Abstract
The dominant model of number processing suggests the existence of a Number Form Area (NFA) in the inferior temporal gyrus (ITG) that supports the processing of Arabic digits as visual symbols of number. However, studies have produced inconsistent evidence for the presence and laterality of digit-specific ITG activity. Furthermore, whether any such activity relates to mathematical competence is unknown. This study investigated these two issues using functional magnetic resonance imaging. Thirty-two adults performed digit and letter detection tasks and reading and math tests. During digit detection, participants determined whether digits were present in a string of letters (e.g., AH3NR versus AHTNR). During letter detection, participants determined whether letters were present in a string of digits (e.g., 93R78 versus 93478). Results showed four clusters in frontal, occipital, and temporal regions for digit detection, including a left ITG cluster. Five clusters in frontal, parietal, occipital, and temporal regions were associated with letter detection, including a left ITG cluster. Digit and letter-related ITG clusters were spatially distinct; however, a direct contrast of digit and letter processing did not reveal greater activity in the left ITG for digit detection. Whole brain correlations showed greater digit-related activity in the right ITG for participants with higher calculation skills, but there was no correlation between letter activity and calculation skills. Together, our results suggest functional localization, but not specialization, for digits in the left ITG and provide the first evidence of a relationship between calculation skills and digit processing in the right ITG.
Keywords: Digit processing, Letter processing, Number Form Area, Mathematical competence
1. Introduction
The dominant neuropsychological model of numerical processing suggests three neural circuits: left-lateralized perisylvian areas that support verbal representations, bilateral intraparietal sulci that support magnitude representations, and the Number Form Area (NFA) – regions in bilateral inferior temporal gyri (ITG) that support processing digits as visual symbols (Dehaene and Cohen, 1995). While there is ample evidence for the neural circuits underlying verbal and magnitude representations (Arsalidou et al., 2017; Arsalidou and Taylor, 2011; Kaufmann et al., 2011; Pollack and Ashby, 2017; Sokolowski et al., 2017), consensus on the existence and function of an NFA is lacking. Additional insight into the neurocognitive mechanisms that support visual encoding of digits is therefore crucial to test and inform models of numerical processing and the role of such processing in numeracy development.
Is there a neural region (e.g., in the ITG) that reliably supports processing digits as visual symbols – in other words, is there functional localization for digit-processing? The few studies that have investigated this question have produced mixed findings. Using functional magnetic resonance imaging (fMRI), one of the first studies to explicitly investigate a potential NFA showed greater brain activation only in the left angular gyrus during passive viewing of digits compared to letters, scrambled digits, and scrambled letters, suggesting the absence of a NFA in the ITG (Price and Ansari, 2011). In contrast, subsequent studies have shown support for a potential NFA, but lack consensus on location. For example, using fMRI, Grotheer et al. (2016b) found greater brain activation in the bilateral ITG for digits than scrambled digits, and for digits than all other symbols combined (i.e., letters, scrambled digits and letters, Fourier randomized digits and letters, objects) during a one-back visual identification task. However, additional studies with fMRI have found only right-lateralized digit-related activity in the ITG (Cui et al., 2013; Gullick and Temple, 2011). Similarly, an fMRI meta-analysis of digit processing suggests concordant activation only in the right ITG for digits compared to other meaningful symbols when experimental and control tasks are closely matched (Yeo et al., 2017). As this literature illustrates, the issue of functional localization of digit-related processing remains unsettled.
The role of attentional mechanisms may shed light on this issue. Attention may modulate brain activation in ventral and inferior temporal cortex related to visual processing, for example, by enhancing neural responses to objects that are targets versus not (Çukur et al., 2013; Vuilleumier and Driver, 2007), and biasing neural processing toward a target category that is present or absent (Peelen et al., 2009). Similarly, object recognition may involve both top-down and bottom-up processes (Bar et al., 2006). This research suggests that functional activation of a potential NFA may require both visual perception of and attention to digits. If so, this may explain why prior active (Grotheer et al., 2016b) but not passive (Price and Ansari, 2011) paradigms have elicited digit-related activity in the ITG. Further, the notion that neural circuits may support symbolic number processing under some conditions (i.e., viewing and attending) and not others (i.e., viewing only) could refine models of numerical processing and the relations among cognitive mechanisms that support numerical development.
Even if there is functional localization of digit processing in a given brain region, however, that region may not be specialized for digit processing (i.e., may not be exclusively involved in digit processing or show greater activation for digits compared to all other stimuli), as implied by longstanding theories (e.g., Dehaene and Cohen, 1995). In other words, functional localization does not imply functional specialization. To date, strong evidence related to functional specialization for digit processing is lacking. Using electrophysiological recording, Shum et al. (2013) showed preferential responses to digits over visually similar symbols (e.g., letters, scrambled digits and letters), and phonologically and semantically related symbols (e.g., “one,” “won”) in right ITG electrodes. However, a study using transcranial magnetic stimulation (TMS) showed that stimulation of the right NFA impaired performance for both digits and letters compared to their scrambled counterparts, leading the authors to suggest that the NFA is involved in visual processing of familiar symbols rather than digits specifically (Grotheer et al., 2016a). Investigations of functional specialization for digit processing (e.g., in the ITG) have the potential to inform ongoing debates regarding category-specific processing in the brain (e.g., Peelen and Downing, 2017), such as for faces and places (e.g., Kanwisher, 2010), body parts (Downing et al., 2001), and letters and objects (Joseph et al., 2006, 2003). Further, knowledge about functional localization and specialization for digit-related brain activity can advance theories of the neurocognitive mechanisms that support learning across educationally-relevant domains, such as the relation between symbol processing for reading (e.g., words, letters) and mathematics (e.g., digits, number words).
Indeed, across both domains, related studies on visual symbol processing suggest the possibility of an association between digit-related brain activity and mathematical competence. Eye-tracking evidence suggests that visual processing of digits relates to mathematical competence beyond behavioral performance metrics (Price et al., 2017). It is possible, therefore, that neural mechanisms supporting early visual processing of digits may relate to mathematics competence in specific ways as well. Importantly, analogous literacy research shows that activity in the visual word form area (VWFA), a region in inferior temporal cortex preferentially responsive to visually-presented words compared to other visual stimuli, relates to reading ability (Dehaene et al., 2015, 2010; Dehaene and Cohen, 2011; Dehaene and Dehaene-Lambertz, 2016; Maisog et al., 2008; Malins et al., 2016; Richlan et al., 2011, 2009). A similar relation between NFA activity and mathematics competence would suggest that neurocognitive mechanisms that support digit identification are important for mathematics performance.
In the present study, we used two familiar symbol sets, digits and letters, to elucidate digit-related processing in the brain and its relation to mathematics performance, in an fMRI study of typically developing adults. First, we investigated the functional localization and specialization of a putative NFA during tasks that require differing levels of attention to digits. Second, we examined the relation between digit-related brain activation across the whole brain and mathematics competence.
2. Materials and Methods
2.1. Participants
Participants were 33 neurologically healthy, right-handed, English speaking 18-23 year old adults (M = 19.42, SD = 1.50, 22 females) recruited from our university and the surrounding community. Participants were recruited via postings to the psychology study pool and listservs, and received course credit or small monetary compensation for participating. All participants gave written consent and the study was approved by the university Institutional Review Board. We excluded data from one participant due to excess head motion in the scanner, resulting in a final sample of 32 participants (Mean age = 19.38, SD = 1.50, 21 females).
2.2. Tasks
2.2.1. fMRI Tasks.
