Abstract
BACKGROUND
Irritable bowel syndrome (IBS) is a bowel disorder involving abdominal pain or discomfort along with irregularity of stool form and passage frequency. The pathophysiology is poorly understood and seems to be multifactorial. Investigations of possible causes of IBS have included only a few colonic transit studies and no simultaneous determination of the colonic faecal content.
AIM
To compare colon transit time and faecal load between IBS-patients and healthy control subjects.
METHODS
The study included 140 patients with IBS, with a mean age of 50.0 years. The control group comprised 44 healthy persons with a mean age of 43.4 years, who were selected at random from the National Civil Register. Both the patient group and the control group underwent a marker study to measure colon transit time (CTT) and to calculate a faecal loading score. The patient group underwent treatment with a combined prokinetic regime, after which their CTT and faecal loading were reassessed. Analyses were performed to compare measurements between the control group and the patient group before and after treatment.
RESULTS
Compared to healthy controls, IBS-patients exhibited a significantly prolonged mean CTT (45.48 h vs 24.75 h, P = 0.0002) and significantly greater mean faecal loading scores in all colonic segments (P < 0.001). Among IBS patients, we found no significant differences between the 48 h and 96 h radiographs. Among patients exhibiting increased CTT and faecal loading, approximately half exhibited a palpable mass in the right iliac fossa. After intervention with a prokinetic treatment, the mean CTT among IBS patients was reduced from 45.48 h to 34.50 h (P = 0.091), with the post-treatment CTT not significantly differing from the CTT among control subjects (P = 0.095). The faecal loading score among IBS patients did not significantly differ before and after treatment (P = 0.442). The post-treatment faecal loading score in IBS patients remained significantly higher compared to that in controls (5.3 vs 4.3, P = 0.014). After treatment, half of the IBS-patients were relieved of bloating, while the majority no longer experienced abdominal pain and achieved a daily consistent stool.
CONCLUSION
IBS-patients exhibited prolonged CTT and heavier faecal loading. These assessments may aid in diagnosis. Faecal retention may contribute to IBS symptoms, which can be treated using a prokinetic regime.
Keywords: Irritable bowel syndrome, Functional bowel disease, Faecal retention, Colon transit time, Faecal load
Core Tip: Patients with irritable bowel syndrome (IBS) exhibit a significant prolonged colon transit time (CTT) and greater faecal loading compared to healthy people. This finding adds to our understanding of IBS since faecal retention may lead to major symptoms like abdominal bloating and pain and defaecation disturbances. The targeted therapy was a prokinetic regime. All the more, CTT/faecal load may serve as a diagnostic procedure.
INTRODUCTION
Irritable bowel syndrome (IBS) is a bowel disorder involving abdominal pain or discomfort along with irregularity of stool form and passage frequency[1]. Its prevalence ranges from 9%-23% of the world population[2]. IBS considerably affects quality of life and imposes a profound burden on patients, physicians, and the health-care system[3,4]. The pathophysiology is poorly understood and seems to be multifactorial. Investigations for possible causes of IBS have included only a few colonic transit studies[5,6], and none have included a simultaneous determination of the colonic faecal content. Therefore, in the present study we aimed to measure colon transit time and faecal load in patients with IBS and to compare these measures with those of a healthy control group.
MATERIALS AND METHODS
This study included 140 patients diagnosed with IBS based on recurrent abdominal pain and abdominal discomfort during the last 3 mo, which was associated with two or more of the following: Improvement with defecation, change in frequency of stool, and change in form (appearance) of stool[7]. The patients were recruited from a database of 281 patients who were referred for abdominal and ano-rectal symptoms[8]. A control group was recruited from a random selection of 372 people over 18 years of age, from the National Civil Register. Screening excluded individuals with gastrointestinal symptoms who took laxatives or strong analgesics and who had previous abdominal surgery. A total of 44 people fulfilled these criteria and were included in the control group. This study was approved by a local ethical and research committee and was conducted in accordance with the Declaration of Helsinki.
