Abstract
Objective
We investigated the efficacy and safety of sugammadex doses calculated using corrected body weight (CBW) for reversing deep rocuronium-induced neuromuscular blockade (NMB) in morbidly obese patients undergoing laparoscopic bariatric surgery.
Methods
One hundred and twenty-five morbidly obese patients were randomly assigned to three groups: (1) a CBW group, n = 50; (2) a total body weight (TBW) group, n = 50; and (3) a control group, n = 25. Deep NMB was maintained using a continuous infusion of rocuronium. At the reappearance of 1 to 2 post-tetanic counts (PTCs), 4 mg/kg sugammadex, calculated using CBW or TBW, were administered.
Results
All the participants in the CBW and TBW groups recovered to a train-of-four (TOF) ratio of 0.9 within 5 minutes. The recovery times from the start of sugammadex administration to a TOF ratio of 0.9 were 2.2 ± 0.7 and 2.0 ± 0.7 minutes in the CBW and TBW groups, respectively. Thus, a sugammadex dose calculated using CBW was not inferior to that calculated using TBW for the reversal of rocuronium-induced deep NMB in morbidly obese patients.
Conclusion
A dose of 4 mg/kg of sugammadex calculated using CBW is efficient and safe for the reversal of deep NMB after a continuous infusion of rocuronium in morbidly obese patients.
Clinical Trial Registration Number
ChiCTR1900028652 (Chinese Clinical Trial Registry, www.chictr.org.cn)
Keywords: Sugammadex, neuromuscular blockade, rocuronium, morbid obesity, laparoscopic bariatric surgery, corrected body weight
Introduction
The increasing prevalence of overweight and obesity (defined as body mass index (BMI) ≥30 kg/m2) has become a major global public health problem in recent years. Globally, the combined prevalence of overweight and obesity in adults has increased by 27.5%, and the number of overweight and obese individuals increased from 921 million to 2.1 billion between 1980 and 2013.1 A previous study predicted that the global prevalence of obesity will reach 18% in men and exceed 21% in women by 2025.2 Many epidemiological studies have confirmed that obesity is associated with multiple chronic diseases, including coronary heart disease, type 2 diabetes, stroke, hypertension, and several cancers.3 Obesity has been reported to be closely associated with high all-cause mortality,4 and morbidly obese individuals (BMI ≥40 kg/m2) have a higher risk of mortality.5 Laparoscopic bariatric surgery is the most effective method of achieving sustainable weight loss and a reduction in weight-associated comorbidities.6,7
Deep neuromuscular blockade (NMB)8 during laparoscopic bariatric surgery facilitates ventilation, provides stable surgical conditions, and is associated with less postoperative pain.9,10 However, general anesthesia with deep NMB in morbidly obese patients creates many challenges for anesthesiologists. Obesity is an independent predictor of difficult mask ventilation and intubation.11 It is also related to the development of obstructive sleep apnea and obesity hypoventilation syndrome, which is associated with excess morbidity and mortality.12 A complete recovery of neuromuscular function after general anesthesia is necessary because postoperative residual curarization (PORC) results in upper airway obstruction, hypoxia, and postoperative pulmonary complications.13,14 Thus, expeditious and reliable reversal of NMB at the end of the surgery is necessary to ensure patient safety and comfort. The non-depolarizing neuromuscular blocking agent rocuronium is frequently used in general anesthesia, and sugammadex (Bridion®, MSD, Oss, The Netherlands) is a novel selective antagonist for the reversal of NMB induced by rocuronium and vecuronium. Sugammadex, a modified γ-cyclodextrin, reduces the concentration of rocuronium at the neuromuscular junction by selectively encapsulating free rocuronium molecules.15 It has been reported that sugammadex is more effective than neostigmine for the rapid, safe, and reliable reversal of rocuronium- and vecuronium-induced NMB.16
The use of sugammadex has changed conventional clinical practice and resolved many problems; however, it has also created new challenges. The effective dose of sugammadex has been determined only in non-obese patients, using their TBW. However, the optimal dose in obese patients, and especially in morbidly obese patients, has yet to be definitively established. Furthermore, some anesthesiologists still hesitate to use sugammadex because of its relatively high cost compared with that of neostigmine. The doses of most drugs are calculated using ideal body weight (IBW) in morbidly obese patients. However, the dose of sugammadex calculated using IBW is insufficient for the reversal of deep NMB in morbidly obese patients.17 Therefore, some authors have suggested that its dose should be calculated on the basis of corrected body weight (CBW), using the formula CBW = IBW+0.4×(TBW−IBW).18,19 We hypothesized that a sugammadex dose calculated on the basis of CBW would be as effective as that calculated on the basis of TBW in terms of the time to recovery of the train-of-four (TOF) ratio after deep NMB. In the present study, we aimed to determine the efficacy and safety of sugammadex doses calculated on the basis of CBW for the reversal of rocuronium-induced deep NMB in morbidly obese patients undergoing laparoscopic bariatric surgery.