Participants completed one 60-minute scanning session during which they performed visual search tasks with strings of digits and letters. Symbols were the digits 1-9 and the letters T, S, N, R, H, E, D, C, and A. During digit detection, participants viewed symbol strings and determined whether a digit was present (i.e., Yes or No). During letter detection, participants viewed symbol strings and indicated whether a letter was present. Each detection task had 54 distinct trials grouped into 27 pairs. Each pair contained an Absent trial with five symbols that were all digits or letters (e.g., A H T N R or 9 3 4 7 8) and a Present trial in which one of the digits was replaced with a letter or vice versa (e.g., A H 3 N R or 9 3 R 7 8). Using present-absent pairs of symbol strings allowed us to examine digit-related activity while subtracting out letter-related activity (e.g., A H 3 N R > A H T N R), and vice-versa. That is, Absent trials provided a high-level control that would reveal brain activity specific to digits (or letters), rather than merely symbol or familiar symbol activity. The use of symbol strings also enabled the manipulation of attentional demands. Participants directed their attention to a specific symbol set while still viewing both letters and digits, which facilitated analyses of active and passive viewing. To ensure participants did not fixate on one location, each replacement digit or letter was used three times, once each in the 2nd, 3rd, and 4th positions of the symbol string. Symbol strings were random subsets of letters and digits, modified so that letter strings contained no words or pseudowords, and digit strings contained no strictly increasing or decreasing number sequences. Each trial ended with an inter-stimulus interval (ISI) of 2000, 4000, or 6000 ms (average ISI of 4000 ms). Each replacement digit (or letter) was matched once with each ISI, with ISIs counterbalanced for position.
Stimuli were presented with PsychoPy 1.84 (Peirce, 2007) using normalized units (i.e., total window size height and width range from −1 to +1) on a computer running Windows 10. Digits and letters were in white Arial font (height = 0.1) on a dark gray background. In the event-related design, each run began with 16 seconds of fixation to improve estimation of the blood-oxygen-level dependent (BOLD) signal baseline. Each trial consisted of a 1000 ms stimulus, followed by a fixation cross during the jittered ISI. For each trial, participants determined whether the symbol string contained a digit (or letter) and indicated Yes or No via a button press with the right index and middle fingers, respectively. Each run ended with 16 seconds of fixation and had a total length of 5 minutes and 2 seconds. Each run was comprised of the same 54 trials, presented in a different pseudorandom order that was the same for all participants. Trial order was adjusted to ensure no more than two sequential equal ISIs or three sequential Yes or No correct responses. Participants completed four sequential runs of digit detection and four sequential runs of letter detection, with digit and letter conditions counterbalanced across participants. Figure 1 presents an example and timing of each task.
Figure 1.
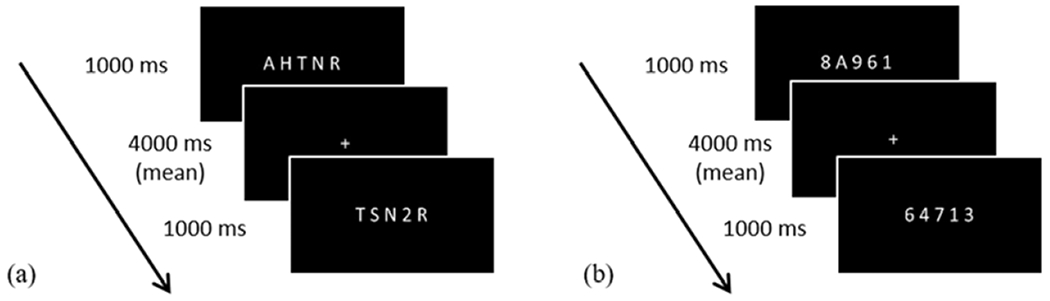
Example task and timing for (a) digit detection and (b) letter detection.
2.2.2. Standardized assessments.
Participants completed two mathematics subtests and one reading subtest of the Woodcock-Johnson III (Woodcock et al., 2001). In the Math Fluency subtest, participants had three minutes to solve single-digit addition, subtraction, and multiplication facts. In the Calculation subtest, participants completed mathematics problems ranging from single-digit arithmetic through calculus. The Math Fluency and Calculation subtests form a Calculation Skills composite, which served as a proxy for mathematics competence. The Letter-Word ID subtest measures word identification skills. During this test, participants read single words that progress in difficulty. This task provided a measure of participants’ ability to engage in symbol processing with letters, but not digits, and served as a proxy for non-mathematical cognitive ability. Participants’ scores on all tests were age-normed.
2.3. fMRI Data Acquisition
Brain images were acquired using a 3T Philips Intera Achieva with a 32-channel head coil. An anatomical scan of the whole brain was acquired in-between the two sets of functional tasks. High-resolution 3D anatomical scans were collected over approximately 6 minutes with the following parameters: TR/TE = 8.1/3.8 ms, flip angle = 5°, field of view (FOV) = 256 mm, and 1mm isotropic voxels. T2*-weighted single-shot echo-planar imaging (EPI) sequence functional images were acquired with TE = 25 ms, TR = 2000 ms, flip angle = 90°, FOV = 240 mm, matrix size = 96 x 96 mm, 2.5 mm isotropic voxels, 40 slices, 3 mm slice thickness, with .25 mm gap between slices, and 151 volumes per run. The scanner discarded 5 dummy volumes at the start of each run to allow for steady-state magnetization.
2.4. Experimental Design and Statistical Analysis
Structural and functional data were analyzed using BrainVoyager 20.4 (Goebel et al., 2006). Head motion was quantified for each run using maximum displacement and three degrees of volume-to-volume displacement (i.e., adjacent volumes and pairs of volumes that were two and three volumes apart), each with a cutoff of 3 mm. One participant was excluded from the analysis due to head motion greater than 3 mm in three runs of the same condition (one run maximum displacement and two runs both maximum and volume-to-volume displacement). Across the remaining 32 participants, three additional runs containing head motion in excess of 3 mm (maximum displacement in one run and both maximum and volume-to-volume displacement in two runs) were excluded from the analysis. All participants in the analysis completed at least three runs each of digit detection and letter detection. Functional images were corrected for slice scan time differences and head motion, and high-pass filtered (GLM approach with Fourier basis set, 2 cycles) to remove linear and non-linear trends. Functional data were co-registered to the structural data, normalized into MNI space, and spatially-smoothed with a Gaussian kernel of 6 mm at full-width half-maximum (FWHM). The expected BOLD signal was modeled using a two-gamma hemodynamic response function. Baselines were calculated from the first and last 16 seconds of each run and the ISIs. Errors were modeled separately and not analyzed.
To examine brain regions that support digit- and letter-related processing, we conducted a group level analysis using a whole-brain random-effects general linear model. A whole brain analysis allowed for the possibility that brain regions other than or in addition to the ITG may process digits as visual symbols (see Price and Ansari, 2011). The model included five regressors of interest, one for present and absent trials for each detection task and one for error trials, and six regressors representing six parameters of head motion. Whole brain analyses were thresholded at an uncorrected alpha of p < .005. Resulting statistical maps were corrected for multiple comparisons using cluster size thresholding (Forman et al., 1995; Goebel et al., 2006). This method uses Monte Carlo simulations (1,000 iterations) to estimate the minimum cluster size (i.e., threshold) that survives correction at a family-wise error (FWE) threshold of p = .05.
To examine digit processing generally, and its potential functional localization or specialization, we conducted a series of whole brain contrasts. We first examined brain activation when participants viewed and attended to digits by contrasting the digit present and absent conditions. We then used three contrasts to investigate whether similar brain regions may support both digit and letter processing (i.e., functional localization versus specialization of digit processing). To do so, we first examined letter processing by contrasting the letter present and letter absent conditions. We then conducted a conjunction analysis of the digit and letter contrasts (i.e., [Digit Present > Digit Absent] & [Letter Present > Letter Absent]), and a double subtraction of the two contrasts (i.e., [Digit Present > Digit Absent] > [Letter Present > Letter Absent]). Finally, to determine whether digit-related brain activity differed when participants attended to different target categories (letters versus digits), we contrasted the letter absent (i.e., all digits) and digit absent (i.e., all letters) conditions. Across these five contrasts, we sought to elucidate localization and specialization of digit-related functional brain activation.
To examine the relation between digit-related activity and mathematics, we conducted a whole brain correlation between digit-related activity and Calculation Skills. We used a whole brain analysis to allow for the possibility that other brain regions involved in digit processing may correlate with mathematics competence in addition to, or instead of, an ITG region. To control for non-mathematical cognitive ability, we first residualized Calculation Skill scores by regressing the Calculation Skills composite on Letter-Word ID scores. We then used the residualized scores in the brain-behavior correlation. Similarly, as a control analysis, we conducted a whole brain correlation between digit-related activity and reading competence. To do so, we first regressed Letter-Word ID scores on Calculation Skill scores to obtain residualized Letter-Word ID scores. We used the same voxel-wise and cluster-wise threshold values as above. Anatomical labels for clusters in all analyses came from the whereami program in AFNI (Cox and Hyde, 1997).
