This systematic review and meta-analysis uses data from studies retrieved from an Ovid Medline, Embase, Scopus, and Cochrane Library database search to investigate survival differences between de novo and inverted papilloma–associated sinonasal squamous cell carcinoma pathogenesis.
Key Points
Question
Are there differences in survival between de novo and inverted papilloma–associated sinonasal squamous cell carcinoma pathogenesis?
Findings
In this systematic review and meta-analysis of 26 studies with 1194 unique patients, those with de novo sinonasal squamous cell carcinoma (dnSCC) had almost a 2-fold increased risk of mortality compared with those with inverted papilloma–associated squamous cell carcinoma (IPSCC).
Meaning
These findings suggest that compared with dnSCC, IPSCC may represent a less aggressive form of malignancy; if future prospective studies corroborate these results, de-escalation of IPSCC treatment may be considered to limit morbidity.
Abstract
Importance
Overall, the prognosis of sinonasal squamous cell carcinoma (SCC) is poor. This malignancy can arise de novo or from inverted papillomas, but it is unclear whether survival differences between the 2 pathologies exist.
Objective
To assess for survival differences between patients with sinonasal de novo SCC (dnSCC) and those with inverted papilloma–associated SCC (IPSCC).
Data Sources
A search of Ovid MEDLINE, Embase, Scopus, and the Cochrane Library from inception to January 23, 2020, with cross-referencing of retrieved studies, was performed. Additional data were requested from authors.
Study Selection
Inclusion and exclusion criteria were designed to capture studies with survival outcomes of adults with sinonasal SCC who underwent regular treatment. Clinical trials, cohort studies, case-control studies, and case series with more than 10 adults aged 18 years or older with sinonasal SCC were included. Exclusion criteria were studies on non-SCC sinonasal neoplasms, studies without histopathologic diagnoses, non-English language articles, nonhuman animal studies, and abstract-only articles. Two blinded investigators (J.J.L., A.M.P., T.W.E., or N.S.W.) screened each abstract and full text, and a third investigator (J.J.L. or P.P.) adjudicated discrepancies. Of 729 unique citations, 26 studies of 1194 total patients were included.
Data Extraction and Synthesis
Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines were followed. The Methodological Index for Nonrandomized Studies (MINORS) criteria were used to assess study quality. Two blinded investigators (J.J.L., A.M.P., T.W.E., or N.S.W.) independently extracted data from each study. Data were pooled using a random-effects model.
Main Outcomes and Measures
The primary outcome was overall survival, and secondary outcomes were disease-free and disease-specific survival. Before data collection, it was hypothesized that the dnSCC cohort would have worse survival outcomes than the IPSCC cohort.
Results
One study of patients with dnSCC, 12 studies of patients with IPSCC, and 5 studies with both cohorts were included in the meta-analysis of overall survival. The pooled 5-year overall survival rate for 255 patients with dnSCC was 56% (95% CI, 41%-71%; I2 = 83.8%) and for 475 patients with IPSCC was 65% (95% CI, 56%-73%; I2 = 75.7%). Five comparative studies of both cohorts totaling 240 patients with dnSCC and 155 patients with IPSCC were included in another meta-analysis. The pooled overall survival hazard ratio was 1.87 (95% CI, 1.24-2.84; I2 = 0%).
Conclusions and Relevance
This systematic review and meta-analysis found that patients with dnSCC had almost a 2-fold increased risk of mortality compared with those with IPSCC. Large, multicenter studies are necessary to validate these findings before considering treatment alterations such as de-escalation based on histopathology.
Introduction
Sinonasal malignancies comprise 5% of all head and neck malignancies and up to 1% of all malignancies.1,2 In the sinonasal cavity, the most common malignancy is squamous cell carcinoma (SCC), with an incidence of 0.32 cases per 100 000 patients.2,3,4 Exposure to nickel, asbestos, arsenic, textile dusts, welding fumes, and smoke have been implicated as risk factors.5 Overall, the prognosis of sinonasal SCC is poor, likely owing to its advanced stage at presentation. The current mainstay of treatment is surgical resection either with or without adjuvant therapy, such as radiotherapy and chemoradiotherapy.2,6 Large national database studies approximate the 5-year overall survival to be 30% to 55% irrespective of treatment modality.2,7
There are 2 distinct methods of sinonasal SCC pathogenesis. Sinonasal SCC can arise either de novo or from inverted papillomas (IPs), which are benign but locally aggressive neoplasms of the nasal cavity or paranasal sinuses.8,9,10 Sinonasal IP may be associated with epithelial atypia, dysplasia, carcinoma in situ, and invasive carcinoma.11,12 Secondary SCC may be synchronous, which is discovered at the same time as a primary IP, or metachronous, which appears at the site of a previously resected IP, indicating malignant transformation.13,14,15 The overall malignancy rate of sinonasal IP is 7% to 11%.14,15,16
Currently, it is unclear whether oncologic outcomes are different between de novo sinonasal SCC (dnSCC) and IP-associated SCC (IPSCC). The majority of the literature on sinonasal SCC survival outcomes is comprised of case series, making direct comparisons with minimal confounding between the 2 cohorts difficult. Only a few retrospective cohort studies have directly compared the 2 distinct pathologies, some of which describe improved survival outcomes in patients with IPSCC compared with those with dnSCC, whereas others report no significant difference.6,17,18,19,20 Furthermore, survival outcomes are heterogeneously reported, and direct comparisons using hazard ratios (HRs) are rare. As a result, clinicians are unable to infer clinically meaningful differences in survival outcomes between the 2 cohorts, and the 2 distinct pathologies are usually treated as if they are the same entity.
If IPSCC does demonstrate improved survival outcomes, treatment de-escalation may spare patients from unnecessary morbidity burden while preserving oncologic outcomes. Thus, the objective of this systematic review and meta-analysis was to qualitatively and quantitatively assess survival outcomes of sinonasal dnSCC and IPSCC and directly compare survival between the 2 cohorts based on the published literature. We hypothesized that the de novo cohort would have worse survival outcomes than the IPSCC cohort in a meta-analysis.
Methods
We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline in completing this systematic review and meta-analysis.21 This review was exempt from the Washington University Institutional Review Board because it used data from published literature. No review protocol was registered for this study.
Eligibility Criteria
Our research focused on the differences in survival and disease characteristics between sinonasal dnSCC and IPSCC. We used the Population, Intervention, Comparator, Outcome, and Study Design (PICOS) framework for this systematic review. The population included adults with sinonasal SCC. The intervention was regular treatment, including surgery, radiotherapy (neoadjuvant, adjuvant, and definitive), or chemotherapy (neoadjuvant, adjuvant, and definitive), of dnSCC, and the comparator intervention was regular treatment of IPSCC. The primary outcome measure was overall survival. Secondary outcomes were disease-free survival (DFS) and disease-specific survival (DSS). Other covariates included age, sex, smoking status, tumor location, T stage, nodal disease, treatment method, and margin status.