Patients and methods
Study design and patient selection
This was a randomized, single-center, parallel-group, safety assessor-blinded study (www.chictr.org.cn; ChiCTR1900028652). The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and current regulatory recommendations; and was approved by the Ethics Committee for Drug Clinical Trials & Study, the Fourth Affiliated Hospital, China Medical University, Shenyang, P. R. China (approval reference: ECDCTS-2019-HS-002, date of approval: 28 October 2019). We have reported the results in accordance with the CONSORT guidelines for randomized trials.
Patients were randomly assigned to treatment groups using computer-generated numbers. Blinding was accomplished using closed envelopes that were opened before a procedure by a research assistant. The participants were blinded to data collection. They were randomized at a 2:2:1 ratio to be administered sugammadex on the basis of TBW or CBW, or not to be administered an antagonist (the control group).
Patients were enrolled if they were aged between 18 and 60 years, had American Society of Anesthesiologists (ASA) physical status I–III, had BMI ≥40 kg/m2, agreed to participate in the study, gave their written informed consent, and were scheduled to undergo laparoscopic bariatric surgery under general anesthesia, with the use of rocuronium for tracheal intubation and the maintenance of deep NMB. The exclusion criteria were as follows: contraindications for the use of aminosteroid muscle relaxants, significant hepatic or renal dysfunction, known or suspected neuromuscular disease, concurrent use of medications known to influence the effect of NMB agents, and allergy to sugammadex or general anesthetic agents. The participants were weighed on the day of surgery to obtain their TBW.
Anesthesia
All the participants were placed in the reverse Trendelenburg position to make their airway management easier. Standard monitoring techniques (electrocardiography, invasive arterial pressure, capnography, pulse oximetry, and gas monitoring) were used, and the bispectral index was measured. The participants were premedicated by intravenous administration of 0.075 mg palonosetron to relieve postoperative nausea and vomiting. Oxygen was administered for 5 minutes before the induction of anesthesia with intravenous sufentanil and propofol. Sevoflurane and a continuous infusion of remifentanil were used to maintain anesthesia and to maintain the bispectral index between 40 and 60. The patients’ central and peripheral body temperatures were maintained above 35°C and 32°C, respectively. Ventilatory support and anesthesia were appropriately maintained until a TOF ratio of ≥0.9 was achieved and the patient was judged by the anesthesiologist to be ready for tracheal extubation.20
Neuromuscular monitoring
Neuromuscular function was monitored using a TOF-Watch® SX acceleromyograph (Organon Ltd., Dublin, Ireland) that was attached to the adductor pollicis muscle immediately after the induction of anesthesia, but before rocuronium administration, according to the Good Clinical Research Practice guidelines for pharmacodynamics studies of neuromuscular blocking agents.21 Following the induction of anesthesia, the TOF-Watch® SX was calibrated and stabilized, as recommended by the manufacturer, in the operating room. Repetitive TOF stimulation was applied to the ulnar nerve every 15 s until the end of the anesthesia or at least until the TOF ratio recovered to 0.9. Neuromuscular data were collected through a transducer affixed to the thumb and transferred to a computer using the TOF-Watch® SX Monitoring Program.
After the TOF-Watch® SX was set up, the participants were administered 0.6 mg/kg rocuronium within a 10-s period by intravenous infusion. Tracheal intubation was performed, then a continuous infusion of 9 µg/kg/minute rocuronium was commenced, with the dose being adjusted to maintain a depth of NMB of zero response to TOF and a post-tetanic count (PTC) ≤2 during surgery. When it was judged to be clinically appropriate by the anesthesiologist, the continuous infusion of rocuronium was stopped and the patient was allowed to recover, until the reappearance of 1 to 2 PTCs.
A single dose of sugammadex of 4 mg/kg, based on the CBW or TBW, was administered within 10 s, and the time was recorded. No antagonist was administered to the control group. The time between the discontinuation of rocuronium administration and the recovery of the TOF ratio to 0.9 was recorded. After admission to the post-anesthesia care unit (PACU), the TOF ratio was again measured by a blinded investigator. The participants were also assessed every 15 minutes for clinical signs of neuromuscular recovery until their discharge to the surgical ward. The assessments included the level of consciousness (awake and oriented, arousable with minimal stimulation, or responsive only to tactile stimulation), muscle strength (ability to independently transfer from surgery table to bed, head tilting, and hand squeezing), and a 5-s head lift test. The respiratory frequency and pattern of the patient were monitored and pulse oximetry was performed in the PACU for at least 2 hours after the recovery of the TOF ratio to 0.9.