Included patients underwent a physical examination with special attention to abdominal signs, as well as a colonic marker study. The patients were on their own diet, and each patient swallowed a capsule containing 24 radiopaque markers (Sitzmark, Konsyl, Pharmaceutical Inc., Fort Worth, TX, United States), and then abdominal X-rays were taken after 48 h and 96 h[9]. Abdominal X-rays were divided into three segments, in a reverse Y-design, formed by the vertical column and two imaginary lines extending from the fifth lumbar vertebra to the right and left pelvic brim, pointing towards the femoral head, which was a modification from earlier studies[10,11]. The three segments include the right, transverse, and left colon and the rectum (Figure 1). The number of markers was counted in each segment and colonic transit time (CTT) was calculated using the following equation: CTT (in hours) = (48/n) × (n48 + n96), where n48 and n96 are the total number of markers observed at 48 h and 96 h after ingestion of n = 24 markers[12]. The control subjects also ingested 24 markers at the same time for 6 d, followed by an abdominal X-ray on day 7. In the control subjects, the number of markers visible on X-ray was then equal to the CTT in hours[12] (Figure 2).
Figure 1.
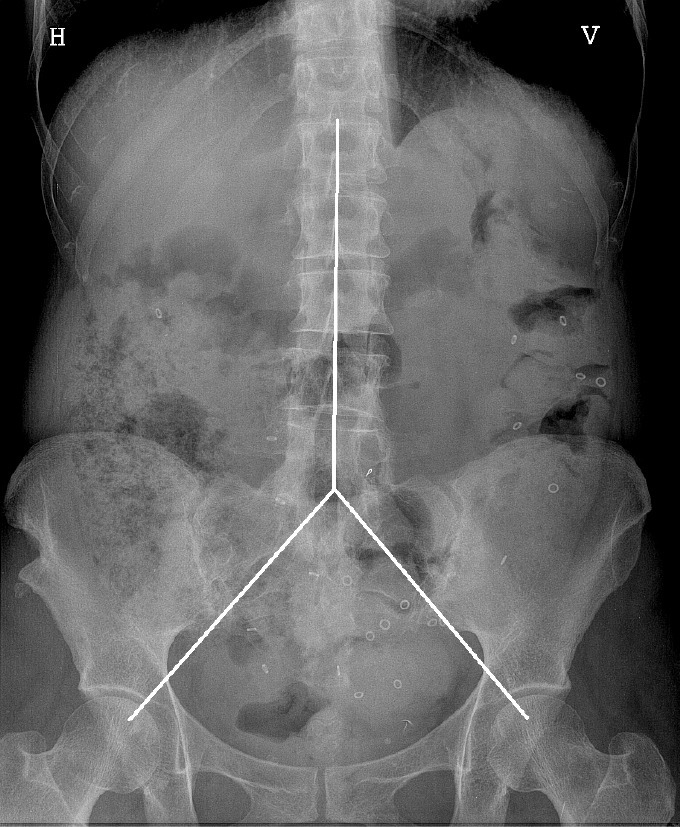
Colon transit study in an irritable bowel syndrome patient. Patient ingested 24 markers, and an X-ray was acquired at 48 h. From the X-ray, we counted the number of markers in each segment: 2 + 8 + 10 = 20; faecal load score: 2 + 2 + 1 = 5 (see text).
Figure 2.
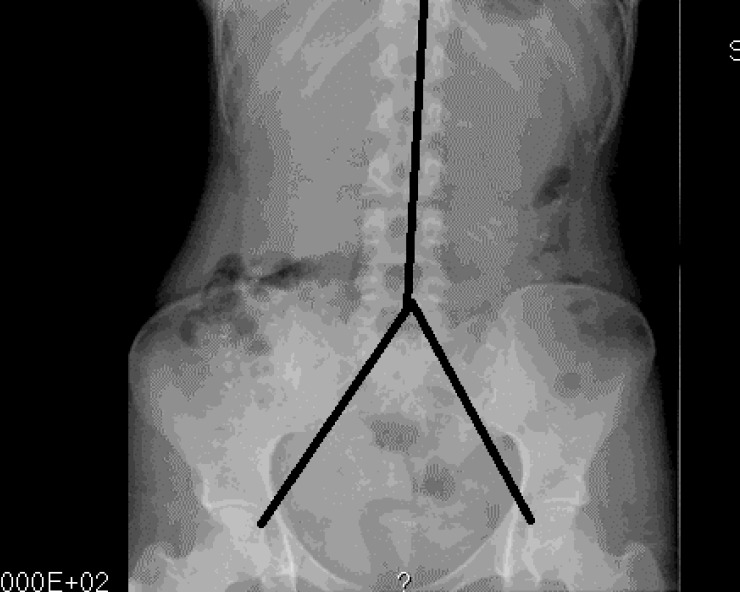
Colon transit study in a healthy control. Subjects ingested the 24 markers for 6 d, and an X-ray was acquired on day 7. From the X-ray we counted the number of markers in each segment: 11 + 6 + 1 = 18; faecal load score: 2 + 1 + 1 = 4 (see text).