Inclusion criteria were clinical trials, cohort studies, case-control studies, or case series with more than 10 participants that reported survival outcomes of adults aged 18 years or older with sinonasal SCC. Exclusion criteria were studies on non-SCC sinonasal neoplasms, studies without histopathologic diagnoses, non-English language articles, nonhuman animal studies, and abstract-only articles. For the IPSCC cohort, both synchronous and metachronous cases confirmed on histopathology were included.
Search Strategy
The published literature was searched using strategies created by a medical librarian (M.M.D.) for outcomes of sinonasal SCC. The search strategies were established using a combination of standardized terms and keywords, including but not limited to nose, sinus, paranasal, sinonasal, papilloma, survival, follow-up, retrospective, and SCC, and were implemented in Ovid MEDLINE (January 1, 1946-January 23, 2020), Embase (January 1, 1947-January 23, 2020), Scopus (January 1, 1960-January 23, 2020), Cochrane Central Register of Controlled Trials (January 1, 1980-January 23, 2020), and Cochrane Database of Systematic Reviews (January 1, 1980-January 23, 2020). All searches were completed June 18, 2019, and then executed again on January 23, 2020. All articles were exported to an EndNote (Clarivate Analytics) library. Reference lists were screened to find any relevant articles not found in the literature search. All corresponding authors of studies comparing survival outcomes between the 2 cohorts of interest were contacted in order to request deidentified individual patient data. The complete search strategy is provided (eText in the Supplement).
Data Collection and Assessment of Study Quality
All stages of the review (title and abstract screen, full text screen, and data extraction) were performed in duplicate by 2 independent reviewers, which included 2 otolaryngology resident physicians or postdoctoral research fellows (J.J.L. and N.S.W.) and 2 predoctoral scholars with a master of science in clinical investigation degrees (A.M.P. and T.W.E.). Each reviewer was blinded to the other reviewer’s results. Discrepancies at each stage of the review were resolved by the first and senior author (J.J.L. and P.P.). Quantitative survival data reported only as Kaplan-Meier curves were extracted with a validated online image analyzer used in previous systematic reviews.22 The quality of observational studies was assessed by 2 independent blinded reviewers (J.J.L. and A.M.P.) using the Methodological Index for Non-Randomized Studies (MINORS), which has a maximum sum score of 16 for noncomparative studies and 24 for comparative studies (eTable in the Supplement).23
Statistical Analysis
We used descriptive statistics to describe the study population, histopathologic diagnosis, tumor morphology, treatment method, and survival outcomes. We assessed median, 1-year, 3-year, and 5-year overall survival, DFS, and DSS for both the dnSCC- and IPSCC-pooled cohorts. We performed a meta-analysis of 5-year survival outcomes. In addition, we performed a meta-analysis of overall survival HRs from comparative studies between dnSCC and IPSCC. When HRs were not reported and unable to be calculated from raw individual data, HRs and 95% CIs were estimated using number of observed events, number of patients in each group, and P values from log-rank tests using published methodology.24 Heterogeneity was calculated using I2 with 25%, 50%, and 75% as respective cutoffs for low, moderate, and high heterogeneity, respectively. Given that all studies were observational, we anticipated high heterogeneity and planned to use a random-effects meta-analysis model a priori. A funnel plot was constructed to assess for publication bias, and the Egger test was utilized to assess for small-study effects. All statistical calculations were performed from June 18, 2020, to November 2, 2020, using STATA, version 15 (StataCorp) software.
Results
Baseline Characteristics of Included Studies
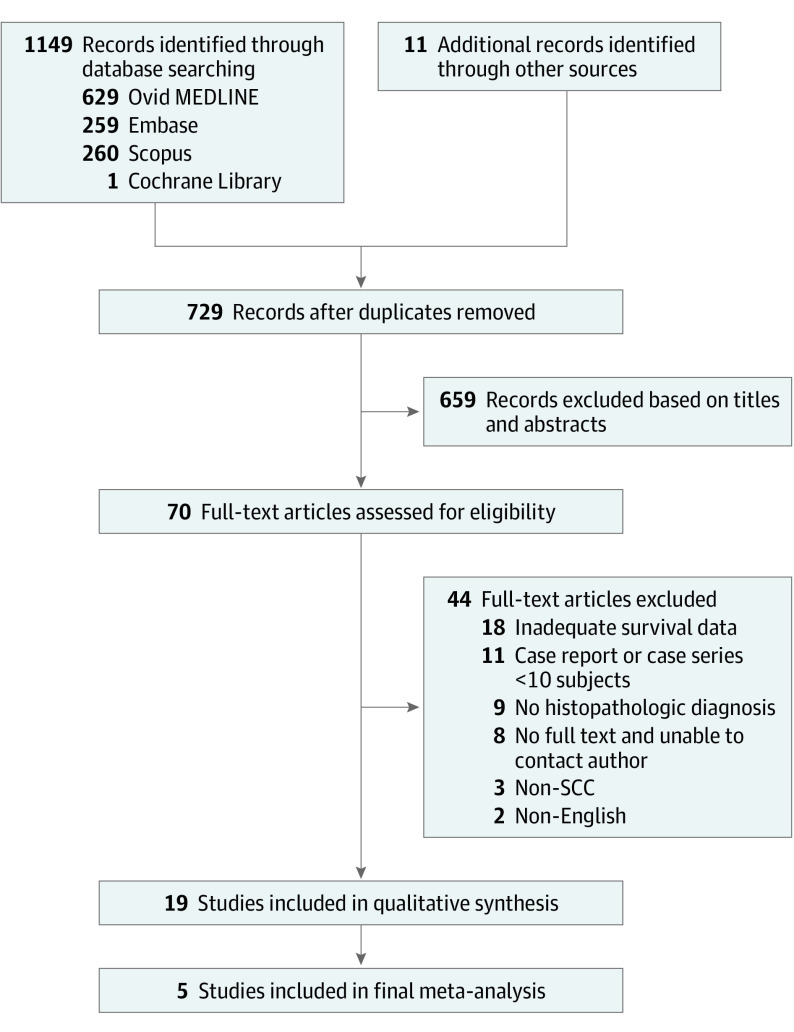
We identified 1149 studies through electronic database searches and 11 studies from other sources, such as reference lists, resulting in 1160 studies in total. After excluding 431 duplicates, we further excluded 659 based on title and abstract screening, resulting in 70 studies for full text review. After additional exclusion of 51 studies, 19 articles remained for qualitative assessment,20,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 of which up to 18 were included in a meta-analysis of 5-year overall survival (Figure 1).6,17,18,19,20 Five were included in a final meta-analysis of overall survival HRs. Baseline study characteristics including descriptions of the study populations and the MINORS total score are shown in Table 1. All 18 included studies involved surgical treatments, including with and without adjuvant therapy. Eight studies (44%) also included a few patients who underwent definitive radiotherapy or chemoradiotherapy without surgery.18,20,28,30,31,34,35,37,40,41,44
Figure 1. Flow Diagram for Assessment of Eligible Studies in the Systematic Review and Meta-Analysis.
SCC indicates squamous cell carcinoma.
