Abstract
Liraglutide is a long-acting human glucagon-like peptide-1 (GLP-1) analogue and an effective treatment for patients with metabolic diseases including type 2 diabetes mellitus (T2DM) and obesity. This review focuses on the mechanism of action of liraglutide as a well-known glucagon-like peptide-1 receptor agonist (GLP-1 RA) in patients with T2DM and obesity. The lower and the higher doses of GLP-1 RAs are used for glycaemic control in T2DM and in obesity respectively. GLP-1 RAs such as liraglutide enhance insulin secretion and inhibit glucagon release via the stimulation of glucagon-like peptide-1 receptors (GLP-1Rs). Liraglutide decreases hemoglobin A1c (HbA1c) in type 2 diabetes (T2D) patients when prescribes as monotherapy or in combination with one or more antidiabetic drugs. Usually, it is well tolerated with minor hypoglycemia in combination therapy. Liraglutide reduces cardiovascular events and related risk factors including improvement of lipid profile and control of blood pressure. Accordingly, it can be cost-effective and may be a budget neutral medication option by considering its protective effect on the cardiovascular system in long-term use in the health care plan. In the near future, by pharmacogenomics approach, prediction of the highest patient’s response with the lowest adverse drug reactions and also rationality of drug development will be possible. Liraglutide can be used as a desirable medicine for glycemic control and obesity. It shows extensive evidence based benefits in diabetes complications. In this narrative review, we have summarized and evaluated studies related to the role of liraglutide in clinical practice.
Keywords: GLP-1 RAs, Liraglutide; Type 2 diabetes; Obesity; Cardiovascular events; Pharmacogenomics
Introduction
Type 2 diabetes mellitus (T2DM) and obesity are major public health burden and their incidence have increased in recent year [1]. Efficacy treatment of these conditions is more challengeable.
Diabetes mellitus (DM) is a complex chronic, progressive, incompletely understood the metabolic condition in the world. T2DM is the most prevalent type which approximately %90 of the population suffering from diabetes [2]. T2DM is a common condition characterized by a combination of peripheral insulin resistance and inadequate insulin secretion by pancreatic beta cells [3]. It is associated with high morbidity and mortality and is one of the diseases with the greatest impact on public health. Patients with T2DM are at high risk for long-term macro and microvascular complications, which lead to frequent hospitalization and complications, including cardiovascular diseases (CVDs) [3]. Furthermore, the mortality rate of diabetes will double in number by 2030 if not given more attention [4].
Eight pathophysiological mechanisms underlying T2DM as an “ominous octet” lead to hyperglycemia [5]. These include (i) decreased insulin secretion from pancreatic beta cells, (ii) increased glucagon secretion from pancreatic α cells, (iii) increased glucose production in the liver, (iv) neurotransmitter dysfunction and insulin resistance in the brain, (v) increased lipolysis, (vi) dysregulated renal glucose handling, (vii) dysfunctional incretin effect, and (viii) impaired glucose uptake in peripheral tissues, mainly in skeletal muscle, liver, and adipose tissue. Currently available glucose-lowering drugs mainly target one of these pathways [5]. Although, the treatment of T2DM has greatly improved, but it is necessary to explore new methods for treating T2DM. While glucose control remains the main priority in managing T2D patients, treatment should always be considered in context of a comprehensive approach to including comorbidity. Obesity as a major global health problem is a complex disease resulting from genetic and environmental factors. Obesity associated with a higher risk of various chronic diseases, including T2DM, hypertension, atherosclerotic cardiovascular diseases (ASCVDs), nonalcoholic fatty liver disease, osteoarthritis and cancers (breast, kidney, endometrium, colon) [6]. Overweight and obesity are commonly estimated by the body mass index (BMI) and waist circumference [6]. Several guidelines recommend that the cornerstones of obesity management are lifestyle and behavioral modifications such as low-calorie intake and increased physical activity. Pharmacotherapy may be added to as an adjunct. Obesity pharmacotherapy is usually indicated for patients with BMI ≥30 kg/m2 or BMI ≥ 27 kg/m2 with obesity-associated comorbidities [7].
Therefore, obesity is associated with many comorbidities so there is an urgent need for new therapeutic strategies too [8, 9]. Pharmacological treatments for obesity are promising, with varying effectiveness for weight loss.
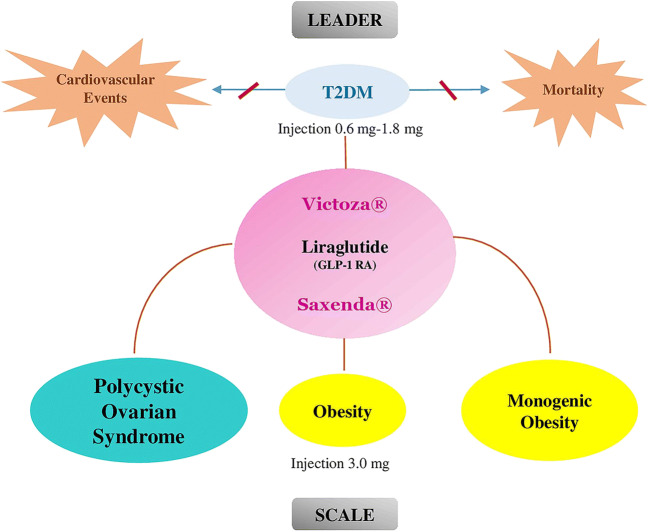
In addition to the insulinotropic activity of glucagon-like peptide-1 (GLP-1) in healthy individuals, glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 play important roles in different biological processes. GLP-1 suppresses glucagon secretion, especially at hyperglycemia. GIP has been stimulating glucagon secretion, particularly at lower glucose concentrations. GLP-1 also decreases hepatic glucose output independent of plasma glucagon level [10]. Therefore, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are useful in the early stages of progression in the natural history of diabetes mellitus with their well-known effects in the improvement of metabolic dysfunction. (Fig. 1). The GLP-1 RAs also demonstrate some differences in their impact on clinical conditions.