Efficacy analyses
The primary efficacy parameter was the length of time between the start of sugammadex administration or the end of rocuronium administration for the control group and the recovery of the TOF ratio to 0.9. The secondary efficacy parameters were the lengths of time from the start of sugammadex administration or the end of rocuronium administration for the control group to the recovery of the TOF ratio to 0.7 or 0.8.
Safety assessments
Safety assessment was performed by a blinded safety assessor who performed physical examination, monitored vital signs, and recorded adverse events (AEs), including serious adverse events, during the period the participants remained in the PACU, during postoperative visits on the day after surgery or ≥10 hours after study drug administration, and on day 8 of the follow-up period. To ensure blinding with respect to participant information, the safety assessor was not involved in the randomization process or the preparation of the trial medication and was not present in the operating room during surgery. In addition, the patients’ mean arterial pressure (MAP) and heart rate (HR) were recorded before, and after 1, 5, 10, and 30 minutes of sugammadex administration.
Statistical analysis
The primary efficacy analysis was performed on an intention-to-treat (ITT) basis; i.e., in all the randomized patients who had been administered sugammadex and had had at least one efficacy assessment made. Imputed recovery times to the TOF ratios of 0.7, 0.8, and 0.9 were used for missing data in the efficacy analysis, and the method used for the imputation was that used previously.20 An all-subjects-treated (AST) group was used for the safety analysis, which included all the randomized patients who were administered a dose of study medication.
The power analysis for this study was based on non-inferiority. For the primary study endpoint, the margin of non-inferiority was set at 0.5 minutes on the basis of previous study findings22 and clinical experience. The mean time for the TOF ratio to recover to 0.9 was predicted to be 2 minutes in the TBW group and the standard deviation was predicted to be 1 minute. With a predicted 10% difference between the CBW and TBW groups and a predicted loss to follow up of 10%, 50 patients in each group were required for a type I error rate of 2.5% (one-sided) and a power of 90%.
The distributions of continuous variables were assessed using the Shapiro–Wilk test. Continuous datasets with skewed distributions are summarized as median and interquartile range; the Mann–Whitney U-test was used to compare continuous data between two groups and the Kruskal–Wallis test was used to compare data among three groups. Variables that were characterized by normally distributed continuous data are summarized as means and SDs or 95% confidence intervals (CIs), and the three groups were compared using one-way ANOVA. Categorical datasets were compared using Pearson’s chi-square test. If at least one cell of the contingency table had an expected count of less than five, the likelihood ratio test was used. Statistical significance was set at P < 0.05. All analyses were performed using the SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
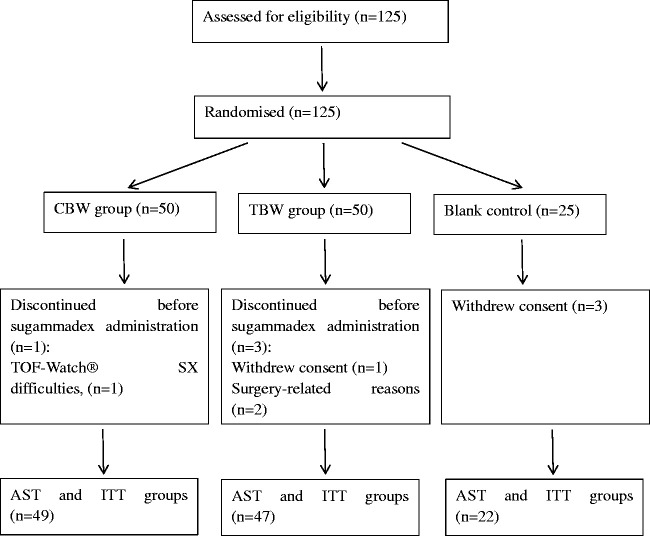
A total of 125 patients were initially randomized: 50 to the CBW group, 50 to the TBW group, and 25 to the control group (Figure 1). Seven patients (CBW group, n = 1; TBW group, n = 3; control group, n = 3) dropped out of the study before they were administered rocuronium or sugammadex (Figure 1) and were not included in the ITT or AST analyses. Thus, 118 participants completed the study (CBW group, n = 49; TBW group, n = 47; control group, n = 22).
Figure 1.