Table 1. Baseline Characteristics of the 19 Included Studiesa.
| Source | Study type | No.b | Age, mean (range), yc | Women, No. (%) | T stage (No. patients) | MINORS criteria score | |||
|---|---|---|---|---|---|---|---|---|---|
| Patients with dnSCC | Patients with IPSCC | dnSCC | IPSCC | dnSCC | IPSCC | ||||
| Direct comparative studies | |||||||||
| Lavertu et al18 | Cohort study | 38 | 10 | 60.3 (23-80) | NA | NA | T1 (3), T2 (9), T3 (20), T4 (16) for both cohorts | NA | 16 |
| de Almeida et al17 | Cohort study | 21 | 13 | 57.0 (SD, 13) | NA | NA | T1 (0), T2 (1), T3 (2), T4 (18) | T1 (2), T2 (2), T3 (2), T4 (7) | 17 |
| Lobo et al6 | Cohort study | 88 | 29 | 63.5 (NA) | 32 (36) | 10 (34) | T1 (8), T2 (10), T3 (9), T4 (52), Tx (9) | T1 (3), T2 (4), T3 (4), T4 (11), Tx (7) | 16 |
| Yan et al19 | Cohort study | 28 | 38 | mean (SD), 62.5 (13.2) for dnSCC, 59.9 (11.5) for IPSCC | 13 (46) | 13 (34) | T1 (7), T2 (4), T3 (6), T4 (11) | T1 (14), T2 (5), T3 (7), T4 (10), Tx (2) | 19 |
| Yu et al20 | Cohort study | 65 | 21 | mean (SD), 58.8 (12.8) for dnSCC, 60.9 (10.3) for IPSCC | 17 (26) | 4 (19) | T1 (3), T2 (9), T3 (22), T4 (31) | T1 (4), T2 (5), T3 (6), T4 (6) | 17 |
| Only dnSCC | |||||||||
| Haraguchi30 | Case series | 15 | 0 | 55.1 | 2 (13) | 0 | NA | T1 (0), T2 (0), T3 (0), T4 (0) | 7 |
| Dulguerov et al28 | Cohort study | 126 | 0 | 56.7 (9-86) | NA | 0 | T1 (18), T2 (26), T3 (27), T4 (52) | T1 (0), T2 (0), T3 (0), T4 (0) | 11 |
| Only IPSCC | |||||||||
| Hug et al32 | Case series | 0 | 18 | 54.5 (21-73) | 0 | 5 (28) | T1 (0), T2 (0), T3 (0), T4 (0) | NA | 8 |
| Lesperance et al36 | Case series | 0 | 14 | 67.0 (51-82) | 0 | 2 (14) | T1 (0), T2 (0), T3 (0), T4 (0) | T1-T3 (7), T4 (7) | 12 |
| Buiret et al25 | Case series | 0 | 11 | 60.0 (42-79) | 0 | 3 (27) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (2), T2 (1), T3 (5), T4 (3) | 9 |
| Choi et al26 | Case series | 0 | 18 | 59.4 (42-86) | 0 | 4 (22) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (5), T2 (2), T3 (8), T4 (3) | 11 |
| Kim et al34 | Cohort study | 0 | 16 | 56.5 (31-74) | 0 | 1 (6) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (1), T2 (4), T3 (2), T4 (9) | 10 |
| Hong et al31 | Cohort study | 0 | 17 | 55.9 (35-77) | 0 | 1 (6) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (1), T2 (3), T3 (8), T4 (5) | 10 |
| Yu et al46 | Cohort study | 0 | 32 | 56.5 (NA) | 0 | 7 (22) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (3), T2 (10), T3 (16), T4 (3) | 11 |
| Liang et al39 | Cohort study | 0 | 87 | 54.0 (17-80) | 0 | 23 (26) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (2), T2 (12), T3 (30), T4 (43) | 10 |
| Karligkiotis et al33 | Cohort study | 0 | 34 | 60.2 (34-80) | 0 | 11 (32) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (11), T2 (7), T3 (11), T4 (5) | 12 |
| Yasumatsu et al45 | Case series | 0 | 15 | 61.0 (39-81) | 0 | 7 (47) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (2), T2 (3), T3 (6), T4 (4) | 10 |
| Li et al38 | Cohort study | 0 | 120 | 54.8 (17-82) | 0 | 33 (28) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (0), T2 (32), T3 (36), T4 (52) | 11 |
| Li et al37 | Cohort study | 0 | 21 | 59.2 (35-81) | 0 | 3 (14) | T1 (0), T2 (0), T3 (0), T4 (0) | T1 (2), T2 (1), T3 (10), T4 (8) | 12 |
Abbreviations: dn, de novo; IP, inverted papilloma; MINORS, methodological index for nonrandomized studies; NA, not available; SCC, squamous cell carcinoma; T, tumor.
Table is divided into studies that directly compare between patients with dnSCC and IPSCC, studies of patients with only dnSCC, and studies of patients with only IPSCC.
No. indicates cohort size.
Values written as mean (range) unless otherwise indicated.
Systematic Review of Survival Outcomes
In total, there were 7 studies6,17,18,19,20,28,30 of a combined 381 patients with sinonasal dnSCC and 17 studies6,17,18,19,20,25,26,31,32,33,34,36,37,38,39,45,46 of a combined 475 patients with sinonasal IPSCC (Table 2), which included 5 studies6,17,18,19,20 of both cohorts. Zero time was defined as the date of first treatment in 2 of 19 (10.5%) studies, date of treatment completion in 2 of 19 (10.5%) studies, date of diagnosis in 3 of 19 (15.8%) studies, date of presentation in 1 of 19 (5.3%) studies, and unspecified in 11 of 19 (57.9%) studies.
Table 2. Data Extraction of Survival Outcomes for dnSCC and IPSCC.