Fig. 1.
Liraglutide and pathophysiological conditions
It is very useful to bring together practical information on the use of liraglutide. The aim of the present narrative review is to describe the emerging role of liraglutide as a therapeutic option mainly used for T2DM and Obesity.
Incretin hormones role in the physiology and pathophysiology of obesity and type 2 diabetes; the therapeutic perspective
Oral glucose induces higher insulin secretory responses than intravenous glucose infusion even when the same plasma glucose concentration profiles (“isoglycaemia”) is achieved in healthy individuals. This effect is uniformly defective in patients with T2DM.
This is the result of the release of incretin hormones (glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1; GIP, GLP-1) from specialized entero-endocrine cells in the gut. Of the three parts of this pathway, substrates such as glucose, incretin hormones, and neural signals transmitted by the autonomic nervous system originating from the gut and reaching the endocrine pancreas, the second one makes the most considerable contribution under physiological circumstances. Accordingly, the physiological stimulation of insulin secretion through incretin hormones is considerably important, and it is so called insulinotropic effect.
The glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like Peptide-1 (GLP-1)
The glucose-dependent insulinotropic polypeptide is an incretin hormone which was purified using a bioassay measuring the inhibition of gastric acid secretion with the old name “Gastric Inhibitory Polypeptide (GIP)”. GIP is produced in K cells of the intestines. The main function of GIP is glucose-dependent augmentation of insulin secretion [10].
Glucagon-like peptide-1 is the other known incretin hormone which was identified as a part of the gene sequence coding for proglucagon that is expressed in L-cells of the intestine.
These two primary incretin hormones secreted from the intestine upon ingestion of glucose and other carbohydrates including sucrose and starch, triglycerides, and some amino acids as well as proteins. Nevertheless, proteins are a comparatively weak stimulus [10–12] and lipids provide a rather robust stimulus for GLP-secretion [11].
Biological effects of GLP-1
GLP-1 exerts their effects by binding to their specific receptors. The glucagon-like peptide-1 receptor (GLP-1R) is a class 2 G protein coupled receptor family. The receptor is commonly distributed in pancreatic islets, kidney, brain, heart and the gastrointestinal tract [11]. Binding GLP-1 to the receptor activates the signal transduction pathway and enhances the level of intracellular cyclic adenosine monophosphate (cyclic AMP) in pancreatic beta-cells, and consequently stimulates insulin secretion in a glucose-dependent manner [11].
The actions exerted by GLP-1 through glucagon like peptide 1 receptor (GLP-1R) were investigated in several studies [13], some of the actions are listed as follow:
Body weight and appetite: GLP-1 as a neurotransmitter can act on both the peripheral nervous system (PNS) and central nervous system (CNS). The latter one is related to satiety and loss of appetite [14]. GLP-1 has been found to reduce appetite and food intake and increases satiety and it seems to be one of the meal-termination signals [15].
Intestinal transition: Exogenous GLP-1 inhibits the gastric emptying, pentagastrin and gastric acid secretion stimulated by food ingestion [16]. In addition to slowing gastrointestinal motility, pancreatic exocrine secretions are reduced, too.
Cardiovascular function: Several effects have been suggested for GLP-1 and GLP-1 RAs regarding their effect on the cardiovascular system. Some of them include inflammatory responses in adipose tissue and blood vessels, heart failure, atherosclerosis progression, inflammation and plaque stability, myocardial ischemia, and cardiac blood supply, which will be discussed later in this article [17].
Pharmacological management of T2DM
The American Diabetes Association (ADA) has released a series of recommendations called Standards of Medical Care in Diabetes to improve diabetes outcomes. The recommendations include lifestyle management, cost-effective screening and pharmacologic approaches to glycemic treatment in order to prevent, delay, or effectively manage T2DM and its life-threatening complications [3]. The main goal of these approaches is to improve quality of life by reducing morbidity and mortality and not necessarily strict glycemic control through different medications. Though, good glycemic control is one of the main principles of T2DM management.
Monotherapy with metformin should be started at the time of T2DM diagnosis; for most of the patients, this will be the main long life anti-diabetic drug in combination with intensive lifestyle management [3, 18].
The other major classes of anti-diabetic drugs include sulfonylureas, thiazolidinediones (TZDs), α-glucosidase inhibitors, meglitinides (glinides), dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, GLP-1 RAs, bile acid sequestrants, dopamine-2 agonists, and amylin mimetics [3]. If the hemoglobin A1c (HbA1c) target is not achieved or rises on combination therapy, insulin initiation should be considered [3, 18]. Food and Drug Administration (FDA) approved oral anti-hyperglycaemic agents with their mechanism actions in treatment of type 2 diabetes is shown in Table 1.
Table 1.
