Abstract
Type 2 diabetes mellitus (T2DM) is global health problem. An estimated 425 million people in the world had diabetes in 2017. It is a major cause of morbidity and mortality worldwide. Although, pathogenesis of T2DM and its complications have been focus of medical research for long, much remains to be learned. A better understanding of molecular pathogenesis is essential for more effective preventive and therapeutic interventions. Role of mitochondria in pathogenesis of metabolic problems such as obesity, metabolic syndrome, and T2DM is the focus of many recent research studies. Mitochondrial dysfunction contributes to the oxidative stress and systemic inflammation leading to insulin resistance (IR). Mitochondria are also essential for pancreatic beta cell insulin secretion. Hence, mitochondria are important players in the pathogenesis of T2DM. In this article, pathogenesis of T2DM is examined from a mitochondrial perspective.
Keywords: Mitochondria, Diabetes, Diabetes mellitus, Metabolic syndrome, Mitochondrial dysfunction, Insulin resistance
Type 2 diabetes mellitus (T2DM) is associated with insulin resistance (IR) in peripheral tissues such as skeletal muscles, and adipose tissues. IR leads to hyperglycemia and overstimulation of pancreatic beta cells. Pancreatic beta cells adapt by increasing insulin secretion. However, persistent overstimulation causes beta cell functional impairment and loss. Loss of beta cells and its functional impairment in genetically predisposed individuals lead to progression from IR to T2DM. Mitochondria play significant roles in the pathogenesis of T2DM, from IR to beta cell impairment and loss.
Mitochondria
Mitochondria and oxidative phosphorylation (OXPHOS)
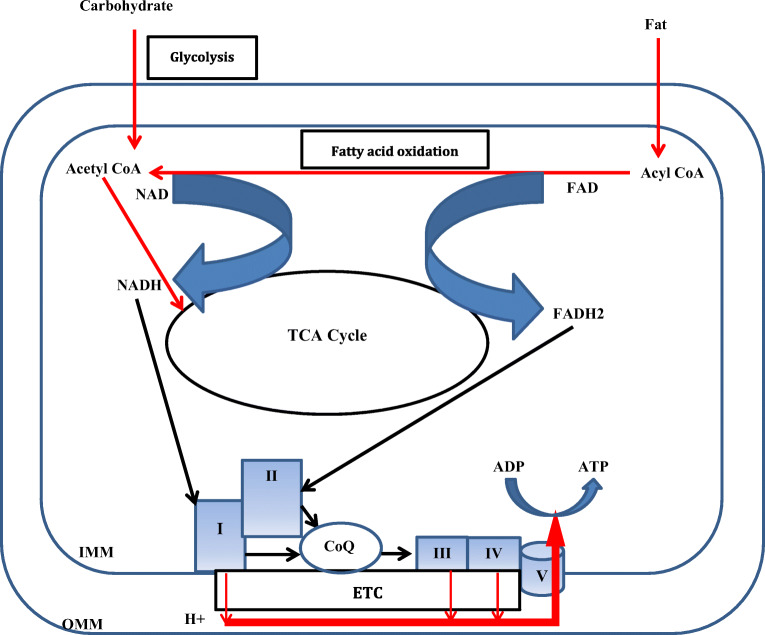
Mitochondria are also called the “power house” of cell as they are the main site of energy (ATP) production. It has a double membranous structure. Outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM) are separated by intermembranous space. Glucose and fatty acids derived from food are oxidized inside mitochondria via tricarboxylic acid (TCA) cycle and beta oxidation, respectively. The energy stored as chemical bonds in these food constituents are released as high energy electrons by these oxidative pathways. These high energy electrons are captured by nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD) molecules leading to generation of NADH and FADH2, respectively [1]. NADH and FADH2 donate these high energy electrons to the electron transport chain (ETC) located in IMM [2]. ETC consists of five complexes - Complex I to V. Complex I receives electron from the NADH. Complex II accepts electrons from FADH2. Complex I and II donate electrons to coenzyme Q (CoQ). CoQ can freely diffuse through the IMM to complex III. Complex III accepts electron from the CoQ and reduces complex IV. Electrons are finally transferred to oxygen by complex IV to generate water. As the electron moves along this chain, free energy is released which is used to pump protons at complexes I, III, and IV from the mitochondrial matrix to the intermembranous space thus creating a proton gradient. Protons diffuse along this electrochemical gradient at complex V where energy released by the proton drive across the IMM is used to generate ATP from ADP (Fig. 1). This process of ATP generation is also called OXPHOS. Thus, mitochondria has critical role in energy metabolism. Beta cells of pancreatic cells are metabolically very active and are primarily dependent on mitochondria for energy production needed for insulin secretion [3].