The estimated faecal load in the colon from each segment on the X-ray was scored from 0-3, where 0 indicated no faeces visible, 1 indicated slight, 2 moderate, and 3 severe faecal loading. We then obtained a segmental score of 0-3 and a total score of 0-9 for each radiograph. Similarly, faecal loading scores were estimated for the controls. The presently used score is a modification of the Leech-score, which details faecal loading from 0-5[13]. The X-ray images were examined by observers who were unaware of the patients’ clinical course.
Intervention
The present study was designed to investigate the pathogenic mechanisms of IBS rather than a therapeutic trial. Thus, the patients received an established bowel stimulatory treatment, which included a low fat and fibre-rich diet and dietician-guided meal planning, in accordance with guidelines of the Danish Nutritional Council. The diet was supplemented with 10-20 g of ispaghula husk per day, and the prokinetic drug, domperidone, 10 mg × 3 a day. Patients were also encouraged to perform 30 min of physical activity on a daily basis. This treatment continued until patients reported relief of symptoms. At this time, CTT and faecal loading were reassessed.
Statistical analysis
The data were entered into a database, and analyses were performed using R 4.0.1 (R Core Team). Patients’ characteristics were expressed using frequency, percentage, mean, range, and standard deviation (SD). Differences (e.g., between the sexes) were calculated using a t-test and a permutations test for independence. The permutation test was also used to calculate differences between CTT values and between faecal loading scores. This test was selected because the variables did not show a normal distribution. Finally, we investigated possible associations of CTT and faecal load with specific symptoms and physical signs. A P of < 0.05 was considered to indicate statistical significance.
RESULTS
Among 140 patients, the mean age was 50.0 years (range 17.0-81.2 years), and 118 patients were female and 22 were male. Mean age did not significantly differ between sexes, 49.6 years vs 52.3 years, P = 0.448. The control group included 44 randomly selected healthy persons with mean age of 43.4 years (range 21.0-67.0 years) and included equal numbers of males and females.
The marker study revealed a mean CTT of 45.48 h among the 140 patients, compared to 24.75 h in the 44 controls, (P = 0.0002). CTT did not significantly differ between male and female patients (41.22 h vs 38.63 h, P = 0.741) or between male and female controls (19.73 h vs 29.77 h, P = 0.111). Patients and controls exhibited significant differences in mean faecal loading scores in all colonic segments at 48 h (right: 2.25 vs 1.80, left: 1.95 vs 1.25, distal: 1.95 vs 1.27; all P < 0.001) and at 96 h (right:2.41 vs 1.80, left: 2.05 vs 1.25, distal: 2.05 vs 1.27; all P < 0.001). Mean loading scores did not significantly differ between the 48 h and 96 h radiographs. Total mean faecal loading scores significantly differed between women and men among patients (5.77 vs 6.40, P = 0.025) but not among controls (4.55 vs 4.09, P = 0.179).
We used linear regression model to examine associations between markers and faecal load. Data from patients´ radiographs at 48 h and 96 h revealed significant associations between markers and faecal load (P < 0.001). These parameters showed the same relationship patterns among controls.
The mean intervention treatment period was 690 d. The mean CTT among patients was reduced from 45.48 h pre-intervention to 34.50 h post-intervention (P = 0.091). The mean CTT did not significantly differ between treated patients and healthy controls (P = 0.095). On the other hand, we found no significant difference between pre-treatment and post-treatment values of total faecal loading score 48 h (P = 0.442) or at 96 h (P = 0.127). Compared to healthy controls, post-treatment patients showed significantly heavier total faecal loading at both 48 h (5.3 vs 4.3, P = 0.014) and 96 h.