3. Results
3.1. Behavioral Results
Table 1 shows descriptive statistics for accuracy and for mean response time of correct responses, by detection task (i.e., digits/letters) and target (i.e., presence/absence). Accuracy rates in Table 1 reflect aggregated errors of commission (i.e., erroneous responses) and omission (i.e., in which participants did not respond to some trials). In Table 1, the lowest accuracy rates for each of the four conditions reflect errors of omission, rather than a failure to understand the task. For example, the participant with the lowest accuracy score for the digit present condition (i.e., 69.44%, see Table 1) made mostly errors of omission. When considering just the trials to which the participant responded, accuracy was 95%. This same pattern of low accuracy rates driven by errors of omission applied to the other three conditions (e.g., digit absent: 53.7% compared to 84%; letter present: 49.38% compared to 86%; letter absent: 55.56% compared to 94%). Given that average response times were in the range of 750 ms, and stimuli were presented for 1000 ms, followed by an average 4000 ms interval during which participants could still respond, we do not think this was due to a time out, in the sense that trial duration was too short and participants needed more time per trial, but rather was likely due to lapses in attending to some trials during a run. This suggests that these participants understood the task, were able to perform it correctly, and should be included in the analyses. For all participants, only correct responses were included in the fMRI analyses.
Table 1.
Mean, standard deviation, and range for in-scanner and behavioral measures. Behavioral measures are expressed as standard scores. Accuracy rates reflect overall accuracy, including both errors of omission and commission.
| Mean | Std Dev. | Range | ||
|---|---|---|---|---|
| In-scanner detection tasks | ||||
| Accuracy (%) | ||||
| Digit present | 92.93 | 8.94 | 69.44-100 | |
| Digit absent | 92.42 | 11.16 | 53.70-100 | |
| Letter present | 92.40 | 11.53 | 49.38-100 | |
| Letter absent | 93.81 | 10.05 | 55.56-100 | |
| Response time (ms) | ||||
| Digit present | 746 | 82 | 630-937 | |
| Digit absent | 771 | 91 | 666-994 | |
| Letter present | 766 | 104 | 638-1079 | |
| Letter absent | 782 | 97 | 641-986 | |
| Standardized measures | ||||
| Math fluency | 114 | 14.36 | 87-149 | |
| Calculation | 120 | 11.58 | 98-146 | |
| Calculation skills | 121 | 12.56 | 93-145 | |
| Letter-Word ID | 112 | 6.27 | 97-122 |
A 2 x 2 repeated measures ANOVA was conducted separately for accuracy and response time, with detection task and target as within subjects factors. For accuracy, there was not a statistically significant main effect of detection task (F(1, 31) = 0.08, p = .78, ) or target presence/absence (F(1, 31) = 1.296, p = .26, ). For response time, there was a statistically significant main effect of detection task (F(1, 31) = 4.636, p = .04, ), in which it took longer on average to respond to letter detection trials. There was a statistically significant main effect of target (F(1, 31) = 8.996, p = .005, ), in which it took longer, on average, to respond to target absent trials. There was no statistically significant interaction (F(1, 31) = .999, p = .33, ).
3.2. fMRI Results
3.2.1. Is there activity in the ITG during digit detection?
We first investigated brain activation when participants viewed and attended to digits. If there were a region in the ITG that is reliably involved in processing digits when the task requires attention to digits (i.e., functional localization for digits), we would expect a cluster of activity in this region that is more active during digit detection when digits are present than when digits are absent, which together would reflect successful digit detection and digit-related processing. To test this, we contrasted brain activation for digit present (e.g., A H 3 N R) versus digit absent (e.g., A H T N R) conditions, which produced four clusters. The first four rows of Table 2 list the clusters with anatomical labels, peak MNI coordinates, and cluster size in anatomical voxels. The first and second clusters in occipital and temporal regions showed greater activation during the digit present condition. The third cluster, in the left ITG, is located in a similar region as Grotheer et al.’s (2016b) left ITG cluster, which suggests this region is a potential NFA that is active when participants view digits while performing a target detection task focused on digits. The fourth cluster in the right inferior frontal gyrus showed greater activity in the digit absent condition. The four clusters are shown in Panel (a) of Figure 2. To show the statistical distribution across subjects, we include boxplots of cluster-level beta-values, by condition, for each cluster, in Figure S1 of the supplementary material.
Table 2.
Anatomical labels (with Brodmann Areas), MNI coordinates, t-statistics, and cluster size, for all contrasts. Results corrected for multiple comparisons at uncorrected p < .005 (voxelwise) and FWE p < .05 (clusterwise).
| Peak MNI | t | Cluster size (mm3) | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Digit Detection | |||||
| Digit Present > Digit Absent | |||||
| 1. Right Superior Occipital Gyrus (BA 7) | 24 | −73 | 40 | 4.82 | 6080 |
| 2. Left Middle Occipital Gyrus (BA 39) | −30 | −73 | 25 | 4.80 | 6111 |
| 3. Left Inferior Temporal Gyrus (BA 37) | −57 | −52 | −11 | 3.87 | 728 |
| Digit Present < Digit Absent | |||||
| 4. Right Inferior Frontal Gyrus (BA 47) | 45 | 29 | 1 | 4.79 | 543 |
| Letter Present > Letter Absent | |||||
| 1. Right Middle Temporal Gyrus (BA 19) | 45 | −70 | 22 | 5.33 | 13246 |
| 2. Left Inferior Parietal Lobule (BA 7) | −30 | −58 | 52 | 5.10 | 4752 |
| 3. Left Middle Frontal Gyrus (BA 6) | −24 | 11 | 58 | 4.76 | 689 |
| 4. Left Inferior Occipital Gyrus (BA 19) | −42 | −64 | −11 | 6.38 | 9736 |
| 5. Left Inferior Parietal Lobule (BA 40) | −36 | −46 | 37 | 5.10 | 1750 |
| (Digit Present > Digit Absent) & (Letter Present > Letter Absent) | |||||
| 1. Right Middle Temporal Gyrus (BA 19) | 42 | −70 | 22 | 4.71 | 1407 |
| 2. Right Superior Parietal Lobule (BA 7) | 21 | −73 | 49 | 4.00 | 753 |
| 3. Left Middle Occipital Gyrus (BA 19) | −33 | −82 | 25 | 4.05 | 697 |
| (Digit Present > Digit Absent) > (Letter Present > Letter Absent) | |||||
| 1. Left Inferior Occipital Gyrus (BA 19) | −42 | −67 | −8 | −4.16 | 1012 |
| Letter Absent > Digit Absent | |||||
| 1. Left Anterior Cingulate Cortex (BA 24) | 0 | 38 | 4 | 5.23 | 641 |
Figure 2.