Flowchart of patient disposition. A total of 125 patients were enrolled in the study. Seven participants (CBW, n=1; TBW, n=3; Control, n=3) dropped out of the study. The CBW group was administered 4 mg/kg sugammadex, calculated on a CBW basis; the TBW group was administered 4 mg/kg sugammadex, calculated on a TBW basis; and the Control group was not administered this drug. Participants who dropped out of the study before being administered the study drug were not included in the ITT or AST analyses; the efficacy and safety data were obtained for the remaining 118 patients (CBW group, n=49; TBW group, n=47; Control group, n=22). The ITT and AST groups comprised 118 patients.
CBW, corrected body weight; TBW, total body weight; ITT, intention to treat; AST, all-subjects-treated.
The demographic characteristics, duration of anesthesia, duration of surgery, and rocuronium dose for the participants are presented in Table 1. No differences were observed with respect to sex, age, body weight, height, BMI, CBW, IBW, ASA class, or duration of surgery among the three groups. The doses of rocuronium used during surgery were also similar in the three groups. However, the duration of anesthesia was significantly longer in the control group than in the CBW and TBW groups (P < 0.001). This was because the lack of antagonist administration lengthened the time taken for the TOF ratio to recover to 0.9. The dose of sugammadex administered to the CBW group was significantly lower than that administered to the TBW group (P < 0.001).
Table 1.
Characteristics of the participants in the three groups and the anesthesia and surgery performed.
| CBW Group (n = 49) | TBW Group (n = 47) | Control group (n = 22) | |
|---|---|---|---|
| Sex | |||
| Male | 18 (36.7%) | 13 (27.7%) | 7 (31.8%) |
| Female | 31 (63.3%) | 34 (72.3%) | 15 (68.2%) |
| Age (years) | 31.4 ± 7.3 | 31 ± 6.8 | 29.7 ± 6.8 |
| Weight (kg) | 132 (121.5–146.5) | 128 (118–144) | 132.5 (122.8–142) |
| Height (cm) | 169 (165–177.5) | 168 (163–174) | 168.5 (164.3–173.3) |
| BMI (kg/m2) | 44.8 (41.9–49.1) | 45.7 (42.8–48.8) | 46.5 (42.9–49.2) |
| CBW (kg) | 94. 2 (84.1–101.7) | 87.8 (83–100) | 94.2 (82.9–99.3) |
| IBW (kg) | 65 (60–77.5) | 63 (58–72) | 63.5 (59.3–73.3) |
| ASA Class | |||
| I | 9 (18.4%) | 11 (23.4%) | 7 (31.8%) |
| II | 38 (77.6%) | 35 (74.5%) | 14 (63.6%) |
| III | 2 (4.1%) | 1 (2.1%) | 1 (4.5%) |
| Duration of anesthesia (minutes) | 114 (92.5–160.5) | 115 (92–140) | 190.5 (175–221.3)*** |
| Duration of surgery (minutes) | 97 (72.5–128.5) | 95 (70–119) | 90 (75–118.5) |
| Rocuronium dose (mg) | 156 (138–180) | 163 (134–183) | 155.5 (142.3–173.5) |
| Sugammadex dose (mg) | 377 (336.4–406.8) | 512 (472–576)### | ——— |
The CBW group was administered 4 mg/kg sugammadex, calculated on a CBW basis; the TBW group was administered 4 mg/kg sugammadex, calculated on a TBW basis; and the Control group was not administered this drug. Data are expressed as median (interquartile range), mean ± SD, or number of patients (%). ***P<0.001 compared with the CBW group and the TBW group (Kruskal–Wallis test) and ###P<0.001 compared with the CBW group (Mann–Whitney U-test).
CBW, corrected body weight; TBW, total body weight, IBW, ideal body weight.