Oral agents approved for treatment of type 2 diabetes
| Class | Mechanism of action | Target organ site |
|---|---|---|
| Biguanides [57] | Decrease in hepatic glucose production; increase in muscle insulin sensitivity by activating AMPK | Liver and intestine |
| Thiazolidinediones (TZDs) [58] | Bind PPAR-γ, decrease insulin resistance and increase glucose utilization | Muscle, adipose tissue, and liver |
| Sulfonylureas [59] | Stimulates beta cell insulin secretion. | Islet cells of pancreas |
| α-glucosidase inhibitors [60] | Reduces absorption of dietary carbohydrate, by inhibiting the enzyme alpha-glucosidase | Intestine |
| Meglitinides (glinides) [61] | Stimulate the release of insulin from the pancreatic beta cells | Islet cells of pancreas |
| DPP-4 inhibitors [62] | Prevent degradation Of GLP-1 | Intestine |
| SGLT-2 inhibitors [63] | Prevent glucose reabsorption and facilitate its excretion in urine by inhibiting SGLT-2 | Kidney |
| GLP-1 receptor agonists [64] | Activate GLP-1 receptor, increase insulin secretion, decrease glucagon secretion | Islet cells of pancreas |
AMPK, AMP-activated protein kinase; PPAR-γ, Peroxisome proliferator-activated receptor gamma; DPP-4, Dipeptidyl peptidase 4; GLP-1, SGLT-2, sodium-glucose cotransporter-2; Glucagon-like peptide-1
GLP-1 RAs for the management of T2DM
Many treatment modalities for T2DM target the hormonal changes that cause glucose disposal weaknesses, including defects in insulin secretion, insulin resistance, and alterations in glucagon secretion, all of which influence on glycemic control. Among them, incretin hormones would be considered as an attractive therapeutic option. Patients with T2DM have appreciably lower GLP-1 concentrations triggered by meal intake when compared with healthy individuals. Furthermore, in prediabetes state, patients show higher GLP-1 concentrations level in comparison with T2DM, but less than who did not have evidence of diabetes [5, 18, 19].
The currently available GLP-1 RAs are divided into the short-acting formulations exenatide and lixisenatide and the longer-acting formulations semaglutide, liraglutide, albiglutide, dulaglutide, and exenatide [12, 20]. All exhibit the same mechanism of action and are resistant to enzymatic degradation by DPP-4. They differ in a number of some characteristics, including (1) treatment dose and frequency (e.g. twice daily vs. once weekly); (2) whether it is a necessity to be taken at a certain time with the meals; (3) optimum use in particular patient groups (e.g. elderly patients or those with liver or kidney conditions); and (4) features of the injection device [12]. The GLP-1 RAs also have some differences in their cardiovascular benefits and in the incidence of side effects such as gastrointestinal symptoms [21].
Liraglutide pharmacology
The natural GLP-1 peptide is short-acting owing to its short half-life, but synthetic GLP-1 RAs are longer acting [22, 23]. Liraglutide (Victoza®) received its initial FDA approval as a “long-acting human GLP-1” analogue in January 2010 as an adjunctive treatment for diet and exercise in patients with T2D [24]. Liraglutide can reversibly bind to albumin through heptamer formation mediated by the fatty acid (Palmitoyl acid) [25]. This binding extends the half-life of its structure. Liraglutide itself encompasses an amino acid sequence that shares %97 similarity with the human endogenous GLP-1 peptide [24, 26].
GLP-1Rs are found in pancreatic alpha and beta cells, Gastrointestinal (GI) tract; the central and peripheral nervous systems, and the heart, lungs. After injection of liraglutide, in the presence of elevated glucose level, liraglutide binds to the GLP-1R and results in an increase in insulin secretion, inhibition of postprandial glucagon secretion and delay of gastric emptying [12].
Liraglutide administered once daily but requires dose titration. It is usually initiated at a dose of 0.6 mg once daily for a week to improve gastrointestinal tolerability and then titrated in a weekly manner. The dose can be increased up to 1.8 mg daily to achieve glycemic goals [3, 12].
Liraglutide is also suggested as monotherapy in adjunct to diet and exercise and when metformin is considered to be unacceptable due to intolerance or contraindications [3].
Liraglutide in clinical practice
The safety, tolerability, and efficacy of liraglutide both as monotherapy and combination with a range of anti-diabetic drugs were investigated in the Liraglutide Effect and Action in Diabetes (LEAD) programs in patients with T2DM in different steps of available treatment. The corresponding efficacy was assessed on glycemic control, body weight, beta cell function, blood pressure, and lipid profile [27]. The LEAD program comprised six randomized controlled phase 3 trials which involving 4456 patients of whom nearly 2700 received liraglutide [27].
In the efficacy concerns, the results of LEAD trials obviously revealed that once-daily administration of liraglutide led to a substantial and sustained reduction in HbA1c. Furthermore, fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) were also effectively decreased across the LEAD trials [25, 27]. Improvement in beta cell function was shown by homeostasis model assessment of β-cell function (HOMA-B) and proinsulin: insulin ratio [25, 27]. In the safety concern no major safety problems with liraglutide were seen in any of the LEAD trials and was generally well tolerated [27].
The progressive nature of T2DM necessitates intensification of treatment in the long run. As an alternative to intensification with prandial insulin in order to successfully improve glycemic control (basal-bolus insulin regimen), basal insulin and GLP-1 RAs can be used in combination. Insulin Degludec/Liraglutide (IDegLira) is the first fixed-ratio grouping of basal insulin and a GLP-1 RA available in a single once-daily injection. Phase 3a trials have been indicated that IDegLira is superior at improving glycemic control compared with insulin degludec or liraglutide alone. IDegLira recommended as an efficacious combination treatment (mean end-of-trial HbA1c was 6.4–%6.9 across the five completed Phase 3 trials) and could help patients to reach their glycemic target (HbA1c < %7 in 60-%81 of patients) [28].