Of the 140 patients, 58 (41.4%) exhibited a palpable faecal mass in the right fossa. Among the 57 patients with an elevated CTT of > 24.75 h (mean among healthy controls), 28 patients (49.1%) had a palpable mass. Similarly, of the 102 patients with a 48 h faecal loading score of > 1.80 (mean among healthy controls), 47 (46.1%) exhibited a palpable mass. Additionally, among 56 patients with an increase in CTT of > 24.75 h, 37 (66.1%) exhibited meteorism (P < 0.001).
After the intervention, 43.9% of the patients were relieved from bloating (P = 0.1083), and 60.9% of patients no longer experienced abdominal pain (P = 0.0193). With regards to defaecation after the intervention, 88.6% of patients achieved normal daily defaecation (P < 0.001), and 74.3% had a formed stool (P < 0.001).
DISCUSSION
To our knowledge, our present study was the first to report the CTT and faecal load in IBS-patients. Our results showed that IBS patients had a prolonged CTT and heavier faecal load in all parts of the colon compared to healthy controls. Prior measurements of the degree of faecal loading have been exclusively described in children, and several systems have been developed to score both the amount of faeces and its localization in different colon segments[14,15]. The Leech-score is a reproducible tool for assessing faecal loading, with high intra-observer and interobserver agreement[13,16-18]. The plain abdominal radiograph has seldom been used in adults[18,19].
In contrast, CTT is widely used as a reproducible method[9]. In particular, CTT is utilized to assess for the presence of slow transit constipation. In our present study of IBS-patients, we utilized a single ingestion of markers to ensure better compliance, and the markers were counted on radiographs acquired at 48 h and 96 h after ingestion. We counted the localized markers in the right, left, and distal parts of the colon, including the rectum. This method was used regardless of bowel outlines that may suggest some other placement of a part of the colon. In the control subjects, we utilized multiple marker ingestion at the same time for 6 consecutive days followed by an abdominal X-ray on day 7, to circumvent the difficulty and unnecessary radiation exposure involved in obtaining two radiographs. With this technique, we measured the mean value of the mean transit times of different boluses of ingested markers, and the numbers of markers visible on the radiograph was equal to the segmental total transit time in hours[12]. This method is analogous to a bolus ingestion of markers visible on successive daily abdominal X-rays, and the two techniques were significantly correlated[9].
CTT has seldom been measured in IBS patients. After eliminating many patients with IBS constipation, Bouchoucha et al[20] found that CTT values in IBS patients significantly differed between male patients (25.7 h; n = 194) and female patients (31.1 h; n = 558). Other reports have also shown sex-based differences in CTT among both IBS-patients and control subjects[21]. However, in our study CTT of patients and controls was not associated with gender. Among healthy adults, CTT reportedly varies between 24.5 h and 45.6 h[20,22-24], and thus it is rather difficult to define a normal CTT. Variations in CTT can be attributed to the population investigated, dietary and fluid intake, physical activity, and study methodology. Notably, the CTT measurements obtained in sitz- or plastic marker studies of patients and controls have decreased over many years. Thus, our present CTT measurements for both IBS-patients and controls are at the lower end compared to prior studies.
Radiopaque markers are not absorbed, do not alter gut metabolism, and have the same specific gravity as gut content and can thus be assumed to travel at the same rate as faeces. Markers are proven to be significantly associated with faecal load. Despite this, we observed great variation. Thus, patients with a heavy load may have few markers, and patients with a high load may have many markers. Additionally, the faecal load determined at 96 h was the same as at 48 h, indicating a stationary condition. A significant difference in faecal load was found between female and male IBS-patients, which was not the case for controls.
The pathophysiology of IBS is poorly understood and appears to be multifactorial, involving the combined impact of food intake, physical activity, mental status, previous infections, and genetics[25]. Recent years have brought emerging insights into the nervous system, and nervous system dysfunction may play a role in IBS[26]. Our increasing understanding of the gut microbiome has also highlighted its potential role in IBS symptoms[27]. In this context, faeces in the colon, and thereby the faecal load, may be viewed as the end result of all of these factors. Here, we found that faecal load was heavier in IBS patients than in healthy persons, and thus appears to be important in IBS. All the more, a palpable faecal mass in the right iliac fossa was found in many patients. The retention was observed irrespective of defaecation patterns (i.e., diarrhoea or constipation) and represents a hidden constipation.