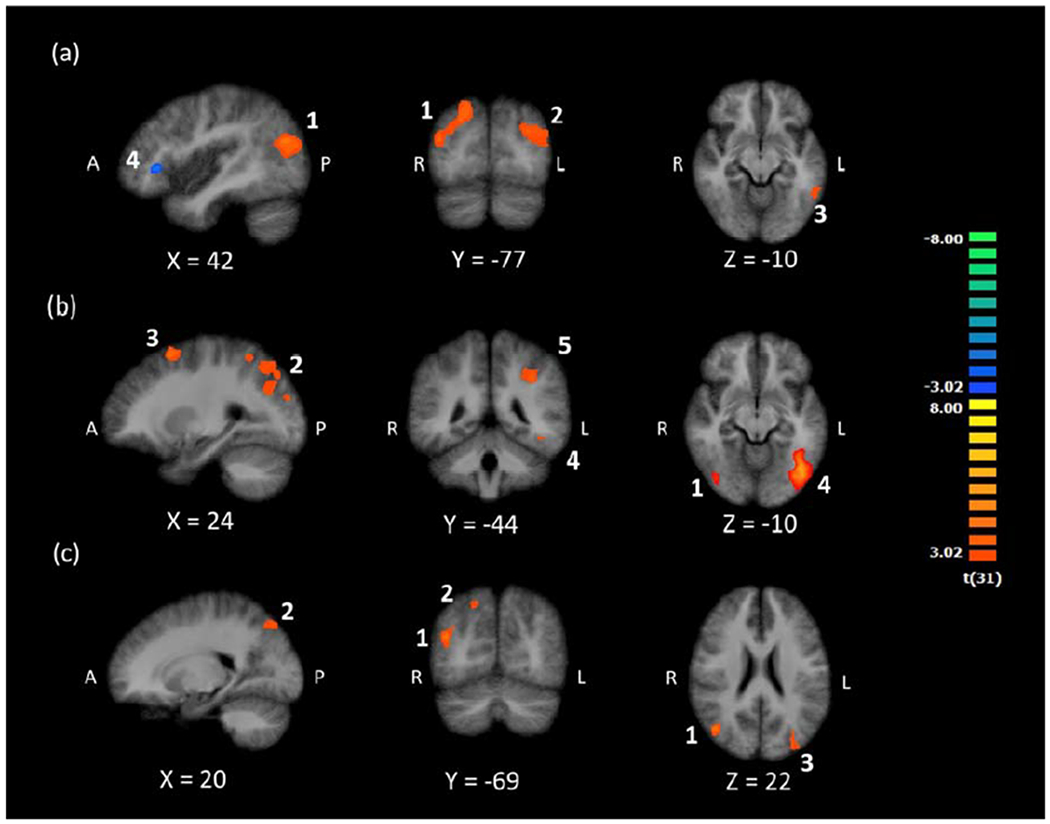
Clusters of activation for (a) Digit Present > Digit Absent and Digit Absent > Digit Present, (b) Letter Present > Letter Absent, and (c) their conjunction. Images are in radiological space (right is left), with MNI labels. Numbered clusters correspond to results of the first four contrasts in Table 2.
3.2.2. Is there shared activation in the left ITG during digit and letter detection?
We next investigated brain regions that showed letter-related activity. If the left ITG region that displayed digit-related activity supported processing of both digits and letters (i.e., lack of functional specialization for digits), we would expect letter-related activity in this region during letter detection. To test this hypothesis, we contrasted letter present (e.g., 9 3 R 7 8) and letter absent (e.g., 9 3 4 7 8) conditions. Table 2 shows the five resulting clusters, which all exhibited greater activity during the letter present condition. One cluster is in right middle temporal gyrus and four are in left frontal, parietal, and occipital regions. The cluster in left inferior occipital gyrus extends into the left ITG. Importantly, this cluster does not overlap with the region in left ITG that is active during digit detection (see Panel (b) of Figure 2 and Figure 3). The lack of overlap between the digit- and letter-related ITG regions could suggest an NFA in the left ITG that is preferentially active when participants simultaneously view and detect digits. We provide boxplots of the cluster-level beta-values for each cluster and contrast in Figure S2 of the supplementary material.
Figure 3.
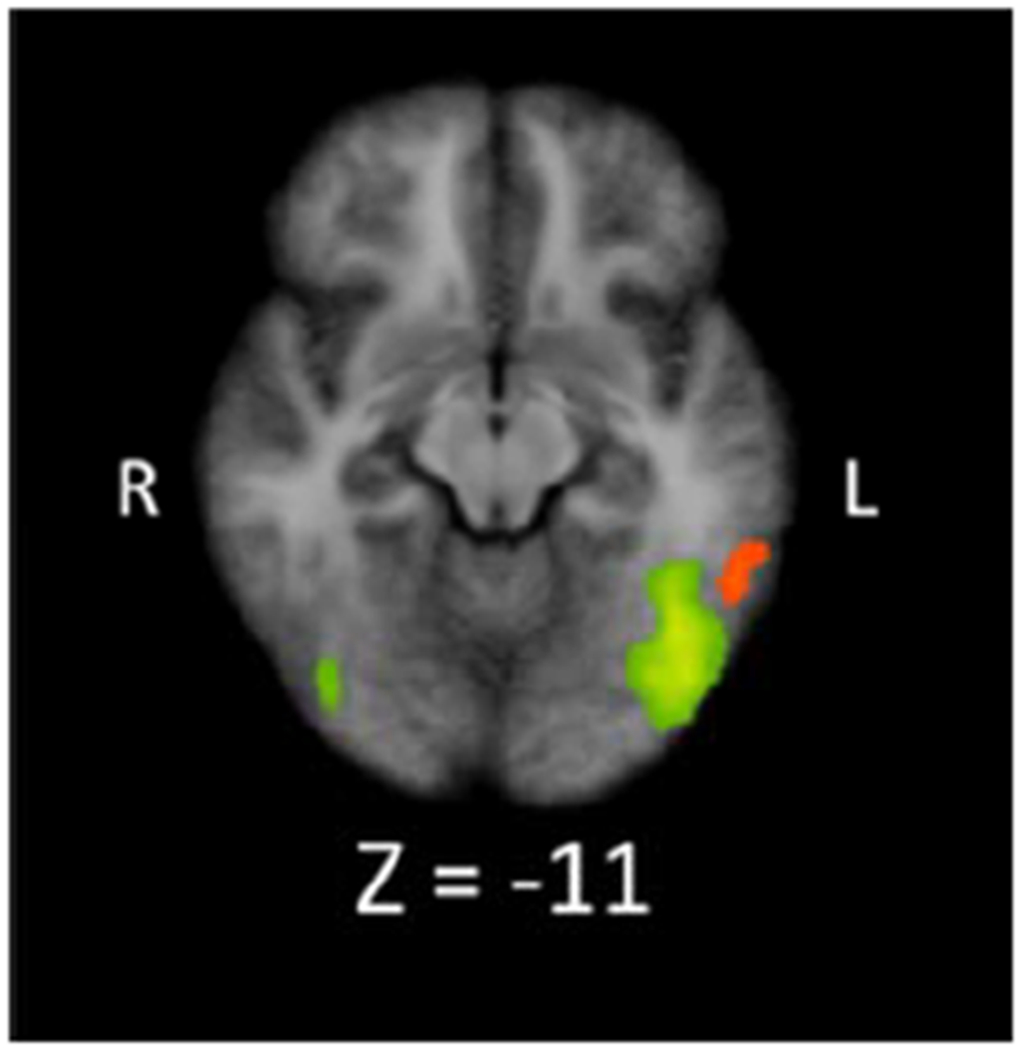
Visual overlay of Digit Present > Digit Absent (orange) and Letter Present > Letter Absent (green) clusters in the ITG region, showing the spatial distinction between left ITG clusters related to digit and letter detection. The orange cluster is the same as cluster 3 in Figure 2, Panel (a). The green clusters are the same as cluster 1 (image right) and 4 (image left) from Figure 2, Panel (b). Note, colors are for distinguishing the two clusters and do not indicate differences in activation level.
We then conducted a conjunction analysis to quantitatively test whether there was overlap in left ITG activation during both digit and letter detection (i.e., (Digits Present > Digits Absent) & (Letter Present > Letter Absent)). The contrast produced three clusters of overlapping activation for digit and letter processing, in right middle temporal gyrus, right superior parietal lobule, and left middle occipital gyrus (see Table 2 and Panel (c) in Figure 2). The conjunction analysis did not provide evidence for shared activation of the ITG for digit processing during digit detection and letter processing during letter detection. Although null results can never be interpreted as definitive proof of the absence of a phenomenon, taken together, the above three contrasts do not provide evidence in support of a left ITG region that is functionally specialized for digits.