Cardiovascular events and mortality in patients with type 2 diabetes
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of morbidity and mortality in patients with T2DM. Diabetic individuals have a two-fold higher risk of developing CVDs than in non-diabetic individuals and the incidence rates of congestive heart failure (HF) in individuals with diabetes are 5-fold and 2.4-fold higher than in women and men without diabetes, respectively [29].
Several cardiovascular outcome trials (CVOTs) had been accomplished for glucose-lowering medications. The long-term effects of liraglutide on cardiovascular outcomes and other clinically important events have been investigated in the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial [30].
The LEADER trial included 9340 patients with T2DM with an age of 50 years or more with established CVD condition (coronary heart disease, cerebrovascular disease, peripheral vascular disease, chronic kidney disease of stage 3 or greater, or chronic heart failure of New York Heart Association class II or III) or greater than 60 years with at least one major CVD risk factors included microalbuminuria or proteinuria, hypertension and left ventricular hypertrophy, left ventricular systolic or diastolic dysfunction, or an ankle-brachial index of <0.9. Patients were randomized to receive 1.8 mg liraglutide (n = 4668) or placebo (n = 4672), both in addition to standard-of-care therapy. The median exposure to study drug and median duration of follow up was 3.5 and 3.8 years respectively [30].
The primary 3-point major adverse cardiovascular events (MACE), composite outcomes included cardiovascular death, non-fatal myocardial infarction or stroke. The primary outcome (MACE) occurred in %13 and %15 of the liraglutide and the placebo treatment group, respectively. The main finding of the LEADER showed that liraglutide significantly reduced the 3-point MACE, cardiovascular and all-cause mortality [30]. One of the secondary endpoints was hypoglycemia. Patients with severe hypoglycemia were at greater risk of cardiovascular events and death, mainly shortly after the hypoglycemic period [31]. The rate of severe hypoglycemia was lower in the liraglutide group.
In a prespecified group analysis the effects of liraglutide on renal outcomes have been investigated too. In this analysis the risk of renal outcomes was determined by an intention-to-treat approach. Change in estimated glomerular filtration rate and albuminuria was also evaluated. The hazard ratio of renal outcomes in the liraglutide group was fewer than in the placebo group. Liraglutide resulted to a lower risk of the composite renal outcomes than placebo that is related to a lower rate of new-onset persistent macroalbuminuria [32].
In a meta-analysis of the LEAD trials, it was seen that the treatment with liraglutide reduces total cholesterol, low-density lipoprotein cholesterol (LDL-C) and triglycerides, compared with standard treatment and in the LEAD-6 a reduction n triglycerides has been observed in comparison with twice-daily exenatide [33].
In a pooled analysis of the LEAD trials, it was observed that liraglutide treatment lead to approximately 2.8 mmHg reduction in systolic blood pressure (SBP), as opposed to 0.5 mmHg with placebo [34].
Potential mediators for the cardiovascular benefit with liraglutide in the LEADER trial have been investigated; HbA1c and, to a lesser extent, urinary albumin-to-creatinine ratio (UACR) were identified as potential mediators of the cardiovascular effects of liraglutide [35].
Liraglutide in obesity treatment
Liraglutide is an obesity medication for chronic weight management that labeled as Saxenda® both in the USA and Europe since 2014 and 2015, respectively [36]. Central nervous system studies confirmed that altered activation of parietal cortex, insula, and putamen which is responsible for appetite behavior in reaction to pictures of desirable food are responsible for reducing hunger and increased fullness in treatment with liraglutide [37].
Liraglutide is prescribed as a subcutaneous injection once daily. The optimal starting dose is 0.6 mg daily and escalated weekly by 0.6 mg to 3.0 mg daily as a therapeutic dosage [38]. The liraglutide’s side effects were investigated in a 56-week, double-blind trial involving 3731 obese patients which randomly patients assigned in a 2:1 ratio that receive once-daily subcutaneous injections of 3.0 mg dose of liraglutide. The most placebo substracted side effects were abdominal pain (%1.7), dyspepsia (%6.4), vomiting (%12.2), constipation (%11.3), diarrhea (%11.6) and nausea (%25.5) [39]. Some areas of the brain contain GLP-1Rs which are targets of GLP-1 analogues are responsible for nausea and vomiting [37]. These side effects usually are mild and tolerable by most of the patients and tolerability increases over time [37, 39].
Liraglutide trials for obesity
The Satiety and Clinical Adiposity-Liraglutide Evidence (SCALE) trials were done to show the efficacy of this drug in different groups of patients who suffer from obesity and included nondiabetic, prediabetic and diabetic people for weight reduction and maintaining the reduced weight and even reduce the complication of obesity such as sleep apnea, resulted in the approval of liraglutide (Saxenda®) as an anti-obesity medication [39–42].
In the SCALE Maintenance, 422 adults obese/overweight without T2DM participants were randomly assigned to receive liraglutide 3.0 mg per day or placebo (subcutaneous administration) for 56 weeks. Liraglutide holds promises for maintenance of weight loss in a placebo-subtracted %32.5 of the patients in the treatment group and an additional placebo-subtracted %6 weight loss. Furthermore, liraglutide induces additional reductions in CVD risk factors including fasting blood glucose (FBG) and systolic blood pressure (SBP) [40].