Fig. 1.
Diagrammatic representation of the role of mitochondria in energy metabolism inside cell. I: Complex I; II: Complex II; III: Complex III, IV: Complex IV; V: Complex V; CoQ: Coenzyme Q; ETC: Electron transport chain; FAD: Flavin adenine dinucleotide; IMM: Inner mitochondrial membrane; NAD: Nicotinamide adenine dinucleotide; OMM: Outer mitochondrial membrane; TCA: Tricarboxylic acid. Energy stored in food constituents are captured by NAD and FAD. The high energy electrons are then transferred to ETC where it gradually moves from high energy state to low energy state. The energy released in this process is harnessed to propel protons across the IMM creating a proton gradient across the IMM. Protons then diffuse along its concentration gradient at V. The energy released in this process is harnessed to generated ATP from ADP
Mitochondrial and cellular oxidative stress (OS)
Apart from the main site of energy production, mitochondria are also important source of reactive oxygen species (ROS) generation [4, 5]. ATP is generated by controlled movement of electrons along the ETC from a high energy state to low energy state in a step wise manner. However, a very small fraction of these high energy electrons (approximately 2%) leak from the ETC and may react directly to oxygen generating superoxide radicals. Superoxide radicals are the main source of ROS inside mitochondria. Superoxide radicals are short lived and get enzymatically or spontaneously converted to hydrogen peroxide. Hydrogen peroxide may react with superoxide radical or undergo Fenton reaction with Iron to produce hydroxyl radicals [6, 7]. In addition, superoxide radicals can react with nitrous oxide to generate peroxynitrite radicals [8]. Peroxynitrite and hydroxyl radicals are highly reactive and can damage numerous cellular constituents such as membranes, proteins, enzymes, and DNA which may cause cellular damage and ultimately cellular death. However, mitochondria have a very efficient antioxidant mechanism. It consists of superoxide dismutase which converts superoxide radicals to hydrogen peroxide which is then converted to water by the enzymes, peroxiredoxins and glutathione peroxidase. Thus, the production of toxic hydroxyl and peroxynitrite radicals is minimized. ETC dysfunction may result in excessive electron leak and thus excessive ROS generation and cellular injury. An overproduction of ROS which exceeds cellular antioxidant defense results in damage of cellular macromolecules and alters cellular functions and viability. This is called OS [9]. OS is considered the most crucial mechanism of beta cell functional impairment and loss, predisposing to T2DM.