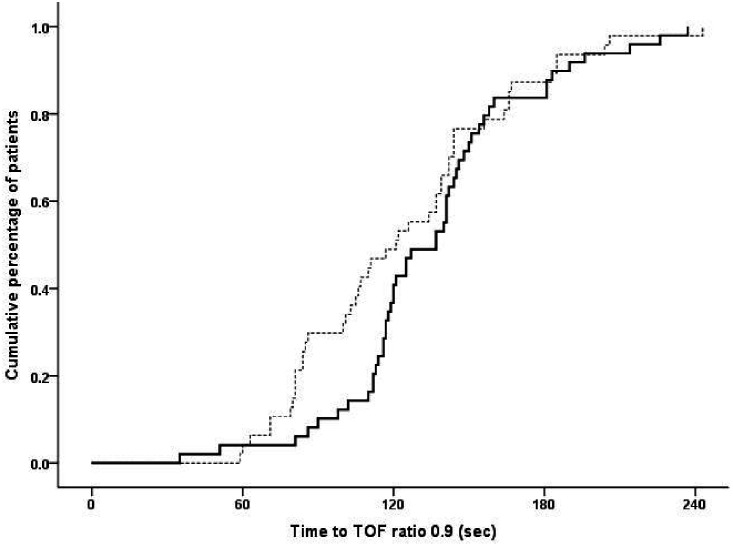
All TOF ratios of all the participants in the CBW and TBW groups recovered to 0.9 within 5 minutes (maximum time: 242 s) (Figure 2). The mean recovery time from the start of sugammadex administration to a TOF ratio of 0.9 was 2.24 ± 0.65 minutes (95% CI, 2.06–2.43 minutes) in the CBW group versus 2.05 ± 0.72 minutes (95% CI, 1.84–2.26 minutes) in the TBW group. The mean recovery time from the start of sugammadex administration to a TOF ratio of 0.8 was 1.87 ± 0.61 minutes (95% CI, 1.69–2.04 minutes) in the CBW group versus 1.65 ± 0.62 minutes (95% CI, 1.46–1.83 minutes) in the TBW group. The mean recovery time from the start of sugammadex administration to a TOF ratio of 0.7 was 1.53 ± 0.57 minutes (95% CI, 1.37–1.69 minutes) in the CBW group versus 1.34 ± 0.55 minutes (95% CI, 1.18–1.50 minutes) in the TBW group. The mean differences in the recovery time from the start of sugammadex administration to TOF ratios of 0.9, 0.8, and 0.7 were 0.20 minutes (95% CI, −0.08 to 0.47 minutes), 0.22 minutes (95% CI, −0.03 to 0.47 minutes), and 0.19 minutes (95% CI, −0.04 to 0.42 minutes) between the CBW and TBW groups, respectively. Because all three upper limits of the 95% CIs were lower than the pre-specified non-inferiority margin (0.5 minutes), non-inferiority was confirmed. These findings imply that the use of a sugammadex dose based on CBW is as effective as one based on TBW at reversing deep NMB. The mean times taken for the TOF ratio to recover to 0.7, 0.8, and 0.9 in the control group were 99.27 ± 19.93, 110.32 ± 19.07, and 121.05 ± 18.97 minutes, respectively (Table 3).
Figure 2.
Cumulative percentages of participants who achieved a TOF ratio of 0.9 after sugammadex administration. The CBW group was administered 4 mg/kg sugammadex, calculated on a CBW basis; the TBW group was administered 4 mg/kg sugammadex, calculated on a TBW basis; and the Control group was not administered this drug.
TBW group (––). CBW group (—). CBW, corrected body weight; TBW, total body weight.
Table 3.
Comparison of the recovery times (minutes) from the start of administration of sugammadex to the achievement of a TOF ratio of 0.7, 0.8, or 0.9.
| CBW Group (n = 49) | TBW Group (n = 47) | Control Group (n = 22) | |
|---|---|---|---|
| TOF 0.7 | |||
| Mean (SD) | 1.53 ± 0.57 | 1.34 ± 0.55 | 99.27 ± 19.93 |
| Median (interquartile range) | 1.43 (1.14–1.81) | 1.30 (0.83–1.67) | 95.50 (82.0–115.0) |
| TOF 0.8 | |||
| Mean (SD) | 1.87 ± 0.61 | 1.65 ± 0.62 | 110.32 ± 19.07 |
| Median (interquartile range) | 1.80 (1.57–2.20) | 1.60 (1.08–2.05) | 108.50 (95.50–124.75) |
| TOF 0.9 | |||
| Mean (SD) | 2.24 ± 0.65 | 2.05 ± 0.72 | 121.05 ± 18.97 |
| Median (interquartile range) | 2.27 (1.90–2.53) | 2.0 (1.38–2.38) | 120.0 (104.50–135.0) |
The CBW group was administered 4 mg/kg sugammadex, calculated on a CBW basis; the TBW group was administered 4 mg/kg sugammadex, calculated on a TBW basis; and the Control group was not administered this drug.
TOF, train-of-four; CBW, corrected body weight; TBW, total body weight.
Regarding the safety of the procedures, 106 patients, comprising 45 (91.8%) in the CBW group, 42 (89.4%) in the TBW group, and 19 (86.4%) in the control group, had at least one AE. The most frequent AEs were pain associated with the procedure, nausea, vomiting, and pharyngolaryngeal pain. AEs that occurred in >5% of at least one group are listed in Table 2. Serious AEs occurred in two patients: one in the CBW group and one in the TBW group. However, neither of these were considered to be related to the use of the study drug.
Table 2.