3.2.3. Does the left ITG exhibit functional specialization for digits?
Even though there was no evidence of overlapping digit- and letter-related activation in the potential NFA, this region may still exhibit sub-threshold letter-related activity, such that levels of digit- and letter-related activation are not statistically significantly different. If the potential NFA is functionally specialized for digits (i.e., is involved exclusively in the processing of digits and therefore shows greater activation for digits compared to all other stimuli, including letters), digit-related activation (i.e., Digit Present > Digit Absent) should be statistically significantly greater than letter-related activation (i.e., Letter Present > Letter Absent) in the left ITG. In other words, in the left ITG, the difference in activation between digit present and digit absent conditions should be greater than the difference in activation between letter present and letter absent conditions. To test this, we conducted a double contrast related to digit and letter detection (i.e., [Digit Present > Digit Absent] > [Letter Present > Letter Absent]). The presence of a resulting cluster that overlaps with the potential NFA would suggest that the region exhibits functional specialization for digits, while the absence of such a cluster would provide no evidence that the region differentially processes digits and letters.
The double contrast revealed one cluster of activation in the left inferior occipital gyrus (See Table 2 and Figure 4). However, activation in this region yielded negative t-values in the contrast, indicating greater activation for detecting letters when searching for a letter than for detecting digits when searching for a digit. In other words, this region appeared to show letter-specific activation. Crucially, this region is distinct from the potential NFA and overlaps with letter-related activity in the left ITG, the latter suggesting functional specialization in that left ITG region for letter processing. Taken together, the above set of contrasts provides evidence that a region of the left ITG, consistent across individuals, supports digit processing, but there is not compelling evidence that this region responds more to digits than letters. Further, as the double contrast provided evidence of functional specialization in a left ITG region for letter-related processing, the lack of evidence for functional specialization for digits is unlikely due to an inability to detect it.
Figure 4.
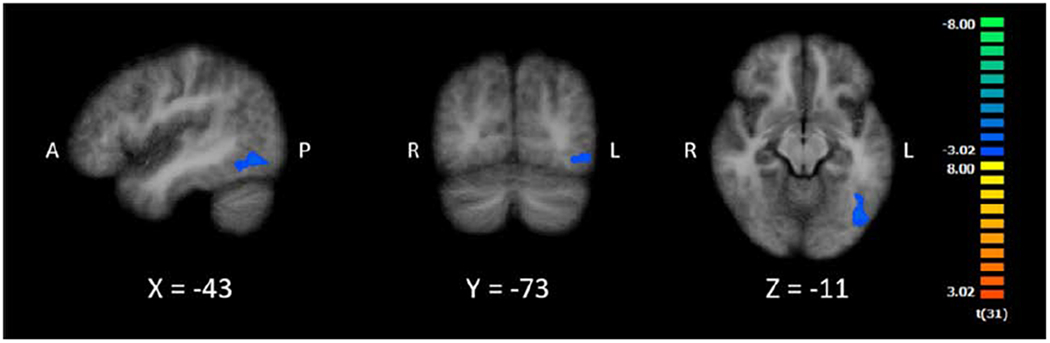
Cluster of activation for the double subtraction of Digit Present > Digit Absent and Letter Present > Letter Absent. Images are in radiological space (right is left), with MNI labels. Cluster corresponds to the fifth contrast in Table 2.
3.2.4. Does digit-related left ITG activation depend on attention to digits?
Next, we sought to determine whether digit-related brain activity differed by attention to different target categories. To do so, we contrasted Letters Absent (i.e., all digits during letter detection) and Digits Absent (i.e., all letters during digit detection). If the left ITG supports digit processing regardless of attention to digits, the Letters Absent (i.e., all digits) > Digits Absent (i.e., all letters) contrast should reveal a left ITG cluster in the potential NFA. However, if digit processing in the ITG depends on attention to digits, we would not expect a cluster in the potential NFA. The contrast produced one cluster of activation in left anterior cingulate cortex (see Table 2, Figure 5, and Figure S3), in which activity was greater for digit viewing. These results suggest that visual perception of digits, while attending to letters, is not enough to elicit activity in the left ITG. Rather, it appears this region only responds to viewing digits when coupled with attentional demands related to digit detection, as opposed to attention directed toward letters.
Figure 5.
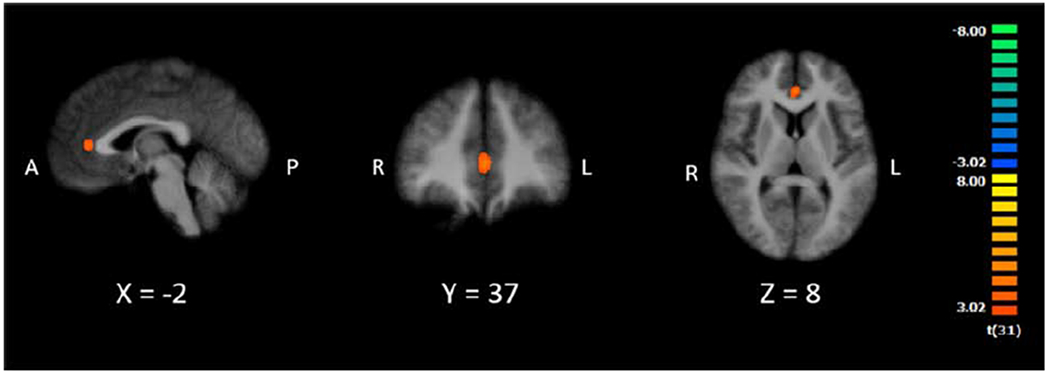
Cluster of activation for Letter Absent (e.g., 9 3 4 7 8) > Digit Absent (e.g., A H T N R). Images are in radiological space (right is left), with MNI labels. Cluster corresponds to last contrast in Table 2.
In sum, the above analyses collectively provide limited evidence of a region in the left ITG that is selective for digits, but only when the associated task requires attention to digits.
3.2.5. Does digit-related activity correlate with mathematical competence?
To address the second aim of this study, we investigated whether neurocognitive mechanisms that support processing digits as visual symbols correlate with mathematics competence. The analysis focused on Calculation Skill scores, a composite of Math Fluency and Calculation, which reflect mathematical fact fluency and problem solving ability. Since mathematics performance may partially reflect performance on non-mathematical cognitive abilities, we used Letter-Word ID, a measure of single word reading ability as a control. We regressed Calculation Skill scores on Letter-Word ID scores to obtain residualized Calculation Skill scores, which we then correlated with digit-related activity during digit detection (Digit Present > Digit Absent). As Figure 6 shows, the analysis produced two clusters of activation, one in the right ITG (54, −52, −14; BA 37) and one in the right cerebellum (33, −76, −29). Interestingly, the right ITG cluster closely overlaps with a candidate NFA (Talairach coordinates: 50, −48, −10) from a recent meta-analysis on digit processing (Yeo et al., 2017). The results of this contrast suggest that greater digit-related activity in the ITG and the cerebellum is associated with higher calculation skills.
Figure 6.
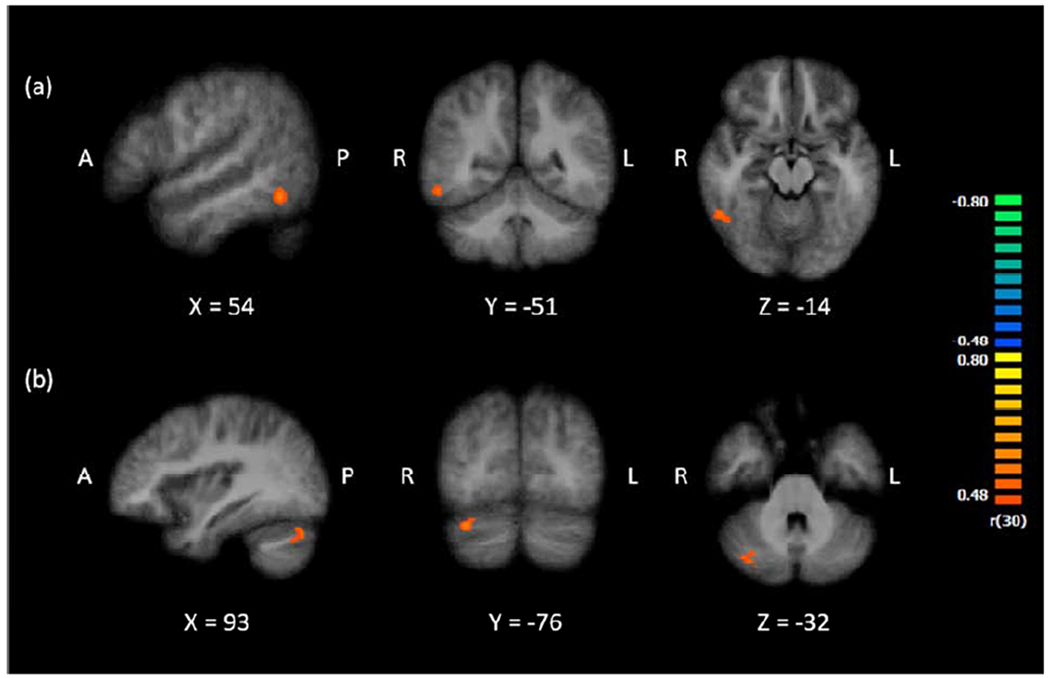
Clusters of activation for the whole brain correlation between digit-related activity (Digit Present > Digit Absent) and residualized Calculation Skills, in (a) the right ITG (54, −52, −14; BA 37) and (b) the right cerebellum (33, −76, −29).