Nearly half of the IBS patients in our study exhibited a palpable faecal mass in the right fossa, which was associated with both increased CTT and heavier faecal load. Moreover, a high proportion of IBS patients with an increased CTT suffered from meteorism. The endogenous source of intestinal gas is the fermentation processes of yeast and bacteria, which produces hydrogen, carbon dioxide, methane, butyric acid, and odoriferous sulphur compounds[28]. In particular, colonic hydrogen production is greater in patients with IBS than controls[29]. Thus, patients’ symptoms of bloating and abdominal pain may be caused by gas distending the colonic wall. This is in agreement with a study showing greater abdominal distension in IBS patients with delayed transit than in those with normal transit[30]. Our present results are consistent with that finding.
The simultaneous determination of CTT and faecal loading may serve as a diagnostic tool for IBS, rather than diagnosing this condition based on a constellation of symptoms alone.
The present study was not a therapeutic trial but rather an investigative study of the mechanisms of IBS. Various IBS treatment concepts have been suggested[31]. Our patients exhibited faecal retention, and the administered treatment was targeted to relieve faecal retention with a dietary and prokinetic regime, including physical activity. Domperidone blocks the inhibitory effect of dopamine in the proximal colon in dogs[32] and thereby facilitates movements. In a placebo-controlled study, domperidone resulted in significantly reduced abdominal pain, flatulence, and abnormal bowel habits[33]. After the intervention, the patients exhibited reduced CTT values that were very close to the CTT values of healthy controls. However, the patients did not exhibit a corresponding reduction of faecal loading, which remained heavier than in the controls. These findings are in good agreement with the fact that only half of our patients experienced relief from bloating after the intervention. Fortunately, the majority of the patients no longer experienced abdominal pain and achieved daily and formed defaecation. It is possible that a treatment including prucalopride may constitute a more effective prokinetic regime for accelerating transit[34].
CONCLUSION
Our present results showed a significantly prolonged CTT and significantly heavier faecal loading in IBS-patients compared to healthy controls. This suggests that faecal retention may contribute to the symptoms in IBS, which could thus be relieved by treatment with a prokinetic regime. Our findings also indicate that the simultaneous determination of CTT and faecal loading may serve as a diagnostic procedure for IBS.
ARTICLE HIGHLIGHTS
Research background
Patients with irritable bowel syndrome (IBS) experience abdominal pain and irregularities of stool form and passage frequency. The prevalence ranges from 9%-23%, and IBS imposes profound burdens on patients, physicians, and the healthcare system. The pathophysiology is poorly understood.
Research motivation
Faecal retention is suspected to play a role in IBS symptoms. However, few colonic transit studies exist, and none have included simultaneous determination of colonic faecal content. Such information would likely have implications for choice of therapeutic decisions.
Research objectives
The present case-control study was performed to compare colonic transit time (CTT) and faecal load between IBS-patients and healthy controls. We further aimed to compare these parameters in patients before and after treatment with a prokinetic regime.
Research methods
CTT and faecal load were measured by performing a marker study. IBS-patients swallowed a capsule containing 24 radiopaque markers, and abdominal X-rays were taken after 48 h and 96 h. Control subjects ingested 24 markers at the same time for 6 d, followed by an X-ray on day 7. For both groups, CTT was calculated in hours, and a faecal load score was estimated.
Research results
Compared to 44 healthy controls, 140 IBS-patients exhibited a significantly prolonged mean CTT (45.48 h vs 24.75 h, P < 0.001) and a significantly greater mean faecal loading scores in each colonic segment (P < 0.001). After the intervention, the mean CTT in IBS-patients was reduced from 45.48 h to 34.50 h (P > 0.05), with the post-treatment CTT not significantly differing from the CTT among control subjects (P > 0.05). Moreover, following treatment, half of the patients were relieved from bloating, and the majority no longer experienced abdominal pain and had achieved a consistent daily stool.