Adverse events related or unrelated to the study drug that occurred in >5% of participants in at least one treatment group.
| CBW Group (n = 49) | TBW Group (n = 47) | Control Group (n = 22) | |
|---|---|---|---|
| Any adverse event | 45 (91.8%) | 42 (89.4%) | 19 (86.4%) |
| Pain associated with the procedure | 34 (69.4%) | 34 (72.3%) | 16 (72.7%) |
| Nausea | 16 (32.7%) | 14 (29.8%) | 7 (31.8%) |
| Vomiting | 13 (26.5%) | 11 (23.4%) | 6 (27.3%) |
| Bradycardia | 5 (10.2%) | 3 (6.4%) | 0 (0) |
| Dizziness | 5 (10.2%) | 6 (12.8%) | 3 (13.6%) |
| Headache | 1 (2%) | 3 (6.4%) | 1 (4.5%) |
| Pharyngolaryngeal pain | 8 (16.3%) | 7 (14.9%) | 4 (18.2%) |
The CBW group was administered 4 mg/kg sugammadex, calculated on a CBW basis; the TBW group was administered 4 mg/kg sugammadex, calculated on a TBW basis; and the Control group was not administered this drug. Data are expressed as number of patients (%).
CBW, corrected body weight; TBW, total body weight.
Ten AEs (five in the CBW group and five in the TBW group) were considered by the investigator to be possibly or probably related to sugammadex administration. The drug-related AEs in the CBW group were bradycardia (n = 5) and those in the TBW group were bradycardia (n = 3), muscle weakness (n = 1), and drug hypersensitivity (n = 1). The clinical symptoms of drug hypersensitivity were facial swelling, facial flushing, and chest skin flushing, all of which disappeared after 20 minutes. In addition, the patient’s blood pressure decreased rapidly from 110/68 mmHg to 65/37 mmHg 2.5 minutes after the administration of sugammadex, and was then 105/63 mmHg after 5 minutes. A case of severe bradycardia was reported in the CBW group, in which the heart rate decreased from 55 beats/minute to 35 beats/minute after the administration of sugammadex, then improved to 65 beats/minute after an intravenous injection of 0.5 mg atropine. No significant differences were observed in the MAP or HR of the CBW and TBW groups at baseline or 1, 5, 10, or 30 minutes after sugammadex administration. The median (range) TOF ratios 15 minutes after the transfer of the participants to the PACU were 106 (95–135) in the CBW group, 103 (93–131) in the TBW group, and 101.5 (94–130) in the control group. No signs of residual NMB or the recurrence of NMB were observed.
Discussion
In the present study, we have shown that a sugammadex dose calculated on the basis of CBW is as effective as one calculated on the basis of TBW for the reversal of deep rocuronium-induced NMB when administered at 1 to 2 PTCs in morbidly obese patients undergoing laparoscopic bariatric surgery. No instances of residual NMB or the recurrence of NMB were observed in the operating theatre or PACU. We have also described the time to recovery from PTC 1 to 2 to a TOF ratio of 0.9 in morbidly obese patients after a continuous intravenous injection of rocuronium. These findings add to knowledge of the pharmacokinetics of rocuronium in morbidly obese patients.
Many studies have been conducted regarding reductions in the dose of sugammadex for the reversal of moderate NMB in morbidly obese patients. The most common form of dose reduction used is based on the patient’s IBW. Duarte et al.23 showed that the time taken for the TOF ratio to return to 0.9 was 225 ± 81 s after the administration of 2 mg/kg sugammadex according to IBW at the reappearance of the second twitch (T2), and they concluded that IBW can be used to calculate the sugammadex dose required to reverse moderate NMB in morbidly obese patients. Abd El-Rahman et al.22 compared the efficacy of sugammadex at doses of 1.5, 2, and 4 mg/kg, calculated according to IBW, for the reversal of moderate rocuronium-induced NMB in laparoscopic bariatric surgery. The lengths of time taken for the recovery of the TOF ratio to 0.9 were significantly longer in the 1.5 and 2 mg/kg groups than in the 4 mg/kg group (P < 0.001 and 0.005, respectively). However, they concluded that sugammadex at a dose of 1.5 mg/kg, calculated according to IBW, was safe and effective for the reversal of moderate NMB induced by rocuronium, as compared with doses of 2 or 4 mg/kg, because although the times to extubation were similar, less sugammadex was required. Sanfilippo et al.24 found that the mean times taken for the TOF ratio to recover to 0.9 after the administration of 2 mg/kg sugammadex were 151 ± 44 s if the dose was calculated on an IBW basis and 121 ± 55 s if it was calculated on a TBW basis (P = 0.07). They concluded that a 2-mg/kg dose of sugammadex, calculated using IBW, is a safe means of ensuring a rapid recovery from moderate rocuronium-induced NMB and the absence of PORC in morbidly obese patients. Loupec et al.25 reported that a 4-mg/kg dose of sugammadex, calculated on an IBW basis, appropriately reversed deep rocuronium-induced NMB and yielded a mean time for recovery of the TOF ratio to 0.9 of 255 ± 63 s. The success rate of recovery from NMB was 93%; however, they defined success as the achievement of a TOF ratio of ≥0.9 in < 10 minutes.