The SCALE Diabetes, 846 adults with overweight or obesity and type 2 diabetes were randomly assigned to receive liraglutide once-daily 3.0 mg, liraglutide 1.8 mg, or placebo for 56 weeks. Weight loss of %5 or greater occurred in %54.3 with liraglutide (3.0 mg) and %40.4 with liraglutide (1.8 mg) vs. %21.4 with placebo at the end of the study [42].
The SCALE Obesity and Prediabetes, a 56-week double-blind trial, 3731 adult obese patients with prediabetes recruited to this study, 2487 patients were assigned to liraglutide 3.0 mg once-daily. In this trial, 3.0 mg of liraglutide in addition to diet and exercise was associated with significant weight loss [39].
In the SCALE Sleep Apnea, 308 obese non-diabetic with moderate to severe obstructive sleep apnea (OSA) were randomized for 32 weeks to ligarutide 3.0 mg or placebo, both an adjunct to diet and exercise. The results showed that liraglutide was well tolerated and produced a greater reduction in HbA1c, apnea-hypopnea index (AHI), body weight, and systolic blood pressure when compared with placebo [42]. By considering the liraglutide trials, liraglutide 3.0 mg once daily seems to show a similar effect on weight loss management without considering previous diet lifestyle management.
In a systematic review and network meta-analysis on FDA weight-loss medications, overall evaluation and comparative analysis of their effects in long-term use on the cardiometabolic risk profile of obese adults were investigated. This meta-analysis indicated that liraglutide was significantly associated with a substantial decrease in HbA1c, FBS and a reduction in the placebo-subtracted waist circumference (WC) of 4 cm in 1.2 to 3.0 mg of liraglutide during 52 to 104 weeks [43].
In a 56-week, randomized, double-blind, placebo-controlled, multinational, multicenter trial a total 396 individuals with overweight or obesity and basal insulin-treated type 2 diabetes were randomized to liraglutide 3.0 mg or placebo combined with intensive behavioral therapy (IBT). In individuals with overweight or obesity and type 2 diabetes treated with basal insulin, liraglutide 3.0 mg as an adjunct to IBT was superior to placebo in terms of weight loss and improved glycemic control despite lower doses of basal insulin [44].
In a recent 56-week randomized, double-blind trial, a total of 125 participants were randomly enrolled (1:1) to receive either liraglutide (3.0 mg) or placebo subcutaneously once daily. The use of liraglutide (3.0 mg) plus lifestyle therapy led to greater decreseae in the BMI standard-deviation score than placebo [45].
Liraglutide in other conditions
Monogenic obesity
The effect of liraglutide 3 mg for 16 weeks was evaluated in 14 monogenic obese individuals with pathogenic melanocortin 4 receptor (MC4R) mutations (BMI: 37.5 ± 6.8 kg/m2) and 28 matched control participants without MC4R mutation (BMI: 36.8 ± 4.8 kg/m2). Melanocortins are expressed in the hypothalamus and play an important role in regulating appetite as one of the mechanisms of GLP1 receptors is appetite inhibition. Administration of liraglutide decreased body weight by %6 in obese with MC4R mutations (6.8 kg) and normal controls (6.1 kg); it shows that GLP-1 receptors are effective in this common form of monogenic obesity [46]. Waist circumferences is reduced by a placebo-subtracted 5.3 and 6.4 cm and fasting insulin by 0.25 and 0.4 mmol/l in the MC4R mutation and control group respectively [46]. Regarding the role of liraglutide in the treatment of monogenic obese individuals with MC4R mutation, it seems that liraglutide will be an appropriate treatment in the most common form of monogenic obesity [46].
Polycystic ovary syndrome (PCOS)
Obesity is one of the most problematic issues in PCOS women, as they show an increased prevalence of obesity compared with normal women [47].
Obese women affecting an estimated reproductive results irrespective of the mode of conception, and greater BMI associates with poorer fertility prognosis [48]. Adiposity affects distinct PCOS phenotypes strongly too [49]. PCOS is also one of the leading causes of infertility in women of reproductive age, being the most common endocrine disorder.
Most of the clinical studies on GLP-1 RAs in obese/overweight PCOS women are related to to the use of liraglutide in patients previously treated with metformin. In a recent review the emerging role of GLP-1 receptor agonists for obese women with PCOS have been shown the weight loss effect of GLP-1 RA as a great treatment available choice for PCOS patients [50].
In a recent meta-analysis, 23 randomized clinical trials reporting on 941 overweight and obese women with PCOS were included and the effectiveness of metformin, inositol, liraglutide and orlistat in reducing body weight were compared. In descending order, the amount of weight loss differed among different class of drugs. Liraglutide 1.2 mg to 3.0 mg lead to 5.2 kg weight loss, followed by 3.2 kg in orlistat, 3.0 kg in liraglutide 1.2 mg plus metformin and 1.34 kg in metformin alone. After 12–36 weeks of treatment with liraglutide waist circumference was also decreased 5.7 cm as compared to other medication [47].
A recent meta-analysis of 8 ranomized trials the efficacy of GLP-1 RAs versus metformin in women with PCOS have been investigated and it is indicated that GLP-1 RA was superior to metformin in reducing BMI and improving insulin sensitivity [51].