To determine whether one of the mathematics subtests was driving the relationship with digit-related activity, we performed the same analysis as above separately with residualized Math Fluency and Calculation subtest scores. There was a positive correlation between digit-related activity and Math Fluency scores in the right middle frontal gyrus (51, 5, 56; BA 6) and the right superior parietal lobule (24, −58, 64; BA 7), in which greater digit-related activity in these regions was associated with higher Math Fluency scores. There was also a positive correlation between digit-related activity and Calculation in the right cerebellum (24, −79, −29) and the left inferior frontal gyrus (−42, 11, 10; BA 44), in which greater digit-related activity was associated with higher calculation skills. Taken together, these results suggest that activity in the right ITG related to calculation skills more broadly, rather than either of the specific subtests.
Finally, to determine whether the above relations were specific to mathematics competence, we correlated digit-related activity (Digit Present > Digit Absent) and word reading ability. We regressed Letter-Word ID scores on the Calculation Skills composite and correlated the residualized word reading scores with digit-related brain activation, at the whole brain level. This analysis showed no relation between word reading and digit-related brain activation, suggesting the prior correlations are mathematics specific.
4. Discussion
The present study examined the neurocognitive mechanisms that underlie processing Arabic digits as visual symbols by contrasting activation for seeing a digit in a letter string versus seeing a letter in a digit string. Furthermore, we investigated the extent to which the neural substrates of asemantic processing of digits correlate with mathematics competence.
4.1. Functional Localization in the ITG for Digits
Our results revealed clusters of activation in frontal, parieto-occipital, and temporo-occipital regions when processing digits as visual symbols, including a cluster in the left ITG. During digit detection, greater activity in the right inferior frontal gyrus when digits were absent versus present could be related to additional effort when looking for, but not finding, a digit. Prior research suggests that activity in frontal regions supports selective attention and may increase during visual search for similar target and distractor items (Anderson et al., 2007). Clusters that spanned the parieto-occipital junction may similarly be involved in attention, as parietal regions may modulate attention during visual perception (Behrmann et al., 2004; Rushworth et al., 2001; Vuilleumier and Driver, 2007). Indeed, there was similar functional activation in parieto-occipital regions during letter detection, and the conjunction analysis revealed activation in temporo-parietal and parieto-occipital regions that supports both digit and letter processing during target detection.
Importantly, left ITG activation during digit detection appeared distinct from the left ITG activation during letter detection and also overlaps with digit-related activation in prior research (Grotheer et al., 2016b). This suggests there is functional localization for digits in the ITG. However, the double contrast suggests there is no statistically significant difference between digit-related and letter-related activity in that same digit-related region. Therefore, the present results do not support functional specialization in the ITG for digit processing. In other words, while there is a spatially consistent region in the ITG that is involved in the visual processing of Arabic digits, that region is not only involved in processing digits, as it appears to be equally involved in the processing of other visual symbols. Indeed, this notion aligns with prior TMS research showing that disruption of a potential NFA region affects both digit and letter processing (Grotheer et al., 2016a), and research suggesting that this region supports mathematical processing across stimuli, including digits, dice, hands, and number-letter morphs (Grotheer et al., 2018). The present findings also align with prior fMRI research that shows that even in elementary school children, the ITG shows word-specific, but not digit-specific activation (Dehaene-Lambertz et al., 2018). The degree of functional specialization required for a region to be considered functionally specific ranges from exclusive processing to merely greater functional activation for one category than another (Cohen and Dehaene, 2004; Kanwisher, 2010; Yeo et al., 2017). The results of the double contrast do not meet the most lenient criteria for functional specialization for digits and accordingly do not suggest that the digit-related left ITG activity found here constitutes a true NFA. In other words, while there may be an ITG region that is reliably involved in processing Arabic digits as visual symbols, that region is not specific to digits.
4.2. Digit-Related Activity in the Left ITG Depends on Attention to Digits
Results of the present study suggest that digit-related activity in the left ITG may be contingent on attention to digits. Left ITG activity was present when participants viewed digits and engaged in digit detection, but absent when participants viewed digits and engaged in letter detection. The notion that attention to digits modulates digit-related activity may explain why prior passive viewing paradigms involving digits have failed to produce reliable functional activation in the ITG (Polk et al., 2002; Price and Ansari, 2011), while tasks that require attention to symbolic stimuli have elicited ITG activation (e.g., Grotheer et al., 2016b). This notion also aligns with research suggesting that digit-related activity in the ITG differs based on how digits are utilized in a given task (Grotheer et al., 2018). Further, in conjunction with Yeo et al.’s (2017) findings, the present results suggest that digit-related ITG activation is detectable using standard fMRI paradigms. Such results challenge arguments that signal dropout from the proximate auditory canal and transverse sinus prevents detection of digit-related ITG activity (see Shum et al., 2013; Grotheer et al., 2016b) and that compensatory MRI acquisition parameters (Abboud et al., 2015) are required to detect digit-related activation in this region.
If digit-related ITG activation requires both perception of and attention to digits, digit processing in the ITG would function differently than letter or word processing in the VWFA, which responds to passive viewing of words or letter strings (Cohen et al., 2002; Mei et al., 2010; Puce et al., 1996). Instead, digit processing may require a combination of bottom-up and top-down processing. Analogous to the Price and Devlin (2011, 2003) theory of orthographic processing, the ITG region that is responsive to digits may be more dynamic, participating in multiple functions (e.g., digit processing, letter processing) in coordination with other brain regions, depending on task demands.
4.3. Digit-related Activation Relates to Mathematics Competence
The present study found that greater activity in the right ITG during digit detection was associated with higher mathematics competence. This right ITG region closely overlaps with a meta-analytically identified candidate NFA (Yeo et al., 2017). The present study shows for the first time that the degree to which this region is active during digit processing relates to calculation skills. Importantly, the correlation between digit-related activation and mathematics skills does not suggest that the right ITG is an NFA. The right ITG may support digit processing, yet not be functionally specialized for digit processing. The present findings differ in approach from prior research investigating the relationship between mathematical competence and ITG response. In a group-level ROI analysis, Amalric and Dehaene (2016) showed that mathematicians had greater left ITG activation for numbers than non-symbolic pictures, compared to non-mathematician controls. This relationship did not hold at the whole brain level and was limited to the left ITG. In contrast, the current study shows that, for non-mathematicians, digit-related activation in the right ITG relates to individual differences in mathematics competence. Such a relationship may be analogous to the positive association between VWFA activation during reading tasks and reading ability (Dehaene et al., 2010; Shaywitz et al., 2002). These findings also support recent evidence that fluent visual processing of digits as symbols may be critical for mathematics skill acquisition, beyond processing numerical magnitude (Price et al., 2017).