| Source | Patients, No.a (%) | Follow-up, median (range), mo | Treatment (No. patients/total No. patients) | Zero time | Median OS, mo | OS, % | Median DFS, mo | DFS, % | Median DSS, mo | DSS, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 y | 3 y | 5 y | 1 y | 3 y | 5 y | 1 y | 3 y | 5 y | ||||||||
| dnSCC | ||||||||||||||||
| Lavertu et al18 | 38 (5.3) | NA (38-120) | Surgery only (3/48), surgery + XRT (30/48), XRT only (15/48)a | Date of treatment completion | 26.0 | 72.7 | 42.8 | 29.8 | NA | NA | NA | NA | NA | NA | NA | NA |
| de Almeida et al17 | 21 (2.9) | 33 (0-111) | Surgery only (10/34), surgery + XRT (12/34), surgery + CRT (12/34)a | Unspecified | >60 | 81.4 | 75.1 | 75.1 | 62.1 | 68.8 | 62.0 | 62.0 | NA | NA | NA | NA |
| Lobo et al6 | 88 (12.2) | 60 (NA) | Surgery +/− adjuvant (74/88), XRT (60/88), chemo (22/88) | Unspecified | 66.0 | 85.7 | 74.4 | 65.8 | 57.6 | 78.9 | 60.3 | 60.3 | NA | NA | NA | NA |
| Yan et al19 | 28 (3.9) | 120 | Surgery only (3/28), surgery + XRT (20/28), surgery + CRT (5/28) | Date of pathologic diagnosis | >120 | 100 | 69.0 | 69.0 | >120 | 67.9 | 52.6 | 52.6 | >120 | 100 | 72.9 | 72.9 |
| Yu et al20 | 65 (9.0) | 47.6 (11-156) | Surgery +/– XRT (37/65), XRT or CRT only (28/65) | Unspecified | 48.9 | 78.6 | 58.6 | 39.5 | NA | NA | NA | NA | 70.2 | 97.9 | 92.3 | 52.8 |
| Haraguchi30 | 15 (2.1) | 60 | Surgery only (3/15), surgery + XRT (7/15), surgery + CRT (2/15), XRT only (2/15), CRT only (1/15) | Date of initial presentation | >60 | 93.3 | 85.7 | 57.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Dulguerov et al28 | 126 (17.5) | 72 (48 to unknown) | Surgery only (32/126), surgery + XRT/CRT (58/126), XRT/CRT only (36/126) | Unspecified | NA | NA | NA | NA | NA | NA | NA | NA | >120 | 83.3 | 64.4 | 60.0 |
| IPSCC | ||||||||||||||||
| Lavertu et al18 | 10 (2.1) | NA (38-120) | Surgery only (3/48), surgery + XRT (30/48), XRT only (15/48)b | Date of treatment completion | >120 | 80.0 | 70.0 | 70.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| de Almeida et al17 | 13 (2.7) | 33 (0-111) | Surgery only (10/34), surgery + XRT (12/34), surgery + CRT (12/34)a | Unspecified | >60 | 100 | 86.0 | 86.0 | >60 | 77.8 | 62.0 | 62.0 | NA | NA | NA | NA |
| Lobo et al6 | 29 (6.1) | 60 (NA) | Surgery +/− adjuvant (28/29), XRT (16/29), chemo (6/29) | Unspecified | 40.8 | 96.7 | 63.2 | 77.0 | 34.8 | 83.5 | 62.6 | 62.6 | NA | NA | NA | NA |
| Yan et al19 | 38 (8.0) | 120 | Surgery only (14/38), surgery + XRT (16/38), surgery + CRT (8/38) | Date of pathologic diagnosis | >120 | 100 | 94.9 | 84.6 | >120 | 90.0 | 67.4 | 62.5 | >120 | 100 | 95.3 | 89.6 |
| Yu et al20 | 21 (4.4) | 47.6 (11-156) | Surgery +/– XRT (19/21), XRT or CRT only (2/21) | Unspecified | 64.9 | 94.7 | 78.4 | 58.3 | NA | NA | NA | NA | 75.9 | 97.6 | 89.1 | 61.5 |
| Hug et al32 | 18 (3.8) | NA (0-120) | Surgery + XRT, surgery + CRT | Unspecified | >120 | 94.4 | 85.0 | 85.0 | >120 | 89.0 | 85.0 | 85.0 | >120 | NA | NA | 88.9 |
| Lesperance et al36 | 14 (2.9) | 53 (6-204) | Surgery only (2/14), surgery + XRT (12/14) | Unspecified | 21.0 | 76.9 | 53.8 | 40.0 | NA | 64.3 | 42.9 | 38.5 | NA | NA | NA | NA |
| Buiret et al25 | 11 (2.3) | 27.6 (4.8-110.4) | Surgery + XRT (10/11), surgery + CRT (1/11) | Date of 1st treatment | 19.5 | 80.7 | 46.2 | 46.2 | 11.6 | 48.5 | 46.2 | 46.2 | NA | NA | NA | NA |
| Choi et al26 | 18 (3.8) | 74.5 (2-212) | Surgery only (7/18), surgery + XRT (9/18), surgery + CRT (2/18) | Unspecified | >150 | 88.2 | 88.2 | 88.2 | >150 | 83.3 | 83.3 | 83.3 | >150 | 88.9 | 88.9 | 88.9 |
| Kim et al34 | 16 (3.4) | 41 (2-141) | Surgery only (4/16), surgery + XRT (5/16), surgery + CRT (2/16), XRT only (3/16), CRT only (2/16) | Date of pathologic diagnosis | 57.4 | 92.9 | 55.7 | 55.7 | 37.3 | NA | NA | NA | >140 | NA | NA | NA |
| Hong et al31 | 17 (3.6) | 47 (8-118) | Surgery only (2/17), surgery + XRT (11/17), surgery + CRT (2/17), CRT only (2/17) | Date of treatment completion | NA | 88.2 | 70.6 | 70.6 | NA | 70.6 | 58.8 | 58.8 | NA | NA | NA | NA |
| Yu et al46 | 32 (6.7) | NA (23-212) | Surgery only (10/32), surgery + XRT (19/32), surgery + CRT (3/32) | Unspecified | 62.2 | 100 | 78.0 | 63.5 | NA | NA | NA | NA | NA | NA | NA | NA |
| Liang et al39 | 87 (18.3) | 40 (7-183) | Surgery only (29/87), surgery + XRT (52/87), surgery + CRT (6/87) | Date of treatment completion | 40.2 | 72.5 | 52.5 | 39.6 | NA | NA | NA | NA | NA | NA | NA | NA |
| Karligkiotis et al33 | 34 (7.2) | 60 (5-166) | Surgery only (21/34), surgery + XRT (11/34), surgery + CRT (2/34) | Date of 1st treatment | >160 | 96.7 | 86.1 | 66.8 | NA | NA | NA | NA | >160 | 97.2 | 86.5 | 71.2 |
| Yasumatsu et al45 | 15 (3.2) | 49 (6-95) | Surgery only (2/15), surgery + XRT (2/15), surgery + CRT (11/15) | Unspecified | >60 | 66.7 | 53.3 | 50.0 | NA | NA | NA | NA | >60 | 75.9 | 63.0 | 58.3 |
| Li et al38 | 81 (17.1) | 62 (6-166) | Surgery only (21/120), surgery + XRT/CRT (92/120), XRT or CRT only (7/120)c | Date of treatment completion | 48.5 | 85.8 | 54.3 | 49.9 | 25.0 | 66.7 | 41.7 | 38.7 | NA | NA | NA | NA |
| Li et al37 | 21 (4.4) | 47.4 (3-123) | Surgery only (4/21), surgery + XRT (7/21), surgery + CRT (2/21), CRT only (8/21) | Date of clinical diagnosis | >120 | 90.5 | 75.4 | 68.5 | >120 | 90.0 | 68.8 | 61.5 | >120 | 90.5 | 80.4 | 80.4 |
Abbreviations: CRT, chemoradiation therapy; DFS, disease-free survival; dnSCC, de novo sinonasal SCC; DSS, disease-specific survival; IPSCC, inverted papilloma–associated SCC; NA, not available; OS, overall survival; SCC, squamous cell carcinoma; XRT, radiation therapy.
No. indicates cohort size.
Treatment rates are of the total combined sample rather than the single cohort.
Treatment rates are of a total sample including patients with carcinoma in situ, which were excluded from our primary analysis.