Pharmacogenomics
Pharmacogenomics is the study of genetic variations, single nucleotide polymorphisms (SNPs) of the drug-metabolizing enzyme, receptors, drug transporters, and drug targets genes, and how these SNPs interact to produce a drug-related phenotype, such as drug response or undesired effects. Pharmacogenomics and pharmacogenetics are often used interchangeably. In pharmacogenetic, the association study of a single genetic variant in a single gene to be investigated with drug response [52]. Defect in insulin secretion in pancreatic β-cells and decreased insulin sensitivity are two mechanisms in T2DM pathogenesis. More than 150 genetic T2DM variants have been introduced and most of them were related to defect in insulin synthesis, processing or secretion [53]. GLP-1R, gastric inhibitory polypeptide receptor (GIPR), transcription factor 7 like 2 (TCF7L2), melatonin receptor 1B (MTNR1B), chymotrypsinogen B1/2 (CTRB1/2), and potassium voltage-gated channel subfamily Q member 1 (KCNQ1) variants seems to be involved in the physiology of incretin secretion [54]. A heterozygous GLP-1R missense (rs6923761; A/G) polymorphism shows potential implication in weight loss and metabolic control and association of this SNP and weight loss after treatment with liraglutide have been investigated. Overweight diabetic subjects with the variant allele (A) of rs6923761 GLP-1R polymorphism had a greater decrease in weight and fat mass after treatment with liraglutide [55].
The endocannabinoid system is another proposed pathway to be involved in the control of food intake and body weight. Cannabinoid type 1 receptor (CNR1) gene is expressed in several brain areas and in a variety of peripheral tissues including adipose tissue. Association of polymorphism (1359 G/A) of the CNR1 gene investigated metabolic changes and weight loss secondary to treatment with liraglutide. Association of the rs1049353 polymorphism A allele with an improvement of insulin resistance secondary to weight loss after liraglutide treatment in obese patients with diabetes has been confirmed [56].
Although major progress has been made in T2DM pharmacogenetics, this field is still needed more trials and research in performing comprehensive assessments of genetic variations in association with drug-phenotype across different ethnicities, sufficiently large sample size, which can be achieved only in the setting of international collaborations. This information has valuable influence in the future of drug development.
Conclusion
In summary, the GLP-1 RAs of an effective class of antihyperglycemic agents that improve the actions of naturally occurring peptide GLP-1. At the present time, GLP-1 RAs are not considered as the first-line therapy for T2DM according to the most diabetes clinical guidelines. However, they are considered as second-line therapy in combination with oral antidiabetic drugs or insulin. Liraglutide is one of the well-known examples of this class. Important randomized trial studies on liraglutide have shown its great impact on glycemic control and diabetes complications in T2DM and also its effect on obesity.
Although, many questions remain to be answered regarding liraglutide impact in clinical practice including: As most of the studies have been performed on individuals who have either had a risk factor or a cardiovascular disease. The main question is that in patients who do not have proven cardiovascular disease, liraglutide still can reduce cardiovascular risk? Another issue is whether liraglutide can reduce cardiovascular events in low risk individuals?
As the most problem in T2DM patients is chronic complication more effort is needed to answer that the long-term treatment with liraglutide whether affects diabetes complications such as retinopathy or neuropathy or not?
Overall, GLP-1 receptor agonists are efficient and innovative agents in patients with type 2 diabetes and some other medical problems.
Abbreviations
- T2DM
Type 2 diabetes mellitus
- DM
Diabetes mellitus
- CVDs
Cardiovascular diseases
- ASCVDs
Atherosclerotic cardiovascular diseases
- BMI
Body mass index
- GLP-1
Glucagon-like peptide-1
- GIP
Glucose-dependent insulinotropic polypeptide
- Cyclic AMP
Cyclic adenosine monophosphate
- GLP-1 RA
Glucagon-like peptide-1 receptor agonist
- GLP-1R
Glucagon-like peptide-1 receptor
- PNS
Peripheral nervous system
- CNS
Central nervous system
- ADA
American Diabetes Association
- TZDs
Thiazolidinediones
- DPP-4
Dipeptidyl peptidase 4
- SGLT-2
Sodium-glucose cotransporter-2
- HbA1c
Hemoglobin A1c
- FDA
Food and Drug Administration
- GI
Gastrointestinal
- LEAD
Liraglutide effect and action in diabetes
- FPG
Fasting plasma glucose
- PPG
Postprandial plasma glucose
- HOMA-B
Homeostasis model assessment of β-cell function
- IDegLira
Insulin Degludec/Liraglutide
- ASCVD
Atherosclerotic cardiovascular disease
- HF
Heart failure
- CVOTs
Cardiovascular outcome trials
- MACE
Major advers cardiovascular events
- LDL-C
Low-density lipoprotein cholesterol
- SBP
- UACR
Urinary albumin-to-creatinine ratio
- SCALE
Satiety and Clinical Adiposity-Liraglutide Evidence
- OSA
Obstructive sleep apnea
- AHI
Apnea-hypopnea index
- IBT
Intensive behavioral therapy
- MC4R
Melanocortin 4 receptor;
- PCOS
Polycystic ovary syndrome
- SNPs
Single nucleotide polymorphisms
- GIPR
Gastric inhibitory polypeptide receptor
- TCF7L2
Transcription factor 7 like 2
- MTNR1B
Melatonin receptor 1B
- CTRB1/2
Chymotrypsinogen B1/2
- KCNQ1
Potassium voltage-gated channel subfamily Q member 1
