lower risk death from any cause. Given the situation that a relatively small number of current clinical randomized controlled studies were needed, major clinical studies that are currently ongoing will hopefully provide more insight and rigorous data.
The detection of heart failure hospitalization benefit attributed to SGLT-2i was a major discovery. Therefore, several theories of the mechanism of SGLT-2i acting on the heart failure have been postulated (24). Firstly, SGLT-2i could convert cardiovascular risk factors, such as improving metabolic patient profile, capable of including reductions in plasma glucose, blood pressure, body weight, and modifications to the lipid profile (25, 26). Secondly, SGLT-2i induce their diuretic effect by reducing interstitial volume to a much greater extent than intravascular volume which is key to the prevention of fluid overload and heart failure exacerbation leading to hospitalization (27). Thirdly, SGLT-2i could produce a hemodynamic response elicited which creates a favorable environment to reduce cardiac hydrostatic pressure to induce ventricular remodeling and hypertrophy (28, 29). Fourthly, SGLT-2i have been shown to inhibit Na+/H+ exchanger 1 on cardiomyocytes which resulted in increased intracellular sodium and calcium, inducing a pro-oxidant and pro-thrombotic state (30). Fifthly, SGLT-2i induce a reduction in epicardial fat and thus providing another possible mechanism for the beneficial heart failure (31, 32). Sixthly, SGLT-2i could protect renal function by multifactorial, involving electrolyte, vascular, and hydrostatic alterations to increases natriuretic and volume contraction (33). This in turn reduces atrial natriuretic peptide causing vasoconstriction of the afferent renal arterioles. Preservation of renal function is of particular importance for patients with heart failure to avoid volume overload and diuretic resistance (27). Finally, SGLT-2i also could result in erythropoiesis and improve oxygen delivery to tissues (34) and modulate inflammatory and oxidative mediators such as leptin, interleukins (IL), tumor necrosis factor α, cyclooxygenase 2 (COX-2) to decrease the frequency of HF-related hospitalizations (35). Therefore, as described above, the beneficial effects of SGLT2i and their effect on heart failure are multidimensional. In all, SGLT2i could significantly improve heart failure-related outcomes and decrease the frequency of heart failure–related hospitalization.
Our study has several strengths. First, in addition to journal reports, we also researched the other web knowledge databases with unpublished clinical trials. Second, we conducted subgroup analyses to explore different SGLT-2i compounds regarding the risk-reduction effect on heart failure hospitalization, MACE, cardiovascular death, and death from any cause. Third, we used the GRADE approach to assess quality of evidence. Fourth, although three published meta-analyses have reported the discovery of SGLT-2i reducing heart failure hospitalization. Yamani et al. study (36) and Singh et al. study (37) both included only three RCTs and three other RCTs have been published after these meta-analyses published. The results of our meta-analysis were inconsistent with these three published meta-analyses but the benefit of heart failure hospitalization in type 2 diabetes patient. For instance, we found that the impact of SGLT-2i on the death from any cause have no statistical significance but SGLT-2i reduced the risk of MACE and cardiovascular death. What’s more, our result of MACE was inconsistent with Sinha et al. study that the events in the pooled analysis of the phase 3 trials reveals for MACE was statistically no significant (HR 0.83, 95% CI 0.66 to1.03, P = 0.10) (38). Therefore, we are the first using meta-analysis of randomized controlled trials who reported heart failure hospitalization, MACE, the cardiovascular death, and death from any cause in type 2 diabetes patient. We also firstly found that the impact of SGLT-2i on the death from any cause have no statistical significance which has not been reported yet.
Our study also has some limitations. There were too few clinical trials of SGLT-2i such as luseogliflozin, remogliflozin, sotagliflozin, and tofogliflozin, that can be used to meta-analyze heart failure hospitalization, MACE, the cardiovascular death, and death from any cause in type 2 diabetes patient. Without enough trials, e.g., ertugliflozin have only one article, the subgroup analysis was unable to differentiate the effects of these SGLT-2i agents.
Conclusion
In summary, the current eight RCTs evidence showed that, SGLT-2i could reduce the risk of heart failure hospitalization, MACE, and cardiovascular death. Therefore, SGLT-2i will be an ideal choice for type 2 diabetes mellitus patient with heart failure. The impact of SGLT-2i on the death from any cause have no statistical significance; more clinical trials may provide important insights on this issue. This meta-analysis will help inform practitioners, patients, and authorities making appropriate choices in hypoglycemic therapy clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material ; further inquiries can be directed to the corresponding author.
Author Contributions
YC and XZ conceptualized and designed the study. AZ, XL, HM, JK, and GQ analyzedand interpreted the data. AZ and XL drafted the manuscript. GQ modified the manuscript. AZ and XL contributed equally to this work. All authors contributed to the article and approved the submitted version.