Research conclusions
IBS-patients were examined by using a new method comprising the simultaneous determination of CTT and faecal load. Our results showed a significantly prolonged CTT and significantly heavier faecal loading in IBS-patients compared to healthy control persons. These findings may contribute to the IBS symptoms, which were relieved to some degree following treatment with a prokinetic regime. Studies are needed to examine further the association between faecal retention and symptoms.
Research perspectives
Simultaneous measurement of CTT and faecal load may serve as a diagnostic tool for investigating IBS-patients and could also be extended for use in patients with other bowel disorders. This method may also be useful for monitoring the effects of different treatment regimens.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Scientific Committee, Department of Gastroenterology and Surgery, Copenhagen University North Sealand Hospital Institutional Review Board.
Informed consent statement: All patients and control persons provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflict of interest.
Data sharing statement: No additional data are available.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: October 20, 2020
First decision: October 27, 2020
Article in press: December 4, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bouchoucha M S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wu YXJ
Contributor Information
Dennis Raahave, Department of Gastroenterology and Surgery, Copenhagen University North Sealand Hospital, Hilleroed 3400, Denmark. dr.dr@dadlnet.dk.
Andreas K Jensen, Faculty of Health Sciences, Section of Biostatistics, University of Copenhagen, Hilleroed 3400, Denmark.
References
- 1.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oświęcimska J, Szymlak A, Roczniak W, Girczys-Połedniok K, Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62:17–30. doi: 10.1016/j.advms.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 3.American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 4.Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansari R, Sohrabi S, Ghanaie O, Amjadi H, Merat S, Vahedi H, Khatibian M. Comparison of colonic transit time between patients with constipation-predominant irritable bowel syndrome and functional constipation. Indian J Gastroenterol. 2010;29:66–68. doi: 10.1007/s12664-010-0015-2. [DOI] [PubMed] [Google Scholar]
- 6.Sadik R, Björnsson E, Simrén M. The relationship between symptoms, body mass index, gastrointestinal transit and stool frequency in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2010;22:102–108. doi: 10.1097/MEG.0b013e32832ffd9b. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 8.Raahave D. Faecal retention: A common cause in functional bowel disorders, appendicitis and haemorrhoids – with medical and surgical therapy (PhD thesis). Faculty of health and medical sciences: University of Copenhagen, 2014. [PubMed] [Google Scholar]
- 9.Bouchoucha M, Devroede G, Arhan P, Strom B, Weber J, Cugnenc PH, Denis P, Barbier JP. What is the meaning of colorectal transit time measurement? Dis Colon Rectum. 1992;35:773–782. doi: 10.1007/BF02050328. [DOI] [PubMed] [Google Scholar]
- 10.Martelli H, Devroede G, Arhan P, Duguay C, Dornic C, Faverdin C. Some parameters of large bowel motility in normal man. Gastroenterology. 1978;75:612–618. [PubMed] [Google Scholar]
- 11.Zaslavsky C, da Silveira TR, Maguilnik I. Total and segmental colonic transit time with radio-opaque markers in adolescents with functional constipation. J Pediatr Gastroenterol Nutr. 1998;27:138–142. doi: 10.1097/00005176-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Devroede G, Bouchoucha M, Steiber W. A simplified way to assess colorectal transit time. Tech Coloproctol . 1999;3:71–73. [Google Scholar]
- 13.Leech SC, McHugh K, Sullivan PB. Evaluation of a method of assessing faecal loading on plain abdominal radiographs in children. Pediatr Radiol. 1999;29:255–258. doi: 10.1007/s002470050583. [DOI] [PubMed] [Google Scholar]