Mitochondria and cell death
Mitochondrial play crucial role in cell death. Cell death can be either programmed (apoptosis) or un-programmed (necrosis). Pathophysiological mechanism of apoptosis consists of activation of a group of proteases called caspases [10]. Caspases cause demolition of numerous cellular substrates leading to cell death. Apoptosis can be initiated by intrinsic and extrinsic pathways [10, 11]. Apoptosis by intrinsic pathway can be initiated by different stimuli such as DNA damage and other ROS mediated cellular injury. The ultimate outcome of these stimuli is mitochondrial outer membrane permeabilization (MOMP) and release of proapoptotic factors such as cytochrome c and apoptosis inducing factor (AIF) from the mitochondrial intermembranous space to the cytoplasm. Cytochrome c activates a cascade of reactions in the cytoplasm leading to caspase 3 (an executioner caspase) activation resulting in apoptosis [12]. AIF localizes to nucleus and causes DNA fragmentation [13]. Apoptosis is coordinated and energy dependent process. However, necrosis is result of energy failure. Excessive ROS injury and intramitochondrial calcium accumulation lead to creation of mitochondrial permeability transition pores (MPTP) in IMM which causes collapse of mitochondrial membrane potential, ATP depletion, mitochondrial swelling, and rapid cell death (necrosis) [14]. Mitochondria play significant role in pancreatic beta cell loss secondary to excessive ROS mediated injury.
Insulin resistance
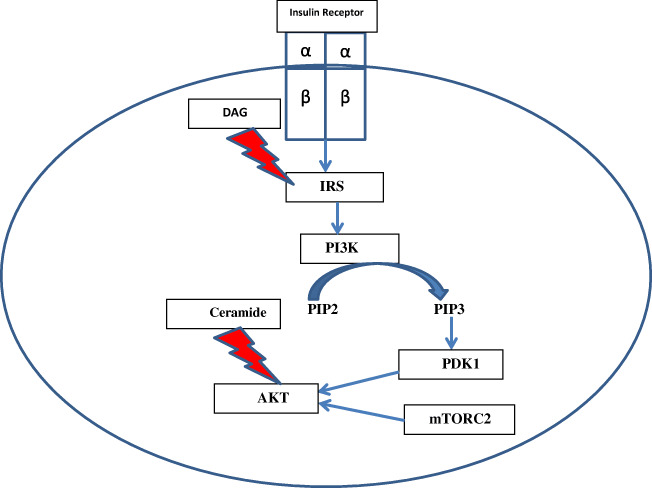
Insulin exerts its effect by binding to insulin receptors on cell surface. Insulin receptor has an extracellular (2 α), and an intracellular domain (2 β) [15]. Intracellular domain has tyrosine kinase activity. Upon binding of insulin to the extracellular domain, intracellular domain is activated which leads to phosphorylation at tyrosine residues of insulin receptor substrate (IRS) [16]. IRS activates phosphoinositide 3-kinase (PI3K) which then catalyzes phosphorylation of phosphatidylinositol bisphosphate (PIP2) to phosphatidylinositol triphosphate (PIP3) [17]. PIP3 activates phosphoinositide dependent protein kinase (PDK) 1. PDK1 together with mammalian target of rapamycin complex 2 (mTORC2), activates serine threonine kinase (AKT) [18]. AKT (also known as protein kinase B or PKB) activation leads to metabolic effects of insulin such as glycogen synthesis and trafficking of glucose transporter type 4 (GLUT4) receptors to cell membrane (Fig. 2) [19].
Fig. 2.