Of the 6 studies6,17,18,19,20,30 in the pooled sample of 255 patients with dnSCC and adequate overall survival data, median overall survival (range) was 81.4% (72.7%-100%) at 1 year, 69.0% (42.8%-75.1%) at 3 years, and 57.0% (29.8%-75.1%) at 5 years (Table 2). Of the 3 studies6,17,19 that reported DFS for 137 total patients with dnSCC, median DFS (range) was 68.8% (67.9%-78.9%) at 1 year, 60.3% (52.6%-62.0%) at 3 years, and 60.3% (52.6%-62.0%) at 5 years. Of the 3 studies19,20,28 that reported DSS for a combined 219 patients with dnSCC, DSS (range) was 97.9% (83.3%-100.0%) at 1 year, 72.9% (64.4%-92.3%) at 3 years, and 60.0% (54.8%-72.9%) at 5 years.
Of the 12 studies25,26,31,32,33,34,36,37,38,39,45,46 in the pooled sample of 475 patients with IPSCC, median overall survival (interquartile range) was 90.2% (80.7%-96.7%) at 1 year, 75.4% (54.3%-85.0%) at 3 years, and 63.5% (50.0%-70.6%) at 5 years (Table 2). Of the 10 studies6,17,19,25,26,31,32,36,37,38 that reported DFS for 260 total IPSCC patients, median DFS (range) was 80.6% (48.5%-90.0%) at 1 year, 62.3% (41.7%-85.0%) at 3 years, and 61.8% (38.5%-85.0%) at 5 years. Of the 7 studies19,20,26,32,33,37,45 that reported DSS for a combined 209 patients with IPSCC, the DSS (range) was 93.9% (75.9%-100.0%) at 1 year, 87.7% (63.0%-95.3%) at 3 years, and 80.4% (58.3%-89.6%) at 5 years.
Meta-analysis of 5-Year Survival Rates
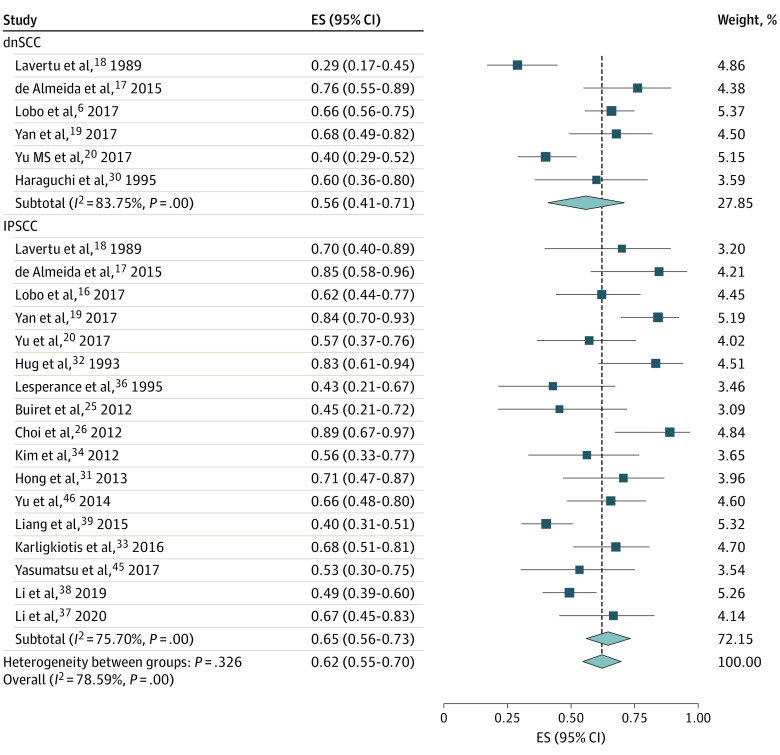
There was 1 study30 of patients with only dnSCC, 12 studies25,26,31,32,33,34,36,37,38,39,45,46 of patients with only IPSCC, and 5 studies6,17,18,19,20 with both cohorts that adequately reported 5-year overall survival rates. Pooled 5-year overall survival rates for 255 patients with dnSCC were 56% (95% CI, 41%-71%; I2 = 83.8%) and for 475 patients with IPSCC were 65% (95% CI, 56%-73%; I2 = 75.7%) (Figure 2). Meanwhile, there were no studies of only patients with dnSCC, 7 studies25,26,31,32,36,37,38 of only patients with IPSCC, and 3 studies6,17,19 with both cohorts that adequately reported 5-year DFS rates. The pooled 5-year DFS for 137 patients with dnSCC was 58% (95% CI, 48%-72%; I2 = 74.0%) and for 260 patients with IPSCC was 60% (95% CI, 48%-72%; I2 = 74.0%) (eFigure 1 in the Supplement). For 5-year DSS rates, there was 1 study28 of patients with only dnSCC, 6 studies26,32,33,34,37,45 of patients with only IPSCC, and 2 studies19,20 with both cohorts. The pooled 5-year DSS for 219 patients with dnSCC was 60% (95% CI, 51%-69%; unable to estimate I2) and for 209 patients with IPSCC was 77% (95% CI, 68%-87%; I2 = 58.6%) (eFigure 2 in the Supplement).
Figure 2. Forest Plot of the Meta-analysis of 5-Year Overall Survival Rates Between de novo Sinonasal Squamous Cell Carcinoma (dnSCC) and Inverted Papilloma–Associated Squamous Cell Carcinoma (IPSCC).
ES indicates effect size.
There were also 7 studies27,29,35,40,41,42,44 that did not explicitly mention IP status, which were excluded in this meta-analysis. However, because de novo etiology represents the majority of sinonasal SCC and there was no mention of IP, a sensitivity analysis including those studies in the meta-analysis was performed. Inclusion resulted in an increase to 12 studies6,17,18,19,20,27,29,30,35,40,41,44 of 568 patients with dnSCC and reported 5-year overall survival rates, 5 studies6,17,19,29,44 of 292 patients with dnSCC and reported 5-year DFS rates, and 5 studies19,20,28,29,42 of 345 patients with dnSCC and reported 5-year DSS rates. Pooled 5-year overall survival for the dnSCC cohort decreased to 48% (95% CI, 39%-58%), DFS decreased to 51% (95% CI, 40%-61%), and DSS decreased to 56% (95% CI, 47%-65%) (eFigures 3, 4, and 5 in the Supplement).
Meta-analysis of Overall Survival HRs
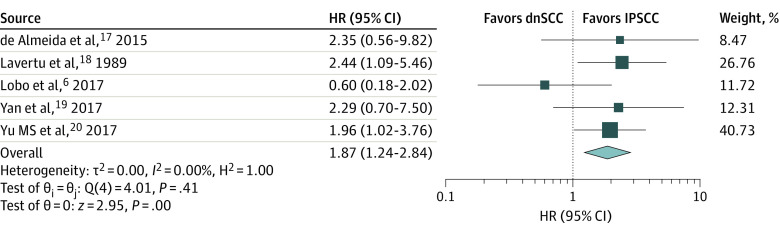
Five studies6,17,18,19,20 directly comparing survival outcomes between a combined 240 patients with dnSCC and 155 patients with IPSCC were included in a separate meta-analysis (Figure 3). Even though heterogeneity was low (I2 = 0%), a random-effects model was used as planned. For 1 study,19 survival HR and 95% CI were calculated using deidentified raw data provided by Yan et al.19 For the other 4 studies,6,17,18,20 estimated overall survival HRs with 95% CIs of each study are shown (Figure 2). The pooled HR was 1.87 (95% CI, 1.24-2.84) for overall survival, suggesting that patients with dnSCC have almost a 2-fold increased risk of mortality compared with patients with IPSCC, which can be as low as a 24% increased risk and almost as high as a 200% increased risk. These results were identical when a fixed-effects model was used. All 5 studies6,17,18,19,20 confirmed IP status pathologically.