Funding
The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.604250/full#supplementary-material
References
- 1. Gerstein H, Pogue J, Mann JF, Lonn E, Dagenais GR, McQueen M, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia (2005) 48:1749–55. 10.1007/s00125-005-1858-4 [DOI] [PubMed] [Google Scholar]
- 2. Sarwar N GP, Seshasai SR. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (2010) 375:2215–22. 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verma S, Sharma A, Kanumilli N, Butler J. Predictors of heart failure development in type 2 diabetes: A practical approach. Curr Opin Cardiol (2019) 34:578–83. 10.1097/HCO.0000000000000647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma A, Green JB, Dunning A, Lokhnygina Y, Al-Khatib SM, Lopes RD, et al. Causes of Death in a Contemporary Cohort of Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease: Insights From the TECOS Trial. Diabetes Care (2017) 40:1763–70. 10.2337/dc17-1091 [DOI] [PubMed] [Google Scholar]
- 5. Lago RM, Singh PP, Nesto RWJL. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet (2007) 370:1129–36. 10.1016/S0140-6736(07)61514-1 [DOI] [PubMed] [Google Scholar]
- 6. Packer M. Worsening Heart Failure During the Use of DPP-4 Inhibitors: Pathophysiological Mechanisms, Clinical Risks, and Potential Influence of Concomitant Antidiabetic Medications. JACC Heart Fail (2018) 6:445–51. 10.1016/j.jchf.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 7. Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, Steg PG, et al. Saxagliptin and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus and Moderate or Severe Renal Impairment: Observations From the SAVOR-TIMI 53 Trial. Diabetes Care (2015) 38:696–705. 10.2337/dc15-0006 [DOI] [PubMed] [Google Scholar]
- 8. Zinman B, Lachin J, Inzucchi S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med (2016) 374:1094. 10.1056/NEJMc1600827 [DOI] [PubMed] [Google Scholar]
- 9. Radholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus. Circulation (2018) 138:458–68. 10.1161/CIRCULATIONAHA.118.034222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med (2019) 381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 11. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med (2020) 383(15):1425–35. 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:889–93. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Norris SL, Meerpohl JJ, Akl EA, Schünemann HJ, Gartlehner G, Chen Y, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol (2016) 79:150–8.e1. 10.1016/j.jclinepi.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 15. Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics (2005) 21:3672–3. 10.1093/bioinformatics/bti536 [DOI] [PubMed] [Google Scholar]
- 16. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA (2006) 295:676–80. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 17. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med (2019) 380:347–57. 10.1056/NEJMc1902837 [DOI] [PubMed] [Google Scholar]
- 18. Kosiborod M, Gause-Nilsson I, Xu J, Sonesson C, Johnsson E. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and concomitant heart failure. J Diabetes Complications (2017) 31:1215–21. 10.1016/j.jdiacomp.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 19. Isreb M, Al-Makki A, Buatois E. Canagliflozin and Renal Outcomes in Diabetic Nephropathy. N Engl J Med (2019) 381:1088. 10.1056/NEJMc1909687 [DOI] [PubMed] [Google Scholar]
- 20. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med (2020) 383(15):1413–24. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 21. Sinha B, Ghosal S. Sodium-Glucose Cotransporter-2 Inhibitors (SGLT-2i) Reduce Hospitalization for Heart Failure Only and Have No Effect on Atherosclerotic Cardiovascular Events: A Meta-Analysis. Diabetes Ther (2019) 10:891–9. 10.1007/s13300-019-0597-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular Outcomes and Risks After Initiation of a Sodium Glucose Cotransporter 2 Inhibitor: Results From the EASEL Population-Based Cohort Study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation (2018) 137:1450–9. 10.1161/CIRCULATIONAHA.117.031227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes care (2020) 43:487–93. 10.1007/s00125-019-05039-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wojcik C, Warden BA. Mechanisms and Evidence for Heart Failure Benefits from SGLT2 Inhibitors. Curr Cardiol Rep (2019) 21:130. 10.1007/s11886-019-1219-4 [DOI] [PubMed] [Google Scholar]
- 25. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia (2018) 61:2108–17. 10.1007/s00125-018-4670-7 [DOI] [PubMed] [Google Scholar]
- 26. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation (2016) 134:752–72. 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 27. Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation (2017) 136:1643–58. 10.1161/CIRCULATIONAHA.117.030012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol (2017) 16:138. 10.1186/s12933-017-0621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, et al. Effect of Empagliflozin on Left Ventricular Mass and Diastolic Function in Individuals With Diabetes: An Important Clue to the EMPA-REG OUTCOME Trial? Diabetes Care (2016) 39:e212–3. 10.2337/dc16-1312 [DOI] [PubMed] [Google Scholar]
- 30. Baartscheer A, Schumacher CA, Wüst RC, Fiolet JW, Stienen GJ, Coronel R, et al. Empagliflozin decreases myocardial cytoplasmic Na+through inhibition of the cardiac Na+/H+exchanger in rats and rabbits. Diabetologia (2017) 60:568–73. 10.1007/s00125-016-4134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol (2018) 17:6. 10.1186/s12933-017-0658-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yagi S, Hirata Y, Ise T, Kusunose K, Yamada H, Fukuda D, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr (2017) 9:78. 10.1186/s13098-017-0275-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zelniker TA, Braunwald E. Cardiac and Renal Effects of Sodium-Glucose Co-Transporter 2 Inhibitors in Diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol (2018) 72:1845–55. 10.1016/j.jacc.2018.06.040 [DOI] [PubMed] [Google Scholar]
- 34. Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased Hematocrit During Sodium-Glucose Cotransporter 2 Inhibitor Therapy Indicates Recovery of Tubulointerstitial Function in Diabetic Kidneys. J Clin Med Res (2016) 8:844–7. 10.14740/jocmr2760w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc Diabetol (2018) 17:101. 10.1186/s12933-018-0745-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamani N, Usman MS, Akhtar T, Fatima K, Asmi N, Khan MS. Sodium-glucose co-transporter 2 inhibitors for the prevention of heart failure in type 2 diabetes: A systematic review and meta-analysis. Eur J Prev Cardiol (2020) 27:667–70. 10.1177/2047487319841936 [DOI] [PubMed] [Google Scholar]
- 37. Singh AK, Singh R. Heart failure hospitalization with SGLT-2 inhibitors: a systematic review and meta-analysis of randomized controlled and observational studies. Expert Rev Clin Pharmacol (2019) 12:299–308. 10.1080/17512433.2019.1588110 [DOI] [PubMed] [Google Scholar]
- 38. Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract (2019) 150:8–16. 10.1016/j.diabres.2019.02.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material ; further inquiries can be directed to the corresponding author.