Some controversy regarding the appropriate dose of sugammadex remains. Monk et al.26 concluded that a sugammadex dose calculated on the basis of TBW provides rapid recovery from NMB in obese patients and that no dose adjustments are required. Furthermore, Llaurado et al.17 reported that the median times taken for the TOF ratio to recover to 0.9 or more were 113 and 167 s for moderate and deep NMB reversed using sugammadex doses of 2 and 4 mg/kg, respectively, calculated using IBW. However, the percentages of the patients that required a second dose of sugammadex because of a lack of recovery of the TOF ratio to >0.9 after 3 minutes, were 23.4% for moderate NMB and 39.5% for deep NMB. They concluded that a sugammadex dose calculated using IBW is insufficient to reverse either moderate or deep NMB in morbidly obese patients. Therefore, the dose of sugammadex was increased by using the CBW. A dose of 2.0 mg/kg, calculated using CBW and administered at the reappearance of T2, was shown to reverse rocuronium-induced NMB faster than neostigmine (2.7 vs. 9.6 minutes) in morbidly obese patients.18 However, to the best of our knowledge, few studies have compared the efficacy of sugammadex at doses calculated using CBW or TBW for the reversal of deep NMB in morbidly obese patients.
In the present study, we compared the recovery time in patients administered sugammadex at doses calculated using CBW or TBW for the reversal of deep NMB (reappearance of 1 to 2 PTCs) in morbidly obese patients, and found that the recovery times from the start of sugammadex administration to the achievement of a TOF ratio of 0.9 were 2.24 ± 0.65 minutes for the CBW group and 2.05 ± 0.72 minutes for the TBW group. Although the recovery time in the CBW group was slightly longer than that in the TBW group, there was no evidence of residual or recurrent NMB in any of the participants, either on the basis of clinical signs or according to neuromuscular monitoring data. Therefore, we consider that a sugammadex dose that is based on CBW is as effective as one based on TBW for the reversal of deep NMB.
The ability of sugammadex to reverse rocuronium-induced deep NMB has important clinical implications for anesthesia during laparoscopic surgery. Anesthesiologists can maintain deep NMB throughout surgery without worrying about residual NMB, thereby ensuring optimal surgical conditions. However, the high cost of sugammadex has prevented its routine use in China. The administration of sugammadex according to CBW would reduce the amount used, thereby reducing the cost to the patient, as well as potentially reducing the incidence of sugammadex-associated complications.
One participant in the present study who received sugammadex had a reaction typical of drug hypersensitivity. Two minutes after the administration of sugammadex, they developed facial swelling, facial flushing, chest skin flushing, and hypotension, but there was no tachycardia or bronchospasm. This reaction was managed using a continuous intravenous infusion of epinephrine at a dose of 0.03 to 0.1 µg/kg/minute for 10 minutes, as well as a bolus injection of norepinephrine 10 µg (total dose of norepinephrine 50 µg), followed by methylprednisolone 80 mg. The patient’s blood pressure had recovered after 5 minutes and the other signs disappeared after 20 minutes. Sugammadex-induced hypersensitivity reactions have been reported previously,27,28 and although the clinical manifestation and severity of the hypersensitivity reactions differed, they occurred within a few minutes of administration.27 Therefore, if hypersensitivity reactions are identified and managed promptly, they should not result in serious clinical problems.
The present study had several limitations. First, it was a single-center study; therefore, the results are not readily generalizable. Second, patients that undergo bariatric surgery are young. Therefore, multi-center studies of patients with a wider range of ages from different populations should be conducted in the future.