- 14.Barr RG, Levine MD, Wilkinson RH, Mulvihill D. Chronic and occult stool retention: a clinical tool for its evaluation in school-aged children. Clin Pediatr (Phila) 1979;18:674, 676, 677–679, passim. doi: 10.1177/000992287901801103. [DOI] [PubMed] [Google Scholar]
- 15.Blethyn AJ, Verrier Jones K, Newcombe R, Roberts GM, Jenkins HR. Radiological assessment of constipation. Arch Dis Child. 1995;73:532–533. doi: 10.1136/adc.73.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Bosch M, Graafmans D, Nievelstein R, Beek E. Systematic assessment of constipation on plain abdominal radiographs in children. Pediatr Radiol. 2006;36:224–226. doi: 10.1007/s00247-005-0065-2. [DOI] [PubMed] [Google Scholar]
- 17.Koh H, Lee MJ, Kim MJ, Shin JI, Chung KS. Simple diagnostic approach to childhood fecal retention using the Leech score and Bristol stool form scale in medical practice. J Gastroenterol Hepatol. 2010;25:334–338. doi: 10.1111/j.1440-1746.2009.06015.x. [DOI] [PubMed] [Google Scholar]
- 18.Park HJ, Noh SE, Kim GD, Joo MC. Plain abdominal radiograph as an evaluation method of bowel dysfunction in patients with spinal cord injury. Ann Rehabil Med. 2013;37:547–555. doi: 10.5535/arm.2013.37.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starreveld JS, Pols MA, Van Wijk HJ, Bogaard JW, Poen H, Smout AJ. The plain abdominal radiograph in the assessment of constipation. Z Gastroenterol. 1990;28:335–338. [PubMed] [Google Scholar]
- 20.Bouchoucha M, Devroede G, Dorval E, Faye A, Arhan P, Arsac M. Different segmental transit times in patients with irritable bowel syndrome and "normal" colonic transit time: is there a correlation with symptoms? Tech Coloproctol. 2006;10:287–296. doi: 10.1007/s10151-006-0295-9. [DOI] [PubMed] [Google Scholar]
- 21.Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003;38:36–42. doi: 10.1080/00365520310000410. [DOI] [PubMed] [Google Scholar]
- 22.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 23.Abrahamsson H, Antov S, Bosaeus I. Gastrointestinal and colonic segmental transit time evaluated by a single abdominal x-ray in healthy subjects and constipated patients. Scand J Gastroenterol Suppl. 1988;152:72–80. doi: 10.3109/00365528809095938. [DOI] [PubMed] [Google Scholar]
- 24.Chan YK, Kwan AC, Yuen H, Yeung YW, Lai KC, Wu J, Wong GS, Leung CM, Cheung WC, Wong CK. Normal colon transit time in healthy Chinese adults in Hong Kong. J Gastroenterol Hepatol. 2004;19:1270–1275. doi: 10.1111/j.1440-1746.2004.03492.x. [DOI] [PubMed] [Google Scholar]
- 25.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133–146. doi: 10.1016/S2468-1253(16)30023-1. [DOI] [PubMed] [Google Scholar]
- 26.Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. J Gastroenterol. 2012;47:1177–1185. doi: 10.1007/s00535-012-0627-7. [DOI] [PubMed] [Google Scholar]
- 27.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suarez F, Furne J, Springfield J, Levitt M. Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol. 1997;272:G1028–G1033. doi: 10.1152/ajpgi.1997.272.5.G1028. [DOI] [PubMed] [Google Scholar]
- 29.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–1189. doi: 10.1016/s0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol. 2009;104:1998–2004. doi: 10.1038/ajg.2009.251. [DOI] [PubMed] [Google Scholar]
- 31.Wall GC, Bryant GA, Bottenberg MM, Maki ED, Miesner AR. Irritable bowel syndrome: a concise review of current treatment concepts. World J Gastroenterol. 2014;20:8796–8806. doi: 10.3748/wjg.v20.i27.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bueno L, Fargeas MJ, Fioramonti J, Honde C. Effects of dopamine and bromocriptine on colonic motility in dog. Br J Pharmacol. 1984;82:35–42. doi: 10.1111/j.1476-5381.1984.tb16439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milo R. Use of the peripheral dopamine antagonist, domperidone, in the management of gastro-intestinal symptoms in patients with irritable bowel syndrome. Curr Med Res Opin. 1980;6:577–584. doi: 10.1185/03007998009109491. [DOI] [PubMed] [Google Scholar]
- 34.Dai L, Zhong LL, Ji G. Irritable bowel syndrome and functional constipation management with integrative medicine: A systematic review. World J Clin Cases. 2019;7:3486–3504. doi: 10.12998/wjcc.v7.i21.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]