- CNR1
Cannabinoid type 1 receptor
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: part I. increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2009;4(2):113–119. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation (IDF). IDF Diabetes Atlas - 8th Edition. 2017. https://diabetesatlas.org/resources/2017-atlas.html. [PubMed]
- 3.Care D. Standards of Medical Care in Diabetesd 2019. Diabetes Care. 2019;42:S81. doi: 10.2337/dc19-S008. [DOI] [PubMed] [Google Scholar]
- 4.Peykari N, Hashemi H, Dinarvand R, Haji-Aghajani M, Malekzadeh R, Sadrolsadat A, Sayyari AA, Asadi-lari M, Delavari A, Farzadfar F, Haghdoost A, Heshmat R, Jamshidi H, Kalantari N, Koosha A, Takian A, Larijani B. National action plan for non-communicable diseases prevention and control in Iran; a response to emerging epidemic. J Diabetes Metab Disord. 2017;16(1):3. doi: 10.1186/s40200-017-0288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFronzo RA. From the triumvirate to the „ominous octet”: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58(4): 773–795. [DOI] [PMC free article] [PubMed]
- 6.Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity: pathophysiology and management. Diabetes Obes Metab. 2018;71(1):69–84. doi: 10.1016/j.jacc.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33(Supplement 1):S62-S9. [DOI] [PMC free article] [PubMed]
- 8.Boutayeb A, Boutayeb S, Boutayeb W. Multi-morbidity of non communicable diseases and equity in WHO eastern Mediterranean countries. Int J Equity Health. 2013;12(1):60. doi: 10.1186/1475-9276-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21. doi: 10.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 12.Romera I, Cebrian-Cuenca A, Alvarez-Guisasola F, Gomez-Peralta F, Reviriego J. A review of practical issues on the use of glucagon-like Peptide-1 receptor agonists for the Management of Type 2 diabetes. Diabetes Ther. 2018;10(1):5–19. doi: 10.1007/s13300-018-0535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips LK, Prins JB. Update on incretin hormones. Ann N Y Acad Sci. 2011;1243(1). [DOI] [PubMed]
- 14.Poon JL, Watson K, Del Olmo-Garcia MI. GLP-1 receptor agonists and cardiovascular disease in patients with type 2 diabetes. Pharmacotherapy. 2018;2018:4020492. doi: 10.1155/2018/4020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holst JJ. Incretin hormones and the satiation signal. Int J Obes. 2013;37(9):1161–1168. doi: 10.1038/ijo.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Ørskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol Endocrinol Metab. 1997;273(5):E981–E9E8. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 17.Bahtiyar G, Pujals-Kury J, Sacerdote A. Cardiovascular effects of different GLP-1 receptor agonists in patients with type 2 diabetes. Curr Diab Rep. 2018;18(10):92. doi: 10.1007/s11892-018-1043-z. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhury A, Duvoor C, Dendi R, Sena V, Kraleti S, Chada A, et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne). 2017;8:6. [DOI] [PMC free article] [PubMed]
- 19.Toft-Nielsen M-B, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86(8):3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 20.Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Intern Med. 2014;25(5):407–414. doi: 10.1016/j.ejim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11(3):202–230. doi: 10.1900/RDS.2014.11.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 23.Hui H, Farilla L, Merkel P, Perfetti R. The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. Eur J Endocrinol. 2002;146(6):863–869. doi: 10.1530/eje.0.1460863. [DOI] [PubMed] [Google Scholar]
- 24.Jackson SH, Martin TS, Jones JD, Seal D, Emanuel F. Liraglutide (victoza): the first once-daily incretin mimetic injection for type-2 diabetes. Pharmacol Ther. 2010;35(9):498. [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma D, Verma S, Vaidya S, Kalia K, Tiwari V. Recent updates on GLP-1 agonists: current advancements & challenges. Biomed Pharmacother. 2018;108:952–962. doi: 10.1016/j.biopha.2018.08.088. [DOI] [PubMed] [Google Scholar]
- 26.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Supplement 2):S279–SS84. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sethi B, Viswanathan V, Kumar A, Chatterjee S, Chandalia H. Liraglutide in clinical practice: insights from LEAD programme. JAPI. 2010;58:18–22. [Google Scholar]
- 28.Gough SC, Jain R, Woo VC. Insulin degludec/liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab. 2016;11(1):7–19. doi: 10.1586/17446651.2016.1113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 30.Buse JB, Committee LS. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J of Med. 2016;375(18):1798. doi: 10.1056/NEJMc1611289. [DOI] [PubMed] [Google Scholar]
- 31.Zinman B, Marso SP, Christiansen E, Calanna S, Rasmussen S, Buse JB. Hypoglycemia, Cardiovascular Outcomes, and Death: The LEADER Experience. Diabetes Care. 2018:dc172677. [DOI] [PubMed]
- 32.Mann JF, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB, LEADER Steering Committee and Investigators Liraglutide and renal outcomes in type 2 diabetes. N Engl J of Med. 2017;377(9):839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 33.Plutzky J, Garber A, Toft A, Poulter N, editors. Meta-analysis demonstrates that liraglutide, a once-daily human GLP-1 analogue, significantly reduces lipids and other markers of cardiovascular risk in type 2 diabetes. Diabetologia; 2009: SPRINGER 233 SPRING ST, NEW YORK, NY 10013 USA.