The positive correlation between right ITG activation and mathematics competence also suggests this region may be an essential component of a neural symbolic number processing system that integrates visual processing of digits and symbol-referent connections. Accordingly, the right ITG cluster may be a candidate region involved in the development of dyscalculia (i.e., mathematics learning disability). Readers with dyslexia show underactivation of the VWFA compared to typical readers (Boros et al., 2016; Richlan et al., 2011). Similarly, it is possible that learners with and without dyscalculia could show differential right ITG activation during digit detection. This could suggest impairment in processing digits as visual symbols, which could in turn contribute to impairment in symbol-referent connections, one candidate etiology for dyscalculia (Rousselle and Noël, 2007; Wilson and Dehaene, 2007).
Results also suggest that the left and right ITG may support digit processing differently. Prior fMRI studies have produced divergent findings on the laterality of digit-related ITG activity, showing bilateral (Grotheer et al., 2016) or right-lateralized (Cui et al., 2013; Gullick and Temple, 2011; Yeo et al., 2017) ITG activation during digit processing. Additionally, some studies using TMS (Grotheer et al., 2016) or electrophysiological recordings (Daitch et al., 2016; Shum et al., 2013) have shown support for digit-related right ITG activity, but did not explicitly examine potential laterality. In the present study, group analyses showed digit-related left ITG activity during digit detection, but a correlational analysis showed that greater right ITG activation related to higher mathematics competence. These results differ from Amalric and Dehaene (2016), who showed that mathematicians had greater activation of left inferior temporal cortex when viewing mathematical formulas, compared to non-mathematician controls. Taken together, this research may suggest differential engagement of the ITG. The left ITG may support digit processing during digit detection generally, and may show differences when group comparisons involve large gaps in mathematics expertise. Yet, the strength of engagement of right ITG during digit detection may relate to individual differences in mathematical competence for those without extensive and specialized mathematics training.
Lastly, results showed a relationship between digit-related activity in the right cerebellum and mathematics competence. Prior research suggests functional activation of the cerebellum during cognitive processes such as language and executive functioning (Buckner, 2013; Stoodley et al., 2012; Stoodley and Schmahmann, 2009), and numerical processing (Arsalidou and Taylor, 2011). While its specific function remains unclear, the cerebellum may support coordination of task-specific goals under tight time constraints (Arsalidou and Taylor, 2011; Buhusi and Meck, 2005), which would have clear implications for math performance.
4.4. Future Directions
Some limitations of the present study suggest avenues for future research. First, the present study focused on processing of digits and digits compared to letters, and accordingly maximized trial-level power for these two stimulus categories. Consequently, there were no scrambled letter and/or digit conditions as in prior research (Grotheer et al., 2016b; Price and Ansari, 2011; Shum et al., 2013), making it impossible for the current data to disentangle the processing of familiar and unfamiliar written symbols. Second, results suggest that attention to digits is required for ITG activation, but this hypothesis requires empirical validation through a direct comparison of active and passive paradigms. Third, the range of Letter-Word ID scores was smaller than the ranges for the measures of mathematical competence. This may have reduced the probability of a correlation between digit detection and reading skills compared to digit detection and mathematics skills. Future studies should confirm the presence of similar brain-behavior correlations with digit processing and whether they are mathematics specific. Fourth, participants took longer on average to respond to target absent trials. Such differences could relate to exhaustive, serial visual search (Treisman and Gelade, 1980; Wolfe, 1994), a possibility that should be explored further in additional studies. Fifth, participants in the present study were relatively high achieving overall, which limits the examination of right ITG activation during digit detection for those with low mathematical competence. Future work should investigate the relation between digit-related right ITG activation and mathematics competence across a wider range of achievement, including those with dyscalculia. Sixth, this study revealed a set of brain regions supporting digit processing, not only the ITG. Future work should investigate whether these regions constitute a true functional network or are simply coactivated. For example, research using electrophysiological recordings suggests that ventral temporal cortex works bidirectionally with parietal regions to support numerical processing (Daitch et al., 2016). Finally, the current study utilized group level analyses, potentially obscuring functional localization in regions with high anatomical variability. It is an open empirical question as to whether alternative regions in the ITG that do not overlap between participants would show true functional specialization for digits. Unfortunately, the current study was not designed for individual subject-level analyses, so future research employing high spatial resolution and high trial-level power should investigate individual differences in the localization of digit-related activity in the ITG.
5. Conclusion
In this study, participants completed digit and letter detection tasks during fMRI, and standardized tests of mathematics and reading competence. Results indicated a left ITG region that demonstrates functional localization, but not functional specialization, for Arabic digit processing. Importantly, results suggest that such functional localization depends on top-down attention mechanisms. Additionally, we observed the first evidence of a positive association between right ITG activation during digit processing and calculation skills, indicating that neural mechanisms underlying the visual identification of digits may play an important role in mathematics skill acquisition. Taken together, these findings illuminate the neurocognitive mechanisms that support processing digits as visual symbols, independent of numerical magnitude processing. These findings inform models of symbolic number processing, which can serve as a reference for and inform our understanding of typical and atypical numerical and mathematical development.
Supplementary Material
Acknowledgements
We thank Benjamin Conrad, Eric Wilkey, and Darren Yeo for their assistance with the analyses and results. Funding: This work was supported by the National Science Foundation [grant number 1660816 to GP].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: none.
References
- Abboud S, Maidenbaum S, Dehaene S, Amedi A, 2015. A number-form area in the blind. Nat. Commun 6, 6026 10.1038/ncomms7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric M, Dehaene S, 2016. Origins of the brain networks for advanced mathematics in expert mathematicians. Proc. Natl. Acad. Sci 113, 4909–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Mannan SK, Husain M, Rees G, Sumner P, Mort DJ, McRobbie D, Kennard C, 2007. Involvement of prefrontal cortex in visual search. Exp. Brain Res 180, 289–302. 10.1007/s00221-007-0860-0 [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Pawliw-Levac M, Sadeghi M, Pascual-Leone J, 2017. Brain areas associated with numbers and calculations in children: Meta-analyses of fMRI studies. Dev. Cogn. Neurosci 10.1016/j.dcn.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Taylor MJ, 2011. Is 2+2=4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage 54, 2382–2393. 10.1016/j.neuroimage.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E, 2006. Top-down facilitation of visual recognition. Proc. Natl. Acad. Sci. U. S. A 103, 449–454. 10.1073/pnas.0507062103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S, 2004. Parietal cortex and attention. Curr. Opin. Neurobiol 14, 212–217. 10.1016/j.conb.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Boros M, Anton J-L, Pech-Georgel C, Grainger J, Szwed M, Ziegler JC, 2016. Orthographic processing deficits in developmental dyslexia: Beyond the ventral visual stream. Neuroimage 128, 316–327. 10.1016/j.neuroimage.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Buckner RL, 2013. The Cerebellum and Cognitive Function: 25 Years of Insight from Anatomy and Neuroimaging. Neuron 80, 807–815. 10.1016/j.neuron.2013.10.044 [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH, 2005. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci 6, 755–765. 10.1038/nrn1764 [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, 2004. Specialization within the ventral stream: the case for the visual word form area. Neuroimage 22, 466–476. 10.1016/j.neuroimage.2003.12.049 [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S, 2002. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain 125, 1054–1069. 10.1093/brain/awf094 [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS, 1997. Software tools for analysis and visualization of fMRI data. NMR Biomed. 10, 171–178. [DOI] [PubMed] [Google Scholar]