Figure 3. Forest Plot of the Meta-analysis of Overall Survival Hazard Ratios (HRs) Between de novo Sinonasal Squamous Cell Carcinoma (dnSCC) and Inverted Papilloma–Associated Squamous Cell Carcinoma (IPSCC).
Hazard ratios greater than 1 suggest worse survival for dnSCC.
SCC indicates squamous cell carcinoma.
Publication Bias
Publication bias was assessed by funnel plot (eFigure 6 in the Supplement). The Egger test for small-study effects was unable to exclude a symmetric distribution of publications, suggesting low risk of publication bias.
Discussion
This systematic review of 719 patients with dnSCC and 475 patients with IPSCC attempted to comprehensively assess survival outcomes of each pathology. Sinonasal dnSCC had lower median 1-year, 3-year, and 5-year overall survival, DFS, and DSS rates compared with IPSCC. Similarly, pooled 5-year overall survival was lower in the dnSCC cohort compared with the IPSCC cohort. The pooled 5-year DFS was similar between the dnSCC cohort and the IPSCC cohort, whereas the pooled 5-year DSS was associated with a substantially lower result in the dnSCC cohort compared with the IPSCC cohort. In addition, findings suggest that a sensitivity analysis including 7 studies27,29,30,35,41,44 that did not explicitly mention IP status further increased the magnitude and precision of the 5-year survival differences between the 2 cohorts.
Our meta-analysis of 240 patients with sinonasal dnSCC and 155 patients with IPSCC found an approximate 2-fold increased risk of mortality in the de novo cohort compared with the IP cohort, which is clinically meaningful. One explanation is that IPSCC may be on a spectrum of mild dysplastic changes to focal invasive carcinoma, which tend to be well or moderately differentiated.47 Focal islands of SCC within benign neoplastic tissue may behave less aggressively than lesions that are completely composed of SCC, which is seen with de novo lesions. Having foci of SCC within a larger IP lesion may also risk overstaging the cancer. In cases of metachronous IPSCC, regular otolaryngologic surveillance of IP with nasal endoscopy may enable clinicians to find these malignancies at an earlier stage or grade before they are even symptomatic. This review’s finding of increased mortality of de novo sinonasal SCC may be used to help guide patient counseling and collaborative decisions on treatment modality. Sinonasal IPSCC may represent a distinct clinical disease compared with dnSCC, and future treatments may need to be tailored accordingly.
Currently, there is conflicting evidence in the existing literature on the prognostic characteristics of dnSCC vs IPSCC on survival outcomes. Owing to the low incidence of sinonasal SCC, the majority of published studies are case series with small sample sizes; thus, assessment of survival outcomes using individual studies is limited. A prior pooled survival analysis of 76 patients with IPSCC demonstrated a median overall survival of 126 months and 5-year overall survival rate of 61%, which was similar to the pooled rate from our meta-analysis.48 To our knowledge, there are no pooled survival data of patients with only sinonasal dnSCC in the current literature. This question also cannot be answered using large national databases such as Surveillance, Epidemiology, and End Results (SEER) and National Cancer Database since they do not capture histopathologic information on whether sinonasal SCC is associated with IP. Therefore, we attempted to address the question with this systematic review and meta-analysis.
Although a few comparative cohort studies exist in the literature regarding survival outcomes between patients with sinonasal dnSCC and IPSCC, they heterogeneously report survival data, including type of survival (ie, overall survival, DFS, DSS, progression-free survival, locoregional recurrence-free survival), duration of follow-up, and summary statistics.6,17,18,19,20 Only 1 study reported an HR,19 which was of DSS adjusted for distant metastasis, orbital involvement, and surgical resection technique. That study’s corresponding author did provide deidentified individual patient data, which we used to calculate overall survival HR with 95% CI. Because time-to-event outcomes are most appropriately analyzed and interpretable using HR, we estimated overall survival HRs with 95% CIs for the other 4 studies6,17,18,20 that reported sufficient data using validated methodology.24 Reporting HRs and 95% CIs allows the readership to interpret both the magnitude and precision of the outcome of dnSCC status compared with IPSCC status, which should assist in making these results more clinically relevant.
We hope these results will promote further studies across multiple institutions. Future directions include a prospective cohort study to confirm improved survival outcomes of sinonasal IPSCC or a multicenter collaboration to share individual patient data and retrospectively perform more accurate pooled survival analyses while controlling for confounders like comorbidity and stage. These studies may also assess the prognostic outcome of amount of SCC burden within IP lesions as measured by absolute size or relative size in comparison with the surrounding papilloma.
If the results of future studies are similar to this current meta-analysis, new studies on treatment de-escalation for IPSCC, such as limiting adjuvant therapy and its subsequent adverse effects, may be considered. De-escalation of radiotherapy, particularly around the orbit, may be vision-sparing, and sinonasal crusting and drainage burden would also improve. In addition, withholding adjuvant chemotherapy would limit systemic adverse effects. A multicenter, randomized trial or prospective cohort study that limits selection bias, such as via propensity score weighting, and has 5-year follow-up outcomes may inform more tailored treatment algorithms for this potentially distinct cancer.
Limitations
To our knowledge, this review is the first meta-analysis of survival outcomes between these 2 distinct pathologies of sinonasal SCC. However, it does have several limitations. Owing to the inherent difficulties in feasibility of conducting a randomized controlled trial to assess survival outcomes of sinonasal SCC, all included studies were observational, including a number of case series of more than 10 patients. Despite this limitation, heterogeneity was low (I2 = 0%) in our final meta-analysis. Sinonasal SCC is also an uncommon pathology, so most of our studies had limited sample sizes of fewer than 100 patients. Another limitation was that a few studies did not distinguish between invasive carcinoma and carcinoma in situ when reporting survival outcomes. Amount of SCC burden within IP was also not readily reported and controlled for. In addition, we were unable to control for confounders such as age; comorbidities; tumor, node, and metastasis (TNM) stage; histologic grade; synchronous vs metachronous status; and treatment modality because of inconsistent reporting of data. The lack of sufficiently reported data and inability to obtain individual patient data precluded our ability to perform a subgroup analysis based on stage and treatment modality.