Insulin signaling pathway. Binding of insulin to the extracellular (α) domain leads to phosphorylation of IRS by the β domain, which in turn activates PI3K leading to conversion of PIP2 to PIP3. PIP3 activates PDK1. AKT is activated by the combined actions of PDK1 and mTORC2. AKT activation results in metabolic actions of insulin. DAG and ceramide inhibits insulin signaling by inhibiting IRS and AKT, respectively. DAG: Diacylglycerol; IRS: insulin receptor substrate; mTORC2: mammalian target of rapamycin complex 2; PDK1: phosphoinositide dependent protein kinase 1; PI3K: phosphoinositide 3-kinase; PIP2: phosphatidylinositol bisphosphate; PIP3: phosphatidylinositol triphosphate
T2DM is usually associated with excessive nutrition and sedentary lifestyle resulting in positive calorie balance. Nutrient excess leads to compensatory increase in mitochondrial fatty acid oxidation. However, chronic nutrition oversupply overwhelms mitochondrial fatty acid oxidation capacity leading to intramyocellular fatty acid deposition. These fatty acids may be channeled towards synthesis of toxic lipids such as ceramide and diacylglerol [20]. Ceramide and diacylglycerol accumulation results in IR in skeletal muscles and adipose tissues by directly inhibiting the insulin signaling pathway (Fig. 2) [21, 22]. IR in adipose tissues augments lipolysis, consequently there is increased availability of free fatty acids to skeletal muscles thus creating a vicious cycle. Moreover, chronic nutritional overload leads to excessive reducing equivalent (NADH and FADH2) generation in mitochondria via TCA cycle and fatty acid oxidation. Nutritional overload leads to over activity of ETC and enhanced ROS production. Excessive ROS production overwhelms the antioxidant defense systems causing OS. ROS impairs mitochondrial function by damaging ETC complexes [23]. Thus substrate handling by mitochondria is impaired. More fatty acids are channeled towards generation of toxic intracellular lipids aggravating the IR. ROS also directly inhibit insulin signaling pathway further contributing to IR [24]. Excessive ROS damage beta cell membranes, and intracellular proteins, enzymes, and DNA which may precipitate beta cell death [25]. Moreover, mitochondrial damage induced by OS leads to enhanced mitochondrial fission and further decline in oxidative phosphorylation as well as aggravation of ROS generation, thus creating a vicious cycle [26, 27].
In addition, sedentary life style associated with T2DM leads to decrease in mitochondrial protein content due to decreased mitochondrial biogenesis contributing to mitochondrial dysfunction [28]. However, decline in mitochondrial function may also be secondary to IR. Insulin resistant cells were shown to have reduced mitochondrial energy production and increased susceptibility to oxidative stress [29]. Thus, mitochondrial dysfunction contributes to IR in several ways. Whether mitochondrial dysfunction is primary contributor to IR or secondary to IR is still debated, but its role in propagation of IR is established. Thus, boosting mitochondrial function is an important and proven strategy to increase insulin sensitivity.
Pancreatic beta cell dysfunction and loss
Mitochondrial dysfunction also plays significant role in pancreatic beta cell failure and progression from insulin resistance to T2DM [30, 31]. Insulin secretion by pancreatic beta cells is tightly coupled to extracellular glucose concentration [32]. After uptake by glucose transporters (GLUT2), glucose is phosphorylated by glucokinase and then metabolized to pyruvate in cytosol. Subsequently, pyruvate enters mitochondria via mitochondrial pyruvate carrier and oxidized via tricarboxylic acid (TCA) cycle leading to generation of NADH and FADH2 [33]. NADH and FADH2 molecules donate these high energy electrons to the ETC where ATP is generated. Mitochondrial ATP is transported to cytosol raising cytosolic ATP/ADP ratio. This leads to closure of ATP-dependent K+ channel on cell membrane of beta cells resulting in its depolarization. This triggers opening of voltage dependent calcium channels on plasma membrane and rise of intracellular calcium which triggers exocytosis of insulin containing vesicles [34].
Although insulin secretion is primarily driven by glucose, other metabolites such as free fatty acids, long chain acyl CoA, and glutamate potentiates insulin secretion [35–37]. Beta cells of pancreas are unique in that most of the pyruvate derived from glucose enters mitochondria to be metabolized by TCA cycle enabling tight coupling of extracellular glucose concentration to insulin secretion [35]. Thus, mitochondrial OXPHOS and resulting ATP generation is the main trigger for insulin secretion. Decreased expression of OXPHOS genes was indeed found in beta cells from patients with T2DM [38].