- 34.Fonseca VA, DeVries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complicat. 2014;28(3):399–405. doi: 10.1016/j.jdiacomp.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buse JB, Bain SC, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Pratley RE, Linder M, Monk Fries T, Ørsted DD, Zinman B, LEADER Trial Investigators Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care. 2020;43:1546–1552. doi: 10.2337/dc19-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matyjaszek-Matuszek B, Szafraniec A, Porada D. Pharmacotherapy of obesity - state of the art. Endokrynol Pol. 2018;69(4). [DOI] [PubMed]
- 37.Farr OM, Sofopoulos M, Tsoukas MA, Dincer F, Thakkar B, Sahin-Efe A, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59(5):954–965. doi: 10.1007/s00125-016-3874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders KH, Umashanker D, Igel LI, Kumar RB, Aronne LJ. Obesity pharmacotherapy. Med Clin North Am. 2018;102(1):135–148. doi: 10.1016/j.mcna.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP, SCALE Obesity and Prediabetes NN8022-1839 Study Group A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J of Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 40.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale P, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes. 2013;37(11):1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 41.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, Andreasen AH, Jensen CB, DeFronzo RA, for the NN8022-1922 Study Group Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 42.Blackman A, Foster G, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond) 2016;40(8):1310. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khera R, Pandey A, Chandar AK, Murad MH, Prokop LJ, Neeland IJ, et al. Effects of weight-loss medications on cardiometabolic risk profiles: a systematic review and network meta-analysis. Gastroenterology. 2018;154(5):1309–1319. doi: 10.1053/j.gastro.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garvey WT, Birkenfeld AL, Dicker D, Mingrone G, Pedersen SD, Satylganova A, Skovgaard D, Sugimoto D, Jensen C, Mosenzon O. Efficacy and safety of Liraglutide 3.0 mg in individuals with overweight or obesity and type 2 diabetes treated with basal insulin: the SCALE insulin randomized controlled trial. Diabetes Care. 2020;43(5):1085–1093. doi: 10.2337/dc19-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, Mastrandrea LD, Prabhu N, Arslanian S, NN8022-4180 Trial Investigators A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117–2128. doi: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 46.Iepsen EW, Zhang J, Thomsen HS, Hansen EL, Hollensted M, Madsbad S, Hansen T, Holst JJ, Holm JC, Torekov SS. Patients with obesity caused by Melanocortin-4 receptor mutations can be treated with a glucagon-like Peptide-1 receptor agonist. Cell Metab. 2018;28:23–32.e3. doi: 10.1016/j.cmet.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Wang FF, Wu Y, Zhu YH, Ding T, Batterham R, Qu F, et al. Pharmacologic therapy to induce weight loss in women who have obesity/overweight with polycystic ovary syndrome: a systematic review and network meta-analysis. Obes Rev. 2018;19(10):1424–1445. doi: 10.1111/obr.12720. [DOI] [PubMed] [Google Scholar]
- 48.Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):498–506. doi: 10.1016/j.bpobgyn.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 49.Yildiz BO. Polycystic ovary syndrome: is obesity a symptom? Womens Health (Lond) 2013;9(6):505–507. doi: 10.2217/whe.13.53. [DOI] [PubMed] [Google Scholar]
- 50.Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome and infertility: a new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. 2020;1(8):105. doi: 10.1210/clinem/dgaa285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod BioMed Online. 2019;39(2):332–342. doi: 10.1016/j.rbmo.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Crews KR, Hicks JK, Pui CH, Relling MV, Evans WE. Pharmacogenomics and individualized medicine: translating science into practice. Clin Pharmacol Ther. 2012;92(4):467–475. doi: 10.1038/clpt.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prasad RB, Groop L. Genetics of type 2 diabetes—pitfalls and possibilities. Genes. 2015;6(1):87–123. doi: 10.3390/genes6010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tkáč I, Gotthardová I. Pharmacogenetic aspects of the treatment of type 2 diabetes with the incretin effect enhancers. Pharmacogenomics. 2016;17(7):795–804. doi: 10.2217/pgs-2016-0011. [DOI] [PubMed] [Google Scholar]
- 55.de Luis DA, Soto GD, Izaola O, Romero E. Evaluation of weight loss and metabolic changes in diabetic patients treated with liraglutide, effect of RS 6923761 gene variant of glucagon-like peptide 1 receptor. J Diabetes Complicat. 2015;29(4):595–598. doi: 10.1016/j.jdiacomp.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 56.de Luis DA, Ovalle HF, Soto GD, Izaola O, de la Fuente B, Romero E. Role of genetic variation in the cannabinoid receptor gene (CNR1)(G1359A polymorphism) on weight loss and cardiovascular risk factors after liraglutide treatment in obese patients with diabetes mellitus type 2. J Investig Med. 2014;62(2):324–327. doi: 10.2310/JIM.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 57.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 58.Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev. 2002;18(Suppl 2):S10–S15. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- 59.Sola D, Rossi L, Schianca GP, Maffioli P, Bigliocca M, Mella R, Corlianò F, Fra GP, Bartoli E, Derosa G. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11(4):840–848. doi: 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 2000;133(1):73–74. doi: 10.7326/0003-4819-133-1-200007040-00016. [DOI] [PubMed] [Google Scholar]
- 61.Guardado-Mendoza R, Prioletta A, Jiménez-Ceja LM, Sosale A, Folli F. The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch Med Sci. 2013;9(5):936–943. doi: 10.5114/aoms.2013.34991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4) Best Pract Res Clin Endocrinol Metab. 2009;23(4):479–486. doi: 10.1016/j.beem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Kalra S. Sodium glucose co-Transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5(2):355–366. doi: 10.1007/s13300-014-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202–210. doi: 10.2337/ds16-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]