Conclusions
In this systematic review and meta-analysis, patients with dnSCC had worse overall survival than those with IPSCC. Pooled survival data also suggest lower DFS and DSS in the dnSCC cohort. Large, multicenter studies are necessary to validate these findings before considering treatment alterations based on histopathology.
eTable. Methodological Index for Nonrandomized Studies (MINORS) Criteria for Each Included Study
eText. Complete Search Strategy
eFigure 1. Forest Plot of the Meta-Analysis of 5-Year Disease-Free Survival Rates Between dnSCC and IPSCC
eFigure 2. Forest Plot of the Meta-Analysis of 5-Year Disease-Specific Survival Rates Between dnSCC and IPSCC
eFigure 3. Forest Plot of the Meta-Analysis of 5-Year Overall Survival Rates Between dnSCC and IPSCC When Including Studies That Did Not Explicitly Mention IP Status Into the De Novo Group
eFigure 4. Forest Plot of the Meta-Analysis of 5-Year Disease-Free Survival Rates Between dnSCC and IPSCC When Including Studies That Did Not Explicitly Mention IP Status Into the De Novo Group
eFigure 5. Forest Plot of the Meta-Analysis of 5-Year Disease-Specific Survival Rates Between dnSCC and IPSCC When Including Studies That Did Not Explicitly Mention IP Status Into the De Novo Group
eFigure 6. Funnel Plot of the Included Studies in the Meta-Analysis With Logarithmic Function of the Hazard Ratio on the X-Axis and Standard Error on the Y-Axis
References
- 1.Dubal PM, Bhojwani A, Patel TD, et al. Squamous cell carcinoma of the maxillary sinus: a population-based analysis. Laryngoscope. 2016;126(2):399-404. doi: 10.1002/lary.25601 [DOI] [PubMed] [Google Scholar]
- 2.Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124(1):76-83. doi: 10.1002/lary.24264 [DOI] [PubMed] [Google Scholar]
- 3.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34(6):877-885. doi: 10.1002/hed.21830 [DOI] [PubMed] [Google Scholar]
- 4.Weber AL, Stanton AC. Malignant tumors of the paranasal sinuses: radiologic, clinical, and histopathologic evaluation of 200 cases. Head Neck Surg. 1984;6(3):761-776. doi: 10.1002/hed.2890060310 [DOI] [PubMed] [Google Scholar]
- 5.Luce D, Leclerc A, Bégin D, et al. Sinonasal cancer and occupational exposures: a pooled analysis of 12 case-control studies. Cancer Causes Control. 2002;13(2):147-157. doi: 10.1023/A:1014350004255 [DOI] [PubMed] [Google Scholar]
- 6.Lobo BCDA, D’Anza B, Farlow JL, et al. Outcomes of sinonasal squamous cell carcinoma with and without association of inverted papilloma: a multi-institutional analysis. Am J Rhinol Allergy. 2017;31(5):305-309. doi: 10.2500/ajra.2017.31.4470 [DOI] [PubMed] [Google Scholar]
- 7.Ansa B, Goodman M, Ward K, et al. Paranasal sinus squamous cell carcinoma incidence and survival based on surveillance, epidemiology, and end results data, 1973 to 2009. Cancer. 2013;119(14):2602-2610. doi: 10.1002/cncr.28108 [DOI] [PubMed] [Google Scholar]
- 8.Chiu AG, Jackman AH, Antunes MB, Feldman MD, Palmer JN. Radiographic and histologic analysis of the bone underlying inverted papillomas. Laryngoscope. 2006;116(9):1617-1620. doi: 10.1097/01.mlg.0000230401.88711.e6 [DOI] [PubMed] [Google Scholar]
- 9.Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80(2):192-206. doi: 10.1177/000348947108000205 [DOI] [PubMed] [Google Scholar]
- 10.Ringertz N. Pathology of malignant tumors arising in the nasal and paranasal cavities and maxilla. Acta Otolaryngol. 1938;27:31–42. [Google Scholar]
- 11.Snyder RN, Perzin KH. Papillomatosis of nasal cavity and paranasal sinuses (inverted papilloma, squamous papilloma). a clinicopathologic study. Cancer. 1972;30(3):668-690. doi: [DOI] [PubMed] [Google Scholar]
- 12.Suh KW, Facer GW, Devine KD, Weiland LH, Zujko RD. Inverting papilloma of the nose and paranasal sinuses. Laryngoscope. 1977;87(1):35-46. doi: 10.1288/00005537-197701000-00005 [DOI] [PubMed] [Google Scholar]
- 13.Christensen WN, Smith RR. Schneiderian papillomas: a clinicopathologic study of 67 cases. Hum Pathol. 1986;17(4):393-400. doi: 10.1016/S0046-8177(86)80463-4 [DOI] [PubMed] [Google Scholar]
- 14.Mirza S, Bradley PJ, Acharya A, Stacey M, Jones NS. Sinonasal inverted papillomas: recurrence, and synchronous and metachronous malignancy. J Laryngol Otol. 2007;121(9):857-864. doi: 10.1017/S002221510700624X [DOI] [PubMed] [Google Scholar]
- 15.Nygren A, Kiss K, von Buchwald C, Bilde A. Rate of recurrence and malignant transformation in 88 cases with inverted papilloma between 1998-2008. Acta Otolaryngol. 2016;136(3):333-336. doi: 10.3109/00016489.2015.1116123 [DOI] [PubMed] [Google Scholar]
- 16.Lawson W, Patel ZM. The evolution of management for inverted papilloma: an analysis of 200 cases. Otolaryngol Head Neck Surg. 2009;140(3):330-335. doi: 10.1016/j.otohns.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 17.de Almeida JRS, Su SY, Koutourousiou M, et al. Endonasal endoscopic surgery for squamous cell carcinoma of the sinonasal cavities and skull base: oncologic outcomes based on treatment strategy and tumor etiology. Head Neck. 2015;37(8):1163-1169. doi: 10.1002/hed.23731 [DOI] [PubMed] [Google Scholar]
- 18.Lavertu P, Roberts JK, Kraus DH, et al. Squamous cell carcinoma of the paranasal sinuses: the Cleveland Clinic experience 1977-1986. Laryngoscope. 1989;99(11):1130-1136. doi: 10.1288/00005537-198911000-00005 [DOI] [PubMed] [Google Scholar]
- 19.Yan CHN, Newman JG, Kennedy DW, Palmer JN, Adappa ND. Clinical outcomes of sinonasal squamous cell carcinomas based on tumor etiology. Int Forum Allergy Rhinol. 2017;7(5):508-513. doi: 10.1002/alr.21899 [DOI] [PubMed] [Google Scholar]
- 20.Yu MS, Lim WS, Lee BJ, Chung YS. Squamous cell carcinoma associated with inverted papilloma of the maxillary sinus: our experience with 21 patients. Clin Otolaryngol. 2017;42(5):1048-1052. doi: 10.1111/coa.12804 [DOI] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 22.Drewry AM, Ablordeppey EA, Murray ET, et al. Antipyretic therapy in critically ill septic patients: a systematic review and meta-analysis. Crit Care Med. 2017;45(5):806-813. doi: 10.1097/CCM.0000000000002285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-716. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 24.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buiret G, Montbarbon X, Fleury B, et al. Inverted papilloma with associated carcinoma of the nasal cavity and paranasal sinuses: treatment outcomes. Acta Otolaryngol. 2012;132(1):80-85. doi: 10.3109/00016489.2011.620001 [DOI] [PubMed] [Google Scholar]