A mitochondrial DNA mutation, m.3243A > G in MT-TL1, leads to pancreatic beta cell failure and diabetes. Clinical manifestation of this mutation depends on the level of heteroplasmy. There are hundreds to thousands of mitochondria in a single cell. Hence, a single cell may harbor mtDNA with pathogenic mutation and wild type mtDNA together. This is called heteroplasmy and measured as percentage of mtDNA with the pathogenic mutation [39]. Heteroplasmy level may vary between different cells of a tissue, between different tissues of an individual, and between different individuals carrying same mtDNA mutation. A higher heteroplasmy level may lead to clinical symptoms while a low level of heteroplasmy may be clinically silent. When present at high level of heteroplasmy, m.3243A > G mutation causes a distinct clinical syndrome called MELAS (Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-like episodes) [40]. Same mutation at low heteroplasmy level may cause maternally inherited diabetes mellitus with or without deafness [41, 42]. Diabetes mellitus is also predominant manifestation of other primary mitochondrial diseases such as Kearns Sayre Syndrome, and MERRF (Myoclonic Epilepsy with Ragged Red Fibers). Diabetes mellitus whether isolated or associated with other clinical features of a mitochondrial disease due to pathogenic mtDNA mutation is also called mitochondrial diabetes [43, 44]. The primary mechanism of mitochondrial diabetes is beta cell failure, although IR may be associated. It is a separate entity from T2DM, but does underscore the role of mitochondrial dysfunction in pancreatic beta cell failure.
As mentioned above, T2DM is preceded by IR and chronic hyperglycemia. Pancreatic beta cells adapt to this by increasing insulin secretion. However, chronic overstimulation of beta cells leads to its decompensation and failure. Increased glucose and fatty acid metabolism in beta cells in the setting of hyperglycemia and hypertriglyceridemia lead to increased ETC activity and consequently increased ROS generation [24, 45]. This results in beta cell OS. OS predisposes to mitochondrial damage and enhanced mitochondrial fission [26, 27, 46]. Accelerated mitochondrial fission leads to further decline in OXPHOS and increase in ROS generation. Progressive mitochondrial damage leads to initiation of apoptosis pathway and beta cell loss [14, 47]. Once the beta cell loss reaches threshold in genetically predisposed individuals, T2DM ensues.
Moreover, the increased demand for insulin synthesis in endoplasmic reticulum(ER) leads to ER stress. There is accumulation of unfolded precursor protein in ER which causes activation of unfolded protein response (UPR). UPR in early stages tends to restore protein folding but when it progresses beyond threshold, it leads to activation of mitochondrial apoptosis pathway and cell death [47, 48]. In addition, Beta cell OS contributes to ER stress and the ER stress leads to further worsening of OS [49, 50]. One consequence of ER stress is increased intracellular calcium due to release of calcium from the ER [51]. Raised intracellular calcium activates calpain mediated apoptosis pathway [52]. There is also increased uptake of calcium in mitochondria at mitochondria associated ER membranes (MAMs) [53]. Increased intramitochondrial calcium aggravates intramitochondrial oxidative stress and triggers apoptosis of beta cells [51].
Conclusion
Role of mitochondria in pathogenesis of T2DM is multifactorial. Mitochondrial dysfunction in skeletal muscle and adipose tissues contributes to IR. On the other hand, pancreatic beta cell mitochondrial dysfunction contributes to impairment of insulin secretion and loss of beta cells. Hence, interventions to increase mitochondrial biogenesis and function have role in treatment of T2DM.
Author contribution
Dr. Pankaj Prasun drafted the initial and all subsequent versions of the manuscript and has approved the article as it is written.
Funding
Dr. Pankaj Prasun does not have any funding sources to declare related to the study and to the article preparation.
Compliance with ethical standards
Declaration of conflicting interests
Dr. Pankaj Prasun has no potential conflicting or competing interests that could in any way affect the conduct of the study, interpretation of results, or preparation of the manuscript.