Conclusion
We recommend that for morbidly obese patients undergoing laparoscopic bariatric surgery, a dose of 4 mg/kg of sugammadex, calculated on the basis of CBW, is an efficient and safe means of reversing deep NMB (PTC 1 to 2) induced by a continuous infusion of rocuronium and does not increase the risk of PORC.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Deming Li https://orcid.org/0000-0002-5625-6203
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197–1209. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Ye Y, Zhang Y, et al. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ 2019; 367: l5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrington De Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010; 363: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Tovar J, Carbajo MA, Jimenez JM, et al. A Long-term follow-up after sleeve gastrectomy versus Roux-en-Y gastric bypass versus one-anastomosis gastric bypass: a prospective randomized comparative study of weight loss and remission of comorbidities. Surg Endosc 2019; 33: 401–410. [DOI] [PubMed] [Google Scholar]
- 7.Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014; 8: CD003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naguib M, Brull SJ, Kopman AF, et al . Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg 2018; 127: 71–80. [DOI] [PubMed] [Google Scholar]
- 9.Torensma B, Martini CH, Boon M, et al. Deep neuromuscular block improves surgical conditions during bariatric surgery and reduces postoperative pain: a randomized double blind controlled trial. PLoS One 2016; 11: e0167907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogunnaike BO, Jones SB, Jones DB, et al . Anesthetic considerations for bariatric surgery. Anesth Analg 2002; 95: 1793–1805. [DOI] [PubMed] [Google Scholar]
- 11.Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology 2006; 105: 885–891. [DOI] [PubMed] [Google Scholar]
- 12.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116: 1–7. [DOI] [PubMed] [Google Scholar]
- 13.Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ 2012; 345: e6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen T, Viby-Mogensen J, Ringsted C. Anesthetic practice and postoperative pulmonary complications. Acta Anesthesiol Scand 1992; 36: 812–818. [DOI] [PubMed] [Google Scholar]
- 15.Bom A, Bradley M, Cameron K, et al. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl 2002; 41: 266–270. [DOI] [PubMed] [Google Scholar]
- 16.Hristovska AM, Duch P, Allingstrup M, et al. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev 2017; 8: CD012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llauradó S, Sabaté A, Ferreres E, et al. Sugammadex ideal body weight dose adjusted by level of neuromuscular blockade in laparoscopic bariatric surgery. Anesthesiology 2012; 117: 93–98. [DOI] [PubMed] [Google Scholar]
- 18.Gaszynski T, Szewczyk T, Gaszynski W. Randomized comparison of sugammadex and neostigmine for reversal of rocuronium-induced muscle relaxation in morbidly obese undergoing general anesthesia. Br J Anesth 2012; 108: 236–239. [DOI] [PubMed] [Google Scholar]
- 19.Gepts E. Pharmacokinetic concepts for TCI anesthesia. Anesthesia 1998; 53: 4–12. [DOI] [PubMed] [Google Scholar]
- 20.Jones RK, Caldwell JE, Brull SJ, et al. Reversal of profound rocuronium-induced blockade with sugammadex. Anesthesiology 2008; 109: 816–824. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs-Buder T, Claudius C, Skovgaard LT, et al. Good clinical research practice in pharmacodynamics studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anesthesiol Scand 2007; 51: 789–808. [DOI] [PubMed] [Google Scholar]
- 22.Abd El-Rahman AM, Othman AH, El Sherif FA, et al. Comparison of three different doses sugammadex based on ideal body weight for reversal of moderate rocuronium-induced neuromuscular blockade in laparoscopic bariatric surgery. Minerva Anestesiol 2017; 83: 138–144. [DOI] [PubMed] [Google Scholar]
- 23.Duarte NMDC, Caetano AMM, Neto SDSC, et al. Sugammadex by ideal body weight versus 20% and 40% corrected weight in bariatric surgery-double-blind randomized clinical trial. Rev Bras Anestesiol 2018; 68: 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanfilippo M, Alessandri F, Wefki Abdelgawwad Shousha AA, et al. Sugammadex and ideal body weight in bariatric surgery. Anesthesiology Res Pract 2013; 2013: 389782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loupec T, Frasca D, Rousseau N, et al. Appropriate dosing of sugammadex to reverse deep rocuronium-induced neuromuscular blockade in morbidly obese patients. Anesthesia 2016; 71: 265–272. [DOI] [PubMed] [Google Scholar]
- 26.Monk TG, Rietbergen H, Woo T, et al . Use of sugammadex in patients with obesity: a pooled analysis. Am J Ther 2017; 24: 507–516. [DOI] [PubMed] [Google Scholar]
- 27.Godai K, Hasegawa-Moriyama M, Kuniyoshi T, et al. Three cases of suspected sugammadex-induced hypersensitivity reactions. Br J Anesth 2012; 109: 216–218. [DOI] [PubMed] [Google Scholar]
- 28.Menéndez-Ozcoidi L, Ortiz-Gómez JR, Olaguibel-Ribero JM, et al. Allergy to low dose sugammadex. Anesthesia 2011; 66: 217–219. [DOI] [PubMed] [Google Scholar]