- Cui J, Yu X, Yang H, Chen C, Liang P, Zhou X, 2013. Neural correlates of quantity processing of numeral classifiers. Neuropsychology 27, 583–594. 10.1037/a0033630 [DOI] [PubMed] [Google Scholar]
- Çukur T, Nishimoto S, Huth AG, Gallant JL, 2013. Attention during natural vision warps semantic representation across the human brain. Nat. Neurosci 16, 763 10.1038/nn.3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch AL, Foster BL, Schrouff J, Rangarajan V, Kaşikçi I, Gattas S, Parvizi J, 2016. Mapping human temporal and parietal neuronal population activity and functional coupling during mathematical cognition. Proc. Natl. Acad. Sci 113, E7277–E7286. 10.1073/pnas.1608434113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, 2011. The unique role of the visual word form area in reading. Trends Cogn. Sci 15, 254–262. 10.1016/j.tics.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, 1995. Towards an anatomical and functional model of number processing. Math. Cogn 1, 83–120. [Google Scholar]
- Dehaene S, Cohen L, Morais J, Kolinsky R, 2015. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci 16, 234–244. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Dehaene-Lambertz G, 2016. Is the brain prewired for letters? Nat. Neurosci 19, 1192–1193. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Filho GN, Jobert A, Dehaene-Lambertz G, Kolinsky R, Morais J, Cohen L, 2010. How Learning to Read Changes the Cortical Networks for Vision and Language. Science 330, 1359–1364. 10.1126/science.1194140 [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Monzalvo K, Dehaene S, 2018. The emergence of the visual word form: Longitudinal evolution of category-specific ventral visual areas during reading acquisition. PLOS Biol. 16, e2004103 10.1371/journal.pbio.2004103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N, 2001. A Cortical Area Selective for Visual Processing of the Human Body. Science 293, 2470–2473. 10.1126/science.1063414 [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC, 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med 33, 636–647. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E, 2006. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp 27, 392–401. 10.1002/hbm.20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotheer M, Ambrus GG, Kovacs G, 2016a. Causal evidence of the involvement of the number form area in the visual detection of numbers and letters. Neuroimage 132, 314–319. 10.1016/j.neuroimage.2016.02.069 [DOI] [PubMed] [Google Scholar]
- Grotheer M, Herrmann K-H, Kovacs G, 2016b. Neuroimaging Evidence of a Bilateral Representation for Visually Presented Numbers. J. Neurosci 36, 88–97. 10.1523/JNEUROSCI.2129-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotheer M, Jeska B, Grill-Spector K, 2018. A preference for mathematical processing outweighs the selectivity for Arabic numbers in the inferior temporal gyrus. NeuroImage 175, 188–200. 10.1016/j.neuroimage.2018.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick MM, Temple E, 2011. Are historic years understood as numbers or events? An fMRI study of numbers with semantic associations. Brain Cogn. 77, 356–364. 10.1016/j.bandc.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Joseph JE, Cerullo MA, Farley AB, Steinmetz NA, Mier CR, 2006. fMRI correlates of cortical specialization and generalization for letter processing. NeuroImage 32, 806–820. 10.1016/j.neuroimage.2006.04.175 [DOI] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, Piper GA, 2003. Shared and dissociated cortical regions for object and letter processing. Cogn. Brain Res 17, 56–67. 10.1016/S0926-6410(03)00080-6 [DOI] [PubMed] [Google Scholar]
- Kanwisher N, 2010. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc. Natl. Acad. Sci 107, 11163–11170. 10.1073/pnas.1005062107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann L, Wood G, Rubinsten O, Henik A, 2011. Meta-analyses of developmental fMRI studies investigating typical and atypical trajectories of number processing and calculation. Dev. Neuropsychol 36, 763–787. 10.1080/87565641.2010.549884 [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF, 2008. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N. Y. Acad. Sci 1145, 237–259. 10.1196/annals.1416.024 [DOI] [PubMed] [Google Scholar]
- Malins JG, Gumkowski N, Buis B, Molfese P, Rueckl JG, Frost SJ, Pugh KR, Morris R, Mencl WE, 2016. Dough, tough, cough, rough: A “fast” fMRI localizer of component processes in reading. Neuropsychologia 91, 394–406. 10.1016/j.neuropsychologia.2016.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xue G, Chen C, Xue F, Zhang M, Dong Q, 2010. The “visual word form area” is involved in successful memory encoding of both words and faces. Neuroimage 52, 371–378. 10.1016/j.neuroimage.2010.03.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Downing PE, 2017. Category selectivity in human visual cortex: Beyond visual object recognition. Neuropsychologia, Special Issue: Concepts, Actions and Objects: Functional and Neural Perspectives 105, 177–183. 10.1016/j.neuropsychologia.2017.03.033 [DOI] [PubMed] [Google Scholar]
- Peelen MV, Fei-Fei L, Kastner S, 2009. Neural mechanisms of rapid natural scene categorization in human visual cortex. Nature 460, 94 10.1038/nature08103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW, 2007. PsychoPy—Psychophysics software in Python. J. Neurosci. Methods 162, 8–13. 10.1016/j.jneumeth.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D’Esposito M, Detre JA, Farah MJ, 2002. Neural specialization for letter recognition. J. Cogn. Neurosci 14, 145–159. 10.1162/089892902317236803 [DOI] [PubMed] [Google Scholar]
- Pollack C, Ashby NC, 2017. Where arithmetic and phonology meet: The meta-analytic convergence of arithmetic and phonological processing in the brain. Dev. Cogn. Neurosci 10.1016/j.dcn.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, 2011. The Interactive Account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci 15, 246–253. 10.1016/j.tics.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, 2003. The myth of the visual word form area. Neuroimage 19, 473–481. 10.1016/S1053-8119(03)00084-3 [DOI] [PubMed] [Google Scholar]
- Price GR, Ansari D, 2011. Symbol processing in the left angular gyrus: Evidence from passive perception of digits. Neuroimage 57, 1205–1211. 10.1016/j.neuroimage.2011.05.035 [DOI] [PubMed] [Google Scholar]
- Price GR, Wilkey ED, Yeo DJ, 2017. Eye-movement patterns during nonsymbolic and symbolic numerical magnitude comparison and their relation to math calculation skills. Acta Psychol. (Amst.) 176, 47–57. 10.1016/j.actpsy.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G, 1996. Differential Sensitivity of Human Visual Cortex to Faces, Letterstrings, and Textures: A Functional Magnetic Resonance Imaging Study. J. Neurosci 16, 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H, 2011. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage 56, 1735–1742. 10.1016/j.neuroimage.2011.02.040 [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H, 2009. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp 30, 3299–3308. 10.1002/hbm.20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle L, Noel M-P, 2007. Basic numerical skills in children with mathematics learning disabilities: a comparison of symbolic vs non-symbolic number magnitude processing. Cognition 102, 361–395. 10.1016/j.cognition.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Paus T, Sipila PK, 2001. Attention Systems and the Organization of the Human Parietal Cortex. J. Neurosci 21, 5262–5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC, 2002. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry 52, 101–110. [DOI] [PubMed] [Google Scholar]
- Shum J, Hermes D, Foster BL, Dastjerdi M, Rangarajan V, Winawer J, Miller KJ, Parvizi J, 2013. A brain area for visual numerals. J. Neurosci 33, 6709–6715. 10.1523/JNEUROSCI.4558-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski HM, Fias W, Mousa A, Ansari D, 2017. Common and distinct brain regions in both parietal and frontal cortex support symbolic and nonsymbolic number processing in humans: A functional neuroimaging meta-analysis. NeuroImage 146, 376–394. 10.1016/j.neuroimage.2016.10.028 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD, 2009. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage 44, 489–501. 10.1016/j.neuroimage.2008.08.039 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD, 2012. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. NeuroImage 59, 1560–1570. 10.1016/j.neuroimage.2011.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G, 1980. A feature-integration theory of attention. Cognit. Psychol 12, 97–136. 10.1016/0010-0285(80)90005-5 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J, 2007. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos. Trans. R. Soc. B Biol. Sci 362, 837–855. 10.1098/rstb.2007.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Dehaene S, 2007. Number sense and developmental dyscalculia, in: Coch D, Dawson G, Fischer KW (Eds.), Human Behavior, Learning, and the Developing Brain: Atypical Development. The Guilford Press, New York; London, pp. 212–237. [Google Scholar]
- Wolfe JM, 1994. Guided Search 2.0 A revised model of visual search. Psychon. Bull. Rev 1, 202–238. 10.3758/BF03200774 [DOI] [PubMed] [Google Scholar]
- Woodcock R, McGrew KS, Mather N, 2001. Woodcock-Johnson-III Tests of Achievement. [Google Scholar]
- Yeo DJ, Wilkey ED, Price GR, 2017. The search for the number form area: A functional neuroimaging meta-analysis. Neurosci. Biobehav. Rev 78, 145–160. 10.1016/j.neubiorev.2017.04.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