This review does not require ethics committee approval at this institution.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Walsh CT, Tu BP, Tang Y. Eight kinetically stable but thermodynamically activated molecules that power cell metabolism. Chem Rev. 2018;118:1460–1494. doi: 10.1021/acs.chemrev.7b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun F, Zhou Q, Pang X, Xu Y, Rao Z. Revealing various coupling of electron transfer and proton pumping in mitochondrial respiratory chain. Curr Opin Struct Biol. 2013;23:526–538. doi: 10.1016/j.sbi.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Maechler P, Li N, Casimir M, Vetterli L, Frigerio F, Brun T. Role of mitochondria in beta-cell function and dysfunction. Adv Exp Med Biol. 2010;654:193–216. doi: 10.1007/978-90-481-3271-3_9. [DOI] [PubMed] [Google Scholar]
- 4.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastor N, Weinstein H, Jamison E, Brenowitz M. A detailed interpretation of OH radical footprints in a TBP-DNA complex reveals the role of dynamics in the mechanism of sequence-specific binding. J Mol Biol. 2000;304:55–68. doi: 10.1006/jmbi.2000.4173. [DOI] [PubMed] [Google Scholar]
- 7.Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxidative Med Cell Longev. 2011;2011:809696. doi: 10.1155/2011/809696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanaan GN, Harper ME. Cellular redox dysfunction in the development of cardiovascular diseases. Biochim Biophys Acta Gen Subj. 1861;2017:2822–2829. doi: 10.1016/j.bbagen.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 10.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons MJ, Green DR. Mitochondria in cell death. Essays Biochem. 2010;47:99–114. doi: 10.1042/bse0470099. [DOI] [PubMed] [Google Scholar]
- 12.Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 2015;112:957–962. doi: 10.1038/bjc.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulbins E, Dreschers S, Bock J. Role of mitochondria in apoptosis. Exp Physiol. 2003;88:85–90. doi: 10.1113/eph8802503. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong JA, Cash NJ, Ouyang Y, Morton JC, Chvanov M, Latawiec D, Awais M, Tepikin AV, Sutton R, Criddle DN. Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift. J Biol Chem. 2018;293:8032–8047. doi: 10.1074/jbc.RA118.003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson KM, Hu C, Lemmon MA. Insulin and epidermal growth factor receptor family members share parallel activation mechanisms. Protein Sci. 2020;29:1331–1344. doi: 10.1002/pro.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaburagi Y, Yamauchi T, Yamamoto-Honda R, et al. The mechanism of insulin-induced signal transduction mediated by the insulin receptor substrate family. Endocr J. 1999;46(Suppl):S25–S34. doi: 10.1507/endocrj.46.suppl_s25. [DOI] [PubMed] [Google Scholar]
- 17.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 18.Paez J, Sellers WR. PI3K/PTEN/Akt pathway. In: Frank DA, editor. Signal transduction in Cancer. Cancer treatment and research. Boston: Springer; 2004. [Google Scholar]
- 19.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6:a009191. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sergi D, Naumovski N, Heilbronn LK, Abeywardena M, O'Callaghan N, Lionetti L, Luscombe-Marsh N. Mitochondrial (Dys)function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front Physiol. 2019;10:532. doi: 10.3389/fphys.2019.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen MC, Shulman GI. Roles of Diacylglycerols and Ceramides in hepatic insulin resistance. Trends Pharmacol Sci. 2017;38:649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- 24.Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biom J. 2017;40:257–262. doi: 10.1016/j.bj.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mailloux RJ. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants (Basel) 2020;9:E472. doi: 10.3390/antiox9060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galloway CA, Lee H, Nejjar S, Jhun BS, Yu T, Hsu W, Yoon Y. Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes. 2012;61:2093–2104. doi: 10.2337/db11-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol. 2017;11:637–645. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burkart AM, Tan K, Warren L, Iovino S, Hughes KJ, Kahn CR, Patti ME. Insulin resistance in human iPS cells reduces mitochondrial size and function. Sci Rep. 2016;6:22788. doi: 10.1038/srep22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diabetes Res. 2012;703538. [DOI] [PMC free article] [PubMed]