- 26.Choi JW, Kim SG, Kim YM, Yoon YH, Kim AY, Rha KS. Clinical and histologic features of inverted papilloma-associated malignancy. Eur Arch Otorhinolaryngol. 2012;269(11):2349-2354. doi: 10.1007/s00405-012-1935-5 [DOI] [PubMed] [Google Scholar]
- 27.Chowdhury N, Alvi S, Kimura K, et al. Outcomes of HPV-related nasal squamous cell carcinoma. Laryngoscope. 2017;127(7):1600-1603. doi: 10.1002/lary.26477 [DOI] [PubMed] [Google Scholar]
- 28.Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? a series of 220 patients and a systematic review. Cancer. 2001;92(12):3012-3029. doi: [DOI] [PubMed] [Google Scholar]
- 29.Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: report of an international collaborative study. Head Neck. 2005;27(7):575-584. doi: 10.1002/hed.20165 [DOI] [PubMed] [Google Scholar]
- 30.Haraguchi H, S Ebihara, M Saikawa, K Mashima, T Haneda, K Hirano. Malignant tumors of the nasal cavity: review of a 60-case series. Jpn J Clin Oncol . 1995;25(5):188-94. [PubMed]
- 31.Hong S-LK, Kim BH, Lee JH, Cho KS, Roh HJ. Smoking and malignancy in sinonasal inverted papilloma. Laryngoscope. 2013;123(5):1087-1091. doi: 10.1002/lary.23876 [DOI] [PubMed] [Google Scholar]
- 32.Hug EBW, Wang CC, Montgomery WW, Goodman ML. Management of inverted papilloma of the nasal cavity and paranasal sinuses: importance of radiation therapy. Int J Radiat Oncol Biol Phys. 1993;26(1):67-72. doi: 10.1016/0360-3016(93)90174-T [DOI] [PubMed] [Google Scholar]
- 33.Karligkiotis A, Lepera D, Volpi L, et al. Survival outcomes after endoscopic resection for sinonasal squamous cell carcinoma arising on inverted papilloma. Head Neck. 2016;38(11):1604-1614. doi: 10.1002/hed.24481 [DOI] [PubMed] [Google Scholar]
- 34.Kim K, Kim D, Koo Y, et al. Sinonasal carcinoma associated with inverted papilloma: a report of 16 cases. J Craniomaxillofac Surg. 2012;40(4):e125-e129. doi: 10.1016/j.jcms.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 35.Lee CH, Hur DG, Roh HJ, et al. Survival rates of sinonasal squamous cell carcinoma with the new AJCC staging system. Arch Otolaryngol Head Neck Surg. 2007;133(2):131-134. doi: 10.1001/archotol.133.2.131 [DOI] [PubMed] [Google Scholar]
- 36.Lesperance MME, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope. 1995;105(2):178-183. doi: 10.1288/00005537-199502000-00013 [DOI] [PubMed] [Google Scholar]
- 37.Li W, Lu H, Zhang H, Sun X, Hu L, Wang D. Squamous cell carcinoma associated with inverted papilloma: Recurrence and prognostic factors. Oncol Lett. 2020;19(1):1082-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Wang C, Wang R, et al. Survival outcomes and prognostic factors of squamous cell carcinomas arising from sinonasal inverted papillomas: a retrospective analysis of 120 patients. Int Forum Allergy Rhinol. 2019;9(11):1367-1373. doi: 10.1002/alr.22400 [DOI] [PubMed] [Google Scholar]
- 39.Liang Q-ZL, Li DZ, Wang XL, Huang H, Xu ZG, Wu YH. Survival outcome of squamous cell carcinoma arising from sinonasal inverted papilloma. Chin Med J (Engl). 2015;128(18):2457-2461. doi: 10.4103/0366-6999.164929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKay SP, Shibuya TY, Armstrong WB, et al. Cell carcinoma of the paranasal sinuses and skull base. Am J Otolaryngol. 2007;28(5):294-301. doi: 10.1016/j.amjoto.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 41.Mine S, Saeki N, Horiguchi K, Hanazawa T, Okamoto Y. Craniofacial Resection for Sinonasal Malignant Tumors: Statistical Analysis of Surgical Outcome over 17 Years at a Single Institution. Skull Base. 2011;21(4):243-248. doi: 10.1055/s-0031-1280686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolai P, Battaglia P, Bignami M, et al. Endoscopic surgery for malignant tumors of the sinonasal tract and adjacent skull base: a 10-year experience. Am J Rhinol. 2008;22(3):308-316. doi: 10.2500/ajr.2008.22.3170 [DOI] [PubMed] [Google Scholar]
- 43.Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8(3):269-286. doi: 10.1007/s12105-014-0527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russo AL, Adams JA, Weyman EA, et al. Long-Term Outcomes After Proton Beam Therapy for Sinonasal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2016;95(1):368-376. doi: 10.1016/j.ijrobp.2016.02.042 [DOI] [PubMed] [Google Scholar]
- 45.Yasumatsu R, Nakashima T, Sato M, et al. Clinical management of squamous cell carcinoma associated with sinonasal inverted papilloma. Auris Nasus Larynx. 2017;44(1):98-103. doi: 10.1016/j.anl.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 46.Yu H-XL, Liu G. Malignant transformation of sinonasal inverted papilloma: a retrospective analysis of 32 cases. Oncol Lett. 2014;8(6):2637-2641. doi: 10.3892/ol.2014.2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirza S, Bradley PJ, Acharya A, Stacey M, Jones NS. Sinonasal inverted papillomas: recurrence, and synchronous and metachronous malignancy. J Laryngol Otol. 2007;121(9):857-864. doi: 10.1017/S002221510700624X [DOI] [PubMed] [Google Scholar]
- 48.Tanvetyanon T, Qin D, Padhya T, Kapoor R, McCaffrey J, Trotti A. Survival outcomes of squamous cell carcinoma arising from sinonasal inverted papilloma: report of 6 cases with systematic review and pooled analysis. Am J Otolaryngol. 2009;30(1):38-43. doi: 10.1016/j.amjoto.2008.02.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Methodological Index for Nonrandomized Studies (MINORS) Criteria for Each Included Study
eText. Complete Search Strategy
eFigure 1. Forest Plot of the Meta-Analysis of 5-Year Disease-Free Survival Rates Between dnSCC and IPSCC
eFigure 2. Forest Plot of the Meta-Analysis of 5-Year Disease-Specific Survival Rates Between dnSCC and IPSCC
eFigure 3. Forest Plot of the Meta-Analysis of 5-Year Overall Survival Rates Between dnSCC and IPSCC When Including Studies That Did Not Explicitly Mention IP Status Into the De Novo Group
eFigure 4. Forest Plot of the Meta-Analysis of 5-Year Disease-Free Survival Rates Between dnSCC and IPSCC When Including Studies That Did Not Explicitly Mention IP Status Into the De Novo Group
eFigure 5. Forest Plot of the Meta-Analysis of 5-Year Disease-Specific Survival Rates Between dnSCC and IPSCC When Including Studies That Did Not Explicitly Mention IP Status Into the De Novo Group
eFigure 6. Funnel Plot of the Included Studies in the Meta-Analysis With Logarithmic Function of the Hazard Ratio on the X-Axis and Standard Error on the Y-Axis