- 31.Fex M, Nicholas LM, Vishnu N, Medina A, Sharoyko VV, Nicholls DG, Spégel P, Mulder H. The pathogenetic role of β-cell mitochondria in type 2 diabetes. J Endocrinol. 2018;236:R145–R159. doi: 10.1530/JOE-17-0367. [DOI] [PubMed] [Google Scholar]
- 32.Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9:25–53. [PMC free article] [PubMed] [Google Scholar]
- 33.Brun T, Maechler P. Beta-cell mitochondrial carriers and the diabetogenic stress response. Biochim Biophys Acta. 1863;2016:2540–2549. doi: 10.1016/j.bbamcr.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman BA, Li C, Soleimanpour SA. Mitochondrial regulation of β-cell function: maintaining the momentum for insulin release. Mol Asp Med. 2015;42:91–104. doi: 10.1016/j.mam.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam MS. Stimulus-secretion coupling in Beta-cells: from basic to bedside. Adv Exp Med Biol. 2020;1131:943–963. doi: 10.1007/978-3-030-12457-1_37. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 37.Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in β cells. J Biol Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 38.Olsson AH, Yang BT, Hall E, Taneera J, Salehi A, Dekker Nitert M, Ling C. Decreased expression of genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2 diabetes. Eur J Endocrinol. 2011;165:589–595. doi: 10.1530/EJE-11-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuppen HA, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim Biophys Acta. 2010;1797:113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 40.El-Hattab AW, Almannai M, Scaglia F. MELAS. 2001 Feb 27 [updated 2018 Nov 29]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [internet]. Seattle: University of Washington, Seattle; 1993-2020.
- 41.Robinson KN, Terrazas S, Giordano-Mooga S, Xavier NA. The role of heteroplasmy in the diagnosis and management of maternally inherited diabetes and defaness. Endocr Pract. 2020;26:241–246. doi: 10.4158/EP-2019-0270. [DOI] [PubMed] [Google Scholar]
- 42.McMillan RP, Stewart S, Budnick JA, et al. Quantitative variation in m.3243A > G mutation produce discrete changes in energy metabolism. Sci Rep. 2019;9:5752. doi: 10.1038/s41598-019-42262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karaa A, Goldstein A. The spectrum of clinical presentation, diagnosis, and management of mitochondrial forms of diabetes. Pediatr Diabetes. 2015;16:1–9. doi: 10.1111/pedi.12223. [DOI] [PubMed] [Google Scholar]
- 44.Maassen JA, T Hart LM, Van Essen E, et al. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53 Suppl 1:S103–S109. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- 45.Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies [published online ahead of print, 2020 Apr 4] Front Med. 2020;14:583–600. doi: 10.1007/s11684-019-0729-1. [DOI] [PubMed] [Google Scholar]
- 46.Archer SL. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 47.Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol. 2016;56:R33–R54. doi: 10.1530/JME-15-0232. [DOI] [PubMed] [Google Scholar]
- 48.Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujii J, Homma T, Kobayashi S, Seo HG. Mutual interaction between oxidative stress and endoplasmic reticulum stress in the pathogenesis of diseases specifically focusing on non-alcoholic fatty liver disease. World J Biol Chem. 2018;9:1–15. doi: 10.4331/wjbc.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med. 2017;49:e291. doi: 10.1038/emm.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Momeni HR. Role of calpain in apoptosis. Cell J. 2011;13:65–72. [PMC free article] [PubMed] [Google Scholar]
- 53.Burgos-Morón E, Abad-Jiménez Z, Marañón AM, Iannantuoni F, Escribano-López I, López-Domènech S, Salom C, Jover A, Mora V, Roldan I, Solá E, Rocha M, Víctor VM. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the Battle continues. J Clin Med. 2019;8:1385. doi: 10.3390/jcm8091385. [DOI] [PMC free article] [PubMed] [Google Scholar]